 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A “wrap-and-wrest” mechanism of fluorescence quenching of CdSe/ZnS quantum dots by surfactant molecules†
Ewelina Kalwarczyk,
Natalia Ziębacz,
Tomasz Kalwarczyk,
Robert Hołyst and
Marcin Fiałkowski*
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: fialkows@ichf.edu.pl
First published on 1st August 2013
Abstract
We identified a mechanism of fluorescence quenching of CdSe/ZnS quantum dots (QDs) coated with two organic layers, octadecylamine and an amphiphilic polymer containing COOH groups, by nonionic polyoxyethylene-based (C12En) surfactants. The surfactant molecules by themselves do not affect the fluorescence of the QDs. For the quenching to occur, “wrapping” of the QDs by a bilayer of the surfactant molecules is necessary. The formation of the bilayer causes an irreversible detachment (“wresting”) of the ligand molecules, accompanied by the creation of quenching sites on the QD surface. Due to its two-stage nature, we refer to the quenching mechanism as the “wrap-and-wrest” mechanism. The adsorption of the surfactant on the QD surface is a relatively slow process, occurring within minutes or hours. Such long quenching times allowed monitoring surfactant adsorption progress in real time. The fluorescence signal decays exponentially, and the decay time is inversely proportional to the surfactant concentration in solution.
1 Introduction
The fluorescence of semiconductor quantum dots (QDs) is associated with radiative transition between their energy levels.1–4 It consists of intrinsic and surface state emission, whose contribution in total emission depends on the structure of QDs.5 Properties of semiconductor QDs are extremely sensitive to the processes taking place on their surface, such as ligand exchange or removal. In particular, such processes can enhance, weaken or quench the fluorescence of QDs.6–14 In the case of quenching of the fluorescence of QDs, the exact mechanism of this phenomenon is still a matter of debate; however it is commonly assumed that it is associated with formation of surface-induced defects. These defects, referred also to as quenching sites, enable non-fluorescent electron–hole recombination.6 It was recently shown15 that in the case of CdSe nanoparticles these quenching sites can be created as a result of desorption of surface ligands upon dilution of the solution containing QDs. This type of fluorescence quenching is a reversible process, as it allows re-attachment of the ligand to the surface of the nanoparticles.Although the role of organic ligands as the quenchers has intensively been investigated,15–18 the effect of non-binding organic molecules on the QD fluorescence has largely been overlooked. In this paper, we present results of our studies on the interaction of nonionic surfactants, polyethylene glycol monododecyl ethers (C12En, n = 8, 9, and 10), with hydrophilic CdSe/ZnS QDs, coated with two organic layers: octadecylamine (ODA) and an amphiphilic polymer possessing COOH surface groups. Surprisingly, although these surfactants by themselves are completely “harmless” to the fluorescence, they were found to induce irreversible quenching of the fluorescence of the CdSe/ZnS QDs. It is the purpose of this paper to investigate and find the mechanism responsible for the fluorescence quenching. We found that the necessary condition for the quenching process to occur is “wrapping” of the QDs by a bilayer of surfactant molecules. It takes place under low pH conditions promoting the formation of hydrogen bonds between the carboxylic groups at the QD surface and the surfactant molecules. After “wrapping” of the QDs with the surfactant bilayer, some primary coating ligands (ODA) are removed (“wrested”) from the surface of QDs and built into the bilayer. This results in the creation of quenching sites. The “wrapping” of QDs by the surfactant molecules is a relatively slow process, occurring over the course of minutes or hours. This allowed us to monitor surfactant adsorption progress in real-time.
2 Experimental
2.1 Chemicals
Hydrophilic core/shell CdSe/ZnS QDs coated with a layer of ODA and a layer of hydrolyzed form of poly(maleic anhydride-alt-1-tetradecene)19,20 – an amphiphilic polymer with carboxylic acid groups on the surface – were purchased from Ocean NanoTech. The structure of the QDs is schematically represented in Fig. 1. The amphiphilic polymer contains long alkyl chains able to bind to the ODA layer (due to hydrophobic interactions), and a sufficient number of COOH groups to provide water solubility. According to the specification, the average number of COOH groups on the surface of the QDs is 120. QDs were supplied in the form of concentrated (8 μM) aqueous solution, and were used as received. The hydrodynamic radius of QDs (measured with DLS, see Fig. S1, ESI†) was 8.6 ± 0.8 nm. The average ζ-potential of the QDs dissolved in deionized water at pH = 7.20 was −41.2 ± 7.0 mV. QDs exhibited absorption in the UV-vis spectral range, with a relatively small maximum at 523 nm and strong emission, the maximum of which falls at 540 nm (FWHM = 30 nm). The intensity of fluorescence of the QDs was high and did not change with time. Quenching of fluorescence of bare QDs in deionized water was not observed even after long time storage (few months) or long (16 h) constant illumination. For absorption and emission spectra of QDs see the ESI, Fig. S2.†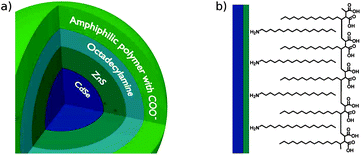 | ||
| Fig. 1 Schematic showing the structure of bare QDs (a) and the chemical structure of the organic layer (ODA and amphiphilic polymer) on the surface of a QD. | ||
Nonionic surfactants: octaethylene glycol monododecyl ether (C12E8) (>98%) and nonaethylene glycol monododecyl ether (C12E9) were bought from Fluka. Decaethylene glycol monododecyl ether (C12E10) was bought from Sigma Aldrich. The values of CMC for C12E8, C12E9 and C12E10 are, respectively, 7.1 × 10−5 M,21 1.6 × 10−4 M,22 and 8.0 × 10−5 M.23 The values of the aggregation numbers for C12E8, C12E9, and C12E10 are, respectively, 120,24 109,25 and 63.26 The hydrodynamic radii of C12En micelles, determined with the DLS method, are presented in Table S1.† Aqueous solutions of C12En exhibit acidic pH. The values of pH measured for different surfactant concentrations are listed in Table S2.†
Tetraethylene glycol (99%), hexaethylene glycol (97%), poly(ethylene glycol) (PEG, Mw = 400), and poly(ethylene glycol) (PEG, Mn from 570 to 630) were bought from Sigma Aldrich. Poly(ethylene glycol) (PEG, Mw ∼ 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was bought from Fluka. Pluronic-F127 (PEG–PPG–PEG triblock copolymer) was bought from Sigma. All chemicals were used as delivered. Water was filtered and demineralized with an ELIX system (Millipore).
000) was bought from Fluka. Pluronic-F127 (PEG–PPG–PEG triblock copolymer) was bought from Sigma. All chemicals were used as delivered. Water was filtered and demineralized with an ELIX system (Millipore).
2.2 Experimental techniques
Dynamic Light Scattering (DLS) measurements were carried out using a Stabilite 2017 argon ion laser (λ = 514 nm) or a He–Ne laser (λ = 633 nm) at selected angles, from 30° to 150°. Fourier transform of the intensity–intensity correlation function g(q, τ) as a function of scattering wave vector, q = (4πn/λ)sin(θ/2), and time, τ, was recorded. ζ-Potential measurements were performed using a Zetasizer Nano ZS apparatus (Malvern Instruments Ltd.). Measurements of pH were made using a pH meter equipped with a glass electrode suitable for viscous solutions (InLab Viscous, Mettler Toledo). Spectral analysis was carried out with an Ocean-Optics USB 2000+ spectrophotometer in the spectral range of 190–1000 nm in a quartz microcuvette (10 mm of path length). The fluorescence was induced using a diode laser (405 nm). For each sample analyzed, fluorescence spectra were recorded automatically every 10 seconds. To reduce possible differences in experimental conditions due to fluctuations of the laser light intensity reaching the sample, and ensure comparability of the results, the following normalizing procedure was applied to each sample analyzed: before the measurements, the fluorescence intensity, Ir, of rhodamine B (0.0001 M water solution) at λmax = 594.8 nm was measured. All spectra recorded for the sample were rescaled by the factor I0/Ir, where I0 was the reference intensity. Fluorescence lifetime imaging microscopy (FLIM) experiments were performed using a PicoQuant fluorescence lifetime system based on a Nikon C1 confocal microscope. The confocal setup uses a Nikon TE-2000 inverted microscope. To excite our samples we used a 481 nm pulse diode laser. Liquid samples were poured into separate cells of LabTek 8-chambered coverglass. We set the focal plane of the microscope at a distance of around 10 micrometers above the surface of the coverglass. FLIM images were acquired using SymphoTime software. We collected 512 × 512 pixel sized images. Each image was accumulated from several frames until the fluorescence intensity (averaged over whole image) was of the order of 100 counts. The fluorescence decay curve was calculated for the whole image and fitted using SymphoTime software. All measurements were carried out at 25 °C.2.3 Verification of factors responsible for quenching of QDs
To verify if the C12En surfactants are the only factor responsible for the quenching, possible effects of (1) metal impurities, (2) photo-induced oxidation, and (3) migration of the polymer COOH groups towards the QD surface were investigated. Metal cations, especially Cu2+ ions, can affect significantly the fluorescence of QDs.8,11,12,27 In the case of C12En surfactants used, they are typical impurities remaining after the synthesis process. The highest possible amount of metal impurities in C12E8, C12E9 and C12E10 (as specified by the manufacturer) was ≤650 mg kg−1 (with Cu2+ ≤50 mg kg−1). To verify the influence of metal impurities, we employed ethylenediaminetetraacetic acid disodium salt (EDTA) – a chelating ligand that complexes metal ions. Firstly, we determined the effect of Cu2+ ions on the fluorescence of QDs. As we checked, the addition of Cu2+ ions to the solution of QDs results in an instantaneous quenching (the top panel of Fig. 2). Next, Cu2+ ions were added to the solution of QDs containing EDTA. In this case the fluorescence of QDs was not affected since all Cu2+ ions were complexed by EDTA (the middle panel of Fig. 2). Finally, the surfactant was added to the solution of QDs containing EDTA in excess (with respect to the highest possible amount of metal impurities in the surfactant solution used). In this case the fluorescence of QDs was also quenched, which confirms that the quenching is caused by the presence of the surfactant molecules itself (the bottom panel of Fig. 2).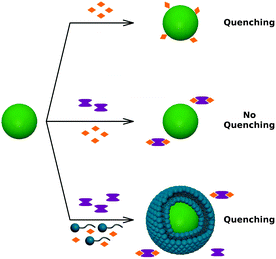 | ||
| Fig. 2 The effect of metal impurities (orange rhombus), metal-chelating ligands, EDTA (marked in violet), and surfactant molecules on the fluorescence of the QDs. | ||
An additional strong confirmation that the presence of surfactants is the only factor responsible for the fluorescence quenching is the fact that the quenching is not observed in C12En solutions at high pH (cf. Fig. 3b). If the fluorescence quenching of QDs resulted from the presence of metal cations it would be observed irrespective of the pH conditions.
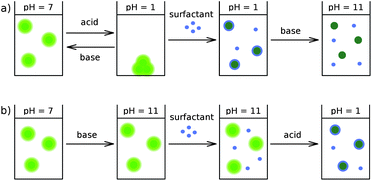 | ||
| Fig. 3 Influence of pH on QDs and a QD–surfactant system. The light-green and dark-green colors correspond, respectively, to fluorescent and non-fluorescent QDs. | ||
Quenching of QDs can also result from photo-induced oxidation.10,28 In such a case fluorescence is quenched if QDs are exposed to daylight, and is not observed when the sample is kept in darkness. In our system, we ruled out the possibility of the photo-induced oxidation because the quenching of QDs in the presence of C12En surfactants was observed regardless of the illumination of the sample.
The quenching may be caused by migration of the COOH groups of the amphiphilic polymer to the surface of the QDs. At low pH values, these groups are not dissociated and do not bear electric charge. Such conditions might thus facilitate their migration through the hydrophobic ODA layer. However, the COOH groups are part of the polymer of the structure shown in Fig. 1b, and their migration towards the QD surface would require a substantial change in the conformation of the whole macromolecule. For this reason, it is unlikely to occur. Also, if this migration was possible, it would result in quenching at low pH even in the absence of the surfactant. As we will show, such fluorescence quenching is not observed. This fact rules out the COOH group migration mechanism.
3 Results and discussion
We studied interactions of nonionic surfactants, polyethylene glycol monododecyl ethers (C12En, n = 8, 9, and 10), with hydrophilic CdSe/ZnS QDs coated with two organic layers – ODA and an amphiphilic polymer with COOH groups. In the following, we demonstrate that the C12En surfactants adsorb on the surface of the QDs to form a bilayer. We also show that the observed quenching of fluorescence of these QDs is caused by the removal of the primary ligand molecules (ODA) by the surfactant bilayer from the surface of the QDs.3.1 Effect of pH
The outer stabilizing layer of QDs is an amphiphilic polymer with ionizable carboxylic acid groups. Thus, the behavior of QDs is strongly pH-dependent. As specified by the manufacturer, QDs are stable in most buffer solutions for pH values from 3 to 14, that is, in the pH range in which most of the COOH groups on the surface of QDs are deprotonated. As we verified, for pH > 3, QDs are well dispersed in water and exhibited strong homogeneous fluorescence. For pH < 3, the QDs aggregated. Importantly, the aggregation process did not affect the fluorescence of the QDs (no change in absorption/emission maxima), and was reversible upon the increase of pH (see Fig. 3a). As demonstrated in the following sections, the fluorescence quenching of QDs is associated with adsorption of the surfactant on the surface of the QDs. According to the literature,29,30 the adsorption of nonionic, polyoxyethylene-based surfactants to various surfaces occurs through the formation of hydrogen bonds. In the case of the QDs used, the formation of hydrogen bonds and the adsorption of surfactants were possible under low pH conditions, when a significant number of COOH groups is protonated. It was found that the addition of C12En to the solution of QDs at low pH results in dissolution of the QD aggregates accompanied by quenching of their fluorescence (cf. Fig. 3a). Remarkably, it was not possible to restore the fluorescence of QDs by increasing the pH, despite the fact that surfactant molecules desorbed from the surface of the QDs under high pH conditions.At high pH the adsorption of the C12En surfactant on the surface of the QDs was inhibited and the fluorescence was unaffected (see Fig. 3b). Under high pH conditions the quenching did not occur even in the presence of the surfactant at high concentration. Lowering the pH value below 3 facilitated the formation of hydrogen bonds leading to the adsorption of the surfactant molecules and the fluorescence quenching.
3.2 DLS studies
To investigate the adsorption of surfactant molecules on the surface of the QDs we performed DLS measurements. Our aim was to determine the hydrodynamic radius, Rh, of the “quenched” QDs that are present in the solution upon the addition of C12E8. Comparison of Rh with the hydrodynamic radius of bare QDs (Rh,QD) and that of the C12E8 micelle (Rh,mic) provides information about the structure of the “quenched” QDs. Unfortunately, determination of Rh directly in a QD solution turned out to be impossible because of screening of the DLS signal by free surfactant micelles. Even in the case of a sample containing the smallest amount of surfactant needed to quench the fluorescence of QDs, the QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) micelle ratio was ∼1
micelle ratio was ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620. Consequently, since the hydrodynamic radii of QDs and micelles are of the same order of magnitude, the measured DLS signal was dominated by that of free micelles. To minimize the screening effect, we took advantage of the fact that the surfactant adsorption is much more efficient under low pH conditions, facilitating the formation of hydrogen bonds. Therefore, instead of using a solution of dispersed QDs, we added dropwise small portions of C12E8 to the acidified (pH ≈ 1), aggregated sample of QDs. That is, we performed the first two steps in the scheme presented in Fig. 3a. The addition of the surfactant led to a successive breakdown of the aggregates and gradual quenching of fluorescence of QDs. We found that for pH ≈ 1 the minimal amount of C12E8 that causes complete fluorescence quenching and dissolution of the aggregates corresponds to a QD
620. Consequently, since the hydrodynamic radii of QDs and micelles are of the same order of magnitude, the measured DLS signal was dominated by that of free micelles. To minimize the screening effect, we took advantage of the fact that the surfactant adsorption is much more efficient under low pH conditions, facilitating the formation of hydrogen bonds. Therefore, instead of using a solution of dispersed QDs, we added dropwise small portions of C12E8 to the acidified (pH ≈ 1), aggregated sample of QDs. That is, we performed the first two steps in the scheme presented in Fig. 3a. The addition of the surfactant led to a successive breakdown of the aggregates and gradual quenching of fluorescence of QDs. We found that for pH ≈ 1 the minimal amount of C12E8 that causes complete fluorescence quenching and dissolution of the aggregates corresponds to a QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) micelle ratio equal to 1
micelle ratio equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. The number of micelles needed to create a bilayer of C12E8 on the surface of the QDs is estimated to be ∼24 (see the ESI†). Such a reduction of the excess of micelles made possible the determination of Rh. The DLS measurements (compare Fig. S3†) yielded Rh = 17.7 ± 0.6 nm. This value of hydrodynamic radius is greater than the hydrodynamic radius of both bare QDs (8.6 ± 0.8 nm) and C12E8 micelles at pH = 1 (3.7 ± 0.1 nm). Within experimental errors, Rh equals to the sum of Rh,QD and doubled Rh,mic, viz. (Rh,QD + 2Rh,mic = (8.6 ± 0.8) nm + (7.4 ± 0.5) nm = (16 ± 2.1) nm). This relation indicates that after the addition of the surfactant to the solution of QDs, the surfactant molecules form a bilayer on the surface of QDs. A schematic representation of the QD with the adsorbed surfactant bilayer is shown in Fig. 4a. After increasing the pH value the measured hydrodynamic radius of the QDs corresponds to that of bare QDs. It follows that the surfactant molecules desorb under basic conditions.
40. The number of micelles needed to create a bilayer of C12E8 on the surface of the QDs is estimated to be ∼24 (see the ESI†). Such a reduction of the excess of micelles made possible the determination of Rh. The DLS measurements (compare Fig. S3†) yielded Rh = 17.7 ± 0.6 nm. This value of hydrodynamic radius is greater than the hydrodynamic radius of both bare QDs (8.6 ± 0.8 nm) and C12E8 micelles at pH = 1 (3.7 ± 0.1 nm). Within experimental errors, Rh equals to the sum of Rh,QD and doubled Rh,mic, viz. (Rh,QD + 2Rh,mic = (8.6 ± 0.8) nm + (7.4 ± 0.5) nm = (16 ± 2.1) nm). This relation indicates that after the addition of the surfactant to the solution of QDs, the surfactant molecules form a bilayer on the surface of QDs. A schematic representation of the QD with the adsorbed surfactant bilayer is shown in Fig. 4a. After increasing the pH value the measured hydrodynamic radius of the QDs corresponds to that of bare QDs. It follows that the surfactant molecules desorb under basic conditions.
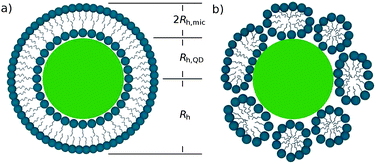 | ||
| Fig. 4 Two possible arrangements of surfactant molecules at the QD surface: (a) uniform bilayer and (b) more realistic patch-like bilayer structure. | ||
As discussed in Section 3.1, quenching of fluorescence of the QDs is enhanced at low pH and inhibited under high pH conditions. This suggests that formation of hydrogen bonds plays an important role in the adsorption process. Under low pH conditions the fraction of protonated COOH groups is increased, which facilitates the formation of hydrogen bonds between the QD surface and the polyoxyethylene units of surfactant molecules. Under high pH conditions formation of hydrogen bonds is inhibited, which prevents the adsorption of surfactants. The C12En surfactants are known31,32 to bind to polymers containing COOH groups. The structure of the surfactant bilayer on the surface of QDs is expected to be similar to that observed in the case of adsorption of (polyoxyethylene-based) surfactants on negatively charged silica particles.30,33 The inner layer of the bilayer is formed owing to adsorption of hydrophilic units of surfactants to the surface of QDs. It is stabilized by hydrogen bonds between protonated carboxylic acid groups on the surface of QDs and the oxyethylene groups of surfactant molecules. The outer layer is formed in a tail-to-tail manner, and is stabilized by hydrophobic interactions between the alkyl chains of surfactant molecules. In the simplest case, these molecules may form a continuous bilayer on the surface of QDs, as schematically shown in Fig. 4a. This, however, is not the only option. The bilayer may possess a patch-like structure consisting of micelles of spherical, ellipsoidal, or cylindrical shape, as shown in Fig. 4b. Small angle neutron scattering data indicate34–38 that various surfactants form micellar aggregates rather than the continuous bilayer on the surface of silica nanoparticles. Importantly, the structure of the micellar aggregates on the surface of nanoparticles may differ in size and shape from the corresponding structures formed in solution. As an example, it was shown36 that C12E5 surfactant molecules form small spherical micelles on the surface of 16 nm sized silica nanoparticles, despite the fact that they form elongated micelles in solution. However, the arrangement of the surfactant molecules in the bilayer does not matter for the discussion presented in the following part of the paper.
Note that the fact that the C12En surfactants form a bilayer on the surface of particles containing COOH groups is of practical importance. As it was reported recently,39 there is an asymmetry in the incorporation of charged nanoparticles (covered with ω-modified thiols containing COOH groups) into the ordered phases formed by a nonionic surfactant, C12E6. Transfer of the positively charged nanoparticles (metal or semiconductor) was less effective than the negatively charged ones. In view of the results obtained, this observation can be attributed to the formation of a bilayer of the surfactant on the surface of the negatively charged nanoparticles. Since the incorporation of the nanoparticles is driven by the geometrical/chemical mismatch between the micelles and polymers, the surfactant-coated nanoparticles, resembling the surfactant micelles, are transferred and built into the ordered surfactant phase much more easily than bare nanoparticles.
3.3 Zeta potential measurements
To provide additional evidence for the “wrapping” of QDs with surfactant molecules, we performed zeta (ζ) potential measurements. For pure QDs dissolved in water the ζ-potential value was negative and equal to −41.2 ± 7.0 mV at pH = 7.20 and −9.2 ± 2.1 mV at pH = 3.0. Upon mixing with 0.01 M aqueous solution of C12E8 (pH = 3.4) the value of ζ-potential changed to −3.5 ± 2.9 mV within 10 minutes. This observation is in line with recent reports30 on adsorption of a polyoxyethylene-based surfactant on silica particles. It was found that under acidic conditions, after the adsorption of the surfactant molecules, the negative charge of naked silica particles is substantially reduced, and the ζ-potential attains a plateau value of about −5.5 mV. ζ-Potential measurements are commonly used to evaluate the stability of colloidal solutions. As we verified, the aqueous solutions of QDs are stable and do not aggregate as long as the pH of the solution is higher than 3. Below this value the QDs precipitate rapidly. Lowering the value of the ζ-potential observed for the QDs in the presence of the surfactant may suggest an increased tendency towards sedimentation. However, we observed that quenching of the fluorescence was not accompanied by any macroscopic aggregation or precipitation, even after a long time of storage (several months) or centrifugation of the quenched sample. This can be explained only by the surfactant adsorption. That is, the surfactant-coated QDs resemble regular surfactant micelles, and their high stability is due to the interactions of the ethylene glycol (EG) units of the surfactant with water.3.4 Role of the amphiphilic structure of the EG-containing molecules in the fluorescence quenching
In the previous sections we showed that the fluorescence quenching is associated with the formation of a dense surfactant coating on the QD surface. To verify whether the quenching is caused by the proximity of the EG units to the QD surface or with the specific bilayer structure of the coating, we compared the effect of EG-containing surfactants and polymers on the fluorescence of QDs (see Fig. 5). We found that PEG consisting of 4, 6, 9, 13, and 464 monomers does not affect the fluorescence of QDs even though they contain the same subunits as those present in the hydrophilic heads of C12En. Interestingly, the addition of Pluronic F-127 – nonionic PEG–PPG–PEG triblock copolymer forming micelles in aqueous solutions40 – resulted in very slow (lasting for two weeks) quenching of the QD fluorescence. Taken together, these results indicate that the amphiphilic structure of the EG-containing molecule is crucial for the quenching of QD fluorescence. The adsorption-mediated fluorescence quenching is observed only if the adsorbing molecule is able to form thick, dense bilayer coating. In the case of C12En surfactants, this type of adsorption is a relatively fast process that results in quenching of the QD fluorescence within minutes/hours. For Pluronic F-127, formation of a bilayer on the surface of QDs requires a change of the conformation of the macromolecule,41 similar to that observed in micelles.40 Probably, this is the reason why the fluorescence quenching caused by Pluronic F-127 occurs significantly slower than in the case of C12En surfactants.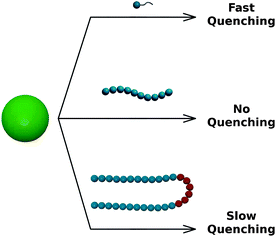 | ||
| Fig. 5 Effect of EG-containing molecules of different structures on the QD fluorescence: surfactants (top), simple polymers (middle), and copolymers (bottom). | ||
3.5 Photoluminescence studies
As we found, C12En surfactants can adsorb on the surface of QDs containing COOH groups. This adsorption is facilitated at low pH and inhibited under high pH conditions. We examined the changes of the fluorescence lifetimes of the QDs under the influence of acidic/basic pH conditions and after addition of the C12E10 surfactant (Fig. S4 and Table S3, ESI†). Under basic conditions (pH ≈ 13) QDs display two different lifetimes: 1.8 and 14.3 ns. Under acidic conditions (pH ≈ 1.5) these lifetimes change to 2.7 and 13.7 ns. For QDs dissolved in C12E10 under basic conditions (pH ≈ 13), the fluorescence lifetimes are 1.6 and 13.7 ns. The most significant change of the lifetimes was observed upon addition of the surfactant under acidic conditions (pH ≈ 1.5), where the fluorescence lifetimes are 1.3 and 6.2 ns. These results prove that the formation of the surfactant coating plays a key role in the quenching process.As long as the “wrapping” of QDs with surfactant is hampered, surfactant molecules remain completely “harmless” for the QD fluorescence. When the formation of the surfactant coating is enabled, the fluorescence intensity decays within minutes/hours, depending on the surfactant type and its concentration. This provided a unique opportunity to monitor the surfactant adsorption process in real time based on the fluorescence intensity changes. We performed real-time fluorescence studies to find time characteristics of the surfactant adsorption process as well as its dependence on the surfactant concentration (cs). We traced the changes of fluorescence intensity of QDs upon mixing with the aqueous solutions of C12En, n = 8, 9, and 10. In a typical experiment, 10 μl of concentrated QD solution (8 μM in water) was mixed with 410 μl of micellar surfactant solution, resulting in 0.19 μM solution of QDs. The as-prepared mixture was then shaken for a few seconds and then the fluorescence intensity was measured under continuous laser illumination. The first spectrum was collected 30 seconds after the addition of QDs, and the subsequent spectra were recorded automatically every 10 seconds. The measurements were carried out for five selected surfactant concentrations: 0.2, 0.1, 0.05, 0.01, and 0.001 M. Because aqueous solutions of C12En surfactants exhibit acidic pH (see Table S2†), the surfactant adsorption process occurred without any pH adjustment upon mixing with the QDs. Typical changes of the emission spectra, and the corresponding decay of the fluorescence intensity of QDs at λmax = 540 nm, in the solution of the nonionic surfactant in time are shown in Fig. 6. The data correspond to the quenching of QDs in the presence of 0.2 M C12E8. As seen in Fig. 6, in the solution of 0.2 M C12E8 the fluorescence of QDs is entirely quenched within less than 10 minutes. The observed fluorescence decay was fitted by a mono-exponential function:
I(t) = I0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τq), exp(−t/τq),
| (1) |
| τq = Acsα, | (2) |
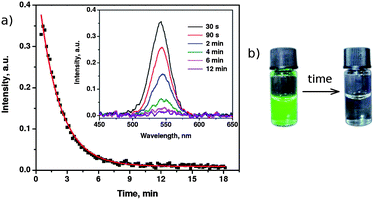 | ||
| Fig. 6 (a) Representative time characteristics of the QD fluorescence quenching, obtained for 0.2 M C12E8 at λmax = 540 nm. The red curve is a mono-exponential fit to the data. Inset: emission spectra recorded for different times of the quenching process. (b) Photographs of a QD solution before and after the quenching taken in the UV light. | ||
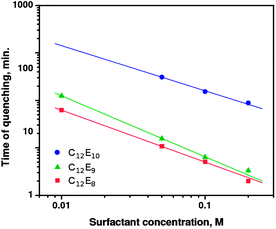 | ||
| Fig. 7 Quenching time of QDs as a function of surfactant concentration. Solid lines represent fits of eqn (2) to the data. | ||
3.6 The “wrap-and-wrest” mechanism of fluorescence quenching
Based on the results obtained, we propose the following “wrap-and-wrest” mechanism governing the fluorescence quenching of the QDs (see Fig. 8): first, the C12En surfactant molecules adsorb on the surface of a QD, “wrapping” it with a bilayer. Then, the ODA molecules (primary coating ligand) are “wrested” from the QD surface, leading to the creation of quenching sites. The ODA molecules, exhibiting strong surfactant properties, are built into the surfactant bilayer. Thus, formation of the surfactant bilayer is crucial to detach the ODA molecules from the QD surface and, consequently, to quench the fluorescence. If the conditions (e.g., high pH) do not allow the adsorption of the surfactant to the QD surface, followed by the detachment of ODA molecules, the fluorescence quenching is not observed.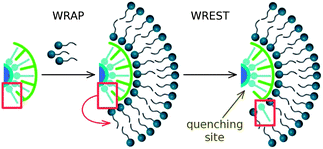 | ||
| Fig. 8 Schematic representation of the proposed “wrap-and-wrest” mechanism of fluorescence quenching of QDs. First, surfactant molecules adsorb on the surface of the QD, “wrapping” it with a bilayer. Then, ODA molecule (primary coating ligand) is “wrested” from the QD surface, leading to the formation of a quenching site. The ODA molecule is built into the surfactant bilayer. | ||
Importantly, within the “wrap-and-wrest” model, the QD does not retain its fluorescence properties when the surfactant coating is desorbed. The reason is that the ODA molecule, once removed from the surface of the QDs, is unlikely to re-adsorb on the QD surface. Instead, it migrates into the solution and is incorporated into the C12En surfactant micelles. Considering the fact that only a few quenching sites are needed to quench the QD fluorescence,15 the observed irreversibility of fluorescence quenching is easily understood.
To provide additional support for the “wrap-and-wrest” mechanism, we measured also the absorption spectra of QDs in water and in the presence of the surfactant. We found that the adsorption process of the surfactant on the surface of the QDs causes changes in the absorption spectra of the latter. After mixing QDs with the surfactant, the UV-vis absorbance of the system decreased, approximately, exponentially in time to reach the level very close to that of pure surfactant solution. The QD absorption peak located at 523 nm vanished completely after the mixing (see Fig. S5†). The characteristic decay times, estimated based on the UV-vis spectra, were of the same order of magnitude as the values of τq obtained for the fluorescence quenching (see Fig. S6†). Importantly, the surfactant itself does not absorb light in the spectral range suitable for excitation of QD emission. This indicates that the quenching of fluorescence of QDs is not caused directly by the presence of surfactant coating.
Hydrophilic QDs are applied in biological imaging42–45 to visualize different areas of a cell. This imaging technique involves contact of the QDs with cell membranes that consist of amphiphilic lipids. According to recent reports,46 the fluorescence of the QDs decreases upon entrapment in phospholipid vesicles. The proposed “wrap-and-wrest” mechanism of the fluorescence quenching may therefore be operative in this process.
3.7 Model of the surfactant adsorption kinetics
The exponential decay of the QD fluorescence observed in the system and the dependence of the quenching time on the surfactant concentration given by the relation (2) can be explained in terms of a simple surfactant adsorption model. The model assumes that the fluorescence intensity, I, of a selected QD decreases linearly with the amount of the surfactant molecules adsorbed on its surface. To quantify this assumption, we denote the degree of saturation of the QD's surface by σ (0 ≤ σ ≤ 1) and the adsorption capacity of the QD by σ0. The quantity σ plays here a role analogous to that of the fractional coverage used in the Langmuir adsorption47 theory. That is, when there are no surfactant molecules attached to the surface σ = 0, and when σ reaches the value of σ0 the QD can no longer adsorb the surfactant molecules. The main difference between the fractional coverage and the degree of saturation is that the latter allows bilayer organization of the adsorbed molecules on the QD surface. Without loss of generality, we can also put σ0 = 1. Thus, the fluorescence intensity depends on σ according to the following formula:| I(σ) = (1 − σ)Ibare, | (3) |
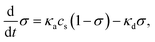 | (4) |
Combining the solution of eqn (4) and the relation (3) gives the following dependence of the fluorescence intensity, I, on time:
I(t) = Ibare![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−κacst). exp(−κacst).
| (5) |
The above relation reproduces the exponential decay of I(t) observed experimentally. In terms of the model parameters, the quenching time is given by the formula
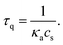 | (6) |
Finally, comparison of eqn (6) and (2) yields α = −1. The obtained value of the exponent α agrees well with that observed experimentally, α = −1.11 ± 0.17, calculated as the average for C12E8, C12E9, and C12E10.
In the adsorption model described above we assumed that the fluorescence signal is quenched successively with an increasing amount of surfactant on the QD surface. Although this process seems to be the most plausible scenario of the quenching, a modified version of the adsorption model should be considered. In the modified model it is assumed that the fluorescence signal of a selected QD is quenched by a portion of surfactant molecules adsorbed upon the first collision with a micelle. That is, it is assumed that the quenching is due to reduction of the population of the bare (fluorescent) QDs in the solution. Let us denote the concentration of the bare QDs by cQD. Assuming – as in the previous adsorption model – that the adsorption of surfactants on the QD surface follows the first-order kinetics with respect to cs, the population of the bare QDs changes as
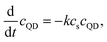 | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−kcst). This relation reproduces also the exponential decay of the fluorescence signal given by eqn (1). For k = κa it becomes identical to eqn (5).
exp(−kcst). This relation reproduces also the exponential decay of the fluorescence signal given by eqn (1). For k = κa it becomes identical to eqn (5).
Finally, a remark on the nature of the adsorption kinetics is in order. For all surfactant systems, the characteristic collision time, τcoll, needed for the QD to collide with a micelle, estimated within the Smoluchowski coagulation theory (see the ESI†), varies in the range from 10−8 to 10−6 s. On the other hand, the observed values of the quenching time, τq, are much larger and span from minutes for C12En to weeks for the copolymer Pluronic F-127. Furthermore, τq for C12E10 and C12E12, having quite similar diffusivity, differs by an order of magnitude. The relation τq ≫ τcoll indicates that the kinetics of the QD–surfactant association is limited by the “merging reaction” between the QD and the micelle.
4 Conclusions
In this paper, we investigated interactions between hydrophilic CdSe/ZnS nanoparticles (QDs) coated with two organic layers (ODA and an amphiphilic polymer containing COOH groups) and a nonionic polyoxyethylene-based surfactant. As the main result, we identified the mechanism of the fluorescence quenching of the QDs by the surfactant molecules. In this process, the surfactant molecules form a bilayer around a QD, and the quenching results from the creation of quenching sites upon detachment of the primary coating ligands (ODA) from the surface of the QD. The ODA molecules “wrested” from the QD surface are built into the surfactant bilayer. When the bilayer is removed, the ODA molecules migrate to the solution along with the surfactant, and are unlikely to re-adsorb to the QD. This makes the fluorescence quenching irreversible. Due to the two-stage nature of the quenching process, we refer to it as the “wrap-and-wrest” mechanism. The most remarkable feature of this mechanism is the fact that the surfactant molecules themselves are completely “harmless” for the fluorescence as long as they do not form a bilayer around the QD. That is, chemical properties of the surfactant molecules are not responsible for the quenching; the fluorescence quenching is caused only by the structure these molecules form.Because the quenching process is quite slow and occurs over a period of minutes or hours it enabled us to monitor the adsorption of the surfactant molecules in real time. We found that the fluorescence signal decays exponentially, and the decay time is inversely proportional to the surfactant concentration. The adsorption of the surfactant molecules on the QD surface was found to follow the first-order kinetics.
Acknowledgements
The work was supported by the project operated within the Foundation for Polish Science Team Programme co-financed by the EU “European Regional Development Fund” Grant No. TEAM/2010-6/4. T.K. thanks the National Science Center for funding the project from the funds granted on the basis of the decision number: DEC1-2011/01/N/ST3/00865. R.H. thanks the National Science Center for funding the project from the funds granted on the basis of the decision number: 2011/02/A/ST3/00143 (Maestro grant).References
- A. P. Alivisatos, J. Phys. Chem., 1996, 100, 13226 CrossRef CAS.
- Nanoparticles: from theory to application, ed. G. Schmid, WILEY-VCH Verlag GmbH & Co. KGaA, 2004 Search PubMed.
- X. Michalet, F. Pinaud, T. D. Lacoste, M. Dahan, M. P. Bruchez, A. P. Alivisatos and S. Weiss, Single Mol., 2001, 2, 261 CrossRef CAS.
- M. A. El-Sayed, Acc. Chem. Res., 2004, 37, 326 CrossRef CAS PubMed.
- G. Morello, M. Anni, P. D. Cozzoli, L. Manna, R. Cingolani and M. D. Giorgi, Nanoscale Res. Lett., 2007, 2, 512 CrossRef CAS.
- A. V. Isarov and J. Chrysochoos, Langmuir, 1997, 13, 3142 CrossRef CAS.
- R. E. Galian and J. Scaiano, Photochem. Photobiol. Sci., 2009, 8, 70 CAS.
- Y. Chen and Z. Rosenzweig, Anal. Chem., 2002, 74, 5132 CrossRef CAS.
- H. Li and X. Wang, Sens. Actuators, B, 2008, 134, 238 CrossRef CAS PubMed.
- X. Hu, P. Zrazhevskiy and X. Gao, Ann. Biomed. Eng., 2009, 37, 1960 CrossRef PubMed.
- K. M. Gattas-Asfura and R. M. Leblanc, Chem. Commun., 2003, 2684 RSC.
- J.-L. Chen and C.-Q. Zhu, Anal. Chim. Acta, 2005, 546, 147 CrossRef CAS PubMed.
- M. Jones, J. Nedeljkovic, R. J. Ellingson, A. J. Nozik and G. Rumbles, J. Phys. Chem. B, 2003, 107, 11346 CrossRef CAS.
- Y. Zhang, P. Jing, Q. Zeng, Y. Sun, H. Su, Y. A. Wang, X. Kong, J. Zhao and H. Zhang, J. Phys. Chem. C, 2009, 113, 1886 CAS.
- L. Hartmann, A. Kumar, M. Welker, A. Fiore, C. Julien-Rabant, M. Gromova, M. Bardet, P. Reiss, P. N. W. Baxter, F. Chandezon and R. B. Pansu, ACS Nano, 2012, 6, 9033 CrossRef CAS PubMed.
- M. J. Ruedas-Rama, A. Orte, E. A. H. Hall, J. M. Alvarez-Pez and E. M. Talavera, ChemPhysChem, 2011, 12, 919 CrossRef CAS PubMed.
- A. Credi, New J. Chem., 2012, 36, 1925 RSC.
- J. Aguilera-Sigalat, V. F. Pais, A. Domenech-Carbo, U. Pischel and R. E. Galian, J. Phys. Chem. C, 2013, 117, 7365 CAS.
- L. Yang, H. Mao, Y. A. Wang, Z. Cao, X. Peng, X. Wang, H. Duan, C. Ni, Q. Yuan, G. Adams, M. Q. Smith, W. C. Wood, X. Gao and S. Nie, Small, 2009, 5, 235 CrossRef CAS PubMed.
- A. M. Smith, H. Duan, M. N. Rhyner, G. Ruan and S. Nie, Phys. Chem. Chem. Phys., 2006, 8, 3895 RSC.
- K. Holmberg, B. Jönsson, B. Kronberg and B. Lindman, Surfactant micellization, in Surfactants and polymers in aqueous solution, John Wiley & Sons Ltd, 2nd edn, 2003, pp. 39–66 Search PubMed.
- A. Dominguez, A. Fernández, N. González, E. Iglesias and L. Montenegro, J. Chem. Educ., 1997, 74, 1227 CrossRef CAS.
- W. H. Noordman, J. H. J. Wachter, G. J. de Boer and D. B. Janssen, J. Biotechnol., 2002, 94, 195 CrossRef CAS.
- C. Tanford, Y. Nozaki and M. F. Rohde, J. Phys. Chem., 1977, 81, 1555 CrossRef CAS.
- Y. Saito, M. Abe and T. Sato, J. Am. Oil Chem. Soc., 1993, 70, 717 CrossRef CAS.
- A. K. Rakshit and B. Sharma, Colloid Polym. Sci., 2003, 281, 45 CAS.
- X.-L. Diao, Y.-S. Xia, T.-L. Zhang, Y. Li and C.-Q. Zhu, Anal. Bioanal. Chem., 2007, 388, 1191 CrossRef CAS PubMed.
- W. G. J. H. M. van Sark, P. L. T. M. Frederix, D. J. V. den Heuvel and H. C. Gerritsen, J. Phys. Chem. B, 2001, 105, 8281 CrossRef CAS.
- T. Rheinlander, E. Klumpp, H. Schlimper and M. J. Schwuger, Langmuir, 2000, 16, 8952 CrossRef.
- P. K. Misra, B. K. Mishra and P. Somasundaran, Colloids Surf., A, 2005, 252, 169 CrossRef CAS PubMed.
- D. F. Anghel, S. Saito, A. Iovescu and A. Baran, Colloids Surf., A, 1994, 90, 89 CrossRef CAS.
- D. F. Anghel, S. Saito, A. Baran, A. Iovescu and M. Cornitescu, Colloid Polym. Sci., 2007, 285, 771 CAS.
- P. K. Misra and P. Somasundaran, J. Surfactants Deterg., 2004, 7, 373 CrossRef CAS.
- G. Despert and J. Oberdisse, Langmuir, 2003, 19, 7604 CrossRef CAS.
- J. Oberdisse, Physica B, 2004, 350, e913 CrossRef CAS PubMed.
- D. Lugo, J. Oberdisse, M. Karg, R. Schweinsc and G. H. Findenegg, Soft Matter, 2009, 5, 2928 RSC.
- D. M. Lugo, J. Oberdisse, A. Lapp and G. H. Findenegg, J. Phys. Chem. B, 2010, 114, 4183 CrossRef CAS PubMed.
- K. P. Sharma, V. K. Aswal and G. Kumaraswamy, J. Phys. Chem. B, 2010, 114, 10986 CrossRef CAS PubMed.
- E. Kalwarczyk, M. Paszewski, X. Xin, E. Gorecka, D. Pociecha, R. Holyst and M. Fialkowski, Langmuir, 2011, 27, 3937 CrossRef CAS PubMed.
- Y.-M. Lam, N. Grigorieff and G. Goldbeck-Wood, Phys. Chem. Chem. Phys., 1999, 1, 3331 RSC.
- M. R. Nejadnik, A. L. J. Olsson, P. K. Sharma, H. C. van der Mei, W. Norde and H. J. Busscher, Langmuir, 2009, 25, 6245 CrossRef CAS PubMed.
- Quantum dots application in biology, ed. M. P. Bruchez and C. Z. Hotz, Humana Press Inc., 2007 Search PubMed.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547 CrossRef CAS PubMed.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS PubMed.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435 CrossRef CAS PubMed.
- R. Generalov, S. K. S. Westrøm, W. Chen, S. Kristensen and P. Juzenas, Int. J. Nanomed., 2011, 6, 1875 CAS.
- R. Masel, Principles of Adsorption and Reaction on Solid Surfaces, Wiley, 1996 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S6, Tables S1–S3, and additional QD characterization. See DOI: 10.1039/c3nr03293k |
| This journal is © The Royal Society of Chemistry 2013 |
