 Open Access Article
Open Access ArticleSurfactant-free synthesis of metal and metal oxide nanomaterials: a perspective
Siavash
Iravani

Faculty of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran. E-mail: siavashira@gmail.com
First published on 6th December 2022
Abstract
Metal and metal oxide nanomaterials have attracted much interest in medical, pharmaceutical, biological, biomedical, and catalytic applications due to their high surface-to-volume ratio and fascinating physicochemical properties. To date, a wide variety of techniques with different advantages and limitations/challenges have been introduced for the preparation of metal and metal oxide nanomaterials. In this context, the corrosion, purity, and stability of nanomaterials as well as the controllability and repeatability of synthesis techniques are important challenging issues. Metal and metal oxide nanoparticles with different sizes and morphologies are prone to loss of reactivity, since they may precipitate or aggregate as bulk metals; thus different stabilizers such as functionalized polymers, dendrimers, inorganic solids (e.g., carbon, metal oxides, sol–gel clays, and zeolites), ligands (e.g., pincer ligands), or ionic surfactants are typically required in their fabrication. Nowadays, several surfactant-free strategies such as laser synthesis, mono-alcohol fabrication, the Co4Cat process, and microplasma-based techniques have been introduced for synthesizing metal and metal oxide nanoparticles with the benefits of cost-effectiveness, simplicity, and environmentally-benign properties, avoiding the utilization of toxic additives or surfactants. However, the optimization of synthesis/reaction conditions, the controllability of size and morphology, stability, and large-scale/commercial production of nanomaterials ought to be comprehensively explored. Herein, the most recent developments pertaining to the surfactant-free synthesis of metal and metal oxide nanomaterials are deliberated, with a focus on important challenges, opportunities, and future perspectives.
Sustainability spotlightThere is a demand for developing safer and sustainable synthesis methods, eliminating the arduousness and complications of often used physicochemical methods. Despite the widespread utilization of surfactants in different synthesis processes, they are actually not required to develop a range of nanomaterials. Avoiding the utilization of surfactants greatly simplifies the production of nanomaterials but also offers cost-effectiveness, simplicity, and environmentally-benign properties. This review aligns with the UN's Sustainable Development Goals, including responsible consumption and production to manage the utilization of toxic chemicals/additives and all wastes throughout their life cycle, in accordance with agreed international frameworks, and to significantly reduce their release into air, water and soil in order to minimize their adverse impacts on human health and the environment. |
1. Introduction
Studies have focused on various strategies for synthesizing metal and metal oxide nanoparticles (NPs) with versatile environmental and biomedical applications.1–6 For instance, colloidal surfactant-free synthesized precious metal nanomaterials have been employed as suitable electrocatalysts for different electrochemical reactions.1 Overall, numerous bottom-up and top-down methods have been introduced for manufacturing metal and metal oxide nanomaterials, including wet chemical techniques, hydrothermal synthesis, templating methods, thermal decomposition, pulsed laser ablation, microwave-assisted synthesis, chemical vapor deposition, combustion methods, gas phase techniques, sol–gel approaches, and solvothermal synthesis.7–14 Notably, synthesis strategies and conditions along with the post-production processes such as isolation/purification, washing, and storage conditions can significantly affect the properties and functionality of these nanomaterials.15,16 In this context, surfactants, stabilizers, ligands, and capping agents such as polyvinyl alcohol, polyvinylpyrrolidone, cetrimonium bromide, etc.17–19 have been widely employed for stabilizing the nanomaterials. One of the mostly applied techniques is colloidal synthesis in which different surfactants have been deployed; these surfactants can negatively affect the properties of nanomaterials such as electrochemical and catalytic features, restricting their catalytic applications by blocking the active surfaces.20Sustainable synthesis techniques demonstrate attractive advantages such as eco-friendliness, mild reaction conditions, less harmful/toxic ingredients, cost-effectiveness, and low power/time consumption.21–25 Indeed, there is a demand for developing safer and sustainable synthesis methods, eliminating the arduousness and complications of often used physicochemical methods.19,26–28 Despite the widespread utilization of surfactants in different synthesis processes, they are actually not required to develop a range of nanomaterials. Avoiding the utilization of surfactants greatly simplifies the production of nanomaterials but also offers cost-effectiveness, simplicity, and environmentally-benign properties. Surfactants with severe environmental impacts can adversely affect water and aquatic microbial populations, diminish the photochemical energy conversion efficiency of plants, and damage aquatic life.29 Thus, the removal of surfactants is highly demanded to provide sustainable synthesis of nanomaterials, avoiding the utilization of materials derived from petroleum resources.30,31 For instance, some synthesis techniques such as shape-selective synthesis of gold (Au) nanoprisms typically required either toxic surfactants or time-consuming purification processes, restricting their functionality and applicability.32 Ramírez-Jiménez et al.32 reported the surfactant-free fabrication and scalable purification of triangular Au nanoprisms via a precipitation technique utilizing a non-toxic glutathione ligand, avoiding the utilization of toxic surfactants/additives and bottleneck purification processes. Despite the sustainability benefits of this technique, the yield of production was also increased.32 In another study, Nb2O5/graphene nanocomposites were synthesized without any surfactants via a microwave irradiation method. These nanocomposites exhibited improved electrochemical conductivity, cycle stability, and specific capacitance.33 On the other hand, after the preparation of NPs using surfactants, they should be removed by applying additional processes such as ozone, heat, chemical, and electrochemical treatments, leading to the need for more steps in the synthesis of nanomaterials and reducing the repeatability of the production process in addition to increasing the cost and reducing the chance of up-scalability.34,35 Thus, there is a vital need for designing simple and reproducible surfactant-free techniques with up-scalable potential for the synthesis of nanomaterials.20,36,37 Several surfactant-free techniques such as laser, polyol, mono-alcohol, plasma, and microwave synthesis techniques have been introduced to avoid the utilization of toxic additives or surfactants, paving a way for reducing the complexity and enhancing the eco-friendliness (Table 1).38–41 Herein, the most recent advances regarding the applications of surfactant-free techniques for manufacturing metal and metal oxide nanomaterials are deliberated, focusing on important challenges, benefits, and future directions. This review aligns with the UN's Sustainable Development Goals, including responsible consumption and production to manage the utilization of toxic chemicals/additives and all wastes throughout their life cycle, in accordance with agreed international frameworks, and to significantly reduce their release into air, water and soil to minimize their adverse impacts on human health and the environment.
| NPs | Techniques | Size (nm) | Morphology | Applications | Ref. |
|---|---|---|---|---|---|
| Iron oxide (Fe3O4) | A surfactant-free co-precipitation method | 13.5–18.1 | Spherical | Magnetic hyperthermia | 42 |
| Iron oxide (Fe3O4) | A surfactant-free electrochemical method | 10–30 | Spherical | — | 43 |
| Platinum (Pt) | The Co4Cat process | 1–2 (1.8 ± 0.6) | Face-centered cubic crystal structure | Electrocatalysis; heterogeneous catalysis; biomedical applications | 44 |
| Zinc peroxide (ZnO2) | A surfactant-free synthesis procedure in methanol solution | 10–20 | Spherical | Antimicrobial applications against methicillin-resistant Staphylococcus aureus and Klebsiella pneumoniae | 45 |
| Tantalum oxide (Ta2O5) | A solvothermal method | ∼2 | Amorphous structure | Computed tomography (CT) imaging; nanocontrast agents (high CT contrast, stability (6 months), and negligible cytotoxicity) | 46 |
| Silica nanosphere-supported ultrafine silver (Ag) nanomaterials | A surfactant-free technique using the hydrolysis of 3-mercaptopropyltrimethoxysilane, providing thiol groups and in situ reduction of Ag+ to Ag0 to form ultrafine Ag NPs on the surface of silica nanospheres | The average diameter of the Ag NPs was 2.5 ± 0.9 nm | Metallic Ag with a face-centered-cubic structure | Antimicrobial applications against Escherichia coli | 47 |
| Zinc oxide (ZnO) microspheres | A surfactant-free microwave-assisted synthesis technique | The thickness was ∼20 nm | Flower-like structure | Antibacterial effects against S. aureus and E. coli | 48 |
| ZnO NPs | A surfactant-free microwave-assisted synthesis technique | ∼10–15 | Spherical | Photocatalytic degradation of methylene blue dye under ultraviolet irradiation | 49 |
| Ternary Cu3SnS4 NPs | A surfactant-free solvothermal process | ∼1.76–4.62 | Orthorhombic crystal phase and sphere-like morphology | — | 50 |
| Au nanorods | A surfactant-free green synthesis technique using an iron/chlorophyll molecular template without other toxic additives/surfactants | ∼18.8–32.42 | Spherical | Photodynamic cancer therapy | 51 |
| Copper (Cu)-1,4-naphthalenedicarboxylic acid-based organic frameworks (Cu-NDCA MOFs) | A surfactant-free solvothermal synthetic technique through a simple protonation–deprotonation method | — | At pH 3.0: irregular flake-like structure; pH 7.0: partial anisotropic structure; pH 9.0: anisotropic structure | Electrocatalytic performance for therapeutic drugs | 52 |
2. Surfactant-free synthesis of metal and metal oxide nanomaterials
A wide variety of surfactant-free synthesis techniques have been introduced for the synthesis of metal and metal oxide nanomaterials.20,37,53 Jiang et al.54 reported the synthesis of tin oxide (SnO2) NPs with tetragonal crystalline structures by heating ethylene glycol solutions containing SnCl2 at atmospheric pressure. By changing the experimental conditions and parameters such as reaction time, pH, and the tin precursor, the size and size distribution could be controlled. These NPs were employed to fabricate carbon-supported PtSnO2 catalysts with high activity for ethanol electrooxidation.54 The optimization of reaction and synthesis conditions is vital for controlling the properties as well as the size and morphology of nanomaterials. For instance, it was revealed that the surface morphology of Cu3SnS4 NPs synthesized through a surfactant-free solvothermal technique was highly affected by the reaction temperature.50 By increasing the reaction temperature, the aggregated particles disappeared and the surface morphology of Cu3SnS4 NPs became more self-assembled.50 Also, pH is another important factor in controlling the size and shape of nanomaterials. In one study, by increasing the pH from 3.0 to 9.0, the morphology of Cu-NDCA MOFs was changed from irregular flake-like to anisotropic structures. This indicated that pH is a crucial factor for the controlled growth of nanomaterials, which could enhance the coordination behavior of organic ligands and metal sites.52 Notably, in aqueous media, the stability of NP colloidal systems can be improved by adjusting the surface charge or zeta potential of NPs. It was revealed that on shifting pH to higher values, the stability of Ta2O5 NPs was improved; on changing the pH from 5.3 to 6.3, the absolute value of the NP zeta potential increased from −35 mV to −52 mV, indicating the significant stability of these NPs.46The fabrication of noble metal nanomaterials using ethylene glycol was reported as a typical surfactant-free technique;55,56 these nanomaterials have been deployed as electrocatalysts for ethanol oxidation reactions.57 For instance, stable Pt, rhodium (Rh), and ruthenium (Ru) metal nanoclusters (∼1–2 nm) were synthesized by heating the corresponding metal hydroxide colloids in ethylene glycol containing NaOH. Accordingly, the Pt nanocluster as a precipitate could be separated from glycol solvent by controlling pH.58 Chen et al.59 synthesized Pt3Fe NPs (∼4 nm) with controlled size and structure through a surfactant-free technique using ethylene glycol–water solution. In this study, potassium chloride (KCl) was an insoluble by-product of the reaction, which acted as a matrix to trap the NPs for avoiding particle agglomerations and controlling the coalescence of NPs during thermal annealing up to 600 °C. The particle size and crystalline order could be independently adjusted by altering the time and temperature of annealing, as well as the molar ratio of metal precursors and KCl.59 In addition, the surfactant-free synthesis of Pt NPs in alkaline ethylene glycol was reported.60 To achieve the size control, one of the key factors was the NaOH/Pt molar ratio, which could affect the kinetics of NP formation. Accordingly, the rate of Pt NP production was slower, and also smaller NPs were produced at higher NaOH/Pt molar ratios.60 Besides, Pt nanoscale cubes (∼3.4–7.1 nm) were prepared using a controllable surfactant-free approach under an atmosphere of 10% carbon monoxide (CO)/90% helium (He) via the adjustment of the potassium bromide (KBr)/Pt proportion (the precursors) and the Pt concentration in ethylene glycol. These nanomaterials could be employed for the oxygen reduction reaction along with the electrochemical oxidation of methanol, offering suitable electrocatalysts.61
Tong et al.62 introduced a self-terminating electroless deposition strategy for the preparation of surfactant-free and monodisperse Pt NPs (∼65 nm) deposited on carbon fiber microelectrodes with hydrogen peroxide (H2O2) electrochemical detection capability in living cells (the linear range was ∼0.5–80 μM and the detection limit was ∼0.17 μM). The Pt NP-modified carbon fiber microelectrodes exhibited suitable reproducibility and sensitivity, offering significant electrocatalytic performance towards H2O2 oxidation. It appears that future explorations ought to be focused on extending this technique to synthesize other metal NPs like Ag and Au, and obtaining surfactant-free and monodisperse metal NP-modified carbon fiber microelectrodes with suitable sensitivity and spatial resolution along with good reproducibility.62
The Co4Cat process with ecological and economic advantages was deployed for the controlled synthesis of precious metal NPs with improved catalytic features.44 Notably, in this strategy, metal precursors such as H2PtCl6 were dissolved in alkaline mono-alcohols (methanol) and reduced to NPs at low temperature (<80 °C) with no requirement of any surfactants. For instance, Pt NPs were synthesized using the Co4Cat process with long-term stability in water (up to 16 months) over a wide range of pH (4–12) and in aqueous buffer solutions, showing the suitability of the Co4Cat process to obtain NPs with electrocatalysis, heterogeneous catalysis, and biomedical applicability.44 Despite being surfactant-free, these Pt NPs exhibited remarkably long-term stability in water (>16 months) in the pH range of 4–12 and in aqueous buffer solutions. This technique displayed good reproducibility and scalability, showing robustness to variations in experimental factors (crucial factors in scale-up production) such as the concentration of H2PtCl6 and the heating time.44 In addition, Pt NPs were synthesized based on the Co4Cat process in a mixture of mono-alcohols and water utilizing alkaline low-boiling-point solvents, leading to the formation of NPs with improved catalytic performances.63 As a result, the control of solvent purity was not required for the formation of stable Pt NP colloids (∼2 nm) with electrocatalytic activity for energy conversion reactions (e.g., methanol oxidation).63
One-pot or seed-mediated fabrication techniques have been widely applied for the synthesis of nanomaterials utilizing surfactants such as poly(N-vinylpyrrolidone) or cetyltrimethylammonium bromide.64 However, the biomedical applications of the synthesized nanomaterials can be restricted due to the potential toxicity of cetyltrimethylammonium bromide, the possible aggregations after multi-step washing and difficult replacement of surfactants throughout the bio-functionalization processes. To overcome these challenging issues, investigations have focused on the surfactant-free fabrication of nanomaterials like in the case of gold nanostars wherein these nanomaterials with unique optical and plasmonic features along with high biocompatibility were synthesized via a simple surfactant-free synthesis technique, and were deployed as efficient contrast agents for in vivo biological imaging.64 In addition, hydrazine- and surfactant-free fabrication of noble metal/graphene nanocomposites was reported wherein the reduction of graphene oxide and noble metals was performed simultaneously in hot water utilizing ascorbic acid (vitamin C) as a reductant (Fig. 1).65 Among the designed composites, palladium (Pd)/graphene nanocomposites exhibited suitable catalytic performance in the Suzuki coupling reaction with good reusability without loss of their activity.65
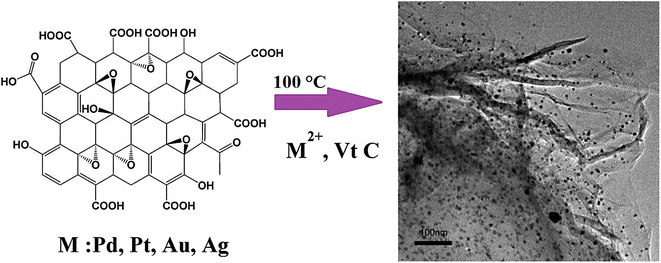 | ||
| Fig. 1 The one-step surfactant-free synthesis of noble metal/graphene nanocomposites using vitamin C (Vt C). Adapted from ref. 65 with permission. Copyright 2012 Elsevier. | ||
Microplasma-based strategies have been deployed for manufacturing different metal and metal oxide NPs.66 Several crucial aspects such as size, size distribution, and chemical composition (especially, the degree of oxidation) in microplasma-based techniques ought to be further explored; these factors can affect the optoelectronic features (e.g., conductivity) along with chemical stability of these NPs.66–69 In one study, surfactant-free well-dispersed metallic Cu NPs (∼8 nm) were synthesized using argon (Ar) + H2 microplasma and a solid Cu precursor. After that, carbon nanotubes/Cu-NP composite structures were prepared by depositing Cu NPs onto porous carbon nanotube ribbons, showing a high degree of surface coverage (Fig. 2).70
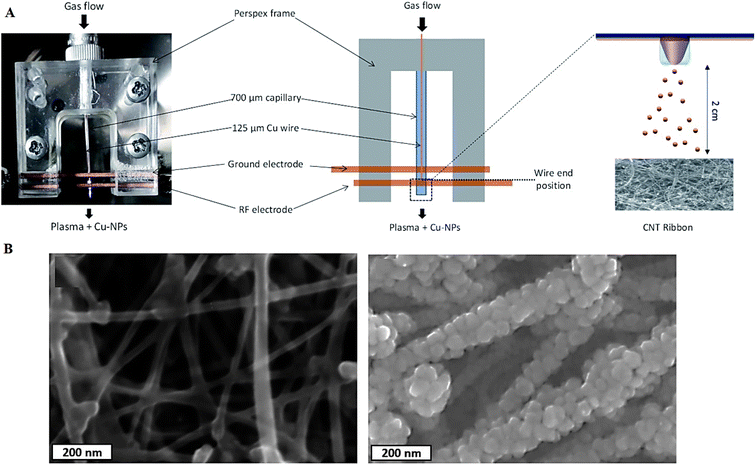 | ||
| Fig. 2 (A) The preparative process of Cu NPs and carbon nanotubes/Cu-NP composites based on the microplasma process. (B) Scanning electron microscopy (SEM) images of (left) pristine carbon nanotube ribbons and (right) decorated carbon nanotube/Cu-NP composites. Adapted from ref. 70 with permission. Copyright 2021 Royal Society of Chemistry (CC BY). | ||
A nanobubble scaffolding self-assembly strategy was introduced for manufacturing three-dimensional (3D) metallic nano-networks by utilizing aqua ammonia as a nanobubble reservoir, avoiding the utilization of any surfactants or polymeric capping agents.71 Accordingly, interlocked metallic nanonetworks could be obtained through the interactions between ammonia and metallic NPs (Cu, gold (Au), Ag, and Pt), as well as the precise control of the anisotropic kinetic growth along with the utilization of robust reducing agents and high concentration of aqua ammonia, providing nanonetworks with a curved geometry and abundant pores.71 The Pt nanonetworks exhibited excellent electrocatalytic performance towards the methanol oxidation reaction, showing the potential of the nanobubble-assisted strategy for surfactant-free synthesis of polyporous nanomaterials.71 In addition, a surfactant-free synthesis technique based on one-step hydrothermal reduction was introduced for the synthesis of Pt nanoclusters (∼2 nm) using 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) (as a non-toxic reducing agent); these NPs could be applied for biological imaging as well as sensing of hypochlorous acid (HClO), with advantages of fast response, high stability, and specificity. As nanoprobes, they exhibited bright blue fluorescence, high stability, and biocompatibility, while their fluorescence could be specifically quenched with hypochlorous acid by a static quenching pathway.72
Mao et al.73 introduced a simple and controllable “mix-and-heat” mechanochemical technique for synthesizing Ag/expanded graphite (EG) composites with high chemical stability under different pH conditions (they could be stored for ∼120 days), providing suitable composites to be applied as reliable surface-enhanced Raman scattering (SERS) active substrates with reusability of at least 4 times. By applying these composites with SERS performance, less than 10 ppb of crystal violet (CV) and methylene blue (MB) could be detected; the trapping of the analytes was mostly accomplished in the substrate in the first 15 min and the kinetics was based on the pseudo-second-order model (Fig. 3).73 Some important challenges for the employment of SERS in biochemical detection are the absence of enough surface roughness along with the presence of organic surfactants.74 The application of surfactants in the preparation of NPs can significantly affect the SERS sensing efficiency, because of the space blocking between SERS substrates and analytes along with the interference of intrinsic Raman signals from the surfactants themselves. Thus, future explorations should focus on the synthesis of nanomaterials based on surfactant-free tactics. In one study, a sensitive and robust SERS hybrid substrate was constructed using Au NPs.74 The introduced technique was based on plasmonic flower-like Au NPs wherein the drastic reduction of Au3+ ions was performed, which triggered the over-growth of Au atoms in the absence of surfactants. The designed hybrid structure was deployed for the in situ recognition of thiabendazole in apples (the limit of detection was ∼8.3 ng mL−1).74 Zeng et al.75 prepared nanosized graphene oxide-coated silver NPs without utilizing any surfactants for SERS sensing applications. Nanosized graphene oxide was suspended in aqueous solution, and silver nitrate (AgNO3) was added to the solution for interacting with the graphene oxide nanosheets within an ice bath. After that, the reducing agent (NaBH4) was added under mild stirring, and the nanocomposites were fabricated. These biocompatible nanocomposites with excellent SERS sensing potential could be applied as nanoprobes for intracellular biosensing; they were also deployed for targeted delivery of anticancer drugs (doxorubicin) with theranostic applicability.75
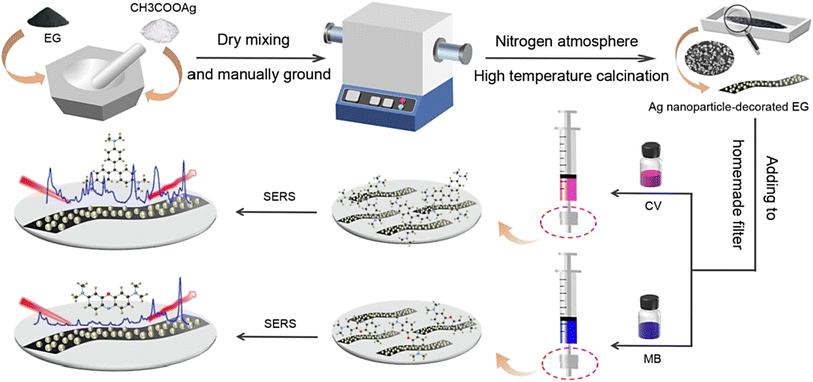 | ||
| Fig. 3 The preparative process of Ag/EG composites with excellent adsorption for SERS performance, in the absence of surfactants. Adapted from ref. 73 with permission. Copyright 2022 Elsevier. | ||
Au–Pt bimetallic NPs were synthesized based on a surfactant-free strategy using a laser-assisted method through the laser ablation and laser irradiation of colloidal mixtures; the surface of NPs was contaminant free without any external ligands and surfactants.76 Compared to the colloidal mixture before the laser irradiation, results revealed that the nonlinear absorption features, non-linear optical susceptibility values, optical limiting efficiency, and non-linear refractive index of these ligand-free bimetallic NPs could be improved after the laser irradiation.76 In addition, a two-step surfactant-free fabrication technique was introduced for manufacturing 3D perovskite titania-based micron-scale motifs in which porous anatase titanium dioxide (TiO2) templates were hydrothermally prepared and then reacted with A(OH)2 precursors (wherein “A” = Ca, Sr, and Ba) under high-temperature annealing conditions (Fig. 4); the designed ATiO3 micron-scale spheres could be applied as catalytic supports for the methanol oxidation reactions.77 A hydrothermal technique was also reported for the synthesis of cupric oxide (CuO) spheres and manganese dioxide (MnO2) nanorods to detect glucose as sensors with a high sensitivity of 2360.81 μA mM−1 cm−2.78 Accordingly, CuO spheres were prepared on an indium tin oxide (ITO)/glass substrate with no need of surfactants. After that, MnO2 nanorods were formed on the exterior of the CuO spheres through a drop-coating technique; MnO2 improved the sensitivity and the linearity of the glucose detection.78
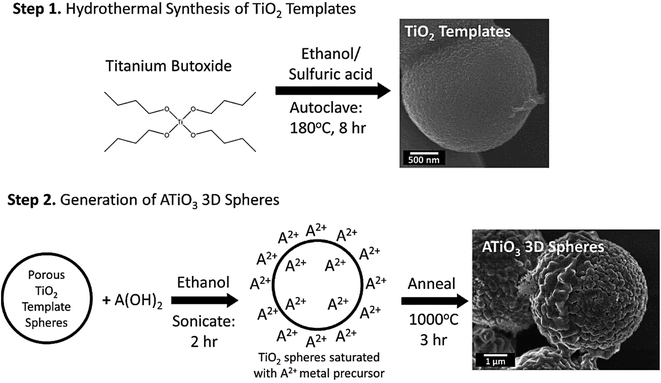 | ||
| Fig. 4 The preparative process of ATiO3 micron-scale spheres. Adapted from ref. 77 with permission. Copyright 2021 Multidisciplinary Digital Publishing Institute (CC BY). | ||
Surfactant-free microemulsions can form in a ternary system of two immiscible fluids and an amphi-solvent.79 The structures and properties of surfactant-free microemulsions are similar to those of traditional surfactant-based microemulsions to some extent.80,81 In one study, this technique was introduced for the synthesis of poly(methyl methacrylate)/Ag nanocomposites using methyl methacrylate as the oil phase and 1-butanol as the amphi-solvent, without using surfactants. These nanocomposites exhibited suitable antibacterial activity similar to the surfactant-based microemulsion system.82 Han et al.83 reported a surfactant-free microemulsion technique for fabricating α-Fe2O3 nanomaterials with great visible light catalytic activity for the removal of a rhodamine B pollutant (the degradation was ∼96.0% in 60 min). The water in oil structure in this technique played an important role of a micro-reactor, which could result in the generation of monodisperse spherical α-Fe2O3 nanomaterials at the controlled temperature and time. The mechanism studies revealed that free Fe3+ captured the electrons and catalyzed to form hydroxyl and superoxide anion radicals.83
Surfactant-free electrodeposition techniques have been employed for synthesis of metal and metal oxide nanomaterials. For instance, Au NPs (∼25–46 nm) were synthesized through a surfactant-free electrodeposition method using porous anodic alumina (as a template) and pulse electrodeposition (Fig. 5).84 Compared to colloidal fabrication approaches, the electrochemical synthesis technique can be applied for manufacturing NPs without surfactants or capping agents to stabilize their sizes and morphologies, paving the way to construct NPs as catalysts without restrictions in their reactive surface sites; this method can also be applied for synthesizing NPs with a controllable inter-pore distance. However, the limitation of this technique was its low yield of production, and future explorations ought to be focused on its up-scalability along with optimization conditions.84 It was revealed that the particle size can be controlled by adjusting the current density and the distance between electrodes.43 In one study, the particle size of magnetite NPs increased by enhancing the current density and reducing the distance between electrodes; the particle formation could not be favored when the distance between electrodes was larger than a critical value.43
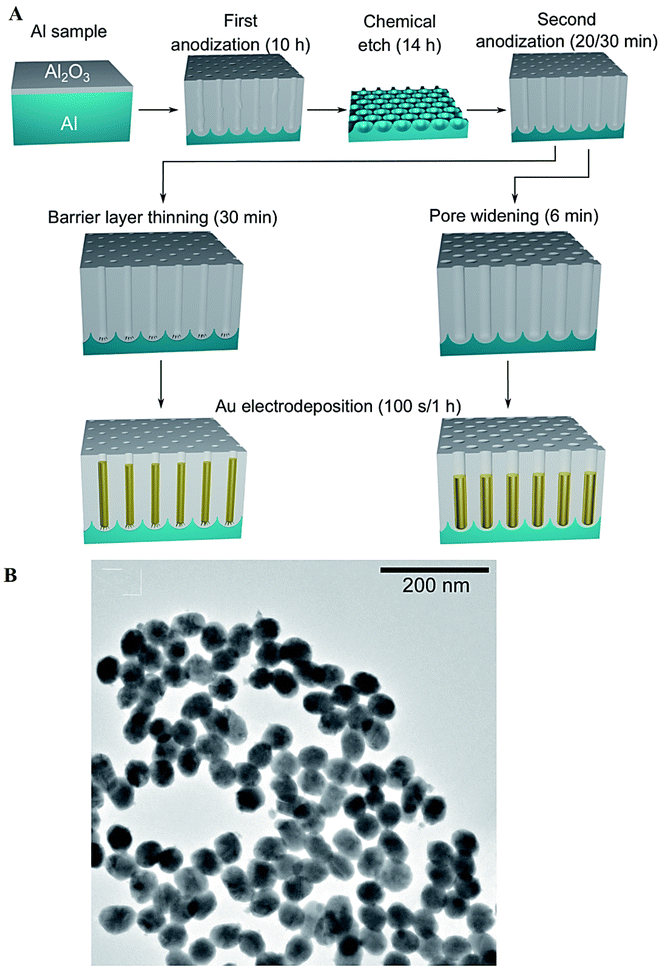 | ||
| Fig. 5 (A) The preparative process of the Au nanostructures using a surfactant-free electrodeposition method. The porous anodic alumina template was treated by pore widening or barrier layer thinning, after the two-step anodization procedure; the nanostructures were branched or round-bottomed, respectively. (B) TEM image of Au NPs produced using a templated and surfactant-free electrodeposition approach (time: 100 s). Adapted from ref. 84 with permission. Copyright 2022 Royal Society of Chemistry (CC BY). | ||
Compared to the conventional synthesis techniques, microwave energy-assisted chemical fabrication of nanomaterials has been proved to be a potential eco-friendly technique with the benefits of high yield of production, very short separation/purification time, and short reaction time, yielding pure nanomaterials with suitable functionality and applicability.85 For instance, surfactant-free fabrication of crystalline Cu nanomaterials with good stability was performed using a microwave irradiation of a solution of copper acetylacetonate in benzyl alcohol.86 In addition, SnO2 nanosheets were fabricated using a microwave hydrothermal technique with no requirement of surfactants and organic solvents.87 The SnO2 nanosheet as an anode with suitable electrochemical performance could be applied in designing a lithium-ion battery, showing an improved discharge capacity of 257.8 mA h g−1 after 50 cycles at a current density of 100 mA g−1.87 Besides, Mallesh et al.88 constructed cube-like shaped α-Fe2O3 nanomaterials with high coercivity using a simple microwave-assisted solvothermal technique without utilizing any surfactants, providing a facile technique to prepare nanostructures with controlled morphology and properties.88
Hybrid metallic nanocomposites with different applicability and functionality have been designed using a variety of surfactant-free techniques.28 For instance, Pd@PdPt/carbon nanotube nanocomposites have been synthesized using a surfactant-free technique in which the core–shell structures of the Pd@PdPt alloy were prepared through surfactant-free monodisperse Pd/carbon nanotube precursors by hydrolysis of tetrachloropalladate(II) ions ([PdCl4]2−) in the presence of carbon nanotubes.89 After that, the hydrogen reduction was accomplished followed by a galvanic replacement reaction. These nanocomposites with surfactant-free electrocatalytic surfaces were deployed for the methanol oxidation reaction, providing electrocatalysts with excellent electrochemical performances and high stability towards methanol oxidation compared to their monometallic counterparts.89 In addition, the hierarchical dahlia-like TiO2/V2O5 composites were synthesized using a facile one-pot surfactant-free strategy.90 These nanocomposites with considerable recyclability (∼5 cycles) exhibited efficient adsorption towards methylene blue owing to physical and electrostatic interactions, offering promising candidates for the removal of organic pollutants in wastewater.90 Cho et al.91 reported an innovative inter-cation redox reaction between two metal cations with various redox capacities to produce Au–TiO2 nanostars with no need of any toxic surfactants, surface-adsorbing polymers, or additives with reducing ability. These nanostars were deployed as photo-anodes with photoelectrochemical potential. The photo-anodes were designed via the deposition of the nanostars onto hydrothermally grown TiO2 nanorods on fluorine-doped tin oxide (SnO2), providing enhanced photoelectrochemical activity for a water-splitting device due to the localized surface plasmon resonance effects in the Au NPs. As a result, these hybrid nanostars on TiO2 nanorods displayed an excellent enhancement (∼33%) in photocurrent density compared to the pristine TiO2 nanorods.91
3. Conclusions and perspectives
Metal and metal oxide nanomaterials with versatile applications ranging from catalysis, medicine, sensing/imaging, cancer theranostics, drug delivery, energy conversion, water remediation/treatment, tissue engineering, antimicrobials/antivirals, etc. have been synthesized using a wide variety of techniques/strategies. Among them, surfactant-free synthesis techniques have attracted researchers due to their cost-effectiveness, simplicity (avoidance of multi-step processes), and environmentally-benign properties. However, the control of size and morphology of metal and metal oxide nanomaterials using surfactant-free synthesis techniques is still an important challenging issue. Also, the stability, time of synthesis, and large-scale/commercial production of NPs still need to be systematically evaluated. The optimization of synthesis conditions by controlling the important criteria (e.g., solvents, pH, temperature, etc.) can help to provide nanomaterials with improved stability and a well-organized size/morphology. On the other hand, transferring the lab-scale surfactant-free synthesis techniques to a large scale is another challenge, which requires careful monitoring and evaluation in various fields such as lower energy requirements, lower temperature, cheap and few chemicals, controllability/repeatability of synthesis processes, ideal solvents, among others. Innovative techniques with optimized conditions for the surfactant-free production of other nanoparticles and advanced nanosystems made of materials such as covalent organic frameworks (COFs), MOFs, graphene, carbon nanotubes, etc. ought to be further explored.Conflicts of interest
The author(s) declare no competing interest.References
- F. Gao, Y. P. Zhang, Z. Y. Wu, H. M. You and Y. K. Du, Coord. Chem. Rev., 2021, 436, 213825 CrossRef CAS.
- R. S. Varma and C. Len, Curr. Opin. Green Sustainable Chem., 2019, 15, 83–90 CrossRef.
- G. Varshney, S. R. Kanel, D. M. Kempisty, V. Varshney, A. Agrawal, E. Sahle-Demessie, R. S. Varma and M.-N. Nadagouda, Coord. Chem. Rev., 2016, 306, 43–64 CrossRef CAS.
- A. Venkateshaiah, D. Silvestri, R. K. Ramakrishnan, S. Wacławek, V. V. T. Padil, M. Černík and R. S. Varma, Molecules, 2019, 24, 3643 CrossRef CAS.
- P. Zhang, D. Hou, D. O'Connor, X. Li, S. O. Pehkonen, R. S. Varma and X. Wang, ACS Sustainable Chem. Eng., 2018, 6, 9229–9236 CrossRef CAS PubMed.
- W. Du, G. Yang, E. Wong, N. A. Deskins, A. I. Frenkel, D. Su and X. Teng, J. Am. Chem. Soc., 2014, 136, 10862–10865 CrossRef CAS.
- S. Iravani, in Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems: Micro and Nano Technologies, ed. K. A. Abd-Elsalam, Elsevier, 2020, pp. 21–32 Search PubMed.
- G. Jamalipour Soufi and S. Iravani, Green Chem., 2020, 22, 2662–2687 RSC.
- N. Rabiee, S. Iravani and R. S. Varma, Molecules, 2022, 27, 6186 CrossRef CAS.
- N. Shafiei, M. Nasrollahzadeh and S. Iravani, Comments Inorg. Chem., 2021, 41, 317–372 CrossRef CAS.
- A. K. Shukla and S. Iravani, Environ. Chem. Lett., 2017, 15, 223–231 CrossRef CAS.
- S. Iravani and G. Jamalipour Soufi, in Nanoparticles in Medicine, ed. A. K. Shukla, Springer, Singapore, 2019, pp. 175–183 Search PubMed.
- A. Shafiee, S. Iravani and R. S. Varma, MedComm, 2022, 3, e118 CrossRef CAS.
- M. Jouyandeh, S. S. Mousavi Khadem, S. Habibzadeh, A. Esmaeili, O. Abida, V. Vatanpour, N. Rabiee, M. Bagherzadeh, S. Iravani, M. R. Saeb and R. S. Varma, Green Chem., 2021, 23, 4931–4954 RSC.
- N. Pinna and M. Niederberger, Angew. Chem., 2008, 47, 5292–5304 CrossRef CAS.
- J. Quinson, S. Kunz and M. Arenz, ChemCatChem, 2021, 13, 1692–1705 CrossRef CAS.
- A. Heuer-Jungemann, N. Feliu, I. Bakaimi, M. Hamaly, A. Alkilany, I. Chakraborty, A. Masood, M. Casula, A. Kostopoulou, E. Oh, K. Susumu, M. H. Stewart, I. L. Medintz, E. Stratakis, W. J. Parak and A. G. Kanaras, Chem. Rev., 2019, 119, 4819–4880 CrossRef CAS PubMed.
- M. R. Chitsazi, H. Korbekandi, G. Asghari, R. Bahri Najafi, A. Badii and S. Iravani, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 328–333 CrossRef CAS.
- H. Korbekandi, M. R. Chitsazi, G. Asghari, R. Bahri Najafi, A. Badii and S. Iravani, Green Process. Synth., 2014, 3, 365–373 CAS.
- J. Quinson, Curr. Opin. Electrochem., 2022, 34, 100977 CrossRef.
- S. Iravani, Green Chem., 2011, 13, 2638–2650 RSC.
- S. Iravani and R. S. Varma, Green Chem., 2019, 21, 4583–4603 RSC.
- S. Iravani and R. S. Varma, Green Chem., 2020, 22, 2643–2661 RSC.
- S. Iravani and R. S. Varma, Green Chem., 2020, 22, 612–636 RSC.
- S. Iravani and R. S. Varma, Environ. Chem. Lett., 2020, 18, 703–727 CrossRef PubMed.
- R. Mohammadinejad, S. Karimi, S. Iravani and R. S. Varma, Green Chem., 2016, 18, 20–52 RSC.
- R. Mohammadinejad, A. Shavandi, D. S. Raie, J. Sangeetha, M. Soleimani, S. S. Hajibehzad, D. Thangadurai, R. Hospet, J. O. Popoola, A. Arzani, M. A. Gómez-Lim, S. Iravani and R. S. Varma, Green Chem., 2019, 21, 1845–1865 RSC.
- M. Nasrollahzadeh, M. Sajjadi, S. Iravani and R. S. Varma, Nanomaterials, 2020, 10, 1784, DOI:10.3390/nano10091784.
- P. Johnson, A. Trybala, V. Starov and V. J. Pinfield, Adv. Colloid Interface Sci., 2021, 288, 102340 CrossRef CAS.
- J. Shi, L. Zhang, Q. Shen, N. Sun and W. Wei, Catal. Lett., 2022 DOI:10.1007/s10562-022-03994-5.
- G. Yim, S. Cho, J. T. Park and H. Jang, Bull. Korean Chem. Soc., 2022, 43, 1002–1006 CrossRef.
- R. Ramírez-Jiménez, Á. Artiga, S. G. Mitchell, R. Martín-Rapún and J. M. de la Fuente, Nanomaterials, 2020, 10, 539 CrossRef.
- P. Nagaraju, R. Vasudevan, A. Alsalme, A. Alghamdi, M. Arivanandhan and R. Jayavel, Nanomaterials, 2020, 10, 160 CrossRef.
- D. G. Li, C. Wang, D. Tripkovic, S. H. Sun, N. M. Markovic and V. R. Stamenkovic, ACS Catal., 2012, 2, 1358–1362 CrossRef.
- P. Trogadas, N. Kapil, G. M. A. Angel, S. Kuhl, P. Strasser, D. J. L. Brett and M. O. Coppens, J. Mater. Chem. A, 2021, 9, 24283–24289 RSC.
- I. A. Safo, C. Dosche and M. Ozaslan, ChemPhysChem, 2019, 20, 3010–3023 CrossRef PubMed.
- J. Quinson, Front. Nanotechnol., 2021, 3, 770281, DOI:10.773389/fnano.772021.770281.
- S. Reichenberger, G. Marzun, M. Muhler and S. Barcikowski, ChemCatChem, 2019, 11, 4489–4518, DOI:10.1002/cctc.201900666.
- S. M. Stavis, J. A. Fagan, M. Stopa and J. A. Liddle, ACS Appl. Nano Mater., 2018, 1, 4358–4385 CrossRef.
- J. Sui, J. Yan, D. Liu, K. Wang and G. Luo, Small, 2020, 16, 1902828, DOI:10.1901002/smll.201902828.
- D. Zhang, B. Gökce and S. Barcikowski, Chem. Rev., 2017, 117, 3990–4103 CrossRef PubMed.
- A. R. Yasemian, M. A. Kashi and A. Ramazani, Mater. Chem. Phys., 2019, 230, 9–16 CrossRef.
- F. Fajaroh, H. Setyawan, W. Widiyastuti and S. Winardi, Adv. Powder Technol., 2012, 23, 328–333 CrossRef.
- J. Quinson, L. Kacenauskaite, J. Bucher, S. B. Simonsen, L. T. Kuhn, M. Oezaslan, S. Kunz and M. Arenz, ChemSusChem, 2019, 12, 1229–1239 CrossRef.
- H. M. Hussein, D. D. Ghafoor and K. M. Omer, Arabian J. Chem., 2021, 14, 103090 CrossRef.
- E. Koshevaya, D. Nazarovskaia, M. Simakov, A. Belousov, V. Morozov, E. Gandalipov, E. Krivoshapkina and P. Krivoshapkin, J. Mater. Chem. B, 2020, 8, 8337–8345 RSC.
- Q. Shen, Y. Shan, Y. Lü, P. Xue, X. Shu, D. Li, Y. Liu and X. Liu, J. Chin. Chem. Soc., 2019, 66, 815–821 CrossRef CAS.
- V. V. Shinde, D. S. Dalavi, S. S. Mali, C. K. Hong, J. H. Kim and P. S. Patil, Appl. Surf. Sci., 2014, 307, 495–502 CrossRef CAS.
- D. Sharma, S. Sharma, B. S. Kaith, J. Rajput and M. Kaur, Appl. Surf. Sci., 2011, 257, 9661–9672 CrossRef.
- B. John, G. G. Silvena and K. V. Kumar, Mater. Today: Proc., 2022, 62, 5071–5074 Search PubMed.
- M.-Y. Liao, T.-C. Huang, Y.-C. Chin, T.-Y. Cheng and G.-M. Lin, ACS Appl. Bio Mater., 2022, 5, 2819–2833 CrossRef PubMed.
- P. Arul, S.-T. Huang, N. S. K. Gowthaman, M. Govindasamy and N. Jeromiyas, Microchim. Acta, 2020, 187, 650 CrossRef PubMed.
- H. Kawasaki, Nanotechnol. Rev., 2013, 2, 5–25 Search PubMed.
- L. Jiang, G. Sun, Z. Zhou, S. Sun, Q. Wang, S. Yan, H. Li, J. Tian, J. Guo, B. Zhou and Q. Xin, J. Phys. Chem. B, 2005, 109, 8774–8778 CrossRef.
- C. Bock, C. Paquet, M. Couillard, G. A. Botton and B. R. MacDougall, J. Am. Chem. Soc., 2004, 126, 8028–8037 CrossRef CAS PubMed.
- D. Shen, Y. Liu, G. Yang, H. Yu, P.-F. Liu and F. Peng, Appl. Catal., B, 2021, 281, 119522 CrossRef CAS.
- W. Du, Q. Wang, D. Saxner, N. A. Deskins, D. Su, J. E. Krzanowski, A. I. Frenkel and X. Teng, J. Am. Chem. Soc., 2011, 133, 15172–15183 CrossRef.
- Y. Wang, J. Ren, K. Deng, L. Gui and Y. Tang, Chem. Mater., 2000, 12, 1622–1627 CrossRef.
- H. Chen, D. Wang, Y. Yu, K. A. Newton, D. A. Muller, H. Abruña and F. J. DiSalvo, J. Am. Chem. Soc., 2012, 134, 18453–18459 CrossRef.
- J. Quinson, A. Dworzak, S. B. Simonsen, L. T. Kuhn, K. M. Ø. Jensen, A. Zana, M. Oezaslan, J. J. K. Kirkensgaard and M. Arenz, Appl. Surf. Sci., 2021, 549, 149263 CrossRef.
- Z. Zhang, G. Yang, H. Wang, Y. Cao, F. Peng and H. Yu, ChemElectroChem, 2022, 9, e202101726 Search PubMed.
- Y. Tong, L. Wang, J. Song, M. Zhang, H. Qi, S. Ding and H. Qi, Anal. Chem., 2021, 93, 16683–16689 CrossRef PubMed.
- J. Quinson, S. B. Simonsen, L. T. Kuhn and M. Arenz, Sustainable Chem., 2021, 2, 1–7 CrossRef.
- H. Yuan, C. G. Khoury, H. Hwang, C. M. Wilson, G. A. Grant and T. Vo-Dinh, Nanotechnology, 2012, 23, 075102 CrossRef.
- S.-H. Kim, G. H. Jeong, D. Choi, S. Yoon, H. B. Jeon, S.-M. Lee and S.-W. Kim, J. Colloid Interface Sci., 2013, 389, 85–90 CrossRef PubMed.
- W.-H. Chiang, D. Mariotti, R. M. Sankaran, J. G. Eden and O. K. Ken, Adv. Mater., 2020, 32, 1905508 CrossRef PubMed.
- G. Jain, M. Macias-Montero, T. Velusamy, P. Maguire and D. Mariotti, Plasma Processes Polym., 2017, 14, 1700052 CrossRef.
- A. U. Haq, S. Askari, A. McLister, S. Rawlinson, J. Davis, S. Chakrabarti, V. Svrcek, P. Maguire, P. Papakonstantinou and D. Mariotti, Nat. Commun., 2019, 10, 817 CrossRef PubMed.
- M. Lozac’h, V. Švrček, S. Askari, D. Mariotti, N. Ohashi, T. Koganezawa, T. Miyadera and K. Matsubara, Mater. Today Energy, 2018, 7, 87–97 CrossRef.
- P. Brunet, R. J. McGlynn, B. Alessi, F. Smail, A. Boies, P. Maguire and D. Mariotti, Nanoscale Adv., 2021, 3, 781–788 RSC.
- J. Li, X. Liang, L. Cai and C. Zhao, Langmuir, 2021, 37, 8323–8330 CrossRef PubMed.
- X. Wang, Y. Wang, L. Yin, Q. Zhang and S. Wang, RSC Adv., 2022, 12, 10395–10400 RSC.
- Y. Mao, B. Yu, H. Zhang, Y. Ma, F. Han, B. Zhou, L. Yang and Z. Han, Appl. Surf. Sci., 2022, 592, 153264 CrossRef.
- Y. Ma, J. Ma, Y. Zhang, Z. Zhao, C. Gu, D. Chen, J. Zhou and T. Jiang, J. Alloys Compd., 2022, 918, 165706 CrossRef.
- F. Zeng, D. Xu, C. Zhan, C. Liang, W. Zhao, J. Zhang, H. Feng and X. Ma, ACS Appl. Nano Mater, 2018, 1, 2748–2753 CrossRef.
- R. Fathima and A. Mujeeb, J. Mol. Liq., 2021, 343, 117711 CrossRef.
- N. Hurley, L. Li, C. Koenigsmann and S. S. Wong, Molecules, 2021, 26(4), 909 CrossRef PubMed.
- F.-R. Juang and T.-M. Wang, Phys. E, 2021, 134, 114831 CrossRef.
- Y. Liu, J. Xu, H. Deng, J. Song and W. Hou, RSC Adv., 2018, 8, 1371–1377 RSC.
- W. Hou and J. Xu, Curr. Opin. Colloid Interface Sci., 2016, 25, 67–74 CrossRef.
- J. Xu, J. Song, H. Deng and W. Hou, Langmuir, 2018, 34, 7776–7783 CrossRef.
- B. Sadat Mirhoseini and A. Salabat, J. Mol. Liq., 2021, 342, 117555 CrossRef.
- Y. Han, N. Pan, S. Liu, J. Chai and D. Li, J. Environ. Chem. Eng., 2022, 10, 108006 CrossRef.
- G. Abbondanza, A. Larsson, W. Linpé, C. Hetherington, F. Carlá, E. Lundgren and G. S. Harlow, Nanoscale Adv., 2022, 4, 2452–2467 RSC.
- C. Singh, V. Khanna and S. Singh, Mater. Today: Proc., 2022 DOI:10.1016/j.matpr.2022.07.216.
- M. Ibrahim Dar, S. Sampath and S. A. Shivashankar, J. Mater. Chem., 2012, 22, 22418–22423 RSC.
- D. Narsimulu, S. Vinoth, E. S. Srinadhu and N. Satyanarayana, Ceram. Int., 2018, 44, 201–207 CrossRef.
- S. Mallesh, D. Narsimulu and K. H. Kim, Phys. Lett. A, 2020, 384, 126038 CrossRef.
- F. Zheng, T.-L. Kwong and K.-F. Yung, Nanomaterials, 2022, 12, 260 CrossRef PubMed.
- D. Jiang, N. Qi, T. Xu and J. Bai, J. Mater. Sci., 2021, 56, 4686–4699 CrossRef.
- S. Cho, G. Yim, J. T. Park and H. Jang, Energy Convers. Manage., 2022, 252, 115038 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |
