Cross-dehydrogenative C–O coupling of oximes with hydrazones: synthesis of fungicidal azo-oxime ethers
Alexander S.
Budnikov
 abc,
Mikhail I.
Shevchenko
abc,
Mikhail I.
Shevchenko
 a,
Igor B.
Krylov
a,
Igor B.
Krylov
 *ab,
Daniil Yu.
Pechen
*ab,
Daniil Yu.
Pechen
 ad,
Anna L.
Alekseenko
ab,
Alexey I.
Ilovaisky
ac and
Alexander O.
Terent'ev
ad,
Anna L.
Alekseenko
ab,
Alexey I.
Ilovaisky
ac and
Alexander O.
Terent'ev
 *ab
*ab
aN. D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences, 47 Leninsky prosp., 119991 Moscow, Russian Federation. E-mail: krylovigor@yandex.ru; terentev@ioc.ac.ru
bD. I. Mendeleev University of Chemical Technology of Russia, 9 Miusskaya Square, 125047 Moscow, Russian Federation
cAll-Russian Research Institute for Phytopathology, B. Vyazyomy, 143050 Moscow Region, Russian Federation
dM. V. Lomonosov Moscow State University, 1 Leninskie Gory, 119991 Moscow, Russian Federation
First published on 7th October 2025
Abstract
The cross-dehydrogenative coupling (CDC) of oximes with hydrazones employing KMnO4 as the oxidant was discovered. Presumably, the reaction proceeds through the selective cross-recombination of oxime and hydrazone-derived free radicals, despite the fact that both of them are known to undergo self-coupling and other processes with C–C, C–O, C–N, and N–N bond formation. The proposed approach is general and applicable to a broad range of oximes and hydrazones. Previously, oxime-derived radicals were mainly involved in intramolecular processes of C–O bond formation, while intermolecular processes remained rare and their scope was limited. Conversely, radical functionalization of hydrazones was generally limited to radical addition and hydrogen substitution reactions of aldehyde hydrazones. The formation of azo compounds in the present work represents a new direction for further development. The synthesized compounds exhibited pronounced fungicidal activity against a wide spectrum of phytopathogenic fungi (Venturia inaequalis, Rhizoctonia solani, Fusarium oxysporum, Fusarium moniliforme, Bipolaris sorokiniana, Sclerotinia sclerotiorum), in some cases surpassing the activity of commercial fungicides triadimefon, kresoxim-methyl, and azoxystrobin. Key factors contributing to the high fungicidal activity were identified as the presence of a small aliphatic substituent at the C–O coupling site and electron-withdrawing substituents in the oxime moiety.
Introduction
To date, cross-dehydrogenative coupling (CDC) has become a robust and versatile tool in modern organic chemistry, enabling the direct formation of C–C or C–heteroatom bonds by coupling two C–H or C–H/X–H partners, respectively, with hydrogen atoms as leaving groups.1–5 Related step-economical dehydrogenative strategies employing redox-active reducible6 or oxidizable7 leaving groups are being actively developed. This strategy eliminates the need for pre-functionalized substrates, providing a more efficient, atom- and step-economical route to complex molecules, including natural products, pharmaceuticals, and agrochemicals.8–10 Among the various CDC strategies developed over the past few decades for the construction of C–X bonds, oxidative C–O coupling stands out as one of the most challenging due to the tendency for competing side reactions, such as hydroxylation, fragmentation, and overoxidation of the starting substrates.11Imine-N-oxyl (oxime) radicals have been widely employed in intramolecular oxidative cyclization reactions of oximes with the functionalization of C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and C–O bond formation.12,13 However, selective intermolecular C–O coupling reactions involving oxime radicals are rare. To date, only a few examples of such transformations have been reported: the oxidative C–O coupling of oximes with dicarbonyl compounds, ketones, esters, acetonitrile, and pyrazolin-5-ones.14–18 These examples highlight the potential of oximes as O-coupling partners in free-radical CDC reactions but also the underexplored nature of this synthetic approach.
C bonds and C–O bond formation.12,13 However, selective intermolecular C–O coupling reactions involving oxime radicals are rare. To date, only a few examples of such transformations have been reported: the oxidative C–O coupling of oximes with dicarbonyl compounds, ketones, esters, acetonitrile, and pyrazolin-5-ones.14–18 These examples highlight the potential of oximes as O-coupling partners in free-radical CDC reactions but also the underexplored nature of this synthetic approach.
Hydrazones are widely utilized in materials science and supramolecular chemistry19–22 and represent common structural motifs in bioactive compounds and pharmaceuticals.23–25 In organic synthesis, hydrazones serve as versatile reagents and valuable intermediates26 for the synthesis of functionalized carbonyl compounds,27,28 amines,29,30 alkenes31 or nitrogen heterocycles.32–34 Over the last few decades, the free-radical chemistry of hydrazones has gained particular attention (Scheme 1A).35–37 However, to date, these transformations have been associated mainly with radical addition to aldehyde-derived hydrazones35,37–39 or oxidative cyclization of β,γ-unsaturated hydrazones.33,34,39 As a rule, these transformations occur with reduction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (amine formation) or without reduction (substituted hydrazone or heterocycle formation). Oxidative C–O coupling employing hydrazones as C-partners is less explored. There are several reports on the formation of unstable geminal azoperoxides, azoxyperoxides or azohydroperoxides by oxidation of hydrazones with a TBHP/Co-Salen complex40 or directly by molecular oxygen (Scheme 1B).41–45 Recently, our group discovered the free-radical C–O coupling of hydrazones with the stable diacetyliminoxyl radical, leading to the formation of azo-oxime ethers (Scheme 1B).46 However, the reported protocol was limited to the use of only the diacetyliminoxyl due to its increased stability and accessibility in an individual form, in contrast to the majority of other oxime radicals.12 The synthesized azo-oxime ethers were discovered as a novel class of highly active fungicides, but a practical approach for their synthesis was not proposed, which hindered their application potential in crop protection. In the present work (Scheme 1C), a universal, atom-efficient and scalable method for oxidative C–O CDC of oximes with hydrazones leading to azo-oxime ethers was proposed, and key structural patterns responsible for the fungicidal activity of the synthesized compounds were determined. Both oximes and hydrazones are known to produce the corresponding free radicals upon oxidation, which then undergo self-coupling reactions or overoxidation. Notably, hydrazone radicals are reported to undergo C–C, C–N, N–N homocoupling and further oxidation.47–51 Oxime radicals dimerize with the formation of C–O, O–N, and N–N bonds, typically producing unstable dimers that undergo further transformations.12,52 It should be noted that oximes and hydrazones are oxidized to the corresponding radicals under similar conditions.34 Despite the fact that oxime and hydrazone radicals are prone to self-coupling and other oxidation processes, the discovered transformation proceeds selectively, delivering oxidative C–O coupling products with good yields. This process is compatible with a wide range of oximes and hydrazones derived from both aldehydes and ketones. The use of inexpensive potassium permanganate—a green, non-toxic and widely available oxidant in both industrial and academic settings53–55—makes the proposed protocol promising for practical applications. This finding is particularly crucial for the gram-scale synthesis of highly active azo-oxime ethers, especially in the context of the growing prevalence of phytopathogenic fungal strains that have developed resistance to conventional synthetic fungicides.56–58
N bond (amine formation) or without reduction (substituted hydrazone or heterocycle formation). Oxidative C–O coupling employing hydrazones as C-partners is less explored. There are several reports on the formation of unstable geminal azoperoxides, azoxyperoxides or azohydroperoxides by oxidation of hydrazones with a TBHP/Co-Salen complex40 or directly by molecular oxygen (Scheme 1B).41–45 Recently, our group discovered the free-radical C–O coupling of hydrazones with the stable diacetyliminoxyl radical, leading to the formation of azo-oxime ethers (Scheme 1B).46 However, the reported protocol was limited to the use of only the diacetyliminoxyl due to its increased stability and accessibility in an individual form, in contrast to the majority of other oxime radicals.12 The synthesized azo-oxime ethers were discovered as a novel class of highly active fungicides, but a practical approach for their synthesis was not proposed, which hindered their application potential in crop protection. In the present work (Scheme 1C), a universal, atom-efficient and scalable method for oxidative C–O CDC of oximes with hydrazones leading to azo-oxime ethers was proposed, and key structural patterns responsible for the fungicidal activity of the synthesized compounds were determined. Both oximes and hydrazones are known to produce the corresponding free radicals upon oxidation, which then undergo self-coupling reactions or overoxidation. Notably, hydrazone radicals are reported to undergo C–C, C–N, N–N homocoupling and further oxidation.47–51 Oxime radicals dimerize with the formation of C–O, O–N, and N–N bonds, typically producing unstable dimers that undergo further transformations.12,52 It should be noted that oximes and hydrazones are oxidized to the corresponding radicals under similar conditions.34 Despite the fact that oxime and hydrazone radicals are prone to self-coupling and other oxidation processes, the discovered transformation proceeds selectively, delivering oxidative C–O coupling products with good yields. This process is compatible with a wide range of oximes and hydrazones derived from both aldehydes and ketones. The use of inexpensive potassium permanganate—a green, non-toxic and widely available oxidant in both industrial and academic settings53–55—makes the proposed protocol promising for practical applications. This finding is particularly crucial for the gram-scale synthesis of highly active azo-oxime ethers, especially in the context of the growing prevalence of phytopathogenic fungal strains that have developed resistance to conventional synthetic fungicides.56–58
Results and discussion
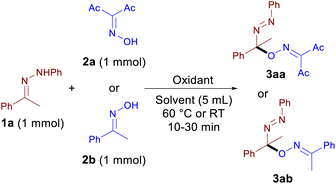
With acetophenone N-phenylhydrazone 1a and diacetyl oxime 2a or acetophenone oxime 2b as the model substrates, the influence of the reaction parameters on the yield of azo-oxime ethers 3aa and 3ab was evaluated (Table 1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Run | Oxidant | Solvent | T, °C | Time, min | Yieldb (%) |
|---|---|---|---|---|---|
| a General reaction conditions: oxidant (63–1096 mg, 0.4–2 mmol) was added to a stirred mixture of acetophenone phenylhydrazone 1a (210 mg, 1 mmol), oxime 2a (129 mg, 1 mmol) or 2b (135 mg, 1 mmol), in solvent (5 mL) at RT (23–25 °C) or 60 °C; stirring was continued at the same temperature for 10–30 min. Stoichiometric amounts of oxidant were used: 2 mmol for one-electron oxidants (Fe(ClO4)3·nH2O, Mn(OAc)3·2H2O, CAN), 1 mmol for two-electron oxidants (Pb(OAc)4, PhI(OAc)2, MnO2), and 0.4 mmol for five-electron oxidants (KMnO4). b Yields were determined by 1H NMR using 1,1,2,2-tetrachloroethane as an internal standard. Isolated yields are given in parentheses. c 1.6 mmol of Mn(OAc)2·4H2O was used. d A solution of CAN in water (2.5 mL) was added dropwise with vigorous stirring for 1–2 min. | |||||
| Oxidative C–O coupling of 1a with diacetyl oxime 2a | |||||
| 1 | Pb(OAc)4 | CH2Cl2 | RT | 30 | 68 |
| 2 | PhI(OAc)2 | CH2Cl2 | RT | 30 | 65 |
| 3 | Fe(ClO4)3·nH2O | MeCN | 60 | 10 | <5 |
| 4 | Mn(OAc)3·2H2O | AcOH | 60 | 10 | 90 |
| 5 | Mn(OAc)3·2H2O | MeCN | 60 | 10 | 95 |
| 6c | Mn(OAc)2·4H2O, KMnO4 | AcOH | 60 | 10 | 75 |
| 7 | KMnO4 | AcOH | 60 | 10 | 85 |
| 8 | KMnO4 | MeCN | 60 | 10 | 19 |
| 9 | KMnO4 | MeCN/AcOH = 19/1 | 60 | 10 | 95 (88) |
| 10 | MnO2 | AcOH | 60 | 10 | 15 |
| 11d | CAN | Acetone | RT | 30 | 59 |
| Oxidative C–O coupling of 1a with acetophenone oxime 2b | |||||
| 12 | Pb(OAc)4 | CH2Cl2 | RT | 30 | 7 |
| 13 | PhI(OAc)2 | CH2Cl2 | RT | 30 | 50 |
| 14 | Fe(ClO4)3·nH2O | MeCN | 60 | 10 | n.d. |
| 15 | Mn(OAc)3·2H2O | AcOH | 60 | 10 | 14 |
| 16 | Mn(OAc)3·2H2O | MeCN | 60 | 10 | 74 |
| 17c | Mn(OAc)2·4H2O, KMnO4 | AcOH | 60 | 10 | 22 |
| 18 | KMnO4 | AcOH | 60 | 10 | 40 |
| 19 | KMnO4 | MeCN | 60 | 10 | 17 |
| 20 | KMnO4 | MeCN/AcOH = 19/1 | 60 | 10 | 75 (63) |
| 21 | MnO2 | AcOH | 60 | 10 | <5 |
| 22d | CAN | Acetone | RT | 30 | 19 |
The oxidative C–O coupling of diacetyl oxime 2a with acetophenone phenylhydrazone 1a proceeds in good to high yields with both single-electron (entries 3–5 and 11) and two- or more electron oxidants (entries 1, 2 and 4–10). The choice of oxidants for screening was based on previous literature reports regarding the CDC reactions of oximes.14–18 Lead(IV) acetate, which was previously used for the quantitative generation of the diacetyliminoxyl radical,16,18,46 afforded the desired product 3aa in a 68% yield (entry 1). The discovered transformation can also be performed under metal-free conditions using (diacetoxyiodo)benzene (PIDA) as the oxidant (entry 2, 65%). However, low yields were obtained when employing iron(III) perchlorate (entry 3) or MnO2 (entry 10) as oxidants, presumably due to rapid oxidation of the starting hydrazone 1a. In contrast, Mn(OAc)3 afforded high yields in both acetic acid and acetonitrile (entries 4 and 5). Notably, the transformation proceeded efficiently with inexpensive KMnO4, achieving yields up to 95% (entries 6 and 7). However, KMnO4 provided high yields only when acetic acid was present (entries 7 and 9), whereas a low yield of 3aa was observed in acetonitrile (entry 8). This demonstrates the requirement of acetate ions for successful oxidation (see below). Interestingly, (NH4)2Ce(NO3)6 (CAN) provided a moderately good yield despite previous reports indicating instability of the diacetyliminoxyl radical (derived from oxime 2a) in the presence of CAN.14
Next, we sought to achieve similar reaction efficiency in the more challenging case of acetophenone oxime 2b, whose oxime radical is far less stable than the diacetyliminoxyl radical. Indeed, employing oxime 2b instead of 2a resulted in generally lower yields of 3ab compared with 3aa (entries 12–22). Low yields were observed not only with iron(III) perchlorate (entry 14), MnO2 (entry 21), and CAN (entry 22), but even for lead tetraacetate (entry 12). Employing PIDA as an oxidant (entry 13) afforded 3ab in 50% yield. Good yields were obtained with Mn(OAc)3 in acetonitrile (entry 16, 74%) but not in acetic acid (entry 15, 14%). The yield of 3ab did not exceed 40% when KMnO4 was used alone (entries 18 and 19) or in combination with Mn(OAc)2 (entry 17). Similar to the case of 3aa (entries 7–9), successful oxidation with KMnO4 requires the presence of acetate ions (entries 17 and 18). To our delight, after careful screening of the acetic acid quantity (see Table S1 in the SI for the complete details), we found that the reaction proceeds efficiently in a MeCN/AcOH (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture, providing good yields (entry 20, 75% by NMR and 63% isolated yield).
1) mixture, providing good yields (entry 20, 75% by NMR and 63% isolated yield).
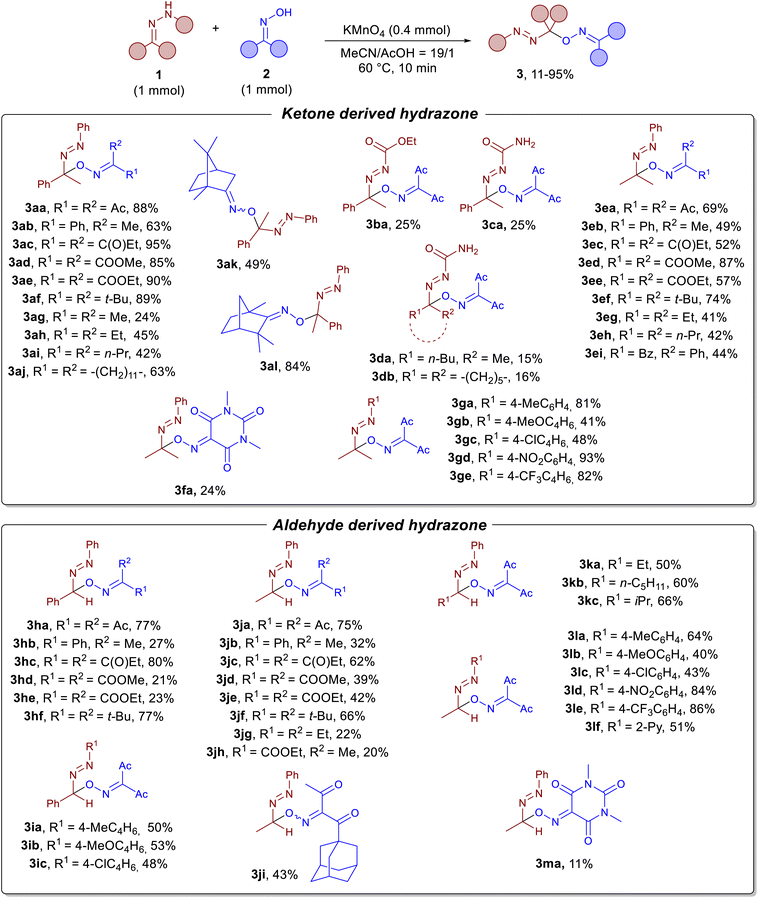
With the optimal conditions in hand (Table 1, entries 9 and 20), we tested the scope of the discovered cross-dehydrogenative C–O coupling (Scheme 2).
The discovered oxidative C–O coupling was applicable to a wide range of hydrazones derived from aromatic and aliphatic ketones (products 3aa–3al, 3ea–3ge) and aldehydes (products 3ha–3ma), yields ranged from 11 to 95%. It is noteworthy that the reaction also proceeds with N-alkoxycarbonyl hydrazones (product 3ba, 25%) and semicarbazones (products 3ca–3db, 15–25%), albeit in lower yields compared to arylhydrazones. Various oximes derived from dicarbonyl compounds or aromatic and aliphatic ketones were well tolerated in this transformation. In general, for the series of oximes, the yield correlates with the stability of the corresponding oxime radicals. Therefore, the highest yields were obtained for diacetyl oxime (products 3aa, 3ea, 3ga–ge, 3ha, 3ia–3ic, 3ja, 3ka–3kc, 3la–3lf), dipropionyl oxime (products 3ac, 3ec, 3hc, 3jc) and di-tert-butyl oxime (3af, 3ef, 3hf, 3jf). The developed protocol is applicable to oximes derived from natural products, including camphor (product 3ak, 49%) and fenchone (product 3al, 84%). For acetone (products 3ga–3ge) and acetaldehyde (products 3la–3lf) hydrazones, we additionally investigated the substitution pattern in the benzene ring at the hydrazone nitrogen atom due to the high fungicidal activity of these compounds.46 In general, higher yields were obtained for hydrazones containing electron-withdrawing substituents on the benzene rings (products 3gd, 3ge, 3ld, and 3le) compared to those with electron-donating ones (products 3gb, and 3la–3lc). Lower yields for hydrazones with electron-donating substituents can be explained by their overoxidation: for optimal C–O coupling, both oxime and hydrazone should be oxidized simultaneously. A 2-pyrdiyl moiety at the nitrogen atom of hydrazone 1l was also tolerated (product 3lf, 51%). It should be noted that the C–H bond of the aldehyde-derived hydrazones remains intact in all studied cases, despite the presence of hydrogen-abstracting species in the reaction medium. For example, N-centered radicals are known to undergo HAT from aldehydes.59,60 DFT calculations on the example of acetaldehyde and its hydrazone (see SI for details, Fig. S1) showed that the C–H bond in the hydrazone is even stronger than that in the aldehyde, whereas the N–H bond in the hydrazone is the easiest to cleave. The final products of the oxidative C–O coupling of oximes with aldehyde-derived hydrazones were shown to be stable to further oxidation under the reaction conditions (see section 2.8 in the SI), despite the presence of the C–H bond adjacent to the oxime and azo fragments. This result is in agreement with our DFT calculations of activation free energy for HAT between the final azo compound and the diacetyliminoxyl, which is as high as 36.7 kcal mol−1 for product 3ha according to the ωB97X-3c61/CPCM(MeCN) level of theory. At the same time, HAT between starting hydrazone 3h and diacetyliminoxyl is predicted to be barrierless (see Fig. S2 in the SI).
The synthetic utility of the discovered C–O CDC was shown by a 40 mmol scale synthesis of 3ja (Scheme 3, eqn (1)). The corresponding product 3ja was obtained in 72% yield (7.83 g, 30 mmol). Additionally, the carbonyl groups in product 3aa can be reduced to hydroxyl groups with the formation of 4 employing sodium borohydride in methanol (Scheme 3, eqn (2)).
