 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Graphene glial-interfaces: challenges and perspectives†
Roberta
Fabbri
,
Emanuela
Saracino
,
Emanuele
Treossi
,
Roberto
Zamboni
,
Vincenzo
Palermo
 * and
Valentina
Benfenati
* and
Valentina
Benfenati
 *
*
Consiglio Nazionale delle Ricerche, Istituto per la Sintesi Organica e la Fotoreattività (CNR-ISOF), via Piero Gobetti 101, 40129 Bologna, Italy. E-mail: valentina.benfenati@isof.cnr.it; Vincenzo.palermo@isof.cnr.it
First published on 26th January 2021
Abstract
Graphene nanosheets are mechanically strong but flexible, electrically conductive and bio-compatible. Thus, due to these unique properties, they are being intensively studied as materials for the next generation of neural interfaces. Most of the literature focused on optimizing the interface between these materials and neurons. However, one of the most common causes of implant failure is the adverse inflammatory reaction of glial cells. These cells are not, as previously considered, just passive and supportive cells, but play a crucial role in the physiology and pathology of the nervous system, and in the interaction with implanted electrodes. Besides providing structural support to neurons, glia are responsible for the modulation of synaptic transmission and control of central and peripheral homeostasis. Accordingly, knowledge on the interaction between glia and biomaterials is essential to develop new implant-based therapies for the treatment of neurological disorders, such as epilepsy, brain tumours, and Alzheimer's and Parkinson's disease. This work provides an overview of the emerging literature on the interaction of graphene-based materials with glial cells, together with a complete description of the different types of glial cells and problems associated with them. We believe that this description will be important for researchers working in materials science and nanotechnology to develop new active materials to interface, measure and stimulate these cells.
1. Introduction
1.1 The state-of-the-art of graphene-based materials for neural implantsTwo dimensional (2D) nanomaterials have been widely used for interfacing with biological systems in recent years due to their diverse potential and unique physicochemical and morphological structures.1 Among them, graphene has emerged due to its outstanding properties. The unique combination of electrochemical, optical and mechanical properties makes graphene a good candidate for biomedical applications in biosensing, bioimaging, drug delivery, phototherapy, and tissue engineering.2
The properties of graphene include large surface area (2630 m2 g−1),3 strong mechanical strength (Young's modulus ∼1100 GPa, fracture strength ∼125 GPa)4 and high thermal conductivity (5000 W m−1 K−1).5 Furthermore, its all-carbon backbone structure can be easily functionalized via standard organic chemistry or electrochemistry.6–9 Mostly, the system of conjugated double sp2 bonds in the graphene plane provides high electronic mobility, giving graphene excellent electrical conductivity and high flexibility. Graphene electrodes can be extremely thin, with a single graphene sheet thickness of ∼0.34 nm.10 Monolayers of graphene can be grown on the centimetre scale and deposited on different rigid or flexible substrates (silicon, quartz, plastic, etc.).6
Mesoscopic electrodes based on multilayer graphene paper can exhibit conductivity >105 S m−1 even after 1 million bending cycles, and thus can be used for high-frequency electronics, for example to replace metallic antennas.11
The outstanding mechanical properties of graphene are also due to the high stability of its sp2 bonds, which form the graphene lattice and oppose changes in its length and angle, allowing a very high resistance to elongation to be achieved. Conversely, the bending of the sheet does not lead to a significant deformation of these bonds, at least for the nanometric radii of curvature that are of practical interest. Thus, graphene electrodes can have high tensile stiffness and strength, and simultaneously, be very flexible, which makes them ideal electrodes to contact soft samples such as brain cells.12 In addition, graphene has high transparency and absorbs 2.3% of incident visible light radiation, with an optical transmittance of 97.7%.13
Interfacing graphene with neural cells is extremely promising for neural engineering. Due to its high electrical conductivity and mechanical flexibility, graphene represents a potentially suitable material as a neuronal interface for electrical stimulation and recording of brain signals. The chemical versatility, and mechanical and electrical properties of graphene allow the fabrication of a variety of graphene-based materials and substrates, offering a new way to interact and dialogue with neural cells and tissues.
The family of graphene nanomaterials can be classified according to the number of layers, chemical modification of the surface, purity, lateral dimensions, density of defects and composition.14 The physical–chemical features of various graphene materials influence their behaviour in the biological environment. A general nomenclature of graphene-related materials (GRMs) has been recently suggested in the framework of the EU project Graphene Flagship guidelines to discriminate single- and few-layered G (1–10 layers; GR), G oxide (single layer, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 C/O ratio; GO), reduced G oxide (rGO), graphite nano- and micro-platelets (more than 10 layers, but <100 nm thickness and average lateral size in the order of the nm and μm, respectively), G and G oxide quantum dots (GQDs and GOQDs, respectively) and a variety of hybridized G nanocomposites.15
1 C/O ratio; GO), reduced G oxide (rGO), graphite nano- and micro-platelets (more than 10 layers, but <100 nm thickness and average lateral size in the order of the nm and μm, respectively), G and G oxide quantum dots (GQDs and GOQDs, respectively) and a variety of hybridized G nanocomposites.15
Exploiting graphene materials to build electrodes or stimulation/recording components in bioelectronic devices can improve the functionality of existing technologies to replace or support conventional silicon and metals materials.2 Due to its high deformability and affinity with biological macromolecules, GRM can improve the long-term stability and biocompatibility of implant devices, promoting cell growth and proliferation and minimizing the formation of fibrous tissue around electrodes.16 The flexibility and mechanical resistance of graphene make it an ideal component for flexible bioelectronic systems, as an integral element or structural reinforcement or coating, able to adapt to biological tissues, limiting the mechanical stress and increasing the lifetime of the device.17,18
Graphene oxide (GO) films (Fig. 1B) with a nanometric thickness can be easily fabricated via spin coating or filtering of aqueous solutions at different concentrations.19 Although GO is non-conductive, it can be transformed to highly conductive and reduced graphene oxide (rGO) via thermal, chemical, and electrochemical treatments, which can be used directly as flexible electrodes for bio-sensing, featuring high affinity with target biomolecules.20–22 GO films can also be used as neural interfaces and integrated in high-performance fully flexible field effect transistors (FET). Recordings of the electrical activity of electrogenic cells using graphene transistors have been reported recently, confirming the enormous potential of graphene for bioelectronics applications, especially in the field of neural prosthesis. The high charge carrier mobility and high interfacial capacitance of graphene result in a high transconductive sensitivity and more effective capacitive stimulation than silicon-based FETs. Graphene-based transistors can be used to either stimulate or record signals from cells.23
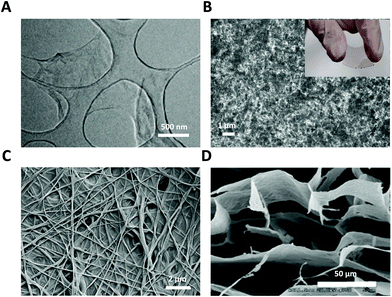 | ||
| Fig. 1 GRM nanomaterials used as glial interfaces. (A) TEM image of GO flakes. Adapted with permission from ref. 29 (Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim). (B) SEM image of GO film; inset: photograph of flexible PET-ITO-GO substrate. (C) FE-SEM image of GO nanofiber hybrid scaffold. Adapted with permission from ref. 30 (Copyright 2014, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim). (D) SEM image of GO 3D scaffold. Adapted with permission from ref. 31 (Copyright 2013, The Royal Society of Chemistry). | ||
In vivo studies have shown that neuronal activity can be stimulated and recorded using graphene-based electrodes. Graphene electrodes produce slightly higher injection charge levels than common metal electrodes. A reduced GO flexible probe incorporated in parylene-C24 was used to stimulate retinal ganglion cells ex vivo and to record neural activity in vivo from the visual cortex of a cat. Graphene electrodes, completely transparent, were, for example, implanted in GCaMP6f mice for electrical brain stimulation and simultaneous optical monitoring of neural activity.25
GRM have been also exploited in tissue engineering applications as electroactive and highly versatile backbones for the design of 3D scaffolds to enhance neuronal regeneration and functional recovery of brain and peripheral injury as effective regenerative therapy in neurological disorders (Fig. 1C and D).26,27 Advancements in the preparation of hybrid nanoscale scaffolds allow the construction of 3D graphene nanostructures, where the presence of surface functional groups is beneficial for the adhesion and growth of cells.28
The conductivity of GRMs strongly depends on their fabrication process, purity, dimension, and defects. A suitable trade-off among conductivity, geometrical factors, fabrication process and flexibility of the device may be difficult to achieve in most cases. Some key quantitative parameters such as the substrate thickness, electrode impedance, charge injection capabilities and signal-to-noise ratio need to be matched to design and engineer novel electrodes for the recording and stimulation of glial cells.17,32
Accordingly, recent studies have revealed that a low impedance and higher adhesion between the material interface highly improve the signal to noise ratio and allow recording in glial cells in vitro.33,34 In addition, the different sensitivities of glial cells to extracellular electrical stimulation protocols compared to neurons can be exploited by using different graphene-based devices for the selective stimulation of glia.
Compared to existing stimulation/recording strategies, the design of bi-functional electrode arrays exploiting the different conductivity properties of GRM coatings to allow the bi-directional sensing and triggering of cell activity may be a possible route to selectively target the molecular mechanisms underlying glial functionality within brain tissue. Additionally, the tuning ability to manage or reduce the concentration of oxygen-containing functional groups by the chemical functionalization of graphene materials, as well as their unique surface properties, which allow the absorption of molecules and ligands, are feasible and suitable to achieve a higher adhesion of glial interfaces and improve their integration with glial cells within neural tissue.32,35
Besides graphene, other biomaterials can successfully meet the current demand for flexible and smart bio-functional devices and probes in neural/glial engineering and neuroregenerative medicine. Among them, organic semiconductor or conducting polymers, including poly(3-hexylthiophene) (P3HT),36,37 (poly(3,4-ethylenedioxythiophene) polystyrene sulfonate) (PEDOT:PSS), and polyaniline (PANI), exhibit a wide range of favourable properties. Their chemical stability and biological affinity, mechanical compliance, intrinsic electrical conductivity and photoexcitability within the visible range and charge photogeneration capability make organic electronics and optoelectronics surprisingly advantageous over the traditional inorganic technologies and suitable to generate novel organic glial interfaces devoted to the recording and recovery of brain functions.33 In addition, the use of conductive carbon nanotubes for the fabrication of scaffolds to drive neural regeneration and glial differentiation can contribute to the repair or replacement of brain tissue structures in the injured central and peripheral nervous system. Due to their high biocompatibility, and good mechanical and functional properties, natural silk fibroin-based substrates have been also explored as bioactive and soft glial platforms for drug-delivery applications and neural prosthesis implantation.33,36 Topographical cues provided by nanofiber alignment of synthetic polymers, such as polycaprolactone (PCL), have shown modulating effects on the adhesion, maturation and differentiation of glial cells, moving biomaterials research and tissue engineering toward the potential development of polymeric glial interfaces that can guide and support brain regenerative processes.33,38
1.2 Bio-compatibility of graphene and related materials
Several in vitro and in vivo studies have been conducted to investigate the biocompatibility of GRMs, a crucial aspect to be evaluated to identify suitable candidates for clinical applications. Despite the general acceptance that graphene is biocompatible, it should be noted that GRMs are a wide class of materials, with tunable flake size, flake thickness, defects, etc.,15 and all these parameters need to be specified to assess the biological activity of a material. Thus, it is not possible to state in a single sentence that GRMs are generally toxic or safe.39–42 The synthetic method, presence of functional groups, type of coating or treatment, size, thickness and structural defects may have different biological impacts.16,43,44 Thickness, which is determined by the number of layers, is directly related to the flexibility of the material, while the lateral dimension is linked to the degree of deformability, and both parameters influence the interactions of graphene with cells, in particular with the plasma membrane.
The simplest electrodes based on GRMs are the those composed of a single layer of graphene grown via chemical vapour deposition (CVD), and then transferred on glass, silicon or flexible polymer substrates. Viability tests were performed to assess the compatibility of CVD graphene-FET with living cells, such as primary retinal ganglion cells, cardiomyocytes, and human embryonic kidney cells. The healthy growth of cells cultured on a transistor array also confirmed the excellent biocompatibility of CVD-graphene.23In vitro studies on primary cultures have demonstrated no changes in the viability of neuronal cells following exposure to graphene.45,46
In contrast to CVD graphene, electrodes based on rGO can be processed in solution, in water, allowing high throughput, low cost and chemical tunability. Interestingly, the published data suggests that a higher degree of oxidation, size reduction, and functionalization of graphene derivatives by macromolecules can contribute to limiting their cytotoxicity and inflammatory responses, favouring their biocompatibility and stability in contact with biological fluids.2,16 The efficient biodegradation of GO sheets was observed using myeloperoxidase (hMPO) derived from human neutrophils40 or recombinant eosinophil peroxidase (EPO) enzyme extracted from human eosinophils.47 However, primary neuronal cultures exposed to GO nanosheets revealed clear alterations in physiological pathways and synaptic transmission processes. A complete analysis of the effects of chronic and acute exposure to GO was performed on cultures of rat primary cortical neurons, evaluating its long-term effects on cell viability and synaptic transmission. Once internalized by the neurons, the graphene nanosheets were found to accumulate preferably in the lysosome. Exposure to GO selectively caused the inhibition of excitatory transmission, in parallel with a reduction in the number of excitatory synaptic contacts and an enhancement in inhibitory activity.45 This was accompanied by autophagocytic activity and alterations in calcium dynamics and homeostasis. These results indicate that although exposure to graphene does not affect neuron viability, it has important effects on neuronal transmission and neural network functions.45,46
1.3 New opportunities to use graphene as interfaces with glial cells
All the above-mentioned evidences confirm the great potentialities of graphene substrates as biomaterial interfaces in neural engineering, aiming at modulating or recovering nervous system function.
This should be placed in a larger context concerning our understanding of how the brain works. This understanding has been significantly modified by evidence provided over the last four decades. The most traditional view assumes that neurons are the only active cells in the brain, with glial cells just working as passive glue supporting neurons. However, currently, increasing experimental evidence suggests that glial cells interact actively with neurons at the molecular, structural and functional level to critically regulate the brain homeostasis and modulate synaptic communication.48–51 Although they are considered electrically silent cells, glial cells express a variety of ion channels, receptors and transporters that allow them to sense the variations in extracellular milieu concentrations and physical properties. Glial cells are also capable of responding to environmental chemo-physical stimuli by ion and water flow through the plasma membrane or by changes in the intracellular calcium concentration ([Ca2+]i), glio-transmitter release or by chemokine and trophic factor secretion.52–55 Thus, glial response critically impacts neural networks and circuity both in physiological and pathological conditions.56,57 Dysfunction of glial cells contributes to the pathogenesis of several neurological disorders, such as epilepsy, spreading depression, brain tumours, and Alzheimer's and Parkinson's diseases.57–59
Currently, the available biomedical technologies aimed at the study, diagnosis and therapy of nervous system (NS) functions and dysfunctions require neurons to be studied both in vitro17,23,60 and in vivo.36 However, despite major efforts, brain implants still need significant improvements in long-term stability and functionality. Accordingly, it is becoming evident that driving and controlling the gliotic inflammatory reaction is critical for the success of therapeutic approaches based on biomaterial implants and devices.51,61,62 Given that glial cells are the first cell type responsible for the reaction of the central nervous system (CNS) to any type of injuries,58,59 studies on glial cells are needed to understand and control the impact of neural interfaces. Thus, there is a major challenge and great potential in biomaterials science and engineering to design and validate glial interfaces and devices that are capable of driving favourable glial growth and selectively modulating glial cell functionality.33 Thus, the impact of biomaterials on glial cell viability, growth, proliferation and functional properties has been investigated.29,33,35,37,54,55,63,64 Notably, traditional neural interfaces based on silicon and metal materials display a series of important technological and mechanical limitations in terms of spatio-temporal resolution, selectivity, mechanical tissue mismatch, and long-term biocompatibility. Graphene substrates, suitably functionalized or in combination with conventional materials, may improve the electrical and mechanical performance of traditional implant devices and their interaction with biological systems.2,16,17
The objective of the present work is to propose glial cells and their biomolecular mechanisms as targets for emerging graphene-based biomedical interfaces and devices.33 Herein, we also critically review the advantages and challenges in using graphene-related nanomaterials and devices for the stimulation and control of the viability, morphology, structure and function of glial cells. Our aim is to provide a fair introductory overview to researchers interested in the applications of graphene in glial engineering33 as electrical stimulation implants (retinal prosthetics and bioelectronics for deep brain stimulation), scaffolds for the repair of injured nerves, and drug delivery systems.
2. Glial cells
There are different types of glial cells, which are located in many organs of the human body with different functions. Here, we provide a brief description of these types of glial cells and the problems associated with them. We believe that this description will be important for researchers working in materials science and nanotechnology to develop new active materials to interface, measure and stimulate these cells.The two major classes of cells that coexist in the nervous system are neurons and glia. Neurons are electrically excitable cells as they can generate and propagate action potentials through the neuronal circuitry, whereas glia are typically considered non-excitable cells given that they are unable to fire action potentials.65–68
The main macro classification of glia includes macroglia and microglia (Fig. 2).65,69 Macroglia are derived from neuroepithelial progenitor cells, which are neuronal progenitor cells giving rise to radial glia. In neurodevelopment, radial glial cells generate most of the cortical neurons, while a gliogenic switch allows the differentiation of radial glial cells into astrocytes or oligodendrocyte precursor cells. After maturation, radial glial cells disappear from many regions of the brain, although they remain in the retina (called Müller glia) and in the cerebellum (called Bergmann glia). Unlike astrocytes and oligodendrocytes, microglia originate from foetal primitive macrophages, which migrate from the periphery to the CNS and give rise to microglia.70
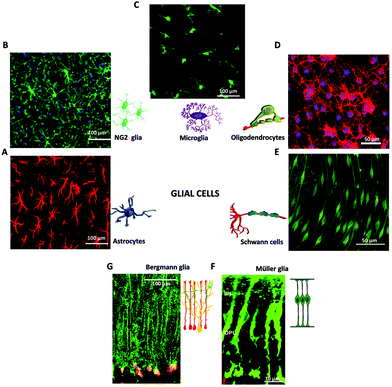 | ||
| Fig. 2 Glial cells in the nervous system. Immunofluorescence images of macroglia (A, B, D, E, F, G) and microglia (C). (A) Fluorescence image of astrocytes, adapted with permission from ref. 71 (licensed under the Creative Commons Attribution License, Copyright 2012 Butenko et al., Public Library of Science). Cartoon adapted with permission from ref. 27 (Copyright 2018, Springer International Publishing AG, part of Springer Nature). (B) Fluorescence image of NG2 glia. Adapted with permission from ref. 72 (Copyright 2018, Elsevier). (C) Fluorescence image of Microglia, adapted with permission from ref. 79 (licensed under the Creative Commons Attribution License, Copyright 2008 Liu et al., Spandidos Publications). Cartoon adapted with permission from ref. 27 (Copyright 2018, Springer International Publishing AG, part of Springer Nature). (D) Fluorescence image of Oligodendrocytes, adapted with permission from ref. 73 (licensed under the Creative Commons Attribution-Non-Commercial-ShareAlike License, Copyright 2014, Wolters Kluwer Medknow Publications). Cartoon adapted with permission from ref. 27 (Copyright 2018, Springer International Publishing AG, part of Springer Nature). (E) Fluorescence image of Schwann cells, adapted with permission from ref. 78 (licensed under the Creative Commons Attribution License, Copyright 2015 Bacallao, Monje, Public Library of Science). Cartoon adapted with permission from ref. 27 (Copyright 2018, Springer International Publishing AG, part of Springer Nature). (F) Fluorescence image of retinal Müller glia, adapted with permission from ref. 74 (Copyright 2007, National Academy of Sciences, USA). Cartoon adapted with permission from ref. 75 (Copyright 2013, The Company of Biologists). (G) Fluorescence image of Bergmann glia present in the cerebellum, adapted with permission from ref. 76 (Copyright 2007, Elsevier). Cartoon adapted with permission from ref. 77 (licensed under the Creative Commons Attribution License, Copyright 2008 Ango et al., Public Library of Science). | ||
2.1 Astrocytes
Astrocytes are macroglial cells in the CNS. The complexity and heterogeneity of astroglial cortical cells is a major difference that distinguishes the structure of an adult human brain from the brain of invertebrates and other mammalians (Fig. 2A). Astrocytes are actively involved in a wide variety of essential physiological and pathological processes in the brain.80,81 (i) They regulate the volume and composition of the cerebral interstitial space. Astrocytic ramified processes envelop neurons forming a neuron-astrocyte interface and establish a close contact with blood vessels at the glio-vascular interface.82 (i) Astrocytes are key elements in the control of brain homeostasis as they regulate the maintenance of ions and molecule concentrations in the brain extracellular environment through the transmembrane flow allowed by numerous ion channels, water channel aquaporins and transporters expressed in selective districts called microdomains.33,81,83 Potassium homeostasis is achieved by astrocytes through the uptake and spatial buffering of potassium (K+) ions.84 This mechanism aims at removing excess K+ from areas of neuronal activity and dissipating it on distal sites in the vicinity of blood vessels, thus stabilizing synaptic activity.48,65 Among the variety of K+ channels expressed by astrocytes, the Kir4.1 inwardly rectifying channels are mainly responsible for potassium homeostasis.81,83 The aquaporin-4 (AQP4) water channel is the predominant channel expressed in astrocytes, which is involved in the regulation of cell volume and K+ spatial buffering as a molecular partner of Kir4.1 to facilitate the movement of water through the plasma membrane.83,85,86 (ii) Astrocytes also contribute to the homeostasis of chloride (Cl−), calcium (Ca2+) and sodium (Na+)81 and regulate extracellular pH.65,83 (iii) Astrocytes participate in the formation, functional maintenance and integrity of the blood brain barrier (BBB), a structural and functional barrier formed by brain microvascular endothelial cells, astrocytes and pericytes, which regulates the bi-directional communication between the cerebral environment and blood vessels and protects the brain from microorganisms and toxins circulating in the blood.87,88 (iv) Astrocytes provide metabolic support for neuronal activity by glucose transporters.89A revolutionary concept in neurophysiology was established when the fundamental role of astrocytes in synaptic connectivity was demonstrated. Subsequently, astrocytes became the third element of the so-called “tripartite synapse”, in which astrocytes actively exchange information with the synaptic elements.49,50,57,82,90 Perisynaptic glia represent a functional component in the transmission and modulation of brain electrical signals. Perisynaptic glia respond to neuronal activity with an increase in intracellular calcium and release of gliotransmitters, which are neuroactive substances that can both directly stimulate postsynaptic and presynaptic neurons, favoring or inhibiting the release of neurotransmitters.90–95 Astrocytes express a variety of neurotransmitter receptors, including receptors for glutamate, γ-aminobutyric acid (GABA), adrenaline, adenosine triphosphate (ATP), serotonin, acetylcholine (Ach), and several peptides. The activation of these receptors evokes astroglial calcium signals, which induce the release of gliotransmitters, such as ATP, GABA, glutamate, D-serine, and lactate.50 Although astrocytes are considered electrically non-excitable cells, their ability to respond to different extracellular chemical–physical stimuli (such as neurotransmitters, temperature, osmotic gradient, mechanical and electrical stimuli) by variations in their [Ca2+]i defines a form of astrocyte excitability.52,53,96–98 However, there is still little evidence regarding the mechanisms underlying the response of astrocytes to physical stimuli such as ultrasound,99 light, and an electric field.54,55
Astrocytes express a variety of ion channels and membrane receptors by which they respond to neuronal activity, modifying their membrane potential and/or by triggering [Ca2+]i variations.86,100 Calcium oscillations can be limited to a single cell or transmitted as “waves” to neighboring astrocytes through gap junctional coupling, mainly formed by connexins (Cx43).101,102 By the propagation of calcium waves, astrocyte networks define an extra-neuronal pathway for the rapid long-distance transmission of brain signals.97,103 Two distinct molecular mechanisms underlie the astroglial [Ca2+]i signals, i.e., an increase in [Ca2+]i caused by the entry of extracellular Ca2+ and the mobilization of Ca2+ from the cytoplasmic stores (Fig. 3).
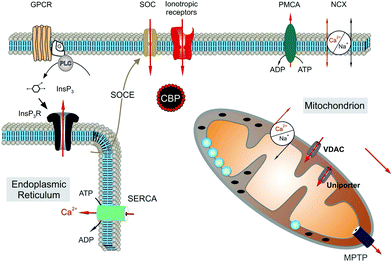 | ||
| Fig. 3 Molecular mechanisms underlying calcium signals in astrocytes. Reproduced with permission from ref. 105 (Copyright 2011, Elsevier Ireland). | ||
The main intracellular Ca2+ sources are the endoplasmic reticulum and mitochondria.53,104,105 Extracellular Ca2+ can mainly enter the cell via ionotropic channels, such as the P2X7 purinergic receptor106 and through members of the transient receptor potential (TRP) family.107 In particular, the transient receptor potential vanilloid 4 (TRPV4) and ankyrin 1 (TRPA1) are the main channels expressed in astrocytes.54,55,108–110 In response to neuronal activity, different calcium dynamics occur in distinct regions of the astrocytes such as the soma, astroglial processes and microdomains of the end feet facing the synapse.111 Slower and long-lasting transients occur when the increase in [Ca2+]i is due to Ca2+ influx from the extracellular compartment, whereas faster, shorter and repetitive oscillations occur when it depends on the release of Ca2+ by the cytoplasmic stores. Previous results have shown the importance of Ca2+ mitochondrial dynamics in the physiology and pathophysiology of astroglial cells. In particular, dysregulation of mitochondrial dynamics is associated with a number of neuropathies. Although the molecular mechanisms underlying pathogenic mitochondrial changes are not fully known, astrocytic mitochondrial networks have shown rapid Ca2+ signalling impairments, metabolic alterations and re-shaping changes in response to injury cues.112
The diversity of astrocyte [Ca2+]i signals has a distinct functional role; however, it is far from being fully understood.113 A major issue is the lack of selective technologies capable of evoking calcium signalling with selective control of distinct components spatially and temporally. Accordingly, the availability of novel and selective technological tools, such as graphene electrodes and interfaces, may represent the optimal solution to trigger or modulate astroglial functionality in the study and therapy of CNS function and dysfunction.
2.2 Radial Müller glial cells in the eye retina
Radial Müller glial cells represent the predominant macroglial cells in the retina, covering 90% of the retinal glia.114,115 They are radially oriented, highly polarized and structured cells, which span the inner retina to the photoreceptor layer in the outer retina (Fig. 2F).The ability of Müller cells to control the concentration of extracellular neuroactive substances, water, ions and pH critically modulate the function of photoreceptors and bipolar and ganglion cells.116 Their processes enwrap retinal blood vessels to maintain the homeostasis of the extracellular space and allow metabolic exchange and waste clearance at the glio-vascular interface. Müller cells are essential structural components that ensure correct retinal formation during retinal development and the integrity of the blood-retinal barrier in the adult retina. Müller glia display many common structural and functional properties with astrocytes,115 as described in the previous section. Importantly, Müller glia and astrocyte communication relies on changes in [Ca2+]i, which represent fundamental biochemical signals that can trigger different processes. Among the protein channels, the TRPV4 calcium channel, the AQP4 water channel, and the Kir4.1 potassium channel are important in retinal function and dysfunction. TRPV4 is a polymodal sensorial channel mediating extracellular calcium entry, which underpins osmosensation and mechanosensation in Müller glia.117,118 A dysfunction of TRPV4 is observed in pathological conditions such as retinal detachment.119 The release of Ca2+ from internal stores such as the endoplasmic reticulum91 is also a crucial signalling pathway for Müller glia that can be triggered by via inositol-3-phosphate (IP3), ryanodine (RyR), and metabotropic glutamate receptors or by mechanical and osmotic stimuli.94,95 [Ca2+]i transients in Müller cells are also triggered by neuronal activity during photostimulation.120
AQP4 is typically involved in the control of water homeostasis in the retina in response to osmotic stress and dysregulation of AQP4 results in an inflammatory response of the retinal tissue. As described for cortical astrocytes,109 synergic cooperation between AQP4 and TRPV4 is essential to promote the volume regulation mechanism through osmosensing, water transport, calcium signalling, and osmolyte efflux.121
Müller glia play a critical role in retinal inflammatory responses to injury or disorders called reactive gliosis (Appendix†), which are also observed in response to implant insertion.36,51 Accordingly, Muller glia ion and water channel functions are a primary target to be considered in engineering more compatible graphene-based retinal prosthesis and/or to trigger the modulation of retinal neurons.
2.3 Radial Bergmann glia in the cerebellum
Radial Bergmann glia (BG) are specialized astrocytes of the cerebellum cortex. BG are unipolar glial cells with cell bodies situated in the Purkinje cell (PC) layer and radial fibers extending through the cerebellum molecular layer (Fig. 2G). BG are critically involved in the fine-tuning of neuronal processing. Ca2+ signalling in BG cells plays a key role in regulating the structural and functional interactions between these cells and PC synapses. Bergmann glia express α-amino-3-idrossi-5-metil-4-idrossazol-propionic acid (AMPA) glutamate receptors (AMPARs) permeable to Ca2+. Genetic deletion of AMPA receptors results in impaired BG calcium signaling, alteration in synaptic transmission, and consequently, the dysfunction of cerebellar motor coordination in adult mice.120,122–124In vivo imaging studies have also described different types of spontaneous and spatially localized Ca2+ signals in the cerebellum of mice depending on their motor behavior. Notably, spontaneous Ca2+ signalling in BG can be amplified in response to electrical stimulation of afferent fibres.124 Thus, the evidence suggests the hypothesis that Ca2+ signals in BG cells can become a possible target for graphene-based glial interfaces and electrodes aiming at stimulating and controlling motorial coordination and the arousal or startling of the brain states.2.4 Microglia
Microglia are immunocompetent cells specifically equipped for surveillance of the CNS environment. Thus, microglial cells represent the endogenous brain immune defence system, which is responsible for CNS protection against all types of pathogenic factors. Their pathological potential has been extensively investigated125,126 also considering being a potential target for neurological treatment. Microglia in the healthy mature CNS, including the brain, spinal cord, and the eye and optic nerve, are in a “resting” state and have a ramified morphology, i.e., a small soma with fine cellular processes (Fig. 2C). Although the role of microglia in the pathological brain is widely recognized, the role of these cells is unknow in healthy brains. It has been demonstrated that microglia can remodel synapses during development and in the adult brain.127 To closely monitor the health of the brain, microglial cells move constantly, scanning the local environment to signal possible lesions of the nervous tissue. They are also equipped with numerous receptors and immune molecule recognition sites, which make them perfect sensors of the status of the CNS tissue.65,68 Sensing of neurotransmitters, e.g., acetylcholine, glutamate, GABA, and ATP, is well documented, as well as the consequent changes in [Ca2+]i, membrane potential, cytokine release, motility and voltage sensitive currents.126,128,129All CNS diseases involve microglia, which typically convert from the resting/surveillant cell type in the normal brain to an activated amoeboid form, specialized to operate within the diseased environment.68,130 Amoeboid or reactive microglia modify their cell shape, gene expression and functional behavior and acquire phagocytic capacity, which is essential for the removal of cellular debris and apoptotic cells and synaptic remodeling.68,70,126,129–131
Microglia-mediated neuroinflammation is one of the hallmarks shared by various neurodegenerative diseases, including Parkinson's disease, Alzheimer's disease, and amyotrophic lateral sclerosis.132,133 Thus, microglia may become an elective target for graphene-based drug delivery approaches aiming at reducing the inflammatory response observed in chronic neuropathology.
2.5 NG2 Glia
A more recently discovered type of glia in the CNS is neuron/glia antigen 2 (NG2). NG2 glia are a type of glial cells that have an oligodendrocyte progenitor cell (OPC) antigenic phenotype and do not express any of the phenotypic markers for mature astrocytes, oligodendrocytes, and microglia.72,134 During development, NG2 immunopositive OPCs give rise to both myelinating oligodendrocytes and a substantial portion (5–10% of all glia) of NG2, which persist throughout the white and grey matter of the mature CNS. NG2-glia are characterized by a small soma and numerous thin, radially oriented processes (Fig. 1B). NG2 glia express voltage-gated Na+, Ca2+ and K+ channels, as well as glutamate and GABA receptors, and they actively communicate with neurons in healthy brains.135 The majority of NG2 glia in the mature and healthy CNS appear to be fully differentiated, but in the adult brain, NG2 glia may generate neurons and astrocytes as well as oligodendrocytes,72,134 acting as multipotent adult neural stem cells. Notably, NG2 glia are highly reactive and rapidly respond to CNS insults by outgrowth and proliferation of processes. Activated NG2 glia participate in glial scar formation together with astrocytes.72,134 The potential of reactive NG2 glia to originate different neural cell types, their ability to shape neuronal synapse formation, their plasticity in response to neuronal disease, and their emerging importance in myelin maintenance and remodelling to repair injured neurons make them a unique group of glial cells and of attractive interest for biomedical engineering.72 Additionally, microglial channels and receptors, which mediate their response to neurotransmitters, growth factors and alteration in the surrounding environment, can become molecular targets for graphene-based scaffolds and devices aiming at regeneration of the function and structure of neural tissue.65,134,1352.6 Oligodendrocytes
Oligodendrocytes are cells that provide the myelin sheath, which is the lipid/protein envelop that surround axons in the CNS and allows saltatory and fast conduction of the action potential propagation (Fig. 2D).65 Given the role of oligodendrocytes in myelination, diseases such as multiple sclerosis and amyotrophic lateral sclerosis, which are characterized by disruption of the myelin and chronic axon degeneration, are also called oligodendropathies.72In a healthy human brain, oligodendrocytes are generated continuously, and they allow the remodeling and homeostasis of myelin throughout their life.68,136 The spatial and functional relationship of oligodendrocytes with other glial cells is critical for myelin homeostasis, the impairment of which can trigger a neuroinflammatory gliotic cascade.72,136 The need to understand the nature and the dynamics of this complex cross-talk occurring during the damage and repair calls for the generation of graphene-based interfaces and electrodes capable of modulating these processes. The use of these devices will increase the global knowledge on demyelinating diseases and may help in the development of novel and more holistic ways to manipulate and improve remyelination of injured neural tissue.
2.7 Schwann cells in the peripheral nervous system
In the peripheral nervous system (PNS), the same myelination task performed by oligodendrocytes in the CNS is in charge of Schwann cells (SC), which enwrap and myelinate peripheral axons.68 SC are key cellular elements in assisting the regeneration of peripheral nerve after injury (Fig. 2E). There are four types of mature SC in the PNS, including myelinating Schwann cells, non-myelinating Schwann cells, perisynaptic Schwann cells of the neuromuscular junctions (NMJs) and terminal Schwann-like cells of the sensory neurites.136,137 Continuous contact with axons during development is particularly important for the survival of SC precursors. Non-myelinating Schwann cells maintain contact with several thin axons during differentiation, whereas myelinating Schwann cells always envelop a single axon with a large diameter. The factors that regulate the fate of SC remain unknown, but they are hypothesized to depend on the local cross-talk between neuronal axons and SC.SC express gap junctions, which are mainly formed by proteins called connexin 32 (Cx32) that are critically involved in SC-axon interactions and in Na+ channels clustering at Ranvier nodes, and thus in stabilizing the structure of the nodal axonal membrane, allowing the conduction of the action potential.65,137 Mature SC can swiftly dedifferentiate and return to the proliferating stage similar to immature cells. This dedifferentiation process underlies the Wallerian degeneration that follows injury of peripheral nerves,65 and thus is a target for neurodegenerative medicine and scaffold engineering. Perisynaptic Schwann cells guide the growth of regenerating presynaptic nerve terminals at adult NMJs. Similar to that observed for astrocytes, Müller glia and Bergmann glia, perisynaptic Schwann cells respond to synaptic activity by means of metabotropic acetylcholine and ATP receptors, which trigger an increase in [Ca2+]i, and consequently the release of transmitters that then target neuronal synapses. The latter cross-talk is critical for the structure and function of NMJs.
Notably, high-frequency electrical stimulation of nerve terminals triggers an increase in [Ca2+]i in neighbouring SC. The Ca2+ signals generated in SC induce feedback in the neuronal terminal, which can potentiate or depress the release of neurotransmitters from the latter. The net effect depends on the frequency of synaptic activation.138 This suggests the possibility to use graphene electrodes to evoke potentiation or depression of SC-mediated synaptic activity by electrical stimulation.
2.8 Glial cells and pathological brain
Studying and possibly controlling the activity of all the glia types described above is very important because their dysfunction is related to a wide range of well-known diseases such as epilepsy, brain tumors, Alzheimer's disease and Parkinson's disease (Table 1). Appendix (ESI†) of the present work provides a detailed overview of the role of different glial cells in specific peripheral and central nervous system pathologies. This is useful to define the proper target cell for each disease and identify the corresponding problems to study them to overcome the limitations of current technology by using graphene-based glial interfaces for neurological intervention.| Type of glia | Morphology | Location | Molecular features (structural proteins, ion channels, and growth factors) | Functional target | Glial pathologies | Application target of graphene interfaces |
|---|---|---|---|---|---|---|
| Astrocytes | Star-shape cells | CNS | GFAP, Kir 4.1, AQP4, TRPA1, TRPV4, P2Y, glutamate and GABA receptors, IP3 receptors, Cx43, TGF, GDNF, LIF | K+ buffering, Ca2+ signalling, water transport, sensing of GABA, glutamate, adrenaline, ATP, serotonin, Ach, release of ATP, GABA, glutamate, D-serine, and lactate | Alzheimer's disease, Parkinson's disease, epilepsy, glioma | Electrodes for electrical stimulation and modulation of glial activity |
| NG2 | Small soma and numerous thin radially oriented processes | CNS | NG2, voltage-gated Na+, Ca2+ and K+ channels, glutamate and GABA receptors, PDGF, EGF, chemokines | Ca2+ signalling in differentiation towards oligodendrocytes, neurons and astrocytes | Demyelinating pathologies | Scaffold for guiding glial differentiation |
| Oligodendrocytes | Myelinated cells | CNS | O4, integrins, Kir 4.1, MAG, NMDA, AMPA receptors, GDNF, BDNF, IGF-1 | Ca2+ signalling in glial mediated myelination processes | Parkinson's disease and demyelinating pathologies | Scaffold for driving glial-mediated myelination |
| Müller glia | Radially oriented cells | Retina | GFAP, vimentin, p75, TRPV4, AQP4, AQP7, AQP9, Kir 4.1, P2Y, NAD+, RyR, glutamate receptors, GDNF, PEDF, IGF-1 | K+ siphoning, Ca2+ signalling, water transport, homeostasis of K+ and neurotransmitters, glucose uptake, mechanical sensing | Retinitis pigmentosa, age-related macular degeneration, glaucoma | Electrodes for electrical stimulation and modulation of glial activity |
| Bergmann glia | Unipolar cells with radial fibers | Cerebellum | GFAP, Glu A1 and Glu A4-AMPA receptors, α1 adrenoceptors | Ca2+ signalling depending on motor behaviour | Parkinson disease and motor dysfunctions | Electrodes for electrical stimulation and modulation of glial activity |
| Schwann cells | Myelinated cells | PNS | S100, Ach receptors, IP3 receptors, Cx32, nestin, actin, NGF, GDNF, BDNF, CNTF | Ca2+ signalling at the neuromuscular junction, upregulation of structural proteins | Peripheral nerve injury | Scaffolds for driving glial-mediated myelination |
| Microglia | Branched shape (resting state) to amoeboid morphology (reactive state) | CNS and PNS | IbA1, Ach, glutamate, GABA and ATP receptors, cytokines, reactive oxygen species | Ca2+ signalling, sensing of neurotransmitters, changes in motility, release of cytokines and reactive oxygen species | All glial pathologies in the CNS and PNS | Electrodes for sensing and controlling inflammatory processes |
3. Glial cells as targets for engineering biomaterials and implants
Interfacing the nervous system with devices (e.g., conductive materials working as electrodes) that can efficiently record or modulate the electrical activity of neuronal cells represents the biggest challenge in neuroscience research and neuroengineering. It is now established that electrodes implanted for the study and therapy of brain function and dysfunction interact with glial cells, not only with neurons (Fig. 4). When a bulk, rigid electrode is inserted in the tissue a pronounced inflammation area, it is characterized by sparse neurons, a high number of reactive astrocytes, and more importantly, the presence of an activated microglia shield around the probe is visible (in red, Fig. 4b). In comparison, a soft and porous electrode will induce an increase in low gliosis and neurodegeneration, and in some circumstances, colonized by cells coming from the surrounding tissue (Fig. 4c). This will give rise to a sort of synthetic/cellular hybrid material, where the maximum electrode/tissue integration is obtained.139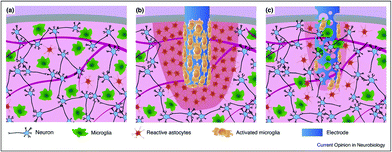 | ||
| Fig. 4 Comparison of the tissue reactivity around a stiff and compact neuronal electrode vs. a probe made of a soft and (micro)porous material. (a) Healthy tissue is characterized by a well-organized neuronal network and a relatively low amount of uniformly dispersed microglial cells and reactive astrocytes; (b) a bulk, rigid electrode inserted in the tissue producing an inflammation area and tan activated microglia shield around the probe (in red); and (c) a soft and porous electrode inducing low gliosis and neurodegeneration, being colonized by cells coming from the surrounding tissue. Adapted with permission from ref. 139 (Copyright 2017, Elsevier). | ||
Activation of microglia and astrocytes is also at the centre of many neuroinflammatory pathways as well as the typical in vivo reaction to the insertion of a neuroprosthetic implant. In the acute phase of gliosis, activated microglia attach to the surface of the electrode and release pro-inflammatory factors, the reaction progresses to a chronic phase where a dense astrocyte encapsulation envelops the electrode, forming a scar, isolating the neural electrode and increasing the distance between the electrode and the target active neurons, thus dramatically affecting the amplitude of recorded or the efficacy of delivered electrical signals to and from neurons. From an electrical interfacing view, astrogliosis is responsible for an increase in electrode impedance, and consequently, a reduction in input/output voltages amplitudes.139,140
Similar to the process described in the brain, Müller glial cells undergo reactive gliosis in response to retinal prosthesis, leading to the formation of a glial scar. The latter severely compromises the device performance as it can envelope the device and the entire retina at the late stages of degeneration.141 Glial fibrillary acidic protein (GFAP) immunostaining of implanted retinal prosthesis based on organic polymers and silk fibroin demonstrated (Fig. 5) that gliosis started immediately after surgery (one week), but then decreased thereafter to return to the baseline five months after surgery.36,142 All these results strongly encourage the development of electrode interfaces, which can reduce glial cell activation and promote anti-inflammatory glial cell phenotypes, resulting in biocompatible and long-term stable neural interfaces. Given the well-established role of astrocytes and microglia and the newly acquired knowledge on oligodendrocyte and NG2 glia susceptibility to inflammation-induced injury, novel strategies and materials should be considered to improve the biological impact of implanted devices.
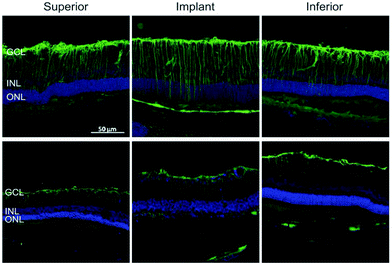 | ||
| Fig. 5 Reactive astrogliosis in retinal implants. GFP reactivity in the inner retina after 7 DPI and 150 DPI. Adapted with permission from ref. 36 (Copyright 2016, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim). | ||
Recent in vivo evidence suggested that glial cells can be a direct target of neural implants for brain stimulation therapeutical approaches.130,143,144 Deep brain stimulation (DBS) has emerged as a powerful surgical therapy for the management of treatment-resistant movement disorders, epilepsy, Parkinson's disease and neuropsychiatric disorders.145 To date, the underlying biophysical mechanism of DBS in the treatment of these disorders is far from understood. The majority of studies on DBS are focused on the change in neuronal activity in the immediate stimulated target area. However, it is apparent that DBS will not only affect neurons, but mainly glial cells, and that a change in both cell types may contribute to its therapeutic effect.143
Although DBS has just been approved by the FDA for the treatment of Parkinson's disease and many tremor diseases, ongoing investigations are directed to study the potential of cerebellum DBS in the restoration of motor functionalities.146,147 Given the involvement of cerebellar Bergmann glia in motor behaviour and the role of Ca2+ signalling in cerebellum synaptic transmission, neuro-plasticity and neuro-modulatory potentialities of DBS can be exploited to stimulate and modulate functional signals and reorganization of glial network in the cerebellar cortex for motor function recovery. Preclinical evidence has shown that the cerebellar DBS of the dentate nucleus can promote synaptic plasticity and improve post-stroke motor recovery in rodent models of stroke. Interestingly, a notable reduction in dystonia by DBS has been reported, highlighting the important role of the cerebellum as a therapeutic target for DBS.147
Similarly, non-invasive electrical brain stimulation by the application of direct current (DCS) has been shown to be an effective treatment to alleviate various neuropsychiatric and neurological disorders and enhance learning and memory formation in humans. DCS promotes plasticity in neuronal networks in vitro and in vivo. This effect has been mainly attributed to the direct modulation of neurons, but the effects of DCS on glial structures have only been sparsely investigated. Recent studies have revealed that astrocytes are principally involved in the DCS mechanism of action, confirming that the direct modulation of glial function by DCS and secondary effects on neuronal plasticity are now conceivable.130,144
Retinal prosthesis technologies capable of providing high-acuity artificial vision are clinically approved biomedical devices to treat people blinded by retina degenerating diseases, such as retinitis pigmentosa and age-related macular degeneration. Current retinal prosthesis is mainly based on arrays of photodetectors, which process images combined with electrode arrays that stimulate these retinal ganglion neurons.148,149 However, the quality of restored vision in current retinal prostheses is still quite low.
Also, in this case the role of Müller glia as a cellular target in biomedical devices aiming at the recovery of vision in degenerated retina is totally unexplored. The need for novel technologies to study glia is also needed to provide further understanding of their role in physiology and not only for pathological meanings. Studying glial cell function with high spatio-temporal resolution and specificity is challenging because they do not generate action potentials.150 Currently, the available tools to study the functional properties of glial cells mainly include electrophysiology, extracellular recording by micro-electrode arrays and calcium imaging techniques. Patch-clamp has become the gold standard technique to stimulate and record whole cell currents in non-excitable glial cells.151,152 However, patch-clamp is an invasive and low-throughput technique with limited spatial distribution as the recording site is restricted to the cell body of the cells. Extracellular recording by planar microelectrode arrays (MEA) provides the opportunity for non-invasive long-term detailed characterization of electrical activity of populations of electrogenic cells cultured on a substrate.34,153–155 However, one of the main challenges of MEA technology is to obtain a low electrode impedance to guarantee an adequate signal to noise ratio, which is necessary to allow the detection of small extracellular signals, especially the slow (theta frequency range) and low (few microvolts) variations of transmembrane potentials typically occurring in non-excitable cells, such as glial cells. Strategies to achieve low electrode impedance focus on increasing the effective surface area by using nanostructured electrodes. Recently, an inorganic glial-silicon nanowire (SiNW) interface was proposed as a bioelectronic platform for the recording of variations in astrocyte transmembrane potentials.63 Similarly, Rocha and colleagues reported an ultra-sensitive detection strategy exploiting a large electrode area maximizing the double-layer capacitance to record the electrical activity of non-electrogenic rat glioma cells.34 The calcium imaging technique represents a common approach used to monitor the Ca2+ responses in astrocytes. It is based on the use of Ca2+ fluorescent dyes, which exhibit fluorescence variations correlating with changes in [Ca2+]i.156–159 The calcium imaging method provides an efficient and versatile tool to spatially and temporally explore different calcium signals in glial cells in vitro54,55 and in vivo.98,160 Recent evidence indicated that extracellular electrical stimulation by the use of the transparent organic cell-stimulating and sensing transistor (O-CST) architecture induces [Ca2+]i responses in rat primary neocortical astrocytes.55 Similarly, photoexcitation of intrinsically light-sensitive, bioorganic polymer interfaces has been shown to cause significant depolarization of the astroglial resting membrane potential, which is associated with an increase in whole-cell chloride conductance.37 The ability to selectively evoke calcium signals and ionic conductance in astrocytes provides substantial proof of the potential of carbon-based bioelectronics in modulating the astroglial functionality.
4. Graphene as a glial interface
4.1 Graphene and astrocytes
Consolidated evidence indicates that FLG and GO flakes do not affect the viability and proliferation of astrocytes in vitro.29,46,64 Injection of rGO flakes in the rodent hippocampus did not induce reactive astrogliosis, indicating that graphene nanomaterials are also well tolerated in vivo.161 However, a limited number of studies have described the effects induced by graphene-related materials on astrocyte functionality. In the study by Chiacchiaretta and colleagues, the consequences of long-term exposure of primary rat astrocytes to pristine graphene (GR) and GO flakes were investigated, demonstrating that GR and GO can interfere with a variety of intracellular processes after their internalization through the endo-lysosomal pathway and improve the homeostatic properties expressed by astrocytes in vitro (Fig. 6).64 Astrocytes exposed to graphene acquire a more differentiated morphology associated with dramatic rearrangement of the cytoskeleton. Mostly, internalization of GO induces profound functional alterations in the upregulation of the Kir channels and increase in glutamate clearance. In addition, astrocytes in contact with GO promote the functional maturation of co-cultured neurons, increasing the intrinsic excitability and density of the GABAergic synapses. This suggests that graphene nanomaterials profoundly influence the physiology of astrocytes in vitro with consequences on the activity of the neuronal network.64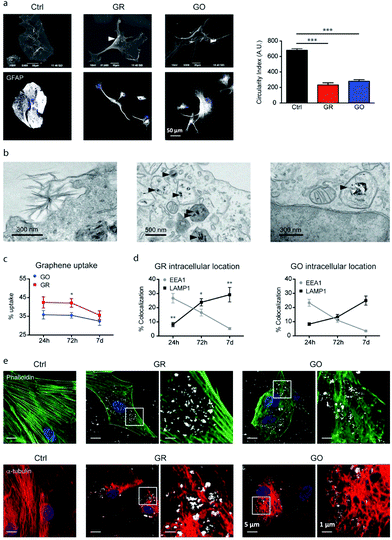 | ||
| Fig. 6 (a–e) Interaction of GR and GO flakes with primary astrocytes. Adapted with permission from ref. 64 (Copyright 2018, the American Chemical Society). | ||
Bramini and collaborators investigated the molecular changes induced in cortical astrocytes by few layer graphene (FLG) and GO flakes (Fig. 9A). Proteomic and lipidomic analyses revealed alterations in several cellular processes, including intracellular calcium homeostasis and cholesterol metabolism.29 The common processes affected by both FLG and GO include cytoskeleton remodelling and lipid binding proteins. The alteration in cytoskeletal proteins is consistent with the changes in the shape observed in astrocytes, which acquire a very elongated morphology upon exposure to graphene flakes. Moreover, GO exposure induces changes in the intracellular protein networks that control Ca2+ signalling, influencing spontaneous and evoked [Ca2+]i signals. This is accompanied by a marked increase in membrane cholesterol levels, which is causally related to the alteration of calcium dynamics.29
To improve the interaction with astrocytes and with cells in general, the chemical versatility of graphene can be exploited for biomimetic approaches, i.e., functionalizing it with (bio)molecules that can enhance its interaction with the membrane of the target cells, which is typically composed of a bilayer of phospholipids (Fig. 7).
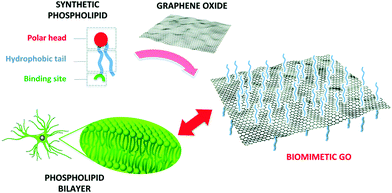 | ||
| Fig. 7 Functionalization of GO with a synthetic phospholipid promotes its interaction with the cell membrane. Reproduced with permission from ref. 35 (licensed under the Creative Commons Attribution 3.0 Unported Licence, Copyright 2018, The Royal Society of Chemistry). | ||
To this aim, we recently demonstrated a new strategy based on the chemical modification of GO with a synthetic phospholipid (PL). The adhesion of primary rat cortical astrocytes on GO-PL substrates significantly increased compared to that on glass substrates coated with standard adhesion agents (e.g., poly-D-lysine, PDL) and compared to that on non-functionalized GO (Fig. 8). Primary rat cortical astrocytes grown on GO-PL showed no significant gliotic reactivity, thus confirming the lack of occurrence of harmful inflammatory reaction at the material interface with the astroglial cells. These results indicate that the reported biomimetic approach can be exploited to promote favourable astrocyte cell growth to the implanted electrode and to avoid glial scar formation in brain implants. Moreover, the high affinity and adhesion of astrocytes to the GO and GO-PL surface is a promising feature to potentially reduce the so-called cleft distance between the GO interface and glial cells, thus allowing the development of devices aimed at detecting or modulating the functional activity of astrocytes.35
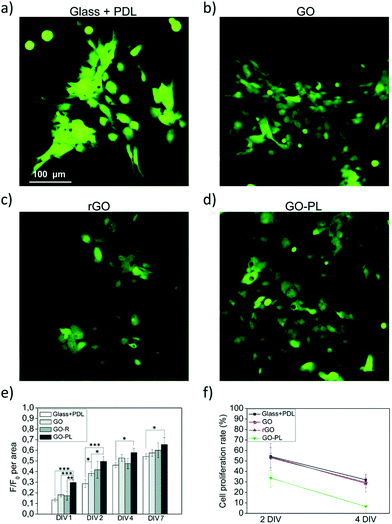 | ||
| Fig. 8 (a–f) Biocompatibility study of astrocytes plated on PDL, GO, rGO and GO-PL substrates. Reproduced with permission from ref. 35 (licensed under the Creative Commons Attribution 3.0 Unported Licence, Copyright 2018, The Royal Society of Chemistry). | ||
Graphene substrates have been used in various neural tissue regeneration studies as promising platforms for neuronal cell survival and differentiation in vitro. Among them, Park and colleagues observed the effective influence of a CVD-grown graphene scaffold on the differentiation of human neural stem cells into neurons rather than glial cells.162 Schmitt and colleagues investigated the effects of macroscopic GO and rGO scaffolds on human astrocyte and microglial cell viability and proliferation as well as the gene expression of neuroinflammation and astrogliosis. In this in vitro model and in ex vivo in organotypic murine brain slices, both the GO- and rGO-based 3D scaffolds showed only small effects on the glial cells responsible for glial scar formation.163 Serrano et al. used 3D GO-based scaffolds for seeding embryonic neural progenitor cells and observed the presence of both neurons and glial cells as well as the formation of interconnected neural networks rich in dendrites, axons, and synaptic connections, highlighting the ability of graphene to create functional neuronal network connectivity (Fig. 9D).31 Despite the potential of graphene to guide neural regeneration, the molecular and functional effects of graphene-based materials on the differentiation of healthy glial cells such as astrocytes, microglia and oligodendrocytes are rarely examined. Thus, further understanding is needed to properly drive the structural and functional recovery of the whole injured neural tissue.
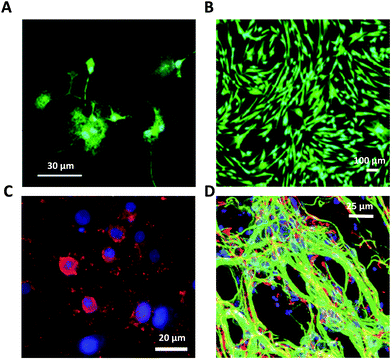 | ||
| Fig. 9 Glial cells on graphene-based glial interfaces. (A) Immunofluorescence images of astrocytes exposed to GO flakes. Adapted with permission from ref. 29 (Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim). (B) Proliferation assay of differentiated Schwann cell-like adipose stem cells on GO films. Adapted with permission from ref. 170 (Copyright 2018, The Authors, The Royal Society of Chemistry). (C) Fluorescence image of differentiated oligodendrocytes on GO nanofiber hybrid scaffold. Adapted with permission from ref. 30 (Copyright 2014, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim). (D) Fluorescence image of differentiated neuron and glia on GO 3D scaffold. Adapted with permission from ref. 31 (Copyright 2013, The Royal Society of Chemistry). | ||
4.2 Graphene and microglia
Despite the rapid advances in using graphene as a neural interface material, it still remains unknown whether graphene can provoke neuroinflammation and whether the topographical features of graphene can trigger the neuroinflammation process. Thus, investigating microglia-mediated inflammation in graphene culturing systems is crucial to understand the effects of the topography on the material-evoked inflammatory responses given that microglia act as the first and main form of active immune defence in the CNS. If the graphene is interfaced with the CNS and triggers inflammatory responses, the major player should be the innate microglia, which represent the perfect cell model to investigate the inflammatory potential of graphene.The inflammatory behaviour of microglia when interfaced with 2D films and 3D-graphene foams was explored by Song and co-workers.164 2D- and 3D-graphene could provide different spatial environments and diverse cell–material interactions for microglia. The graphene, especially 3D graphene, supported microglia growth and showed comparable biocompatibility to commercial tissue culture polystyrene substrates and evoked milder neuroinflammation in the microglia in comparison to 2D graphene, suggesting that the topographical structures of the materials may affect the inflammatory behaviours.164 Recent in vivo evidence showed that electrospun microfiber scaffolds coated with self-assembled colloidal graphene were implanted into the striatum or into the subventricular zone of adult rats.165 Microglia and astrocyte activation levels were suppressed with graphene functionalization. In addition, self-assembled graphene implants prevented glial scarring in the brain 7 weeks following implantation. Astrocyte directional guidance within the scaffold and redirection of neuroblasts from the subventricular zone along the implants was also demonstrated.165 A mouse spinal organotypic culture was used by Musto et al. to investigate the inflammatory response of the spinal cord tissue to chronic accumulation of s-GO.166 The patch-clamp analyses performed by the authors indicated that the post-synaptic current frequency, not amplitude, was lowered by the accumulation of small GO nanosheets (<200 nm; s-GO) in the organotypic ventral horns. Moreover, after 14 days of incubation with a high s-GO dose, microglial cells increased in number in the organotypic preparation, an effect that was not accompanied by a significant increase in the production and release of chemokines and cytokines. The data provided on microglial spinal cord primary culture evidenced that s-GO exposure induces microglial proliferation. Moreover, the phagocytic ability of microglia possibly favours the internalization, accumulation and aggregation of graphene inside cells.166
These findings confirm the functional evidence for the potential use of graphene nanosheets as a therapeutic drug delivery platform and scaffold to support CNS regeneration and suggest the importance of investigating the role of glial structures in neural tissue engineering. However, compared to the more accurate evidence provided for other materials,61,72,167 the scarceness in the literature on the impact of graphene-based films and bioelectronic devices on microglial cells in vivo calls for more selective and in-depth studies on this topic.
4.3 Graphene and oligodendrocytes
A number of current approaches in regenerative medicine have been employed to guide the differentiation of human neural stem cells into neurons, including genetic modification, growth factors, cytokines, substrate topography and nanomaterials.162 However, oligodendrocyte differentiation has been proven to be much more elusive, resulting in only a small percentage of differentiated cell population. Shah et al. reported the use of GO as a coating material for the design of hybrid nanofibrous scaffolds to selectively guide neural stem cell differentiation into oligodendrocytes (Fig. 9C).30 By varying the amount of GO coating on the nanofibers, a GO concentration-dependent change was observed in the expression of key neural markers, while coating with a higher concentration of GO was seen to promote differentiation into mature oligodendrocytes. Further investigation into the role of the GO-coating on the nanofibrous scaffolds showed the overexpression of a number of key integrin-related intracellular signalling molecules that are known to promote oligodendrocyte differentiation in normal development. This hybrid scaffold is novel because it combines the well-established properties of nanofibers and graphene-based nanomaterials, including ideal topography for directing nerve growth and promoting axonal regeneration, permissive surfaces for protein and cell adhesion and high conductivity to mediate electrical stimulation for supporting neuronal electrophysiology.304.4 Graphene and Schwann cells
Peripheral nerve regeneration and functional recovery remain a significant clinical challenge. Currently, most clinical repairs of nerve damage are performed using autologous nerve transplantation. Although this method can promote nerve repair, autologous nerve transplants are still limited by the source of donor grafts, post-surgery complications, poor regenerative capacity and sometimes the risk of secondary operative intervention. According to the mechanism of peripheral nerve regeneration and the principle of biocompatibility, it is important to explore the potential of bioactive materials to modulate Schwann cell functions and promote nerve regeneration.168 Many efforts are directed towards the fabrication of bioengineered conduits to promote and guide peripheral nerve regeneration and investigating whether graphene and related nanomaterials can be useful in the fabrication of these conduits. The possibility of combining the physicochemical properties of GO with nanofibers to develop bioactive scaffolds for nerve regeneration was explored by Wang and coworkers.169 GO was coated on composite Antheraea pernyi silk fibroin (ApF)/(poly(L-lactic acid-co-caprolactone)) (PLCL) scaffolds and in vitro and in vivo studies were carried out to demonstrate the potentials of this platform toward nerve regeneration. In vitro, the GO-coated scaffolds could enhance the biological response of Schwann cells including migration, proliferation, and myelination, and the secretions from Schwann cells could induce PC12 cells differentiation. In vivo, the GO-ApF/PLCL nerve conduits could successfully repair a 10 mm sciatic nerve defect, suggesting the potential of GO-based scaffold to efficiently modulate biological behaviours and promote nerve regeneration.169In the study by Zhao and colleagues, a composite hydrogel named polyacrylamide/graphene oxide/gelatin/sodium alginate (PAM/GO/Gel/SA) was fabricated to evaluate its effects on Schwann cell growth and the related molecular mechanism.168 The characterization of the physicochemical properties showed that the prepared PAM/GO/Gel/SA composite hydrogels displayed different colours as a function of variations in their components. The surface morphology, components, swelling ratio, mechanical properties, and porosity were all changed with a change in the concentration of each ingredient, but no obvious degradation behaviour was observed, indicating a controllable physicochemical property. The in vitro analyses showed that the composite hydrogels could well support the attachment and proliferation of Schwann cells, providing an important theoretical and experimental basis for the design and development of hydrogel scaffolds for nerve tissue engineering application.168 As a clinically viable alternative, mesenchymal stem cells and adipose-derived mesenchymal stem cells (ASCs) have been differentiated in vitro towards a Schwann-like cells phenotype. These differentiated adipose stem cells (dASCs) express glial markers such as GFAP, S100 and p75, and express myelin proteins and structures when co-cultured with neurons. When implanted in bioengineered conduits to repair murine peripheral nerve gaps in vivo, dASCs have demonstrated the promotion of nerve regeneration, reduction of muscle atrophy, increased nerve conduction velocity and higher rates of myelination. The study by Verre et al. aimed at the biological characterization of GO and rGO to verify if these materials can sustain the dASC phenotype, which is rapidly lost following the withdrawal of growth factors. The results show an increase in the expression of neurotrophins and filament proteins on the rGO and GO substrates, which can be used as functional surfaces to increase the glial differentiation of ASCs at an earlier stage (Fig. 9B).170
5. Conclusions
We described the most recent knowledge on the mechanisms underlying the functionality of glial cells, highlighting their potential as targets for the development of selective technologies for nervous system investigations and for therapeutics in neurological implants. It is evident that the crucial role of glia in brain homeostasis maintenance and information processing can no longer be ignored in biomaterials science and neural engineering. Astrocytes, microglia and NG2 glia are cells mainly involved in the mediation of inflammatory responses occurring in acute and chronic insults of the CNS, and a consequence of device implantation.We also provided evidence highlighting the fundamental importance of selective investigation of molecular signals and physiological processes underlying the functionality of glial cells and networks. Novel devices enabling the control and modulation of glial signalling may have significant potential in the study and treatment of neurodegenerative diseases affecting CNS, PNS or sensorial function such as vision and equilibrium.
We suggested using recent results that interfacing graphene nanomaterials with glial cells may be the optimal strategy to achieve a combination of selectivity, resolution, mechanical flexibility and biocompatibility to be successfully exploited in the engineering of advanced nanoscale glial interfaces devoted to diagnostic and therapeutic applications.171–173
Graphene-based glial engineering and glia interfaces can be useful for uncovering the unexplored domain of the role of glial cells in brain and sensory circuits, where deepening our knowledge on the function of calcium signalling, ion channels and aquaporins, we may achieve a wider comprehension of glial functionality in an attempt to possibly trigger and control their mechanisms and functional properties in brain function and dysfunction.
Nonetheless, graphene-based glial engineering and glia interfaces may generate a novel class of bidirectional brain machine interfaces for the diagnosis and therapy of clinically intractable neuropathological conditions. Accordingly, graphene-based glial interfaces may represent a novel bioelectronic approach for the therapy of epilepsy, brain tumours, spreading depression, edema and stroke, and Alzheimer's and Parkinson's disease, as well as of the next generation of prosthesis aiming at the recovery of vision and functionalities hampered by retinal degeneration, demyelinating diseases and motor disabilities.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the AFOSR Research Projects FA95501910370: “Decoding astrocytes rhythm”; ASTROMAT: FA9550-17-1-0502; 3D NEUROGLIA: FA9550-18-1-0255; ASTRONIR: FA9550-17-1-0052; ASTROLIGHT: FA9550-20-1-0386, and MSCA-ITN-2020-ASTROTECH(GA956325) and by Graphene Flagship. The research leading to these results has received funding from the European Union's Horizon 2020 research and innovation programme under GrapheneCore3 881603 – Graphene Flagship. Dr Sofi Bin-Salamon, AFOSR Biophysics Programm Manager, is acknowledged for the trust in the vision, the discussions and the support. JCM Italia-USA bilateral commission and Italian Embassy in the USA are also acknowledged.References
- O. Erol, I. Uyan, M. Hatip, C. Yilmaz, A. B. Tekinay and M. O. Guler, Nanomedicine, 2018, 14, 2433 CrossRef CAS.
- Y. Qu, F. He, C. Yu, X. Liang, D. Liang, L. Ma, Q. Zhang, J. Lv and J. Wu, Mater. Sci. Eng., C, 2018, 90, 764 CrossRef CAS.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498 CrossRef CAS.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385 CrossRef CAS.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902 CrossRef CAS.
- Z. Xia, F. Leonardi, M. Gobbi, Y. Liu, V. Bellani, A. Liscio, A. Kovtun, R. Li, X. Feng, E. Orgiu, P. Samorì, E. Treossi and V. Palermo, ACS Nano, 2016, 10, 7125 CrossRef CAS.
- M. Melucci, M. Durso, M. Zambianchi, E. Treossi, Z. Xia, I. Manet, G. Giambastiani, L. Ortolani, V. Morandi, F. De Angelis and V. Palermo, J. Mater. Chem., 2012, 22, 18237 RSC.
- M. Melucci, E. Treossi, L. Ortolani, G. Giambastiani, V. Morandi, P. Klar, C. Casiraghi, P. Samorì and V. Palermo, J. Mater. Chem., 2010, 20, 9052 RSC.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156 CrossRef CAS.
- A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS.
- A. Scidà, S. Haque, E. Treossi, A. Robinson, S. Smerzi, S. Ravesi, S. Borini and V. Palermo, Mater. Today, 2018, 21, 223 CrossRef.
- V. Palermo, I. A. Kinloch, S. Ligi and N. M. Pugno, Adv. Mater., 2016, 28, 6232 CrossRef CAS.
- P. Kang, M. C. Wang and W. S. Nam, Microelectron. Eng., 2016, 161, 18 CrossRef CAS.
- S. Goenka, V. Sant and S. Sant, J. Controlled Release, 2014, 173, 75 CrossRef CAS.
- P. Wick, A. E. Louw-Gaume, M. Kucki, H. F. Krug, K. Kostarelos, B. Fadeel, K. A. Dawson, A. Salvati, E. Vázquez, L. Ballerini, M. Tretiach, F. Benfenati, E. Flahaut, L. Gauthier, M. Prato and A. Bianco, Angew. Chem., Int. Ed., 2014, 53, 7714 CrossRef CAS.
- M. Bramini, G. Alberini, E. Colombo, M. Chiacchiaretta, M. L. Di Francesco, J. F. Maya-Vetencourt, L. Maragliano, F. Benfenati and F. Cesca, Front. Syst. Neurosci., 2018, 12, 1662 Search PubMed.
- K. Kostarelos, V. Melissa, C. Hebert and J. A. Garrido, Adv. Mater., 2017, 29, 1700909 CrossRef.
- G. Reina, J. M. Gonzàlez-Domìnguez, A. Criado, E. Vàzquez, A. Bianco and M. Prato, Chem. Soc. Rev., 2017, 46, 4400 RSC.
- A. Liscio, G. P. Veronese, E. Treossi, F. Suriano, F. Rossella, V. Bellani, R. Rizzoli, P. Samorì and V. Palermo, J. Mater. Chem., 2011, 21, 2924 RSC.
- G. Maccaferri, F. Terzi, Z. Xia, F. Vulcano, A. Liscio, V. Palermo and C. Zanardi, Sens. Actuators, B, 2019, 281, 739 CrossRef CAS.
- G. Maccaferri, C. Zanardi, Z. Xia, A. Kovtun, A. Liscio, F. Terzi, V. Palermo and R. Seeber, Carbon, 2017, 120, 165 CrossRef CAS.
- F. Vulcano, A. Kovtun, C. Bettini, Z. Xia, A. Liscio, F. Terzi, A. Heras, A. Colina, B. Zanfrognini and M. Melucci, 2D Mater., 2020, 7, 024007 CrossRef CAS.
- L. H. Hess, M. Seifert and J. A. Garrido, Proc. IEEE, 2013, 101, 1780 CAS.
- N. V. Apollo, I. M. Maturana, W. Tong, D. A. X. Nayagam, M. N. Shivdasani, J. Foroughi, G. G. Wallace, S. Prawer, M. R. Ibbotson and D. J. Garrett, Adv. Funct. Mater., 2015, 25, 3551 CrossRef CAS.
- D. W. Park, J. P. Ness, S. K. Brodnick, C. Esquibel, J. Novello, F. Atry, D. H. Baek, H. Kim, J. Bong, K. I. Swanson, A. J. Suminski, K. J. Otto, R. Pashaie, J. C. Williams and Z. Ma, ACS Nano, 2018, 12, 148 CrossRef CAS.
- O. Akhavan, J. Mater. Chem. B, 2016, 4, 3169 RSC.
- T. Aydin, C. Gurcan, H. Taheri and A. Yilmazer, Adv. Exp. Med. Biol., 2018, 1107, 129 CrossRef CAS.
- P. Orsu and K. Arun, Mater. Today Commun., 2019, 22, 100823 CrossRef.
- M. Bramini, M. Chiacchiaretta, A. Armirotti, A. Rocchi, D. D. Kale, C. Martin, E. Vázquez, T. Bandiera, S. Ferroni, F. Cesca and F. Benfenati, Small, 2019, 15, e1900147 CrossRef.
- S. Shah, P. T. Yin, T. M. Uehara, S. T. D. Chueng, L. Yang and K. B. Lee, Adv. Mater., 2014, 26, 3673 CrossRef CAS.
- M. Serrano, J. Patiño, C. García-Rama, M. Ferrer, J. Fierro, A. Tamayo, J. Collazos-Castro, F. Del Monte and M. Gutierrez, J. Mater. Chem. B, 2014, 2, 5698 RSC.
- A. Bourrier, P. Shkorbatova, M. Bonizzato, E. Rey, Q. Barraud, G. Courtine, R. Othmen, V. Reita, V. Bouchiat and C. Delacour, Adv. Healthcare Mater., 2019, 8, 1801331 CrossRef.
- L. Maiolo, V. Guarino, E. Saracino, A. Convertino, M. Melucci, M. Muccini, L. Ambrosio, R. Zamboni and V. Benfenati, Adv. Healthcare Mater., 2020, 2001268 Search PubMed.
- P. R. F. Rocha, M. C. R. Medeiros, U. Kintzel, J. Vogt, I. M. Araújo, A. L. G. Mestre, V. Mailänder, P. Schlett, M. Dröge, L. Schneider, F. Biscarini, D. M. de Leeuw and H. L. Gomes, Sci. Adv., 2016, 2, 1600516 CrossRef.
- M. Durso, A. I. Borrachero-Conejo, C. Bettini, E. Treossi, A. Scidà, E. Saracino, M. Gazzano, M. Christian, V. Morandi, G. Tuci, G. Giambastiani, L. Ottaviano, F. Perrozzi, V. Benfenati, M. Melucci and V. Palermo, J. Mater. Chem. B, 2018, 6, 5335 RSC.
- M. R. Antognazza, M. Di Paolo, D. Ghezzi, M. Mete, S. Di Marco, J. F. Maya-Vetencourt, R. Maccarone, A. Desii, F. Di Fonzo, M. Bramini, A. Russo, L. Laudato, I. Donelli, M. Cilli, G. Freddi, G. Pertile, G. Lanzani, S. Bisti and F. Benfenati, Adv. Healthcare Mater., 2016, 5, 2271 CrossRef CAS.
- V. Benfenati, N. Martino, M. R. Antognazza, A. Pistone, S. Toffanin, S. Ferroni, G. Lanzani and M. Muccini, Adv. Healthcare Mater., 2014, 3, 392 CrossRef CAS.
- E. Saracino, V. Cirillo, M. Marrese, V. Guarino, V. Benfenati, R. Zamboni and L. Ambrosio, Mater. Sci. Eng., C, 2021, 118, 111363 CrossRef CAS.
- A. Bianco, Angew. Chem., Int. Ed., 2013, 52, 4986 CrossRef CAS.
- R. Kurapati, J. Russier, M. A. Squillaci, E. Treossi, C. Ménard-Moyon, A. E. Del Rio-Castillo, E. Vazquez, P. Samorì, V. Palermo and A. Bianco, Small, 2015, 11, 3985 CrossRef CAS.
- J. Russier, V. León, M. Orecchioni, E. Hirata, P. Virdis, C. Fozza, F. Sgarrella, G. Cuniberti, M. Prato, E. Vázquez, A. Bianco and L. G. Delogu, Angew. Chem., Int. Ed., 2017, 56, 3014 CrossRef CAS.
- J. Russier, E. Treossi, A. Scarsi, F. Perrozzi, H. Dumortier, L. Ottaviano, M. Meneghetti, V. Palermo and A. Bianco, Nanoscale, 2013, 5, 11234 RSC.
- S. Gurunathan and J. H. Kim, Int. J. Nanomed., 2016, 11, 1927 CrossRef CAS.
- K. Yang, L. Feng, X. Shi and Z. Liu, Chem. Soc. Rev., 2013, 42, 530 RSC.
- M. Bramini, S. Sacchetti, A. Armirotti, A. Rocchi, E. Vázquez, V. L. Castellano, T. Bandiera, F. Cesca and F. Benfenati, ACS Nano, 2016, 10, 7154 CrossRef CAS.
- R. Rauti, N. Lozano, V. León, D. Scaini, M. Musto, I. Rago, F. P. U. Severino, A. Fabbro, L. Casalis, E. Vázquez, K. Kostarelos, M. Prato and L. Ballerini, ACS Nano, 2016, 10, 4459 CrossRef CAS.
- R. Kurapati, C. Martin, V. Palermo, Y. Nishina and A. Bianco, Faraday Discuss., 2020 10.1039/C9FD00094A.
- H. K. Kimelberg and M. Nedergaard, Neurotherapeutics, 2010, 7, 338 CrossRef CAS.
- E. A. Newman, Trends Neurosci., 2003, 26, 536 CrossRef CAS.
- U. Pannasch and N. Rouach, Trends Neurosci., 2013, 36, 405 CrossRef CAS.
- J. W. Salatino, K. A. Ludwig, T. D. Y. Kozai and E. K. Purcell, Nat. Biomed. Eng., 2017, 1, 862 CrossRef CAS.
- M. Navarrete, G. Perea, L. Maglio, J. Pastor, R. G. de Sola and A. Araque, Cereb. Cortex, 2013, 23, 1240 CrossRef.
- E. Scemes and C. Giaume, Glia, 2006, 54, 716 CrossRef.
- A. I. Borrachero-Conejo, E. Saracino, M. Natali, F. Prescimone, S. Karges, S. Bonetti, G. P. Nicchia, F. Formaggio, M. Caprini, R. Zamboni, F. Mercuri, S. Toffanin, M. Muccini and V. Benfenati, Adv. Healthcare Mater., 2019, 8, 1801139 CrossRef.
- A. I. Borrachero-Conejo, W. R. Adams, E. Saracino, M. G. Mola, M. Wang, T. Posati, F. Formaggio, M. De Bellis, A. Frigeri, M. Caprini, M. Hutchinson, M. Muccini, R. Zamboni, G. P. Nicchia, A. Mahadevan-Jansen and V. Benfenati, FASEB J., 2020, 34, 6539 CrossRef CAS.
- C. Agulhon, J. Petravicz, A. B. McMullen, E. J. Sweger, S. K. Minton, S. R. Taves, K. B. Casper, T. A. Fiacco and K. D. McCarthy, Neuron, 2008, 59, 932 CrossRef CAS.
- M. V. Sofroniew and H. V. Vinters, Acta Neuropathol., 2010, 119, 7 CrossRef.
- E. Dossi, F. Vasile and N. Rouach, Brain Res. Bull., 2018, 136, 139 CrossRef CAS.
- A. Chvátal, M. Anděrová, H. Neprašová, I. Prajerová, J. Benešová, O. Butenko and A. Verkhratsky, Physiol. Res., 2008, 57, S101 Search PubMed.
- V. Benfenati, S. Toffanin, S. Bonetti, G. Turatti, A. Pistone, M. Chiappalone, A. Sagnella, A. Stefani, G. Generali, G. Ruani, D. Saguatti, R. Zamboni and M. Muccini, Nat. Mater., 2013, 12, 672 CrossRef CAS.
- T. D. Kozai, A. S. Jaquins-Gerstl, A. L. Vazquez, A. C. Michael and X. T. Cui, ACS Chem. Neurosci., 2015, 6, 48 CrossRef CAS.
- B. Tian and C. M. Lieber, Chem. Rev., 2019, 119, 9136 CrossRef CAS.
- E. Saracino, L. Maiolo, D. Polese, M. Semprini, A. I. Borrachero-Conejo, J. Gasparetto, S. Murtagh, M. Sola, L. Tomasi, F. Valle, L. Pazzini, F. Formaggio, M. Chiappalone, S. Hussain, M. Caprini, M. Muccini, L. Ambrosio, G. Fortunato, R. Zamboni, A. Convertino and V. Benfenati, Adv. Biosyst., 2020, 4, 1900264 CrossRef CAS.
- M. Chiacchiaretta, M. Bramini, A. Rocchi, A. Armirotti, E. Giordano, E. Vázquez, T. Bandiera, S. Ferroni, F. Cesca and F. Benfenati, Nano Lett., 2018, 18, 5827 CrossRef CAS.
- A. Verkhratsky and A. Butt, Glial Neurobiology: A Textbook, Chichester, Wiley, 2007 Search PubMed.
- A. Verkhratsky, Pflugers Arch.: Eur. J. Physiol., 2006, 453, 411 CrossRef CAS.
- G. G. Somjen, Glia, 1998, 1, 2 CrossRef.
- H. Kettenmann and A. Verkhratsky, in Neuroscience in the 21st Century, ed. D. W. Pfaff and N. D. Volkow, Springer, New York, 2013, pp. 547–578 Search PubMed.
- B. Di Benedetto and R. Rupprecht, Curr. Neuropharmacol., 2013, 11, 171 CrossRef CAS.
- N. J. Allen and D. A. Lyons, Science, 2018, 362, 181 CrossRef CAS.
- O. Butenko, D. Dzamba, J. Benesova, P. Honsa, V. Benfenati, V. Rusnakova, S. Ferroni and M. Anderova, PLoS One, 2012, 7, e39959 CrossRef CAS.
- S. M. Wellman, F. Cambi and T. D. Kozai, Biomaterials, 2018, 183, 200 CrossRef CAS.
- C. Fan, H. Wang, D. Chen, X. Cheng, K. Xiong, X. Luo and Q. Cao, Neural Regen. Res., 2014, 9, 119 CrossRef CAS.
- K. Franze, J. Grosche, S. N. Skatchkov, S. Schinkinger, C. Foja, D. Schild, O. Uckermann, K. Travis, A. Reichenbach and J. Guck, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8287 CrossRef CAS.
- N. Surzenko, T. Crowl, A. Bachleda, L. Langer and L. Pevny, Development, 2013, 140, 1445 CrossRef CAS.
- O. Komine, M. Nagaoka, K. Watase, D. H. Gutmann, K. Tanigaki, T. Honjo, T. Radtke, T. Saito, S. Chiba and K. Tanaka, Dev. Biol., 2007, 311, 238 CrossRef CAS.
- F. Ango, C. Wu, J. J. Van der Want, P. Wu, M. Schachner and Z. J. Huang, PLoS Biol., 2008, 6, 103 CrossRef.
- K. Bacallao and P. V. Monje, PLoS One, 2015, 10, e0116948 CrossRef.
- L. Liu, X. G. Luo, H. M. Yu, Y. Feng, Y. Ren, Y. F. Yin, H. Shang and Z. Y. He, Mol. Med. Rep., 2015, 12, 7271 CrossRef CAS.
- N. A. Oberheim, X. Wang, S. Goldman and M. Nedergaard, Trends Neurosci., 2006, 29, 547 CrossRef CAS.
- A. Verkhratsky and M. Nedergaard, Physiol. Rev., 2018, 98, 239 CrossRef CAS.
- M. Nedergaard, B. Ransom and S. A. Goldman, Trends Neurosci., 2003, 26, 523 CrossRef CAS.
- V. Benfenati and S. Ferroni, Neuroscience, 2010, 168, 926 CrossRef CAS.
- P. Kofuji and E. A. Newman, Neuroscience, 2004, 129, 1045 CrossRef CAS.
- E. A. Nagelhus, T. M. Mathiisen and O. P. Ottersen, Neuroscience, 2004, 129, 905 CrossRef CAS.
- V. Benfenati, G. P. Nicchia, M. Svelto, C. Rapisarda, A. Frigeri and S. Ferroni, J. Neurochem., 2007, 100, 87 CrossRef CAS.
- K. S. Kim, Nat. Rev. Microbiol., 2008, 6, 625 CrossRef CAS.
- N. J. Abbott, L. Rönnbäck and E. Hansson, Nat. Rev. Neurosci., 2006, 7, 41 CrossRef CAS.
- P. J. Magistretti, Am. J. Clin. Nutr., 2009, 90, 875S CrossRef CAS.
- A. Araque, V. Parpura, R. P. Sanzgiri and P. G. Haydon, Trends Neurosci., 1999, 22, 208 CrossRef CAS.
- A. Araque, R. P. Sanzgiri, V. Parpura and P. G. Haydon, J. Neurosci., 1998, 18, 6822 CrossRef CAS.
- A. Araque, E. D. Martín, G. Perea, J. I. Arellano and W. Buño, J. Neurosci., 2002, 22, 2443 CrossRef CAS.
- V. Parpura, T. A. Basarsky, F. Liu, K. Jeftinija, S. Jeftinija and P. G. Haydon, Nature, 1994, 369, 744 CrossRef CAS.
- E. A. Newman, J. Neurosci., 2005, 25, 5502 CrossRef CAS.
- E. A. Newman and K. R. Zahs, J. Neurosci., 1998, 18, 4022 CrossRef CAS.
- N. Kuga, T. Sasaki, Y. Takahara, N. Matsuki and Y. Ikegaya, J. Neurosci., 2011, 31, 2607 CrossRef CAS.
- A. H. Cornell-Bell, S. M. Finkbeiner, M. S. Cooper and S. J. Smith, Science, 1990, 247, 470 CrossRef CAS.
- H. Hirase, L. Qian, P. Barthó and G. Buzsáki, PLoS Biol., 2004, 2, 96 CrossRef.
- M. Shi, F. Du, Y. Liu, L. Li, J. Cai, G. F. Zhang, X. F. Xu, T. Lin, H. R. Cheng, X. D. Liu, L. Z. Xiong and G. Zhao, Acta Neuropathol., 2013, 126, 725 CrossRef CAS.
- B. A. Barres, L. L. Y. Chun and D. P. Corei, Annu. Rev. Neurosci., 1990, 13, 441 CrossRef CAS.
- A. Volterra, N. Liaudet and I. Savtchouk, Nat. Rev. Neurosci., 2014, 15, 327 CrossRef CAS.
- L. Leybaert and M. J. Sanderson, Physiol. Rev., 2012, 92, 1359 CrossRef CAS.
- A. C. Charles, J. E. Merrill, E. R. Dirksen and M. J. Sanderson, Neuron, 1991, 6, 983 CrossRef CAS.
- P. B. Simpson, S. Mehotra, G. D. Lange and J. T. Russell, J. Biol. Chem., 1997, 272, 22654 CrossRef CAS.
- A. Verkhratsky, J. J. Rodríguez and V. Parpura, Mol. Cell. Endocrinol., 2012, 353, 45 CrossRef CAS.
- P. Ballerini, R. Ciccarelli, F. Caciagli, M. P. Rathbone, E. S. Werstiuk, U. Traversa, S. Buccella, P. Giuliani, S. Jiang, E. Nargi, D. Visini, C. Santavenere and P. Di lorio, Int. J. Immunopathol. Pharmacol., 2005, 18, 417 CrossRef CAS.
- A. Verkhratsky, R. C. Reyes and V. Parpura, Rev. Physiol., Biochem. Pharmacol., 2014, 166, 1 CAS.
- E. Shigetomi, X. Tong, K. Y. Kwan, D. P. Corey and B. S. Khakh, Nat. Neurosci., 2012, 15, 70 CrossRef CAS.
- V. Benfenati, M. Caprini, M. Dovizio, M. N. Mylonakou, S. Ferroni, O. P. Ottersen and M. Amiry-Moghaddam, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2563 CrossRef CAS.
- V. Benfenati, M. Amiry-Moghaddam, M. Caprini, M. N. Mylonakou, C. Rapisarda, O. P. Ottersen and S. Ferroni, Neuroscience, 2007, 148, 876 CrossRef CAS.
- K. Kanemaru, H. Sekiya, M. Xu, K. Satoh, N. Kitajima, K. Yoshida, Y. Okubo, T. Sasaki, S. Moritoh, H. Hasuwa, M. Mimura, K. Horikawa, K. Matsui, T. Nagai, M. Iino and K. F. Tanaka, Cell Rep., 2014, 8, 311 CrossRef CAS.
- J. Göbel, E. Motori and M. Bergami, Biochem. Biophys. Res. Commun., 2018, 500, 17 CrossRef.
- N. Bazargani and D. Attwell, Nat. Neurosci., 2016, 19, 182 CrossRef CAS.
- E. Vecino, F. D. Rodriguez, N. Ruzafa, X. Pereiro and S. C. Sharma, Prog. Retinal Eye Res., 2016, 51, 1 CrossRef CAS.
- A. Bringmann, T. Pannicke, J. Grosche, M. Francke, P. Wiedemann, S. N. Skatchkov, N. N. Osborne and A. Reichenbach, Prog. Retinal Eye Res., 2006, 25, 397 CrossRef CAS.
- N. C. Connors and P. Kofuji, Glia, 2006, 53, 124 CrossRef.
- D. A. Ryskamp, A. O. Jo, A. M. Frye, F. Vazquez-Chona, N. MacAulay, W. B. Thoreson and D. Križaj, J. Neurosci., 2014, 34, 15689 CrossRef.
- M. Lakk, O. Yarishkin, J. M. Baumann, A. Iuso and D. Križaj, Glia, 2017, 65, 2038 CrossRef.
- H. Matsumoto, S. Sugio, F. Seghers, D. Krizaj, H. Akiyama, Y. Ishizaki, P. Gailly and K. Shibasaki, J. Neurosci., 2018, 38, 8745 CrossRef CAS.
- M. R. Metea and E. A. Newman, Glia, 2006, 54, 650 CrossRef.
- A. O. Jo, D. A. Ryskamp, T. T. T. Phuong, A. S. Verkman, O. Yarishkin, N. MacAulay and D. Križaj, Neuroscience, 2015, 35, 13525 CrossRef CAS.
- A. S. Saab, A. Neumeyer, H. M. Jahn, A. Cupido, A. A. M. Šimek, H. J. Boele, A. Scheller, K. Le Meur, M. Götz, H. Monyer, R. Sprengel, M. E. Rubio, J. W. Deitmer, C. De Zeeuw and F. Kirchhoff, Science, 2012, 337, 749 CrossRef CAS.
- T. C. Bellamy, Cerebellum, 2006, 5, 116 CrossRef.
- B. S. Khakh and M. V. Sofroniew, Nat. Neurosci., 2015, 18, 942 CrossRef CAS.
- U. K. Hanisch and H. Kettenmann, Nat. Neurosci., 2007, 10, 1387 CrossRef CAS.
- H. Kettenmann, U. K. Hanisch, M. Noda and A. Verkhratsky, Physiol. Rev., 2011, 91, 461 CrossRef CAS.
- K. M. Lenz and L. H. Nelson, Front. Immunol., 2018, 9, 698 CrossRef.
- K. Biber, H. Neumann, K. Inoue and H. W. Boddeke, Trends Neurosci., 2007, 30, 596 CrossRef CAS.
- D. P. Schafer, E. K. Lehrman and B. Stevens, Glia, 2013, 61, 24 CrossRef.
- A. K. Gellner, J. Reis and B. Fritsch, Front. Cell. Neurosci., 2016, 10, 188 Search PubMed.
- Y. Tang and W. Le, Mol. Neurobiol., 2016, 53, 1181 CrossRef CAS.
- S. B. Beynon and F. R. Walker, Neuroscience, 2012, 225, 162 CrossRef CAS.
- C. K. Glass, K. Saijo, B. Winner, M. C. Marchetto and F. H. Gage, Cell, 2010, 140, 918 CrossRef CAS.
- L. Dimou and M. Götz, Physiol. Rev., 2014, 94, 709 CrossRef CAS.
- J. Eugenín-von Bernhardi and L. Dimou, Adv. Exp. Med. Biol., 2016, 949, 27 CrossRef.
- H. S. Domingues, C. C. Portugal, R. Socodato and J. B. Relvas, Front. Cell Dev. Biol., 2016, 4, 71 Search PubMed.
- K. Bhatheja and J. Field, Int. J. Biochem. Cell Biol., 2006, 38, 1995 CrossRef CAS.
- R. M. G. Menorca, T. S. Fussell and J. C. Elfar, Hand Clin., 2013, 29, 317 CrossRef.
- D. Scaini and L. Ballerini, Curr. Opin. Neurobiol., 2018, 50, 50 CrossRef CAS.
- M. Jorfi, J. L. Skousen, C. Weder and J. R. Capadona, J. Neural Eng., 2015, 12, 011001 CrossRef.
- B. I. Gallego, J. J. Salazar, R. Hoz, B. Rojas, A. I. Ramírez, M. Salinas-Navarro, A. Ortín-Martínez, F. J. Valiente-Soriano, M. Avilés-Trigueros, M. P. Villegas-Perez, M. Vidal-Sanz, A. Triviño and J. M. Ramírez, J. Neuroinflammation, 2012, 9, 92 CAS.
- J. F. Maya-Vetencourt, D. Ghezzi, M. R. Antognazza, E. Colombo, M. Mete, P. Feyen, A. Desii, A. Buschiazzo, M. Di Paolo, S. Di Marco, F. Ticconi, L. Emionite, D. Shmal, C. Marini, I. Donelli, G. Freddi, R. Maccarone, S. Bisti, G. Sambuceti, G. Pertile, G. Lanzani and F. Benfenati, Nat. Mater., 2017, 16, 681 CrossRef CAS.
- V. Vedam-Mai, E. Y. van Battum, W. Kamphuis, M. G. P. Feenstra, D. Denys, B. A. Reynolds, M. S. Okun and E. M. Hol, Mol. Psychiatry, 2012, 17, 124 CrossRef CAS.
- H. Monai, M. Ohkura, M. Tanaka, Y. Oe, A. Konno, H. Hirai, K. Mikoshiba, S. Itohara, J. Nakai, Y. Iwai and H. Hirase, Nat. Commun., 2016, 7, 11100 CrossRef CAS.
- J. S. Perlmutter and J. W. Mink, Annu. Rev. Neurosci., 2006, 29, 229 CrossRef CAS.
- C. A. Wathen, L. A. Frizon, T. K. Maiti, K. B. Baker and A. G. Machado, Neurosurg. Focus, 2018, 45, E13 Search PubMed.
- E. G. Brown, I. O. Bledsoe, N. S. Luthra, S. Miocinovic, P. A. Starr and J. L. Ostrem, Mov. Disord. Clin. Pract., 2020, 7, 188 CrossRef.
- G. Dagnelie, Curr. Opin. Neurol., 2012, 25, 67 CrossRef.
- S. Picaud and J. A. Sahel, C. R. Biol., 2014, 337, 214 CrossRef.
- D. Li, C. Agulhon, E. Schmidt, M. Oheim and N. Ropert, Front. Cell. Neurosci., 2013, 7, 193 Search PubMed.
- E. Neher and B. Sakmann, Nature, 1976, 260, 799 CrossRef CAS.
- S. W. Kuffler and D. D. Potter, J. Neurophysiol., 1964, 27, 290 CrossRef CAS.
- C. A. Thomas Jr., P. A. Springer, G. E. Loeb, Y. Berwald-Netters and L. Okun, Exp. Cell Res., 1972, 74, 61 CrossRef CAS.
- E. Masi, E. Azzarello and S. Mancuso, Plant Electrophysiology, ed. A. G. Volkov, Springer, Berlin, 2012 Search PubMed.
- J. M. Keller and M. Frega, in Advances in Neurobiology, ed. M. Chiappalone, V. Pasquale and M. Frega, Springer, Cham, 2019, vol. 22, p. 3 Search PubMed.
- M. D. Bootman, K. Rietdorf, T. Collins, S. Walker and M. Sanderson, Cold Spring Harb. Protoc., 2013, 2013, 83 Search PubMed.
- R. Y. Tsien, Nature, 1981, 290, 527 CrossRef CAS.
- R. Srinivasan, B. S. Huang, S. Venugopal, A. D. Johnston, H. Chai, H. Zeng, P. Golshani and B. S. Khakh, Nat. Neurosci., 2015, 18, 708 CrossRef CAS.
- K. E. Vogt, S. Gerharz, J. Graham and M. Canepari, J. Physiol., 2011, 589, 489 CrossRef CAS.
- A. Pérez-Alvarez, A. Araque and E. D. Martín, Front. Cell. Neurosci., 2013, 7, 51 Search PubMed.
- M. C. P. Mendonça, E. S. Soares, M. B. De Jesus, H. J. Ceragioli, S. P. Irazusta, A. G. Batista, M. A. R. Vinolo, M. R. Maróstica Jr. and M. A. Da Cruz-Höfling, J. Nanobiotechnol., 2016, 14, 53 CrossRef.
- S. Y. Park, J. Park, S. H. Sim, M. G. Sung, K. S. Kim, B. H. Hong and S. Hong, Adv. Mater., 2011, 23, H263 CrossRef CAS.
- C. Schmitt, F. Rasch, F. Cossais, J. Held-Feindt, R. Lucius, A. R. Vazquez, A. S. Nia, M. R. Lohe, X. Feng, Y. K. Mishra, R. Adelung, F. Schütt and K. Hattermann, Biomed. Mater., 2020, 16, 015008 CrossRef.
- Q. Song, Z. Jiang, N. Li, P. Liu, L. Liu, M. Tang and G. Cheng, Biomaterials, 2014, 35, 6930 CrossRef CAS.
- K. Zhou, S. Motamed, G. A. Thouas, C. C. Bernard, D. Li, H. C. Parkington, H. A. Coleman, D. I. Finkelstein and J. S. Forsythe, PLoS One, 2016, 11, e0151589 CrossRef.
- M. Musto, R. Rauti, A. F. Rodrigues, E. Bonechi, C. Ballerini, K. Kostarelos and L. Ballerini, Front. Syst. Neurosci., 2019, 13, 1 CrossRef CAS.
- J. M. Zuidema, R. J. Gilbert and M. K. Gottipati, Cells Tissues Organs, 2018, 205, 372 CrossRef.
- Y. Zhao, Y. Wang, C. Niu, L. Zhang, G. Li and Y. Yang, J. Biomed. Mater. Res., 2018, 106, 1951 CrossRef CAS.
- J. Wang, W. Zheng, L. Chen, T. Zhu, W. Shen, C. Fan, H. Wang and X. Mo, ACS Biomater. Sci. Eng., 2019, 5, 2444 CrossRef CAS.
- A. F. Verre, A. Faroni, M. Iliut, C. Silva, C. Muryn, A. J. Reid and A. Vijayaraghavan, Interface Focus, 2018, 8, 20180002 CrossRef.
- M. D'Angelo, V. Castelli, E. Benedetti, A. Antonosante, M. Catanesi, R. Dominguez-Benot, G. Pitari, R. Ippoliti and A. Cimini, Front. Bioeng. Biotechnol., 2019, 7, 325 CrossRef.
- G. Liu, H. Shen, J. Mao, L. Zhang, Z. Jiang, T. Sun, L. Qing and Z. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 6909 CrossRef CAS.
- Y. Zhu, G. Koley, K. Walsh, A. Galloway and P. Ortinski, Application of ion-senstitive field effect transistors for measuring glial cell K+ transport, IEEE SENSORS, Orlando, FL, 2016, pp. 1–3 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nr07824g |
| This journal is © The Royal Society of Chemistry 2021 |
