Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review on terpenes for treatment of gastric cancer: current status and nanotechnology-enabled future†
Komal
Attri
ad,
Deepinder
Sharda
a,
Bhupendra
Chudasama
bd,
Roop L.
Mahajan
*cd and
Diptiman
Choudhury
 *ad
*ad
aSchool of Chemistry and Biochemistry, Thapar Institute of Engineering and Technology, Patiala, 147004, Punjab, India
bSchool of Physics and Material Sciences, Thapar Institute of Engineering and Technology, Patiala, 147004, Punjab, India
cDepartment of Mechanical Engineering, Virginia TechInstitute for Critical Technology and Applied Science, Virginia Tech, Blacksburg, VA 24061, USA. E-mail: mahajanr@vt.edu; Tel: +1-5402312597
dThapar Institute of Engineering and Technology-Virginia Tech Centre of Excellence for Emerging Materials, Thapar Institute of Engineering and Technology, Patiala-147004, Punjab, India. E-mail: diptiman@thapar.edu; Tel: +91-8196949843
First published on 29th May 2023
Abstract
Eighty-five percent of gastric cancer is caused by Helicobacter pylori infection. Delays in detection, limited efficacy, and significant side effects of the available treatments lead to a 5-year survival chance of only 32%. Therefore, better remedies are required. Numerous studies have been published on herbal medications offering an edge over conventional medicines. Secondary metabolites such as different polyphenolic compounds including terpenes are key players for therapeutic advantages. The antimicrobial, anticarcinogenic, anti-inflammatory, etc. activities of biocompatible active ingredients make these compounds suitable for therapeutic use. Despite such advantages, the use of herbal medicine in gastric cancer treatment is limited. In this article, we describe the therapeutic potential and limitations of terpenes followed by the potential advantages offered by the combinatorial effects of terpenes with their nanoconjugates. These include increasing the anticancer and antimicrobial potency of drugs as well as resolving drawbacks including targeted delivery, stability, half-life, etc, thus making them suitable for gastric cancer treatment. The article concludes with a detailed discussion on the challenges encountered in deploying targeted secondary metabolites and their future developmental prospects to provide ideas and insights for future research.
Sustainability spotlightGlobally, cancer is the second most deadly disease after heart disease. Among all forms of cancer, gastric cancer is the 4th most deadly after lung, breast, and colon cancer, costing 769![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lives in 2020, with more than 1.09 million new cases being diagnosed. Helicobacter pylori is associated with ∼85% of the tumors. H. pylori infection is directly linked to the population with a poor economy and hygiene, and so is gastric cancer. This review deals with managing gastric cancer with natural products such as terpenes. Nature is full of resources in the form of secondary metabolites that humankind has used for a long time to manage many diseases, including gastric cancer. Many of these herbal formulations are already in use in different forms of medicine, such as Ayurveda, Unani, Siddha, and homoeopathy. Terpenes are a diverse group of compounds consisting of around fifty-five thousand members, of which many have selective anticancer, anti-microbial, and anti-inflammatory properties. Due to their abundance, fewer side effects, and cost-effectiveness, they have been popularised in traditional medicines. But problems such as low stability, solubility, and shelf life restricted their use in modern medicine. This review describes the potential role of terpenes in gastric cancer management. It suggests how terpenes may be explored in the development of practical, safer, cheaper, and sustainable modern medicine with the help of nanomedicine. 000 lives in 2020, with more than 1.09 million new cases being diagnosed. Helicobacter pylori is associated with ∼85% of the tumors. H. pylori infection is directly linked to the population with a poor economy and hygiene, and so is gastric cancer. This review deals with managing gastric cancer with natural products such as terpenes. Nature is full of resources in the form of secondary metabolites that humankind has used for a long time to manage many diseases, including gastric cancer. Many of these herbal formulations are already in use in different forms of medicine, such as Ayurveda, Unani, Siddha, and homoeopathy. Terpenes are a diverse group of compounds consisting of around fifty-five thousand members, of which many have selective anticancer, anti-microbial, and anti-inflammatory properties. Due to their abundance, fewer side effects, and cost-effectiveness, they have been popularised in traditional medicines. But problems such as low stability, solubility, and shelf life restricted their use in modern medicine. This review describes the potential role of terpenes in gastric cancer management. It suggests how terpenes may be explored in the development of practical, safer, cheaper, and sustainable modern medicine with the help of nanomedicine.
|
1. Introduction
Globally, cancer is the second most deadly disease after heart disease.1 In cancer, the body cells lose the property of normal division and undergo uncontrolled division giving rise to abnormal cells that collectively leads to tumor formation.2 The primary causative agents of cancer are ultraviolet radiation or ionizing radiation, bacterial infections, viral infections, parasitic infections, etc.3. Among different forms of cancer, gastric cancer is the 4th most deadly after lung, breast, and colon cancer, costing 769![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lives in 2020, with more than 1.09 million new cases being diagnosed.4,5 The primary cause of gastric cancer is infection with Helicobacter pylori (H. pylori),6 a Gram-negative, microaerophilic, motile, flagellated spiral-shaped bacterium associated with 70–85% of gastric cancer and considered a type I carcinogen for the disease. It is the most common etiologic agent that is associated with other cancers, such as duodenal cancers (90–95%), and is responsible for 25% of all infection-associated cancers.7 Its pathogenicity is due to its genes, including CagA, VacA, BabA, SabA, OipA, DupA, and FlaA.6 Approximately half of the world's population is infected with H. pylori, and most such colonized individuals develop coexisting chronic inflammation. Apart from H. pylori, various other factors, including smoking and consumption of tobacco-related products, a low-fiber diet, long-term stomach inflammation, obesity, family history, and a diet high in salty and smoked foods, increase the risk of gastric cancer.8
000 lives in 2020, with more than 1.09 million new cases being diagnosed.4,5 The primary cause of gastric cancer is infection with Helicobacter pylori (H. pylori),6 a Gram-negative, microaerophilic, motile, flagellated spiral-shaped bacterium associated with 70–85% of gastric cancer and considered a type I carcinogen for the disease. It is the most common etiologic agent that is associated with other cancers, such as duodenal cancers (90–95%), and is responsible for 25% of all infection-associated cancers.7 Its pathogenicity is due to its genes, including CagA, VacA, BabA, SabA, OipA, DupA, and FlaA.6 Approximately half of the world's population is infected with H. pylori, and most such colonized individuals develop coexisting chronic inflammation. Apart from H. pylori, various other factors, including smoking and consumption of tobacco-related products, a low-fiber diet, long-term stomach inflammation, obesity, family history, and a diet high in salty and smoked foods, increase the risk of gastric cancer.8
Unfortunately, in most cases, the diagnosis of gastric cancer is delayed due to the absence of early specific symptoms and the five-year survival rate is only 15%.9 This low rate is despite different treatment modalities for gastric cancer, such as chemotherapy, surgical treatments, radiation therapy, and immunotherapy. Therefore, prevention is a more efficient way of gastric cancer management than treating it. Furthermore, available treatments involving modern chemotherapeutic agents show many side effects, including hair and weight loss, diarrhea, anemia, blood clots, and heart, kidney, and liver damage, compromising the patient's quality of life.10 Furthermore, the effectiveness of surgery and radiation therapy is primarily limited to the early stages of gastric cancer progression.11 An alternative approach to managing the disease is thus urgently needed. One potential alternative is the use of herbal formulations. To understand their viability, a brief look at the role of inflammation and the body's natural response to it is in order.
From any damaging stimuli such as pathogens, wounds, foreign bodies, or infection, a biological response of inflammation ensues.12 The organism tries to self-protect or remove that inflammation using a natural body process – self-defense. In many cases, the body produces cytokines against a particular disease or foreign substance. In a few instances, these cytokines are overexpressed by the body's immune system. Gastric cancer is one of the best examples of human inflammation-associated cancer.13 Synthetic anti-inflammatory drugs are being used to suppress or inhibit these mediators. These synthetic drugs have benefits and side effects that compromise patients' quality of life. For this reason, currently, people are expecting or even preferring treatments that involve natural products with safe toxicological profiles, leading to the exploration of these for alternative drug delivery treatments.14
Nature is full of resources in the form of secondary metabolites that humankind has used for a long time to manage many diseases, including gastric cancer. The formulations are well documented in few cases, but additional documentation is needed for most. Many of these herbal formulations are already in use in India, China, Vietnam, and Africa, in various forms through different systems of medicine such as Ayurveda, Unani, Siddha, and homoeopathy.15 These medicines are mostly a mixture of various plant secondary metabolites, including phenolics, alkaloids, saponins, terpenes, sterols, and sphingolipids. For example, in many plants, the extract has anti H. pylori activity, suggesting their potential for treating gastric cancer. In addition, many of the compounds present in such herbal formulations can be administered orally to work on the target site without being absorbed by the intestine or stomach. The net result is targeted delivery and site-specific activity, which increase their potential for treatment with reduced side effects. In other words, herbs play a crucial role in controlling the growth and progression of cancer and can help patients overcome the side effects caused by chemotherapeutics. They enhance the body's immunity through naturally occurring compounds in them and thus prevent the spread of cancer by any means, including growth, metastasis, and invasion. Therefore, using these formulations instead of radiation and chemotherapy will be a better option as they do not cause any of the ill effects that conventional therapies cause, such as fatigue, hair loss, mouth sores, nausea, vomiting, and organ failure.16
In this framework, a class of natural compounds named terpenes, a highly diverse family of natural products, has drawn attention due to their capability of selectively killing tumor cells without hampering normal cells. These are synthesized by plants and are also called secondary metabolites. Terpenes have 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 members with different chemical structures and curing potentials.17 They suppress or inhibit the growth of several cancers, such as breast, skin, stomach, pancreatic, colon, and prostate, without damaging or contributing any toxicity to normal cells as they exhibit pleiotropic actions by acting on a target in multiple ways. They also inhibit protein synthesis by targeting the main events crucial for the process. They are among the leading herbal formulations in cancer treatment and are discussed in detail in Section 2. However, despite the remedial effect and other advantages such as biocompatibility, safety, non-invasiveness, convenience, and low cost in the treatment of cancer as compared to conventional chemotherapy,18 many natural formulations, including terpenes, have not been widely deployed in modern medicine due to certain problems associated with their transport across the body. Conventionally, they are given as such via different ways through food or in the form of supplements or directly as herbal medication but they have certain pharmacological and chemical issues associated with them such as low water solubility, low permeability, stability, reduced bioavailability, sensitivity to environmental conditions (temperature, pH, and humidity), chemical degradation or volatilization being a few of them.19 This is precisely where nanotechnology can help overcome these disadvantages of herbal medicines.
000 members with different chemical structures and curing potentials.17 They suppress or inhibit the growth of several cancers, such as breast, skin, stomach, pancreatic, colon, and prostate, without damaging or contributing any toxicity to normal cells as they exhibit pleiotropic actions by acting on a target in multiple ways. They also inhibit protein synthesis by targeting the main events crucial for the process. They are among the leading herbal formulations in cancer treatment and are discussed in detail in Section 2. However, despite the remedial effect and other advantages such as biocompatibility, safety, non-invasiveness, convenience, and low cost in the treatment of cancer as compared to conventional chemotherapy,18 many natural formulations, including terpenes, have not been widely deployed in modern medicine due to certain problems associated with their transport across the body. Conventionally, they are given as such via different ways through food or in the form of supplements or directly as herbal medication but they have certain pharmacological and chemical issues associated with them such as low water solubility, low permeability, stability, reduced bioavailability, sensitivity to environmental conditions (temperature, pH, and humidity), chemical degradation or volatilization being a few of them.19 This is precisely where nanotechnology can help overcome these disadvantages of herbal medicines.
Nanotechnology involves the utilization of critical properties of materials at the nanoscale. It consists of developing nanomaterials between 1–100 nm by manipulating the structures at the atomic level. Nanomaterials have unique electrical, optical and magnetic properties and a large surface area to volume ratio and are governed by the laws of quantum mechanics.20 Due to their large surface area to volume ratio, they exhibit specific properties such as enhanced catalytic activity, biological activity, non-linear optical performance, and thermal conductivity. They can be delivered as liposomes, vesicular systems, nano-emulsions, nanostructured lipid carriers and solid lipid nanoparticles. Moreover, the use of these terpenes in the form of nano-formulations has been shown to enhance their solubility, stability and bioavailability, anti-proliferative activity, prevention from biological and chemical degradability and cell toxicity, enhanced specificity and pharmacological activity, and improved distribution of tissue macrophages along with sustained release.21–23 Nano-delivery systems have been used to deliver all the herbal compounds irrespective of their hydrophobic or hydrophilic nature and thus can be used for transfer of hydrophobic terpenes via encapsulating them with nanoparticles which enhance their potency. Some of the terpenes are generally recognised as safe penetration enhancers. As a result, they can serve as excipients for other drugs or phytochemicals and make them suitable for administration through topical routes.19 For instance, the bioavailability of celastrol was increased after loading it into poly (ethylene glycol)-block-poly(ε-caprolactone) nano-polymeric micelles. These micelles play a crucial role in inhibiting the growth of SO–Rb 50 retinoblastoma in a mouse xenograft model in a dose dependent manner. The nano-encapsulated celastrol was found to exhibit the antitumor activity by reducing the expression of Bcl-2, NF-κB, and phospho–NF–κB p65 proteins at 54.4 μg ml−1 after 48 h of the treatment.24 The anti-proliferative activity of celastrol loaded poly(ε-caprolactone) nanoparticles (CS-NPs) has also been examined using different prostate cancer cell lines (LNCaP, DU-145, and PC3) by exposing the cells to a 0.5–2.0 μM concentration of CS-NPs.25 The results showed inhibition of concentration-dependent proliferation. In a nutshell, nanotechnology has fundamentally changed the way many diseases, including gastric cancer, are diagnosed and treated. To this end, in this review article, we focus on different active natural terpenes obtained from natural sources, their role in controlling gastric cancer, and directions for future research in gastric cancer management.
2. Natural terpenes for treating gastric cancer
An active area of global research is to develop effective anti-cancerous products from natural materials such as medicinal plants or effective medicinal components.26,27 Of all the available human medications, one-third are derived from herbal plants or extracts. Based on their positive aspects, their consumption is encouraged by physicians and researchers. Apart from cancer, they are widely used in different applications. Several dietary products such as fruits, vegetables, cereals, and spices exhibit anti-cancerous activity to a great extent, and it was found that there is an inverse relationship between the intake of natural products and cancer cases.28–30 The primary factor responsible for this salutary action is the presence of different phytochemicals, which promote various mechanisms to prevent gastric cancer. The effective means for gastric cancer are cell proliferation or H. pylori inhibition, autophagy, apoptosis, anti-angiogenesis, or suppressed cell metastasis.30–322.1 Natural terpenes and their role in gastric cancer treatment
Terpenes are the largest and most diverse category of naturally occurring family of ∼30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different polymeric compounds made up of isoprenes, or 2-methyl-1,3-butadiene (CH2
000 different polymeric compounds made up of isoprenes, or 2-methyl-1,3-butadiene (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–CH
C(CH3)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)n where n is the number of isoprene units (where n ≥ 2). Terpenes play an essential role in plants' defense mechanisms, protect them from herbivores, and generate disease resistance. They play different roles in plants, including serving as thermoprotectants and performing signaling and medicinal functions. They also work as solvents or flavoring agents.33 In addition, they have antimicrobial, anticarcinogenic, anti-inflammatory, antiallergic, and neuroprotective properties.34 Not surprisingly, numerous studies have shown that they play an equally essential role in supporting human health.
CH2)n where n is the number of isoprene units (where n ≥ 2). Terpenes play an essential role in plants' defense mechanisms, protect them from herbivores, and generate disease resistance. They play different roles in plants, including serving as thermoprotectants and performing signaling and medicinal functions. They also work as solvents or flavoring agents.33 In addition, they have antimicrobial, anticarcinogenic, anti-inflammatory, antiallergic, and neuroprotective properties.34 Not surprisingly, numerous studies have shown that they play an equally essential role in supporting human health.
Based on the number of isoprene units, they are classified into monoterpenes, diterpenes, triterpenes, and sesquiterpenes.33 A brief description of these follows.
2.2 Monoterpenes
They comprise a large group that performs numerous biological activities and act as antioxidants, anti-inflammatory, anti-diabetic, and antitumor agents. They also assist in neuroprotection, hepatoprotection, and cardio-protection.35,36 Chemically, they consist of 2 isoprene units formed by five carbons joined together head-to-tail. These biochemically active units are diphosphate esters, isopentyl diphosphate, and dimethylallyl diphosphate. Monoterpenes are further subdivided into acyclic, monocyclic, bicyclic, and iridoid glycosides.37 These are mainly found as active ingredients in essential oils and fixed oils from plants and related sources.35 Besides their biological activities, they are also used as fragrant sources for making cosmetics and perfumes due to their strong aroma and odor.38 Some of the most common monoterpenes employed for anticancer activity are described below.2.3 Diterpenes
They belong to a naturally occurring class of chemical constituents comprising four isoprene units combined, thus having a core skeleton of 20 carbons. They are derivatives of geranyl pyrophosphate50 and are produced by plants, animals, and fungi via 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase pathway 3.51 They exhibit several biological activities such as antioxidation, antimicrobial, anti-inflammatory, anticarcinogenic and neurobiological.52 Compared with monoterpenes and sesquiterpenes, they are fewer in number and less volatile.53 Some of the significant diterpenes are discussed below.2.4 Triterpenes
They consist of 6 isoprene units having 30 carbons in which squalene epoxide units are arranged in the chair-chair-chair-boat confirmation after the condensation process.62 The primary sources of triterpenes are plants and fungi.63 These are the main plant membrane structure constituents responsible for stabilizing the phospholipid bilayer in the cell membrane.62 They perform numerous biological functions ranging from anti-oxidant, antiviral, antibacterial, anti-fungal, anti-inflammatory, and anticancerous activities, influencing several signaling pathways in cancer treatment.63 Pentacyclic terpenes are plants' primary and secondary metabolites from their leaves, roots, stem barks, and fruits.64 The four triterpenes commonly used in gastric treatment are described below.2.5 Sesquiterpenes
Many sesquiterpene compounds are found naturally and play a significant role in biological activities and other human uses. They are very diverse and have two distinct characteristics – the presence of a 15 carbon skeleton that makes up the backbone and the arrangement of different functional groups and substituents in layers on the structural scaffolds. The constituents are arranged in diverse regiospecific and stereo-specific manners.73 They can be monocyclic, bicyclic, or tricyclic. When compared with terpenes, they are less volatile. They have a more pungent odor and show anti-inflammatory and antibacterial properties.74,75 After oxidation, sesquiterpenes get converted into sesquiterpenols.76 The α-methylene-γ-lactone group (αMγL) of sesquiterpenes is responsible for anticarcinogenic and anti-inflammatory activity, whereas helenalin causes anti-inflammatory effects only and parthenolide accounts for antitumor activity. Furthermore, sesquiterpene lactone artemisinin exerts antimicrobial effects.77 A few of the common sesquiterpenes for gastric cancer are described below.The structures of monoterpenes, sesquiterpenes, diterpenes, and triterpenes are given in Fig. 1. The details of different terpenes, their properties, sources, and modes of action against cancers are shown in Table 1.
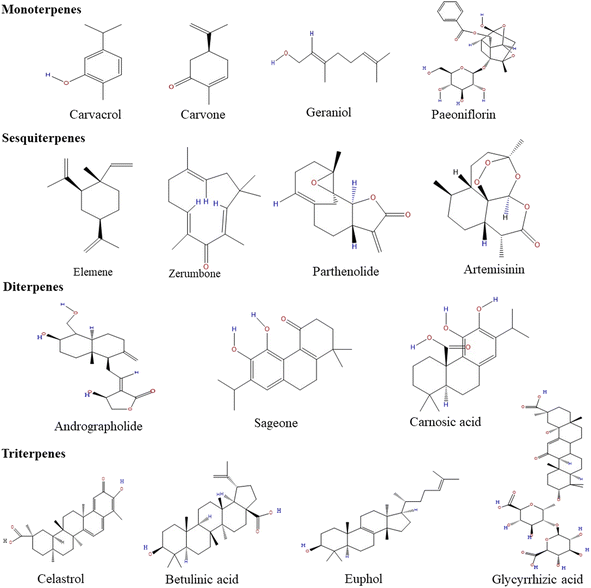 | ||
| Fig. 1 The chemical structures of different monoterpenes, sesquiterpenes, diterpenes and triterpenes. | ||
| Compounds | Biological properties | Source | Cell line | Dose |
|---|---|---|---|---|
| Carvacrol | Anti-microbial, antitumor, anti-inflammatory, anti-mutagenic, analgesic, and antiparasitic88 | Thymus vulgaris, Origanum vulgare, Lepidium flavum, and Citrus89 | AGS34,43 | 0–600 μmol l−1 (ref. 43) |
| Geraniol | Antitumor | Lemon, geranium | AGS44,90 | 5–35 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μM ml−1 (ref. 44) μM ml−1 (ref. 44) |
| Anti-inflammatory | ||||
| d-Carvone | Antimicrobial, antioxidant, anti-inflammatory, and anti-cancer91 | Caraway and dill weeds | AGS46 | 5–35 μM (ref. 46) |
| Paeoniflorin | Antitumor | P. lactiflora | SGC-7901, MGC-803,48 HepG2 (liver cancer), SMMC-7721 (liver cancer),92 SGC-7901 (gastric adenocarcinoma cell),93 and MCF-7 (breast cancer cell)94 | 80–324 μg ml−1 (ref. 93) |
| Elemene | Anticancer | Rhizoma curcumae 80 | BGC-823 (ref. 80) | 0.02–0.16 mg ml−1 (ref. 80) |
| Zerumbone | Anticancer and anti-angiogenic | Ginger78 | SGC-7901 (ref. 78) | 0.1–50 μM ml−1 (ref. 78) |
| Parthenolide | Antitumor | Feverfew (Tanacetum parthenium L. Schulz Bip.)95 | SGC7901,83 colon carcinoma cells, lung cancer85 | 5–200 μmol l−1 (ref. 83) |
| Artemisinin | Antitumor, antimicrobial, antioxidant | Artemisia annua 96 | AGS and MKN7487 brain cancer, breast cancer, cervical, colorectal, gastric, lung, and ovarian cancer97 | 0–5 μM (ref. 87) |
| Andrographolide | Anticancer | Andrographis paniculata | SGC 7901 cells55, Jurkat, PC-3, HepG2, and colon 205 tumor cells, and normal peripheral blood mononuclear cells (PBMCs)98 | 5–40 μg ml−1 (ref. 55) |
| Anti-inflammatory | ||||
| Sageone | Anticancer | Rosmarinus officinalis | Gastric adenocarcinoma cell lines AGS, SNU-1, and SNU-16 (ref. 59) | 6.25–100 μM (ref. 58) |
| Carnosic acid | Antitumor | Rosmarinus officinalis | AGS and MKN-45 cells61 | 6.25–100 μg ml−1 (ref. 61) |
| Celastrol | Anti-inflammatory and antitumor | Tripterygium wilfordii (Thunder god Vine), Celastrus orbiculatus, Celastrus aculeatus, Celastrus reglii, and Celastrus scandens99 | MKN45 (ref. 65) melanoma tumor cells68 | 0–20 μM (ref. 65) |
| Euphol | Antiviral, anti-inflammatory, and anti-cancer69 | Euphorbia tirucalli 69 | CS12 (ref. 69) | 2–60 μg ml−1 (ref. 69) |
| Glycyrrhizic acid | Immunomodulatory | Licorice Glycyrrhiza glabra L.100 | MGC-803, BGC-823, SGC-7901 (ref. 70) KATO III, HL 60, DU-145 & LNCaP101,102 | 0–4 mg ml−1 (ref. 70) |
| Antitumor | ||||
| Betulinic acid | Antiviral, antibacterial, antimalarial, anti-inflammatory, and anticancerous71 | Jujubee seeds and white birch bark71 | SNU-16 and NCI–N87 (ref. 71) | 0–80 μM (ref. 71) |
3. Nanotechnology and herbal formulations
With conventional therapeutic and diagnostic tools for cancer, there is an increase in systemic toxicities and refractoriness. Due to this, various other strategies are used to enhance diagnostic abilities and combat disease severity.103 Among these, nanotechnology has gained attraction due to its many inherent advantages. Various nanotechnology-based devices are being developed for their beneficial roles in detection, drug delivery, gene & targeted therapy, biomarker mapping, bioimaging, early diagnosis, and rapid testing.103–105 Recent advancements in nanotechnology, especially in the synthesis of nanoparticles, have helped address some issues that were otherwise not possible, such as low target specificity, and improved disease diagnostic ability in terms of sensitivity, quality, speed, early detection, biocompatibility, stability, and effectiveness of drugs.106Nanoparticles can be synthesized by various means, such as physical, chemical, and green methods. Chemical methods comprise high energy consumption, high cost, low yield, and environmental degradation through harsh reducing agents.107 Physical techniques are also not preferred since they involve high cost, high pressure & high temperature and are not amenable to control the size & shape of nanoparticles.108 However, because of the use of toxic chemicals in synthesizing nanoparticles, greener methods are preferred in which the particles are synthesized by following a redox reaction in which the reduction of metal ions to stabilize nanoparticles is carried out by using natural extracts or components of an organism. Recently, research on green synthesized nanoparticles has increased exponentially worldwide due to the widespread use of different plant species, isolated compounds, and extracts. Furthermore, green synthesized nanoparticles have low cost, environment-friendly behavior, energy efficiency, easy synthesis, better bio-activities, high catalytic activities, and low toxicity. For instance, a large number of green nanoparticles have been synthesised using extracts of different plants having terpenes as secondary metabolites. Their mode of action on different gastric cancer cell lines and H. pylori has been investigated.109–114 For example, leaf extract of Artemisia turcomanica upregulates BAX mRNA expression and inhibits Bcl2 expression and increases the number of early and late apoptotic cells,109 Artemisia ciniformis up-regulates pro-apoptotic genes, caspase-3, caspase-9, and Bax, down-regulates the Bcl2 gene, inhibits the expression of cyclin D1 and MMP2 genes and causes the arrest of cancer cells in the G0/G1 phase,110C. monogyna induces apoptosis and ROS generation,111Cardiospermum halicacabum induces apoptosis via inhibiting antiapoptotic proteins as well as proapoptotic proteins,112Cassia fistula leaf extract causes bacterial cytoskeleton disruption and DNA damage in H. pylori,113 and Vitex negundo ethanolic extract augments expression of caspase-3, caspase-9, Bid and Bax and reduces expression of Bcl-XL.114 Similarly, quantum clusters and quantum dots are being synthesized using different proteins, natural polymers, etc. For instance, insulin-protected quantum clusters are made using other metal ions, such as nickel, zinc, etc., for their role in developing biomarkers using the green synthesis method.115,116
Similarly, biomarkers can be developed to detect cancer more precisely & efficiently.103,117 Coal-GO (graphene oxide) exhibits higher interaction with single-stranded DNA aptamers, resulting in chemiluminescence resonance energy transfer-based biosensors with higher sensitivity.118 Also, apigenin, a natural flavone, exhibits synergistic anticancer activity with curcumin after binding with tubulin at different sites.119 Ginger, which has been used since time immemorial as a traditional medicine, shows antiproliferative activity when used as an aqueous extract by disrupting the microtubule network of cancer cells.120 A bacterioboat-based drug carrier consisting of surface-encapsulated mesoporous nanoparticles on L. reuteri was developed for suitable oral administration of drugs.121
Nanomaterials also offer an advantage in minimizing the limitations of various imaging techniques, such as MRI (magnetic resonance imaging), X-ray, CT-scan (computed tomography scan), and ultrasound that are commonly deployed to enhance the diagnostic accuracy and progression of the disease. These techniques can only examine the alterations on the surface of the tissue, which is also relatively late in the progression of the disease. In addition, when used with imaging contrast agents, such as small molecules with a fast metabolism, unwanted toxic side effects are commonly experienced. By comparison, nanomaterials can be synthesized to act as powerful contrast agents in all imaging techniques due to their ability to show less toxicity and more permeability in tissues. For example, quantum dots are widely used for specific cellular imaging, and iron oxide particles are used for tumor imaging.20,122–124 Following this, the development of nanoscale range drug delivery tools using nanotechnology can be used for targeting cancer tissue more precisely and with little or no side effects. Nanomaterials are also widely used in drug delivery due to their biological nature, ability to cross cell barriers easily, and active & passive targeting nature.103,125,126 Using these, various novel methods for drug delivery are being developed that have been proven clinically effective. For instance, paclitaxel, incorporated with polymeric mPEG-PLA micelles, is used for treating metastatic breast cancer chemotherapeutically.20,127
Furthermore, anticancer therapies are often considered superior only when the therapeutic agent can reach the target site without causing side effects. With nanoparticles used as carriers, this can be relatively easily achieved by chemically modifying carrier surfaces. For example, incorporating PEG (polyethylene glycol) or polyethylene oxide at the surface of nanoparticles enhances tumor targeting ability because PEG prevents nanoparticle detection as an antigen by the body's immune system, thus making it easy to circulate in the bloodstream. Another example, Abraxane, a nanoparticle formulation consisting of albumin-stabilized paclitaxel, is FDA (Food and Drug Administration) approved for treating breast cancer.20,128
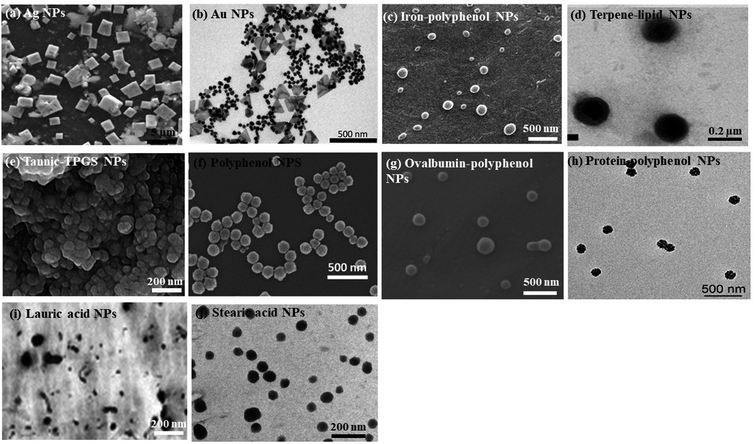
In a nutshell, different nano-formulations utilized for curing various cancers can be exploited for treating one of the deadliest diseases, gastric cancer. Several preclinical studies prove that synthetic and naturally occurring terpenes have therapeutic and chemopreventive effects against cancer. They act by exerting their effects on various developmental stages of the tumor via inhibiting initiation and promotion of carcinogenesis, inducing tumor cell differentiation and apoptosis, and suppressing tumor angiogenesis, invasion and metastasis by regulating various growth factors, transcription factors, and intracellular signaling pathways.129–131 Thus, nanotechnology can be employed for developing new formulations using different herbal secondary metabolites for treating gastric cancer. Several nano-formulations have been made using plant extracts, with metal ions that exhibit antioxidant, anti-cancerous, anti-inflammatory, antiviral, and antibacterial properties.132–135 SEM (scanning electron microscope) and TEM (transmission electron microscope) images of some of the green synthesized nanoparticles are given in Fig. 2. The various applications of green synthesized nanoparticles are given in Fig. 3.
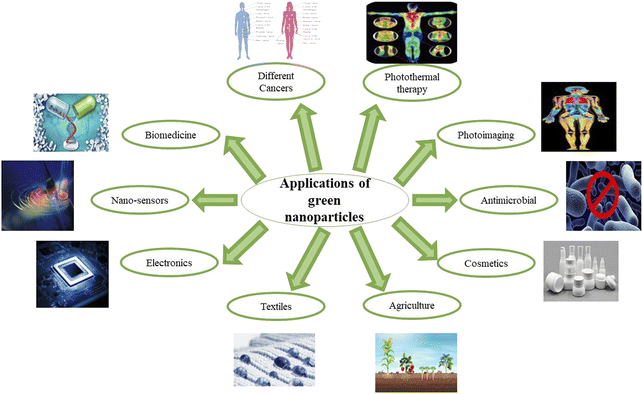 | ||
| Fig. 3 The significant fields in which green synthesized nano-formulations have been used for the past few years. | ||
3.1 FDA-approved drugs for gastric cancer
FDA is a federal agency of the Department of Health and Human Services. It protects public health by assuring the safety and efficacy of human and veterinary drugs, medical devices, food supplies, and biological products. Since the last 50 years, more than 100 anticancer drugs have been approved for various cancers by the FDA. The approval of drugs is based on 3 distinct parameters: overall survival or patient related outcomes, progression-free survival involving the time until cancer worsens or occurs again, and the response rate which involves the percentage of people experiencing tumor shrinkage. The drugs are divided into two categories depending upon their mode of action: cytotoxic or targeted agents. Cytotoxic drugs target the components of the mitotic or DNA replication pathways thus killing rapidly dividing cells. They include mainly alkylating agents, topoisomerase inhibitors or anti-microtubule agents. On the other side the targeted agents interact with the molecular targets involved in the pathways related to growth, progression and spread of cancer, eventually blocking the growth and spread of cancer. This category comprises mainly of signal transduction inhibitors, apoptosis inducers, gene expression modulators, monoclonal antibodies, and hormone therapies.136 For treatment of gastric cancer, some of the mainly consumed anti-gastric cancer drugs are listed in ESI Table 1,† which includes their structure, mechanisms of action, and side effects. A brief description of the first few drugs listed in the table is discussed next. Everolimus is a mTOR inhibitor, which results in G0/G1 arrest by preventing the mTOR mediated phosphorylation of p70S6K and 4E-BP1.137 It also decreases the proliferation and attenuates the production of HIF-1α and VEGF in gastric cancer cells in vitro. Ramucirumab, on the other hand, is a human monoclonal antibody IgG1, which acts by targeting vascular endothelial growth factor receptor-2 (VEGFR-2). It prevents VEGF ligand binding to VEGFR-2 and receptor-mediated pathway activation in the case of endothelial cells. Furthermore, it also results in macrophage inhibition which causes decreased tumor immune infiltration and cytokine-chemokine release which results in decreased tumor growth and proliferation. It is the only FDA approved antiangiogenic agent for gastric or gastroesophageal junction adenocarcinoma.138,139 Another FDA approved drug, trastuzumab, is also a humanized monoclonal antibody which is directed against epidermal growth factor receptor 2 (HER2). It treats multiple oncologic conditions, metastatic gastric cancer being one of them. It acts by binding to the extracellular domain of HER2 and follows various mechanisms including downregulation of the DNA repair pathway and angiogenesis, abrogation of oncogenic cellular signaling, activation of antibody dependent cell-mediated cytotoxicity, and inhibition of extracellular HER2 domain cleavage to trigger tumor-suppressive action.140 For the FDA-approved drugs, the reader is referred to ESI Table 1.†4. Concluding remarks and directions for future research
Gastric cancer cases are reported worldwide, with maximum prevalence in Asia and Latin America; the rate is also high from northern to central America, Europe, and central Africa. But even countries with good economies and advanced medical facilities have not been able to reduce the death rate considerably. The 5-year survival rate in the USA for gastric cancer is 31%, and for the European nations it is around 26%. The situation is similar or worse in countries with weak economies and poor hygiene. Therefore, there is an urgent need and high demand for the development of abundant, effective, and economically favorable medicines. Furthermore, available chemotherapy, including 5-fluorouracil, capecitabine, carboplatin, cisplatin, docetaxel, epirubicin, oxaliplatin, etc., has considerable side effects such as anemia, shortness of breath, nausea, diarrhea, fatigue, loss of appetite, hair loss, etc., which compromise the quality of life of the patient. Therefore, more research is needed to develop suitable treatments/drugs with fewer side effects.Since almost 89% of gastric cancer directly relates to H. pylori infection, the most crucial step in gastric cancer management is to control the infectivity. Plants such as Hedyotis diffusa Willd, Aloe vera, Rhizophora mangle, Astragalus membranaceus, etc., are effective against the pathogen due to the presence of phenolics, terpenes, alkaloids, and polyketides in them. Many of those plants grow in the tropical, subtropical, temperate, and Mediterranean regions of the world and are native to a particular geographical location such as India, China, Japan, South Africa, Iraq, Iran, Vietnam, the USA, and other European countries and show excellent anti-H. pylori activity. Extensive research is needed to invent, purify, characterize, and formulate safer drugs, including natural products. Attention should also be given to the composition, stability, and pharmaceutical efficiency of those formulations as the concentration and the stability of the lead compounds may greatly vary based on many factors, including soil composition, pH, macro and micronutrient content, humidity, porosity, weather, availability of sunlight, etc. In this regard advancement of nanotechnology may play a critical role in maintaining the quality and efficacy of herbal formulations. Many metallic nanoparticles, including silver, gold, selenium, zinc oxide, etc., have anti-cancer properties. Enhanced uptake of nanoparticles by cancer cells has been reported for many cancer lines, including MCF-7 (human breast carcinoma cell line),141 Hep G2 (human liver cancer cell line),142 and AGS (human gastric carcinoma cell line).110 Nanoparticles and these natural compounds may show synergistic anticancer activity by generating various stresses, including oxidative damage, DNA damage, and mitochondrial dysfunctions.
Furthermore, many nanoparticles also show anti H. pylori activity. In addition, due to the high surface area to volume ratio, nanoparticles can interact with and stabilize bioactive polyphenols on their surfaces, enabling the delivery of a large quantity of the drugs at the target site. Cancer cell specific delivery of herbal medicines will make them safer and side effect free. Furthermore, polyphenolic surface-protected ferromagnetic nanoparticles will allow the detection and imaging of the cancer tissue using MRI imaging techniques and help develop an effective therapeutic approach using hyperthermia.
In conclusion, medicinal plants show promise as potential candidates for cancer treatment due to their beneficial properties. Knowledge of active secondary metabolites from extracts of different plant parts having anti-cancerous activities and their mode of action against critical targets paves the way for further research. The current information on various herbs and their modes of action against different diseases can be further exploited to develop more efficient and less harmful drugs. At a minimum, when used with conventional treatments, they can increase the effectiveness of these treatments in fighting cancer. Moreover, isolating active compounds and finding their chemical structure further enables molecular and pharmacological comparison with the currently used chemicals and pharmaceutical products. In the case of H. pylori, which has become resistant to conventional antibiotics, green nano-formulations with high biocompatibility and bactericidal potency can be utilized as a replacement. We conclude this review article by outlining directions for future research on NT (nanotechnology)-enabled herbal formulations for treating cancer, particularly gastric cancer.
We believe that herbal formulations using the unique properties of nanomaterials offer a potentially winning proposition. It is our hope that this review article will spur interest among interdisciplinary teams of researchers in developing safer and more effective treatments for gastric cancer.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
KA acknowledges TIET-VT CEEMS- for a fellowship. DC thanks TIET-VT CEEMS for funding.References
- F. M. Millimouno, J. Dong, L. Yang, J. Li and X. Li, Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature, Cancer Prev. Res., 2014, 7, 1081–1107 CrossRef CAS PubMed.
- M. Esteller, Epigenetics in cancer, N. Engl. J. Med., 2008, 358, 1148–1159 CrossRef CAS PubMed.
- K. I. Block, C. Gyllenhaal, L. Lowe, A. Amedei, A. R. M. Ruhul Amin, A. Amin, K. Aquilano, J. Arbiser, A. Arreola, A. Arzumanyan, S. Salman Ashraf, A. S. Azmi, F. Benencia, D. Bhakta, A. Bilsland, A. Bishayee, S. W. Blain, P. B. Block, C. S. Boosani, T. E. Carey, A. Carnero, M. Carotenuto, S. C. Casey, M. Chakrabarti, R. Chaturvedi, G. Z. Chen, H. Chen, S. Chen, Y. C. Chen, B. K. Choi, M. R. Ciriolo, H. M. Coley, A. R. Collins, M. Connell, S. Crawford, C. S. Curran, C. Dabrosin, G. Damia, S. Dasgupta, R. J. DeBerardinis, W. K. Decker, P. Dhawan, A. M. E. Diehl, J. T. Dong, Q. P. Dou, J. E. Drewa, E. Elkord, B. El-Rayes, M. A. Feitelson, D. W. Felsheru, L. R. Ferguson, C. Fimognari, G. L. Firestone, C. Frezza, H. Fujii, M. M. Fuster, D. Generali, A. G. Georgakilas, F. Gieseler, M. Gilbertson, M. F. Green, B. Grue, G. Guhal, D. Halicka, W. G. Helferich, P. Heneberg, P. Hentosh, M. D. Hirschey, L. J. Hofseth, R. F. Holcombe, K. Honoki, H. Y. Hsu, G. S. Huang, L. D. Jensen, W. G. Jiang, L. W. Jones, P. A. Karpowicz, W. N. Keith, S. P. Kerkar, G. N. Khan, M. Khatami, Y. H. Ko, O. Kucuk, R. J. Kulathinal, N. B. Kumar, B. S. Kwon, A. Leb, M. A. Leab, H. Y. Lee, T. Lichtor, L. T. Lin, J. W. Locasale, B. L. Lokeshwar, V. D. Longo, C. A. Lyssiotis, K. L. MacKenzie, M. Malhotra, M. Marino, M. L. Martinez-Chantar, A. Matheu, C. Maxwellx, E. McDonnell, A. K. Meeker, M. Mehrmohamadi, K. Mehta, G. A. Michelotti, R. M. Mohammad, S. I. Mohammed, D. J. Morre, I. Muqbil, V. Muralidharcq, M. P. Murphy, G. P. Nagaraju, R. Nahta, E. Niccolai, S. Nowsheen, C. Panis, F. Pantano, V. R. Parslow, G. Pawelec, P. L. Pedersen, B. Poore, D. Poudyal, S. Prakash, M. Prince, L. Raffaghello, J. C. Rathmell, W. K. Rathmell, S. K. Ray, J. Reichrath, S. Rezazadeh, D. Ribatti, L. Ricciardiello, R. B. Robeydf, F. Rodierdh, H. P. V. Rupasinghe, G. L. Russo, E. P. Ryan, A. K. Samadi, I. Sanchez-Garcia, A. J. Sanders, D. Santini, M. Sarkar, T. Sasada, N. K. Saxena, R. E. Shackelford, H. M. C. Shantha Kumara, D. Sharma, D. M. Shin, D. Sidransky, M. D. Siegelin, E. Signori, N. Singh, S. Sivanand, D. Sliva, C. Smythe, C. Spagnuolo, D. M. Stafforini, J. Stagg, P. R. Subbarayan, T. Sundin, W. H. Talib, S. K. Thompson, P. T. Tran, H. Ungefroren, M. G. Vander Heiden, V. Venkateswaran, D. S. Vinay, P. J. Vlachostergios, Z. Wang, K. E. Wellen, R. L. Whelan, E. S. Yang, H. Yang, X. Yang, P. Yaswen, C. Yedjou, X. Yin, J. Zhu and M. Zollo, Designing a broad-spectrum integrative approach for cancer prevention and treatment, Semin. Cancer Biol., 2015, 35, S276–S304 CrossRef PubMed.
- F. Bray, J. S. Ren, E. Masuyer and J. Ferlay, Global estimates of cancer prevalence for 27 sites in the adult population in 2008, Int. J. Cancer, 2013, 132, 1133–1145 CrossRef CAS PubMed.
- J. Feng, K. Li, G. Liu, Y. Feng, H. Shi and X. Zhang, Precision hyperthermia-induced miRNA-409–3p upregulation inhibits migration, invasion, and EMT of gastric cancer cells by targeting KLF17, Biochem. Biophys. Res. Commun., 2021, 549, 113–119 CrossRef CAS PubMed.
- L. E. Wroblewski, R. M. Peek and K. T. Wilson, Helicobacter pylori and gastric cancer: Factors that modulate disease risk, Clin. Microbiol. Rev., 2010, 23, 713–739 CrossRef CAS PubMed.
- H. H. Hartgrink, E. P. Jansen, N. C. van Grieken and C. J. van de Velde, Gastric cancer, Lancet, 2009, 374, 477–490 CrossRef PubMed.
- K. D. Crew and A. I. Neugut, Epidemiology of gastric cancer, World J. Gastroenterol., 2006, 12, 354–362 CrossRef PubMed.
- S. M. Mbulaiteye, M. Hisada and E. M. El-Omar, Helicobacter Pylori associated global gastric cancer burden, Front. Biosci., 2009, 14, 1490–1504 CrossRef CAS PubMed.
- İ. Altun and A. Sonkaya, The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy, Iran. J. Public Health, 2018, 47, 1218 Search PubMed.
- M. Orditura, G. Galizia, V. Sforza, V. Gambardella, A. Fabozzi, M. M. Laterza, F. Andreozzi, J. Ventriglia, B. Savastano, A. Mabilia, E. Lieto, F. Ciardiello and F. De Vita, Treatment of gastric cancer, World J. Gastroenterol., 2014, 20, 1635–1649 CrossRef CAS PubMed.
- C. Chong, Y. Wang, A. Fathi, R. Parungao, P. K. Maitz and Z. Li, Skin wound repair: Results of a pre-clinical study to evaluate electropsun collagen–elastin–PCL scaffolds as dermal substitutes, Burns, 2019, 45, 1639–1648 CrossRef PubMed.
- K. Şenol, M. B. Özkan, S. Vural and M. Tez, The role of inflammation in gastric cancer, Adv. Exp. Med. Biol., 2014, 816, 235–257 CrossRef PubMed.
- M. L. Del Prado-Audelo, H. Cortés, I. H. Caballero-Florán, M. González-Torres, L. Escutia-Guadarrama, S. A. Bernal-Chávez, D. M. Giraldo-Gomez, J. J. Magaña and G. Leyva-Gómez, Therapeutic Applications of Terpenes on Inflammatory Diseases, Front. Pharmacol., 2021, 12, 2114 CrossRef PubMed.
- H. Yuan, Q. Ma, L. Ye and G. Piao, The Traditional Medicine and Modern Medicine from Natural Products, Molecules, 2016, 21, 559 CrossRef PubMed.
- M. Jermini, J. Dubois, P. Y. Rodondi, K. Zaman, T. Buclin, C. Csajka, A. Orcurto and L. E. Rothuizen, Complementary medicine use during cancer treatment and potential herb-drug interactions from a cross-sectional study in an academic centre, Sci. Rep., 2019, 9, 1–11 CrossRef CAS PubMed.
- R. J. S. Vega, N. C. Xolalpa, A. J. A. Castro, C. P. González, J. P. Ramos and S. P. Gutiérrez, Terpenes from Natural Products with Potential Anti-Inflammatory Activity, Terpenes and Terpenoids, 2018, pp. 60–85, DOI:10.5772/INTECHOPEN.73215.
- M. Wink, Modes of Action of Herbal Medicines and Plant Secondary Metabolites, Med, 2015, 2, 251–286 CAS.
- M. A. Gómez-Favela, D. U. Santos-Ballardo, M. E. Bergés-Tiznado and D. L. Ambriz-Pérez, Nanoformulations applied to the delivery of terpenes, Phytochem. Nanodelivery Syst. as Potential Biopharm., 2023, pp. 221–256 Search PubMed.
- S. Sim and N. K. Wong, Nanotechnology and its use in imaging and drug delivery (Review), Biomed. Rep., 2021, 14(5), 42 CrossRef CAS PubMed.
- M. Huang, J. J. Lu, M. Q. Huang, J. L. Bao, X. P. Chen and Y. T. Wang, Terpenoids: natural products for cancer therapy, Expert Opin. Invest. Drugs, 2012, 21, 1801–1818 CrossRef CAS PubMed.
- B. Chopra, A. K. Dhingra, K. L. Dhar and K. Nepali, Emerging Role of Terpenoids for the Treatment of Cancer: A Review, Mini-Rev. Med. Chem., 2021, 21, 2300–2336 CrossRef CAS PubMed.
- C. El-Baba, A. Baassiri, G. Kiriako, B. Dia, S. Fadlallah, S. Moodad and N. Darwiche, Terpenoids' anti-cancer effects: focus on autophagy, Apoptosis, 2021, 26, 491–511 CrossRef CAS PubMed.
- Z. Li, X. Wu, J. Li, L. Yao, L. Sun, Y. Shi, W. Zhang, J. Lin, D. Liang and Y. Li, Antitumor activity of celastrol nanoparticles in a xenograft retinoblastoma tumor model, Int. J. Nanomed., 2012, 7, 2389 CrossRef CAS PubMed.
- V. Sanna, J. C. Chamcheu, N. Pala, H. Mukhtar, M. Sechi and I. A. Siddiqui, Nanoencapsulation of natural triterpenoid celastrol for prostate cancer treatment, Int. J. Nanomed., 2015, 10, 6835–6846 CrossRef CAS PubMed.
- N. Hasima and B. B. Aggarwal, Cancer-linked targets modulated by curcumin, Int. J. Biochem. Mol. Biol., 2012, 3, 328 CAS.
- E. Aleebrahim-Dehkordy, H. Nasri, A. Baradaran, P. Nasri, M. R. Tamadon, M. Hedaiaty, S. Beigrezaei and M. Rafieian-Kopaei, Medicinal Plants, Effective Plant Compounds (Compositions) and their Effects on Stomach Cancer, Int. J. Prev. Med., 2017, 8, 96 Search PubMed.
- Y. Li, S. Li, X. Meng, R. Gan, J. Zhang and H. Li, Dietary natural products for prevention and treatment of breast cancer, Nutrients, 2017, 9(7), 728 CrossRef PubMed.
- J. Zheng, Y. Zhou, Y. Li, D. Xu, S. Li and H. Li, Spices for prevention and treatment of cancers, Nutrients, 2016, 8(8), 495 CrossRef PubMed.
- X. Fang, J. Wei, X. He, P. An, H. Wang, L. Jiang, D. Shao, H. Liang, Y. Li, F. Wang and J. Min, Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies, Eur. J. Cancer, 2015, 51, 2820–2832 CrossRef PubMed.
- P. Zou, Y. Xia, J. Ji, W. Chen, J. Zhang, X. Chen, V. Rajamanickam, G. Chen, Z. Wang, L. Chen, Y. Wang, S. Yang and G. Liang, Piperlongumine as a direct TrxR1 inhibitor with suppressive activity against gastric cancer, Cancer Lett., 2016, 375, 114–126 CrossRef CAS PubMed.
- T. Kim, S. Lee, M. Kim, C. Cheon and S. Ko, Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells, Cell Death Dis., 2018, 9(9), 875 CrossRef PubMed.
- D. Cox-Georgian, N. Ramadoss, C. Dona and C. Basu, Med. Plants, 2019, 333–359 Search PubMed.
- A. Masyita, R. Mustika Sari, A. Dwi Astuti, B. Yasir, N. Rahma Rumata, T. Bin Emran, F. Nainu and J. Simal-Gandara, Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives, Food Chem.: X, 2022, 13, 100217 CAS.
- J. S. S. Quintans, S. Shanmugam, L. Heimfarth, A. A. S. Araújo, J. R. G. d. S. Almeida, L. Picot and L. J. Quintans-Júnior, Monoterpenes modulating cytokines - A review, Food Chem. Toxicol., 2019, 123, 233–257 CrossRef CAS PubMed.
- M. Jakaria, D. Y. Cho, M. E. Haque, G. Karthivashan, I. S. Kim, P. Ganesan and D. K. Choi, Neuropharmacological potential and delivery prospects of thymoquinone for neurological disorders, Oxid. Med. Cell. Longevity, 2018, 1209801 Search PubMed.
- M. Ashrafizadeh, Z. Ahmadi, R. Mohammadinejad, N. Kaviyani and S. Tavakol, Monoterpenes modulating autophagy: A review study, Basic Clin. Pharmacol. Toxicol., 2020, 126, 9–20 CrossRef CAS PubMed.
- M. V. Sobral, A. L. Xavier, T. C. Lima and D. P. De Sousa, Antitumor Activity of Monoterpenes Found in Essential Oils, Sci. World J., 2014, 953451 Search PubMed.
- M. De Vincenzi, A. Stammati, A. De Vincenzi and M. Silano, Constituents of aromatic plants: carvacrol, Fitoterapia, 2004, 75, 801–804 CrossRef CAS PubMed.
- L. A. Sampaio, L. T. S. Pina, M. R. Serafini, D. S. Tavares and A. G. Guimarães, Antitumor Effects of Carvacrol and Thymol: A Systematic Review, Front. Pharmacol., 2021, 12, 1673 Search PubMed.
- A. Mondal, S. Bose, K. Mazumder and R. Khanra, Carvacrol (Origanum vulgare): Therapeutic Properties and Molecular Mechanisms, Adv. Struct. Mater., 2021, 140, 437–462 Search PubMed.
- Z. Taghizadeh, S. Rakhshani, V. Jahani, O. Rajabi, H. M. Haghighi and M. Abbaspour, Preparation and in vitro characterization of carvacrol pellets by combination of liquisolid technique and extrusion-spheronization, J. Drug Delivery Sci. Technol., 2021, 61, 102232 CrossRef CAS.
- A. Günes-Bayir, H. S. Kiziltan, A. Kocyigit, E. M. Güler, E. Karataş and A. Toprak, Effects of natural phenolic compound carvacrol on the human gastric adenocarcinoma (AGS) cells in vitro, Anticancer Drugs, 2017, 28, 522–530 CrossRef PubMed.
- H. Yang, G. Liu, H. Zhao, X. Dong and Z. Yang, Inhibiting the JNK/ERK signaling pathway with geraniol for attenuating the proliferation of human gastric adenocarcinoma AGS cells, J. Biochem. Mol. Toxicol., 2021, 35, e22818 CAS.
- S. Sharma, P. Gao and V. E. Steele, Quantitative Morphometry of Respiratory Tract Epithelial Cells as a Tool for Testing Chemopreventive Agent Efficacy, Anticancer Res., 2010, 30, 737–742 CAS.
- L. Lv, N. Yang, Y. Cao, J. Dang, L. Cheng, M. A. El-Sheikh and Y. Zhang, d-Carvone inhibits the JAK/STAT3 signaling pathway and induced the apoptotic cell death in the human gastric cancer AGS cells, J. Biochem. Mol. Toxicol., 2021, 35, e22746 CAS.
- R. Javed, M. A. Hanif, R. Rehman, M. Hanif and B. T. Tung, Med. Plants South Asia Nov. Sources Drug Discov., Caraway, 2020, pp. 87–100 Search PubMed.
- Y. Xiang, Q. Zhang, S. Wei, C. Huang, Z. Li and Y. Gao, Paeoniflorin: a monoterpene glycoside from plants of Paeoniaceae family with diverse anticancer activities, J. Pharm. Pharmacol., 2020, 72, 483–495 CrossRef CAS PubMed.
- J. Zhang, K. Yu, X. Han, L. Zhen, M. Liu, X. Zhang, Y. Ren and J. Shi, Paeoniflorin influences breast cancer cell proliferation and invasion via inhibition of the Notch-1 signaling pathway, Mol. Med. Rep., 2018, 17, 1321–1325 CAS.
- A. Pasdaran and A. Hamedi, Natural Products as Source of New Antimicrobial Compounds for Skin Infections, Microbiol. Ski. Soft Tissue, Bone Jt. Infect., 2017, pp. 223–253 Search PubMed.
- J. Bohlmann and J. Gershenzon, Old substrates for new enzymes of terpenoid biosynthesis, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10402–10403 CrossRef CAS PubMed.
- M. T. Islam, C. B. Da Silva, M. V. O. B. De Alencar, M. F. C. J. Paz, F. R. D. C. Almeida and A. A. D. C. Melo-Cavalcante, Diterpenes: Advances in Neurobiological Drug Research, Phyther. Res., 2016, 30, 915–928 CrossRef PubMed.
- G. B. Lockwood, GAS CHROMATOGRAPHY | Terpenoids, Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2013, DOI:10.1016/B978-0-12-409547-2.04768-5.
- J. Rysz, M. Banach, R. A. Stolarek, J. Pasnik, A. C. Rysz, R. Koktysz, M. Piechota and Z. Baj, Serum matrix metalloproteinases MMP-2 and MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy, J. Nephrol., 2007, 20, 444–452 CAS.
- L. Dai, G. Wang and W. Pan, Andrographolide inhibits proliferation and metastasis of SGC7901 gastric cancer cells, BioMed Res. Int., 2017 DOI:10.1155/2017/6242103.
- M. Hidalgo, A. Romero, J. Figueroa, P. Cortes, I. I. Concha, J. L. Hancke and R. A. Burgos, Andrographolide interferes with binding of nuclear factor-κB to DNA in HL-60-derived neutrophilic cells, Br. J. Pharmacol., 2005, 144, 680–686 CrossRef CAS PubMed.
- A. Allegra, A. Tonacci, G. Pioggia, C. Musolino and S. Gangemi, Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials, Nutrients, 2020, 12, 1–25 CrossRef PubMed.
- S. Shrestha, Y. W. Song, H. Kim, D. S. Lee and S. K. Cho, Sageone, a diterpene from Rosmarinus officinalis, synergizes with cisplatin cytotoxicity in SNU-1 human gastric cancer cells, Phytomedicine, 2016, 23, 1671–1679 CrossRef CAS PubMed.
- T. J. Fan, L. H. Han, R. S. Cong and J. Liang, Caspase Family Proteases and Apoptosis, Acta Biochim. Biophys. Sin., 2005, 37, 719–727 CrossRef CAS PubMed.
- S. Bahri, S. Jameleddine and V. Shlyonsky, Relevance of carnosic acid to the treatment of several health disorders: Molecular targets and mechanisms, Biomed. Pharmacother., 2016, 84, 569–582 CrossRef CAS PubMed.
- W. El-Huneidi, K. Bajbouj, J. S. Muhammad, A. Vinod, J. Shafarin, G. Khoder, M. A. Saleh, J. Taneera and E. Abu-Gharbieh, Carnosic Acid Induces Apoptosis and Inhibits Akt/mTOR Signaling in Human Gastric Cancer Cell Lines, Pharm, 2021, 14, 230 CAS.
- K. T. Liby, M. M. Yore and M. B. Sporn, Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer, Nat. Rev. Cancer, 2007, 7, 357–369 CrossRef CAS PubMed.
- M. C. Yin, Anti-glycative potential of triterpenes: A mini-review, Biomedicine, 2012, 2, 2–9 CrossRef CAS.
- S. Jäger, H. Trojan, T. Kopp, M. N. Laszczyk and A. Scheffler, Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts, Molecules, 2009, 14, 2016–2031 CrossRef PubMed.
- S. S. Yao, L. Han, Z. Bin Tian, Y. N. Yu, Q. Zhang, X. Y. Li, T. Mao and L. Yang, Celastrol inhibits growth and metastasis of human gastric cancer cell MKN45 by down-regulating microRNA-21, Phyther. Res., 2019, 33, 1706–1716 CrossRef CAS PubMed.
- H. W. Lee, K. S. Bin Jang, H. J. Choi, A. Jo, J. H. Cheong and K. H. Chun, Celastrol inhibits gastric cancer growth by induction of apoptosis and autophagy, BMB Rep., 2014, 47, 697 CrossRef PubMed.
- X. Chen, Y. Zhao, W. Luo, S. Chen, F. Lin, X. Zhang, S. Fan, X. Shen, Y. Wang and G. Liang, Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells, Theranostics, 2020, 10, 10290 CrossRef CAS PubMed.
- A. Petronellia, G. Pannitterib and U. Testaa, Triterpenoids as new promising anticancer drugs, Anticancer Drugs, 2009, 20, 880–892 CrossRef PubMed.
- M. W. Lin, A. S. Lin, D. C. Wu, S. S. W. Wang, F. R. Chang, Y. C. Wu and Y. Bin Huang, Euphol from Euphorbia tirucalli selectively inhibits human gastric cancer cell growth through the induction of ERK1/2-mediated apoptosis, Food Chem. Toxicol., 2012, 50, 4333–4339 CrossRef CAS PubMed.
- H. Wang, X. Ge, H. Qu, N. Wang, J. Zhou, W. Xu, J. Xie, Y. Zhou, L. Shi, Z. Qin, Z. Jiang, W. Yin and J. Xia, Glycyrrhizic Acid Inhibits Proliferation of Gastric Cancer Cells by Inducing Cell Cycle Arrest and Apoptosis, Cancer Manage. Res., 2020, 12, 2853 CrossRef CAS PubMed.
- Y. Chen, X. Wu, C. Liu and Y. Zhou, Betulinic acid triggers apoptosis and inhibits migration and invasion of gastric cancer cells by impairing EMT progress, Cell Biochem. Funct., 2020, 38, 702 CrossRef CAS PubMed.
- H. Wang, H. Wang, L. Ge, Y. Zhao, K. Zhu, Z. Chen, Q. Wu, Y. Xin and J. Guo, Betulinic acid targets drug-resistant human gastric cancer cells by inducing autophagic cell death, suppresses cell migration and invasion, and modulates the ERK/MEK signaling pathway, Acta Biochim. Pol., 2022, 69, 25–30 CAS.
- B. M. Fraga, Natural sesquiterpenoids, Nat. Prod. Rep., 2008, 25, 1180–1209 RSC.
- Z. Lorigooini, F. Jamshidi-kia and S. Dodman, Analysis of sesquiterpenes and sesquiterpenoids, Recent Adv. Nat. Prod. Anal., 2020, 289–312 Search PubMed.
- K. Ishnava and J. Chauhan, Anticariogenic and phytochemical evaluation of Eucalyptus globules Labill, Saudi J. Biol. Sci., 2013, 20, 69–74 CrossRef CAS PubMed.
- J. Buckle, Basic Plant Taxonomy, Basic Essential Oil Chemistry, Extraction, Biosynthesis, and Analysis, Clin. Aromather., 2015, pp. 37–72 Search PubMed.
- M. Chadwick, H. Trewin, F. Gawthrop and C. Wagstaff, Sesquiterpenoids Lactones: Benefits to Plants and People, Int. J. Mol. Sci., 2013, 14, 12780–12805 CrossRef PubMed.
- D. Wang, Y. Li, P. Cui, Q. Zhao, B. bo Tan, Z. dong Zhang, Y. Liu and N. Jia, Zerumbone induces gastric cancer cells apoptosis: Involving cyclophilin A, Biomed. Pharmacother., 2016, 83, 740–745 CrossRef CAS PubMed.
- K. Tsuboi, Y. Matsuo, T. Shamoto, T. Shibata, S. Koide, M. Morimoto, S. Guha, B. Sung, B. B. Aggarwal, H. Takahashi and H. Takeyama, Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer, Oncol. Rep., 2014, 31, 57–64 CrossRef CAS PubMed.
- P. Li, X. Zhou, W. Sun, W. Sheng, Y. Tu, Y. Yu, J. Dong, B. Ye, Z. Zheng and M. Lu, Elemene Induces Apoptosis of Human Gastric Cancer Cell Line BGC-823 via Extracellular Signal-Regulated Kinase (ERK) 1/2 Signaling Pathway, Med. Sci. Monit., 2017, 23, 809 CrossRef CAS PubMed.
- T. Tan, J. Li, R. Luo, R. Wang, L. Yin, M. Liu, Y. Zeng, Z. Zeng and T. Xie, Recent Advances in Understanding the Mechanisms of Elemene in Reversing Drug Resistance in Tumor Cells: A Review, Molecules, 2021, 26(19), 5792 CrossRef CAS PubMed.
- J. Wen, K. You, S. Lee, C. Song and D. Kim, Oxidative stress-mediated apoptosis: the anticancer effect of the sesquiterpene lactone parthenolide, J. Biol. Chem., 2002, 277, 38954–389564 CrossRef CAS PubMed.
- L. J. Zhao, Y. H. Xu and Y. Li, Effect of parthenolide on proliferation and apoptosis in gastric cancer cell line SGC7901, J. Dig. Dis., 2009, 10, 172–180 CrossRef CAS PubMed.
- M. Liu, Y. Yang, D. Liu, Y. Cao and Y. Li, Parthenolide increases the sensitivity of gastric cancer cells to chemotherapy, J. Tradit. Chin. Med., 2020, 40, 908–916 Search PubMed.
- W. H. Talib and L. T. Al Kury, Effect of parthenolide on proliferation and apoptosis in gastric cancer cell line SGC7901, Biomed. Pharmacother., 2018, 107, 1488–1495 CrossRef CAS PubMed.
- T. Efferth, H. Dunstan, A. Sauerbrey, H. Miyachi and C. R. Chitambar, The anti-malarial artesunate is also active against cancer, Int. J. Oncol., 2021, 18, 767–773 Search PubMed.
- H. T. Zhang, Y. L. Wang, J. Zhang and Q. X. Zhang, Artemisinin inhibits gastric cancer cell proliferation through upregulation of p53, Tumor Biol., 2014, 35, 1403–1409 CrossRef CAS PubMed.
- K. Can Baser, Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils, Curr. Pharm. Des., 2008, 14, 3106–3119 CrossRef PubMed.
- M. Sharifi-Rad, E. M. Varoni, M. Iriti, M. Martorell, W. N. Setzer, M. del Mar Contreras, B. Salehi, A. Soltani-Nejad, S. Rajabi, M. Tajbakhsh and J. Sharifi-Rad, Carvacrol and human health: A comprehensive review, Phytother. Res., 2018, 32, 1675–1687 CrossRef CAS PubMed.
- N. Ortiz, M. F. Jiménez, C. Chaverri, J. F. Cicció and C. Díaz, Effect on cell growth, viability and migration of geraniol and geraniol-containing essential oil from Lippia alba (Verbenaceae) on gastric carcinoma cells, J. Essent. Oil Res., 2021, 34, 65–76 CrossRef.
- A. Bouyahya, H. Mechchate, T. Benali, R. Ghchime, S. Charfi, A. Balahbib, P. Burkov, M. A. Shariati, J. M. Lorenzo and N. El Omari, Health Benefits and Pharmacological Properties of Carvone, Biomolecules, 2021, 11(12), 1803 CrossRef CAS PubMed.
- J. J. Wu, W. Y. Sun, S. S. Hu, S. Zhang and W. Wei, A standardized extract from Paeonia lactiflora and Astragalus membranaceus induces apoptosis and inhibits the proliferation, migration and invasion of human hepatoma cell lines, Int. J. Oncol., 2013, 43, 1643–1651 CrossRef PubMed.
- S. Fang, W. Zhu, Y. Zhang, Y. Shu and P. Liu, Paeoniflorin modulates multidrug resistance of a human gastric cancer cell line via the inhibition of NF-κB activation, Mol. Med. Rep., 2012, 5, 351–356 CAS.
- Q. Zhang, Y. Yuan, J. Cui, T. Xiao and D. Jiang, Paeoniflorin inhibits proliferation and invasion of breast cancer cells through suppressing Notch-1 signaling pathway, Biomed. Pharmacother., 2016, 78, 197–203 CrossRef CAS PubMed.
- M. Majdi, Q. Liu, G. Karimzadeh, M. A. Malboobi, J. Beekwilder, K. Cankar, R. De Vos, S. Todorović, A. Simonović and H. Bouwmeester, Biosynthesis and localization of parthenolide in glandular trichomes of feverfew (Tanacetum parthenium L. Schulz Bip.), Phytochemistry, 2011, 72, 1739–1750 CrossRef CAS PubMed.
- N. K. B. K. Ikram and H. T. Simonsen, A Review of Biotechnological Artemisinin Production in Plants, Front. Plant Sci., 2017, 1966 CrossRef PubMed.
- H. C. Lai, N. P. Singh and T. Sasaki, Development of artemisinin compounds for cancer treatment, Invest. New Drugs, 2012, 31, 230–246 CrossRef PubMed.
- M. Geethangili, Y. K. Rao, S. H. Fang and Y. M. Tzeng, Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in Jurkat cells, Phyther. Res., 2008, 22, 1336–1341 CrossRef CAS PubMed.
- S. H. Venkatesha and K. D. Moudgil, Celastrol and Its Role in Controlling Chronic Diseases, Adv. Exp. Med. Biol., 2016, 928, 267–289 CrossRef CAS PubMed.
- G. Kuttan, P. Pratheeshkumar, K. A. Manu and R. Kuttan, Inhibition of tumor progression by naturally occurring terpenoids, Pharm. Biol., 2011, 49, 995–1007 CrossRef CAS PubMed.
- H. Hibasami, H. Iwase, K. Yoshioka and H. Takahashi, Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells, Int. J. Mol. Med., 2005, 16, 233–236 CAS.
- S. Thirugnanam, L. Xu, K. Ramaswamy and M. Gnanasekar, Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP, Oncol. Rep., 2008, 20, 1387–1392 CAS.
- C. Jin, K. Wang, A. Oppong-Gyebi and J. Hu, Application of Nanotechnology in Cancer Diagnosis and Therapy - A Mini-Review, Int. J. Med. Sci., 2020, 17, 2964 CrossRef CAS PubMed.
- J. Hu, W. Huang, S. Huang, Q. Zhuge, K. Jin, Y. Zhao and S.-V. Berlin, Magnetically active Fe3O4 nanorods loaded with tissue plasminogen activator for enhanced thrombolysis, Nano Res., 2016, 9, 2652–2661 CrossRef CAS.
- Z. Rafiq, P. Patel, S. Kumar, H. S. Sofi, J. Macossay and F. A. Sheikh, Advancements of Nanotechnology in Diagnostic Applications, Appl. Nanotechnol. Biomed. Sci., 2020, 1–15 Search PubMed.
- M. Kumar Teli, S. Mutalik and G. K. Rajanikant, Nanotechnology and Nanomedicine: Going Small Means Aiming Big, Curr. Pharm. Des., 2010, 16, 1882–1892 CrossRef PubMed.
- G. Pal, P. Rai and A. Pandey, Green synthesis of nanoparticles: A greener approach for a cleaner future, Green Synth. Charact. Appl. Nanoparticles, 2019, pp. 1–26 Search PubMed.
- K. Bloch, K. Pardesi, C. Satriano and S. Ghosh, Bacteriogenic Platinum Nanoparticles for Application in Nanomedicine, Front. Chem., 2021, 9 DOI:10.3389/FCHEM.2021.624344.
- B. Mousavi, F. Tafvizi and S. Z. Bostanabad, Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line, Artif. Cells Nanomed. Biotechnol., 2018, 46, 499–510 CrossRef CAS PubMed.
- S. Aslany, F. Tafvizi and V. Naseh, Characterization and evaluation of cytotoxic and apoptotic effects of green synthesis of silver nanoparticles using Artemisia Ciniformis on human gastric adenocarcinoma, Mater. Today Commun., 2020, 24, 101011 CrossRef CAS.
- M. Shirzadi-Ahodashti, S. Mortazavi-Derazkola and M. A. Ebrahimzadeh, Biosynthesis of noble metal nanoparticles using crataegus monogyna leaf extract (CML@X-NPs, X= Ag, Au): Antibacterial and cytotoxic activities against breast and gastric cancer cell lines, Surf. Interfaces, 2020, 21, 100697 CrossRef CAS.
- C. Li, Y. Wang, H. Zhang, M. Li, Z. Zhu and Y. Xue, An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized gold nanoparticles using Cardiospermum halicacabum on AGS gastric carcinoma cells, Int. J. Nanomed., 2019, 14, 951 CrossRef CAS PubMed.
- M. Naseer, R. Ramadan, J. Xing and N. A. Samak, Facile green synthesis of copper oxide nanoparticles for the eradication of multidrug resistant Klebsiella pneumonia and Helicobacter pylori biofilms, Int. Biodeterior. Biodegrad., 2021, 159, 105201 CrossRef CAS.
- Z. Yun, A. Chinnathambi, S. A. Alharbi and Z. Jin, Biosynthesis of gold nanoparticles using Vetex negundo and evaluation of pro-apoptotic effect on human gastric cancer cell lines, J. Photochem. Photobiol., B, 2020, 203, 111749 CrossRef CAS PubMed.
- D. Sharda, K. Attri, P. Kaur and D. Choudhury, Protection of lead-induced cytotoxicity using paramagnetic nickel-insulin quantum clusters, RSC Adv., 2021, 11, 24656–24668 RSC.
- P. Kaur and D. Choudhury, Functionality of receptor targeted zinc-insulin quantum clusters in skin tissue augmentation and bioimaging, J. Drug Targeting, 2021, 29, 541–550 CrossRef CAS PubMed.
- S. Tran, P. DeGiovanni, B. Piel and P. Rai, Cancer nanomedicine: a review of recent success in drug delivery, Clin. Transl. Med., 2017, 6(1) DOI:10.1186/S40169-017-0175-0.
- S. Y. Lee and R. L. Mahajan, A facile method for coal to graphene oxide and its application to a biosensor, Carbon, 2021, 181, 408–420 CrossRef CAS.
- D. Choudhury, A. Ganguli, D. G. Dastidar, B. R. Acharya, A. Das and G. Chakrabarti, Apigenin shows synergistic anticancer activity with curcumin by binding at different sites of tubulin, Biochimie, 2013, 95, 1297–1309 CrossRef CAS PubMed.
- D. Choudhury, A. Das, A. Bhattacharya and G. Chakrabarti, Aqueous extract of ginger shows antiproliferative activity through disruption of microtubule network of cancer cells, Food Chem. Toxicol., 2010, 48, 2872–2880 CrossRef CAS PubMed.
- P. Kaur, S. Ghosh, A. Bhowmick, K. Gadhave, S. Datta, A. Ghosh, N. Garg, R. L. Mahajan, B. Basu and D. Choudhury, Bacterioboat-A novel tool to increase the half-life period of the orally administered drug, Sci. Adv., 2022, 8 DOI:10.1126/SCIADV.ABH1419.
- C. Medina, M. J. Santos-Martinez, A. Radomski, O. I. Corrigan and M. W. Radomski, Nanoparticles: pharmacological and toxicological significance, Wiley Online Libr., 2007, vol. 150, pp. 552–558 Search PubMed.
- S. A. Wickline and G. M. Lanza, Nanotechnology for molecular imaging and targeted therapy, Circulation, 2003, 107, 1092–1095 CrossRef PubMed.
- S. Lanone and J. Boczkowski, Biomedical Applications and Potential Health Risks of Nanomaterials: Molecular Mechanisms, Curr. Mol. Med., 2006, 6, 651–663 CrossRef CAS PubMed.
- J. Hu, S. Huang, L. Zhu, W. Huang, Y. Zhao, K. Jin and Q. Zhuge, Tissue plasminogen activator-porous magnetic microrods for targeted thrombolytic therapy after ischemic stroke, ACS Appl. Mater. Interfaces, 2018, 10, 32988–32997 CrossRef CAS PubMed.
- V. Chaturvedi, A. Singh, V. K. Singh and M. P. Singh, Cancer nanotechnology: a new revolution for cancer diagnosis and therapy, Curr. Drug Metab., 2019, 20, 416–429 CrossRef CAS PubMed.
- N. Ochekpe, P. Olorunfemi and N. C. Ngwuluka, Nanotechnology and drug delivery part 1: background and applications, Trop. J. Pharm. Res., 2009, 8, 265–274 CAS.
- A. J. Montero, B. Adams, C. M. Diaz-Montero and S. Glück, Nab-paclitaxel in the treatment of metastatic breast cancer: A comprehensive review, Expert Rev. Clin. Pharmacol., 2011, 4, 329–334 CrossRef CAS PubMed.
- K. Liby, M. Yore and M. B. Sporn, Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer, Nat. Rev. Cancer, 2007, 7, 357–369 CrossRef CAS PubMed.
- J. M. Patlolla and C. V. Rao, Triterpenoids for cancer prevention and treatment: current status and future prospects, Curr. Pharm. Biotechnol., 2012, 13, 147–155 CAS.
- J. Liu, Oleanolic acid and ursolic acid: Research perspectives, J. Ethnopharmacol., 2005, 100, 92–94 CrossRef CAS PubMed.
- G. Marslin, K. Siram, Q. Maqbool, R. K. Selvakesavan, D. Kruszka, P. Kachlicki and G. Franklin, Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles, Mater., 2018, 11 DOI:10.3390/MA11060940.
- K. B. Narayanan and N. Sakthivel, Biological synthesis of metal nanoparticles by microbes, Adv. Colloid Interface Sci., 2010, 156, 1–13 CrossRef CAS PubMed.
- V. Kumar and S. K. Yadav, Plant-mediated synthesis of silver and gold nanoparticles and their applications, J. Chem. Technol. Biotechnol., 2009, 84, 151–157 CrossRef CAS.
- R. G. Haverkamp and A. T. Marshall, The mechanism of metal nanoparticle formation in plants: limits on accumulation, J. Nanopart. Res., 2008, 116, 1453–1463 Search PubMed.
- J. Sun, Q. Wei, Y. Zhou, J. Wang, Q. Liu and H. Xu, A systematic analysis of FDA-approved anticancer drugs, BMC Syst. Biol., 2017, 11 DOI:10.1186/S12918-017-0464-7.
- E. Y. Chen, V. Raghunathan and V. Prasad, An Overview of Cancer Drugs Approved by the US Food and Drug Administration Based on the Surrogate End Point of Response Rate, JAMA Intern. Med., 2019, 179, 915–921 CrossRef PubMed.
- S. J. Casak, I. Fashoyin-Aje, S. J. Lemery, L. Zhang, R. Jin, H. Li, L. Zhao, H. Zhao, H. Zhang, H. Chen, K. He, M. Dougherty, R. Novak, S. Kennett, S. Khasar, W. Helms, P. Keegan and R. Pazdur, FDA Approval Summary: Ramucirumab for Gastric Cancer, Clin. Cancer Res., 2015, 21, 3372–3376 CrossRef CAS PubMed.
- M. Javle, E. C. Smyth and I. Chau, Ramucirumab: successfully targeting angiogenesis in gastric cancer, Clin. Cancer Res., 2014, 20, 5875–5881 CrossRef CAS PubMed.
- N. Mohan, J. Jiang, M. Dokmanovic and W. J. Wu, Trastuzumab-mediated cardiotoxicity: current understanding, challenges, and frontiers, Antibiot. Ther., 2018, 1, 13–17 CAS.
- S. R. Abulateefeh, S. G. Spain, K. J. Thurecht, J. W. Aylott, W. C. Chan, M. C. Garnett and C. Alexander, Enhanced uptake of nanoparticle drug carriers via a thermoresponsive shell enhances cytotoxicity in a cancer cell line, Biomater. Sci., 2013, 1, 434–442 RSC.
- J. Zhao and M. H. Stenzel, Entry of Nanoparticles into Cells: the Importance of Nanoparticle Properties, Polym. Chem., 2018, 9, 259–272 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2su00137c |
| This journal is © The Royal Society of Chemistry 2023 |