The canonical behavior of the entropic component of thermodynamic effective molarity. An attempt at unifying covalent and noncovalent cyclizations
Abstract
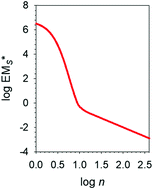
This review article is concerned with the measurement, significance and applications of the concept of effective molarity (EM) in a large variety of cyclization reactions ranging from the formation of giant macromolecules in polymeric equilibrates to the self-assembly of cyclic supermolecules and supramolecular aggregates. Based on a dissection of EM into enthalpic and entropic components (EM = EMH × EMS), a careful examination of a large number of often overlooked quantitative studies of reversible cyclizations led to the definition of a set of “canonical” values of the entropic component EMS, expressed in graphical form by the plot of EMS* vs. n, where the asterisk denotes statistically corrected quantities, and n is the number of single bonds in the ring product. It is proposed that, to a useful approximation, all cyclization reactions comply with the “canonical” EMS* values, independent of the chemical nature of end groups and of the intervening chain, but solely dependent on the number n of rotatable bonds. The entropic component EMS* is approximately the same for cyclizations carried out under kinetic or thermodynamic control, and does not appear to be altered to a very significant extent by replacement of covalent with noncovalent bonds.
- This article is part of the themed collections: 2019 PCCP HOT Articles and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...