Analysis of metabonomic profiling alterations in a mouse model of colitis-associated cancer and 2-deoxy-D-glucose treatment†
Peng Yang‡
a,
Zongwei Li‡*a,
Lichao Zhanga,
Hanqing Lib and
Zhuoyu Li*ac
aInstitute of Biotechnology, Key Laboratory of Chemical Biology and Molecular Engineering of National Ministry of Education, Shanxi University, Taiyuan 030006, China. E-mail: zongweili@sxu.edu.cn; lzy@sxu.edu.cn; Fax: +86 351 7018268
bCollege of Life Science, Shanxi University, Taiyuan 030006, China
cCollege of Life Science, Zhejiang Chinese Medical University, Hangzhou 310053, China
First published on 13th June 2016
Abstract
Inflammation is well recognized to be associated with tumorigenesis, cancer progression and tumor metabolism of colorectal cancer (CRC). 2-Deoxy-D-glucose (2-DG), a glycolytic inhibitor, has been reported to possess anticancer properties and is considered to be a promising treatment for tumors. However, the metabolic alteration in tumorigenesis caused by inflammation and 2-DG prevention remains elusive. In this study, a gas chromatography time-of-flight mass spectrometry (GC-TOF/MS) analysis was applied to investigate the anticancer activity of 2-DG on the alteration of metabolites in a colitis-associated cancer model induced by azoxymethane (AOM) and dextran sodium sulfate (DSS). The data showed that 2-DG obviously decreased the incidence of tumor formation induced by AOM and DSS. 14 metabolites were significantly decreased in the AOM/DSS group, while all these metabolites were reversed by 2-DG treatment. Furthermore, metabolic pathway analysis (MetPA) was introduced to reveal the involvement of four metabolic networks including linoleic acid metabolism, pentose phosphate pathway, nicotinate and nicotinamide metabolism and inositol phosphate metabolism. The significantly altered metabolites of linoleic acid, nicotinamide, ribose-5-phosphate and myo-inositol were involved in these four pathways. Moreover, the expression of PKM2, which was induced by AOM and DSS, was attenuated by 2-DG treatment. Together, this study provides an insight into how 2-DG shows anticancer effects and may serve as a therapeutic agent for colitis-associated cancer.
1. Introduction
As one of the most common diagnosed malignancies, colorectal cancer (CRC) is the third most lethal cancer around the world.1 More than 1 million new cases of CRC worldwide are diagnosed and nearly 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people die from this disease each year.2 The occurrence of CRC is a complex multistep process which involves the accumulation of genetic and epigenetic changes.3 Epidemiological studies show that about 80% of CRC is attributed to chronic intestinal inflammation, environmental mutagens, bad dietary habits and intestinal commensals.4 Mounting evidence suggests that chronic inflammation plays a critical role in the pathogenesis of CRC.5,6 Inflammatory bowel disease (IBD) is a well-known example of the connection between inflammation and tumorigenesis, and one of the consequences is ulcerative colitis (UC), which represents a greatly increased risk factor in the progression of CRC.7 Although the relationship between inflammation and CRC progression has drawn intensive research, the underlying mechanisms remain poorly understood.
000 people die from this disease each year.2 The occurrence of CRC is a complex multistep process which involves the accumulation of genetic and epigenetic changes.3 Epidemiological studies show that about 80% of CRC is attributed to chronic intestinal inflammation, environmental mutagens, bad dietary habits and intestinal commensals.4 Mounting evidence suggests that chronic inflammation plays a critical role in the pathogenesis of CRC.5,6 Inflammatory bowel disease (IBD) is a well-known example of the connection between inflammation and tumorigenesis, and one of the consequences is ulcerative colitis (UC), which represents a greatly increased risk factor in the progression of CRC.7 Although the relationship between inflammation and CRC progression has drawn intensive research, the underlying mechanisms remain poorly understood.
Azoxymethane (AOM) is a colonic genotoxic carcinogen that leads to DNA damage, DNA mismatch repair of colon epithelial cells and tumorigenesis.8 The combination of AOM and inflammatory agent dextran sodium sulfate (DSS) is widely used in mouse models to mimic colon carcinogenesis driven by chronic inflammation.9 The C57BL/6J mouse in AOM/DSS model is chosen because it is widely used in metabolic research and is prone to colitis-associated cancer.9 2-Deoxy-D-glucose (2-DG), whose C-2-hydroxyl group is replaced by hydrogen, is a well-known glycolytic inhibitor.10 2-DG competitively suppresses glucose uptake and is phosphorylated by hexokinase to produce 2-deoxyglucose 6-phosphate (2-DG-6P) which cannot be further metabolized.11 Cancer cells rely mainly on glycolysis for energy production. 2-DG is considered to be a promising chemotherapeutic strategy because it inhibits glucose transport, hexokinase and glycolysis of cancer cells.12 However, the corresponding metabolic changes associated with inflammation, colorectal carcinogenesis and 2-DG treatment has not been elucidated yet.
In the present study, we applied a GC-TOFMS based metabonomic approach coupled with MetPA and western blot to characterize the metabolic signature induced by AOM and DSS in C57BL/6J mouse. The intervention effects of 2-DG against carcinogenesis on the metabolic alteration were also detected by this strategy. This study provides new insights into the development of tumor at the metabolic level and maybe helpful to understand the underlying molecular mechanisms of 2-DG treatment.
2. Materials and methods
2.1. Antibodies and reagents
AOM and 2-DG were obtained from Sigma (St. Louis, USA). DSS was purchased from ICN Biochemicals (Aurora, OH). β-Actin antibody was from Abmart (Shanghai, China). PKM2 antibody was purchased from Cell Signaling Technology (Beverly, MA). HRP-conjugated secondary antibodies were obtained from Invitrogen (Carlsbad, CA). The enhanced chemiluminescence detection kit was purchased from Engreen Biosystem (Beijing, China).2.2. Animal experiment
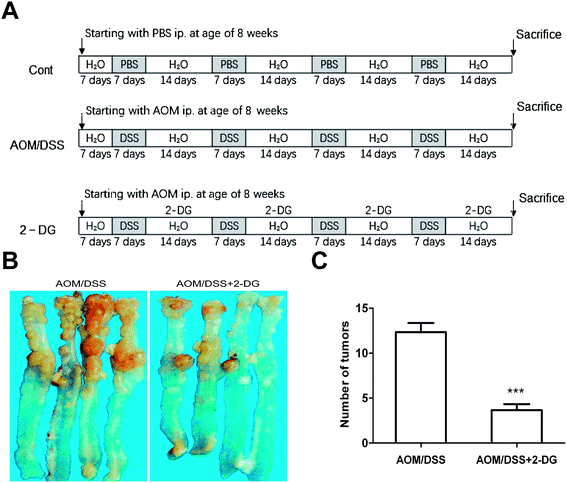
Mouse colitis-associated cancer was induced as previously described.13,14 A total of 30 7 week-old male C57BL/6J mice were purchased from Beijing Vital River Laboratories Co. The animals were acclimated to new environment for one week before the start of experiments. All animals were kept under controlled conditions of temperature (24 ± 1 °C), humidity (45 ± 15%) and light (a 12/12 light–dark cycle with lights on at 8:00 a.m.). Mice had free access to a pelleted basal diet and drinking water for one-week acclimation period. Experiments were conducted in accordance with the European Directive (2010/63/EU) on the protection of animals used for experimental and other scientific purposes. Our study was approved by the Committee on the Ethics of Animal Experiments of Shanxi University. Then, the mice were randomized into three groups: the control group, the AOM/DSS group and the 2-DG-AOM/DSS group (n = 10/each group). In the AOM/DSS group, the mice were intraperitoneal injected with 10 mg kg−1 AOM on day 1. One week after AOM injection, mice were given four cycles of DSS treatment. For each cycle, 1.25% DSS was added in their drinking water for 7 days, and then normal water was given for 14 days of recovery. The control group was provided with basal diet, water and injected with same volume of saline as processed in AOM/DSS group. In 2-DG-AOM/DSS group, 2-DG (10 mg/200 μl/mouse) was injected subcutaneously during recovery period. At the end of the second recovery period, four mice of each group were killed for histopathologic evaluation. At the end of the experimental procedure, six mice of each group were sacrificed and intestinal tissue samples were collected for GC-TOF/MS analysis.2.3. Sample preparation
Sample collection was performed as previously described.15 The entire colon from cecum to anus was removed and opened longitudinally along with the main axis and washed with saline. The large bowel was macroscopically observed and then used for histological analysis. Intestinal tissue samples were then submerged in liquid nitrogen followed by storage at −80 °C for GC-TOF/MS analysis.2.4. Metabolites extraction
Tissue samples for GC-TOF/MS analysis were pretreated according to previous method.16 In brief, 100 mg of each frozen intestinal tissue was powdered with 50 μl of L-2-chlorophenylalanine (0.1 mg ml−1 stock in ddH2O) and then submerged in mixed solvent solution (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform = 3
chloroform = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The whole mixture was ultrasonicated for 10 min and then stored at −20 °C for 20 min. Subsequently, the samples were centrifuged at 14
1, v/v). The whole mixture was ultrasonicated for 10 min and then stored at −20 °C for 20 min. Subsequently, the samples were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min at 4 °C. The supernatants were carefully transferred to a vial containing an internal standard, L-2-chlorophenylalanine and then dried by vacuum. A total of 80 μl methoxamine hydrochloride pyridine was added to the dried residues and kept at 37 °C for 90 min, followed by 80 μl bis-(trimethylsilyl)-trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) at 70 °C for 1 h. The samples were cooled to room temperature and prepared for GC-TOF/MS analysis.
000 g for 10 min at 4 °C. The supernatants were carefully transferred to a vial containing an internal standard, L-2-chlorophenylalanine and then dried by vacuum. A total of 80 μl methoxamine hydrochloride pyridine was added to the dried residues and kept at 37 °C for 90 min, followed by 80 μl bis-(trimethylsilyl)-trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) at 70 °C for 1 h. The samples were cooled to room temperature and prepared for GC-TOF/MS analysis.
2.5. GC-TOF/MS analysis
GC-TOF/MS analysis was performed on the Agilent 7890 gas chromatograph system coupled with a Pegasus HT time-of-flight mass spectrometer. Each 1 μl aliquot of the analyte was injected in splitless mode. The samples of control group, AOM/DSS group and 2-DG-AOM/DSS group were run respectively, to minimize systematic analytical deviations. The ultra-pure helium was used as the carrier gas at a constant flow rate of 1 ml min−1. The temperature of injection, transfer interface, and ion source was set to 270 °C, 260 °C and 200 °C, respectively. The electron energy was −70 eV and the mass data were acquired in full scan mode (m/z 20–600).2.6. Protein extraction and western blot
The extraction of total protein was performed as previously described.17,18 In brief, frozen samples were minced and lysed in RIPA buffer with 2 mM phenylmethylsulfonyl fluoride on ice for 30 min. After centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 15 min at 4 °C, supernatants were collected and protein concentrations were measured by BCA protein assay. Equal amounts of proteins were subjected to SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skim milk for 1 h, and then incubated overnight at 4 °C with the PKM2 and β-actin antibody, respectively. Subsequently, the membranes were incubated with HRP-conjugated secondary antibody for 1 h at room temperature. The bands were detected with an enhanced chemiluminescence detection kit and radiographic film.
000 g for 15 min at 4 °C, supernatants were collected and protein concentrations were measured by BCA protein assay. Equal amounts of proteins were subjected to SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skim milk for 1 h, and then incubated overnight at 4 °C with the PKM2 and β-actin antibody, respectively. Subsequently, the membranes were incubated with HRP-conjugated secondary antibody for 1 h at room temperature. The bands were detected with an enhanced chemiluminescence detection kit and radiographic film.
2.7. Data analysis
The acquired MS files from GC-TOF/MS analysis were performed with the Chroma TOF4.3X software (LECO) and LECO-Fiehn Rtx5 database as previously described.19 Briefly, the processing employed raw peaks exacting, the data baselines filtering and calibration of the baseline, peak alignment, deconvolution analysis, peak identification and integration of the peak area. SIMCA-P 13.0 software package (Umetrics, Umea, Sweden) was used for principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA) and orthogonal projections to latent structures-discriminant analysis (OPLS-DA). PCA was used to show the distribution of origin data. The PLS-DA model was estimated by 10-fold cross validation to check its validity. OPLS-DA was performed to identify the significantly different metabolites among control group, AOM/DSS group and 2-DG-AOM/DSS group. To refine this analysis, the first principal component of variable importance projection (VIP) was obtained. Metabolites with a p < 0.05 and VIP > 1.0 were considered to be statistically significant. The LECO/Fiehn Metabolomics Library was used to identify the compounds. It gives a similarity value for the compound identification accuracy. If the similarity is >700, the metabolite identification is reliable. If the similarity is <200, the library only uses “analyte” for the compound name. If the similarity is between 200 and 700, the compound name is putative annotation. The metabolic pathways were identified by the Kyoto Encyclopedia of Genes and Genomes (KEGG) and MetPA.2.8. Statistical analysis
The number of tumors/mouse ± standard deviation was calculated for each group. Differences among groups were tested by one-way analysis of variance (ANOVA). Differences between two groups were tested by Student's t-test. A value of p < 0.05 was considered statistically significant.3. Results
3.1. Preventive effects of 2-DG on AOM/DSS induced colitis-associated tumorigenesis
The shift to glycolysis is a key feature of metabolism reprogramming of cancer cell.20 As a glycolytic inhibitor, 2-DG may serve as a molecular cancer therapeutic.11 Although a few reports focused on the relationship between inflammation and CRC progression, the role of 2-DG in the metabolic alteration of colitis associated tumorigenesis has not been elucidated. Therefore, C57BL/6J mice were given a low dose of AOM followed by four cycles of 1.25% DSS administration for colon tumorigenesis (Fig. 1A). As shown in Fig. 1B and C, the mice in 2-DG-AOM/DSS group showed significantly less number of tumors in the colon compared to AOM/DSS group. The results demonstrated that 2-DG obviously suppressed AOM/DSS induced colitis-associated tumorigenesis.3.2. GC-TOF/MS metabolite profiling
Representative GC-TOF/MS total ion current (TIC) chromatograms from control group, AOM/DSS group and 2-DG-AOM/DSS group were respectively displayed in Fig. 2. The majority of the peaks in the TIC chromatograms were identified by their retention times, mass spectra characteristics and the LECO/Fiehn Metabolomics Library. The peaks in TIC profiles among the three groups were various differences, which indicated that the TIC chromatograms could reflect the discrimination among the three groups.3.3. Statistical comparison of metabolites in the three groups
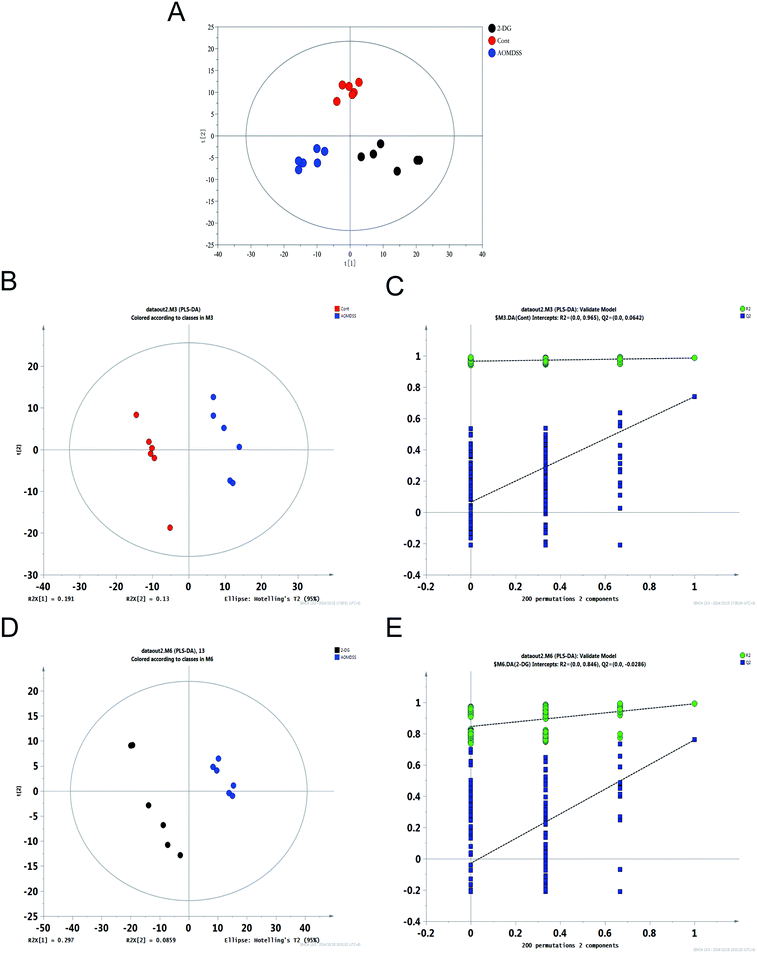
The resulted three-dimensional data involving the peak number, sample name, and normalized peak area were fed to SIMCA-P 13.0 software package for PCA, PLS-DA and OPLS-DA. PLS-DA and OPLS-DA were applied to obtain a higher level of group separation and get a better understanding of variables responsible for classification.21 The PCA scores plot and PLS-DA showed that control group, AOM/DSS group and 2-DG-AOM/DSS group were divided into three different regions (Fig. S1† and 3A). The PLS-DA model parameters were as follows: R2X = 0.321, R2Y = 0.986 and Q2 = 0.739 for control group vs. AOM/DSS group; R2X = 0.383, R2Y = 0.99 and Q2 = 0.76 for 2-DG-AOM/DSS group vs. AOM/DSS group (Fig. 3B and D). It suggested that the model was stable and good to fitness and prediction. 10-fold cross validation was used to estimate the robustness and the predictive ability of our model, such permutation test was proceeded in order to further validate the model.22 As shown in Fig. 3C and E, the R2 and Q2 intercept values were 0.965 and 0.0642, 0.846 and −0.0286 after 200 permutations for control group vs. AOM/DSS group and 2-DG-AOM/DSS group vs. AOM/DSS group, respectively. The low values of Q2 intercept indicated the robustness of the models, and thus showed a low risk of over fitting and reliable.19 The scores plot of OPLS-DA model further showed that 2-DG-AOM/DSS group and AOM/DSS group were all clearly separated from the controls (Fig. S2†). The results suggested that the OPLS-DA model can be used to identify the difference among the three groups.3.4. Metabolite variations and key different metabolic pathways among the three groups
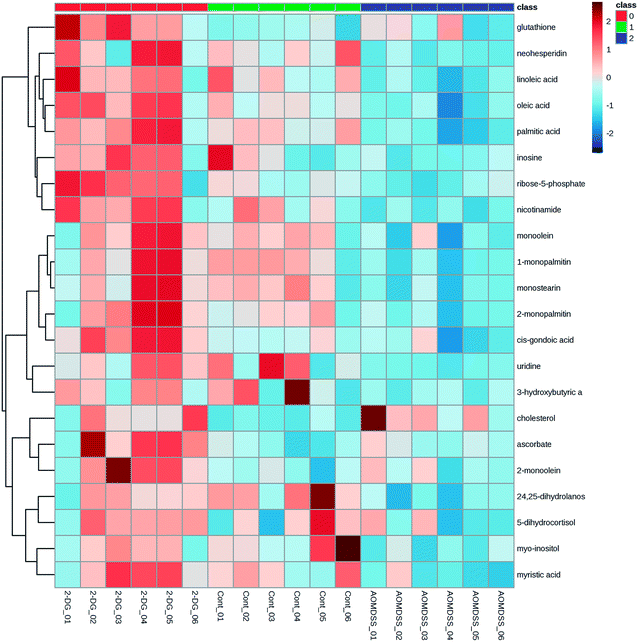
Based on the VIP values from the OPLS-DA models (VIP ≥ 1) and the P values from the two-tailed student's t test (P < 0.05), 24 significant metabolites were ultimately obtained (Table 1). There were only 14 variables common to all three groups. As shown in Table 1, oleic acid, linoleic acid, uridine, 1-monopalmitin, monostearin, palmitic acid, 2-monopalmitin, myo-inositol, ribose-5-phosphate, monoolein, myristic acid, 24,25-dihydrolanosterol, neohesperidin and nicotinamide in AOM/DSS group were significantly decreased relative to the control group. However, these 14 metabolites in 2-DG-AOM/DSS group displayed higher levels than that of AOM/DSS group. The heatmap in Fig. 4 further showed that the significantly attenuated metabolites in AOM/DSS group were reversed in 2-DG-AOM/DSS group. To explore which pathways involved in the preventive effect of 2-DG on AOM/DSS induced colitis-associated tumorigenesis, MetPA was used. Fig. 5 showed that the enriched pathways for 14 common metabolites of the three groups were biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, linoleic acid metabolism, ascorbate and aldarate metabolism, nicotinate and nicotinamide metabolism, pentose phosphate pathway, galactose metabolism, fatty acid elongation in mitochondria, inositol phosphate metabolism, steroid biosynthesis, fatty acid metabolism, pyrimidine metabolism and purine metabolism. According to the pathway impact value higher than 0.1, four disturbed metabolic pathways including linoleic acid metabolism, pentose phosphate pathway, nicotinate and nicotinamide metabolism and inositol phosphate metabolism were revealed (Fig. 5 and Table 2). The metabolites of linoleic acid, nicotinamide, ribose-5-phosphate and myo-inositol were involved in these four significantly relevant pathways.| Metabolite | Similarity | Rt (min) | Control group vs. AOM/DSS group | 2-DG-AOM/DSS group vs. AOM/DSS group | ||||
|---|---|---|---|---|---|---|---|---|
| VIP | p-Value | Fold change | VIP | p-Value | Fold change | |||
| Oleic acid | 976 | 20.7098 | 1.88604 | 0.003735136 | 1.471243872 | 1.7207 | 0.00071257 | 1.951210302 |
| Linoleic acid | 957 | 20.6259 | 1.86727 | 0.004361213 | 1.815133187 | 1.5711 | 0.006434742 | 2.120799824 |
| Uridine | 954 | 22.6546 | 1.5408 | 0.045264612 | 1.690020994 | 1.64937 | 0.005663319 | 1.716079716 |
| 1-Monopalmitin | 938 | 23.6594 | 1.84342 | 0.005636521 | 2.05589318 | 1.50376 | 0.011233764 | 2.381927261 |
| Monostearin | 933 | 25.0592 | 1.74963 | 0.009146697 | 1.832940249 | 1.50201 | 0.009974206 | 2.172065728 |
| Palmitic acid | 928 | 19.1308 | 2.11333 | 0.000200406 | 1.492331233 | 1.71903 | 0.001181451 | 1.75291404 |
| 2-Monopalmitin | 914 | 23.3935 | 1.60113 | 0.018729912 | 1.642543332 | 1.46829 | 0.013676516 | 2.343641168 |
| Myo-inositol | 870 | 19.554 | 1.51719 | 0.039616923 | 1.468769141 | 1.30115 | 0.025672345 | 1.302553926 |
| Ribose-5-phosphate | 819 | 19.6324 | 1.67121 | 0.013757499 | 1.418078552 | 1.52643 | 0.010184359 | 2.243239649 |
| Monoolein | 814 | 24.91785 | 1.53857 | 0.026064798 | 1.537440018 | 1.40824 | 0.013304874 | 1.846397358 |
| Myristic acid | 785 | 17.164 | 1.61705 | 0.017020786 | 1.40686955 | 1.50408 | 0.006869831 | 1.60076873 |
| 24,25-Dihydrolanosterol | 767 | 28.8301 | 1.83108 | 0.006909951 | 1.698949988 | 1.46539 | 0.007960147 | 1.444306128 |
| Neohesperidin | 765 | 22.9512 | 1.89 | 0.005731407 | 2.938875183 | 1.41062 | 0.024361224 | 3.702263901 |
| Nicotinamide | 709 | 13.167 | 1.66746 | 0.019935185 | 1.459151329 | 1.66724 | 0.003478964 | 1.858921027 |
| 6-Deoxy-D-glucose | 800 | 15.796 | 1.90087 | 0.002273401 | 2.446490924 | |||
| Cholesterol | 792 | 27.9519 | 1.70412 | 0.020752995 | 0.220261336 | |||
| Ascorbate | 943 | 18.1354 | 1.33668 | 0.031903481 | 1.730867664 | |||
| Inosine | 911 | 23.5665 | 1.57085 | 0.006426088 | 3.0558897 | |||
| Glutathione | 889 | 20.2289 | 1.3413 | 0.019056917 | 1.66314291 | |||
| cis-Gondoic acid | 863 | 22.3528 | 1.627 | 0.001636193 | 2.229625454 | |||
| 2-Monoolein | 914 | 24.6392 | 1.25917 | 0.036185917 | 1.973423515 | |||
| 3-Hydroxybutyric acid | 800 | 8.5187 | 1.39747 | 0.019761634 | 1.875615914 | |||
| Pantothenic acid | 796 | 18.5693 | 1.35871 | 0.023657372 | 1.410777285 | |||
| 5-Dihydrocortisol | 762 | 27.8043 | 1.25497 | 0.033653403 | 1.206007385 | |||
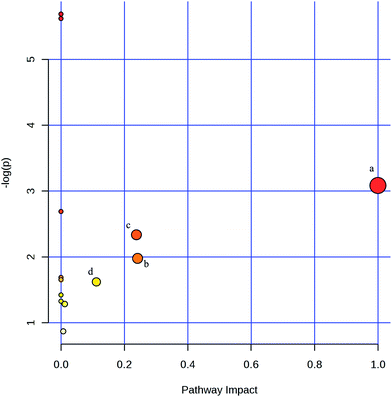 | ||
| Fig. 5 Summary of pathway analysis with MetPA. (a) Linoleic acid metabolism; (b) pentose phosphate pathway; (c) nicotinate and nicotinamide metabolism; (d) inositol phosphate metabolism. | ||
| Pathway name | Total | Hits | Raw P | −log(P) | Impact |
|---|---|---|---|---|---|
| Biosynthesis of unsaturated fatty acids | 42 | 3 | 0.0033883 | 5.6874 | 0 |
| Fatty acid biosynthesis | 43 | 3 | 0.0036268 | 5.6194 | 0 |
| Linoleic acid metabolism | 6 | 1 | 0.045762 | 3.0843 | 1 |
| Ascorbate and aldarate metabolism | 9 | 1 | 0.067921 | 2.6894 | 0 |
| Nicotinate and nicotinamide metabolism | 13 | 1 | 0.09674 | 2.3357 | 0.2381 |
| Pentose phosphate pathway | 19 | 1 | 0.13845 | 1.9772 | 0.24174 |
| Galactose metabolism | 26 | 1 | 0.1849 | 1.688 | 0 |
| Fatty acid elongation in mitochondria | 27 | 1 | 0.19134 | 1.6537 | 0 |
| Inositol phosphate metabolism | 28 | 1 | 0.19774 | 1.6208 | 0.11163 |
| Steroid biosynthesis | 35 | 1 | 0.24126 | 1.4219 | 0 |
| Fatty acid metabolism | 39 | 1 | 0.26516 | 1.3274 | 0 |
| Pyrimidine metabolism | 41 | 1 | 0.27685 | 1.2843 | 0.01202 |
| Purine metabolism | 68 | 1 | 0.41896 | 0.86999 | 0.00719 |
3.5. Pyruvate kinase M2 is involved in the preventive effect of 2-DG on AOM/DSS induced metabolite variations
Pyruvate kinase M2 (PKM2), which highly expressed in tumor cells, is a key enzyme in the final rate-limiting reaction in glycolysis.17 PKM2 has been reported to be tightly associated with aerobic glycolysis, pentose phosphate pathway, serine synthesis pathway and glutaminolysis pathway.23 Our previous study demonstrated that PKM2 played an important role in inflammatory cytokine production in CRC progression.24 Therefore, we were interested to explore if PKM2 was related to AOM/DSS induced metabolite variations. As shown in Fig. 6, the expression of PKM2 was significantly upregulated in AOM/DSS group. However, the increase of PKM2 caused by the induction of AOM/DSS was reversed by 2-DG treatment. The results indicated that PKM2 was involved in the suppression effect of 2-DG on AOM/DSS induced colitis-associated tumorigenesis and metabolite variations.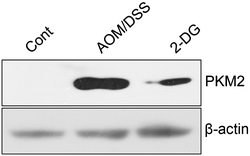 | ||
| Fig. 6 2-DG represses AOM/DSS induced PKM2 expression. The expressions of PKM2 in AOM/DSS group and 2-DG group were estimated using western blot. β-Actin was used as an internal control. | ||
4. Discussion
Chronic inflammation is an important risk factor for prompting the carcinogenesis, tumor growth and progression of CRC.25 People suffering from IBD are at increased risk of developing CRC.26 Because of the close link between inflammation and carcinogenesis, it is urgent to clarify the underlying molecular mechanisms. Due to respond to the biological systems to disease and nutrition stress quickly and sensitively, metabolomics is a very beneficial method to understand the biosystem's physiological process.27 2-DG, which is the glucose analog and glycolytic inhibitor, has been widely used as an anticancer drug.28 In this study, metabonomic approach was used to evaluate metabolic changes induced by AOM, DSS and the preventive effect of 2-DG, in addition to MetPA and western blot analysis. 14 potential biomarkers associated with AOM, DSS and the preventive effect of 2-DG were identified. MetPA was applied to show the significantly altered metabolites including linoleic acid, nicotinamide, ribose-5-phosphate and myo-inositol, which involved in the linoleic acid metabolism, pentose phosphate pathway, nicotinate and nicotinamide metabolism and inositol phosphate metabolism. 2-DG could attenuate the number of tumors through the alteration of PKM2 expression and the disturbed metabolic pathways.As a polyunsaturated fatty acid, linoleic acid is the precursor of arachidonic acid and is known to exert critical role in maintaining cell membrane structure and function.29 Linoleic acid has potential to inhibit inflammatory responses by lowering the production of inflammatory cytokine, such as TNF-α and IL-1β.30 In addition, the main reason of anti-inflammatory effect of linoleic acid is that linoleic acid could suppress the activity of the peroxisome proliferator-activated receptor-α (PPAR-α).31 Our results showed that AOM/DSS group had down-regulated levels of linoleic acid and facilitate PKM2 expression (Table 1 and Fig. 6). The results are supported by the study that the suppression of the activity of PPAR-α could upregulate PKM2 expression and promote glycolysis.32 Our results demonstrated that the decrease of linoleic acid enhanced arachidonic acid synthesis and suppressed the activity of PPAR-α activity to promote PKM2 expression in AOM/DSS induced tumor group.
Myo-inositol, which is an isomer of glucose, plays an indispensable role as the structural basis for a second messenger system and the phosphoinositide lipid signaling molecules.33 Myo-inositol is also known as phosphatidylinositol 3-kinase (PI3K) inhibitor, which is involved in signal pathways with cell proliferation and tumor metabolic reprogramming.34 Other reports showed that the decrease of myo-inositol is accompanied with increased glycolysis and augments the oncogenesis.35 It has also been reported that inflammatory cytokine of TNF-α and IL-1β could suppress the myo-inositol accumulation.36 Our previous studies showed that PKM2 serve as a key inflammatory mediator to facilitate TNF-α and IL-1β production.24 These results indicated that lower levels of myo-inositol in AOM/DSS group may be a result of elevated PKM2 expression.
Nicotinamide, also referred to as vitamin B3, is a precursor of the coenzymes NADH and NADPH and is tightly associated with energy metabolism.37 Nicotinamide has also been reported to show anti-inflammatory effects and scavenge oxygen radicals.38 Recent studies showed that the acetylated PKM2 is augment by nicotinamide and PKM2 protein is degraded subsequently.39 Consistent with these findings, the present results showed that the depletion of nicotinamide may be indicative of AOM/DSS induced tumor.
In summary, we investigated metabolic changes of 14 potential biomarkers in AOM/DSS induced model involving linoleic acid metabolism, pentose phosphate pathway, nicotinate and nicotinamide metabolism and inositol phosphate metabolism. 2-DG is able to attenuate AOM/DSS induced PKM2 expression and metabolic alterations of 14 potential biomarkers. Collectively, our data suggested that 2-DG can be exploited as a potent therapeutic agent for the treatment of colitis-associated cancer.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31271516, No. 31201072), Research Project Supported by Shanxi Scholarship Council of China (2015-2), Zhejiang Province Science Foundation (LY15H280008), The R&D Infrastructure and Facility Development Program of Shanxi Province (2015091015), and Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2015175).References
- E. Martino-Echarri, B. R. Henderson and M. G. Brocardo, Oncotarget, 2014, 5, 9889–9900 CrossRef PubMed
.
- R. Siegel, D. Naishadham and A. Jemal, Ca-Cancer J. Clin., 2012, 62, 10–29 CrossRef PubMed
.
- N. Zhang, X. Li, C. W. Wu, Y. Dong, M. Cai, M. T. Mok, H. Wang, J. Chen, S. S. Ng, M. Chen, J. J. Sung and J. Yu, Oncogene, 2012 DOI:10.1038/onc.2012.526
.
- J. Terzic, S. Grivennikov, E. Karin and M. Karin, Gastroenterology, 2010, 138, 2101–2114 CrossRef CAS PubMed
e2105.
- T. Cooks, I. S. Pateras, O. Tarcic, H. Solomon, A. J. Schetter, S. Wilder, G. Lozano, E. Pikarsky, T. Forshew, N. Rosenfeld, N. Harpaz, S. Itzkowitz, C. C. Harris, V. Rotter, V. G. Gorgoulis and M. Oren, Cancer Cell, 2013, 23, 634–646 CrossRef CAS PubMed
.
- Y. Ben-Neriah and M. Karin, Nat. Immunol., 2011, 12, 715–723 CrossRef CAS PubMed
.
- J. Liang, M. Nagahashi, E. Y. Kim, K. B. Harikumar, A. Yamada, W. C. Huang, N. C. Hait, J. C. Allegood, M. M. Price, D. Avni, K. Takabe, T. Kordula, S. Milstien and S. Spiegel, Cancer Cell, 2013, 23, 107–120 CrossRef CAS PubMed
.
- R. Suzuki, H. Kohno, S. Sugie, H. Nakagama and T. Tanaka, Carcinogenesis, 2006, 27, 162–169 CrossRef CAS PubMed
.
- M. W. Zimmerman, G. E. Homanics and J. S. Lazo, PLoS One, 2013, 8, e58300 CAS
.
- D. Zhang, J. Li, F. Wang, J. Hu, S. Wang and Y. Sun, Cancer Lett., 2014, 355, 176–183 CrossRef CAS PubMed
.
- J. Wang, Z. Jiang, L. Xiang, Y. Li, M. Ou, X. Yang, J. Shao, Y. Lu, L. Lin, J. Chen, Y. Dai and L. Jia, Sci. Rep., 2014, 4, 5006 CrossRef PubMed
.
- X. Li, Y. Gao, M. Yang, Q. Zhao, G. Wang, Y. M. Yang, Y. Yang, H. Liu and Y. Zhang, PLoS One, 2014, 9, e95347 Search PubMed
.
- M. Tanaka, Y. Masaki, K. Tanaka, M. Miyazaki, M. Kato, R. Sugimoto, K. Nakamura, S. Aishima, K. Shirabe, M. Nakamuta, M. Enjoji, K. Kotoh and R. Takayanagi, Mol. Med. Rep., 2013, 7, 365–370 CAS
.
- A. Hernandez-Aguilera, A. Rull, E. Rodriguez-Gallego, M. Riera-Borrull, F. Luciano-Mateo, J. Camps, J. A. Menendez and J. Joven, Mediators Inflammation, 2013, 2013, 135698 CrossRef PubMed
.
- Y. Gao, X. Li, M. Yang, Q. Zhao, X. Liu, G. Wang, X. Lu, Q. Wu, J. Wu, Y. Yang and Y. Zhang, Carcinogenesis, 2013, 34, 1861–1869 CrossRef CAS PubMed
.
- W. Liao, H. Wei, X. Wang, Y. Qiu, X. Gou, X. Zhang, M. Zhou, J. Wu, T. Wu, F. Kou, Y. Zhang, Z. Bian, G. Xie and W. Jia, J. Proteome Res., 2012, 11, 3436–3448 CrossRef CAS PubMed
.
- P. Yang, Z. Li, R. Fu and H. Wu, Cell. Signalling, 2014, 26, 1853–1862 CrossRef CAS PubMed
.
- S. Kasiri, K. I. Ansari, I. Hussain, A. Bhan and S. S. Mandal, RSC Adv., 2013, 3, 3260–3269 RSC
.
- H. Z. Sun, D. M. Wang, B. Wang, J. K. Wang, H. Y. Liu, L. Guan le and J. X. Liu, J. Proteome Res., 2015, 14, 1287–1298 CrossRef CAS PubMed
.
- P. Yang, Z. Li, Y. Wang, L. Zhang and H. Wu, Biochem. Biophys. Res. Commun., 2015, 459, 327–332 CrossRef CAS PubMed
.
- H. Wu, T. Liu, C. Ma, R. Xue, C. Deng, H. Zeng and X. Shen, Anal. Bioanal. Chem., 2011, 401, 635–646 CrossRef CAS PubMed
.
- C. M. Rubingh, S. Bijlsma, E. P. Derks, I. Bobeldijk, E. R. Verheij, S. Kochhar and A. K. Smilde, Metabolomics, 2006, 2, 53–61 CrossRef CAS PubMed
.
- Z. Li and P. Yang, Biochim. Biophys. Acta, 2014, 1846, 285–296 CAS
.
- P. Yang, Z. Li, H. Li, Y. Lu and H. Wu, Cell. Signalling, 2015, 27, 1525–1532 CrossRef CAS PubMed
.
- L. Hartnett and L. J. Egan, Carcinogenesis, 2012, 33, 723–731 CrossRef CAS PubMed
.
- J. M. Carethers, Dig. Dis. Sci., 2015, 60, 711–721 CrossRef PubMed
.
- M. H. Abu Bakar, M. R. Sarmidi, K. K. Cheng, A. Ali Khan, C. L. Suan, H. Zaman Huri and H. Yaakob, Mol. BioSyst., 2015, 11, 1742–1774 RSC
.
- H. Xi, M. Kurtoglu, H. Liu, M. Wangpaichitr, M. You, X. Liu, N. Savaraj and T. J. Lampidis, Cancer Chemother. Pharmacol., 2011, 67, 899–910 CrossRef CAS PubMed
.
- P. R. Baker, F. J. Schopfer, S. Sweeney and B. A. Freeman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11577–11582 CrossRef CAS PubMed
.
- M. Milanski, G. Degasperi, A. Coope, J. Morari, R. Denis, D. E. Cintra, D. M. Tsukumo, G. Anhe, M. E. Amaral, H. K. Takahashi, R. Curi, H. C. Oliveira, J. B. Carvalheira, S. Bordin, M. J. Saad and L. A. Velloso, J. Neurosci., 2009, 29, 359–370 CrossRef CAS PubMed
.
- M. Y. Sheu, A. J. Fowler, J. Kao, M. Schmuth, K. Schoonjans, J. Auwerx, J. W. Fluhr, M. Q. Man, P. M. Elias and K. R. Feingold, J. Invest. Dermatol., 2002, 118, 94–101 CrossRef CAS PubMed
.
- D. Han, W. Wei, X. Chen, Y. Zhang, Y. Wang, J. Zhang, X. Wang, T. Yu, Q. Hu, N. Liu and Y. You, Oncotarget, 2015, 6, 26119–26228 CrossRef PubMed
.
- J. T. Bjerrum, O. H. Nielsen, F. Hao, H. Tang, J. K. Nicholson, Y. Wang and J. Olsen, J. Proteome Res., 2010, 9, 954–962 CrossRef CAS PubMed
.
- D. Beyoglu, S. Imbeaud, O. Maurhofer, P. Bioulac-Sage, J. Zucman-Rossi, J. F. Dufour and J. R. Idle, Hepatology, 2013, 58, 229–238 CrossRef CAS PubMed
.
- J. Wei, G. Xie, S. Ge, Y. Qiu, W. Liu, A. Lu, T. Chen, H. Li, Z. Zhou and W. Jia, J. Proteome Res., 2012, 11, 1302–1316 CrossRef CAS PubMed
.
- H. W. Kim, J. H. Kim, H. S. An, K. K. Park, B. K. Kim and T. Park, Life Sci., 2003, 73, 2477–2489 CrossRef CAS PubMed
.
- M. Chen, Y. Ni, H. Duan, Y. Qiu, C. Guo, Y. Jiao, H. Shi, M. Su and W. Jia, Chem. Res. Toxicol., 2008, 21, 288–294 CrossRef CAS PubMed
.
- S. Ganti, S. L. Taylor, O. Abu Aboud, J. Yang, C. Evans, M. V. Osier, D. C. Alexander, K. Kim and R. H. Weiss, Cancer Res., 2012, 72, 3471–3479 CrossRef CAS PubMed
.
- L. Lv, D. Li, D. Zhao, R. Lin, Y. Chu, H. Zhang, Z. Zha, Y. Liu, Z. Li, Y. Xu, G. Wang, Y. Huang, Y. Xiong, K. L. Guan and Q. Y. Lei, Mol. Cell, 2011, 42, 719–730 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01718e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |