The pseudo-capacitive deionization behaviour of CuAl-mixed metal oxides†
Abstract
In this work, a laminated CuAl-mixed metal oxide (CuAl-LDO) has been prepared and proposed as an electrode to interact with chloride ions for pseudo-capacitive deionization (P-CDI). To constitute a full P-CDI cell, CuAl-LDO and HNO3-treated activated carbon (AC-HNO3) were employed as the anode and cathode, respectively. When operating in constant voltage mode, the CuAl-LDO‖AC-HNO3 P-CDI cell shows a high salt removal capacity of 39.08 mg g−1 in NaCl solution with an initial concentration of 500 mg L−1 at an applied voltage of 1.2 V after 20 cycles. Meanwhile, the charge efficiency is calculated to be 80.72%. Moreover, the crystal phase evolution of CuAl-LDO as well as corresponding electrochemical spectra recorded during the P-CDI process illustrated that chloride ions intercalated into the CuAl-LDO electrode via chemical bonds, resulting in good P-CDI performance. Besides, it is found that Cu(OH,Cl)2·2H2O may be formed as the number of P-CDI cycles increases, which is responsible for the salt removal capacity decay after long-term cycling.
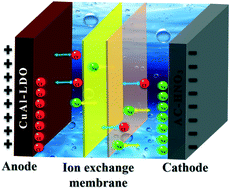
- This article is part of the themed collections: Environmental Science: Water Research & Technology Cover Art and Capacitive deionisation and electrosorption 2020


 Please wait while we load your content...
Please wait while we load your content...