Carbon nanotube-penetrated mesoporous V2O5 microspheres as high-performance cathode materials for lithium-ion batteries†
Xilai Jiaab,
Liqiang Zhangb,
Rufan Zhanga,
Yunfeng Lu*c and
Fei Wei*a
aBeijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, P. R. China. E-mail: wf-dce@tsinghua.edu.cn; Fax: +86 10-6277-2051
bState Key Laboratory of Heavy Oil Processing, China University of Petroleum, Changping, Beijing 102249, P. R. China
cDepartment of Chemical and Biomolecular Engineering, University of California, Los Angeles, CA 90095, USA. E-mail: luucla@ucla.edu
First published on 11th March 2014
Abstract
A three-dimensional nanoarchitecture consisting of mesoporous V2O5 and penetrating CNTs was synthesized via an aerosol-spray drying process followed by two-step thermal annealing. The nanocomposites show a hierarchical structure, effective for ion and electron transport, making their lithium-ion battery electrodes show superhigh capacity and rate-capability.
High-performance electrochemical energy storage devices, such as lithium-ion batteries, are of great importance for a broad range of applications.1 During their charging and discharging processes, the shuttling of ions and the transport of electrons cause chemical energy and electrical energy, respectively, to be reversibly transformed. However, current battery technologies are mostly limited by such kinetic problems.2 Vanadium pentoxide (V2O5) shows a high theoretical capacity (∼296 mA h g−1 based on the intercalation of two lithium ions) and can be synthesized at a low cost, properties which make it a promising cathode candidate for batteries. However, V2O5 exhibits slow ion diffusion and poor electronic conductivity. The design of the ion and electron transport systems is therefore the most important consideration for better performance.
In this context, low-dimensional V2O5 materials such as nanoparticles,3 nanowires,4 nanosheets,5 nanobelts6 and nanorods,7 have been synthesized and have achieved improved performances, since their low dimensionalities have shortened the ion diffusion length. However, the use of such low-dimensional V2O5 structures always leads to the undesired problems of loading, interfaces and handling.2 Based on the low-dimensional building blocks, 3D nanostructured V2O5 architectures have been synthesized, including nanostructured fibers,8 arrays,9 films,10 microspheres,11 microflowers12 and foams.13 Accordingly, interconnected pore channels for electrolyte transport and shortened distances for ion diffusion are built into those architectures; however, effective electron transport is still required for further improvement.14 Nanocarbon materials such as carbon nanotubes (CNTs) and graphene have therefore emerged to replace traditional carbon blacks in battery synthesis techniques,15 since they can offer long-range conductivity, better interfacial contact, and more robust structures. To date, various approaches (e.g., direct mechanical mixing,16 in situ hydrothermal growth,17 sol–gel,18 atomic layer deposition (ALD),19 etc.) have been proposed to construct such nanocomposites, such as the commonly made “entangled network” nanocomposites, with V2O5 entangled with CNTs,16 and “core–shell” hybrid nanocomposites, with V2O5 coated onto CNT surfaces.20 Nevertheless, most of those nanocomposites cannot form 3D structures, and thus only facilitate charge transfer locally. Some 3D nanocomposites have been fabricated using the ALD technique;19 such deposition processes, however, require expensive precursors and facilities, and are difficult to implement in large-scale production.
Herein, we propose the construction of a novel 3D nanoarchitecture, using a facile aerosol-spray drying process, that consists of nanostructured mesoporous V2O5 and penetrating CNTs, for use as a high-performance cathode material (Fig. 1a). In contrast to prior work on V2O5-based electrodes,11 our design, firstly, presents two levels of ion transport in the nanocomposites, including interconnected pore networks for facile electrolyte access and the mesoporous structure of V2O5, with shortened distances for ion diffusion. Additionally, CNTs are directly assembled within V2O5 for efficient electron transport. Moreover, the structured pores of the nanocomposites are able to accommodate the volume changes during the lithium ion insertion–extraction process, which is desirable for cycling stability. Therefore, the nanocomposites simultaneously form robust ion and electron transport networks, and are expected to produce high-performance batteries.
 | ||
| Fig. 1 (a) Schematic illustration and (b) SEM image of the structure of the CNT–V2O5 nanocomposite microspheres. | ||
To fabricate the nanocomposites, we started by dispersing the CNTs into a precursor solution of V2O5, forming a homogeneous dispersion. Simultaneously, a surfactant (F127, polyethylene glycol, propoxylated) was also introduced into the dispersion for templating. The atomization process, using nitrogen as the carrier gas, continuously generated precursor droplets containing the CNTs. After passing through a heated tube furnace, evaporation of the solvent from the droplets enriched the precursor and condensed the CNTs into solid particles. Subsequent two-step thermal treatments, using hydrogen and air annealing in sequence, converted the collected particles into the final CNT–V2O5 nanocomposite microspheres (Fig. 1b), which are made from networks of V2O5, penetrated throughout by CNTs.
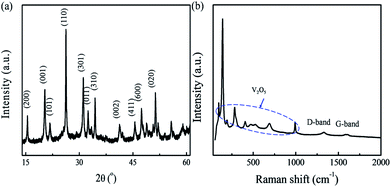
The morphologies and structures of the nanocomposites were first investigated using X-ray diffraction (XRD), Raman spectroscopy and electron microscopic techniques. Fig. 2a shows the XRD pattern of the nanocomposites. It exhibits intense diffraction peaks characteristic of the orthorhombic V2O5 phase (space group Pmmn, JCPDS card no. 41-1426), suggesting the successful transformation of the V-precursor into V2O5. Note that the characteristic CNT diffraction peak at 26.2° is not visible; however, the Raman spectrum (Fig. 2b) of the nanocomposites detects carbon peaks with characteristic disorder-induced D and graphitic G bands at 1336 and 1582 cm−1, respectively. The results suggest two phases, namely V2O5 and carbon, coexist in the nanocomposites. The elemental mapping of the nanocomposites was performed by energy dispersive X-ray spectroscopy (EDX, Fig. S1†), and confirmed their distributions. The chemical composition was further determined by thermogravimetric analysis as displayed in Fig. S2,† which reveals a composition of 94 wt% V2O5 and 6 wt% carbon.
Scanning electron microscopy (SEM) imaging shows that the nanocomposite microspheres are spherical in shape and polydisperse, with submicron sizes (Fig. S3†). Such nanocomposites can circumvent the limitations of other nanoparticles and offer easier handling in actual production.2 It is worth mentioning that direct thermal treatment of the collected spray-dried particles at 400 °C in air has failed to produce the nanocomposites, because the nanostructured microspherical morphologies are destroyed to a certain degree and the CNTs are completely oxidized, as indicated by Fig. S4.† The thermal treatment at a lower temperature, such as 300 °C in air, however, cannot fully achieve the formation of V2O5 (Fig. S5†). Note that some CNT–V2O5 nanocomposites have been obtained under treatment at 400 °C in air;21 which could be ascribed to the nanotubes used and the structural properties of the nanocomposites.
Transmission electron microscopy (TEM, Fig. 3a) imaging further reveals the interconnected 3D networks of the nanocomposites. Close TEM observation (Fig. 3b) shows the direct interfacial contacts between the CNTs and the active materials. As expected, the CNTs were assembled into the V2O5 with no phase separation. Such direct interfacial contacts facilitate effective electron transport, a key factor affecting the electrode's rate performance. In comparison, nanocomposites obtained by direct mechanical-mixing16 and recently developed interpenetrating nanocomposites22 can hardly provide such intimate interfacial contacts. TEM observation further reveals the mesoporous structure of V2O5 (Fig. 3c). It shows that V2O5 has abundant mesopores of 2–4 nm, which should be produced by the decomposition of the surfactant. Those distributed mesopores serve as electrolyte storage points for locally efficient ion transport. Moreover, Fig. 3c suggests that V2O5 microspheres are composed of a layered crystalline structure, with a layer-to-layer distance of ∼2.1 Å, which can accommodate facile ion transport. Selected area electron diffraction (SAED, inset of Fig. 3c) further reveals their polycrystalline nature.
 | ||
| Fig. 3 (a–c) TEM images of the CNT–V2O5 nanocomposite microspheres. (d) Nitrogen isotherms of the nanocomposite microspheres. | ||
The 3D structure of the CNT–V2O5 nanocomposites was further confirmed using nitrogen adsorption measurements (Fig. 3d). A high Brunauer–Emmett–Teller (BET) surface area of 95.8 m2 g−1 was exhibited. The significant nitrogen uptake at a high relative pressure of nitrogen, as well as the absence of adsorption–desorption hysteresis, suggests the formation of interconnected porous channels. The nitrogen adsorption–desorption isotherms also suggest that the nanocomposites exhibit hierarchically structured pores with a broad pore size distribution ranging from several nanometers to nearly one hundred nanometers (inset of Fig. 3d). More specifically, the continuous pore channels around 4–60 nm could be summarily ascribed to the interconnected pores; while the abundant pores below 4 nm should be produced from the decomposition of surfactant after annealing. In short, based on the structural characterization, it is convincing that the designed nanocomposite microspheres are effective for ion and electron transport.
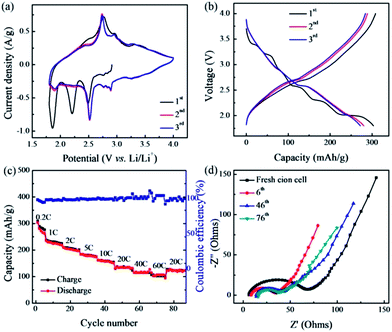
The cathode performance of the CNT–V2O5 nanocomposites is shown in Fig. 4. Their charge storage behaviour was characterized by cyclic voltammetry (CV, Fig. 4a). The electrochemical Li+ insertion–extraction process can be expressed by V2O5 + xLi+ + xe− ↔ LixV2O5, where x is the mole fraction of the inserted Li ions. For the first cathodic scan, multiple reduction peaks located at 2.9, 2.5, 2.2, 1.9 V are observed due to the phase transformations associated with Li insertion, which relate to the ε-LixV2O5, δ-LixV2O5, ω-LixV2O5, and γ-LixV2O5 phases, respectively.23 Note that the formation of the ω-LixV2O5 and γ-LixV2O5 phases is irreversible due to the deep Li insertion. However, the following cycles are highly reversible, suggesting facile phase transformations at the nanoscale. Consistent with the CV curves, the charge–discharge curves at a current density of 50 mA g−1 showed that the first charge and discharge capacities based on the total mass of electrode were 308 and 303 mA h g−1, respectively (Fig. 4b). This corresponds to an initial coulombic efficiency of 98%, which is extremely high for lithium-ion batteries. When increasing the current densities (Fig. S6a†), the improved kinetics of the Li insertion–extraction were maintained due to the hierarchical structure of the nanocomposites and, more importantly, increased conductivity contributed by the CNTs. The capacity contribution of the CNTs within the nanocomposite electrode was relatively small, compared to that of the V2O5, between the voltage limits of 1.8–4.0 V (Fig. S7†). Based on the mass of the V2O5 component, it displayed a discharge capacity of ∼442 mA h g−1 at 50 mA g−1, which is markedly higher than those of reported V2O5-based cathodes.24 Obviously, the designed nanocomposites can optimize the performance of the active materials.
Fig. 4c shows the rate and cycling performance of the CNT–V2O5 electrode at different current densities. In spite of a capacity decrease of ca. 10% during the initial cycles, as is commonly observed for V-oxides,25 the electrode shows a stable capacity at various C rates. The total discharge capacity of the electrode reached ∼300 mA h g−1 at 0.2 C. Even at high rates of 20 and 60 C, the electrode still delivered a reversible capacity of 132 and 104 mA h g−1. In comparison, the electrode made from aerosol-synthesized V2O5 microspheres showed a slightly lower rate-capability due to the inefficient transport of electrons (Fig. S6b†). Such rate capability is really impressive. Furthermore, upon returning back to the rate of 20 C after 75 cycles at different rates, the nanocomposite electrode still delivered a discharge capacity of 130 mA h g−1. Such an electrode still also presents a capacity over 210 mA h g−1 after 60 cycles at the charge–discharge rate of 0.5 C (Fig. S8†), suggesting a moderate stability. In comparison with several high-rate V2O5 electrodes from recent work,21,26 the designed nanocomposite electrode shows comparable or much better performance. Importantly, the performance is based on the total weight of the active nanocomposite materials, binders and conductive additives. With the demand for efficient batteries, there is usually a compromise in terms of storage capability and discharge rate. Herein, high energy and power densities were combined by 3D nanostructured composites, making them promising for high-power devices.
Electrochemical impedance spectroscopy (EIS) was carried out to probe the structural properties of the nanocomposite electrode, along with cycling. Nyquist plots of the electrode at different cycling stages are shown in Fig. 4d. It was found that the fresh electrode exhibited a relatively large single semicircle and an intercept in the high frequency range, which was associated with a combination of ohmic and charge-transfer resistance, and a low-frequency Warburg tail, associated with ion diffusion resistance. Interestingly, the diameter of the semicircle decreases and the slope of the Warburg tail increases after 5 cycles at 0.2 C, indicating a decreased charge-transfer resistance and improved lithium-diffusion rate. This may be due to removal of the protons from the electrodes and better CNT–V2O5 interfaces, induced by the charge–discharge processes.22 The impedance behaviour shows similar features during the following cycles, confirming the robust structure of the nanocomposites. Even after 75 cycles, the charge transport resistance remains small and, accordingly, the capacity is maintained with high stability.
In summary, we have demonstrated a novel 3D CNT–V2O5 nanoarchitecture consisting of hierarchically structured V2O5 and penetrating CNTs. The nanocomposites are realized by an efficient aerosol-spray drying process followed by annealing processes. Due to their nanostructured features, such nanocomposites display effective charge transport, thus offering batteries with high capacities and rate-capabilities. The synthesis method may be extended to synthesize other cathode and anode electrode materials for energy storage devices.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, 2011CB932602) and the Natural Scientific Foundation of China (no. 21306102), and was partially supported by the Science Foundation of China University of Petroleum, Beijing (no. 2462013YJRC028).Notes and references
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed.
- X.-F. Zhang, K.-X. Wang, X. Wei and J.-S. Chen, Chem. Mater., 2011, 23, 5290 CrossRef CAS; M. Koltypin, V. Pol, A. Gedanken and D. Aurbach, J. Electrochem. Soc., 2007, 154, A605 CrossRef PubMed.
- J. W. Lee, S. Y. Lim, H. M. Jeong, T. H. Hwang, J. K. Kang and J. W. Choi, Energy Environ. Sci., 2012, 5, 9889 CAS; H. Liu and W. Yang, Energy Environ. Sci., 2011, 4, 4000 Search PubMed.
- Y. Li, J. Yao, E. Uchaker, J. Yang, Y. Huang, M. Zhang and G. Cao, Adv. Energy Mater., 2013, 3, 1171 CrossRef CAS; X. Rui, Z. Lu, H. Yu, D. Yang, H. H. Hng, T. M. Lim and Q. Yan, Nanoscale, 2013, 5, 556 RSC.
- G. Li, S. Pang, L. Jiang, Z. Guo and Z. Zhang, J. Phys. Chem. B, 2006, 110, 9383 CrossRef CAS PubMed.
- A. M. Glushenkov, M. F. Hassan, V. I. Stukachev, Z. Guo, H. K. Liu, G. G. Kuvshinov and Y. Chen, J. Solid State Electrochem., 2010, 14, 1841 CrossRef CAS PubMed.
- H. G. Wang, D. L. Ma, Y. Huang and X. B. Zhang, Chem. – Eur. J., 2012, 18, 8987 CrossRef CAS PubMed; D. Yu, C. Chen, S. Xie, Y. Liu, K. Park, X. Zhou, Q. Zhang, J. Li and G. Cao, Energy Environ. Sci., 2011, 4, 858 Search PubMed; L. Mai, L. Xu, C. Han, X. Xu, Y. Luo, S. Zhao and Y. Zhao, Nano Lett., 2010, 10, 4750 CrossRef PubMed.
- X. Yu, Z. Lu, G. Zhang, X. Lei, J. Liu, L. Wang and X. Sun, RSC Adv., 2013, 3, 19937 RSC.
- X. Chen, E. Pomerantseva, K. Gregorczyk, R. Ghodssi and G. Rubloff, RSC Adv., 2013, 3, 4294 RSC; X. Chen, E. Pomerantseva, P. Banerjee, K. Gregorczyk, R. Ghodssi and G. Rubloff, Chem. Mater., 2012, 24, 1255 CrossRef CAS.
- J. Shao, X. Li, Z. Wan, L. Zhang, Y. Ding, L. Zhang, Q. Qu and H. Zheng, ACS Appl. Mater. Interfaces, 2013, 5, 7671 Search PubMed; A. Pan, H. B. Wu, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2013, 125, 2282 CrossRef CAS; A. M. Cao, J. S. Hu, H. P. Liang and L. J. Wan, Angew. Chem., Int. Ed., 2005, 44, 4391 CrossRef PubMed; J. Liu, Y. Zhou, J. Wang, Y. Pan and D. Xue, Chem. Commun., 2011, 47, 10380 RSC; H. Pang, P. Cheng, H. Yang, J. Lu, C. X. Guo, G. Ning and C. M. Li, Chem. Commun., 2013, 49, 1536 RSC; S. Wang, S. Li, Y. Sun, X. Feng and C. Chen, Energy Environ. Sci., 2011, 4, 2854 Search PubMed; C. Zhang, Z. Chen, Z. Guo and X. W. D. Lou, Energy Environ. Sci., 2013, 6, 974 Search PubMed.
- A. Pan, H. B. Wu, L. Yu, T. Zhu and X. W. Lou, ACS Appl. Mater. Interfaces, 2012, 4, 3874 CAS.
- J. Zhu, L. Cao, Y. Wu, Y. Gong, Z. Liu, H. E. Hoster, Y. Zhang, S. Zhang, S. Yang, Q. Yan, P. M. Ajayan and R. Vajtai, Nano Lett., 2013, 13, 5408 CrossRef CAS PubMed.
- J. Liu and D. Xue, Nanoscale Res. Lett., 2010, 5, 1525 CrossRef CAS PubMed.
- R. Yu, C. Zhang, Q. Meng, Z. Chen, H. Liu and Z. Guo, ACS Appl. Mater. Interfaces, 2013, 5, 12394 Search PubMed; X. Chen, H. Zhu, Y.-C. Chen, Y. Shang, A. Cao, L. Hu and G. W. Rubloff, ACS Nano, 2012, 6, 7948 CrossRef CAS PubMed; A. Ghosh, E. J. Ra, M. Jin, H. K. Jeong, T. H. Kim, C. Biswas and Y. H. Lee, Adv. Funct. Mater., 2011, 21, 2541 CrossRef; Z.-L. Wang, D. Xu, Y. Huang, Z. Wu, L.-M. Wang and X.-B. Zhang, Chem. Commun., 2012, 48, 976 RSC; Q. Zhu, Z. Li, S. Huang, X. Zhang, W. Chen and G. S. Zakharova, Electrochim. Acta, 2012, 81, 25 CrossRef PubMed; H. Gwon, H.-S. Kim, K. U. Lee, D.-H. Seo, Y. C. Park, Y.-S. Lee, B. T. Ahn and K. Kang, Energy Environ. Sci., 2011, 4, 1277 Search PubMed; W. Tang, X. Gao, Y. Zhu, Y. Yue, Y. Shi, Y. Wu and K. Zhu, J. Mater. Chem., 2012, 22, 20143 RSC; X. Zhou, G. Wu, J. Wu, H. Yang, J. Wang, G. Gao, R. Cai and Q. Yan, J. Mater. Chem. A, 2013, 1, 15459 Search PubMed; X. Rui, J. Zhu, D. Sim, C. Xu, Y. Zeng, H. H. Hng, T. M. Lim and Q. Yan, Nanoscale, 2011, 3, 4752 RSC; J. S. Sakamoto and B. Dunn, J. Electrochem. Soc., 2002, 149, A26 CrossRef PubMed; W. Lu, A. Goering, L. Qu and L. Dai, Phys. Chem. Chem. Phys., 2012, 14, 12099 RSC; G. Du, K. H. Seng, Z. Guo, J. Liu, W. Li, D. Jia, C. Cook, Z. Liu and H. Liu, RSC Adv., 2011, 1, 690 RSC; S. Li, D. Wu, C. Cheng, J. Wang, F. Zhang, Y. Su and X. Feng, Angew. Chem., 2013, 125, 12327 CrossRef; V. V. Kulish, M.-F. Ng, O. I. Malyi, P. Wu and Z. Chen, RSC Adv., 2013, 3, 8446 RSC; Y. Su, S. Li, D. Wu, F. Zhang, H. Liang, P. Gao, C. Cheng and X. Feng, ACS Nano, 2012, 6, 8349 CrossRef PubMed; C. Cheng, S. Li, J. Zhao, X. Li, Z. Liu, L. Ma, X. Zhang, S. Sun and C. Zhao, Chem. Eng. J., 2013, 228, 468 CrossRef PubMed; T. Chen, L. Pan, X. Liu, K. Yu and Z. Sun, RSC Adv., 2012, 2, 11719 RSC; X. Jia, C. Yan, Z. Chen, R. Wang, Q. Zhang, L. Guo, F. Wei and Y. Lu, Chem. Commun., 2011, 47, 9669 RSC.
- K. H. Seng, J. Liu, Z. P. Guo, Z. X. Chen, D. Jia and H. K. Liu, Electrochem. Commun., 2011, 13, 383 CrossRef CAS PubMed.
- Z. Chen, Y. Qin, D. Weng, Q. Xiao, Y. Peng, X. Wang, H. Li, F. Wei and Y. Lu, Adv. Funct. Mater., 2009, 19, 3420 CrossRef CAS.
- S. Liang, M. Qin, J. Liu, Q. Zhang, T. Chen, Y. Tang and W. Wang, Mater. Lett., 2013, 93, 435 CrossRef CAS PubMed.
- S. Boukhalfa, K. Evanoff and G. Yushin, Energy Environ. Sci., 2012, 5, 6872 CAS.
- M. Sathiya, A. S. Prakash, K. Ramesha, J. M. Tarascon and A. K. Shukla, J. Am. Chem. Soc., 2011, 133, 16291 CrossRef CAS PubMed.
- Y. S. Hu, X. Liu, J. O. Müller, R. Schlögl, J. Maier and D. S. Su, Angew. Chem., Int. Ed., 2009, 48, 210 CrossRef CAS PubMed.
- X. Jia, Z. Chen, A. Suwarnasarn, L. Rice, X. Wang, H. Sohn, Q. Zhang, B. M. Wu, F. Wei and Y. Lu, Energy Environ. Sci., 2012, 5, 6845 CAS.
- C. K. Chan, H. Peng, R. D. Twesten, K. Jarausch, X. F. Zhang and Y. Cui, Nano Lett., 2007, 7, 490 CrossRef CAS PubMed; J. Liu, H. Xia, D. Xue and L. Lu, J. Am. Chem. Soc., 2009, 131, 12086 CrossRef PubMed.
- T. Zhai, H. Liu, H. Li, X. Fang, M. Liao, L. Li, H. Zhou, Y. Koide, Y. Bando and D. Golberg, Adv. Mater., 2010, 22, 2547 CrossRef CAS PubMed; S. Wang, Z. Lu, D. Wang, C. Li, C. Chen and Y. Yin, J. Mater. Chem., 2011, 21, 6365 RSC.
- Z. Cao and B. Wei, Nano Energy, 2013, 2, 481 CrossRef CAS PubMed; F. Carn, M. Morcrette, B. Desport and R. Backov, Solid State Sci., 2013, 17, 134 CrossRef PubMed.
- H. Yamada, K. Tagawa, M. Komatsu, I. Moriguchi and T. Kudo, J. Phys. Chem. C, 2007, 111, 8397 Search PubMed; A. Pan, J.-G. Zhang, Z. Nie, G. Cao, B. W. Arey, G. Li, S.-Q. Liang and J. Liu, J. Mater. Chem., 2010, 20, 9193 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01316f |
| This journal is © The Royal Society of Chemistry 2014 |