Open Access Article
Open Access ArticleHydrometallurgical recycling technologies for NMC Li-ion battery cathodes: current industrial practice and new R&D trends†
Krystal
Davis
*ab and
George P.
Demopoulos
 *a
*a
aMining and Materials Engineering, McGill University, 3610 University Street, Montreal, QC H3A 0C5, Canada. E-mail: George.demopoulos@mcgill.ca
bEnergy, Mining and Environment, National Research Council of Canada, 1200 Montreal Rd., Ottawa, ON K1A 0R6, Canada. E-mail: Krystal.Davis@nrc-cnrc.gc.ca
First published on 16th October 2023
Abstract
The supply chain for raw materials needed to fulfill the demand for lithium-ion battery (LIB) manufacturing is less than certain. With the stress and uncertainty of securing the raw materials, predicted price increases in metals such as lithium and nickel could jeopardize the economics in EV battery production. Lithium-ion battery recycling could help alleviate the demands on critical virgin materials. This would realize a price parity goal, $100 per kW h, for internal combustion engines (ICE) and EVs. Simultaneously, recycling could reduce waste in landfill sites as 1 M tonnes of LIBs are set to retire by 2030. In this context the recycling of LIBs becomes imperative, but this industrial activity must be done in full compliance with sustainability principles. For example, current industrial recycling technologies tend to generate a lot of waste products as only some value metals are recovered while simultaneously tend to be energy intensive with heavy chemical usage and risks of water and soil contamination. In this review we focus on spent nickel-manganese-cobalt (NMC) lithium-ion batteries that currently dominate the EV market examining primarily their recycling by hydrometallurgical processing as this route seems to be the most advocated. Our review compares the different hydrometallurgical process flowsheets seeking to highlight areas for chemical and technological improvement as they are proposed in recent patented R&D developments. The review concludes with the strong endorsement of hydrometallurgy-based direct recycling approach as the most sustainable route.
Sustainability spotlightNi–Mn–Co oxides (NMC) are currently the preferred cathode materials in Li-ion batteries (LIB) powering the electric vehicle (EV) revolution. The composition chemistry of these cathodes constantly evolves while at the same time the volume of spent batteries exponentially increases demanding new advanced sustainable recycling technologies. Currently the industrial NMC recycling practice targets mainly the recovery of the valuable metals by pyrometallurgical and hydrometallurgical processes, or a combination of both. However, these have several limitations, including high energy and/or chemical consumption, incomplete recovery of metals, and GHG emissions. To overcome these limitations, there are new R&D developments in hydrometallurgical recycling technologies. One area of development is the use of novel solvents to improve metal recovery rates. Another exciting development is a paradigm-shift towards direct recycling of the active cathode material (CAM) enabled by innovative hydrometallurgical treatment that avoids breaking it down into its constituent elements. Direct recycling may be combined with upcycling, thereby further promoting circularity and less dependency on raw material extraction. Our review relates to the following UN SDGs: affordable and clean energy (SDG 7), industry & innovation (SDG 9), responsible consumption and production (SDG 12), and climate action (SDG 13). |
Introduction
Lithium-ion battery production is projected to reach 440 GWh by 2025 as a result of the decarbonisation efforts of the transportation sector which contribute 27 percent of the total GHG emissions.1 A lithium-ion battery is deemed “spent” when it has reached a state of health which is less than 80 percent, typically after 10 years of use.2 Recycling lithium-ion batteries has been recognized as a way to secure the supply chain and reduce environmental impacts if they go to landfill sites.3 Recycling can contribute to an overall reduction in the GHG emissions that are associated with the battery industry.4Battery chemistries have evolved over the years, two factors have driven research and innovation; increasing density, thus battery life and range, and reducing material costs.5 The most common battery chemistry in EV LIB cathodes are lithium, nickel, manganese and cobalt oxide (NMC) batteries. The cathode can be any mixture of LiNixMnyCozO2, NMC (where x, y and z = 1). Other chemistries such as lithium iron phosphate (LFP), LiNixCoyAlzO2, NCA (where x, y and z = 1), lithium manganese oxide (LMO) and lithium cobalt oxide (NCO), are commercially available but for this study we will look at the NMC battery only.6 The NMC cathode has become economically more desirable, the cobalt content has decreased (NMC 111, NMC 523, NMC 622, NMC 811) improving not only overall cost but also lessening the environmental and ethical impacts. The density has also improved with the increase in nickel content, NMC 811, improving the range.7
Some of the LIB raw material have been listed as critical in countries like Canada, USA, Europe, Japan and South Korea, making recycling equally critical from economic and environmental perspectives.8 Sourcing and securing a sustainable supply chain for manufacturing LIBs means social issues are meaningful. The Democratic Republic of the Congo (DRC) holds 60 percent of the worlds' cobalt resource. The country has artisanal and small-scale mines (ASM) dominated by Chinese owned companies. This gives the Chinese LIB manufactures security over international manufacturers for cobalt supply. For years, human's rights groups have reported severe issues in mining operations in the DRC including, child labour, fatal accidents, and high corruption. Companies in need of cobalt supply are invested in improving the ASM standard for mine safety and child labor to ensure cobalt is mined responsibly.9
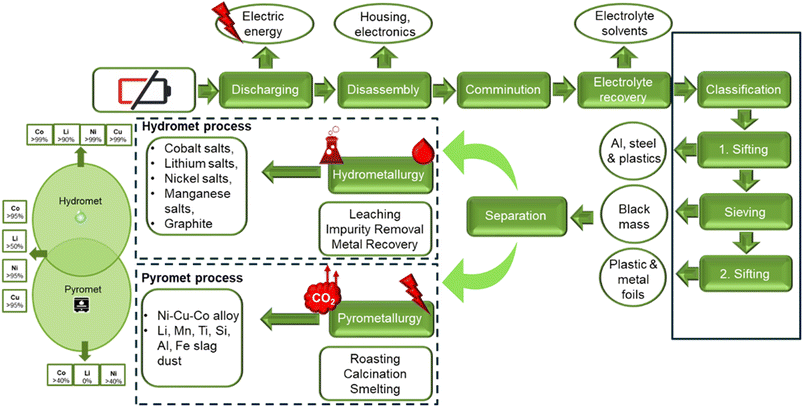
In order to enable LIB recycling, pathways for other types of batteries (NiMH, alkaline, and lead-acid) are modified to support the circular economy system. The components of LIBs, their structures, and their charge-storage mechanisms are used to design the recycling process.5 The current commercial approach to recycling NMC cathodes which offers the greatest extraction and lowest energy impacts, is the hydrometallurgical processing route.10 Several recycling facilities are operating globally and recovering critical metals like Li, Co, and Ni on a commercial scale using this approach. Although pyro- and hydro-metallurgical LIB recycling processes are commercialized, they aren't without fault. They involve high temperature smelting, pyrometallurgy, and the use of large quantities of chemicals, hydrometallurgy. Lately, research has focused on developing a greener recycling approach to decrease the environmental impact and operation costs associated with the current industrial methods. The promising direct recycling approach does not decompose the cathode active material into the elemental products which need to be completely re-manufactured like the pyro- and hydro-met routes do. Instead, the direct recycling approach applies “soft” means to induce relithiation of the cathode compound, e.g., NMC leaving the structure intact and re-functionalized.10 Hydrometallurgy is expected to play a key role towards this end as pointed out in the perspective section of the present paper. In this review a systematic compilation of the most important state-of-the-art hydrometallurgical recycling processes applicable to the dominant EV NMC cathode material family is presented by sourcing technical information from the recent patent literature and industrial news complementing the underlying chemistry discussion. Many of the presented processes are at the pilot scale, yet to be commercialized. Some of the processes described recover only cathode materials (Ni, Co, Li, Mn), while others are targeting a circular economy approach, recovering all components of the spent battery (graphite, Cu, Al, electrolyte, plastics etc.).
Demand of lithium-ion batteries
As climate change takes the forefront of many countries' roadmaps, greenhouse gas emissions reduction becomes a main priority. The Paris Agreement was born as a legally binding international treaty on climate change that includes 191 countries and the European Union. The goal to decrease the global warming temperature to 1.5 degrees Celsius brings challenges to countries to achieve climate neutrality. According to the World Bank, the transportation sector contributes 20 percent of the GHG emissions globally. This number could grow to 60 percent by 2050. The reduction of climate impact from the transportation sector is critical. Electric vehicles (EVs) have the potential to significantly reduce GHG emissions. Renewable energy sources such as LIBs are important to put an end to the era of fossil fuels. This has driven a lot of research and development within battery technology. The main objectives are to increase the battery range and decrease the cost in order to strongly compete with internal combustion engine cars (ICE). The data shows that the adoption of EVs has taken off exponentially. The sales of EV cars have increased by 297 percent, between 2016 and 2020. After a decade of growth, in 2020 the global electric car stock hit 10 million, shown in Fig. 1. Although China had the largest fleet in 2020, 4.5 million EVs, Europe had the largest annual increase, 3.2 million EVs.11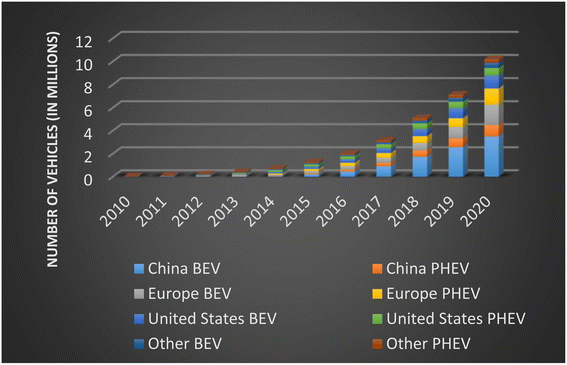 | ||
| Fig. 1 Global electric passenger car stock, 2010–2020 (data sourced from ref. 12). | ||
According to the latest EV outlook report from BloombergNEF, 60 percent of new car sales worldwide must be electric by 2030 if a net-zero scenario is to be achieved.12
LIBs are a source of critical materials
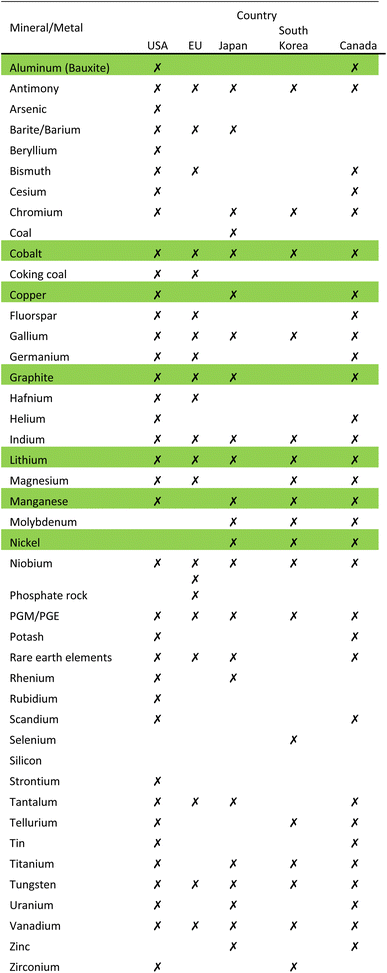
Raw materials that have been deemed economically important and have a high supply risk, are identified as critical raw materials. Countries have developed a list of critical raw materials that require strategic planning to achieve sustainability and resource security. With the onset of the rapid change from ICE to EV in the automotive industry, the demand on raw metals and minerals that make up a battery is surging.13Demand for raw materials like graphite, lithium and cobalt is predicted to increase 500 percent by 2050, according to the World Bank.1 Several countries have revisited their list of critical materials in light of the EV era. The USA, EU, Japan, South Korea and Canada have released a list of critical minerals/metals.8
Table 1 shows 44 minerals/metals identified as critical, 9 of which are consistent between all countries: antimony, cobalt, gallium, indium, lithium, niobium, PGE/PGM, tungsten and vanadium. The metals lithium, nickel, cobalt, manganese, aluminum, and copper along with graphite that are required to manufacture LIBs are highlighted in green in Table 1.8 The EU Commission estimates that demand for cobalt and lithium will increase by a factor of 4 and 10, respectively, by 2030; and 10 and 40, respectively, by 2050.14 Cobalt is mainly mined as a by-product in nickel and copper mines. The supply of cobalt is of real concern because it is limited to a few countries such as DRC, Australia, Cuba, Philippines, Russia and Canada.15Fig. 2 shows the Global Cobalt Reserves which are dominated by DRC, 3.6 M tons, and Australia, 1.4 M tons. Although Australia has substantial reserves, they only mine 4 percent of the global production of cobalt. Due to the ethical and environmental concerns of mining in the DRC, the need to eliminate global dependence on this metal is crucial.9
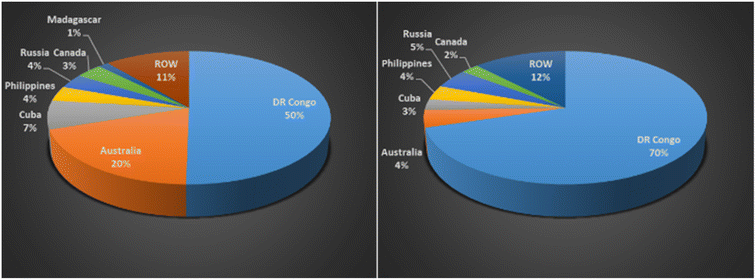 | ||
| Fig. 2 (Left) global cobalt reserves 2020, by country (in metric tons), (Right) global production of cobalt 2020, by country (% share of production) (data sourced from ref. 15). | ||
The raw materials needed to fulfill the supply that is being demanded to achieve net-zero emissions is not possible through mining alone. The current mines and projects that are under construction will only be able to produce 50 percent of the projected lithium and cobalt, and 80 percent of the required copper by 2030.16 Spent lithium-ion batteries contain between 5-20 weight percent of cobalt, 5–7 weight percent of lithium, and 5–7 weight percent of nickel, these concentrations are higher than what is naturally available from raw materials. Recycling is an economical solution to the raw material shortage.17
The estimation of material intensity of NMC lithium-ion battery packs (kg kW−1 h−1) has been analyzed by Argonne National Laboratory using their BatPaC modeling software with the main metrics presented in Table 2.18 The weight of lithium includes both the electrolyte and cathode. The cathode contains nickel, cobalt and manganese. Aluminum weights include current collectors, cell terminals, thermal conductors and module and battery enclosures. Copper weight is derived from the cell current collectors, terminals, thermal conductors and module and battery enclosures. The anode is responsible for the graphite weight.18
| Material | NMC 111 | NMC 532 | NMC 622 | NMC 811 |
|---|---|---|---|---|
| Lithium | 0.141 | 0.136 | 0.118 | 0.1 |
| Nickel | 0.351 | 0.508 | 0.531 | 0.6 |
| Cobalt | 0.352 | 0.204 | 0.178 | 0.75 |
| Manganese | 0.328 | 0.285 | 0.166 | 0.07 |
| Aluminum | 3.11 | 3.07 | 3.017 | 2.921 |
| Copper | 0.677 | 0.661 | 0.605 | 0.549 |
| Graphite | 0.978 | 0.981 | 0.96 | 0.961 |
The change in NMC battery chemistry has evolved from high cobalt NMC 111 to high nickel NMC 811, from 2010 to predicted 2030, see Fig. 3. Cathode chemistry advancements can be credited for the reduction in cost of an EV battery.19
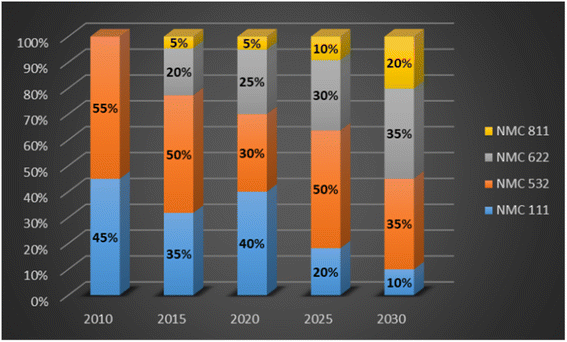 | ||
| Fig. 3 NMC cathode chemistry evolution (data sourced from ref. 19). | ||
The average cost to produce a lithium-ion battery for an electric vehicle (EV) has significantly declined from $1200 per kW h in 2010, to $132 per kW h in 2021. S&P Global Platts has forecasted the EV battery manufacturing cost will reach price parity with ICE, at $100 per kW h by 2026.20 BloombergNEF has broken down the overall cost of a battery cell, see in Fig. 4. The cathode accounts for the half of the average cell cost, while the anode is 12 percent of the cell cost.
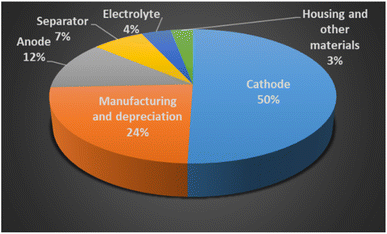 | ||
| Fig. 4 The cost of a lithium-ion battery (reproduced from ref. 12 with permission from RCS, copyright 2021). | ||
The battery metals supply chain, particularly metals that make up the cathode, are experiencing pricing increases. Metals like lithium, nickel and cobalt have seen extreme increase in prices between 2020 and 2022, while manganese, aluminum, copper and graphite have also increased since 2020, refer to Table 3. Lithium pricing exploded by 496.7 percent between 2020 and 2021.21 Platts Analytics was expecting prices to stop rising in 2022 as the supply chain becomes more secure.20
Current industrial hydrometallurgical recycling processes
General process chemistry
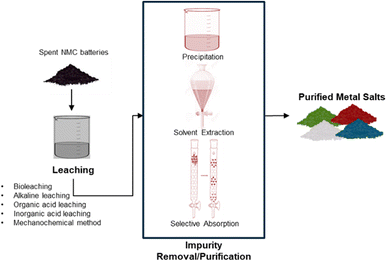
Hydrometallurgical recycling processing uses aqueous chemistry and follows typically three main steps, leaching of spent cathode material, impurity removal/purification of leach solution, and recovery of the metal salts, see Fig. 5. Leaching uses acids (or bases) at low temperatures in an oxidizing or reducing environment to dissolve valuable metals. The impurity removal and purification separate the valuable metals from unwanted metals/impurities via selective chemical reactions. Methods used are typically hydrolytic precipitation (pH adjustment), liquid–liquid reactions (solvent extraction), and selective absorption (ion exchange). The production of precursor metal salts can be done by crystallization/precipitation or via metal deposition (by gas reduction or electrolytic reduction) and redissolution and crystallization.3The main advantage of hydrometallurgical recycling over pyrometallurgy is the recovery of high purity pay metals, Ni, Co, Mn, Li, with high recoveries, greater than 99 percent, low impurity content and a lower energy consumption (Fig. 6).17 Lithium is only recovered with hydrometallurgy, as lithium carbonate. Another benefit of the hydromet processing route is that it can adapt to a mixed cathode feed stock, eliminating the need to separate the spent batteries based on chemistry.10 The pyrometallurgical processing route, can only recover Co, Ni and Cu as alloys. The alloys require further refinement using hydrometallurgy.29
When recycling lithium-ion batteries, the most common hydrometallurgical method uses sulfuric acid and hydrogen peroxide. The following chemical equation, eqn (1), describes the leaching process.
| 2LiMO2(s) + 3H2SO4 + H2O2 → 2MSO4(aq) + Li2SO4 + 4H2O + O2 | (1) |
Cathode transition metals, such as Co and Mn, have low solubility because in a discharged cathode they are in the +3/+4 valence states. The strong M–O bonds make leaching difficult. Reducing the metals to a divalent state weakens the M–O bond allowing for effective leaching. It is understood that the hydrogen peroxide, H2O2, behaves as a reducing agent which converts Co3+ into the soluble Co2+ metal ion. The addition of peroxide has improved the leaching efficiency to greater than 95 percent.30 The by-product from the addition of H2O2 is water and oxygen gas. This reaction is graphically depicted in Fig. 7. This is a greener alternative than using other reducing agents such as sulfur dioxide, SO2, which produces sulfurous acid.
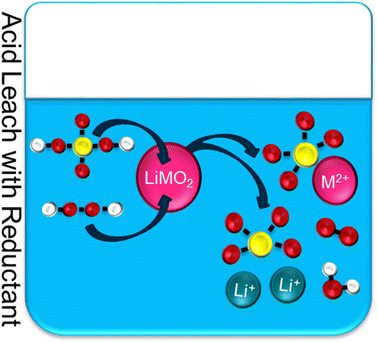 | ||
| Fig. 7 Typical acid leach with reductant for NMC recycling, where yellow = sulfur, red = oxygen, white = hydrogen, M = metal (Ni–Mn–Co), M2+ = metal ions (divalent). | ||
Lithium leaching efficiency is directly correlated with the strength of acid. Displacement of Li+ with H+ allows for the lithium to become easily soluble during leaching.29 Once the leached metals, Li+, Ni2+, Mn2+, Co2+, Cu2+, Fe3+, and Al3+, are in solution, they are selectively addressed in the impurity removal and purification stage. Typically, multiple precipitation and solvent extraction stages are used to separate and purify the solution prior to the recovery of the metals. Metals such as Cu, Fe and Al, are considered to be impurities in this process. These metals can co-precipitate with value metals. The solubility of metal hydroxides in the NMC leach solution can be evaluated with the help of solubility diagrams, Fig. 8a and b. Sequential hydrolytic precipitation of metal hydroxides may be brought upon by Incremental pH adjustments following the solubility curves using a base, such as NaOH for example. The solution separation of Ni, Mn and Co is difficult to achieve using pH due to closeness of their respective solubility lines resulting in co-precipitation, and therefore a mixed metal hydroxide precipitate that requires further refinement by re-dissolution adding complexity and cost to the operation. Lithium hydroxide has a high solubility and thus often is precipitated with sodium carbonate, Na2CO3, as Li2CO3 (Fig. 8c and d). The fundamentals of metal ion compound precipitation from the standpoint of particle growth and cleanliness using supersaturation control methodologies are described by Demopoulos.31
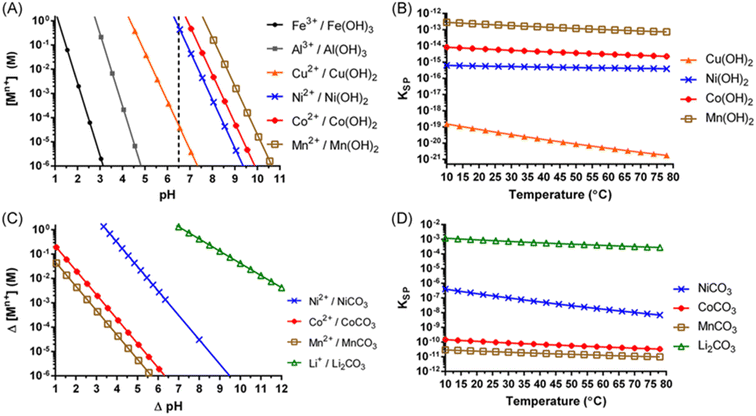 | ||
| Fig. 8 Solubility diagram for metal hydroxides at 25 °C (A) temperature effect on metal hydroxide solubility (B) solubility diagram for metal carbonates at 25 °C (C) and temperature effect on metal carbonate solubility (D) (reproduced from ref. 30 with permission from ACS, copyright 2022). | ||
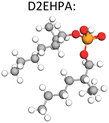
The Ni–Mn–Co mixed metal solution following the impurity (Fe, Al, Cu) removal stage (by hydrolytic precipitation) is advanced to purification where the metals can be separated into individual purified solutions. Most hydromet processes use solvent extraction to separate the dissolved metals. The typical extraction reaction is given by, eqn (2), where (HA)2 is the dimer form of an acidic extractant like D2EHPA. The reverse reaction represents stripping of the metal ions into an acidic solution from which metal recovery is affected.
| Maq2+ + Aorg− + 2(HA)2org ↔ MA2n3HAorg + Haq+ | (2) |
The selection of solvent extraction reagents (extractants) depends on composition of feedstock solution and the ionic state of key elements.39 Various extractants have been researched to separate and purify critical battery metals. The process uses time, pH, aqueous/organic (A/O) ratios, and concentration of extractant to control which metal ion will be selectively removed. Table 4 illustrates certain examples of different extraction conditions that have been reported. Many extractants have been used. By far as per well established practice of Co/Ni solvent extraction (consult for example the seminal book by G. M. Ritchey and A. W. Ashbrooke “Solvent Extraction: Principles & Applications to Process Metallurgy)” in commercial use for metallurgical nickel extraction plants the organophosphorus acid extractant family attracts the biggest interest.
| Category | Extractant | Applications | Solution composition (g L−1) | Extraction conditions | Extraction efficiency | Ref. |
|---|---|---|---|---|---|---|
| Organic phosphoric acid extractant |
|
• Mn extraction and impurity ions | Co: 0.175, Ni: 0.099, Mn: 5.269, Li: 1.248 | T: 300 s, pH: 5, A/O: 0.5, 20% vol D2EHPA, 70–75% saponification | Mn: 97% | 34 |
| • Co-extraction Co–Ni–Mn from Li | Co: 6.438, Ni: 0.089, Mn: 6.312, Li: 1.602 | T: 300 s, pH: 3.5, A/O: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15% vol Mextral272P 1, 15% vol Mextral272P |
Mn: 97.1% | 35 | ||
| • Separate Ni and Co | ||||||
| Organic phosphonic acid extractant |
|
• Separate Ni | Ni: 2.54, Li: 4.82 | pH: 6.5, A/O: 1, 0.15 M PC88 A | Ni: 99.9% | 36 and 37 |
|
• Separate Ni and Co | Co: 20, Ni: 0.5, Li: 2.5 | pH: 3.5, A/O: 1.5, 25% vol P507 | Co: 95%, <5% Ni and Li co-extraction | 38 and 36 | |
| Organic phosphinic acid extractant |
|
• Separate Co | Co: 7.18, Ni: 4.29, Mn: 0.045, Li: 1.49 | T: 300 s, pH: 4.5, A/O: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20% vol Ni loaded Mextral272P 1, 20% vol Ni loaded Mextral272P |
Co: 97.8% | 39 |
| Blended extractants |
|
• Separate Co/Li and Co/Ni | Co: 13.8, Ni: 0.015, Mn: 0.011, Li: 2.04 | pH: 5.5–6, A/O: 0.5, 0.4 M cyanex 272, 50% saponification | Co: 95–98%, ∼1% Ni extraction | 40 |
| D2EHPA + versatic 10 acid | — | Co: 11.4, Ni: 12.2, Mn: 11.7, Li: 5.3 | pH: 4.5, A/O: 1, 0.43 M D2EHPA and 0.7 M versatic 10 acid | Mn: 98.33%, Co: 1.06% Ni: 4.11% and Li: 0.25% co-extraction | 41 |
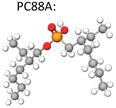
There are three types of organophosphorus acids (see their structures in Table 4): organic phosphoric, exemplified by D2EHPA (di-(2-ethylhexyl) phosphoric acid); organic phosphonic such as 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester, marketed as PC88A or P507; and organic phosphinic, bi(2,4,4-trimethylpentyl) phosphinic acid, marketed as Cyanex 272P or Mextral 272P. The organophosphorus acids may be graded according to their acidity (pKa) as follows: phosphoric > phosphonic > phosphinic. The more acidic the extractant the lower the pH at which can extract a metal, the faster the extraction rate, the lower the separation efficiency. For example, in Co/Ni separation, the extraction of Co follows the order D2EHPA > Phosphonic > Phosphinic, while the reverse happens in terms of Co/Ni separation efficiency Co: phosphinic > phosphonic > D2EHPA. A sample of extraction results with these reagents reported from NMC recycling studies is provided in Table 4.
The above results can be understood on the basis of the respective metal extraction equilibria which provide the selectivity series. The phosphinic acid (Cyanex 272/Mextral 272), which is commonly used to separate cobalt from nickel in sulphate media exhibits the selectivity series illustrated in Fig. 9.42
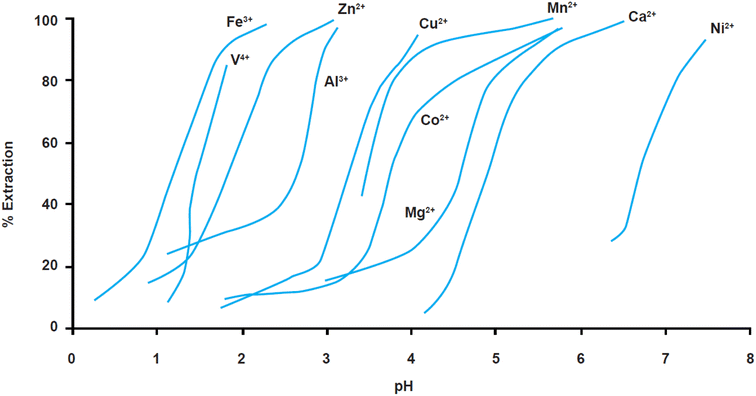 | ||
| Fig. 9 Extraction equilibria of metals (in%) using cyanex 272 vs. pH in sulphate solutions (reproduced from ref. 43 with permission from KISTI, copyright 2015). | ||
Whereas D2EHPA is commonly used to separate Mn from Co, Ni and Li. The respective selectivity series can be seen in Fig. 10.
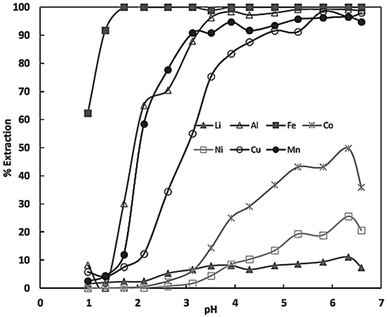 | ||
| Fig. 10 Extraction equilibria of metals (in%) using D2EHPA vs. pH in sulphate solutions (reproduced from ref. 44 with permission from MDPI, copyright 2022). | ||
Metal separations are often enhanced by using a blend of two extractants, as is the example shown in Table 4, where a carboxylic acid extractant (Versatic 10, neodecanoic acid) was used in mixture with D2EHPA.41
Commercial facilities
There are several commercial hydrometallurgical LIB recycling facilities in operation today. Selected process flowsheets are presented in the ESI.† Recovering valuable metals from spent LIBs started with the “Toxco” process in 1994, rebranded as Retriev Technologies company in 2013.45,46 The Toxco facility, located in Canada, treats the spent batteries with liquid nitrogen, −196 °C, to deactivate them (see ESI Fig. S1†). Once frozen, the batteries are shredded and then report to an aqueous solution with high pH.45 The material is then sent through a hammer mill, the metallic components are screened and treated with a shaker table to separate aluminum from plastics. The cathode material is sent to the filter tank. The filter cake is rich in graphite and metal oxides, this is referred to as black mass. Metal oxides are then subjected to hydrometallurgical processing. Lithium is recovered as lithium carbonate or lithium phosphate.30 The overall process recycles 60 percent of the battery pack materials and has an annual capacity of 4500 tonnes.47On a pilot scale, Retriev has patented a process (Fig. S2†) that further treats and regenerates the cathode material. The black mass undergoes thermal treatment to remove the binder and a froth flotation treatment to remove the graphite anode. The metal oxides from the cathode material are recovered in the non-floated portion and regenerated with LiOH addition to correct lithium concentration and heating in a furnace (700–815 °C), milling and screening to achieve a particle size between 5 µm and 25 µm.48 The purity of the reactivated cathode material has not been reported but judging from their latest development activities involving the addition of hydrometallurgical purification steps it is deduced that this was not battery-grade. The latest addition has yet to be commercialized.
