Open Access Article
Open Access ArticleAcrylated soybean oil: a key intermediate for more sustainable elastomeric materials from silicones†
Miguel
Melendez-Zamudio
 ,
Erin
Donahue-Boyle
,
Erin
Donahue-Boyle
 ,
Yang
Chen
,
Yang
Chen
 and
Michael A.
Brook
and
Michael A.
Brook
 *
*
Department of Chemistry and Chemical Biology, McMaster University, 1280 Main St. W., Hamilton, ON L8S 4M1, Canada. E-mail: mabrook@mcmaster.ca
First published on 13th December 2022
Abstract
Silicone elastomers are widely used because of their myriad useful properties. However, their synthesis requires a high energy input. We report that the amount of silicone, per application, can be significantly reduced by the creation of silicone composites derived from soybean oil. Acrylated soybean oil, prepared by addition of acrylic acid to epoxidized soybean oil, was linked to aminoalkylsilicones using a catalyst-free aza-Michael reaction in the absence of solvents; the reaction takes <1–12 hours depending on reaction temperature (room temperature to 60 °C). The resulting opaque elastomers behave very similarly to silicone elastomers with respect to durometer, surface energy and thermal stability. Although stable to boiling water, the products readily undergo degradation by basic alcoholysis in ethanol to give processable oils.
Introduction
Sustainability hinges on the ability to close the life cycle of materials. This requirement is encoded in Green Chemistry Rules 7 and 10 that call for both the use of natural starting materials and the degradation of end products to feedstock materials once back in the environment. Silicones are entirely synthetic polymers with a very broad range of applications. While the end-of-life degradation of silicone oils in the environment, to water, CO2 and sand is relatively rapid, their synthesis involves a relatively unsustainable high energy expenditure.1,2 Can one render silicone elastomers more sustainable by finding a compromise between synthetic and natural feedstocks that would reduce the impact of the energy cost?Silicone polymers that contain organic functional groups including alcohols, thiols, and amines are readily available. The latter are potent nucleophiles. The aza-Michael reaction is a particularly attractive strategy to link aminoalkylsilicones to natural materials, as the process is catalyst free, with excellent atom economy, and occurs at low to medium temperatures in a few hours in excellent yield.3–5
Vegetable oils – triglycerides – constitute a class of molecules typically destined for food, but are also available post consumer use and from plant sources that are not used as food. Thus, they constitute useful, natural feedstocks that need not compromise access to food.
Triglycerides are typically polyunsaturated materials. Soybean oil, a commonly used vegetable oil, can be directly modified for further use through reaction at the alkene groups.6 More commonly, the alkenes are rendered subject to nucleophilic attack by first conversion to epoxides7,8 or cyclic carbonates.9 For example, diethylamine easily ring-opens soybean oil epoxides in the presence of zinc chloride.10 Related cyclic carbonates are useful feedstocks for naturally derived, isocyanate free polyurethanes.11 An isocyanate free polyurethane was prepared by opening cyclic carbonates with aminosilane coupling agents.12
We reasoned that widely available telechelic aminoalkylsilicones could participate in aza-Michael reactions with acrylated soybean oil formed first by epoxidation and then acrylation.8 We report the ability to prepare libraries of silicone elastomers based on soybean oil, and their facile degradation under basic conditions, when desired.
Results
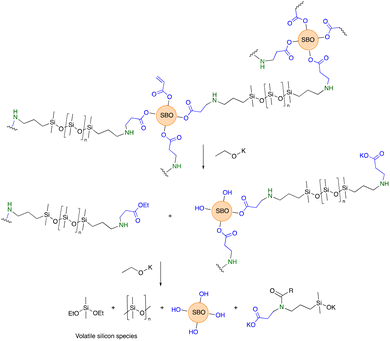
Epoxidized soybean oil 1 was readily prepared in 88% yield by the H2O2-facilitated oxidation of soybean oil at 55 °C in the presence of a solid catalyst Amberlite acid resin, which is easily removed by filtration at reaction end. The oxidant is attractive because the by-product is water. Note that epoxidized soybean and other vegetable oils are increasingly available from several chemical suppliers.The ring-opening of epoxides is well-established in industrial processes.13 When good nucleophiles like amines are used, as with epoxy-based resins, accelerators are common; co-catalysts are also frequently exploited under acidic conditions, including with fatty acid-derived epoxides.14 A systematic examination of the reaction of 1 with acrylic acid demonstrated that, by controlling both concentrations of reagents and temperature, catalysts were not required (Fig. 1A). The oligo-acrylated product 2 was formed in 86% yield in 12 hours at 120 °C. It would be desirable to reduce both reaction time and temperatures for, among other reasons, energy consumption. However, survey experiments showed that higher temperatures were associated with degradation of the soybean oil constituent, as easily seen from the color changes. The use of, for example, Brønsted acids also led to degradation. Lewis acids did not significantly reduce the reaction temperatures required and were eventually rejected because of the need, at reaction end, to remove the catalyst.
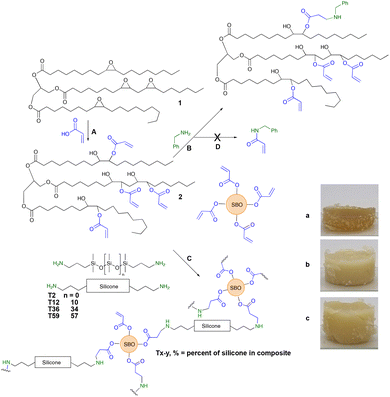 | ||
| Fig. 1 (A) Formation of soybean acrylates from the epoxide; (B) Model aza-Michael reaction with benzylamine (Fig. S1, ESI†); and (C) crosslinked soybean silicones following the aza-Michael reaction between acrylated soybean oil and telechelic silicones. Photos of elastomers formed from different aminosilicones (a) T12-25, (b) T36-25 and (c) T59-25. Note: although the soybean oil sample had 4.2 alkenes per molecule, only 4 alkenes are drawn for clarity. | ||
The aza-Michael reaction between aminosilicones and simple acrylates is very efficient; catalysts are not required and the reaction is particularly facile when there is an alcohol in the beta-position of the ester.5 Model studies that involved reacting 2 with benzylamine showed that, on demand, 25–100% of the acrylates could be consumed at room temperature in <12 hours simply by adding the appropriate quantity of the amine and mixing (Fig. 1B). Thus, if desired, acrylate groups can remain in the product for further elaboration.
The same process was then used to crosslink telechelic aminoalkylsilicones with 2. As can be seen in Fig. 1C, the combination of the two materials leads to opaque, yellow → brown elastomers. Since 2 is yellow/brown, depending on batch and age, the elastomers formed with higher concentrations of 2 were darker brown; with an increase in the silicone content they can become off white. Silicones are both hydro- and oleophobic15 and, therefore are expected to be only partly miscible with 2 and its products. Thus, the materials are opaque unless very low concentrations of 2 are used within the silicone matrix; it is possible that the use of surfactants could lead to much small domains of the two materials, reducing opacity.
The rate of crosslinking was affected by the length of the siloxane chain and the concentrations of 2 used (Table 1). Silicones with relatively higher concentrations of amines underwent more rapid crosslinking; T2 ∼1 hour and ∼4 hours for T12 (nomenclature: Tn, T = telechelic, n = number of Me2SiO monomers in the chain). However, reaction rates slowed as the amines were diluted by more Me2SiO backbone monomers; samples derived from T36 or T59 required heated overnight in a 60 °C oven to achieve complete cure. IR-ATR characterization of model T2 materials showed a decrease in peaks at 1633, 1406 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 981 cm−1 (vibration of H–C in C
CH–), 981 cm−1 (vibration of H–C in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) from the acrylic group (Fig. S2, ESI†). Peaks corresponding to the –Si–O–Si– bond that appear in the region between 1130–1000 cm−1 were not seen with the T2 derivatives, but showed clearly within higher molecular weight aminoalkylsilicone products (Fig. S2, ESI†).16,17 Unsurprisingly, both surface energy (from contact angle) and hardness (Shore OO) decreased with an increase in silicone content. T2-derived samples did not follow this trend. We attribute this to inefficient grafting at both ends of the telechelic chains due to more challenges in forming bridges between soybean acrylates. These materials are thus both softer and less hydrophobic because of a higher proportion of dangling chains with amino termini; pure silicone elastomers typically have sessile drop water contact angles near 100–110°.18 The chain length also plays a crucial role in the hardness of the materials. The presence of longer chain siloxanes will produce softer materials even if the composite contains the same concentration of silicone (Fig. 2).
C–H) from the acrylic group (Fig. S2, ESI†). Peaks corresponding to the –Si–O–Si– bond that appear in the region between 1130–1000 cm−1 were not seen with the T2 derivatives, but showed clearly within higher molecular weight aminoalkylsilicone products (Fig. S2, ESI†).16,17 Unsurprisingly, both surface energy (from contact angle) and hardness (Shore OO) decreased with an increase in silicone content. T2-derived samples did not follow this trend. We attribute this to inefficient grafting at both ends of the telechelic chains due to more challenges in forming bridges between soybean acrylates. These materials are thus both softer and less hydrophobic because of a higher proportion of dangling chains with amino termini; pure silicone elastomers typically have sessile drop water contact angles near 100–110°.18 The chain length also plays a crucial role in the hardness of the materials. The presence of longer chain siloxanes will produce softer materials even if the composite contains the same concentration of silicone (Fig. 2).
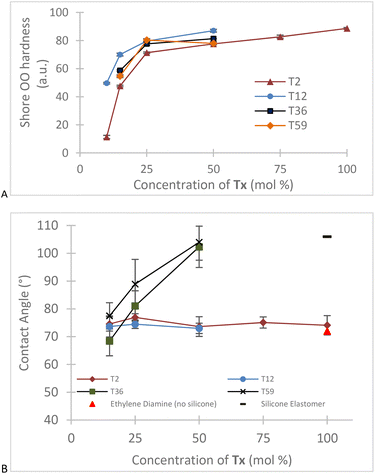 | ||
| Fig. 2 Changes in (A): hardness and (B): contact angle with different ratios of 2 to a series of aminosilicones. | ||
| Acrylated soybean oil, g (mmol) | Silicones | Product | Concentration, mg (mmol) | t | %Db |
|---|---|---|---|---|---|
| a Curing time in hours. b %D units (Me2SiO) in the product. c Heated at 60 °C in an oven. | |||||
| 2 (0.809) | T2 | T2-10 | 10% 20 (0.080) | 12 | 8.3 |
| T2-15 | 15% 30 (0.121) | 8 | 11.5 | ||
| T2-25 | 25% 50 (0.202) | 4 | 16.7 | ||
| T2-50 | 50% 100 (0.404) | 0.5 | 25.0 | ||
| T2-75 | 75% 150 (0.607) | 0.5 | 30.0 | ||
| T2-100 | 100% 201 (0.809) | 0.5 | 33.3 | ||
| 2 (0.809) | T12 | T12-10 | 10% 71.8 (0.0809) | 12 | 43.2 |
| T12-15 | 15% 108 (0.121) | 3 | 52.1 | ||
| T12-25 | 25% 179 (0.202) | 2 | 62.0 | ||
| T12-50 | 50% 359 (0.404) | 1 | 72.4 | ||
| 2 (0.809) | T36 | T36-10 | 10% 215 (0.0809) | — | 74.4 |
| T36-15 | 15% 323 (0.121) | 12c | 80.3 | ||
| T36-25 | 25% 538 (0.202) | 12c | 85.7 | ||
| T36-50 | 50% 1077 (0.404) | 12c | 90.2 | ||
| 2 (0.809) | T59 | T59-10 | 10% 353 (0.0809) | — | 82.9 |
| T59-15 | 15% 529 (0.121) | 12c | 87.2 | ||
| T59-25 | 25% 0.883 (0.202) | 12c | 90.9 | ||
| T59-50 | 50% 1766 (0.404) | 12c | 93.9 | ||
One interesting feature offered by these materials is the ability to tailor the crosslinking density simply by changing the concentration of aminoalkylsilicone that is added, leaving different quantities of unreacted acrylic groups that can be modified in secondary reactions (e.g., another aza-Michael reaction, radical polymerization, etc.). 1H NMR of the swollen materials clearly showed residual acrylics present in the sample (Fig. S3, ESI†).
A selection of the soybean silicone elastomers was characterized for thermal stability using TGA under argon. There is a small loss of mass up to 211–215 °C, but significant thermal degradation only starts at about 330–345 °C and continues to about 480 °C. Essentially complete combustion is observed up to 500 °C, with residual mass of only a few percent (Fig. S7, ESI†).
It is well known that reinforced silicones possess outstanding mechanical properties but, in the absence of fillers, the elastomers are comparatively weak. We wished to directly compare the physical properties of a traditional unfilled silicone with the soybean acrylate silicone prepared here. Dogbones of a hydrosilylation-cured silicone elastomer (R3SiH + H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSi-Silicone → R3SiH2CCH2Si-Silicone) with a Shore OO hardness of 72 were compared with two different unfilled formulations prepared from 2 with aminosilicones T12-50 and T36-50, with Shore harnesses of 87 and 81, respectively. The presence of the soybean constituent decreased elongation at break in proportion to its presence in the polymer (Fig. 3). Samples with longer siloxane chains (T36, T59) are more flexible and have better mechanical properties than samples with smaller chains (T2 & T12) that are harder and stiffer.
CHSi-Silicone → R3SiH2CCH2Si-Silicone) with a Shore OO hardness of 72 were compared with two different unfilled formulations prepared from 2 with aminosilicones T12-50 and T36-50, with Shore harnesses of 87 and 81, respectively. The presence of the soybean constituent decreased elongation at break in proportion to its presence in the polymer (Fig. 3). Samples with longer siloxane chains (T36, T59) are more flexible and have better mechanical properties than samples with smaller chains (T2 & T12) that are harder and stiffer.
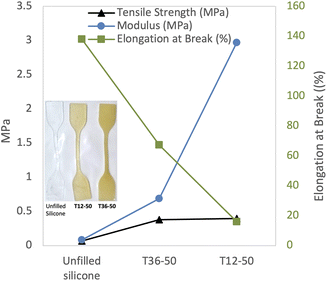 | ||
| Fig. 3 Comparison of % elongation at break of: unfilled silicone, T36-50 and T12-50. Note: the lines are only to facilitate reading the chart. | ||
Polyesters and silicones are both condensation polymers and could be expected to undergo hydrolysis, even in the absence of catalysts; it is noted that the amine present could be expected to act as such a catalyst. Hydrolytic stability of the materials was tested for two samples by boiling the T12-25 and T12-50, respectively, in water for 24 hours. Approximately 95% of the mass was recovered in each case, consistent with little or no degradative chemistry resulting from the treatment.
Enforced degradation of both ester19 and silicone20 groups is facilitated by catalysts. To date, experiments with enzyme catalysts such as lipase for the ester groups have not been successful. However, aqueous base catalysis using EtOH as a cosolvent was very effective. After 1 hour at 40 °C the sample had undergone complete degradation leading to an aqueous dispersion; after removal of solvents and redispersion in water/CDCl3 (used for convenience in the NMR) the upper aqueous phase in fatty acid, alcohols, and traces of silicones, while the organic lower phase contained mostly silicones (determined by 1H and 13C, Fig. S4 and S6, ESI†). The product silicones were of higher molar mass than the starting silicone material (degradation products from T59-50Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640 g mol−1, Mw = 25
640 g mol−1, Mw = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 665 g mol−1, ĐM = 2.20 vs. starting material T59Mn = 2215 g mol−1, Mw = 4220 g mol−1, ĐM = 1.90) consistent with some residual coupling between silicones and soybean oil constituents (on average 1 lipid for each 2 or 3 silicone chains). Amides were seen as products of this degradation in the infrared spectrum (Fig. S5, ESI†). After a second degradation using base, all the silicone products had been converted to volatile products and were not observed in the residual product. Thus, simple base cleavage allows facile separation of hydrophobic silicone constituents from the soybean oil-derived products (Fig. 4).
665 g mol−1, ĐM = 2.20 vs. starting material T59Mn = 2215 g mol−1, Mw = 4220 g mol−1, ĐM = 1.90) consistent with some residual coupling between silicones and soybean oil constituents (on average 1 lipid for each 2 or 3 silicone chains). Amides were seen as products of this degradation in the infrared spectrum (Fig. S5, ESI†). After a second degradation using base, all the silicone products had been converted to volatile products and were not observed in the residual product. Thus, simple base cleavage allows facile separation of hydrophobic silicone constituents from the soybean oil-derived products (Fig. 4).
Discussion
Simply mixing of the two precursors: 2 and an aminoalkylsilicone; led under mild conditions to efficient conversion to elastomers. Two variables in particular allow the tuning of the desired physical properties of the composite: ratio of [acrylic]/[amine] and length of the telechelic silicone. As the ratio approaches 1 the samples are harder, an effect amplified if the telechelic polymer is shorter; more effective crosslinking is observed (Fig. 2). It is possible to prepare composites that contain from about 10–95% silicone using this process.Composites with low silicone content, e.g., derived from T2, exhibited contact angles consistent with the soybean oil-derived product that, because of residual alcohols, will have higher energy than silicones. Water contact angles rose in proportion to the amount of silicone present. This was expected, as silicones normally ‘win the fight’ to get to air interfaces and manifest in very high water contact angles.21
Silicones are both oleophobic and hydrophobic and it is entirely expected that they will be immiscible with the soybean oil constituents.15 Silicones have refractive indices (∼1.41) that are quite different from vegetable oils such as soybean oil (∼1.46). Therefore, unless special attention is paid to refractive index matching, or additional ingredients like surfactants are used, opaque elastomers are to be expected; the yellow-brown color is a consequence of the soybean oil.
The opacity further reinforces the observation of incomplete miscibility of the two constituents. Relatively large domains must be present and to these are ascribed the somewhat lower than expected water contact angles for the less silicone rich composites; the domains derived from 2 must also be presenting at the air interface – the silicone is unable to completely coat the surface.
The thermal stability of silicone polymers is perceived to be quite good; silicone polymers typically only start to degrade at ∼385 °C,22,23 and much higher temperature stability has been reported.24 For an organic molecule, the soybean oil is reasonably thermally stable; most reports suggest that the degradation of the oil starts after ∼300 °C. Soybean oil polyurethanes also exhibit quite good thermal stability.25
Silicone polymers are known to provide additional thermal stability to composite polymer systems. For example, simple polyurethanes, depending on structure, undergo thermal decomposition (TGA) starting well below 400 °C,26 while silicone polyurethane copolymers begin to decompose well above 400 °C.27,28 That thermal stabilizing effect is similarly exhibited with the silicone/soybean copolymers (Fig. S7, ESI†), which start decomposition at much higher temperatures than pure organic polymers.
The main objective of this research was to dilute the energy impact of silicone (via silicon) synthesis by the incorporation of derivatives of natural materials. That objective was met, with the synthesis of composites of wide-ranging silicone content from about 10 to 95% to give flexible silicone elastomers. During synthesis, the aza-Michael addition to the acrylate, used to create the linkage, was preferred over amidation of the ester, a process that would have cleaved the two constituents (Fig. 1D). While the ester groups binding them will inevitably succumb to hydrolysis, they do not react readily; boiling in water did not lead to significant changes in the properties of the silicone/soybean composite. It is proposed, therefore, that applications could easily be found for elastomers that possess reasonable thermal and hydrolytic stability (providing color and opacity do not compromise requirements).
Previous studies with soybean oil-derived materials and silicones exploited silylated soybean oil as a filler and trialkoxy-siloxanes as crosslinkers in a room temperature vulcanization (RTV) catalyzed by dibutyltin dilaurate (DBTL).6,29 The known toxicity of the tin catalyst is disadvantageous, particularly compared to the catalyst free formulation reported here. The RTV system showed that an increase in the concentration of soybean oil inside the material led to more brittle materials. Perhaps because there is some phase segregation in these composite materials, the materials even at low silicone content are stiff but not brittle and soften with increasing silicone content.
Elastomers are frequently difficult to reprocess at end of life, controlled degradation is desirable so that the constituents can be repurposed in the original form, or at least recovered for use, typically, in a lower value application. Mild base degradation led to conversion of the elastomers to oils. It is hoped that analogous degradation processes can be elicited by enzymes, an examination of which is underway. However, this outcome is already quite encouraging, as processability and degradation processes will speed up as the surface areas of the constituents are increased. Although the silicone/soybean composites may be degraded under mild conditions, complete degradation is facilitated by more aggressive conditions via both ester hydrolysis and depolymerization of the silicone products. No attempt was made to identify the volatile silicone constituents, but these are inferred to be volatile cyclic silicones and alkoxysilanes (Fig. 4).30
One of the drivers for this research was learning if one could replace significant quantities of silicone by other, less energy intensive, preferably natural feedstocks without significantly degrading the beneficial properties that silicones bring. The silicone constituents in silicone/soybean elastomers manifest their properties in the composite with respect to thermal stability and surface energy. As has been shown, these materials can be easily degraded, and the oils can be separated easily by a simple extraction where one can obtain in one phase the siloxane polymer and in the other the degraded products of the soybean oil.
The proposed systems in this paper have an advantage compared to alternative systems in that linking soybean oil to silicones involves clean, facile nucleophilic addition that can be done without the need for any type of catalyst or solvent, and without the production of any type of byproduct. Additionally, the quantity of reacted acrylics can be controlled, which provides an opportunity to tailor the final mechanical and other properties of the material. Finally, to a degree, the method allows the incorporation of silicone properties in an elastomer with much lower silicone content.
Experimental
Materials
Soybean oil (SBO), ethylenediamine, hydrogen peroxide, Amberlite® IR-120, 1,3,5-trimethoxybenzene (≥99%), acetic acid, anhydrous Na2SO4, acrylic acid and hydroquinone were obtained from Sigma-Aldrich. Potassium hydroxide was obtained from Caledon Laboratories Ltd. Ethanol (95%) was obtained from Thermo-Fisher. Telechelic aminopropyl-terminated silicones (H2N(CH2)3Me2SiO(Me2SiO)nSiMe2(CH2)3-NH2): bis(3-aminopropyltetramethyldisiloxane (T2, n = 0, Mn = 248.52 g mol−1), DMS-A11 (T12, n = 10, Mn = 890 g mol−1), DMS-A15 (T36, n = 34, Mn ∼2665 g mol−1), DMS-A21 (T59, n = 57, Mn ∼4365 g mol−1); vinylsiloxane-containing silicone (VDT-5035 48–52% vinyl, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and HMe2Si-terminated silicone (DMS-H03, 450 g mol−1), were obtained from Gelest, Inc. and were used as received. The solvents used in this study were dried by passing through activated alumina column under a nitrogen stream before use.
000 g mol−1) and HMe2Si-terminated silicone (DMS-H03, 450 g mol−1), were obtained from Gelest, Inc. and were used as received. The solvents used in this study were dried by passing through activated alumina column under a nitrogen stream before use.
Methods
1H and 29Si NMR spectra were recorded using a Bruker AV-600 spectrometer at room temperature with CDCl3 as solvent and analyzed using Bruker Topspin software. For 29Si NMR, chromium(III) acetylacetonate was used as a paramagnetic relaxation agent. Infrared spectroscopy was performed with a Thermo Scientific Nicolet 6700 FT-IR spectrometer using a Smart iTX attenuated total reflectance (ATR) attachment. Gel Permeation Chromatography was performed on a Waters 1500-series HPLC pump coupled to a Waters 2414 refractive index detector. Calibration was done using polystyrene calibration kit S-M-10 (Lot 85) from Polymer Laboratories; HPLC grade toluene was used as eluent. Thermogravimetric analyses were done using a Mettler Toledo TGA-DSC 3+ using 70 μL alumina pans (2 to 5 mg of sample). A 30 cm3 min−1 argon purge was applied and the furnace was heated from 25 °C to 500 °C at 10 °C min−1 (Fig. S7†) Tensile tests were performed on a Instron 4411C5983 with 4400 series electronics at room temperature with a 50 N load cell; the specimen gauge length was ∼20.00 mm and a tensile speed of 25 mm min−1. A Shore OO durometer (Rex Gauge Company, Inc., US) was used to characterize the hardnesses of the elastomers. Sessile drop water contact angles were measured by taking photographs of the sessile drop processing using ImageJ.Synthesis of epoxidized soybean oil
To a mixture of soybean oil (100.0 g, 0.475 mol of alkenes; note: this sample of soybean oil had 4.2 alkenes per mol as measured by 1H NMR using 1,3,5-trimethoxylbenzene as internal standard.) and acetic acid (14.269 g, 0.238 mol) in toluene (100 mL), Amberlite® IR-120 hydrogen form (9.509 g) was added and the mixture was stirred at 55 °C. Once that temperature was reached, hydrogen peroxide (30%, 80.825 g, 0.713 mol) was added dropwise over 1 h through a dropping funnel; the reaction mixture was stirred at 55 °C for 24 h. The reaction mixture was filtered to remove the Amberlite® IR-120 and the resulting toluene solution was washed three times with water (3 × 100 mL) and dried over anhydrous Na2SO4. After filtration and removal of solvents, 94.7 g of light-yellow oil (88% yield) was obtained.
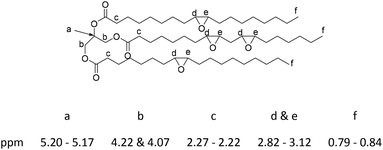
1H NMR (δ, 600.13 MHz, CDCl3): 0.79–0.84 (m, 8H), 0.95–0.99 (m, 1H), 1.18–1.69 (m, 67H), 2.22–2.27 (m, 6H), 2.82–3.12 (m, 9H), 4.07 (dd, 2H, J = 5.88, 11.85 Hz), 4.22 (dd, 2H, J = 4.20, 11.88 Hz), 5.17–5.20 (m, 1H) ppm. 13C NMR (δ, 150.9 MHz, CDCl3): 13.9, 14.0, 14.1, 22.6, 22.7, 22.8, 24.8, 24.8, 24.9, 26.1–27.9 (m), 29.0–29.7 (m), 31.6, 31.8, 31.9, 33.9, 34.0, 34.1, 54.2, 54.3, 56.6–57.2 (m) (epoxy C), 62.1, 68.9, 172.7, 173.2, 173.3 ppm (Fig. S8 and S9, ESI†).
Synthesis of the acrylated soybean oil
In a 500 mL round-bottomed flask epoxidized soybean oil (60 g, 0.063 moles) was added and, with stirring at room temperature, acrylic acid (19.55 mL, 0.284 mol) was added via syringe. Hydroquinone (0.1 mol%, 0.284 mmol) was also added as an inhibitor to suppress radical polymerization. The mixture was stirred for 10 min and then heated to 120 °C in an oil bath for 12 h with constant stirring. Purification of the acrylated soybean oil was performed by dissolving the oil in toluene and then washing the organic phase with NaHCO3 (0.1 M aq. × 3) and brine. The organic phase was collected, Na2SO4 was added to dry the solution, which was then filtered. The product was concentrated using rotatory evaporation to give the acrylated soybean oil 68 g (86%).1H NMR (δ, 600.13 MHz, CDCl3): 0.84–0.87 (m, 9H), 1.18–1.67 (m, 70H), 2.27–2.31 (m, 6H), 2.62–2.74 (m, 4H), 4.09–4.15 (m, 2H), 4.23–4.30 (m, 2H), 4.33–4.47 (m, 4H), 5.20–5.27 (m, 1H), 5.78–5.92 (m, 3H), 6.04–6.17 (m, 3H), 6.33–6.49 (m, 3H) ppm. 13C NMR (δ, 150.9 MHz, CDCl3): 13.9, 22.6, 24.6, 28.7–29.6 (m), 31.8, 34.0, 53.2, 59.8, 61.9, 68.8, 128.2, 131.2, 165.9, 170.3, 173.3 (Fig. S10 and S11, ESI†).
Model reaction with benzylamine
In a general procedure, 2 (0.5 g, 0.404 mmol) was transferred to a 20 mL vial and was dissolved in toluene (3 mL) to decrease the viscosity of the mixture. After 5 min of stirring benzylamine was added (from 0.101→0.404 mmol – 25–100% based on acrylate, Table S1 ESI†). The mixture was stirred for 12 h at room temperature and characterized by 1H NMR to follow the decrease of the acrylate groups (Fig. S1, ESI†).Synthesis of acrylated soybean oil elastomers crosslinked with telechelic amino silicones (T12 samples): general procedure
Acrylated soybean oil 2 (1 g, 0.809 mmol) was transferred to a 24 wellplate via syringe. Then T12 (0.359 g, 0.404 mmol) was added stirred to give an opaque yellow mixture; crosslinking to produce the elastomer was complete within 1 h. Related elastomers were prepared using other telechelic aminosilicones (Table 1 and Table S2†). *Note: the crosslinking rate increases in proportion to the concentration of the crosslinker; higher molar mass materials required more time to crosslink (Table 1).For IR data and an NMR spectrum, see Fig. S2 and Fig. S3 (ESI†); for Shore OO hardness and contact angle, see Fig. 2.
Structural characterization of remaining acrylic groups on the elastomers
Samples T2-25 and T12-50 were separately ground using mortar and pestle with liquid N2 until a fine dust formed. The particulate was placed inside an NMR tube and swollen using CDCl3 by 2 h after which 1H NMR of were taken (Fig. S3, ESI†).Hydrolytic stability of the acrylated soybean oil crosslinked with amino silicones
Samples T12-25 and T12-50, respectively, were weighed Winitial and then placed in 50 mL round-bottomed flasks with 20 mL of distilled water. The samples were refluxed at 100 °C for 24 h using an oil bath. After reflux, the samples were weighed and then placed in an oven at 60 °C for 24 h and then weighed again (Wfinal).T12-25: Winitial = 637 mg, Wfinal = 600 mg, recovery Wi/Wf = 94%. T12-50: Winitial = 561 mg, Wfinal = 533 mg, recovery = 95%.
Model, unfilled silicone elastomer
Vinyl siloxane copolymer (9 g (56.2 mmol), VDT-5035 48–52% vinyl, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was placed in a FlackTek 20 cup with DMS-H03 (1.60 g (2.25 mmol), Mn = 753.84 g mol−1) and speedmixed in a FlackTek mixer for 1 min at 3300 rpm. Karstedt's catalyst (3 μL, 6.71 mmol) was then directly added from the bottle and speed mixed for 1 min at 3300 rpm. The sample was left to cure for 12 h at 60 °C to give a Shore OO durometer of 72.
000 g mol−1) was placed in a FlackTek 20 cup with DMS-H03 (1.60 g (2.25 mmol), Mn = 753.84 g mol−1) and speedmixed in a FlackTek mixer for 1 min at 3300 rpm. Karstedt's catalyst (3 μL, 6.71 mmol) was then directly added from the bottle and speed mixed for 1 min at 3300 rpm. The sample was left to cure for 12 h at 60 °C to give a Shore OO durometer of 72.
Degradation of the acrylated soybean oil and aminosilicone elastomers
For the degradation tests, potassium hydroxide (0.28 g, 5 mmol) was dissolved in ethanol (40 mL) in a 50 mL round-bottomed flask. Sample T59-50 (1.01 g) was added and the flask was placed in an oil bath and heated at 40 °C for 1 h. The degradation products were concentrated using rotatory evaporation. After removal of the solvents, a brownish viscous oil was obtained. CDCl3 was added, to give a translucent solution. The brownish viscous oil residue was dispersed in D2O. NMR spectra of both phases were measured. The phases were separated, solvents removed under reduced pressure and then characterized using 1H, 13C (Fig. S4, S5 and S6 ESI†) and GPC.Mass1 = 0.344 g (dispersed in CDCl3)
Mass2 = 0.519 g (dispersed In D2O)
Total of mass recovered = 0.863 g (85% of the original mass)
Conclusions
Soybean (or other vegetable) oils are readily converted first to epoxy and then acrylated analogues by reaction with acrylic acid. Silicone elastomers based on the acrylated soybean oil, with silicone contents ranging from about 90 down to 10%, were readily prepared by the catalyst-free, aza-Michael reaction with aminoalkylsilicones. The opaque elastomers retain many of the physical properties associated with silicone elastomers, including thermal stability, durometer and surface energy. The materials are more sustainable than pure silicone elastomers, as: less silicone (which has a high energy input) is required for a given application, and the products readily undergo degradation to silicone oils by base hydrolysis of the esters, which should facilitate recovery and reuse.Author contributions
MAB, YC and MMZ were jointly responsible for supervisorship. MAB was responsible for obtaining resources and, with MMZ, wrote and modified the paper. The project was conceptualized by MAB, YC and MMZ. MMZ, YC, and EDB performed all the syntheses and analyses.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada for financial support and Dr Paul Zelisko, Brock University, for helpful discussions and some preliminary degradation experiments with lipase.References
- M. A. Brook, in Silicon in Organic, Organometallic and Polymer Chemistry, John Wiley & Sons, Inc., New York, 2000, ch. 12, pp. 381–458 Search PubMed.
- M. A. Brook, Silicones, in Silicon in Organic, Organometallic and Polymer Chemistry, Wiley, New York, 2000, ch. 9 and 12, pp 256–308, 381–458 Search PubMed.
- A. Genest, D. Portinha, E. Fleury and F. Ganachaud, The aza-Michael reaction as an alternative strategy to generate advanced silicon-based (macro)molecules and materials, Prog. Polym. Sci., 2017, 72, 61–110, DOI:10.1016/j.progpolymsci.2017.02.002.
- A. Genest, S. Binauld, E. Pouget, F. Ganachaud, E. Fleury and D. Portinha, Going beyond the barriers of aza-Michael reactions: controlling the selectivity of acrylates towards primary amino-PDMS, Polym. Chem., 2017, 8(3), 624–630, 10.1039/C6PY01802E.
- G. Lu, A. Yepremyan, S. Godfrey, C. Mohr, M. Herrlein and M. A. Brook, Aza-Michael silicone cure is accelerated by β-hydroxyalkyl esters, J. Polym. Sci., 2021, 59(17), 1935–1941, DOI:10.1002/pol.20210390.
- C. B. Gale, B. Chin, C. Tambe, D. Graiver and M. A. Brook, Silicone Structurants for Soybean Oil: Foams, Elastomers, and Candles, ACS Sustainable Chem. Eng., 2019, 7(1), 1347–1352, DOI:10.1021/acssuschemeng.8b05047.
- R. P. Wool, Properties of Triglyceride-based Thermosets, in Bio-Based Polymers and Composites, ed. R. P. Wool and X. S. Sun, Academic Press, Burlington, 2005, pp. 202–255 Search PubMed.
- R. P. Wool, Polymers and Composite Resins from Plant Oils, in Bio-Based Polymers and Composites, ed. R. P. Wool and X. S. Sun, Academic Press, Burlington, 2005, pp. 56–113 Search PubMed.
- A. J. Kamphuis, F. Picchioni and P. P. Pescarmona, CO2-fixation into cyclic and polymeric carbonates: principles and applications, Green Chem., 2019, 21(3), 406–448, 10.1039/C8GC03086C.
- A. Biswas, A. Adhvaryu, S. H. Gordon, S. Z. Erhan and J. L. Willett, Synthesis of Diethylamine-Functionalized Soybean Oil, J. Agric. Food Chem., 2005, 53(24), 9485–9490, DOI:10.1021/jf050731o.
- H. Gholami and H. Yeganeh, Soybean oil-derived non-isocyanate polyurethanes containing azetidinium groups as antibacterial wound dressing membranes, Eur. Polym. J., 2021, 142, 110142, DOI:10.1016/j.eurpolymj.2020.110142.
- A. Lee and Y. Deng, Green polyurethane from lignin and soybean oil through non-isocyanate reactions, Eur. Polym. J., 2015, 63, 67–73, DOI:10.1016/j.eurpolymj.2014.11.023.
- H. Q. Pham and M. J. Marks, Epoxy Resins, in Kirk–Othmer Encyclopedia of Chemical Technology, Wiley, 2004 Search PubMed.
- B. R. Moser, S. C. Cermak, K. M. Doll, J. A. Kenar and B. K. Sharma, A review of fatty epoxide ring opening reactions: Chemistry, recent advances, and applications, J. Am. Oil Chem. Soc., 2022, 99(10), 801–842, DOI:10.1002/aocs.12623.
- A. J. O'Lenick Jr. and J. K. Parkinson, Three-Dimensional HLB: This revolutionary development helps formulators choose surfactants for stable oil, water and silicone emulsions, Cosmet. Toiletries, 1996, 111, 37–44 Search PubMed.
- D. Behera and A. K. Banthia, Synthesis, characterization, and kinetics study of thermal decomposition of epoxidized soybean oil acrylate, J. Appl. Polym. Sci., 2008, 109(4), 2583–2590, DOI:10.1002/app.28350.
- L. Feng, H. Yang, X. Dong, H. Lei and D. Chen, pH-sensitive polymeric particles as smart carriers for rebar inhibitors delivery in alkaline condition, J. Appl. Polym. Sci., 2018, 135(8), 45886, DOI:10.1002/app.45886.
- M. J. Owen, Siloxane Surface Activity, in Silicon-Based Polymer Science: A Comprehensive Resource, ed. J. M. Zeigler and F. W. G. Fearon, American Chemical Society, Washington, D.C., 1990, pp. 705–739 Search PubMed.
- P. G. M. Wuts and T. W. Greene, Greene's Protective Groups in Organic Synthesis, Wiley-Interscience, New Jersey, 4th edn, 2006 Search PubMed.
- M. A. Brook, in Silicon in Organic, Organometallic, and Polymer Chemistry, John Wiley & Sons, Inc., New York, 2000, ch. 9, p. 256 Search PubMed.
- T. Rambarran, F. Gonzaga, M. A. Brook, F. Lasowski and H. Sheardown, Amphiphilic thermoset elastomers from metal–free, click crosslinking of PEG–grafted silicone surfactants, J. Polym. Sci., Part A: Polym. Chem., 2015, 53(9), 1082–1093, DOI:10.1002/pola.27539.
- L. Yang, H. Dai, A. Yi, B. Lin and G. Li, Structure and properties of partially epoxidized soybean oil, J. Therm. Anal. Calorim., 2008, 93(3), 875–879, DOI:10.1007/s10973-008-9043-x.
- N. Grassie and I. G. Macfarlane, The thermal degradation of polysiloxanes—I. Poly(dimethylsiloxane), Eur. Polym. J., 1978, 14(11), 875–884, DOI:10.1016/0014-3057(78)90084-8.
- F. de Buyl and S. Yoshida, Degradation Mechanisms of Silicones, in Reliability of Organic Compounds in Microelectronics and Optoelectronics: From Physics-of-Failure to Physics-of-Degradation, ed. W. D. van Driel and M. Yazdan Mehr, Springer International Publishing, Cham, 2022, pp. 1–31 Search PubMed.
- K. Mizera and J. Ryszkowska, Thermal properties of polyurethane elastomers from soybean oil-based polyol with a different isocyanate index, J. Elastomers Plastics, 2018, 51(2), 157–174, DOI:10.1177/0095244318772323.
- E. G. Bajsić and V. Rek, Thermal stability of polyurethane elastomers before and after UV irradiation, J. Appl. Polym. Sci., 2001, 79(5), 864–873, DOI:10.1002/1097-4628(20010131)79:5<864::AID-APP110>3.0.CO;2-D.
- S. H. Liu, M. Y. Shen, C. F. Kuan, H. A.-O. Kuan, C. Y. Ke and C. L. Chiang, Improving Thermal Stability of Polyurethane through the Addition of Hyperbranched Polysiloxane, Polymers, 2019, 11(4), 697, DOI:10.3390/polym11040697.
- C.-A. Xu, M. Lu, Z. Tan, Z. Qu, K. Wu and J. Shi, Study on the surface properties and thermal stability of polysiloxane-based polyurethane elastomers with aliphatic and aromatic diisocyanate structures, Colloid Polym. Sci., 2020, 298(9), 1215–1226, DOI:10.1007/s00396-020-04695-4.
- C. Tambe, J. Kaufmann, D. Graiver and R. Narayan, Reactive blends derived from modified soybean oil and silicone, J. Polym. Sci., Part A: Polym. Chem., 2016, 54(19), 3086–3093, DOI:10.1002/pola.28192.
- B. Rupasinghe and J. C. Furgal, Degradation of silicone-based materials as a driving force for recyclability, Polym. Int., 2022, 71(5), 521–531, DOI:10.1002/pi.6340.
Footnote |
| † Electronic supplementary information (ESI) available: Model aza-Michael reaction formulations; IR and NMR data of starting material, 2, model reactions; elastomers and degraded elastomer products; table of silicone elastomer content; TGA data for 3 elastomers. See DOI: https://doi.org/10.1039/d2gc04073e |
| This journal is © The Royal Society of Chemistry 2023 |