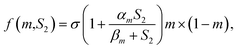Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biological properties of calcium phosphate biomaterials for bone repair: a review
Jingyi Lu
 abc,
Huijun Yu
*abc and
Chuanzhong Chen
*ad
abc,
Huijun Yu
*abc and
Chuanzhong Chen
*ad
aShenzhen Research Institute of Shandong University, Shenzhen 518057, Guangdong, P. R. China. E-mail: yhj2001@sdu.edu.cn; czchen@sdu.edu.cn; Fax: +86 531 88395991; Tel: +86 531 88395991
bKey Laboratory of High-Efficiency and Clean Mechanical Manufacture (Shandong University), Ministry of Education, School of Mechanical Engineering, Shandong University, Ji'nan 250061, Shandong, P. R. China
cNational Demonstration Center for Experimental Mechanical Engineering Education (Shandong University), School of Mechanical Engineering, Shandong University, Ji'nan 250061, Shandong, P. R. China
dKey Laboratory for Liquid-Solid Structural Evolution and Processing of Materials (Ministry of Education), School of Materials Science and Engineering, Shandong University, Ji'nan 250061, Shandong, P. R. China
First published on 9th January 2018
Abstract
Bone defects are a common disease threatening the health of many people. Calcium phosphate (CaP) is an ideal bone substitutive material that is widely used for bone repair due to its excellent biological properties including osteoinductivity, osteoconductivity and biodegradability. For this reason, investigation of these properties and the effects of various influencing factors is vital for modulating calcium phosphate during the design process to maximally satisfy clinical requirements. In this study, the latest studies on the biological properties of CaP biomaterials, including hydroxyapatite (HA), tricalcium phosphate (TCP), and biphasic calcium phosphate (BCP), have been summarized. Moreover, recent advances on how these properties are altered by different factors are reviewed. Considering the limited mechanical strength of CaP materials, this study also reviews CaP composites with different materials as improvement measures. Finally, perspectives regarding future developments of CaP materials are also provided.
1. Introduction
Nowadays, bone defects are among the most common diseases in clinical orthopedics and are mainly caused by infections, defects, tumors, and congenital diseases.1 Generally, these defects need bone grafts since they cannot heal by themselves, and inappropriate treatment can lead to death or invalidity.2 Researchers have, therefore, tried to find novel materials for bone repair and substitution.Autologous bone is still the most commonly used bone material, but it is accompanied by some limitations such as requiring additional surgical incisions.3,4 Even though the allograft or heterogeneous bone has a richer source than autogenous bone, it lacks osteogenetic potential and may be rejected or may spread disease and are therefore not ideal candidates for bone repair.5 Moreover, metal scaffolds and polymeric materials lack bioactivity, even if they have shown great potential regarding mechanical properties.4,6 In magnesium implants, for example, the rapid degradation rate in vivo has become the main limitation hindering their applications;7 thus, they cannot be considered as prominent candidates for use in bone reconstruction.
Recently, calcium phosphate materials have been gradually attracting significant attention due to their excellent abilities to induce osteoblast differentiation into bone cells,8,9 causing them to grow on the surface of scaffolds10,11 and degrade at proper rates,12,13 and even be totally replaced by newly formed bone tissue, in addition to withstanding stress at the defect site.14 Within the calcium phosphate family, HA (hydroxyapatite) has been widely studied since the 1980s,15 and its inorganic composition is similar to that of natural bone. The crystalline network of the stoichiometric HA can be described as a compact assemblage of tetrahedral PO4 groups, where P5+ ions are in the center of the tetrahedrons and whose tops are occupied by 4 oxygen atoms. Each PO4 tetrahedron is shared by a column and delimits two types of unconnected channels.16 The most common ways to prepare HA are by the solution-precipitation method17–19 and the sol–gel method.20,21 Endowed with a composition and appropriate porosity similar to natural bone, HA exhibits great osteoconductivity and vasculogenesis, thus serving as an outstanding material for achieving strong bonds with the host bone, and prompting vascular formation; however, its poor mechanical properties hinder its applications in load-bearing circumstances.
As another extensively studied form of calcium phosphate, tricalcium phosphate (TCP) has three polycrystalline morphologies: α, β and ά, which exist at different temperatures and exhibit great bioactivity as well as high degradability.22 ά lacks practical interest because it only exists at temperatures >1430 °C and reverts almost instantaneously to α-TCP on cooling below the transition temperature. In contrast, β-TCP is stable at room temperature and transforms reconstructively at ∼1125 °C to α-TCP, which can be retained during cooling to room temperature.23 α-TCP has better solubility than β-TCP in aqueous solutions24 and it is not commonly used for the preparation of bioceramics, but is used in the preparations of calcium phosphate bone cement and calcium phosphate composite ceramics.25 β-TCP is a metastable phase that occurs during the cooling process, which shows high stability at room temperature and has excellent bioactivity properties, especially in inducing apatite deposition.23 Jarcho et al.26 reported that the solubility of TCP is 22.3 times that of HA in alkaline solution. As for mechanical properties, they can be altered by various factors such as porosity, sintering temperature and the shape of the CaP scaffold; for example, the compressive strength of the HTCP (hierarchically porous tricalcium phosphate) scaffold is 5.06 ± 1.21 MPa,27 whereas a porous TCP with porosities of 82% and 49% are 0.4 MPa and 40 MPa, respectively.28 To make it clear, some major properties of HA and TCP are reviewed in Table 1.
| Material | Stoichiometry | Crystallography | Ca/P ratio | Solubility at 25 °C | |
|---|---|---|---|---|---|
−log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ks Ks |
g l−1 | ||||
| HA | Ca10(PO4)6(OH)2 | Hexagonal | 1.67 | 116.8 | 0.00010 |
| β-TCP | β-Ca3(PO4)2 | Rhombohedral | 1.50 | 28.9 | 0.20 |
| α-TCP | α-Ca3(PO4)2 | Monoclinic | 1.50 | 25.5 | 0.97 |
| ά-TCP | ά-Ca3(PO4)2 | Hexagonal | 1.50 | 25.5 | 0.97 |
There is another important type of calcium phosphate called biphasic calcium phosphate (BCP), consisting of HA and TCP. Due to the advantages of BCP achieved by adjusting the ratio of HA/TCP, the degradation rate can be modified to match the degree of new bone formation.31,32
It is important for researchers to investigate the biological abilities of scaffolds, since both the general and detailed conditions of bone repair are reflected by osteogenic activity.8 One of the most crucial biological properties is osteoinductivity, which is normally used to demonstrate the ability of the implant to induce bone growth in an ectopic site. The mechanism of inducing osteogenesis is that Ca and P ions existing in body fluids gather on the surface of the implant and form an ion layer to integrate with proteins close to the bone cells; therefore, the implant material is able to firmly connect with the host through this ion layer.33 In this case, a deep investigation of the interaction between the material surface and various proteins and cells can lead us to a better understanding of the osteoinduction process as well as its requirements for an ideal biomaterial. Unlike osteoinduction, osteoconduction is a phenomenon where after bioactive materials are implanted into the bone environment, bone tissue will grow along the surface or internal pore of the implant. Therefore, proper porosity is desirable for achieving great osteoconductivity by providing space for cells to crawl and grow through the channel inside the scaffold. Osteoconductivity is usually reflected in bone coverage measurement, which can be achieved by SEM analysis and micro-computed tomography.34 Biodegradability refers to the biological property of a material dissolving with time after implantation into the body, accompanied by the decrease of mechanical properties of the implanted materials.35 So far, most of the research has concentrated on understanding how the physiological environment changes the material. It is equally important to understand how the surrounding cells react to the degrading material.
Researchers are able to modulate the performance of bone substitute material by studying the influence of various factors on these biological properties. For instance, different porosities of calcium phosphate scaffolds lead to various degrees of healing of segmental bone,36,37 degradation of materials38 and drug release kinetics.39 The optimization of the phase composition is thought to improve the osteoinductivity of the CaP ceramics, thus helping the restoration of the bone defect. In this case, a combination of the influences of the above factors is usually necessary, as is an assessment of whether each factor needs to be analyzed separately, or if a combined analysis is reasonable.
The factors that considerably influence the biological properties of CaP materials have not been systematically investigated in previous reports. Therefore, in this paper we discuss several important biological properties including osteoinduction, osteoconduction and biodegradation of calcium phosphate materials as well as their influencing factors from several aspects. Some improvements made by researchers to overcome the limitations of CaP in their mechanical properties are also reviewed.
2. Biological properties of calcium phosphate biomaterials
Calcium phosphate biomaterials are attracting attention in the field of bone regeneration, particularly due to their excellent biological properties. As aforementioned, the highly complex environment of the body requires that calcium phosphate biomaterial must be able to degrade at a suitable rate to achieve a balance with the regeneration of new bone tissue, and simultaneously provide an ideal place for cell growth. Generally, there are three main properties of calcium phosphate materials that have been widely studied by researchers.2.1. Osteoinductivity
Osteoinduction implies the recruitment of immature cells and the stimulation of these cells to develop into preosteoblasts. In a bone healing situation such as that from a fracture, the majority of bone healing is dependent on osteoinduction.40Interestingly, some osteogenic agents including TGB-β, BMPs, Wnt and other growth factors via related signaling pathways are fundamentally important throughout this osteoinduction process. They exhibit versatile regulatory functions in modulating signal transduction, gene expression and osteoblastic differentiation of various cells.
Transforming growth factor-beta (TGF-β)/bone morphogenic protein (BMP) signaling is involved in the vast majority of cellular processes and is fundamentally important throughout life. TGF-β/BMPs have widely recognized roles in bone formation during mammalian development and exhibit versatile regulatory functions in the body.41
The transforming growth factor-beta (TGF-β) superfamily is comprised of over forty members, such as TGF-βs, nodal, activin, and bone morphogenetic proteins (BMPs). TGF-β signaling first transmits signals across the plasma membrane through the formation of heteromeric complexes of specific type I and type II serine/threonine kinase receptors. The type I receptor is phosphorylated following the activation of specific type II receptors. Activated type I receptors initiate intracellular signaling through phosphorylation of specific Smad proteins, R-Smads. Activated R-Smads form a complex with co-Smad and Smad4 and then translocate into the nucleus to direct the transcriptional response (Fig. 1).42
 | ||
| Fig. 1 TGF-β signaling and negative regulation in bone formation. Reprinted with permission from ref. 41 (Copyright © 2012, IVYSPRING INTERNATIONAL PUBLISHER). | ||
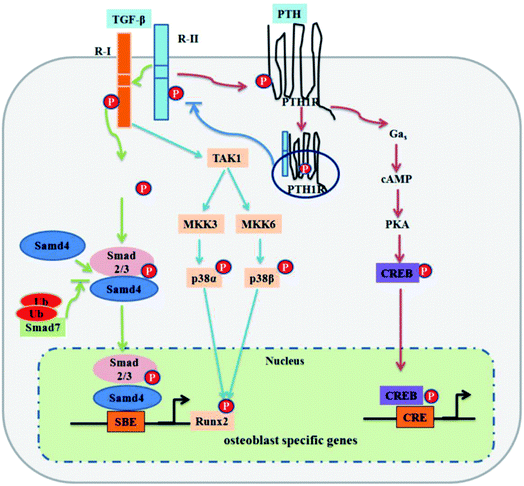
There is another signal molecule called Wnt, which is capable of modulating new bone formation alone or synergistically with BMP via its two kinds of signaling pathways: canonical and noncanonical pathways (signaling pathway can be seen in Fig. 2).43 Wnts are a family of secreted glycoproteins that regulate many processes in skeletal development. Wnt proteins bind to their cognate receptor frizzled (Fz) and LRP-5/6 co-receptors, and activate distinct signaling pathways, including the canonical β-catenin pathway. In the absence of Wnt signaling, β-catenin is degraded by the proteasome system after GSK3β dependent phosphorylation. In the presence of Wnt signaling, unphosphorylated β-catenin accumulates in the cytoplasm and translocates into the nucleus where it associates with Tcf/LEF transcription factors to regulate the expression of target genes.44 Canonical Wnt signaling stabilizes nuclear β-catenin levels and targets gene activation, whereas non-canonical Wnt signaling activates c-Jun N-terminal kinase (JNK) or calcium/calmodulin-dependent kinase 2, which results in convergent extension movements and cellular polarization.45,46
 | ||
| Fig. 2 Extracellular regulators of the Wnt signaling pathway: (A) Wnt ligands use diverse co-receptors to activate and modulate different downstream signals in the Wnt signaling pathway. (B) After the binding of Wnt ligands to the frizzled receptor and LRPs co-receptors, Wnt signaling is activated and causes the transcription of gene targets. (C) Models of Wnt signaling inhibition. Reprinted with permission from ref. 46 (Copyright © 2017, Elsevier). | ||
Many studies have demonstrated the vital effect of the Wnt signaling pathway on osteogenesis.47,48 For example, the deletion of mouse β-catenin in early mesenchymal precursors results in the loss of Runx2 and Sp7 expression and a corresponding failure of bone formation, suggesting that osteogenesis requires Wnt/β-catenin signaling.49 According to Okamoto et al.,50 Wnt5a-induced noncanonical signaling cooperates with Wnt/beta-catenin signaling to achieve proper bone formation, since noncanonical Wnt5a is proved to enhance Wnt/beta-catenin signaling during osteoblastogenesis. Besides, according to Zhang et al.,44 BMP9 and Wnt3A may act synergistically to induce osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Using an in vivo stem cell implantation assay, they found that while BMP9-transduced SCAPs induce robust ectopic bone formation, SCAPs stimulated with both BMP9 and Wnt3A exhibit more mature and highly mineralized trabecular bone formation. It is conceivable that TGF-β, BMPs and/or Wnt3A may be explored as efficacious biofactors for odontogenic regeneration and tooth engineering.
Wnt3a reduced osteoclast formation when applied to early bone-marrow macrophage (BMM) osteoclast differentiation cultures, whereas late addition did not suppress osteoclast formation. Results indicate that Wnt3a directly suppresses osteoclast differentiation through both canonical (β-catenin) and noncanonical (cAMP/PKA) pathways in osteoclast precursors, osteoblast numbers as well as trabecular bone mass.51 This is opposite to the conclusion of previously published studies that the Wnt signaling pathway promotes osteoblastic proliferation.52 Thus, the specific mechanism related to the complexity of various signaling pathways still needs to be explored.
Unlike most metallic materials lacking osteogenesis ability, some calcium phosphate ceramics have been reported to induce bone formation in ectopic sites without adding any more osteogenic agents (e.g., TGF, BMPs or Wnt) to the material. This phenomenon is called “osteoinduction,” and the capacity for osteoinduction is called “osteoinductivity” or “osteoinductive potential”. Normally, whether a material is bone induced depends on whether it can still function well, even in the situation of ectopic implantation such as subcutaneous injection53 and intramuscular implantation.54
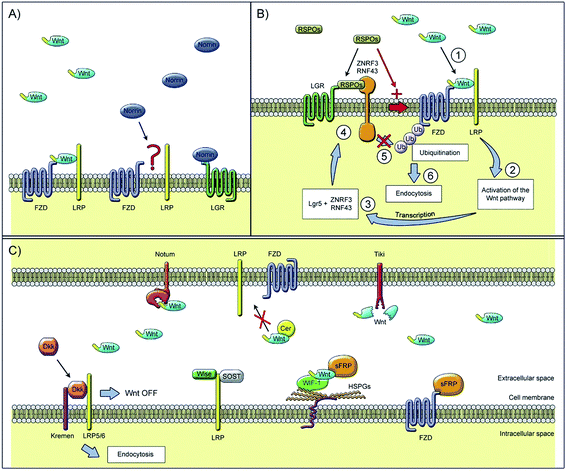
Among numerous biomaterials, calcium phosphate materials, such as HA, TCP and BCP have been regarded as prominent candidates for bone reconstruction due to their great osteoinductivity, which is of importance for the adsorption and differentiation of mesenchymal cells into osteoblasts and osteocytes, further enhancing the vascular formation as well as bone tissue regeneration.55,56 With osteoinductive ability, these CaP ceramics were able to achieve better bone regeneration in vivo, even in a critical size defect, without the addition of cells or growth factors.5 Implanting porous nano-crystalline HA into the back region of mini pigs18 was found to result in a phenomenon of ectopic osteogenesis characterized by the formation of bone-like structures and tendon-like structures with bone marrow and focal chondrogenesis; bone formation was better in the subcutaneous than in the intramuscular implantation sites.57 TCP microspheres have excellent bioactivity properties, specifically in inducing apatite deposition,22 which can achieve selective absorption of serum protein so as to enhance the adhesion of osteoblasts and the secretion of collagen fibers.33,58 The BCP scaffold can provide a suitable environment for the differentiation and activity of osteoblasts according to the extracellular matrix secreted by osteoblasts, observed in the pores of hydroxyapatite/tricalcium phosphate scaffold, which can be seen as an important marker for osteogenesis induction (Fig. 3).59
 | ||
| Fig. 3 Scanning electron microscopy technique: (a) stem cells adhered in the pores of the scaffold and (b) extracellular matrix as the deposition of granular products secreted by differentiated osteoblasts in the pores of the hydroxyapatite/tricalcium phosphate scaffold. Reprinted with permission from ref. 59 (Copyright © 2016, Wolters Kluwer India Pvt. Ltd). | ||
Based on the extensive research work surrounding these biomaterials, the intriguing phenomena have been categorized as those influenced by material factors, and those by biological factors. It is well known that osteoinduction is highly dependent on several material parameters including porosity, granule size, phase composition and even sintering temperature, which will be discussed in Section 3.
As for biological factors, the osteoinduction of calcium phosphate reportedly varies with different animals including dogs, mice, rabbits and pigs. Interestingly, the same material may exhibit discrepancies on different animals. Cheng et al.14 compared the osteoinductivity of calcium phosphate ceramics in four kinds of animals. Results showed that CaP ceramics have good biocompatibility and biological safety, and the degree of ease of osteogenesis was as follows: mouse > dog > rabbit > rat.
Based on conditions mentioned before, graft materials played a significant role in inducing bone cells to grow and differentiate, but the mechanism of osteoinduction was not well explored, including the source of stem cells, the type of signaling molecules and transforming factors, since most of the molecular biotechniques were only based on rodents.8 Thus, it is essential for researchers to focus their studies of osteoinduction assessment on large animals, continuing to detail the mechanisms of osteoinduction on a molecular level.
2.2. Osteoconductibility
There is a phenomenon where after implanting bioactive materials into a bone environment, bone tissue will grow along the surface or internal pore of the implant, which is called bone conduction.The most common and effective way to characterize the osteoconductivity of biomaterials is using bone coverage measurement, which can be induced from SEM images and biopsy analysis including micro-computed tomography, non-calcified histology and histomorphometry.34 The more tissue and bone cells that are grown on substitute implants, the better will be the osteoconductivity.
Recently, Fern et al.60 established a constitutive equation that is able to simulate the osteoconduction model based on a numerical analysis. This reaction–diffusion equation incorporates mass matrix, osteogenic cells and osteoblasts; growth factors considering these variables are closely related to multiphysics interactions associated with the bone-implant interface. Let m, S2 ∈ R be the density of osteogenic cells and the concentration of osteogenic growth factors (BMPs, TGB-β), respectively. For the density of the osteogenic cells, m, the flux is modelled with a linear diffusion term and a linear chemotaxis term along the gradient of the growth factor S2; the kinetics is represented by a proliferative term consisting of a logistic growth with a natural linear rate, and there is a linear term related to the differentiation into osteoblasts and natural cell death such that
Various factors have effects on this osteoconduction process. As mentioned in Section 3.1, osteoconduction is influenced by material-dependent factors as well as the conditions of defect sites. For instance, bone conduction is seen as a characteristic of biomaterials that are not regarded as ideal materials from the point of view of biocompatibility, e.g., stainless steel and titanium,61 but as biomaterials having the ability to acquire a particular morphology with proper inner structure and toughness to provide ideal placement for cell events. According to the results of Yu et al.,62 different channel sizes induce different vascularization (Fig. 4): in the porous CaP scaffold, the channel with the size of 250 pm increases the expression of the representative angiogenic factors HIF1 alpha (hypoxia-inducible factor 1), PLGF (placental growth factor) and migration factor CXCR4 (C-X-C chemokine receptor type 4), which promote the formation of small vessels, while the channel with the size of 500 pm enhances VEGF-A (vascular endothelial growth factor A) expression, which benefits the development of large vessels. Not only does the size of the interconnecting channels have an obvious effect on tissue formation, but it is thought that macroporosity (pores > 50 μm) determines cell colonization and accordingly, the growth of vascular and bone tissue, while the microporosity (<50 μm) of bioceramics increases its protein adsorption, which can in turn determine cell fate.
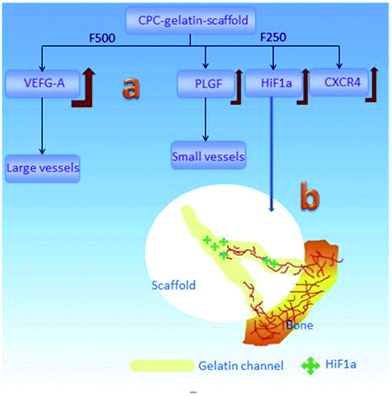 | ||
| Fig. 4 Schematic of the vascularization strategy within the channels of the CaP scaffold: (a) different channel diameters induced different expression behaviors for growth factors and then induced the different vessel formation; (b) the gradually-increased HIF1α expression in the channels induced the in-growth of blood vessels into its host. Reprinted with permission from ref. 62 (Copyright © 2016, Elsevier). | ||
On the other hand, bone growth depends on the conditions of the defect sites, such as the action of differentiated bone cells, which may originate in osteoblasts activated by trauma, or in cells recruited from primitive mesenchymal cells by osteoinduction.63 As suggested in the results of Johari et al.,64 who prepared a cell-seeded scaffold for the repair of calvarial bone with defects, and a blank scaffold as comparison, the number of new cells stained by H&E (hematoxylin and eosin) in cell-seeded grafts was significantly higher than the defect filled with blank scaffold.
Results have shown that calcium phosphate appears to have the advantages of good osteoconductivity and has been investigated in the clinical treatment of rabbits,65,66 dogs11,62,67,68 and rats,69,70 Yu et al.62 implanted CaP ceramics with interconnected channels into the defect sites of rats; the results indicated that the in-growth of blood vessels was observed in the border of the scaffold. nHA-coated BCP ceramics seeded with mesenchymal stem cells (MSCs) were prepared and shown to enhance the formation of new bone tissue in the BCP ceramics after being implanted into rabbits for 12 weeks. Based on the references collected in this paper, it can be concluded that calcium phosphate functions well in human patients. According to Friedmann et al.,71 five patients benefited from three augmentation regimens, the percentage of bone coverage of graft particles for all biopsies ranged from 27.83% to 80.17% (average at 55.39%), which indicated that a close contact between the graft particles and newly formed bone had been achieved. Yang et al.72 demonstrated that the metal implant coated with the CaP layer promotes migration of osteoprogenitor cells along its surface, and thus accelerates osteogenesis in relation to implants so coated. Still, only a few studies have been reported about in vivo osteoconduction of CaP used for human patients, thus, CaP osteoconductibility in human cases still needs to be assessed and characterized with suitable approaches.
2.3. Biodegradability
Biodegradable material will dissolve with time after being implanted in the body, accompanying a decrease in mechanical properties of the implanted materials; the loads will gradually transfer from the implants to human bones and soft tissues to avoid the stress shielding effect.There are two kinds of explanations for the mechanism of calcium phosphate biodegradability, namely, “dispersing material into particles” and “dissolving material into ions”. The former concept is on the basis that implanted material is first dispersed into tiny particles or debris until the debris is transferred by phagocytes or osteoclasts;73 the latter concept is based on the premise that the implanted material dissolves and releases Ca2+ and HPO42−, which are then absorbed by the cells for bone repair and reconstruction, and the formation of new bone.74 Subsequently, it was found that these two kinds of mechanisms seem to be able to coexist in the process of osteogenesis, and may be applied to different conditions according to different environments and materials.
Sheikh et al.75 summarized the degradation process of implanted materials as three reactions (physical, chemical, biological response) and two stages (stage 1: early dissolution; stage 2: cell-mediated absorption).
The physical reaction is the process of degradation via dissolving of materials, where the biomaterial surface transforms into bone-like apatite through dissolving, deposition, ion exchange and a series of reactions before the material finally collapses into tiny particles; during this period, mechanical strength and density remarkably decrease.
For the biological response, the degradation and absorption of material involve the mutual participation of cells including osteoclasts,73,76,77 osteoblasts, fibroblasts, macrophages78 and multinucleated giant cells (Fig. 5).79 During bone injury, monocytes and macrophages play diverse roles in repair, modulating the acute inflammatory response, producing growth factors such as BMP-2 and PDGF-BB, and inducing osteogenesis of mesenchymal progenitor cells. Osteoclasts are giant multinucleated bone-resorbing cells differentiated from precursors of the monocyte/macrophage lineage. Their bone resorbing activity is essential for controlling bone development as well as calcium homeostasis.77 Osteoblasts and fibroblasts are generally employed after receiving the growing factors released by monocytes/macrophages, and play a significant role in forming new bone tissue.59,80
 | ||
| Fig. 5 Osteoclasts function on the surface of CaP. Reprinted with permission from ref. 80 (Copyright © 2017, Elsevier). | ||
Two important properties of an ideal bioactive bone substitution biomaterial are reportedly thought to be exhibiting the same biomechanical competence and regenerating in the same fashion as autologous bone.73 This requires materials to degrade at the same speed at which osteoblasts lay down new bone on their surfaces, until the material is completely replaced by new, living bone.81 Ideally, controlled degradation of a biomaterial leads to the consecutive loss of the mechanical strength of the device, which in turn leads to slowly rising forces in the healing tissue, thereby enhancing the healing process and avoiding the unwanted consequences such as stress shielding.
Many biomaterials have been investigated to prove whether they can be employed as absorbable implants. Numerous metals and polymers35 cannot degrade properly with time after implantation, while calcium phosphate, especially TCP, has excellent biodegradability. It has been reported that soaking the calcium phosphate cement in DMEM (Dulbecco's minimum essential medium) for an hour results in the precipitated concentration of phosphate ions peaking at (4.380 ± 0.019) mmol L−1, calcium ions increase slowly to (1.690 ± 0.064) mmol L−1 after 6 h. According to the law of cell proliferation, only when the concentrations of phosphorus and calcium ions become stable at a certain value can the proliferation of cells reach the best state. Thus, controlling the solubility of the substitute bone material in the body is vital both for bone repair and strength enhancement.
Additionally, Wang et al.82 recently confirmed the correlation between the biodegradation and osteoinductive capacity of BCP ceramic. The ceramic itself and its degradation products can induce macrophages to express and secrete various signaling molecules (TNF-α, IL-6, MCP-1, MCP-1a, MCP-1b and MDC), which then recruit and promote the MSCs to differentiate into osteoblasts. Other studies also mentioned this relation. For example, Wang et al.83 found that the pro-inflammatory cytokines including TNF-α and IL-6 were less expressed and the bone repair related cytokine of TGF-β1 was up-regulated by macrophages in MCPC (magnesium–calcium phosphate cement) extract. However, there are few detailed mechanisms and much complexity between osteoinduction and biodegradation, which need to be addressed in the future in order to design promising scaffolds for bone repair.
3. The factors influencing biological properties
The biological performance of calcium phosphate bioceramics varies with the nature of the material itself, the processing technology and the changes in the external environment. The most important impact on the biological properties of calcium phosphate bioceramics is caused by the intrinsic properties of the material, which are relatively easy to measure and analyse, according to a large number of relevant reports available for study. The influence of the external environment is more complicated, since fluid composition is too complex to control. Given that studying the effect of a particular component usually causes changes in the other ingredients, the effects of the external factors on the properties of calcium phosphate have not been systematically studied.3.1. Intrinsic factors
The CaP phase always varies with its Ca/P ratio. Zhang et al.84 obtained calcium phosphate cement with different Ca/P molar ratios (1.50, 1.60, 1.67, 1.80) by adding CaCl2 to increase the concentration of Ca in the calcium phosphate cement. Ergun et al.85 found that for the samples with Ca/P ratios of 0.5 and 0.75, TCP and Ca2P2O7 phases were observed, while those with ratios of 1.5 and 1.6 had TCP and HA, respectively, as their dominant phases. In terms of the BCP samples, HA50TCP50 and HA100TCP0 have Ca/P ratios of 1.59 and 1.67, respectively.86 Moreover, the experiment done by Ergun et al.85 showed that higher Ca/P ratios (up to 2.5) could enhance increased osteoblast adhesion on calcium phosphate, and the interactions of human bone marrow mesenchymal stem cells (BMSCs) with the Ca–P samples.
The optimization of the phase composition is thought to improve the osteoinductivity and other biological abilities of CaP ceramics, thus helping the restoration of the bone defect. Wang et al.87 found that the group of BCP with 30% HA and 70% TCP promoted the highest expression of BMP-2 and then showed the strongest osteoinduction in mice, compared to several other groups with different phase compositions. HA50TCP50 (Ca/P ratio at 1.59) markedly enhanced cell spreading, proliferation and expression of the extracellular matrix (ECM) genes such as α-smooth muscle actin (α-SMA) and fibronectin (FN), compared with HA100TCP0 (Ca/P = 1.67).86 It was also reported that the ceramics with BCP (HA/β-TCP = 9/1) can cause bone-like apatite to form in shorter immersion time, and with the increase of the β-TCP amount, the bone-like apatite formation is easier.88 Besides, it was demonstrated that the degradation rate of the carrier and the drug release kinetics could be made tunable within the time scale of 1–2 h for the most soluble CaP phase, monocalcium phosphate (Ca(H2PO4)2), compared to 1–2 years for the least soluble one, HA. From the standpoint of antibiotic therapy for osteomyelitis, typically lasting for 6 weeks, the most promising CaP powder was amorphous CaP.89 Similarly, Chen et al.90 employed amorphous CaP as an effective carrier, loading it with IgY molecules (chicken immunoglobulin Y), which indicated the potential applications in dental care, especially in the prevention and treatment of dental caries. Results showed that the nanospheres exhibited significant antibacterial activity against Streptococcus mutans (S. mutans), and the as-prepared ACP nanospheres showed a relatively high IgY protein adsorption ability and sustained release behavior.
A common way to obtain a porous structure in calcium phosphate is by the sacrificial template method. During this method, slurry is absorbed into a porous template (such as polyurethane sponge91–93 or micron/nano-sized graphite94) with desired porosity, then it is dried into a solid template for a certain time, followed by sintering the template until it is burnt away, leaving the desired porous structure.
Calcium phosphate porosity plays a significant role in modulating its biological properties including degradation, osteoconductivity and osteoinductivity. Woodard et al.95 suggested that the cause of a greater decrease in the strength of MP scaffolds (CaP with microporosity and macroporosity) after implantation, in comparison to the NMP (CaP only with macroporosity) scaffolds may be the greater osteoclast-induced degradation at the chemically reactive grain boundaries than that of NMP. Kasuya et al.96 prepared CPC (calcium phosphate ceramic) mixed with gelatin powder to create different porosities at 10% (C10) and 15% (C15) respectively, and the residual composite area was observed to decrease from 65% to 31% in C10, and 70% to 20% in C15, which indicated that the degradation degree of CPC was positively related to the porosity. Besides, it has been demonstrated that the large specific surface area, which can be achieved by increasing the number of micropores, is essential for osteoconductivity for bone regeneration. According to Woodard et al.,95 bone was formed only in the CaP scaffolds containing microporosity, demonstrating superior osteoconductivity compared to those without. This can be attributed to microporosity-improved growth factor retention, upon which bone formation largely depends in ectopic sites. Similarly, the group HA-nG-25% that contained more micropores was possibly more favorable for allowing the full in-growth of cells.94 In general, the nanoporosity in scaffolds could significantly promote osteoinductivity during bone tissue engineering by enhancing osteogenic differentiation.94 The greater bone formation was seen in scaffolds with increased strut porosity (from 20% to 30%) in an ovine ectopic model (Fig. 6).97 Generally, microporosity is necessary to achieve excellent osteoinductivity resulting from inner pores providing a larger surface area, which is believed to contribute to higher bone induction and protein adsorption as well as ion exchange and bone-like apatite formation by dissolution and reprecipitation.38,39 Tsukanaka et al.98 observed osteoinduction in half of the β-TCP materials with 60% porosity implanted into mice, whereas there was no osteoinduction in the 75% porosity group, although the latter exhibited the greatest number of vessels.
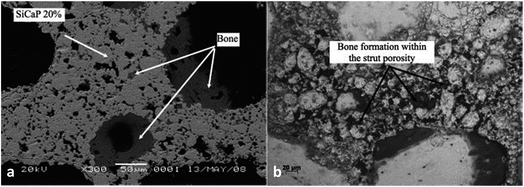 | ||
| Fig. 6 A scanning electron micrograph showing bone formation within (a) the SiCaP-20 scaffold; (b) the SiCaP-30 scaffold. Reprinted with permission from ref. 97 (Copyright © 2012, John Wiley and Sons). | ||
Localized drug/growth factor release requires the biomaterials to have permeable porous structures as scaffolds and carriers. The CaP composite prepared by Yang et al.99 had a unique three-dimensional structure with interconnected nanopores and exhibits liquid permeability and absorbability.
On the other hand, the increase in porosity will sacrifice the mechanical strength. The BCP powders (60% HA, 40% β-TCP) obtained by Kim et al.100 showed that with the porosity increasing from 43.0% to 45.9%, the compressive strength decreased accordingly (46.8 to 33.1 MPa). As such, further studies are needed to focus on the relationship between porosity and the performance of calcium phosphate materials, and to investigate the optimal porosity for bone regeneration.
It is worth mentioning that element concentration may have notable effects on the microstructure and porosity of composite biomaterials. For instance, in CaAl–CaP composites, the CaAl rich sample has a micro closed-pore structure, while the CaP rich sample has a nano open-pore structure, and the CaP rich sample has smaller nanoplatelets than the CaAl rich sample.99 Other additives such as pore phosphate-based glass, which could be used as a sintering aid,101 can also influence the porosity of ceramics.
3.2. Dopants
The addition of biologically active inorganic ions into the calcium phosphate matrix to stimulate cellular reactions is a promising strategy to accelerate bone defect healing via the addition of various dopants inside the crystalline matrix to adjust its crystal structure and thus affect its properties.According to Schamel et al.,111 the cell experiments with hMSC revealed the positive influence of the modification with 50 mmol Cr3+ and – to a less extent – with 10 mmol Cu2+ on cytocompatibility; the modification with Co2+ resulted in the suppression of cell growth and osteogenic differentiation (Fig. 7). However, in the experiment of Albayrak et al.,112 injecting Co-doped calcium phosphate microparticles into the intra-bone marrow of osteoporotic rats was found to increase the bone mineral density (BMD).
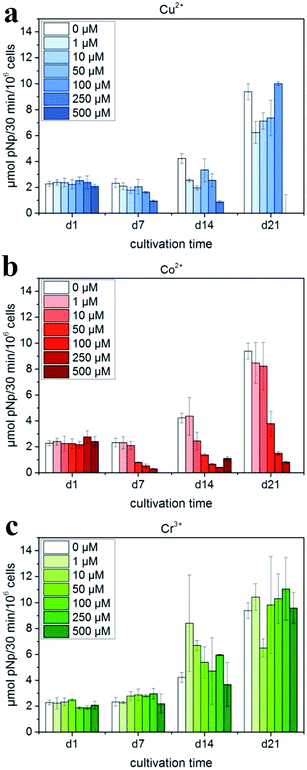 | ||
| Fig. 7 ALP activity of hMSC cultivated in the presence of Cu2+, Co2+ and Cr3+ ions added to cell culture medium (with OS) in various concentrations: (a) Cu2+: 0–500 μM (μmol L−1); (b) Co2+: 0–500 μM; and (c) Cr3+: 0–500 μM. Reprinted with permission from ref. 111 (Copyright © 2017, Elsevier). | ||
As many researchers have reported, doping Ag into calcium phosphate cement can enhance antibacterial properties.113–116 However, the incorporation of silver must be strictly controlled during the synthesis, since this influences the silver release kinetics. Range et al.117 compared two kinds of silver-containing biomaterials, namely, stoichiometric silver phosphate and silver-doped calcium phosphate; antimicrobial studies showed that both had a high bactericidal effect. However, due to a high release rate of silver ions in stoichiometric silver phosphate, it leads to a cytotoxic effect on eukaryotic cells as well, making the silver phosphate unsuitable as a silver-containing ceramic material. Interestingly, Rodriguez et al.118 found that the calcium phosphate coatings with 2.7% of selenium also resulted in a significant anti-proliferative effect (p < 0.01) on cancerous osteoblasts (MG63) in a preliminary study, and anti-biofilm properties (p < 0.01) against Staphylococcus epidermidis and Staphylococcus aureus bacterial strains. In this case, it is reasonable to believe that more studies using Se-doped calcium phosphate as prominent antibacterial materials need to be done in the future, taking cost into consideration.
The effects of Li, Fe, Mg and Zn doped in CaP on biomedical applications have also been investigated. Recent studies suggest that the presence of Fe3+ affects the crystallinity and solubility of HA,119,120 while small amounts of iron were found to have a positive impact on the biomedical properties of HA.121,122 Micro-CT showed better repair of bone defects in Li-doped CPC groups, compared to the blank group.123 Dual addition of bivalent magnesium (Mg2+) and cobalt (Co2+) ion dopants to hydroxyapatite had a significantly higher protein absorption capacity in comparison to pure hydroxyapatite.124 Detailed analysis pertaining to the bone cell (MG-63) compatibility and differentiation revealed that this doping process significantly promoted cell proliferation and differentiation.124 Apart from having a similar function, Zn ions are thought to also possess a potent and selective inhibitory effect on osteoclastic bone resorption.125
3.3. Surface modification
To endow calcium phosphate with better bioactivity, coating bioactive membranes on CaP matrix have been devised and applied to clinical treatment for a long time.Chitosan is a type of natural biopolymer with complete biocompatibility, thus allowing it to be applied in various medical fields. The study done by Coelho et al.126 showed that the Ca–P/chitosan coated on metals could generate sufficient adhesion with substrate, more so than the pure chitosan coating. Chitosan-coated Mg–Zn–tricalcium phosphate composite was found to slow down the in vivo degradation of the composite after surgery, improve the concrescence of the bone tissues and play a unique role in enhancing the corrosion resistance of the implant.127
Due to the improvement of cell adhesion and excellent biocompatibility, polydopamine (PDA) has been widely used in the surface modification of biomaterials. For instance, Ryu et al.128 introduced dopamine (DA) solution, oxidized for two days, into CPC, and formed PDA on the surface of CPC utilizing non-oxidized DA, by which it was possible to obtain faster mineralization and formation of bone-like hydroxyapatite with nano-micro structure. The formation mechanism of apatite on the substrate with PDA coating is shown in Fig. 8.128
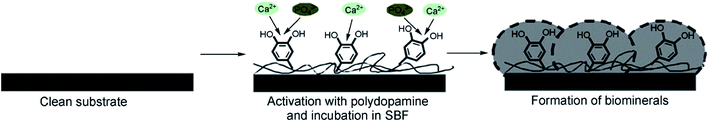 | ||
| Fig. 8 Formation mechanism of apatite deposited on the substrate with PDA coating. Reprinted with permission from ref. 128 (Copyright © 2010, John Wiley and Sons). | ||
The calcium phosphate substitute covered with autogenous periosteum has the ability to provide a sufficient supply of bone tissue, osteoblasts and cell growth factors for biomaterials to promote and stimulate the formation of new bone. The bending strength test showed that the sample covered with autogenous periosteum is much better than the one without periosteum, and reached half of the strength of normal bone after six months of implanting. Histology observations also confirmed that the sample covered with autogenous periosteum exhibited a remarkably higher osteogenesis rate and osteogenesis quality than the pure CaP substitute.54
As one of the most abundant structural proteins in hard tissues and a well-known mediator of osteoblast cellular functions such as initial attachment, proliferation, and differentiation,129 collagen has been widely explored as a coating on calcium phosphate scaffolds, including unmodified/modified microporous BCP130 and HA.129,131
Although these various coatings provide the scaffold with the unique enhancement of different properties such as improved osteoinductivity and adsorption of bone morphogenetic proteins, some coating solutions used for impregnating scaffolds may lead to the considerably decreased porosity of the scaffold, due to the high viscosity, which could impact the in-growth of bone cells into the scaffold.132 Thus, further research is necessary to deal with the problems caused by sticky coating solutions.
3.4. Environmental factors
When a bone graft substitute is implanted into the body, there will be a process of interaction between the implant and the surrounding environment including body fluid, which consists of various non-organic ions (Ca2+, Mg2+, Cl− etc.), organic substances (glucose, protein, ATP etc.) and some gases (CO2, N2, O2 etc.). After the dissolution of the surfaces of the biomaterials in this environment, the increase of substances such as Ca2+ and PO43− in body fluid will gather at the graft surface, followed by their acting as the raw materials for the formation of apatite-like phosphate, absorbing proteins and other growth factors that trigger the regeneration of new bone tissue. Besides, there are also some other factors influencing the bioactivity of bone graft, such as the temperature, the fluid flow rate, the special pressure and various growth factors.To elucidate what effects these factors will have on an implant in vivo is hard to achieve because of the high requirements of techniques, expensive costs of animal tests and the long periods of experiments, etc. Thus, it is necessary to create a special environment, identical to body fluid, so that the whole process of the degradation of the implant can be simulated. Simulated body fluid (SBF) contains all kinds of ions whose concentrations are at the same level as those in human blood plasma. Moreover, the pH value is also adjusted to the normal level of body fluid. By investigating the influences of different factors in SBF on the performance of biomaterial, people are able to approximately estimate the real situation of implants in vivo.
In a large number of studies, it has been assumed that the in vitro apatite-forming ability measured by the simulated body fluid (SBF) test is a predictor of in vivo bioactivity. In the experiment done by Wolke et al.,133 the in vitro test suggested that all of the porous calcium phosphate coatings induced the formation of homogeneous and adherent CaP precipitation layers, and in vivo tests then found that all coatings became surrounded by a dense, fibrous tissue capsule after implantation. Zhang et al.134 found that increasing the concentration of Mg2+ in simulated body fluid (SBF) would inhibit the growth process of apatite according to the results of EDS of the composite suggesting that the Ca content in SBF increased with higher Mg2+ concentration (from 1× Mg to 10× Mg). This finding is in accordance with other studies reporting that magnesium ions can kinetically hinder the nucleation and growth of hydroxyapatite (HA). However, Mg may also exhibit some positive performances such as enhancing the phase stability of β-TCP when Mg is added into biphasic ceramics.135,136 In addition, NaCl-free or low NaCl-content 5 SBF solution led to the earlier apatite precipitation in this solution, compared to the normal 5 SBF solution. The HCO3− content strongly affected the supersaturation and Ca–P structure by increasing the pH of the solution due to its buffering capacity. Furthermore, HCO3− favored the attachment of CaP mineral on Ti6Al4V by decreasing the CaP crystal size, resulting in the better physical attachment of the CaP coating on the Ti6Al4V substrate.137,138 According to Sakaguchi et al.,139 apatite precipitation did not occur in the SBF with a low Na+ concentration, whereas Ca2+ had little effect on the initial apatite precipitation.
Few researchers have investigated the influence of the temperature of SBF on the biological behavior of materials, whereas several studies have been reported for metallic biomaterials. High temperature and high concentration of SBF can greatly accelerate the deposition and increase the size of HA formed on the surface of titanium, and even affect the morphology of deposited HA. As shown in the experiment by Li et al.,140 the diameter of the spherical HA exceeds 30 μm after soaking at 57 °C (20 °C higher than the human body), in 3 SBF for only 1 day. Since the mechanisms of apatite layer formation on metallic and CaP materials are identical, it is reasonable to deduce that the temperature of SBF may have a similar effect on CaP. The studies with results of optimal temperature for metallic implants can also provide valuable references and statistics that would be of great help for further research using SBF.
Regarding the influence of SBF flow rate, Ca–P formation on aporous β-TCP/PLLA scaffold surfaces in dynamic simulated body fluid (dSBF) occurred slower than in static simulated body fluid (sSBF), and became more difficult with increasing the flow rate of dSBF. Apatite formations were analyzed based on classical crystallization theories of thermodynamics and kinetics. In sSBF, the Ca2+, PO43− from the scaffolds diffused with difficulty into sSBF solution so that the concentration of Ca and P increased to the threshold of nucleation and formed crystal nuclei. With dSBF (the schematic is shown in Fig. 9), the Ca2+, PO43− can easily diffuse into solution and be brought out of the sample chamber by the flow of SBF. Therefore, on the surface of the specimens, it was hard to achieve the threshold concentration of nucleation, but in the inner pore, the dissolved ions were hard to remove and there was easy accumulation of high Ca2+ and PO43− concentrations for nucleation. Therefore, spherical apatite individual crystals can be easily found on the walls of inner pores.141
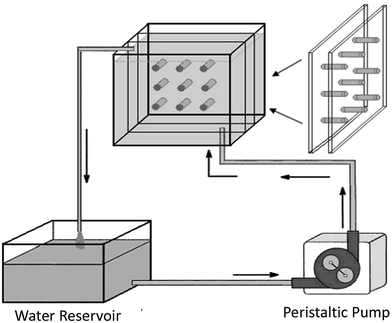 | ||
| Fig. 9 Schematic showing the apparatus for studying the degradation of scaffolds under conditions of fluid flow.141 Reprinted with permission from ref. 141 (Copyright © 2007, Trans Tech Publications). | ||
Calcium phosphate biomaterials with excellent mechanical strength are widely employed in the fields of load-bearing bone repair such as joint impact and the fracture of tibia. Thus, immersing materials in SBF in conjunction with a simulation of load conditions is necessary to evaluate whether the implant will be able to simultaneously function as a bioactive scaffold and a support. Kang et al.142 examined the effects of mechanical loading on the in vitro degradation characteristics and kinetics of porous PLLA/β-TCP scaffolds. They found that the porosity and decrease of the compressive strength under static compressive loading were lower than that of a non-loading case, and so was the mass loss rate. This might be due to the loading retarding the penetration, absorption and transfer of simulated body fluid.
Apart from acting as a predictor for in vivo tests, SBF can also promote CPC mineralization and improve the bioactivity to better integrate with the host when immersing the ceramics in SBF prior to implantation. In fact, proteins, along with other organic molecules, are also active players in the regulation of the biomineralization processes in vivo. Huang et al.9 found that BCP pre-incubated in SBF and BSA–SBF (bovine serum albumin–simulated body fluid) up-regulated ALP activity and osteogenic related genes and proteins, thus testifying to the positive effect of SBF and BSA–SBF. Moreover, the special SBF that was prepared with the addition of BSA remarkably enhanced the cell growth.
There still are limitations to in vitro tests regarding the complex ingredients of SBF and possible reactions between them, and it is therefore hard to define the exact effect of each factor on the bioactivity of CaPs.
4. Limitations and improvements of CaP
Resorbable CaP ceramics are attractive materials for bone regeneration, but they are intrinsically brittle and thus unsuitable for use in load-bearing sites. Moreover, introducing high porosity is required to encourage better cellular in-growth into bone regeneration scaffolds and is detrimental to the mechanical strength of the material.143 By coating CaP ceramic on biocompatible metallic implants with high mechanical strength, an advanced composite material or incorporating CaP with polymers, excellent load-bearing performance and bioactivity can be achieved in vivo.4.1. Coating CaP on metallic materials
Titanium and its alloys are bio-inert, having a high corrosion resistance, and sufficient mechanical strength for most indications.144,145 In this case, Ti is commonly employed as the substrate, coated with brittle, but bioactive CaP. Gross et al.146 found that titanium promoted the plastic deformation of the coating compared to stainless steel and Co–Cr substrate during contact nanofatigue. Kaemmerer et al.144 prepared a titanium implant with biphasic CaP coating and found that this compound exhibited great osteoinductivity and drug delivery potential as well as mechanical stability.As for the surface of the coated substitute, increasing the surface area and porosity of the implant can improve bone in-growth and the coefficient of friction between the bone and implant, thereby reducing micromotion and increasing osseointegration. An anchor-like surface topography with a secondary interconnected porous coating was prepared by a direct metal laser sintering method.147 This special surface modification was proved to significantly improve primary implant fixation and bone in-growth, and decreased the micromotion amplitude process in both in vitro and large animal in vivo studies. Lan et al.148 combined acid etching with UV exposure to alter the structure and surface energy. The resulting surface improved osteoblast ALP production and deposited mineralization, while decreasing the presence of S. aureus and S. epidermidis by approximately 70%. Besides, the morphology of pure calcium phosphate layers can be changed when Ag and/or Zn components are introduced into the basic electrolyte (Fig. 10).149 When silver and/or zinc particles are incorporated, the CaP particles become smaller and, in some cases, flake-like aggregates are formed. In addition, CaP coatings consisting of a mixture of different calcium phosphate phases such as DCP and HAp can be obtained.
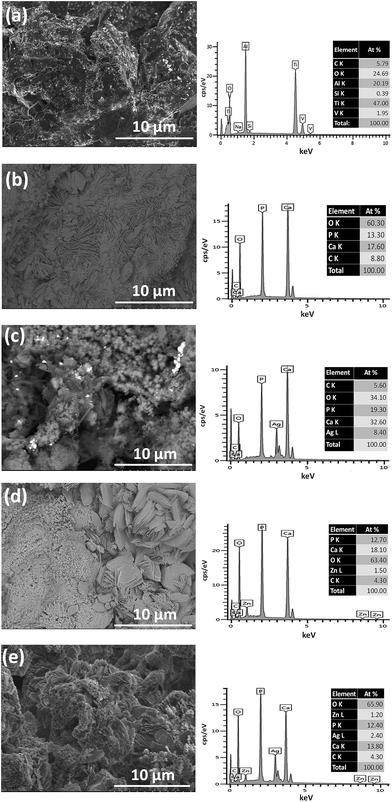 | ||
| Fig. 10 SEM and the corresponding EDX measurements on the Ti–6Al–4V substrate (a) on a CaP coating (b), on a Ag_CaP coating (c), on a Zn_CaP coating (d) as well as on a AgZn_CaP coating (e). Reprinted with permission from ref. 149 (Copyright © 2016, Elsevier). | ||
Doping additives such as Ag and Zn alter the morphology of the coatings whilst promoting the antimicrobial properties and bioactivity150 of implant materials. However, these may lead to a loss of corrosion resistance of at least one order of magnitude.149 The coated surfaces doped with Ag or Zn inhibited the growth, colonization and adherence of P. gingivalis, resulting in the reduced thickness of biofilms and bacterial inhibition in the culture medium, as compared to the uncoated materials.150 Sr was added by Geng et al.151 as a binary dopant to reduce the cytotoxicity of Ag, while maintaining good antibacterial properties.
Saeed et al.146 proposed a contact nanofatigue testing method, a rapid approach, to reveal the combination strength of CaP-coated implants. It is widely known that the mechanical loading history of the coated implanted prosthesis includes (i) abrasion during insertion inside a bone cavity and (ii) dynamic loading during physical activity of the recipient. While abrasion assesses particulate generation from the coating surface, cyclic loading determines the resistance of the entire coating to cracking and delamination. Therefore, unlike conventional testing methods such as bond-strength testing, which is time-consuming, cyclic nanoindentation has been utilized to quickly assess the effect of cyclical loading on the HA coating on the titanium substrate. Results suggest that contact nanofatigue on an amorphous calcium phosphate splat offers a possible means to evaluate crack growth during cyclic loading and to compare materials with different characteristics (i.e. crystallinity, composition, grain size, etc.).
Our group has also been researching the preparation and characterization of CaP coatings on Ti or Mg alloys. For example, we prepared calcium phosphate coatings on the surface of self-designed Mg–Zn–Ca–Mn alloys via micro-arc oxidation technology. The SBF immersion test proved that our related biomaterials achieved excellent bioactivity, with the evidence of bone-like apatite being formed on the surfaces.152,153
4.2. Incorporating CaP with polymers
Introducing CaP into nanoscale ductile polymers is widely thought of as an attempt at mimicking the structure of natural bone, where nanocrystallites of CaP ceramic are bonded by thin collagen layers.Generally, CaP coating is incorporated with polymer via coating on the polymer surface or mixing these two ingredients and then shaping using desired scaffolds. Coated composites are usually prepared by electrochemically assisted co-deposition154 or two-step biomimetic methods;155,156 the mixed composites are fabricated via the 3D printing technique,157 extrusion or direct injection into the bone defect site. Interestingly, the CaP-coated polymer nanofiber was recently fabricated by Junginger et al.158 via the mineralization process, and it is thought to add value to the synthesis of advanced hybrid materials for bone repair.
According to Poh et al.,159 PCL scaffolds coated with CaP have a negligible effect on the scaffold's porosity and compressive Young's modulus, compared to three other groups, namely, the PCL (control), PCL/50-45S5 and PCL/50-SrBG scaffolds. However, in the study of Birgani et al.,160 CaP-coated PLA and PLA/CaP composites did not exhibit significant differences in enzymatic alkaline phosphatase activity as well as the mRNA expression of bone morphogenetic protein-2, osteopontin and osteocalcin. Thus, the method of incorporation into the hybrid material plays a less prominent role in osteogenic differentiation.
Aghyarian et al.161 studied two novel composite (PMMA–CaP) bone cements including PMMA–HA and PMMA–brushite using an anatomically accurate human cadaveric vertebroplasty model. The mechanical testing included monotonic compression and cyclical fatigue tests, in which up to 57% of both PMMA–brushite and PMMA–HA reached sequence 4, demonstrating efficient reinforcement of the fractured vertebrae through stiffness restoration.161 In their following research,162 the biological performance of two PMMA–CaP cements were characterized. ALP assays showed no inhibition of osteoblast differentiation on the cement surface. Histological analysis indicated bone formation around the defect for the case of PMMA–HA and PMMA–brushite composite cements, but not for the PMMA–K cement (dark green lamellar bone was present around the defect in Fig. 11).
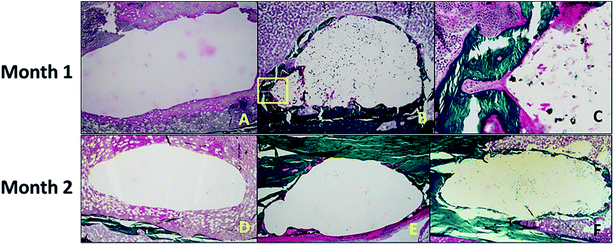 | ||
| Fig. 11 (Top) After 1 month of healing, (A) there was no bone formation around the defect in the negative control. (B and C) For the PMMA–brushite cement, osseointegration was observed with initial bone remodeling indicated by the formation of a cutting cone (area highlighted in the yellow square in (B), and magnified in (C)). (Bottom) The histology slides are shown for the cement test groups after 2 months of healing. Bone formation was only observed around the composite (E) PMMA–HA and (F) PMMA–brushite cements with no bone formation shown around (D) PMMA–K. Reprinted with permission from ref. 162 (Copyright © 2017, American Chemical Society). | ||
Except for several polymers mentioned above, PLLA was also explored by Cecen et al.163 with respect to the biocompatibility and biomechanical characteristics of loofah-based scaffolds combined with hydroxyapatite (HA), cellulose, poly-L-lactic acid (PLLA) with chondrocyte-like cells. Obvious improvements on the mechanical properties could principally be recognized in the strong interaction formed between loofah, PLLA and HA. Cells in all scaffolds produced an extracellular matrix that defined proteoglycan and type I–II collagens, suggesting that the loofah-based scaffold with desirable properties can be considered as an ideal candidate for cartilage tissue engineering applications. Similar conclusions about CaP/PLA were made by Birgani et al.160 Besides, polyelectrolyte multilayers (PEM),164 polyurethane (PU)154 and PHBV165 were all explored for incorporation with CaP, especially HA, in manufacturing scaffolds, and all have achieved both high mechanical strength and excellent osteointegration.
Additionally, doping some elements such as Sr166 into CaP coatings on polymer scaffolds is thought to be an effective measure to improve bone fixation and in vivo stability. It is worth mentioning that a mild annealing treatment at 130 °C for 6 h played a significant role in the fabrication process. The amorphous coatings were transformed into nanocrystalline HA films incorporating Sr (2+) with Sr/Ca molar ratios close to those of the as-deposited films. The annealing did not affect the topography and the roughness of the coatings, but did improve the hardness of the films. Recently, Zan et al.164 found that modulating the roughness of the surface of the PEMs/CaP–Col coatings can achieve optimal MSC proliferation and MSC osteogenesis, which further demonstrated that the roughness was a critical factor for bone formation.
5. Summary and future prospects
The most frequently used materials, biological properties and influencing factors of the biological properties of calcium phosphate, which have attracted great attention in the field of bone repair, were reviewed. As an ideal bone substitute, calcium phosphate is endowed with sufficient osteoconductivity and osteoinductivity, which ensure that sufficient body tissue forms and grows on the surface of the scaffold. Another significant property, the biodegradation of CaP, refers to various fields that are so complex that researchers have not yet come to an agreement on the mechanism. The biological properties of CaP remarkably depend on various factors. Optimal phase composition is essential to improve osteoinductivity, while the porosity is more relevant to osteoconductivity and biodegradability. Doping bioactive elements into calcium phosphate ceramics and different surface modification methods are considered as effective ways to enhance cell growth. Besides, altering the conditions of SBF can promote implant bioactivity by forming bone-like apatite on the surface. Due to the limited mechanical strength of CaP, some effective attempts including coating it on metallic materials and incorporating it with polymers have been taken to consideration, and these advanced products have been shown to achieve excellent bioactivities while maintaining mechanical stability.The insights on several significant biological properties of CaP materials are invaluable in exploring ideal bone substitutes for employment in all sorts of clinical applications. However, some more attempts are needed to gain a better understanding of CaP and the series of reactions or biological changes after its implantation into bone defect sites. The following are some guidelines concerning the future pre-design principles and characterization methods of biological CaP materials:
(1) Apart from HA, TCP and BCP, more research is required on other CaP materials such as polycalcium phosphate.
(2) More investigations are needed on the dominant factors in CaP that stimulate cells to secrete signal molecules and how the various cytokine networks function.
(3) Other factors that influence the osteoinductivity of CaP need to be determined.
(4) More research is needed to focus on exploring both antibacterial and corrosion resistant additives added into CaP substrates.
(5) The characterization of the osteoconduction of CaP requires more reliable methods such as building numerical models concerning different variables.
(6) The biodegradation process after CaP is implanted into bone defect sites needs to be investigated on the cytobiological level.
(7) The complex correlation between osteoinduction and biodegradation is needed; for example, the possibility that degradation products may initiate the osteogenesis process should be considered.
(8) More attempts at coating polymers with CaP are required.
(9) The implantation experiments should not be done only on animals such as dogs or mice, but should also be applied to larger animals such as sheep and calves, which are considered similar to humans in terms of their environmental influences on implants.
(10) More research on the degradable performance of CaP implants under conditions of pressure and cyclic loadings are needed to simulate the environment of load-bearing bone defects.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Shenzhen Strategic Emerging Industry Development Special Funds (Grant no: JCYJ20150626095244634).References
- I. Denry and L. T. Kuhn, Dent. Mater., 2016, 32, 43–53 CrossRef CAS PubMed.
- R. Shao, R. Quan, L. Zhang, X. Wei, D. Yang and S. Xie, J. Ceram. Soc. Jpn., 2015, 123, 17–20 CrossRef.
- E. J. Lee, F. K. Kasper and A. G. Mikos, Ann. Biomed. Eng., 2014, 42, 323–337 CrossRef PubMed.
- C. Liu and R. Tang, Chin. J. Inorg. Chem., 2014, 30, 1–9 CAS.
- Z. Tang, Y. Tan, Y. Ni, J. Wang, X. Zhu, Y. Fan, X. Chen, X. Yang and X. Zhang, Mater. Sci. Eng., C, 2017, 70, 1000–1010 CrossRef CAS PubMed.
- R. A. Perez, J. Won, J. C. Knowles and H. Kim, Adv. Drug Delivery Rev., 2013, 65, 471–496 CrossRef CAS PubMed.
- H. Yang, K. Xia, T. Wang, J. Niu, Y. Song, Z. Xiong, K. Zheng, S. Wei and W. Lu, J. Alloys Compd., 2016, 672, 366–373 CrossRef CAS.
- R. Yang, F. Ye, L. Cheng, J. Wang, X. Lu, Y. Shi, H. Fan, X. Zhang and H. Bu, J. Zhejiang Univ., Sci., B, 2011, 12, 582–590 CrossRef CAS PubMed.
- L. Huang, B. Zhou, H. Wu, L. Zheng and J. Zhao, Mater. Sci. Eng., C, 2017, 70, 955–961 CrossRef CAS PubMed.
- C. Yang, Y. Dong and L. Meng, Chin. J. Exp. Surg., 2005, 22, 837–838 Search PubMed.
- R. Duan, D. Barbieri, X. Luo, J. Weng, J. D. de Bruijn and H. Yuan, J. Orthop. Res., 2016, 34, 1865–1873 CrossRef CAS PubMed.
- A. F. Schilling, W. Linhart, S. Filke, M. Gebauer, T. Schinke, J. M. Rueger and M. Amling, Biomaterials, 2004, 25, 3963–3972 CrossRef CAS PubMed.
- S. Chiba, T. Anada, K. Suzuki, K. Saito, Y. Shiwaku, N. Miyatake, K. Baba, H. Imaizumi, M. Hosaka, E. Itoi and O. Suzuki, J. Biomed. Mater. Res., Part A, 2016, 104, 2833–2842 CrossRef CAS PubMed.
- L. Cheng, T. Wang, J. Zhu and P. Cai, Transplant. Proc., 2016, 48, 1309–1314 CrossRef CAS PubMed.
- Y. Lei, Z. Xu, Q. Ke, W. Yin, Y. Chen, C. Zhang and Y. Guo, Mater. Sci. Eng., C, 2017, 72, 134–142 CrossRef CAS PubMed.
- A. Fihri, C. Len, R. S. Varma and A. Solhy, Coord. Chem. Rev., 2017, 347, 48–76 CrossRef CAS.
- W. Yu, X. Wang, J. Zhao, Q. Tang, M. Wang and X. Ning, Ceram. Int., 2015, 41, 10600–10606 CrossRef CAS.
- M. Mirzaee, M. Vaezi and Y. Palizdar, Mater. Sci. Eng., C, 2016, 69, 675–684 CrossRef CAS PubMed.
- Y. Y. Ozbek, F. E. Bastan, N. Canikoglu and U. Ozsarac, J. Therm. Anal. Calorim., 2016, 125, 651–658 CrossRef CAS.
- B. A. E. Ben-Arfa, I. M. Miranda Salvado, J. M. F. Ferreira and R. C. Pullar, Mater. Sci. Eng., C, 2017, 70, 796–804 CrossRef CAS PubMed.
- M. H. Fathi and A. Hanifi, Mater. Lett., 2007, 61, 3978–3983 CrossRef CAS.
- B. Li, Z. Liu, J. Yang, Z. Yi, W. Xiao, X. Liu, X. Yang, W. Xu and X. Liao, Mater. Sci. Eng., C, 2017, 70, 1200–1205 CrossRef CAS PubMed.
- R. G. Carrodeguas and S. De Aza, Acta Biomater., 2011, 7, 3536–3546 CrossRef CAS PubMed.
- N. Kobayashi, Y. Hashimoto, A. Otaka, T. Yamaoka and S. Morita, Materials, 2016, 9, 853 CrossRef PubMed.
- M. B. Thuermer, C. E. Diehl and L. A. Loureiro Dos Santos, Ceram. Int., 2016, 42, 18094–18099 CrossRef.
- M. Jarcho, Dent. Clin. North Am., 1986, 30, 25–47 CAS.
- S. Feng, F. He and J. Ye, Mater. Sci. Eng., C, 2018, 82, 217–224 CrossRef CAS PubMed.
- S. Flauder, U. Gbureck and F. A. Mueller, Acta Biomater., 2014, 10, 5148–5155 CrossRef CAS PubMed.
- S. V. Dorozhkin, Acta Biomater., 2012, 8, 963–977 CrossRef CAS PubMed.
- M. Ebrahimi, M. G. Botelho and S. V. Dorozhkin, Mater. Sci. Eng., C, 2017, 71, 1293–1312 CrossRef CAS PubMed.
- M. Ebrahimi, M. G. Botelho and S. V. Dorozhkin, Mater. Sci. Eng., C, 2017, 71, 1293–1312 CrossRef CAS PubMed.
- K. H. Kim, J. Y. Park, H. S. Park, K. S. Kim, D. K. Chin, Y. E. Cho and S. U. Kuh, Yonsei Med. J., 2017, 58, 407–414 CrossRef PubMed.
- J. Tu, F. Guo, C. Lu and B. Li, J. Biomed. Eng., 2011, 28, 99–103 CAS.
- P. Weiss, P. Layrolle, L. P. Clergeau, B. Enckel, P. Pilet, Y. Amouriq, G. Daculsi and B. Giumelli, Biomaterials, 2007, 28, 3295–3305 CrossRef CAS PubMed.
- D. Das, Z. Zhang, T. Winkler, M. Mour, C. I. Guenter, M. M. Morlock, H. Machens and A. F. Schilling, in Advances in Biochemical Engineering-Biotechnology, ed. C. Kasper, F. Witte and R. Portner, 2012, pp. 317–333 Search PubMed.
- Y. Zhao, J. Fan, Z. Li, Y. Liu, Y. Wu and J. Liu, Artif. Organs, 2017, 41, 199–204 CrossRef CAS PubMed.
- J. Schnieders, U. Gbureck, E. Vorndran, M. Schossig and T. Kissel, J. Biomed. Mater. Res., Part B, 2012, 100, 890 CrossRef.
- H. Wang, J. Ding, X. Zhu, H. Fan and X. Zhang, J. Funct. Mater., 2010, 41, 1727–1730 CAS.
- J. Schnieders, U. Gbureck, E. Vorndran, M. Schossig and T. Kissel, J. Biomed. Mater. Res., Part B, 2011, 99, 391–398 CrossRef PubMed.
- T. Albrektsson and C. Johansson, Eur. Spine J., 2001, 10, S96–S101 CrossRef PubMed.
- G. Chen, C. Deng and Y. Li, Int. J. Biol. Sci., 2012, 8, 272–288 CrossRef CAS PubMed.
- J. J. Yi, A. P. Barnes, R. Hand, F. Polleux and M. D. Ehlers, Cell, 2010, 142, 144–157 CrossRef CAS PubMed.
- G. G. Tortelote, R. R. Reis, F. D. A. Mendes and J. G. Abreu, Cell. Signalling, 2017, 40, 30–43 CrossRef CAS PubMed.
- H. Zhang, J. Wang, F. Deng, E. Huang and Z. Yan, Biomaterials, 2015, 39, 145–154 CrossRef CAS PubMed.
- S. Jang, H. Cho, J. Park and H. Jeong, Neurosci. Lett., 2017, 660, 68–73 CrossRef CAS PubMed.
- M. E. Rieger, B. Zhou, N. Solomon, M. Sunohara, C. Li, N. Cu, Y. Liu, J. Pan, P. Minoo, E. D. Crandall, S. L. Brody, M. Kahn and Z. Borok, J. Biol. Chem., 2016, 291, 6569–6582 CrossRef CAS PubMed.
- O. A. Vega, C. M. J. Lucero, H. F. Araya, S. Jerez, J. C. Tapia, M. Antonelli, F. Salazar-Onfray, F. Las Heras, R. Thaler, S. M. Riester, G. S. Stein, A. J. van Wijnen and M. A. Galindo, J. Cell. Biochem., 2017, 118, 3662–3674 CrossRef CAS PubMed.
- G. Nalesso, J. Sherwood, J. Bertrand, T. Pap, M. Ramachandran, C. De Bari, C. Pitzalis and F. Dell'Accio, Int. J. Exp. Pathol., 2012, 93, A8 Search PubMed.
- S. Stewart, A. W. Gomez, B. E. Armstrong, A. Henner and K. Stankunas, Cell Rep., 2014, 6, 482–498 CrossRef CAS PubMed.
- M. Okamoto, N. Udagawa, S. Uehara, K. Maeda, T. Yamashita, Y. Nakamichi, H. Kato, N. Saito, Y. Minami, N. Takahashi and Y. Kobayashi, Sci. Rep., 2014, 4, 4493 CrossRef PubMed.
- M. M. Weivoda, M. Ruan, C. M. Hachfeld, L. Pederson, A. Howe, R. A. Davey, J. D. Zajac, Y. Kobayashi, B. O. Williams, J. J. Westendorf, S. Khosla and M. J. Oursler, J. Bone Miner. Res., 2016, 31, 65–75 CrossRef CAS PubMed.
- Z. Jing, Z. Chunlei, X. Fang and C. Hong, China Cattle Science, 2012, 38, 33–37 Search PubMed.
- K. Oh, Y. Ko, S. Jaiswal and I. Whang, J. Mater. Sci.: Mater. Med., 2016, 27, 179 CrossRef PubMed.
- C. Zhang, J. Wang, B. Lu, Y. Yao, H. Fen, X. Jiang, G. Deng, L. Liu, D. Heng and X. Zhang, Med. J. Natl. Defending Forces Northwest China, 2003, 14–17 Search PubMed.
- K. M. Scheufler and D. Diesing, Orthopade, 2015, 44, 146–153 CrossRef PubMed.
- S. Ishack, A. Mediero, T. Wilder, J. L. Ricci and B. N. Cronstein, J. Biomed. Mater. Res., Part B, 2017, 105, 366–375 CrossRef CAS PubMed.
- W. Goetz, S. Lenz, C. Reichert, K. Henkel, V. Bienengraeber, L. Pernicka, K. K. H. Gundlach, T. Gredes, T. Gerber, T. Gedrange and F. Heinemann, Folia Histochem. Cytobiol., 2010, 48, 589–596 Search PubMed.
- N. L. D'Elia, N. Gravina, J. M. Ruso, J. L. Marco-Brown, J. M. Sieben and P. V. Messina, J. Colloid Interface Sci., 2017, 494, 345–354 CrossRef PubMed.
- B. Hashemibeni, L. Dehghani, F. Sadeghi, E. Esfandiari, M. Gorbani, A. Akhavan, S. T. Tahani, H. Bahramian and V. Goharian, Int. J. Prev. Med., 2016, 7, 62 CrossRef PubMed.
- A. Fern, J. E. Ndez, R. J. Manuel Garcia-Aznar and M. Masid, Z. Angew. Math. Mech., 2017, 97, 1050–1063 CrossRef.
- T. Albrektsson and C. Johansson, Eur. Spine J., 2001, 10, S96–S101 CrossRef PubMed.
- T. Yu, C. Dong, Z. Shen, Y. Chen, B. Yu, H. Shi, C. Zhou and J. Ye, Mater. Sci. Eng., C, 2016, 68, 153–162 CrossRef CAS PubMed.
- J. M. Bouler, P. Pilet, O. Gauthier and E. Verron, Acta Biomater., 2017, 5, 1–12 CrossRef PubMed.
- B. Johari, M. Ahmadzadehzarajabad, M. Azami, M. Kazemi, M. Soleimani, S. Kargozar, S. Hajighasemlou, M. M. Farajollahi and A. Samadikuchaksaraei, J. Biomed. Mater. Res., Part A, 2016, 104, 1770–1778 CrossRef CAS PubMed.
- J. Z. Hu, Z. M. Yang, Y. C. Zhou, Y. Liu, K. Y. Li and H. B. Lu, J. Mater. Sci.: Mater. Med., 2015, 26, 257 CrossRef PubMed.
- K. Emilov-Velev, C. Clemente-de-Arriba, M. Á. Alobera-García, E. M. Moreno-Sansalvador and J. Campo-Loarte, Revista Española de Cirugía Ortopédica y Traumatología, 2015, 59, 200–210 CrossRef CAS PubMed.
- D. Barbieri, H. Yuan, A. Ismailoglu and J. de Bruijn, Tissue Eng., Part A, 2017, 23, 1310–1320 CrossRef CAS PubMed.
- J. Carrel, A. Wiskott, S. Scherrer and S. Durual, Clin. Implant Dent. R., 2016, 18, 1183–1192 CrossRef PubMed.
- S. Frasca, F. Norol, C. Le Visage, J. Collombet, D. Letourneur, X. Holy and E. Sari Ali, J. Mater. Sci.: Mater. Med., 2017, 28, 35 CrossRef PubMed.
- S. Bose, S. Tarafder and A. Bandyopadhyay, Ann. Biomed. Eng., 2017, 45, 261–272 CrossRef PubMed.
- A. Friedmann, M. Dard, B. M. Kleber, J. P. Bernimoulin and D. D. Bosshardt, Clin. Oral Implants Res., 2009, 20, 708–714 CrossRef PubMed.
- C. Yang, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 2001, 92, 606–609 CrossRef CAS PubMed.
- A. F. Schilling, S. Filke, S. Brink, H. Korbmacher, M. Amling and J. M. Rueger, Eur. J. Trauma, 2006, 32, 107–113 CrossRef.
- L. Tan, X. Yu, P. Wan and K. Yang, J. Mater. Sci. Technol., 2013, 29, 503–513 CAS.
- Z. Sheikh, S. Najeeb, Z. Khurshid, V. Verma, H. Rashid and M. Glogauer, Materials, 2015, 8, 5744–5794 CrossRef PubMed.
- V. Perrotti, B. M. Nicholls, M. A. Horton and A. Piattelli, J. Bone Miner. Res., 2007, 22, 1127–1128 Search PubMed.
- M. Nakamura, T. Hentunen, J. Salonen, A. Nagai and K. Yamashita, J. Biomed. Mater. Res., Part A, 2013, 101, 3141–3151 Search PubMed.
- M. E. Ogle, C. E. Segar, S. Sridhar and E. A. Botchwey, Exp. Biol. Med., 2016, 241, 1084–1097 CrossRef CAS PubMed.
- G. Ciapetti, G. Di Pompo, S. Avnet, D. Martini, A. Diez-Escudero, E. B. Montufar, M. Ginebra and N. Baldini, Acta Biomater., 2017, 50, 102–113 CrossRef CAS PubMed.
- B. Neuhaus, B. Tosun, O. Rotan, A. Frede, A. M. Westendorf and M. Epple, RSC Adv., 2016, 6, 18102–18112 RSC.
- T. Winkler, E. Hoenig, R. Gildenhaar, G. Berger, D. Fritsch, R. Janssen, M. M. Morlock and A. F. Schilling, Acta Biomater., 2010, 6, 4127–4135 CrossRef CAS PubMed.
- J. Wang, D. Liu, B. Guo, X. Yang and X. Chen, Acta Biomater., 2017, 51, 447–460 CrossRef CAS PubMed.
- M. Wang, Y. Yu, K. Dai, Z. Ma and Y. Liu, Biomater. Sci., 2016, 4, 1574–1583 RSC.
- T. Zhang, J. Gao, S. Qu, M. Li and J. Weng, Chin. J. Inorg. Chem., 2010, 26, 957–962 CAS.
- C. Ergun, H. Liu, T. J. Webster, E. Olcay, S. Yilmaz and F. C. Sahin, J. Biomed. Mater. Res., Part A, 2008, 85, 236–241 CrossRef PubMed.
- I. Bajpai, D. Y. Kim, J. Kyong-Jin, I. Song and S. Kim, J. Biomed. Mater. Res., Part B, 2017, 105, 72–80 CrossRef CAS PubMed.
- J. Wang, Y. Chen, X. Zhu, T. Yuan, Y. Tan, Y. Fan and X. Zhang, J. Biomed. Mater. Res., Part A, 2014, 102, 4234–4243 Search PubMed.
- X. Y. Lu, J. G. Ran and L. Gou, Rare Met. Mater. Eng., 2005, 34, 601–604 CAS.
- V. Uskokovic and T. A. Desai, J. Biomed. Mater. Res., Part A, 2013, 101, 1416–1426 CrossRef PubMed.
- F. Chen, B. Yang, C. Qi, T. Sun, Y. Jiang, J. Wu, X. Chen and Y. Zhu, RSC Adv., 2015, 5, 100682–100688 RSC.
- P. Pripatnanont, P. Praserttham, S. Suttapreyasri, N. Leepong and N. Monmaturapoj, Int. J. Oral Maxillofac. Implants, 2016, 31, 294–303 CrossRef PubMed.
- S. Baradararan, M. Hamdi and I. H. Metselaar, Adv. Appl. Ceram., 2012, 111, 367–373 CrossRef CAS.
- J. Lee, H. Choi, S. Yoon, B. Kim and H. Park, J. Ceram. Process Res., 2013, 14, 544–548 Search PubMed.
- Z. Chen, X. Zhang, Y. Yang, K. Zhou, N. Wragg, Y. Liu, M. Lewis and C. Liu, Ceram. Int., 2017, 43, 336–344 CrossRef CAS.
- J. R. Woodard, A. J. Hilldore, S. K. Lan, C. J. Park, A. W. Morgan, J. A. C. Eurell, S. G. Clark, M. B. Wheeler, R. D. Jamison and A. J. W. Johnson, Biomaterials, 2007, 28, 45–54 CrossRef CAS PubMed.
- A. Kasuya, S. Sobajima and M. Kinoshita, J. Orthop. Res., 2012, 30, 1103–1111 CrossRef CAS PubMed.
- M. J. Coathup, K. A. Hing, S. Samizadeh, O. Chan, Y. S. Fang, C. Campion, T. Buckland and G. W. Blunn, J. Biomed. Mater. Res., Part A, 2012, 100, 1550–1555 CrossRef PubMed.
- M. Tsukanaka, S. Fujibayashi, B. Otsuki, M. Takemoto and S. Matsuda, J. Mater. Sci.: Mater. Med., 2015, 26, 132 CrossRef PubMed.
- J. Yang, X. Hu, J. Huang, K. Chen, Z. Huang, Y. Liu, M. Fang and X. Sun, Nanoscale, 2016, 8, 3599–3606 RSC.
- D. Kim, K. Kim, H. Chun, T. Kim, H. Park and S. Yoon, Ceram. Int., 2014, 40, 8293–8300 CrossRef CAS.
- Y. Yang, F. He and J. Ye, Mater. Sci. Eng., C, 2016, 69, 1004–1009 CrossRef CAS PubMed.
- L. Xia, K. Lin, X. Jiang, B. Fang, Y. Xu, J. Liu, D. Zeng, M. Zhang, X. Zhang, J. Chang and Z. Zhang, Biomaterials, 2014, 35, 8514–8527 CrossRef CAS PubMed.
- M. J. Coathup, Q. Cai, C. Campion, T. Buckland and G. W. Blunn, J. Biomed. Mater. Res., Part B, 2013, 101, 902–910 CrossRef PubMed.
- L. Wang, D. Barbieri, H. Zhou, J. D. de Bruijn, C. Bao and H. Yuan, J. Biomed. Mater. Res., Part A, 2015, 103, 1919–1929 CrossRef CAS PubMed.
- V. Uskokovic, S. S. Batarni, J. Schweicher, A. King and T. A. Desai, ACS Appl. Mater. Interfaces, 2013, 5, 2422–2431 CAS.
- S. Lee, W. F. Regnault, J. M. Antonucci and D. Skrtic, J. Biomed. Mater. Res., Part B, 2007, 80, 11–17 CrossRef PubMed.
- J. Yang and J. Ye, Bull. Chin. Ceram. Soc., 2008, 27, 213–219 CAS.
- S. Chernousova, J. Klesing, N. Soklakova and M. Epple, RSC Adv., 2013, 3, 11155–11161 RSC.
- W. Moo-Chin, C. Hui-Ting, S. Wei-Jen, C. Hsin-Fang, H. Min-Hsiung and H. I-Ming, Ceram. Int., 2015, 41, 2999–3008 CrossRef.
- J. S. Sun, H. C. Liu, W. Chang, J. Li, F. H. Lin and H. C. Tai, J. Biomed. Mater. Res., 1998, 39, 390–397 CrossRef CAS PubMed.
- M. Schamel, A. Bernhardt, M. Quade, C. Wurkner, U. Gbureck, C. Moseke, M. Gelinsky and A. Lode, Mater. Sci. Eng., C, 2017, 73, 99–110 CrossRef CAS PubMed.
- O. Albayrak, Mater. Charact., 2016, 113, 82–89 CrossRef CAS.
- J. V. Rau, M. Fosca, V. Graziani, A. A. Egorov, Y. V. Zobkov, A. Y. Fedotov, M. Ortenzi, R. Caminiti, A. E. Baranchikov and V. S. Komlev, J. Funct. Biomater., 2016, 7, 10–15 CrossRef PubMed.
- A. Costescu, C. S. Ciobanu, S. L. Iconaru, R. V. Ghita, C. M. Chifiriuc, L. G. Marutescu and D. Predoi, J. Nanomater., 2013, 2013, 1–9 CrossRef.
- R. B. Bostancioglu, C. Peksen, H. Genc, M. Gurbuz, F. B. Karel, A. S. Koparal, A. Dogan, N. Kose and A. T. Koparal, Biomed. Mater., 2015, 10, 045024 CrossRef PubMed.
- M. E. Ureyen, A. Dogan and A. S. Koparal, Text. Res. J., 2012, 82, 1731–1742 CrossRef.
- S. Range, D. Hagmeyer, O. Rotan, V. Sokolova, J. Verheyen, B. Siebers and M. Epple, RSC Adv., 2015, 5, 43172–43177 RSC.
- C. Rodriguez-Valencia, P. Freixeiro, J. Serra, C. M. Ferreiros, P. Gonzalez and M. Lopez-Alvarez, Biomed. Mater., 2017, 12, 15028 CrossRef CAS PubMed.
- O. Kaygili, S. V. Dorozhkin, T. Ates, A. A. Al-Ghamdi and F. Yakuphanoglu, Ceram. Int., 2014, 40, 9395–9402 CrossRef CAS.
- S. Gomes, A. Kaur, J. Greneche, J. Nedelec and G. Renaudin, Acta Biomater., 2017, 50, 78–88 CrossRef CAS PubMed.
- Y. Li, J. Widodo, S. Lim and C. P. Ooi, J. Mater. Sci., 2012, 47, 754–763 CrossRef CAS.
- M. Iafisco, M. Sandri, S. Panseri, J. Manuel Delgado-Lopez, J. Gomez-Morales and A. Tampieri, Chem. Mater., 2013, 25, 2610–2617 CrossRef CAS.
- L. Li, Y. Qin, G. Ma and B. Li, J. South. Med. Univ., 2016, 36, 824–828 Search PubMed.
- H. Wu, R. Zhang, X. Li, J. Ni, C. Zhao, Y. Song, J. Wang, S. Zhang, Y. Zheng and X. Zhang, Prog. Nat. Sci.: Mater. Int., 2014, 24, 479–485 CrossRef CAS.
- Gunawan, I. Sopyan, Suryanto and A. Naqshbandi, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2014, 53, 152–158 Search PubMed.
- W. T. Coelho, J. M. Fernandes, R. S. Vieira, M. B. Thurmer and L. A. Santos, in Materials Science Forum, ed. L. Salgado and F. Ambrozio, 2012, pp. 1181–1186 Search PubMed.
- J. Zhao, L. Chen, K. Yu, C. Chen, Y. Dai, X. Qiao and Y. Yan, Biointerphases, 2014, 9, 31004 CrossRef PubMed.
- J. Ryu, S. H. Ku, H. Lee and C. B. Park, Adv. Funct. Mater., 2010, 20, 2132–2139 CrossRef CAS.
- J. Guan, J. Yang, J. Dai, Y. Qin, Y. Wang, Y. Guo, Q. Ke and C. Zhang, RSC Adv., 2015, 5, 36175–36184 RSC.
- M. Lee, C. You and K. Kim, Materials, 2015, 8, 1150–1161 CrossRef PubMed.
- M. R. Kumar and M. S. Freund, RSC Adv., 2015, 5, 57318–57327 RSC.
- N. A. S. Zairani, M. Jaafar, N. Ahmad and K. A. Razak, Ceram. Int., 2016, 42, 5141–5147 CrossRef CAS.
- R. P. F. Lanao, J. W. M. Hoekstra, J. G. C. Wolke, S. C. G. Leeuwenburgh, A. S. Plachokova, O. C. Boerman, J. J. J. P. Beucken and J. A. Jansen, J. Tissue Eng. Regener. Med., 2014, 8, 473–482 CrossRef PubMed.
- J. Zhang, C. Dai, J. Wei, Z. Wen, S. Zhang and L. Lin, Appl. Surf. Sci., 2013, 280, 256–262 CrossRef CAS.
- S. Kannan, I. Lemos, J. Rocha and J. Ferreira, J. Solid State Chem., 2005, 178, 3190–3196 CrossRef CAS.
- K. Salma-Ancane, L. Stipniece, A. Putnins and L. Berzina-Cimdina, Ceram. Int., 2015, 41, 4996–5004 CrossRef CAS.
- H. Pan, X. Zhao, B. W. Darvell and W. W. Lu, Acta Biomater., 2010, 6, 4181–4188 CrossRef CAS PubMed.
- S. Jalota, S. B. Bhaduri and A. C. Tas, J. Mater. Sci.: Mater. Med., 2006, 17, 697–707 CrossRef CAS PubMed.
- A. Sakaguchi, M. Nakano, J. Hieda, N. Ohtake and H. Akasaka, Appl. Surf. Sci., 2015, 347, 610–618 CrossRef CAS.
- N. Li, G. Xiao, B. Liu, Z. Wang, R. Zhu and Y. Lu, Surf. Coat. Technol., 2016, 301, 121–125 CrossRef CAS.
- K. Yunqing, Y. Guangfu, W. Kefeng, L. Lin, L. Li and Y. Yadong, Key Eng. Mater., 2007, 330–332, 483–486 Search PubMed.
- Y. Kang, G. Yin, L. Luo, K. Wang and Y. Zhang, in Key Engineering Materials, ed. Y. H. Kim, C. S. Cho, I. K. Kang, S. Y. Kim and O. H. Kwon, 2007, p. 273 Search PubMed.
- I. Gotman, S. K. Swain, A. Sharipova and E. Y. Gutmanas, in AIP Conference Proceedings, ed. V. E. Panin, S. G. Psakhie and V. M. Fomin, 2016 Search PubMed.
- T. A. Kaemmerer, V. Palarie, E. Schiegnitz, V. Topalo, A. Schroeter, B. Al-Nawas and P. W. Kaemmerer, J. Oral Pathol. Med., 2017, 46, 61–66 CrossRef CAS PubMed.
- A. Krzakala, A. Kazek-Kesik and W. Simka, RSC Adv., 2013, 3, 19725–19743 RSC.
- S. Saber-Samandari and K. A. Gross, Acta Biomater., 2013, 9, 5788–5794 CrossRef CAS PubMed.
- J. Raphel, M. Holodniy, S. B. Goodman and S. C. Heilshorn, Biomaterials, 2016, 84, 301–314 CrossRef CAS PubMed.
- G. Lan, M. Li, Y. Tan, L. Li, X. Yang, L. Ma, Q. Yin, H. Xia, Y. Zhang, G. Tan and C. Ning, J. Mater. Sci. Technol., 2015, 31, 182–190 Search PubMed.
- M. Furko, Y. Jiang, T. Wilkins and C. Balazsi, Ceram. Int., 2016, 42, 4924–4931 CrossRef CAS.
- A. K. Aranya, S. Pushalkar, M. Zhao, R. Z. LeGeros, Y. Zhang and D. Saxena, J. Biomed. Mater. Res., Part A, 2017, 105, 2218–2227 CrossRef PubMed.
- Z. Geng, R. Wang, X. Zhuo, Z. Li, Y. Huang, L. Ma, Z. Cui, S. Zhu, Y. Liang, Y. Liu, H. Bao, X. Li, Q. Huo, Z. Liu and X. Yang, Mater. Sci. Eng., C, 2017, 71, 852–861 CrossRef CAS PubMed.
- J. Dou, Q. You, G. Gu, C. Chen and X. Zhang, Biointerphases, 2016, 11, 031006 CrossRef PubMed.
- J. Dou, G. Gu and C. Chen, Mater. Lett., 2017, 196, 42–45 CrossRef CAS.
- M. Meskinfam, S. Bertoldi, N. Albanese, A. Cerri, M. C. Tanzi, R. Imani, N. Baheiraei, M. Farokhi and S. Fare, Mater. Sci. Eng., C, 2018, 82, 130–140 CrossRef CAS PubMed.
- X. Zhao, H. Li, Z. Xu, K. Li, S. Cao and G. Jiang, Surf. Coat. Technol., 2017, 322, 227–237 CrossRef CAS.
- T. Mai, S. Boye, J. Yuan, A. Voelkel, M. Graewert, C. Guenter, A. Lederer and A. Taubert, RSC Adv., 2015, 5, 103494–103505 RSC.
- J. B. Vella, R. P. Trombetta, M. D. Hoffman, J. Inzana, H. Awad and D. S. W. Benoit, J. Biomed. Mater. Res., Part A, 2017 DOI:10.1002/jbm.a.36270.
- M. Junginger, C. Kuebel, F. H. Schacher, A. H. E. Mueller and A. Taubert, RSC Adv., 2013, 3, 11301–11308 RSC.
- P. S. P. Poh, D. W. Hutmacher, B. M. Holzapfel, A. K. Solanki, M. M. Stevens and M. A. Woodruff, Acta Biomater., 2016, 30, 319–333 CrossRef CAS PubMed.
- Z. T. Birgani, C. A. van Blitterswijk and P. Habibovic, J. Mater. Sci.: Mater. Med., 2016, 27, 54 CrossRef PubMed.
- S. Aghyarian, X. Hu, R. Haddas, I. H. Lieberman, V. Kosmopoulos, H. K. W. Kim and D. C. Rodrigues, J. Orthop. Res., 2017, 35, 2067–2074 CrossRef CAS PubMed.
- S. Aghyarian, E. Bentley, T. N. Hoang, I. M. Gindri, V. Kosrnopoulos, H. K. W. Kim and D. C. Rodrigues, ACS Biomater. Sci. Eng., 2017, 3, 2267–2277 CrossRef CAS.
- B. Cecen, L. D. Kozaci, M. Yuksel, O. Ustun, B. U. Ergur and H. Havitcioglu, Mater. Sci. Eng., C, 2016, 69, 437–446 CrossRef CAS PubMed.
- X. Zan, P. Sitasuwan, S. Feng and Q. Wang, Langmuir, 2016, 32, 1808–1817 CrossRef CAS PubMed.
- O. Rasoga, L. Sima, M. Chiritoiu, G. Popescu-Pelin, O. Fufa, V. Grumezescu, M. Socol, A. Stanculescu, I. Zgura and G. Socol, Appl. Surf. Sci., 2017, 417, 204–212 CrossRef CAS.
- M. Bianchi, L. Degli Esposti, A. Ballardini, F. Liscio, M. Berni, A. Gambardella, S. C. G. Leeuwenburgh, S. Sprio, A. Tampieri and M. Iafisco, Surf. Coat. Technol., 2017, 319, 191–199 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |