Smart thermo-triggered squirting capsules for nanoparticle delivery†
Li
Liu
,
Wei
Wang
,
Xiao-Jie
Ju
,
Rui
Xie
and
Liang-Yin
Chu
*
School of Chemical Engineering, Sichuan University, Chengdu, Sichuan, China. E-mail: chuly@scu.edu.cn; Tel: +86 28 8546 0682
First published on 27th April 2010
Abstract
A squirting capsule is designed to deliver nanoparticles inspired by the squirting cucumber ejecting its seeds. The capsule has a thermo-sensitive hydrogel shell, and encapsulates nanoparticles by emulsifying the nanoparticle aqueous suspension in the water-in-oil emulsion core. The squirting capsule can completely squirt out the encapsulated nanoparticles with a high momentum, just like a nanoparticle bomb, by the dramatic shrinkage and sudden rupture of the capsule membrane upon heating.
Introduction
Nowadays nanoparticles are getting more and more prevalent in the fields of disease diagnosis and therapy;1 however, several challenges still remain for their practical applications, such as how to protect nanoparticles from premature degradation2 or unwanted interaction with biological molecules before reaching target site,3 how to selectively deliver nanoparticles only to diseased tissue,4 and how to achieve a wide distribution of nanoparticles at the target site.5 Here we report on a novel strategy for nanoparticle delivery, in which nanoparticles are encapsulated in hydrogel capsules and delivered via thermo-induced squirting, on demand. Such squirting capsules can protect the encapsulated nanoparticles within a hydrogel membrane until thermo-triggered delivery. As a temperature stimulus is convenient to manipulate and can be generated remotely, site-specific targeted delivery of nanoparticles can be achieved with such capsules. We demonstrate that the proposed capsules work like nanoparticle bombs that squirt nanoparticles with a high momentum when the hydrogel membrane breaks upon heating, which provides a novel mode for drug and diagnostic reagent delivery systems.Some promising nanoparticle candidates for drug delivery systems, such as protein nanoparticles, liposomes, and micelles, usually exhibit low physical and chemical stability.6 Besides, micelles disassemble if they are diluted below their critical micelle concentration. Thus, nanoparticle delivery carriers are desired to protect the encapsulated nanoparticles and prevent them from degrading prematurely before delivery, and to deliver them only at the targeted site. For stimuli-triggered site-targeting delivery, the delivery triggered by physical contact may not be practical in the human body,7 and delivery triggered remotely is more preferable.8 Furthermore, some biological tissues present diffusion obstacles for nanoparticles and/or their surrounding media are quite viscous,9 in which situations higher initial momentum for the nanoparticle delivery is very important.
Recently, De Geest et al.10 have succeeded in developing microcapsules that can project nanoparticles into the local environment at high speed under alkaline conditions. They filled a crosslinked layer-by-layer polyelectrolyte microcapsule with a degradable dextran-hydroxyethyl methacrylate (dex-HEMA) polymeric network and nanoparticles. When the environmental pH value was increased to 13–14 by the addition of 1 M NaOH solution, the encapsulated polymeric network rapidly decomposed and the osmotic pressure inside the capsule increased sharply, which caused the capsule to explode within a few seconds and project the nanoparticles at a very high speed. These microcapsules offer a new route to introduce nanoparticles into a tumor or into organs with a broader distribution than the free diffusion mode does.11 However, the extremely high pH value to trigger the capsule explosion is quite difficult to achieve safely under physiological conditions, because the physiological pH is not higher than 7.4 in the human body.11 Soon after, Bedard et al.12 reported a new mechanism to induce nanoparticle release from capsules by constructing polyelectrolyte microcapsules on dex-HEMA microgels and functionalizing capsule membranes with gold nanoparticles. Upon selectively irradiating a given region of the capsule's wall with an infrared laser beam, the capsule was able to release encapsulated materials in a pre-determined direction. It is a significant advance to use an infrared laser as a remotely addressable stimuli-trigger, meanwhile the oriented release makes a lot of sense in practice. Unfortunately, 1 M sodium hydroxide is still necessary in this system to dissolve the microgel core to generate a suitable osmotic pressure for the capsule explosion, which might be harmful to the encapsulated substances in practical applications. More feasible stimuli-triggered modes for delivering nanoparticles are still necessary and essential for promoting the application of such self-exploding carriers.
