Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of iminium ions during the processing of metal halide perovskites†
Jakob
Möbs
 ab,
Ajna
Tomori
a and
Johanna
Heine
ab,
Ajna
Tomori
a and
Johanna
Heine
 *a
*a
aDepartment of Chemistry, Philipps-University Marburg, Hans-Meerwein-Straße 4, 35043 Marburg, Germany. E-mail: johanna.heine@chemie.uni-marburg.de
bDepartment of Physics, University of Oxford, Parks Road, OX1 3PU Oxford, UK
First published on 24th May 2024
Abstract
Ammonium ions are a key component of many organic–inorganic metal halide materials. We show that the hexagonal perovskite (Me2NH2)PbI3 is rapidly transformed to iminium-based perovskites (Me2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMeR)PbI3 (R = Me, Et), simply by stirring the material in the respective ketone at room temperature, triggering clear changes in the materials’ photophysical properties.
NMeR)PbI3 (R = Me, Et), simply by stirring the material in the respective ketone at room temperature, triggering clear changes in the materials’ photophysical properties.
Metal halide perovskites, most prominently (MeNH3)PbI3 (MAPI) and related compounds, have become a major research focus since 2009, as they are highly efficient solar absorbers for the next generation of solar cells1 as well as promising materials for other semiconductor applications.2 Unfortunately, many of these perovskites are sensitive towards moisture, temperature and voltage in their pure forms - an issue that can be mitigated by adding small amounts of other organic cations.3 A commonly used additive in this regard is dimethyl ammonium, (Me2NH2)+, which can be added either in the form of its halide salts4 or by decomposition of the solvent DMF under acidic conditions.5 However, a potential problem arises when (Me2NH2)+-containing compounds are further processed with acetone, for example in washing procedures, as secondary amines can form iminium ions as condensation products with ketones under acidic conditions.6 In early works on iminium cations, halogenido metalate ions were used to produce isolable compounds containing these reactive cations,7,8 but the possibility of an unintentional formation is sometimes disregarded in metal halide perovskite research.
Using iodido plumbates as examples, we show that metalates with dimethyl ammonium cations quickly undergo a transformation to iminium compounds when treated with acetone or butanone and that the optical properties of the resulting compounds differ significantly from those of the starting materials, despite having similar crystal structures.
We prepared the literature-known compound (Me2NH2)PbI39,10 and reacted it with acetone to give (MeIm)PbI3 (1, MeIm = C5H12N+, N-(propan-2-ylidene)-N-methylmethanaminium) and butanone to give (EtIm)PbI3 (2, C6H14N+, EtIm = N-(butan-2-ylidene)-N-methylmethanaminium). The ketones react with the dimethyl amine following Scheme 1.
(Me2NH2)PbI3 readily reacts with acetone or butanone when suspended in the solvents to give near quantitative yields of the corresponding iminium salts. To qualitatively investigate the speed of the conversion, we left a suspension of 74 mg of (Me2NH2)PbI3 in 20 mL of acetone stirring at room temperature and took samples of the solid powder every 10 min. The ratio between the ammonium and iminium compound was determined by Rietveld refinement. The results are displayed in Fig. 1 as well as the ESI.† Already after 10 min about 10% of the mixture are made up of 1 and after 70 min the conversion is complete. At 65 °C the full conversion had already taken place after 30 min.
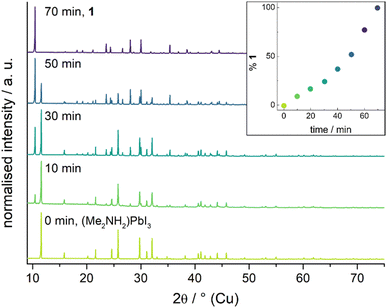 | ||
| Fig. 1 Diffraction patterns of the powder taken from a suspension of (Me2NH2)PbI3 in acetone. Percentage of 1 in the mixture as determined by Rietveld refinement shown as an inset. | ||
2 can be prepare similarly in butanone instead of acetone. Both can also be prepared from PbI2 and Me2NH2I directly. Detailed procedures are given in the ESI.†
1 crystallises in the hexagonal spacegroup P63/m as faintly yellow needles with six formula units in the unit cell. The structural motif is closely related to that of hexagonal perovskites, for example the prototypical BaNiO3.11 The anionic motif consists one-dimensional chains of face-sharing {PbI6}-octahedra with the cations separating the chains. Two different types of anionic chains can be observed. The ones situated at the corners of the unit cell feature near perfect octahedra, which are just slightly elongated along the crystallographic c-axis, while for the chains in the centre of the cell the shared faces (those perpendicular to the c-axis) are twisted 22° about the c-axis compared to an ideal octahedron. This gives I–Pb⋯Pb–I torsion angles of 49° and 71° instead of the ideal 60°. At 100 K the cations are disordered in a way that the two quaternary atomic position are both partly occupied by N and C. At higher temperatures this disorder gets stronger while the overall crystal structure stays the same safe for a slight lattice expansion (see ESI† for details). Fig. 2 shows excerpts of the crystal structure of 1.
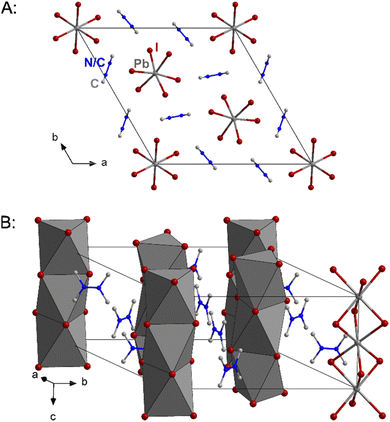 | ||
| Fig. 2 Unit cell of 1 from different viewing directions. The atomic positions denoted with N/C are partly occupied by nitrogen and carbon atoms. Hydrogen atoms are omitted for clarity. | ||
The crystal structure of 1 is very similar to that of the parent compound (Me2NH2)PbI3. For (Me2NH2)PbI3 a phase transition between room temperature and 100 K has been observed. Both phases feature a hexagonal perovskite-like motif of face-sharing {PbI6}-octahedra.10 The Pb–I distances of 1 are with 3.232 Å–3.239 Å at 100 K and 3.241 Å–2.246 Å at 273 K well in line with the corresponding values observed in (Me2NH2)PbI3. However, due to the smaller cation, the anionic chains are packed much more tightly together in (Me2NH2)PbI3 than in 1, which can be illustrated by comparing the shortest I⋯I distance between chains. In 1 this value is 4.855 Å/5.013 Å at 100 K/273 K, respectively, while for (Me2NH2)PbI3 it is 4.084 Å/4.409 Å at 100 K/293 K.
Compound 2 crystallises in the orthorhombic spacegroup Pnma as faintly yellow needles with four formula units in the unit cell. Its structure is closely related to those of 1 and (Me2NH2)PbI3 and shows the same anionic motif with Pb–I ranging from 3.178 Å–3.250 Å at 100 K. This is a wider range than in 1 but not as wide as in (Me2NH2)PbI3. In (Me2NH2)PbI3 García-Fernandez et al. observed a shift of the Pb atom towards one of the faces of the octahedron. This is also the case in 2, however not as strong, and the reason for the wider range of Pb–I distances.
As a result of the bulkier cation the packing of the anionic chains is also the widest of the three compounds with the shortest inter-chain I⋯I distance being 5.515 Å. Fig. 3 shows excerpts of the crystal structure of 2.
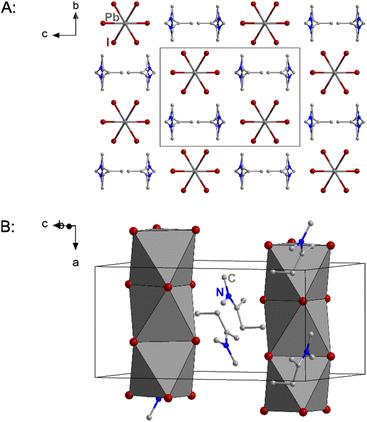 | ||
| Fig. 3 Excerpts of the crystal structure of 2. Hydrogen atoms and in part B the disorder are not shown for clarity. | ||
The optical properties of halogenido metalates of heavy main group elements are, in general, considered to be mostly dependent on the metal/halogen combination12 and the anion motif.13 For compounds featuring the same anion motif only small differences in their optical properties are expected unless the organic cation is involved, for example through charge transfer phenomena.14
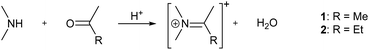
This is also the case for the compounds investigated here. However, the differences are already discernible to the eye and far from insignificant when application in optoelectronic devices is considered. To quantify them we investigated optical absorption at room temperature and photoluminescence (PL) at room temperature as well as 85 K of (Me2NH2)PbI3, 1 and 2. Key values of the spectroscopic measurements are summarised in Table 1. Fig. 4 shows absorption and PL spectra.
| (Me2NH2)PbI3 | 1 | 2 | |
|---|---|---|---|
| E g | 3.26 eV | 3.48 eV | 3.37 eV |
| E exc | 3.24 eV | 3.28 eV | 3.32 eV |
| λ onset | 443 nm | 417 nm | 436 nm |
| PL293 K | 1.55 eV | 1.76 eV | 1.78 eV |
| PL85 K | 1.82 eV | 1.71 eV | 1.78 eV |
For all three compounds analysis of Tauc-plots reveals a direct band gap and a strong and distinct excitonic absorption band at energies slightly below the band edge.
The samples also show strong, Gaussian shaped photoluminescence signals. The centres of these signals vary little with temperature for 1 and 2, while for (Me2NH2)PbI3, the centre is shifted about 0.3 eV to higher energies when cooled down. We attribute this shift to the aforementioned phase transition the compound undergoes. It should also be noted that that the PL signal of 2 at 293 K does not appear to be of Gaussian shape at first glance. However, 2 is the only of the three compounds that shows two emission peaks in the investigated range. At room temperature the two peaks strongly overlap, but the picture becomes clear at 85 K, where the difference in intensity of the two peak is increased and the weaker peak is blue-shifted (see Fig. S8–S14, ESI† for details).
The spectroscopic features of (Me2NH2)PbI3, 1 and 2 are consistent with literature reports on 1D lead iodide perovskites.15,16 The broad, strongly Stokes-shifted luminescence band observed for all three compounds is typically assigned to self-trapped exciton (STE) emission, while higher energy features, as observed for 2 at 85 K, are attributed to free exciton (FE) emission. The observation of FE emission appears to strongly depend on specific structural details and is sometimes only observed at very low temperatures.16
Summarising, we have presented two novel iminium iodido plumbates that form when (Me2NH2)PbI3 is processed using ketones and compared their crystal structures and optical properties to those of the parent compound. We have demonstrated that the formation happens very fast even at room temperature in suspension. As similar reactions are feasible for any kind of halogenido metalates featuring primary or secondary ammonium ions,8,17 our investigations shows that great care needs to be taken when ketones are used as solvents in this context. In any case the composition of the final compound needs to be carefully checked, as it might be the original ammonium compound, a newly formed iminium compound or a mixture or double salt of the two, as shown in a recent example.18
However, we also want to emphasize the potential of iminium ions: They may be useful as surface modification agents for metal halide perovskite thin films or nanoparticles and they represent an easily accessible extension and targeted modification of the typically used ammonium cations for the intentional synthesis of new halogenido metalate compounds, extending the rich structural chemistry of these materials even further.
This work was supported by the German Research Foundation DFG (505757318 and via the Collaborative Research Center No. 223848855-SFB 1083).
Conflicts of interest
There are no conflicts to declare.References
- (a) A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS PubMed; (b) A. K. Jena, A. Kulkarni and T. Miyasaka, Chem. Rev., 2019, 119, 3036 CrossRef CAS PubMed.
- (a) W. Zheng, X. Wang, X. Zhang, B. Chen, H. Suo, Z. Xing, Y. Wang, H.-L. Wei, J. Chen, Y. Guo and F. Wang, Adv. Mater., 2023, 35, e2205410 CrossRef PubMed; (b) W. Song, G. Qi and B. Liu, J. Mater. Chem. A, 2023, 11, 12482 RSC; (c) K. Sakhatskyi, B. Turedi, G. J. Matt, E. Wu, A. Sakhatska, V. Bartosh, M. N. Lintangpradipto, R. Naphade, I. Shorubalko, O. F. Mohammed, S. Yakunin, O. M. Bakr and M. V. Kovalenko, Nat. Photon., 2023, 17, 510 CrossRef CAS; (d) W. Chen, Z. Huang, H. Yao, Y. Liu, Y. Zhang, Z. Li, H. Zhou, P. Xiao, T. Chen, H. Sun, J. Huang and Z. Xiao, Nat. Photon., 2023, 17, 401 CrossRef CAS.
- H. Zhu, S. Teale, M. N. Lintangpradipto, S. Mahesh, B. Chen, M. D. McGehee, E. H. Sargent and O. M. Bakr, Nat. Rev. Mater., 2023, 8, 569 CrossRef.
- (a) C. Anelli, M. R. Chierotti, S. Bordignon, P. Quadrelli, D. Marongiu, G. Bongiovanni and L. Malavasi, Inorg. Chem., 2019, 58, 944 CrossRef CAS PubMed; (b) W. M. J. Franssen, C. M. M. van Heumen and A. P. M. Kentgens, Inorg. Chem., 2020, 59, 3730 CrossRef CAS PubMed; (c) A. Ray, B. Martín-García, A. Moliterni, N. Casati, K. M. Boopathi, D. Spirito, L. Goldoni, M. Prato, C. Giacobbe, C. Giannini, F. Di Stasio, R. Krahne, L. Manna and A. L. Abdelhady, Adv. Mater., 2022, 34, e2106160 CrossRef PubMed; (d) S. Jariwala, R. E. Kumar, G. E. Eperon, Y. Shi, D. P. Fenning and D. S. Ginger, ACS Energy Lett., 2022, 7, 204 CrossRef CAS; (e) D. P. McMeekin, P. Holzhey, S. O. Fürer, S. P. Harvey, L. T. Schelhas, J. M. Ball, S. Mahesh, S. Seo, N. Hawkins, J. Lu, M. B. Johnston, J. J. Berry, U. Bach and H. J. Snaith, Nat. Mater., 2023, 22, 73 CrossRef CAS PubMed.
- (a) M. Daub and H. Hillebrecht, Z. Anorg. Allg. Chem., 2018, 644, 1393 CrossRef CAS; (b) W. Ke, I. Spanopoulos, C. C. Stoumpos and M. G. Kanatzidis, Nat. Commun., 2018, 9, 4785 CrossRef PubMed.
- A. Erkkilä, I. Majander and P. M. Pihko, Chem. Rev., 2007, 107, 5416 CrossRef PubMed.
- M. Lamchen, W. Pugh and A. M. Stephen, J. Chem. Soc., 1954, 4418 RSC.
- R. Kuhn and H. Schretzmann, Chem. Ber., 1957, 90, 557 CrossRef CAS.
- A. Mancini, P. Quadrelli, G. Amoroso, C. Milanese, M. Boiocchi, A. Sironi, M. Patrini, G. Guizzetti and L. Malavasi, J. Solid State Chem., 2016, 240, 55 CrossRef CAS.
- A. García-Fernández, J. M. Bermúdez-García, S. Castro-García, A. L. Llamas-Saiz, R. Artiaga, J. López-Beceiro, S. Hu, W. Ren, A. Stroppa, M. Sánchez-Andújar and M. A. Señarís-Rodríguez, Inorg. Chem., 2017, 56, 4918 CrossRef PubMed.
- J. J. Lander, Acta Crystallogr., 1951, 4, 148 CrossRef CAS.
- (a) C. K. Möller, Nature, 1958, 182, 1436 CrossRef; (b) N. Dehnhardt, M. Axt, J. Zimmermann, M. Yang, G. Mette and J. Heine, Chem. Mater., 2020, 32, 4801 CrossRef CAS.
- (a) D. H. Cao, C. C. Stoumpos, O. K. Farha, J. T. Hupp and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 7843 CrossRef CAS PubMed; (b) J. Möbs, G. Stuhrmann, F. Weigend and J. Heine, Chem. – Eur. J., 2023, 29, e202202931 CrossRef PubMed.
- A. E. Maughan, J. A. Kurzman and J. R. Neilson, Inorg. Chem., 2015, 54, 370 CrossRef CAS PubMed.
- J. A. Zienkiewicz, K. Kałduńska, K. Fedoruk, A. J. Barros Dos Santos, M. Stefanski, W. Paraguassu, T. M. Muzioł and M. Ptak, Inorg. Chem., 2022, 61, 20886 CrossRef CAS PubMed.
- S. Maqbool, T. Sheikh, Z. Thekkayil, S. Deswal, R. Boomishankar, A. Nag and P. Mandal, J. Phys. Chem. C, 2021, 125, 22674 CrossRef CAS.
- O. Knop, T. S. Cameron, P. K. Bakshi, W. Kwiatkowski, S. C. Choi and D. Adhikesavalu, Can. J. Chem., 1993, 71, 1495 CrossRef CAS.
- J. Cheng, G. Yi, Z. Zhang, Y. Long, H. Zeng, L. Huang, G. Zou and Z. Lin, Angew. Chem., Int. Ed., 2024, 63, e202318385 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details on experimental procedures, crystallography (single crystal and powder), UV-Vis and photoluminescence spectroscopy. CCDC 2346572–2346575. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc01735h |
| This journal is © The Royal Society of Chemistry 2024 |