Open Access Article
Open Access ArticleElectrophilic aromatic substitution in eutectic-type mixtures: from an old concept to new sustainable horizons
Tatiana
Martí†
a,
Xavier
Marset†
 *a,
Celia
Guillem
b,
Diego J.
Ramón
*a,
Celia
Guillem
b,
Diego J.
Ramón
 a and
Gabriela
Guillena
a and
Gabriela
Guillena
 *a
*a
aDepartamento de Química Orgánica, Instituto de Síntesis Orgánica (ISO), Facultad de Ciencias, Universidad de Alicante, Apdo. 99, 03080 Alicante, Spain. E-mail: xavier.marset@ua.es; gabriela.guillena@ua.es
bKongsberg Discovery Spain, Partida Atalayes 20, Villajoyosa, 03570 Alicante, Spain
First published on 21st March 2024
Abstract
The Friedel–Crafts reaction is present in many fields of chemistry, being especially relevant in industrial applications, where it is one of the key methodologies to transform raw materials into functionalized aromatic products. During the last few years, the industry has been compelled to modify the conditions applied in its processes owing to the demand for more environmentally conscious and safe production techniques. In this review, some alternatives to conventional solvents, such as the use of deep eutectic solvents (DESs) as new reaction media with compatible catalysts, commonly used in Friedel–Crafts reactions are reported. These new conditions represent a major step towards sustainability in comparison to traditional methods, which employ stoichiometric amounts of Lewis acids and organic volatile solvents as reaction media.
Sustainability spotlightElectrophilic aromatic substitution is one of the most relevant organic transformations in the chemical industry that generally take place at the initial steps of any synthetic route. However, traditional methods to perform these reactions involve the use of stoichiometric amounts of strong acids or/and transitions metals, as well as dangerous petrol-derived volatile organic solvents as reaction media. In addition, an aqueous work-up is needed to neutralize the mixture, generating more by-products and contaminated water. This review covers the use of novel eutectic-type mixtures, which greatly reduce the environmental impact of these procedures (goal 12, sustainable consumption and production) by employing bio-renewable components (goal 13, climate action) and recyclable catalytic systems. The generated waste is thus reduced, and water contamination minimized (goal 14, life below water). Also, the future challenges of this process are highlighted. |
Introduction
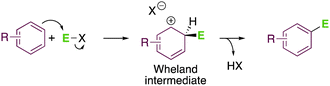
While undertaking any organic chemistry course, one of the first reactions that students learn is electrophilic aromatic substitution. This transformation is one of the oldest C–C bond-forming reactions, which dates back to 1877,1 and is conceptually simple enough to start understanding organic chemistry.2 In a nutshell, SE(Ar), which stands for substitution of electrophilic arenium, is a bi-molecular reaction in which an aromatic ring reacts with an electrophile via a Wheland intermediate to generate a new C–C bond after proton elimination (Scheme 1).3Although this reaction has been known for almost 150 years and has found many applications in industrial processes, new advancements are still being proposed.4 The classical protocols, using stoichiometric amounts of strong acids and halogenated solvents, are far away from the current sustainability standards. These protocols rely on the use of volatile organic solvents (VOCs) in combination with strong Lewis or Brønsted acids, requiring an extra step of neutralization once the reaction is completed. Thus, the number of steps and, therefore, reagents are increased, generating salts as by-products that should be treated as waste, thus leading to unacceptable values of the E-factor of the product.5 Therefore, modern approaches should improve the E-factor of the processes by designing procedures that generate less waste.
Recently, some efforts have been made to improve this aspect. In this sense, the use of ionic liquids (ILs) was proposed as an alternative to VOCs as reaction media.6 Over the years, ILs have been found to offer some interesting properties, but they are now excluded as a green alternative to VOC solvents as they tend to be toxic,7,8 and their synthesis generates loads of waste and uses non-renewable feedstocks. In contrast, deep eutectic solvents (DESs) were proposed as alternative sustainable solvent systems, which maintain most of the advantages of ILs while greatly improving their sustainability aspects and decreasing their manufacturing costs.9 Although it was not until the beginning of this decade that deep eutectic solvents became popular in the scientific community as organic reaction media, the formation of eutectic mixtures has been known for decades.
DESs are mixtures of two or more components with enthalpic-driven negative deviation from thermodynamic ideality.10 Although a myriad of mixtures have been described over the last decade11 and their toxicological properties must be individually addressed,12 in general these mixtures are formed from readily available and/or (bio)renewable components. Their preparation typically involves only the mixing of components with slight heating or grinding, without using any additional solvents, with the E-factor being zero and the atom economy 100%.
There are five types of DES described in the literature,13 with types I, II and IV containing metal halides in their structure. These metallic salts can catalyse certain reactions while being part of a recyclable solvent system. On the other hand, type III and V DESs are formed by mixtures of organic HBD (hydrogen bond donor group) and HBA (hydrogen bond acceptor group), containing salts in their structure (type III) or only non-ionic compounds (type V). Over the last years, DESs have found many applications in organic synthesis,14 being used as innocent reaction media in transition-metal catalysed processes,15 or as catalysts/promoters of certain reactions,16 as well as in organocatalysis.17 However, even if a number of reports of increasingly complex transformation are being reported in these solvents, traditional reactivities are also explored in DESs. Although the nature of some DES, specially acidic ones,18 would apparently be ideal to perform SEAr transformations, their implementation in this type of processes has been scarcely explored and is mainly applied to highly activated aromatic substrates. In this review, we aim to highlight the possibilities offered by these green and sustainable media to perform this transformation and also to show the limitations encountered during their application.