Similar delivery behaviours can be found in some plants in nature when they eject seeds from fruits for the widest possible distribution. For example, the ripe fruit of ecballium elaterium (Fig. 1A), also called squirting cucumber or exploding cucumber, is highly turgid. Due to its own ripeness or being disturbed by sniffing animals or whatsoever, the ripe fruit squirts a stream of mucilaginous liquid containing its seeds into air for a considerable long distance by sudden contraction of the wall of the fruit (Fig. 1B).
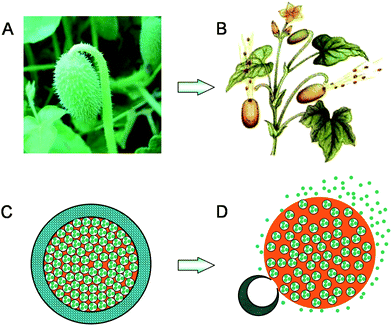 | ||
| Fig. 1 (A) A picture of squirting cucumber (http://www.lnkp.gov.cn/huanbao/c1/200905/7861.html); (B) A schematic illustration of squirting cucumbers ejecting seeds together with a stream of mucilaginous liquid (http://xyzw.plantlib.net/plant/plant/09/0902.htm); (C) A microcapsule with crosslinked PNIPAM hydrogel shell containing nanoparticles in the inner water phase of W/O emulsion core at a temperature below the LCST; (D) Nanoparticles being squirted out from the microcapsule together with the oil phase stream due to the dramatic shrinkage and sudden rupture of the PNIPAM hydrogel shell triggered by increasing the environmental temperature above the LCST. | ||
Inspired by the squirting cucumber, herein we design a novel, thermo-triggered squirting capsule for nanoparticle delivery. The proposed microcapsule is composed of a crosslinked poly(N-isopropylacrylamide) (PNIPAM) hydrogel shell, and encapsulates water-based nanoparticles by dispersing the aqueous phase, which contains the nanoparticles, into its oil phase core (Fig. 1C). PNIPAM is a well-known thermo-responsive polymer with a dramatic phase transition property when environmental temperature changes across its lower critical solution temperature (LCST). When the temperature is lower than the LCST, the PNIPAM hydrogel exhibits a swollen and hydrophilic state, while it shrinks dramatically and turns to hydrophobic when the temperature is higher than the LCST.13 Due to such fantastic and reversible thermo-responsive properties, PNIPAM hydrogels have been intensively investigated as temperature-triggered systems for the controlled release of drugs and/or chemicals. However, the concept of delivering nanoparticles from a thermo-triggered squirting capsule has never been reported before. In our proposed system, because the encapsulated nanoparticles exist in the water phase of the water-in-oil (W/O) emulsion core inside the microcapsule, the swollen and hydrophilic PNIPAM hydrogel membrane of the microcapsule can protect the encapsulated nanoparticles when the temperature is below the LCST (Fig. 1C). To eject the encapsulated nanoparticles, all we need to do is apply a heat stimulus to increase the local environmental temperature above the LCST. Upon heating, the PNIPAM hydrogel shell rapidly shrinks, which results in a sudden increase of the liquid pressure inside the microcapsule because both the continuous oil phase and the dispersed water phase in the capsule are incompressible. When the internal pressure increases to a critical value, the PNIPAM hydrogel shell ruptures suddenly, due to its limited mechanical strength, at the same time, the encapsulated nanoparticles are squirted from the microcapsule together with the oil phase stream into the environment with a high momentum (Fig. 1D), just like the seed-ejecting of ripe squirting cucumber.
Experimental
Materials
3 mL of water containing 0.2% yellow-green fluorescent (505/515) carboxylate-modified FluoSphere® polystyrene beads (200 nm, Invitrogen F8811) was employed as the water phase, and 7 mL of soybean oil containing 0.56 g polyglycerol polyricinoleate (PGPR 90, Danisco) as surfactant was employed as the oil phase. The two phases were mixed by magnetic agitation for 10 min and then homogenized (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 1 min) by a BRT homogenizer (B25 10 mm head).
000 rpm, 1 min) by a BRT homogenizer (B25 10 mm head).
Microfluidic device
The microfluidic device for fabrication of (W/O)/W/O emulsions was assembled according to our previous method.14 Briefly, three cylindrical capillaries were used as the injection, transition, and collection tubes by aligning them coaxially inside the square capillaries (see ESI for a schematic illustration of the microfluidic device†). The outer diameter of the cylindrical capillaries was 1.0 mm, and the inner dimension of the square capillary tubes was 1.0 mm. The inner diameters of the injection, transition, and collection tubes were 580, 250, and 580 μm, respectively. The ends of injection and transition tubes were tapered by a micropuller (Narishige, Japan) and then adjusted by a microforge (Narishige, Japan). The tapered orifice diameters of the injection and transition tubes were 60 and 200 μm, respectively.Microfluidic emulsification
The W/O primary emulsion mentioned above was employed as the inner fluid. The middle fluid was monomer aqueous solution containing surfactant Pluronic F127 (1% (w/v), Sigma-Aldrich), monomer NIPAM (1 mol L−1, Kohjin), crosslinker N,N′-methylenebisacrylamide (MBA) (0.02 mol L−1), and initiator 2,2′-azobis(2-amidinopropane) dihydrochloride) (0.005 mol L−1). Soybean oil containing 8% (w/v) PGPR 90 was employed as the outer fluid. The inner, middle and outer fluids were separately pumped into the injection, transition and collection tubes of the microfluidic device. (W/O)/W/O emulsions generated in the collection tube were collected in soybean oil containing 2% (w/v) 2,2-dimethoxy-2-phenylacetophenone (BDK) as the photo initiator.UV-initiated polymerization
The collected (W/O)/W/O emulsions were converted into microcapsules by polymerization with UV irradiation for 10 min in an ice-water bath. Under UV light, the activated photo-initiator BDK diffused to the interface between the outer oil phase and middle aqueous phase, where it initiated the polymerization of the NIPAM monomer and MBA crosslinker in the middle aqueous phase to build the hydrogel shell of the microcapsules. A 250 W UV lamp with an illuminance spectrum of 250–450 nm was employed to produce UV light. The ice-water bath was introduced to ensure the polymerization was carried out at temperature below the LCST of PNIPAM. The microcapsules were separated from the oil by adding deionized water into the container. When the oil phase and water phase had completely separated, the capsules settled into the bottom water layer and the upper oil layer was removed. The microcapsules were washed with deionized water several times and then dispersed in deionized water.Characterization
Optical microscope images were obtained by an Olympus optical microscope (BX 61). The thermo-triggered squirting behaviors were observed by an optical microscope equipped with a thermostatic stage system (TS 62, Instec, USA) and a CCD camera. After microcapsules were equilibrated in deionized water at 20 °C, the temperature was increased to 50 °C within ∼90 s, at the same time the thermo-induced shrinkage and sudden squirting behaviors of the microcapsules were recorded by the CCD camera that was mounted on the optical microscope. Fluorescent images and corresponding density profiles of capsules before and after squirting the encapsulated nanoparticles were obtained by a confocal laser scanning microscope (CLSM) (Leica Microsystem SP5).Results and discussion
In this study, carboxylate-modified yellow-green fluorescent FluoSphere® beads (200 nm, Invitrogen F8811) are used as model nanoparticles. The nanoparticle aqueous suspension is emulsified in soybean oil by a homogenizer to form W/O primary emulsion which contains nanoparticles in its inner water phase. The confocal laser scanning microscope (CLSM) images of the primary emulsion show that the nanoparticle aqueous suspension forms droplets with sizes ranging from 570 nm to 920 nm, and disperse well in the emulsion without coalescence (Fig. 2A and 2B). The prepared W/O primary emulsion containing nanoparticles is employed as the inner fluid and the N-isopropylacrylamide (NIPAM) monomer solution with crosslinker is employed as the middle fluid in the following process to prepare (water-in-oil)-in-water-in-oil ((W/O)/W/O) emulsions with a microfluidic emulsification method.14 The obtained (W/O)/W/O emulsions serve as templates for synthesis of hydrogel capsules. The (W/O)/W/O emulsion droplets (Fig. 2C) are collected in soybean oil containing a photo initiator and then subjected to UV light to initiate the polymerization of NIPAM monomer. The polymerized capsules are separated from the oil and redispersed in deionized water.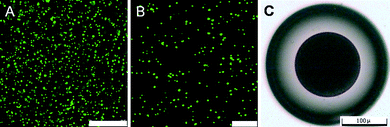 | ||
| Fig. 2 (A and B) CLSM images of primary W/O emulsion containing nanoparticles in the inner water phase at different magnification. Scale bars: (A) 25 μm; (B) 10 μm. To see the emulsion droplets more clearly, the emulsion shown here is diluted with soybean oil to one third of the original concentration before CLSM observation. (C) An optical microscope image of prepared (W/O)/W/O emulsion containing nanoparticles in its innermost water phase. The scale bar is 100 μm. | ||
In the bright field under the microscope at room temperature (below the LCST), the PNIPAM hydrogel shell is transparent, while the inner primary emulsion is dark (Fig. 3A). Actually, the thickness of hydrogel membrane of the prepared microcapsule is not perfectly uniform along the circumference, as seen in Fig. 3A. This membrane thickness difference resulted from the density difference between the encapsulated W/O emulsion and the NIPAM monomer solution in the (W/O)/W/O emulsion. Although we have adjusted the density of both the inner W/O phase and the middle aqueous monomer phase carefully, slight density differences do still exist, so the encapsulated W/O emulsion is not in the exact center of the (W/O)/W/O emulsion droplet. Consequently, the thickness of polymerized hydrogel membrane is a little thicker on one side but a little thinner on the other side of the microcapsule. The CLSM fluorescent images illustrate that no leakage of nanoparticles from the prepared hydrogel capsule is observed (Fig. 3B and C). This result is also convinced by the fluorescence intensity profile (Fig. 3D). Inside the microcapsule the intensity is quite high (from 50 to 260), whereas the intensity outside is nearly zero. The innermost nanoparticle suspension is separated from the hydrogel shell by the continuous oil phase of the W/O emulsion inside the capsule, and the oil phase cannot permeate through the PNIPAM hydrogel membrane and then prevent the encapsulated nanoparticles from leaking.
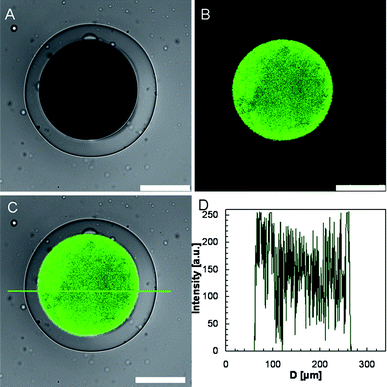 | ||
| Fig. 3 CLSM images of the prepared microcapsule at room temperature, in which (A) shows the transmission channel image, (B) shows the green channel image, and (C) shows the overlay of green channel and transmission channel images. The scale bars are 100 μm. (D) The fluorescence intensity profile corresponding to (C). | ||
To observe the squirting of nanoparticles from the prepared microcapsules upon heating, a glass slide with a drop of microcapsule suspension is placed on a thermostatic stage under a microscope. When the temperature increases from 20 °C to 50 °C, the hydrogel shell of the microcapsule shrinks rapidly. The inner oil phase cannot permeate through the shrinking hydrogel membrane, leading to deformation of the capsule. During the deformation in the thermo-triggered squirting process, the encapsulated W/O phase tends to breach the thinner side of the hydrogel membrane, which is stretched by the incompressible inner oil phase. When the shrinkage reaches a high degree, the hydrogel membrane turns into an “8” shape, of which one head (the side with thinner membrane) is full of the encapsulated W/O primary emulsion and the hydrogel shell becomes extremely thin. When the inner pressure reaches a critical value, the hydrogel membrane ruptures and the contained oil phase, together with the encapsulated nanoparticles, is squirted out to the surrounding water (Fig. 4A). Fig. 4B shows the snapshots of the thermo-triggered squirting process in dark field (see ESI for a movie of the thermo-triggered squirting process†). Because the squirting direction is upwards, the “8” deformation is not as obvious as that shown in Fig. 4A. During the squirting process, the large bright area indicates the considerable wide distribution of the squirted substance.
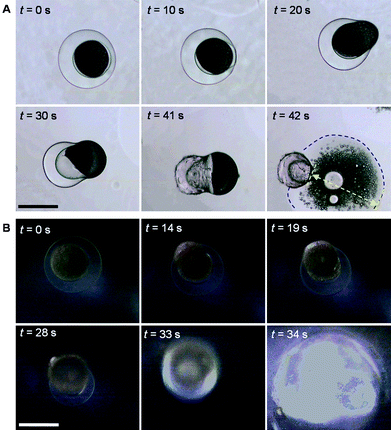 | ||
| Fig. 4 Bright-field (A) and dark-field (B) microscope snapshots of thermo-triggered squirting of nanoparticles from microcapsules by increasing the environmental temperature from 20 °C to 50 °C. The blue dashed line in (A) indicates the propagating front of the squirted liquid containing nanoparticles, and the yellow arrow shows the propagating distance of the squirted liquid containing nanoparticles. The scale bars are 200 μm. | ||
Fig. 5A shows a CLSM image of the typical distribution of the nanoparticles after the squirting release. The fluorescent intensity in the central area is very low (from 50 to 100), while around the shrunken and ruptured capsule the fluorescent intensity is quite high (about 250) and gradually fades away at 200 μm from the center of the microcapsule (Fig. 5B), which indicates that encapsulated nanoparticles are completely squirted out from the capsule. We find that capsules squirt out nanoparticles in different directions. Some capsules squirt nanoparticles sideways, for example the capsule shown in Fig. 4A. In this case, the fluorescent trail of nanoparticles indicates the squirting direction (Fig. 5C), and the fluorescent intensity in the squirting direction is as high as about 250 while that in the opposite direction is only about 130 (Fig. 5D). We also observe an interesting phenomenon that the squirted nanoparticles are distributed like a vortex (Fig. 5E and 5F). From Fig. 5F, we can see that at least two peaks of the fluorescent intensity reach about 250 on the left side of the microcapsule. This may be caused by rotation of capsules during the squirting process. We presume the rotation in this case is caused by squirting in tangential direction; however, the nanoparticles are usually squirted out in a radial direction in other cases.
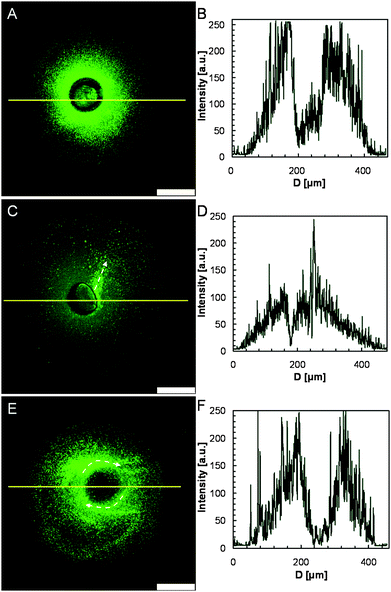 | ||
| Fig. 5 CLSM images (A, C, E) of diverse distributions of nanoparticles squirted from microcapsules triggered by local heating, and their fluorescence intensity profiles (B, D, F), respectively. The white arrows indicate the squirting direction. The scale bars are 100 μm. | ||
To estimate the squirting speed of the nanoparticles from the capsule, we compare the distance that nanoparticles travel by squirting from capsules with that by free diffusion. According to the Stokes–Einstein equation (eqn (1)), the diffusion coefficient (D) of 200 nm sized nanoparticles in water at 50 °C is 2.156 μm2 s−1.
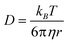 | (1) |
The diffusion length (x) for 200 nm nanoparticle within 1 s by one-dimensional diffusion is calculated to be 2.94 μm, (eqn (2)).
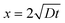 | (2) |
However, in the bight-field snapshots (Fig. 4A), the squirted nanoparticles travel 359 μm within the first 1 s after the hydrogel membrane ruptures. This distance is 122 times longer than that in the above-mentioned diffusion case. For our proposed capsule system, the release speed of nanoparticles from the capsule can be controlled by varying the polymeric crosslinkage of the shell, the shell thickness, the viscosity of the encapsulated oil phase, and the heating rate.
Conclusions
We have succeeded in developing smart, thermo-triggered squirting capsules for nanoparticle delivery. Nanoparticles are encapsulated in the hydrogel capsules without any leakage before delivering on demand. The delivery of the nanoparticles is triggered by simply increasing the environmental temperature, which can be addressed remotely. Nanoparticles are completely squirted out and no residue remains inside the ruptured capsules. Besides this, the squirting from the proposed capsule also provides the nanoparticles with a very high momentum, which is beneficial to achieve a wide distribution. To make these squirting capsules more suitable for practical use, the LCST of the PNIPAM hydrogel shell can be easily adjusted by simply copolymerizing the NIPAM monomer with hydrophilic15 or hydrophobic15c,16 monomers. For example, we can tune the LCST of the PNIPAM-based capsule shell in our system to be 40 °C or higher just by adding 7 mol% or more acrylamide into the NIPAM monomer solution in the polymerization recipe.15c Thus, the LCST is several degrees higher than the normal physiological temperature, therefore our proposed capsules will remain in a swollen state and protect the inner encapsulated substances from leaking under physiological temperature, and slight temperature fluctuation within the physiological range will never cause capsule rupture. Upon site-specific local thermotherapy, for instance, local treatment with infrared irradiation, microwave or ultrasound, the site-specific local temperature is increased to be higher than the LCST of the capsule shell materials; as a result, the hydrogel capsule shell undergoes volume phase transition, which causes the squirting delivery of nanoparticles from the capsule on demand. Future work must focus on refining the system proposed here to allow the full potential of the technique to be realized. In addition, applications of these systems to biomedical fields would require a smaller capsule size, which can be achieved with developing the microfluidic and nanofluidic techniques.17 We believe that such a novel and feasible mode of nanoparticle delivery provided by the squirting capsule has great potential in biomedical fields.Acknowledgements
The authors gratefully acknowledge support from the National Natural Science Foundation of China (20825622), the National Basic Research Program of China (2009CB623407), the Specialized Research Fund for the Doctoral Program of Higher Education by the Ministry of Education of China (200806100038), and Sichuan Youth Science and Technology Foundation for Distinguished Young Scholars (08ZQ026-042). The authors are grateful to the Kohjin Co., Ltd., Japan, for kindly supplying the N-isopropylacrylamide.References
- (a) T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818 CrossRef CAS; (b) J. D. Byrne, T. Betancourt and L. Brannon-Peppas, Adv. Drug Delivery Rev., 2008, 60, 1615 CrossRef CAS; (c) K. A. Howard, Adv. Drug Delivery Rev., 2009, 61, 710 CrossRef CAS.
- D. A. Giljohann, D. S. Seferos, A. E. Prigodich, P. C. Patel and C. A. Mirkin, J. Am. Chem. Soc., 2009, 131, 2072 CrossRef CAS.
- J. Blummel, N. Perschmann, D. Aydin, J. Drinjakovic, T. Surrey, M. Lopez-Garcia, H. Kessler and J. P. Spatz, Biomaterials, 2007, 28, 4739 CrossRef.
- (a) R. Mortera, J. Vivero-Escoto, I. I. Slowing, E. Garrone, B. Onida and V. S. Y. Lin, Chem. Commun., 2009,(22), 3219 RSC; (b) B. M. Budhlall, M. Marquez and O. D. Velev, Langmuir, 2008, 24, 11959 CrossRef CAS; (c) T. Y. Liu, S. H. Hu, D. M. Liu, S. Y. Chen and I. W. Chen, Nano Today, 2009, 4, 52 CrossRef CAS.
- J. K. Vasir and V. Labhasetwar, Biomaterials, 2008, 29, 4244 CrossRef CAS.
- B. M. Discher, Y. Y. Won, D. S. Ege, J. C. M. Lee, F. S. Bates, D. E. Discher and D. A. Hammer, Science, 1999, 284, 1143 CrossRef.
- S. H. Hu, D. M. Liu, W. L. Tung, C. F. Liao and S. Y. Chen, Adv. Funct. Mater., 2008, 18, 2946 CrossRef CAS.
- D. V. Volodkin, A. G. Skirtach and H. Mohwald, Angew. Chem., Int. Ed., 2009, 48, 1807 CrossRef CAS; D. V. Volodkin, A. G. Skirtach and H. Mohwald, Angew. Chem., 2009, 121, 1839 CrossRef.
- (a) W. M. Pardridge, Adv. Drug Delivery Rev., 2007, 59, 141 CrossRef CAS; (b) J. Suh, M. Dawson and J. Hanes, Adv. Drug Delivery Rev., 2005, 57, 63 CrossRef CAS.
- B. G. De Geest, M. J. McShane, J. Demeester, S. C. De Smedt and W. E. Hennink, J. Am. Chem. Soc., 2008, 130, 14480 CrossRef.
- L. Dahne, Angew. Chem., Int. Ed., 2009, 48, 4106 CrossRef; L. Dahne, Angew. Chem., 2009, 121, 4169 CrossRef.
- M. F. Bedard, B. G. De Geest, H. Moehwald, G. B. Sukhorukov and A. G. Skirtach, Soft Matter, 2009, 5, 3927 RSC.
- (a) S. Katayama, Y. Hirokawa and T. Tanaka, Macromolecules, 1984, 17, 2641 CrossRef; (b) T. Tanaka, E. Sato, Y. Hirokawa, S. Hirotsu and J. Peetermans, Phys. Rev. Lett., 1985, 55, 2455 CrossRef CAS.
- (a) L. Y. Chu, A. S. Utada, R. K. Shah, J. W. Kim and D. A. Weitz, Angew. Chem., Int. Ed., 2007, 46, 8970 CrossRef CAS; L. Y. Chu, A. S. Utada, R. K. Shah, J. W. Kim and D. A. Weitz, Angew. Chem., 2007, 119, 9128 CrossRef; (b) W. Wang, L. Liu, X. J. Ju, D. Zerrouki, R. Xie, L. H. Yang and L. Y. Chu, ChemPhysChem, 2009, 10, 2405 CrossRef CAS.
- (a) F. Eeckman, A. J. Moes and K. Amighi, Eur. Polym. J., 2004, 40, 873 CrossRef CAS; (b) M. Shibayama, S. Mizutani and S. Nomura, Macromolecules, 1996, 29, 2019 CrossRef; (c) R. Xie, Y. Li and L. Y. Chu, J. Membr. Sci., 2007, 289, 76 CrossRef CAS.
- (a) D. Shao and C. H. Ni, J. Appl. Polym. Sci., 2007, 105, 2299 CrossRef CAS; (b) X. J. Ju, L. Y. Chu, P. Mi, H. Song and Y. M. Lee, Macromol. Rapid Commun., 2006, 27, 2072 CrossRef CAS; (c) I. Soutar, L. Swanson, P. G. Adamson and N. J. Flint, Macromolecules, 2009, 42, 9153 CrossRef CAS.
- G. M. Whitesides, Nature, 2006, 442, 368 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Schematic illustration of the microfluidic device, and a movie to show the thermo-induced burst delivery process of nanoparticles from the smart squirting capsule. See DOI: 10.1039/c002231d |
| This journal is © The Royal Society of Chemistry 2010 |
