Open Access Article
Open Access ArticleAcetaldehyde in the indoor environment
Tunga
Salthammer
 *
*
Fraunhofer WKI, Department of Material Analysis and Indoor Chemistry, 38108 Braunschweig, Germany. E-mail: tunga.salthammer@wki.fraunhofer.de
First published on 10th December 2022
Abstract
Acetaldehyde is a very volatile carbonyl compound with a boiling point of 20.1 °C. The industrial importance of acetaldehyde is comparatively small and tends to decrease. Nevertheless, the substance is ubiquitous in the environment, because acetaldehyde occurs in many chemical and biological processes as an intermediate and byproduct. Acetaldehyde plays an important role in atmospheric chemistry, it is formed during combustion and from the oxidation of fats and oils. Acetaldehyde primarily occurs in the metabolism of plant and animal organisms. Due to the diverse chemical reactions, there are a large number of potential sources, which means that acetaldehyde is also important for the indoor environment. Building products are often rich in fatty acids, which slowly decompose under the formation of aldehydes. Sources from human activities include the preparation of food, burning of candles, wood, and ethanol, as well as the consumption of cigarettes and e-cigarettes. Many other products and devices can release acetaldehyde, the human respiratory gas must not be disregarded, and acetaldehyde is present in outdoor air. Several organizations and institutions regard acetaldehyde as a priority indoor pollutant. This is due to its acute and chronic effects, but also to its classification as a carcinogenic substance. There are sufficient data for acetaldehyde in the atmosphere, as the substance is easily accessible analytically using the DNPH method and is thus often recorded in measurements. Nevertheless, to date, there has been no scientific work that comprehensively characterizes and evaluates acetaldehyde indoors. From the point of view of the necessity of such a summary, an overview of the properties of acetaldehyde and the most important reaction mechanisms is given, followed by a discussion of potential sources and indoor air concentrations. Finally, a health-related assessment of the substance is provided on the basis of indoor guide values.
Environmental significanceAcetaldehyde is a substance that is of great importance in environmentally relevant processes in both living and non-living nature. At the same time, the occurrence and thus the health effects of acetaldehyde on humans are difficult to evaluate, since it is an intermediate and byproduct whose formation and release depends on many factors. On average, exposure to acetaldehyde indoors is significantly higher than outdoors. It therefore seems appropriate and necessary to carry out an assessment of acetaldehyde for the indoor environment. |
1 Introduction
A large number of organic indoor air pollutants have been identified up to the present day. However, the scientific and public interest in specific substances and substance groups changes over certain periods of time. There are many reasons for this, including their use, substitution and bans in industrial products, new toxicological classifications, and the introduction or updating of guide values.1 For many years, acetaldehyde was only considered in classical atmospheric chemistry because it is a byproduct from the oxidation of ethane and a precursor to the formation of peroxyacetyl nitrate (PAN).2 Interest in acetaldehyde as an indoor pollutant began with a WHO report from 1989,3 which states cigarette smoke as the main source. In the early 1990s, it was shown that there are a number of other sources of acetaldehyde indoors. The substance is easily accessible analytically via the DNPH method and has therefore often been measured. However, it mostly went under the radar, since at that time the interest in very volatile and volatile organic compounds (VVOCs and VOCs) was dominated by formaldehyde, solvents, terpenes, and higher aldehydes. The acetaldehyde concentrations measured indoors are often inconspicuous and are pushed into the background by other carbonyl compounds.In 2001, an expert group of the European Union classified acetaldehyde as one of the 14 indoor pollutants, which need a detailed assessment.4 Logue et al.5 evaluated the results from 77 published studies reporting measurements of chemical pollutants in residences in the United States. Acetaldehyde was one of the 9 substances that were identified as priority hazards based on the robustness of measured concentration data and the fraction of residences that appeared to be impacted. This assessment of Logue et al.5 is essentially based on the U.S. EPA's Reference Concentration for Chronic Inhalation Exposure (RfC) of 0.009 mg m−3. In his 2009 publication on changes in indoor pollutants since the 1950s, Weschler1 names acetaldehyde as one of the five VVOCs, but states that the trend regarding its importance for the indoor environment is undetermined. On the basis of pending reassessments by IARC and the EU with regard to carcinogenicity, Salthammer6 later came to the conclusion that in the future, acetaldehyde will receive a lot more attention in indoor air studies.
With the exception of formaldehyde, the group of VVOCs, which includes acetaldehyde, was not considered in the first approaches for the assessment of building materials.7 It took until 2013 that acetaldehyde was recognized as a priority pollutant in the harmonization framework for health-based evaluation of indoor emissions from construction products in the European Union and an LCI (lowest concentration of interest) value was derived.8 Despite the growing interest in indoor exposure to acetaldehyde, there is still no comprehensive overview of this substance. This may also be due to the fact that acetaldehyde is difficult to classify as a typical intermediate and reaction product. There is only vague information on the respective sources and emission levels, the same applies to the room air concentrations. In the present work, the physical and health-related properties of acetaldehyde are first described. After an outline of the analytical methods, the second part focuses on the emission sources and indoor air concentrations with reference to guide values. The cited literature was chosen to be representative for the respective topic. With the information presented, the reader has the opportunity to better assess the emission of and exposure to acetaldehyde indoors.
2 Properties of acetaldehyde
2.1 Molecular properties
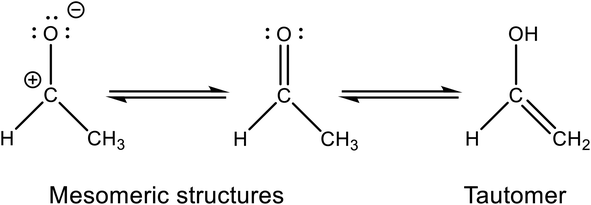
The mesomeric boundary structures of the aprotic compound acetaldehyde are shown in Fig. 1. Its tautomer vinyl alcohol (CH2 = CH–OH) is not stable at room temperature. The absorption maximum at 290 nm results from an n → π* transition, and the absorption cross section at this wavelength is σ(298 K) = 4.86 × 10−20 cm2 per molecule.9 In the atmosphere, acetaldehyde is an important precursor for peroxyacetyl nitrate (PAN). In the first step (see eqn (1)), the main reaction with the OH radical leads to the acetyl radical. PAN is then formed in the well-known subsequent reactions with oxygen and nitrogen dioxide.2| CH3–CHO + ȮH → H3C–ĊO + H2O | (1) |
At 20 °C, acetaldehyde is a low-viscosity, flammable and colorless liquid. The substance is completely miscible with water, at low concentrations it causes a sharp and fruity odor. According to Ruth,10 the odor thresholds are between 0.0002 mg m−3 and 4.14 mg m−3. Amoore and Hautala11 state an odor threshold in air of 0.050 ppm. Devos et al.12 evaluated various studies and arrived at an odor threshold of 0.19 ppm (0.34 mg m−3). Because of its fruity aroma, acetaldehyde is used as a flavoring agent.13 The molecular properties of acetaldehyde are summarized in Table 1. The LFER (linear free energy relationship) parameters are Abraham coefficients. They allow kinetic and thermodynamic molecular properties to be calculated using QSAR approaches.14 Acetaldehyde is a reactive compound. At room temperature, the trimeric molecule paraldehyde is formed by cyclization in the presence of acids. Furthermore, acetaldehyde tends to undergo aldol addition and aldol condensation. Acetaldehyde also forms hydrates in aqueous solution.
| Parameter | Value | Ref. |
|---|---|---|
| Systematic IUPAC name | Ethanal | |
| Molecular formula | CH3CHO | |
| CAS | 75-07-0 | |
| SMILES | CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
|
| Molecular weight (MW) | 44.05 g mol−1 | 15 |
| Melting point (m.p.) | −123.4 °C | 15 |
| Boiling point (b.p.) | 20.8 °C | 15 |
| Density (ρ) | 0.785 g cm−3 (20 °C) | 15 |
| Dipole moment (μ) | 2.750 D | 15 |
| Polarizability (α) | 4.30 × 10−24 cm3 | 16 |
| Refraction index (nD) | 1.3316 (20 °C) | 15 |
| Dielectric constant (ε) | 21.0 (20 °C) | 15 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW KOW |
0.45 | 17 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA KOA |
1.98 | 18 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KHdA (L) KHdA (L) |
1.22 | 18 |
| Henry constant (H) | 1.3 × 10−1 mol (m3 Pa)−1 | 19 |
| Antoine coefficients (219–313 K) |
A = 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814 814 |
20 |
| B = 1070.6 K | ||
| C = −37.15 K | ||
| ΔvapH | 25.8 kJ mol−1 (293.3 K) | 21 |
| LFER parameters | A = 0.00 | 22 |
| B = 0.45 | ||
| E = 0.21 | ||
| S = 0.67 | ||
| L = 1.23 | ||
| V = 0.4061 | ||
| Proton affinity | 768.5 kJ mol−1 | 23 |
| k (proton transfer) | 3.36 × 10−9 cm3 s−1 | 16 |
| k (OH) | 14.7 × 10−12 cm3 per molecule per s (298 K) | 24 |
| k (O3) | 3.4 × 10−20 cm3 per molecule per s (298 K) | 24 |
| Conversion factor (T = 298 K, p = 1013 mbar) | 1 ppb = 1.801 μg m−3 |
2.2 Health-related properties
According to the Globally Harmonized System of Classification and Labeling of Chemicals, acetaldehyde is flammable (GHS02), harmful (GHS07), and a health hazard (GHS08). The European Chemicals Agency (ECHA) classifies acetaldehyde as carcinogenic (category 1B) and suspected to be mutagenic (category 2). The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 2B (possibly carcinogenic to humans) carcinogen on the basis of inadequate evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals. However, if associated with the consumption of alcoholic beverages, the classification is Group 1 (carcinogenic to humans). In 2019, IARC concluded that there were sufficient data for a reassessment. Acetaldehyde is acutely irritating to the eyes and respiratory tract and has central nervous system effects. The occupational exposure limit (OEL) in the European Union is 91 mg m−3 (50 ppm). The agreed EU-LCI value (with status 2022) is 300 μg m−3.25 The previous value as derived in 2013 is 1200 μg m−3.8 EU-LCI values are health-based reference concentrations of volatile organic compounds for inhalation exposure used to assess emissions after 28 days from a single product during a laboratory test chamber procedure as defined in the EN 16516.26The most comprehensive study on the toxicology of acetaldehyde with reference to the indoor environment was presented by the ad hoc working group of the German Federal Environment Agency for the derivation of indoor air guide values.27 The study by Dorman et al.28 was used as the starting point for deriving the LOAEC (lowest observed adverse effect concentration). After subchronic inhalation exposure of rats to 270 mg m−3 (150 ppm), Dorman et al.28 observed degeneration and vacuole formation in the olfactory nasal epithelium with loss of olfactory neurons. Note that despite the carcinogenic properties of acetaldehyde, guide values are derived. It is assumed that at concentrations below these guide values, no significant contribution to the cancer risk for humans is to be expected. A detailed discussion of indoor guide values is provided in Section 6.
3 Production and formation of acetaldehyde
3.1 Industrial production
Acetaldehyde was first synthesized by Carl Wilhelm Scheele in the 18th century when he was trying to oxidize ethanol. However, it was Justus Liebig who characterized the compound as a dehydrated alcohol and coined the name “aldehyde”. The classic synthesis with ethanol involves the use of strong oxidizing agents such as chromic acid. For large-scale synthesis, acetaldehyde is produced using the Wacker process by catalytic water addition to ethene with simultaneous air oxidation over fixed-bed catalysts. Eqn (2) describes the formal reaction.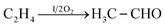 | (2) |
The annual global production of acetaldehyde varies between one and two million tons, depending on its importance and demand for the raw material market. It essentially serves as an intermediate in the production of several industrial chemicals.29
3.2 Formation via natural processes
Combustion processes are the main source of acetaldehyde in outdoor air. These include road traffic30 and emissions in the industrial31 and private32 sectors. The basic mechanisms of potential indoor sources are explained below. Specific sources are discussed in Section 5.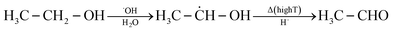 | (3) |
Ethanol combustion also takes place indoors, mostly with open flames. Typical sources are ethanol stoves and fondue burners.35,36
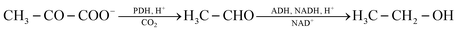 | (4) |
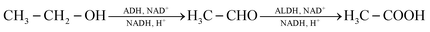 | (5) |
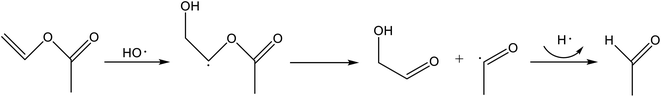 | ||
| Fig. 2 Photocatalytic formation of acetaldehyde from vinyl acetate as proposed by Gandolfo et al.69 | ||
4 Sampling and analysis of acetaldehyde
The concentration of short-chain aldehydes in the gas phase can be precisely measured using a variety of methods. A good overview is provided by Vairavamurthy et al.70 For the specific determination of individual components, however, colorimetric methods using UV detection are not recommended, since the sum values of different carbonyls are essentially determined. This applies both to derivatization with the Hantzsch reagent 3,5-diacetyl-1,4-dihydrolutidine (DDL) and to 3-methyl-2-benzothiazolonehydrazone (MBTH).71 The DNPH method and online mass spectrometry are of major practical importance today. These methods are discussed in detail below.4.1 DNPH method
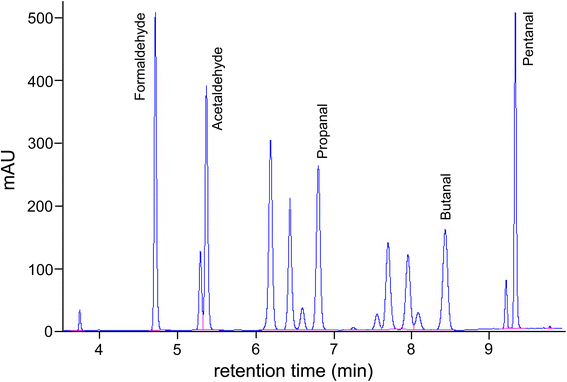
As shown in Fig. 3, carbonyl compounds can react with 2,4-dinitrophenylhydrazine (DNPH) to form the hydrazone by elimination of water. The derivatives are analyzed utilizing high-performance liquid chromatography (HPLC) with UV detection or diode array detection. This method, as described in ISO 16000-3,72 is used most frequently worldwide to determine acetaldehyde in indoor air. Cartridges packed with silica gel are used to sample aldehydes and ketones. The silica gel is coated with dinitrophenylhydrazine as the derivatization reagent. Approximately 50–100 L of air is drawn through the cartridge with a volume flow of 0.5–1.5 L min−1. The derivatization, therefore, takes place directly on the cartridge. The hydrazones formed are then eluted with acetonitrile. This eluate is used for the HPLC determination. The chromatographic separation is carried out using a reverse phase C18 column and a water/acetonitrile or water/methanol solvent combination as the mobile phase. The absorption maxima for the different hydrazones are in the range of 345–425 nm.70 For the acetaldehyde DNPH derivative the absorption maximum is 363 nm. The precise analytical parameters for the DNPH method can also be found in ISO 16000-3.72Fig. 4 shows the chromatogram of a multistandard. Modern HPLC devices allow complete spectra to be recorded, which significantly improves the sensitivity of the method. Interference of ozone73 can be avoided by the use of an ozone scrubber placed in front of the cartridge. Nitrogen dioxide (NO2) also interferes by forming 2,4-dinitrophenyl azide.74 An automated sampling and analysis system based on DNPH was introduced by Aiello and McLaren.75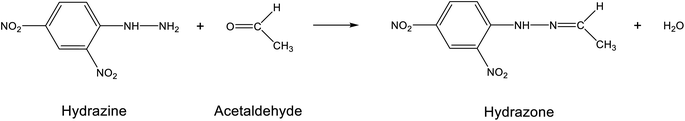 | ||
| Fig. 3 Formation of 2,4-dinitrophenylhydrazone from DNPH and acetaldehyde. See ISO 16000-3 (ref. 72) for the analytical parameters. | ||
For standardized analysis, the usual detection limits are in the range of 0.5–1.0 μg m−3. Note that the DNPH method is primarily suitable for short-chain aldehydes C1–C4. From C5 (pentanal), sampling on Tenax TA followed by thermal desorption gas chromatography/mass spectrometry (GC/MS) is preferable.76 Acrolein cannot be reliably analyzed by the DNPH method.77 DNPH is also suitable for passive sampling. The collection rates for acetaldehyde and other carbonyl compounds were determined by Birmili et al.78
4.2 PTR-MS and SIFT-MS
If a high time resolution is required, proton transfer reaction mass spectrometry (PTR-MS) can be used to measure acetaldehyde. In this method, the corresponding compound of molecular weight MW is protonated by H3O+ according to eqn (6), so the target ion is m/z [MW + 1].| CH3–CHO + H3O+ → C2H5O+ + H2O | (6) |
Reaction (6) takes place because the proton affinity of acetaldehyde, at 768.5 kJ mol−1,23 is significantly higher than that of water, at 691 kJ mol−1.79 The essential components of a PTR-MS instrument are the ion source, the drift tube and the analysis system (quadrupole mass analyzer or time-of-flight (TOF) mass spectrometer). Commercially available PTR-MS devices have a response time of about 100 ms, which enables a high temporal resolution, and reach a detection limit in the lower ppt range. The theoretical and technical details of the technique are described in detail in the book by Ellis and Mayhew.80
With a molecular weight of 44.05 g mol−1, the nominal mass of the target ion when detected with a low-resolution quadrupole mass filter is m/z 45. It is advantageous that only comparatively few substances such as carbon dioxide, propane, and ethylene oxide can interfere with this m/z value. At 541 kJ mol−1, carbon dioxide has a lower proton affinity than water and is therefore hardly protonated, but the CO2H+ ion can still cause interference due to the high concentration of carbon dioxide in the air. The proton affinity of propane is 626 kJ mol−1. However, the concentration in the outside and inside air can vary greatly,81 since propane is a component of liquid gas and is also released through combustion processes. Ethylene oxide is not common in indoor air. Price et al.82 discussed correction factors for acetaldehyde when using a quadrupole PTR-MS to account for possible interference from the ethylene glycol fragment HO–CH2–ĊH2. An exact assignment can be made with high-resolution TOF mass spectrometry, which also allows the analysis of isotopes and complex mixtures of substances, which usually occur in the indoor environment.83 The monoisotopic mass of acetaldehyde (sum of the most abundant stable isotope masses of each atom in the molecule) is 44.026 Da. A comparison of the quadrupole and the TOF technique for indoor applications is provided by Schripp et al.84
A much-discussed question concerns the calibration of the PTR-MS. The use of standard compounds is possible, but usually the calibration is done via the proton transfer reaction rate constant kpt according to eqn (7).
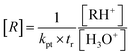 | (7) |
A closely related technique is selected-ion-flow-tube-mass-spectrometry (SIFT-MS). The main difference is that a quadrupole mass filter selects the ions produced in the source based on their mass-to-charge ratio. As a result, only certain ions get into the reaction chamber. The ions are generated by plasma discharge from cleaned, humidified air using a microwave resonator. This means that other ions such as NO+, O+2, etc. are available for selection in addition to H3O+. Fundamentals and applications are detailed in the review by Smith and Španěl.87
4.3 Other methods
Many other methods for measuring acetaldehyde in the atmosphere have been published, but these are practically irrelevant for indoor use and will not be discussed further here. A compilation of methods based on colorimetry, fluorescence, and gas chromatography, specific and non-specific for acetaldehyde, is offered by Vairavamurthy et al.70 Various reagents for the derivatization of aldehydes are described by Vogel et al.885 Indoor emission rates and sources
The basic mechanisms of acetaldehyde formation have already been described in Section 3. This part now deals with the categorization of the specific indoor sources as compiled in Table 2. The influence of the outside air is discussed in a later section.| Source | Comment | Ref. |
|---|---|---|
| Candles (scented, unscented) | 24 candles; 8 m3 chamber; range ≤16–82 μg per candle per h | 89 |
| Candles (scented) | 5 candles, chamber; max. emission rate: 1.12 μg g−1 | 90 |
| Mosquito coils and candles | 5 mosquito coils; 18 m3 chamber emission rate: 1000–2000 μg g−1 | 91 |
| Ethanol fireplaces | Different fuels; 42 m3 chamber concentration: 150–570 μg m−3 | 35 |
| Wood-burning fireplace ovens | 7 private homes; range 12–89 μg m−3 | 92 |
| Wood combustion | Different wood types and ovens emission rate: 1.3–1704 mg kg−1 | 37, 38, 93 and 94 |
| Environmental tobacco smoke | Conventional cigarettes emission rate: 2360 μg per cig.; 2480 μg per cig. | 95 |
| e-cigarettes | Formation from propylene glycol: 50–1650 μg m−3 in puffs | 96 and 97 |
| e-cigarettes | Formation from triacetin | 45 |
| e-cigarettes | Release per puff maximum emission rate: 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468 ng per puff 468 ng per puff |
98 |
| Heated tobacco products | 3 heatsticks, 200 L chamber emission rates: 18–181 μg per heatstick | 99 |
| Cooking | Laboratory kitchen, different food types emission rates: 19–110 μg (goil h)−1 | 48 |
| Cooking | Laboratory kitchen, different food types concentrations in fume: 7–274 μg m−3 | 47 |
| Building materials | 23 products; 23 L chamber emission rates: ≤1.3–500 μg (m2 h)−1 | 100 |
| Plaster with vegetable oil | Different oils, 20 L chamber emission rate: 8.0–523 μg (m2 h)−1 | 101 |
| Wood-based materials | 20 L chamber emission rates in dependency of time | 102 |
| Wood, wood-based materials | 34 samples; 1 m3 chamber concentration: ≤3–245 μg m−3 | 103 |
| Particleboard | 4 samples; 1 m3 chamber emission in dependency of surface coating | 104 |
| Leather | Emissions at 80 °C | 105 |
| Photocatalytic wall paint | 3 products; 1 m3 chamber maximum concentration: 106 μg m−3 | 64 |
| Photocatalytic wall paint | 6 products; 27 L chamber concentration (2 h): 15–898 μg m−3 | 65 |
| Photocatalytic wall paint | 3 products, flow tube photoreactor emission rate: 257 μg (m2 h)−1 | 69 |
| Photocatalytic air cleaners | 4 devices; 24 m3 and 48 m3 chamber maximum concentration: 8–67 μg m−3 | 66 |
| Photocatalytic air cleaner | 1 device, model room: 9.2–189 μmol h−1 | 106 |
| Exhaled human breath | 10 probands range: 29–82 ppb | 52 |
| Exhaled human breath | 30 probands: 50-P = 22 ppb; 95-P = 52 ppb | 107 |
Combustion processes are certainly the strongest and most important indoor sources. With regard to the starting materials, these can be roughly divided into candles, ethanol, and wood. Tobacco products are more complex in their application. In addition to the classic cigarette, there are electronic cigarettes, heat-not-burn techniques, and water pipes. However, individual product groups can also show significant differences in their emission behavior, as will be demonstrated using the example of candles. In order of an optimized burning performance, fuel, additives, wick, and candle geometry must be matched to each other. Common fuels are paraffin, stearin, wax, and palm. With scented candles, different types of additives are added. Salthammer et al.89 have detailed the constituents of various fragrances. It was found that the release of acetaldehyde from burning candles depended largely on their chemical composition. This is shown in Fig. 5, where the use of the “Fresh” and “Fruit” fragrances leads to significantly increased acetaldehyde emission rates of up to 82 μg per candle per h. These two fragrances already contain different aldehydes, esters, and oils in their formulation.89 If the candle does not contain any fragrances, only low acetaldehyde emissions result. All investigations were carried out in an 8 m3 chamber under identical conditions. In their test chamber investigations, Derudi et al.90 arrived at emission rates of between approximately 0.5 μg g−1 and 1.12 μg g−1 for five scented candles. The authors state that their results are in the range of similar studies. Lee and Wang91 examined the emissions from mosquito coils and measured high emission rates for acetaldehyde of around 1000–2000 μg g−1. The result could be expected since mosquito coils usually smolder more than they burn, which leads to the formation of byproducts. Habarta et al.35 measured acetaldehyde concentrations between 150 μg m−3 and 570 μg m−3 in 42 m3 chamber experiments on ethanol fireplaces. Wegscheider et al.108 studied the emissions of sauna essences on hot stones at 500 °C and found release rates for acetaldehyde up to 61 mg ml−1 essence.
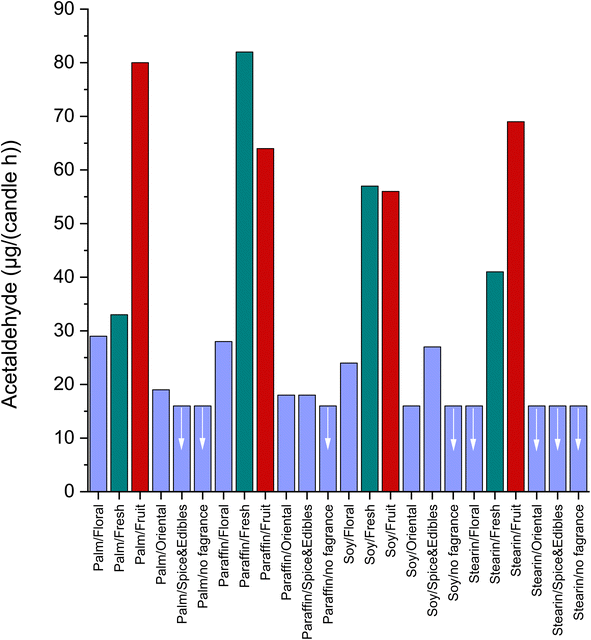 | ||
| Fig. 5 Acetaldehyde emission rates from burning candles in dependence of fuel and fragrance. Green bars represent “Fresh”, red bars represent “Fruit” and blue bars represent all other fuel/fragrance combinations. The arrows indicate an emission rate ≤16 μg (candle per h). See Salthammer et al.89 for details on candles and experimental conditions. | ||
Various works have been published on the formation of acetaldehyde during wood combustion. A summary is provided by Reda et al.38 on the basis of their own data with reference to the publications by Schauer et al.,93 Hedberg et al.94 and Cerqueira et al.37 The results differed widely with emission rates between 1.3 mg kg−1 (pellet boiler) and 1704 mg kg−1 (fireplace). The most important influencing factors were the type of wood, the wood moisture content, and the combustion process. All measurements were carried out in the exhaust gas section of the chimney. The direct impact of wood burning on indoor air quality was studied by Salthammer et al.92 When measurements were taken in a total of seven living rooms, the acetaldehyde concentration was between 12 μg m−3 and 80 μg m−3. Gustafson et al.109 also found that domestic wood burning increases the acetaldehyde concentration in living spaces.
A frequently discussed topic is emissions from smoking and vaping. The classic cigarettes are studied in detail, and the amounts of pollutants, including acetaldehyde, in mainstream, sidestream, and environmental tobacco smoke (ETS), are well known. Singer et al.95 measured acetaldehyde emission rates of 2360 μg per cigarette and 2480 μg per cigarette in ETS of a 50 m3 room. The formation of acetaldehyde in e-cigarettes is also well studied. Sleiman et al.97 as well as Azimi et al.96 assume that the first step is a dehydrogenation of propylene glycol. The methylglyoxal thus formed then thermally decomposes to formaldehyde and acetaldehyde. The acetaldehyde concentrations in the e-cigarette puffs varied between 50 μg m−3 and 1650 μg m−3. Vreeke et al.45 found that triacetin enhances the levels of acetaldehyde and other substances in the aerosol of e-cigarettes. The publication by Beauval et al.98 contains a compilation of literature data on the release of acetaldehyde from e-cigarettes with emission rates up to 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468 ng per puff. Geiss et al.110 found a significant increase in the release of acetaldehyde from e-cigarettes when the electrical power of the heating coil was increased.
468 ng per puff. Geiss et al.110 found a significant increase in the release of acetaldehyde from e-cigarettes when the electrical power of the heating coil was increased.
With some technologies, tobacco is just heated. Such so-called heat-not-burn products were investigated by Cancelada et al.99 Acetaldehyde emission rates of 151–181 μg per heatstick were measured in the mainstream and emission rates of 19–24 μg per heatstick in the sidestream. The authors conclude that exposure to acetaldehyde is significantly higher for heat-not-burn products than for e-cigarettes. Other studies also compared the release of pollutants when using indoor combustion sources, e-cigarettes, and tobacco heating systems under realistic conditions. Mitova et al.111 found an increase in acetaldehyde concentration when using a tobacco heating system, but the highest values were achieved with conventional cigarettes. Ruprecht et al.112 came to analogous results.
It has long been known that food preparation can also be a strong indoor source for acetaldehyde. A comprehensive study was published by Zhang et al.48 The emissions of carbonyl compounds from heating 5 edible oils, 3 seasonings, and 2 dishes were examined in a laboratory kitchen. The sampling took place in the air duct above the ventilation range hood. The emission rates for acetaldehyde ranged from 19 μg (goil h)−1 (heating soybean oil and chili powder) to 110 μg (goil h)−1 (heating rapeseed oil). A study with a similar design was previously performed by Peng et al.47 The aldehyde emissions in cooking oil fumes were examined. The results are essentially the same as those of Zhang et al.,48 but only the concentrations are given. For acetaldehyde, these range from 7 μg m−3 (stir frying of soybean oil) to 274 μg m−3 (deep frying of rapeseed oil). Acetaldehyde is also a flavor component in bread113 and orange juice.114
The release of acetaldehyde from building products varies greatly. Plaisance et al.100 examined the emission of 23 building products using chamber studies. Two finishing plasters were responsible for the by far highest emission rates of 291 μg (m2 h)−1 and 500 μg (m2 h)−1. In contrast, two other finishing plasters were unremarkable with regard to the release of acetaldehyde. Odaka et al.101 investigated the release of acetaldehyde from the plaster in dependence of their vegetable oil content. The highest emission rates were found when using linseed oil and perilla oil with the suspected precursor substance linolenic acid. For pine wood and plywood, Plaisance et al.100 measured 57 μg (m2 h)−1 and 32 μg (m2 h)−1, respectively. For all other products, the emission rate was ≤15 μg (m2 h)−1. Suzuki et al.102 have studied the acetaldehyde emission rates of wood-based materials as a function of time, temperature, and humidity. With initial values between about 10 μg (m2 h)−1 and 35 μg (m2 h)−1, a clear decay behavior was observed within 14 days. In their chamber studies on preventing VOC diffusion through surface-coated particleboard, Pibiri et al.104 also found a rapid decay in the acetaldehyde concentration that was independent of the layer. Schieweck103 tested wood and wood-based materials in a 1 m3 chamber under standard conditions (T = 23 °C, RH = 50%, AER = 0.4 h−1) and realistic loading rates. The chamber concentrations within 72 h were between ≤3 μg m−3 and 245 μg m−3.
Ammenn et al.105 reported that acetaldehyde, although not actively used, is a relevant substance in the manufacture of leather. The authors postulate that acetaldehyde is bound in the form of an imine through lysine residues in collagen and is released when the leather surface is thermally stressed. This can lead to high concentrations, especially in the automotive sector.
One phenomenon was the release of acetaldehyde and other pollutants from photocatalytic wall paints, which are supposed to clean the indoor air of such substances. It was quickly shown that the pollutants origin from the paint itself.64,65 Typical recipes contain the photocatalyst titanium dioxide and blends from organic binders. The OH radicals and O−2 ions formed by TiO2 upon irradiation oxidize the organic components of the binder. Salthammer and Fuhrmann,64 as well as Auvinen and Wirtanen,65 measured considerable concentrations of acetaldehyde in their chamber investigations. Based on the results of Ye et al.,115 Geiss et al.116 postulate that higher aldehydes and acids are first formed from hydrocarbon groups, which then decompose to lower aldehydes. Polyvinyl acetate is a common component of organic binders in paints, so that the reaction scheme presented by Gandolfo et al.69 (see Fig. 2) is also plausible here. Fiorentino et al.117 carried out measurements in an office room and found a significant increase in the acetaldehyde concentration in the presence of paints containing titanium dioxide and under the influence of UV light.
Another point is air-cleaning devices that work on a photocatalytic basis. Destaillats et al.118 showed that the effectiveness of many devices for acetaldehyde is insufficient, and the degradation rate is less than 50%. Hodgson et al.106 found a net production of acetaldehyde from the ultraviolet photocatalytic oxidation of typical indoor VOCs. Gunschera et al.66 conducted test chamber experiments with air purifiers and various test substances. Acetaldehyde was formed and released in relevant amounts. Some air cleaning devices work with ozone. It should therefore be noted at this point that components of the air and the ingredients of many building products such as paints59 and carpets61 form aldehydes in the presence of ozone.
The last source to be discussed here concerns human respiratory gas. Acetaldehyde is a natural component, but its concentration is highly dependent on an individual's metabolism. In experiments lasting 35 minutes each, Riess et al.52 measured typical concentrations of 30 ppb to 50 ppb acetaldehyde in the respiratory gas of 10 healthy volunteers during light physical exertion. For a person who had previously consumed alcohol, the concentration was 82 ppb. Turner et al.107 came to analogous results earlier in respiratory gas studies on 30 subjects. This can be relevant to the indoor environment. At 37 °C and 1013 mbar, 50 ppb acetaldehyde corresponds to approx. 87 μg m−3. With an assumed respiratory minute volume of 8 l at rest, the emission rate is 42 μg h−1 per person.
There are only a few studies that deal with the reverse case, i.e. with the removal of acetaldehyde from room air. Yamashita et al.119 describe the chemisorption through L-cysteine. Reactions between carbonyl compounds and amino groups are common and lead to the formation of a Schiff base. Krou et al.120 investigated the reactivity between acetaldehyde and cement-based hydrates. An overview of passive removal materials for indoor air pollutants is provided by Shayegan et al.121
6 Indoor guide values
Guide and reference values are valuable tools for assessing indoor air quality and exposure for evaluating potential health hazards and from a statistical point of view. However, it must be clear which criteria are used to derive guide and reference values and under which conditions they can be applied.122 For acetaldehyde, country and organization-specific guide values are available. Most are listed in the Toyinbo et al. database.123 Others, such as the reference exposure limits (REL), can be found on the OEHHA (Office of Environmental Health Hazard Assessment) website. Table 3 provides a compilation of published guide values for acetaldehyde.| Value | Comment | Country | Ref. |
|---|---|---|---|
| a Ref. Toyinbo et al.123 was accessed on 28.10.2022. b ISHRAE: India Society of Heating, Refrigerating and Air Conditioning Engineers. c REL = OEHHA Reference Exposure Limit; see https://oehha.ca.gov/air/chemicals/acetaldehyde, assessed on 28.10.2022. | |||
| 48 μg m−3 (0.03 ppm) | Chronic effects | Japan | 123 |
| 0.10 mg m−3 | GV I (precautionary value) | Germany | 129 |
| 1.0 mg m−3 | GV II (effect related) | Germany | 129 |
| 1420 μg m−3 (795 ppb) | Acute effects (1 hour) | Canada | 124 |
| 280 μg m−3 (157 ppb) | Chronic effects (24 hours) | Canada | 124 |
| 1420 μg m−3 | Acute effects (1 hour) | UK | 130 |
| 280 μg m−3 | Chronic effects (24 hours) | UK | 130 |
| 140 μg m−3 | ISHRAE standard 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001: 2019 001: 2019 |
India | 123 |
| 470 μg m−3 | Acute REL | United States | OEHHA |
| 300 μg m−3 | Inhalation REL (8 hours) | United States | OEHHA |
| 140 μg m−3 | Chronic inhalation REL | United States | OEHHA |
Health Canada124 uses a Reference Concentration (RfC) extracted from key toxicological, controlled human exposure, and indoor epidemiological studies as a starting point. For the short-term guideline, this is the 95-P lower confidence limit of 142 mg m−3 from a study on asthmatic subjects. With uncertainty factors of 10 to account for a use of the lowest observed adverse effects level (LOAEL) and 10 to account for additional sensitivity in the human population, a short-term RfC of 1420 μg m−3 is obtained, which is also interpreted as a short-term guide value. A concentration of 120 mg m−3 is used as starting point for the long-term value, which is based on a study of the olfactory epithelium in rats. This value is adjusted for continuous exposure, to account for toxicodynamic differences between animals and humans, for additional sensitivity in the human population, and for uncertainty in the shape of the lower region of the concentration-response curve. The resulting long-term RfC is 280 μg m−3, which is interpreted as a long-term guide value. For acetaldehyde, the United Kingdom (UK) has adopted the guide values derived from Health Canada.125
The procedure for deriving the German guide values is similar but not identical.27 It is interesting that both the German and Canadian approach is based on the same study. However, the German ad hoc working group assumes the lowest observed adverse effect concentration (LOAEC) of 48 mg m−3 (subchronic, rat) by extrapolating the intermittent value of Dorman et al.28 to continuous exposure. Taking into account factors for subchronic/chronic, interspecies differences, interindividual variability, and the physiology of children, an effect-related guide value GV II of 1.0 mg m−3 (rounded) results. A safety factor of 10 was considered appropriate for the derivation of the precautionary guide value. This results in GV I = 0.10 mg m−3 (rounded). The basic scheme for deriving guideline values in Germany is published in the Bundesgesundheitsblatt.126
In Japan, an indoor guideline value of 48 μg m−3 was published, but without a consistent derivation. Effects on the nasal olfactory epithelium in rats are mentioned as the toxicological endpoint. The Indian value of 140 μg m−3 is consistent with the OEHHA chronic inhalation reference exposure limit (REL). Each REL (acute, 8 hours, chronic) is derived toxicologically, where essentially the studies by Appelman et al.127,128 were used.
According to Table 3, the indoor guide values can be roughly divided into two ranges: 50–250 μg m−3 for chronic effects and 500–1500 μg m−3 for acute effects. It is noteworthy that the guideline values have not yet been withdrawn, despite the classification of acetaldehyde as a substance that is carcinogenic to humans.
7 Acetaldehyde concentrations in indoor and outdoor air
After formation mechanisms, indoor sources, and guide values have been explained, the indoor concentrations can now be discussed and evaluated. Note that the concentration unit used in the original work (ppb or μg m−3) is given in each case. This is due to the fact that temperature and air pressure are needed to convert from ppb to μg m−3 and vice versa. Before 1990, the interest in acetaldehyde in indoor air was low. Grosjean et al.131 measured indoor air concentrations in Brazil, but the reason for this was the noticeably high values in urban ambient air. The first systematic measurements of acetaldehyde concentrations in indoor air were carried out by Reiss et al.132 and Zhang et al.133 Since then acetaldehyde was measured frequently in the room air. The studies discussed in this section provide a representative overview of exposure to acetaldehyde under various conditions. In Table 4, only studies are considered that were not cause-related, comprised at least 10 data points, and were based on percentiles or the geometric mean (GM) for the statistical analysis. Under the premise of a logarithmic normal distribution,134 it is assumed that 50-P and GM are largely identical. Studies whose discussion is based on the arithmetic mean are not included but are cited.135–137 Outside air concentrations are only given in Table 4 if they were measured at the same time as the room air concentration.| Location and no. of samples | μg m−3 | Ref. | ||
|---|---|---|---|---|
| 50-P/GM | 95-P | Max. | ||
| Indoor, US homes (N = 353) | 18.6 | 50.2 | 138 | |
| Indoor, California childhood education (N = 40) | 7.5 | 23.3 | 139 | |
| Indoor, US residences (N = 15) | 55.4 | 140 | ||
| Indoor, California kitchen (N = 340) | 8.0 | 23 | 141 | |
| Indoor, California bedrooms (N = 340) | 7.9 | 23 | 141 | |
| Indoor, California commercial buildings (N = 40) | 8.9 | 73 | 142 | |
| Indoor, Canadian residences (N = 59) | 18.9 | 79.1 | 143 | |
| Indoor, Italian homes (N = 59) | 8.4 | 38.8 | 144 | |
| Indoor European private houses (N = 96) | 11.2 | 24.8 | 41.3 | 145 |
| Indoor European public buildings/schools (N = 186) | 7.2 | 18.8 | 29.1 | 145 |
| Indoor European office buildings in summer (N = 143) | 6.1 | 10 | 16 | 146 |
| Indoor European office buildings in winter (N = 140) | 4.5 | 8.3 | 12 | 146 |
| Indoor, German homes (GerES IV, N = 586) | 15.5 | 50.3 | 863 | 147 |
| Indoor, German homes (GerES V, N = 639) | 5.5 | 16.8 | 49.9 | 78 |
| Indoor, French dwellings (N = 554) | 11.5 | 95 | 148 | |
| Indoor, French homes (N = 162) | 9.3 | 19.1 | 149 | |
| Indoor, French schools (N = 10) | 5.2 | 150 | ||
| Indoor, Portugiese classrooms (N = 73) | 7.7 | 64.6 | 151 | |
| Indoor, Spanish living rooms (N = 25) | 14.3 | 49.6 | 152 | |
| Indoor, Spanish bedrooms (N = 25) | 15.8 | 57.9 | 152 | |
| Indoor, Japanese households, 1.5 years (N = 4843) | 12 | 37 | 150 | 153 |
| Indoor, Japanese households, 3 years (N = 4843) | 12 | 35 | 170 | 153 |
| Indoor, Japanese households, winter (N = 602) | 15 | 230 | 154 | |
| Indoor, Japanese households, summer (N = 602) | 13 | 210 | 154 | |
| Indoor, Chinese residences (N = 27) | 18.7 | 99.7 | 155 | |
| Indoor, Malaysian private residences (N = 39) | 5.2 | 67.6 | 156 | |
| Indoor, Australian dwellings (N = 76) | 7.1 | 157 | ||
| Outdoor, US (N = 353) | 5.4 | 14.9 | 138 | |
| Outdoor, California (N = 19) | 1.8 | 6.5 | 139 | |
| Outdoor, California (N = 178) | 1.4 | 4.6 | 141 | |
| Outdoor, Italy (N = 27) | 3.2 | 11.9 | 144 | |
| Outdoor Europe (N = 105) | 1.8 | 4.2 | 5.1 | 145 |
| Outdoor, France (N = 10) | 1.4 | 150 | ||
| Outdoor, Spain (N = 25) | 2.2 | 4.3 | 152 | |
| Outdoor, Japan (N = 4843) | 0.9 | 1.8 | 39 | 153 |
| Outdoor, Japan (N = 4843) | 1.1 | 1.9 | 52 | 153 |
| Outdoor, Japan winter (N = 602) | 2.2 | 14 | 154 | |
| Outdoor, Japan summer (N = 602) | 3.1 | 11 | 154 | |
| Outdoor, Australia (N = 69) | 0.7 | 157 | ||
The outdoor air concentrations of acetaldehyde vary greatly. Finlayson-Pitts and Pitts2 give typical values for different areas of ≤0.22 ppb (remote), 0.1–4 ppb (rural-suburban), and 1–18 ppb (urban). This is also reflected by the outdoor values in Table 4. Very high values usually have specific causes. Corrêa et al.33 measured peak concentrations of 46 ppb in Rio de Janeiro in 2001, which were due to direct emissions from vehicles and photochemically initiated oxidation of organic compounds. Duan et al.158 recorded maximum values of 53 μg m−3 during haze days in Beijing, and Delikhoon et al.159 reported maximum concentrations of 34 μg m−3 in Shiraz, Iran. Loh et al.160 state that indoor acetaldehyde mainly comes from outdoor sources. However, with the data presented in this work, it is clear that outdoor air is an important source, but there are also many relevant indoor sources that can lead to high concentrations of acetaldehyde. This is especially true in the winter months as shown by Wang et al.161 in an indoor/outdoor study in China.
As already explained, the results of the studies listed in Table 4 are not case-related. Some come from surveys, others from specific research projects. The acetaldehyde results of the environmental survey (GerES V)78 shown in Fig. 6 are representative for Germany. The concentrations measured with passive samplers are 7-day mean values. The 50-P is 5.5 μg m−3 and the 95-P is 16.8 μg m−3.
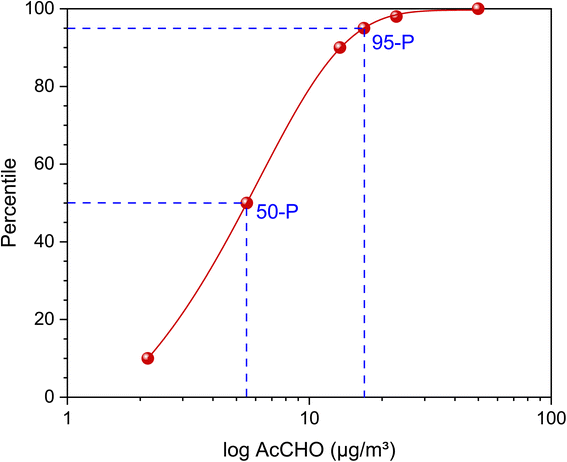 | ||
| Fig. 6 Plot of log acetaldehyde concentrations versus percentile (639 households) for the results from the German Environmental Survey 2014–2017 (GerES V).78 The data were fitted with a sigmoidal curve. The 50-P value is 5.5 μg m−3 and the 95-P is 16.8 μg m−3. | ||
The data presented in Table 4 are largely identical to the literature review by Shrubsole et al.125 Logue et al.162 assumed a population-average indoor acetaldehyde concentration of 22 μg m−3. The 50-P values or geometric means (GM) from Table 4 determined under normal living conditions are between approximately 5 μg m−3 and 19 μg m−3. The highest 95-P value is 50 μg m−3. The maximum value assumed was 230 μg m−3. The conspicuous maximum of 863 μg m−3 from the German 2003–2006 study GerES IV was not taken into account. This value seems to be extreme or questionable, the 98-P of GerES IV is 60 μg m−3. The large differences between the GerES IV and GerES V studies are also unusual. The data from Table 4 can be used to roughly classify the concentrations, which is shown in Fig. 7. The upper limit of the “low range” was assumed to be 10 μg m−3, about half of the highest 50-P. The highest value of the 95-P was chosen as the upper limit of the “medium range”. The classification of the outdoor air values is based on Finlayson-Pitts and Pitts,2 but takes into account the extreme values measured by Duan et al.158 and Corrêa et al.33 The assumed ranges of the guide values have already been discussed.
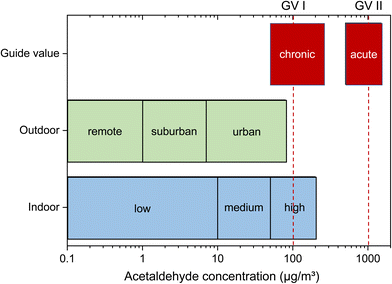 | ||
| Fig. 7 Classification of indoor air and outdoor air concentrations of acetaldehyde. The ranges of guideline values based on acute and chronic effects (see Table 3) are also given. The German guide values GV I and GV II are specially marked because they were justified most carefully. The classification was based on the evaluation of the cited studies. | ||
From the statistical data of the cited studies, it is difficult to evaluate how the room air concentrations relate to the guide values. The highest 95-P values are in the range of the smallest acute value of 48 μg m−3 (see Tables 3 and 4). Even taking into account the maximum values, exceeding 50 μg m−3 appears to be unlikely under normal living conditions. On the basis of a conservative estimate, also taking into account published percentiles that are not listed in Table 4, the proportion of concentrations of ≥50 μg m−3 is below 1%. Liu et al.163 evaluated studies on indoor measurements in China published between 2001 and 2021. With a 50-P of 12.9 μg m−3 for acetaldehyde in residences, small differences with a slightly increasing trend were found between different periods. However, the number of sample sizes was small and therefore hardly assessable. On average, indoor concentrations between 10 μg m−3 and 20 μg m−3 can be assumed to be plausible globally.
Nevertheless, the question still arises which level the acetaldehyde concentrations in the indoor environment can achieve under extreme conditions. Naddafi et al.164 measured acetaldehyde concentrations in 40 cafés serving water pipes in Ardabil, Iran. For fruit-flavored tobacco the concentrations were between 64 μg m−3 and 563 μg m−3, for regular tobacco between 31 μg m−3 and 183 μg m−3. Sousa et al.165 measured acetaldehyde concentrations between 12 μg m−3 and 55 μg m−3 in two Brazilian hospitals. In other Brazilian hospitals, the values were significantly higher with 17 μg m−3 to 263 μg m−3. In a Chinese hospital, on the other hand, the concentrations were unremarkable. The high acetaldehyde concentrations between 53 μg m−3 and 110 μg m−3 resulting from the burning of incense sticks in Hong Kong temples are not surprising.166 A much-discussed topic is the pollution of the indoor air in new buildings shortly after construction. Shinohara et al.167 conducted measurements in 19 temporary houses built after the Fukushima earthquake before residents moved in. The results were very different. In some cases, concentrations around 100 μg m−3 or higher were measured, while other houses showed significantly lower values. Derbez et al.168 found that acetaldehyde concentrations in newly built, energy-efficient houses in France were slightly higher than average values, but the differences were small. Schieweck103 examined the acetaldehyde concentrations in German prefabricated houses during different phases (P1: after completion of the shell, P2: after completion of interior work, P3: during the occupation). High concentrations of up to 350 μg m−3 were measured during P1 and P2. At P3, normal concentrations were reached by manual ventilation or when the ventilation system was switched on.
8 Final discussion and conclusion
The frequent detection of acetaldehyde in the environment is not primarily due to its use as an industrial chemical. The production quantities are comparatively small and the importance as a starting material in chemical synthesis is declining. However, acetaldehyde is a product of metabolic processes in animalia, plantae and fungi, combustion chemistry, thermal degradation, and photochemistry. In the outside air, the acetaldehyde concentration essentially depends on road traffic, photosmog, and fire incidents. This also leads to concentrations that fluctuate diurnally. Duan et al.158 were able to show that on haze days the acetaldehyde outdoor concentration in Beijing correlates with the ozone concentration. It was also found that the concentrations of formaldehyde, acetaldehyde, and acetone correlate. From their findings, Duan et al.158 concluded that the three abundant compounds were formed in photochemical reactions. In this respect, the concentrations given in Table 4 for the outside air only represent background concentrations for urban areas, but these levels cannot generally be expected and will vary greatly from region to region.Outdoor air is certainly an important source of indoor acetaldehyde, but a relationship is not necessarily given. This was investigated by Santarsiero and Fuselli169 using principal component analysis. Indoor concentrations measured in different rooms of Italian apartments were strongly correlated but independent of the outdoor concentration. In any case, high acetaldehyde concentrations in the indoor environment can only be explained by the outdoor air concentration in exceptional cases, but usually indoor-specific sources are active. Looking at the individual sources in detail, a problem becomes obvious. As a rule, acetaldehyde is not emitted directly but is created by chemical and thermal processes. In some cases, such as wood burning and cooking, acetaldehyde emissions can be removed via exhaust gas ducts. In contrast, candles, ethanol burners, and incense sticks are designed for indoor use. So when these products are used, exposure to acetaldehyde and other emitted pollutants is accepted at the same time. Thus, many sources of acetaldehyde are linked to human activities, and indeed Cheng et al.157 found that acetaldehyde was elevated in dwellings with higher occupant densities. Pagonis et al.170 speculated that elevated acetaldehyde concentrations in a museum might be due to the alcohol consumption of attendees.
The use of conventional cigarettes and e-cigarettes is no longer an issue in public buildings and the population is well informed about the disadvantages of passive smoking and passive vaping. Visiting a smoker's pub or a shisha bar is voluntary, but the question still arises if visitors can be expected to deal with high concentrations of pollutants or whether the owner must ensure adequate ventilation. However, it must also be made clear that acetaldehyde plays only a minor role in the emissions from these products.
The release of acetaldehyde from photocatalytic reactions is still a problem as the substance is one of the main components here. As long as organic polymers, such as PVAC, are used as binders, the formation of acetaldehyde cannot be prevented. In the case of photocatalytic air cleaners, the reaction rarely leads to the complete mineralization of the pollutants, and the formation of byproducts is more the rule than the exception. Whether acetaldehyde and other substances are formed depends on the technical design of the respective device.
An ambivalent situation concerns the construction and consumer products. The xylans contained in hardwood are rich in acetyl groups (H3C–C(O)⋯).40 Therefore, such woods can release acetaldehyde. Other substances derived from xylan structure of hardwood are formic acid and acetic acid.104 On the other hand, hardwood contains few terpenes. Softwood xylans are comparatively low in acetyl groups, but mono- and sesquiterpenes dominate, the release of which into the room air is also only desired to a limited extent. When adding vegetable oil to plaster, the release of acetaldehyde can be limited by selecting certain oils, but this leads to an increased emission of higher aldehydes such as hexanal.101 These examples show that substitution can be quite difficult since by regulating one substance one accepts the higher emission of another substance. Leather is potentially a strong source of acetaldehyde, but relevant emissions occur at temperatures above 50 °C, affecting particular automotive applications. Here, too, there is the problem that the precursor substances are already present in the leather matrix.
The essential question to be addressed is whether indoor exposure to acetaldehyde poses a health risk to occupants. The main result of practically all surveys is that acetaldehyde only rarely dominates the spectrum of indoor air pollutants. Nevertheless, health-based guide values were derived for the substance, with the help of which an assessment can be made. None of the studies listed in Table 4 significantly exceeded the Japanese guide value of 48 μg m−3 for chronic effects in the 95-P; in most cases, the measured value is significantly lower. The toxicologically based German guideline value GV I = 0.10 mg m−3 for chronic exposure is well above the 95-P. The maximum values listed in Table 4 are still below the chronic inhalation REL of the OEHHA and the chronic 24-hour value of Health Canada. With the exception of one concentration in the GerES IV study, the guide values for acute exposure are never reached. Consequently, the risk of acute and chronic health impairments from indoor acetaldehyde can be considered to be low under normal living conditions. Koistinen et al.4 also concluded that people in Europe do not experience increased health hazards associated with acetaldehyde levels in their homes. Acetaldehyde concentrations under extreme conditions have already been discussed in Section 7. The increased values in prefabricated houses measured by Schieweck103 during the construction phase can be regarded as typical when using new wood-based materials. However, the release of acetaldehyde from wood decreases rapidly104 and with adequate ventilation after the residents moved in, the concentrations are in the usual range. The high values in a shisha bar,164 which are in the region of the OEEHA acute REL, must be viewed as significantly more critical. The acetaldehyde concentration in vehicle cabins should also be considered, as measurements by Wang et al.171 on leather-containing fabrics at 25 °C and 65 °C have shown.
In summary, it can be said that acetaldehyde is inevitable in indoor air, which is essentially due to the natural formation of the substance. However, the concentration can be kept in the non-critical range through responsible handling of products and living behavior. A sufficiently high air exchange from a hygienic point of view is also advantageous in reducing high concentrations of pollutants, but this must not be understood as a request to combat high emission rates with high air exchange rates. Moreover, it is up to the people themselves if they are a potential acetaldehyde source, since the concentration in the respiratory gas correlates closely with the consumption of ethanol. Statistically based studies and surveys with randomized selection of the measuring sites have shown that acetaldehyde hardly poses acute and chronic health problems under normal living conditions. Nevertheless, the compound definitely belongs to the list of priority pollutants due to its ubiquity and carcinogenic potential and should always be included in indoor measurement programs.
Conflicts of interest
The author has no conflicts of interest to declare.Acknowledgements
The 3D structure image in the Graphical Abstract was taken from PubChem® (CID 177) https://pubchem.ncbi.nlm.nih.gov/.References
- C. J. Weschler, Changes in indoor pollutants since the 1950s, Atmos. Environ., 2009, 43, 153–169 CrossRef CAS.
- B. J. Finlayson-Pitts and J. N. Pitts, Chemistry of the Upper and Lower Atmosphere, Academic Press, San Diego, 2000 Search PubMed.
- World Health Organization, Indoor Air Quality: Organic Pollutants, EURO Reports and Studies, Copenhagen, 1989 Search PubMed.
- K. Koistinen, D. Kotzias, S. Kephalopoulos, C. Schlitt, P. Carrer, M. Jantunen, S. Kirchner, J. McLaughlin, L. Mølhave, E. O. Fernandes and B. Seifert, The INDEX project: executive summary of a European Union project on indoor air pollutants, Allergy, 2008, 63, 810–819 CrossRef CAS PubMed.
- J. M. Logue, T. E. McKone, M. H. Sherman and B. C. Singer, Hazard assessment of chemical air contaminants measured in residences, Indoor Air, 2011, 21, 92–109 CrossRef CAS PubMed.
- T. Salthammer, Very volatile organic compounds: an understudied class of indoor air pollutants, Indoor Air, 2016, 26, 25–38 CrossRef CAS PubMed.
- Commission of the European Communities, Evaluation of VOC Emissions from Building Products, Indoor Air Quality and its Impact on Man, Report No. 18 EUR 17334 EN, Publications Office of the European Union, Luxembourg, 1997 Search PubMed.
- European Collaborative Action, Harmonisation Framework for Health Based Evaluation of Indoor Emissions from Construction Products in the European Union Using the EU-LCI Concept; Urban Air, Indoor Environment and Human Exposure, Report No. 29 EUR 26168 EN, Publications Office of the European Union, Luxembourg, 2013 Search PubMed.
- H. Akimoto, Atmospheric Reaction Chemistry, Springer Verlag, Tokyo, 2014 Search PubMed.
- J. H. Ruth, Odor thresholds and irritation levels of several chemical substances: a review, Am. Ind. Hyg. Assoc. J., 1986, 47, A142–A151 CrossRef CAS PubMed.
- J. E. Amoore and E. Hautala, Odor as an ald to chemical safety: odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution, J. Appl. Toxicol., 1983, 3, 272–290 CrossRef CAS PubMed.
- M. Devos, F. Patte, J. Rouault, P. Laffort and L. Van Gemert, Standardized Human Olfactory Thresholds, Oxford University Press, Oxford, 1992 Search PubMed.
- R. G. Berger, Flavours and Fragrances, Springer Verlag, Berlin, 2007 Search PubMed.
- R. P. Schwarzenbach, P. M. Gschwend and D. M. Imboden, Environmental Organic Chemistry, John Wiley & Sons, Hoboken, 2017 Search PubMed.
- J. R. Rumble, T. J. Bruno and M. J. Doa, Handbook of Chemistry and Physics, CRC Press, Boca Raton, 101st edn, 2020 Search PubMed.
- J. Zhao and R. Zhang, Proton transfer reaction rate constants between hydronium ion (H3O+) and volatile organic compounds, Atmos. Environ., 2004, 38, 2177–2185 CrossRef CAS.
- J. Sangster, Octanol-water Partition Coefficients: Fundamentals and Physical Chemistry, John Wiley & Sons Ltd., Chichester, 1997 Search PubMed.
- E. M. Duffy and W. L. Jorgensen, Prediction of properties from simulations: free energies of solvation in hexadecane, octanol, and water, J. Am. Chem. Soc., 2000, 122, 2878–2888 CrossRef CAS.
- R. Sander, Compilation of Henry's law constants (version 4.0) for water as solvent, Atmos. Chem. Phys., 2015, 15, 4399–4981 CrossRef CAS.
- J. Dykyj, J. Svoboda, R. Wilhoit, M. Frenkel and K. Hall in, Vapor Pressure of Chemicals. Subvolume B: Vapor Pressure and Antoine Constants for Oxygen Containing Organic Compounds, ed. K. Hall, Springer Verlag, Berlin, 2000, vol. 20 Search PubMed.
- V. Majer and V. Svoboda, Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation, Blackwell Scientific Publications, Oxford, 1985 Search PubMed.
- N. Ulrich, S. Endo, T. Brown, N. Watanabe, G. Bronner, M. H. Abraham and K.-U. Goss, UFZ-LSER Database V 3.2.1, Helmholtz Centre for Environmental Research, Leipzig, 2017 Search PubMed.
- E. P. L. Hunter and S. G. Lias, Evaluated gas phase basicities and proton affinities of molecules: an update, J. Phys. Chem. Ref. Data, 1998, 27, 413–656 CrossRef CAS.
- J. Calvert, R. Atkinson, J. Kerr, S. Madronich, K. Moortgat, T. Wallington and G. Yarwood, The Mechanisms of Atmospheric Oxidation of the Alkenes, Oxford University Press, New York, 2000 Search PubMed.
- The EU-LCI Working Group, Agreed EU-LCI values, European Commision, https://ec.europa.eu/docsroom/documents/49239, assessed 26.10.2022, 2021 Search PubMed.
- EN 16516, Construction Products – Assessment of Release of Dangerous Substances – Determination of Emissions into Indoor Air, Beuth Verlag, Berlin, 2017 Search PubMed.
- Ad hoc Working Group, Richtwerte für Acetaldehyd in der Innenraumluft, Bundesgesundheitsbl., 2013, 56, 1434–1447 CrossRef.
- D. C. Dorman, M. F. Struve, B. A. Wong, E. A. Gross, C. Parkinson, G. A. Willson, Y.-M. Tan, J. L. Campbell, J. G. Teeguarden, H. J. Clewell and M. E. Andersen, Derivation of an inhalation reference concentration based upon olfactory neuronal loss in male rats following subchronic acetaldehyde inhalation, Inhalation Toxicol., 2008, 20, 245–256 CrossRef CAS PubMed.
- M. Eckert, G. Fleischmann, R. Jira, H. M. Bolt and K. Golka, Acetaldehyde; Ullmanns Encyclopedia of Industrial Chemisty, Wiley-VCH, Weinheim, 2006, vol. 1, pp. 191–207 Search PubMed.
- G. A. Ban-Weiss, J. P. McLaughlin, R. A. Harley, A. J. Kean, E. Grosjean and D. Grosjean, Carbonyl and Nitrogen Dioxide Emissions From Gasoline- and Diesel-Powered Motor Vehicles, Environ. Sci. Technol., 2008, 42, 3944–3950 CrossRef CAS PubMed.
- K. Kohse-Höinghaus, P. Oßwald, T. Cool, T. Kasper, N. Hansen, F. Qi, C. Westbrook and P. Westmoreland, Biofuel Combustion Chemistry: From Ethanol to Biodiesel, Angew. Chem., Int. Ed., 2010, 49, 3572–3597 CrossRef PubMed.
- B. Languille, et al., Wood burning: A major source of Volatile Organic Compounds during wintertime in the Paris region, Sci. Total Environ., 2020, 711, 135055 CrossRef CAS PubMed.
- S. M. Corrêa, E. M. Martins and G. Arbilla, Formaldehyde and acetaldehyde in a high traffic street of Rio de Janeiro, Brazil, Atmos. Environ., 2003, 37, 23–29 CrossRef.
- S. M. Sarathy, P. Oßwald, N. Hansen and K. Kohse-Höinghaus, Alcohol combustion chemistry, Prog. Energy Combust. Sci., 2014, 44, 40–102 CrossRef.
- C. Habarta, M. Sysoitseva, S. Eckert, L. Fembacher, A. Frenzen, J. Wolf, R. Winterhalter, C. Scheu and H. Fromme, Ethanol fireplaces – effects on indoor air quality, Gefahrstoffe – Reinhalt. Luft, 2018, 78, 375–384 CAS.
- T. Schripp, T. Salthammer, S. Wientzek and M. Wensing, Chamber studies on nonvented decorative fireplaces using liquid or gelled ethanol fuel, Environ. Sci. Technol., 2014, 48, 3583–3590 CrossRef CAS PubMed.
- M. Cerqueira, L. Gomes, L. Tarelho and C. Pio, Formaldehyde and acetaldehyde emissions from residential wood combustion in Portugal, Atmos. Environ., 2013, 72, 171–176 CrossRef CAS.
- A. A. Reda, H. Czech, J. Schnelle-Kreis, O. Sippula, J. Orasche, B. Weggler, G. Abbaszade, J. M. Arteaga-Salas, M. Kortelainen, J. Tissari, J. Jokiniemi, T. Streibel and R. Zimmermann, Analysis of Gas-Phase Carbonyl Compounds in Emissions from Modern Wood Combustion Appliances: Influence of Wood Type and Combustion Appliance, Energy Fuels, 2015, 29, 3897–3907 CrossRef CAS.
- X. Zhang, W. Yang and W. Blasiak, Thermal decomposition mechanism of levoglucosan during cellulose pyrolysis, J. Anal. Appl. Pyrolysis, 2012, 96, 110–119 CrossRef CAS.
- D. Fengel and G. Wegener, Wood – Chemistry, Ultrastructure, Reactions, Walter de Gruyter, Berlin, 1989 Search PubMed.
- J. I. Seeman, M. Dixon and H.-J. Haussmann, Acetaldehyde in mainstream tobacco smoke: Formation and occurrence in smoke and bioavailability in the smoker, Chem. Res. Toxicol., 2002, 15, 1331–1350 Search PubMed.
- Z. Geng, M. Zhang and Y. Yu, Theoretical investigation on pyrolysis mechanism of glycerol, Fuel, 2012, 93, 92–98 CrossRef CAS.
- R. Talhout, A. Opperhuizen and J. G. van Amsterdam, Role of acetaldehyde in tobacco smoke addiction, Eur. Neuropsychopharmacol., 2007, 17, 627–636 CrossRef CAS PubMed.
- D. J. Conklin, M. A. Ogunwale, Y. Chen, W. S. Theis, M. H. Nantz, X.-A. Fu, L.-C. Chen, D. W. Riggs, P. Lorkiewicz, A. Bhatnagar and S. Srivastava, Electronic cigarette-generated aldehydes: the contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure, Aerosol Sci. Technol., 2018, 52, 1219–1232 CrossRef CAS PubMed.
- S. Vreeke, D. H. Peyton and R. M. Strongin, Triacetin Enhances Levels of Acrolein, Formaldehyde Hemiacetals, and Acetaldehyde in Electronic Cigarette Aerosols, ACS Omega, 2018, 3, 7165–7170 CrossRef CAS PubMed.
- A. Khlystov and V. Samburova, Flavoring compounds dominate toxic aldehyde production during Eigarette vaping, Environ. Sci. Technol., 2016, 50, 13080–13085 CrossRef CAS PubMed.
- C.-Y. Peng, C.-H. Lan, P.-C. Lin and Y.-C. Kuo, Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes, J. Hazard. Mater., 2017, 324, 160–167 CrossRef CAS PubMed.
- W. Zhang, Z. Bai, L. Shi, J. H. Son, L. Li, L. Wang and J. Chen, Investigating aldehyde and ketone compounds produced from indoor cooking emissions and assessing their health risk to human beings, J. Environ. Sci., 2023, 127, 389–398 CrossRef PubMed.
- H.-D. Belitz, W. Grosch and P. Schieberle, Food Chemistry, Springer Verlag, Berlin, 2009 Search PubMed.
- H.-S. Jeong, H. Chung, S.-H. Song, C.-I. Kim, J.-G. Lee and Y.-S. Kim, Validation and determination of the contents of acetaldehyde and formaldehyde in foods, Toxicol. Res., 2015, 31, 273–278 CrossRef CAS PubMed.
- D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, W.H. Freeman, New York, 6th edn, 2012 Search PubMed.
- U. Riess, U. Tegtbur, C. Fauck, F. Fuhrmann, D. Markewitz and T. Salthammer, Experimental setup and analytical methods for the non-invasive determination of volatile organic compounds, formaldehyde and NO in exhaled human breath, Anal. Chim. Acta, 2010, 669, 53–62 CrossRef CAS PubMed.
- R. Koppmann, Volatile Organic Compunds in the Atmosphere, Blackwell Publishing, Oxford, 2007 Search PubMed.
- J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics, John Wiley & Sons Inc., Hoboken, 2016 Search PubMed.
- R. Sommariva, J. A. de Gouw, M. Trainer, E. Atlas, P. D. Goldan, W. C. Kuster, C. Warneke and F. C. Fehsenfeld, Emissions and photochemistry of oxygenated VOCs in urban plumes in the Northeastern United States, Atmos. Chem. Phys., 2011, 11, 7081–7096 CrossRef CAS.
- A. Calogirou, B. Larsen and D. Kotzias, Gas-phase terpene oxidation products: a review, Atmos. Environ., 1999, 33, 1423–1439 CrossRef CAS.
- A. Lee, A. H. Goldstein, M. D. Keywood, S. Gao, V. Varutbangkul, R. Bahreini, N. L. Ng, R. C. Flagan and J. H. Seinfeld, Gas-phase products and secondary aerosol yields from the ozonolysis of ten different terpenes, J. Geophys. Res., 2006, 111, D07302 Search PubMed.
- F. Rancière, C. Dassonville, C. Roda, A.-M. Laurent, Y. L. Moullec and I. Momas, Contribution of ozone to airborne aldehyde formation in Paris homes, Sci. Total Environ., 2011, 409, 4480–4483 CrossRef PubMed.
- R. Reiss, P. B. Ryan, P. Koutrakis and S. J. Tibbetts, Ozone Reactive Chemistry on Interior Latex Paint, Environ. Sci. Technol., 1995, 29, 1906–1912 CrossRef CAS PubMed.
- C. J. Weschler, A. T. Hodgson and J. D. Wooley, Indoor chemistry: ozone, volatile organic compounds, and carpets, Environ. Sci. Technol., 1992, 26, 2371–2377 CrossRef CAS.
- G. C. Morrison and W. W. Nazaroff, Ozone Interactions with Carpet: Secondary Emissions of Aldehydes, Environ. Sci. Technol., 2002, 36, 2185–2192 CrossRef CAS PubMed.
- A. Nørgaard, J. Kudal, V. Kofoed-Sørensen, I. Koponen and P. Wolkoff, Ozone-initiated VOC and particle emissions from a cleaning agent and an air freshener: risk assessment of acute airway effects, Environ. Int., 2014, 68, 209–218 CrossRef PubMed.
- J. Palmisani, A. W. Nørgaard, V. Kofoed-Sørensen, P. A. Clausen, G. de Gennaro and P. Wolkoff, Formation of ozone-initiated VOCs and secondary organic aerosol following application of a carpet deodorizer, Atmos. Environ., 2020, 222, 117149 CrossRef CAS.
- T. Salthammer and F. Fuhrmann, Photocatalytic Surface Reactions on Indoor Wall Paint, Environ. Sci. Technol., 2007, 41, 6573–6578 CrossRef CAS PubMed.
- J. Auvinen and L. Wirtanen, The influence of photocatalytic interior paints on indoor air quality, Atmos. Environ., 2008, 42, 4101–4112 CrossRef CAS.
- J. Gunschera, D. Markewitz, B. Bansen, T. Salthammer and H. Ding, Portable photocatalytic air cleaners: efficiencies and by-product generation, Environ. Sci. Pollut. Res., 2016, 23, 7482–7493 CrossRef CAS PubMed.
- B.-Å. Sultan and E. Sörvik, Thermal degradation of EVA and EBA–A comparison. I. Volatile decomposition products, J. Appl. Polym. Sci., 1991, 43, 1737–1745 CrossRef CAS.
- K. Buchanan and W. McGill, Photodegradation of poly(vinyl esters)—III. Photolysis mechanism for both polymeric and low molecular mass esters, Eur. Polym. J., 1980, 16, 319–324 CrossRef CAS.
- A. Gandolfo, S. Marque, B. Temime-Roussel, R. Gemayel, H. Wortham, D. Truffier-Boutry, V. Bartolomei and S. Gligorovski, Unexpectedly high levels of organic compounds released by indoor photocatalytic paints, Environ. Sci. Technol., 2018, 52, 11328–11337 CrossRef CAS PubMed.
- A. Vairavamurthy, J. M. Roberts and L. Newman, Methods for determination of low molecular weight carbonyl compounds in the atmosphere: a review, Atmos. Environ., 1992, 26, 1965–1993 CrossRef.
- R. Giesen, T. Schripp, D. Markewitz, B. Meyer, H. Schwab, E. Uhde and T. Salthammer, Comparison of methods for the determination of formaldehyde in air, Anal. Lett., 2016, 49, 1613–1621 CrossRef CAS.
- ISO 16000-3, Indoor Air – Part 3: Determination Of Formaldehyde and Other Carbonyl Compounds in Indoor Air and Test Chamber Air – active Sampling Method, Beuth Verlag, Berlin, 2011 Search PubMed.
- R. R. Arnts and S. B. Tejada, 2,4-Dinitrophenylhydrazine-coated silica gel cartridge method for determination of formaldehyde in air: identification of an ozone interference, Environ. Sci. Technol., 1989, 23, 1428–1430 CrossRef CAS.
- U. Karst, N. Binding, K. Cammann and U. Witting, Interferences of nitrogen dioxide in the determination of aldehydes and ketones by sampling on 2,4-dinitrophenylhydrazine-coated solid sorbent, Fresenius. J. Anal. Chem., 1993, 345, 48–52 CrossRef CAS.
- M. Aiello and R. McLaren, Measurement of airborne carbonyls using an automated sampling and analysis system, Environ. Sci. Technol., 2009, 43, 8901–8907 CrossRef CAS PubMed.
- T. Salthammer and S. Mentese, Comparison of analytical techniques for the determination of aldehydes in test chambers, Chemosphere, 2008, 73, 1351–1356 CrossRef CAS PubMed.
- A. Schieweck, E. Uhde and T. Salthammer, Determination of acrolein in ambient air and in the atmosphere of environmental test chambers, Environ. Sci.: Processes Impacts, 2021, 23, 1729–1746 RSC.
- W. Birmili, A. Daniels, R. Bethke, N. Schechner, G. Brasse, A. Conrad, M. Kolossa-Gehring, M. Debiak, J. Hurraß, E. Uhde, A. Omelan and T. Salthammer, Formaldehyde, aliphatic aldehydes (C2-C11), furfural, and benzaldehyde in the residential indoor air of children and adolescents during the German Environmental Survey 2014–2017 (GerES V), Indoor Air, 2022, 32, e12927 CrossRef CAS PubMed.
- R. S. Blake, P. S. Monks and A. M. Ellis, Proton-transfer reaction mass spectrometry, Chem. Rev., 2009, 109, 861–896 CrossRef CAS PubMed.
- A. M. Ellis and C. A. Mayhew, Proton Transfer Reaction Mass Spectrometry: Principles and Applications, John Wiley & Sons, Chichester, 2013 Search PubMed.
- A. Borbon, N. Locoge, M. Veillerot, J. C. Galloo and R. Guillermo, Characterisation of NMHCs in a French urban atmosphere: overview of the main sources, Sci. Total Environ., 2002, 292, 177–191 CrossRef CAS PubMed.
- D. J. Price, D. A. Day, D. Pagonis, H. Stark, L. B. Algrim, A. V. Handschy, S. Liu, J. E. Krechmer, S. L. Miller, J. F. Hunter, J. A. de Gouw, P. J. Ziemann and J. L. Jimenez, Budgets of organic carbon composition and oxidation in indoor air, Environ. Sci. Technol., 2019, 53, 13053–13063 CrossRef CAS PubMed.
- D. M. Lunderberg, P. K. Misztal, Y. Liu, C. Arata, Y. Tian, K. Kristensen, R. J. Weber, W. W. Nazaroff and A. H. Goldstein, High-Resolution Exposure Assessment for Volatile Organic Compounds in Two California Residences, Environ. Sci. Technol., 2021, 55, 6740–6751 CrossRef CAS PubMed.
- T. Schripp, S. Etienne, C. Fauck, F. Fuhrmann, L. Märk and T. Salthammer, Application of proton-transfer-reaction-mass-spectrometry for indoor air quality research, Indoor Air, 2014, 24, 178–189 CrossRef CAS PubMed.
- T. Su and M. T. Bowers, Theory of ion-polar molecule collisions. Comparison with experimental charge transfer reactions of rare gas ions to geometric isomers of difluorobenzene and dichloroethylene, J. Chem. Phys., 1973, 58, 3027–3037 CrossRef.
- L. Cappellin, T. Karl, M. Probst, O. Ismailova, P. M. Winkler, C. Soukoulis, E. Aprea, T. D. Märk, F. Gasperi and F. Biasioli, On Quantitative Determination of Volatile Organic Compound Concentrations Using Proton Transfer Reaction Time-of-Flight Mass Spectrometry, Environ. Sci. Technol., 2012, 46, 2283–2290 CrossRef CAS PubMed.
- D. Smith and P. Španěl, Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis, Mass Spectrom. Rev., 2005, 24, 661–700 CrossRef CAS PubMed.
- M. Vogel, A. Büldt and U. Karst, Hydrazine reagents as derivatizing agents in environmental analysis - a critical review, Fresenius. J. Anal. Chem., 2000, 366, 781–791 CrossRef CAS PubMed.
- T. Salthammer, J. Gu, S. Wientzek, R. Harrington and S. Thomann, Measurement and evaluation of gaseous and particulate emissions from burning scented and unscented candles, Environ. Int., 2021, 155, 106590 CrossRef CAS PubMed.
- M. Derudi, S. Gelosa, A. Sliepcevich, A. Cattaneo, R. Rota, D. Cavallo and G. Nano, Emissions of air pollutants from scented candles burning in a test chamber, Atmos. Environ., 2012, 55, 257–262 CrossRef CAS.
- S. Lee and B. Wang, Characteristics of emissions of air pollutants from mosquito coils and candles burning in a large environmental chamber, Atmos. Environ., 2006, 40, 2128–2138 CrossRef CAS.
- T. Salthammer, T. Schripp, S. Wientzek and M. Wensing, Impact of operating wood-burning fireplace ovens on indoor air quality, Chemosphere, 2014, 103, 205–211 CrossRef CAS PubMed.
- J. J. Schauer, M. J. Kleeman, G. R. Cass and B. R. T. Simoneit, Measurement of emissions from air pollution sources. 3. C1-C29 organic compounds from fireplace combustion of wood, Environ. Sci. Technol., 2001, 35, 1716–1728 CrossRef CAS PubMed.
- E. Hedberg, A. Kristensson, M. Ohlsson, C. Johansson, P.-Å. Johansson, E. Swietlicki, V. Vesely, U. Wideqvist and R. Westerholm, Chemical and physical characterization of emissions from birch wood combustion in a wood stove, Atmos. Environ., 2002, 36, 4823–4837 CrossRef CAS.
- B. C. Singer, A. T. Hodgson and W. W. Nazaroff, Gas-phase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposures from habitual smoking, Atmos. Environ., 2003, 37, 5551–5561 CrossRef CAS.
- P. Azimi, Z. Keshavarz, M. L. Luna, J. G. C. Laurent, J. Vallarino, D. C. Christiani and J. G. Allen, An unrecognized hazard in e-cigarette vapor: preliminary quantification of methylglyoxal formation from propylene glycol in e-cigarettes, Int. J. Environ. Res. Public Health, 2021, 18, 385 CrossRef CAS PubMed.
- M. Sleiman, J. M. Logue, V. N. Montesinos, M. L. Russell, M. I. Litter, L. A. Gundel and H. Destaillats, Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals, Environ. Sci. Technol., 2016, 50, 9644–9651 CrossRef CAS PubMed.
- N. Beauval, M. Verrièle, A. Garat, I. Fronval, R. Dusautoir, S. Anthérieu, G. Garçon, J.-M. Lo-Guidice, D. Allorge and N. Locoge, Influence of puffing conditions on the carbonyl composition of e-cigarette aerosols, Int. J. Hyg. Environ. Health, 2019, 222, 136–146 CrossRef CAS PubMed.
- L. Cancelada, M. Sleiman, X. Tang, M. L. Russell, V. N. Montesinos, M. I. Litter, L. A. Gundel and H. Destaillats, Heated tobacco products: volatile emissions and their predicted impact on indoor air quality, Environ. Sci. Technol., 2019, 53, 7866–7876 CrossRef CAS PubMed.
- H. Plaisance, A. Blondel, V. Desauziers and P. Mocho, Hierarchical cluster analysis of carbonyl compounds emission profiles from building and furniture materials, Build Environ., 2014, 75, 40–45 CrossRef.
- Y. Odaka, H. Seto, H. Nakaoka, M. Hanazato, E. Todaka and C. Mori, Aldehyde emissions from lime plaster containing vegetable oil, Indoor Built Environ., 2014, 25, 254–261 CrossRef.
- M. Suzuki, H. Akitsu, K. Miyamoto, S. ichiro Tohmura and A. Inoue, Effects of time, temperature, and humidity on acetaldehyde emission from wood-based materials, J. Wood Sci., 2014, 60, 207–214 CrossRef CAS.
- A. Schieweck, Very Volatile Organic Compounds (VVOC) as emissions from wooden materials and in indoor air of new prefabricated wooden houses, Build Environ., 2021, 190, 107537 CrossRef.
- E. Pibiri, A. Omelan, E. Uhde and T. Salthammer, Effect of surface covering on the release of formaldehyde, acetaldehyde, formic acid and acetic acid from particleboard, Build Environ., 2020, 178, 106947 CrossRef.
- J. Ammenn, B. Wegner and B. Dannheim, Recent findings in acetaldehyde emission from leather, J. Am. Leather Chem. Assoc., 2017, 112, 259–262 Search PubMed.
- A. T. Hodgson, H. Destaillats, D. P. Sullivan and W. J. Fisk, Performance of ultraviolet photocatalytic oxidation for indoor air cleaning applications, Indoor Air, 2007, 17, 305–316 CrossRef CAS PubMed.
- C. Turner, P. Španěl and D. Smith, A longitudinal study of ethanol andacetaldehyde in the exhaled breath of healthy volunteers using selected-ion flow-tube mass spectrometry, Rapid Commun. Mass Spectrom., 2006, 20, 61–68 CrossRef CAS PubMed.
- W. Wegscheider, B. Heinrich, A. Albrecht, H. Assenmacher, D. Fendler, H. Kübler, G. Naujoks and B. Scheibner, Saunaaufgüsse: Thermische Reaktionsprodukte und(Formaldehyd-)Exposition, Gefahrstoffe – Reinhalt. Luft, 2017, 77, 332–341 Search PubMed.
- P. Gustafson, L. Barregard, B. Strandberg and G. Sällsten, The impact of domestic wood burning on personal, indoor and outdoor levels of 1,3-butadiene, benzene, formaldehyde and acetaldehyde, J. Environ. Monit., 2007, 9, 23–32 RSC.
- O. Geiss, I. Bianchi and J. Barrero-Moreno, Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours, Int. J. Hyg. Environ. Health, 2016, 219, 268–277 CrossRef CAS PubMed.
- M. I. Mitova, P. B. Campelos, C. G. Goujon-Ginglinger, S. Maeder, N. Mottier, E. G. Rouget, M. Tharin and A. R. Tricker, Comparison of the impact of the Tobacco Heating System 2.2 and a cigarette on indoor air quality, Regul. Toxicol. Pharmacol., 2016, 80, 91–101 CrossRef CAS PubMed.
- A. A. Ruprecht, et al., Environmental pollution and emission factors of electronic cigarettes, heat-not-burn tobacco products, and conventional cigarettes, Aerosol Sci. Technol., 2017, 51, 674–684 CrossRef CAS.
- D. Xu, H. Zhang, J. Xi, Y. Jin, Y. Chen, L. Guo, Z. Jin and X. Xu, Improving bread aroma using low-temperature sourdough fermentation, Food Biosci., 2020, 37, 100704 CrossRef CAS.
- A. Hinterholzer and P. Schieberle, Identification of the most odour-active volatiles in fresh, hand-extracted juice of Valencia late oranges by odour dilution techniques, Flavour Fragrance J., 1998, 13, 49–55 CrossRef CAS.
- X. Ye, D. Chen, J. Gossage and K. Li, Photocatalytic oxidation of aldehydes: byproduct identification and reaction pathway, J. Photochem. Photobiol., A, 2006, 183, 35–40 CrossRef CAS.
- O. Geiss, C. Cacho, J. Barrero-Moreno and D. Kotzias, Photocatalytic degradation of organic paint constituents-formation of carbonyls, Build Environ., 2012, 48, 107–112 CrossRef.
- E.-A. Fiorentino, H. Chen, A. Gandolfo, V. Lannuque, K. Sartelet and H. Wortham, Measurements and Modelling of OH and Peroxy Radicals in an Indoor Environment Under Different Light Conditions and VOC Levels, Atmos. Environ., 2023, 292, 119398 CrossRef CAS.
- H. Destaillats, M. Sleiman, D. P. Sullivan, C. Jacquiod, J. Sablayrolles and L. Molins, Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions, Appl. Catal., B, 2012, 128, 159–170 CrossRef CAS.
- K. Yamashita, M. Noguchi, A. Mizukoshi and Y. Yanagisawa, Acetaldehyde removal from indoor air through chemical absorption using L-cysteine, Int. J. Environ. Res. Public Health, 2010, 7, 3489–3498 CrossRef CAS PubMed.
- N. Krou, I. Batonneau-Gener, T. Belin, S. Mignard, I. Javierre, I. Dubois-Brugger and M. Horgnies, Reactivity of volatile organic compounds with hydrated cement paste containing activated carbon, Build Environ., 2015, 87, 102–107 CrossRef.
- Z. Shayegan, M. Bahri and F. Haghighat, A review on an emerging solution to improve indoor air quality: application of passive removal materials, Build Environ., 2022, 219, 109228 CrossRef.
- T. Salthammer, Critical evaluation of approaches in setting indoor air quality guidelines and reference values, Chemosphere, 2011, 82, 1507–1517 CrossRef CAS PubMed.
- O. Toyinbo, L. Hägerhed, S. Dimitroulopoulou, M. Dudzinska, S. Emmerich, D. Hemming, J.-H. Park and U. Haverinen-Shaughnessy, Open database for international and national indoor environmental quality guidelines, Indoor Air, 2022, 32, e13028 CrossRef PubMed.
- Health Canada, Residential Indoor Air Quality Guideline: Acetaldehyde, Government of Canada, Ottawa, 2017 Search PubMed.
- C. Shrubsole, S. Dimitroulopoulou, K. Foxall, B. Gadeberg and A. Doutsi, IAQ guidelines for selected volatile organic compounds (VOCs) in the UK, Build Environ., 2019, 165, 106382 CrossRef.
- Ad hoc Working Group, Richtwerte für die Innenraumluft: erste Fortschreibung des Basisschemas, Bundesgesundheitsblatt., 2012, 55, 279–290 CrossRef PubMed.
- L. Appelman, R. Woutersen and V. Feron, Inhalation toxicity of acetaldehyde in rats. I. Acute and subacute studies, Toxicology, 1982, 23, 293–307 CrossRef CAS PubMed.
- L. M. Appelman, R. A. Woutersen, V. J. Fcron, R. N. Hooftman and W. R. F. Notten, Effect of variable versus fixed exposure levels on the toxicity of acetaldehyde in rats, J. Appl. Toxicol., 1986, 6, 331–336 CrossRef CAS PubMed.
- H. Fromme, M. Debiak, H. Sagunski, C. Röhl, M. Kraft and M. Kolossa-Gehring, The German approach to regulate indoor air contaminants, Int. J. Hyg. Environ. Health, 2019, 222, 347–354 CrossRef CAS PubMed.
- Public Health England, Indoor Air Quality Guidelines Forselected Volatile Organic Compounds(VOCs) in the UK, PHE Publications, London, 2019 Search PubMed.
- D. Grosjean, A. H. Miguel and T. M. Tavares, Urban air pollution in Brazil: acetaldehyde and other carbonyls, Atmos. Environ., 1990, 24, 101–106 CrossRef.
- R. Reiss, P. B. Ryan, S. J. Tibbetts and P. Koutrakis, Measurement of organic acids, aldehydes, and ketones in residential environments and their relation to ozone, J. Air Waste Manage. Assoc., 1995, 45, 811–822 CrossRef CAS PubMed.
- J. Zhang, P. J. Lioy and Q. He, Characteristics of aldehydes: concentrations, sources, and exposures for indoor and outdoor residential microenvironments, Environ. Sci. Technol., 1994, 28, 146–152 CrossRef CAS PubMed.
- W. R. Ott, A physical explanation of the lognormality of pollutant concentrations, J. Air Waste Manage. Assoc., 1990, 40, 1378–1383 CrossRef CAS PubMed.
- K. Azuma, I. Uchiyama, S. Uchiyama and N. Kunugita, Assessment of inhalation exposure to indoor air pollutants: screening for health risks of multiple pollutants in Japanese dwellings, Environ. Res., 2016, 145, 39–49 CrossRef CAS PubMed.
- C. Marchand, S. L. Calvé, P. Mirabel, N. Glasser, A. Casset, N. Schneider and F. de Blay, Concentrations and determinants of gaseous aldehydes in 162 homes in Strasbourg (France), Atmos. Environ., 2008, 42, 505–516 CrossRef CAS.
- D. A. Sarigiannis, S. P. Karakitsios, A. Gotti, I. L. Liakos and A. Katsoyiannis, Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk, Environ. Int., 2011, 37, 743–765 CrossRef CAS PubMed.
- W. Liu, J. Zhang, L. Zhang, B. J. Turpin, C. P. Weisel, M. T. Morandi, T. H. Stock, S. Colome and L. R. Korn, Estimating contributions of indoor and outdoor sources to indoor carbonyl concentrations in three urban areas of the United States, Atmos. Environ., 2006, 40, 2202–2214 CrossRef CAS.
- A. Bradman, F. Gaspar, R. Castorina, J. Williams, T. Hoang, P. L. Jenkins, T. E. McKone and R. Maddalena, Formaldehyde and acetaldehyde exposure and risk characterization in California early childhood education environments, Indoor Air, 2017, 27, 104–113 CrossRef CAS PubMed.
- E. L. Hult, H. Willem, P. N. Price, T. Hotchi, M. L. Russell and B. C. Singer, Formaldehyde and acetaldehyde exposure mitigation in US residences: in-home measurements of ventilation control and source control, Indoor Air, 2015, 25, 523–535 CrossRef CAS PubMed.
- N. A. Mullen, J. Li, M. L. Russell, M. Spears, B. D. Less and B. C. Singer, Resultsof the California Healthy Homes Indoor Air Quality Study of 2011–2013: impact of natural gas appliances on air pollutant concentrations, Indoor Air, 2016, 26, 231–245 CrossRef CAS PubMed.
- X. M. Wu, M. G. Apte, R. Maddalena and D. H. Bennett, Volatile organic compounds in small- and medium-sized commercial buildings in California, Environ. Sci. Technol., 2011, 45, 9075–9083 CrossRef CAS PubMed.
- N. L. Gilbert, M. Guay, J. D. Miller, S. Judek, C. C. Chan and R. E. Dales, Levels and determinants of formaldehyde, acetaldehyde, and acrolein in residential indoor air in Prince Edward Island, Canada, Environ. Res., 2005, 99, 11–17 CrossRef CAS PubMed.
- P. Lovreglio, A. Carrus, S. Iavicoli, I. Drago, B. Persechino and L. Soleo, Indoor formaldehyde and acetaldehyde levels in the province of Bari, South Italy, and estimated health risk, J. Environ. Monit., 2009, 11, 955–961 RSC.
- O. Geiss, G. Giannopoulos, S. Tirendi, J. Barrero-Moreno, B. R. Larsen and D. Kotzias, The AIRMEX study – VOC measurements in public buildings and schools/kindergartens in eleven European cities: statistical analysis of the data, Atmos. Environ., 2011, 45, 3676–3684 CrossRef CAS.
- C. Mandin, et al., Assessment of indoor air quality in office buildings across Europe – The OFFICAIR study, Sci. Total Environ., 2017, 579, 169–178 CrossRef CAS PubMed.
- Umweltbundesamt, Vergleichswerte für flüchtige organische Verbindungen (VOC und Aldehyde) in der Innenraumluft von Haushalten in Deutschland, Bundesgesundheitsbla., 2008, 51, 109–112 CrossRef PubMed.
- S. Langer, O. Ramalho, M. Derbez, J. Ribéron, S. Kirchner and C. Mandin, Indoor environmental quality in French dwellings and building characteristics, Atmos. Environ., 2016, 128, 82–91 CrossRef CAS.
- C. Dassonville, C. Demattei, A.-M. Laurent, Y. L. Moullec, N. Seta and I. Momas, Assessment and predictor determination of indoor aldehyde levels in Paris newborn babies' homes, Indoor Air, 2009, 19, 314–323 CrossRef CAS PubMed.
- M. Verriele, C. Schoemaecker, B. Hanoune, N. Leclerc, S. Germain, V. Gaudion and N. Locoge, The MERMAID study: indoor and outdoor average pollutant concentrations in 10 low-energy school buildings in France, Indoor Air, 2015, 26, 702–713 CrossRef PubMed.
- J. Madureira, I. Paciência, J. Rufo, E. Ramos, H. Barros, J. P. Teixeira and E. de Oliveira Fernandes, Indoor air quality in schools and its relationship with children's respiratory symptoms, Atmos. Environ., 2015, 118, 145–156 CrossRef CAS.
- F. Villanueva, S. Lara, A. Notario, M. Amo-Salas and B. Cabañas, Formaldehyde, acrolein and other carbonyls in dwellings of university students. Levels and source characterization, Chemosphere, 2022, 288, 132429 CrossRef CAS PubMed.
- C.-R. Jung, Y. Nishihama, S. F. Nakayama, K. Tamura, T. Isobe, T. Michikawa, M. Iwai-Shimada, Y. Kobayashi, M. Sekiyama, Y. Taniguchi and S. Yamazaki, Indoor air quality of 5,000 households and its determinants. Part B: volatile organic compounds and inorganic gaseous pollutants in the Japan Environment and Children's study, Environ. Res., 2021, 197, 111135 CrossRef CAS PubMed.
- S. Uchiyama, T. Tomizawa, A. Tokoro, M. Aoki, M. Hishiki, T. Yamada, R. Tanaka, H. Sakamoto, T. Yoshida, K. Bekki, Y. Inaba, H. Nakagome and N. Kunugita, Gaseous chemical compounds in indoor and outdoor air of 602 houses throughout Japan in winter and summer, Environ. Res., 2015, 137, 364–372 CrossRef CAS PubMed.
- L. Huang, H. Qian, S. Deng, J. Guo, Y. Li, W. Zhao and Y. Yue, Urban residential indoor volatile organic compounds in summer, Beijing: Profile, concentration and source characterization, Atmos. Environ., 2018, 188, 1–11 CrossRef CAS.
- N. Sakai, S. Yamamoto, Y. Matsui, M. F. Khan, M. T. Latif, M. A. Mohd and M. Yoneda, Characterization and source profiling of volatile organic compounds in indoor air of private residences in Selangor State, Malaysia, Sci. Total Environ., 2017, 1279–1286 CrossRef CAS PubMed.
- M. Cheng, I. E. Galbally, S. B. Molloy, P. W. Selleck, M. D. Keywood, S. J. Lawson, J. C. Powell, R. W. Gillett and E. Dunne, Factors controlling volatile organic compounds in dwellings in Melbourne, Australia, Indoor Air, 2016, 26, 219–230 CrossRef CAS PubMed.
- J. Duan, S. Guo, J. Tan, S. Wang and F. Chai, Characteristics of atmospheric carbonyls during haze days in Beijing, China, Atmos. Res., 2012, 114–115, 17–27 CrossRef CAS.
- M. Delikhoon, M. Fazlzadeh, A. Sorooshian, A. N. Baghani, M. Golaki, Q. Ashournejad and A. Barkhordari, Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran, Environ. Pollut., 2018, 242, 938–951 CrossRef CAS PubMed.
- M. M. Loh, J. I. Levy, J. D. Spengler, E. A. Houseman and D. H. Bennett, Ranking cancer risks of organic hazardous air pollutants in the United States, Environ. Health Perspect., 2007, 115, 1160–1168 CrossRef CAS PubMed.
- B. Wang, S. Lee and K. Ho, Characteristics of carbonyls: Concentrations and source strengths for indoor and outdoor residential microenvironments in China, Atmos. Environ., 2007, 41, 2851–2861 CrossRef CAS.
- J. M. Logue, P. N. Price, M. H. Sherman and B. C. Singer, A method to estimate the chronic health impact of air pollutants in U.S. Residences, Environ. Health Perspect., 2012, 120, 216–222 CrossRef CAS PubMed.
- N. Liu, et al., Indoor exposure levels and risk assessment of volatile organic compounds in residences, schools, and offices in China from 2000 to 2021: a systematic review, Indoor Air, 2022, 32, e13091 CAS.
- K. Naddafi, R. Nabizadeh, R. Rostami, H. R. Ghaffari and M. Fazlzadeh, Formaldehyde and acetaldehyde in the indoor air of waterpipe cafés: measuring exposures and assessing health effects, Build Environ., 2019, 165, 106392 CrossRef.
- F. W. Sousa, I. B. Caracas, R. F. Nascimento and R. M. Cavalcante, Exposure and cancer risk assessment for formaldehyde and acetaldehyde in the hospitals, Fortaleza-Brazil, Build Environ., 2011, 46, 2115–2120 CrossRef.
- B. Wang, S. Lee, K. Ho and Y. Kang, Characteristics of emissions of air pollutants from burning of incense in temples, Hong Kong, Sci. Total Environ., 2007, 377, 52–60 CrossRef CAS PubMed.
- N. Shinohara, M. Tokumura, M. Kazama, H. Yoshino, S. Ochiai and A. Mizukoshi, Indoor air quality, air exchange rates, and radioactivity in new built temporary houses following the Great East Japan Earthquake in Minamisoma, Fukushima, Indoor Air, 2013, 23, 332–341 CrossRef CAS PubMed.
- M. Derbez, B. Berthineau, V. Cochet, M. Lethrosne, C. Pignon, J. Riberon and S. Kirchner, Indoor air quality and comfort in seven newly built, energy-efficient houses in France, Build Environ., 2014, 72, 173–187 CrossRef.
- A. Santarsiero and S. Fuselli, Indoor and outdoor air carbonyl compounds correlation elucidated by principal component analysis, Environ. Res., 2008, 106, 139–147 CrossRef CAS PubMed.
- D. Pagonis, D. J. Price, L. B. Algrim, D. A. Day, A. V. Handschy, H. Stark, S. L. Miller, J. de Gouw, J. L. Jimenez and P. J. Ziemann, Time-resolved measurements of indoor chemical emissions, deposition, and reactions in a university art museum, Environ. Sci. Technol., 2019, 53, 4794–4802 CrossRef CAS PubMed.
- H. Wang, J. Zheng, T. Yang, Z. He, P. Zhang, X. Liu, M. Zhang, L. Sun, X. Yu, J. Zhao, X. Liu, B. Xu, L. Tong and J. Xiong, Predicting the emission characteristics of VOCs in a simulated vehicle cabin environment based on small-scale chamber tests: parameter determination and validation, Environ. Int., 2020, 142, 105817 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |