Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metabolism of different dietary phenolic compounds by the urolithin-producing human-gut bacteria Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens†
Rocío
García-Villalba
 ,
David
Beltrán
,
María D.
Frutos
,
María V.
Selma
,
Juan C.
Espín
,
David
Beltrán
,
María D.
Frutos
,
María V.
Selma
,
Juan C.
Espín
 and
Francisco A.
Tomás-Barberán
and
Francisco A.
Tomás-Barberán
 *
*
Laboratory of Food & Health, Research Group on Quality, Safety, and Bioactivity of Plant Foods, Department of Food Science and Technology, CEBAS-CSIC, P.O. Box 164, 30100 Campus de Espinardo, Murcia, Spain. E-mail: fatomas@cebas.csic.es
First published on 22nd July 2020
Abstract
Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens are two human gut bacterial species that convert ellagic acid into urolithins. Urolithins are bioactive postbiotics produced by dehydroxylation reactions catalyzed by different catechol-dehydroxylases. The metabolic ability of these anaerobic bacteria on other dietary-phenolic compounds is unknown. In the present study, we evaluated the metabolism of flavonoids (quercetin, hesperetin, hesperidin, nobiletin, catechin, isoxanthohumol), isoflavonoids (daidzein), coumarins (esculetin, umbelliferone, scoparone), phenylpropanoids [caffeic acid; 3-(3′,4′-dihydroxyphenyl)propanoic acid (dihydrocaffeic acid); rosmarinic acid, and chlorogenic acid], benzoic acid derivatives (gallic acid, ellagic acid), lignans (secoisolariciresinol diglucoside), stilbenes (resveratrol), and secoiridoids (oleuropein) by G. urolithinfaciens DSM 27213T and E. isourolithinifaciens DSM 104140T. Both strains metabolized ellagic acid leading to the characteristic urolithins. They also metabolized caffeic, dihydrocaffeic, rosmarinic, and chlorogenic acids. The rest of the phenolic compounds were not transformed. Catechol dehydroxylation and double bond reduction were prominent transformations observed during the incubations. The enzymatic activities seem to have a narrow substrate scope as many catechol- (quercetin, catechin, esculetin, gallic acid) and double bond-containing (resveratrol, esculetin, scoparone, umbelliferone) phenolics were not metabolized. The catechol-dehydroxylase activity was more efficient in E. isourolithinifaciens, while the reductase activity was more relevant in G. urolithinfaciens.
Introduction
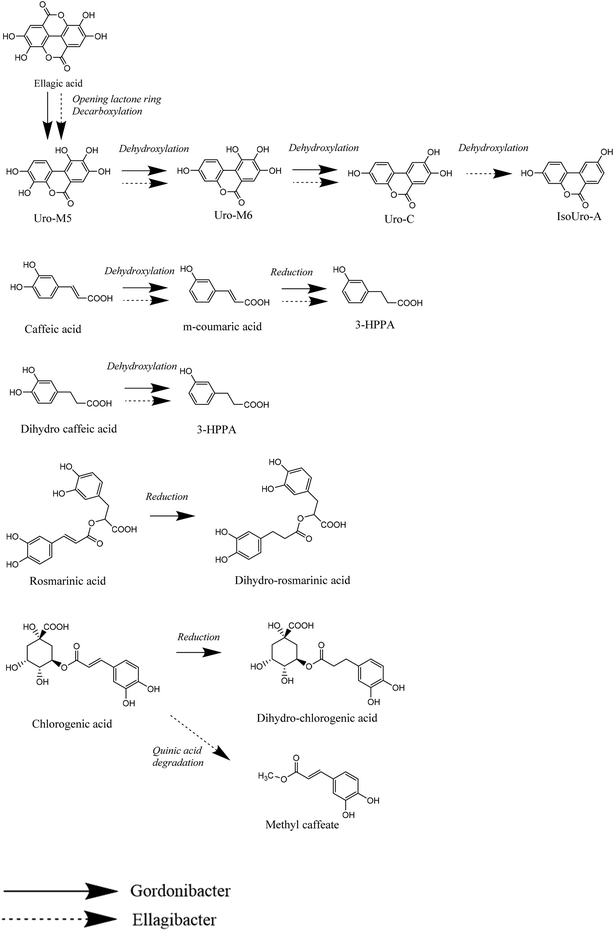
The bioavailability of dietary (poly)phenols (PPs) is very low.1 Most of them reach the colon where they are catabolized by the gut microbes producing metabolites, recently named as postbiotics,2–4 that are much better absorbed than the original PPs, show relevant biological effects in the colon and systemically, and persist in the body for long periods (sometimes up to three or four days).5In the case of ellagitannins and ellagic acid, the original PPs are converted to urolithins,6 and different metabolites are produced by the opening and decarboxylation of one of the two lactone rings in the ellagic acid molecule and the sequential removal of phenolic hydroxyls that lead to different urolithin metabolites.7 These biochemical conversions involve dehydroxylation by different catechol-dehydroxylases.
Three consistent metabotypes for the production of urolithins have been reported in humans,8 and bacterial strains producing this metabolic conversion have been discovered, characterized, and deposited in bacterial banks. Gordonibacter urolithinfaciens and Gordonibacter pamelaeae, the first bacterial species that were reported to produce urolithins from ellagic acid, were able to provide urolithins M5 (3,4,8,9,10-pentahydroxy urolithin), M6 (3,8,9,10-tetrahydroxy urolithin) and C (3,8,9-trihydroxy urolithin),9,10 and the abundance of this genus within a complex human gut bacterial community positively correlated with the metabotype A, while correlated negatively with metabotype B.11 However, the recently discovered Ellagibacter isourolithinifaciens12,13 was able to produce isourolithin A (3,9-dihydroxy urolithin) and positively correlated with metabotype B.14
Although enzymes with catechol-dehydroxylase activity are well-known, some of these enzymes have only been recently isolated and characterized from human gut microbes.15,16 Thus, an enzyme for the regioselective p-dehydroxylation of dopamine to produce m-tyramine has been described from Eggerthella lenta strains.15 The removal of a p-hydroxyl group from hydrocaffeic acid [3-(3′,4′-dihydroxyphenyl)-propanoic acid] and 3,4-dihydroxyphenylacetic acid (DOPAC) has also been recently reported.16 Another study has characterized the dehydroxylation of bidesmethyl-secoisolariciresinol to yield the mammalian lignan enterodiol by G. pamelaeae.17 Besides, recent research has demonstrated considerable variability in the catechol-dehydroxylase activity across closely related gut microbiota strains, with a very narrow substrate scope, suggesting that different enzymes can dehydroxylate various catechol-containing phenolics.16
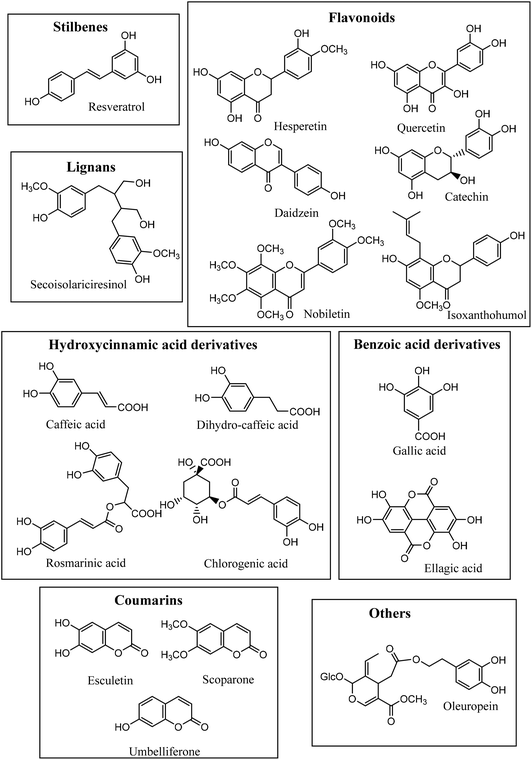
In the present study, we aimed to evaluate the metabolism of eighteen dietary phenolics, representative of different phenolic classes, by G. urolithinfaciens and E. isourolithinifaciens, and identify the metabolites produced.
Materials and methods
Chemicals
The PP standards selected (and their main dietary sources) were: gallic acid (3,4,5-trihydroxybenzoic acid, many foods and wine), hesperetin (5,7,3′-trihydroxy,4′-methoxyflavanone, citrus), hesperidin (5,7,3′-trihydroxy-4′-methoxyflavanone 7-O-rhamnosyl(1–6)glucoside, citrus fruits), quercetin (3,5,7,3′,4′-pentahydroxyflavone, onion), daidzein (7,4′-dihydroxy-isoflavone, soybean), catechin (flavan-3-ol, many foods, tea), secoisolariciresinol diglucoside (lignan, flaxseed), isoxanthohumol (prenylated flavanone, hops and beer), resveratrol (stilbene, grapes, and wine); caffeic acid (hydroxycinnamic acid, coffee); rosmarinic acid (caffeic acid ester, rosemary), dihydrocaffeic acid [3-(3′,4′-dihydroxyphenyl)propanoic acid] (gut microbiota metabolite derived from many dietary phenolics), oleuropein (dihydroxy-phenylethanol ester, olives and olive oil), esculetin (coumarin, barley), scoparone (coumarin methyl ether, citrus), umbelliferone (coumarin, carrot), nobiletin (5,6,7,8,3′,4′-hexamethoxyflavone, citrus fruits), chlorogenic acid (caffeoyl-quinic derivative, coffee, and widespread in food), ellagic acid (gallic acid dimer, strawberry, pomegranate) (Fig. 1). Ferulic acid (3′-methoxy-4′-hydroxycinnamic acid), isoferulic acid (3′-hydroxy-4′-methoxy-cinnamic acid), p-coumaric acid (4′-hydroxy cinnamic acid), m-coumaric acid (3′-hydroxy cinnamic acid), 3-(4′-hydroxyphenyl)-propanoic acid, and 3-(3′-hydroxypheny)-propanoic acid were used for chromatographic comparisons. All standards were purchased from Sigma-Aldrich (St Louis, MO, USA). Methyl-caffeate was obtained by heating caffeic acid dissolved in methanol at 80 °C in a stoppered pressure tube under N2 atmosphere. Stock solutions for each PP were prepared in methanol UPLC-MS quality (3 mM), and filtered through 0.22 μm polyvinylidene fluoride (PVDF) (Merck Millipore, Cork, Ireland) syringe filter for sterilization. The standards were injected independently in the analytical conditions (50 μM) to evaluate their response in DAD and MS detectors.Bacterial strains
The two strains used in this study were Gordonibacter urolithinfaciens (DSM 27213T = CCUG 64261T)9,10 and Ellagibacter isourolithinifaciens (DSM 104140T = CCUG 70284T).12,13In vitro fermentation method
Preparation of bacterial strains and subsequent culturing experiments were made in an anaerobic chamber (Concept 400, Baker Ruskin Technologies, Ltd, Bridgend, South Wales, UK) with an atmosphere composed of N2/H2/CO2 (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at 37 °C. Both bacterial strains from an early stationary phase culture, were grown separately or in co-culture with an initial concentration of 7 log(cfu mL−1) (determined by plate counting with an automatic counter), were inoculated into 5 mL of pre-reduced, anaerobe basal broth (Oxoid, Basingstoke, Hampshire, UK) containing 30 μM (a concentration reachable in the colon after dietary interventions) of the different PPs. Triplicate samples were done for each compound. As control samples, PPs were incubated without bacteria, and the bacterial strains were incubated without added PPs. All samples (5 mL) were incubated for seven days.
10) at 37 °C. Both bacterial strains from an early stationary phase culture, were grown separately or in co-culture with an initial concentration of 7 log(cfu mL−1) (determined by plate counting with an automatic counter), were inoculated into 5 mL of pre-reduced, anaerobe basal broth (Oxoid, Basingstoke, Hampshire, UK) containing 30 μM (a concentration reachable in the colon after dietary interventions) of the different PPs. Triplicate samples were done for each compound. As control samples, PPs were incubated without bacteria, and the bacterial strains were incubated without added PPs. All samples (5 mL) were incubated for seven days.
HPLC-DAD-MS analyses of the microbial metabolites
After incubations, 5 mL medium was extracted with 5 mL ethyl acetate LC-MS (Scharlau, Barcelona, Spain) acidified with 1.5% of formic acid (Panreac Química, Barcelona, Spain) using a refrigerated thermoblock shaker (VWR lnternational, LLC, Radnor, PA, USA) at 20 °C for 10 min and 1500 rpm. Samples were centrifuged at 3500g and 4 °C for 10 min, and the supernatants evaporated in a speed vacuum concentrator (Savant SPD121P, ThermoScientific, Spain). The evaporated samples were re-dissolved in 250 μL methanol and filtered through a 0.22 μm PVDF filter before injecting into the HPLC-DAD-ESI-IT equipment.Analyses were carried out on an Agilent 1100 HPLC system coupled in series to an ultraviolet-visible diode array detector (UV–Vis DAD) (Agilent Technologies, Waldbronn, Germany) and an ion trap (IT) mass spectrometer detector (model Esquire 1100 equipped with an electrospray interface (ESI)) (Brüker Daltoniks). The separation was achieved on a reverse-phase Poroshell 120 EC-18 column (100 × 3 mm, 2.7 μm particle size) (Agilent Technologies, Waldbronn, Germany), operating at room temperature and a flow rate of 0.5 mL min−1. A volume of 5 μL of the sample was injected. The mobile phases used were water with formic acid (1%) (phase A), and acetonitrile (phase B) and the solvent gradient changed according to the following conditions: 0 min, 1% B, 0 to 15 min, 1–25% B; 15 to 28 min, 25–40% B; 28–30 min, 40–90% B; 30 to 31 min, 90% B; 31 to 32 min, 90 to 1% B and maintained at 1% 5 min. The UV–Vis spectra were acquired in the range of 200 to 550 nm, and the chromatograms were recorded at 360, 320, 305, and 280 nm. In the mass spectrometry detection, nitrogen was used as a drying and nebulizing gas with the following parameters: nebulizer pressure was set at 50 psi, dry gas flow 10 L min−1, and dry gas temperature 350 °C. Mass spectrometry data were acquired in the negative ionization mode, with a capillary voltage of 4 kV. Mass scan (MS) and MS-MS spectra were measured in the range of m/z 100–1100 with a target mass of 400 and compound stability of 75%. The maximum accumulation time of the ion trap was 50 ms. Auto MS-MS mode was applied with the following conditions: width of the isolation, 4.0; fragmentation amplitude, 1.00 V; and the number of parents, 3. The identification of the compounds was carried out through their elution order in the HPLC chromatograms, UV–Vis spectra, molecular weight, their MS-MS fragments, and, whenever possible, chromatographic comparison with authentic standards.
Results
Screening of (poly)phenols conversion
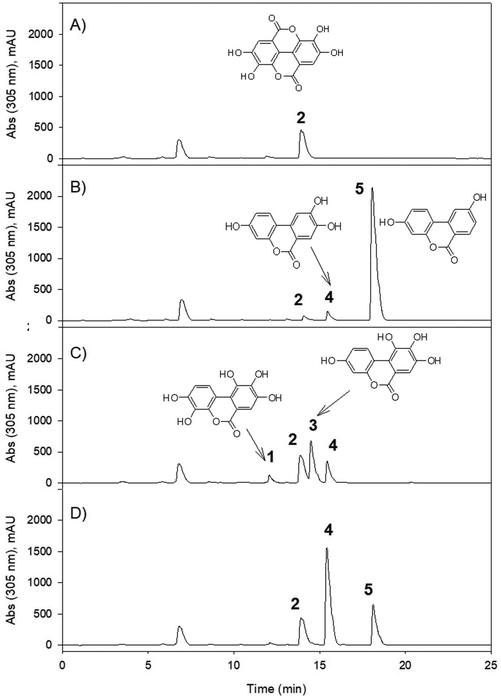
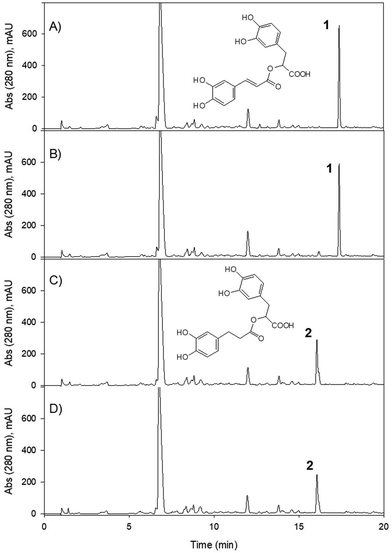
For the majority of the assayed PPs, the chromatographic integration of the PP peaks in the control incubations (PP in medium) and the bacterial incubations (PP with each bacterial strain and with a co-culture of both strains) were the same. The study showed that despite the prolonged exposure of the PP to the bacterial strains (7 days), most of them were not converted into other metabolites. When the chromatographic profiles of the control samples and those incubated with the bacterial isolates were compared at different UV wavelengths, no new peaks were observed (potential new metabolites produced) for most of the PPs. The MS analyses also showed no new metabolites after the incubation. As a positive control, both bacterial strains were active in the conversion of ellagic acid to produce the expected urolithin metabolites (Fig. 2), as was previously reported.6,9,10,12,13G. urolithinfaciens yielded urolithin M5 (3,4,8,9,10 pentahydroxy urolithin), urolithin M6 (3,8,9,10-tetrahydroxy urolithin), and urolithin C (3,8,9-trihydroxy urolithin) while E. isourolithinifaciens transformed ellagic acid into urolithins M5, M6, C, and isourolithin A (3,9-dihydroxy urolithin). The incubations were repeated three times with identical results.For the rest of the incubations with the selected PPs, new metabolites were only found after the incubation of caffeic (3′,4′-dihydroxy-cinnamic acid), dihydrocaffeic [3-(3′,4′-dihydroxyphenyl) propanoic], rosmarinic, and chlorogenic (5-caffeoyl-quinic) acids (Table 1). The study suggested that under the assayed conditions, both strains showed catechol-dehydroxylase, reductase, and lactonase/decarboxylase activities on some of the PPs. However, they did not show demethylase-, glucosidase-, rhamnosidase-, or single hydroxyl dehydroxylase-mediated conversions.
| Phenolics | Catabolic reactions | E. isourolithinifaciens | G. urolithinfaciens | Co-culture |
|---|---|---|---|---|
| 3-HPPA: 3-(3-Hydroxyphenyl)propionic acid. (−) no metabolite produced. Other (poly)phenols tested that were not metabolized under the assayed conditions: coumarins (esculetin, umbelliferone, scoparone), flavonoids (quercetin, catechin, hesperidin, hesperetin, nobiletin, isoxanthohumol), isoflavones (daidzein), lignans (secoisolariciresinol diglucoside), stilbenes (resveratrol), other (oleuropein and hydroxytyrosol). | ||||
| Ellagic acid | Decarboxylation | Urolithin M5 | Urolithin M5 | Urolithin M5 |
| Dehydroxylation | Urolithin C, isourolithin A | Urolithin M6, | Urolithin C | |
| Urolithin C | isourolithin A | |||
| Caffeic acid | Dehydroxylation | m-Coumaric acid | m-Coumaric acid | m-Coumaric acid |
| Reduction | 3-HPPA | 3-HPPA | 3-HPPA | |
| Dihydrocaffeic acid | Dehydroxylation | 3-HPPA | 3-HPPA | 3-HPPA |
| Rosmarinic acid | Reduction | — | Dihydro rosmarinic acid | Dihydro rosmarinic acid |
| Chlorogenic acid | Quinic acid degradation | Methyl caffeate | — | — |
| Reduction | — | Dihydro chlorogenic acid | Dihydro chlorogenic acid | |
| Dehydroxylation | 3-HPPA | 3-HPPA | 3-HPPA | |
G. urolithinfaciens and E. isourolithinifaciens showed different conversion rates on the phenolics that were metabolized by these bacterial species. In Table 2, the percentage of conversion of the phenolic compounds by each bacterial species and the co-culture are included. Interestingly the percentage of conversion of ellagic acid into urolithins was much more relevant in E. isourolithinifaciens (89%) than in G. urolithinfaciens (7%) and in the co-culture (2%). These differences should be further studied to understand better this biochemical process.
| PPs | Ellagibacter isourolithinifaciens | Gordonibacter urolithinfaciens | Co-culture |
|---|---|---|---|
| Ellagic acid | 89 | 7 | 2 |
| Caffeic acid | 100 | 48 | 69 |
| Dihydrocaffeic acid | 100 | 100 | 100 |
| Rosmarinic acid | 8 | 100 | 100 |
| Chlorogenic acid | 65 | 100 | 100 |
Identification of the metabolites
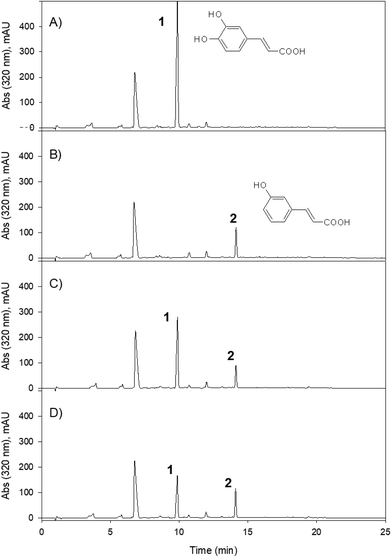
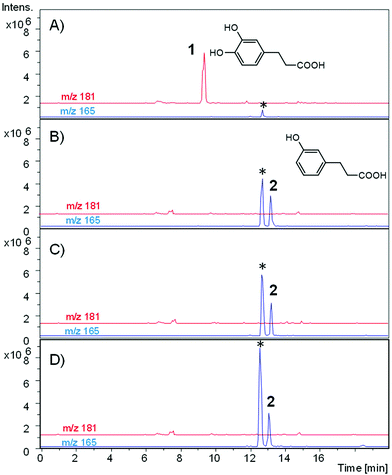
From caffeic acid (Rt 9.84 min), a new metabolite was produced (Rt 13.79) by both bacterial species and the co-culture, and E. isourilithinifaciens was much more active producing the metabolite (100% conversion) than G. urolithinfaciens (48% conversion), as was evident by the remaining caffeic acid after the seven days of incubation (Fig. 3, Table 2). Caffeic acid decreased in the presence of G. urolithinfaciens, although it disappeared entirely in the presence of E. isourilithinifaciens, while a new metabolite appeared as a prominent peak in the chromatogram. The new metabolite had a [M − H]− at m/z 163, with a fragmentation profile 163/119 (–COO), while the fragmentation profile of caffeic acid was 179/135 (–COO). The new metabolite showed a difference of 16 mass units with caffeic acid, suggesting that the metabolite was a dehydroxylated derivative. The samples were also injected into a high-resolution UPLC-ESI-QTOF, and a peak of mass 163.0399 with a molecular formula C9H8O3 consistent with a hydroxy-cinnamic acid was found. Following the two potential dehydroxylations of caffeic acid, the possible metabolites produced from caffeic acid by both strains were p-coumaric acid (4′-hydroxycinnamic acid) and m-coumaric acid (3′-hydroxycinnamic acid). The UV spectrum of the metabolite (UV max nm 278, 316 sh) showed clearly that it was very different from that of p-coumaric acid (UV max nm 290 sh, 310), and the metabolite was confirmed to be m-coumaric acid by chromatographic comparison with an authentic standard. Besides, 3-(3′-hydroxy-phenyl) propanoic acid (3HPP) was also detected after the incubation with both species. However, dihydrocaffeic [3-(3′,4′-dihydroxyphenyl) propanoic acid] was not detected, suggesting that the reductase activity was particularly active on m-coumaric acid as a substrate and not on caffeic acid.Dihydrocaffeic acid [3-(3,4-dihydroxy-phenyl) propanoic acid] eluted at 9.2 min. Both bacterial species were very active to metabolize dihydrocaffeic (Table 2) yielding a metabolite eluting at 12.99 min, with a UV spectrum with maxima at 272, 276 sh nm, and a mass with [M − H]− at m/z 165 consistent with a loss of one hydroxyl from dihydrocaffeic [M − H]− (m/z 181). The chromatographic comparison with 3-hydroxy phenyl propanoic acid (UV 272, 276 sh nm) and 4-hydroxy phenyl propanoic acid (UV max 276 nm), and its UV spectrum clearly showed that the metabolite produced was the 3-hydroxy derivative (Fig. 4).
Rosmarinic acid, a dimeric molecule, including a caffeic acid residue and a dihydrocaffeic acid residue, was not metabolized by these bacterial species to produce dehydroxylated derivatives. The results show that although both species were able to dehydroxylate both caffeic and dihydrocaffeic acids, they were not able to dehydroxylate the dimer, suggesting that the rosmarinic acid molecule was probably too large to interact with the active center of the catechol-dehydroxylase (Fig. 5), or to be transported inside the cell for metabolism.
Rosmarinic acid (Rt 17.39 min) was only slightly metabolized by E. isourolithinifaciens (8% conversion, Table 2). However, it was completely converted (100%) by G. urolithinfaciens into a new metabolite at 16.06 min with a UV maximum at 280 nm and an [M − H]− at m/z 361 suggesting that the hydroxycinnamic residue was converted to a hydroxy-phenyl propanoic acid derivative by a reduction of the double bond (Fig. 5). This agrees with the changes observed in the UV spectrum of rosmarinic acid (328, 298sh nm) to yield a metabolite with UV spectrum with a maximum at 280 nm, and in the addition of two mass units (the two H added, m/z− 361) to the mass of rosmarinic acid (m/z− 359). The hydrogenation activity (reductase) is new for Gordonibacter and deserves further study. It seems that G. urolithinfaciens has a prominent reductase activity, although for E. isourolithinifaciens it does not seem to be a relevant metabolic transformation.
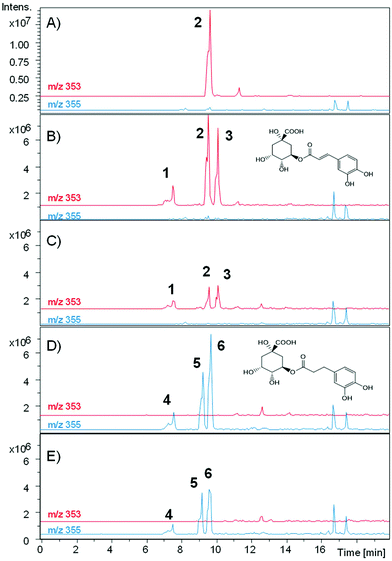
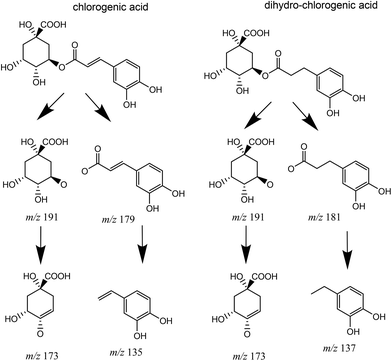
The metabolism of chlorogenic acid (5-caffeoyl-quinic acid) [Rt 9.5 min, [M − H]− at m/z 353, and MS-MS fragment at 191 (quinic acid)] was more complex. Its incubation as a control under the conditions used for conversion, but without the bacterial inoculum, showed that chlorogenic acid was partially isomerized into two different isomers. The isomers showed the same UV spectrum as chlorogenic acid, the same [M − H]− with m/z 353, and similar, although not identical, MS-MS fragments (ESI Fig. 1†). Thus, an isomer at 7.6 min showed MS/MS fragments at 191 (quinic acid), 179 (caffeic acid), and 135 (caffeic-COO). The isomer at 10.0 min showed fragments at 173 (quinic acid –H2O) and 135, and other minor fragments at 191 and 179 (Fig. 6). A similar conversion was reported in a previous study with chlorogenic acid incubation with complex gut microbiota communities from different volunteers,19 and was also reported to occur after incubation at different pH values.20 The Rts of the isomers suggested that they could be 3-caffeoyl quinic and 4-caffeoyl quinic isomers of 5-caffeoyl quinic acid (chlorogenic acid). These metabolites were identified by comparison with a green coffee bean extract,21 using this extract as a surrogate standard.22
After incubation with E. isourolithinifaciens, chlorogenic acid and the isomers were substantially metabolized (65%, Table 2). A new metabolite appeared at 16.4 min, with the same UV spectrum of chlorogenic and caffeic acids and a [M − H]− ion at m/z 193. The UPLC-Q-TOF analysis showed a high-resolution mass of 193.0512 and a molecular formula of C10H10O4. The mass, UV, and retention time suggest that the metabolite could be ferulic acid, isoferulic acid, or methyl caffeate. This conversion was consistently observed in three different incubation experiments. Chromatographic comparisons with authentic standards showed that this was methyl caffeate. This metabolite was only produced in the incubation with E. isourolithinifaciens, and it was not detected in its co-culture with G. urolithinfaciens, when chlorogenic acid was only incubated with G. urolithinfaciens, and in the control. An explanation for the formation of this metabolite is that E. isourolithinifaciens could have the ability to break the carbon–carbon bonds in the quinic acid residue, leaving finally a methyl fragment linked to the acid group of the caffeic acid. In addition, 3-(3-hydroxy-phenyl) propanoic acid (Rt 12.76 min) was also produced and confirmed that E. isourolithinifaciens can dehydroxylate catechol-containing groups and reduce the double bonds.
The incubation with G. urolithinfaciens showed the full conversion of chlorogenic acid and their isomers produced during the incubation (Table 2). It also showed the production of 3-(3-hydroxy-phenyl) propanoic acid (Rt 12.98 min). Three new peaks (Rts 7.4, 9.1, and 9.5 min, with [M − H]− at m/z 355) suggested the reduction of the double bond of the caffeic acid residue of the three caffeoyl-quinic acid isomers (Rts 7.6, 9.5 and 10.0 min) (Fig. 7). This was also consistent with the slight decrease in Rt observed when comparing the chromatographic mobility of caffeic acid with that of dihydrocaffeic acid. The UV spectra of the new metabolites (UV max 280 nm) were also similar to that of dihydrocaffeic acid. The MS analysis showed the production of these metabolites only in the media incubated with G. urolithinfaciens (Fig. 6). The relative amount of the three metabolites also followed the same quantitative trend of the different isomers formed from chlorogenic acid in the incubation medium without bacterial inoculum (Fig. 7). The MS/MS fragments of the different dihydro chlorogenic acids [Rt 7.4, m/z 181 (dihydrocaffeic) and 137 (dihydrocaffeic-COO); Rt 9.1, m/z 181 (low), 173 (quinic acid–H2O); Rt 9.5, 191 (quinic acid), 173 (quinic acid–H2O)] were also consistent with those observed for their precursors (see above) (Fig. 6).
Discussion
The present study confirms the ability of E. isourolithinifaciens and G. urolithinfaciens to convert ellagic acid into isourolithin A (3,9-dihydroxy urolithin), and urolithin C (3,8,9-trihydroxy urolithin), respectively, through successive lactonase and decarboxylase activities, and sequential specific dehydroxylations that remove one hydroxyl group of different catechol moieties.Remarkably, these human gut bacterial species can also catabolize other dietary-relevant phenolic compounds. Thus, E. isourolithinifaciens was able to dehydroxylate some catechol-containing phenolics, such as caffeic and dihydrocaffeic acids, preferentially by removing the hydroxyl group at the para position of the aromatic ring. The dehydroxylation, however, was not observed in related catechol-containing phenolics, such as rosmarinic acid and chlorogenic acid, showing that the size of the molecule can affect the interaction with the enzyme. This could be due to a limited uptake of the substrate by the bacterial strain and(or) by a decreased catalytic efficiency of the enzyme towards the substrates. No dehydroxylation was observed either in other catechol-containing phenolics as quercetin, catechin, oleuropein, and esculetin or in pyrogallol-containing molecules like gallic acid (Fig. 8).
The regioselective removal of the hydroxyl in the p-position of the side chain of the catechol agrees with the metabolic conversions observed after the complex gut microbial communities’ metabolism of catechol-phenylpropanoids produced after the breakdown of most flavonoids,18 and the catechol derivatives produced during the conversion of lignans,17 suggesting that both bacterial strains can be among those gut microbes responsible for this important conversion. A catechol-dehydroxylase for the dehydroxylation of dihydrocaffeic acid (hcdh) has recently been characterized from Egerthella lenta.16
The results showed that E. isourolithinifaciens was also able to convert chlorogenic acid into methyl caffeate, which involved the ring-breakdown of the quinic acid cycle and the removal of carbon–carbon bonds. Something similar was already reported when chlorogenic acid was incubated with human gut microbiota of different volunteers as caffeoyl-glycerol was detected after the fermentation, and suggested that the quinic acid part of the molecule can be partially degraded by the colonic bacterial communities.19 This activity deserves to be studied in future work.
G. urolithinfaciens, although had a catechol-dehydroxylase activity, also had a double-bond reductase activity as it was demonstrated for rosmarinic, chlorogenic, and caffeic acids. However, the double bond reduction was not observed on other phenolics that could potentially be reduced, like resveratrol and the coumarins esculetin, scoparone, and umbelliferone. It seems that this reduction is particularly active on larger molecules. The double-bond reductase activity had not been reported before in this bacterial genus.
Double bond reduction by some gut bacterial species had been reported for relevant biological transformations. Thus, Eggerthella lenta, also a member of the family Eggerthellaceae has been reported to be responsible for converting urocanate to imidazole propionate through the enzyme urocanate reductase, which reduced a double bond. This is a very relevant conversion as the production of imidazole propionate was associated with the development of type 2 diabetes.23E. lenta was also more abundant in subjects with type 2 diabetes. The same enzymatic activity was also observed in Adlercreutzia equolifaciens, another member of the Eggerthellaceae.22E. lenta was also responsible for the reduction of a double bond in digoxin, leading to its inactivation.24,25 In a similar way, Slackia equolifaciens, and A. equolifacients (with lower activity than Slackia), both members of the same family, were reported to be responsible for the conversion of the stilbene resveratrol to dihydro resveratrol.26 Therefore, the double bond reductase activity observed in G. urolithinfaciens is relevant and could be a common feature of the Eggerthellaceae family. However, this activity could have a narrow substrate scope since no reduction of the double bond of resveratrol by G. urolithinfaciens or E. isourolithinfaciens was found in the present study.
The dehydroxylation of catechol-type PPs is a very unusual metabolic feature only observed in anaerobic bacteria occurring in the gastrointestinal tract.16 The enzyme responsible for the dehydroxylation that converts dopamine into m-tyramine, a molybdenum-dependent dehydroxylase (Dadh), has recently been described in E. lenta.15 This conversion, however, was not done by G. pamelaeae, which as well as G. urolithinfaciens and E. isourolithinifaciens, is known to remove hydroxyl groups and to replace them with a hydrogen atom during the catabolism of ellagic acid into urolithins.9 However, Gordonibacter species can also dehydroxylate the lignan intermediate bi-desmethyl-secoisolariciresinol to enable the synthesis of the active metabolite enterodiol,17 and E. lenta is known to open the tetrahydrofuran rings in pinoresinol and lariciresinol leading to secoisolariciresinol.27–29
Our results showed that non-catechol phenolics (such as resveratrol, daidzein, isoxanthohumol, and umbelliferone) were not substrates for the catechol-dehydroxylase activity of both strains. However, the dehydroxylation of monohydroxy phenolics is catalyzed by the xanthine-oxidase related p-hydroxy-benzoyl-CoA reductase, from anaerobic soil bacteria.30 To the best of our knowledge, this activity has not been described in phenolic-metabolizing gut microbial strains so far.
Another relevant enzymatic activity of both bacterial species is the lactone-ring opening and decarboxylation activity that are the first steps in ellagic acid metabolism. This activity was not observed on other phenolics bearing a lactone ring as the coumarins that were not metabolized. Recent studies have demonstrated that this decarboxylase activity to convert ellagic acid into urolithin M5 (3,4,8,9,10-pentahydroxy urolithin) can be completed by cell-free extracts, while the further dehydroxylations to produce urolithin C (3,8,9-trihydroxy urolithin) need the bacterial cells.16,31 The study of this catabolic activity also deserves further research.
Conclusions
In addition to ellagic acid, the urolithin-producing bacterial species G. urolithinfaciens and E. isourolithinifaciens can catabolize other dietary phenolics through the removal of hydroxyls groups from catechol moieties, and also by reducing double bonds. These activities, however, are not promiscuous and are specific in the metabolism of some phenolic compounds. Therefore, these bacterial species can be involved in some relevant steps of the metabolism of dietary phenolics when they are present in the gut bacterial ecology of individuals.The abundance of several genera of the Eggerthellaeaceae family previously reported to be involved in polyphenol metabolism differ widely between individuals.18 Among them, Gordonibacter and Ellagibacter abundances in the human gut vary from 0 to 0.9% with average values of 0.03% and 0.08%, respectively.14
There are large inter-individual differences in the occurrence of both bacterial genera (Gordonibacter and Ellagibacter), and they are differentially distributed in the so-called ‘urolithin metabotypes’. Therefore, they could also contribute to the different responses of individuals to (poly)phenols consumption in randomized controlled trials.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work has been supported by the Projects 19900/GERM/15 (Fundación Séneca, Spain), CSIC 201870E014, and PID2019-103914RB-I00 and AGL2015-73107-EXP (MINECO, Spain).References
- C. Manach, G. Williamson, C. Morand, A. Scalbert and C. Remesy, Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies, Am. J. Clin. Nutr., 2005, 80, 230S–242S CrossRef.
- A. Cortés-Martín, M. V. Selma, F. A. Tomás-Barberán, A. González-Sarrías and J. C. Espín, Where to look into the puzzle of polyphenols and health? The postbiotics and the gut microbiota associated with human metabotypes, Mol. Nutr. Food Res., 2020, e1900952 CrossRef PubMed.
- K. Tsliugiri and M. Rescisno, Postbiotics: What else?, Benefic. Microbes, 2013, 4, 101–107 CrossRef.
- J. N. Malagón-Rojas, A. Mantziari, S. Salminen and H. Szajewska, Postbiotics for preventing and treating common infectious diseases in children: A systematic review, Nutrients, 2020, 12, 389 CrossRef.
- A. González-Sarrías, J. C. Espín and F. A. Tomas-Barberan, Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects, Trends Food Sci. Technol., 2017, 69, 281–288 CrossRef.
- F. A. Tomás-Barberán, A. González-Sarrías, R. García-Villalba, M. A. Núñez-Sánchez, M. V. Selma, M. T. García-Conesa and J. C. Espín, Urolithins, the rescue of ‘old’ metabolites to understand a ‘new’ concept; metabotypes as a nexus between phenolic metabolism, microbiota dysbiosis and host health status, Mol. Nutr. Food Res., 2017, 61, 1500901 CrossRef.
- R. García-Villalba, M. V. Selma, J. C. Espín and F. A. Tomás-Barberán, Identification of novel urolithin metabolites in human feces and urine after the intake of a pomegranate extract, J. Agric. Food Chem., 2019, 67, 11099–11107 CrossRef.
- F. A. Tomás-Barberán, R. García-Villalba, A. González-Sarrías, M. V. Selma and J. C. Espín, Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status, J. Agric. Food Chem., 2014, 62, 6535–6538 CrossRef PubMed.
- M. V. Selma, D. Beltrán, R. García-Villalba, J. C. Espín and F. A. Tomás-Barberán, Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species, Food Funct., 2014, 5, 1779–1784 RSC.
- M. V. Selma, F. A. Tomás-Barberán, D. Beltrán, R. García-Villalba and J. C. Espín, Gordonibacter urolithinfaciens sp. nov., a urolithin-producing bacterium isolated from the human gut, Int. J. Syst. Evol. Microbiol., 2014, 64, 2346–2352 CrossRef CAS PubMed.
- M. V. Selma, M. Romo-Vaquero, R. García-Villalba, A. González-Sarrías, F. A. Tomás-Barberán and J. C. Espín, The human gut microbial ecology associated to overweight and obesity determines ellagic acid metabolism, Food Funct., 2016, 7, 1769–1774 RSC.
- M. V. Selma, D. Beltrán, M. C. Luna, M. Romo-Vaquero, R. García-Villalba, A. Mira, J. C. Espín and F. A. Tomás-Barberán, Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin A from ellagic acid, Front. Microbiol., 2017, 8, 1521 CrossRef PubMed.
- D. Beltrán, M. Romo-Vaquero, J. C. Espín, F. A. Tomás-Barberán and M. V. Selma, Ellagibacter isourolithinifaciens gen. Nov., sp. Nov. a new member of the family Egerthellaceae, isolated from human gut, Int. J. Syst. Evol. Microbiol., 2018, 68, 1707–1712 CrossRef PubMed.
- M. Romo-Vaquero, A. Cortés-Martín, V. Loria-Kohen, A. Ramirez-de-Molina, I. García-Mastrana, M. C. Collado, J. C. Espín and M. V. Selma, Deciphering the human gut microbiome of urolithin metabotypes: Associations with enterotypes and potential cardiometabolic health implications, Mol. Nutr. Food Res., 2019, 63, 1800958 CrossRef.
- V. M. Rekdal, E. N. Bess, J. E. Bisanz, P. J. Turnbaugh and E. P. Balskus, Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism, Science, 2019, 364, 1055 Search PubMed.
- V. M. Rekdal, P. N. Bernardino, M. U. Luescher, S. Kiamehr, C. Le, J. E. Bisanz, P. J. Turnbaugh, E. N. Bess and E. P. Balskus, A widely distributed metalloenzyme class enables gut microbial metabolism of host- and diet-derived catechols, eLife, 2020, 9, e50845, DOI:10.7554/eLife.50845.
- E. N. Bess, J. E. Bisanz, F. Yarza, A. Bustion, B. E. Rich, X. Li, S. Kitamura, E. Kaligurski, Q. Y. Ang, D. L. Alba, P. Spagiannopoulos, S. Nayfach, S. K. Koliwad, D. W. Wolan, A. A. Franke and P. J. Turnbaugh, Genetics basis for the cooperative bioactivation of plant lignans by Eggethella lenta and other human gut bacteria, Nat. Microbiol., 2020, 5, 56–66 CrossRef CAS.
- M. V. Selma, J. C. Espín and F. A. Tomás-Barberán, Interaction between phenolics and gut microbiota: Role in human health, J. Agric. Food Chem., 2009, 57, 6485–6501 CrossRef CAS PubMed.
- F. A. Tomás-Barberán, R. Garcia-Villalba, A. Quartieri, S. Raimondi, A. Amaretti, A. Leonardi and M. Rossi, In vitro transformations of chlorogenic acid by human gut microbiota, Mol. Nutr. Food Res., 2014, 58, 1122–1131 CrossRef.
- S. Deshpande, R. Jaiswal, M. F. Matei and N. Kuhnert, Investigation of acyl migration in mono- and di-caffeoyl quinic acids under aqueous basic, aqueous acid, and dry roasting conditions, J. Agric. Food Chem., 2014, 62, 9160–9170 CrossRef CAS PubMed.
- F. A. Tomás-Barberán, J. Loaiza-Velarde, A. Bonfanti and M. E. Saltveit, Early wound- and ethylene-induced changes in phenylpropanoid metabolism in harvested lettuce, J. Am. Soc. Hortic. Sci., 1997, 122, 399–404 Search PubMed.
- M. N. Clifford and N. E. Madala, Surrogate standards: A cost-effective strategy for identification of phytochemicals, J. Agric. Food Chem., 2017, 65, 3589–3590 CrossRef CAS.
- A. Koh, A. Molinaro, A. Stalman, M. T. Khan, C. Schmidt, L. Manneras-Holm, H. Wu, A. Carreras, H. Jeong, L. E. Olofsson, P. O. Bergh, V. Gerdes, A. Hartstra, M. de Brauw, R. Perkins, M. Nieuwdorp, G. Bergstrom and F. Backhed, Microbially produced imidazole propionate impairs insulin signalling through mTORC1, Cell, 2018, 175, 947–961 CrossRef CAS.
- J. R. Saha, V. P. Butler, M. C. Neu and J. Linderbaum, Digoxin-inactivating bacteria: identification in human gut flora, Science, 1983, 220, 325–327 CrossRef CAS.
- P. Spagianopoulos, E. N. Bess, R. N. Carmody and P. J. Turnbaugh, The microbial pharmacist within us: a metagenomics view of xenobiotic metabolism, Nat. Rev. Microbiol., 2016, 14, 273–287 CrossRef.
- L. M. Bode, D. Bunzel, M. Huch, G. S. Cho, D. Ruhland, M. Bunzel, A. Bub, C. M. Franz and S. E. Kulling, In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota, Am. J. Clin. Nutr., 2013, 97, 295–309 CrossRef CAS.
- K. Struijs, J. P. Vincken and H. Gruppen, Bacterial conversion of secoioslariciresinol and anhydrosecosiolariciresinol, J. Appl. Microbiol., 2009, 107, 308–317 CrossRef CAS PubMed.
- T. Clavel, G. Henderson, W. Engst, J. Doré and M. Blaut, Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside, FEMS Microbiol. Ecol., 2006, 55, 471–478 CrossRef CAS PubMed.
- J. S. Jin, Y. F. Zhao, N. Nakamura, T. Aka, N. Kakiuchi, B. S. Min and M. Hattori, Enantioselective degradation of enterodiol and enterolactone precursors by human intestinal bacteria, Biol. Pharm. Bull., 2007, 30, 2113–2119 CrossRef CAS.
- M. Unciuleac, E. Warkentin, C. C. Page, M. Ball and U. Ermler, Structure of a xanthine oxidase-mediated 4-hydroxybenzoyl-CoA reductase with an additional [4Fe-4S] cluster and an inverted electron flow, Structure, 2004, 12, 2249–2256 CrossRef CAS.
- H. Watanabe, S. Kishino, M. Kudoh, H. Yamamoto and J. Ogawa, Evaluation of electron-transferring cofactor mediating enzyme systems involved in urolithin dehydroxylation in Gordonibacter urolithinfaciens DSM 27213, J. Biosci. Bioeng., 2020, 129, 552–557 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0fo01649g |
| This journal is © The Royal Society of Chemistry 2020 |