Eucalmaidials A and B, phloroglucinol-coupled sesquiterpenoids from the juvenile leaves of Eucalyptus maideni†
Abstract
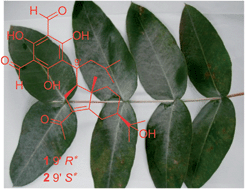
Two new phloroglucinol-coupled sesquiterpenoids, eucalmaidials A and B (1 and 2), were isolated from the juvenile leaves of Eucalyptus maideni, along with eight known macrocarpals (3–10), eucalyptone (11), and three known triterpenoids (12–14). Eucalmaidials A and B represent a new skeleton of phloroglucinol-coupled iphionane. Their structures were elucidated by extensive NMR spectroscopic analysis and theoretical calculation of the 13C NMR chemical shifts. The biosynthetic pathway of 1 and 2 was also postulated. Compounds 1, 3, 5, 7, 8, and 10–14 were evaluated for their antifungal and antibacterial activities. Compound 1 exhibited antifungal activity against Candida glabrata with an IC50 value of 0.75 μg mL−1.

 Please wait while we load your content...
Please wait while we load your content...