To gain insight into the reaction mechanism, control experiments were conducted (Scheme 4). The reaction was significantly inhibited by the addition of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT), and the C–O coupling product 3ja was isolated in 11% and 36% yield, respectively (Scheme 4, eqn (1) and (2)). TEMPO-adduct 5 derived from interception of the C-radical generated from hydrazone was isolated in 60% yield and characterized by 1H and 13C NMR, IR and HRMS. A BHT-adduct with hydrazone was detected by HRMS. Additionally, the C–O coupling product of diacetyl oxime with BHT 6 was isolated in 13% yield.62
N,N-Diphenylhydrazone 8 containing no N–H bond for the formation of a hydrazone radical was introduced into the reaction with diacetyl oxime 2a under the standard reaction conditions (Scheme 4, eqn (3)). No C–O coupling product was observed by 1H NMR analysis of the crude reaction mixture. Thus, hydrogen atom abstraction from the nitrogen atom of hydrazone 1 is possibly the crucial step, rather than the addition of an oxime radical at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond of the hydrazone. To elucidate the formation of radicals upon hydrazone oxidation, experiments in the absence of an oxime as a C–O coupling partner were conducted (Scheme 4, eqn (4a) and (b)). HRMS analysis of the crude reaction mixture revealed the formation of dimers 9a and 9b, derived from 1e and 1j, respectively. The formation of oxime radicals upon oxidation of oximes with KMnO4 was shown earlier.14,16 To sum up, the collected results indicate that the discovered C–O coupling may take place via the recombination of radicals derived from oximes and hydrazones.
N double bond of the hydrazone. To elucidate the formation of radicals upon hydrazone oxidation, experiments in the absence of an oxime as a C–O coupling partner were conducted (Scheme 4, eqn (4a) and (b)). HRMS analysis of the crude reaction mixture revealed the formation of dimers 9a and 9b, derived from 1e and 1j, respectively. The formation of oxime radicals upon oxidation of oximes with KMnO4 was shown earlier.14,16 To sum up, the collected results indicate that the discovered C–O coupling may take place via the recombination of radicals derived from oximes and hydrazones.
On the basis of control experiments, and our previous study on diacetyliminoxyl, including DFT calculations,46 a plausible reaction pathway is depicted in Scheme 5. The reaction proceeds through the oxidation of hydrazones 1 and oximes 2 by Mnn+ species to radicals A and B, respectively. We speculate that the oxidation of hydrazone 1 can occur either by hydrogen atom abstraction by oxime radical B or by direct oxidation with Mnn+ species. The possibility of hydrogen atom transfer (HAT) depends on the structures of the oximes and hydrazones. The thermodynamic driving force for this process can be quantitatively characterized by the difference between the bond dissociation enthalpies (BDEs) in an oxime and a hydrazone. We estimated the O–H and N–H BDEs for oximes and hydrazones from the reaction scope in the present study using the ALPHABET machine learning method63 and DFT calculations61,64,65 (see SI for details). Based on these results, the OH BDE in oximes is ca. 75–85 kcal mol−1 and the NH BDE in hydrazones is ca. 80–95 kcal mol−1, which corresponds to a very large variation in the reactivity of the corresponding starting materials and intermediate radicals. At least for some combinations, the HAT process between iminoxyl radical A and hydrazone 2 is thermodynamically unfavored. In the final step, radical cross-recombination of hydrazonyl A and oxime radical B delivers the oxidative C–O coupling product 3.
In vitro fungicidal activity of the synthesized azo compounds
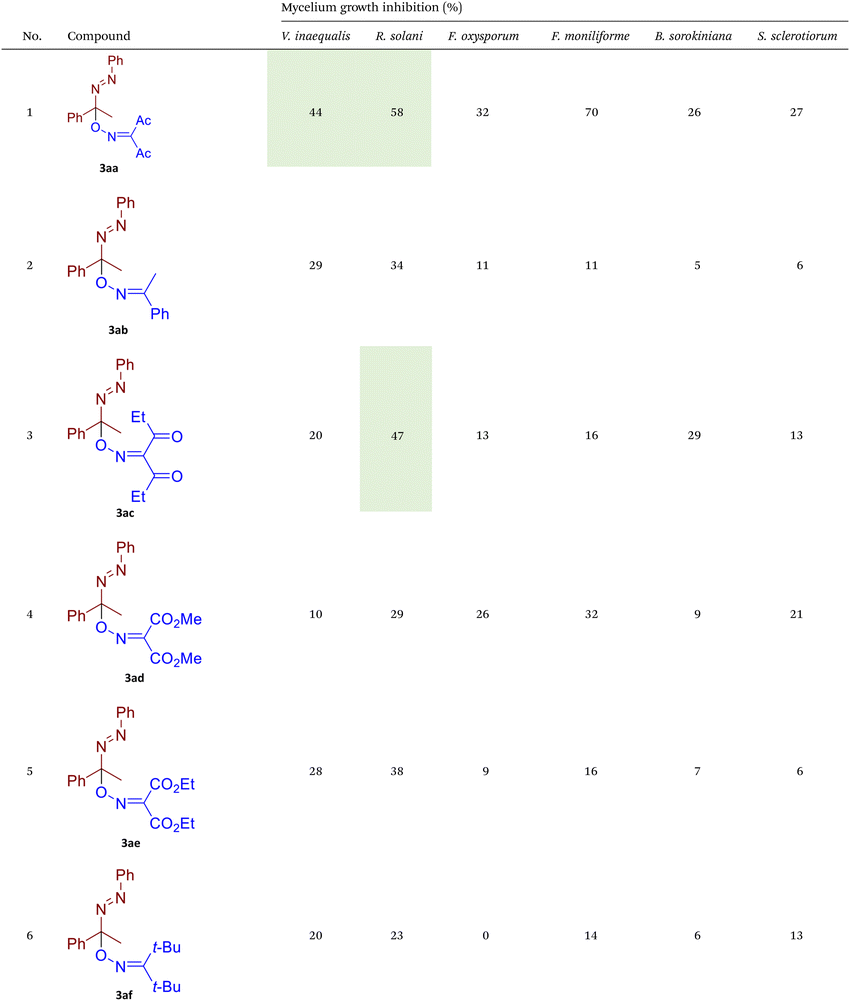
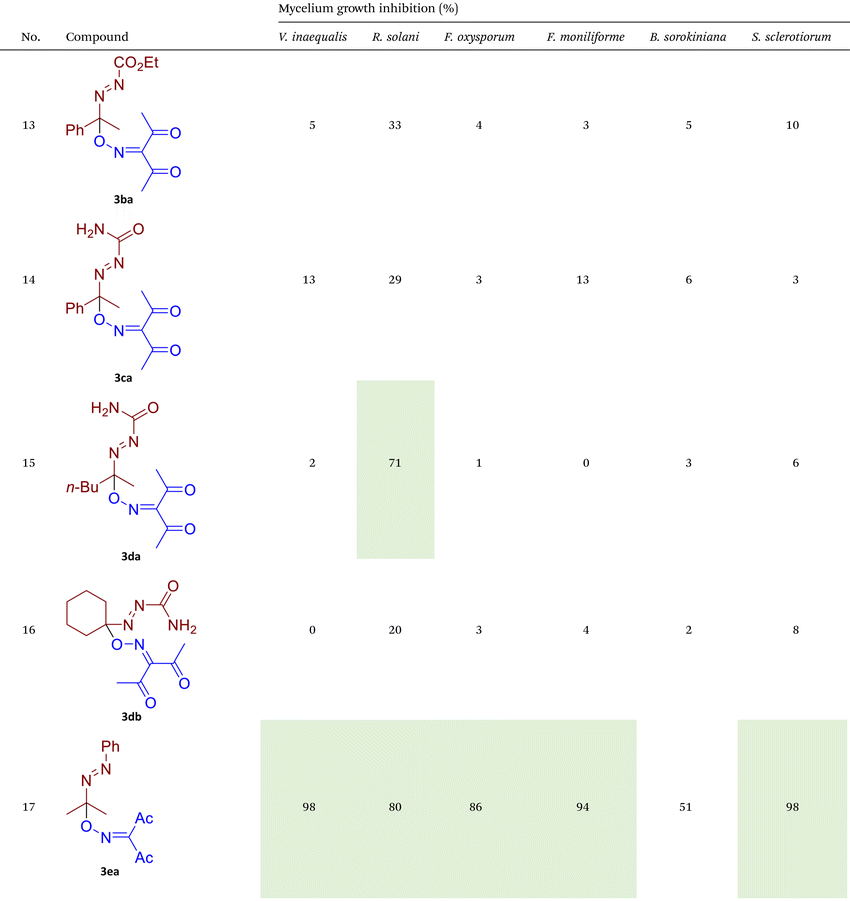
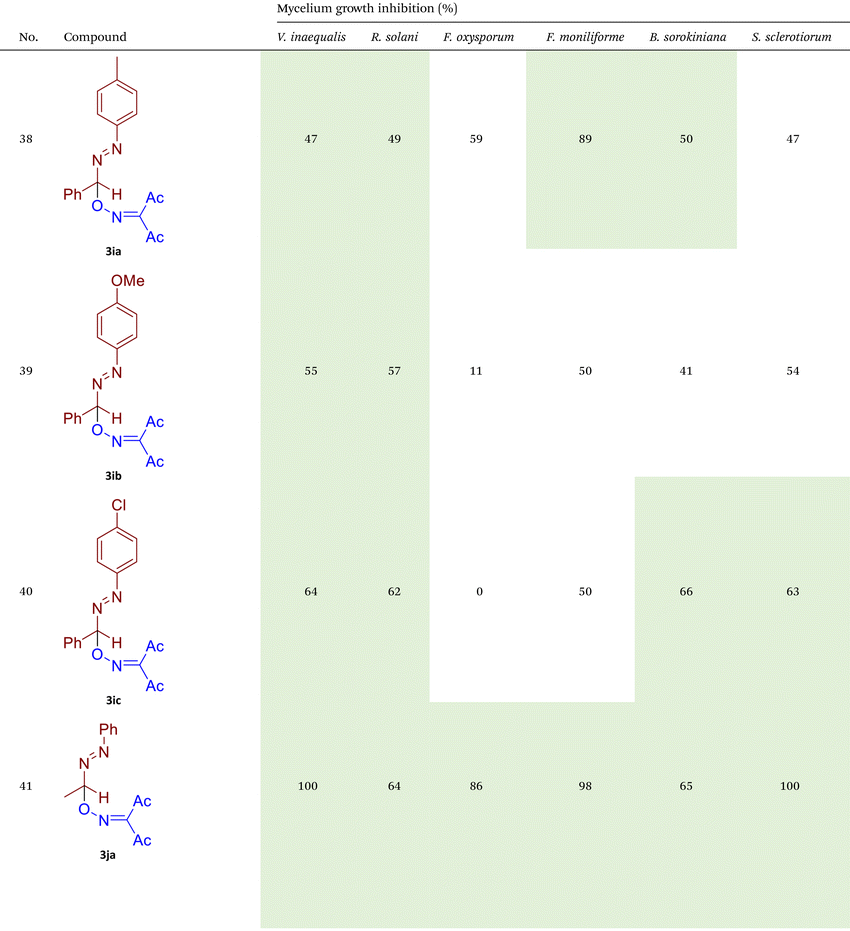
Phytopathogenic fungi currently represent one of the most serious challenges to global crop production and public health.66–69 Fungicides remain the most effective tool for protecting agricultural crops from fungal infections and preventing poisoning by mycotoxins.70–72 However, the majority of commercially available fungicides belong to a narrow range of chemical classes and exhibit similar modes of action. This has resulted in the widespread emergence of resistance to these established fungicidal compounds, both in agricultural and medical areas.56–58 Consequently, the identification and development of novel fungicidal agents with unique mechanisms of action represent a critical objective in scientific research.73–76 In the second part of our study, the synthesized azo-oxime ethers 3 were tested for fungicidal activity against six phytopathogenic fungi from different taxonomic classes that pose a high threat to agricultural production (Table 2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
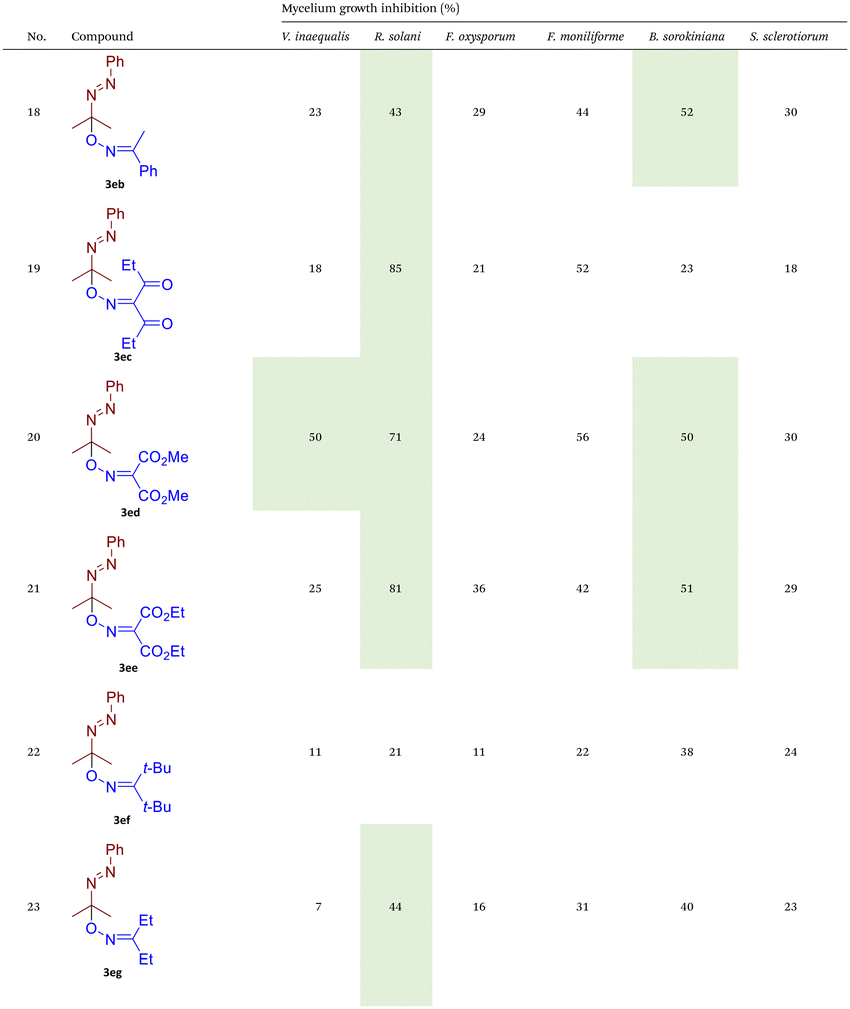
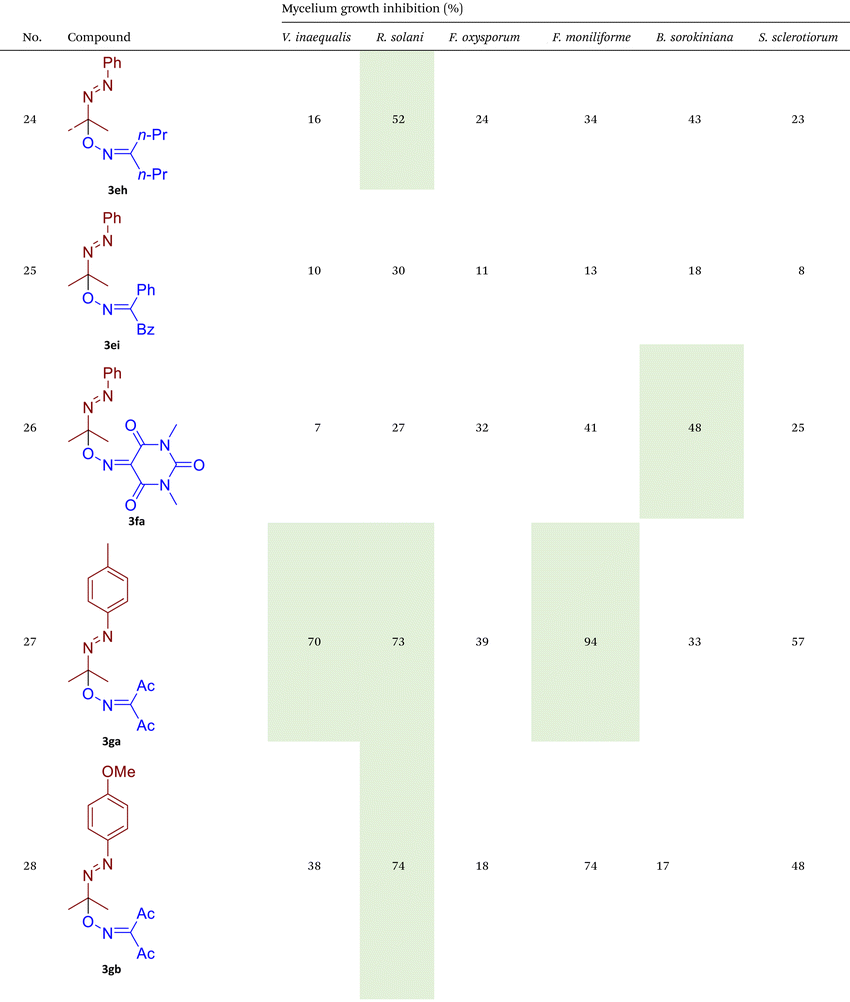
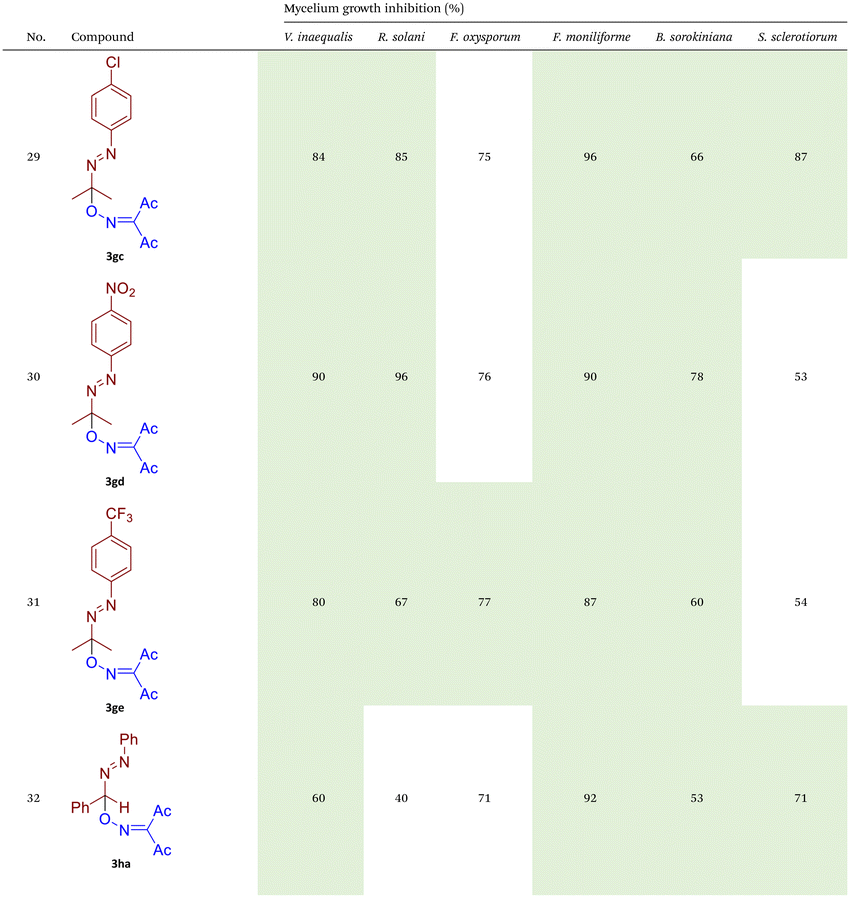
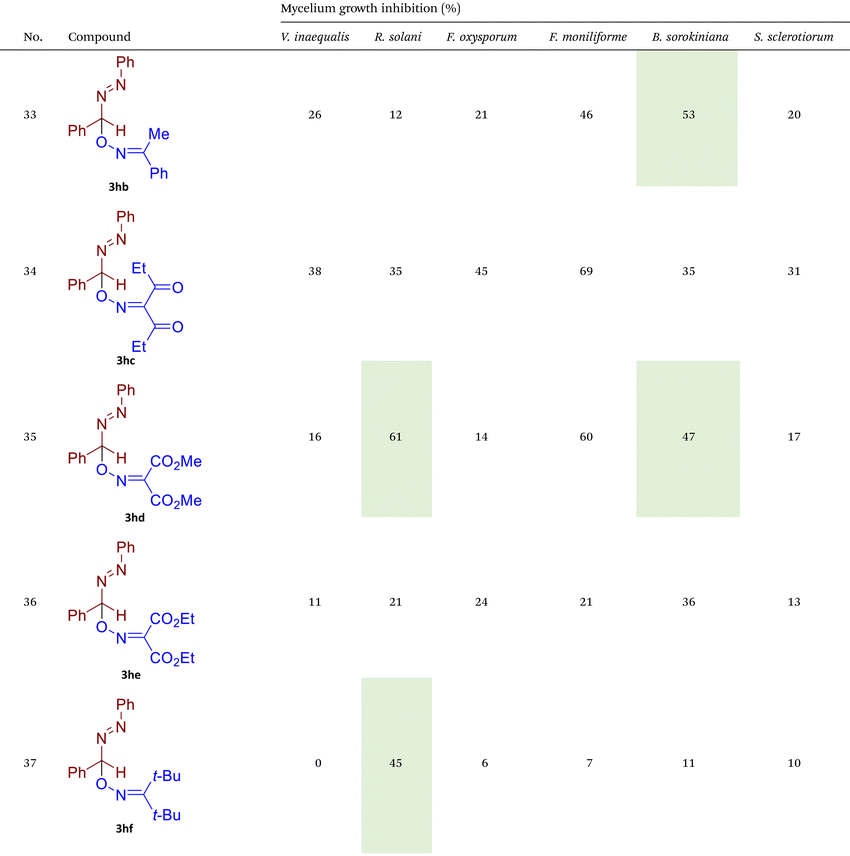
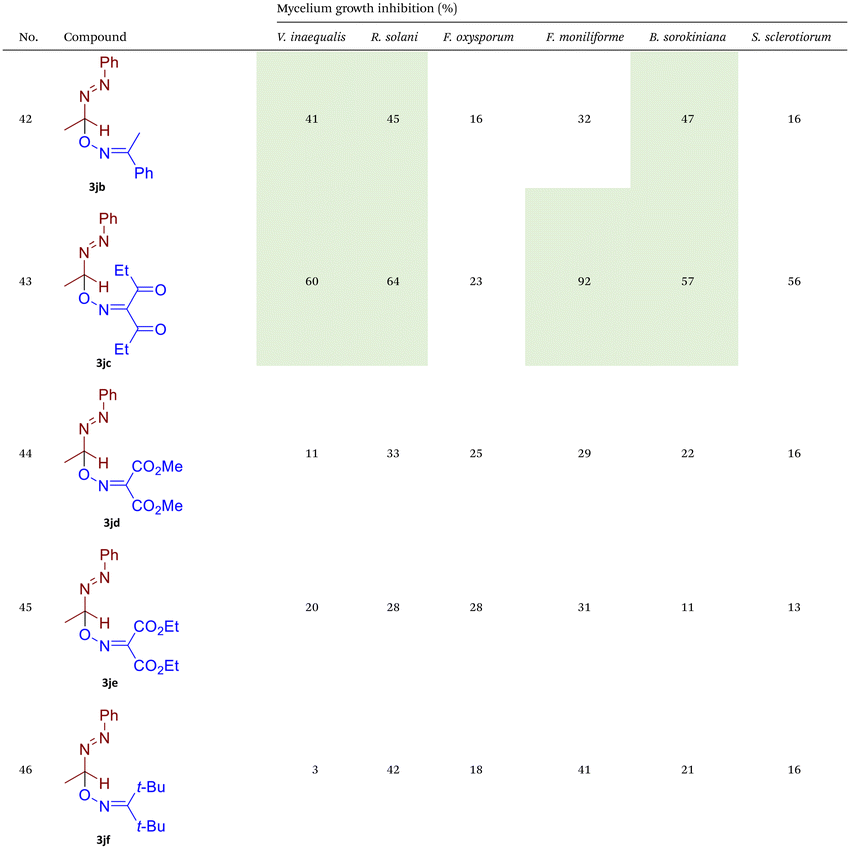
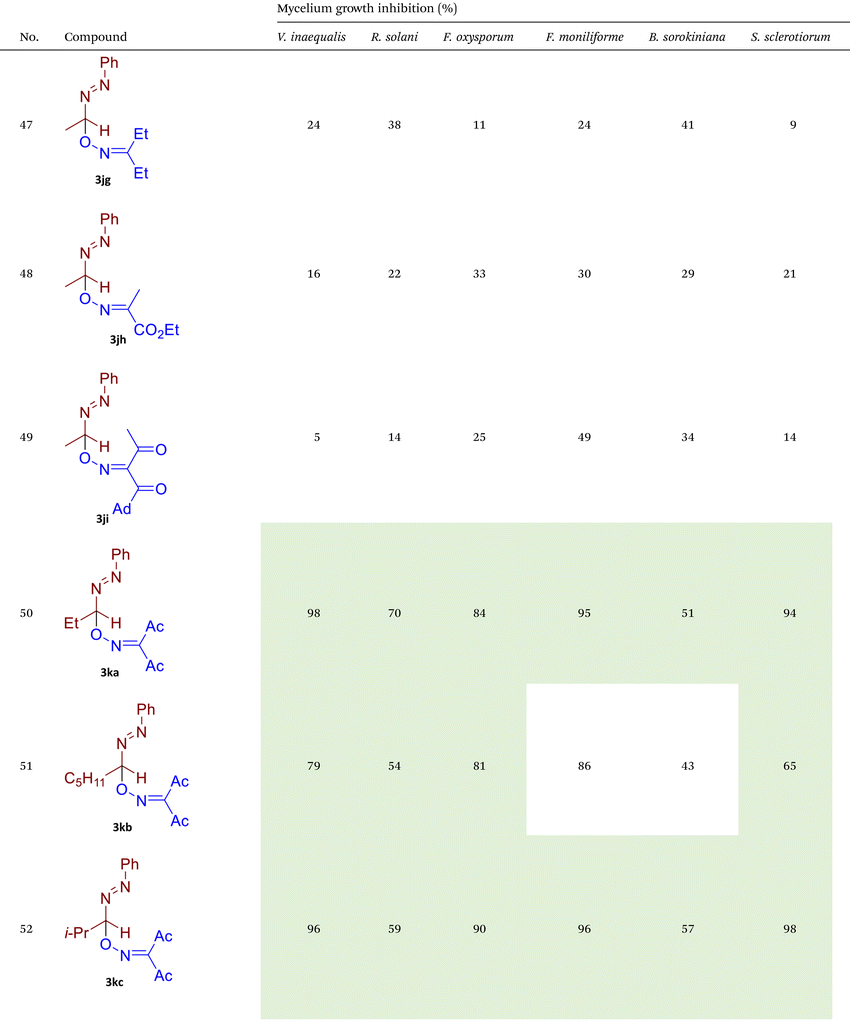
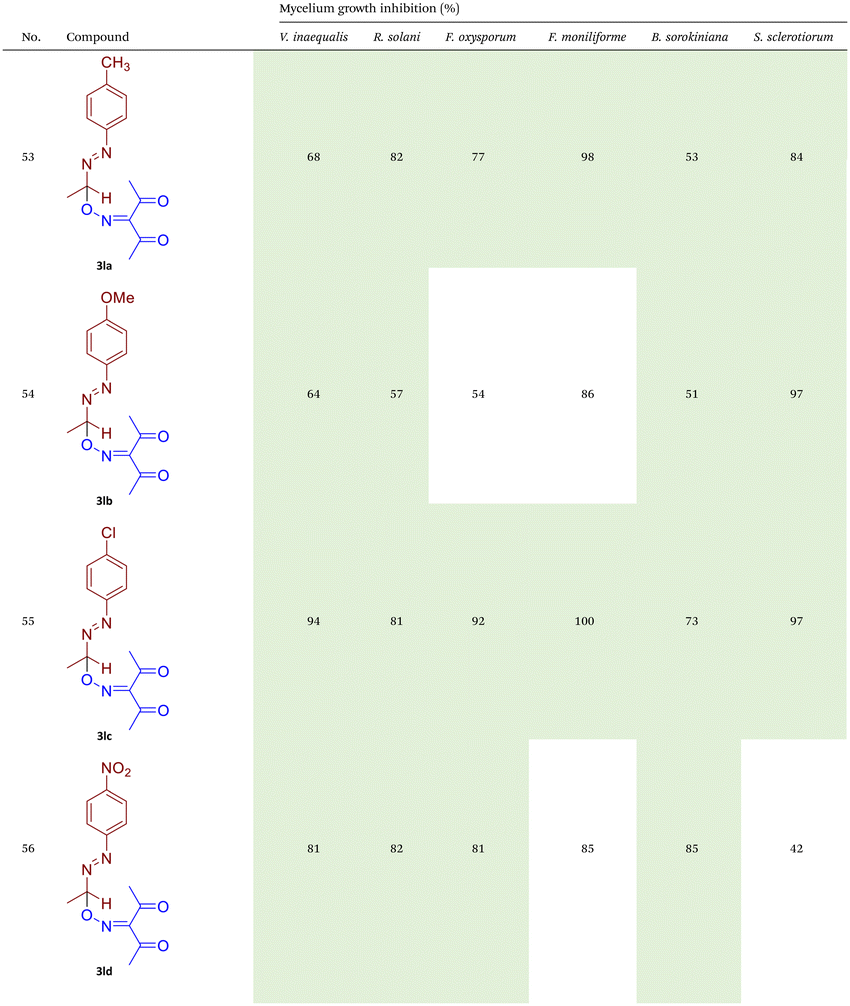
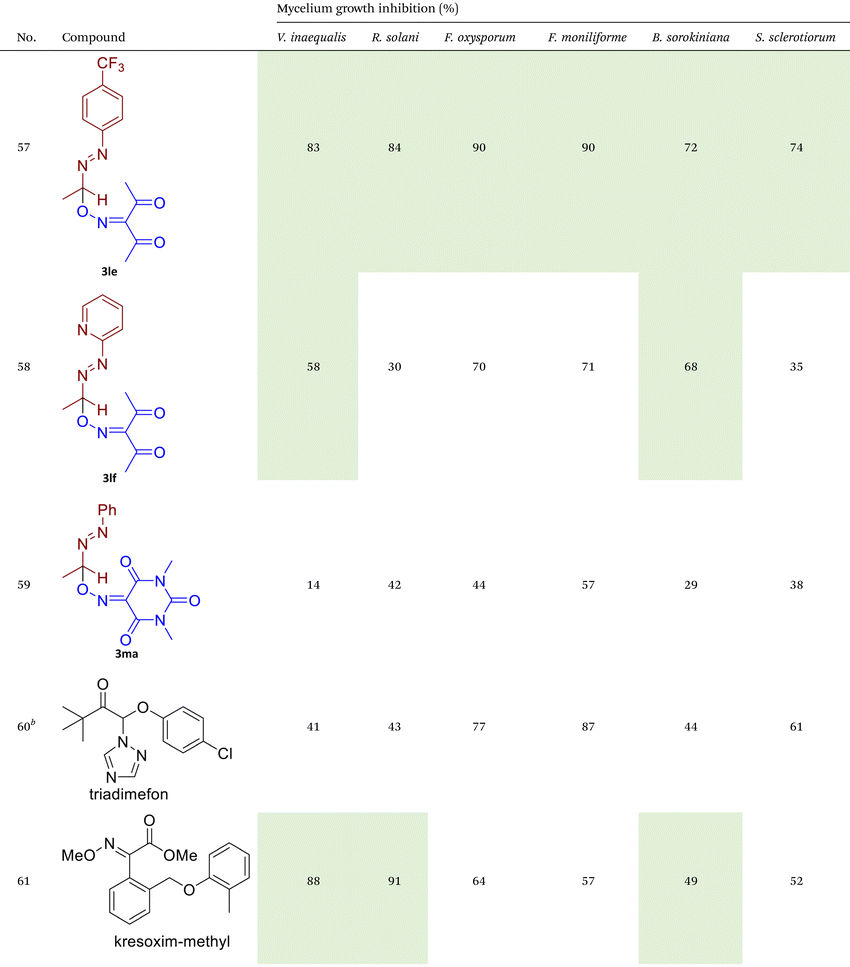
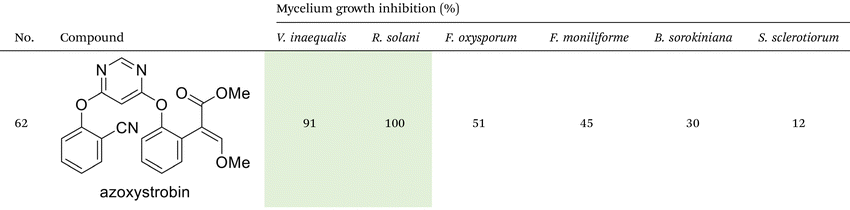
As illustrated in Table 2, compounds 3gc, 3ge, 3ja, 3ka, 3kc, and 3lc demonstrate the highest activity against the tested phytopathogenic fungi. In general, azo-oxime ethers featuring relatively small aliphatic substituents at the C–O coupling site (3ea–3ei and 3jb–3jI, 3ka–3kc) exhibit greater activity compared to those with aromatic substituents at the same position (3aa–3al and 3ha–3hf, 3ia–3ic). Furthermore, aldehyde-derived azo-oxime ethers (3ha–3hf, 3ia–3ic and 3jb–3jI, 3ka–3kc) generally show superior activity relative to their ketone-derived counterparts (3aa–3al and 3ea–3ei). Variation in the oxime fragment revealed that acyclic compounds with electron-withdrawing substituents at the oxime moiety generally exhibit the highest activity. Specifically, azo-ethers synthesized from oximes derived from 1,3-diketones (3aa, 3ac, 3ea, 3ec, 3ga–3ge, 3ha, 3hc, 3ia–3ic, 3ja, 3jc, 3ka–3kc, 3la–3lf) and malonic esters (3ad, 3ae, 3ed, 3ee, 3hd, 3he, 3jd, 3je) demonstrated higher activity than those derived from either aliphatic or aromatic oximes (3ab, 3af–3aj, 3eb, 3ef–3ei, 3hb, 3hf, 3jb, 3jf, 3jg). In all cases, the products synthesized from diacetyl oxime (3aa, 3ea, 3ga–3ge, 3ha, 3ia–3ic, 3ja, 3ka–3kc, 3la–3lf) showed the greatest activity among the other oximes. Azo-compounds derived from N-alkoxycarbonyl hydrazones (3ba), and semicarbazones (3ca–3db) did not show significant activity. Similarly, azo-ethers obtained from fenchone (3ak), camphor oximes (3al), and N,N′-dimethylbarbituric acid oxime (3fa and 3ma) showed negligible activity. For azo-oxime ethers derived from acetone (3ea) and acetaldehyde (3ja) hydrazones, the introduction of substituents into the benzene ring resulted in a slight reduction in activity compared to their unsubstituted derivatives (3ga–3ge and 3la–3le). This effect was particularly pronounced in the case of electron-donating substituents, such as methyl (3ga and, 3la) or methoxy (3gb and 3lb) groups. Additionally, replacing the phenyl substituent with a 2-pyridyl group (3lf) led to a significant decrease in fungicidal activity. The activity of azo-oxime ethers 3ea, 3gc–ge, 3ja, 3ka–kc, 3la, 3lc, and 3le is similar to or higher than that of the widely employed commercial fungicides triadimefon, kresoxim-methyl, and azoxystrobin, which are used in crop protection.
Conclusions
In summary, we discovered cross-dehydrogenative C–O coupling between oximes and hydrazones. The proposed protocol is scalable, employs cost-effective potassium permanganate as an oxidant, and is applicable to a broad range of oximes and keto- or aldehyde-derived hydrazones. The synthesized azo-oxime ethers exhibit fungicidal activity comparable to or superior to that of commercially available fungicides. Key structural motifs contributing to enhanced fungicidal activity were identified, including electron-withdrawing substituents at the oxime moiety and small aliphatic groups at the carbon atom and a phenyl group at the nitrogen atom of the hydrazone residue. The present methodology provides a powerful and sustainable approach for the large-scale synthesis of azo-oxime ethers, offering significant potential for their application in crop protection.Author contributions
I. B. and A. S.: conceived the project; A. S., M. I. and D. Y.: carried out the synthetic experiments and analysed the experimental data; A. S. and I. B.: performed the manuscript writing with input from the other co-authors; A. L.: performed fungicidal activity tests; A. I.: performed proofreading, and A. O.: supervised the project.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details, compound characterization, computational details and NMR spectra. See DOI: https://doi.org/10.1039/d5qo01151e.Additional references are cited in the SI.77–96
Acknowledgements
This work was supported by the Russian Science Foundation (Grant 19-73-20190).References
- H. Diao, Y. Chen and F. Liu, Iron-Catalyzed Cross-Dehydrogenative Coupling, Molecules, 2025, 30, 250 CrossRef CAS.
- A. M. F. Phillips and A. J. L. Pombeiro, Recent Developments in Transition Metal–Catalyzed Cross–Dehydrogenative Coupling Reactions of Ethers and Thioethers, ChemCatChem, 2018, 10, 3354–3383 CrossRef.
- T. Tian, Z. Li and C.-J. Li, Cross-dehydrogenative coupling: a sustainable reaction for C–C bond formations, Green Chem., 2021, 23, 6789–6862 RSC.
- H. Wang, X. Gao, Z. Lv, T. Abdelilah and A. Lei, Recent Advances in Oxidative R1-H/R2-H Cross-Coupling with Hydrogen Evolution via Photo-/Electrochemistry: Focus Review, Chem. Rev., 2019, 119, 6769–6787 CrossRef CAS PubMed.
- W.-T. Ouyang, J. Jiang, Y.-F. Jiang, T. Li, Y.-Y. Liu, H.-T. Ji, L.-J. Ou and W.-M. He, Sono-photocatalytic amination of quinoxalin-2(1H)-ones with aliphatic amines, Chin. Chem. Lett., 2024, 35, 110038 CrossRef CAS.
- H. Xu, X. Li, J. Ma, J. Zuo, X. Song, J. Lv and D. Yang, An electron donor–acceptor photoactivation strategy for the synthesis of S-aryl dithiocarbamates using thianthrenium salts under mild aqueous micellar conditions, Chin. Chem. Lett., 2023, 34, 108403 CrossRef CAS.
- H.-Y. Song, J. Jiang, C. Wu, J.-C. Hou, Y.-H. Lu, K.-L. Wang, T.-B. Yang and W.-M. He, Semi-heterogeneous g-C3N4/NaI dual catalytic C–C bond formation under visible light, Green Chem., 2023, 25, 3292–3296 RSC.
- J. Xu, L.-D. Shao, D. Li, X. Deng, Y.-C. Liu, Q.-S. Zhao and C. Xia, Construction of Tetracyclic 3-Spirooxindole through Cross-Dehydrogenation of Pyridinium: Applications in Facile Synthesis of (±)-Corynoxine and (±)-Corynoxine B, J. Am. Chem. Soc., 2014, 136, 17962–17965 CrossRef CAS PubMed.
- Z. Wu and H. Xu, Synthesis of C3−Fluorinated Oxindoles through Reagent–Free Cross–Dehydrogenative Coupling, Angew. Chem., Int. Ed., 2017, 56, 4734–4738 CrossRef CAS.
- D. Srimani, Y. Ben-David and D. Milstein, Direct Synthesis of Pyrroles by Dehydrogenative Coupling of β–Aminoalcohols with Secondary Alcohols Catalyzed by Ruthenium Pincer Complexes, Angew. Chem., Int. Ed., 2013, 52, 4012–4015 CrossRef CAS.
- I. B. Krylov, V. A. Vil’ and A. O. Terent'ev, Cross-dehydrogenative coupling for the intermolecular C–O bond formation, Beilstein J. Org. Chem., 2015, 11, 92–146 CrossRef PubMed.
- I. B. Krylov, S. A. Paveliev, A. S. Budnikov and A. O. Terent'ev, Oxime radicals: generation, properties and application in organic synthesis, Beilstein J. Org. Chem., 2020, 16, 1234–1276 CrossRef CAS PubMed.
- J. Liao, L. Ouyang, Q. Jin, J. Zhang and R. Luo, Recent advances in the oxime-participating synthesis of isoxazolines, Org. Biomol. Chem., 2020, 18, 4709–4716 RSC.
- I. B. Krylov, A. O. Terent'ev, V. P. Timofeev, B. N. Shelimov, R. A. Novikov, V. M. Merkulova and G. I. Nikishin, Iminoxyl Radical–Based Strategy for Intermolecular C-O Bond Formation: Cross–Dehydrogenative Coupling of 1,3−Dicarbonyl Compounds with Oximes, Adv. Synth. Catal., 2014, 356, 2266–2280 CrossRef CAS.
- I. B. Krylov, S. A. Paveliev, N. S. Shumakova, M. A. Syroeshkin, B. N. Shelimov, G. I. Nikishin and A. O. Terent'ev, Iminoxyl radicals vs. tert-butylperoxyl radical in competitive oxidative C–O coupling with β-dicarbonyl compounds. Oxime ether formation prevails over Kharasch peroxidation, RSC Adv., 2018, 8, 5670–5677 RSC.
- I. B. Krylov, S. A. Paveliev, A. S. Budnikov, O. O. Segida, V. M. Merkulova, V. A. Vil’, G. I. Nikishin and A. O. Terent'ev, Hidden Reactivity of Barbituric and Meldrum's Acids: Atom-Efficient Free-Radical C–O Coupling with N-Hydroxy Compounds, Synthesis, 2022, 54, 506–516 CrossRef CAS.
- Z. Chen, H. Liang, R. Chen, L. Chen, X. Tang, M. Yan and X. Zhang, Cross–Dehydrogenative C−O Coupling of Oximes with Acetonitrile, Ketones and Esters, Adv. Synth. Catal., 2019, 361, 3324–3330 CrossRef CAS.
- I. B. Krylov, S. A. Paveliev, B. N. Shelimov, B. V. Lokshin, I. A. Garbuzova, V. A. Tafeenko, V. V. Chernyshev, A. S. Budnikov, G. I. Nikishin and A. O. Terent'ev, Selective cross-dehydrogenative C–O coupling of N-hydroxy compounds with pyrazolones. Introduction of the diacetyliminoxyl radical into the practice of organic synthesis, Org. Chem. Front., 2017, 4, 1947–1957 RSC.
- L. A. Tatum, X. Su and I. Aprahamian, Simple Hydrazone Building Blocks for Complicated Functional Materials, Acc. Chem. Res., 2014, 47, 2141–2149 CrossRef CAS.
- T. A. Khattab, From chromic switchable hydrazones to smart materials, Mater. Chem. Phys., 2020, 254, 123456 CrossRef CAS.
- G. Vantomme and J. Lehn, Photo– and Thermoresponsive Supramolecular Assemblies: Reversible Photorelease of K+ Ions and Constitutional Dynamics, Angew. Chem., Int. Ed., 2013, 52, 3940–3943 CrossRef CAS.
- D. Ramimoghadam, D. J. Eyckens, R. A. Evans, G. Moad, S. Holmes and R. Simons, Towards Sustainable Materials: A Review of Acylhydrazone Chemistry for Reversible Polymers, Chem. – Eur. J., 2024, 30, e202401728 CrossRef CAS.
- P. C. Sharma, D. Sharma, A. Sharma, N. Saini, R. Goyal, M. Ola, R. Chawla and V. K. Thakur, Hydrazone comprising compounds as promising anti-infective agents: chemistry and structure-property relationship, Mater. Today Chem., 2020, 18, 100349 CrossRef CAS.
- R. Çakmak, E. Başaran, K. Sahin, M. Şentürk and S. Durdağı, Synthesis of Novel Hydrazide–Hydrazone Compounds and In Vitro and In Silico Investigation of Their Biological Activities against AChE, BChE, and hCA I and II, ACS Omega, 2024, 9, 20030–20041 CrossRef PubMed.
- J. Wahbeh and S. Milkowski, The Use of Hydrazones for Biomedical Applications, SLAS Technol., 2019, 24, 161–168 CrossRef CAS.
- R. Lazny and A. Nodzewska, N,N-Dialkylhydrazones in Organic Synthesis. From Simple N,N-Dimethylhydrazones to Supported Chiral Auxiliaries, Chem. Rev., 2010, 110, 1386–1434 CrossRef CAS PubMed.
- D. Enders, L. Wortmann and R. Peters, Recovery of Carbonyl Compounds from N,N-Dialkylhydrazones, Acc. Chem. Res., 2000, 33, 157–169 CrossRef CAS.
- D. Lim and D. M. Coltart, Simple and Efficient Asymmetric α–Alkylation and α,α–Bisalkylation of Acyclic Ketones by Using Chiral N–Amino Cyclic Carbamate Hydrazones, Angew. Chem., Int. Ed., 2008, 47, 5207–5210 CrossRef CAS.
- G. K. Friestad, Chiral N–Acylhydrazones: Versatile Imino Acceptors for Asymmetric Amine Synthesis, Eur. J. Org. Chem., 2005, 3157–3172 CrossRef CAS.
- M. Sugiura and S. Kobayashi, N–Acylhydrazones as Versatile Electrophiles for the Synthesis of Nitrogen–Containing Compounds, Angew. Chem., Int. Ed., 2005, 44, 5176–5186 CrossRef CAS PubMed.
- Z. Shao and H. Zhang, N-Tosylhydrazones: versatile reagents for metal-catalyzed and metal-free cross-coupling reactions, Chem. Soc. Rev., 2012, 41, 560–572 RSC.
- A. Claraz, Harnessing the versatility of hydrazones through electrosynthetic oxidative transformations, Beilstein J. Org. Chem., 2024, 20, 1988–2004 CrossRef CAS PubMed.
- Y. Lv, J. Meng, C. Li, X. Wang, Y. Ye and K. Sun, Update on the Synthesis of N–Heterocycles via Cyclization of Hydrazones (2017–2021), Adv. Synth. Catal., 2021, 363, 5235–5265 CrossRef CAS.
- Y. Si, Q. Lv and B. Yu, Radical Cascade Reactions of β,γ–Unsaturated Hydrazones/Oximes, Adv. Synth. Catal., 2021, 363, 4640–4666 CrossRef CAS.
- X. Xu, J. Zhang, H. Xia and J. Wu, C(sp2)–H functionalization of aldehyde-derived hydrazones via a radical process, Org. Biomol. Chem., 2018, 16, 1227–1241 RSC.
- P. Xu, W. Li, J. Xie and C. Zhu, Exploration of C–H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond, Acc. Chem. Res., 2018, 51, 484–495 CrossRef CAS PubMed.
- A. Prieto, D. Bouyssi and N. Monteiro, Radical–Mediated Formal C(sp2)–H Functionalization of Aldehyde–Derived N, N –Dialkylhydrazones, Eur. J. Org. Chem., 2018, 2378–2393 CrossRef CAS.
- B. A. Van Der Worp, M. D. Kosobokov, V. V. Levin and A. D. Dilman, Photoredox Fluoroalkylation of Hydrazones in Neutral and Reductive Modes, Adv. Synth. Catal., 2021, 363, 1152–1158 CrossRef CAS.
- M. Latrache and N. Hoffmann, Photochemical radical cyclization reactions with imines, hydrazones, oximes and related compounds, Chem. Soc. Rev., 2021, 50, 7418–7435 RSC.
- A. Nishinaga, S. Yamazakhi, H. Nogusa, T. Shimoyam and T. Matsuura, Oxidation of phenols and hydrazones with t-butyl hydroperoxide and catalysis by Co(salen)., Nippon Kagaku Kaishi, 1985, 378–386 CrossRef CAS.
- T. Tezuka and S. Ando, Novel Substituent Effect Controlling the Stability of α-Azohydroperoxides, Chem. Lett., 1986, 15, 1671–1674 CrossRef.
- M. Schulz, U. Missol and H. Bohm, Azoperoxide. I Synthese von trans–α–Hydroxy–dialkyldiazenen aus α–Alkylazo–alkylhydroperoxiden, J. Prakt. Chem., 1974, 316, 47–53 CrossRef CAS.
- A. L. Baumstark and P. C. Vasquez, Oxygen–atom transfer chemistry of α–AZO hydroperoxides: Effect of competitive intramolecular hydrogen bonding and α–methyl substitution, J. Phys. Org. Chem., 1988, 1, 259–265 CrossRef CAS.
- M. Harej and D. Dolenc, Autoxidation of Hydrazones. Some New Insights, J. Org. Chem., 2007, 72, 7214–7221 CrossRef CAS PubMed.
- A. S. Nazran and J. Warkentin, Concerted homolysis in thermal decomposition of peresters from .alpha.-hydroperoxydiazenes, J. Am. Chem. Soc., 1982, 104, 6405–6407 CrossRef CAS.
- A. S. Budnikov, I. B. Krylov, M. I. Shevchenko, O. O. Segida, A. V. Lastovko, A. L. Alekseenko, A. I. Ilovaisky, G. I. Nikishin and A. O. Terent'ev, C–O Coupling of Hydrazones with Diacetyliminoxyl Radical Leading to Azo Oxime Ethers—Novel Antifungal Agents, Molecules, 2023, 28, 7863 CrossRef CAS PubMed.
- I. Bhatnagar and M. V. George, Oxidation of phenylhydrazones with manganese dioxide, J. Org. Chem., 1967, 32, 2252–2256 CrossRef CAS.
- R. B. Woodward and C. Wintner, On the oxidation products or benzaldehyde phenylhydrazone and the isomers of benzil osazone, Tetrahedron Lett., 1969, 10, 2697–2700 CrossRef.
- H. Minato, H. Tateno and H. Yokokawa, The Chemistry of Polyazanes. V. The Mechanism of the Oxidation of Benzalphenylhydrazone, Bull. Chem. Soc. Jpn., 1966, 39, 2724–2728 CrossRef CAS.
- T. W. Milligan and B. C. Minor, Oxidative Dimers of Benzaldehyde Phenylhydrazone, J. Org. Chem., 1962, 27, 4663–4665 CrossRef CAS.
- K. Titenkova, A. D. Shuvaev, F. E. Teslenko, E. S. Zhilin and L. L. Fershtat, Empowering strategies of electrochemical N–N bond forming reactions: direct access to previously neglected 1,2,3-triazole 1-oxides, Green Chem., 2023, 25, 6686–6693 RSC.
- J. L. Brokenshire, J. R. Roberts and K. U. Ingold, Kinetic applications of electron paramagnetic resonance spectroscopy. VII. Self-reactions of iminoxy radicals, J. Am. Chem. Soc., 1972, 94, 7040–7049 CrossRef CAS.
- N. Singh and D. G. Lee, Permanganate: A Green and Versatile Industrial Oxidant, Org. Process Res. Dev., 2001, 5, 599–603 CrossRef CAS.
- S. Dash, S. Patel and B. K. Mishra, Oxidation by permanganate: synthetic and mechanistic aspects, Tetrahedron, 2009, 65, 707–739 CrossRef CAS.
- J. R. Laszakovits, A. Kerr and A. A. MacKay, Permanganate Oxidation of Organic Contaminants and Model Compounds, Environ. Sci. Technol., 2022, 56, 4728–4748 CrossRef CAS PubMed.
- N. Umetsu and Y. Shirai, Development of novel pesticides in the 21st century, J. Pestic. Sci., 2020, 45, 54–74 CrossRef CAS.
- T. C. Sparks and R. J. Bryant, Crop protection compounds – trends and perspective, Pest Manage. Sci., 2021, 77, 3608–3616 CrossRef CAS.
- P. Jeschke, Progress of modern agricultural chemistry and future prospects: Progress of modern agricultural chemistry and future prospects, Pest Manage. Sci., 2016, 72, 433–455 CrossRef CAS.
- H.-S. Wang, L. Li, X. Chen, J.-L. Wu, K. Sun, X.-L. Chen, L.-B. Qu and B. Yu, Recent advances in amidyl radical-mediated photocatalytic direct intermolecular hydrogen atom transfer, Beilstein J. Org. Chem., 2025, 21, 1306–1323 CrossRef CAS PubMed.
- H.-S. Wang, H.-C. Li, X.-Y. Yuan, K. Sun, X.-L. Chen, L. Qu and B. Yu, A hydrogen atom transfer-enabled photocatalytic system for direct heteroarylation of C(sp3)–H and C(sp2)–H bonds, Green Chem., 2025, 27, 4655–4663 RSC.
- M. Müller, A. Hansen and S. Grimme, ω,B97X-3c: A composite range-separated hybrid DFT method with a molecule-optimized polarized valence double- ζ basis set, J. Chem. Phys., 2023, 158, 014103 CrossRef.
- A. S. Budnikov, I. B. Krylov, I. V. Kuzmin, O. O. Segida, A. V. Lastovko, M. I. Shevchenko, G. I. Nikishin and A. O. Terent'ev, Diacetyliminoxyl as a selective radical reagent for organic synthesis: dehydrogenation and dehydrogenative C–O coupling reactions, Org. Chem. Front., 2023, 10, 388–398 RSC.
- P. C. St John, Y. Guan, Y. Kim, S. Kim and R. S. Paton, Prediction of organic homolytic bond dissociation enthalpies at near chemical accuracy with sub-second computational cost, Nat. Commun., 2020, 11, 2328 CrossRef CAS PubMed.
- F. Neese, Software Update: The ORCA Program System—Version 6.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2025, 15, e70019 Search PubMed.
- C. Bannwarth, S. Ehlert and S. Grimme, GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions, J. Chem. Theory Comput., 2019, 15, 1652–1671 CrossRef CAS PubMed.
- V. S. Brauer, C. P. Rezende, A. M. Pessoni, R. G. De Paula, K. S. Rangappa, S. C. Nayaka, V. K. Gupta and F. Almeida, Antifungal Agents in Agriculture: Friends and Foes of Public Health, Biomolecules, 2019, 9, 521 CrossRef CAS PubMed.
- E.-C. Oerke, Crop losses to pests, J. Agric. Sci., 2006, 144, 31–43 CrossRef.
- M. C. Fisher, D. A. Henk, C. J. Briggs, J. S. Brownstein, L. C. Madoff, S. L. McCraw and S. J. Gurr, Emerging fungal threats to animal, plant and ecosystem health, Nature, 2012, 484, 186–194 CrossRef CAS PubMed.
- A. B. Tleuova, E. Wielogorska, V. S. S. L. P. Talluri, F. Štěpánek, C. T. Elliott and D. O. Grigoriev, Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture, J. Controlled Release, 2020, 326, 468–481 CrossRef CAS.
- J. Cooper and H. Dobson, The benefits of pesticides to mankind and the environment, Crop Prot., 2007, 26, 1337–1348 CrossRef CAS.
- X. Chen, M. F. Abdallah, S. Landschoot, K. Audenaert, S. De Saeger, X. Chen and A. Rajkovic, Aspergillus flavus and Fusarium verticillioides and Their Main Mycotoxins: Global Distribution and Scenarios of Interactions in Maize, Toxins, 2023, 15, 577 CrossRef CAS PubMed.
- G. P. Munkvold, R. H. Proctor and A. Moretti, Mycotoxin Production in Fusarium According to Contemporary Species Concepts, Annu. Rev. Phytopathol., 2021, 59, 373–402 CrossRef CAS PubMed.
- S. Wang, Z. Zhao, Y. Wang, H. Zheng, Q. Zhang, L. Zhang, J. Zhang, H. Lu and Y. Dong, Design, Synthesis, and Bioactivity Determination of Novel Malononitrile Derivatives Containing 1,2,3-Triazole, J. Agric. Food Chem., 2024, 72, 19274–19285 CrossRef CAS.
- S. Wang, Z. Zhao, L. Zhang, J. Zhang, H. Lu and Y. Dong, The design, synthesis, and biological activity assay of malononitrile oxime ether compounds as effective fungicides, New J. Chem., 2023, 47, 22134–22145 RSC.
- W. Liu, Z. Zhang, Y. Wang, Y. Ni, X. Sun, H. Liu, H. Bin, Z. Yu and X. Li, Design, Synthesis, and Antifungal Activity of Novel Stilbene Cyclohexyl Sulfonamide Derivatives against Botrytis cinerea, J. Agric. Food Chem., 2025, 73, 15356–15365 CrossRef CAS.
- A. S. Budnikov, E. R. Lopat'eva, I. B. Krylov, O. O. Segida, A. V. Lastovko, A. I. Ilovaisky, G. I. Nikishin, A. P. Glinushkin and A. O. Terent'ev, 4-Nitropyrazolin-5-ones as Readily Available Fungicides of the Novel Structural Type for Crop Protection: Atom-Efficient Scalable Synthesis and Key Structural Features Responsible for Activity, J. Agric. Food Chem., 2022, 70, 4572–4581 CrossRef CAS.
- R. Chowdhury and A. Mendoza, N-Hydroxyphthalimidyl diazoacetate (NHPI-DA): a modular methylene linchpin for the C–H alkylation of indoles, Chem. Commun., 2021, 57, 4532–4535 RSC.
- G. Kumar, B. Saroha, R. Kumar, M. Kumari, S. Dalal and S. Kumar, Design, synthesis, biological evaluation, and molecular docking studies of some novel N,N–dimethylaminopropoxy–substituted aurones, J. Heterocycl. Chem., 2022, 59, 297–308 CrossRef CAS.
- J. Mokhtari, M. R. Naimi-Jamal, H. Hamzeali, M. G. Dekamin and G. Kaupp, Kneading Ball–Milling and Stoichiometric Melts for the Quantitative Derivatization of Carbonyl Compounds with Gas–Solid Recovery, ChemSusChem, 2009, 2, 248–254 CrossRef CAS.
- I. R. Siddiqui, R. Rahila, P. Rai, H. Sagir and M. A. Waseem, Molecular iodine catalysed domino cyclization in aqueous medium: a simple and efficient synthetic route to 1,4-dihydropyridazines, RSC Adv., 2015, 5, 52355–52360 RSC.
- X. Wang, D.-P. Chen, W.-P. Wang, C.-H. Yang, M. Li, W.-B. Xu, X.-C. Wang and Z.-J. Quan, Hydrazone Phosphaketene as a Synthetic Platform To Obtain Three Classes of 1,2,4-Diazaphosphol Derivatives by Switchable Chemoselectivity Strategies, Org. Lett., 2024, 26, 3575–3580 CrossRef CAS.
- D. I. Fomenkov, R. A. Budekhin, P. S. Radulov, A. I. Fomenkov, L.-N. He, I. A. Yaremenko and A. O. Terent'ev, C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N Bond Ozonolysis and β-Scission: A Breakthrough Approach to the Synthesis of ω-Functionalized Compounds from Carbonyl Derivatives, Org. Lett., 2024, 26, 8095–8099 CrossRef CAS.
N Bond Ozonolysis and β-Scission: A Breakthrough Approach to the Synthesis of ω-Functionalized Compounds from Carbonyl Derivatives, Org. Lett., 2024, 26, 8095–8099 CrossRef CAS. - Z. Dong, C. Chen, J. Wang, J. Xu and Z. Yang, Dual roles of bisphosphines and epoxides: Rh-catalyzed highly chemoselective and diastereoselective (3 + 2) transannulations of 1,2,3-thiadiazoles with cyanoepoxides, Org. Chem. Front., 2021, 8, 6687–6698 RSC.
- B. M. Eisenhauer, M. Wang, H. Labaziewicz, M. Ngo and G. D. Mendenhall, Limitations on the Persistence of Iminoxyls: Isolation of tert-Butyl-1,1-Diethylpropyl Ketiminoxyl and Related Radicals, J. Org. Chem., 1997, 62, 2050–2053 CrossRef CAS.
- Y. E. Jad, S. N. Khattab, B. G. De La Torre, T. Govender, H. G. Kruger, A. El-Faham and F. Albericio, Oxyma-B, an excellent racemization suppressor for peptide synthesis, Org. Biomol. Chem., 2014, 12, 8379–8385 RSC.
- Y. Xue and S. Wang, Generation of Carbonyl Compounds from Oximes through Electrooxidative Deoximation, J. Org. Chem., 2024, 89, 4199–4204 CrossRef CAS PubMed.
- A. S. Pelegrí, M. R. J. Elsegood, V. McKee and G. W. Weaver, Unexpected Ring Expansion of an Enantiopure Imidazoline Carbene Ligand, Org. Lett., 2006, 8, 3049–3051 CrossRef.
- O. D. Dhingra and J. B. Sinclair, Basic plant pathology methods, CRC Press, Inc, Boca Raton, 1987 Search PubMed.
- H. Xu and L. Fan, Antifungal agents. Part 4: Synthesis and antifungal activities of novel indole[1,2-c]-1,2,4-benzotriazine derivatives against phytopathogenic fungi in vitro, Eur. J. Med. Chem., 2011, 46, 364–369 CrossRef CAS.
- P. K. Singh, Synthesis and Fungicidal Activity of Novel 3-(Substituted/unsubstituted phenylselenonyl)-1-ribosyl/deoxyribosyl-1H-1,2,4-triazole, J. Agric. Food Chem., 2012, 60, 5813–5818 CrossRef CAS PubMed.
- H. Itoh, H. Kajino, T. Tsukiyama, J. Tobitsuka, H. Ohta, Y. Takahi, M. Tsuda and H. Takeshiba, Synthesis of silicon-containing azole derivatives with magnesium bromide diethyl etherate, and an investigation of their fungicidal activities, Bioorg. Med. Chem., 2002, 10, 4029–4034 CrossRef CAS PubMed.
- S. V. Popkov, L. V. Kovalenko, M. M. Bobylev, O. Yu. Molchanov, M. Z. Krimer, V. P. Tashchi and Y. G. Putsykin, The Synthesis and Fungicidal Activity of 2-Substituted 1-Azol-1-ylmethyl-6-arylidenecyclohexanols, Pestic. Sci., 1997, 49, 125–129 CrossRef CAS.
- D. A. Pratt, J. A. Blake, P. Mulder, J. C. Walton, H.-G. Korth and K. U. Ingold, O−H Bond Dissociation Enthalpies in Oximes: Order Restored, J. Am. Chem. Soc., 2004, 126, 10667–10675 CrossRef CAS PubMed.
- O. Kondo and S. W. Benson, Kinetics and equilibria in the system Br + MeOOH ⇌ HBr + MeOO. An upper limit for the heat of formation of the methylperoxy radical, J. Phys. Chem., 1984, 88, 6675–6680 CrossRef CAS.
- B. Ruscic, A. F. Wagner, L. B. Harding, R. L. Asher, D. Feller, D. A. Dixon, K. A. Peterson, Y. Song, X. Qian, C.-Y. Ng, J. Liu, W. Chen and D. W. Schwenke, On the Enthalpy of Formation of Hydroxyl Radical and Gas-Phase Bond Dissociation Energies of Water and Hydroxyl, J. Phys. Chem. A, 2002, 106, 2727–2747 CrossRef CAS.
- H. Neugebauer, B. Bädorf, S. Ehlert, A. Hansen and S. Grimme, High–throughput screening of spin states for transition metal complexes with spin–polarized extended tight–binding methods, J. Comput. Chem., 2023, 44, 2120–2129 CrossRef CAS.
| This journal is © the Partner Organisations 2026 |