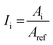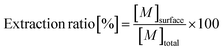Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Assessing sources and fractions of metals associated with environmental plastics: a case study in Lake Como (Italy)†
Stefano
Carnati
a,
Andrea
Pozzi
a,
Davide
Spanu
a,
Damiano
Monticelli
a,
Roberta
Bettinetti
b,
Ginevra
Boldrocchi
b,
Luca
Nizzetto
cd and
Gilberto
Binda
 *ac
*ac
aDepartment of Science and High Technology, University of Insubria, Via Valleggio 11, Como 22100, Italy
bDepartment of Human and Innovation for the Territory, University of Insubria, Via Valleggio 11, Como 22100, Italy
cNorwegian Institute for Water Research (NIVA), Økernveien 94, Oslo 0579, Norway. E-mail: gilberto.binda@uninsubria.it
dRECETOX, Masaryk University, Kamenice 753/5, Brno 625 00, Czech Republic
First published on 6th November 2023
Abstract
Understanding plastic–metal interactions is paramount to unveil the ecological risks of plastic pollution. Besides including a (variable) amount of metal-containing additives, plastic objects can adsorb metals on their surface in the environment. This work aims at measuring and assessing the possible origin of metals in environmental plastics deposited along the shores of Lake Como (Italy). Samples were characterized through Fourier-transformed infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and water contact angle. Then, the total metal load was analysed by acid digestion. Surface extraction with nitric acid was also performed to detect labile metals and a three-step extraction scheme enabled the determination of physisorbed, carbonate-bonded and organic matter-bonded metals, respectively. Eighteen metals (Al, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Sr, Ag, Cd, Sn, Ba, Pb and U) were analysed in total. Newly produced plastic items were also analysed as a reference. Our findings revealed that environmental samples retained a higher concentration of metals compared to virgin ones, especially in the loosely bonded acid-extractable fractions, indicating their potential bioavailability. The source of metals on plastics was extremely variable: some metals were predominantly sorbed from the environment (e.g., Mn and Pb), and others were mainly leached from the plastic matrix (Ba, Cu and Ti) or had a mixed origin (Zn, Fe, Sn, Sr and Al). This work shed light on the changes in bioavailability of metals induced by plastic environmental ageing, set baseline values for a freshwater site, and provided insights into the potential bioavailability exerted by metals associated with plastic litter.
Environmental significanceThe interactions between plastic and metals in the environment are of increasing interest owing to the possible environmental implications, such as the change in environmental fate and bioavailability of metals. Metals in plastics can be present as additives (e.g., dyes and catalysts), but can also sorb on plastic surfaces under environmental conditions. In this study, we investigated the content and speciation of different metals in environmental samples of plastic litter and in pristine plastic objects. This permitted the recognition of the likely source of metals in the environmental samples, fostering the investigation of plastic–metal interactions in the environment and shedding light on the potential implications of these processes. |
1. Introduction
Plastic polymers are used in a great number of products and applications (e.g., industrial, agricultural and technological) owing to their versatility, durability and low cost.1 The short usage lifespan of most plastic articles in commerce and the increasing production rates (worldwide production is expected to exceed 33 billion tons by 2050)2–4 led to the ubiquitous accumulation of plastics in environmental matrices, despite the effort in some regions to improve waste disposal and management.5,6 Microplastics (MPs), defined as plastic particles having a size <5 mm, are of foremost concern due to their widespread distribution and potential environmental impacts.7 They have been found in various ecosystems, ranging from water to terrestrial and atmospheric compartments,8–10 as well as in food products for human consumption.11–13MPs can induce several direct negative effects on the biota, such as entanglement or cellular inflammation following uptake.14–17 Still, other indirect and subtle effects which can put ecosystems at risk are still not thoroughly understood.18 As an example, MPs can act as vectors of metals and other inorganic chemicals in the environment:19–21 These particles can, in fact, adsorb and accumulate metals from the surrounding media.22,23 Furthermore, plastic formulations contain metal-based additives which are added for a variety of purposes, such as plasticizers, flame retardants, antioxidants, pigments and more.24–26 Many metals exhibit toxicity at low concentrations and can severely threaten organisms at different trophic levels, leading to both acute and chronic effects. Ingestion of metal-enriched plastics can possibly enhance their bioaccumulation.4
The sorption–desorption processes of metals on MPs can be affected by various factors. Besides the polymer type and its physicochemical features, a key role in the plastic–metal interaction is played by ageing processes.27,28 Naturally aged MPs generally show greater sorption of metals compared to pristine polymers.29–31 Environmental ageing is also observed to increase the leaching of metal-containing additives.32 Environmental factors such as ultraviolet (UV) radiation exposure, oxidative reactions, mechanical deterioration, and biofouling23,33 indeed induce polymer embrittlement, increase surface area and enhance polymer wettability. All these alterations enhance the sorption and desorption of metals. Likewise, water physicochemical conditions (e.g., pH, salinity, temperature, redox potential, suspended solids and dissolved organic matter) affect the sorption and desorption processes.10,34–37 Given the complex framework governing the sorption–desorption processes, unveiling the consequences for the ecosystem is challenging.35,38
The analysis of metals on plastic samples collected from environmental matrices can represent an initial step to assess the relevance of this interaction in environmental settings and to help in assessing the environmental risk posed by metal-containing additives leaching from aged plastics, as well as by the metals adsorbed on MPs from the environment. Several approaches have been proposed to examine metals on plastic samples. Acid digestion with strong acid mixtures is still the most employed method: it targets the total content of metals in plastics through the complete dissolution of the plastic polymers. This approach does not allow comparison of the chemical species sorbed on MPs and the metals present in the bulk polymer, limiting the information for an effective bioavailability and exposure assessment.39–41 Thereby, other methods have been applied to specifically investigate loosely bonded or bioavailable fractions of metals, including single and sequential extractions.29,42–46
In our study, we tested a comprehensive analytical approach including total acid digestion, a single-step extraction and a sequential extraction protocol to assess the metal content and fractionation on MP samples. This approach was performed on environmental plastic litter found on the shores of Lake Como (Lombardy, Italy) and, for comparison, on equivalent virgin plastic objects obtained from local grocery stores. The comparison between virgin and environmental samples helped to size the metal pool originally present in the plastic formulations and, by comparison, the potential changes in (bio)availability (i.e., the release from the polymer matrix or the sorption from the environment). In addition, we investigated the role of plastic properties and environmental ageing in the changes in metal availability through their characterization using Fourier-transformed infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and water contact angle. This study focused on a freshwater body since freshwater ecosystems have recently been reported as sinks for MPs, but they are still limitedly studied concerning the interaction of MPs and metals.39,47–49 Data associated with these environments are therefore limited and fragmented.50,51
2. Materials and methods
2.1 Study area
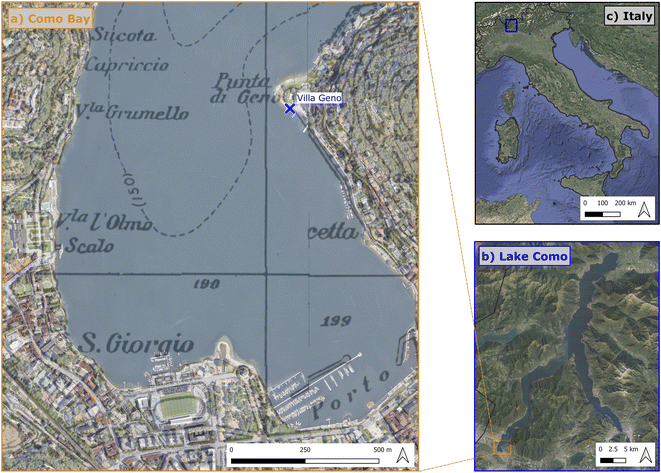
The study area for the collection of environmental plastic and water samples encompassed Como shores (Italy). This site is located at the end of the southwestern branch of Como Lake: a glacial, meso-eutrophic lake in northern Italy.52,53 Water chemistry in Lake Como is dominated by Mg2+, Ca2+ and HCO3− ions.54In this part, the lake has no effluents. Two main streams (Valduce and Cosia), instead, drain from Como city to the lake. The main sources of plastic litter in the Bay were observed to be direct littering on the beaches and longitudinal transport of floating litter from the northern part of the lake induced by local winds.55 We collected plastic litter samples around Villa Geno (45.823° N, 9.076° E, Fig. 1) since this site was observed to be a major hot spot of plastic litter in the area.55
2.2 Plastic sample collection and pre-treatment
In this study, environmental and pristine macroscopic plastic objects and fragments (>5 mm) were used to generate simulated MPs after a grinding process. This approach enables the analysis of the abundant and homogenous sample in the MP size range, which in turn opens the way to analyse both the total amount of metals and the fractionation through different extraction protocols from a single sample batch.46We manually collected macroscopic plastic fragments characterized by a visible advanced ageing state. Sample collection was done wearing nitrile gloves and the samples were stored in virgin polyethylene (PE) bags to avoid metal contamination. Once in the laboratory, environmental samples were sorted according to the object category of litter (e.g., bottles, glasses, caps, toys, packaging), colour and polymer type. The latter was assigned after visual sorting in case recycling codes were still visible and validated through FTIR analysis (see Section 2.3).
Samples of virgin plastics were obtained from new plastic objects representing the same categories, colours, and polymer types as the environmental samples. These samples were analysed in order to: (i) evaluate the concentrations of metals voluntarily used as additives in new objects during manufacturing, and (ii) control potential contamination during sample processing.
All plastic samples (virgin and environmental) were in fact processed in the same way. They were first rinsed with ultrapure water to remove other solids loosely attached to the surface (e.g., sand) and air-dried for approximately 24 hours. Samples were then cut into smaller pieces, ground with a commercial mixer with stainless-steel blades, and sieved to <2 mm. This treatment yielded enough homogeneous samples in the form of fragments with the size scale of MPs (<5 mm), needed for the following analytical steps. In total, 3 samples of PE, 3 of polypropylene (PP) and 3 of polyethylene terephthalate (PET) were prepared for metal analysis. For every polymer category, one pristine and two environmental samples were analysed (Table 1).
| Polymer type | Sample label | Type of sample | Item | Colour |
|---|---|---|---|---|
| PE | PEvir1 | Virgin | Cap | Blue, green, and white |
| PEenv2 | Environmental | Cap | Blue, green, and white | |
| PEenv3 | Environmental | Vase | Grey | |
| PP | PPvir1 | Virgin | Cup | Transparent |
| PPenv2 | Environmental | Cup | Transparent | |
| PPenv3 | Environmental | Cup | White | |
| PET | PETvir1 | Virgin | Bottle | Transparent |
| PETenv2 | Environmental | Bottle | Transparent | |
| PETenv3 | Environmental | Bottle | Transparent |
2.3 Plastic sample characterization
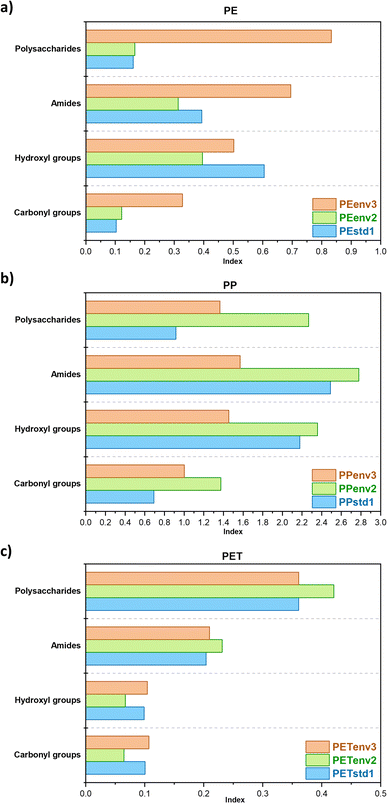
Samples were analysed for specific surface functional groups with a Thermo Scientific™ (Waltham, MA, USA) Nicolet™ iS™ 10 attenuated total reflectance FTIR spectrometer, in order to: (i) determine the polymer composition and (ii) assess the changes of surface functional groups induced by environmental ageing and biofilm development. Thirty-two scans were performed for every sample in the 4000–650 cm−1 spectral interval, with a resolution of 0.482 cm−1. A background spectrum was recorded prior to every measurement. All collected FTIR spectra were smoothed using the Savitzky–Golay filter (30 points of the window) and normalized on the maximum absorbance peak using Origin 2018 software (OriginLab Corporation). A range of indexes were calculated for a quantitative comparison of FTIR data. The following generalized equation was applied for calculating the indexes of different functional groups:in which Ii represents the index for the surface group analysed, namely the carbonyl group at 1715 cm−1, hydroxyl groups at 3500 cm−1, amides at 1650 cm−1, and polysaccharides at 1040 cm−1. Ai is the absorbance value at the specific wavelength, whereas Aref stands for the absorbance values at specific reference bands selected for the different polymer types: the C–H band at 1465 cm−1 for PE,56 the reference peak at 1892 cm−1 for PP,57 and the band absorbing at 721 cm−1 for PET.58
In addition to FTIR spectra, SEM images were collected to examine the surface micromorphology using a Philips® (Netherlands) field emission gun-scanning electron microscope (FEG-SEM), with a 20 keV beam under high vacuum conditions. Before SEM analysis, samples were made more conductive by covering them with a gold layer using a Cressington (UK) 108 auto vacuum sputter coater.
Static water contact angles were also measured to assess the surface charge of plastic samples. Briefly, 5 μL of ultrapure water was deposited on the sample surface and pictures were collected via a smartphone camera system. Then, contact angles were processed using ImageJ software.59
2.4 Metal analyses
The total metal concentration in all plastic samples was assessed by acid digestion.44,60 Approximately 60 milligrams of the prepared sample were weighed and put into a Teflon vessel, where 4 mL of ultrapure concentrated nitric acid were added. The vessels were placed in a microwave oven (Milestone ETHOS One), with a 10 minute initial heating up to 180 °C and a following isotherm of 15 minutes. After digestion, the solutions were transferred into LDPE bottles, diluted with ultrapure water and stored at 4 °C in a refrigerator.Surface extraction with ultrapure nitric acid (HNO3) 2% v/v in an ultrasonic bath for 10 minutes at 120 W was also applied to desorb all loosely bonded metals.27 In addition, a recently proposed sequential extraction scheme was performed on the samples to analyse three different metal fractions on MPs: (i) the physisorbed and readily soluble fraction of metals with ammonium nitrate 1 M;61 (ii) the acid-soluble fraction of metals with acid acetic 0.1 M; and (iii) the oxidable and bonded to organic matter fraction of metals with hydrogen peroxide 30% v/v. A detailed description of this methodology is available elsewhere.46
Solutions obtained by acid digestions and different extraction methods were analysed through inductively coupled plasma – mass spectrometry (ICP-MS Thermo Scientific™ Icap-Q, USA) for the detection and quantification of 18 metals, namely: aluminium (Al), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), strontium (Sr), silver (Ag), cadmium (Cd), tin (Sn), barium (Ba), lead (Pb) and uranium (U).
2.5 Water sample collection and chemical analysis
Along with the collection of plastic items, lake surface water was sampled to analyse the physicochemical conditions and the metal concentration in the water phase, potentially affecting sorption processes. Physicochemical parameters (pH, temperature, and electrical conductivity) were measured in situ using handheld probes (HI 9025 pH meter and HI 9033 conductivity meter, HANNA Instruments, Italy). For metal analyses, water samples were directly filtered in situ and stored in pre-cleaned LDPE bottles (Nalgene®).62 These bottles were kept in the dark and transported to the laboratory, where samples were stored in a refrigerator at 4 °C. The analysis of trace elements was done via ICP-MS, as explained in Section 2.3. Before ICP-MS analysis, samples were acidified by the addition of ultra-pure distilled nitric acid reaching a final concentration of 2% v/v. Samples were analysed in three replicates, monitoring the relative standard deviation (RSD) to be below ±5%. Major anions and cations (F−, Cl−, Br−, NO3−, PO43−, SO42−, Li+, Na+, NH4+, K+, Mg2+, and Ca2+) were instead determined through ion chromatography (Eco IC Metrohm, Switzerland). More details concerning water sampling methods, analysis and QA/QC protocols are reported elsewhere.632.6 Reagents, QA/QC protocols and data analysis
Quality control (QA/QC) measures were applied to ensure reliable and accurate measurements of metals. First, all operations were carried out under a linear flow hood to avoid airborne contamination (aura HZ72T BIOAIR, Italy). The solutions were prepared from analytical grade reagents and ultrapure water (obtained by a Sartorius Arium® mini, Germany; resistivity: 18.8 M Ω cm; TOC: <5 ppb). Ultrapure nitric acid 67–69% was produced through purification of analytical grade acid (Carlo Erba reagents, Italy) using sub-boiling distillation in a Milestone (USA) DuoPur system.64 Ammonium nitrate solution was prepared by dissolution of its crystalline salt (Carlo Erba reagents, Italy). Acetic acid solution was obtained from dilution of glacial acetic acid (Carlo Erba reagents, Italy), while hydrogen peroxide 30% v/v was purchased as it is (Sigma-Aldrich, USA). Before use, all the lab containers were cleaned with a detergent solution (NALGENE® L900) first, then soaked in a 2% nitric acid bath, washed with ultrapure water, and lastly air dried under a laminar flow hood. To assure precision and accuracy in the quantification during ICP-MS analysis, a multi-element standard was used for external calibration. This was prepared by dilution of Sigma-Aldrich (USA) multi-elemental standard. Analytical blanks were employed to take any source of possible contamination into account. To avoid cross-contamination during the grinding process, the blender was rinsed twice with ultrapure water between any sample processed.All the measurements were performed in replicates to determine their uncertainty. Method detection limits (MDLs) were estimated using method blanks: they were calculated as the mean determined concentration plus three times the standard deviation of a set of method blanks. During data analysis, values which were below the method detection limits were substituted with MDL/2 value.65
The final data were reported as μg of the extracted metal by a unit of volume (cm3) of the dry sample. This method of displaying results allowed all values coming from different polymer types to be compared with each other. To this end, reference density values chosen for weight-to-volume conversions were 0.94 μg cm−3 for PE, 0.90 μg cm−3 for PP and 1.41 μg cm−3 for PET.66
To evaluate the available fractions (extractable from the surface) against the total metal quantities in different samples, extraction ratios were calculated as follows:
Descriptive statistics (25th–75th percentiles, median and maximum) were applied to summarize the dataset regarding acid digestion, surface extraction and sequential extractions for all samples. Calculations and other statistics (non-parametric Mann–Whitney test) were performed with Origin 2018 software (OriginLab Corporation, Northampton, MA, USA).
3. Results and discussion
3.1 Total and extractable concentrations of metals from plastic samples
The total load of metals in the samples showed pronounced variability with concentrations ranging from values greater than 1000 μg cm−3 to values below the limit of detection (Table S1 and Fig. S1†). Specifically, metals such as Sn, Fe, Al, Ba and Zn were detected at the highest concentrations (average values ranged from ∼10 μg cm−3 to ∼200 μg cm−3), Mn, Cu, Sr and Ti exhibited one order of magnitude lower concentrations (average values from ∼1 μg cm−3 to ∼10 μg cm−3), while Ni, Pb, As, Cr, Ag, Co, V, Cd and U were less abundant (averagely below 1 μg cm−3, Table S1†). This heterogeneous trend was also observed in virgin samples: no statistically meaningful differences in the total metal concentration were observed between environmental and pristine samples for most of the analysed elements (Mn and Pb were the only two exceptions, Fig. S1†). The high total concentrations of several metals in virgin samples indicate the abundant use of inorganic and metal–organic additives and fillers in the formulation of plastic items (see detailed discussion in Section 3.4).High variance of total metals in environmental plastics was reported for other freshwater settings.39,49 Variance in metal content can stem from sample polymer type and level of ageing, environmental conditions of the sampling areas and extraction methods used for metal quantification. This high variance also hampers a proper comparison with our data. These outcomes confirm the great chemical heterogeneity of plastic materials, as well as the complexity of the interactions with other chemicals they are exposed to in the environment. The interpretation of the total digestion data from environmental plastic, therefore, should be carefully investigated in order to avoid potential overestimations of the environmental exposure and bioavailability.
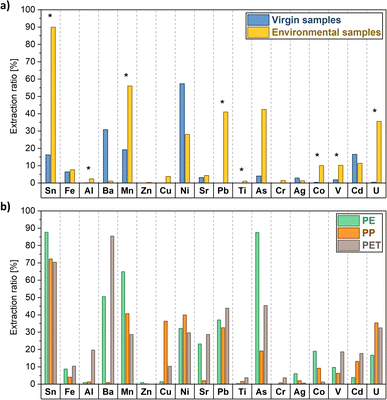
The extraction ratios measured through data from the surface extraction (HNO3 2%, targeting loosely bound metals on the surface of the objects) revealed a clearer trend. Environmental samples of every polymer displayed, in fact, generally higher extraction ratios compared to virgin samples for the corresponding metals, with significant differences for 8 of the analysed metals (i.e., Sn, Al, Mn, Pb, Ti, Co, V and U, Fig. 2a). Considering the environmental samples, Sn, Pb, Mn and U were the metals desorbing from the surface in a greater quantity relative to their total content (extraction ratios ranged from 30% to 90%). By contrast, other metals yielded <10% in extraction ratios (i.e., Fe, Cr, Ag, Cu, Sr and Zn), indicating that they were likely more present (and more strongly retained) in the polymer matrix, most probably already in origin. The significant increase of extraction ratios of environmental samples compared to virgin ones can be potentially related to the increased leaching of metal additives after polymer ageing: experimental evidence in the laboratory highlighted that the polymer ageing process (after UV irradiation) increased extraction ratios of different plastic types, reaching values up to the 75%.67
Ni, Cd and Ba showed instead (not statistically significant) higher values of extraction ratios for virgin plastics in comparison to environmental samples. This observation coincided however with extremely low concentrations of these elements in both the total digestion and the direct extraction of several pristine MPs (Table S1†), which may more easily lead to an overestimation of the extraction ratios. Considering the absolute values of surface extractions with HNO3 2% (Table S2 and Fig. S2†), in fact, environmental samples show a significantly higher absolute concentration of these metals in comparison to virgin samples.
3.2 Metal fractionation on plastic samples
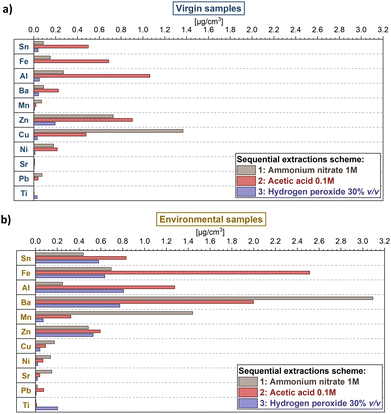
Sequential extractions provided further insights concerning the fractions of labile metals, helping in discriminating the possible sources of the metals on plastic samples.The discussion of metal fractionation here is limited to the 11 more abundant analysed elements since the others (i.e., Ag, As, Cd, Co, Cr, V, U) presented values below the detection limits for more than 50% of the samples, hampering a significant comparison of data. Expectedly, as for other extractions, there were marked differences between environmental and pristine plastic samples (Fig. 3 and Table S2†). Virgin samples yielded lower concentrations of metals in all three extraction steps (Fig. 3a) in comparison to environmental samples (Fig. 3b) for most of the analysed elements, with the exceptions of Cu and Zn. Results were in line with the concentrations obtained from surface extraction.
The fractionation pattern of the metals differed between virgin and environmental samples, too. In the case of virgin samples, the second extraction step (acetic acid 0.1 M) provided higher quantities for most metals (Mn, Cu, Pb and Ti were the only exceptions), whereas the third extraction step (hydrogen peroxide 30% v/v) yielded the lowest amount (or below detection limits), with the only exception of Ti. Environmental samples showed, instead, several changes in metal fractionation. For example, the third extraction step produced a significant increase in the case of Sn, Fe, Al and Zn: this effect could be due to the sorption of metals in the organic matter (or biofilms) present on the surface of plastics obtained from the environment, and absent on pristine plastics.68–70 In addition, other metals such as Ni and Sr showed higher concentrations in the first step (which extracted metals bonded through more labile forms of bonding compared to the other steps). For Al, Fe, Zn and Pb instead the extraction step with acetic acid yielded the highest quantity of metals. These results were in line with previous studies, indicating a pH-dependent leaching of these metals.25,27,71 The high variability of changes in metal fractionation proves again that plastic–metals interactions are complex processes, both dependent on metal properties (e.g., the source as additive or the sorption in the environment and its chemical properties) and the environmental conditions.
3.3 The role of polymer type and ageing in regulating plastic–metal interactions
The differences observed between environmental and pristine samples indicate that MP environmental ageing processes are key in defining metal sorption–desorption equilibria, confirming the results of other case studies investigating MP–metal interactions.72–76 The physicochemical characterization of the plastic samples helped in shedding light on the factors affecting sorption–desorption processes.FTIR analysis guided the identification of the polymers composing the environmental and pristine samples (i.e., PE, PP and PET, Fig. S3†) through the analysis of the fingerprinting absorption bands (Table S3†). These polymers are indeed the most used in everyday items (PE and PP are the two most manufactured types worldwide), as also found in other settings.77 In addition, the abundance of PE and PP is representative of beached litter, which is generally composed of low-density polymers, which float on water and are transported by surface currents and winds.55
The FTIR spectra of environmental samples also showed some clear changes in functional groups induced by the environmental ageing of polymers in comparison to the virgin samples.78 The changes in the indexes for specific aging bands are shown in Fig. 4: the main variations were observed in the regions of hydroxyl (3600–3000 cm−1) and carbonyl groups (1300–1000 cm−1), confirming the superficial oxidation due to a series of co-occurring environmental processes, such as UV exposure, atmospheric oxygen and thermal effects. This is particularly evident for polymers mainly composed of aliphatic chains like PE and PP. These changes in functional groups on the surface of polymers may affect metal sorption, with the potential development of either coordination complexes or non-covalent interactions.2,38,79 Another process that can modify polymer FTIR spectra is biofouling.68,69 The presence of a biofilm on the environmental samples can be inferred from characteristic absorption peaks indicative of either proteins (1650 cm−1 and 1550 cm−1 regions, respectively for amide I and II stretching), or polysaccharides (1040 cm−1 band of C–O stretching).81 These bands were noticeable in 2 out of 6 environmental samples (PEenv3 and PPenv2, respectively), while clearly lacking in virgin samples. These two samples, in fact, showed higher concentrations of Al, Mn, Fe and Pb compared to most of the other pristine and environmental samples in the most labile fractions (steps 1 and 2, Table S2†). This organic layer may play a pivotal role in the sorption of metals from the environment and in the potential release of additives. The different surface groups may induce chemical sorption of several metals (e.g., through complexation with extracellular polysaccharides), while microorganisms may also actively absorb metals from water in solution.68,69,82–84 Biofilms may mediate metal additive release from plastic, too.85 Unfortunately, the lack of a quantitative analysis of biofilm coverage on plastics hampered a clear assessment of this process in our study.
Beyond surface functional groups, environmental ageing of plastic affected the surface micromorphology of the generated MPs. SEM images (Fig. 5) showed, in fact, evident differences between the images of virgin samples and environmental samples, regardless of the type of polymer composing the sample. The surfaces of pristine samples appeared smooth and homogeneous, with limited irregularities, whereas those of environmental samples were typically rugged, and wrinkled, with several evident pits and cracks (Fig. S4†). These changes may indeed increase both the sorption of metals from the environment and the release of metal additives due to the increase of the specific surface area of plastic fragments, as already largely documented in prior studies.23,27,49,86 Another noteworthy feature of environmental samples was the presence of several organisms composing the biofilm community on the surface of environmental samples: the residuals of diatom frustules are evident in some of the samples (Fig. 5b and f), as well as cocci and filamentous-shaped cells colonizing the surfaces (shown in Fig. 5d). These organisms can actively absorb metals in their cytoplasm and entrap ions in their extracellular matrices, favouring the sorption from the environment.87
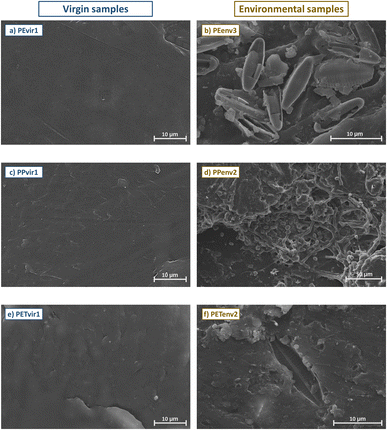 | ||
| Fig. 5 SEM images of one virgin (a, c, and e) versus one environmental (b, d, and f) sample for every polymer type (i.e., PE, PP and PET) at various magnifications. | ||
Finally, the effects of ageing on the surface properties of plastic samples are corroborated by the water contact angle measurements (Fig. S5†). The values of contact angle are influenced by the polymer type in virgin samples: PP and PE samples are hydrophobic, while PET is slightly hydrophilic (i.e., contact angle <90°). All the environmental samples, however, showed a decrease in contact angle values of at least 10° in comparison to the virgin sample of the same polymer, regardless of the polymer type. This confirms the effect of ageing and biofouling, making the surface of plastics richer in polar surface functional groups and increasing wettability. This increase in wettability can both favour metal sorption processes82 and facilitate the release of metal-containing additives.72,80
3.4 Assessing the potential sources of elements in plastics
The combination of the information obtained by the analysis of total, surface extracted and sequentially extracted metals on the plastic samples helped in assessing the origin of metals in the samples; the characterization of plastic samples and the analysis of water samples gave insights into the chemical features of plastics and the water chemical conditions affecting sorption–desorption processes. The analysis of metals in the water phase further helped in investigating which metals were potentially enriched from the environment (Tables S4 and S5†).The changes in metal fractionation between virgin and environmental samples can give further insights into understanding their origin. For example, Fe, Mn, Zn and Al exhibited a marked increase in the third extraction step, indicating a strong effect of the organic layer or the biofilm formed on plastic in the sorption of these metals from the environment. The high concentration of these elements in this step for the samples PEenv3 and PPenv2, which showed also higher absorbance in the bands representing biofilms (Fig. 4), further corroborates this hypothesis.
For Pb and Mn a significantly higher concentration was observed in environmental samples by acid digestion, too. The significantly higher total concentration further suggests an enrichment of these elements on the surface of environmental plastics in comparison with virgin samples.
Some of the abovementioned metals were also abundant in the water of Como Bay (e.g., Sr: 146.39 μg L−1; Zn: 20.90 μg L−1; Al: 15.8 μg L−1; Fe: 3.14 μg L−1): this is consistent with the hypothesis of their prevailing environmental origin.
Some metals with potential enrichment from the environment show low extraction ratios in environmental samples, too (i.e., Zn, Al, Zn, Fe and Sr). All these metals also showed non-significant differences in the total concentration from acid digestion between environmental and virgin samples (Fig. S1†). These elements may therefore have a contribution from the plastic polymer matrix too, indicating a mixed source (i.e., a contribution of both sorption from the environment and additive leaching) in environmental samples.
As a final note, the linkage between plastic colours and metal content indicated the extensive use of metal-based pigments for plastic products. For example, Pb and Ba are used as yellow pigments, while Cu, Pb and Cr-based pigments are, instead, often mixed to obtain darker variants.25,92 This is evident observing the acid digestion results of our samples: the blue and green PEvir1 and PEenv2 showed a higher abundance of Fe, Zn, Cu, Ba and Cr compared to other samples (Table S1†). This is also in accordance with other reports.19,21,93 In our study we also assessed an increase in the concentration of these elements in the most labile fraction (i.e., step 1 of the sequential extraction). This evidence clearly shows that plastic ageing processes also make metal additives more likely to be released, and, in turn, more bioavailable.
3.5 Lessons learned in this study towards a refined risk assessment of metals and environmental plastics
The combination of different analytical approaches in this study helped in gaining insights into the origin of metals in environmental plastics. This approach can improve the understanding of sorption–desorption processes from the environment and help in investigating the likelihood of additive release during plastic environmental ageing. This in turn can help in improving assessment of the risk of MPs as metal vectors.37,73,94–96The results observed from the acid digestion of plastic samples in our study (Section 3.1) showed, instead, that the total load of metals is often similar in virgin and environmental samples. This indicates a potential overestimation of the risk when exposure assessment is based upon the total acid digestion data:97,98 the potential bioavailability of the metals strongly bonded within the polymer matrix relies on the kinetics of additive release, the polymer type and the grade of environmental ageing.99
The results of this study also remark on the complexity of plastic–metal interactions in the environment. Despite the complete analytical assessment of the analysed environmental samples, the results showed high variance and several unclear trends. This indicates that plastic–metal interactions are driven by several factors at stake, such as the chemical property of the metal, polymer type, degree of ageing and the water chemical conditions. The assessment of the origin and, consequently, the potential availability of metals in plastics are therefore far from straightforward: even a thorough analytical approach as the one used in this study led to some unclear trends. Several metals (e.g., Al, Fe, Sn, Sr), in fact, not surprisingly showed a possible mixed origin.
The results of this study also highlight the increase in the concentration of metals in the extractable phases in environmental samples compared to virgin ones, regardless of their origin. The processes of environmental ageing are key to affect the potential bioavailability of metals on plastic, both by increasing the sorption capacity of metals from the environment and promoting the release of additives from the polymeric matrix. Therefore, we suggest future studies to assess the potential factors changing metal bioavailability in more simplified systems (e.g., micro- or mesocosms), in order to test the effects of different factors avoiding confounding factors and uncontrollable conditions (e.g., adding plastics with a known amount of metal additives).
4. Conclusions
In this study, we examined metals associated with beached plastics sampled from Lake Como shores. We applied a multi-tiered analytical approach to differentiate the origin of metals on environmental plastic samples, possibly discerning the metals enriched by sorption processes and the additives released by plastic polymers. The analysis of the total concentration, the labile fraction and the different fractionation by extraction techniques shed light on the speciation of metals in different samples and the comparison of environmental samples and virgin plastic objects helped in assessing the background concentrations of metals in plastic polymers.The combination of this information also helped in understanding the more likely origin of the analysed metals in the environmental samples: Mn and Pb were mostly enriched from the environment; Ba, Cu and Ti were mainly released from the polymer matrix; Zn, Sn, Sr, Fe and Al instead likely originated from a mixed source.
This study also showed that environmental samples generally present higher concentrations of labile metals, which indicates a potential increased environmental risk of metal bonded to plastics induced by polymer ageing processes. The sequential extraction results also indicated an increase of several metals (e.g., Al, Fe, Zn) in the organic matter bonded fraction, confirming the important role of organic matter and biofilms in mediating the sorption process of metals from the environment. Overall, our findings highlighted the role of plastics as potential carriers for the transfer of metals in freshwater media and confirmed that field studies can give important insights into the environmental risk related to this issue.
Author contributions
Conceptualization: SC and GB (Gilberto Binda); methodology: SC, DS, DM, and GB (Gilberto Binda); investigation: SC, DS, and GB (Ginevra Boldrocchi); data curation: SC, AP, and RB; writing (original draft): SC; writing (review and editing): AP, DS, DM, RB, GB (Ginevra Boldrocchi), LN, and GB (Gilberto Binda); supervision: AP, RB, and LN; funding acquisition: LN and GB (Gilberto Binda).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the European Commission under the H2020 MSCA-IF project “PLANET-understanding PLAstic pollutioN effects on the biogeochemical cycle of ElemenTs” (grant number 101023603). The authors would also acknowledge the three anonymous reviewers for their helpful comments and suggestions.Notes and references
- A. L. Andrady, Mar. Pollut. Bull., 2011, 62, 1596–1605 CrossRef CAS PubMed.
- S. Liu, J. Huang, W. Zhang, L. Shi, K. Yi, H. Yu, C. Zhang, S. Li and J. Li, J. Environ. Manage., 2022, 302, 113995 CrossRef CAS PubMed.
- C. M. Rochman, M. A. Browne, B. S. Halpern, B. T. Hentschel, E. Hoh, H. K. Karapanagioti, L. M. Rios-Mendoza, H. Takada, S. Teh and R. C. Thompson, Nature, 2013, 494, 169–171 CrossRef CAS PubMed.
- T. Zhang, B. Jiang, Y. Xing, H. Ya, M. Lv and X. Wang, Environ. Sci. Pollut. Res., 2022, 29, 16830–16859 CrossRef CAS PubMed.
- W. Steffen, Å. Persson, L. Deutsch, J. Zalasiewicz, M. Williams, K. Richardson, C. Crumley, P. Crutzen, C. Folke, L. Gordon, M. Molina, V. Ramanathan, J. Rockström, M. Scheffer, H. J. Schellnhuber and U. Svedin, Ambio, 2011, 40, 739–761 CrossRef PubMed.
- J. Zalasiewicz, C. N. Waters, J. A. Ivar do Sul, P. L. Corcoran, A. D. Barnosky, A. Cearreta, M. Edgeworth, A. Gałuszka, C. Jeandel, R. Leinfelder, J. R. McNeill, W. Steffen, C. Summerhayes, M. Wagreich, M. Williams, A. P. Wolfe and Y. Yonan, Anthropocene, 2016, 13, 4–17 CrossRef.
- Plastic Debris in the Ocean: The Characterization of Marine Plastics and Their Environmental Impacts, Situation Analysis Report, ed. F. Thevenon, C. Carroll and J. Sousa, International Union for Conservation of Nature, 2015 Search PubMed.
- D. Fu, Q. Zhang, Z. Fan, H. Qi, Z. Wang and L. Peng, Aquat. Toxicol., 2019, 216, 105319 CrossRef CAS PubMed.
- X. Guo and J. Wang, Mar. Pollut. Bull., 2019, 142, 1–14 CrossRef CAS PubMed.
- J. Zou, X. Liu, D. Zhang and X. Yuan, Chemosphere, 2020, 248, 126064 CrossRef CAS PubMed.
- G. Kutralam-Muniasamy, F. Pérez-Guevara, I. Elizalde-Martínez and V. C. Shruti, Sci. Total Environ., 2020, 714, 136823 CrossRef CAS PubMed.
- D. Peixoto, C. Pinheiro, J. Amorim, L. Oliva-Teles, L. Guilhermino and M. N. Vieira, Estuarine, Coastal Shelf Sci., 2019, 219, 161–168 CrossRef CAS.
- V. C. Shruti, F. Pérez-Guevara, I. Elizalde-Martínez and G. Kutralam-Muniasamy, Sci. Total Environ., 2020, 726, 138580 CrossRef CAS PubMed.
- G. Chen, Q. Fu, X. Tan, H. Yang, Y. Luo, M. Shen and Y. Gu, Environ. Pollut., 2022, 295, 118695 CrossRef CAS PubMed.
- A. P. da Costa Araújo, N. F. S. de Melo, A. G. de Oliveira Junior, F. P. Rodrigues, T. Fernandes, J. E. de Andrade Vieira, T. L. Rocha and G. Malafaia, J. Hazard. Mater., 2020, 382, 121066 CrossRef PubMed.
- J.-P. W. Desforges, M. Galbraith and P. S. Ross, Arch. Environ. Contam. Toxicol., 2015, 69, 320–330 CrossRef CAS PubMed.
- M. Long, B. Moriceau, M. Gallinari, C. Lambert, A. Huvet, J. Raffray and P. Soudant, Mar. Chem., 2015, 175, 39–46 CrossRef CAS.
- X. Li, Y. Chen, S. Zhang, Y. Dong, Q. Pang, I. Lynch, C. Xie, Z. Guo and P. Zhang, Ecotoxicol. Environ. Saf., 2023, 251, 114564 CrossRef CAS PubMed.
- K. Ashton, L. Holmes and A. Turner, Mar. Pollut. Bull., 2010, 60, 2050–2055 CrossRef CAS PubMed.
- L. A. Holmes, A. Turner and R. C. Thompson, Environ. Pollut., 2012, 160, 42–48 CrossRef CAS PubMed.
- A. Turner and L. A. Holmes, Environ. Chem., 2015, 12, 600 CrossRef CAS.
- Y. Dong, M. Gao, Z. Song and W. Qiu, Environ. Pollut., 2019, 254, 112950 CrossRef CAS PubMed.
- P. Liu, K. Lu, J. Li, X. Wu, L. Qian, M. Wang and S. Gao, J. Hazard. Mater., 2020, 384, 121193 CrossRef CAS PubMed.
- V. Ambrogi, C. Carfagna, P. Cerruti and V. Marturano, in Modification of Polymer Properties, ed. C. F. Jasso-Gastinel and J. M. Kenny, William Andrew Publishing, 2017, pp. 87–108 Search PubMed.
- C. Catrouillet, M. Davranche, I. Khatib, C. Fauny, A. Wahl and J. Gigault, Environ. Sci.: Processes Impacts, 2021, 23, 553–558 RSC.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, J. Hazard. Mater., 2018, 344, 179–199 CrossRef CAS PubMed.
- F. Gao, J. Li, C. Sun, L. Zhang, F. Jiang, W. Cao and L. Zheng, Mar. Pollut. Bull., 2019, 144, 61–67 CrossRef CAS PubMed.
- J. P. Rodrigues, A. C. Duarte and J. Santos-Echeandía, J. Hazard. Mater. Adv., 2022, 6, 100072 CrossRef CAS.
- G. Chen, Q. Fu, X. Tan, H. Yang, Y. Luo, M. Shen and Y. Gu, Environ. Pollut., 2022, 295, 118695 CrossRef CAS PubMed.
- G. Kalčíková, T. Skalar, G. Marolt and A. Jemec Kokalj, Water Res., 2020, 175, 115644 CrossRef PubMed.
- M. Salehi, C. T. Jafvert, J. A. Howarter and A. J. Whelton, J. Hazard. Mater., 2018, 347, 242–251 CrossRef CAS PubMed.
- J. Meng, B. Xu, F. Liu, W. Li, N. Sy, X. Zhou and B. Yan, Chemosphere, 2021, 283, 131274 CrossRef CAS PubMed.
- G. Binda, G. Zanetti, A. Bellasi, D. Spanu, G. Boldrocchi, R. Bettinetti, A. Pozzi and L. Nizzetto, Environ. Sci. Pollut. Res., 2023, 30, 6298–6312 CrossRef CAS PubMed.
- M. Ahechti, M. Benomar, M. El Alami and C. Mendiguchía, Int. J. Environ. Anal. Chem., 2022, 102, 1118–1125 CrossRef CAS.
- G. Binda, D. Spanu, D. Monticelli, A. Pozzi, A. Bellasi, R. Bettinetti, S. Carnati and L. Nizzetto, Water Res., 2021, 204, 117637 CrossRef CAS PubMed.
- V. Godoy, G. Blázquez, M. Calero, L. Quesada and M. A. Martín-Lara, Environ. Pollut., 2019, 255, 113363 CrossRef CAS PubMed.
- L. A. Holmes, A. Turner and R. C. Thompson, Mar. Chem., 2014, 167, 25–32 CrossRef CAS.
- Y. Cao, M. Zhao, X. Ma, Y. Song, S. Zuo, H. Li and W. Deng, Sci. Total Environ., 2021, 788, 147620 CrossRef CAS PubMed.
- C. Campanale, I. Savino, C. Massarelli and V. F. Uricchio, NanoImpact, 2022, 28, 100438 CrossRef CAS PubMed.
- C. Catrouillet, M. Davranche, I. Khatib, C. Fauny, A. Wahl and J. Gigault, Environ. Sci.: Processes Impacts, 2021, 23, 553–558 RSC.
- I. Savino, C. Campanale, P. Trotti, C. Massarelli, G. Corriero and V. F. Uricchio, Polymers, 2022, 14, 1958 CrossRef CAS PubMed.
- M. E. Hodson, C. A. Duffus-Hodson, A. Clark, M. T. Prendergast-Miller and K. L. Thorpe, Environ. Sci. Technol., 2017, 51, 4714–4721 CrossRef CAS PubMed.
- L. A. Holmes, R. C. Thompson and A. Turner, Environ. Pollut., 2020, 261, 114107 CrossRef CAS PubMed.
- G. Kutralam-Muniasamy, F. Pérez-Guevara, I. E. Martínez and V. C. Shruti, J. Hazard. Mater., 2021, 415, 125755 CrossRef CAS PubMed.
- A. Turner, Environ. Pollut., 2018, 236, 1020–1026 CrossRef CAS PubMed.
- G. Binda, S. Carnati, D. Spanu, A. Bellasi, R. Hurley, R. Bettinetti, D. Monticelli, A. Pozzi and L. Nizzetto, J. Hazard. Mater., 2023, 452, 131330 CrossRef CAS PubMed.
- W. Li, H.-S. Lo, H.-M. Wong, M. Zhou, C.-Y. Wong, N. F.-Y. Tam and S.-G. Cheung, Mar. Pollut. Bull., 2020, 153, 110977 CrossRef CAS PubMed.
- A. T. Ta and S. Babel, Chemosphere, 2020, 257, 127234 CrossRef CAS PubMed.
- J. Wang, J. Peng, Z. Tan, Y. Gao, Z. Zhan, Q. Chen and L. Cai, Chemosphere, 2017, 171, 248–258 CrossRef CAS PubMed.
- A. Bellasi, G. Binda, A. Pozzi, S. Galafassi, P. Volta and R. Bettinetti, Environments, 2020, 7, 30 CrossRef.
- R. Leiser, G.-M. Wu, T. R. Neu and K. Wendt-Potthoff, Water Res., 2020, 176, 115748 CrossRef CAS PubMed.
- D. Copetti, N. Guyennon and F. Buzzi, Sci. Total Environ., 2020, 749, 141587 CrossRef CAS PubMed.
- W. Scheffler and G. Morabito, J. Limnol., 2003, 62, 47–60 CrossRef.
- G. Binda, F. Frascoli, D. Spanu, M. F. Ferrario, S. Terrana, R. Gambillara, S. Trotta, P. J. Noble, F. A. Livio, A. Pozzi and A. M. Michetti, Water, 2022, 14, 124 CrossRef CAS.
- A. Bellasi, G. Binda, G. Boldrocchi, A. Pozzi and R. Bettinetti, Appl. Sci., 2022, 12, 5388 CrossRef CAS.
- K. I. Martínez, R. González-Mota, J. J. Soto-Bernal and I. Rosales-Candelas, J. Appl. Polym. Sci., 2021, 138, 50158 CrossRef.
- A. Jansson, K. Möller and T. Gevert, Polym. Degrad. Stab., 2003, 82, 37–46 CrossRef CAS.
- X. Bai, F. Li, L. Ma and C. Li, Sci. Total Environ., 2022, 804, 150168 CrossRef CAS PubMed.
- W. Han, J. Shin and J. Ho Shin, HardwareX, 2022, 12, e00327 CrossRef PubMed.
- L. Hildebrandt, M. von der Au, T. Zimmermann, A. Reese, J. Ludwig and D. Pröfrock, PLoS ONE, 2020, 15, 1–18 CrossRef PubMed.
- M. Pueyo, J. F. López-Sánchez and G. Rauret, Anal. Chim. Acta, 2004, 504, 217–226 CrossRef CAS.
- G. Binda, A. Pozzi, F. Livio, P. Piasini and C. Zhang, J. Geochem. Explor., 2018, 190, 58–68 CrossRef CAS.
- M. R. Rosen, G. Binda, C. Archer, A. Pozzi, A. M. Michetti and P. J. Noble, Water Resour. Res., 2018, 54, 5225–5244 CrossRef CAS.
- D. Monticelli, A. Castelletti, D. Civati, S. Recchia and C. Dossi, Int. J. Anal. Chem., 2019, 2019, 1–5 CrossRef PubMed.
- R. A. Hites, Environ. Sci. Technol., 2019, 53, 11059–11060 CrossRef CAS PubMed.
- A. Bellasi, G. Binda, A. Pozzi, G. Boldrocchi and R. Bettinetti, Chemosphere, 2021, 278, 130357 CrossRef CAS PubMed.
- W. Feng, C. Huang, X. Tan, N. Tang, L. Zhang, H. Li, X. Xu and J. Peng, Ecotoxicology, 2022, 31, 75–84 CrossRef CAS PubMed.
- S. He, M. Jia, Y. Xiang, B. Song, W. Xiong, J. Cao, H. Peng, Y. Yang, W. Wang, Z. Yang and G. Zeng, J. Hazard. Mater., 2022, 424, 127286 CrossRef CAS PubMed.
- W. Qiongjie, Z. Yong, Z. Yangyang, L. Zhouqi, W. Jinxiaoxue and C. Huijuan, J. Hazard. Mater., 2022, 424, 127340 CrossRef PubMed.
- H. Richard, E. J. Carpenter, T. Komada, P. T. Palmer and C. M. Rochman, Sci. Total Environ., 2019, 683, 600–608 CrossRef CAS PubMed.
- M. Horváth, G. Halász, E. Kucanová, B. Kuciková, I. Fekete, D. Remeteiová, G. Heltai and K. Flórián, Microchem. J., 2013, 107, 121–125 CrossRef.
- Q. Fu, X. Tan, S. Ye, L. Ma, Y. Gu, P. Zhang, Q. Chen, Y. Yang and Y. Tang, Chemosphere, 2021, 270, 128624 CrossRef CAS PubMed.
- L. Gao, D. Fu, J. Zhao, W. Wu, Z. Wang, Y. Su and L. Peng, Mar. Pollut. Bull., 2021, 169, 112480 CrossRef CAS PubMed.
- L. Wang, C. Guo, Q. Qian, D. Lang, R. Wu, S. Abliz, W. Wang and J. Wang, Chemosphere, 2023, 313, 137439 CrossRef CAS PubMed.
- Q. Wang, Y. Zhang, X. Wangjin, Y. Wang, G. Meng and Y. Chen, J. Environ. Sci., 2020, 87, 272–280 CrossRef CAS PubMed.
- Y. Xia, S. Niu, T. Wang and J. Wu, Mar. Pollut. Bull., 2023, 187, 114588 CrossRef CAS PubMed.
- Y.-B. Ma, Z.-Y. Xie, N. Hamid, Q.-P. Tang, J.-Y. Deng, L. Luo and D.-S. Pei, Aquat. Toxicol., 2023, 261, 106597 CrossRef CAS PubMed.
- M. R. Jung, F. D. Horgen, S. V. Orski, V. Rodriguez C., K. L. Beers, G. H. Balazs, T. T. Jones, T. M. Work, K. C. Brignac, S.-J. Royer, K. D. Hyrenbach, B. A. Jensen and J. M. Lynch, Mar. Pollut. Bull., 2018, 127, 704–716 CrossRef CAS PubMed.
- S. Tang, L. Lin, X. Wang, A. Feng and A. Yu, J. Hazard. Mater., 2020, 386, 121960 CrossRef CAS PubMed.
- K. Aghilinasrollahabadi, M. Salehi and T. Fujiwara, J. Hazard. Mater., 2021, 408, 124439 CrossRef CAS PubMed.
- M. M. Rahman, S. Al-Sulaimi and A. M. Farooque, Appl. Water Sci., 2018, 8, 183 CrossRef.
- G. Binda, M. Costa, L. Supraha, D. Spanu, C. Vogelsang, E. Leu and L. Nizzetto, Sci. Total Environ., 2023, 893, 164807 CrossRef CAS PubMed.
- K. Qi, N. Lu, S. Zhang, W. Wang, Z. Wang and J. Guan, J. Hazard. Mater., 2021, 411, 125115 CrossRef CAS PubMed.
- Q. Xie, H. Li, Z. Li, H. Zhang, M. Yuan, M. Wu, H. Li and X. Xu, Gondwana Res., 2022, 108, 181–192 CrossRef CAS.
- G. Peng, Z. Pu, F. Chen, H. Xu, X. Cao, C. Chun Chen, J. Wang, Y. Liao, X. Zhu and K. Pan, Environ. Int., 2023, 177, 107988 CrossRef CAS PubMed.
- R. Mao, M. Lang, X. Yu, R. Wu, X. Yang and X. Guo, J. Hazard. Mater., 2020, 393, 122515 CrossRef CAS PubMed.
- M. S. Dodhia, K. L. Rogers, V. Fernández-Juárez, J. A. Carreres-Calabuig, C. R. Löscher, A. A. Tisserand, N. Keulen, L. Riemann, Y. Shashoua and N. R. Posth, Front. Mar. Sci., 2023, 10, 1134815 CrossRef.
- H. K. Imhof, C. Laforsch, A. C. Wiesheu, J. Schmid, P. M. Anger, R. Niessner and N. P. Ivleva, Water Res., 2016, 98, 64–74 CrossRef CAS PubMed.
- C. Arcos, L. Muñoz, D. Cordova, H. Muñoz, M. Walter, M. I. Azócar, Á. Leiva, M. Sancy and G. Rodríguez-Grau, Polymers, 2022, 14, 5220 CrossRef CAS PubMed.
- A. Turner and M. Filella, Mar. Pollut. Bull., 2020, 158, 111352 CrossRef CAS PubMed.
- H. Luo, Y. Xiang, Y. Li, Y. Zhao and X. Pan, Sci. Total Environ., 2020, 729, 139083 CrossRef CAS PubMed.
- H. El Hadri, J. Gigault, S. Mounicou, B. Grassl and S. Reynaud, Mar. Pollut. Bull., 2020, 160, 111716 CrossRef CAS PubMed.
- S. Dobaradaran, T. C. Schmidt, I. Nabipour, N. Khajeahmadi, S. Tajbakhsh, R. Saeedi, M. Javad Mohammadi, M. Keshtkar, M. Khorsand and F. Faraji Ghasemi, Waste Manage., 2018, 78, 649–658 CrossRef CAS PubMed.
- D. Brennecke, B. Duarte, F. Paiva, I. Caçador and J. Canning-Clode, Estuarine, Coastal Shelf Sci., 2016, 178, 189–195 CrossRef CAS.
- M. Kedzierski, M. D'Almeida, A. Magueresse, A. Le Grand, H. Duval, G. César, O. Sire, S. Bruzaud and V. Le Tilly, Mar. Pollut. Bull., 2018, 127, 684–694 CrossRef CAS PubMed.
- C. Campanale, C. Massarelli, I. Savino, V. Locaputo and V. F. Uricchio, Int. J. Environ. Res. Public Health, 2020, 17, 1212 CrossRef CAS PubMed.
- P. Klöckner, T. Reemtsma and S. Wagner, Sci. Total Environ., 2021, 764, 142870 CrossRef PubMed.
- A. Turner and M. Filella, Environ. Int., 2021, 156, 106622 CrossRef CAS PubMed.
- A. Turner, L. Holmes, R. C. Thompson and A. S. Fisher, Water Res., 2020, 173, 115577 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1–S5 and Fig. S1–S5. See DOI: https://doi.org/10.1039/d3va00254c |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: