Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical recycling of PET to value-added products
Zixian
Jia
 *a,
Lin
Gao
a,
Lijiao
Qin
a and
Jianzhong
Yin
*a,
Lin
Gao
a,
Lijiao
Qin
a and
Jianzhong
Yin
 *b
*b
aSINOPEC Dalian Research Institute of Petroleum and Petrochemicals Co., Ltd., Dalian 116045, China. E-mail: jiazixian.fshy@sinopec.com
bSchool of Chemical Engineering, Dalian University of Technology, Dalian, Liaoning, China. E-mail: jzyin@dlut.edu.cn
First published on 19th October 2023
Abstract
The term 'plastic' includes various materials (PP, PE, PET, PVC, and more), but most of them, but most of them take very long period of time to decompose and continue to cause harm to the environment. Currently, only a small percentage of plastic waste is recycled, usually for energy or the production of lower-quality products. Chemical recycling and upcycling offers a solution where plastic can be broken down into its original building blocks and transformed into new and high-quality plastics. When compared to traditional mechanical recycling, polymer upcycling systems may provide more energy-efficient pathways and have less impact on the environment. It is heartening to see recycling and upcycling techniques gain traction in industry, and creating new catalyst-driven technologies is critical to encourage better plastics recycling. This review highlights recent advancements in the development of efficient catalysts and effective strategies for the chemical recycling of polyethylene terephthalate (PET) into monomers, fine chemicals, and carbon materials.
Sustainability spotlightThe term 'plastic' includes various materials (PP, PE, PET, PVC, and more), but most of them, but most of them take very long period of time to decompose and continue to cause harm to the environment. At present, only a small proportion of plastic waste is recycled, often for energy generation or to manufacture lower-grade goods. This review is focused on the concept of upcycling, which involves utilizing PET waste as a raw material for the production of value-added products, such as monomers, fine chemicals, and hydrogen or carbon materials. Chemical recycling, coupled with the rational design, and optimization of catalysis, provides a necessary addition to current recycling methods. Our study emphasizes the importance of the following UN sustainable development goals of industry, innovation, and infrastructure (SDG 9), and “Ensure sustainable consumption and production patterns (SDG12). |
1. Introduction
Due to issues, such as inadequate handling, lack of infrastructure, and low civic awareness, discarded plastic has become a major environmental problem worldwide. Since the 1950s, a total of 8.3 billion tons of non-degradable plastic has been produced worldwide, with only 11% of it being recycled.1 In 2021, the worldwide polymer production was 390.7 Mt with an increase of 8% over the previous year. Polyethylene terephthalate (PET) production accounts for 8% of it.2 PET has excellent properties, such as high tensile strength, good size consistency, low thermal expansion, and chemical resistances. Owing to these features, PET has emerged as the most common thermoplastic polymer resin of the polyester family used for bottle beverages, food packaging, textiles, medical devices, and electrical application. One of the most widely used applications of PET is single-use beverage packaging, which is produced on a large scale and consumed globally on a massive scale every day. It is worth mentioning that PET bottles are one of plastic waste that are well collected, e.g., 94% in Germany3 and 90% in China.4 Consequently, the clean and properly collected PET waste can be an ideal raw material for industrial purposes.The properly collected and clean PET waste can serve as an excellent raw material for industrial purposes. In contrast, a circular plastic economy adopts a sustainable approach by ensuring that plastics are continuously reused and recycled in a closed loop. This involves extracting resources from products that have reached the end of their life cycle and reintroducing them into the production process. The objective is to minimize waste and promote a regenerative system. Scientists and researchers are increasingly interested in repurposing waste materials with diverse chemical properties for advanced applications. With the advancements in science and technology, these wastes offer potential solutions to various issues such as healthcare, industrial waste management, unemployment, income generation, and contribute to a cleaner, greener, and safer environment when used at a large scale.
In the past five years, a significant number of high-quality reviews on the chemical recycling of PET have been published. These reviews primarily focus on different depolymerization technologies and catalysts, as indicated in Table 1. However, instead of concentrating on the catalytic processes like most reviews, the aim of this study is to focus on the final products obtained from the depolymerization of PET. Monomers, oligomers, fine chemicals, and carbon materials are investigated, considering their environmental impact, economic attractiveness, and industrial relevance.
| Title | Key points | References |
|---|---|---|
| Current developments in the chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: a review | ✓ Review major PET depolymerization methods: alcoholysis, hydrolysis, aminolysis, ammonolysis, and glycolysis | Raheem et al. (2019)5 |
| ✓ Highlight the glycolysis in view of reaction process and conditions, kinetics of reactions, catalysts used, products of degradations, and their potential applications | ||
| Chemical recycling of PET: a stepping-stone toward sustainability | ✓ Provide a detailed study of chemical recycling of PET | Shojaei et al. (2020)6 |
| ✓ The most influential factors: time, temperature, catalyst species, the quantity of catalyst, the ratio of catalyst to PET, and the ratio of PET to reagent | ||
| The chemical recycling of polyesters for a circular plastics economy: challenges and emerging opportunities | ✓ Summarize recent advancements in the chemical recycling of poly(lactic acid) (PLA) and poly(ethylene terephthalate) (PET) | Payne and D. Jones (2021)7 |
| ✓ Focus on the upcycling and the use of metal-based catalysts | ||
| ✓ Suggest five objectives to promote the advancement of commercially feasible and environment-friendly chemical recycling methods | ||
| Chemolytic depolymerization of PET: a review | ✓ Propose three green chemistry metrics: the energy economy (ε coefficient), environmental factor (e), and the combined impact of both factors | Barnard et al. (2021)8 |
| ✓ Compare various studies numerically and assess their relative feasibility | ||
| Progress in the catalytic glycolysis of polyethylene terephthalate | ✓ Summarize the key factors that drive the PET glycolysis process, with a specific focus on ionic solvents such as ionic liquids (ILs) and deep eutectic solvents (DESs) | Xin et al. (2021)9 |
| ✓ Proposes a catalytic mechanism for ionic solvent catalysts | ||
| ✓ Provide a summary of the glycolysis kinetic analysis, which helps to understand the depolymerization process both in homogeneous and heterogeneous conditions | ||
| Strategic possibility routes of recycled PET | ✓ Review the recent developments of chemical recycling PET, especially, combination of glycolysis–hydrolysis, glycolysis–methanolysis, and methanolysis–hydrolysis | Damayanti and Wu (2021)10 |
| ✓ Investigate theoretically and experimentally the reaction kinetics and reaction conditions | ||
| Chemical upcycling of poly(Ethylene terephthalate) waste: moving to a circular model | ✓ Discuss different updated state of the art works about recycling of PET from mechanical and chemical approach | Caputto et al. (2022)11 |
| ✓ Promising approaches for these recycling systems were introduced, which involved the use of eutectic solvents, organocatalytic agents, or microwave-assisted technology, under sustainable reaction conditions | ||
| Upcycling hazardous metals and PET waste-derived metal–organic frameworks: a review on recent progresses and prospects | ✓ Focus on the recycling of solid wastes containing metals and organic linkers for the synthesis of MOFs | Shanmugam et al. (2022)12 |
| ✓ Explores the potential applications of these synthesized MOFs in addressing environmental issues such as CO2 adsorption, H2 production, and photoreduction | ||
| Recent advances in chemical recycling of polyethylene terephthalate waste into value added products for sustainable coating solutions – hope vs. hype | ✓ The recent advancements in recycling PET waste into value-added products and their subsequent use in the field of organic coatings | Ghosal and Nayak13 |
| ✓ The existing challenges in this field associated with the upscaling of these processes in industry | ||
| Chemical recycling processes of waste polyethylene terephthalate using solid catalysts | ✓ Summarize current progresses on the heterogeneously catalyzed chemical recycling of PET | Bohre et al. (2023)14 |
| ✓ Describe four key pathways for PET depolymerization including, glycolysis, pyrolysis, alcoholysis, and reductive depolymerization | ||
| ✓ Outline the catalyst function, active sites, and structure–activity correlations |
1.1. PET production and properties
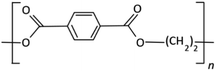
The development of PET resin began in the 1950s and 1960s with the discovery of terephthalic acid (TPA) and dimethyl terephthalate (DMT), and the first patent was done by John Rex Whinfield.15 To produce PET, these compounds were combined with ethylene glycol to form bis(hydroxyethyl)terephthalate (BHET) based on the esterification and trans-esterification reaction. The pre-polymerization step follows in which BHET is polymerized to a degree of polymerization of up to 30. The final polycondensation process is to produce suitable molecular weight (Mw) or intrinsic viscosity for fibers, sheets, or bottle applications. In the 1990s and beyond, the use of PET resin continued to expand into new industries such as food packaging, medical equipment, and textiles due to the main physical and chemical properties of PET resin presented in Table 2.| Property | Valuea |
|---|---|
| a Data from ref. 16–18. | |
| PET repeating unit |
|
| Molecular weight of repeating unit (g mol−1) | 192 |
| M w (g mol−1) | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–20 000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fiber-grade 000 fiber-grade |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–36 000–36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bottle-grade 000 bottle-grade |
|
| Density (g cm−3) | 1.45 |
| Glass transition temperature (°C) | 65–80 |
| Melting point (°C) | 240–270 |
| O2 permeability | 0.114 |
| CO2 permeability | 0.46 |
| E-Modulus (GPa) | 2.1–2.2 |
| Yield stress (MPa) | 50–60 |
1.2. PET recycling policies and regulations
In response to the environmental pressures imposed by waste management, the application of PET recycling technology has emerged, and various countries have established PET recycling plans.19 The United States,20 the European Union,21 South Korea,22 China,23 and other countries or regions24–26 have clearly stipulated that recycled plastic can be used as food contact materials and have formulated relevant regulatory measures.The environmental management policies and regulatory systems in the United States are primarily divided into the federal level and local level. The United States' Environmental Protection Agency (EPA) issued the Resource Conservation and Recovery Act (RCRA), which established the 4R principle (Recovery, Recycle, Reuse, Reduction). This principle regulates the management and treatment of waste, including the recycling and utilization of PET bottles.27 The Superfund Law provides detailed provisions on waste treatment technologies, coordination of regulations among states, expansion of EPA authority, and increased national funding investment. It has played a significant role in promoting comprehensive recycling and utilization of waste and national environmental protection in the United States. In 10 states, including California, a deposit return system (DRS) is implemented. This means that an additional charge is made when purchasing a product, and a refund is given when the product is returned. The implementation mainly includes packaging for soft drinks, mineral water, beer, malt bottles, and more. The recycling rate reaches 80%, which is twice as high as states where DRS is not implemented.
The European Union places significant importance on the recycling of plastics, with the aim of fundamentally transforming the plastic market from a linear economy to a circular economy and eventually transitioning to a green economy.28 Currently, the EU has established a policy and regulatory system consisting of one strategy (European Plastics Strategy), five regulations (Packaging and Packaging Waste Directive, Waste Framework Directive, EU Single-Use Plastics Directive, EU Plastic Bags Directive, and Directive on the Shipment of Plastic Waste), and two proposals (EU Plastic Tax and EU Carbon Border Adjustment Mechanism). The European Plastics Strategy clearly states the target for the recycling rate of plastic waste: 50% by 2025 and 55% by 2030. Germany, Sweden, and Norway have implemented an environmental deposit system for beverage packaging.
Plastic waste is a major concern in China. In 2018, China introduced the National Sword Policy, which banned the import of 24 types of solid waste, including certain types of plastic waste. This policy aimed to reduce the amount of plastic waste entering the country and encourage domestic recycling. Since 2010, China has become the largest consumer of PET bottles in the world. Informal collectors account for 90% of the collection of post-consumed PET bottles, according to a study by Zhang et al.4 Currently, China is responsible for treating 53% of the global collection of PET bottles, with approximately 30% of the country's total production of polyester fiber sourced from recycled PET bottles. In December 2021, the Ministry of Industry and Information Technology issued the “14th Five-Year Plan for Green Industrial Development”, which proposes to encourage the chemical recycling of plastic waste and promote the application of low-value plastic waste pyrolysis technology. It also aims to promote the high-value recycling of recycled resources and cultivate leading backbone enterprises in the recycling of major recycled resources such as plastic waste.
Although some countries have higher rates of recycling, regulations only address contaminant levels and, in some cases, limit the amount of recycled PET allowed. More regulations are needed to mandate recycling, specify properties of recycled bottles, and provide processing information for improved recycling. It should be pointed out that during the resumed fifth session of the UN Environment Assembly, a significant resolution was passed to establish an international legally binding agreement on plastic pollution. The international cooperation would be the only way to end plastics pollution.
1.3. PET recycling methods
The various techniques used for plastic recycling are categorized as primary, secondary (mechanical), tertiary (chemical), and quaternary (energy recovery) recycling.Primary recycling involves reprocessing plastic to create an item that can be used for the same purpose as the original plastic item. This method, known as “closed-loop recycling”, requires clean and high-quality waste materials, such as process scrap or post-consumer items with a known origin. An excellent example of primary recycling is the production of plastic bottles made from a mixture of recycled PET (rPET) and virgin PET.
Secondary (mechanical) recycling is the easiest method to implement in the industry as it utilizes the same infrastructure as the production from virgin materials. It also reduces economic losses caused by plastic shrinkage during the production process. However, mechanical recycling requires the prior separation of the polymers from the post-consumer plastic waste, followed by cleaning to avoid contamination of the container content (food, chemicals, etc.) during the recycling process. A major drawback of mechanical recycling is the degradation of PET properties with each cycle, leading to a decrease in elasticity and viscosity.29 This results in the recycled polymer losing value and eventually being sent to landfills after several cycles.
Quaternary recycling involves the incineration of plastics to recover energy for electricity generation. Unlike other recycling methods, this process cannot produce reusable plastics. However, it is suitable for heavily contaminated municipal plastic waste or complex multicomponent materials or when primary to tertiary recycling infrastructure and logistics are not available.30
To achieve the purpose of circular economy, tertiary recycling refers to the use of a chemical process to recover petrochemical components in plastics, which could be a solution. However, this method is not commonly used on an industrial scale due to high energy requirements. Nevertheless, the recovery of pure monomers through chemical recycling could separate polymer prices from oil prices. Therefore, efforts are being made to develop mild processes for the catalytic conversion of polymers directly into monomers or new polymers. As shown in Fig. 1, low-value PET waste can be used as feedstock to produce economic value-added products. In addition to economic added value, recycling PET is expected to bring further value both environmentally and socially by reducing waste to a minimum and creating jobs. Although the use of post-consumer PET to produce new chemical products through chemical recycling is an interesting avenue, these new recycling processes should be more sustainable than traditional methods in the context of circular economy. By performing studies on life-cycle assessment (LCA)31 and techno-economic analysis (TEA),32 future research should strive to define the environmental and financial advantages of these techniques. It is also critical to keep creating chemistry that can withstand contaminants like additives, dyes, missorted polymers, and multilayer goods that are found in post-consumer waste streams.
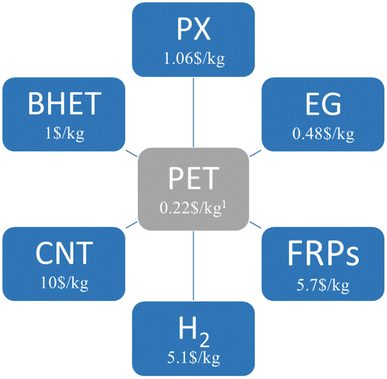 | ||
| Fig. 1 The valued added products produced by the chemical upcycling of PET waste (PX: paraxylene; EG: ethylene glycol; FRP: fiberglass-reinforced plastics; CNT: carbon nanotube; BHET: bis(2-hydroxyethyl)terephthalate).1 All prices are based on the Chinese market. | ||
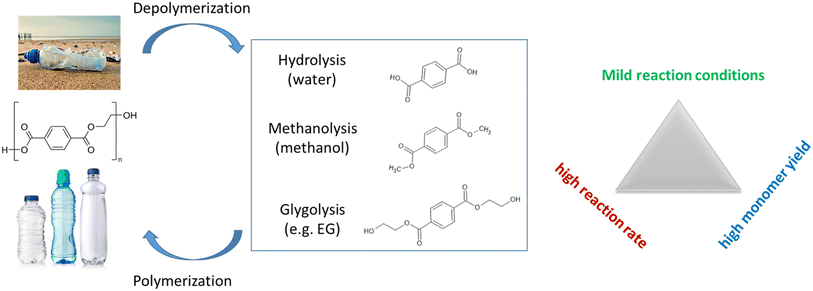
2. PET to monomers for closed-loop recycling
PET has been extensively studied as a potential substance for breaking down into smaller molecules through depolymerization. This is mainly due to the presence of an ester group that is chemically susceptible as well as the existence of reliable systems for collecting and sorting this type of plastic. Various solvents have been used, with or without a catalyst, for the successful dissolution of PET. These solvents include water,33,34 amines,35,36 and alcohol,9,37,38 as illustrated in Fig. 2. Among them, glycolysis is regarded as one of the most promising techniques for depolymerizing PET on an industrial scale among the several chemical recycling processes. This is because it uses a less volatile alcoholysis reagent called EG (ethylene glycol) and mild reaction conditions. PET undergoes solvolytic breakdown, in which the ester bonds are broken and replaced by hydroxyl functionalities. Companies including DuPont, Dow Chemicals, Shell Polyester, and others have created a number of commercial or demonstration glycolysis-based plants.39 This process produces useful monomers, with BHET being a particularly common one due to its ability to create various high-value products.40–42 The usual approach involves using ethylene glycol, PET waste, and a catalyst (such as zinc acetate) for depolymerization. Studies have shown that PET glycolysis outperforms other processes in terms of economic and environmental performance.43 In this section, we will explore this process in greater detail, focusing on achieving high monomer yields, fast reaction rates, and mild reaction conditions. The development of new catalysts is the key factor in overcoming the challenges depicted in Fig. 2.To reduce the reaction time, Liu et al.44 conducted a study investigating the impact of different solvents on the conversion of PET to BHET. They discovered that DMSO was the most effective solvent for the co-solvation of EG (ethylene glycol) and PET, resulting in an 82% increase in BHET yield. This represented a significant improvement compared to the yield of 20% obtained without the addition of DMSO. The reaction only required 1 minute at 190 °C and atmospheric pressure, yielding remarkably short reaction times. The glycolysis of PET was achieved using microwave irradiation, which exhibited a lower required activation energy (Ea) compared to conventional heating. Furthermore, microwave irradiation increased the reaction rate, resulting in the complete depolymerization of PET in just 2 minutes with DEG (diethylene glycol) as the solvent, whereas it took 8 hours under conventional heating to achieve complete depolymerization.45
To enhance the monomer yield, a new catalyst known as titanium(IV) phosphate46 was employed at a rate of 0.3 wt%, a temperature of 190 °C, and a reaction time of 150 minutes. This approach achieved a BHET selectivity of 97.5%. Through ultrasound irradiation, a straightforward single-step method for synthesizing GO–Mn3O4 nanocomposites47 was successfully accomplished. The catalyst demonstrated excellent performance in PET depolymerization, with a BHET monomer yield of over 96%. Additionally, this procedure offered ecological and economic advantages, including the elimination of hazardous reagents, reduced synthesis time, and lower energy consumption. Al-Sabagh et al.48 introduced multiwalled carbon nanotubes (MWCNT) as Fe3O4–MWCNT to develop an efficient, sustainable, and easily recoverable catalyst for PET glycolysis processes. The synergetic effect between magnetite and MWCNT greatly enhanced the catalytic glycolysis process, nearly reaching a BHET yield of 100%. Moreover, the utilized catalyst could be easily separated and reused due to the presence of magnetite. The introduction of an iron-containing ionic liquid onto silica-coated magnetic Fe3O4 nanoparticles resulted in the creation of a magnetically recoverable nanocatalyst Fe3O4@SiO2@(mim)[FeCl4].49 This catalyst was used for the conversion of PET into BHET via glycolysis using standard heating techniques. It exhibited nearly complete yield and selectivity for twelve consecutive reaction cycles at 180 °C and was easily recovered without requiring complex purification procedures. Further analysis indicated that the quantity of lost catalyst after each cycle was negligible, and no iron residue was detected in the final purified BHET product. Organocatalysts can encourage transesterification reactions that result in very small molecules, which are then suited for further PET polymerization. Fukushima et al.50 firstly reported the glycolysis of PET using a potent guanidine base, 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD). At 190 °C, waste PET pellets were completely degraded in 3.5 hours with a 78% yield of BHET. The most remarkable findings were achieved with a catalyst formed by an equal amount of triazabicyclodecene (TBD) and methanesulfonic acid (MSA). This catalyst fully depolymerizes PET in under 2 hours, resulting in a 91% yield of highly pure BHET.
For the mild depolymerization of PET, Tournier et al.51 utilized computer-aided enzyme engineering to achieve a conversion rate of 90% in less than 10 hours for enzyme-catalyzed PET depolymerization. Furthermore, they achieved a mean productivity of 16.7 g TA per liter per hour with a 200 gram per kilogram PET suspension. The purified terephthalic acid monomers obtained were used for PET synthesis, which were then converted into bottles, establishing a closed-loop system within the circular economy. Employing bionic catalysis, Zhang et al.52 developed a binuclear zinc catalyst for polyester depolymerization. This catalyst contained Zn–Zn sites that bound the plastic substrate and nucleophile together, facilitating intramolecular hydrolysis. This proximity effect significantly enhanced the reaction rate under mild conditions, with a high specific activity of up to 577 gPET d−1 gcatal−1 and less demanding conditions than conventional chemical recycling. Additionally, the binuclear catalyst displayed compatibility with complex mixtures of plastic waste and had a broad substrate scope. This study is expected to inspire further research into the development of more efficient catalysts for plastic waste degradation, particularly considering the versatile structure of the binuclear zinc complex (Table 3).
| Monomer | Catalysts | Reaction time (h) | Temp. (°C) | Monomer yield (%) | Key messages | Ref. |
|---|---|---|---|---|---|---|
| BHET | Zn(OAc)2 | 0.017 | 190 | 82 | Short reaction time: 1 min | 44 |
| BHET | Mn(CH3COO)2 | 0.033 | 180 | Not reported | Short reaction time: 2 min | 45 |
| BHET | Zn(OAc)2 | 0.083 | 240 | 96.3 | Short reaction time: 5 min | 53 |
| BHET | Titanium(IV)-phosphate | 2.5 | 190 | 97 | High BHET yield: 97.5% | 46 |
| BHET | GO–Mn3O4 | 1.25 | 300 | 96 | High BHET yield: 96% | 47 |
| BHET | Fe3O4–MWCNT | 2 | 190 | 100 | High BHET yield: 100% | 48 |
| BHET | Fe3O4 | 2.5 | 210 | 93 | High BHET yield: 93% | 54 |
| BHET | Fe3O4@SiO2@(mim)[FeCl4] | 24 | 180 | 100 | High BHET yield: 100% | 49 |
| BHET | TBD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MSA (1 MSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
<2 | 180 | 91 | High BHET yield: 91% | 55 |
| TPA | Enzyme | 10 | 72 | 96 | Mild condition and high monomer yield | 51 |
| TPA | Zn2/C | 4200 | 30–90 | 100 | Mild condition and high monomer yield | 52 |
| TPA | KOH | 0.5 | 35 | 99 | Mild condition and high monomer yield | 56 |
Although the use of catalysts allows the reaction to take place under milder conditions, the catalyst free strategy cannot be ignored. The latter can save on catalyst costs and reduce separation steps. Under supercritical conditions and without a catalyst, polyethylene terephthalate (PET) can be depolymerized into monomers, dimers, and oligomers using water,57 methanol,58 and ethanol.59 The temperature, weight ratio of methanol to PET, and reaction time were identified as significant factors affecting both the yield of monomer and the degree of PET depolymerization.60,61 In the early stages of PET depolymerization in supercritical methanol, random scission primarily occurs in the heterogeneous phase where mass transfer plays an important role. Consequently, the addition of CO2 in supercritical methanol37 enhances PET depolymerization and the production of dimethyl terephthalate (DMT). By operating significantly far from equilibrium, an electrified selective thermal heating (STH)-based depolymerization process can produce monomers from commodity plastics.62 This selective depolymerization is achieved in two ways: through a spatial temperature gradient and a time–temperature heating curve. The monomer yield is 36% for polypropylene (PP) and 43% for PET.
In conclusion, the back to monomer strategy for the closed-loop recycling of PET has attracted great attention since big companies are transitioning to 100% rPET. Chemical recycling processes have the potential to provide a solution by offering monomers that can be used to create new polymers. It is expected to obtain high monomer yield under mild reaction condition with short reaction time for industrial applications. Using microwave heating or DMSO as co-solvent, the reaction time could be reduce to several minutes. The design of catalytic process enables the high monomer yield and mild reaction conditions. Future studies should continue to focus on new catalytic systems to enhance the economic competitiveness of PET conversion under mild conditions. This can be achieved by improving the conversion efficiency, product selectivity, product value, and substrate versatility. Furthermore, reactor design and process optimization necessitate particular attention to enhance the separation and purification of products. Scaling up the lab-scale reaction system is crucial to increase the processing capacity for industrial applications.
3. PET to fine chemicals
Besides the monomers for the closed-loop recycling of PET, chemicals are another appealing target for upcycling PET waste. As depicted in Fig. 3, low-value residual PET waste can be transformed into high value-added fine chemicals.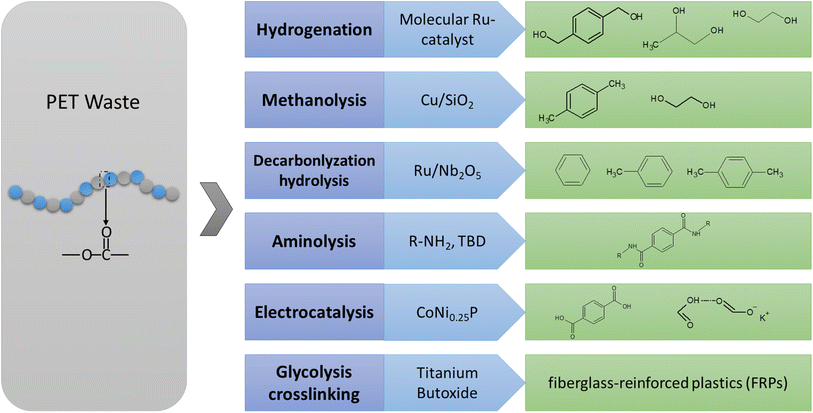 | ||
| Fig. 3 Summary of the chemical reactions, reaction conditions, and catalysts for PET to fine chemicals. | ||
To enhance the value of PET plastic waste, the hydrogenation and hydrosilation of PET by transition metals results in the production of multifunctional small molecules with high yields (64–99%).63,64 Catalytic hydrogenation is a chemical reaction that involves the addition of hydrogen (H2) to a molecule in the presence of a catalyst. With H2, Yan et al.65 utilized Ru/Nb2O5 catalyst for the hydrogenolysis of various aromatic plastic wastes containing C–O and/or C–C linkages into arenes. The reaction was conducted at a temperature of 473 K and with 0.3H2 for 12 hours in water as the solvent. The highest yield was achieved by Ru/Nb2O5 with a remarkable selectivity of 87.1% toward arenes and an overall yield of 95.2% for arenes and cyclic hydrocarbons. Using a molecular ruthenium complex, various abundant polyester and polycarbonate polymers can be selectively catalyzed into diols.64
To achieve H2-free approaches, Ru/Nb2O5 was utilized as a catalyst for converting PET into benzene, toluene, and xylene (BTX) without the use of external H2.66 Ru/Nb2O5 gave a much higher selectivity to BTX (>99.9%). Moreover, the total yield of toluene and p-xylene over Ru/Nb2O5 reached 66.7%, significantly higher than that over Ru/NiAl2O4 (19.3%). Hence, Ru/Nb2O5 preferred the hydrogenolysis of PET into toluene and p-xylene rather than decarboxylation into benzene. The superior hydrogenolysis capability of Ru/Nb2O5 was partially attributed to the strong oxygen affinity of NbOx species for C–O bond activation. Gao et al.67 developed a low-cost process to convert PET into p-xylene (PX) and ethylene glycol (EG). This is achieved using modified Cu/SiO2 catalyst with methanol serving as both the solvent and hydrogen donor. Kinetic and in situ Fourier-transform infrared spectroscopy (FTIR) studies show that PET is degraded into PX through PET methanolysis and dimethyl terephthalate (DMT) selective hydro-deoxygenation (HDO) steps, with in situ production of H2 from methanol decomposition at 210 °C. This one-pot approach, which requires no hydrogen, directly produces gasoline fuels and antifreeze components from waste polyester plastic.
Furthermore, the production of terephthalamides, which are valuable building blocks, can be accomplished via aminolysis as an alternative to using phosgene or its derivatives.68 This method can yield up to 92% without the need for purification and can be completed within minutes. These terephthalamides can then serve as monomers for various materials such as polybenzoxazines or polyionenes, which possess unique properties based on their functionality. These materials find diverse applications such as strong adhesives, treating infections, and creating antimicrobial and antifungal materials.68–70 However, some of these methods involve high temperatures and/or pressures, and purification steps, which complicate the scaling-up process. Nevertheless, this approach offers a sustainable advantage over traditional methods that employ highly toxic compounds.
A strategy for upcycling plastics by combining reclaimed PET with bio-based monomers was investigated.71 This approach aims to save energy and reduce greenhouse gas emissions using renewable feedstock and creating long-lasting, high-performance materials. The process involves glycolyzing rPET with diols and converting them into UPE (unsaturated polyester) or vinyl esters, which are then dissolved in a reactive diluent and applied to a fiberglass mat to produce FRPs.
Electrocatalysis driven by renewable energy sources offers a sustainable strategy for upcycling PET waste under mild conditions. Electrocatalysis was applied to transform PET waste into valuable chemicals such as potassium diformate (KDF) and TPA, while also generating hydrogen gas.72 The process involves the use of a bifunctional CoNi0.25P electrocatalyst in a KOH electrolyte solution. Using the same concept, with cobalt as the electrocatalyst and at a low onset potential of 1.27 V vs. RHE, terephthalic acid (TPA) with a yield of 100% and potassium diformate (KDF) with an approximately 80% selectivity were obtained.73 Concerted and selective electro-reforming of ethylene glycol derived from PET into high-value glycolic acid and H2 fuel was successfully carried out at an industry-level current density (600 mA cm−2 at 1.15 V vs. RHE) using a Pd–Ni(OH)2 catalyst.74 Importantly, stable electrolysis over 200 hours was achieved in a flow electrolyzer, surpassing the performance of all existing Pd-based catalysts.
4. PET to carbon materials
Due to its high carbon content, PET waste, which contains at least 62.5% carbon, holds promise as a valuable source for producing carbon-based materials as a polymer. Since the first research on converting plastic waste into porous carbon by László et al.,75 subsequent studies have demonstrated the synthesis of various forms of carbon materials from plastics waste. These include carbon nanotubes (CNTs), carbon microspheres, 2D graphene-based materials, carbon nanofibers (CNFs), graphite, and fullerene,76,77 which could be further used as materials of energy-related applications.78 As shown in Table 4, these studies investigated how reactor design, catalyst type, and process parameters impact the characteristics, structure, and amount of carbon nanomaterials.| Products | Method | Catalysts | Condition | Application | Ref. |
|---|---|---|---|---|---|
| Graphene | Green-CVD | Ni foil | 900 °C; N2; 5 min | Not reported | 80 |
| Pyrolysis and CVD carbonization | Ni foil | 1050 °C; N2, H2; 20 min | Electrochemical cells | 81 | |
| Flash joule heating | No | E = 120 V; I = 180 A; t = 100 ms | Not reported | 82 | |
| Flash joule heating | No | E = 400 V; I = 1000 A; t = 100 ms | Not reported | 83 | |
| CNT | Arc discharge | No | 1700–3000 °C; N2 | Not reported | 85 |
| Single-stage pyrolysis | Ferrocene | 800 °C; 20 h | Not reported | 86 | |
| Two-step pyrolysis | Co–Fe, Co–Ni, and Fe–Ni | 500 °C and 800 °C; N2; 5 min | Adsorption of metal cation | 88 | |
| Two-step pyrolysis | Ni/CaCO3 | 600 °C (plastic); 500/800 °C (cat.); N2; 30 min | Not reported | 94 | |
| Porous carbon | Single-stage pyrolysis | No | 700 °C; N2 | Phenol removal | 75 |
| Sequential pyrolysis | No | 400 °C; N2 and 925 °C; CO2 | PAH and phenol removal | 89 | |
| Single-stage pyrolysis | H2SO4 | 500–800 °C | Methylene blue and iodine removal | 90 | |
| Single-stage pyrolysis | KOH | 500 °C; N2 | CO2 capture | 91 | |
| ZnO–C | Single-stage pyrolysis | Zn | 700 °C; air | Photocatalysis | 93 |
| Graphite | Single-stage pyrolysis | B | Carbonization: 900 °C; N2; 1 h | Not reported | 95 |
| Graphitization: 2400 °C; He; 1 h |
4.1. Graphene
Monolayer graphene refers to a single layer of carbon atoms arranged in a hexagonal lattice. It is a two-dimensional material with unique properties that make it highly desirable for various applications in electronics, energy storage, and other fields.79 The synthesis of monolayer graphene is a challenge. You et al.80 have developed a green chemical vapor deposition process that can be carried out at atmospheric pressure and does not require the use of pure hydrogen gas. In this study, monolayer graphene was only produced when PET plastic waste was directly placed onto the substrate. The researchers developed a method where they ground PET bottles into powder and placed them between two Ni foils. They then thermally annealed the set-up at 900 °C in a N2 flow under atmospheric pressure. This process resulted in high-quality monolayer graphene (I2D/IG above 2.6) forming on the backside of the bottom foil.Cui et al.81 used PET bottle as sources of carbon in order to produce freestanding graphene foil. In this study, PET bottle was used as a source of hydrocarbon gas in a CVD system containing an Ni foil substrate. After cooling, FeCl3/HCl was used to etch and remove the Ni from the resulting multi-layer graphene foil. The thickness of the foil is microscale but consists of a defined number of countable graphene layers with extended lateral dimension, making it a multi-layer graphene. Raman spectroscopy showed that the final product had negligible structural defects, leading to high electrical conductivity (up to 3824 S cm−1) due to compact interlayer stacking and ordered structure. The resulting graphene foils were used as anode materials for lithium-ion batteries, making it the first study to report the application of 2D graphene-based materials produced from plastics in the electrochemical and energy field.
To produce flash graphene, Luong et al.82 developed a method called flash joule heating (FJH). This method utilizes electrical energy and resistance to quickly generate exceedingly high temperatures (approximately 3000 K) within a very short time frame (0.05–3 s), allowing to convert low-value solid materials into high value carbon materials. In a related study, Algozeeb et al.83 from the same research group focused on obtaining high-quality and high-yield flash graphene from mixed plastic wastes (20% PET). They used an alternating current (AC) pulse discharge and conducted experiments to investigate the impact of particle size, resistivity, and thermal stability of the plastic waste on the yield of flash graphene. The results showed that as the particle size and thermal stability increased, the resistivity decreased, while the yield also increased. Flash Joule heating conversion can create flash graphene with higher efficiency and in less time than traditional pyrolysis. For this, FJH is one of the most promising techniques for larger-scale graphene synthesis with a prototype manufacturing line operational by Universal Matter Inc.
4.2. CNT
Carbon nanotubes have unique electrical, thermal, and mechanical properties, making them highly valuable in various fields of science and technology.84 However, the production of carbon nanotubes on a large scale is still challenging and expensive.An innovative method85 for producing nano-channeled ultrafine carbon tubes and multi-walled carbon nanotubes from PET waste, without the need for catalysts or solvents, technique involves utilizing rotating cathode arc discharge at high temperatures, resulting in the formation of predominantly tube-shaped carbon structures with nano-sized channels. At lower temperatures, solid spheres are observed instead. A stainless-steel autoclave was employed by EI Essawy et al.86 to carry out the thermal decomposition of crushed PET bottles waste, using ferrocene catalyst, at 800 °C, and autogenic pressure. According to the TEM analysis, a small quantity of MWCNTs was observed in the carbon powder sample produced by decomposing with 20 mL 30% H2O2 as an oxidizing agent for 22 hours. Conversely, when utilizing carbon powder with 15 mL 30% H2O2 for 20 hours, SWCNTs containing fullerenes were detected. MWCNTs were successfully produced using a three-stage catalytic pyrolysis/gasification (CVD) process.87 The non-condensable gases derived from recycling different plastic packaging, including PET with varying percentages (11.8% and 27.5%), along with other plastics, were utilized in the process. The production was carried out at a temperature of 700 °C in the presence of Fe2O3 loaded on zeolite (7.5 g) and Ni loaded on CaCO3 catalyst (9.0 g). CO2 was used as the gasification agent. The study observed that the properties of the MWCNTs obtained varied depending on the type of plastic waste used. Furthermore, it demonstrated that this particular approach effectively converted this type of plastic waste into MWCNTs. However, due to a high content of CO2 in product gases, Yao et al.88 propose that the PET bottle was not suitable for CNT production.
4.3. Porous carbon
Porous carbons are a type of carbon material that possess a high degree of porosity. They are characterized by having a large number of interconnected pores and a high surface area, which make them useful in a wide range of applications. They are commonly used as adsorbents for gas and liquid purification, as catalyst supports, and as electrodes in energy storage devices such as supercapacitors and batteries.To achieve carbon materials with high adsorption capacity, László et al.75 carbonized waste PET and poly acrylonitrile (PAN) at a temperature of 700 °C with a flow of nitrogen gas, and then activated the resulting material with water vapor at 900 °C. This process yielded porous carbons with BET surface areas of 1254 and 544 m2 g−1 for PET and PAN, respectively. Parra et al.89 pyrolyzed PET waste at 400 °C under a flow of N2 gas and then activated the remaining solid with CO2 at 925 °C in order to enhance the porosity of the adsorbents. Another study by Kartel et al.90 involved impregnating the PET precursor with sulfuric acid prior to steam activation. This method produced carbon adsorbents with a BET surface area of 1030 m2 g−1 and well-developed pores. Arenillas et al.91 investigated the CO2 capture capacities of KOH-activated carbons derived from PET waste obtained from post-consumer soft-drink bottles that were carbonized at 500 °C. The carbon matrix was co-carbonized with acridine, carbazole, and urea to enrich it with nitrogen. The highest CO2 capture capacities, as determined via thermogravimetric analysis, were reported as 1.09 mmol g−1 at 25 °C and 0.272 mmol g−1 at 100 °C. Using the same strategy (KOH activation), the maximum CO2 uptake capacity of 2.31 mmol g−1 was achieved by PET-3-700 at 30 °C and 100% CO2 flow.92 Mohamed et al.93 introduced a novel type of advanced 3D mesoporous carbon nanocomposites with high surface area derived from a mixture of Zn dust and PET bottle waste. Upon thermal treatment at 700 °C, the Zn metal transformed into ZnO nanoparticles while the PET bottle waste converted into porous carbon materials, resulting in the creation of waste-based nanocomposite featuring a large surface area (up to 684.5 m2 g−1) with a pore size distribution of 18.47–16.88 nm. Additionally, it was observed that the created nanocomposite showed enhanced photocatalytic properties toward the degradation of organic dyes such as methylene blue and malachite green.
To sum up, a significant stride toward a circular economy is being made by transforming plastic waste into carbon-based material. Various techniques including anoxic pyrolysis, catalytic pyrolysis, arc discharge, and FJH have been applied. Among them, pyrolysis is the most common method for thermochemical decomposition process. The reactor design, catalyst, and process parameters influenced the carbon-based products on its quality, quantity, and morphology. To scale up, metal foil or foams could be investigated instead of the catalyst powder for this process. Moreover, FJH is one of the most promising techniques for larger-scale graphene synthesis due to its high efficiency and simple input materials. For a further industrial development, it might be helpful to have knowledge and expertise from the industry on current facilities, such as those for recycling, gasification/pyrolysis, as well as fluid catalytic cracking and hydrocracking plants.
5. PET to hydrogen
Plastic materials typically contain approximately 8–14 wt% of hydrogen, which makes them valuable as a source of hydrogen-rich energy feedstock. When plastic waste is pyrolyzed, hydrogen is produced along with carbon materials or fuel.96–100PET plastics have been less studied compared to polyolefins due to their low hydrogen content (11.3 wt%). The amount of hydrogen produced is greatly influenced by the type of plastic waste used as feedstock. Among plastic waste, steam reforming and gasification result in the highest hydrogen yields for PE and PP, followed by PET and polystyrene. This is because these plastics do not fully convert into vapor products.101 A pyrolysis-catalytic-dry reforming process using a Ni–Co–Al catalyst was employed to produce syngas, and it was observed that PET led to significantly reduced syngas concentrations.102 Using the photoreforming of the PET approach, an CN–CNT–NM (carbon nitride–carbon nanotube–NiMo) proved to be an efficient and stable catalyst for PET photoreforming. CN–CNTs–NM exhibited remarkable H2 production in plastics photoreforming, which was 14 times higher than CN. Through autogenic pressure pyrolysis and KOH activation, waste PET was effectively transformed into activated carbon and pyrolysis gas.103 The resulting pyrolysis gas consisted of CO2, CH4, CO, and H2. By eliminating CO2, the pyrolysis gas displayed a notable calorific value of 29.2 MJ m−3, making it suitable for use as a supplementary fuel source or as a feedstock for chemical production.
The production of hydrogen from polyethylene terephthalate (PET) dissolved in phenol has also been widely investigated. Nabgan et al.104 conducted a parametric study using a fixed bed reactor with Ni over La2O3–Al2O3 support (various parameters including temperature, feed flow rate, mass flow, phenol concentration, and concentration of PET solution were investigated). The results revealed that all the main independent variables had a significant influence on the dependent variables, with phenol conversion ranging from 47.24% to 97.6% and hydrogen selectivity ranging from 49% to 70.96%. Using Ni–Co/ZrO2 nanostructured catalysts,68 PET–phenol conversion and hydrogen yield of 67.6% and 64.8% were achieved, respectively. In another study, a fixed bed reactor was employed for the catalytic steam reforming of PET dissolved in phenol using a Ni–Pt/Al–Ti catalyst.69 The reaction achieved phenol conversion rates of 94%, resulting in high hydrogen yields of 78%. It has also been found that the PET–phenol steam reforming reaction can successfully produce valuable liquid fuels such as styrene, acetic acid, benzoic acid, benzene, and various other components.
6. Last trends
The simultaneous conversion of CO2 and plastics into valuable products through solar energy presents an opportunity to create a sustainable circular economy. However, integrating these processes poses significant challenges.To achieve this, one approach is to use the products (e.g., methanol produced from the hydrogenation of CO2) to further react with PET waste. A novel catalytic process for converting polyethylene terephthalate (PET) and carbon dioxide (CO2) into high-value chemicals was investigated.105 This process involves the synergistic coupling of three reactions: CO2 hydrogenation, PET methanolysis, and dimethyl terephthalate (DMT) hydrogenation. It was demonstrated that the chemical equilibria of both reactions are shifted forward, leading to enhanced PET depolymerization and increased methanol yield from CO2 hydrogenation. By optimizing the catalyst composition and reaction conditions, high yields of desired products, such as DMCD (dimethyl-trans-1,4-cyclohexanedicarboxylate), were achieved.
Another approach to achieve the simultaneous conversion of CO2 and plastics is to combine the CO2 reduction reaction with PET reforming. An electrocatalytic integrating strategy for the valorization of poly(ethylene terephthalate) (PET) plastic and CO2 to simultaneously produce formic acid at both the anode and cathode was achieved.106 A NiCo2O4 electrocatalyst was used for the oxidation of PET hydrolysate, and a SnO2 electrocatalyst was used for the reduction of CO2. The assembled electrolyzer exhibited a low cell voltage of 1.55 V and achieved a high faradaic efficiency of 155% for formic acid production. This strategy offers a promising approach for the efficient upcycling of PET plastic waste and the production of value-added chemicals.
Combining photochemistry and electrochemistry, a new photoelectrochemical platform was developed, enabling the simultaneous conversion of CO2 and plastic waste into valuable products.107 This versatile system operates with the help of an internal chemical bias under zero applied voltage. A Pd–Cu alloy anode was used to selectively reform polyethylene terephthalate (PET) plastics into glycolate in an alkaline solution. The photoelectrochemical system demonstrates efficient CO2-to-fuel production coupled with plastic-to-chemical conversion, offering a promising and sustainable technology powered by sunlight. The results show high production rates and selectivity, outperforming photocatalytic suspension processes.
7. Conclusion and future directions
In order to promote a sustainable plastics economy, there are numerous challenging obstacles that must be addressed due to their complexity and magnitude. This review has focused on the concept of upcycling, which involves utilizing PET waste as a raw material for the production of value-added products such as monomers, fine chemicals, hydrogen, or carbon materials. Chemical recycling and coupling with rational design and optimization of catalysis provide a necessary addition to current recycling methods. Research and development activities have primarily focused on process engineering, neglecting catalyst design, and molecular-level understanding. As a result, yields have been suboptimal and susceptibility to contaminants like sulfur or chlorine-containing compounds has been high. These limitations highlight the significant potential for technological improvement. Furthermore, the absence of standardized testing protocols presents a challenge in accurately comparing performance results across various laboratories, impeding necessary progress that must be tackled.From the point of view of the product, value-added products can be obtained from PET waste. To break down the plastic waste into monomers for closed-loop recycling, the polyesters polymers can be chemically recycled more efficiently than polyolefin due to the low reaction barriers and near-neutral reaction free energies of C–O bond. Solvolysis, which includes hydrolysis, methanolysis, and glycolysis, is a fundamental method used to breakdown the C–O band. It involves the use of nucleophilic solvents that react with the carbonyl groups present in the plastic, resulting in the formation of products that contain both the monomer and the nucleophile. This process in greater detail focuses on achieving high monomer yields, fast reaction rates, and mild reaction conditions. The development of new catalysts is the key factor in overcoming the challenges. For fine chemicals' production from PET waste, when designing the process, the final value of the product must be considered. The hydrogenation process with metal-based catalyst was proved to be a useful strategy for PET to obtain aromatic compounds. The key to producing aromatic compounds is to break the C–O bond in the polymer chains while keeping the aryl groups intact. Aminolysis has been performed on PET with a variety of amine-containing substrates to generate amide-containing terephthalate-based monomers, which could then be used to make high-value amide-based polymers. Carbon materials, such as carbon nanotubes, graphene, and porous carbon can be obtained from PET waste by cleaving the C–H bonds with the formation of hydrogen gas. The simultaneous conversion of CO2 and plastics into valuable products through solar energy presents an opportunity to create a sustainable circular economy.
For an industrial prospect, ‘plastics refinery’ could be a future option. To determine a suitable process for plastic waste, factors such as environmental impact, industrial feasibility, and economic relevance should be taken into consideration, with the help of life cycle assessment (LCA), techno-economic analysis (TEA), and material flow analysis (MFA). The endeavor to commercialize chemical recycling methods for plastic waste in the industrial sector sheds light on the intricate challenges of separating and purifying real-world waste streams. These streams range from mixed plastic waste to metal-plastic components with multiple components to comprehensive municipal waste. Despite the complexity of the issue, significant progress has been made in obtaining impressive yields of desired products such as monomers, gases, oils, and solids by skillfully customizing catalysts, solvents, temperature stages, residence times, and initial feedstock.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The funding provided by China Petrochemical Technology Company Limited (Sinopec) is grateful acknowledged.References
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef.
- A. M. Atta, A. F. El-Kafrawy, M. H. Aly and A.-A. A. Abdel-Azim, Prog. Org. Coat., 2007, 58, 13–22 CrossRef CAS.
- S. Schmidt, D. Laner, E. Van Eygen and N. Stanisavljevic, Waste Manage., 2020, 110, 74–86 CrossRef CAS PubMed.
- H. Zhang and Z.-G. Wen, Waste Manage., 2014, 34, 987–998 CrossRef CAS PubMed.
- A. B. Raheem, Z. Z. Noor, A. Hassan, M. K. Abd Hamid, S. A. Samsudin and A. H. Sabeen, J. Cleaner Prod., 2019, 225, 1052–1064 CrossRef CAS.
- B. Shojaei, M. Abtahi and M. Najafi, Polym. Adv. Technol., 2020, 31, 2912–2938 CrossRef CAS.
- J. Payne and M. D. Jones, ChemSusChem, 2021, 14, 4041–4070 CrossRef CAS PubMed.
- E. Barnard, J. J. Rubio Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3789 RSC.
- J. Xin, Q. Zhang, J. Huang, R. Huang, Q. Z. Jaffery, D. Yan, Q. Zhou, J. Xu and X. Lu, J. Environ. Manage., 2021, 296, 113267 CrossRef CAS PubMed.
- Damayanti and H.-S. Wu, Polymers, 2021, 13, 1475 CrossRef CAS PubMed.
- M. D. de Dios Caputto, R. Navarro, J. L. Valentín and Á. Marcos-Fernández, J. Polym. Sci., 2022, 60, 3269–3283 CrossRef CAS.
- M. Shanmugam, C. Chuaicham, A. Augustin, K. Sasaki, P. J. J. Sagayaraj and K. Sekar, New J. Chem., 2022, 46, 15776–15794 RSC.
- K. Ghosal and C. Nayak, Mater. Adv., 2022, 3, 1974–1992 RSC.
- A. Bohre, P. R. Jadhao, K. Tripathi, K. K. Pant, B. Likozar and B. Saha, ChemSusChem, 2023, 16(14), e202300142 CrossRef CAS PubMed.
- W. J. Rex and D. James Tennant, US Pat., 2465319, 1945 Search PubMed.
- F. Awaja and D. Pavel, Eur. Polym. J., 2005, 41, 1453–1477 CrossRef CAS.
- H. Nakajima, P. Dijkstra and K. Loos, Polymers, 2017, 9, 523 CrossRef PubMed.
- U. Göschel, in Handbook of Thermoplastic Polyesters, John Wiley & Sons, Ltd, 2002, pp. 289–316 Search PubMed.
- K. G. Gopalakrishna and N. Reddy, in Recycling of Polyethylene Terephthalate Bottles, ed. S. Thomas, A. Rane, K. Kanny, A. Vayyaprontavida Kaliyathan and M. G. Thomas, William Andrew Publishing, 2019, pp. 23–35 Search PubMed.
- K. L. Law, N. Starr, T. R. Siegler, J. R. Jambeck, N. J. Mallos and G. H. Leonard, Sci. Adv., 2020, 6, eabd0288 CrossRef PubMed.
- W. Leal Filho, U. Saari, M. Fedoruk, A. Iital, H. Moora, M. Klöga and V. Voronova, J. Cleaner Prod., 2019, 214, 550–558 CrossRef.
- Y.-C. Jang, G. Lee, Y. Kwon, J. Lim and J. Jeong, Resour., Conserv. Recycl., 2020, 158, 104798 CrossRef.
- Y. Wang, Y. Gu, Y. Wu, G. Zhou, H. Wang, H. Han and T. Chang, Resour., Conserv. Recycl., 2020, 162, 105014 CrossRef.
- P. Dauvergne, Global Environ. Change, 2018, 51, 22–31 CrossRef.
- S. Sakai, H. Yoshida, Y. Hirai, M. Asari, H. Takigami, S. Takahashi, K. Tomoda, M. V. Peeler, J. Wejchert, T. Schmid-Unterseh, A. R. Douvan, R. Hathaway, L. D. Hylander, C. Fischer, G. J. Oh, L. Jinhui and N. K. Chi, J. Mater. Cycles Waste Manage., 2011, 13, 86–102 CrossRef CAS.
- E. Schwanse, Waste Manage. Res., 2011, 29, 973–981 CrossRef.
- R. L. Smith, S. Takkellapati and R. C. Riegerix, ACS Sustainable Chem. Eng., 2022, 10, 2084–2096 CrossRef CAS PubMed.
- W. McDowall, Y. Geng, B. Huang, E. Barteková, R. Bleischwitz, S. Türkeli, R. Kemp and T. Doménech, J. Ind. Ecol., 2017, 21, 651–661 CrossRef.
- M. Frounchi, Macromol. Symp., 1999, 144, 465–469 CrossRef CAS.
- C. Palacios-Mateo, Y. van der Meer and G. Seide, Environ. Sci. Eur., 2021, 33, 2 CrossRef CAS PubMed.
- A. Vlasopoulos, J. Malinauskaite, A. Żabnieńska-Góra and H. Jouhara, Energy, 2023, 277, 127576 CrossRef CAS.
- V. Cappello, P. Sun, G. Zang, S. Kumar, R. Hackler, H. E. Delgado, A. Elgowainy, M. Delferro and T. Krause, Green Chem., 2022, 24, 6306–6318 RSC.
- S. Ügdüler, K. M. V. Geem, R. Denolf, M. Roosen, N. Mys, K. Ragaert and S. D. Meester, Green Chem., 2020, 22, 5376–5394 RSC.
- T. Yoshioka, T. Motoki and A. Okuwaki, Ind. Eng. Chem. Res., 2001, 40, 75–79 CrossRef CAS.
- S. M. Elsaeed and R. K. Farag, J. Appl. Polym. Sci., 2009, 112, 3327–3336 CrossRef CAS.
- R. V. Shah and S. R. Shukla, J. Appl. Polym. Sci., 2012, 125, 3666–3675 CrossRef CAS.
- J. Liu and J. Yin, Ind. Eng. Chem. Res., 2022, 61, 6813–6819 CrossRef CAS.
- J. Rodrigues Fernandes, L. Pereira Amaro, E. Curti Muniz, S. L. Favaro and E. Radovanovic, J. Supercrit. Fluids, 2020, 158, 104715 CrossRef CAS.
- T. Thiounn and R. C. Smith, J. Polym. Sci., 2020, 58, 1347–1364 CrossRef CAS.
- E. Mendiburu-Valor, T. Calvo-Correas, L. Martin, I. Harismendy, C. Peña-Rodriguez and A. Eceiza, J. Cleaner Prod., 2023, 400, 136749 CrossRef CAS.
- K. Kirshanov, R. Toms, P. Melnikov and A. Gervald, Polymers, 2022, 14(8), 1602 CrossRef CAS PubMed.
- T. Erceg, N. Vukić, O. Šovljanski, V. Teofilović, S. Porobić, S. Baloš, S. Kojić, P. Terek, B. Banjanin and S. Rakić, Cellulose, 2023, 30, 5825–5844 CrossRef CAS.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, ACS Sustainable Chem. Eng., 2023, 11, 965–978 CrossRef CAS.
- B. Liu, X. Lu, Z. Ju, P. Sun, J. Xin, X. Yao, Q. Zhou and S. Zhang, Ind. Eng. Chem. Res., 2018, 57, 16239–16245 CrossRef CAS.
- D. S. Achilias, H. H. Redhwi, M. N. Siddiqui, A. K. Nikolaidis, D. N. Bikiaris and G. P. Karayannidis, J. Appl. Polym. Sci., 2010, 118, 3066–3073 CrossRef CAS.
- K. Troev, G. Grancharov, R. Tsevi and I. Gitsov, J. Appl. Polym. Sci., 2003, 90, 1148–1152 CrossRef CAS.
- G. Park, L. Bartolome, K. G. Lee, S. J. Lee, D. H. Kim and T. J. Park, Nanoscale, 2012, 4, 3879–3885 RSC.
- A. Al-Sabagh, F. Yehia, D. R. Harding, G. Eshaq and A. ElMetwally, Green Chem., 2016, 18, 3997–4003 RSC.
- I. Cano, C. Martin, J. A. Fernandes, R. W. Lodge, J. Dupont, F. A. Casado-Carmona, R. Lucena, S. Cardenas, V. Sans and I. de Pedro, Appl. Catal., B, 2020, 260, 118110 CrossRef CAS.
- K. Fukushima, O. Coulembier, J. M. Lecuyer, H. A. Almegren, A. M. Alabdulrahman, F. D. Alsewailem, M. A. Mcneil, P. Dubois, R. M. Waymouth, H. W. Horn, J. E. Rice and J. L. Hedrick, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1273–1281 CrossRef CAS.
- V. Tournier, C. M. Topham, A. Gilles, B. David, C. Folgoas, E. Moya-Leclair, E. Kamionka, M.-L. Desrousseaux, H. Texier, S. Gavalda, M. Cot, E. Guémard, M. Dalibey, J. Nomme, G. Cioci, S. Barbe, M. Chateau, I. André, S. Duquesne and A. Marty, Nature, 2020, 580, 216–219 CrossRef CAS PubMed.
- S. Zhang, Q. Hu, Y.-X. Zhang, H. Guo, Y. Wu, M. Sun, X. Zhu, J. Zhang, S. Gong, P. Liu and Z. Niu, Nat. Sustain., 2023, 1–9 Search PubMed.
- S. Mohammadi, M. G. Bouldo and M. Enayati, ACS Appl. Polym. Mater., 2023, 5(8), 6574–6584 CrossRef CAS PubMed.
- Q. Sun, Y.-Y. Zheng, L.-X. Yun, H. Wu, R.-K. Liu, J.-T. Du, Y.-H. Gu, Z.-G. Shen and J.-X. Wang, ACS Sustainable Chem. Eng., 2023, 11, 7586–7595 CrossRef CAS.
- C. Jehanno, I. Flores, A. P. Dove, A. J. Müller, F. Ruipérez and H. Sardon, Green Chem., 2018, 20, 1205–1212 RSC.
- S. Zhang, W. Xu, R. Du, W. An, X. Liu, S. Xu and Y.-Z. Wang, Chem. Eng. J., 2023, 470, 144032 CrossRef CAS.
- M. Ajiri, K. Machida, K. Arai, O. Sato and I. Saito, Kagaku Kogaku Ronbunshu, 1997, 23(4) 10.1039/d3su00311f.
- Y. Yang, Y. Lu, H. Xiang, Y. Xu and Y. Li, Polym. Degrad. Stab., 2002, 75, 185–191 CrossRef CAS.
- P. Lozano-Martinez, T. Torres-Zapata and N. Martin-Sanchez, ACS Sustainable Chem. Eng., 2021, 9, 9846–9853 CrossRef CAS.
- Q. Liu, R. Li and T. Fang, Chem. Eng. J., 2015, 270, 535–541 CrossRef CAS.
- M. Yan, Y. Yang, T. Shen, N. Grisdanurak, A. Pariatamby, M. Khalid, D. Hantoko and H. Wibowo, Process Saf. Environ. Prot., 2023, 169, 212–219 CrossRef CAS.
- Q. Dong, A. D. Lele, X. Zhao, S. Li, S. Cheng, Y. Wang, M. Cui, M. Guo, A. H. Brozena, Y. Lin, T. Li, L. Xu, A. Qi, I. G. Kevrekidis, J. Mei, X. Pan, D. Liu, Y. Ju and L. Hu, Nature, 2023, 616, 488–494 CrossRef CAS PubMed.
- L. Monsigny, J.-C. Berthet and T. Cantat, ACS Sustainable Chem. Eng., 2018, 6, 10481–10488 CrossRef CAS.
- S. Westhues, J. Idel and J. Klankermayer, Sci. Adv., 2018, 4, eaat9669 CrossRef CAS PubMed.
- Y. Jing, Y. Wang, S. Furukawa, J. Xia, C. Sun, M. J. Hülsey, H. Wang, Y. Guo, X. Liu and N. Yan, Angew Chem. Int. Ed. Engl., 2021, 60, 5527–5535 CrossRef CAS PubMed.
- S. Lu, Y. Jing, B. Feng, Y. Guo, X. Liu and Y. Wang, ChemSusChem, 2021, 14, 4242–4250 CrossRef CAS PubMed.
- Z. Gao, B. Ma, S. Chen, J. Tian and C. Zhao, Nat. Commun., 2022, 13, 3343 CrossRef CAS PubMed.
- K. Fukushima, J. M. Lecuyer, D. S. Wei, H. W. Horn, G. O. Jones, H. A. Al-Megren, A. M. Alabdulrahman, F. D. Alsewailem, M. A. McNeil, J. E. Rice and J. L. Hedrick, Polym. Chem., 2013, 4, 1610–1616 RSC.
- P. Sharma, B. Lochab, D. Kumar and P. K. Roy, ACS Sustainable Chem. Eng., 2016, 4, 1085–1093 CrossRef CAS.
- J. P. K. Tan, J. Tan, N. Park, K. Xu, E. D. Chan, C. Yang, V. A. Piunova, Z. Ji, A. Lim, J. Shao, A. Bai, X. Bai, D. Mantione, H. Sardon, Y. Y. Yang and J. L. Hedrick, Macromolecules, 2019, 52, 7878–7885 CrossRef CAS.
- N. A. Rorrer, S. Nicholson, A. Carpenter, M. J. Biddy, N. J. Grundl and G. T. Beckham, Joule, 2019, 3, 1006–1027 CrossRef CAS.
- H. Zhou, Y. Ren, Z. Li, M. Xu, Y. Wang, R. Ge, X. Kong, L. Zheng and H. Duan, Nat. Commun., 2021, 12, 4679 CrossRef CAS PubMed.
- S. Behera, S. Dinda, R. Saha and B. Mondal, ACS Catal., 2023, 13, 469–474 CrossRef CAS.
- F. Liu, X. Gao, R. Shi, Z. Guo, E. C. M. Tse and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202300094 CrossRef CAS PubMed.
- K. László, A. Bóta, L. G. Nagy and I. Cabasso, Colloids Surf., A, 1999, 151, 311–320 CrossRef.
- O. Vieira, R. S. Ribeiro, J. L. Diaz de Tuesta, H. T. Gomes and A. M. T. Silva, Chem. Eng. J., 2022, 428, 131399 CrossRef CAS.
- S. Sharifian and N. Asasian-Kolur, J. Anal. Appl. Pyrolysis, 2022, 163, 105496 CrossRef CAS.
- J. Gong, X. Chen and T. Tang, Prog. Polym. Sci., 2019, 94, 1–32 CrossRef CAS.
- K. S. Novoselov, V. I. Fal′ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- Y. You, M. Mayyas, S. Xu, I. Mansuri, V. Gaikwad, P. Munroe, V. Sahajwalla and R. K. Joshi, Green Chem., 2017, 19, 5599–5607 RSC.
- L. Cui, X. Wang, N. Chen, B. Ji and L. Qu, Nanoscale, 2017, 9, 9089–9094 RSC.
- D. X. Luong, K. V. Bets, W. A. Algozeeb, M. G. Stanford, C. Kittrell, W. Chen, R. V. Salvatierra, M. Ren, E. A. McHugh, P. A. Advincula, Z. Wang, M. Bhatt, H. Guo, V. Mancevski, R. Shahsavari, B. I. Yakobson and J. M. Tour, Nature, 2020, 577, 647–651 CrossRef CAS PubMed.
- W. A. Algozeeb, P. E. Savas, D. X. Luong, W. Chen, C. Kittrell, M. Bhat, R. Shahsavari and J. M. Tour, ACS Nano, 2020, 14, 15595–15604 CrossRef CAS PubMed.
- S. Rathinavel, K. Priyadharshini and D. Panda, Mater. Sci. Eng., B, 2021, 268, 115095 CrossRef CAS.
- A. Joseph Berkmans, M. Jagannatham, S. Priyanka and P. Haridoss, Waste Manage., 2014, 34, 2139–2145 CrossRef CAS PubMed.
- N. A. El Essawy, A. H. Konsowa, M. Elnouby and H. A. Farag, J. Air Waste Manage. Assoc., 2017, 67, 358–370 CrossRef CAS PubMed.
- M. Kusenberg, A. Zayoud, M. Roosen, H. D. Thi, M. S. Abbas-Abadi, A. Eschenbacher, U. Kresovic, S. De Meester and K. M. Van Geem, Fuel Process. Technol., 2022, 227, 107090 CrossRef CAS.
- D. Yao, H. Li, B. C. Mohan, A. K. Prabhakar, Y. Dai and C.-H. Wang, ACS Sustainable Chem. Eng., 2022, 10, 1125–1136 CrossRef CAS.
- J. B. Parra, C. O. Ania, A. Arenillas, F. Rubiera and J. J. Pis, Appl. Surf. Sci., 2004, 238, 304–308 CrossRef CAS.
- M. T. Kartel, N. V. Sych, M. M. Tsyba and V. V. Strelko, Carbon, 2006, 44, 1019–1022 CrossRef CAS.
- A. Arenillas, F. Rubiera, J. B. Parra, C. O. Ania and J. J. Pis, Appl. Surf. Sci., 2005, 252, 619–624 CrossRef CAS.
- B. Kaur, J. Singh, R. K. Gupta and H. Bhunia, J. Environ. Manage., 2019, 242, 68–80 CrossRef CAS PubMed.
- H. H. Mohamed, A. A. Alsanea, N. A. Alomair, S. Akhtar and D. W. Bahnemann, Environ. Sci. Pollut. Res., 2019, 26, 12288–12301 CrossRef CAS PubMed.
- A. Veksha, A. Giannis and V. W.-C. Chang, J. Anal. Appl. Pyrolysis, 2017, 124, 16–24 CrossRef CAS.
- S. Ko, Y. J. Kwon, J. U. Lee and Y.-P. Jeon, J. Ind. Eng. Chem., 2020, 83, 449–458 CrossRef CAS.
- J. Chattopadhyay, T. S. Pathak, R. Srivastava and A. C. Singh, Energy, 2016, 103, 513–521 CrossRef CAS.
- S. C. Wijayasekera, K. Hewage, O. Siddiqui, P. Hettiaratchi and R. Sadiq, Int. J. Hydrogen Energy, 2022, 47, 5842–5870 CrossRef CAS.
- R. Mishra, H. C. Ong and C.-W. Lin, Energy Convers. Manage., 2023, 284, 116983 CrossRef CAS.
- P. T. Williams, Waste Biomass Valorization, 2021, 12, 1–28 CrossRef CAS.
- A. S. Al-Fatesh, N. Y. A. AL-Garadi, A. I. Osman, F. S. Al-Mubaddel, A. A. Ibrahim, W. U. Khan, Y. M. Alanazi, M. M. Alrashed and O. Y. Alothman, Fuel, 2023, 344, 128107 CrossRef CAS.
- T. Tsuji and A. Hatayama, J. Mater. Cycles Waste Manage., 2009, 11, 144–147 CrossRef CAS.
- J. M. Saad and P. T. Williams, Energy Fuels, 2016, 30, 3198–3204 CrossRef CAS.
- H. Zhang, X.-L. Zhou, L.-M. Shao, F. Lü and P.-J. He, Sci. Total Environ., 2021, 772, 145309 CrossRef CAS PubMed.
- B. Nabgan, W. Nabgan, T. A. Tuan Abdullah, M. Tahir, Y. Gambo, M. Ibrahim and W. Syie Luing, Energy Convers. Manage., 2017, 142, 127–142 CrossRef CAS.
- Y. Li, M. Wang, X. Liu, C. Hu, D. Xiao and D. Ma, Angew. Chem., Int. Ed., 2022, 61, e202117205 CrossRef CAS PubMed.
- J. Wang, X. Li, M. Wang, T. Zhang, X. Chai, J. Lu, T. Wang, Y. Zhao and D. Ma, ACS Catal., 2022, 12, 6722–6728 CrossRef CAS.
- S. Bhattacharjee, M. Rahaman, V. Andrei, M. Miller, S. Rodríguez-Jiménez, E. Lam, C. Pornrungroj and E. Reisner, Nat. Synth., 2023, 2, 182–192 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |