Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A perspective on task-specific ionic liquids for the separation of rare earth elements
Chi-Linh
Do-Thanh
 a,
Huimin
Luo
b and
Sheng
Dai
a,
Huimin
Luo
b and
Sheng
Dai
 *ac
*ac
aDepartment of Chemistry, Institute for Advanced Materials and Manufacturing, University of Tennessee, Knoxville, TN 37996, USA. E-mail: dais@ornl.gov
bManufacturing Science Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
cChemical Sciences Division, USA
First published on 19th May 2023
Abstract
Rare earth elements (REEs) play essential roles in various technological applications. With the demand for these materials increasing globally, numerous efforts have been put into the separation and recovery of REEs. Liquid–liquid extraction is one of the most studied methods for separating and recycling these elements, with ionic liquids (ILs) having been proven as effective extraction solvents. This review looks at recent progress in solvent extraction systems using task-specific ionic liquids (TSILs) as extractants for REE separation.
Sustainability spotlightRare earth elements (REEs) are critical materials in consumer products used in daily life due to their unique catalytic, magnetic, and phosphorescent properties. Among their various applications is clean energy technology where they are found in hybrid and electric vehicles, lighting, solar cells, and wind turbines. With rising global demand for REEs, their separation and recovery from primary and secondary sources have become increasingly important. In line with the UN's “Affordable and Clean Energy” goal (SDG 7), this review paper presents an overview of recent advances in systems involving solvent extraction, one of the most promising techniques for separating REEs, and task-specific ionic liquids as extractants. |
1. Introduction
Rare earth elements (REEs) are a set of metals that contains the 15 lanthanides, scandium, and yttrium. REEs are used in a variety of green and low-carbon applications in modern science and technology such as magnets, lighting, catalysts, and rechargeable batteries.1 Due to their prominent role as materials in everyday consumer products, the demand for REEs keeps rising, and therefore, efficient methods are needed to separate and recover these elements.2–4Among the most frequently studied separation techniques for REEs is liquid–liquid extraction which involves the use of extractants in various solvents.5,6 This method utilizes a mixture of REEs, most frequently as nitrates or chlorides, in a low-pH, acidic aqueous solution that is combined with an immiscible organic solvent containing the extractant. In general, REEs are in the cationic form at low pH values while they hydrolyze around pH > 5 and precipitate out of solution as hydroxides.7 Accordingly, the common acidity range for extractions is at pH 1–5.
As green alternatives to traditional organic solvents, ionic liquids (ILs) have been increasingly used in solvent extractions to enhance the separation performances of known extractants8–11 due to their beneficial properties such as low vapor pressure, low flammability, the ability to dissolve both organic and inorganic compounds, high thermal stability, high conductivity, and wide electrochemical windows.12–16 Generally, the typical IL-based extraction process starts with an aqueous solution of REE ions that comes into contact with an IL layer that includes the extractant. The REE ions are then chelated by a combination of the extractant and cations and/or anions from the IL, followed by traveling from the aqueous phase to the IL phase. After separating the two layers, if needed the IL phase can undergo a stripping procedure in which the REEs are taken away from the extractant and IL to a solution with higher affinity for REEs, usually an acidic aqueous solution containing nitric acid or hydrochloric acid. The remaining IL phase can be reused for additional new extractions.
However, it should be noted that even hydrophobic ILs such as those with the commonly used bis(trifluoromethanesulfonyl)imide ([NTf2]−) anion have partial solubilities in water, and therefore, portions of them can be lost to the aqueous phases during extractions. IL process design rarely deals with waste streams containing dissolved [NTf2]− in water,17 which can cause ecotoxicological issues due to the low biodegradability of the anion.18,19 To help avoid this loss, the ILs can be made more hydrophobic by using cations with long alkyl chains.20
Besides acting as diluents in extractions, ILs can also contribute to separation processes through solvent interactions21 and ion-exchange mechanisms,22,23 which allows for tuning the properties of specific extractants.24 Consequently, functionalized ILs known as task-specific ionic liquids (TSILs)25 have attracted increased attention in extraction methods. TSILs are made of conventional ILs that include functional groups tailored to provide suitable physicochemical properties to the ILs as extractants or solvents. Due to the ion-exchange mechanisms, these TSILs can avoid the drawback of partial dissolution in the aqueous phase that conventional ILs face.16
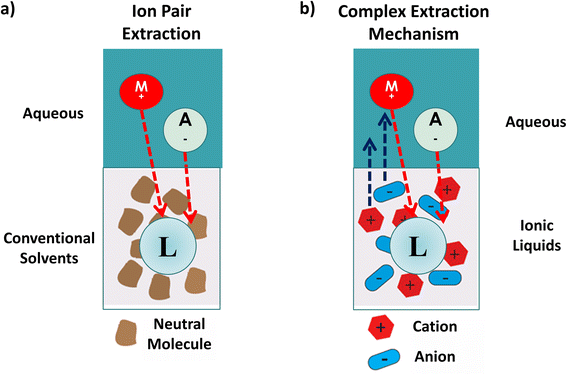
In a typical ion pair extraction involving the target metal ion in an aqueous solution and a neutral ligand as the extractant in a conventional organic solvent, the cations and anions can travel from the aqueous phase to the organic phase to interact with the ligand (Fig. 1a). In contrast, when an ionic liquid is used instead of an organic solvent, there is additional cooperativity coming from the cationic and anionic parts of the IL which can enter the aqueous layer and also interact with the metal ion (Fig. 1b), allowing for stronger solvation.8,22
More specifically, various extraction mechanisms are possible when cations or anions from ILs are transferred to the aqueous phase during extraction. In the neutral exchange mechanism, which is similar to that in extractions using conventional organic solvents, hydrophobic ILs can extract neutral complexes made of the REE cation and anionic ligands. In this case, the IL simply acts as a polar non-aqueous solvent. The extraction for a trivalent REE (Ln3+) with a monobasic Brønsted acid ligand (HL) can be shown in the following equation:
| Ln3+(aq) + 3HL(IL) ⇌ LnL3(IL) + 3H+(aq) | (1) |
In the cation-exchange mechanism, the IL acts as a liquid ion exchanger with cationic complexes that are formed between the REE cation and a certain number (x) of neutral ligands (L). Cations from the IL are transferred to the aqueous phase while the REE cation moves to the IL phase to form the complex as expressed in the following example:
| Ln3+(aq) + xL(IL) + 3IL-cation+(IL) ⇌ LnLx3+(IL) + 3IL-cation+(aq) | (2) |
| Ln3+(aq) + xHL(IL) + (x − 3)IL-anion−(IL) ⇌ LnLx(x−3)−(IL) + xH+(aq) + (x − 3)IL-anion−(aq), x > 3 | (3) |
Another possible pathway is the ion-association mechanism which can be found in the presence of long alkyl chains on ILs, which help avoid ion exchange, and other anions (A−) in the aqueous phase such as chloride and nitrate coming from salts or their conjugate acids. In this case, both cations and anions from the IL take part in the REE extraction, resulting in a hydrophobic neutral complex16 as follows:
| Ln3+(aq) + x[IL-cation][IL-anion](IL) + 3A−(aq) ⇌ LnA3·x[IL-cation][IL-anion](IL) | (4) |
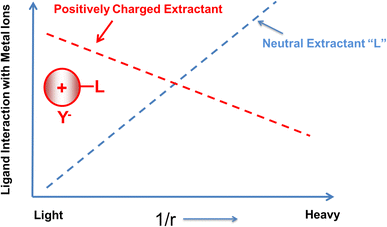
Additionally, by utilizing TSILs as ligands, the extractants themselves become charged, thereby enabling another way to interact with the metal and influence the extraction process. For example, one strategy to explore is whether a positively charged extractant having an IL cation from a TSIL can change extraction efficiencies and selectivities. Neutral extractants favor the smaller and heavier metals, showing a linear dependence of extraction efficiencies on 1/r, the inverse radius of the metal ion. With the introduction of a cation into the extractant, the question becomes whether this linear dependence can be attenuated (Fig. 2).
Broader aspects of extraction systems with ILs and TSILs have recently been covered in several review articles.26,27 In this review, we describe recent progress in liquid–liquid extraction systems using TSILs as novel extractants for the separation of REEs.
2. Task-specific ionic liquids as extractants
2.1 Early extraction work with task-specific ionic liquids
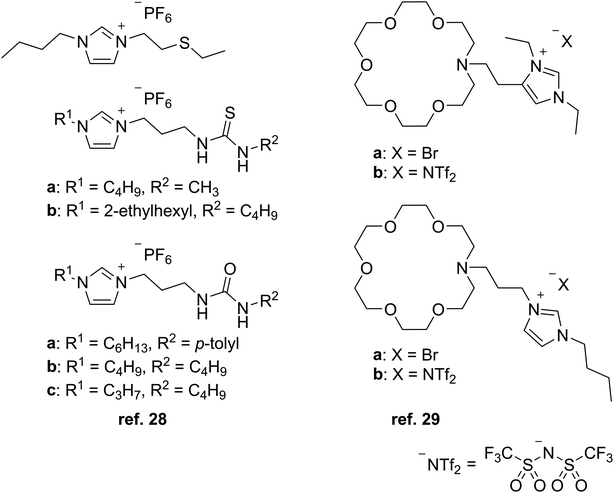
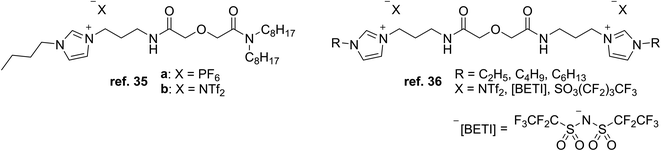
Initial research efforts by Visser et al. focused on extracting heavy metal ions Cd2+ and Hg2+ from water with TSILs made of dialkylimidazolium cations that have been derivatized with thioether, thiourea, and urea functional groups and combined with the PF6− anion (Fig. 3) in order to act as both the extractant and the hydrophobic solvent in liquid–liquid extractions.28 The more hydrophobic environment provided by the extractant aimed to facilitate the transport of the metal ion out of the aqueous phase, and having the metal-binding group tethered to the imidazolium cation would lower the chance of losing the extractant to the aqueous phase. The attached functional groups and alkyl chains influenced the extraction performance, with the thiourea and urea groups together with longer alkyls giving the highest distribution ratios (D values) for each metal ion.Another study from our group utilized TSILs consisting of aza-crown ethers (Fig. 3) bound to imidazolium cations to extract Cs+ and Sr2+ from aqueous solutions.29 Compared to the neutral extractants dicyclohexano-18-crown-6 and N-octyl monoaza-18-crown-6, the TSILs showed lower extraction and recovery efficiencies due to charge repulsion between the imidazolium groups and metal cations. These results indicated that a stronger complexation capability may be needed in the extractant to overcome the repulsion. Among the TSILs, the binding ability of the aza-crown ether group was observed to be affected by the substitution pattern of the alkyl groups on the attached imidazolium ring.
2.2 Recent extraction systems using task-specific ionic liquids
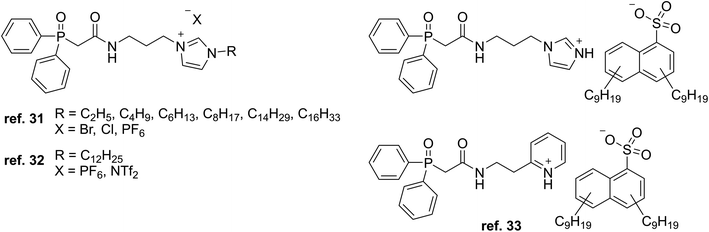
Mohapatra et al. used a similar CMPO-based TSIL with different alkyl chain lengths and anions (Fig. 4) for the solvent extraction of Eu3+ and actinide ions Pu4+, Am3+, and UO22+ from acidic solutions mixed with various imidazolium ILs and n-dodecane as solvents.32 For Eu3+, the extraction efficiencies with the TSIL were lower than those with the neutral CMPO ligand (N,N-diisobutylcarbamoylmethyl)octylphenylphosphine oxide, while the systems using the IL solvent with the shortest alkyl chain, [C4mim][NTf2], gave the highest distribution ratios compared to the other solvents, likely due to ILs being less able to go through ion exchange with having longer alkyl chains and being more hydrophobic.
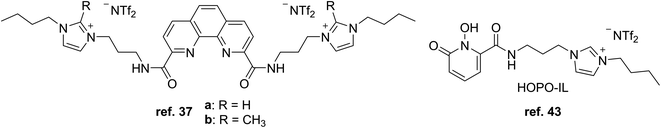
More recently, Turanov et al. prepared TSILs made of CMPO and protonated imidazolium and pyridinium cations alongside the dinonylnaphthalenesulfonate anion (Fig. 4) for extracting lanthanide ions from nitric acid solutions with 1,2-dichloroethane as the organic solvent.33 The cations were designed for coordination solvation of the lanthanide ions, while the anion contributes to the hydrophobicity to help the target ions transfer into the organic phase. The extraction efficiency of the TSILs decreased across the lanthanide series as the atomic number increased. In contrast, the corresponding neutral CMPO analogues showed increasing efficiencies with increasing atomic number while overall being lower than those for the TSILs. The neutral conjugate acid of the anion also had lower distribution ratios, indicating the presence of the “inner synergistic effect”34 for the TSILs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal-ion
2 metal-ion![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) extractant stoichiometry with an additional nitrate ion present. Efficient stripping of the extracted ions was possible via a modified solvent system using dilution and buffered complexing agents such as ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetic acid (DTPA), and the radiolytic stability of the TSILs was superior to that of other DGA-based or IL-based solvent systems.
extractant stoichiometry with an additional nitrate ion present. Efficient stripping of the extracted ions was possible via a modified solvent system using dilution and buffered complexing agents such as ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetic acid (DTPA), and the radiolytic stability of the TSILs was superior to that of other DGA-based or IL-based solvent systems.
A set of dicationic DGA-based TSILs consisting of two imidazolium groups (Fig. 5) was also synthesized and evaluated by Y. Wu et al. for extracting lanthanides in an imidazolium IL solvent.36 Unfortunately, no lanthanide extraction from the aqueous phase into the IL solvent was possible at any acidity, indicating that the TSILs suppressed extraction by forming water-soluble complexes.
Phenanthroline ligands are known in the literature40,41 to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal
2 metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
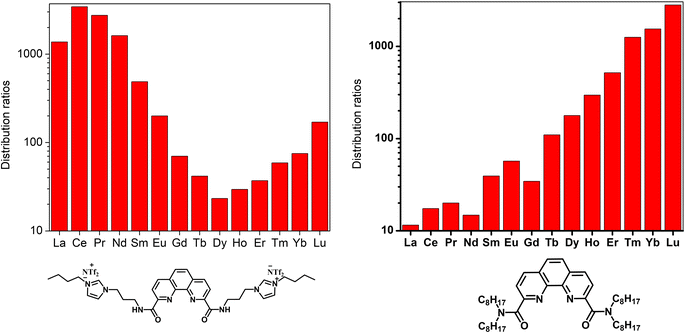
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand complexes that can also include a nitrate anion ligand, similar to the aforementioned DGA-TSIL system. Computational modeling for the complexes with the TSILs revealed strong hydrogen bonding between the amide hydrogen and a sulfuryl group from the NTf2− anions that are paired with the imidazolium cations, allowing these anions to fit between the phenanthroline core and imidazolium groups, which can compensate for the positive charge of the lanthanide ion in the center of the complex and still leave enough space for the nitrate ligand. Energy calculations suggested that nitrate addition is energetically unfavorable for the heavy lanthanides. In comparison, the complexes with neutral ligands did not show a reaction energy barrier due to the lack of cations that would electrostatically interact with nitrate in the outer second coordination sphere. These results implied that metal–ligand coordination can be tuned via coulombic interactions in the outer coordination sphere, allowing the cationic charges from the TSIL to control the separation properties of the extractant.
ligand complexes that can also include a nitrate anion ligand, similar to the aforementioned DGA-TSIL system. Computational modeling for the complexes with the TSILs revealed strong hydrogen bonding between the amide hydrogen and a sulfuryl group from the NTf2− anions that are paired with the imidazolium cations, allowing these anions to fit between the phenanthroline core and imidazolium groups, which can compensate for the positive charge of the lanthanide ion in the center of the complex and still leave enough space for the nitrate ligand. Energy calculations suggested that nitrate addition is energetically unfavorable for the heavy lanthanides. In comparison, the complexes with neutral ligands did not show a reaction energy barrier due to the lack of cations that would electrostatically interact with nitrate in the outer second coordination sphere. These results implied that metal–ligand coordination can be tuned via coulombic interactions in the outer coordination sphere, allowing the cationic charges from the TSIL to control the separation properties of the extractant.
The effect of acidity in the aqueous phase on extraction was also analyzed with varying concentrations of nitric acid. Extraction efficiencies dropped sharply after increasing the acid concentration to around 0.01 M, making it possible to develop stripping strategies for recovering the lanthanide ions under strongly acidic conditions.
Boyd et al. developed new preorganized 1,2-diamide-functionalized bidentate ligand-embedded hydrophobic ILs (DAILs) consisting of morpholinium or ammonium head groups, a rigid 2,3-diketopiperazine middle unit with the 1,2-diamide moiety for binding REEs, and a hydrophobic decyl tail (Fig. 8).45 These highly viscous DAILs were mixed with the phosphonium IL [P6,6,6,14][NTf2] to extract lanthanides from an aqueous feed. Higher D values were observed for the heavy lanthanides while dilute HCl at pH 0.5 was enough for stripping off all complexed ions. An NMR titration was carried out to analyze complexation with Lu3+, showing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 metal
3 metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DAIL complex. The hydrolytic stability of the DAILs was also monitored by NMR spectroscopy in an 80
DAIL complex. The hydrolytic stability of the DAILs was also monitored by NMR spectroscopy in an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v CD3OD
20 v/v CD3OD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O solvent mixture saturated with HCl, and no decomposition was found after one week.
D2O solvent mixture saturated with HCl, and no decomposition was found after one week.
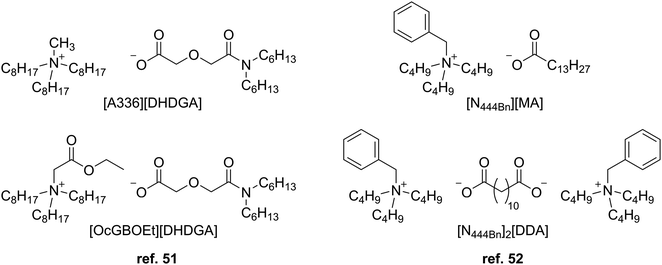
More recently, Khodakarami and Alagha synthesized ammonium-based TSILs with dihexyldiglycolamate (DHDGA) anions (Fig. 9) for extracting REEs from aqueous solutions with hexane as a suitable diluent.51 The extraction efficiencies of the TSILs for Eu3+ were higher than those of their precursors, supporting the inner synergistic effect of both cationic and anionic parts. FTIR spectroscopy suggested an ion-association mechanism for extraction, and slope analysis showed stoichiometric metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratios of 1
ligand ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, with the latter for the [OcGBOEt][DHDGA] TSIL variant that has additional oxygen atoms in the cation structure. Both TSILs exhibited higher affinities towards the middle and heavy REEs and were reusable for extraction after stripping with around 0.3 M nitric acid.
2, with the latter for the [OcGBOEt][DHDGA] TSIL variant that has additional oxygen atoms in the cation structure. Both TSILs exhibited higher affinities towards the middle and heavy REEs and were reusable for extraction after stripping with around 0.3 M nitric acid.
Another set of ammonium-based TSILs was prepared by Yu et al. with carboxylate anions containing long alkyl chains for the extraction of REEs from a chloride aqueous solution.52 One of these TSILs utilized a dicarboxylate dianion to pair with two ammonium cations (Fig. 9). Again, an ion-association extraction mechanism was proposed from FTIR characterization from complexing Y3+, and slope analysis indicated the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 or 1
1.5 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal
2 metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand complexes. D values were higher for the heavy lanthanides with the monocationic [N444Bn][MA] TSIL having better selectivity overall while most extracted ions were stripped off at 0.04 M HCl (Table 1).
ligand complexes. D values were higher for the heavy lanthanides with the monocationic [N444Bn][MA] TSIL having better selectivity overall while most extracted ions were stripped off at 0.04 M HCl (Table 1).
| REE ions | Extractants, cation, anion | Extraction solvents | Metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand stoichiometry ligand stoichiometry |
Selectivity | Extraction mechanism | Ref. |
|---|---|---|---|---|---|---|
| Eu3+ | CMPO, imidazolium, PF6, NTf2 | [Cnmim][NTf2] (n = 4, 6, 8) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cation-exchange | 32 | |
| Ln3+ | CMPO, imidazolium, pyridinium, DNNS | 1,2-Dichloroethane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Light Ln3+ | 33 | |
| Eu3+ | DGA, imidazolium, PF6, NTf2 | None | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Cation-exchange | 35 | |
| Ln3+ | Phenanthroline, amide, imidazolium, NTf2 | [Cnmim][NTf2] (n = 4, 6, 8) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Light Ln3+ | 37 | |
| Ln3+ | HOPO, imidazolium, NTf2 | [Cnmim][NTf2] (n = 4, 6, 8, 10), [C4mim][BETI], [BMPip][NTf2], [B4Pic][NTf2] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 1 3 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Middle and heavy Ln3+ | Cation-exchange | 43 |
| Ln3+ | TPTNA, imidazolium, NTf2 | [C6mim][NTf2] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Heavy Ln3+ | Cation-exchange | 44 |
| Ln3+ | 1,2-Diamide, ammonium, morpholinium, NTf2 | [P6,6,6,14][NTf2] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Heavy Ln3+ | 45 | |
| Y3+ | Pyridinium, carboxylate, ammonium, NTf2 | [C4mim][NTf2] | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cation-exchange | 46 | |
| Ln3+ | Ammonium, DHDGA | Hexane | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Middle and heavy Ln3+ | Ion-association | 51 |
| Ln3+ | Ammonium, carboxylate | Sulfonated kerosene, n-octanol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 or 1 1.5 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Heavy Ln3+ | Ion-association | 52 |
3. Conclusion and outlook
An overview of recent advances in TSIL-based extraction systems for the separation of REEs has been presented. While still a fairly new approach to extraction systems, TSILs have already offered vast improvements in extraction over conventional neutral ligands in some cases due to their variety and flexibility of being used as ligands and a pure IL phase, and being soluble in other ILs. With an expanded library of cations and anions available to fine-tune IL properties such as hydrophobicity, viscosity, and radiolytic stability, there still are plenty of structures that have untapped potential to be used as extractants. And even though many IL-based extraction systems have been studied and characterized to date, certain aspects such as investigating the molecular structure of ILs or tuning the electrostatic interactions in the second coordination sphere remain largely underexplored.Furthermore, computational modeling53 and machine learning54 can help accelerate the discovery of new extractants and optimize the separation and selectivities for REEs while another separation application involves the electrodeposition of REEs from ILs.55 Other ongoing challenges include selectivity for a specific ion among adjacent elements, high viscosity, slow extraction kinetics, and economic viability. Besides reusing the IL phase after stripping procedures, other recovery and purification methods of ILs56 may be necessary to make these systems more cost-effective. It remains challenging to come up with a more environmentally friendly extraction method to replace the traditional liquid–liquid extraction involving conventional solvents on a commercial scale57 due to high viscosities, high costs for synthesis and purification, and waste disposal concerns because of the degradation and toxicity of ILs.58 Most of these issues will need to be expanded upon and improved in order for these extraction systems to find applications in industrial separation and purification settings.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Basic Energy Science Program of the Office of Science, U.S. Department of Energy, under contract DE-AC05-0096OR22725 with the Oak Ridge National Laboratory, managed by UT-Battelle, LLC.References
- K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton and M. Buchert, J. Cleaner Prod., 2013, 51, 1–22 CrossRef CAS.
- R. F. Service, Science, 2010, 327, 1596–1597 CrossRef CAS PubMed.
- A. Cho, Science, 2013, 340, 914–918 CrossRef CAS PubMed.
- T. Cheisson and E. J. Schelter, Science, 2019, 363, 489–493 CrossRef CAS PubMed.
- M. L. P. Reddy, T. Prasada Rao and A. D. Damodaran, Miner. Process. Extr. Metall. Rev., 1993, 12, 91–113 CrossRef.
- A. P. Paiva and P. Malik, J. Radioanal. Nucl. Chem., 2004, 261, 485–496 CrossRef CAS.
- V. A. Anagnostopoulos and B. D. Symeopoulos, Desalin. Water Treat., 2016, 57, 3957–3963 CrossRef CAS.
- X. Q. Sun, H. M. Luo and S. Dai, Chem. Rev., 2012, 112, 2100–2128 CrossRef CAS PubMed.
- A. N. Turanov, V. K. Karandashev and M. Boltoeva, Hydrometallurgy, 2020, 195, 105367 CrossRef CAS.
- Q. Chen, C. Lu, Y. Hu, Y. Liu, Y. Zhou, C. Jiao, M. Zhang, H. Hou, Y. Gao and G. Tian, J. Radioanal. Nucl. Chem., 2021, 327, 565–573 CrossRef CAS.
- T. Liu and J. Chen, Sep. Purif. Technol., 2021, 276, 119263 CrossRef CAS.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- Y.-L. Wang, B. Li, S. Sarman, F. Mocci, Z.-Y. Lu, J. Yuan, A. Laaksonen and M. D. Fayer, Chem. Rev., 2020, 120, 5798–5877 CrossRef CAS PubMed.
- O. Nordness and J. F. Brennecke, Chem. Rev., 2020, 120, 12873–12902 CrossRef CAS PubMed.
- B. J. Mincher and J. F. Wishart, Solvent Extr. Ion Exch., 2014, 32, 563–583 CrossRef CAS.
- K. Wang, H. Adidharma, M. Radosz, P. Wan, X. Xu, C. K. Russell, H. Tian, M. Fan and J. Yu, Green Chem., 2017, 19, 4469–4493 RSC.
- C. J. Clarke, L. Bui-Le and J. Hallett, Anal. Methods, 2020, 12, 2244–2252 RSC.
- E. Diaz, V. Monsalvo, J. Palomar and A. F. Mohedano, in Encyclopedia of Inorganic and Bioinorganic Chemistry, 2016, pp. 1–12, DOI: DOI:10.1002/9781119951438.eibc2393.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132–7189 CrossRef CAS PubMed.
- E. Quijada-Maldonado and J. Romero, Curr. Opin. Green Sustainable Chem., 2021, 27, 100428 CrossRef CAS.
- S. Dai, Y. H. Ju and C. E. Barnes, J. Chem. Soc., Dalton Trans., 1999, 1201–1202 RSC.
- M. L. Dietz and J. A. Dzielawa, Chem. Commun., 2001, 2124–2125 RSC.
- H. M. Luo, S. Dai, P. V. Bonnesen, T. J. Haverlock, B. A. Moyer and A. C. Buchanan, Solvent Extr. Ion Exch., 2006, 24, 19–31 CrossRef CAS.
- F. Kubota and M. Goto, Solvent Extr. Res. Dev., Jpn., 2006, 13, 23–36 CAS.
- J. H. Davis, Chem. Lett., 2004, 33, 1072–1077 CrossRef CAS.
- H. Okamura and N. Hirayama, Anal. Sci., 2021, 37, 119–130 CrossRef CAS PubMed.
- M. Iqbal, K. Waheed, S. B. Rahat, T. Mehmood and M. S. Lee, J. Radioanal. Nucl. Chem., 2020, 325, 1–31 CrossRef CAS.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, R. Mayton, S. Sheff, A. Wierzbicki, J. H. Davis and R. D. Rogers, Chem. Commun., 2001, 135–136 RSC.
- H. M. Luo, S. Dai, P. V. Bonnesen and A. C. Buchanan, J. Alloys Compd., 2006, 418, 195–199 CrossRef CAS.
- K. Nakashima, F. Kubota, T. Maruyama and M. Goto, Anal. Sci., 2003, 19, 1097–1098 CrossRef CAS PubMed.
- I. L. Odinets, E. V. Sharova, O. I. Artyshin, K. A. Lyssenko, Y. V. Nelyubina, G. V. Myasoedova, N. P. Molochnikova and E. A. Zakharchenro, Dalton Trans., 2010, 39, 4170–4178 RSC.
- P. K. Mohapatra, P. Kandwal, M. Iqbal, J. Huskens, M. S. Murali and W. Verboom, Dalton Trans., 2013, 42, 4343–4347 RSC.
- A. N. Turanov, V. K. Karandashev, O. I. Artyushin and E. V. Sharova, Solvent Extr. Ion Exch., 2022, 40, 203–215 CrossRef CAS.
- X. Q. Sun, Y. Ji, F. C. Hu, B. He, J. Chen and D. Q. Li, Talanta, 2010, 81, 1877–1883 CrossRef CAS PubMed.
- P. K. Mohapatra, A. Sengupta, M. Iqbal, J. Huskens and W. Verboom, Chem. Eur. J., 2013, 19, 3230–3238 CrossRef CAS PubMed.
- Y. Wu, Y. Zhang, F. Fan, H. M. Luo, P. Hu and Y. L. Shen, J. Radioanal. Nucl. Chem., 2014, 299, 1213–1218 CrossRef.
- J. Dehaudt, N. J. Williams, I. A. Shkrob, H. M. Luo and S. Dai, Dalton Trans., 2016, 45, 11624–11627 RSC.
- K. Shimojo, K. Kurahashi and H. Naganawa, Dalton Trans., 2008, 37, 5083–5088 RSC.
- I. Billard, A. Ouadi and C. Gaillard, Anal. Bioanal. Chem., 2011, 400, 1555–1566 CrossRef CAS PubMed.
- M. Galletta, S. Scaravaggi, E. Macerata, A. Famulari, A. Mele, W. Panzeri, F. Sansone, A. Casnati and M. Mariani, Dalton Trans., 2013, 42, 16930–16938 RSC.
- C. Ekberg, E. Löfström-Engdahl, E. Aneheim, M. R. S. Foreman, A. Geist, D. Lundberg, M. Denecke and I. Persson, Dalton Trans., 2015, 44, 18395–18402 RSC.
- W. Yantasee, G. E. Fryxell, Y. Lin, H. Wu, K. N. Raymond and J. Xu, J. Nanosci. Nanotechnol., 2005, 5, 527–529 CrossRef CAS PubMed.
- C.-L. Do-Thanh, H. M. Luo, J. A. Gaugler and S. Dai, Sep. Purif. Technol., 2022, 301, 121939 CrossRef CAS.
- H. Wu, X. Zhang, X. Yin, I. Yusuke, H. Miki and K. Takeshita, Chem. Lett., 2018, 47, 732–735 CrossRef CAS.
- R. Boyd, L. Jin, P. Nockemann, P. K. J. Robertson, L. Stella, R. Ruhela, K. R. Seddon and H. Q. N. Gunaratne, Green Chem., 2019, 21, 2583–2588 RSC.
- K. Hu, H. Gao, Y. Nie, H. Dong, J. Yan, X. Zhang and F. Li, Sep. Purif. Technol., 2021, 269, 118774 CrossRef CAS.
- A. Rout, J. Kotlarska, W. Dehaen and K. Binnemans, Phys. Chem. Chem. Phys., 2013, 15, 16533–16541 RSC.
- X. Q. Sun, H. M. Luo and S. Dai, Talanta, 2012, 90, 132–137 CrossRef CAS PubMed.
- X. Q. Sun, C.-L. Do-Thanh, H. M. Luo and S. Dai, Chem. Eng. J., 2014, 239, 392–398 CrossRef CAS.
- C. W. Abney, C. Do, H. M. Luo, J. Wright, L. He and S. Dai, J. Phys. Chem. Lett., 2017, 8, 2049–2054 CrossRef CAS PubMed.
- M. Khodakarami and L. Alagha, Sep. Purif. Technol., 2020, 232, 115952 CrossRef CAS.
- Y. Deng, Y. Ding, Z. Huang, Y. Yu, J. He and Y. Zhang, J. Mol. Liq., 2021, 329, 115549 CrossRef CAS.
- D. A. Penchoff, E. Valeev, H. Jagode, P. Luszczek, A. Danalis, G. Bosilca, R. J. Harrison, J. Dongarra and T. L. Windus, in Rare Earth Elements and Actinides: Progress in Computational Science Applications, American Chemical Society, 2021, ch. 1, vol. 1388, pp. 3–53 Search PubMed.
- T. Liu, K. R. Johnson, S. Jansone-Popova and D.-e. Jiang, JACS Au, 2022, 2, 1428–1434 CrossRef CAS PubMed.
- E. Bourbos, I. Giannopoulou, A. Karantonis, I. Paspaliaris and D. Panias, in Rare Earths Industry, ed. I. Borges De Lima and W. Leal Filho, Elsevier, Boston, 2016, pp. 199–207, DOI: DOI:10.1016/B978-0-12-802328-0.00013-9.
- J. Zhou, H. Sui, Z. Jia, Z. Yang, L. He and X. Li, RSC Adv., 2018, 8, 32832–32864 RSC.
- H. Pereira Neves, G. Max Dias Ferreira, G. Max Dias Ferreira, L. Rodrigues de Lemos, G. Dias Rodrigues, V. Albis Leão and A. Barbosa Mageste, Sep. Purif. Technol., 2022, 282, 120064 CrossRef CAS.
- H. B. Trinh, J.-c. Lee and J. Lee, in Ionic Liquid-Based Technologies for Environmental Sustainability, ed. M. Jawaid, A. Ahmad and A. V. B. Reddy, Elsevier, 2022, pp. 101–121, DOI: DOI:10.1016/B978-0-12-824545-3.00007-6.
| This journal is © The Royal Society of Chemistry 2023 |