Optimising oxygen diffusion in non-cubic, non-dilute perovskite oxides based on BiFeO3†
Abstract
An ion-conducting crystal possessing non-cubic crystal symmetry and a non-dilute composition will in general display a multiplicity of different migration paths and different site energies for the mobile ions. Predicting the macroscopic rate of ion transport, and in particular, identifying substituent cations that optimise this rate, is therefore challenging. In this study, molecular dynamics simulations employing pair potentials were used to calculate the oxygen tracer diffusion coefficient 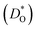 as a function of temperature in various substituted systems based on the rhombohedral perovskite oxide bismuth ferrite, BiFeO3. Substituent cations that maximise (or minimise)
as a function of temperature in various substituted systems based on the rhombohedral perovskite oxide bismuth ferrite, BiFeO3. Substituent cations that maximise (or minimise) 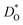 are identified. The results also reveal the limits of the standard crystal-chemical approach to maximising
are identified. The results also reveal the limits of the standard crystal-chemical approach to maximising 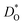 by matching the size of the substituent cation to that of the host's. The implications for the use of BiFeO3 as a high-performance oxide-ion conductor or as a multiferroic medium are discussed.
by matching the size of the substituent cation to that of the host's. The implications for the use of BiFeO3 as a high-performance oxide-ion conductor or as a multiferroic medium are discussed.

- This article is part of the themed collection: Editor’s Choice: Solid-state ion conductors


 Please wait while we load your content...
Please wait while we load your content...