Umicore (Belgium) has the largest capacity, 7000 tonnes/year, amongst the commercial recycling plants using a hydrometallurgical method. The process recovers nickel and cobalt using a combination of pyrometallurgy and hydrometallurgy. Lithium and aluminum are lost as slag in the shaft furnace. There appears to be a two-stage leaching process (Fig. S3†) with the first stage (H2SO4) serving to remove selectively iron and copper impurities and the second stage in HCl solution allowing the separation of Ni and Co into Ni(OH)2 and CoCl2 products. A key environmental step of the Umicore process is the use of a high temperature step to treat waste gases, avoiding toxic emissions. Overall, the process seems to recover ∼70 percent of the metals, 10 percent graphite and 15 percent of the plastics. The earlier grade of the recovered metals was not suitable for new battery manufacturing,47 but this appears to have been overcome with process improvements reported from pilot scale testing.48
Brunp Recycling/CATL (China) process has an annual capacity to treat 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year of spent LIBS.48 The recycling process (Fig. S4†) includes discharging, thermal pre-treatment, mechanical treatment and complex hydrometallurgy (acid leaching with H2SO4 and HCl, solvent extraction for the separation of Ni and Co, crystallization of metal sulphate and chloride salts and a mixed intermediate hydroxide precipitate). The process, it is claimed to have a 99.3 percent metal recovery of nickel, cobalt and manganese, but this is difficult to verify. Additionally, it is unclear if the copper, aluminum and graphite is recovered in their process.
000 t per year of spent LIBS.48 The recycling process (Fig. S4†) includes discharging, thermal pre-treatment, mechanical treatment and complex hydrometallurgy (acid leaching with H2SO4 and HCl, solvent extraction for the separation of Ni and Co, crystallization of metal sulphate and chloride salts and a mixed intermediate hydroxide precipitate). The process, it is claimed to have a 99.3 percent metal recovery of nickel, cobalt and manganese, but this is difficult to verify. Additionally, it is unclear if the copper, aluminum and graphite is recovered in their process.
GEM Ltd, also known as Green Eco-Manufacturer Hi-Tech Co., Ltd, is located in China. The recycling company has an annual production of 5000 tonnes per year. The process begins with pre-treatment which sorts the major battery components using comminution. Metals such as, copper, aluminum, iron, cobalt and nickel, are separated by complex hydrometallurgical processing steps (of which little is known, Fig. S5†) and then synthesized into salts.6 The electrolyte is not recovered in this process.
Accurec (Germany) operates a LIB recycling process that includes thermal, mechanical, pyrometallurgy and hydrometallurgy steps (Fig. S6†). The recycling plant treats 3000 tonnes of lithium-ion batteries annually. Accurec has new technology being implemented in the current recycling process that will allow for the recovery of lithium and graphite.6
Nickelhütte Aue GmbH (NHA) has been recycling Li-ion batteries in Germany since 2011.6 Thermal pre-treatment, pyrometallurgy processing, and hydrometallurgy methods make up the recycling process. The flow diagram, shown in Fig. S7,† depicts NHA's process.49 The pyromet process smelts the spent batteries in a batch-wise-furnace to obtain Ni–Co–Mn matte product which subsequently is subjected to leaching to recover various metal salts. A rotary kiln is used to thermally pre-treat the batteries. Limited information is available regarding the specifics of the hydrometallurgy treatment. Unless there have been recent improvements the produced metal salts may not be of battery-grade but rather used in other fields like electroplating shops. The overall process capacity is 7000 tonnes per year.6
The Duesenfeld lithium-ion battery recycling process, LithoRec, completely discharges the battery packs before physical separation. As shown in Fig. S8,† the process isolates the electrode coatings and removes fluoride before leaching. The metals are then separated from graphite. Then lithium, cobalt, nickel and manganese are separated using various extraction methods. The metals are then purified and recovered as battery-grade salts, ready for new cathode application. The mechanical recycling rate is 72 percent, the recovery of electrolyte and graphite bring the overall recycling efficiency to 91 percent. The separator film and high boiler portion of the electrolyte make up the unrecycled 9 percent. The process located in Germany has an annual capacity of 3000 tonnes.6
SungEel HiTech, located in Korea, have a recycling plant capacity that treats 8000 tonnes of spent LIBS annually. The process scheme, shown in Fig. S9,† follows the general approach encountered in the previous plants starting with mechanical separation and followed by hydrometallurgical purification. There is not specific information available on the hydrometallurgy operations but judging from the products listed in the published flowsheet (Fig. 16) that includes salts and metal ingots, it is expected to be a complex one ranging from leaching to solution purification and recovery of metal salts by crystallization and metals by electrolysis. The company announced in 2020, it will be expanding operations to a new site that will triple the current capacity to 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year.6,50
000 tonnes per year.6,50
Dowa Holdings Co. Ltd (Japan) currently treats 1000 tonnes of spent LIBs a year. The process includes disassembly, thermal pre-treatment (rotary kiln), pyromet and hydromet processing. Details regarding the process technology, efficiency and recovery rates are not available.6
New research developments
The life cycle analysis of pyro- and hydro-metallurgy recycling methods was compared to the direct recycling method by Argonne National Laboratory using the EverBatt model as shown in Fig. 11.51 The hydrometallurgy method consumed the highest energy, a result of high material throughput.10 Greenhouse gas emissions between pyro- and hydro-met were around 2 kg kg−1 cell. The economics were better for hydrometallurgy when compared to pyrometallurgy route because of the higher and more efficient recoveries of high value cathode materials. The advantages for investing research into direct recycling are illustrated in all three sectors of the analysis, energy consumption, GHG emissions and revenue potential. Many of the new processes under development aim towards more efficient hydrometallurgy-based recycling technologies and in particular towards adoption of the new direct recycling approach. Advanced examples of such developments that have reached the pilot-plant stage are reviewed in this section.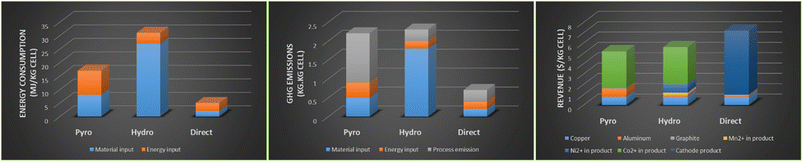 | ||
| Fig. 11 Life cycle analysis for pyrometallurgy, hydrometallurgy and direct recycling (pyro, hydro, direct) (left) energy consumption, (middle) GHG emissions and (right) revenue from outputs, (reproduced from ref. 10 with permission from MDPI, copyright 2022). | ||
New hydrometallurgy recycling technologies
France is home to Recupyl, now TES-AMM recycling company, this process uses a multi comminution approach. The spent batteries are crushed under an inert gas blanket. The material is then separated by size (to separate black mass in a fine powder from shredded battery components like collector foils, separator and casings), by magnetic separation (removes steel casings) and density separation (separates electrode foils from plastics). Finally, the separated material is subjected to dual leaching treatment to yield partially-delithiated metal oxide cathode and Li3PO4 as by-product (see Fig. 12). Any remaining lithium can be precipitated as Li3PO4 using H3PO4. The process can also recover cobalt as a hydroxide or elemental cobalt via electrolysis.48 The annual capacity of the Recupyl process is 110 tonnes.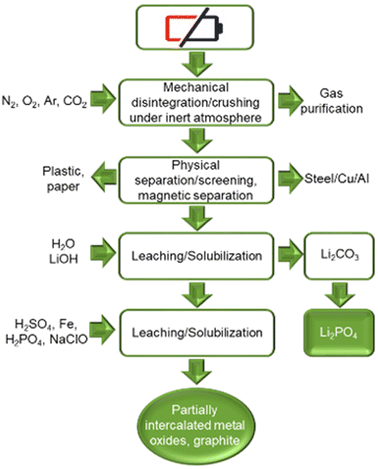 | ||
| Fig. 12 Recupyl LIB recycling process (reproduced from ref. 48 with permission from MDPI, copyright 2021). | ||
In another development, Volkswagen (VW) adopted the LithoRec process from Duesenfeld and is now recycling LIBs on a pilot scale (Fig. S10†). This is the automaker's attempt to secure the raw materials needed and solve the supply chain shortage in house. The recycling of spent batteries will save VW from expensive procurement and disposal of raw materials. They have a target to recycle over 90 percent of spent batteries at the Salzgitter plant in Germany.52
Aalto University (Finland) has developed a comprehensive LIB recycling process that has been demonstrated in a laboratory scale (Fig. 13). The process targets 99 percent overall recovery using an improved pyro- and hydro-metallurgical method.53 The process involves mechanical separation of spent LIBs followed by screening of cathode material from metal foils.7 The cathode material is subjected to acid leaching with sulfuric acid because of the economic advantages. There is though provision for extra alkali leaching, using sodium hydroxide, in case recovery of rare earth elements as alkali double sulfates-is justified. The manganese is oxidatively precipitated with potassium permanganate. The iron impurity is removed with sodium hydroxide addition to pH 5.5. The cobalt, nickel and lithium continue into solvent extraction section. An important feature of the Aalto process is the treatment of sodium hydroxide and its in-plant utilization for REE precipitation to improve the overall economic and environmental impact.54
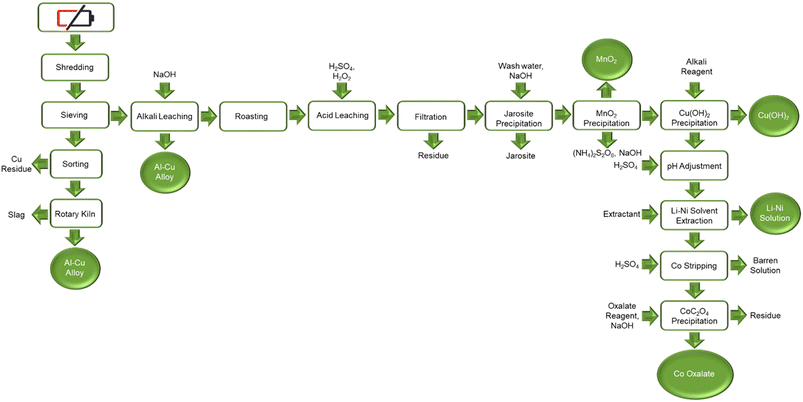 | ||
| Fig. 13 Aalto University suggested NMC recycling process (reproduced from ref. 48 with permission from MDPI, copyright 2021). | ||
American Technology Company, located in the USA, uses the automated de-manufacturing of batteries (high separation of low-value by-products, targeted removal of contaminants), targeted hydrometallurgical proprietary chemical extraction train which extracts Li, Ni, Co and Mn and upgrades into battery grade (simplified IR, highly selective recovery of products, battery grade purity cathode products).55 The commercial recycling facility is under construction. The facility will treat 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of spent LIBs annually.
000 tonnes of spent LIBs annually.
Lithion Recycling received $125 M in funding to build the first LIB recycling plant in Quebec, Canada. Commercialization of the plant is expected to begin in 2023, following the successful demonstration plant. The hydrometallurgical process operations are outlined in Fig. 14A, the leaching circuit, and in Fig. 14B, the metal separation steps. Leaching is done in H2SO4 followed by removal of Fe and Al impurities by neutralization and copper by sulfide precipitation before Li/Ni/Co/Mn are separated via a combination of precipitation and solvent extraction steps. Following solution purification, the cathode metals are recovered as lithium carbonate (by crystallization), nickel hydroxide (by neutralization) and cobalt metal (by electrowinning). The company claims their hydrometallurgical technology, has a recovery rate of 95 percent battery metals, which are purified for re-entry into the battery production chain56.
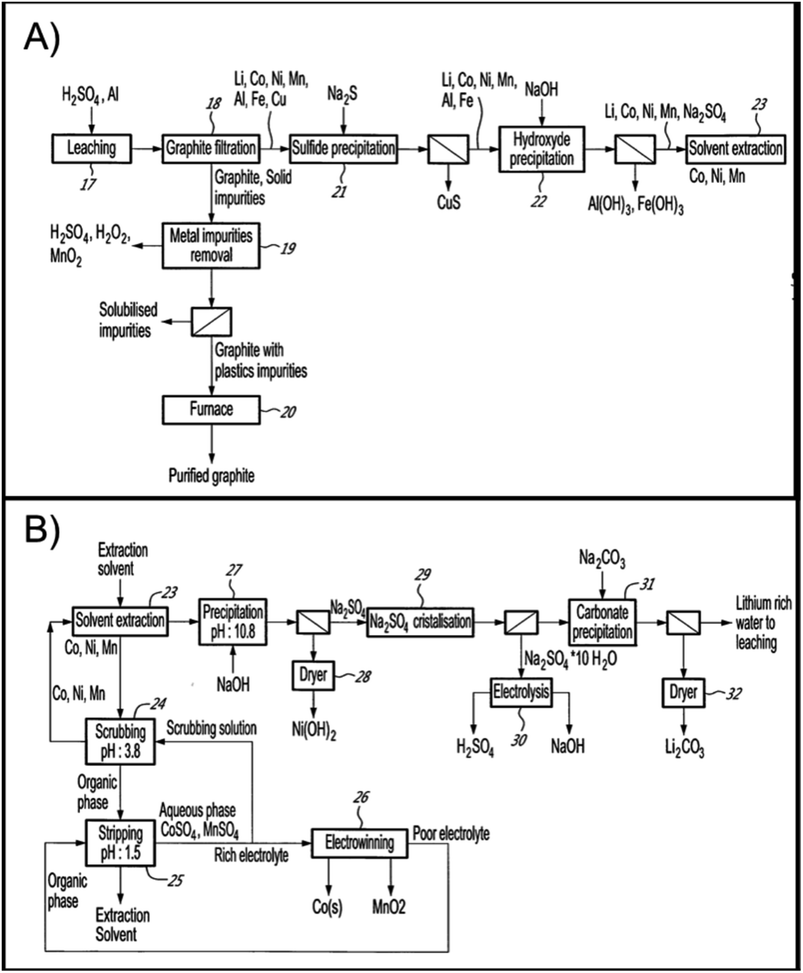 | ||
| Fig. 14 Lithion recycling patented hydromet process (A) leaching circuit and (B) metal separation for recovery of all value metals and graphite from spent NMC.57 | ||
Li Cycle is another LIB recycling company located in Canada developing a spoke and hub operation for the North American market. The facility treats all types of LIBs and brings them from a charged state to inert product. A combination of mechanical separation and hydrometallurgical resource recovery techniques are used. The mechanical separation and size reduction produces a black mass free of foil components. Then the black mass follows a hydrometallurgical flowsheet for recovery of both cathode and anode materials. The graphite and copper sulfide are recovered in the first two stages of the patented process. Then manganese is recovered through solvent extraction and a secondary mixing stage as a carbonate. Cobalt and nickel are both recovered through solvent extraction and crystallization as sulphate salts. Sodium sulphate is then recovered through the crystallization process. Finally, lithium is treated and crystallized as a carbonate. Li-Cycle plans to have seven spokes and one hub in North America and Europe by the end of 2023. The Li Cycle process will have greater than ninety five percent recovery rate according to their patent.58
Direct recycling concept
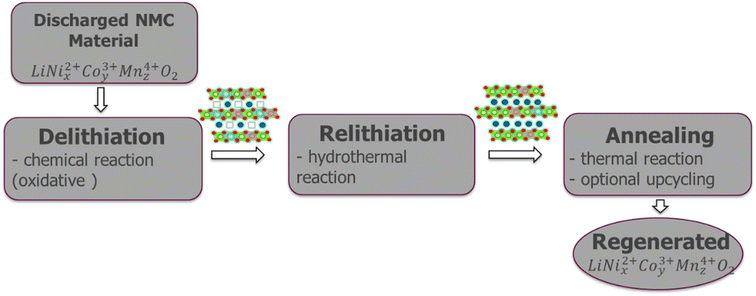
Direct recycling has emerged as a more efficient means to recycle NMC cathodes. The traditional recycling processes (hydrometallurgical and pyrometallurgical) decompose the cathode into high purity elemental products. The separated elements then need to be remanufactured into new cathodes, which is energy-intensive and has a high chemical consumption. Direct recycling maintains the cathode's chemical compound structure through selective regeneration treatment and separation of non-active components enabling its reuse in new battery assembly.51 Sloop et al. first patented this concept in 2016.59,60 As per ReCell center's modeling work direct recycling has the lowest impacts for all cost and environmental categories.51 This modeling was done for NMC 111 chemistry, which now is evolving to higher Ni and lower Co content, e.g., NMC 532, 622 and 811, in order to reduce dependency on cobalt and thereby decrease the overall cost and environmental footprint, with the added benefit increased capacity/energy density.61 Typically, direct recycling (Fig. 15) consists of three processing steps:1 selective chemical de-lithiation (if NMC cathode in discharged state),2 hydrothermal re-lithiation, and3 thermal processing/annealing.62Hydrothermal treatment to induce re-lithiation10 of the de-lithiated NMC cathode is done in an autoclave with lithium hydroxide solution, to bring the Li back to ideal stoichiometry, 1.0.63 as per the following reaction (eqn (3)).
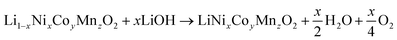 | (3) |
Once the target Li concentration has been achieved, a final short annealing treatment is required to restore the layered crystal structure. To compensate for lithium loss during the high temperature annealing, generally 5 mol% excess lithium as carbonate salt is added.10
This method is non-destructive to the active NMC particles and regenerates their functionality in terms of lithium capacity and conductivity.64
An interesting variation of the direct recycling approach is the upcycling method which in addition to regenerating NMC it seeks to change its composition from low-Ni to Ni-rich stoichiometries, i.e., from NMC 111 to NMC 622. Upcycling (Ni-enrichment) typically is done during the thermal/annealing stage with the addition and mixing of extra Ni salt to hydrothermally re-lithiated NMC material.63 In the following section the latest hydromet-based direct recycling developments involving NMC cathodes are briefly introduced.
Direct recycling developments
American Manganese is a recycling company that is using a direct recycling/upcycling approach. The patented process, RecycLiCo™, has achieved up to 99 percent recovery of lithium, cobalt, nickel and manganese. The process comprises a leach stage, co-precipitation stage and a lithium recovery and regeneration stage. The cathode material is not decomposed/dissolved like other hydrometallurgical processes but is preserved in compound form and regenerated as cathode active material (CAM).65 Lithium is recovered by electrodialysis. A three-compartment cell regenerates lithium hydroxide and sulfuric acid from lithium sulfate solution (Fig. 16). The Li+ moves to the cathode compartment and the SO42− moves to the anode compartment, as shown in Fig. 16. The upcycling method includes the conversion of spent NMC chemistry to the desired Ni-rich composition (ex. NMC 111 to NMC 622) and allows for a direct integration into the re-manufacturing of LIBS. The demonstration plant is located in Vancouver, Canada and has exceeded the original capacity of 500 kg of scrap NMC cathode material per day, with an impressive 99% extraction of valuable materials such as lithium, cobalt, nickel and manganese.66 This will be the first hydrometallurgical cathode waste recycling and upcycling plant in North America.67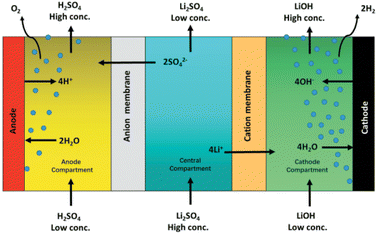 | ||
| Fig. 16 American Manganese RecycLiCo™ process, a three compartment membrane electrodialysis cell for generating LiOH and H2SO4 (reproduced from ref. 67 with permission from Taylor and friends, copyright 2021). | ||
Battery Resources, now Ascend Elements, opened a 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 square foot facility which can process 30
000 square foot facility which can process 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of LIBs waste per year in Georgia, USA.68 Using a hydrometallurgical and direct recycling approach, the process has shown superior performance of recycled cathode materials.69 The patented Hydro-CathodeTM process claims that upcycled battery materials have 50 percent longer cycle life and 88 percent higher power capacity.70 The material from the lithium battery recycling stream undergoes an acid leach, which produces the leached solids. The solids are heated while being heated with a strong acid to remove any remaining cathode and separator components. By changing from aluminum oxide to aluminum sulfate, the strong acid eliminates any remaining aluminum oxide from the separator. Washing the acid-treated solids removes water-soluble impurities, such as aluminum sulfate formed when aluminum oxide and sulfuric acid interacted and produces graphite that is essentially pure (99%). The elemental composition of the cathode material is adjusted to suit industry needs. The process can either leave as a mixed NMC hydroxide (precursor), or a sinter (CAM) into various NMC chemistries (111, 532, 622, 811).71
000 tonnes of LIBs waste per year in Georgia, USA.68 Using a hydrometallurgical and direct recycling approach, the process has shown superior performance of recycled cathode materials.69 The patented Hydro-CathodeTM process claims that upcycled battery materials have 50 percent longer cycle life and 88 percent higher power capacity.70 The material from the lithium battery recycling stream undergoes an acid leach, which produces the leached solids. The solids are heated while being heated with a strong acid to remove any remaining cathode and separator components. By changing from aluminum oxide to aluminum sulfate, the strong acid eliminates any remaining aluminum oxide from the separator. Washing the acid-treated solids removes water-soluble impurities, such as aluminum sulfate formed when aluminum oxide and sulfuric acid interacted and produces graphite that is essentially pure (99%). The elemental composition of the cathode material is adjusted to suit industry needs. The process can either leave as a mixed NMC hydroxide (precursor), or a sinter (CAM) into various NMC chemistries (111, 532, 622, 811).71
OnTo Technology LLC has been a pioneer in direct recycling technology. Their patented process is called Cathode-HealingTM Direct Recycling. The process includes, extraction of electrolyte using supercritical CO2, shredding, mechanical electrode harvesting, flotation, Cathode-HealingTM, and rebuilding cells using recycled cathode and anode materials.53 The cathode healing method, classified as a direct recycling process, uses two-steps as described above, hydrothermal and heat treatment. The NMC cathode is regenerated by using lithium solution to add Li+ back into the structure, as well as repair the microstructure defects using heat.64 The regeneration mechanism of the cathode material structure is graphically illustrated in Fig. 17.
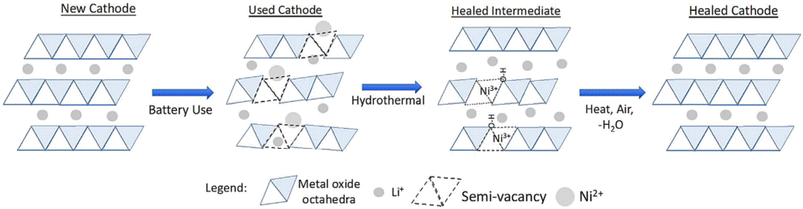 | ||
| Fig. 17 OnTo technology's advanced battery recycling process for LIBs: cathode-healing™ impacts structure by mixing Ni2+ and Li+ to regenerate the full layered structure (reproduced from ref. 64 with permission from Elsevier, copyright 2019). | ||
Discussion: challenges and trends
Currently the recovery of expensive materials, primarily cathode, has been the focus of commercial recycling companies using pyro- and hydro-metallurgy.17 With recent supply chain security issues, more companies are looking to improve the current commercial recycling methods by enhancing recovery rates and reducing environmental footprint. Hydrometallurgy-based processes tend to achieve the highest recovery of metals such as: nickel, cobalt, and lithium compared to the pyrometallurgical methods. Both type of processes involves high temperature (pyro) and or high use of chemicals (hydro), creating environmental issues and operating costs.72 Companies such as Retriev Technologies, Umicore, Brunp/CATL, GEM Ltd, Accurec, Duesenfeld and SungEel HiTech all use a hydrometallurgical route to recycle spent LIBs. A combination of pyro/hydrometallurgy is used by Nickelhütte Aue GmbH and Dowa Holdings.6Table 5 summarizes the current commercial lithium-ion battery recyclers that use hydrometallurgy. Duesenfeld is the only company that recovers the electrolyte while Retriev and Duesenfeld both show the recovery of graphite in their processes.6,48 All summarized in Table 5.| Company | Location | Capacity (tonnes per year) | Method used to expose active materials | Method used to recover recyclable materials | Primary recovery material | Secondary recovery material | Lost material | References |
|---|---|---|---|---|---|---|---|---|
| Retriev technologies | Canada | 4500 | Wet mechanical treatment | Flotation, alkaline precipitation | Li2CO3, MeO | Steel, Cu, Co, Al, graphite | Plastics | 48 |
| Umicore | Belgium | 7000 | Dismantling/physical separation | Acid leaching, solvent extraction | Co, Ni, Cu, Fe, CoCl2 | Slags: Al, Si, Ca, Fe, Li, Mn, REE | Electrolyte, plastics, graphite | 48 |
| Brunp/CATL | China | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Discharging, thermal pre-treatment, mechanical treatment | Hydromet: H2SO4/H2O2, Na2S, or NaHS reductive acid leaching, SX | Ni–Mn–Co hydroxides, Co–Ni sulfates, Co chloride | Unknown | Unknown | 6 |
| GEM Ltd | China | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Sorting, comminution | Hydrometallurgy, high temperature treatment | Co, Ni, Cu, Al, Fe | Unknown | Electrolyte, binder | 6 |
| Accurec | Germany | 4000 | Sort, dismantle, milling, separation, agglomeration | Vacuum thermal treatment, reductive leaching H2SO4 | Li2CO3, Co-Alloy | Metallic alloy | Electrolyte, polymers, graphite | 48 |
| Nickelhutte Aue GmbH | Germany | 3000 | Thermal pre-treatment | Pyro- and hydro- metallurgical treatment | Ni, Cu, Co | Slags | Unknown | 6 |
| Duesenfeld | Germany | 2000 | Discharge, inert physical separation: two-stage crushing, air classification | Drying, calcination, leaching | Li2CO3, metal oxides | Al–Cu, plastics, electrolyte | Graphite | 6 and 48 |
| SungEel HiTech | Korea | 8000 | Mechanical pre-treatment in water | Hydrometallurgical process | Co, Mn, Ni, Li2CO3 | Cu, Al | Unknown | 6 |
| Dowa holdings | Japan | 1000 | Dismantle, thermal pre-treatment | Pyro- and hydro-metallurgical treatment | Co, Ni, Cu | Unknown | Unknown | 6 |
In many operations a common physical disassembly and separation approach is to mix several different battery components and types together. The black mass becomes cross contaminated with several impurity metals (aluminum, copper, titanium etc.) and organics (like the fluorine containing PVDF binder) that need to be treated downstream. Hydromet processing is effectively able to separate and purify the pay metals but in a very chemically intense manner. OnTo Technology has made advancements in this physical separation process by adding a supercritical CO2 process step to recover the organic electrolyte solvent. They have implemented a froth flotation process to separate anode and cathode material. This arguably provides a more suitable method by mitigating the anode and electrolyte from the downstream cathode recycling process that is more important economically.53
The cathode materials recovered in each of the processes listed in Table 5 only account for 50 percent of the total battery material. Valuable components are lost such as graphite, which makes up 12 percent (0.978 kg kW−1 h−1) of the total for a NMC 111 battery, and aluminum and copper, which account for 3.11 kg kW−1 h−1 and 0.677 kg kW−1 h−1 respectively.73 There is much need for improvement in the current LIB recycling practice by embracing circular economy approaches. Among the challenges of the current hydromet processes are manganese oxidative state control, fluorine (electrolyte) recovery, recovery of battery-grade lithium, nickel, and cobalt salts and graphite in a marketable form.10
A closed-loop recycling process of lithium-ion batteries could save up to 51.3 percent of mined raw materials.74 The environmental impacts can be lowered with a 70 percent reduction in CO2 emissions and up to 70 percent energy consumption savings according to the EverBatt model, developed by Argonne National Laboratory.51 Emerging recycling technology, direct recycling/upcycling, will need more research to determine the realization of commercialization and compete with current hydro-pyro- metallurgy processes. Hydrometallurgical-based unit operations like hydrothermal relithiation10,75 or electrochemical cathodic relithiation76 are examples of how direct recycling can benefit by innovative process integration. Direct recycling technology though still doesn't address one of the major challenges with LIB recycling, the need to sort based on battery chemistry. There is no evidence that this process can handle a “mixed cathode” feed stock.51 Until improvements to battery sorting, state of health (SOH), state of charge (SOC), is implemented through policy and mandates, the commercial recycling processes will not be 100% free of producing harmful by-products or additional toxins that need to be treated.77 There has not been enough research on the long term affects and environmental risk associated with metal and organic contaminants building up in the processing plant. The new state-of-the-art recycling technologies incorporating hydrometallurgical innovations including direct recycling are summarized in Table 6.
| Company | Location | Capacity (tonnes per year) | Stage | Method used to expose active materials | Method used to recover recyclable materials | Primary recovery material | Secondary recovery material | Lost material | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Recupyl | France | 110 | Active | Dry mechanical treatment under inert atmosphere | H2SO4/H2O2 reductive acid leach, selective precipitation | Li, metal oxides, graphite | Steel: Cu/Al, plastic | Unknown | 31 and 32 |
| Volkswagen | Germany | Unknown | Pilot scale | Physical separation, shredding, drying, sieving | Hydro-metallurgy | Co, Ni, Mn, Li | Steel, separator, Al, Cu | Electrolyte, plastics | 19 |
| Aalto University | Finland | Unknown | Concept laboratory process | Shredding, sieving | Al smelter, leaching | CoC2O4, Al–Cu Alloy | Jarosite, MnO2, Cu(OH)2, Li–Ni solution | Graphite, binder, plastic, Cu, water | 48 and 21 |
| American battery technology | USA | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Under construction | Automated de-manufacturing process | Hydro-metallurgy: Impurity removal | Unknown | Unknown | Unknown | 22 |
| Lithion | Canada | 5000 | Under construction | Physical separation, shredding, drying, sieving | Hydro-metallurgy, distillation | Ni, Co, Mn, Li | Unknown | Unknown | 23 and 24 |
| Li-Cycle | Canada | 5000 | Planned | Mechanical separation, size reduction | Hydro-metallurgy | Ni, Co, Mn, Li | Steel: Cu/Al, graphite | Electrolyte, plastics | 25 |
| American manganese | Canada | 180 | Planned | Shred, separate | Direct recycling/Hydro upcycling | NMC hydroxide, Li2CO3 | Unknown | Unknown | 26 and 27 |
| Battery resourcers | USA | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Planned | Discharge, shred, mag sep, sieve, dense media, ambient | Hydromet/Direct recycling: NaOH, H2O2, H2SO4 and Na2CO3 | Li2CO3, cathode powder | Ferrous metals | Electrolyte | 48 |
| OnTo technology LLC | USA | Unknown | Unknown | Supercritical CO2, physical disassembly | Hydromet/Direct recycling: Heat treatment, LiOH leaching | Cathode powder, Li2CO3 | Electrolyte, graphite, Fe, Al, Cu, plastics | Unknown | 20, 30 and 33 |
To achieve effective and sustainable recycling of lithium-ion batteries (LIBs), several key areas of R&D must be considered. Reducing pollutant emissions and adopting non-toxic solvents are crucial for minimizing environmental impact.78 Recycling efforts should go beyond metal recovery and also reclaim electrolytes, separators, and negative carbon materials.79 As the cobalt content in cathodes decreases, current recycling processes may become uneconomical, necessitating advancements in recycling technologies.80 The current and state of the art R&D processes shown in both Tables 5 and 6 are not without deficiencies. Technology innovation is essential to develop low-waste and high-efficiency processes. Environmental and safety aspects should not be overlooked, and efforts should focus on reducing heavy metal content, switching to environmentally friendly binders and electrolytes, and treating and mitigating wastewater, gas, and residues produced during the recycling process to minimize emissions.81
Success in battery recycling will also depend on non-technical factors, such as implementing legislation and policies for proper collection, transportation, and storage of end-of-life batteries. To ensure proper tracking and accountability, governments should implement a battery passport system that monitors each stage of the recycling process and ensures compliance with recycling responsibilities.80
Direct recycling methods should ensure high purity of recovered materials by carefully analyzing cell component chemistries, state of charge (SOC), and state of health (SOH) before disassembly. Fan et al. emphasize the need for technical advancements, including automated disassembly, simplified recycling processes, and the development of new battery chemistries with reduced dependence on critical minerals.81
Conclusion and perspective
In summary, the need to secure a reliable supply chain that is sustainable for lithium-ion battery manufacturing is imperative. The current EV market demand creates an exponential increase in materials needed to manufacture LIBs. Mining raw materials will not be sufficient to meet this demand. Hydrometallury has been in recent years the most widely adopted recycling method, it is an effective route to recover/recycle high value metals from cathode materials (in particular NMCs) but comes at a cost, high energy consumption, heavy usage of chemicals and waste water treatment needs while still generates greenhouse gas emissions. Improvement to the process is also required to produce battery-grade precursor salts in fewer steps in addition to embracing closed loop processing. The new direct recycling method is by far the most promising green recycling technology. The process is simpler and more effective in repairing the cathode materials, without causing structural changes, and restoring the original electrochemical performance. Hydrometallurgy is expected to play a key role in developing direct recycling technologies as are for example the lithiation and relithiation steps by adopting non-polluting reagents and hybrid low temperature treatments. There are currently no commercialized facilities for direct recycling. The challenge all current recycling methods face is the feed stock needs to be indiscriminate to the different LIB cathode compositions. A technology that is applicable to all battery chemistries has yet to be discovered.In conclusion, the development of physical recycling processes, including stabilization, opening, and separation, is crucial for sustainable LIB recycling. Separating the black mass following cell disassembly enables direct recycling and reduces waste, contributing to a more environmentally friendly and efficient recycling approach.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was performed through the Energy Mining and Environment (EME) and sponsored by the Advance Clean Energy (ACE) Program at the National Research Council of Canada. The presented research was conducted as part of the M. Sc. thesis of KD at McGill University. This work has been contributed to by the Canadian Government employees and their work is in the public domain in Canada. The authors greatly acknowledge the support from the NRC (KD) and NSERC (GPD).References
- World Bank Group, Minerals for Climate Action: the Mineral Intensity of the Clean Energy Transition. Washington, 2020 Search PubMed.
- M. Chen, X. Ma, B. Chen, R. Arsenault, P. Karlson and N. Simon, et al., Recycling end of life electric vehicle lithium-ion batteries, Joule, 2019, 3(11), 2622–2646 CrossRef CAS.
- R. Ciez and J. F. Whitacre, Examining different recycling processes for lithium-ion batteries, Nat Sustainability, 2019, 2, 148–156 CrossRef.
- M. Iturrondobeitia, C. Vallejo, M. Berroci, O. Akizu-Gardoki and R. Minguez, Environmental Impact Assessment of LiNi1/3Mn1/3Co1/3O2 Hydrometallurgical Cathode Recycling from Spent Lithium-ion Batteries, ACS Sustain. Chem. Eng., 2022, 10, 9798–9810 CrossRef CAS.
- L. Marins, L. Guimaraes, A. Botelho, J. Tenorio and D. Espinosa, Electric car battery: An overview on global demand, recycling and future approaches towards sustainability, J. Environ. Manage., 2021, 295 DOI:10.1016/j.jenvman.2021.113091.
- R. Sojka, P. Qiaoyan and L. Billmann, Comparative Study of Li-Ion Battery Recycling Processes, ACCUREC Recycling GmbH, 2020 Search PubMed.
- J. Piqtek, S. Afyon, T. Budnyak, S. Budnyk, M. Sipponen and A. Slabon, Sustainable li-ion batteries:chemistry and recycling, Adv. Energy Mater., 2021, 11 Search PubMed.
- Resourceful Paths, Canada's critical minerals list is out. Now what?, 2021, available from: http://www.resourcefulpaths.com/blog/2021/3/21/canadas-critical-minerals-list-is-out-now-what Search PubMed.
- Council on Foreign Relations, Why cobalt mining in DRC needs urgent attention, 2020, available from: https://www.cfr.org/blog/why-cobalt-mining-drc-needs-urgent-attention Search PubMed.
- P. Xu, Z. Yang, X. Yu, J. Holoubek, H. Gao and M. Li, et al., Design and optimization of the direct recycling of spent li-ion battery cathode materials, ACS Sustain. Chem. Eng., 2021, 9, 4543–4553 CrossRef CAS.
- B. Schmidt, Three global trends that will affect the electric car transition in 2022 (thedriven.io), The Driven, 2022, https://thedriven.io Search PubMed.
- BloombergNEF, EVO Report 2021|BloombergNEF|Bloomberg Finance LP (bnef.com), 2021, available from: https://about.bnef.com Search PubMed.
- P. Slowik, N. Lutsey and C. W. Hsu, How Technology, Recycling, and Policy Can Mitigate Supply Risks to the Long-Term Transition to Zero-Emission Vehicles, Washington, 2020 Search PubMed.
- European Commission, CORDIS EU research results, 2005, available from: https://cordis.europa.eu/project/id/G1RD-CT-2000-00232 Search PubMed.
- Statista, Mine Production of Cobalt in the Democratic of Congo from 2010 to 2021, 2021, Cobalt production DR Congo 2020|Statista, available from: https://www.statista.com/statistics/339834/mine-production-of-cobalt-in-dr-congo/ Search PubMed.
- International Energy Agency, The role of critical minerals in clean energy transitions, 2021, available from: https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions Search PubMed.
- Y. Wang, N. An and L. Wen, et al., Recent progress on the recycling technology of li-ion batteries, J. Energy Chem., 2021, 55, 391–419 CrossRef CAS.
- Argonne National Laboratory, BatPaC: Battery Manufacturing Cost Estimation, 2020, available from: https://www.anl.gov/partnerships/batpac-battery-manufacturing-cost-estimation Search PubMed.
- C. Pillot, The rechargeable battery market and main trends, in International Battery Seminar and Exhibit, Fort Lauderdale, 2017 Search PubMed.
- S&P Global, Global light duty EV sales to rise to 26.8 M by 2030:Platts Analytics, 2022, available from: https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/energy-transition/021622-global-light-duty-ev-sales-to-rise-to-268-mil-by-2030-platts-analytics Search PubMed.
- Elements, How metals prices performed in 2021, 2022, available from: https://elements.visualcapitalist.com/how-metals-prices-performed-in-2021/ Search PubMed.
- Benchmark Minerals, Lithium carbonate prices break through $40/kg barrier, 2022, available from: https://www.benchmarkminerals.com/membership/lithium-carbonate-prices-break-through-40-kg-barrier/ Search PubMed.
- Markets Insider, Business Insider Commodities: nickel price, 2022, available from: https://markets.businessinsider.com/commodities/nickel-price Search PubMed.
- Statista, Futures price of cobalt worldwide from August 2019 to March 2022, 2022, available from: https://www.statista.com/statistics/1171975/global-monthly-price-of-cobalt/ Search PubMed.
- SMM, Price metal-Manganese, 2022, available from: https://price.metal.com/Manganese Search PubMed.
- Markets Insider, Business Insider Commodities: Aluminum price, 2022, available from: https://markets.businessinsider.com/commodities/aluminum-price Search PubMed.
- Markets Insider, Business insider commodities: copper price, 2022, available from: https://markets.businessinsider.com/commodities/copper-price Search PubMed.
- Fastmarkets IM. Graphite pricing, 2022, available from: https://www.indmin.com/Article/5094474/Graphite-PricingNews/Tight-supply-drives-up-European-flake-amorphous-graphite-prices.html Search PubMed.
- W. Lv, Z. Wang, H. Cao, Y. Sun, Y. Zhang and Z. Sun, A critical review and analysis on the recycling of spent lithium-ion batteries, ACS Sustain. Chem. Eng., 2018, 6, 1504–1521 CrossRef CAS.
- T. Or, S. W. D. Gourley, K. Kaliyappan, A. Yu and Z. Chen, Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook, Carbon Energy, 2020, 2, 6–43 CrossRef CAS.
- G. P. Demopoulos, Aqueous precipitation and crystallization forthe production of particulate solids with desired properties, Hydrometallurgy, 2009, 96(3), 199–214 CrossRef CAS.
- Y. Yao, M. Zhu, Z. Zhao, B. Tong, Y. Fan and Z. Hua, Hydrometallurgical Process for Recycling Spent Lithium-ion Batteries: A Critical Review, ACS Sustain. Chem. Eng., 2018, 6, 13611–13627 CrossRef CAS.
- S. Lei, W. Sun and Y. Yang, Solvent extraction for recycling of spent lithium-ion batteries, J. Hazard. Mater., 2022, 424 DOI:10.1016/j.jhazmat.2021.127654.
- X. P. Chen, K. J. R. Zhou, H. X. Fang and Y. B. Chen, Separation and recovery of metal values from leach liquor of waste lithium nickel cobalt manganese oxide based cathode, Sep. Purif. Technol., 2015, 141, 76–83 CrossRef CAS.
- X. P. Chen, Y. B. Chen, T. Zhou, D. P. Liu, H. Hu and S. Y. Fan, Hydrometallurgical recovery of metal values from sulfuric liquor of spent lithium-ion batteries, Waste Manage., 2015, 38, 349–356 CrossRef CAS PubMed.
- S. H. Joo, S. M. Shin, Shin, C. Oh and J. P. Wang, Extractive separation studies of manganese from spent lithium battery leachate using mixture of PC88A and Versatic 10 acid in kerosen, Hydrometallurgy, 2015, 156, 136–141 CrossRef CAS.
- V. T. Nguyen, Jc Lee, J. Jeong, B. S. Kim and B. D. Pandey, The separation and recovery of nickel and lithium from the sulfate leach liquor of spent lithium ion batteries using PC-88A, Korean Chem. Eng. Res., 2015, 53(2), 137–144 CrossRef CAS.
- L. Chen, X. C. Tang, Y. Zhang, L. X. Li, Z. W. Zeng and Y. Zhang, Process for the recovery of cobalt oxalate from spent lithium-ion batteries, Hydrometallurgy, 2011, 108, 80–86 CrossRef CAS.
- X. P. Chen, B. Xu, T. Zhou, D. P. Liu, H. Hu and S. Y. Fan, Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries, Sep. Purif. Technol., 2015, 144, 197–205 CrossRef CAS.
- K. Jingu, S. Gamini, S. Jeongsoo and M. S. Shun, Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272, Hydrometallurgy, 2010, 100(3–4), 168–171 Search PubMed.
- S. H. Joo, D. Shin, C. Oh, J. P. Wang and S. M. Shin, Extraction of manganese by alkyl monocarboxylic acid in a mixed extractant from a leaching solution of spent lithium-ion battery ternary cathodic material, J. Power Sources, 2016, 305, 175–181 CrossRef CAS.
- Solyvay, 2023, available from: https://www.solvay.com/en/product/cyanex-272 Search PubMed.
- S. Cho, G. H. Lee, C. G. Lee and S. Uhm, Extraction/Separation of Cobalt by Solvent Extraction: A Review, Appl. Chem. Eng., 2015, 26(6), 631–639 CrossRef.
- P. Meshram, S. Virolainen, A. Sainio and T. Sainio, Solvent Extraction for Separation of 99.9% Pure Cobalt and Recovery of Li, Ni, Fe, Cu, Al from Spent LIBs, Metals, 2022, 12 DOI:10.3390/met12061056.
- W. Maclaughlin, Method for neutralization of hazardous materials, US Pat.5345033, 1994 Search PubMed.
- R. Sommerville, R. Zhu, P. Rajaeifar, O. Heidrich, V. Goodship and E. Kendrick, A qualitative assessment of lithium-ion battery recycling processes, Resour., Conserv. Recycl., 2021 DOI:10.1016/j.resconrec.2020.105219.
- L. Gaines, J. Sullivan, A. Burnham and I. Belharouak, Life-cycle analysis for lithium-ion battery production and recycling, in Transportation Research Board 90th Annual Meeting, Washington, 2010 Search PubMed.
- O. Velazquez-Martinez, J. Valio, A. Santasalo-Aarnio, M. Reuter and R. Serna-Guerrero, A critical review of lithium-ion battery recycling processes from a circular economy perspective, Batteries, 2019, 5(4), 68 CrossRef CAS.
- J. Diekmann, Ecologically friendly recycling of lithium-ion batteries-the lithorec process, ECS Trans., 2016, 73(1), 1–9 CrossRef CAS.
- SungEel HiTec, SMC Recycling, 2020, available from: http://www.sungeel.com/page/history.php Search PubMed.
- L. Gaines, D. Qiang, J. Vaughey and S. Gillard, Direct recycling r&d at the Recell Center, Recycling, 2021, 6(31) DOI:10.3390/recycling6020031.
- Volkswagen, Volkswagen Aktiengesellschaft, 2019, available from: https://www.volkswagenag.com/en/news/stories/2019/02/lithium-to-lithium-manganese-to-manganese.html Search PubMed.
- S. Sloop, et al., A direct recycling case study from a lithium-ion battery recall, Sustainable Mater. Technol., 2020, 25 DOI:10.1016/j.susmat.2020.e00152.
- F. Liu, et al., Synergistic recovery of valuable metals from spent nickel-metal hydride batteries, ACS Sustain. Chem. Eng., 2019, 7, 16103–16111 CrossRef CAS.
- United States Securities and Exchange Commission, American battery metals corporation Form 8-K, 2021, available from: https://sec.report/Document/0001493152-21-018376/ Search PubMed.
- Lithion Recycling, Lithion, 2022, available from: https://www.lithionrecycling.com/lithium-ion-battery-recycling-plant/ Search PubMed.
- D. Morin, C. Gagne-Bourque, E. Nadeau and B. Couture, Lithium-ion Batteries Recycling Process, WO 2019/060996A1, 2018.
- Li-Cycle. Lithium-ion battery recycling, 2022, available from: https://investors.li-cycle.com/overview/default.aspx Search PubMed.
- S. E. Sloop, Recycling of Battery Electrode Materials, US Pat.12/709144, 2016 Search PubMed.
- S. E. Sloop, Reintroduction of lithium into recycled battery materials, US Pat.12/390364, 2016 Search PubMed.
- T. Wang, H. Luo, F. Juntian, P. T. Bishnu, B. Ilias and B. Yaocai, et al., Flux upcycling of spent NMC 111 to nickel-rich NMC cathodes in reciprocal ternary molten salts, iScience, 2022, 25 DOI:10.1016/j.isci.2022.103801.
- J. Yi, E. E. Kpodzro, C. T. Jafvert and F. Zhao, Direct recycling technologies of cathode in spent lithium-ion batteries, Clean Technologies and Recycling, 2021, 1, 124–151 Search PubMed.
- Y. Shi, G. Chen, F. Liu, X. Yue and Z. Chen, Resolving the compositional and structural defects of degraded LiNixCoyMnzO2 particles to directly regenerate high performance lithium battery cathodes, ACS Energy Lett., 2018, 3, 1683–1692 CrossRef CAS.
- S. Sloop, L. Crandon, M. Allen, M. Lerner, H. Zhang and W. Sirisaksoontorn, et al., Cathode healing methods for recycling of lithium-ion batteries, Sustainable Mater. Technol., 2019, 22 DOI:10.1016/j.susmat.2019.e00113.
- D. Morin, C. Gagne-Bourque, E. Nadeau and B. Couture, Lithium-ion Batteries Recycling Process, Canada Pat.3076688, 2019 Search PubMed.
- RecycLiCo. RecycLiCo., 2023, available from: https://recyclico.com/recyclicos-demonstration-plant-testing-produces-bulk-quantities-of-battery-grade-lithium-carbonate/ Search PubMed.
- Y. Chung and J. Jung, A novel closed loop process for recycling spent li-ion battery cathode materials, Int. J. Green Energy, 2021, 18(15), 1597–1612 CrossRef.
- Ascend Elements. Cision PR Newswire, 2023, available from: https://www.prnewswire.com/news-releases/ascend-elements-opens-north-americas-largest-electric-vehicle-battery-recycling-facility-in-georgia-301786245.html Search PubMed.
- Y. Wang, et al., Recycled cathode materials enabled superior performance for lithium-ion batteries, Joule, 2021, 5(11), 2955–2970 CrossRef.
- Ascend Elements. Ascend Elements, 2022, available from: https://ascendelements.com/innovation/ Search PubMed.
- E. Gratz and Z. Zhangfeng, Anode Recovery in Recycled Batteries, US Pat.20210384563A1, 2021 Search PubMed.
- Y. Wang, A. Ning, W. Lei, W. Lei, J. Xiatong and F. Hou, et al., Recent progress on the recycling technology of li-ion batteries, J. Energy Chem., 2021, 55, 391–419 CrossRef CAS.
- J. Dunn, A. K. Slattery, H. Ambrose and S. Shen, Circularity of lithium-ion battery materials in electric vehicles, Environ. Sci. Technol., 2021, 55, 5189–5198 CrossRef CAS PubMed.
- J. Heelan, E. Gratz, Z. Zhangfeng, Q. Wang, M. Chen and D. Apelian, et al., Current and prospective li-ion battery recycling and recovery processes, JOM, 2016, 68, 2632–2638 CrossRef CAS.
- P. Xu, D. H. S. Tan, B. Jiao, H. Gao, X. Yu and Z. Chen, A materials perspective on direct recycling of lithium-ion batteries: principles, challenges and opportunities, Adv. Funct. Mater., 2023, 33(14) DOI:10.1002/adfm.202213168.
- K. Amouzegar, A. Vijh, G. P. Demopoulos and F. Larouche, Ambient-temperature Aqueous Electrochemical Route for Direct Re-functionalization of LFP Cathodes, 2023 Search PubMed.
- R. Sommerville, J. Shaw-Stewart, V. Goodship, N. Rowson and E. Kendrick, A review of physical processes used in the safe recycling of lithium ion batteries, Sustain. Mater. Technol., 2020, 25, e00197 CAS.
- C. M. Costa, J. C. Barbosa, R. Goncalves, H. Castro, F. J. Del Campo and S. Lanceros-Mendez, Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities, Energy Stor. Mater., 2021, 37, 433–465 Search PubMed.
- S. Jin, D. Mu, Z. Lu, R. Li, Z. Liu and Y. Wang, et al., A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances, J. Clean. Prod., 2022, 340, 130535 CrossRef CAS.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater and R. Stolkin, Recycling lithium-ion batteries from electric vehicles, Nature, 2019, 575, 75–86 CrossRef CAS PubMed.
- E. Fan, L. Li, Z. Wang, J. Lin, Y. Huang and Y. Yao, et al., Sustainable recycling technology for li-ion batteries and beyond: challenges and future prospects, Chem. Rev., 2020, 120, 720–7063 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00142c |
| This journal is © The Royal Society of Chemistry 2023 |