Friedel–Crafts alkylation
The direct alkylation of aromatic compounds is a straightforward way to produce substituted aromatic molecules which are the starting materials not only of pharmaceuticals and materials but also of surfactant components.Early examples of Friedel–Crafts alkylation
Although the possible application of DES as media to several organic reactions was demonstrated in the first decade of the 20th century, there is an early report of a Friedel–Crafts alkylation process where the formation of eutectic mixtures is shown to be crucial for the success of the process. This example dates back to 1960 when the reaction between o-xylylene dibromide and benzene in the presence of aluminium chloride provided a mixture of anthracene and diphenylmethane (Scheme 2).19 When this condensation was extended to other benzene derivatives, it was found that o-xylylene dibromide and toluene reacted to afford a mixture of several compounds. The reaction began with the formation of hydrobromic acid and 9,10-dihydroanthracene, which after the loss of 2 protons led to 3,4′-dimethyldiphenylmethane as well as 2,6- and 2,7-dimethylanthracenes. This reaction proceeded without the need of solvents, but the authors observed the formation of an eutectic mixture coming from the mixture of the generated products, which was suggested to be one of the driving forces of the reaction.Synthesis of bis(indolyl)methanes
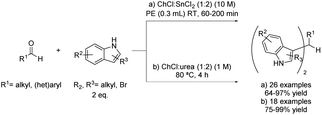
More recently, eutectic-type mixtures, and more specifically DESs, have been proposed as reaction media in which Friedel–Crafts alkylations can be performed. One of the most studied examples in this sense is the synthesis of bis(indolyl)methane through an electrophilic substitution reaction of electron-rich indole derivatives with several aldehydes.In 2012, an interesting variety of aromatic, heterocyclic and aliphatic aldehydes, as well as simple ketones, were tested using 0.1 mL of DES choline chloride and tin chloride mixture in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio at room temperature in the presence of 0.3 mL of polyethylene glycol (PEG) (Scheme 3a).20 Thus, the DES was used as a catalyst and co-solvent, with PEG being a co-solvent of the process. This method offers reaction yields similar to the ones reported with MeCN employing Zr-based catalysts21 or molecular iodine,22 among others, with the advantage of being a recyclable catalytic system. Regarding the DES, it contains a metal halide with Lewis acidity character fulfilling a double role, as it acts as a component of the deep eutectic mixture but also as a catalyst for the reaction. Therefore, the corresponding bis(indolyl)methane products were obtained in good to excellent yields. Furthermore, the scope of the reaction was extended to many commercial indole derivatives, getting the desired products in excellent yields.
2 ratio at room temperature in the presence of 0.3 mL of polyethylene glycol (PEG) (Scheme 3a).20 Thus, the DES was used as a catalyst and co-solvent, with PEG being a co-solvent of the process. This method offers reaction yields similar to the ones reported with MeCN employing Zr-based catalysts21 or molecular iodine,22 among others, with the advantage of being a recyclable catalytic system. Regarding the DES, it contains a metal halide with Lewis acidity character fulfilling a double role, as it acts as a component of the deep eutectic mixture but also as a catalyst for the reaction. Therefore, the corresponding bis(indolyl)methane products were obtained in good to excellent yields. Furthermore, the scope of the reaction was extended to many commercial indole derivatives, getting the desired products in excellent yields.
A couple of years later, the same reaction was described using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the solvent at 80 °C for 4 hours (Scheme 3b) without the need of an additional acid to act as a catalyst for this transformation.23 Therefore, comparing both processes, the use of a type I DES in the first described process was replaced by a type III in the second, which translates into the substitution of a metal halide component by urea, an inexpensive and biodegradable organic component. In this sense, the reaction was more efficient from a sustainable point of view, although part of the catalytic efficiency of the previous mixture was lost. In this case, different aromatic and alkenyl substituted aldehydes were reacted with unsubstituted indole to test the scope of the reaction. After product isolation through extraction with an organic solvent, the desired products were obtained in good to excellent yields. However, the aromatic substitution was ineffective when applied to aliphatic aldehydes, probably due to an aldol-type reaction competition. Likewise, the use of ketones as electrophiles did not take place with the starting material being recovered unreacted.
2) as the solvent at 80 °C for 4 hours (Scheme 3b) without the need of an additional acid to act as a catalyst for this transformation.23 Therefore, comparing both processes, the use of a type I DES in the first described process was replaced by a type III in the second, which translates into the substitution of a metal halide component by urea, an inexpensive and biodegradable organic component. In this sense, the reaction was more efficient from a sustainable point of view, although part of the catalytic efficiency of the previous mixture was lost. In this case, different aromatic and alkenyl substituted aldehydes were reacted with unsubstituted indole to test the scope of the reaction. After product isolation through extraction with an organic solvent, the desired products were obtained in good to excellent yields. However, the aromatic substitution was ineffective when applied to aliphatic aldehydes, probably due to an aldol-type reaction competition. Likewise, the use of ketones as electrophiles did not take place with the starting material being recovered unreacted.
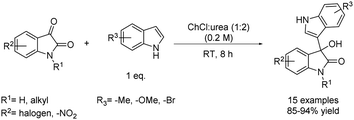
The same DES was used lately for the C-3 functionalization of indoles with electron-deficient carbonyl compounds such as isatins. The achieved 3-substituted-3-hydroxyoxindoles are interesting compounds in the field of pharmaceutical and natural products. The scope of the reaction was extended not only to different isatins substituted in the benzene ring but also to N-substituted isatins that reacted with substituted indoles at room temperature (Scheme 4).24 All the tested starting materials were well tolerated regardless of the fact of being decorated with different functional groups, proving the robustness of the method by leading to several 3-hydroxy-3-indolylindolin-2-ones in excellent yields.
Synthesis of monoalkylbenzenes
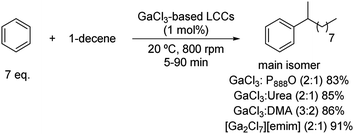
The SE(Ar) can also take place between olefins and arenes. When this reaction is performed under controlled conditions, linear monoalkyl arenes can be synthetized. In this sense, monoalkyl benzenes containing chains with 10 to 14 carbons are valuable intermediates for the synthesis of anionic detergents.25,26 These compounds are obtained through a Friedel–Crafts alkylation reaction with benzene and alpha olefins such as 1-decene. Several GaCl3-based liquid coordination complexes (LCC) were tested as catalysts, with benzene being used as the starting material but also as the solvent of the reaction.These liquid coordination complexes are liquid Lewis acids formed by anionic, cationic and neutral coordination complexes. These media, similar in structure to a DES, are obtained after mixing a donor molecule, such as amides, amines, phosphines or thioureas, with an excess of an anhydrous metal halide [commonly Al(III) or Ga(III)].27
Full conversions of 1-decene were achieved using tiny amounts of these LCC catalysts (0.25–2.00 mol% per mol of olefin) at room temperature, with the excess of benzene being the solvent of the reaction (Scheme 5).28 Nonetheless, there is room for improvement in terms of selectivity towards 2-phenyldecane (up to 47%), which is the most biodegradable isomer. This fact can be explained through a competition between the direct attack of the arene on the generated carbocation on 1-decene and a previous carbocation migration on to the most stable isomer. Regarding the donor ligands tested to afford these LCC catalysts, dimethylacetamide, urea, trioctylphosphine oxide and 1-ethyl-3-methylimidazolium chloride were employed, all of them offering yields from 83 to 91%. These excellent yields are remarkable since the aromatic substrate is not activated towards the electrophilic substitution and the catalytic LCC loading is rather low, being one of the rare examples of this transformation using DES applied to non-rich aromatic rings.
Synthesis of 1,1-diarylalkanes
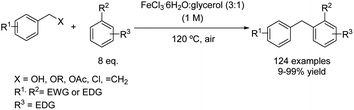
Very recently, the use of a DES composed of iron(III) chloride hexahydrate and glycerol (DES type IV) as a solvent and catalyst has been reported for a wide variety of Friedel–Crafts alkylations.29 This strategy is compatible with benzylating reagents such as styrenes, alcohols, acetates, ethers and chlorides, leading to 1,1-diarylalkanes in good yields even when arenes bearing deactivating groups were used as nucleophiles (Scheme 6). In this case, an excess of the nucleophilic arene was employed, allowing in most cases the formation of two different liquid phases, which were separated without the need of using any external work-up solvent, and the product could be purified by distillation. In this way, no VOC solvents were employed during the whole process and the generation of waste was minimized.Synthesis of triarylmethanes
In contrast to the previous sections, there are certain occasions in which the polyarylation led to the desired reaction product. An interesting methodology to obtain triarylmethanes and diarylalkanes (quite important scaffolds in medicinal30,31 and materials chemistry32) is through an electrophilic aromatic substitution such as Friedel–Crafts alkylation of arenes with aldehydes. For instance, an electron-rich arene such as 1,2,4-trimethoxybenzene was alkylated with several aromatic aldehydes decorated with electron-withdrawing and -donating groups using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1
zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the solvent and catalyst of the reaction (Scheme 7).33 In addition, aliphatic aldehydes were also compatible with these reaction conditions, affording the corresponding diarylalkanes in good to excellent yields.
2) as the solvent and catalyst of the reaction (Scheme 7).33 In addition, aliphatic aldehydes were also compatible with these reaction conditions, affording the corresponding diarylalkanes in good to excellent yields.
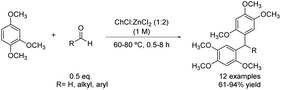 | ||
Scheme 7 Friedel–Crafts alkylation of electron-rich arene with aldehydes using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1 zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
The scope of this reaction was later expanded under the same mild conditions for the synthesis of other triarylmethanes. In this case, the alkylation of different arenes was performed using benzaldehyde as the coupling partner (Scheme 8).33 However, the lower nucleophilicity and electron density on the aromatic ring compared with the previous example notably decreased the yield of the reaction in some examples, which could be overcome by increasing the reaction time.
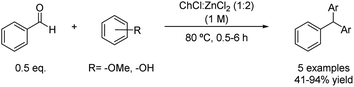 | ||
Scheme 8 Friedel–Crafts alkylation of benzaldehyde with nucleophiles using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1 zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
Arylation of β-nitrostyrene derivatives
Changing the electrophile to β-nitrostyrene derivatives allows the synthesis of interesting compounds. The arylation of β-nitrostyrenes with highly nucleophilic mono- and dialkyl anilines has been carried out using 10 mol% of the same DES as in the example before at 70 °C (Scheme 9).34 The use of water as the solvent, however, did not yield any product, while employing neat ZnCl2 instead of the corresponding eutectic mixture provoked a three times decrease in the reaction yield. In this instance, a versatile scope of the reaction was examined through the test of different β-nitrostyrenes substituted by electron-donor and -withdrawing groups as well as distinct N,N-alkyl and aryl aniline derivatives. Good to excellent yields were obtained in all cases except for the N-phenylaniline, whose corresponding product was formed in only 46% yield, probably due to its lower nucleophilicity. Excellent regioselectivity was denoted for these conditions as ortho or N-alkylation products were observed just in trace amounts, while the most activated and less sterically hindered para-position was mainly alkylated and isolated. It is worth noting that in this case the DES is employed in a catalytic amount. Therefore, the role of the eutectic mixture is only as a reaction promoter and not as a solvent, which further decreases the generated waste by employing neat conditions.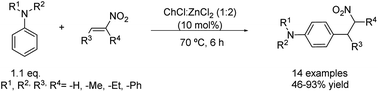 | ||
Scheme 9 Friedel–Crafts alkylation of several nitroalkenes with N,N-dialkyl anilines using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1 zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
Multicomponent reactions
Multicomponent reactions have also been described in this field as in the case of the domino Henry–Friedel–Crafts alkylation process of aldehydes, nitromethane and N-substituted anilines using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1
zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) described below (Scheme 10).35 The versatility of the reaction was explored, with benzaldehydes containing electron-withdrawing and -donating groups providing the corresponding products in good yields. Heterocyclic aldehydes were also tolerated, except for the case of 4-pyridine carboxaldehyde being the expected product not detected probably due to the lower electron density of the pyridine ring. It is worth mentioning that the reaction was tested by the same authors using VOC solvents such as EtOH, MeCN or DMSO, as well as using water and ZnCl2 as additives, not leading to the formation of the desired product or affording only marginal yields.
1) described below (Scheme 10).35 The versatility of the reaction was explored, with benzaldehydes containing electron-withdrawing and -donating groups providing the corresponding products in good yields. Heterocyclic aldehydes were also tolerated, except for the case of 4-pyridine carboxaldehyde being the expected product not detected probably due to the lower electron density of the pyridine ring. It is worth mentioning that the reaction was tested by the same authors using VOC solvents such as EtOH, MeCN or DMSO, as well as using water and ZnCl2 as additives, not leading to the formation of the desired product or affording only marginal yields.
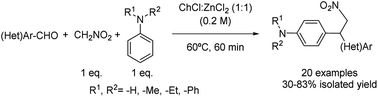 | ||
Scheme 10 One pot domino Henry–Friedel–Crafts alkylation mediated by choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1 zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Besides, secondary, and tertiary anilines showed moderate to good yields and regioselectivity as only para-alkylated products were obtained.
The alkylation of phenol with tert-butyl alcohol to synthesise tert-butyl phenols has been described using choline bisulfate and p-toluenesulfonic acid mixture in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (Scheme 11).36 Milder reaction conditions were needed to carry out this alkylation, with the reaction taking place only at 30 °C, to give a high conversion ratio of tert-butyl alcohol, most probably due to the high acidic activity of the solvent.
1 molar ratio (Scheme 11).36 Milder reaction conditions were needed to carry out this alkylation, with the reaction taking place only at 30 °C, to give a high conversion ratio of tert-butyl alcohol, most probably due to the high acidic activity of the solvent.
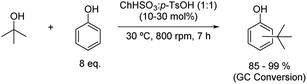 | ||
Scheme 11 Alkylation of phenol with tert-butyl alcohol using choline bisulfate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-toluenesulfonic acid (1 p-toluenesulfonic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
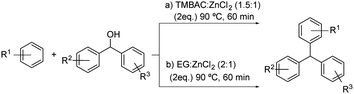
This kind of process is also described in patents as the one shown in Scheme 12. The preparation method for triarylmethane compounds through a Friedel–Crafts alkylation using the DES formed by benzyltrimethylammonium chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1.5
zinc chloride (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was developed (Scheme 12a).37 Different electron-rich aromatic compounds and a variety of diarylcarbinols were tested, obtaining the desired products in high selectivity and reaction yield. The same conditions were reported when using the DES formed by ethylene glycol
1) was developed (Scheme 12a).37 Different electron-rich aromatic compounds and a variety of diarylcarbinols were tested, obtaining the desired products in high selectivity and reaction yield. The same conditions were reported when using the DES formed by ethylene glycol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (2
zinc chloride (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).38
1).38
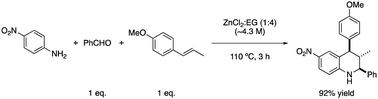
Another process in which the eutectic mixture formed by ZnCl2 and ethylene glycol has been used as both a co-solvent and catalyst is the multicomponent synthesis of tetrahydroquinolines.39 This reaction involves a [4 + 2] cycloaddition between N-arylimines and electron-rich olefins through a Lewis acid catalyst, i.e., zinc chloride (Scheme 13).
In addition, the reaction can be performed under microwave irradiation, fulfilling another green chemistry principle related to the design of reactions for increased energy efficiency, as well as the ones related to the use of DES, which are safer solvents and auxiliaries, and the use of renewable feedstocks.
Friedel–Crafts acylation
Likewise, deep eutectic solvents have been used for the Friedel–Crafts acylation of interesting compounds. As mentioned before, these green solvents play a dual role as acid catalysts and solvents. This fact has allowed the substitution of the traditional moisture-sensitive Lewis acids used in this type of reaction and also the conventional solvents by these eutectic mixtures, thereby increasing the sustainability of the process.One of the first Friedel–Crafts acylations using a deep eutectic solvent as a reaction medium was described in 2016. The acylation of aromatic compounds has been reported using propionic and benzoic anhydrides as acylating reagents. The eutectic mixture formed by choline chloride and zinc chloride in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio (35 mol%) was used as a catalyst under microwave irradiation (Scheme 14).40
3 molar ratio (35 mol%) was used as a catalyst under microwave irradiation (Scheme 14).40
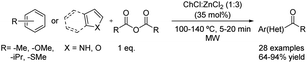 | ||
Scheme 14 Friedel–Crafts acylation of several aromatic compounds using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1 zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). 3). | ||
To expand the applicability of this methodology, the scope of the reaction was developed comprising 28 examples varying the acylating reagent used, temperature (100–140 °C) and the reaction time (5–20 min) but also a great amount of different aromatic compounds was tested. Aromatic compounds containing electron-donating groups such as methoxy substituents were benzoylated in good to excellent yields. Even though longer reaction time and higher temperatures compared with the previous example were needed, alkyl-substituted benzenes also reacted in good yields. Furthermore, thioanisole was benzoylated in excellent yield. However, the application of this reaction condition to non-activated substrates was not tested.
In addition, the regioselective Friedel–Crafts propionylation at position 3 of several electron-rich heteroaromatic scaffolds such as pyrrole, benzofurane or indoles decorated with both electron-withdrawing and electron-donating groups was possible under these conditions with good to excellent yields, even without the need of NH protection.
The acylation of electron-rich arenes using carboxylic acids is an interesting approach since water is the only by-product of the reaction, increasing the eco-scale values. This process has been reported by using the combination of the same deep eutectic solvent as in the previous example with the ionic liquid [AMIm]OTf (1-allyl-3-methylimidazolium trifluoromethanesulfonate) (Scheme 15).41
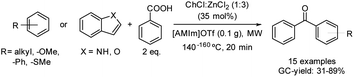 | ||
Scheme 15 Friedel–Crafts acylation of different arenes with carboxylic acids using a binary mixture formed by ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and [AMIm]OTf. 3) and [AMIm]OTf. | ||
Regarding the scope of this reaction, the benzoylation of anisole and thioanisole gave mainly the p-substituted product in moderate yield. Acylation of electron-rich arenes afforded the corresponding products in moderate to good yields with high selectivity. Naphthalene derivatives were also tolerated in moderate yields. Furthermore, fluorene and biphenyl were benzoylated in moderate yields at the 3-position and p-position, respectively.
In addition, the acylation of indole was carried out in moderate yield with high selectivity for the 3-position. Oxygen-containing heterocycles such as benzofuran gave mixtures of 2- and 3- benzoylated products. Note that in all cases the yield and selectivity were determined by GC using naphthalene as an internal standard.
Another example of F–C acylation was reported in 2017 when the acylation of 1,2,4-trimethoxybenzene using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1
zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was carried out at 70 °C (Scheme 16).42 Several electrophilic reagents were tested, obtaining the desired product in moderate to good yields, except for a couple of cases in which the yield was quite lower.
2) was carried out at 70 °C (Scheme 16).42 Several electrophilic reagents were tested, obtaining the desired product in moderate to good yields, except for a couple of cases in which the yield was quite lower.
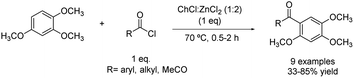 | ||
Scheme 16 Friedel–Crafts acylation of 1,2,4-trimethoxybenzene with different acylating reagents using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1 zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
The reaction between benzoyl chloride and the aforementioned substrate showed a higher yield under these conditions (85%). Regarding linear acyl chloride, it was demonstrated that the yield obtained decreased with increasing the chain length. Concurrently, other acid chlorides besides acyl chloride produced a notable decrease in the yield to 33%. Predictably, anhydrides showed less reactivity than acyl halides and even some anhydrides such as succinic anhydride did lead to the desired product. Finally, p-toluenesulfonyl chloride reacted with the substrate, obtaining the target product in 56%, probably due to the important steric hindrance but also the weakened electron-withdrawing ability.
The acylation of several arenes with benzoyl chloride matching the conditions used in the previous case was reported by the same authors (Scheme 17).42 The scope of the reaction denoted that the product yields were affected by electron-donating ability but also by the steric hindrance of the arenes. Unsurprisingly, the best results were obtained when using arenes with strong electron-donating substituents, increasing the yield when substituents directing to ortho and para positions are present at the aromatic ring. Nevertheless, the target products were not obtained when reacting aromatic compounds with weakly activating electron-donating substituents but neither with electron-withdrawing groups.
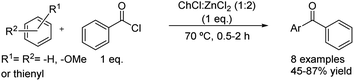 | ||
Scheme 17 Friedel–Crafts acylation of different arenes with benzoyl chloride using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride (1 zinc chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
An interesting alternative to the traditional Lewis acids commonly used in Friedel–Crafts acylation is metal triflates. These compounds are greener than traditional ones. Furthermore, metal triflates present high Lewis acidity and elevated tolerance to water, which makes them suitable for their use in organic solvent media but also aqueous media.
Very recently, an acylation reaction of different electron-rich arenes has been reported using praseodymium triflate as a catalyst and a mixture of choline chloride and pyrrole in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 molar ratio as a solvent. The reaction took place using 1 equivalent of benzoic anhydride as the acylating reagent at 100 °C under microwave conditions (Scheme 18).43
7 molar ratio as a solvent. The reaction took place using 1 equivalent of benzoic anhydride as the acylating reagent at 100 °C under microwave conditions (Scheme 18).43
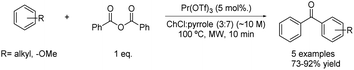 | ||
Scheme 18 Friedel–Crafts acylation using choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrrole (3 pyrrole (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) combined with praseodymium triflate. 7) combined with praseodymium triflate. | ||
The synthesis of five aromatic ketones was developed using these optimized conditions. Different arenes with electron-donating substituents such as methoxy- or methyl-groups were tested, obtaining the desired products in good yields for all cases.
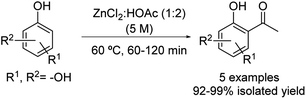
The Friedel–Crafts acylation of substituted phenols was freshly reported using the eutectic mixture formed by zinc chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid in a 1
acetic acid in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio under mild conditions (Scheme 19).44 The scope of the reaction includes several phenol derivatives such as catechol, resorcinol, hydroquinone and phloroglucinol. The desired products were obtained in all cases in excellent yields, apart from the case of phenol. In this occasion, after 180 minutes of reaction, a mixture composed of the unreacted phenol, phenyl ester and hydroxyl-aryl ketone products was observed probably due to the lower electron density on the benzene ring.
2 molar ratio under mild conditions (Scheme 19).44 The scope of the reaction includes several phenol derivatives such as catechol, resorcinol, hydroquinone and phloroglucinol. The desired products were obtained in all cases in excellent yields, apart from the case of phenol. In this occasion, after 180 minutes of reaction, a mixture composed of the unreacted phenol, phenyl ester and hydroxyl-aryl ketone products was observed probably due to the lower electron density on the benzene ring.
Assessment of green metrics
In this review, the use of alternative eutectic-based solvents has been described. Most of these solvents fulfil a dual role, acting as reaction media and catalysts at the same time, and greatly improving the sustainability of the methods over traditional approaches thanks to the use of less toxic, safer to use (non-volatile, non-flammable) and in some cases biorenewable solvent systems. In addition to these qualitative improvements, other quantitative parameters can be analysed. Table 1 summarizes some of the most important aspects of the previously discussed transformations and adds the E-factor and atom economy values. In general, the atom economy of this kind of reaction is very high, since most of the atoms contained in the starting materials can be found in the final products. Thus, the Friedel–Crafts approach is highly desirable from the sustainability point of view. However, atom economy does not consider some other parameters such as work-up solvents or purification methods. For this reason, E-factor has been also included in Table 1. In general, most of the presented results show E-factor values within which can be expected for fine chemicals or pharmaceutical products, although some of them show high values mainly due to the use of excess work-up solvents. The case of entry 4 is particularly interesting, in which a very low E-value is obtained thanks to the thoroughly designed process in which no additional work-up solvents are required. This type of approach should be used as inspiration to develop more sustainable processes in the future by considering every aspect of the reaction, including work-up processes.| Entry | Reaction | Yields | DES employed/DES-type | Starting materials | E-Factor | Atom economy |
|---|---|---|---|---|---|---|
| a E-Factor not included due to a certain lack of information in the original source. b Acetic acid was part of the solvent and therefore was not included in the atom economy calculation. | ||||||
| 1 | Scheme 3a | 64–97% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/type I 2)/type I |
PhCHO, indole | 67.1 | 94.7 |
| 2 | Scheme 3b | 77–99% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/type III 2)/type III |
4-Anisaldehyde, indole | 35.7 | 95.1 |
| 3 | Scheme 4 | 85–94% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/type III 2)/type III |
1-Methylisatin, indole | 48.0 | 99.9 |
| 4 | Scheme 6 | 9–99% | FeCl3·6H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (3 glycerol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/type IV 1)/type IV |
4-Chlorostyrene, o-xylene | 8.7 | 94.3 |
| 5 | Scheme 7 | 61–94% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/type I 2)/type I |
PhCHO, 1,3,6-trimethoxybenzene | 203.7 | 95.2 |
| 6 | Scheme 9 | 46–93% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/type I 2)/type I |
Nitrostyrene, Me2NPh | 110.2 | 99.9 |
| 7 | Scheme 10 | 30–83% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/type I 2)/type I |
PhCHO, MeNO2, Me2NPh | 4249.8 | 93.7 |
| 8 | Scheme 11 | 85–99% | ChHSO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-TsOH (1 p-TsOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/type III 1)/type III |
t BuOH, PhOH | —a | 98.9 |
| 9 | Scheme 13 | 92% | ZnCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (1 EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)/type IV 4)/type IV |
p-Nitroaniline, PhCHO, trans-anethol | —a | 95.4 |
| 10 | Scheme 14 | 64–94% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/type I 3)/type I |
Anisole, benzoic anhydride | 269.0 | 63.4 |
| 11 | Scheme 15 | 31–89% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/type I 3)/type I |
Trimethylbenzene, PhCO2H | 217.6 | 92.5 |
| 12 | Scheme 16 | 33–85% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/type I 2)/type I |
Trimethoxybenzene, PhCOCl | 44.2 | 88.1 |
| 13 | Scheme 18 | 73–92% | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrrole (3 pyrrole (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7)/type III 7)/type III |
Anisole, benzoic anhydride | 166.7 | 63.4 |
| 14 | Scheme 19 | 92–99% | ZnCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/type IV 2)/type IV |
Phloroglucinol, AcOH | 38.0 | 133.2b |
Another conclusion that can be drawn from Table 1 is the preference to use type 1 DESs for these transformations. Type 1 DESs present a metal halide in their structure combined with a quaternary ammonium salt (entries 1, 5–7, and 10–12). The presence of these metal salts endows the solvent with a Lewis acid character, promoting the use of the DES as a catalyst. Similarly, type IV DESs contain a metal halide in combination with a hydrogen bond donor molecule, like glycerol (entry 4) or ethylene glycol (entry 13). These solvents have been less explored so far but offer some of the best results and should be taken into account in the future when designing a new reaction process. Finally, type III DESs do not contain any metal salt in their structure (entries 2, 3, 8 and 13), and are still able to promote the desired transformations with or without the addition of an additional catalyst. In this sense, type III DESs can be seen as the less toxic mixtures, but the high efficiency and the possibility to be recycled of types I, II and IV make them also interesting to be used.
Conclusions
The pursuit of more sustainable procedures has impregnated every aspect of synthetic chemistry, not only when developing ground-breaking transformations, but also revisiting some of the most important traditional transformations, as is the Friedel–Crafts reaction. In this sense, eutectic-type mixtures proved to be efficient alternatives to traditional reaction media, fulfilling in some cases a double role as solvents and catalysts. Thus, the safety of these processes is improved while reducing the generated waste, with some of these processes being already patented and used on an industrial scale.However, loads of effort is still needed to unravel the full potential of these mixtures. Although in general good yields are reported in this review, the substrates are mostly limited to electron-rich arenes. Inactivated aromatic rings have shown limited or null reactivity so far, and novel strategies employing less sustainable media still have to be adapted to eutectic mixtures. Thus, further research is still needed to achieve better yields without compromising the sustainable aspect.
In addition, eutectic mixtures containing a component with a strong Lewis acid character have proven to act as an efficient catalyst for this kind of transformation. However, only a few archetypical DES-like mixtures have been tested. A systematic study testing several mixtures with different Lewis acid components and their catalytic ability is still missing, and it could pave the way for further research.
Finally, these eutectic mixtures can act as innocent solvents or even catalysts, but the use of some DES components as electrophiles has not been explored in this type of transformation. If this strategy is followed, an excess of the electrophilic reagent will be used, as it would be part of the solvent mixture, but due to the recycling capability of these solvents, no additional waste would be produced while the efficiency of the reaction is maximised.
In conclusion, eutectic-type mixtures have been found to be efficient solvents and catalysts to perform Friedel–Crafts transformations while improving the sustainability of the process. However, more research should be conducted to make these methods achieve the highest standards.
Author contributions
T. M. and X. M. processed the data and wrote the original draft. D. J. R.; C. G.; and G. G. obtained the resources needed for this research and reviewed the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research has been made possible with the financial support received from the University of Alicante (VIGROB-316FI) and the Spanish Ministerio de Ciencia e Innovación (PID2021-127332NB-I00). T. M. thanks to the University of Alicante for a fellowship (UAIND21-11C).Notes and references
- C. Friedel and J.-M. Crafts, C. R. Chim., 1877, 84, 1392–1395 Search PubMed.
- J. J. Li, in Name Reactions: A Collection of Detailed Reaction Mechanisms, ed. J. J. Li, Springer Berlin Heidelberg, Berlin, Heidelberg, 2006, pp. 240–242, DOI:10.1007/3-540-30031-7_107.
- R. Valiulin, in Organic Chemistry: 100 Must-Know Mechanisms, De Gruyter, Berlin, Boston, 2020, pp. 92–93, DOI:10.1515/9783110608373-039.
- A. H. Mohamed and N. Masurier, Org. Chem. Front., 2023, 10, 1847–1866 RSC.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- G. I. Borodkin and V. G. Shubin, Russ. J. Org. Chem., 2006, 42, 1745–1770 CrossRef CAS.
- K. S. Egorova and V. P. Ananikov, ChemSusChem, 2014, 7, 336–360 CrossRef CAS.
- T. P. T. Pham, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352–372 CrossRef CAS.
- D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramón, Eur. J. Org Chem., 2016, 2016, 612–632 CrossRef CAS.
- D. O. Abranches and J. A. P. Coutinho, Annu. Rev. Chem. Biomol. Eng., 2023, 14, 141–163 CrossRef CAS PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- J. Torregrosa-Crespo, X. Marset, G. Guillena, D. J. Ramón and R. M. Martínez-Espinosa, Sci. Total Environ., 2020, 704, 135382 CrossRef CAS PubMed.
- D. O. Abranches and J. A. P. Coutinho, Curr. Opin. Green Sustainable Chem., 2022, 35, 100612 CrossRef CAS.
- F. M. Perna, P. Vitale and V. Capriati, in Deep Eutectic Solvents, 2019, pp. 111–134, DOI:10.1002/9783527818488.ch7.
- X. Marset and G. Guillena, Molecules, 2022, 27, 8445 CrossRef CAS PubMed.
- S. E. Hooshmand, S. Kumar, I. Bahadur, T. Singh and R. S. Varma, J. Mol. Liq., 2023, 371, 121013 CrossRef CAS.
- D. A. Alonso, S.-J. Burlingham, R. Chinchilla, G. Guillena, D. J. Ramón and M. Tiecco, Eur. J. Org Chem., 2021, 2021, 4065–4071 CrossRef CAS.
- H. Qin, X. Hu, J. Wang, H. Cheng, L. Chen and Z. Qi, Green Energy Environ., 2020, 5, 8–21 CrossRef.
- K. Sisido, U. YôKo, T. Nakamura and H. Nozaki, J. Org. Chem., 1961, 26, 1368–1371 CrossRef.
- N. Azizi and Z. Manocheri, Res. Chem. Intermed., 2012, 38, 1495–1500 CrossRef CAS.
- Z.-H. Zhang, L. Yin and Y.-M. Wang, Synthesis, 2005, 2005, 1949–1954 CrossRef.
- B. P. Bandgar and K. A. Shaikh, Tetrahedron Lett., 2003, 44, 1959–1961 CrossRef CAS.
- S. Handy and N. M. Westbrook, Tetrahedron Lett., 2014, 55, 4969–4971 CrossRef CAS.
- A. Kumar, R. D. Shukla, D. Yadav and L. P. Gupta, RSC Adv., 2015, 5, 52062–52065 RSC.
- J. A. Kocal, B. V. Vora and T. Imai, Appl. Catal., A, 2001, 221, 295–301 CrossRef CAS.
- A. Shokri and S. Karimi, Russ. J. Appl. Chem., 2021, 94, 1546–1559 CrossRef CAS.
- J. M. Hogg, F. Coleman, A. Ferrer-Ugalde, M. P. Atkins and M. Swadźba-Kwaśny, Green Chem., 2015, 17, 1831–1841 RSC.
- K. Matuszek, A. Chrobok, J. M. Hogg, F. Coleman and M. Swadźba-Kwaśny, Green Chem., 2015, 17, 4255–4262 RSC.
- A. Presa Soto, M. Ramos-Martín and J. García-Álvarez, ChemRxiv, 2024, preprint, DOI:10.26434/chemrxiv-2024-pvzsr.
- A. V. Cheltsov, M. Aoyagi, A. Aleshin, E. C.-W. Yu, T. Gilliland, D. Zhai, A. A. Bobkov, J. C. Reed, R. C. Liddington and R. Abagyan, J. Med. Chem., 2010, 53, 3899–3906 CrossRef CAS PubMed.
- S. Mondal and G. Panda, RSC Adv., 2014, 4, 28317–28358 RSC.
- Y.-Q. Xu, J.-M. Lu, N.-J. Li, F. Yan, X.-W. Xia and Q.-F. Xu, Eur. Polym. J., 2008, 44, 2404–2411 CrossRef CAS.
- A. Wang, P. Xing, X. Zheng, H. Cao, G. Yang and X. Zheng, RSC Adv., 2015, 5, 59022–59026 RSC.
- M. A. Ranjbari, H. Tavakol and M. Manoukian, Res. Chem. Intermed., 2021, 47, 709–721 CrossRef CAS.
- Z. Hu, G. Jiang, Z. Zhu, B. Gong, Z. Xie and Z. Le, Chin. J. Org. Chem., 2021, 41, 325–332 CrossRef CAS.
- J. Xiong, D. Zhang, G. Yang and Z. Zhang, Ind. Eng. Chem. Res., 2021, 60, 13204–13213 CrossRef CAS.
- A. Wang, P. Xing and X. Zheng, China Pat., CN201510582680A, 2015.
- A. Wang, P. Xing and X. Zheng, China Pat., CN105237362A, 2016.
- W. J. Quintero, M. C. Ávila and C. Ochoa-Puentes, J. Chem. Educ., 2023, 100, 4763–4771 CrossRef CAS.
- P. H. Tran, H. T. Nguyen, P. E. Hansen and T. N. Le, RSC Adv., 2016, 6, 37031–37038 RSC.
- H. T. Nguyen, N. Le, Y. Kawazoe, N.-N. Pham-Tran and P. H. Tran, ChemistrySelect, 2022, 7, e202103708 CrossRef CAS.
- X. Jin, A. Wang, H. Cao, S. Zhang, L. Wang, X. Zheng and X. Zheng, Res. Chem. Intermed., 2018, 44, 5521–5530 CrossRef CAS.
- M.-T. T. Nguyen, N. Le, H. T. Nguyen, T. D. V. Luong, V. K. T. Nguyen, Y. Kawazoe, P. H. Tran and N.-N. Pham-Tran, ACS Omega, 2023, 8, 271–278 CrossRef CAS PubMed.
- F. Tamaddon and H. Rashidi, Res. Chem. Intermed., 2023, 49, 3589–3603 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |