Evaluating the photophysical and photochemical characteristics of green-emitting cerium(iii) mono-cyclooctatetraenide complexes†
Abstract
The photophysical and photochemical reactivity of the organometallic mono(cyclooctatetraenide)-Ce(III) complexes: [(C8H8)Ce(μ-X)(THF)2]2 X = O3SCF3− (1), Cl− (2), were studied. In the course of these studies, a new polymorph of [(C8H8)Ce(μ-O3SCF3)(THF)2]2 (1) was described. Photoluminescence (PL) studies of 1 and 2 showed characteristic excitation and emission bands in the visible region, with bright green light emission under light irradiation from states corresponding to the 5d → 4f transition. Complexes 1 and 2 exhibit relatively long lifetime of 205.4 ± 0.2 and 145.8 ± 0.4 ns respectively. And the quantum yields (Φ) for 1 and 2 are 0.180 and 0.068 respectively. Electrochemical studies were performed on complexes 1 and 2 with a reversible Ce(III)/Ce(IV) redox couple recorded at E1/2 = −1.53 V versus the Fc/Fc+ for 2. Complex 1 shows an irreversible Ce(III/IV) oxidation wave. Complexes 1 and 2 revealed strongly-reducing, estimated, excited state reduction potentials 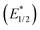 of −3.31 and −4.02 V versus the Fc/Fc+ respectively, with small Stokes shifts of 0.12 eV. With their associated relatively long lifetimes, small Stokes shifts and large, negative
of −3.31 and −4.02 V versus the Fc/Fc+ respectively, with small Stokes shifts of 0.12 eV. With their associated relatively long lifetimes, small Stokes shifts and large, negative 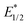 values, both complexes were evaluated as potential photosensitisers for halogen atom transfer (XAT) using a test reaction of the dehalogenation of benzyl chloride.
values, both complexes were evaluated as potential photosensitisers for halogen atom transfer (XAT) using a test reaction of the dehalogenation of benzyl chloride.

- This article is part of the themed collection: Spotlight Collection: Photoinduced redox chemistry


 Please wait while we load your content...
Please wait while we load your content...
