Open Access Article
Open Access ArticleSyntheses, characterizations and functions of cationic polyethers with imidazolium-based ionic liquid moieties†
Shigetaka
Hayano
 *,
Keisuke
Ota
and
Hoang The
Ban‡
*,
Keisuke
Ota
and
Hoang The
Ban‡
Zeon Corporation R&D Center, 1-2-1 Yako, Kawasaki-ward, Kawasaki-city, Kanagawa-pref. 210-9507, Japan. E-mail: S.Hayano@zeon.co.jp
First published on 24th January 2018
Abstract
Cationic polyethers with imidazolium-based ionic liquid moieties were synthesized and characterized thoroughly for their properties and functions. Poly(epichlorohydrin) (poly(ECH)) was quaternized by 1-methylimidazole to provide poly(N-glycidyl-N′-methylimidazolium chloride) (poly(ECH-MeIm+Cl−)). Anion exchange of the poly(ECH-MeIm+Cl−) with Li salts provided other cationic polyethers: hydrophobic poly(N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide) (poly(ECH-MeIm+TFSI−)) and hydrophilic poly(N-glycidyl-N′-methylimidazolium tetrafluoroborate) (poly(ECH-MeIm+BF4−)). The 5% thermal decomposition temperatures of these cationic polyethers are quite high (300–400 °C). Contrary to poly(ECH) (glass transition temperature (Tg) = −49 °C), the poly(ECH-MeIm+Cl−) is a polymer plastic (Tg = 92 °C) when completely dry. Poly(ECH-MeIm+TFSI−) is elastomeric (Tg = −12 °C), whereas poly(ECH-MeIm+BF4−) is a plastic (Tg = 67 °C). Poly(ECH-MeIm+Cl−) rapidly absorbed a large amount of moisture from the atmosphere, changing its appearance from solid to a viscous fluid even under relatively dry conditions (23 °C, and 40% relative humidity (RH); water uptake = 29 wt%), suggesting its highly deliquescent nature. The hydrated poly(ECH-MeIm+Cl−) possesses neither a glass transition nor a phase transition between −60 °C and 80 °C. The ionic conductivity of the hydrated polymer is as high as 1.8 × 10−2 S cm−1 under humid conditions (23 °C, and 80% RH; water uptake = 72 wt%) and is dependent mainly on water uptake. The miscibility between the cationic polyethers and ionic liquids is quite high; mixtures of ethylmethylimidazolium bis(trifluoromethanesulfonyl)imide and the cationic polyethers had no phase transitions between −70 °C and 100 °C. Since the cationic polyethers were miscible with each other to exchange anions, random copolymers with desired copolymerization ratios can be prepared through simple mixing.
Introduction
An ionic liquid is generally defined as a molten organic salt with a melting point lower than 100 °C.1 Since their discovery, ionic liquids have been extensively studied due to their high ionic densities, fluidities and negligible vapor pressures.2–4 Recent reports show that the properties of ionic liquids largely depend on the combination of the cation and anion that are chosen from the ionic liquid library.An ionic polymer is comprised of a polymer chain and a positively or negatively charged ionic moiety in each repeating unit. A poly(ionic liquid) (poly(IL)) is recognized as a specific type of ionic polymer in which the ionic group is composed of an organic cation and anion.5–7 The synthesis and properties of poly(IL)s have been extensively studied in the past two decades, and now it is established as an emerging field in chemistry.8–21 The development of poly(IL)s not only allowed combining the unique properties of ionic liquids with the processability and solidity of polymer materials, but also allowed for the attainment of new functions. There are two basic methods to synthesize poly(IL)s. One is the direct polymerization of ionic liquid monomers, and the other is the quaternization of existing polymer materials. Most poly(IL)s are vinyl polymers, having quaternary ammonium salts as side groups. Since propagation radicals are stable in the presence of ionic moieties and polar impurities, the poly(IL)s are synthesized using free radical polymerization or the controlled radical polymerization of vinyl monomers with ionic liquid groups. Consequently, conventional poly(IL)s mostly have hydrocarbon main chains which work as hydrophobic and rigid components for the ionic polymer materials. However, methodologies to synthesize a poly(IL) with a polar main chain have still been quite limited. One can expect that the polar backbone would enhance the polarity of the polymer, and increase the ionic conductivity and the hydrophilicity.
Polyethers are versatile polar elastic materials with hydrophilic and flexible chains. For instance, non-substituted poly(ethylene oxide) is a hydrophilic crystalline elastomer, while substituted ones such as poly(propylene oxide) and poly(epichlorohydrin) (poly(ECH)) are hydrophobic elastomers due to their non-polar side groups. Poly(ethylene oxide) is commonly used in a variety of applications due to its hydrophilicity, high solubility of ionic materials, ionic conductivity and low toxicity, originating from the poly(oxirane) backbone. Poly(ethylene oxide) is generally synthesized via the living anionic polymerization of ethylene oxide using alkaline metal-based initiators.22 Substituted polyethers such as poly(ECH) and its copolymers are mainly applied as synthetic rubbers. These polyethers are prepared using coordination anionic polymerization catalysts.23–25 Due to the limited ability of coordination anionic polymerization catalysts to precisely control the polymerization, the existing poly(ECH) and ECH-copolymers have extremely high molecular weights (MW) and broad molecular weight distributions (MWD). Recently, many researchers have developed new Al-based catalysts for living polymerizations of ethylene oxide and propylene oxide with high initiation efficiencies.26–28 Deffieux's group29,30 and our group31 have independently developed the living anionic polymerization of functional epoxides such as epichlorohydrin (ECH). This method appeared to be a useful tool to accomplish the control of the molecular weight, molecular weight distribution and end functionality of polyethers. Poly(ECH), with a controlled structure, could be a useful precursor for a new type of well-defined poly(IL) if the chloromethyl group of each repeating unit can be quaternized by a tertiary amine such as 1-methylimidazole in a quantitative manner.
The cationization of high MW poly(ECH) and ECH-copolymers has not been thoroughly investigated. Stoica’s group studied the quaternization of a commercially available ECH-copolymer using a mixture of 1-azabicyclo-[2.2.2]-octane and 1,4-diazabicyclo-[2.2.2]-octane. Unfortunately, the cationization ratio appeared to be around 10% and the obtained polymer was not fully characterized.32,33 In our unpublished data, the quaternization of commercially available poly(ECH) was quite difficult because of its high MW.34 Recently, the quaternization of poly(ECH) with a well-controlled MW was studied by Baker et al.35,36 and by our group.37,38 Baker's group focused on the synthesis and properties of cationic polyether with less polar butylimidazolium bis(trifluoromethanesulfonyl)imide in each repeating unit, however, the molecular structure has not been fully characterized. To our knowledge there is no report concerning cationized poly(ECH) with highly polar methylimidazolium chloride. Furthermore, it can be expected that imidazolium-based poly(IL)s with ether groups would be unique materials due to competitive interactions between the ether oxygens and anions with the imidazolium protons.39 In particular, such competitive interactions would weaken the cation–anion interactions of the ionic groups and change various functions of the poly(IL), including the thermal properties, ionic conductivity and affinity to CO2.20,36
Here we report on well-defined cationic polyethers with imidazolium-based ionic liquid moieties in each repeating unit, with narrow MWDs. Living anionic polymerization of epichlorohydrin was conducted first, and then quaternization of the obtained poly(ECH) was carried out. As a result, well-defined poly(ECH) was successfully quaternized using 1-methylimidazole to produce a highly hydrophilic cationic polyether, poly(N-glycidyl-N′-methylimidazolium chloride) (poly(ECH-MeIm+Cl−)), with desirable outcomes. It is worthy to note that poly(ECH-MeIm+Cl−) is a highly deliquescent polymer. The hydrated poly(ECH-MeIm+Cl−) was characterized with high ionic conductivity and high water retention. Furthermore, anion exchanges of the poly(ECH-MeIm+Cl−) were conducted using lithium salts to form poly(N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide) (poly(ECH-MeIm+TFSI−)) and poly(N-glycidyl-N′-methylimidazolium tetrafluoroborate) (poly(ECH-MeIm+BF4−)). The variety of the properties of these cationized polyethers depended mainly on the combination of cation and anion. These cationized polyethers showed high miscibility with imidazolium-based ionic liquids. The cationic polyethers were miscible with each other to exchange anions, therefore a random copolymer with a desired copolymerization ratio was prepared using simple mixing.
Experimental
General remarks
All operations were carried out in a glove-box (nitrogen atmosphere, O2 < 1 ppm and H2O < 1 ppm) or under an argon atmosphere using standard Schlenk techniques. Me3Al (Kanto Chemical), Et3Al (Kanto Chemical), i-Bu3Al (Kanto Chemical), n-Oc3Al (Kanto Chemical), Me4NBr (Tokyo Kasei), n-Bu4NBr (Tokyo Kasei), n-Oc4NBr (Tokyo Kasei), lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) (Kanto Chemical), lithium tetrafluoroborate (LiBF4) (Kanto Chemical), ethylmethylimidazolium bis(trifluoromethanesulfonyl)imide ([EMIm+][TFSI−]) (Tokyo Kasei), butylmethylimidazolium bis(trifluoromethanesulfonyl)imide ([BMIm+][TFSI−]) (Tokyo Kasei), ethylmethylimidazolium chloride ([EMIm+][Cl−]) (Tokyo Kasei), butylmethylimidazolium chloride ([BMIm+][Cl−]) (Tokyo Kasei), ethylmethylimidazolium tetrafluoroborate ([EMIm+][BF4−]) (Aldrich) and butylmethylimidazolium tetrafluoroborate ([BMIm+][BF4−]) (Aldrich) were used as received. Dehydrated reaction solvents such as toluene, tetrahydrofuran (THF), acetonitrile (CH3CN), dimethylsulfoxide (DMSO) and 1,4-dioxane were used without further purification. Epichlorohydrin (Tokyo Kasei) and 1-methylimidazole (Wako Chemicals) were distilled from calcium hydride. Chloroform-d (CDCl3) and dimethylsulfoxide-d6 (DMSO-d6) were dehydrated using activated molecular sieves 3A prior to use. n-Hexane, chloroform (CHCl3), dimethylformamide (DMF), acetone and methanol (MeOH) were used as received. Water was purified using a deionizer.Analysis of the polymers
1H and 13C NMR spectra were obtained using a JEOL JNM-EX400WB spectrometer (399.78 MHz for 1H and 100.53 MHz for 13C) at 25 °C or on a Bruker Avance III 500 MHz spectrometer (500.13 MHz for 1H and 125.77 MHz for 13C) at 27 °C. Chemical shifts were determined using tetramethylsilane (δ 0.00 ppm), dimethylsulfoxide (δ 2.50 ppm for 1H, δ 39.5 ppm for 13C) or chloroform (δ 7.24 ppm for 1H, δ 77.2 ppm for 13C) as references. The molecular weights (MW) of the polymers were estimated using a gel-permeation chromatograph (GPC) (Tosoh HLC-8220 GPC; eluent THF, or Tosoh HLC-8320 GPC; eluent 0.1 M NaNO3 aq). The relative number- and weight-average molecular weights (Mn and Mw, respectively) and molecular weight distribution (MWD; Mw/Mn) were acquired from calibration curves obtained using polystyrene or poly(ethylene oxide) standards. Differential scanning calorimetry (DSC) measurements were performed on a SII NanoTechnology X-DSC7000 under a dry nitrogen stream. Thermogravimetry (TG/DTA) was recorded on a SII NanoTechnology TG/DTA7200 under a dry nitrogen stream. Water uptake was measured using gravimetry in a thermo-hygrostat chamber (Espec Corp., TBL-2HW2P3A). Ionic conductivity values were estimated using electrochemical impedance spectroscopy, using a Solartron 1281 Multiplexer (Toyo Technica) and a Solartron 1287 Electrochemical Interphase (Toyo Technica) in the frequency range of 32 MHz to 0.1 Hz at 25 °C. The samples for the impedance spectroscopy were placed between aluminum electrodes in a thermo-hygrostat chamber, and then sealed prior to the measurement.Syntheses of the polymers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†). The polymer yield was determined using gravimetric measurement.
000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†). The polymer yield was determined using gravimetric measurement.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†) and 1-methylimidazole, equimolar to the ECH units, were introduced in a glass flask equipped with a three-way stopcock. The mixture containing 26 wt% of the poly(ECH) was then heated to the reaction temperature. The quaternization reaction of the poly(ECH) was carried out at 80 °C for 96 h with stirring. At the end of the polymer reaction, a fully cationized polyether reprecipitated as an oil. After the quaternization, the reaction mixture was cooled to room temperature. The resulting poly(N-glycidyl-N′-methylimidazolium chloride), poly(ECH-MeIm+Cl−) (Mn(calc) = 18
000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†) and 1-methylimidazole, equimolar to the ECH units, were introduced in a glass flask equipped with a three-way stopcock. The mixture containing 26 wt% of the poly(ECH) was then heated to the reaction temperature. The quaternization reaction of the poly(ECH) was carried out at 80 °C for 96 h with stirring. At the end of the polymer reaction, a fully cationized polyether reprecipitated as an oil. After the quaternization, the reaction mixture was cooled to room temperature. The resulting poly(N-glycidyl-N′-methylimidazolium chloride), poly(ECH-MeIm+Cl−) (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108), was washed with acetone to remove residual acetonitrile and 1-methylimidazole, and dried in vacuo at 80 °C for 24 h. 1H NMR and 13C NMR spectra of the obtained poly(ECH-MeIm+Cl−) are illustrated in Fig. S1(a) and (b),† respectively. A theoretically predicted number-average molecular weight of the poly(ECH-MeIm+Cl−) was obtained using the following equation: Mn(calc) = (MW of N-glycidyl-N′-methylimidazolium chloride) × (DP of the poly(ECH) as a starting material).
900, and DP = 108), was washed with acetone to remove residual acetonitrile and 1-methylimidazole, and dried in vacuo at 80 °C for 24 h. 1H NMR and 13C NMR spectra of the obtained poly(ECH-MeIm+Cl−) are illustrated in Fig. S1(a) and (b),† respectively. A theoretically predicted number-average molecular weight of the poly(ECH-MeIm+Cl−) was obtained using the following equation: Mn(calc) = (MW of N-glycidyl-N′-methylimidazolium chloride) × (DP of the poly(ECH) as a starting material).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108) (10 wt%) were mixed at room temperature and stirred for 30 min. The mole ratio of LiTFSI and MeIm+Cl− groups was equivalent. The resulting polymer precipitated as a viscous oil just after mixing. After separating the viscous oil compound from the aqueous LiCl solution by decantation, the polymer was dried in vacuo at 80 °C for 24 h to produce poly(N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide) (poly(ECH-MeIm+TFSI−)) (Mn(calc) = 45
900, and DP = 108) (10 wt%) were mixed at room temperature and stirred for 30 min. The mole ratio of LiTFSI and MeIm+Cl− groups was equivalent. The resulting polymer precipitated as a viscous oil just after mixing. After separating the viscous oil compound from the aqueous LiCl solution by decantation, the polymer was dried in vacuo at 80 °C for 24 h to produce poly(N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide) (poly(ECH-MeIm+TFSI−)) (Mn(calc) = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, and DP = 108) as a viscous, transparent oil. 1H NMR and 13C NMR spectra of the obtained poly(ECH-MeIm+TFSI−) are described in Fig. S2(a) and (b),† respectively. A theoretically predicted number-average molecular weight of the poly(ECH-MeIm+TFSI−) was obtained using the following equation: Mn(calc) = (MW of N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide) × (DP of the poly(ECH) as a starting material).
400, and DP = 108) as a viscous, transparent oil. 1H NMR and 13C NMR spectra of the obtained poly(ECH-MeIm+TFSI−) are described in Fig. S2(a) and (b),† respectively. A theoretically predicted number-average molecular weight of the poly(ECH-MeIm+TFSI−) was obtained using the following equation: Mn(calc) = (MW of N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide) × (DP of the poly(ECH) as a starting material).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108) (10 wt%) were mixed at room temperature and stirred for 30 min. The mole ratio of LiBF4 and MeIm+Cl− groups was equivalent. The resulting polymer precipitated as a viscous oil just after mixing. After separating the viscous oil compound from the methanol LiCl solution by decantation, the polymer was dried in vacuo at 80 °C for 24 h to produce poly(N-glycidyl-N′-methylimidazolium tetrafluoroborate) (poly(ECH-MeIm+BF4−)) (Mn(calc) = 24
900, and DP = 108) (10 wt%) were mixed at room temperature and stirred for 30 min. The mole ratio of LiBF4 and MeIm+Cl− groups was equivalent. The resulting polymer precipitated as a viscous oil just after mixing. After separating the viscous oil compound from the methanol LiCl solution by decantation, the polymer was dried in vacuo at 80 °C for 24 h to produce poly(N-glycidyl-N′-methylimidazolium tetrafluoroborate) (poly(ECH-MeIm+BF4−)) (Mn(calc) = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and DP = 108) as a brittle, transparent resin. 1H NMR and 13C NMR spectra of the obtained poly(ECH-MeIm+BF4−) are described in Fig. S3(a) and (b),† respectively. A theoretically predicted number-average molecular weight of the poly(ECH-MeIm+BF4−) was calculated based on the following equation: Mn(calc) = (MW of N-glycidyl-N′-methylimidazolium tetrafluoroborate) × (DP of the poly(ECH) as a starting material).
500, and DP = 108) as a brittle, transparent resin. 1H NMR and 13C NMR spectra of the obtained poly(ECH-MeIm+BF4−) are described in Fig. S3(a) and (b),† respectively. A theoretically predicted number-average molecular weight of the poly(ECH-MeIm+BF4−) was calculated based on the following equation: Mn(calc) = (MW of N-glycidyl-N′-methylimidazolium tetrafluoroborate) × (DP of the poly(ECH) as a starting material).
Results and discussion
Synthesis of cationic polyether: quaternization of poly(ECH) and successive anion exchange
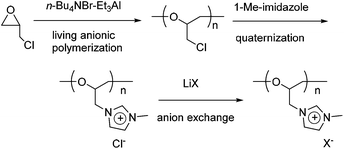
Cationic polyethers with imidazolium-based ionic liquid moieties were synthesized using the living anionic polymerization of epichlorohydrin (ECH), followed by quaternization of the chloromethyl groups of poly(epichlorohydrin) (poly(ECH)) with 1-methylimidazole, and anion exchange of the formed imidazolium chloride groups (Scheme 1). In this section, the quaternization and anion exchange processes are outlined in detail, while the living polymerization of ECH is described in the ESI.†It is well known that the quaternization reaction between a tertiary amine and an alkyl halide can be accelerated in polar solvents such as CH3CN and DMF. Thus, it appears relevant that the polarity of a solvent would influence the quaternization of the chloromethyl groups in poly(ECH). Therefore, we investigated the effect of reaction solvent on the quaternization of poly(ECH) (Mn = 1200, and Mw/Mn = 1.22; Table S1, run 5†) using 1-methylimidazole first, with the results summarized in Fig. S8.† When toluene was used as the solvent, all of the polymers precipitated during the early period of quaternization, and as a result, the cationization reaction levelled off at a low quaternization ratio. The quaternization proceeded slowly to a certain extent in THF, however the reaction mixture became heterogeneous during the middle period of the reaction. In contrast, polar solvents were shown to be suitable for full quaternization of poly(ECH). The polymer reaction proceeded smoothly and steadily in CH3CN, and eventually a fully cationized polymer precipitated as an oil at the end of the polymer reaction. DMSO and 1,4-dioxane were also found to be good solvents for the quaternization of poly(ECH). Cationization proceeded relatively fast when poly(ECH) and 1-methylimidazole were mixed and heated in bulk. In this study, we decided to employ CH3CN as the reaction solvent for the quaternization of the polymer.
To describe the cationization in detail, the poly(ECH) (Mn = 1200; degree of polymerization (DP) = 13; Mw/Mn = 1.22 (Table S1, run 5†)) was quaternized in the presence of 2 equivalents of 1-methylimidazole for each chloromethyl group, in CH3CN at 80 °C.40 The quaternization ended within 96 h resulting in fully cationized polyether, precipitated as an oil. The obtained oily compound was first washed with acetone in order to extract 1-methylimidazole and CH3CN, and then dried in vacuo at 80 °C for 24 h to remove residual volatiles and to isolate poly(N-glycidyl-N′-methylimidazolium chloride) (poly(ECH-MeIm+Cl−)) as a brittle polymer plastic (a macroscopic view is shown in Fig. 5(a)). Fig. 1 shows the 1H NMR spectra and GPC traces of the poly(ECH) before and after quaternization. In Fig. 1(b), proton signals of the poly(ECH-MeIm+Cl−) are observed between 8–10 ppm. The chemical shifts of the imidazolium protons are similar to those of [EMIm+][Cl−] and appear in a lower magnetic field compared to those of 1-methylimidazole (Fig. S9† illustrates selected 1H NMR spectra of [EMIm+][Cl−] and poly(ECH-MeIm+Cl−)). The integration ratios between the protons in the imidazolium ring and the protons in the main chain suggest the full conversion of the chloromethyl groups to form the imidazolium chloride groups. It is interesting that there are no proton signals attributable to compounds derived from side reactions, including the Hofmann elimination. The proton signal at 6 ppm is attributed to the terminal OH group. Fig. 1 also illustrates GPC traces of the poly(ECH) before (c) and after (d) the quaternization. THF was chosen as the eluent for poly(ECH), while 0.1 M NaNO3 aq. was chosen for poly(ECH-MeIm+Cl−). Suitable GPC columns were employed for each condition. It is noteworthy that the obtained cationic polyether kept a narrow MWD during the polymer reaction at 80 °C for 96 h, suggesting no degradation of the main chain. In contrast, when poly(ECH) was quaternized at 150 °C, the GPC elution curve of the cationized poly(ECH) became broad, suggesting degradation of the main chain. Therefore it can be concluded that poly(ECH-MeIm+Cl−) is unstable at high temperatures when it is in a solution, and that the quaternization must not be performed at high temperature.
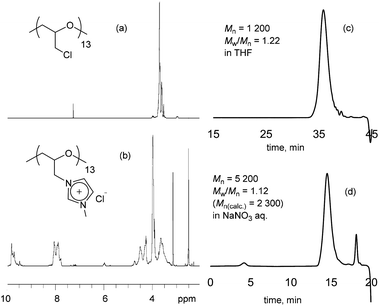 | ||
| Fig. 1 1H NMR spectra of (a) poly(ECH) (in CDCl3; Mn = 1200; DP = 13, as described in Table S1, run 5†) and (b) poly(ECH-MeIm+Cl−) (in DMSO-d6; Mn(calc) = 2300; DP = 13), and GPC chromatograms of (c) poly(ECH) and (d) poly(ECH-MeIm+Cl−). | ||
To summarize, the data in Fig. 1 indicate that cationic polyether, poly(ECH-MeIm+Cl−), was successfully produced. The estimated Mn of poly(ECH) from GPC (PSt calibration) is almost identical to the predicted Mn value based on the following equation: Mn(calc) = (ECH loading/g)/(n-Bu4NBr loading/mol). On the other hand, the relative Mn of poly(ECH-MeIm+Cl−) collected using GPC is 5200, as acquired using poly(ethylene glycol) calibration, which differs from the predicted value (Mn(calc) = 2300; DP = 13). At this moment, the origin of the inaccurate MW reading of poly(ECH-MeIm+Cl−) from GPC is not evident. It is presumable that repulsion of the ionic moiety or partial anion exchange of the poly(ECH-MeIm+Cl−) with NaNO3 in the eluent, or a combination of both, might have influenced the hydrodynamic volume of the polymer. We performed additional GPC analyses of poly(ECH-MeIm+Cl−) samples with different MWs ((a) Mn(calc) = 1900; DP = 11, (b) Mn(calc) = 5800; DP = 33, and (c) Mn(calc) = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200; DP = 92) produced from poly(ECH) samples of different MW. GPC elution curves of the poly(ECH-MeIm+Cl−) samples are depicted in Fig. S10.† All of the polymers retained their narrow MWDs after the cationization reaction, irrespective of their MWs. A peak shift was observed for the retention time when the MW of the starting polymer changed. The results implied that the hydrodynamic volumes of the cationic polyethers are dependent on their molecular weights. In conclusion, cationic polyether, poly(ECH-MeIm+Cl−), was successfully produced.
200; DP = 92) produced from poly(ECH) samples of different MW. GPC elution curves of the poly(ECH-MeIm+Cl−) samples are depicted in Fig. S10.† All of the polymers retained their narrow MWDs after the cationization reaction, irrespective of their MWs. A peak shift was observed for the retention time when the MW of the starting polymer changed. The results implied that the hydrodynamic volumes of the cationic polyethers are dependent on their molecular weights. In conclusion, cationic polyether, poly(ECH-MeIm+Cl−), was successfully produced.
Anion exchange of poly(ECH-MeIm+Cl−) with LiTFSI was investigated. Aqueous solutions of LiTFSI and poly(ECH-MeIm+Cl−) (Mn(calc) = 2300; DP = 13), depicted in Fig. 1(b) and (d), were mixed at room temperature and stirred for 30 min with an equal mole ratio between the LiTFSI and MeIm+Cl− groups. A viscous oily compound precipitated just after mixing these two aqueous solutions. After separation of the resulting compound from the LiCl aqueous solution by decantation, the viscous oil was dried in vacuo at 80 °C for 24 h to give poly(N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide) (poly(ECH-MeIm+TFSI−)) as a transparent viscous oil (Fig. 5(c) shows a macroscopic view). The predicted value of Mn for poly(ECH-MeIm+TFSI−) was 5500. Similarly, poly(N-glycidyl-N′-methylimidazolium tetrafluoroborate) (poly(ECH-MeIm+BF4−)) was also produced via the anion exchange of poly(ECH-MeIm+Cl−) with LiBF4. Fig. 2 shows selected 1H NMR spectra of (a) poly(ECH-MeIm+Cl−), (b) poly(ECH-MeIm+TFSI−) and (c) poly(ECH-MeIm+BF4−).
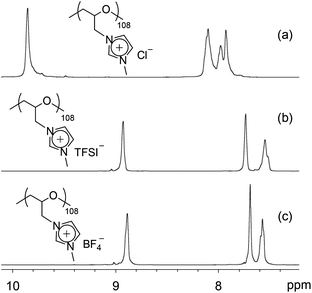 | ||
Fig. 2 Selected 1H NMR spectra of (a) poly(ECH-MeIm+Cl−) (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108), (b) poly(ECH-MeIm+TFSI−) (Mn(calc) = 45 900, and DP = 108), (b) poly(ECH-MeIm+TFSI−) (Mn(calc) = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, and DP = 108), and (c) poly(ECH-MeIm+BF4−) (Mn(calc) = 24 400, and DP = 108), and (c) poly(ECH-MeIm+BF4−) (Mn(calc) = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and DP = 108), as described in Fig. S1(a), S2(a), and S3(a),† respectively (recorded in DMSO-d6 at 27 °C). 500, and DP = 108), as described in Fig. S1(a), S2(a), and S3(a),† respectively (recorded in DMSO-d6 at 27 °C). | ||
The proton signals of the imidazolium ring shifted to the higher magnetic field after the anion exchange reaction, whilst keeping the integration ratios of the imidazolium protons and the main chain protons constant. These observations are in good agreement with the fact that the proton signals of the imidazolium ring of [EMIm+][TFSI−] are in a higher magnetic field compared to those of [EMIm+][Cl−] (Fig. S9†). These results suggest that the poly(ECH-MeIm+TFSI−) and the poly(ECH-MeIm+BF4−) were successfully produced via the anion exchange reactions.
Furthermore, anion exchange was conducted for poly(ECH-MeIm+Cl−) samples of varied MWs ((a) Mn(calc) = 1900, and DP = 11; (b) Mn(calc) = 5800, and DP = 33; and (c) Mn(calc) = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, and DP = 92) which were obtained from poly(ECH) samples of different MWs. Similarly, tacky oil products were formed through anion exchange with LiTFSI. NMR analyses of the obtained oily products (Fig. S11†) confirmed that the TFSI anions were introduced to the poly(ECH-MeIm+Cl−) samples to yield poly(ECH-MeIm+TFSI−) samples, irrespective of MW. In addition, C–F coupling was observed in the 13C NMR spectra in Fig. S2(b),† indicating the CF3 carbon of the TFSI anion. To summarize, anion exchanges were conducted successfully.
200, and DP = 92) which were obtained from poly(ECH) samples of different MWs. Similarly, tacky oil products were formed through anion exchange with LiTFSI. NMR analyses of the obtained oily products (Fig. S11†) confirmed that the TFSI anions were introduced to the poly(ECH-MeIm+Cl−) samples to yield poly(ECH-MeIm+TFSI−) samples, irrespective of MW. In addition, C–F coupling was observed in the 13C NMR spectra in Fig. S2(b),† indicating the CF3 carbon of the TFSI anion. To summarize, anion exchanges were conducted successfully.
Fundamental properties of cationic polyethers
Next, the solubility of poly(ECH), poly(ECH-MeIm+Cl−), poly(ECH-MeIm+TFSI−) and poly(ECH-MeIm+BF4−), as shown in Fig. 2, was studied and the results are summarized in Table 1. Poly(ECH) dissolves in ordinary organic solvents including toluene and THF. In contrast, poly(ECH-MeIm+Cl−) is soluble only in polar solvents (e.g., H2O, MeOH and DMSO), and therefore is proven to be a highly polar material. Poly(ECH-MeIm+TFSI−) is soluble in mid-polar solvents such as acetone, CH3CN, THF and DMF, while it is insoluble in both non-polar and highly polar solvents. Poly(ECH-MeIm+BF4−) appears to have limited solubility; the polymer is soluble in only H2O and aprotic polar solvents.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†), poly(ECH-MeIm+Cl−) (Mn(calc) = 18
000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†), poly(ECH-MeIm+Cl−) (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108), poly(ECH-MeIm+TFSI−)(Mn(calc) = 45
900, and DP = 108), poly(ECH-MeIm+TFSI−)(Mn(calc) = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, and DP = 108) and poly(ECH-MeIm+BF4−) (Mn(calc) = 24
400, and DP = 108) and poly(ECH-MeIm+BF4−) (Mn(calc) = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and DP = 108), as shown in Fig. 2
500, and DP = 108), as shown in Fig. 2
| Poly(ECH) | Poly(ECH-MeIm+Cl−) | Poly(ECH-MeIm+TFSI−) | Poly(ECH-MeIm+BF4−) | |
|---|---|---|---|---|
| n-Hexane | × | × | × | × |
| Toluene | Soluble | × | × | × |
| CHCl3 | Soluble | × | × | × |
| THF | Soluble | × | Soluble | × |
| CH3CN | Soluble | × | Soluble | Soluble |
| Acetone | × | × | Soluble | × |
| MeOH | × | Soluble | × | × |
| H2O | × | Soluble | × | Soluble |
| DMF | Soluble | × | Soluble | Soluble |
| DMSO | Soluble | Soluble | Soluble | Soluble |
The miscibility of ionic liquids with water or organic solvents changes with a side chain on the cation and with the choice of anion. More specifically, [EMIm+][TFSI−] is hydrophobic, while [EMIm+][Cl−] and [EMIm+][BF4−] are hydrophilic. The solubility of these cationic polyethers might be influenced by the choice of anion. It is noteworthy that the cationic polyethers are miscible with various ionic liquids; the results are detailed below.
The thermal properties of the cationic polyethers were studied. Fig. 3 illustrates the DSC thermograms of poly(ECH), poly(ECH-MeIm+Cl−), poly(ECH-MeIm+TFSI−), and poly(ECH-MeIm+BF4−), as depicted in Fig. 2 and Table 1. As expected, poly(ECH) had the lowest glass transition point (Tg = −45 °C). The dry poly(ECH-MeIm+Cl−) shown in Fig. 5(a) appeared as a brittle resin. To prove this speculation, its glass transition point was higher than room temperature (Tg = 90 °C). This result indicates that the ionic interaction and the hydrogen bonding between the ionic groups and polyether chain decreased the mobility of the polymer. The poly(ECH-MeIm+TFSI−) has a glass transition point at −12 °C and no melting point. The macroscopic view shown in Fig. 5(c) indicates that poly(ECH-MeIm+TFSI−) is a liquid rubber, and the DSC data supports this idea. The Tg of the poly(ECH-MeIm+TFSI−) is comparable with that of poly(N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide) (Tg = −14 °C) reported by Baker et al.36 The DSC thermogram of poly(ECH-MeIm+BF4−) shows a glass transition at 65 °C, not a phase transition, which appears to be like a melting point peak. It is interesting that a very large relaxation of the constrained poly(ECH-MeIm+BF4−) was observed at the glass transition point, but we do not have any experimental data to explain this phenomena. To summarize, all of the cationic polyethers are amorphous polymers, in which the glass transition points are dependent on the counter anions.
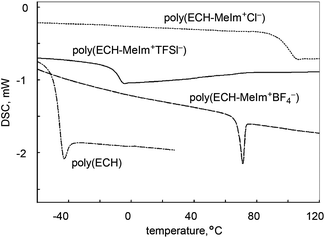 | ||
Fig. 3 DSC thermograms (heating steps) of poly(ECH) (Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†), poly(ECH-MeIm+Cl−) (Mn(calc) = 18 000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†), poly(ECH-MeIm+Cl−) (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108), poly(ECH-MeIm+TFSI−) (Mn(calc) = 45 900, and DP = 108), poly(ECH-MeIm+TFSI−) (Mn(calc) = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, and DP = 108), and poly(ECH-MeIm+BF4−) (Mn(calc) = 24 400, and DP = 108), and poly(ECH-MeIm+BF4−) (Mn(calc) = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and DP = 108), as described in Fig. 2 and Table 1 (determined under N2, at 10 °C min−1 for each step). 500, and DP = 108), as described in Fig. 2 and Table 1 (determined under N2, at 10 °C min−1 for each step). | ||
Furthermore, the thermal stability of the cationic polyethers was studied using TG/DTA measurement. Fig. 4 summarizes the DTA analysis under an N2 stream for poly(ECH), poly(ECH-MeIm+Cl−), poly(ECH-MeIm+TFSI−), and poly(ECH-MeIm+BF4−), which were described in Fig. 3. The poly(ECH) started thermal decomposition above 300 °C and the 5% thermal decomposition temperature (Td5%) was 330 °C. The Td of ethylmethylimidazolium bromide and [BMIm+][Cl−] are reported to be around 220 °C and 240 °C, respectively, and the low thermal stability may be attributed to the nucleophilic attack of the halide anion on the imidazolium ring. It is interesting that Td5% of the poly(ECH-MeIm+Cl−) is as high as 298 °C. At first, the water confined in the poly(ECH-MeIm+Cl−) started to evaporate at 60 °C. Then, the weight of the poly(ECH-MeIm+Cl−) became almost constant above 100 °C. Finally, the poly(ECH-MeIm+Cl−) started to decompose around 300 °C.41 In contrast, Stoica et al. reported that the partially cationized polyether started to decompose at 220 °C.32 This can be explained by the idea that densely branched ionic liquid groups were stabilized by each other and by the polyether chain. This phenomenon was also observed not only in another polymerized ionic liquid but also in another type of ionic liquid complex.42,43 Poly(ECH-MeIm+TFSI−) is highly stable at elevated temperatures. The Td5% of [EMIm+][TFSI−] is reported to be above 400 °C. Similar to the case of the Td5% of poly(N-glycidyl-N′-butyllimidazolium bis(trifluoromethanesulfonyl)imide) reported by Baker et al.,36 the Td5% of the poly(ECH-MeIm+TFSI−) is around 400 °C. In this case the polyether chain was thermally stabilized by the MeIm+TFSI− group. It is interesting to note that the Td5% of the poly(ECH-MeIm+BF4−) is also high at around 350 °C. Note, that the Td5% of [EMIm+][BF4−] was reported at around 350 °C. We conducted TG-DTA analysis of commercially available [EMIm+][BF4−] without purification, and found that the Td5% was 265 °C. Bearing the thermal stabilities of poly(ECH) and [EMIm+][BF4−] in mind, it can be said that the polyether chain and the MeIm+BF4− group of poly(ECH-MeIm+BF4−) stabilize each other. To summarize, the cationic polyethers are thermally stable.
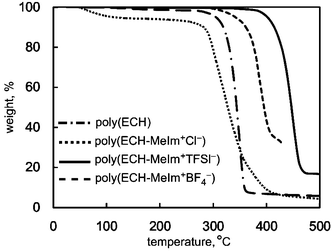 | ||
Fig. 4 TG/DTA thermograms of poly(ECH) (Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†), poly(ECH-MeIm+Cl−) (Mn(calc) = 18 000, Mw/Mn = 1.13, and DP = 108; Table S1, run 7†), poly(ECH-MeIm+Cl−) (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108), poly(ECH-MeIm+TFSI−) (Mn(calc) = 45 900, and DP = 108), poly(ECH-MeIm+TFSI−) (Mn(calc) = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, and DP = 108), and poly(ECH-MeIm+BF4−) (Mn(calc) = 24 400, and DP = 108), and poly(ECH-MeIm+BF4−) (Mn(calc) = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and DP = 108), as described in Fig. 2, Fig. 3 and Table 1 (determined under N2, at 10 °C min−1 for each). 500, and DP = 108), as described in Fig. 2, Fig. 3 and Table 1 (determined under N2, at 10 °C min−1 for each). | ||
Deliquescent nature of poly(ECH-MeIm+Cl−)
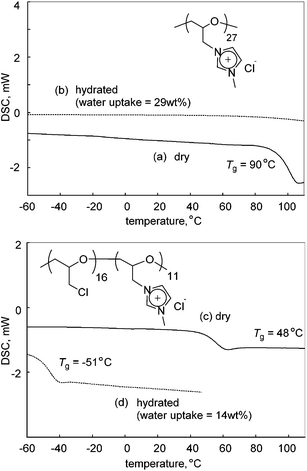
One of the most significant properties of poly(ECH-MeIm+Cl−) is a deliquescence feature coming from its high polarity. Fig. 5 illustrates macroscopic views of (a) poly(ECH-MeIm+Cl−) (Mn(calc) = 4700, and DP = 27; as described in Table 2 below) which was kept under a dry atmosphere, and (b) the hydrated poly(ECH-MeIm+Cl−) after being exposed to air under ambient conditions (23 °C, and 40% relative humidity (RH)) for 30 min. The poly(ECH-MeIm+Cl−) appears as an amorphous resin when it is completely dry (Fig. 5(a)). In general, polyethers, aside from crystalline poly(ethylene oxide), are recognized as elastomers. It is presumed that the ionic interaction and hydrogen bonding between the ionic groups strongly suppressed the molecular mobility of the polymer chain, and as a result, increased the glass transition point of the poly(ECH-MeIm+Cl−). When the poly(ECH-MeIm+Cl−) was exposed to air, it absorbed large amounts of moisture to be hydrated under relatively dry conditions (23 °C, 40% RH, and a water uptake = 29 wt%). The water-absorption process took less than 30 min when 0.5 g of the polymer powder was in contact with air. First, the surface of the polymer powder absorbed moisture to become wet. Then, the polymer continued to absorb water in air to change its appearance to something like a viscous fluid. Finally, the hydration of the polymer saturated to become an aqueous solution where the viscosity seemed very low (Fig. 5(b)). This result suggests that poly(ECH-MeIm+Cl−) is a deliquescent polymer. This character is supposed to be derived mainly from the specific property of the imidazolium chloride-based ionic liquid moiety; [EMIm+][Cl−] exhibits a high moisture absorption capacity. In addition, the hydrophilic polyether chain might play an important role in the deliquescent nature of poly(ECH-MeIm+Cl−).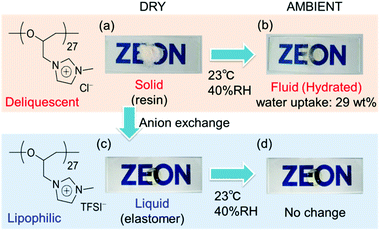 | ||
Fig. 5 Macroscopic views of (a) poly(ECH-MeIm+Cl−) (Mn(calc) = 4700, and DP = 27) prepared from poly(ECH) (Mn = 2500, and DP = 27; Table S1, run 6†) kept under a dry atmosphere, (b) poly(ECH-MeIm+Cl−) (Mn(calc) = 4700, and DP = 27) after being exposed to air (23 °C, and 40% RH) for 30 min, (c) the dry poly(ECH-MeIm+TFSI−) (Mn(calc) = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, and DP = 27) prepared via anion exchange with poly(ECH-MeIm+Cl−) (Mn(calc) = 4700, and DP = 27) and (d) poly(ECH-MeIm+TFSI−) (Mn(calc) = 11 300, and DP = 27) prepared via anion exchange with poly(ECH-MeIm+Cl−) (Mn(calc) = 4700, and DP = 27) and (d) poly(ECH-MeIm+TFSI−) (Mn(calc) = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, and DP = 27) after being exposed to air (23 °C, and 40% RH) for 24 h. 300, and DP = 27) after being exposed to air (23 °C, and 40% RH) for 24 h. | ||
| Run | DP | RHb (%) | Appearance | Water uptakec (wt%) | [H2O]/[cation]d | Ionic conductivityc (S cm−1) |
|---|---|---|---|---|---|---|
a Poly(ECH-MeIm+Cl−) (Mn(calc) = 4700; DP = 27) was prepared from poly(ECH) (Mn = 2500; DP = 27; Table S1, run 6†). Poly(ECH-MeIm+Cl−) (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900; DP = 108) was a cationized poly(ECH) (Mn = 10 900; DP = 108) was a cationized poly(ECH) (Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; DP = 108; Table S1, run 7†).
b The polymers were exposed to air at 23 °C for 6 h.
c The ionic conductivity was determined using impedance spectroscopy at 20 °C.
d Mole ratio. 000; DP = 108; Table S1, run 7†).
b The polymers were exposed to air at 23 °C for 6 h.
c The ionic conductivity was determined using impedance spectroscopy at 20 °C.
d Mole ratio.
|
||||||
| 1 | 27 | 0 | Solid | 0 | 0 | <10−10 |
| 2 | 27 | 20 | Liquid | 18 | 1.8 | 5.7 × 10−4 |
| 3 | 27 | 40 | Liquid | 29 | 2.8 | 2.7 × 10−3 |
| 4 | 27 | 60 | Liquid | 45 | 4.4 | 8.4 × 10−3 |
| 5 | 27 | 80 | Liquid | 72 | 7.0 | 1.8 × 10−2 |
| 6 | 108 | 0 | Solid | 0 | 0 | < 10−10 |
| 7 | 108 | 20 | Liquid | 18 | 1.8 | 5.1 × 10−4 |
| 8 | 108 | 40 | Liquid | 29 | 2.8 | 3.2 × 10−3 |
| 9 | 108 | 60 | Liquid | 44 | 4.3 | 5.9 × 10−3 |
| 10 | 108 | 80 | Liquid | 69 | 6.7 | 1.6 × 10−2 |
To the best of our knowledge, not much has been reported regarding deliquescent polymers.44 The water-uptake behavior, solubility and hydrophilicity of various poly(IL)s have been thoroughly studied in the past, however they were not deliquescent.5–8 For instance, we synthesized poly(methylvinylimidazolium iodide) according to the literature and exposed it to air under ambient conditions (23 °C, and 60% RH). It was confirmed to be hygroscopic but not deliquescent. Highly hygroscopic poly(acrylic acid) sodium salt (Mw = 5100) absorbed moisture in air to a certain extent (23 °C, and 60% RH; water uptake = 55 wt%) but did not display any fluidity. This suggests that poly(acrylic acid) sodium salt is not deliquescent but just hygroscopic under less-humid conditions. A partially cationized polyether was reported to swell in water, but it was not deliquescent.32,33 In contrast, fully cationized polyethers with imidazolium halide groups such as the low MW poly(ECH-MeIm+Cl−) (Mn(calc) = 4700, and DP = 27), the high MW one (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108) and poly(N-glycidyl-N′-methylimidazolium iodide) (Mn(calc) = 7200, and DP = 27) absorbed moisture to become fully plasticized and fluid (23 °C, and 60% RH; water uptake = 45 wt%, 44 wt% and 14 wt%, respectively). Note that poly(acrylic acid) sodium salt and poly(ethylene oxide) were reported to absorb small amounts of moisture under relatively dry conditions (30 °C, and 40% RH; water uptake <5 wt%),45 in contrast to the poly(ECH-MeIm+Cl−) which is deliquescent under similar conditions.
900, and DP = 108) and poly(N-glycidyl-N′-methylimidazolium iodide) (Mn(calc) = 7200, and DP = 27) absorbed moisture to become fully plasticized and fluid (23 °C, and 60% RH; water uptake = 45 wt%, 44 wt% and 14 wt%, respectively). Note that poly(acrylic acid) sodium salt and poly(ethylene oxide) were reported to absorb small amounts of moisture under relatively dry conditions (30 °C, and 40% RH; water uptake <5 wt%),45 in contrast to the poly(ECH-MeIm+Cl−) which is deliquescent under similar conditions.
With regards to the contribution of the counter anion to deliquescence, poly(ECH-MeIm+TFSI−) is hydrophobic (23 °C, and 40% RH; water uptake <0.2 wt%). The macroscopic view of the completely dry poly(ECH-MeIm+TFSI−) (Fig. 5(c)) didn't change after being exposed to air (Fig. 5(d)), indicating a low water absorbency for poly(ECH-MeIm+TFSI−). Poly(ECH-MeIm+BF4−) (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900, and DP = 108) is hygroscopic (23 °C, and 40% RH; water uptake = 3.3 wt%, 23 °C, and 60% RH; water uptake = 6.8 wt%) but not deliquescent. To summarize, the deliquescent character of the present cationic polyethers with imidazolium halide groups is supposed to be derived from the combination of both the imidazolium chloride groups and the polyether chain.46
900, and DP = 108) is hygroscopic (23 °C, and 40% RH; water uptake = 3.3 wt%, 23 °C, and 60% RH; water uptake = 6.8 wt%) but not deliquescent. To summarize, the deliquescent character of the present cationic polyethers with imidazolium halide groups is supposed to be derived from the combination of both the imidazolium chloride groups and the polyether chain.46
The relationship between water uptake and Mn of poly(ECH-MeIm+Cl−) was investigated to understand the effect of the polymer's MW on the hydration behavior. We prepared poly(ECH-MeIm+Cl−) with the following MWs (Mn(calc) = 2300, and DP = 13; Mn(calc) = 9400, and DP = 54; Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, and DP = 108) and allowed them to be exposed to air (0.5 g, 23 °C, and 40% RH). Similar to the case of the poly(ECH-MeIm+Cl−) stated above (Mn(calc) = 4700 and DP = 27, at 23 °C and 40% RH; water uptake = 29 wt%), the water-absorption process only needed 30–60 min. The hydration rate of each polymer will not be discussed here, because the surface area of each polymer powder seemed unclear. However, the saturated water uptake of each polymer was verified to be almost identical at around 29 wt% (water uptake = 30 wt% for Mn(calc) = 2300, 30 wt% for Mn(calc) = 9400, and 29 wt% for Mn(calc) = 18
800, and DP = 108) and allowed them to be exposed to air (0.5 g, 23 °C, and 40% RH). Similar to the case of the poly(ECH-MeIm+Cl−) stated above (Mn(calc) = 4700 and DP = 27, at 23 °C and 40% RH; water uptake = 29 wt%), the water-absorption process only needed 30–60 min. The hydration rate of each polymer will not be discussed here, because the surface area of each polymer powder seemed unclear. However, the saturated water uptake of each polymer was verified to be almost identical at around 29 wt% (water uptake = 30 wt% for Mn(calc) = 2300, 30 wt% for Mn(calc) = 9400, and 29 wt% for Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800). It can be said that the water uptake depends not on the molecular weight but on the molecular structure of the repeating units.
800). It can be said that the water uptake depends not on the molecular weight but on the molecular structure of the repeating units.
Effects of hydration on the properties of poly(ECH-MeIm+Cl−)
In order to obtain information regarding the effects of hydration on the thermal properties of the cationic polyethers, DSC measurements of poly(ECH-MeIm+Cl−), the hydrated poly(ECH-MeIm+Cl−), poly(ECH60-ran-(N-glycidyl-N′-methylimidazolium chloride)40) (poly(ECH-MeIm+Cl−40%)) and the hydrated poly(ECH-MeIm+Cl−40%) were conducted next (Fig. 6). The dry poly(ECH-MeIm+Cl−) shown in Fig. 5(a) appeared to be a brittle resin. As evidence of the speculation, its glass transition point was shown to be higher than room temperature (Tg = 90 °C; Fig. 6(a)). On the other hand, it is interesting that the hydrated poly(ECH-MeIm+Cl−) (23 °C, 40% RH, water uptake = 29 wt%, and [H2O]/[MeIm+Cl− group] = 2.8 mol/mol, in reference to Table 3) possessed neither a glass transition nor a phase transition between −60 °C and 80 °C (Fig. 6(b)).47 This suggests that the confined water does not freeze, and that the hydrated poly(ECH-MeIm+Cl−) can be regarded as an elastomer with a very low glass transition point. More specifically, the absorbed water might have a strong interaction with the MeIm+Cl− group of the present polymer and, as a result, the poly(ECH-MeIm+Cl−) was plasticized by the water, while the water molecule became non-freezing.| Ionization | 0 mol% | 20 mol% | 40 mol% | 60 mol% | 80 mol% | 100 mol% |
|---|---|---|---|---|---|---|
| a At 23 °C. b The ionic conductivity was measured using impedance spectroscopy at 20 °C. | ||||||
| RHa | Water uptake, wt% | |||||
| [H2O]/[cation], mol/mol | ||||||
Ionic conductivity, S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
||||||
| 20% | 0 | 1.7 | 6.3 | 13 | 15 | 18 |
| 0 | 0.5 | 1.1 | 1.7 | 1.6 | 1.8 | |
| 5.4 × 10−10 | 1.3 × 10−7 | 1.3 × 10−6 | 1.0 × 10−4 | 2.5 × 10−4 | 5.7 × 10−4 | |
| 40% | 4.1 | 14 | 21 | 25 | 29 | |
| 1.2 | 2.4 | 2.8 | 2.7 | 2.8 | ||
| 4.5 × 10−5 | 4.2 × 10−4 | 8.8 × 10−4 | 1.1 × 10−3 | 2.7 × 10−3 | ||
| 60% | 10 | 25 | 35 | 40 | 45 | |
| 3.0 | 4.4 | 4.6 | 4.4 | 4.4 | ||
| 6.2 × 10−4 | 2.4 × 10−3 | 2.7 × 10−3 | 4.5 × 10−3 | 8.4 × 10−3 | ||
| 80% | 22 | 43 | 59 | 66 | 72 | |
| 6.7 | 7.5 | 7.7 | 7.3 | 7.0 | ||
| 2.3 × 10−3 | 5.5 × 10−3 | 5.9 × 10−3 | 9.8 × 10−3 | 1.8 × 10−2 | ||
The 40% cationized poly(ECH), poly(ECH-MeIm+Cl−40%), was prepared from poly(ECH) (Mn = 2500, Mw/Mn = 1.20, and DP = 27; Table S1, run 6†). The dry poly(ECH-MeIm+Cl−40%) exhibited a glass transition point at 48 °C which is lower than that of the fully cationized polymer (Fig. 6(c)). This suggests a decrease of the ionic aggregation of the imidazolium groups and an increase in the molecular mobility of the chain. The hydrated poly(ECH-MeIm+Cl−40%) (23 °C, and 40% RH; water uptake = 14 wt%, and [H2O]/[MeIm+Cl− group] = 2.4 mol/mol, in reference to Table 3) had a very low glass transition point at −51 °C, and didn't have a phase transition point relating to the fusion of H2O (Fig. 6(d)). This result also supports the hypothesis above.
We found that the hydrated poly(ECH-MeIm+Cl−) was characterized to be highly conductive. Table 2 summarizes the water uptake and ionic conductivity of the hydrated poly(ECH-MeIm+Cl−) samples measured using dielectric spectroscopy under various RHs (Fig. S12† shows selected Nyquist plots of the poly(ECH-MeIm+Cl−) after being exposed to air with different RHs). Poly(ECH-MeIm+Cl−) is an insulator (conductivity < 10−10 S cm−1) if it is entirely dry. When the polymer was kept under humid conditions (23 °C, and 80% RH), the water uptake increased up to 72 wt% and the conductivity at 20 °C also elevated up to 1.8 × 10−2 S cm−1. To understand the effect of the incorporated water molecules on the ionic conductivity, the varied RH of the atmosphere was studied. Under relatively dry conditions (23 °C, and 20% RH), the water uptake remained at 18 wt% and the conductivity at 20 °C was 5.4 × 10−4 S cm−1. To roughly summarize, the water uptake increased with increasing RH, while the conductivity also increased with increasing water uptake. In contrast, the molecular weight had little effect on the water-uptake and the ionic conductivity. The properties of the fully cationized poly(ECH-MeIm+Cl−) samples with two different DPs are compared in Table 2. The water-uptake and the conductivity are dependent not on molecular weight but on RH.
Furthermore, we prepared cationized polyethers with different ionization ratios. Table 3 shows the RH dependence of the water uptake and the ionic conductivity of partially cationized polyethers. The [H2O]/[cation] ratio was dependent not on the cationization ratio but on the relative humidity, particularly when the ionization ratio and RH were not very low. The ionic conductivity was influenced by the [H2O]/[cation] ratio, while it was largely influenced by the water uptake. To provide an insight to these results, the water incorporated in the cationic polyethers appears to play an essential role in increasing the ionic conductivity. The origin of the high ionic conductivity of the hydrated polymers can be explained by a water-assisted transport mechanism that has been well studied for poly(IL)s and Nafions.11,12,48
Miscibility of cationic polyethers and ionic liquids
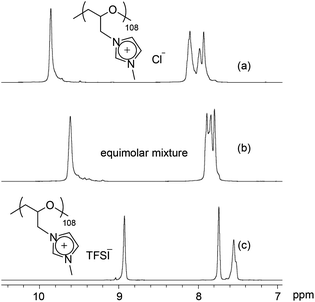
Cationic polyethers and ionic liquids are ionic materials. Thus far, the miscibility of ionic liquids and the properties of the formed ionic liquid mixtures have been well-studied.49–52 In general, ionic liquids are not highly soluble in organic solvents such as toluene or methanol, while they are miscible in each other. Polymer materials are less soluble in ionic liquids. In this part, we have studied the miscibility of the cationic polyethers in themselves and in ionic liquids.At first, the miscibility of poly(ECH-MeIm+Cl−) and poly(ECH-MeIm+TFSI−) was investigated. A poly(ECH-MeIm+Cl−) solution in methanol was added dropwise into equimolar poly(ECH-MeIm+TFSI−) in acetone under constant stirring. As a result, no precipitation was observed in the formed homogeneous solution. The organic solvents of the solution were evaporated at 50 °C for 24 h so that only a brittle transparent polymer, an equimolar mixture, remained behind. Fig. 7 shows selected 1H NMR spectra of the poly(ECH-MeIm+Cl−), the poly(ECH-MeIm+TFSI−) and the equimolar mixture of the two. It is interesting to note that the signals of the imidazolium protons of the equimolar mixture are observed between those of the poly(ECH-MeIm+Cl−) and the poly(ECH-MeIm+TFSI−). If the equimolar mixture is just a mixture of the two polymers, four distinct proton signals from the poly(ECH-MeIm+Cl−) and the poly(ECH-MeIm+TFSI−) would be observable in the aromatic region. This strongly implies that the equimolar mixture was a uniform compound.
To understand further about the uniform compound (equimolar mixture), the thermal properties and the solubility were studied (a DSC thermogram of the mixture is depicted in Fig. S13†). Between −20 °C and 110 °C, only one glass transition point was observed at 37 °C. To put this into context, we note that the Tg of the poly(ECH-MeIm+Cl−) and the poly(ECH-MeIm+TFSI−) were 90 °C and −12 °C, respectively. Furthermore, the solubility of the mixture was studied. Table S2† summarizes the solubility of the poly(ECH-MeIm+Cl−), the poly(ECH-MeIm+TFSI−) and the equimolar mixture. The solubility was different based on the starting materials: the mixed compound was completely insoluble in acetone or water and soluble in alcohols such as MeOH. The water uptake of the mixture was moderate compared with those of the poly(ECH-MeIm+Cl−) and the poly(ECH-MeIm+TFSI−) (23 °C, and 40% RH; water uptake = 6.9 wt%, and at 60% RH; water uptake = 11.6 wt%). From these results, it is proven that a random copolymer, poly(N-glycidyl-N′-methylimidazolium bis(trifluoromethanesulfonyl)imide-ran-N-glycidyl-N′-methylimidazolium chloride), poly(ECH-MeIm+Cl−-ran-ECH-MeIm+TFSI−), was formed just by mixing the poly(ECH-MeIm+Cl−) and the poly(ECH-MeIm+TFSI−), as suggested in Scheme 2. Anion exchange between cationic polyethers would proceed very fast even though the counter cation is a polymeric material. To summarize, the present cationic polyethers are miscible in each other, and form a corresponding random copolymer by anion exchange.
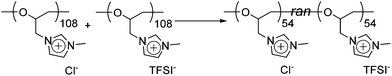 | ||
| Scheme 2 Synthesis of poly(ECH-MeIm+Cl−-ran-ECH-MeIm+TFSI−) just by mixing poly(ECH-MeIm+Cl−) and poly(ECH-MeIm+TFSI−). | ||
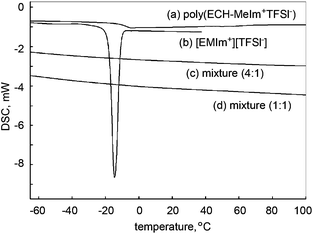
Next, the miscibility of the cationic polyethers in ionic liquids was investigated. In the previous section, the water incorporated in poly(ECH-MeIm+Cl−) was verified to be non-freezing. That is, the hydrated poly(ECH-MeIm+Cl−) possessed neither a glass transition nor a phase transition between −60 °C and 80 °C. Taking this significant result into account, we investigated the interaction between the apolar cationic polyether, poly(ECH-MeIm+TFSI−) (Mn(calc) = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, and DP = 108), and a typical ionic liquid, [EMIm+][TFSI−], first. The results are summarized in Fig. 8. [EMIm+][TFSI−] and the poly(ECH-MeIm+TFSI−) are entirely miscible in each other at room temperature. It is interesting that 1
400, and DP = 108), and a typical ionic liquid, [EMIm+][TFSI−], first. The results are summarized in Fig. 8. [EMIm+][TFSI−] and the poly(ECH-MeIm+TFSI−) are entirely miscible in each other at room temperature. It is interesting that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt mixtures of [EMIm+][TFSI−] and the poly(ECH-MeIm+TFSI−) have neither a melting point nor a glass transition point. [EMIm+][TFSI−] is a molten salt with an experimentally observed melting point at around −15 °C (Fig. 8(b)). When 20 wt% of the poly(ECH-MeIm+TFSI−) was added to 80 wt% of [EMIm+][TFSI−], both the melting point of [EMIm+][TFSI−] and the glass transition point of the poly(ECH-MeIm+TFSI−) entirely disappeared between −70 °C and 100 °C (Fig. 8(c)). The mixture of equivalent weights of [EMIm+][TFSI−] and the poly(ECH-MeIm+TFSI−) also displayed neither a melting point nor a glass transition (Fig. 8(d)) in the cooling and heating steps. To summarize this result, the poly(ECH-MeIm+TFSI−) was miscible in [EMIm+][TFSI−] even at low temperature, and it played a role as a freezing point depressant for [EMIm+][TFSI−].
1 wt/wt mixtures of [EMIm+][TFSI−] and the poly(ECH-MeIm+TFSI−) have neither a melting point nor a glass transition point. [EMIm+][TFSI−] is a molten salt with an experimentally observed melting point at around −15 °C (Fig. 8(b)). When 20 wt% of the poly(ECH-MeIm+TFSI−) was added to 80 wt% of [EMIm+][TFSI−], both the melting point of [EMIm+][TFSI−] and the glass transition point of the poly(ECH-MeIm+TFSI−) entirely disappeared between −70 °C and 100 °C (Fig. 8(c)). The mixture of equivalent weights of [EMIm+][TFSI−] and the poly(ECH-MeIm+TFSI−) also displayed neither a melting point nor a glass transition (Fig. 8(d)) in the cooling and heating steps. To summarize this result, the poly(ECH-MeIm+TFSI−) was miscible in [EMIm+][TFSI−] even at low temperature, and it played a role as a freezing point depressant for [EMIm+][TFSI−].
Furthermore, three cationic polyethers were mixed in imidazolium-based ionic liquids. Table 4 summarizes the miscibility and the thermal properties of the mixtures of ionic liquids and cationic polyethers. Poly(ECH-MeIm+TFSI−) was miscible in [EMIm+][TFSI−] and in [BMIm+][TFSI−]. From our results a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt mixture of [EMIm+][TFSI−] and [BMIm+][TFSI−] froze at −23 °C. As stated above, a 4
1 wt/wt mixture of [EMIm+][TFSI−] and [BMIm+][TFSI−] froze at −23 °C. As stated above, a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt mixture of [EMIm+][TFSI−] and the poly(ECH-MeIm+TFSI−) was non-freezing. One of the significant characteristics of the poly(ECH-MeIm+Cl−) is the freezing point depression of [BMIm+][Cl−]. A mixture of [BMIm+][Cl−] and the poly(ECH-MeIm+Cl−) (4
1 wt/wt mixture of [EMIm+][TFSI−] and the poly(ECH-MeIm+TFSI−) was non-freezing. One of the significant characteristics of the poly(ECH-MeIm+Cl−) is the freezing point depression of [BMIm+][Cl−]. A mixture of [BMIm+][Cl−] and the poly(ECH-MeIm+Cl−) (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt) had a low glass transition point at −48 °C and no phase transition. In contrast, a 4
1 wt/wt) had a low glass transition point at −48 °C and no phase transition. In contrast, a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt mixture of [BMIm+][Cl−] and [EMIm+][TFSI−] showed cold-crystallization at 35 °C and melted at 59 °C, suggesting phase separation at low temperature. Similarly, 4
1 wt/wt mixture of [BMIm+][Cl−] and [EMIm+][TFSI−] showed cold-crystallization at 35 °C and melted at 59 °C, suggesting phase separation at low temperature. Similarly, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt mixtures of [EMIm+][BF4−] with the poly(ECH-MeIm+BF4−), [EMIm+][TFSI−] with the poly(ECH-MeIm+Cl−), and [EMIm+][TFSI−] with the poly(ECH-MeIm+BF4−) were found to be non-freezing. It has been already reported that [EMIm+][TFSI−], [EMIm+][Cl−] and [EMIm+][BF4−] are highly miscible in each other even at low temperature.49–52
1 wt/wt mixtures of [EMIm+][BF4−] with the poly(ECH-MeIm+BF4−), [EMIm+][TFSI−] with the poly(ECH-MeIm+Cl−), and [EMIm+][TFSI−] with the poly(ECH-MeIm+BF4−) were found to be non-freezing. It has been already reported that [EMIm+][TFSI−], [EMIm+][Cl−] and [EMIm+][BF4−] are highly miscible in each other even at low temperature.49–52
| Run | Ionic compounds/weight ratio | Thermal behaviorb | |
|---|---|---|---|
a Poly(ECH-MeIm+Cl−) (Mn(calc) = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900; DP = 108), poly(ECH-MeIm+TFSI−) (Mn(calc) = 45 900; DP = 108), poly(ECH-MeIm+TFSI−) (Mn(calc) = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400; DP = 108) and poly(ECH-MeIm+BF4−) (Mn(calc) = 24 400; DP = 108) and poly(ECH-MeIm+BF4−) (Mn(calc) = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500; DP = 108) as described in Fig. 2–4 were employed.
b Determined under N2, at 5 °C min−1 between −80 °C and 150 °C.
c
T
cc: Cold crystallization temperature.
d
T
m: Melting point. 500; DP = 108) as described in Fig. 2–4 were employed.
b Determined under N2, at 5 °C min−1 between −80 °C and 150 °C.
c
T
cc: Cold crystallization temperature.
d
T
m: Melting point.
|
|||
| 1 | [EMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(ECH-MeIm+TFSI−) poly(ECH-MeIm+TFSI−) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 2 | [EMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(ECH-MeIm+TFSI−) poly(ECH-MeIm+TFSI−) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 3 | [BMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(ECH-MeIm+TFSI−) poly(ECH-MeIm+TFSI−) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 4 | [EMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BMIm+][TFSI−] [BMIm+][TFSI−] |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 5 | [EMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BMIm+][TFSI−] [BMIm+][TFSI−] |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
T cc = −35 °C,cTm = −23 °Cd |
| 6 | [BMIm+][Cl−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(ECH-MeIm+Cl−) poly(ECH-MeIm+Cl−) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
T g = −48 °C |
| 7 | [BMIm+][Cl−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EMIm+][Cl−] [EMIm+][Cl−] |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
T g = −44 °C, Tcc = 35 °C, Tm = 59 °C |
| 8 | [EMIm+][BF4−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(ECH-MeIm+BF4−) poly(ECH-MeIm+BF4−) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 9 | [EMIm+][BF4−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BMIm+][BF4−] [BMIm+][BF4−] |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 10 | [EMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(ECH-MeIm+Cl−) poly(ECH-MeIm+Cl−) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 11 | [EMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EMIm+][Cl−] [EMIm+][Cl−] |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 12 | [EMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(ECH-MeIm+BF4−) poly(ECH-MeIm+BF4−) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
| 13 | [EMIm+][TFSI−]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EMIm+][BF4−] [EMIm+][BF4−] |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Non-freezing |
To conclude this section, the miscibility of the cationic polyethers in ionic liquids was identical to or higher than those of ionic liquids. Since the melting points of the ionic liquids are not very low, the fluidity and the ionic conductivity of the ionic liquids sometimes decrease at low temperature. Similarly, the ionic conductivities of polymer electrolytes are always modest at low temperature. In terms of ionic liquids, the melting points disappeared by adding cationic polyethers. In the meantime, the glass transition points of the cationic polyethers disappeared with the addition of ionic liquids. Therefore, the present composite of ionic liquid and cationic polyether can be regarded as a new class of ionic material which can eliminate the drawbacks of conventional ionic liquids and ionomers. Now, further study is in progress to uncover the fundamental properties of the present ionic liquid composites and elucidate the basic requirements for this unique phenomenon.
Conclusions
Cationic polyethers were successfully synthesized and their properties and functions were characterized. Cationic polyethers were prepared by the living ring-opening anionic polymerization of epichlorohydrin followed by quantitative quaternization of the chloromethyl group by 1-methylimidazole and by anion exchange. Consequently, the cationic polyethers had both an ionic liquid group and a polyether chain in each repeating unit. Various functions are dependent on the choice of the ionic moiety. In fact, poly(ECH-MeIm+Cl−) was characterized to be highly deliquescent in nature and have an excellent ionic conductivity when hydrated. The water confined in poly(ECH-MeIm+Cl−) was non-freezing water. The cationic polyethers generally displayed high thermal stability. The miscibility of the cationic polyethers in themselves and in ionic liquids was higher than that of ionic liquids. The cationic polyethers played a role as freezing point depressants for ionic liquids. It can be emphasized that the present cationic polyethers are very unique compounds, having extraordinary functions that come from the combination of ionic liquid moieties and the main chain of polyoxyethylene. We have already investigated another type of cationic polyether with various onium groups and various counter anions. Moreover, a cross-linkable cationic polyether was also developed by the cationization of a copolymer of ECH and unsaturated epoxide. The functions and properties of further cationic polyethers will be detailed in a following report.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to express appreciation to the Tosoh Corporation for the GPC analyses of the poly(ECH-MeIm+Cl−) samples. SH thanks Mr Yuta Makita for the helpful discussion. We are grateful to Dr Renata Drozdzak for the scientific support.Notes and references
- J. S. Wilkes, Green Chem., 2002, 4, 73 RSC.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- T. Welton, Chem. Rev., 1999, 99(8), 2071 CrossRef CAS PubMed.
- J. S. Wilkes and M. J. Zawarotko, J. Chem. Soc., Chem. Commun., 1992, 965 RSC.
- J. Yuan and M. Antonietti, Polymer, 2011, 52, 1469 CrossRef CAS.
- D. Mecerreyes, Prog. Polym. Sci., 2011, 36, 1629 CrossRef CAS.
- J. Yuan, D. Mecerreyes and M. Antonietti, Prog. Polym. Sci., 2013, 38, 1009 CrossRef CAS.
- J. C. Salamone, S. C. Israel, P. Tailor and B. Snider, Polymer, 1973, 14, 639 CrossRef CAS.
- R. L. Weber, Y. Ye, S. M. Banik, Y. A. Elabd, M. A. Hickner and M. K. Mahantthappa, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1287 CrossRef CAS.
- K. M. Meek and Y. A. Elabd, Macromolecules, 2015, 48, 7071 CrossRef CAS.
- K. M. Meek, S. Sharick, Y. Ye, K. I. Winey and Y. A. Elabd, Macromolecules, 2015, 48, 4850 CrossRef CAS.
- Y. Ye, S. Sharick, E. M. Davis, K. I. Winey and Y. A. Elabd, ACS Macro Lett., 2013, 2, 575 CrossRef CAS.
- U. H. Choi, Y. Ye, D. S. Cruz, W. Liu, K. I. Winey, Y. A. Elabd, J. Runt and R. H. Colby, Macromolecules, 2014, 47, 777 CrossRef CAS.
- J.-H. H. Wang, C. H.-C. Yang, H. Masser, H.-S. Shiau, M. V. O'Reilly, K. I. Winey, J. Runt, P. C. Painter and R. H. Colby, Macromolecules, 2015, 48, 7273 CrossRef CAS.
- C. M. Evans, C. R. Bridges, G. E. Sanoja, J. Bartels and R. A. Segalman, ACS Macro Lett., 2016, 5, 925 CrossRef CAS.
- C. M. Evans, G. E. Sanoja, B. C. Popere and R. A. Segalman, Macromolecules, 2016, 49, 395 CrossRef CAS.
- K. Ueno, J. Murai, K. Ikeda, S. Tsuzuki, M. Tsuchiya, R. Tatara, T. Mandai, Y. Umebayashi, K. Dokko and M. Watanabe, J. Phys. Chem. C, 2016, 120, 15792 CAS.
- H. Asai, K. Fujii, T. Ueki, T. Sakai, U. Chung, M. Watanabe, Y.-S. Han, T.-H. Kim and M. Shibayama, Macromolecules, 2012, 45, 3902 CrossRef CAS.
- H. Yoshimitsu, A. Kanazawa, S. Kanaoka and S. Aoshima, Macromolecules, 2012, 45, 9427 CrossRef CAS.
- T. K. Carlisle, E. F. Wiesenauer, G. D. Nicodemus, D. L. Gin and R. D. Noble, Ind. Eng. Chem. Res., 2013, 52, 1023 CrossRef CAS.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Polym. Chem., 2015, 6, 2163 RSC.
- For a book: N. Hadjichristidis and A. Hirao, Anionic Polymerization: Principles, Practice, Strength, Consequences and Application, Springer, Japan, 2015 Search PubMed.
- E. J. Vandenberg, J. Polym. Sci., 1960, 47, 486 CrossRef CAS.
- J. Furukawa, T. Tsuruta, R. Sakata, T. Saegusa and A. Kawasaki, Makromol.Chem., 1959, 32, 90 CrossRef CAS.
- S. Kambara and A. Takahashi, Makromol. Chem., 1963, 63, 89 CrossRef CAS.
- T. Aida, Y. Maekawa, S. Asano and S. Inoue, Macromolecules, 1988, 21, 1195 CrossRef CAS.
- N. Emig, H. Nguyen, H. Krautschield, R. Reau, J.-B. Cazaux and G. Bertrand, Organometallics, 1998, 17, 3599 CrossRef CAS.
- W. Braune and J. Okuda, Angew. Chem., Int. Ed., 2003, 42, 64 CrossRef CAS PubMed.
- C. Billouard, S. Carlotti, P. Desbois and A. Deffieux, Macromolecules, 2004, 37, 4038 CrossRef CAS.
- S. Carlotti, A. Labbé, V. Rejsek, S. Doutaz, M. Gervais and A. Deffieux, Macromolecules, 2008, 41, 7058 CrossRef CAS.
- S. Hayano and Y. Tsunogae, Japanese Patent5229464, 2008 Search PubMed.
- D. Stoica, L. Ogier, L. Akrour, F. Alloin and J.-F. Fauvarque, Electrochim. Acta, 2007, 53, 1596 CrossRef CAS.
- D. Stoica, F. Alloin, S. Marais, D. Langevin, C. Chappy and P. Judeinstein, J. Phys. Chem. B, 2008, 112, 12338 CrossRef CAS PubMed.
- Before starting this study, we tried to quaternize commercially available poly(ECH), of which the MW was higher than 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The quaternization reaction was very sluggish: for example, only 20% of the chloromethyl was cationized by 2 equivalents of 1-imidazole after 10 days reaction at 100 °C.
000. The quaternization reaction was very sluggish: for example, only 20% of the chloromethyl was cationized by 2 equivalents of 1-imidazole after 10 days reaction at 100 °C. - H. Hu, W. Yuan, Z. Jia and G. L. Baker, RSC Adv., 2015, 5, 3135 RSC.
- H. Hu, W. Yuan, L. Lu, H. Zhao, Z. Jia and G. L. Baker, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2104 CrossRef CAS.
- S. Hayano, H. Yonemaru and K. Ohta, WO2012/133786.
- S. Hayano, K. Ohta and T. B. Hoang, Chem. Lett., 2017, 46, 1033 CrossRef CAS.
- G. D. Smith, O. Borodin, L. Li, H. Kim, Q. Lin, J. E. Bara, D. L. Gin and R. Noble, Phys. Chem. Chem. Phys., 2008, 10, 6301 RSC.
- It should be noted that the present poly(ECH) samples have chloromethyl groups at the repeating units and one bromomethyl group at the initiation end which came from n-Bu4NBr as the initiator. The bromomethyl group was also cationized by 1-methylimidazole during the quaternization. Therefore each cationized polymer has one imidazolium group at the initiation end. We have omitted the end groups in Scheme 1 for the clarity of the focus in this study.
- All of the polymer samples were dried in vacuo for 48 h at first, and after that they were put in a sample pan and covered up under N2 in a glove-box. The lid of the sample pan was removed quickly just before loading in the TG/DTA apparatus. Unfortunately our TG/DTA was situated not in a dry room but in a regular laboratory. Therefore all of the samples were exposed to air slightly just before loading in to the equipment. As a result the deliquescent poly(ECH-MeIm+Cl−) was hydrated slightly and, in turn, the TG/DTA measurement detected the evaporation of water. Thus, in the case of the poly(ECH-MeIm+Cl−), the Td5% was estimated excluding the weight-loss from the evaporation of the confined water.
- K. Tanaka, F. Ishiguro and Y. Chujo, J. Am. Chem. Soc., 2010, 132, 17649 CrossRef CAS PubMed.
- K. Tanaka and Y. Chujo, Polym. J., 2013, 45, 247 CrossRef CAS.
- L. Tian, X. Shu and J. Zhu, Adv. Mater., 2007, 19, 4548 CrossRef CAS.
- H. M. L. Thijs, C. R. Becer, C. Guerrero-Sanchez, D. Fournier, R. Hoogenboom and U. S. Schubert, J. Mater. Chem., 2007, 17, 4864 RSC.
- For instance, ionic liquids such as [EMIm+][Cl−] exhibit high capacities for moisture absorption, and poly(ethylene oxide) is well known as an absorbent and hydrophilic polymer.
- The aluminum sample pan for the DSC measurement was covered up tightly with the aluminum pan lid, in order to not observe an endothermic peak from the evaporation of the incorporated water. As a result, the incorporated water didn't evaporate easily at around 60 °C. A slight endotherm was observable in the DSC thermogram of the hydrated poly(ECH-MeIm+Cl−) above 80 °C, probably due to the slow evaporation of the water inside of the sealed DSC pan. In contrast, the TG/DTA of the lightly hydrated poly(ECH-MeIm+Cl−) was measured after removing the lid of the sample pan, therefore the water easily evaporated to observe the weight loss above 60 °C.
- T. Okada, G. Xie, O. Gorseth, S. Kjelstrup, N. Nakamura and T. Arimura, Electrochim. Acta, 1998, 43, 3741 CrossRef CAS.
- R. P. Matthews, I. J. Viller-Garcia, C. C. Weber, J. Griffith, F. Cameron, P. A. Hunt and T. Welton, Phys. Chem. Chem. Phys., 2016, 18, 8608 RSC.
- S. Omer, J. Lemus, E. Ruiz, V. R. Ferro, J. Ortega and J. Palomar, J. Phys. Chem. B, 2014, 118, 2442 CrossRef PubMed.
- G. Annat, M. Forsyth and D. R. MacFarlane, J. Phys. Chem. B, 2012, 116, 8251 CrossRef CAS PubMed.
- H. Niedermeyer, J. P. Hallett, I. J. Viller-Garcia, P. A. Hunt and T. Welton, Chem. Soc. Rev., 2012, 41, 7780 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the living polymerization of ECH, schemes, figures and tables. See DOI: 10.1039/c7py01985h |
| ‡ Present address: Saigon Hi-Tech Park Incubation Center, K1-G3, D1 Road, Dist. 9, HCMC, Vietnam. |
| This journal is © The Royal Society of Chemistry 2018 |