 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silylation: a reproducible method for characterization of non-extractable residues (NER) of organic chemicals in the assessment of persistence†
Dieter
Hennecke
 *a,
Mike
Kruse
a,
Joana
Bräutigam
a,
Boris
Meisterjahn
*a,
Mike
Kruse
a,
Joana
Bräutigam
a,
Boris
Meisterjahn
 a,
Judith
Klein
a,
Daniela
Claßen
be,
Stefan
Trapp
c,
Matthias
Kästner
d,
Andreas Libonati
Brock
a,
Judith
Klein
a,
Daniela
Claßen
be,
Stefan
Trapp
c,
Matthias
Kästner
d,
Andreas Libonati
Brock
 c and
Andreas
Schäffer
c and
Andreas
Schäffer
 *e
*e
aFraunhofer Institute for Molecular Biology and Applied Ecology, 57392 Schmallenberg, Germany. E-mail: dieter.hennecke@ime.fraunhofer.de
bGerman Environment Agency, Section Chemicals, 06844 Dessau-Roßlau, Germany
cTechnical University of Denmark, Department of Environmental Engineering, 2800 Kgs. Lyngby, Denmark
dUFZ, Helmholtz Centre for Environmental Research, Department Environmental Biotechnology, 04318 Leipzig, Germany
eInstitute for Environmental Research, RWTH Aachen University, 52074 Aachen, Germany. E-mail: andreas.schaeffer@bio5.rwth-aachen.de
First published on 20th January 2023
Abstract
Most, if not all, chemicals, biocides, pharmaceuticals and pesticides are known to produce non-extractable residues (NER) in solid environmental media like soil and sediment during degradation testing to various extents. Since it has been found that parent substances and relevant metabolites can be contained and potentially released from NER there is currently much debate on how to include NER in the environmental persistence assessment. Using radioactive or stable isotope labelled test substances, three types of NER can be experimentally discriminated: entrapped, sorbed or heavily sorbed (type I) having the potential to be released from the matrix. Type II NER, i.e. residues covalently bound to organic matter in soils or sediments, are being considered to have very low remobilisation potential. Type III NER (bioNER) are formed after degradation of the xenobiotic chemical and incorporation into natural biomolecules (anabolism) like amino acids and other biomass compounds, and are, thus, of no environmental concern. Silylation has been suggested as a methodology to differentiate types I and II NER but concern has been addressed that this procedure is not suitable for routine analysis, e.g. in the context of studies for authorisation and registration of chemicals. Here, we describe a readily applicable and reproducible experimental procedure to apply this method for the analysis of NER derived from bromoxynil, sulfadiazine and isoproturon, respectively. This method is able to distinguish between heavily sorbed and covalently bound residues of chemicals, biocides, pharmaceuticals and pesticides in soils and to subsequently identify residues of the parent substance entrapped in type I NER.
Environmental significanceThe manuscript describes a procedure to distinguish the fraction of bound residues without environmental concern from the fraction with a potential concern. It enables chemical regulation to identify chemicals generating bound residues with significant fractions of environmental concern, which was not possible so far as appropriate methods are lacking. In order to set appropriate limits for use and release of those chemicals to the environment, regulation needs stand-ard procedures to determine reliable data in laboratory routine testing. The publication of applicable and reproducible laboratory methods for determining relevant parameters is a first step towards standardizing these methods. |
Introduction
The fate of chemicals in the environment – degradation, transport, bioaccumulation and partitioning – depends on the physical–chemical properties of the respective substance and the environmental matrix including its (micro-) biological status, and climatic conditions. Typically, in degradation studies on soils, organic chemicals are extracted from the solid matrix using appropriate solvents. Extractability of an organic chemical is somehow connected with its bioavailability. At the 10th SETAC Europe Special Science Symposium (BIOAVAILABILITY OF ORGANIC CHEMICALS, 14.-15. October 2014 in Brussels) a concept was developed.40 Besides extractable, mineralised and volatile products, most if not all chemicals are prone to form non-extractable residues (NER) in solid environmental media such as soils, sediments, plants, and other organisms. Despite this widespread immobilization process, knowledge on the identity, the binding mechanisms and the long-term fate of NER are available only for a small number of the vast majority of chemicals spanning a huge variety of physical, chemical and biological properties.1 Nevertheless, NER are part of the concept published.40According to the IUPAC definition,2 NER in soils are defined as species originating from chemicals, that remain unextracted by methods which do not significantly change the chemical nature of these residues or the structure of the respective matrix.3 Although guidance documents4–7 describe some principles on the assessment of NER, clear definitions for the extraction procedure of the environmental matrix to obtain NER and, especially, of techniques to characterize NER and NER-subtypes are missing or vague.
Regulatory views on NER formation differ considerably, either defining them as degraded residues of no environmental concern as in the case of pesticides,8,9 or as potentially bioavailable and non-degraded residues (“parent substance”) in the regulation of general industrial chemicals4,6,10 if it has not been demonstrated that the chemical is completely degraded or irreversibly bound. In this respect the terminology of NER is used as either safe sink or as hidden hazard.11
In most investigations, NER have been only quantified by use of isotope labelled test substances. For quantification, the solid sample containing NER is combusted and the formed isotope labelled carbon dioxide can be trapped and measured. In the case of radiolabelled substances this is by liquid scintillation counting (LSC).12 In the case of stable isotope labelling this is conducted by use of compound specific high-resolution mass spectrometry (HR-MS).13 NER have been characterized using spectrometric techniques after labelling the molecule with suitable stable isotopes, e.g.13C or 15N for corresponding NMR analysis14–17 or HR-MS18–24 or 14C-labelling combined with LC-MS.25,26 However, in the case of stable isotopes this is often at elevated concentrations of the test substances and this might have led to significantly decreased degradation.27,28
Regarding the persistence assessment of chemicals under REACH, it has been suggested29 to consider unknown total NER as remobilisable parent or transformation products, if no characterization or additional information has been provided. Different subtypes have been proposed,39 type I NER defined as sorbed and physically entrapped parent substance and transformation products. Type II NER is defined as parent or metabolites covalently bound to the matrix. Type III bioNER comprising natural compounds like amino acids, nucleic acids or phospholipids formed by microbial anabolism and assimilation of the carbon from the environmental pollutant and eventually incorporated into the soil organic matter or humic fraction of the respective matrix.29 If type III/bioNER and type II NER have been identified, these fractions should be considered as 'safe sink',29 since (i) biomolecules are of no environmental concern and (ii) covalently bound NER have a very low remobilisation potential due to stable chemical bonds,30,31 unless evidence for remobilisation has been provided. Whether or not type I NER have to be considered in the persistence assessment of chemicals, is currently under debate32 and the European Chemicals Agency seems in favour of.33
A standardized and reliable technology is needed to clearly differentiate the type I and II fractions and to unroll the safe sink vs. hidden hazard presumption of NER in environmental risk assessment. Silylation of soils and sediments is a proposed method to differentiate types I and II.29 Alternatively, an EDTA based extraction method has been proposed.41 These authors admitted that silylation may provide a clear differentiation of entrapped and covalently bound residues. Since there have been strong reservations about the silylation method in routine laboratory work, the methods have only been compared occasionally. However, as shown here, silylation is a fit for purpose method for characterisation of NER.
Silylation with trimethylchlorosilane (TMCS) leads to the exchange of protons in soil/sediment functional groups like –OH, –COOH, –NH2 with trimethylsilyl residues, thereby hindering the formation of hydrogen bridges. This results in the fragmentation of the humic matter associates29 and release of entrapped, heavily sorbed residues (type I NER). If feasible, the silylation extract containing the released residues can be analyzed for the presence of the parent molecule and degradation products. Examples for both extremes have been published recently (see discussion). However, in the framework of regulatory degradation studies concern about the proposed methodology has been addressed that this procedure is not easily manageable for routine analysis. In the present paper we describe a standardized, readily applicable, and reproducible procedure to employ silylation for analysis of NER in soil fate and turnover studies, in order to distinguish type I and type II NER derived from chemicals, biocides, pharmaceuticals and pesticides. It should serve as basis for future studies to compare and evaluate the results of silylation and EDTA-extraction to decide on the best procedure for type I NER determination.
Data shown were obtained within the scope of a project funded by the German Environment Agency (UBA), which was set up in order to investigate the formation and characterization of NER of the herbicides bromoxynil and isoproturon and the antibiotic sulfadiazine by use of the silylation procedure.
Materials and methods
Setup of soil degradation studies
Soil samples containing NER were obtained from three soil degradation studies performed with 14C-labelled sulfadiazine, bromoxynil, and isoproturon, according to the OECD 307 protocol.34 Each study was performed with one selected soil material (see ESI, Table S1†). Samples subjected to silylation procedures after exhaustive extraction (see below) were taken at five sampling dates (7 d, 14 d, 28 d, 60 d, 120 d). In addition, at 14 d and 120 d sterile samples were sampled and analysed. For each sampling date, duplicate samples were prepared. Samples were incubated at 20 °C in the dark in a flow-through setup. A constant stream of water saturated air was passed over the individual samples in order to maintain aerobic conditions. The outgoing gas was bubbled through 1 N NaOH in order to capture 14CO2 formed and to assess the extent of mineralization. A sacrificial sampling strategy was followed.Procedure to obtain NER containing matrix
Since clear definitions for NER with regard to procedures to determine NER are still missing, a pragmatic approach was followed in this project starting from the requirements of OECD guideline 307 for soil degradation studies.34 These studies demand extraction recoveries between 90% and 110% of the applied amount of label (in the case of radiolabelled studies) to be valid, and specific extraction methods were developed for each test substance. After shaking extractions with organic solvents, the soil residue was diluted with diatomaceous earth at a known ratio (20–30% depending on soil properties until soil was pourable) and was subjected to pressurized liquid extraction (PLE). Tables S2 and S3 (ESI†) summarize the extraction procedures for the three test substances. The PLE extraction residue was ground to a uniform consistency using a mortar mill and NER were quantified by combustion analysis followed by LSC measurements.Pre-test: extractability by silylation solvents
Experiments with spiked soils (see “verification of substance stability and recovery”) showed that radioactivity from the sulfadiazine spiked soil was only partially recovered by the described procedure due to limited solubility in the applied solvents. Thus, additional extractions were performed with the sulfadiazine-silylation residue with methanol as a more polar solvent. Extraction was conducted three times with 15 mL of methanol, two times shaking for 20 min and the last extraction shaking for 60 min. For isoproturon the silylation residue of samples from the degradation experiment were extracted once with 10 mL of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 80
water, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v, shaking for 60 min. About the same amount of radioactivity was found in the methanol
20, v/v, shaking for 60 min. About the same amount of radioactivity was found in the methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water extract (6.4% of the applied radioactivity) as in the silylation extract (6.7% of the applied radioactivity). However, radioactivity recovered with methanol
water extract (6.4% of the applied radioactivity) as in the silylation extract (6.7% of the applied radioactivity). However, radioactivity recovered with methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water was predominantly due to isoproturon degradation products, since the recovery from the soil spiked with parent isoproturon was 100% in the silylation extract.
water was predominantly due to isoproturon degradation products, since the recovery from the soil spiked with parent isoproturon was 100% in the silylation extract.
Verification of substance stability and recovery
The silylation reagent TMCS will react not only with the humic substances in the soil matrix but also with test substances if they carry corresponding functional groups with exchangeable protons such as hydroxy, carboxy or amino groups. This has to be evaluated before any silylation procedure on real samples is performed. In order to get conditions as realistic as possible, a stability test was conducted with the respective test soil material that was subjected to the same extraction and grinding procedures as the test samples from the OECD 307 studies. Aliquots of 1.5 g of this soil material were spiked with 4.7 kBq to 9.7 kBq of the radiolabelled test substances using dilutions of the application solutions from the OECD 307 tests. For each substance duplicate samples were prepared. After evaporation of the solvent the samples were subjected to the silylation procedure as described in the following.Silylation procedure
First, duplicate samples of 1.5 g each of the air dried and ground extracted soil with added diatomaceous earth was weighed (per soil sample from each sampling date, resulting in four soil aliquots per sampling date) into 250 mL Schlenk flasks and an oval magnetic stir bar was added (20 × 10 mm, VWR). To remove any remaining moisture, the samples were dried for 30 min at 105 °C in an oven because water would hydrolyze the silylation agent. Afterwards, the samples were placed on a magnetic stirrer and 30 mL of dry chloroform (ChemSolute, p.a., dried with a molecular sieve, 0.3 nm), 1.5 g NaOH micro granulate (ChemSolute, ≥99.5%) and 15 mL trimethylchlorosilane (TMCS, Sigma Aldrich, ≥99%) were added. In order to get a moisture-free inert atmosphere the reaction flask was flushed with argon immediately after the addition of TMCS. A gas bag (Linde PLASTIGAS® bag 5.5 L) filled with argon was connected with a silicon tube to the reaction flask in order to allow a pressure balance for the HCl gas produced and to maintain the protective gas atmosphere (Fig. 1).During reaction, the samples were stirred at room temperature at 100–200 rpm to maintain a homogeneous suspension. After three hours a further 10 mL of TMCS and 1.5 g NaOH were added to each sample. For addition of the reagents, plugs were opened only for a short time. Slight pressing of the gas bags helped to maintain the inert atmosphere in the reaction flasks during the addition. Then, the plugs were secured again with a clamp and the samples were further stirred overnight at room temperature. The gas bags were emptied directly after the reaction was finished to prevent corrosion. At the slightest suspicion of a leak, the bags were replaced with new ones. In total 42 samples (5 biotic and 2 sterile samples in duplicate per substance) were silylated in duplicate which corresponds to 84 individual silylation results.
Preparation of silylation extract
The reaction suspension was transferred into a centrifugation tube (Sarstedt vials, 50 mL) by a 5 mL pipette and centrifuged for 10 min at 3000 g. The supernatant was transferred to a 100 mL screw cap bottle (Schott, Teflon coated screw cap). The reaction flasks and the stir bars were rinsed with 10 mL of acetone (ChemSolute ≥99.8%). The washing solution was transferred to the soil residues in the centrifuge tubes, shaken for 5 minutes and centrifuged. This washing step was repeated twice with 10 mL of acetone and a further three times with 10 mL of chloroform each. The washing solutions were combined with the initial supernatant and the resulting clear, slightly yellow solution was called “silylation extract”. The silylation extract was stored closed at −20 °C in the dark until further analysis.Analysis of silylation extract and residues
The silylation extract was analysed by 14C-radio-thin layer chromatography (TLC) and LC-MS.All extracts were initially analysed by liquid scintillation (LSC) counting for the total extracted radioactivity (Hidex 600 SL, Hidex, Turku, Finland). For further work-up the silylation extract was transferred into a 50 mL Sarstedt vial and concentrated to about 15 mL of extract volume by a gentle stream of nitrogen. The additional extracts of the silylation residues in the cases of sulfadiazine and isoproturon (see above) were added to the corresponding silylation extracts and concentrated again by a gentle stream of nitrogen to around 15 mL final of extract volume. The resulting extract was filled up with methanol to 20 mL total volume and an aliquot was analysed by LSC. The recovery of radioactivity during concentration was mostly between 90% and 100%. The bromoxynil silylation extract could be directly analyzed by radio-TLC without any further treatment, as no extraction of the silylation residue was needed because bromoxynil is soluble enough in chloroform. The radio-TLC application volumes were adjusted in a way that the spots contained about 2.5 Bq to 10 Bq of extracted radioactivity, respectively. Exposure time was 7 d. The TLC conditions used are stated in ESI Table S4.† All sample runs on radio-TLC were converted into chromatograms and integrated using Aida Version 3.44 software. An example is shown in Fig. 4. For quality control purpose in this study we analysed the silylation residue for remaining radioactivity by combustion analysis in order to establish a mass balance for the silylation procedure.
In addition, soil degradation experiments with 13C-labelled sulfadiazine, bromoxynil and isoproturon have been performed. The chemical analysis from 13C-silylation extracts was performed by substance-specific LC-MS analysis for extracted parent. The data were compared with the parent substance recovered in the 14C- experiments. Details on LC-MS analysis are stated in ESI (Tables S5 and Table S6†).
Results and discussion
Fate of sulfadiazine, bromoxynil, and isoproturon in soil
All three compounds dissipated in soil but at different rates. The individual half-lives, calculated based on the parent concentrations in the extractable fractions by use of the open access software CAKE (Tessella Ltd, Abingdon, Oxfordshire, UK), were 8, 7, and 54 days for sulfadiazine, bromoxynil and isoproturon, respectively (calculation and plots see ESI Fig. S7–S9†). Mineralization rates after 120 days of incubation differed considerably with sulfadiazine at the lower end (1.7% of the applied radioactivity), bromoxynil with the highest level (29% of the applied radioactivity) and isoproturon being in the middle (17% of the applied radioactivity). In each case high amounts of NER were formed: sulfadiazine (up to 92.5% of the applied radioactivity), bromoxynil (up to 71.1% of the applied radioactivity) and isoproturon (up to 54.3% of the applied radioactivity Table 1).| substance | Sampling day | In % of the applied radioactivity | |||
|---|---|---|---|---|---|
| Extractable | Mineralised | NER | Mass balance | ||
| a One replicate not entirely sterile, data from sterile replicate only. | |||||
| Bromoxynil | 0 | 108.3 | — | 3.4 | 111.8 |
| 1 | 95.3 | 0.3 | 8.6 | 104.3 | |
| 2 | 87.7 | 0.7 | 14.1 | 102.5 | |
| 7 | 68.0 | 3.9 | 30.1 | 101.9 | |
| 10 | 57.7 | 6.0 | 41.2 | 104.9 | |
| 14 | 45.0 | 8.5 | 49.4 | 102.9 | |
| 27 | 17.2 | 16.1 | 71.1 | 104.4 | |
| 62 | 7.5 | 22.0 | 70.3 | 99.8 | |
| 120 | 4.9 | 28.8 | 65.5 | 99.2 | |
| Bromoxynil sterile samples | 14 | 102.1 | — | 7.8 | 109.9 |
| 119a | 99.6 | — | 6.3 | 108.9 | |
| Sulfadiazine | 0 | 95.9 | — | 4.1 | 100.0 |
| 1 | 85.0 | 0.0 | 16.8 | 101.8 | |
| 2 | 73.3 | 0.1 | 25.5 | 98.9 | |
| 3 | 66.3 | 0.2 | 36.5 | 103.0 | |
| 7 | 53.1 | 0.3 | 43.4 | 96.8 | |
| 10 | 44.9 | 0.5 | 56.9 | 102.3 | |
| 14 | 33.0 | 0.6 | 68.6 | 102.2 | |
| 28 | 20.7 | 0.9 | 72.5 | 94.2 | |
| 58 | 12.7 | 1.0 | 92.5 | 106.1 | |
| 120 | 6.0 | 1.7 | 82.8 | 90.5 | |
| Sulfadiazine sterile samples | 14 | 59.2 | — | 44.4 | 103.5 |
| 120 | 18.0 | — | 81.5 | 99.5 | |
| Isoproturon | 0 | 100.3 | — | 1.2 | 101.5 |
| 1 | 90.5 | 0.2 | 1.9 | 92.6 | |
| 2 | 94.3 | 0.3 | 2.8 | 97.3 | |
| 3 | 91.1 | 0.4 | 4.0 | 95.4 | |
| 7 | 89.9 | 0.7 | 4.8 | 95.4 | |
| 11 | 86.0 | 1.0 | 6.2 | 93.2 | |
| 14 | 83.2 | 1.6 | 8.7 | 93.5 | |
| 29 | 71.9 | 3.5 | 17.4 | 92.7 | |
| 58 | 55.0 | 9.2 | 32.5 | 96.7 | |
| 98 | 30.2 | 18.1 | 41.7 | 90.0 | |
| 120 | 21.2 | 17.0 | 54.3 | 92.5 | |
| Isoproturon sterile samples | 15 | 97.7 | — | 2.8 | 100.4 |
| 120 | 92.5 | — | 3.2 | 95.7 | |
Silylation of NER containing soil
In order to characterize the high amounts of NER of each test substances, the extracted soil samples were silylated with trimethylchlorosilane. Due to the formation of sodium chloride precipitates during silylation, the weight increase of the extracted solid samples has to be measured and compared to the original sample weight before silylation, for establishing correct mass balances.To analyse the reproducibility of the method, the recoveries of radioactivity of both replicate samples are compared using the Mann–Whitney U test (also known as Wilcoxon rank-sum test). Calculation was performed using the R function wilcox.test.35 There is no significant difference between both replicate samples (p-value 0.78 > 0.05). Thus, the two distributions are stochastically equivalent and reproducibility of the method is confirmed. In addition the variation of both replicate samples was similar (Levene test, F(1, 82) = 0.014, p = 0.91 > 0.05).36 The data used for this analysis and results of the statistical evaluation are shown in Tables S7 and S8 in the ESI.†
For all three test substances stability tests in spiked soil were performed and high recoveries of radioactivity were proven, i.e., for bromoxynil between 93% and 95% and for isoproturon 103%. The silylation extract from sulfadiazine spiked soil in the stability test contained only 13.1% of the spiked radioactivity, but in the methanol extract up to 83% were additionally recovered so that the mass balance could be accepted with a total recovery of 92–96%. The chemical analysis of the extracts by radio-TLC showed different results (Fig. S4–S6†). While bromoxynil was stable against silylation (100% parent in the recovered radioactivity, Fig. S4†), isoproturon showed some losses (82.5% of the recovered radioactivity identified as parent, Fig. S6†) and sulfadiazine degraded significantly with recoveries of 34.1% and 40.8% parent in the recovered radioactivity (Fig. S5†).
For the NER-containing extracted soil samples from the OECD 307 soil degradation test, significant amounts of radioactivity were released from all samples by silylation (Fig. 2).
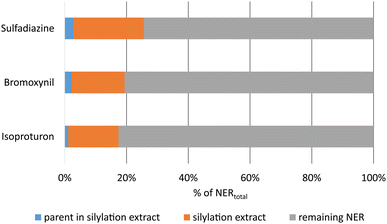 | ||
| Fig. 2 Release of radioactivity and of parent equivalents by silylation of thoroughly extracted soil, normalized to the respective total amounts of NER after 120 days of incubation. Absolute NER amounts see Table 1. | ||
As shown in Fig. 2, between 17% and 25% of the total unextractable radioactivity were released by silylation from the soil matrix. The remobilized fraction may include also biogenic NER and other degradation products besides the parent compounds. Chemical analysis by radio-TLC proved that only minor amounts of 1% to 3% of the total NER contained the parent test substances, which was also confirmed by LC-MS analyses (Table S6†). As an example, the TLC analysis of silylation extracts of soil incubated with bromoxynil is shown in Fig. 3 and 4.
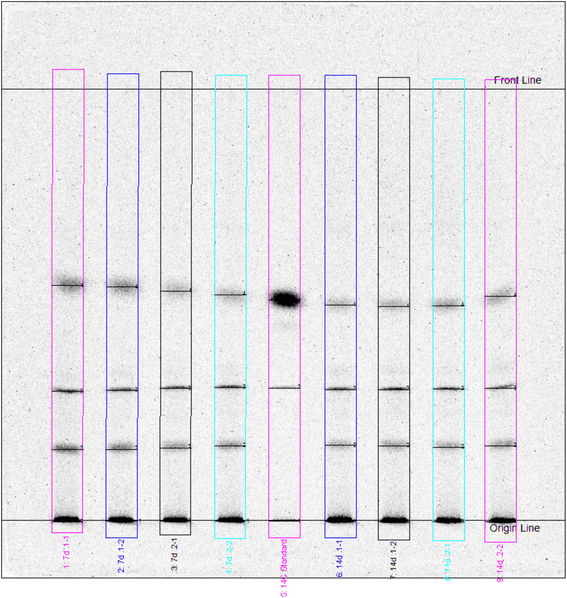 | ||
| Fig. 3 Radio-TLC analysis of silylation extracts from 7 d and 14 d replicates of bromoxynil incubated soil. The track in the middle (“standard”) represents the analytical bromoxynil standard. | ||
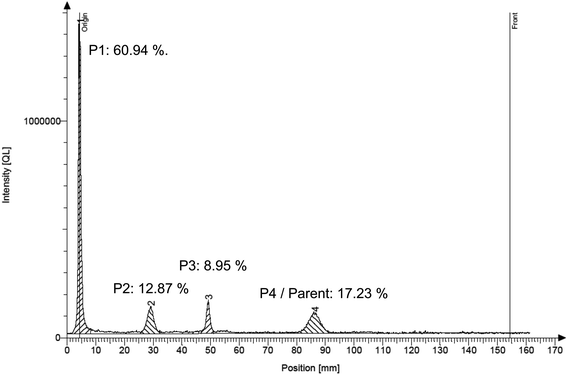 | ||
| Fig. 4 Radioanalytical thin-layer chromatogram of the silylation extract of soil incubated with bromoxynil for 7 days. The chromatogram resembles the left track of the TLC plate picture shown in Fig. 3 (track “7 d 1–1”). Peak number 4 represents the parent compound bromoxynil. Percentages shown are the respective percentages of the individual peaks compared to the total peak area. | ||
The LC-MS analysis of the 13C-silylation extracts were very comparable to the results of radio-TLC from the 14C-silylation extracts. In no case significant amounts of parent test substance were detected in the silylation extracts (for details see Table S6 in the ESI†). However, the sulfadiazine data may not be as reliable due to the observed sensitivity of sulfadiazine against the silylation procedure.
Chemical analysis of the silylation extract is essential for a realistic assessment of the hazard of the released parent substance from NER over time that may occur under natural conditions. For two test substances the environmental risk from NER release is considered to be low. Sulfadiazine results are not reliable due to the instability of the substance against the silylation procedure. In such cases, an alternative method for NER characterization like EDTA extraction41 can be used, but unfortunately, sulfadiazine proved to be not stable under such conditions either (data not shown). However, for other compounds the released parent amounts can be significant, for instance in the case of bisphenol S. Bisphenol S (a substitute of bisphenol A) forms high amounts of NER (45% of the applied amount) in soil; about half of this amount has been shown to comprise type I NER and about one third type II NER. Chemical analysis of the silylation extract representing type I NER showed the dominant presence of the parent substance.37 Regarding the persistence assessment of bisphenol S, the NER fraction does considerably increase its degradation half-life, if the parent substance released by silylation has to be added to the amounts released by extraction with organic solvents in the context of degradation experiments. On the other hand, pendimethalin, forming about one third of the applied amount as NER, was shown to predominantly belong to the type II fraction with only low potential of remobilization under natural conditions and thus being of little environmental concern.38
Conclusions
Since all chemicals form NER in solid environmental matrices in various amounts, investigation of these residues has to be included for their environmental risk assessment. Using three test substances, sulfadiazine, bromoxynil, and isoproturon, we showed that silylation of thoroughly pre-extracted soil containing only NER, can be performed readily and reproducibly. Handling, laboratory safety issues and usually available equipment in analytical labs allows application of the method even under routine conditions. No additional safety measures are required, since such silylation studies are carried out anyway in 14C radiation protection areas, in which higher demands are put forward towards work safety than in normal routine laboratories. Working with 13C labeling for regulatory purposes, which could also be done in routine labs, will remain the exception. Despite the harsh chemical conditions on a complex matrix the resulting silylation extracts did not contain high matrix load and remain suitable for subsequent chemical analyses. However, solvent exchange may be necessary depending on the solubility of the test substance. This will be determined in the initial test silylation using a soil matrix spiked with the parent test substance. This test is crucial to prove recovery and stability of the test substance under silylation conditions.Radio-TLC has proven to be a suitable analytical method for quantification of 14C-labelled parent test substances. The LC-MS analyses of the 13C-samples proved that silylation extracts are also appropriate for LC-MS analysis. No matrix effects of the silylation extract matrix were observed in LC-MS analyses. This is important if silylation is considered to become part of the routine characterisation of NER. Though NER are determined with isotope-labelled test substances only, LC-MS can serve as confirmatory analytical method.
The final aim of NER research was to provide a unified approach for NER characterisation and quantification to be incorporated in the persistence and environmental hazard assessment guidelines for REACH chemicals and biocides, human and veterinary pharmaceuticals, and pesticides, irrespective of the different regulatory frameworks. For this purpose, we here present a reliable methodology to quantify type I and type II NER and to identify remobilisable residues.
Author contributions
Dieter Hennecke: funding acquisition; project administration; investigation; visualization; writing – original draft; writing – review & editing. Andreas Schäffer: funding acquisition; conceptualization; methodology; supervision; writing – original draft; writing – review & editing. Mike Kruse: data curation; investigation, resources; formal analysis. Joana Bräutigam: visualization; data curation; investigation, resources; formal analysis. Boris Meisterjahn: project administration; methodology; investigation; visualization; writing – original draft. Judith Klein: formal analysis; data curation; validation; writing – original draft. Daniela Classen: conceptualization, supervision; methodology. Stefan Trapp: funding acquisition; conceptualisation, data curation; formal analysis; software; writing – original draft. Matthias Kästner: funding acquisition; conceptualization; supervision; writing – original draft; investigation. Andreas Libonati Brock: formal analysis; writing-original draft.Conflicts of interest
Fraunhofer Institute for Molecular Biology and Applied Ecology conducts commercial environmental fate studies that include NER characterisation work if applicable. German Federal Environment Agency evaluates environmental fate studies for regulatory purpose.Acknowledgements
This study was supported by the German Environment Agency project FKZ 3718 65 407 0 “Berücksichtigung nicht-extrahierbarer Rückstände (NER) in der PBT-Bewertung” (Consideration of non-extractable residues (NER) in the PBT assessment, 2018–2020). Particular thanks to Ulrich Jöhnke, Astrid Wiemann, Jana Schmidt, Gunther Speichert and Anna Pissarello from UBA.Notes and references
- Z. Y. Wang, G. W. Walker, D. C. G. Muir and K. Nagatani-Yoshida, Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories, Environ. Sci. Technol., 2020, 54(5), 2575–2584 CrossRef CAS PubMed.
- T. R. Roberts, Non-extractable pesticide residues in soils and plants, Pure Appl. Chem., 1984, 56(7), 945–956 CrossRef.
- F. Führ, H. Ophoff, P. Burauel, U. Wanner and K. Haider, Modification of definition of bound residues, in. Pesticide Bound Residues in Soil, ed. F. Fuhr and H. Ophoff, Wiley-VCH, Weinheim, 1998, pp. 175–6 Search PubMed.
- ECHA_2017_R.7b, Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.7b: Endpoint Specific Guidance, Version 4.0, 2017 Search PubMed.
- ECHA_2017_R.7c, Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.7c: Endpoint Specific Guidance, Version 3.0, 2017 Search PubMed.
- ECHA_2017_R.11, Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.11: Endpoint Specific Guidance (PBT/vPvB Assessment), Version 3.0, 2017 Search PubMed.
- European_Food_Safety_Authority, Options to Address Non-extractable Residues in Regulatory Persistence Assessment, 2019, pp. 1–5 Search PubMed.
- DG_SANCO_2012, Working Document on "Evidence Needed to Identify POP, PBT and vPvB Properties for Pesticides", 2012 Search PubMed.
- FOCUS, Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, Report of the FOCUS Work Group on Degradation Kinetics, EC Document Reference Sanco/10058/2005 version 2.0, 2014, p. 434 Search PubMed.
- EC_1907_2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Search PubMed.
- J. Harmsen, Measuring bioavailability: From a scientific approach to standard methods, J. Environ. Qual., 2007, 36(5), 1420–1428 CrossRef CAS PubMed.
- E. Barriuso, P. Benoit and I. G. Dubus, Formation of pesticide nonextractable (bound) residues in soil: Magnitude, controlling factors and reversibility, Environ. Sci. Technol., 2008, 42(6), 1845–1854 CrossRef CAS PubMed.
- M. Kaestner, K. M. Nowak, A. Miltner and A. Schaeffer, (Multiple) Isotope probing approaches to trace the fate of environmental chemicals and the formation of non-extractable 'bound' residues, Curr. Opin. Biotechnol., 2016, 41, 73–82 CrossRef CAS PubMed.
- A. Berns, R. Vinken, M. Bertmer, A. Breitschwerdt and A. Schaffer, Use of N-15-depleted artificial compost in bound residue studies, Chemosphere, 2005, 59(5), 649–658 CrossRef CAS PubMed.
- A. E. Berns, M. Bertmer, A. Schaffer, R. J. Meier, H. Vereecken and H. Lewandowski, The N-15-CPMAS spectra of simazine and its metabolites: measurements and quantum chemical calculations, Eur. J. Soil Sci., 2007, 58(4), 882–888 CrossRef CAS.
- T. Ertunc, N. Hartlieb, A. Berns, W. Kliens and A. Schaeffer, Investigations on the binding mechanism of the herbicide simazine to dissolved organic matter in leachates of compost, Chemosphere, 2002, 49(6), 597–604 CrossRef CAS PubMed.
- P. Riefer, T. Klausmeyer, A. Adams, B. Schmidt, A. Schaeffer and J. Schwarzbauer, Incorporation Mechanisms of a Branched Nonylphenol Isomer in Soil-Derived Organo-Clay Complexes during a 180-Day Experiment, Environ. Sci. Technol., 2013, 47(13), 7155–7162 CrossRef CAS PubMed.
- C. Girardi, K. M. Nowak, O. Carranza-Diaz, B. Lewkow, A. Miltner and M. Gehre, et al., Microbial degradation of the pharmaceutical ibuprofen and the herbicide 2,4-D in water and soil - Use and limits of data obtained from aqueous systems for predicting their fate in soil, Sci. Total Environ., 2013, 444, 32–42 CrossRef CAS PubMed.
- K. M. Nowak, C. Girardi, A. Miltner, M. Gehre, A. Schaffer and M. Kastner, Contribution of microorganisms to non-extractable residue formation during biodegradation of ibuprofen in soil, Sci. Total Environ., 2013, 445, 377–384 CrossRef PubMed.
- K. M. Nowak, V. N. Kouloumbos, A. Schaffer and P. F. X. Corvini, Effect of sludge treatment on the bioaccumulation of nonylphenol in grass grown on sludge-amended soil, Environ. Chem. Lett., 2008, 6(1), 53–58 CrossRef CAS.
- K. M. Nowak, A. Miltner, M. Gehre, A. Schaffer and M. Kastner, Formation and Fate of Bound Residues from Microbial Biomass during 2,4-D Degradation in Soil, Environ. Sci. Technol., 2011, 45(3), 999–1006 CrossRef CAS PubMed.
- S. Wang, A. Miltner, M. Kaestner, A. Schaeffer and K. M. Nowak, Transformation of metamitron in water-sediment systems: Detailed insight into the biodegradation processes, Sci. Total Environ., 2017, 578, 100–108 CrossRef CAS PubMed.
- S. Wang, B. Seiwert, M. Kaestner, A. Miltner, A. Schaeffer and T. Reemtsma, et al., (Bio)degradation of glyphosate in water-sediment microcosms - A stable isotope co-labeling approach, Water Res., 2016, 99, 91–100 CrossRef CAS PubMed.
- S. Z. Wang, A. Miltner and K. M. Nowak, Identification of degradation routes of metamitron in soil microcosms using C-13-isotope labeling, Environ. Pollut., 2017, 220, 927–935 CrossRef CAS PubMed.
- T. Junge, N. Classen, A. Schaffer and B. Schmidt, Fate of the veterinary antibiotic C-14-difloxacin in soil including simultaneous amendment of pig manure with the focus on non-extractable residues, J. Environ. Sci. Health - B Pestic. Food Contam. Agric. Wastes, 2012, 47(9), 858–868 CrossRef CAS.
- T. Junge, K. C. Meyer, K. Ciecielski, A. Adams, A. Schaffer and B. Schmidt, Characterization of non-extractable C-14- and C-13-sulfadiazine residues in soil including simultaneous amendment of pig manure, J. Environ. Sci. Health - B Pestic. Food Contam. Agric. Wastes, 2011, 46(2), 137–149 CrossRef CAS PubMed.
- J. Balesdent, C. Chenu and M. Balabane, Relationship of soil organic matter dynamics to physical protection and tillage, Soil Tillage Res., 2000, 53(3–4), 215–230 CrossRef.
- P. Fogg, A. B. A. Boxall and A. Walker, Degradation of pesticides in biobeds: The effect of concentration and pesticide mixtures, J. Agric. Food Chem., 2003, 51(18), 5344–5349 CrossRef CAS PubMed.
- A. Schaeffer, M. Kaestner and S. Trapp, A unified approach for including non-extractable residues (NER) of chemicals and pesticides in the assessment of persistence, Environ. Sci. Eur., 2018, 30, 51 CrossRef PubMed.
- A. Eschenbach, R. Wienberg and B. Mahro, Fate and stability of nonextractable residues of 14C-PAH in contaminated soils under environmental stress conditions, Environ. Sci. Technol., 1998, 32(17), 2585–2590 CrossRef CAS.
- A. Gulkowska, M. Sander, J. Hollender and M. Krauss, Covalent Binding of Sulfamethazine to Natural and Synthetic Humic Acids: Assessing Laccase Catalysis and Covalent Bond Stability, Environ. Sci. Technol., 2013, 47(13), 6916–6924 CrossRef CAS PubMed.
- A. Schaffer, K. Fenner and Z. Y. Wang, To be or not to be degraded: in defense of persistence assessment of chemicals, Environ. Sci.: Processes Impacts, 2022, 24, 1104–1109 RSC.
- ECHA, Options to Address Non-extractable Residues in Regulatory Persistence Assessment, 2019 Search PubMed.
- OECD_307, Aerobic and Anaerobic Transformation in Soil, 2002 Search PubMed.
- R-Core-Team, A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2020, https://www.R-project.org/ Search PubMed.
- J. Fox and S. Weisberg, An R Companion to Applied Regression, Sage Publications, Thousand Oaks, California, United States, 3 edn, 2019 Search PubMed.
- S. Cao, S. Wang, Y. Zhao, L. Wang, Y. Maa and A. Schäffer, et al., Fate of bisphenol S (BPS) and characterization of non-extractable residues in soil: Insights into persistence of BPS, Environ. Int., 2020, 143, 105908 CrossRef CAS PubMed.
- A. K. Luks, T. Zegarski, K. M. Nowak, A. Miltner, M. Kästner and M. Matthies, et al., Fate of Pendimethalin in Soil and Characterization of Non-extractable Residues (NER), Sci. Total Environ., 2020, 753, 141870 CrossRef PubMed.
- M. Kaestner, K. M. Nowak, A. Miltner, S. Trapp and A. Schaeffer, Classification and Modelling of Nonextractable Residue (NER) Formation of Xenobiotics in Soil - A Synthesis, Crit. Rev. Environ. Sci. Technol., 2014, 44(19), 2107–2171 CrossRef CAS.
- J.-J. Ortega-Calvo, J. Harmsen, J. R. Parsons, K. T. Semple, M. D. Aitken and C. Ajao, et al., From Bioavailability Science to Regulation of Organic Chemicals, Environ. Sci. Technol., 2015, 49(17), 10255–10264 CrossRef CAS PubMed.
- A. Eschenbach and K. Oing, Erarbeitung eines gestuften Extraktionsverfahrens zur Bewertung gebundener Rückstände, Expert Opinion for UBA FKZ 360 01 070, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2va00314g |
| This journal is © The Royal Society of Chemistry 2023 |

