Very fast catalytic reduction of 4-nitrophenol, methylene blue and eosin Y in natural waters using green chemistry: p(tannic acid)–Cu ionic liquid composites
Nurettin Sahiner*ab,
Selin Sagbasa and
Nahit Aktasc
aFaculty of Science & Arts, Chemistry Department, Canakkale Onsekiz Mart University, Terzioglu Campus, 17100 Canakkale, Turkey. E-mail: sahiner71@gmail.com; Fax: +90-2862181948; Tel: +90-2862180018/+90-2862182041
bNanoscience and Technology Research and Application Center (NANORAC), Canakkale Onsekiz Mart University, Terzioglu Campus, 17100 Canakkale, Turkey
cChemical Engineering Department, Yuzuncu Yil University, 65080, Van, Turkey
First published on 3rd February 2015
Abstract
Using tannic acid (TA) as a biopolymer, poly(tannic Acid) (p(TA)) microgels were obtained by cross-linking TA with trimethylolpropane triglycidyl ether (TMPGDE) as cross-linker in a water-in-oil micro emulsion system. Ionic liquid forms of p(TA) micro particles were prepared as Ionic Liquid Colloids (ILC) by post chemical modification of p(TA) particles using quaternization agents such as 3-chloro-2-hydroxypropyl trimethyl ammonium chloride (CHPACl) and ammonia (NH3) in aqueous solution to generate positively-charged ammonium salts on the network. Then the modified p(TA) micro particles were used as template for Co, Ni, and Cu metal nanoparticle preparation in situ after loading of metal salts such as CoCl2, NiCl2, and CuCl2 from ethyl alcohol solution into the p(TA) network, and consequent reduction with sodium borohydride (NaBH4). The prepared metal nanoparticle-containing ILCs of p(TA) microgel composites were used as catalysts in the reduction of toxic organic compounds such as 4-nitrophenol (4-NP), eosin Y (EY), and methylene blue (MB). Various parameters affecting the 4-NP and MB reduction were investigated. The activation energy, enthalpy, and entropy for the reduction of 4-NP to 4-AP catalyzed by ILC p(TA)-co composite catalyst system were calculated as 26.19 kJ mol−1, 23.12 kJ mol−1, and −182.35 J mol−1 K−1, respectively.
1. Introduction
Ionic liquids (ILs), sometimes referred to as molten salts, consist of organic cations and organic/inorganic anions at room temperature.1 Due to their intriguing properties, such as high thermal stability, high thermal conductivity, high polarity, high flame resistance, low interface energy, low melting points, almost zero vapor pressure and volatility related to their effective ion–ion interaction, low pollution, ease of recycling, etc.2–4 ILs are promising materials in many applications, such as synthesis,5 catalysis,6 electrochemistry,7 energy,8 and separation.9 Recently ILs have been especially employed in the colloid and interface sciences because their hydrophilic and hydrophobic structure can be controlled by changing the cations or anions.10 The most common ILs consist of organic cations such as imidazolium, pyridinium, tetraalkylphosphonium [C4P]+, tetraalkyammonium [C4N]+, pyrrolidinium, and organic/inorganic anions such as hexafluorophosphate [PF6]−, tetraafluoroborate [BF4]−, nitrate [NO3]− and dicyanamide [N(CN)2]−.11Recently, different ionic liquid-based materials such as poly crystalline, polymers,5 ceramics, glasses and gels5 have been used for advanced material technology.12,13 Micron-sized hydrogels that swell in a good solvent14 can respond to changes in their environment or external stimuli such as temperature,15 pH, ionic strength of the medium,16 magnetic field,17 electric field, and light18 can be combined with ILs. The hydrogels with various functional groups offer unique properties, widely used for various applications such as biomedical, pharmaceutical, tissue engineering, drug delivery,19 purification and separation,20 biosensor,21 and catalysis.22,23 Biopolymers are generally derived from renewable, non-toxic, and inexhaustible natural biological resources.24,25 Tannic acid (TA) is a common polyphenolic compound and is a natural biopolymer with strong affinity for enzymes,26 proteins,27,28 carbohydrates, lipids,29 metal ions,30 and toxic molecules31 due to the abundant number of –OH on their phenolic structure. It is known that TA molecules can react with various metal ions such as Cu, Fe, Ni, Pd, Cr, Ar, Zn and Mn, via complex formation,32,33 ion exchange and reduction processes.34,35
In addition to synthetic polymers, natural polymers can be used to create ILs properties. Therefore, herein p(TA) micro particles were synthesized using trimethylolpropane triglycidyl ether (TMPGDE) as crosslinker. The prepared p(TA) microgels were used in ionic liquid colloids of p(TA) particle preparation by exposure to various modification agents. Moreover, the prepared p(TA)-based ILs materials were used as template for in situ metal nanoparticle preparation. Some metal salts such as CoCl2, NiCl2, CuCl2, FeCl2 and PdCl2 are commonly used in metal source catalysis,8,11,36 biomedical, and environmental applications.11,37 Therefore, modified p(TA) micro particles were interacted with Cu(II), Co(II) and Ni(II) solutions in ethanol to prepare ILCs of p(TA)–M composites (M: Co, Ni, Cu etc.). The metal nanoparticle-containing composite materials provide high catalytic activity, and reusability in various applications such as hydrogen storage, fuel cell, ammonia decomposition, catalytic reform, waste water treatment, nitro compound reduction and so on.37–43 As reported, ILs have promising potential in eliminating water pollutants such as phenolic compounds, polycyclic aromatic hydrocarbons and dyes from aquatic media.37,38 Therefore, the synthesized ILC of p(TA)–M composite containing different metals was used as catalyst in the reduction of toxic organic compounds such as 4-nitrophenol (4-NP) and dyes e.g. MB and EY.
2. Materials and methods
2.1. Materials
Tannic acid (TA) (ACS grade, 97% Sigma-Aldrich) as monomer, trimethylolpropane triglycidyl ether (TMPGDE) (technical grade, Sigma-Aldrich) as crosslinker and l-alpha-lecithin, granular (Acros Organics) as surfactant were used as received. Gasoline as an organic solvent was procured from a local vender. The modifying agents, 3-chloro-2-hydroxypropyl trimethyl ammonium chloride solution (CHPACl, 65%, Fluka) and ammonia solution (32%, Merck) were used. Cobalt(II) chloride hexahydrate (CoCl2·6H2O, 99% Sigma-Aldrich), nickel(II) chloride hexahydrate (NiCl2·6H2O, 97%, Riedel-de Haën), and CuCl2·6H2O (98%, Sigma) were used as metal ion sources. Sodium borohydride (NaBH4, 98%, Merck) was used for preparation of metal nanoparticles and in catalytic reduction reactions. Organic compounds such as 4-nitrophenol (4-NP, 98%, Merck), eosin Y disodium salt (85%, Sigma-Aldrich), and methylene blue hydrate (97%, Sigma-Aldrich) were used as received. Absolute ethyl alcohol (99.7%) was used for preparation of metal salt solutions and washing processes. NaOH (99%) and HCl (37.5%) were purchased from Aldrich. Seawater, Saricay (river) water, and tap water were obtained from Canakkale city in Turkey. Ultra pure distilled water 18.2 MΩ cm (Millipore Direct-Q UV3) was used throughout the experiments.2.2. Synthesis of tannic acid particles
Tannic acid, 2.5 g, was dissolved in 5 mL 0.2 M NaOH solution. This solution was put into 150 mL of 0.1 M lecithin in gasoline under vigorous stirring at 1200 rpm for 15 min at 50 °C. The crosslinker, TMPGDE 1.15 mL (30 mol% of gallic acid unit), as crosslinker was subsequently added as network-forming agent to this mixture under fast stirring for 12 h at 50 °C. The obtained p(TA) particles were purified by centrifugation at 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 544g for 2 min at 20 °C. The supernatant solution was decanted and p(TA) particles were washed with cyclohexane to remove lecithin. Again, the particles were washed with ethanol two times to remove the unreacted species of TA, surfactant and crosslinker. Finally, the obtained p(TA) particles were dried with a heat gun and kept in a closed container for further use.
544g for 2 min at 20 °C. The supernatant solution was decanted and p(TA) particles were washed with cyclohexane to remove lecithin. Again, the particles were washed with ethanol two times to remove the unreacted species of TA, surfactant and crosslinker. Finally, the obtained p(TA) particles were dried with a heat gun and kept in a closed container for further use.
2.3. Preparation of modified p(TA) particles
Synthesized p(TA) particles were modified with 3-chloro-2-hydroxypropyl trimethyl ammonium chloride and ammonia aqueous solution.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 544g for 2 min at 20 °C, dried with a heat gun and kept in a closed container for further use.
544g for 2 min at 20 °C, dried with a heat gun and kept in a closed container for further use.2.4. The in situ metal nanoparticle preparation within modified p(TA) microgel particles
For the preparation of metal salt-loaded IL p(TA) particles, 0.5 g modified p(TA) particles were placed into 1000 ppm 250 mL metal chloride salts (CoCl2, NiCl2, and CuCl2) in ethyl alcohol for 12 h. The metal salt-loaded p(TA) particles were washed with ethanol several times to remove unbound metal salts. For the reduction of metal salts inside the particle, metal salt-loaded IL p(TA) particles were treated with 0.1 M 50 mL NaBH4 solution for 2 h to reduce the metal salts to their corresponding metal nanoparticles. The prepared composite IL p(TA)–M (M: Co, Ni, Cu) particles were dried with a heat gun and kept in a closed container for further use.2.5. Characterization of p(TA)-based particles
The p(TA) particles were imaged by scanning electron microscopy (SEM, Jeol JSM-5600 LV) by placing the particles onto carbon tape-attached aluminum SEM stubs, after coating with gold to a thickness of a few nanometers, in a vacuum using an operating voltage of 20 kV.To determine point zero charges/pKa of particles, the pH potentiometric titration of 100 mg tannic acid (as control) and p(TA) particles were placed into 50 mL 0.01 M KCl solution, and 0.1 M NaOH solution was added drop wise under constant stirring. A change in pH was measured after equilibrium (within 10 min) within the pH range of 2–10 (Sartorius) under N2 gas.
The functional groups of tannic acid-based materials were analyzed using FT-IR spectroscopy (Themoscientific, FT-IR 100) in the spectral range of 4000–650 cm−1 with a resolution of 4 cm−1, using the ATR technique.
Zeta potential measurements of bare and modified p(TA) particles were conducted with a zetasizer (Zeta-Pals Zeta Potential Analyzer BIC) in 0.001 M KCl solution at different pHs in DI water.
The metal amount in ILs p(TA)–metal composites were determined by using atomic absorption spectroscopy (AAS, Thermo, ICA 3500 AA SPECTRO) and thermogravimetric analysis measurements. The thermal degradation and the metal content of ILs p(TA)-based particles were investigated using a thermogravimetric analyzer (SII TG/DTA 6300). About 5 mg of particles were placed in a ceramic crucible and the weight lost was recorded over the temperature range of 50–1000 °C at a heating rate of 10 °C min−1 under a dry flow of N2 of 100 mL min−1. To determine the exact amounts of M within modified p(TA)–M composites, 0.1 g was treated with 10 mL 1 M HCl solution to dissolve metal nanoparticles embedded in p(TA)–M composites, and the amount of metal ions ins solutions were determined using by AAS measurements.
2.6. Reduction of 4-NP, eosin Y, and MB by p(TA)–M based composite particles
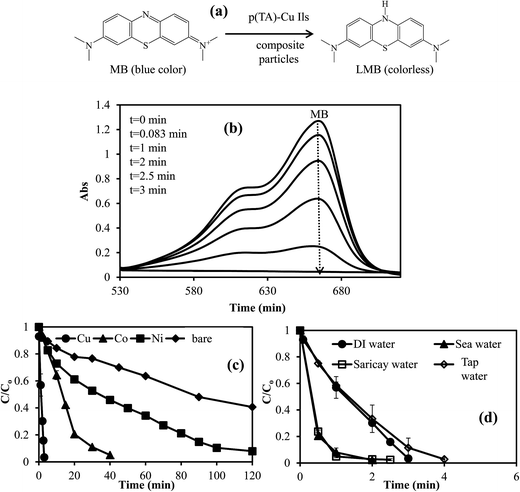
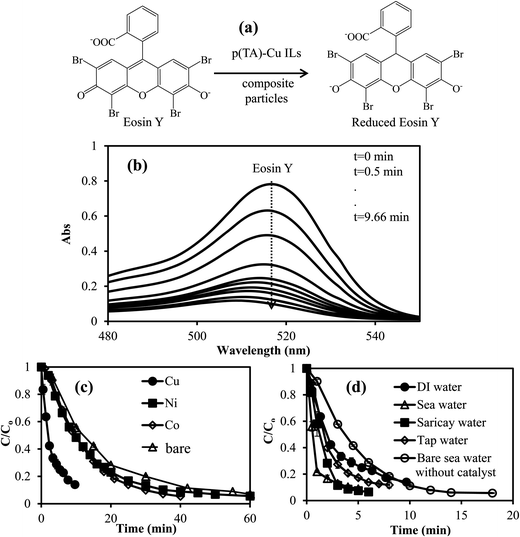
Separately, 0.01 M 4-NP, 8 × 10−4 M Eosin Y, and 3.2 × 10−4 M MB solutions were prepared and used in reduction. For the reduction of 4-NP to 4-AP, certain amounts of ILs p(TA)–M composite particles without NaBH4 treatment and reduced p(TA)–M composite with NaBH4 (M: Co, Ni, Cu; each 0.059 mmol) containing the same amount of metal nanoparticles were put into 0.01 M 50 mL 4-NP solution at 30 ± 2 °C. Then, 0.4 M (0.77 g) of NaBH4 was added quickly to this mixture under constant stirring rate of 800 rpm. At certain time intervals, 0.1 mL amount of this solution was taken and diluted 80 fold. The conversion of 4-NP to 4-AP reaction was monitored with the change in the intensity of 4-NP at the maximum absorption wavelength of 400 nm, and at 290 nm for the increase in the absorption maximum of 4-AP with respect to time by employing a UV-vis spectrophotometer (T80+UV/VIS Spectrometer, PG Ins. Ltd). To investigate the kinetic parameters of the reduction of 4-NP to 4-AP, a certain amount of p(TA)–Co particles containing 0.059 mmol of metal was used as catalyst by running the reaction at five different temperatures between 30 and 70 °C under the same conditions. In order to determined the reusability of the catalysts system, the same p(TA)–Cu composite particles were used in the reduction of 4-NP five times consecutively under the same catalytic conditions one day. The composite particle was washed with DI water before each usage.For the reduction of EY or MB, composite ILs p(TA)–M particles containing 0.0039 mmol Co, Ni, Cu nanoparticles (prepared without NaBH4 treatment) was placed into 8 × 10−4 M 100 mL EY or 3.2 × 10−4 M 100 mL MB solution. Immediately, 0.075 g (2 × 10−2 M NaBH4) was added to this mixture under constant stirring rate of 800 rpm. At certain times, 0.5 mL amount of this solution was removed and diluted 5 fold. Then, the reduction rate of EY or MB was measured with the change in EY (at maximum absorption wavelength 514 nm) or MB (at 664 nm) peak intensity with respect to time by employing a UV-vis spectrophotometer. In order to determine the effect of the media on the reduction rate of EY and MB, the same molar amount of EY and MB solutions were prepared in DI water, tap water, seawater, and creek water (Saricay). Then, the reduction of EY and MB were performed under the same reaction conditions. All the experiments were done in triplicate, the mean values were calculated and the results are given with standard deviation.
3. Results and discussion
3.1. Characterization of p(TA)-based particles
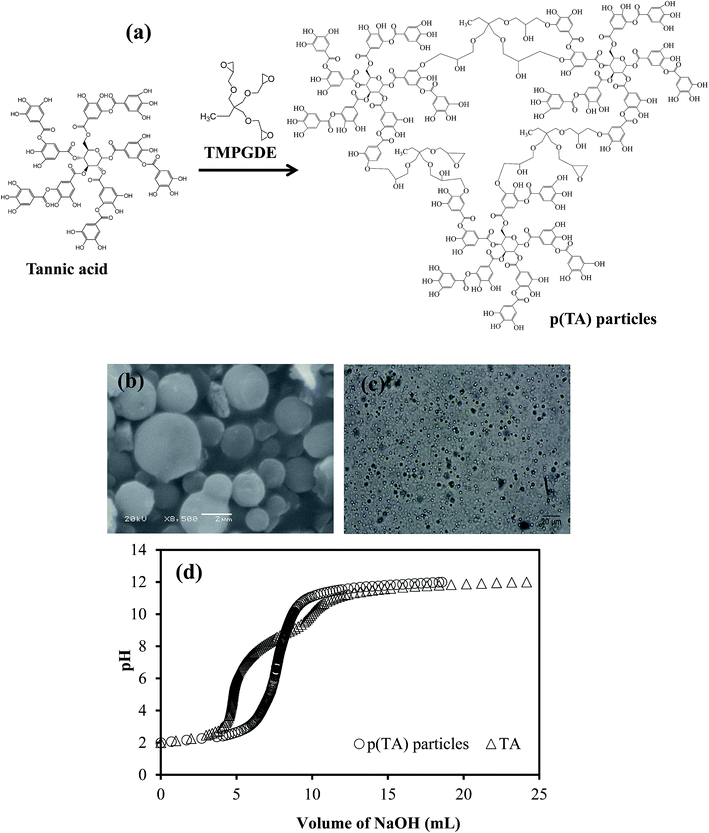
It is known that TA molecules interact with metal ions due to the existence of many –OH functional groups in gallic moieties repeating groups for different purposes, e.g., in environmental applications (Fig. 1a). In this study, TA molecules were crosslinked to form p(TA) microgel particles via simple micro emulsion technique with high yield (73 ± 6%). The shape, size and size distribution of the prepared p(TA) particles were visualized by optic microscope and SEM images, and it was found that the average diameter of the particles is broad, varying between 1–5 μm represented in Fig. 1b and c from SEM images and optical images in water, respectively. Because of the ionizable acidic nature of –OH groups, potentiometric titration of TA molecules and p(TA) particles in 0.01 M 50 mL KCl solution with 0.1 M NaOH was completed. The corresponding graph of pH versus amount of added NaOH is illustrated in Fig. 1d. The potentiometric titration results indicate that TA molecules have two inflection points at pH 4.8 ± 0.1 and pH 9.6 ± 0.2, coming from the presence of some carboxylic groups and phenolic/hydroxyl groups, respectively44 as gallic units are readily hydrolysable in acidic and basic environments. As supported by the literature, carboxylic groups and phenolic/hydroxyl groups have pKa values ranging from 2 to 6 and 8 to 10, respectively. However, the inflection point of p(TA) particles was found at pH 7.1 ± 0.1 because most of the –OH groups were crosslinked with TMPGDE crosslinker.Upon the modification of p(TA) particles with CHPACl, positive charges are generated on the p(TA) network, and upon treating with ammonia aqueous solution, ammonium salts of p(TA) particles are formed due to the neutralization of galloyl groups. Then these modified ILs p(TA) particles were used for in situ metal nanoparticle preparation. As presented in Fig. 2a, some –OH groups on p(TA) particles react with the modification agent (CHPACl) with their –OH groups. Therefore, modified p(TA) particles become positively charged coming from CHPACl, or upon neutralization with NH3 generating ammonia molecules and negatively charged pheoxylates on their structure. FT-IR spectra of p(TA) particles, and modified p(TA) particles with CHPACl and NH3 are shown in Fig. 2b. In the FT-IR spectra for p(TA) particles, a broad band at 3600–3000 cm−1 is due to –OH groups of polyphenols, and the peak at 1700 cm−1 can be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations from TA molecules. Three characteristic peaks at 1605, 1532, and 1444 cm−1 come from aromatic compound stretching, and the peaks at 1183, 1078, and 1016 cm−1 are ascribed to the vibration of substituted benzene rings.45 The peak at 1050 cm−1 is due to the CH2–O stretching because of formation ether linkage upon the crosslinking reaction. Modified p(TA) particles with CHPACl have two characteristic peaks at 1476 and 1340 cm−1 corresponding to C–N stretching of CHPACl molecules.14 In the spectra of NH3 p(TA) particles, the peak at 1430 cm−1 is stretching vibration coming from NH4+ salts on the polymer network after the modification reaction. That peak is shifted a little right in CHPACl modified p(TA) particles, as the quaternary amine groups are attached to the structure (not free). Therefore, these results demonstrate that ionic liquid colloids of p(TA) particles can be successfully prepared by CHPACl and ammonia modification. Additionally, to further confirm the modification reactions, zeta potential measurements of bare and modified p(TA) particles in 0.001 M KCl solution were carried out and are summarized in Table 1. The value of zeta potential of bare p(TA) particles, and CHPACl and NH3 modified p(TA) particles were found as −27 ± 1, −18 ± 3, and −13 ± 4 mV, respectively. These results show that modified particles possess some positive charges on the network of p(TA) and their zeta potentials increased or shifted toward more positive values in comparison to unmodified p(TA) particles.
O stretching vibrations from TA molecules. Three characteristic peaks at 1605, 1532, and 1444 cm−1 come from aromatic compound stretching, and the peaks at 1183, 1078, and 1016 cm−1 are ascribed to the vibration of substituted benzene rings.45 The peak at 1050 cm−1 is due to the CH2–O stretching because of formation ether linkage upon the crosslinking reaction. Modified p(TA) particles with CHPACl have two characteristic peaks at 1476 and 1340 cm−1 corresponding to C–N stretching of CHPACl molecules.14 In the spectra of NH3 p(TA) particles, the peak at 1430 cm−1 is stretching vibration coming from NH4+ salts on the polymer network after the modification reaction. That peak is shifted a little right in CHPACl modified p(TA) particles, as the quaternary amine groups are attached to the structure (not free). Therefore, these results demonstrate that ionic liquid colloids of p(TA) particles can be successfully prepared by CHPACl and ammonia modification. Additionally, to further confirm the modification reactions, zeta potential measurements of bare and modified p(TA) particles in 0.001 M KCl solution were carried out and are summarized in Table 1. The value of zeta potential of bare p(TA) particles, and CHPACl and NH3 modified p(TA) particles were found as −27 ± 1, −18 ± 3, and −13 ± 4 mV, respectively. These results show that modified particles possess some positive charges on the network of p(TA) and their zeta potentials increased or shifted toward more positive values in comparison to unmodified p(TA) particles.
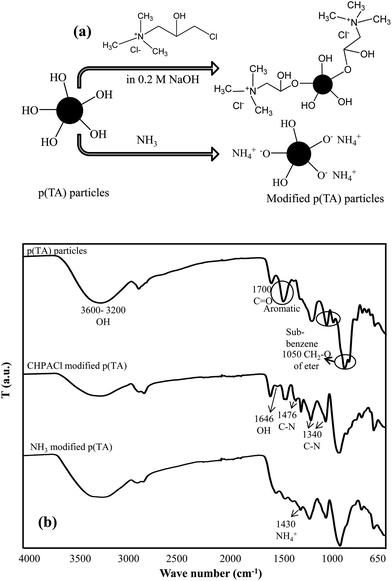 | ||
| Fig. 2 (a) The schematic representation of the modification mechanism of p(TA) particles, and (b) FT-IR spectra of p(TA) and modified p(TA) particles. | ||
| Materials | Zeta potential (mV) |
|---|---|
| p(TA) particles | −27 ± 1 |
| Modified p(TA) particles with CHPACl | −18 ± 3 |
| Modified p(TA) particles with ammonia | −13 ± 4 |
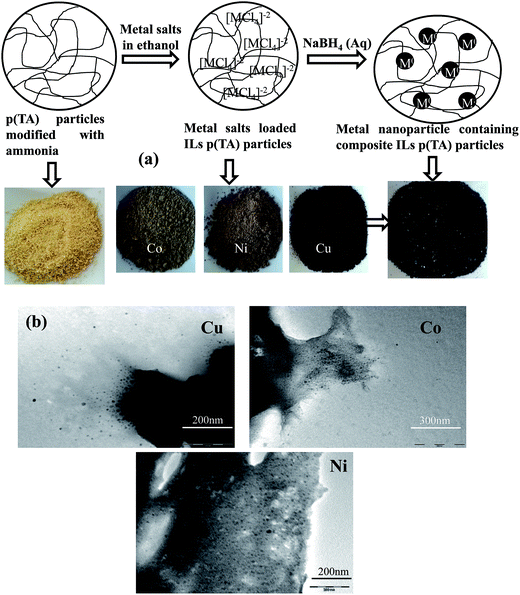
The modified p(TA) particles can be used as template for in situ metal nanoparticle preparation after loading metal salts, such as CoCl2, NiCl2, and CuCl2 in ethyl alcohol, into the microgels. As presented in Fig. 3a, modified p(TA) particles were laden with metal salts (CoCl2, NiCl2, CuCl2) in ethyl alcohol to form IL colloids because of the electrostatic interaction between metal salts and charged groups on p(TA). It is reported that TA molecules have strong affinity for metal ions due to the –OH groups on phenol groups in the TA structure. Das and Velusamy determined that phenolic compounds such as hydroxyl and ketonic groups are able to bind to metal ions because of the nucleophilic character of the aromatic ring in the molecular structure.46 Flavonoids such as TA are also known for effective scavenging properties for reactive oxygen species. It was reported that metal nanoparticles formation mechanism in modified p(TA) particle network is facilitated due to complex formation between the metal ions and –O− groups in TA molecules to their corresponding zero valent forms.47 Therefore, the colors of metal salt-loaded particles were different from the p(TA) particles without metal ions, and are darker as an indication of particle formation. In addition to the reduction ability of TA molecules, the metal salt-containing p(TA) particles were exposed to NaBH4 solution for complete reduction of the metal chlorides to the corresponding metal nanoparticles. The color of in situ metal nanoparticle-containing composite ILs p(TA)–M particles turned to an even darker black color as illustrated in the bottom right of Fig. 3a. The TEM images of the metal nanoparticles within p(TA)–M IL composites are shown in Fig. 3b. As can be seen from TEM images; Co, Ni or Cu nanoparticles are spherical in shape with the sizes ranging from about 5 nm to few of tens of nanometer.
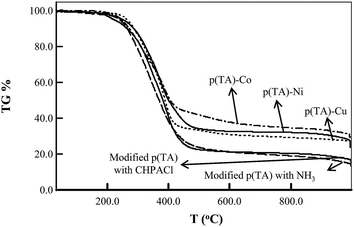
The thermal stability of modified p(TA) and metal-loaded ILs composite of p(TA)–M particles are illustrated in Fig. 4. The onset thermal degradation of the p(TA) modified with ammonia started at about 75 °C and continued up to 140 °C with slight degradation of about 1.3% weight, most probably due to the loss of bound water. The main thermal degradation range was observed between 210 to 496 °C with 76.1 wt% loss. The degradation of p(TA) particles modified with CHPACl was similar to p(TA) particles treated with ammonia. The metal salt-loaded composite thermograms also exhibited similar thermal degradation profile to the modified p(TA) particles. Heating up to 993 °C, modified p(TA) particles and the ILs composite of p(TA)–[CuCl4]−2, p(TA)–[NiCl4]−2 and p(TA)–[CoCl4]−2 particles displayed 85.4% 75.6%, 73.4% and 72.9% weight loss, respectively. The TGA results show that addition of metal salts increases the thermal stability of p(TA) particles. The metal contents of the IL composite particles of p(TA)–M were estimated at 9.7 wt% Cu, 11.9 wt% Ni, and 12.4 wt% Co in comparison to modified p(TA) particles as given in Table 2. In order to determine the exact amount of metals in composite ILs p(TA) particles, the metal nanoparticles were treated with 10 mL 1 M HCl for 24 h, and the metal ion concentration was measured by AAS. According to the AAS results given in Tables 2 and 1 g IL composite of p(TA)–M particles contains 45.8 ± 5 mg Co, 36.5 ± 3 mg Ni and 25.1 ± 2 mg Cu ions, whereas, unmodified p(TA)–M composite particles have 40.0 ± 3 mg Co, 35.5 ± 0 mg Ni and 27.1 ± 1 mg Cu ions.
| Metal species | p(TA) particles | Modified p(TA) particles with CHPACl | Modified p(TA) particles with ammonia | |
|---|---|---|---|---|
| AAS results (mg g−1) | AAS results (mg g−1) | AAS results (mg g−1) | TG (residue %) | |
| Co | 40.0 ± 3 | 38.4 ± 2 | 45.8 ± 5 | 9.7 |
| Ni | 35.5 ± 0 | 32.3 ± 1 | 36.5 ± 3 | 11.9 |
| Cu | 27.1 ± 1 | 26.7 ± 1 | 25.1 ± 2 | 12.4 |
3.2. Catalytic activity of p(TA)–M ILs composite particles
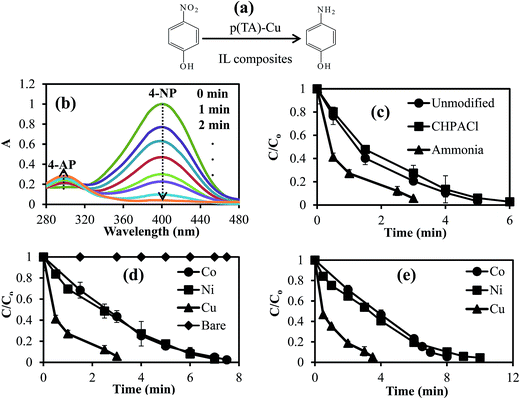
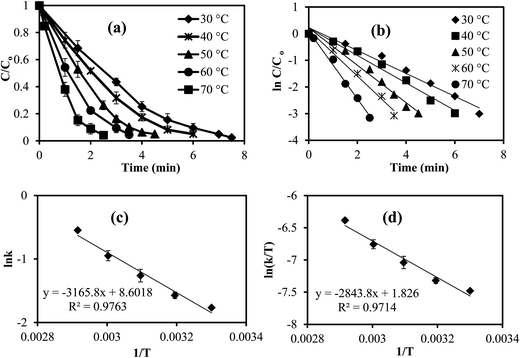
Nitro aromatic compounds and dyes are present in most surface and ground waters, and are also identified as toxic pollutant materials, as they are discharged or effluents from many industrial wastewater streams from textile, paper, plastic, leather and pharmaceutical industries,40 and some of these compounds are recognized as carcinogens. One of the toxic compounds, 4-NP, can be reduced to 4-AP which is non toxic and a useful material for several pharmaceutical applications. Metal nanoparticles and metal alloys of Co, Ni, Cu, Fe, Pd, Pt, Au, and Ag nanoparticles have been reported as catalyst for diverse reactions including nitro compounds and various dye reductions with high catalytic activity.41–43 The materials prepared here as green or environmentally-friendly IL composites of p(TA)–M particles were shown to be very useful catalysts in the reduction of toxic organic compounds, such as 4-NP and dyes e.g., MB and EY.As 4-NP reduction by the p(TA)–Cu composites is very fast, we investigated the effect of temperature on the reduction of 4-NP catalyzed by p(TA)–Co particles at five different temperatures (30, 40, 50, 60, and 70 °C) under the same reaction conditions. Fig. 6a demonstrates the decrease in the concentration of 4-NP with time at different temperatures. The reduction rate constants (k) values are given in Table 3. As can be clearly seen from the results, the reduction rate constant of the reaction increased with increasing temperature, and the 4-NP reduction rate is found to follow pseudo first order reaction kinetics because of a linear correlation of the natural logarithmic of concentration decease with time, as shown in Fig. 6b. The activation energy of 4-NP reactions at five different temperatures were calculated using the Arrhenius equation (eqn (1))
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − (Ea/RT) A − (Ea/RT)
| (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1/T as shown in Fig. 6c and Table 3. The kinetic parameters such as activation enthalpy (ΔH#) and activation entropy (ΔS#) were calculated using the Eyring equation (eqn (2)).
k vs. 1/T as shown in Fig. 6c and Table 3. The kinetic parameters such as activation enthalpy (ΔH#) and activation entropy (ΔS#) were calculated using the Eyring equation (eqn (2)).| ln(k/T) = ln(kB/h) + ΔS#/R – ΔH#/R(1/T) | (2) |
| Compound | T (°C) | K (min−1) | Total turn over frequency (mol 4-NP mol per catalyst per min) | Ea (kJ mol−1) | ΔH# (kJ mol−1) | ΔS# (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| 4-NP | 30 | 0.43 | 1.12 | 26.19 | 23.12 | −182.35 |
| 40 | 0.52 | 1.41 | ||||
| 50 | 0.70 | 1.88 | ||||
| 60 | 0.87 | 2.42 | ||||
| 70 | 1.27 | 3.38 | ||||
| MB | 30 | 0.68 | 3.28 | 38.89 | 35.43 | −127.54 |
| 40 | 1.50 | 8.20 | ||||
| 50 | 1.92 | 10.94 | ||||
| 60 | 3.69 | 16.41 | ||||
| 70 | 4.12 | 23.44 | ||||
| EY | 30 | 0.30 | 2.93 | 22.88 | 19.76 | −188.31 |
| 40 | 0.42 | 4.10 | ||||
| 50 | 0.45 | 5.86 | ||||
| 60 | 0.78 | 10.25 | ||||
| 70 | 0.84 | 16.41 |
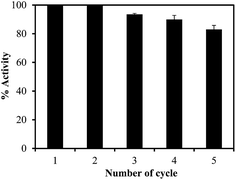
The reusability of catalyst is an important subject to determine the faith of catalyst in true industrial applications. Therefore, the p(TA)–Cu composite ILs particles was used in the same 4-NP reduction repetitively five time in one day, and their % activity of catalyst system was determined and graphed in Fig. 7. The % catalytic activity was calculated based on the % ratio of reduction rates after each use to the first use. It is obvious that the catalytic activity of p(TA)–Cu composite ILs particles is the same after 2nd usage, but then the catalytic activity was found decrease to 83% at the end of the 5th use. Therefore, p(TA)–Cu composite IL particles has great potential in real industrial applications.
| Compound | MB | EY | ||
|---|---|---|---|---|
| pH | Conductivity (μs cm−1) | pH | Conductivity (μs cm−1) | |
| DI water | 6.15 ± 0.04 | 0.61 ± 0.1 | 5.50 ± 0.06 | 0.44 ± 0.06 |
| Tap water | 8.08 ± 0.05 | 6.27 ± 1.2 | 7.60 ± 0.03 | 5.87 ± 0.8 |
| Saricay water | 8.12 ± 0.04 | 285.8 ± 4 | 8.36 ± 0.04 | 202.6 ± 12 |
| Seawater | 8.40 ± 0.09 | 439.2 ± 7 | 7.95 ± 0.02 | 483 ± 8 |
| Compound | K (min−1) | Total turn over frequency (mol compound mol per catalyst per min) |
|---|---|---|
| MB in DI water | 0.95 | 2.74 |
| MB in tap water | 0.86 | 2.05 |
| MB in Saricay water | 1.40 | 3.28 |
| MB in seawater | 1.83 | 4.10 |
| Eosin Y in DI water | 0.22 | 1.85 |
| Eosin Y in tap water | 0.34 | 2.34 |
| Eosin Y Saricay water | 0.57 | 3.19 |
| Eosin Y in seawater | 0.66 | 3.74 |
To investigated the thermodynamic parameters for MB and EY reductions, the reaction rate constants (k), TOF (mol MB or EY mol per catalyst per min) values, activation energy (Ea), enthalpy (ΔH) and entropies (ΔS) were calculated catalyzed by p(TA)–Cu composite IL particles for the reaction carried out at five different temperatures: 30, 40, 50, 60, and 70 °C, under the same reaction conditions, and the results are summarized in Table 3. The reduction rate constants (k) values for MB and EY reactions were calculated as 0.68 and 0.30 min−1 at 30 °C, whereas, rate constant (k) values were increased to 4.12 and 0.84 min−1 for MB and EY reactions at 70 °C. Moreover, the TOF values for MB and EY reductions catalyzed by p(TA)–Cu were increased to 23.44 mol MB mol per catalyst per min and 16.41 mol EY mol per catalyst per min at 70 °C from 3.28 MB mol per catalyst per min, and 2.93 mol EY mol per catalyst per min at 30 °C. From Table 3, rate constants and TOF values were increased with increasing temperature. The activation energy, enthalpy, and entropy were calculated as 38.89 kJ mol−1, 35.43 kJ mol−1, and −127.54 J mol−1 K−1, respectively, for the reduction of MB dye. These values for EY reduction catalyzed by p(TA)–Cu composite ILC particles were found as 22.88 kJ mol−1 for Ea, 19.76 kJ mol−1 for ΔH, and −188.31 J mol−1 K−1 for ΔS, respectively. Therefore, it can be presume that, EY reduction reaction by the p(TA)–Cu catalyst is resulted in better catalytic activity than MB reduction with the same catalyst. Gupta et al. reported that the activation energy of MB reduction with gold catalyst was found to be about 38.42 kJ mol−1.52,53 Moreover, Srivastava et al. demonstrated that the activation energy of EY reduction catalyzed by gold nanorod was determined as 32.84 kJ mol−1.54 Therefore, this current research that employed p(TA)–Cu catalyst system provided better catalytic performances on MB and EY reductions in comparison to the similar studies in the literature.53,54
4. Conclusions
Herein, a simple synthesis of p(TA) particles in a single step is reported by using epoxy-based cross linker, TMPGDE, with a high yield (73 ± 6%). These prepared natural particles were found to be very useful for in situ metal nanoparticle preparation due to the self-reducing ability of TA molecules for different metal ions such as Cu, Co, and Ni. p(TA) microgels were modified by chemical treatments to form ILs. To sum up;- p(TA) particles can be modified with 3-chloro-2-hydroxypropyl ammonium chloride and ammonia aqueous solution to generate positive charges on p(TA), and neutralized with NH3 to form ammonium salt of p(TA) as natural ionic liquid polymeric particles.
- The modified p(TA) particles can be used for in situ metal nanoparticle preparation after loading metal salts such as CoCl2, NiCl2, and CuCl2 in ethyl alcohol into NH3-modified p(TA) ILCs.
- It was found that without NaBH4 treatments of the metal salt-laden p(TA) particles, TA molecules reduce the metal salts to the corresponding metal nanoparticles readily, and provide better catalytic performances in the catalytic reduction of nitro compounds and organic dyes.
- Kinetic parameters such as energy, enthalpy and entropy of the 4-NP reduction catalyzed by p(TA)–Co composites were calculated as 26.19 kJ mol−1 for Ea, 23.12 kJ mol−1 for ΔH and −182.35 J mol−1 K−1 for ΔS, respectively.
- The reduction of toxic organic compounds in addition to 4-nitrophenol, such as EY and MB, catalyzed by p(TA)–M IL composite were shown to be very efficient. In addition, interestingly, it was found that MB and EY reduction in natural waters such as seawater and creek waters was much faster than the reduction reactions in DI and tap waters, suggesting the great potential for the real application of p(TA)–M IL composite to clean organic nitro compound- or dye-contaminated waters.
Acknowledgements
This work is supported by The Scientific and Technological Research Council of Turkey (113Z238).Notes and references
- J. Saienand and S. Asadabadi, Colloids Surf., A, 2014, 444, 138 CrossRef PubMed.
- T. Sun, S. Gao, Q. Chen and X. Shen, Colloids Surf., A, 2014, 456, 18 CrossRef CAS PubMed.
- C. P. Fredlake, J. M. Crosthwaite, D. G. Hert, S. N. V. K. Aki and J. F. Brennecke, J. Chem. Eng. Data, 2004, 49, 954 CrossRef CAS.
- C. H. Liang, W. Y. Ho, L. H. Yeh, Y. S. Chen and T. H. Chou, Colloids Surf., A, 2013, 436, 1083 CrossRef CAS PubMed.
- Y. Men, D. Kuzmicz and J. Yuan, Curr. Opin. Colloid Interface Sci., 2014, 19, 76 CrossRef CAS PubMed.
- D. B. Zhao, M. Wu, Y. Kou and E. Z. Min, Catal. Today, 2002, 74, 157 CrossRef CAS.
- W. Suna, L. Dong, Y. Lu, Y. Deng, J. Yu, X. Sun and Q. Zhu, Sens. Actuators, B, 2014, 199, 36 CrossRef PubMed.
- N. Sahiner, T. Turhan and L. A. Lyon, Energy, 2014, 66, 256 CrossRef CAS PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef CAS PubMed.
- M. Armada, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621 CrossRef PubMed.
- M. P. Singh, R. K. Singh and S. Chandra, Prog. Mater. Sci., 2014, 64, 73 CrossRef CAS PubMed.
- J. Lu, F. Yan and J. Texter, Prog. Polym. Sci., 2009, 34, 431 CrossRef CAS PubMed.
- T. Ueki and M. Watanabe, Langmuir, 2007, 23, 988 CrossRef CAS PubMed.
- S. Sagbas, S. Butun and N. Sahiner, Carbohydr. Polym., 2012, 87, 2718 CrossRef CAS PubMed.
- B. H. Tan, R. H. Pelton and K. C. Tam, Polymer, 2010, 51, 3238 CrossRef CAS PubMed.
- S. Sagbas, C. Kantar and N. Sahiner, Water, Air, Soil Pollut., 2014, 225, 1809 CrossRef.
- N. Sahiner and S. Sagbas, J. Power Sources, 2014, 246, 55 CrossRef CAS PubMed.
- D. Klinger and K. Landfester, Polymer, 2012, 53, 5209 CrossRef CAS PubMed.
- N. Sahiner, C. Silan, S. Sagbas, P. Ilgin, S. Butun, H. Erdugan and R. S. Ayyala, Microporous Mesoporous Mater., 2012, 155, 124 CrossRef CAS PubMed.
- H. Kawaguchi and K. Fujimoto, Bioseparation, 1999, 7, 253 CrossRef CAS.
- Z. Hu, Y. Chen, C. Wang, Y. Zheng and Y. Li, Nature, 1998, 393, 149 CrossRef CAS PubMed.
- S. Sagbas and N. Sahiner, Int. J. Hydrogen Energy, 2012, 37, 18944 CrossRef CAS PubMed.
- N. Sahiner, Prog. Polym. Sci., 2013, 38, 1329 CrossRef CAS PubMed.
- N. Sahiner and S. Sagbas, Mater. Sci. Eng., C, 2014, 40, 366–372 CrossRef CAS PubMed.
- N. Sahiner, S. Sagbas and M. Turk, Int. J. Biol. Macromol., 2014, 66, 236 CrossRef CAS PubMed.
- V. Krajka-Kuźniak, H. Szaefer and W. Baer-Dubowska, Toxicol. Lett., 2004, 152, 117 Search PubMed.
- S. A. Chowdhury, R. Vijayaraghavan and D. R. MacFarlane, Green Chem., 2010, 12, 1023 RSC.
- J. C. Isenburg, D. T. Simionescu and N. R. Vyavahare, Biomaterials, 2004, 25, 3293 CrossRef CAS PubMed.
- T. Yugarani, B. K. H. Tan, M. Teh and N. P. Das, Lipids, 1992, 27, 181 CrossRef CAS.
- G. K. B. Lopes, H. M. Schulman and M. Hermes-Lima, Biochim. Biophys. Acta, 1999, 472, 142 CrossRef.
- K. Tikoo, M. S. Sane and C. Gupta, Toxicol. Appl. Pharmacol., 2011, 251, 191 CrossRef CAS PubMed.
- A. Üçer, A. Uyanik and Ş. F. Aygün, Sep. Purif. Technol., 2006, 47, 113 CrossRef PubMed.
- W. Li, X. Gong, X. Li, D. Zhang and H. Gong, Bioresour. Technol., 2012, 113, 106 CrossRef CAS PubMed.
- A. Dutta and S. K. Dolui, Appl. Surf. Sci., 2011, 257, 6889 CrossRef CAS PubMed.
- M. M. Kumari, S. A. Aromal and D. Philip, Spectrochim. Acta, Part A, 2013, 103, 130 CrossRef PubMed.
- N. Sahiner, S. Demir and S. Yildiz, Colloids Surf., A, 2014, 449, 87 CrossRef CAS PubMed.
- S. Demirci and N. Sahiner, J. Mol. Liq., 2014, 194, 85 CrossRef CAS PubMed.
- J. Ma and X. Hong, J. Environ. Manage., 2012, 99, 104 CrossRef CAS PubMed.
- N. Sahiner and S. Sagbas, Colloids Surf., A, 2013, 418, 76 CrossRef CAS PubMed.
- Z. Dong, X. Le, C. Dong, W. Zhang, X. Li and J. Ma, Appl. Catal., B, 2015, 162, 372 CrossRef CAS PubMed.
- J. Zhang, G. Chen, M. Chaker, F. Rosei and D. Ma, Appl. Catal., B, 2013, 132–133, 107 CrossRef CAS PubMed.
- N. Sahiner, H. Ozay, O. Ozay and N. Aktas, Appl. Catal., B, 2010, 101, 137 CrossRef CAS PubMed.
- W. Wojtków, A. M. Trzeciak, R. Choukroun and J. L. Pellegatta, J. Mol. Catal. A: Chem., 2004, 224, 81 CrossRef PubMed.
- P. Kraal, B. Jansen, K. G. J. Nierop and J. M. Verstraten, Chemosphere, 2006, 65, 2193 CrossRef CAS PubMed.
- A. Janošević, G. Ćirić-Marjanović, B. S. Paunković, I. Pašti, S. Trifunović, B. Marjanović and J. Stejskal, Synth. Met., 2012, 162, 843–856 CrossRef PubMed.
- J. Das and P. Velusamy, J. Taiwan Inst. Chem. Eng., 2014, 45, 2280 CrossRef CAS PubMed.
- T. Ahmad, J. Nanotechnol., 2014, 954206 Search PubMed.
- M. Ajmal, M. Siddiq, H. Al-Lohedan and N. Sahiner, RSC Adv., 2014, 4, 59562 RSC.
- S. Butun and N. Sahiner, Polymer, 2011, 52, 4834 CrossRef CAS PubMed.
- M. Tang, G. Huang, S. Zhang, Y. Liu, X. Li, X. Wang, X. Pang and H. Qiu, Mater. Chem. Phys., 2014, 145, 418 CrossRef CAS PubMed.
- N. Sahiner, A. Kaynak and S. Butun, J. Non-Cryst. Solids, 2012, 358, 758 CrossRef CAS PubMed.
- V. K. Vidhu and D. Philip, Micron, 2014, 56, 54 CrossRef CAS PubMed.
- N. Gupta, H. P. Singh and R. K. Sharma, Colloids Surf., A, 2010, 367, 102 CrossRef CAS PubMed.
- S. Srivastava, S. K. Sharma and R. K. Sharma, Colloids Surf., A, 2011, 373, 61 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |