Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A life cycle assessment of greenhouse gas emissions from direct air capture and Fischer–Tropsch fuel production†
Caroline M.
Liu
 a,
Navjot K.
Sandhu
b,
Sean T.
McCoy
a,
Navjot K.
Sandhu
b,
Sean T.
McCoy
 a and
Joule A.
Bergerson
a and
Joule A.
Bergerson
 *a
*a
aChemical and Petroleum Engineering, Schulich School of Engineering, University of Calgary, 2500 University Dr. NW, Calgary, Alberta, Canada. E-mail: jbergers@ucalgary.ca
bCarbon Engineering Ltd., PO Box 187, 37321 Galbraith Road, Squamish, British Columbia, Canada
First published on 15th April 2020
Abstract
Direct air capture (DAC) separates carbon dioxide (CO2) from ambient air either chemically or physically. As such, it could be a potential climate mitigation tool when paired with geological sequestration of CO2 or downstream conversion to produce products with low life cycle carbon intensities. Of particular interest is the ability to pair CO2 from DAC with electrolytic hydrogen powered by renewable electricity to synthesize liquid hydrocarbons that can be used in transportation (often referred to as “e-fuels”). This presents a pathway additional to electric and fuel cell vehicles to harness renewable electricity for use in the transportation sector and may present an attractive opportunity as costs of renewable electricity and electrolysis equipment continue to fall. We conduct a life cycle assessment (LCA) of the greenhouse gas (GHG) emissions of a DAC system paired with Fischer–Tropsch synthesis (FTS) to produce transportation fuel (i.e., diesel). This is the first LCA study of a DAC-to-fuel process based on data from an operating DAC pilot plant. We estimate the system emits 0.51 gCO2e per gCO2 captured from air or 29 gCO2e per MJ FTS fuel combusted in the baseline scenario, in which the electricity emissions factor used in the process is relatively low. This carbon intensity (CI) is extremely sensitive to changes in the electricity emissions factor. We find that an electricity emissions factor of less than 139 g CO2e per kW h is required for this pathway to provide a climate benefit over conventional diesel fuel. If a low carbon source of electricity is used, this pathway can deliver transport fuels at a CI lower than conventional diesel production and several biofuel pathways. This analysis suggests that fuel synthesis facilities need to be located in regions with very low grid emissions factors, or preferentially, co-located with new-build renewable electricity.
Introduction
The recent IPCC special report on the impacts of global warming of 1.5 °C estimates that we must emit less than 420 GtCO2 from 2018 onwards to have a 67% chance of limiting global average temperature increase to 1.5 °C, which is the principle goal of the Paris agreement.1 Anthropogenic emissions amounted to 42 GtCO2 in 2017,2 which means that potentially less than one decade remains in this carbon budget at current rates. Even with strong measures to reduce global greenhouse gas emissions, removing CO2 from the atmosphere will likely be required. In their review of 1.5 °C consistent emission reduction pathways, the IPCC concluded that they all “project the use of carbon dioxide removal (CDR) on the order of 100–1000 GtCO2 over the 21st century”.1Direct air capture (DAC) is the process of separating CO2 from ambient air, either chemically (e.g., using solvents or solid sorbents) or physically (e.g., through phase changes). There are many industrial separation processes that remove CO2 from gas streams, such as natural gas processing, and there has been a substantial focus on development of “CO2 capture” systems in recent decades.3 However, DAC differs from such processes in that the initial concentration of CO2 in the air is roughly 100 times lower than in these other situations. This implies a relatively large energy requirement for separation and necessitates use of different materials and substantially different process designs.4 Research and development has advanced the design of practical DAC processes, but scale-up, financial, and policy implementation must continue to enable its large-scale deployment.5,6 The primary benefit of DAC – and other carbon dioxide removal approaches – is that they create space in the carbon budget for difficult to decarbonize activities, such as long-haul transportation, aviation, and agriculture. However, DAC could also be coupled with downstream processes to produce valuable, low CI materials or fuels that mitigate emissions by substituting for their fossil equivalent.7
In this paper, we perform a life cycle assessment (LCA) to examine the production of synthetic fuel (i.e., diesel) from a DAC source of CO2. The products of this process are referred to as e-fuels. The goal of this study is to assess the potential of DAC and fuel synthesized from DAC CO2 to mitigate atmospheric and transportation GHG emissions. LCA is a tool used to evaluate the environmental impacts of a product or process across the supply chain throughout its lifetime (i.e., from raw materials to end use and disposal). This assessment is based on data provided by Carbon Engineering Ltd. (CE), a Canadian company commercializing a solvent-based DAC technology, and literature sources for a downstream Fischer–Tropsch synthesis (FTS) process to produce synthetic fuel. A baseline scenario is estimated and then a detailed sensitivity analysis is performed to test the impact on the performance of the system due to variation in parameters such as the CI of the electricity used as well as the uncertainty associated with projected performance at commercial scale.
Literature review
The concept of using engineered systems to remove CO2 from the air has a long history.8 The earliest proposals involved stripping CO2 from the air to produce hydrocarbon fuels (using nuclear energy) in response to concerns about fossil fuel availability.9 The first assessment of DAC as a climate change mitigation option followed in the late 1990s10 and, since this time, many assessments have been carried out.4,6,11–17 Notably, the first peer-reviewed assessment of the technical and economic cost of CO2 removal via DAC based on pilot plant data was published in late-2018 by CE.18 The study provided an engineering cost estimate for a commercial 1.1 MtCO2 per year plant, and estimated a levelized cost of $94–232 per tCO2 captured from atmosphere, which is lower than previously estimated.4,16,17 Most recently, a report from the US National Academies reviewed the approaches to DAC and past studies, in which they performed an independent assessment of the energy demands and cost of CO2 removal, and identified pressing research needs.6 The National Academies' results are within the same order of magnitude (estimated net removed cost of $199–357 per tCO2) with those of the CE study and point out that – among other things – there is a need to apply LCA to understand the environmental impacts of system design choices. de Jonge et al.19 assessed the life cycle GHG emissions from an alkali solvent-based DAC system (from which the CO2 is geologically stored), and concluded that more carbon is captured than emitted in their baseline scenario. However, the impacts of upstream emissions on the overall carbon footprint of a DAC process have not been thoroughly assessed and reported.CO2 captured from the air can be sequestered in geological formations or utilized to produce low-carbon products. Geologic sequestration of CO2 removed from the air would result in “negative emissions”, creating additional space in the carbon budget; utilization to produce fuels could avoid emissions from fossil fuel use and, when the energy inputs to conversion are largely renewable, serve as a renewable energy vector. The existing markets that provide near-term value to support technology deployment are largely tied to a tangible product, rather than negative emissions, per se. Near-term product-based markets include: enhanced Oil Recovery (EOR), CO2 supply to greenhouses and for beverage carbonation, and production of “renewable” fuels such as methane and “drop-in” transportation fuels.5 This latter category of uses for CO2 has attracted considerable attention, as various policies (e.g., the California Low Carbon Fuel Standard, LCFS) provide incentives for low-carbon fuels or conversion of CO2 to fuel products. At the current time, CE has piloted an AIR TO FUELS™ process based on coupling their DAC technology18 with FTS to produce synthetic fuel (i.e., diesel),20 but in practice a variety of thermo-chemical technologies can be used based on demands of regional markets.
FTS has been investigated to a greater extent than DAC, as it is a mature technology and commercially used today. First developed in the 1920s to convert coal to liquid fuels, the largest single facility employing FTS today is located in Qatar, and produces 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bpd of liquid fuels from natural gas.20 The reactions in FTS convert a synthesis gas – a mixture of carbon monoxide (CO) and hydrogen (H2) – to a mixture of hydrocarbons and other oxygenated products (e.g., alcohols, carboxylic acids). FTS is exothermic and requires use of a catalyst, predominantly cobalt or iron. The FTS product is a synthetic crude oil that can then be refined to produce finished products, such as fuels, waxes, and lubricants. This refining process is an integral part of commercial FTS processes used today. In this study, it is assumed that the blended product or synthetic fuel is equivalent to diesel. References to synthetic fuel or fuel throughout this paper refer to a diesel equivalent, unless otherwise specified.
000 bpd of liquid fuels from natural gas.20 The reactions in FTS convert a synthesis gas – a mixture of carbon monoxide (CO) and hydrogen (H2) – to a mixture of hydrocarbons and other oxygenated products (e.g., alcohols, carboxylic acids). FTS is exothermic and requires use of a catalyst, predominantly cobalt or iron. The FTS product is a synthetic crude oil that can then be refined to produce finished products, such as fuels, waxes, and lubricants. This refining process is an integral part of commercial FTS processes used today. In this study, it is assumed that the blended product or synthetic fuel is equivalent to diesel. References to synthetic fuel or fuel throughout this paper refer to a diesel equivalent, unless otherwise specified.
Studies have evaluated the life cycle environmental impacts of FTS processes. Typically, feedstock for FTS processes is coal or natural gas, but previous studies have also evaluated the use of biomass.21–23 A coal feedstock used to produce FT diesel will emit more than conventional diesel production, whereas natural gas to liquids can have a similar CI.22 Biodiesel from FTS could have life cycle emissions as low as 21 gCO2e per MJ diesel (roughly 5 times lower emissions than conventional diesel life cycle emissions),23 but there are few FTS-based biomass-to-liquids plants24 and considerable uncertainty over the indirect emissions (and other impacts) arising from cultivation of bioenergy crops.25 CO2 is not used as a feedstock for FTS processes today, thus, there are few LCA studies of conversion of CO2 to liquid fuels via FTS. van der Giesen et al.26 performed a LCA for production of liquid fuels from CO2via FTS where the CO2 is supplied by a DAC process powered by a carbon-free electricity supply portfolio (i.e., a 100% photovoltaic source). A low-temperature (moisture-swing) DAC process27 is combined with conversion of CO2 to CO and the FTS reaction. To convert CO2 to CO for use in the FTS process, they model a process based on the reverse Water Gas Shift (rWGS) reaction. In this reaction, CO2 is reacted with H2 over a catalyst to produce water and CO, and the resulting CO is then mixed with additional H2 to produce a suitable syngas for FTS. The performance of the FTS process in this LCA is based on the Shell Middle Distillate Synthesis process used in Shell's Pearl Gas-to-Liquids facility.
While both DAC and FTS have been studied in literature, there are only a few studies that investigate their combination and none that is based on data from a pilot-scale DAC process. We fill this gap by using data from CE's pilot-scale DAC process18 and combining it with published information on a commercial-scale FTS process26 – as will be described in the following sections. This allows for a more in-depth analysis of actual operations, a better understanding of upstream emissions (e.g., catalyst production), and a detailed sensitivity analysis.
Methods
We estimate the GHG emissions of CO2 capture via DAC and the associated liquid fuel production using two different metrics of evaluation (i.e., functional units): per tonne (t) of CO2 captured from the atmosphere and per megajoule (MJ) of synthetic fuel combusted. Life cycle emissions from the system are normalized by each of these two values to analyze the drivers of life cycle impacts (e.g., process energy use emissions, construction and decommissioning emissions). The first functional unit is a metric of the DAC system performance and the denominator does not include additional CO2 captured from fossil-fuel use in the DAC process. This is an important metric for the examination of the effectiveness of DAC as a carbon dioxide removal tool and to compare against other carbon capture methods. The second functional unit looks at the CI of the synthetic fuel and is relevant to existing and emerging regulatory schemes (e.g., the California LCFS, EU Fuel Quality Directive, and Canadian Clean Fuel Standard).The LCA we performed has a cradle-to-grave scope, in that emissions associated with the entire system from raw materials supply (e.g., natural gas for the calciner, cobalt for the catalyst, potassium hydroxide (KOH) for the air contactor) to end use (e.g., fuel combustion) are included. Fig. 1 illustrates the principle process groups of interest in this LCA, including those associated with DAC and FTS. The DAC process studied here is described in detail elsewhere.18 In summary, ambient air is brought into contact with a KOH solution in the air contactor. This solution absorbs the CO2 to form carbonate (CO32−) ions, and is then sent to the pellet reactor, where addition of calcium hydroxide (Ca(OH)2) causes precipitation of calcium carbonate (CaCO3) pellets. The finished CaCO3 pellets are calcined in a natural gas and oxygen-fired circulating fluidized bed, liberating CO2. The remaining calcium oxide (CaO, or quicklime) is slaked with water and returned to the pellet reactor. The CO2 is compressed to 3 MPa, dehydrated, and directed to fuel synthesis.
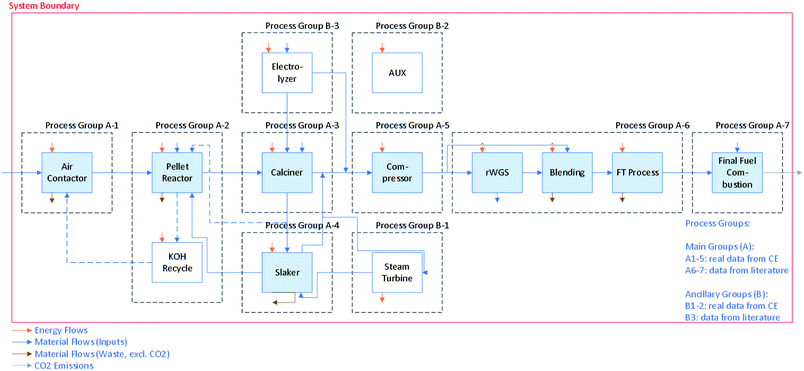 | ||
| Fig. 1 Block flow diagram with process groups and system boundaries of the combined direct air capture (DAC) and Fischer–Tropsch synthesis (FTS) process. | ||
Fuel synthesis starts with conversion of the CO2 to CO using the rWGS reaction. An electrolyzer, considered here as part of the FTS process, is used to produce H2 for the rWGS and FTS reactions, as well as oxygen (O2) for the oxy-fired calciner in the DAC process. Syngas is then created by blending the CO with additional H2 to achieve the ratio required to operate the FTS. The syngas is directed to FTS to produce synthetic crude oil, which can then be hydrocracked and fractionated into middle distillates, kerosene, naphtha, and light ends (e.g., methane, ethane, propane). For this case study, it is assumed that the synthetic fuel produced is equivalent to diesel. This fuel is then combusted in vehicles for transportation etc., emitting CO2 to the atmosphere. The baseline scenario assumes capacity for this process is 1.1 MtCO2 captured from air per year, which is converted (along with CO2 captured from natural gas combustion) into 20 PJ of synthetic diesel (9800 bpd).
The O2 and H2 required by the DAC and FTS processes, respectively, are provided by alkaline water electrolysis. We assume that electricity and natural gas of varying GHG emissions intensity is sourced from an independent supplier. We include emissions associated with the construction of and materials used in the equipment shown in Fig. 1. Detailed assumptions and process modelling are explained in Methods and Model sections respectively.
It is important to note that the material and energy flows for the DAC process as described here are for a full-scale plant, capturing approximately 1.1 MtCO2 per year from the air. The current CE pilot plant in Squamish, British Columbia (BC), Canada captures about 1 tCO2 per day from the air, implying a technology readiness level of 6.28 In 2017, CE incorporated fuel synthesis capability into the DAC pilot. When operating, the pilot produces roughly 1 bpd of synthetic fuel. To achieve full-scale operation as modelled by Keith et al.,18 equipment would need to be scaled-up, and certain low-risk process changes implemented (e.g., a slaker instead of a stirred tank reactor, continuous operation of the calciner). While the pilot-plant is believed to be representative of the full-scale process, scale-up and integration may result in unanticipated changes to the process design and resulting performance (both positive and negative). For this reason, we investigate performance and energy consumption uncertainties in both the major and low-risk units through a sensitivity analysis.
We summarize key parameters for the baseline scenario (i.e., DAC using an oxy-fired calciner coupled to FTS), in Table 1. The baseline scenario assumes the electricity comes from a generic low-CI grid (such as hydro-dominated grids that exist in locations like BC and Quebec in Canada, or a nuclear-dominated region such as France). While relatively few electricity grids have carbon intensities as low as the above-mentioned grids today, in future energy scenarios consistent with a 2 °C (or lower) target, average global CI of electricity generation falls to around zero by 2050.29 Furthermore, direct air capture and fuel synthesis could be located in regions where wind resource or solar insolation are most abundant, but lack of transmission capacity or isolation from global demand centers necessitate that the energy be converted to fuels in order to realize viable economic value. This baseline provides indicative CI values for the same fuel production process powered by the new-build renewables, although the economics would be different. The impact of electricity CI is explored in the sensitivity analysis, and we identify a CI threshold under which the DAC system can still deliver a GHG emissions reduction compared to conventional diesel production.
| Parameter | Value | Reference |
|---|---|---|
| a The electricity CI for the baseline scenario is assumed to be that of the BC grid electricity, the GHG impact from Canada's National Greenhouse Gas Inventory.30 CI for existing low-carbon grids, such as BC is indicative of CI for new-build renewables and potentially future grids with increased renewable penetration. The variability of electricity emission intensities due to different generation mixes is investigated in the sensitivity analysis. b The value is an assumption based on commonly used technologies or operating parameters and does not have a specific source. These values are investigated in the sensitivity analysis due to the uncertainty associated with the assumptions. | ||
| Electricity carbon intensity | BC, 13 gCO2e per kW ha | National Inventory Report30 |
| H2 production method | Electrolysis, 57 kW h per kg H2 | Bhandari et al.31 |
| O2 production method | Electrolysis, 7.1 kW h per kg O2 | Bhandari et al.31 |
| Electrolysis type | Alkaline water electrolysis | Author's estimateb |
| Synthetic fuel heating value, lower heating value (LHV) | 43 MJ per kg synthetic fuel | van der Giesen et al.26 |
| Synthetic fuel density | 820 kg per m | |
| End use fuel combustion emissions, LHV | 75 gCO2e per MJ fuel | Han et al.32 |
| Natural gas, LHV | 47 MJ per kg | Natural Resources Canada33 |
| H2 to CO produced ratio (rWGS) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio 1 molar ratio |
van der Giesen et al.26 |
| H2 to CO ratio (FT) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio 1 molar ratio |
van der Giesen et al.26 |
| Capture efficiency | 75% from air | Keith et al.18 |
| Material and energy use | As per references (right) for DAC and Fischer–Tropsch respectively | Keith et al.,18 van der Giesen et al.26 |
| Electrolysis emissions allocation | 100% to hydrogen | Keith et al.18 |
| Calciner type | Natural gas | Keith et al.18 |
| Electric heating efficiency | 100% | Carbon Engineering Ltd.34 |
| Construction and decommissioning emissions | EIO-LCA with 33% reduction in power grid emissions | EIO-LCA,35 Schivley et al.36 |
| Chemical production emissions factors | SimaPro 8.0.2 | SimaPro |
| CO2 pressure to FTS | 3 MPa | Keith et al.18 |
| CE plant scenario | Scenario D | Keith et al.,18 Carbon Engineering Ltd.37 |
| Catalyst amount (rWGS and FTS) | 2.0 t per year | Author's estimateb |
| FT temperature | 220 °C | Author's estimateb |
| FT pressure (CO2 pressure) | 3 MPa | Keith et al.18 |
| FT space velocity | 340 per h | Author's estimateb |
Keith et al. present four different scenarios for the CE DAC process:18 Scenario A, in which electricity used in the DAC plant is generated onsite using a gas-turbine based combined heat and power plant; Scenario B is an economic variation on Scenario A with reduced “Nth plant” capital and operating costs; and, Scenarios C and D, are process variations on Scenario A, in which the onsite combined heat and power facility is omitted, and supplementary electricity and natural gas (for the oxy-fired calciner) are imported. Further, Scenario D is optimized to provide CO2 for fuel synthesis. In this configuration, H2 for fuel synthesis is provided by electrolysis and co-produced O2 is sufficient to supply the oxy-fired calciner in the DAC plant, thus the air separation unit (ASU) is not needed. The baseline scenario for this LCA is similar to Scenario D, except that CO2 is assumed to be delivered to FTS at 3 MPa instead of at atmospheric pressure.
The Shell Middle Distillate Synthesis process modeled here is optimized for diesel production, and converts 80% (on an energy basis) of the syngas into liquid products (e.g., naphtha, kerosene, gasoil, and base oil) suitable for blending directly into finished fuel.20,26,38 The other 20% are low-value products (e.g., gases), which are consumed on-site to generate electricity and heat for the plant. The conversion ratios by mass can be found in Table S1 in the ESI.† We use the average heating value of the product slate (i.e., MJ per kg) reported by van der Giesen et al.26 to normalize the mass of FTS products to the heating value(MJ) of synthetic fuel. We assume the blended product is functionally equivalent to diesel.
Electrolysis is an important source of emissions due to the consumption of electricity. In the baseline scenario, we follow the assumption made by Keith et al. in their Scenario D18 and attribute the emissions associated with electricity generation for electrolysis to the H2 product that is consumed in FTS. However, when a natural gas fired calciner is used in the DAC system, some of the O2 co-produced in electrolysis is used by DAC. There are multiple methods of allocation that could be applied to distribute the environmental burdens of electrolysis in this multi-product system,39 and we investigate several: mass, market value, mole, and volume. The avoided burden and displacement by co-producing O2 through electrolysis instead of separately from an ASU is also examined.
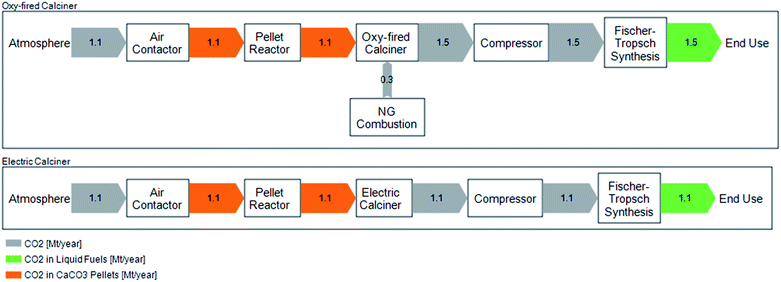
In the baseline scenario, CO2 released from natural gas combustion to provide the heat for calcination is captured and combined with atmospheric CO2 released from decomposition of CaCO3. The combined CO2 stream is then delivered to the FTS system. However, CE is also investigating use of an electric calciner as opposed to a natural gas and O2 fired calciner for the full-scale facility. We, thus, evaluate a variant of the baseline scenario, in which the calciner is heated electrically rather than by combusting natural gas. The difference in carbon flows between the oxy-fired (baseline) and electric calciner scenarios are shown in Fig. 2.
In the electric calciner case, CO2 delivered to the FTS is comprised only of CO2 captured from air. This case assumes that the calciner heat requirement can be met solely through electric heating which does not otherwise impact the overall process efficiency. We assume that the heat duty supplied by natural gas (in the oxy-fired calciner case) is equivalent to that required through electric heating for the electric calciner case.
We assume that minimal transportation is required for both process and building materials that are not produced on-site. However, the emissions incurred during construction and decommissioning and the production of chemicals consumed in the DAC process are included in the analysis.
Modeling
To model the DAC and FTS processes, we compiled a material and energy balance in Microsoft Excel (Table S2 in the ESI†). Material inputs include air, water, KOH, CaCO3, and Ca(OH)2 for DAC. We modeled catalyst consumption by assuming an average annual use rate (e.g., if 2 tonnes of catalyst were required every 2 years, the annual rate assumed would be 1 t per year). The energy inputs are electricity, natural gas, and steam. Steam is generated within the DAC process and circulated, and there is also water make-up for quicklime rehydration.The emissions factors used in this analysis and their sources can be found in the ESI, Table S3.† For the metal catalysts, the emissions factor for the metal with the highest composition in the catalyst (e.g., copper and cobalt) was used. This emission factor includes the metal production, but does not include the steps for catalyst fabrication.
Emissions associated with construction and decommissioning are quantified using two different methods, shown in Fig. S1 in the ESI.† Economic input-output LCA (EIO-LCA)35 is used to estimate the economy-wide impacts and emissions associated with products or processes. The benefit of this method is it encapsulates the economy-wide emissions, the downside is the model aggregates the sectors it can analyse. Thus, approximations must be made (e.g., a pellet reactor is modelled as a generic metal tank). Furthermore, the EIO-LCA model is based on the 2002 United States of America (USA) economy. The average USA electricity grid CI is approximately one-third less than it was in 2002 (ref. 36), thus an adjustment to the EIO-LCA GHG emissions was made. The assumptions and modelling methods associated with EIO-LCA are listed in Table S4.† For comparison, the EIO-LCA results were also compared to the construction and decommissioning emissions estimated for a comparable type of plant. A steam methane reforming (SMR) plant was used as a proxy, as it is of similar unit number and complexity to DAC and FTS processes.
A model of FTS was created in Aspen Plus™ based on the work of Mansouri et al.40 (discussed further in the ESI†) to verify process results as well as perform a sensitivity analysis on important and uncertain parameters. This validated the extent of conversion of syngas to fuel and examined the effects of changes in process parameters. Process parameters may change due to changes made in operations to maximize production of products with a particular composition or adjust to unexpected plant performance (e.g., fouling that reduces conversion yield or efficiency).
Results and analysis
Fig. 3 shows life cycle GHG emissions estimates on the basis of two different functional units: per gCO2 captured from air and per MJ fuel combusted. Note that only the baseline scenario emissions are displayed here; uncertainties and sensitivities are presented in Fig. 4. The four inset panels display only the emissions associated with the DAC and FTS processes to more clearly identify their individual contributions, while the two main panels in Fig. 3 show emissions over the full life cycle (i.e., both the CO2 captured (negative) and fuel combustion emissions (positive)). Recall the fuel is assumed to be functionally equivalent to diesel so a standard diesel combustion emissions factor is used. The net emissions are shown as black diamonds in the two main panels. The bars in each main panel show the results for the oxy-fired and electrically heated calciner in the DAC process. The functional unit in the upper panel is the mass of GHGs (gCO2e) emitted for each mass unit of CO2 (gCO2) captured from air by the DAC process. The lower panel presents the mass of GHGs (gCO2e) emitted for each unit of FTS fuel (MJ).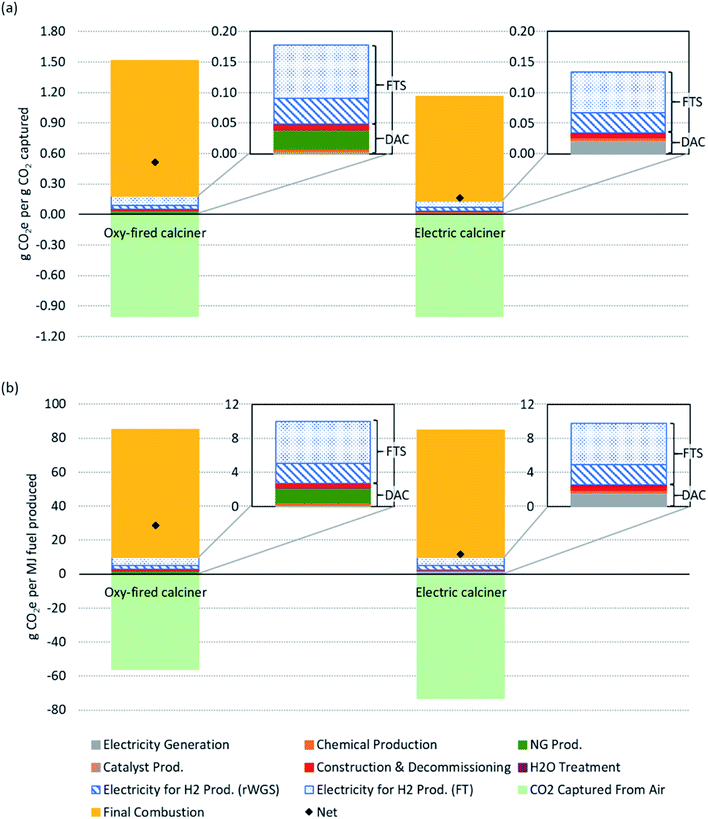 | ||
| Fig. 3 Process and full life cycle emissions for the baseline scenario (described in Table 1) of Fischer–Tropsch synthesis (FTS) fuel produced from the combined direct air capture (DAC) and FTS process. Two cases: oxy-fired calciner (left bars) and electric calciner (right bars) are shown with two functional units each, per gCO2e captured from air (a) and per MJ fuel combusted (b). For a sensitivity analysis on the emissions, refer to Fig. 4. The inset images highlight the process emissions associated with DAC and FTS (H2 and synthetic fuel production). This includes direct and indirect emissions associated with process. | ||
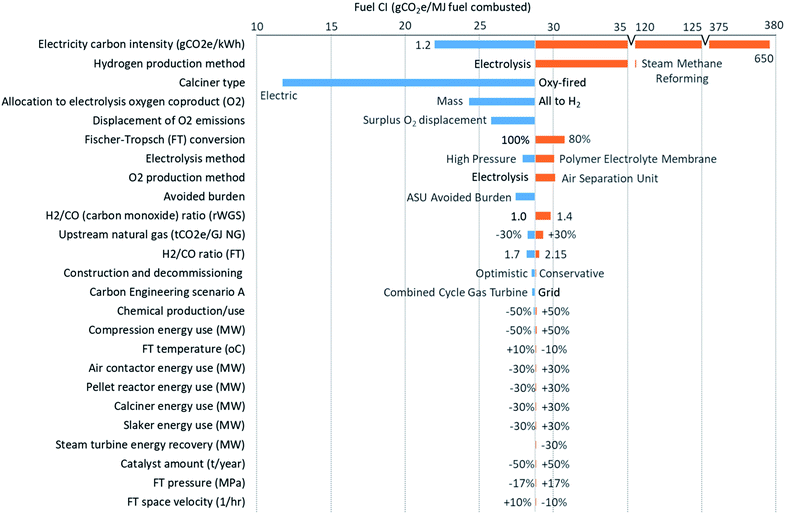 | ||
| Fig. 4 Sensitivity analysis of various parameters on the direct air capture (DAC) and Fischer–Tropsch synthesis (FTS) processing emissions, with the change in parameter labelled. Baseline scenario assumptions can be found in Table 1 and on the graph for H2 and O2 production methods, allocation, CE scenario, and H2/CO ratio for rWGS. | ||
Fig. 3(a) shows that we estimate the full life cycle net emissions in the baseline scenario of 0.51 gCO2e emitted per gCO2 captured from air using an oxy-fired calciner (shown on the left) and 0.16 gCO2e emitted per gCO2 captured from air for the electric calciner alternative (shown on the right). This figure shows that the DAC and FTS process emissions (i.e., material and energy emissions consumed by DAC and FTS processes, excluding the negative contribution from carbon capture from air and the combustion emissions from fuel use) contribute a small fraction of emissions (excluding CO2 capture): 8% and 26%, respectively, in the oxy-fired case; and, 3% and 9%, respectively, in the electric case. The emissions associated with the combustion of diesel contribute the remaining 66% (88% for the electric calciner) of emissions. Note that combustion emissions are lower (measured per gCO2 captured from air) when an electric calciner is used. This is due to the ratio between MJ fuel combusted (or produced) and gCO2 captured: in the oxy-fired case, CO2 is produced in the calciner and, thus, more fuel is produced (higher ratio of fuel produced to CO2 captured from air); whereas, in the electric alternative, the ratio is closer to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The emissions from DAC, FTS, and fuel use are partially offset by the capture of CO2 from air, reducing the 1.5 and 1.1 gCO2e emitted per gCO2 captured to a net of 0.51 and 0.16 gCO2e emitted per gCO2 captured respectively for the oxy-fired and electric calciner cases. Large amounts of H2 are required in the FTS process and, in the baseline scenario, are assumed to be produced through alkaline water electrolysis.
1. The emissions from DAC, FTS, and fuel use are partially offset by the capture of CO2 from air, reducing the 1.5 and 1.1 gCO2e emitted per gCO2 captured to a net of 0.51 and 0.16 gCO2e emitted per gCO2 captured respectively for the oxy-fired and electric calciner cases. Large amounts of H2 are required in the FTS process and, in the baseline scenario, are assumed to be produced through alkaline water electrolysis.
Fig. 3(b) shows net life cycle emissions of 29 and 12 gCO2e per MJ fuel for the oxy-fired and electric calciner respectively. The amount of CO2 captured from air and emitted from combustion is nearly the same in the electric calciner case (approximately 73 gCO2), resulting in a lower net emissions value. The difference between the two cases is primarily the carbon captured from air, as there is little difference in process emissions. Using an oxy-fired calciner, CO2 is both captured from the atmosphere and produced by combusting natural gas in the calciner are processed into synthetic fuel. In this case, only the CO2 removed from the atmosphere is considered as negative emissions. When using an electric calciner, all the CO2 in the system that is processed into fuel is taken from the atmosphere. This results in a higher carbon captured value when normalizing by MJ fuel combusted. Using an electric calciner over an oxy-fired calciner avoids further fossil fuel use and results in a lower fuel CI on a per MJ basis.
Combined process emissions for the DAC and FTS are 0.18 and 0.14 gCO2e emitted per gCO2 captured from air for the oxy-fired and electric calciner cases, respectively, as shown in Fig. 3(b). Based on fuel energy content, these process emissions become 10 and 9.8 gCO2e emitted per MJ (two inset panels of Fig. 3(b)). In the baseline scenario, FTS contributes 0.13 gCO2e emitted per gCO2 captured in the oxy-fired calciner case and 0.10 gCO2e emitted per gCO2 captured with the electric calciner. The FTS CI is 7.3 gCO2e per MJ fuel in both cases, contributing 73 to 74% of the total process emissions. This includes electricity used to produce H2. The next largest process emissions category is the calciner heating, whether by natural gas at 18% (0.03 gCO2e emitted per gCO2 captured, 1.8 gCO2e per MJ fuel) or electrically at 14% (0.02 gCO2e emitted per gCO2 captured, 1.4 gCO2e per MJ fuel). Following these categories is construction and decommissioning at 5% (0.01 gCO2e emitted per gCO2 captured, 0.49 gCO2e per MJ fuel) for the oxy-fired calciner and 6% (0.01 gCO2e emitted per gCO2 captured, 0.63 gCO2e per MJ fuel) for the electric calciner. The remainder of the emissions are from chemical and catalyst production (2%), electricity generation for general plant operation (2%, e.g., pumping, mixers, fans), and water treatment (less than 1%).
Fig. 4 shows the sensitivity of the life cycle emissions estimates on the basis of fuel energy content to important input parameters. Sensitivity analyses for the life cycle emissions on a per gCO2 captured from air for the combined DAC and FTS, and DAC-only process emissions can be found in the ESI in Fig. S2 and S3† respectively. Additionally, an FTS-only process emissions sensitivity on a fuel energy content basis can be found in Fig. S4.† The impact of each parameter is ranked from largest to smallest. The impact of changes to the electricity CI is larger than variation in any other parameter by more than an order of magnitude. This is due to the electricity consumption for H2 production via electrolysis for the rWGS (32% of total electricity use) and FTS (65%) reactions, followed by electricity consumed in the DAC and CO2 compression process (3%).
In addition to the electricity emission factor, Fig. 4 shows that the method of H2 production, and the choice of calciner heating can have a substantial impact on the net CI. SMR and a cryogenic ASU were considered as an alternative to electrolysis (which produces both H2 and O2) in the baseline scenario, as they are the most common production methods for H2 and O2 respectively. Using H2 produced by SMR results in synthetic fuel produced with a higher CI than conventional fossil fuels, making this approach not viable. Carbon capture and sequestration (CCS) has been implemented at SMR facilities41 and could reduce upwards of 90% of the process emissions of CO2,42 making SMR competitive with electrolysis in terms of emissions at the assumed baseline electricity emissions intensity. However, converting natural gas to H2, thus liberating CO2 (and necessitating geological storage or conversion), only to recombine it with atmospheric CO2 to produce a liquid fuel is difficult to rationalize from a thermodynamic and/or resource efficiency standpoint. If low-CI H2 could be imported, an ASU could be used to produce O2 for use in the oxy-fired calciner.
If using electrolysis, the emissions associated with the production of H2 contribute between 23% and 28% of the net CI of synthetic fuel depending on the type of electrolysis. The higher the electricity CI, the more important the type (and efficiency) of the electrolysis process employed. Development of more efficient electrolysis techniques will, thus, allow for a higher CI of the electricity than assumed in the baseline scenario for the same emissions and still provide mitigation benefit. For example, pressurized alkaline electrolysis can reduce energy use for electrolysis and H2 compression by 12%. On the other hand, PEM electrolysis has a higher specific energy demand and investment cost compared to alkaline electrolysis, 67–89 kW h per kg H2 (ref. 31) and approximately 1000–1800 USD per kW (ref. 43) compared to 50–78 kW h per kg H2 (ref. 31) and 500–1400 USD per kW,43 but it also has better flexibility with regards to variable power supply (e.g., intermittent renewables).
As previously discussed, use of electric calciner could reduce life cycle emissions by 60% from 29 to 12 gCO2e per MJ fuel. However, should the electric heating efficiency of the calciner be lower by 20% than assumed for the baseline scenario, then the CI with an electric calciner may only decrease by 58% (i.e., a 2% difference from assuming no decrease in efficiency). Another uncertainty associated with scale-up is the fraction of CO2 delivered from DAC that can be converted to the final product (diesel). A reduction of CO2 conversion in FTS would increase the resulting fuel CI. However, this relationship may not be linear due to gas recycle or changes in the product slate. Increases in the energy use of the major units or the energy recovery by the steam turbine have negligible emissions impacts, especially when using low CI electricity.
Fig. 4 also shows that the impact of different allocation methods can change the DAC and FTS emissions by up to 45%. Allocation of emissions from the electrolysis process to H2 and O2 (the two co-products of the process) is required, as meeting the demand for H2 in FTS will produce all the O2 required by DAC, plus surplus O2 that could be sold. If this O2 has a lower CI than the alternative method of producing O2, a potential displacement credit is possible. Four methods are investigated, namely allocation based on the mass, market value, volume, and mole fraction of the co-products. Another method considered is the estimate of avoided burden, calculated by producing O2 using an alternative process. There is no single accepted method to assign emissions to each product, but the results can be greatly affected. For example, mass allocation, shown in Fig. 4, results in a 45% or 4.5 gCO2 per MJ fuel decrease in combined DAC and FTS emissions compared the baseline scenario (where all emissions are allocated to H2). The other allocation methods do not change the CI as much as comparing mass allocation with all emissions allocated to hydrogen. Volume has the lowest decrease in CI of 4% or 0.4 gCO2 per MJ fuel in DAC and FTS emissions. The avoided burden resulting from the co-production of O2 rather than employing an ASU will reduce the synthetic fuel life cycle CI from 29 to 28 gCO2 per MJ fuel. Since surplus O2 is also produced and could be sold, 1.3 gCO2 per MJ fuel could be displaced from the O2 market.
The DAC stage of the LCA is based on a modeled performance of CE's proposed full-scale plant. The model for this 1.1 MtCO2 per year plant is based on data acquired from the pilot-scale plant (which captures 1 tCO2 per day), data from vendors of commercial-scale equipment, and Aspen simulation of process flows at a reference commercial scale.18 Extrapolating performance from the pilot-scale to full-scale requires many assumptions. For example, CE assumes the pressure drop in their full-scale air contactor will be 30% lower than observed in the pilot-scale system based on computer simulations of advanced packing designs and future process optimization. Changes to operating conditions and equipment that will occur during scale-up mean that energy requirements will not be exactly as projected. Thus, while these results are indicative of future performance, they will need to be re-evaluated as new data is collected from successively larger facilities.
The FTS system is modeled as a stand-alone facility in the LCA (sharing only electrolysis), which ignores potential process integration opportunities with the DAC processes. The FTS system needs to be matched appropriately to handle a 1.1 MtCO2 per year feed and the higher space velocities through the reactors. If not, incomplete conversion of CO2 and CO (and thus higher recycle flows) and/or shorter hydrocarbon chains (i.e., less valuable products) from FTS could result. Depending on market conditions, different combinations of products might be targeted. While this study does not model the system with sufficient detail to investigate this potential variation, the life cycle CI may be affected by the extent of conversion in the FT (and rWGS) reactor.
Some variation in the sensitivity analysis, such as the H2/CO ratios in rWGS and FTS, may not be operationally feasible. From literature, a range of H2/CO ratios exist and result in different products (e.g., a ratio greater than 2 in FTS would drive shorter hydrocarbon chain production, likely closer to gasoline). The ratios could also change depending on the plant operation. If for some reason too much wax is being produced, operations may choose to increase the H2 going to the reactor. Other investigated parameters have negligible effects and can be seen in Fig. 4.
There was insufficient data available to perform a sensitivity analysis on the alternative products that could be produced through the FTS process. The FTS process generally can produce a range of products that can be blended into a range of different fuels (in addition to light hydrocarbons, lubricant oils, and waxes), which may not have the same emissions intensity as diesel. Also, the FTS process modeled here is optimized for diesel yield, but there are other fuel production possibilities. However, to change the product slate of FTS, the FT reactor itself and the processing units (e.g., hydrotreating, cracking, and isomerization) would need to be reconfigured. Having said this, simply changing the energy content of the final product (assuming that the process emissions are the same) from 40 to 46 LHV MJ per kg fuel (representing pure fuel oil and gasoline respectively), results in an 8% increase to 6% decrease from the baseline scenario in process emissions on a per MJ fuel basis.
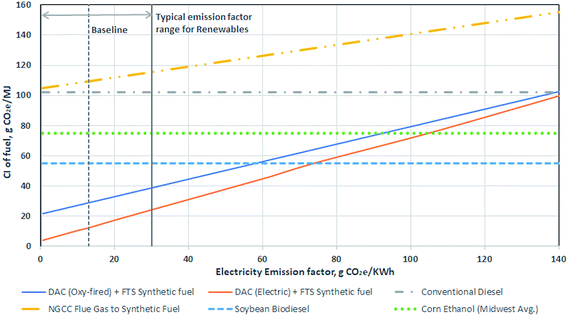
Fig. 5 presents the life cycle CIs associated with different methods of producing diesel fuel. The different diesel production methods include conventional petroleum production and refining, biodiesel, natural gas combined cycle (NGCC) power plant flue gas capture to fuels, and synthetic fuel from DAC. The combustion emissions for all methods assume a common emissions factor for diesel fuel at 75 gCO2e per MJ. These results show that production of fuel from DAC cannot be considered a net negative emissions pathway,48 but the CI of the resulting fuel can be much lower conventional production.
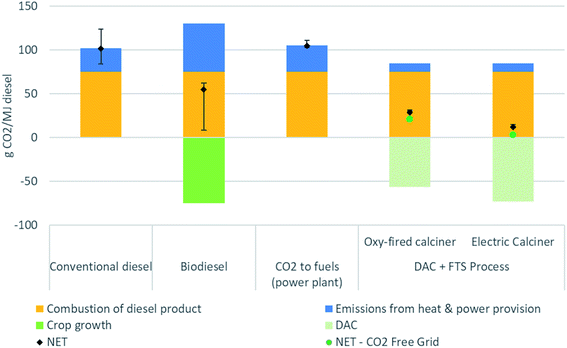 | ||
| Fig. 5 Comparison of carbon intensity of different diesel production methods: conventional,32 biodiesel,44 CO2 to fuels,18,32 and the combined direct air capture (DAC) and Fischer–Tropsch synthesis (FTS) process. Whiskers show the variability from the selected main or baseline scenario process, with the exception of the DAC + FTS process being similar to Fig. 3, where the sensitivity analysis can be found in Fig. 4. | ||
Multiple factors affect the magnitude of benefits possible using DAC and FTS. Note that there are no whiskers on the DAC + FTS process bars in Fig. 5, which represent the baseline scenario emissions. The sensitivity analysis is shown in Fig. 4.
In the baseline scenario, synthetic fuel has a lower CI at 29 gCO2e per MJ than conventional diesel at 104 gCO2e per MJ.49 The electricity supply CI could be as high as 139 gCO2e per kW h before the CI of the DAC synthetic diesel exceeds that of conventional diesel. Using CO2 free electricity supply (green dots), the CI decreases to 21 gCO2e per MJ. With an electric calciner, the net CI with the baseline scenario BC grid emissions intensity would be 12 gCO2e per MJ. A CO2 free electricity supply would result in a net CI of 3 gCO2e per MJ. As seen, there is some flexibility with regards to the electricity source emissions intensity used for the DAC and FTS process to produce synthetic fuel.
In the case where a natural gas combined cycle power plant (NGCC) power plant is built to serve as the source of the CO2 input to a FTS process to produce diesel, the resulting fuel has a net CI of 105 gCO2e per MJ (higher than the conventional diesel case).26 In this case, the electricity required to produce hydrogen via electrolysis exceeds the total production from the NGCC power plant. Thus, all the electricity from the NGCC plant plus electricity purchased from the grid is needed to satisfy the electricity requirements of the system. As such, the process does not avoid fossil CO2 emissions from power generation, nor does it remove CO2 from the atmosphere. Such a system is unlikely to be built, but we include it here to demonstrate that this combination of technologies is not sensible. Having said this, there are other combinations of stationary CO2 capture and conversion systems, some of which may be sensible, and should be explored in future work.
Soybean diesel was chosen for a biofuel comparison, as it comprises approximately 50% by mass of all biodiesel production in the USA. Other feedstocks (e.g., canola oil or recycled oils) each make up between 10 to 15% of the remainder.50 The various biodiesel pathways from the California Air Resources Board database of LCFS pathways51 have a range of 50 to 60 gCO2e per MJ, with an average of 54 gCO2e per MJ. The whisker contains the various biodiesel production methods. The lowest CI methods are attributed to used cooking oil feedstocks, which can range between 8 and 30 gCO2e per MJ. However, many of these pathways are limited in their scale, and represent provisional CIs that may be subject to change.
Fig. 6 further expands on the Fig. 4 sensitivity analysis to showcase the impact of electricity emissions factor on synthetic fuel CI. Vertical gridlines in the figure are example scenarios of low CI electricity, at which the DAC + FTS process can deliver fuels with a GHG impact lower than conventional diesel. The figure also presents the impact of electricity CI on other transportation fuel production pathways. The DAC and FTS process is the most sensitive to the electricity CI, with the electric calciner being more sensitive than the oxy-fired calciner. NGCC flue gas to fuel is capturing CO2 from a power plant and converting it to synthetic fuel. This is also sensitive to the electricity CI, as the same CO2 processing is used (FTS). The other three pathways, conventional, corn ethanol, and soybean biodiesel, are all assumed to be negligibly impacted by increasing electricity CI.
An electricity emissions factor of 56 gCO2e per kW h using an oxy-fired calciner is enough to make DAC and FTS synthetic fuel comparable to soybean biodiesel. If an electric calciner is used, a CI of 74 gCO2e per kW h makes it comparable. To reach the emissions of corn ethanol and conventional diesel, the electricity emission factor would be 93 and 139 gCO2e per kW h respectively for an oxy-fired calciner and 105 and 144 gCO2e per kW h for an electric calciner respectively.
Conventional CO2 capture (i.e., on flue gas streams using either pre or post combustion scrubbing techniques), or CCS, is conceptually similar to DAC in that CO2 is separated from a gas. However, we treat the carbon accounting in DAC and CCS differently. In the case of DAC, CO2 already present in the atmosphere is removed, thus creating a physical flow of carbon from the atmosphere. Whereas, in conventional capture, CO2 emissions from fossil fuel use are avoided, but no CO2 is removed from the atmosphere. While at the current time, there is no practical difference between avoided fossil emissions and removed atmospheric CO2 – as global emissions are large and positive – in a net zero future, avoidance and removal should be treated differently and we do so here. In either case, if the captured CO2 is converted to fuels and used, the carbon is ultimately released to the atmosphere and the entire process can not be seen as “net negative”.45 As a result, conventional capture with FTS, once system-wide emissions of fossil carbon are allocated to the fuel, would have a CI of 70 to 83 gCO2e per MJ higher than the DAC with FTS process across the range of electricity CIs in Fig. 6. The difference in CI changes as there are other factors impacted by the electricity CI in the DAC and FTS process. It is also likely that the CI for conventional diesel and corn ethanol change with electricity CI, however it would be negligible compared to the changes in the DAC and FTS process and flue gas capture.
While estimating the cost of the resulting fuels is not within the scope of this work, the results suggest that the cost of electricity will have a major impact on the cost of fuel production. Other studies have investigated the techno-economics of similar or related synthetic fuel production pathways and concluded that the costs of electricity and water electrolysis for H2 production contribute the highest share to total production cost.46,47 The near-term business case for fuels from DAC, thus, rests upon sourcing low-cost renewable electricity and finding markets that value the environmental attributes of the resulting fuel in addition to its commodity value. Examples such as California's LCFS provide motivation to continue development and scale-up of the technology. In a system such as the California LCFS, which creates a value for fuels with a CI below a declining baseline,48 and in which compliance credits were trading near $200 per tCO2e at the time of writing, the fuel produced by such a facility would generate compliance credits worth $1.7 per gal ($13 per GJ).49 This highlights the importance of improvements in technology that might increase efficiency of electrolysis, but also for policy in creating markets that encourage development of sustainable, low-CI fuels.
These results highlight that factors specific both to DAC (and associated CO2 conversion) process design and the context in which they are used have a substantial influence on the emissions reduction benefit. In addition, they clearly illustrate the thermodynamic reality that, while separation of CO2 from the air is energetically costly, it is an order of magnitude less costly than reduction of CO2 to hydrocarbons. Thus, while the emissions benefit of DAC is sensitive to the CI of the electricity inputs (if any),6,19 fuel synthesis is much more sensitive to changes in the CI of electricity supply.
Discussion
In this paper, we present a LCA of a DAC paired with electrolysis for conversion of CO2 to synthetic fuel through FTS. We show that, while this pathway does not deliver negative emissions, it can produce fuel with a CI that is lower than conventional petroleum fuels when low-carbon electricity is used. Specifically, in our baseline scenario, we find that 0.51 gCO2e are emitted for each g of CO2 captured from air on a life cycle basis. The resulting fuel has a CI of 29 gCO2e per MJ. Compared to conventional petroleum diesel fuel, this synthetic fuel from DAC has a CI that is lower by 75 gCO2e per MJ fuel.As shown in Fig. 4 and further illustrated in Fig. 6, the synthetic fuel CI is dictated by the electricity emission factor; the lower the electricity CI, the lower is the GHG impact of the fuel produced. For the baseline scenario, we assume an electricity CI of 13 gCO2e per kW h. Low CI values, such as this, are indicative of clean but not fully renewable electricity supply, and can be found in hydro-dominated grids, such as BC and Quebec, or in future renewable dominated grids. On the other hand, using fossil derived electricity doesn't result in similar climate benefits and yields a fuel CI an order of magnitude higher than the baseline. While it is possible to use such electricity, in essence, it negates the motivation to produce synthetic fuels, which is to produce fuels with a low GHG impact. Using low carbon electricity supply technologies is essential to this climate benefit of synthetic fuel.
In the baseline scenario, the electricity CI must be lower than 139 gCO2e per kW h for the synthetic fuel to have a lower CI than conventional petroleum diesel. The electricity requirements, and thus emissions, can be decreased with improvements in electrolysis or by using different electrolysis types, such as pressurized alkaline water electrolysis. There are also other products and uses for CO2, such as EOR, that require lesser amounts of (or no) H2, which would reduce the sensitivity of the product CI to electricity emissions factor.
The baseline scenario DAC process defined in this study employs an oxy-fired natural gas calciner, and no emissions are allocated to the surplus O2 produced from electrolysis, with the produced CO2 then being used as a feedstock for FTS. If an electric calciner is used, the life cycle CI of the fuel produced in the baseline scenario falls from 29 to 12 gCO2e per MJ fuel. In both cases, the same amount of CO2 is captured from the atmosphere, but, where an oxy-fired calciner is used, additional fossil CO2 is supplied to be converted into a fuel. The production and combustion of this additional fossil-CO2 based fuel generates emissions that can not be offset by capture from the DAC system.
Uncertainty or variability in the allocation method of emissions between H2 and O2 in electrolysis (as well as displacement and avoided burden), FTS yield of synthetic fuel, H2/CO reaction ratios, and upstream natural gas emissions could also affect the process emissions, but to a lesser extent than electricity CI, electrolysis energy intensity, and the choice of calciner. Other parameters, such as FTS operating conditions, DAC unit energy use, construction and decommissioning emissions, different DAC plant scenarios from CE, and catalyst and chemical use have negligible effects in the overall LCA picture.
Synthetic fuels are energy dense carriers which provide an attractive alternative to store and move bulk renewable energy. Consequentially, large-scale fuel production will require large amounts of electricity. To quantify this scale, the baseline scenario assessed in the paper (configuration captures 1.1 MtCO2 captured per year from air, which produces 9800 bpd synthetic fuel) requires 11 TW h per year (0.57 kW h per MJ fuel) of electricity. Electricity providers in existing renewable grids like BC and Quebec generated nearly 62 TW h and 212 TW h in 2017, respectively.41,43 Care must be taken to ensure that demand for CO2 conversion does not compete for renewable electricity that could otherwise be used more effectively to decarbonize the energy system in the near term, or more importantly, result in continuing operation of fossil-based generating assets. Policymakers will need to carefully craft incentives for clean fuels (and other policies) to avoid such suboptimal outcomes.
One way around this issue is to focus on developing renewable resources specifically for synthetic fuel production. There remains a vast potential of renewable resources untapped across the world, many due to lack of local demand or transmission capacity. These renewable resources are prime candidates for development in conjunction with fuel synthesis facilities, which enable the energy to be stored in fuels and then transported to global demand centers. This provides the option to locate synthetic fuel plants wherever renewable energy is abundant and cheap. In the United States, for example, the levelized cost of electricity from renewables (i.e., excluding tax credits and other incentives) has fallen to around $40 per MWh in recent years for both utility scale wind and solar installations.51,52 The lowest costs for solar PV projects in the US are generally found in regions with the strongest solar irradiance, such as southern California, the southwest, and west Texas, while low cost wind projects are found in the interior of the US, where winds are strongest and most constant. Elsewhere in the world, Argentina has an abundance of both solar and wind resources, with an estimated terrestrial wind generation potential of 17.9 PWh per year.53 While these examples illustrate the scale and cost of renewable energy available in some regions of the world today, actual plant deployment decisions would also need to consider power requirements, fuel production volumes, and capital requirements; the study of which are beyond the scope of this paper.
This work sets the stage for additional LCA of DAC and the technologies it could be paired with. In future work, the broader consequences of deploying this technology at scale could be investigated. The DAC plus FTS system could be optimized at the process unit level to maximize emissions reductions with normalized energy and material flows. Other combinations of DAC with CO2 conversions or EOR to identify optimal pathways for life cycle emissions reductions (or negative emissions) should be considered. Lastly, analysis should be conducted to identify contexts where DAC plus conversion to fuel is preferred over direct use of the renewable energy inputs,54 understanding the economic and environmental competitiveness for different products (e.g., jet fuel compared to diesel fuel) and in different geographic regions, or the relative attractiveness of DAC compared to other routes to carbon dioxide removal.
Conflicts of interest
Navjot Sandhu is an employee of Carbon Engineering and has an ownership stake in the form of options; Carbon Engineering is developing direct air capture and fuel synthesis technologies.Acknowledgements
The authors would like to acknowledge Carbon Engineering for their technical knowledge and collaboration, and the financial support from the Natural Sciences and Engineering Research Council (NSERC) of Canada, and the Canada First Research Excellence Fund (CFREF). We would also like to thank Geoff Holmes of Carbon Engineering for helpful discussion and advice.References
- IPCC, An IPCC special report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty, in Global Warming of 1.5 °C, ed. V. Masson-Delmotte, P. Zhai,H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor and T. Waterfield, World Meteorological Organization, Geneva, Switzerland, 2018 Search PubMed.
- C. Le Quéré, R. M. Andrew, P. Friedlingstein, S. Sitch, J. Hauck, J. Pongratz, P. A. Pickers, J. I. Korsbakken, G. P. Peters, J. G. Canadell, A. Arneth, V. K. Arora, L. Barbero, A. Bastos, L. Bopp, F. Chevallier, L. P. Chini, P. Ciais, S. C. Doney, T. Gkritzalis, D. S. Goll, I. Harris, V. Haverd, F. M. Hoffman, M. Hoppema, R. A. Houghton, G. Hurtt, T. Ilyina, A. K. Jain, T. Johannessen, C. D. Jones, E. Kato, R. F. Keeling, K. K. Goldewijk, P. Landschützer, N. Lefèvre, S. Lienert, Z. Liu, D. Lombardozzi, N. Metzl, D. R. Munro, J. E. M. S. Nabel, S. Nakaoka, C. Neill, A. Olsen, T. Ono, P. Patra, A. Peregon, W. Peters, P. Peylin, B. Pfeil, D. Pierrot, B. Poulter, G. Rehder, L. Resplandy, E. Robertson, M. Rocher, C. Rödenbeck, U. Schuster, J. Schwinger, R. Séférian, I. Skjelvan, T. Steinhoff, A. Sutton, P. P. Tans, H. Tian, B. Tilbrook, F. N. Tubiello, I. T. van der Laan-Luijkx, G. R. van der Werf, N. Viovy, A. P. Walker, A. J. Wiltshire, R. Wright, S. Zaehle and B. Zheng, Earth System Science Data, 2018, 10, 2141–2194 CrossRef.
- M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell, J. R. Fernández, M.-C. Ferrari, R. Gross, J. P. Hallett, R. S. Haszeldine, P. Heptonstall, A. Lyngfelt, Z. Makuch, E. Mangano, R. T. J. Porter, M. Pourkashanian, G. T. Rochelle, N. Shah, J. G. Yao and P. S. Fennell, Energy Environ. Sci., 2014, 7, 130–189 RSC.
- R. Socolow, M. Desmond, R. Aines, J. Blackstock, O. Bolland, T. Kaarsberg, N. Lewis, M. Mazzotti, A. Pfeffer, K. Sawyer, J. Siirola, B. Smit and J. Wilcox, Direct Air Capture of CO2 with Chemicals, American Physical Society, 2011 Search PubMed.
- D. Sandalow, J. S. Friedman, C. McCormick and S. McCoy, Direct Air Capture of Carbon Dioxide: 2018 ICEF Roadmap, Innovation for Cool Earth Forum, Tokyo, Japan, 2018 Search PubMed.
- E. National Academies of Sciences, Negative Emissions Technologies and Reliable Sequestration: A Research Agenda, 2018 Search PubMed.
- F. S. Zeman and D. W. Keith, Philos. Trans. R. Soc., A, 2008, 366, 3901–3918 CrossRef CAS PubMed.
- National Research Council, Climate Intervention: Carbon Dioxide Removal and Reliable Sequestration, National Academies Press, Washington, D.C., 2015 Search PubMed.
- M. Steinberg, Fuel, 1978, 57, 460–468 CrossRef CAS.
- K. S. Lackner, H.-J. Ziock and P. Grimes, Carbon dioxide extraction from air: Is it an option?, 24th International Technical Conference on Coal Utilization and Fuel Systems, 1999 Search PubMed.
- R. Baciocchi, G. Storti and M. Mazzotti, Chem. Eng. Process., 2006, 45, 1047–1058 CrossRef CAS.
- K. Z. House, C. F. Harvey, M. J. Aziz and D. P. Schrag, Energy Environ. Sci., 2009, 2, 193–205 RSC.
- K. Z. House, A. C. Baclig, M. Ranjan, E. A. van Nierop, J. Wilcox and H. J. Herzog, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20428–20433 CrossRef CAS PubMed.
- F. Zeman, Environ. Sci. Technol., 2007, 41, 7558–7563 CrossRef CAS PubMed.
- J. K. Stolaroff, D. W. Keith and G. V. Lowry, Environ. Sci. Technol., 2008, 42, 2728–2735 CrossRef CAS PubMed.
- M. Mazzotti, R. Baciocchi, M. J. Desmond and R. H. Socolow, Clim. Change, 2013, 118, 119–135 CrossRef CAS.
- F. Zeman, Environ. Sci. Technol., 2014, 48, 11730–11735 CrossRef CAS PubMed.
- D. W. Keith, G. Holmes, D. S. Angelo and K. Heidel, Joule, 2018, 2, 1573–1594 CrossRef CAS.
- M. M. J. de Jonge, J. Daemen, J. M. Loriaux, Z. J. N. Steinmann and M. A. J. Huijbregts, Int. J. Greenhouse Gas Control, 2019, 80, 25–31 CrossRef CAS.
- A. de Klerk, Fischer-Tropsch refining, Wiley-VCH, Weinheim, 2011 Search PubMed.
- J. J. Marano and J. P. Ciferno, Life-Cycle Greenhouse-Gas Emissions Inventory For Fischer-Tropsch Fuels, National Energy Technology Laboratory, Morgantown, WV, 2001 Search PubMed.
- O. P. R. van Vliet, A. P. C. Faaij and W. C. Turkenburg, Energy Convers. Manage., 2009, 50, 855–876 CrossRef CAS.
- D. Iribarren, A. Susmozas and J. Dufour, Renewable Energy, 2013, 59, 229–236 CrossRef CAS.
- S. S. Ail and S. Dasappa, Renewable Sustainable Energy Rev., 2016, 58, 267–286 CrossRef CAS.
- R. J. Plevin, J. Beckman, A. A. Golub, J. Witcover and M. O'Hare, Environ. Sci. Technol., 2015, 49, 2656–2664 CrossRef CAS.
- C. van der Giesen, R. Kleijn and G. J. Kramer, Environ. Sci. Technol., 2014, 48, 7111–7121 CrossRef CAS PubMed.
- K. S. Lackner, Eur. Phys. J.: Spec. Top., 2009, 176, 93–106 Search PubMed.
- J. C. Mankins, Acta Astronaut., 2009, 65, 1216–1223 CrossRef.
- J. Rogelj, S. Drew, K. Jiang, S. Fifita, P. M. Forster, V. Ginzberg, C. Handa, H. S. Kheshgi, S. Kobayshi, E. Kriegler, L. Mundaca, R. Séférian and M. V. Vilarino, An IPCC special report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty, in Global Warming of 1.5 °C, ed. V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor and T. Waterfield, Geneva, Switzerland, 2018 Search PubMed.
- Environment and Climate Change Canada, Canada's greenhouse gas inventory, https://www.canada.ca/en/environment-climate-change/services/climate-change/greenhouse-gas-emissions/inventory.html, accessed February 23, 2019.
- R. Bhandari, C. A. Trudewind and P. Zapp, J. Cleaner Prod., 2014, 85, 151–163 CrossRef CAS.
- J. Han, G. S. Forman, A. Elgowainy, H. Cai, M. Wang and V. B. DiVita, Fuel, 2015, 157, 292–298 CrossRef CAS.
- N. R. Canada, Natural Gas, https://www.nrcan.gc.ca/energy/natural-gas/5641, accessed April 23, 2019.
- N. Sandhu, personal communication, Carbon Engineering, 2018.
- Carnegie Mellon University Green Design Institute, Economic Input-Output Life Cycle Assessment (EIO-LCA) US 2002 (428 sectors) Producer model, http://www.eiolca.net, accessed September 10, 2018.
- G. Schivley, I. Azevedo and C. Samaras, Environ. Res. Lett., 2018, 13, 064018 CrossRef.
- N. Sandhu, personal communication, Carbon Engineering, 2018.
- J. Eilers, S. A. Posthuma and S. T. Sie, Catal. Lett., 1990, 7, 253–269 CrossRef CAS.
- ISO, Environmental management—Life cycle assessment—Requirements and guidelines, International Standards Organization, 2006 Search PubMed.
- M. Mansouri, H. Atashi and A. A. Mirzaei, J. Thermodyn. Catal., 2012, 03 Search PubMed.
- Shell, Quest Carbon Capture and Storage Project: Annual Summary Report- Alberta Department of Energy: 2017, Shell Canada Energy, Calgary, Alberta, 2018 Search PubMed.
- IEAGHG, Techno-Economic Evaluation of SMR Based Standalone (Merchant) Hydrogen Plant with CCS, IEA Greenhouse Gas R&D Program, Cheltenham, UK, 2017 Search PubMed.
- IEA, The Future of Hydrogen: Seizing today's opportunities, International Energy Agency, Paris, France, 2019 Search PubMed.
- H. Huo, M. Wang, C. Bloyd and V. Putsche, Environ. Sci. Technol., 2009, 43, 750–756 CrossRef CAS PubMed.
- S. E. Tanzer and A. Ramirez, Energy Environ. Sci., 2019, 12, 1210–1218 RSC.
- P. Schmidt, V. Batteiger, A. Roth, W. Weindorf and T. Raksha, Chem. Ing. Tech., 2018, 90, 127–140 CrossRef CAS.
- M. Fasihi, D. Bogdanov and C. Breyer, Energy Procedia, 2016, 99, 243–268 CrossRef CAS.
- S. Yeh, J. Witcover, G. E. Lade and D. Sperling, Energy Policy, 2016, 97, 220–234 CrossRef.
- California Air Resources Board, LCFS Dashboard, https://www.arb.ca.gov/fuels/lcfs/dashboard/dashboard.htm, accessed April 29, 2019.
- S. C. Government of Canada, Table 25-10-0020-01, Electric power, annual generation by class of producer, https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=2510002001, accessed March 28, 2019.
- M. Bolinger and J. Seel, Utility-Scale Solar: Empirical Trends in Project Technology, Cost, Performance, and PPA Pricing in the United States – 2018 Edition, 2018 Search PubMed.
- R. H. Wiser and M. Bolinger, 2018 Wind Technologies Market Report, 2019 Search PubMed.
- K. Eurek, P. Sullivan, M. Gleason, D. Hettinger, D. Heimiller and A. Lopez, Energy Economics, 2017, 64, 552–567 CrossRef.
- J. C. Abanades, E. S. Rubin, M. Mazzotti and H. J. Herzog, Energy Environ. Sci., 2017, 10, 2491–2499 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9se00479c |
| This journal is © The Royal Society of Chemistry 2020 |