Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrocarbons from kraft pulp pre-hydrolysis liquors in two steps using heterogeneous catalysis†
Daria
Lebedeva
and
Joseph S. M.
Samec
 *
*
Stockholm University, Department of Organic Chemistry, Stockholm, Sweden. E-mail: joseph.samec@su.se
First published on 14th June 2023
Abstract
Valorizing industrial side-streams that are burnt to a low value are important to make biorefining more sustainable. In this work, hemicellulose obtained as a pre-hydrolysis liquor from kraft pulping was converted to furfural in a 19 wt% yield based on hemicellulose content in raw biomass using a beta zeolite as a catalyst. Furfural was then directly hydro-processed to yield pentane and higher hydrocarbons in 60%. It was found that a combination of Pd/C and ZSM-5 gave full hydrodeoxygenation. Depending on reaction conditions, aromatic or aliphatic compounds could be generated. By using pentane as a carrier liquid, a 100% renewable hydrocarbon stream is possible. Off gases, comprising <C5 hydrocarbons (23% yield), could be reformed to green hydrogen to yield renewable hydrocarbon products. This way, the pulp mill is de-bottlenecked and thus can increase the production of pulp, and at the same time, another valuable product stream is generated.
Introduction
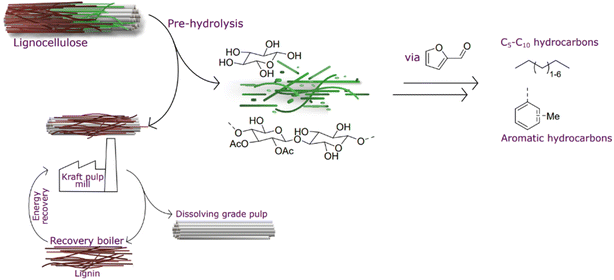
Meeting this century's major counteracting challenges – resource depletion1,2 and population growth3 – without negatively affecting climate change,4 land use,5,6 or water scarcity7 is perplexing. In only 40 years from now, predictions estimate 25% population growth and exhaustion of crude oil. To address these challenges, it is pivotal to valorize streams that are either discarded or burnt to a low value.Regenerated cellulose, that is textile fiber from lignocellulosic biomass,8 has been predicted to be an important source to meet an increased demand, where cotton manufacture cannot be increased from current production due to land use and water scarcity.9 The production of regenerated cellulose can be performed using kraft pulping; however, the hemicellulose needs to be removed by hydrolysis before delignification. This generated pre-hydrolysis liquor is currently not upgraded but burnt in a recovery boiler.10 This additional pressure on the recovery boiler results in a lower production capacity as the recovery boiler is the bottleneck of the pulping process. Therefore, it would be beneficial to remove this hemicellulose fraction from the recovery process in order to debottleneck the process.11 Furthermore, there is potential to upgrade this fraction beyond its heating value (Fig. 1).
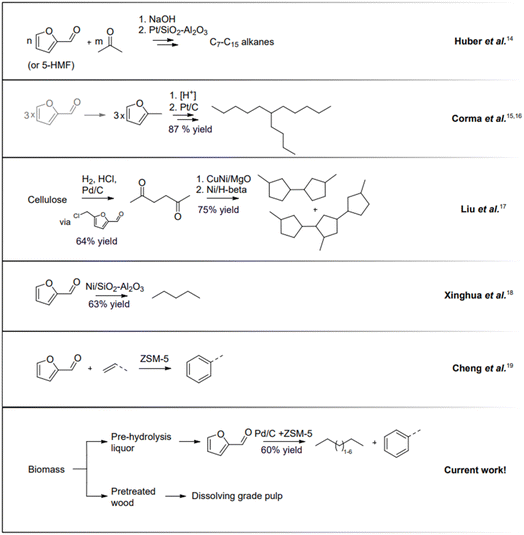
Hydrocarbons that today are produced from fossil resources will need to be replaced during this century. Pentane is indispensable in certain applications and is currently formed during cracking in oil refineries. Higher hydrocarbons below C16 are important components in fuels. In the last couple of decades, there has been intense research and development to convert bio-based carbohydrates to liquid hydrocarbons.12,13 The first cutting-edge work reported by Huber et al.14 described the transformation of carbohydrate-derived molecules into C7–C15 alkanes (Fig. 2). Sugar-derived furfural and 5-HMF undergo condensation with acetone yielding C7–C15 molecules that are successively converted to alkanes by hydroprocessing. Another innovative approach, the Sylvan process, was reported by Corma et al.15,16 in which three molecules of 2-methylfuran form C15 precursors for liquid hydrocarbon fuels. A promising approach was proposed by Liu et al., where cellulose can be converted to C9 and C15 hydrocarbons in several steps with an overall yield of 48%.17 The reports that mentioned direct conversion of pentose-derived furfural into C5 alkanes are scarce. For direct Ni-catalysed furfural hydrodeoxygenation, 63% conversion was reported with selectivity to pentane.18 Cheng et al. described the synthesis of aromatic compounds when furan derivatives are co-fed with olefins.19 More research concerning the transformation of bio-derived feeds, including a furanic platform, to hydrocarbons is described in the review by Muldoon et al.20
We hypothesized a value chain using pre-hydrolysis liquors obtained in a kraft mill to generate furfural that then is directly hydroprocessed to generate pentanes and other liquid hydrocarbons that are potential candidates to be used as liquid transportation fuel components.
Experimental
Materials and methods
All chemicals were purchased from Sigma-Aldrich if not stated otherwise. Zeolites were purchased from Zeolyst. Zeolites were calcined at 550 °C for 4 h. Birch sawdust was dried at 60 °C overnight prior to analyses and reactions. Furfural was distilled prior to use. GC-MS/FID analyses were performed on a QP2020 system (SHIMADZU, Japan) equipped with two parallel HP-5MS columns (30 m × 0.25 mm × 0.25 μm). HPLC analyses were performed on an Agilent 1200 Series HPLC system equipped with an Aminex HPX-87P column (300 mm × 7.8 mm).General procedure for the pre-hydrolysis of birch sawdust
Birch sawdust (0.5 g) and 5 mL of DI water (a water/wood ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added to an autoclave and stirred at elevated temperatures (140–200 °C) for 20–60 min. After completion, the solids were filtered off, washed with DI water, dried at 60 °C overnight, and weighed. Pre-treated wood samples were analyzed for glucan, xylan, and lignin contents as described below. Pre-hydrolysis liquor was analyzed for sugar content (ESI, Fig. S1†) and submitted to subsequent zeolite-assisted conversion to furfural.
1) were added to an autoclave and stirred at elevated temperatures (140–200 °C) for 20–60 min. After completion, the solids were filtered off, washed with DI water, dried at 60 °C overnight, and weighed. Pre-treated wood samples were analyzed for glucan, xylan, and lignin contents as described below. Pre-hydrolysis liquor was analyzed for sugar content (ESI, Fig. S1†) and submitted to subsequent zeolite-assisted conversion to furfural.
Sugar and lignin content determination
Sugar and lignin contents in raw biomass and pre-treated samples were measured following the procedure from the standard NREL protocol.21 According to the protocol, two-step hydrolysis of biomass samples was performed. Each biomass sample (300 mg) was treated with 72% H2SO4 (3 mL) at 30 °C for 1 hour in a sealed pressure tube under stirring. After completion, the mixture was diluted with 84 mL of DI water to get 4% H2SO4, and heated at 120 °C for 1 hour in the sealed pressure tube under stirring. The mixture was cooled down to room temperature and filtered. After filtration, an aliquot of the filtrate was taken and neutralized (pH 6–7) by adding CaCO3. An aliquot was filtered and subjected to HPLC analysis for sugar determination and quantification using calibration curves for each sugar (ESI, Fig. S2–S6†).The solid residue was washed with DI water, dried overnight, weighed, and incinerated at 575 °C for ash content determination. The ash was weighed, and the weight difference was considered acid-insoluble lignin (AIL) content.
Furfural formation from xylose
Reactions were performed in autoclaves. D-(+)-xylose (75 mg, 0.5 mmol) was added to a H2O/dioxane mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), followed by the addition of a zeolite catalyst (25 mg). Reactions were performed at elevated temperatures for 1 hour. After completion, the standard (dodecane) was added. A reaction mixture was filtrated and subjected to GC-MS/FID analysis. The yields were quantified by GC-FID using a calibration curve for furfural (ESI, Fig. S7†).
20), followed by the addition of a zeolite catalyst (25 mg). Reactions were performed at elevated temperatures for 1 hour. After completion, the standard (dodecane) was added. A reaction mixture was filtrated and subjected to GC-MS/FID analysis. The yields were quantified by GC-FID using a calibration curve for furfural (ESI, Fig. S7†).
Direct furfural formation from hydrolysis liquor
After the pre-hydrolysis of the raw wood was completed (conditions: 200 °C, 40 min, and 0.5 g of wood), the mixture was filtered and washed with approx. 1 mL of DI water, giving 5 mL of hydrolysate liquor. Dioxane (5 mL) was added to 5 mL of hydrolysate liquor resulting in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dioxane/water solution. H-Beta zeolite (50 mg) was added to the mixture. The reaction was performed at 180 °C for 2 h. After completion, the standard (dodecane) was added. The product was extracted by using EtOAc (3 × 10 mL). The EtOAc layers were combined and dried over Na2SO4. An aliquot was taken, filtered, and subjected to GC-MS/FID analysis to determine the yield.
1 dioxane/water solution. H-Beta zeolite (50 mg) was added to the mixture. The reaction was performed at 180 °C for 2 h. After completion, the standard (dodecane) was added. The product was extracted by using EtOAc (3 × 10 mL). The EtOAc layers were combined and dried over Na2SO4. An aliquot was taken, filtered, and subjected to GC-MS/FID analysis to determine the yield.
Furfural HDO
Freshly distilled furfural (200 mg, 2.08 mmol), a transition metal catalyst (20 mg (Pd/C: Pd content of 5 wt%; 0.45 mol%)), a co-catalyst (ZSM-5, 100 mg, 50 wt%) and a carrier liquid (pentane or cyclohexane, 5 mL) were loaded into a reactor (ESI, Fig. S8†). The reactor was thoroughly flushed with nitrogen to remove any oxygen. The initial hydrogen pressure was applied (10–25 bar). The reactions were run in a sand bath at elevated temperatures (340–400 °C, the temperature of the sand bath). After reaction completion, the reactor was cooled down to room temperature, and pressure was slowly released through the pressure valve. Dodecane was added as an internal standard. The crude mixture was dried over NaSO4, filtered, and subjected to GC-MS/FID analysis. The characterization and quantification of products are described in detail in the ESI, and shown in Fig. S9–S17.†Results and discussion
Birch wood was chosen for feedstock as it is a favoured species for the production of pre-hydrolysis kraft pulp. Composition analysis using a standardized protocol21 disclosed xylan as the major sugar from hemicellulose (25 wt% based on wood). Other major components were glucan 32 wt% and lignin 19 wt% (Table 1).| Reaction conditions | Mass of wood after the reaction, wt% of raw biomass | Composition of pre-treated wood, wt% | ||
|---|---|---|---|---|
| Glucan | Xylan | Lignin | ||
| a For each entry, the weight of the pre-treated wood is given as a percentage of the weight of the raw biomass, taking into account the weight loss of biomass after the reaction. The content of glucan, xylan, and lignin in the treated wood is given in absolute values without regard to weight loss. | ||||
| No reaction | 100 | 32.4 | 25.0 | 19.0 |
| 140 °C, 40 min | 96.3 | 32.3 | 24.9 | 18.9 |
| 140 °C, 60 min | 95.3 | 34.7 | 23.6 | 20.0 |
| 160 °C, 20 min | 98.2 | 33.8 | 24.1 | 19.5 |
| 160 °C, 40 min | 79.3 | 29.6 | 18.5 | 21.4 |
| 160 °C, 60 min | 74.2 | 45.5 | 11.9 | 23.3 |
| 180 °C, 20 min | 76.3 | 45.5 | 12.6 | 22.5 |
| 180 °C, 40 min | 64.4 | 48.9 | 6.4 | 28.4 |
| 180 °C, 60 min | 64.0 | 51.8 | 5.6 | 28.2 |
| 200 °C, 20 min | 61.4 | 53.7 | 3.6 | 27.6 |
| 200 °C, 40 min | 60.1 | 55.3 | 1.3 | 31.1 |
The pre-hydrolysis temperature was screened for xylan solubilization that was measured by performing sugar analysis of the resulting biomass and correlating the sugar content to the composition of the raw material considering weight loss of biomass during the reaction (Table 1). Performing pre-hydrolysis at 140 °C, initial 4% solubilization of xylan was observed after 40 min based on the raw material (Fig. 3A). After 60 min, pre-hydrolysis yielded in 10% xylan recovery. At 160 °C, only 20 min was needed to reach a similar result: 5% solubilization was observed. After 40 min at the same temperature, the pre-hydrolysis had progressed to 41% xylan solubilization, and after 60 min to 65%. When the pre-hydrolysis temperature was raised to 180 °C, solubilization was more facile, and after 1 hour, 85% was solubilized. At 200 °C, the solubilization was rapid and after 20 min 91% was observed. Within 40 min, 97% solubilization was reached and only 1% of xylan remained in the generated pre-treated pulp based on the pre-treated sample weight (Table 1). The resulting pre-hydrolysis liquor was directly analysed by HPLC and only 0.4% of glucan was observed based on pre-treated wood. Thus, the prehydrolysis was selective for removing xylose. The resulting pulps differed in color, where a correlation between a dark color and efficient pre-hydrolysis was observed (Fig. 3B). This is due to oxidation of the lignin on the remaining pulp.
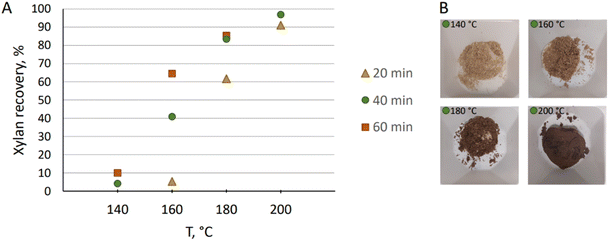 | ||
| Fig. 3 (A) Hemicellulose recovery from the biomass during pre-treatment at various temperatures and reaction times; (B) wood after 40 min of pre-treatment at different temperatures. | ||
We recently found that zeolites can transform sugars from hemicellulose to furfural and levulinic acid.22 Other groups have also reported this transformation.23,24 What is noteworthy is that by selecting a zeolite with a defined pore-size, a confinement control is possible. This is very efficient, as a known side-product from hydrolysis of hemicellulose is humin resulting from bimolecular condensation reactions.
First, we studied the effect of the SiO2/Al2O3 molar ratio, associated with the Lewis and Brønsted acidity, on the furfural yield. When decreasing the SiO2/Al2O3 ratio in ZSM-5 zeolites from 280 mol% to 23 mol%, the furfural yields significantly increased from 10 to 72 mol% yield (Table 2, entries 1–3). Then two Y zeolites differing only in SiO2/Al2O3 ratios were screened. The yield of furfural was not improved (Table 2, entries 4 and 5). However, the yield was not decreased compared to ZSM-5 (SiO2/Al2O3 molar ratio of 23), which was expected as the pore size was almost doubled. Interestingly, the SiO2/Al2O3 ratio did not affect the product yield for Y zeolites. When using a beta zeolite with a SiO2/Al2O3 ratio of 25, the conversion of xylose to furfural at 180 °C for 60 minutes gave 83% conversion to furfural (Table 2, entry 6). The reactions were performed in dioxane/water (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Running the reaction in water, we observed a low furfural yield of only 33% (Table 2, entry 7).
1). Running the reaction in water, we observed a low furfural yield of only 33% (Table 2, entry 7).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20)
20)
| Entry | Catalyst | Surface area, m2 g−1 | SiO2/Al2O3 mole ratio | Furfural yield,b % |
|---|---|---|---|---|
| a Reaction performed in water as a solvent. b Yields calculated from GC-FID. Yield, % = mol (prod.)/mol (sm) × 100. | ||||
| 1 | ZSM-5 | 400 | 280 | 10 |
| 2 | ZSM-5 | 425 | 80 | 54 |
| 3 | ZSM-5 | 425 | 23 | 72 |
| 4 | Y | 780 | 80 | 66 |
| 5 | Y | 780 | 30 | 67 |
| 6 | Beta | 680 | 25 | 83 |
| 7a | Beta | 680 | 25 | 33 |
With the optimized reaction conditions in hand, we ran the transformation using the pre-hydrolysis liquor. Before starting the reaction, the xylose content was measured by HPLC to be 8% of the initial wood (ESI, Fig. S1†), corresponding to 32% of the hemicellulose. The rest of the hemicellulose is expected to stay in an oligomeric form in the pre-hydrolysis liquor. The yield of furfural from xylose in pre-hydrolysis liquor was measured to be 94% after two hours. It is important to note that the non-transformed residues from the pre-hydrolysis liquor will be transferred back to the recovery in the kraft process, resulting in no overall losses.
We hypothesized a direct hydrotreatment of the generated furfural to yield a mixture of pentanes and higher hydrocarbons. A challenge when hydrotreating bio-feeds is the oxygen content that leads to uncontrolled exothermic reactions.25,26 Controlling these exothermic reactions are pivotal for preventing coking, especially using an industrial set-up with a catalyst bed. Therefore, co-processing with hydrocarbon carrier liquid is for example employed in the production of hydrogenated vegetable oils (HVOs). We argued that pentanes would be a preferred carrier liquid as parts of the furfural would generate pentanes, that could be recycled, and this would result in an 100% bio-based feed.
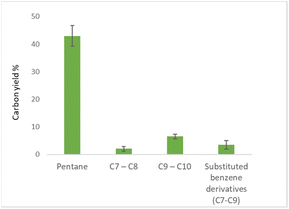
Running the hydrotreatment at 400 °C and 10 bar initial hydrogen pressure gave full consumption of furfural (Table 3, entry 1). However, only fully hydro-dearomatized oxygenates were observed (ESI, Fig. S9†). The addition of the zeolite, using the same reaction conditions, gave full consumption of the starting material. Interestingly, and in contrast to the reaction performed with only Pd/C, full deoxygenation was observed (Table 3, entry 2). We achieved conversion of furfural to pentane in 46% carbon yield and higher (C7–C10) hydrocarbons in 14% carbon yield. Interestingly, aromatic compounds were detected in 5% carbon yield, disclosing the possible [2 + 4] addition of reaction intermediates. When pentane was used as a carrier liquid, the yield of hydrocarbons above C5 was found to be 12% (Table 3, entry 3) with similar aliphatic/aromatic product distribution as for the reaction run in cyclohexane. Reducing the zeolite loading of substrate/zeolite to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, we observed the formation of oxygenated products (Table 3, entry 4). When the temperature was lowered to 340 °C and the initial pressure was increased to 25 bar, marginally lower yields of hydrocarbons were observed; however, no aromatics were detected (Fig. 3, entry 5). Changing the catalyst to Pt/Al2O3, Pt/Mo/TiO2, or Ni/Al2O3 led to conversion to oxygenates and no hydrocarbons were detected (Table 3, entries 6–8).
1, we observed the formation of oxygenated products (Table 3, entry 4). When the temperature was lowered to 340 °C and the initial pressure was increased to 25 bar, marginally lower yields of hydrocarbons were observed; however, no aromatics were detected (Fig. 3, entry 5). Changing the catalyst to Pt/Al2O3, Pt/Mo/TiO2, or Ni/Al2O3 led to conversion to oxygenates and no hydrocarbons were detected (Table 3, entries 6–8).
| Entry | Catalyst | Sub/Me-cat/zeolite mass ratio | T, °C | P, bar | Liquid hydrocarbons, carbon yield % | ||
|---|---|---|---|---|---|---|---|
| Pentane | C7–C10 (incl. aromatics) | Oxygenates | |||||
| a Yields calculated from GC-FID. Carbon yield, % = mol (prod.)/mol (sm) × n (carbon atoms in prod.)/n (carbon atoms in sm) × 100. b Reaction was performed in cyclohexane; NA = not applicable (for those reactions performed in pentane carrier liquid); n.d. = not detected. | |||||||
| 1b | Pd/C | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
400 | 10 | <1 | n.d. | 51 |
| 2b | Pd/C + ZSM-5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:5 1:5 |
400 | 10 | 46 | 14(5) | n.d. |
| 3 | Pd/C + ZSM-5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:5 1:5 |
400 | 10 | NA | 12(3) | n.d. |
| 4b | Pd/C + ZSM-5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:1 1:1 |
400 | 10 | 1 | n.d. | 38 |
| 5 | Pd/C + ZSM-5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:5 1:5 |
340 | 25 | NA | 6(n.d.) | n.d. |
| 6 | Pt/Al2O3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
340 | 25 | NA | n.d. | 24 |
| 7 | Pt/Mo/TiO2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
340 | 25 | NA | n.d. | 55 |
| 8 | Ni/Al2O3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
340 | 25 | NA | n.d. | 43 |
Furthermore, product distribution and experimental error were determined (Fig. 4).
Mass balance
The mass balance of the hemicellulose transformation process was calculated according to the results obtained from the zeolite-assisted transformation of pre-hydrolysis liquor and the subsequent HDO reaction (400 °C, 10 bar initial hydrogen pressure; Table 3, entry 2). The carbon yield of liquid hydrocarbons after HDO was 60%, which corresponds to 44.6 wt% based on furfural and 8.34 wt% based on hemicellulose (Fig. 5). To complete the mass balance, the yield of off-gases was studied. To determine off-gases, the amount of butane was estimated by following the formation of C4 intermediates during the HDO reaction. After 2 h reaction, the formation of C4 and C5 intermediates was observed (ESI, Fig. S17†). The product ratio of C4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C5 was 1
C5 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Thus, we assume that C4 intermediates formed via furfural decarbonylation are converted to C4 alkanes. Therefore, we expect the butane yield to be around 23%, which corresponds to 2.6 wt% for butane and 0.72 wt% for methane as the associated gas (Fig. 5). The carbon yield from the HDO reaction was 83%. This corresponds to an overall yield of 11.66 wt% from hemicellulose, of which 8.34 wt% is pentane and BTX..
2. Thus, we assume that C4 intermediates formed via furfural decarbonylation are converted to C4 alkanes. Therefore, we expect the butane yield to be around 23%, which corresponds to 2.6 wt% for butane and 0.72 wt% for methane as the associated gas (Fig. 5). The carbon yield from the HDO reaction was 83%. This corresponds to an overall yield of 11.66 wt% from hemicellulose, of which 8.34 wt% is pentane and BTX..
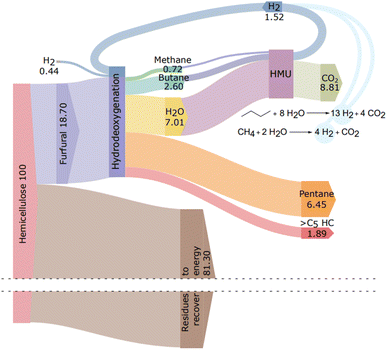 | ||
| Fig. 5 Sankey diagram of hemicellulose transformation to hydrocarbons. HMU (hydrogen manufacturing unit) is shown to describe hydrogen recovery. | ||
Conclusions
In this work we demonstrated a two-step conversion of prehydrolysis liquors, generated when pulp mills produce textile fibers, to hydrocarbons. The generation of both saturated and unsaturated compounds was demonstrated. Compared to state of the art, comparable yields were obtained; however in the current work we demonstrate that pre-hydrolysis liquors can be used directly to obtain hydrocarbons in a two-step process. For 500 kton per year pulp mill, 73 ktons of liquid hydrocarbons would be co-produced. Taking into account that 18.7 wt% of the hemicellulose is transformed to value-added products and 81.3 wt% is transferred back to the pulp mill, effective debottlenecking of the pulp mill corresponds to 10.6%; thus the pulp mill could increase its production from 500 kton to 553 kton. Thus, the potential revenue for the pulp mill stems from both the increased pulp production and the production of liquid hydrocarbons. We showed that different hydrocarbons can be generated depending on the reaction parameters employed. We hope that this concept study will inspire other researchers to pursue further development on upgrading on pre-hydrolysis liquors.Author contributions
Joseph Samec: conceptualization, supervision, and writing the manuscript; Daria Lebedeva: conceptualization, investigation, analysis, and writing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was funded by: Municipal of Stockholm (Region Stockholm) (grant RS 2019-0843), Mistra SafeChem (grant 2018/11), Swedish Research Council VR (2020-04143) and Swedish Energy Agency (grant 45903-1).Notes and references
- Proved Reserves of Crude Oil and Natural Gas in the United States, Year-End 2021, https://www.eia.gov/naturalgas/crudeoilreserves/, accessed February 14, 2023 Search PubMed.
- Statistical Review of World Energy|Energy economics|Home, https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html, accessed February 14, 2023 Search PubMed.
- W. Lutz and S. KC, Science, 2011, 333, 587–592 CrossRef CAS PubMed.
- D. I. Armstrong McKay, A. Staal, J. F. Abrams, R. Winkelmann, B. Sakschewski, S. Loriani, I. Fetzer, S. E. Cornell, J. Rockström and T. M. Lenton, Science, 2022, 377, eabn7950 CrossRef PubMed.
- A. Marques, I. S. Martins, T. Kastner, C. Plutzar, M. C. Theurl, N. Eisenmenger, M. A. J. Huijbregts, R. Wood, K. Stadler, M. Bruckner, J. Canelas, J. P. Hilbers, A. Tukker, K. Erb and H. M. Pereira, Nat. Ecol. Evol., 2019, 3, 628–637 CrossRef PubMed.
- O. Venter, E. W. Sanderson, A. Magrach, J. R. Allan, J. Beher, K. R. Jones, H. P. Possingham, W. F. Laurance, P. Wood, B. M. Fekete, M. A. Levy and J. E. M. Watson, Nat. Commun., 2016, 7, 12558 CrossRef CAS PubMed.
- M. M. Mekonnen and A. Y. Hoekstra, Sci. Adv., 2016, 2, e1500323 CrossRef PubMed.
- A. Basit, W. Latif, S. A. Baig, A. Rehman, M. Hashim and M. Zia Ur Rehman, Mater. Sci., 2018, 24, 230–235 CrossRef.
- H. Sixta, M. Iakovlev, L. Testova, A. Roselli, M. Hummel, M. Borrega, A. van Heiningen, C. Froschauer and H. Schottenberger, Cellulose, 2013, 20, 1547–1561 CrossRef CAS.
- V. Lundberg, J. Bood, L. Nilsson, E. Axelsson, T. Berntsson and E. Svensson, Clean Technol. Environ. Policy, 2014, 16, 1411–1422 CrossRef CAS.
- E. Axelsson, M. R. Olsson and T. Berntsson, Nord. Pulp Pap. Res. J., 2006, 21, 485–492 CrossRef CAS.
- Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering|Chemical Reviews, https://pubs.acs.org/doi/full/10.1021/cr068360d, accessed February 14, 2023 Search PubMed.
- J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164–7183 CrossRef CAS PubMed.
- G. W. Huber, J. N. Chheda, C. J. Barrett and J. A. Dumesic, Science, 2005, 308, 1446–1450 CrossRef CAS PubMed.
- A. Corma, O. de la Torre, M. Renz and N. Villandier, Angew. Chem., Int. Ed., 2011, 50, 2375–2378 CrossRef CAS PubMed.
- A. Corma, O. de la Torre and M. Renz, Energy Environ. Sci., 2012, 5, 6328–6344 RSC.
- Y. Liu, G. Li, Y. Hu, A. Wang, F. Lu, J.-J. Zou, Y. Cong, N. Li and T. Zhang, Joule, 2019, 3, 1028–1036 CrossRef CAS.
- Z. Xinghua, W. Tiejun, M. Longlong and W. Chuangzhi, Fuel, 2010, 89, 2697–2702 CrossRef.
- Y.-T. Cheng and G. W. Huber, Green Chem., 2012, 14, 3114–3125 RSC.
- J. A. Muldoon and B. G. Harvey, ChemSusChem, 2020, 13, 5777–5807 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Determination of Structural Carbohydrates and Lignin in Biomass Report NREL/TP-510-42618, 2012 Search PubMed.
- E. Subbotina, A. Velty, J. S. M. Samec and A. Corma, ChemSusChem, 2020, 13, 4528–4536 CrossRef CAS PubMed.
- S. B. Kim, S. J. You, Y. T. Kim, S. Lee, H. Lee, K. Park and E. D. Park, Korean J. Chem. Eng., 2011, 28, 710–716 CrossRef CAS.
- I. Mongkolpichayarak, D. Jiraroj, W. Anutrasakda, C. Ngamcharussrivichai, J. S. M. Samec and D. N. Tungasmita, J. Catal., 2022, 405, 373–384 CrossRef CAS.
- D. D. Francesco, C. Dahlstrand, J. Löfstedt, A. Orebom, J. Verendel, C. Carrick, Å. Håkansson, S. Eriksson, H. Rådberg, H. Wallmo, M. Wimby, F. Huber, C. Federsel, M. Backmark and J. S. M. Samec, ChemSusChem, 2021, 14, 2414–2425 CrossRef PubMed.
- J. Löfstedt, C. Dahlstrand, A. Orebom, G. Meuzelaar, S. Sawadjoon, M. V. Galkin, P. Agback, M. Wimby, E. Corresa, Y. Mathieu, L. Sauvanaud, S. Eriksson, A. Corma and J. S. M. Samec, ChemSusChem, 2016, 9, 1392–1396 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se00616f |
| This journal is © The Royal Society of Chemistry 2023 |