Open Access Article
Open Access ArticleBiofuels and hydrogen production: back to the Langmuir–Blodgett approach for large-scale structuration of Bi-based photoelectrodes
Claire
Dazon
 *,
Márcio César
Pereira
*,
Márcio César
Pereira
 and
Douglas Santos
Monteiro
and
Douglas Santos
Monteiro

Institute of Science, Engineering, and Technology (ICET), Federal University of Jequitinhonha and Mucuri Valleys (UFVJM) – Campus Mucuri, 39803-371 Teófilo Otoni, Minas Gerais, Brazil. E-mail: dazonclaire@gmail.com
First published on 1st May 2024
Abstract
In light of global warming and climate change, the sustainable production of biofuels and hydrogen for energy applications, notably transportation, is an urgent issue. Among the various compounds, Bi-based nanomaterials have gained considerable attention as photoelectrocatalysts for hydrogen production through water splitting or organic matter decomposition. However, despite their promising applications, the challenges associated with shaping and scaling-up these compounds remain unsolved. This perspective aims to describe and highlight a potentially promising approach based on Langmuir–Blodgett thin films, which could lead to efficient, stable, and scalable production of Bi-based nanostructured photoelectrodes.
1. Introduction
Climate change, global warming, and depletion of natural resources are pressing issues that require immediate attention. Recent statistics from the Environmental Protection Agency (EPA) have revealed the ongoing degradation of our planet, with several indicators: a week earlier first bloom date, a rise in hot daily lows (nighttime), and the release of over 50 billion metric tons of carbon dioxide equivalents by global greenhouses to quote some of the 50 indicators of climate change.1–3 While humans require energy for survival (transportation, food, industries, health, etc.), particularly with the help of digital tools and AI, the need for power cannot come at the expense of further damage to the planet.4 Therefore, finding ways to maintain energy consumption with minimal environmental impact should not be a dream, provided intelligent solutions will be proposed in the coming years. Fortunately, several promising options have popped up for years: biofuels and hydrogen.Biofuels and hydrogen have the potential to contribute to a green economy by substituting or complementing gasoline and diesel without producing toxic gases.5–10 However, their production levels are still low, and the current technologies have ecological drawbacks incompatible with future energy models intended to preserve the environment. For example, the production of hydrogen through steam reforming of natural gas is one of the most cost-effective technologies; however, it results in the production of a mixture of hydrogen and carbon monoxide (syngas), which requires further treatment to produce more hydrogen and carbon dioxide, making it a non-environmentally friendly option.11
The road sector is the sole consumer of 2.1 Gtep of fuel, whose only 91 Mtep are biofuels, representing less than 5% of alternative fuels.9,10 In the aviation sector, nine types of biokerosene are considered potential alternatives, but data collection is ongoing to determine the extent of their global implementation. Most current biofuels are derived from edible biomass, such as sugar cane, soybeans, corn, and wheat, contributing to deforestation and CO2 release. However, large-scale production of biofuels and hydrogen can also be produced from non-edible biomass sources such as agricultural and human waste, algal biomass, and other intelligent breakthrough technologies, such as engineered algae and bioinspired systems. Then, the challenge is to produce safe, industrial-scale fuels, limiting the impact on the environment. A bright frame from Rosa et al.12 illustrates it and is reproduced in Fig. 1 with additional information from our study.
 | ||
| Fig. 1 Development timelines for (photo)electrocatalysts and large-scale implementation in electrolysis applications for biofuels and hydrogen production (adapted from Rosa et al.12). | ||
The idea is to use renewable energy sources such as solar, wind, or hydraulic power to feed an electrolyzer that ignites oxido-reduction reactions to convert organic matter (Biomass) into biofuels or reusable gas. Another option is to use water electrolysis to produce hydrogen. An efficient and stable photo(electro)catalyst must be combined at the electrodes to achieve this virtuous cycle. Materials such as TiO2, Cu2O, Fe2O3, and BiVO4 have been widely studied in recent years and are expected to become available for technology soon,13,14 as indicated on the colored scale in Fig. 1. The deposition of these materials at electrodes must be structured in a well-designed manner to ensure high fuel productivity. Recent advancements in the development of photoelectrocatalysts have focused on transition metal oxides,13,14 particularly scheelite-type monoclinic BiVO4, sometimes completed by carbon moieties as co-catalysts (GO, MWCNT), owing to their advantages in scaling up photoelectrodes, such as an adapted band gap for solar absorption (2.4 eV (VIS)), efficient charge separation, and atomic element availability.15–21 Although these compounds are still under study, ongoing research aims to validate their efficiency and long-term stability under real working conditions.
Notwithstanding the challenges above, the core aspect of this concept is large-scale electrocatalysis. This part is tedious because of the necessity of designing a complex electrode to ensure the efficiency and long-term stability of the photocatalyst, electrical conductivity, mechanical stability, and chemical stability (corrosion resistance). Therefore, the development of this field is not at the same level as that of photocatalysts. Several approaches have been employed to design such electrodes, including vacuum methods (PVD, CVD), atomic layer deposition, electrodeposition, hydrothermal, spray pyrolysis, and dip-coating. Patil et al.22 recently designed BiVO4 photoanodes on Ti porous transport layers through an electrodeposition approach and tested several geometries to determine their impact on photocatalytic performance. The results demonstrated a reduced loss of performance between small (1 cm2) and large (100 cm2) photoanodes, and a combination with a commercial polycrystalline Si PV led to 6 h of stability in photocurrent production (208 mA), representing a 2.2% yield. These results are encouraging but raise questions regarding scalability and cost, as the design requires large surface areas of Ti porous layers, long-term stability (several days and months), and exploration of other porous layer materials, as indicated by the authors as perspectives. Finally, no golden deposition technique has been identified to obtain a well-structured photoelectrode containing BiVO4 or other photocatalysts for the large-scale production of biofuels and hydrogen. Have all possible methods for enhancing the scalability of photoelectrodes been exhausted? Is there any previously overlooked technique that merits reexamination? Perhaps an old and almost forgotten deposition technique deserves to be highlighted: the Langmuir–Blodgett.
It is what we intend in this perspective article: we propose reviving the Langmuir–Blodgett method as a potential solution for scaling up BiVO4 photoanodes for biofuel and hydrogen production. By reviewing the literature and considering the potential of this technique in Brazilian's large-scale biofuel and hydrogen development, we aimed to assess the pros and cons of the Langmuir–Blodgett technique and provide recommendations for accelerating its development in the context of ongoing energy transition.
2. Biofuels and hydrogen in Brazil: looking for soft energy path
Among countries valorizing biofuels and hydrogen, the Brazilian case is interesting because it gathers by itself all the resources motivating the acceleration of photocatalyst scale-up given the soft energy production: renewable energy through dense sunny days per year, wind along the coasts, a large amount of edible and non-edible biomass for biofuels/hydrogen production, and primary matter for photocatalysts. The concept of a soft energy path was introduced by Lovins23 over more than 40 years, claiming clean energy production and intelligent consumption. In this section, we intend to demonstrate that, at a local scale, it is plausible to consider an energetic transition based on photocatalyst materials scaling up to modify edible and non-edible biomass to produce biofuels for transportation applications, the second area responsible for CO2 release (24% of global emissions10). Just before this, a slight recording of how it is possible to transform biomass into energy/heat/fuel is proposed in Fig. 2.24 Biomass is available everywhere, from vegetal, animal, and microorganisms, and a considerable part can be encountered in waste. Several as said eco-friendly approaches can transform organic molecules, such as alcohols, sugars, fatty acids, and triglycerides, into fuels (liquid or gas). Thermal processes (direct burning, pyrolysis, and hydrothermal) consume much energy and release CO2 but can be faster than fermentation, digestion, or hydrolysis processes. Here, the topic addresses biomass conversion into fuel (liquid or gas) in a softer and faster way, directly using solar energy for conversion: the photoelectrochemical approach.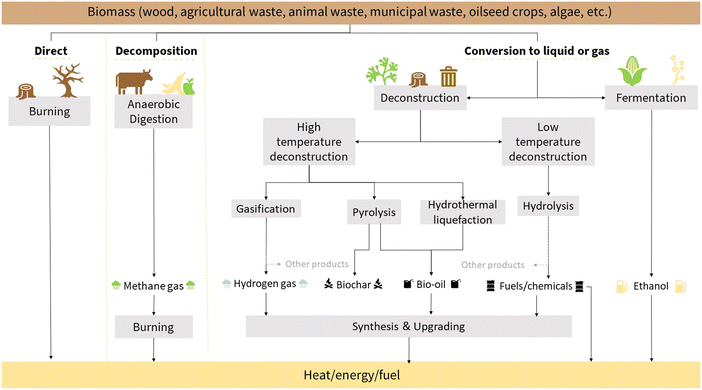 | ||
| Fig. 2 Global view of transformations of biomass to biofuel processes.24 | ||
2.1. Biofuels potential in Brazil: feedstocks availability and underexploited biomass from vegetal waste and animal fats
Several recent reviews have demonstrated Brazil's high potential for producing biofuels, mainly for transportation applications (cars, trucks, and aircraft).5,8,9,25–30 These biofuels are obtained through different processes depending on the nature of the biomass used as the primary reactive compound. Biomass can be accurately divided into several categories, as summarized in Fig. 3 by Dessalle et al.31 Plant biomass is encountered mainly through forest crops, aquatic biomass, and agricultural harvesting. Wood, straw, leaves, fruit peels, and shells are enriched in different sugar and alcohol molecules; cellulose, hemicellulose, and lignin are the most abundant, and fatty acids can also be encountered.32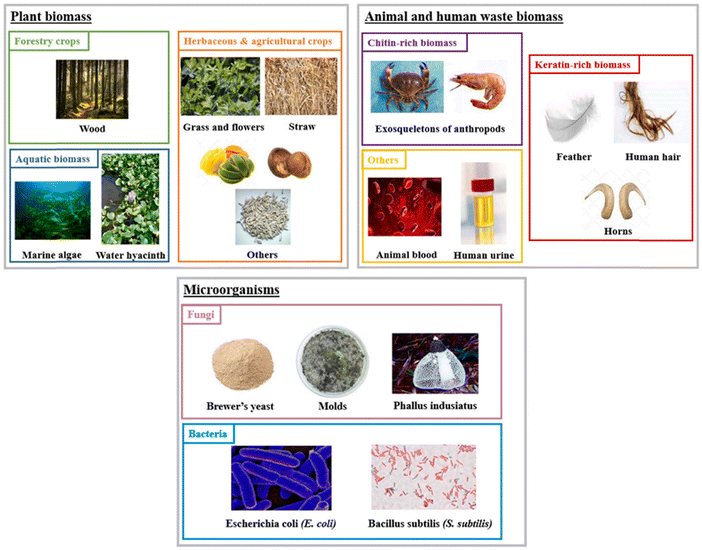 | ||
| Fig. 3 Categories of Biomass from Dessalle et al.31 | ||
Animal and human wastes are an exciting source of fats (triglycerides) or polysaccharides (chitin) encountered notably in seafood shells.31,32 Microorganisms are a great source of chitin and are expected to be an abundant source of biofuel precursors. Regarding biofuel conversion, another classification is possible according to “biomass generation” (Fig. 4), which is the classification we privileged in this study because it better highlights Brazil's potential to harvest all accessible biomass in the country.
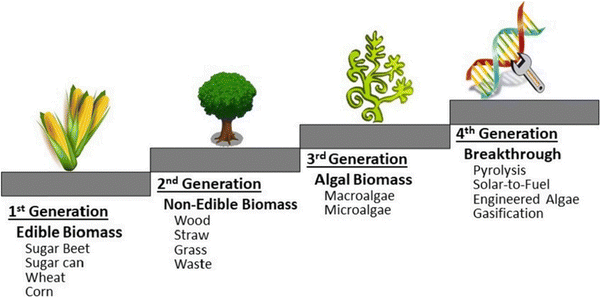 | ||
| Fig. 4 Biomass generation from Khan et al.5 | ||
According to Khan et al.,5 biomass can be classified as edible (Biomass that can be eaten, first generation), non-edible (a waste category including vegetal, animal, and human waste, second generation), the specific case of algal biomass (third generation), and advanced biomass qualified as a breakthrough (fourth generation). Upon closer examination of the Brazilian case, it becomes evident that only the first and second generations have been explored and implemented for biofuel production, albeit with significant disparities.8,28,29 However, with the latter approach, sufficient biofuel production can be envisaged. Brazilian biodiesel production is currently around 7.1 billion liters, whereas biodiesel consumption is forecasted at 7.15 billion liters. Thus, Brazil can be considered independent in this view, and the remaining key point would be safe biofuel production for both road and bio-jet uses. For the latter, efforts should be made.
As explained by Carvalho et al.8 a geoeconomic study focusing on prospects for aviation fuels demonstrates the following points:
• Sugar cane, rice, soybeans, maize, eucalyptus, pinus, and wheat represent the primary edible biomass throughout the country (>70% used);
• Few data on the non-edible biomass (waste of the crops above) are available in the biofuel production;
• The south region (Paraná, Santa Catarina, Rio Grande do Sul states) and midwest region (Mato Grosso, Mato Grosso do Sul, Goiás states) display the highest bioenergy potential for independence, with notable production sites close to the airports (less than 100 km)
• Their calculations, according to different scenarios, show a real cost-benefit using biofuels due to the low cost of biomass;
• CO2 taxes should be proposed as the total life-cycle assessment of bio-jet production to motivate smaller production plants and review all the biomass availability (edible and non-edible) to reduce environmental costs.
While these hotspot regions have been utilizing edible biomass, the non-edible biomass is abundantly present in all plant residues. However, it remains underutilized. Brazil can use 86 Mt of straw residue to produce biofuels,5 but several other parts are enriched in lignin, hemicellulose, and cellulose, such as grass, wheat grasses, corn cobs, and nut shells. Newspapers as human waste can be considered to have a content of these compounds between 25 and 50%, depending on the residues. Fats from animals are also a great source of fatty acids, which can be considered in this view. Animal fats (pork lard, beef tallow, mutton tallow, and poultry fat) generally contain more than 30% saturated, monosaturated, and polysaturated fatty acids, the most common being stearic (∼20%), palmitic (∼20–25%), and oleic (∼30–40%) fatty acids. The animal fats exploitation as a non-edible biomass could boost and ensure biofuel production in Brazil, including bio-jet, notably since the country consumes a lot of this animal food with 9.85 million metric tons for instance, for poultry, and for beef, 7.3 million ton.27
Given the soft energy pathway, cleaner biodiesel and biojet production must be powered by more research and development on efficient and eco-friendly production technologies to include more non-edible biomass and eliminate the disparities highlighted in Brazil between the different regions. This diversification is expected to provide the technologies to transform this non-edible biomass to ensure significant yields and cost benefits for the environment and society.
2.2. Hydrogen potential in Brazil: perspectives and actual context of H2 production
The recent study by Chantre et al.6 demonstrated the great potential of Brazil to move toward a hydrogen economy shared with others that is already in progress. According to this study, in the medium (6–10 years) and long term (>11 years), producing and consuming green hydrogen in Brazil is a real future, providing several challenges are addressed: scaling up, cost reduction, and adoption and sustainable growth of hydrogen-based technologies. Beyond this, several rules should be stated, such as standards and regulations on demand, and mobility should not be a unique consumer of such fuels.The first step in a green hydrogen economy is production. We do not treat the subject of storage and consumption here, which are also serious areas to consider. Producing green hydrogen requires at least two actors: a renewable energy system and a catalyst to boost the (electro)chemical reaction. Table 1, inspired by Macedo and Peyerl7 and Santana et al.33 summarizes some possibilities with only renewable and reduced polluting resources identified. Brazil has several advantages because the country displays sun, wind, a vast framework of urban waste to transform in the big cities, notably, crop economy, to exploit the biomass, and hydroelectricity has already been a great opportunity to furnish the energy required for H2 production (in 2020, more than 60% of hydroelectricity is included in the energy grid33). However, the choice of renewable resources should be adapted depending on the region. Indeed, the regions along the coasts can benefit from sun and wind, whereas the center and the north are limited to hydroelectricity and biomass resources.
| Energy source | Potential system for H2 production | Brazil region with potential implementation |
|---|---|---|
| Hydroelectricity | Water electrolysis | Country |
| Solar | Northeast, southeast | |
| Wind | Northeast, southeast, and south | |
| Biomass (edible, non-edible) | Photodegradation of biomass | Country |
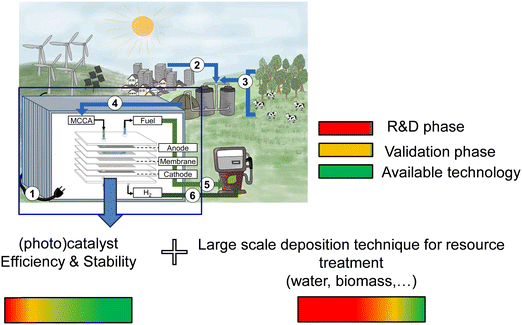
While no single method holds exclusive merit for hydrogen (H2) production, water electrolysis in an alkaline medium emerges as the most advanced, potent, and rewarding approach. This assertion remains valid even when contemplating biomass conversion, as it demands comparatively less energy expenditure.7,35 Santana et al.33 showed that combining sun and wind as renewable resources cannot withstand the H2 demand and consumption in a prospective scenario. However, a recent study by Butburee et al.34 attempted to demonstrate that the scaling up of a complete chain of soft energy, notably through mimicking photosynthesis, is a promising option for biorefinery, aiming at producing H2 as well as biofuels or biofuel precursors. Such photosynthetic alternatives require biomass (primary matter), the sun (renewable energy igniting the (electro)chemical reactions), and a photocatalyst, as illustrated in Fig. 5.34 Photocatalysts and their implementation in real production systems are a long row. If many photocatalyst materials are available, their implementation and stability in a prototype system must be tested. Thus, an efficient photocatalyst is mandatory, but not only. The whole design from the resources (Biomass notably) should be accurately proposed to ensure charge separation efficiency, electron and hole conductivity, (electrocatalysts) stability, yield, and cost-effectiveness, and if any, encompass ecological aspects.
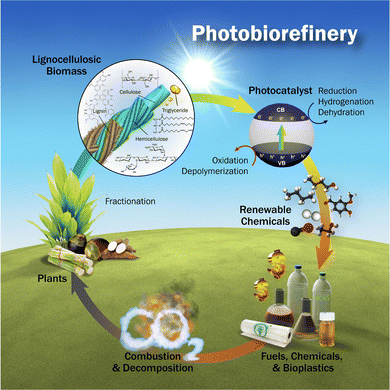 | ||
| Fig. 5 The concept of biorefinery proposed by Butburee et al.34 | ||
These perspectives for H2 production could appear out of goal regarding the energy demand complex to implement, but there is some good news to highlight here for Brazil. Indeed, Brazil's energy matrix comprises 48% renewables, with hydropower and wind energy being pivotal contributors, making its energy infrastructure among the least carbon-intensive globally.35 The onshore wind sector, with a capacity of 21.5 GW, is the fastest-growing energy generation industry. In 2021, Brazil secured a significant position in wind power installations, surpassed only by China and the USA. On the other hand, the discovery of substantial seaward oil and gas (O&G) reserves in Brazil, particularly within pre-salt, deep-water basins, has solidified its status as a leading O&G province globally. Despite limited natural gas export infrastructure, Brazil strategically aims to become a prominent offshore gas producer, comparable to the USA and Qatar, especially given the sanctions on Russian natural gas. Brazil is actively decarbonizing its energy sector to sustain O&G production, which is necessary for meeting domestic and international demands while adhering to net-zero objectives. Achieving such an aim involves deploying green technologies and capitalizing on extensive renewable energy resources. Although Brazil possesses untapped offshore wind potential, no offshore wind farms have been established. In January 2022, the Brazilian Government issued a decree regulating territorial waters for power generation, envisioning that floating offshore wind power could substantially reduce the O&G industry's carbon footprint, mainly if turbines are used to supply power to production and exploration units. Brazil boasts a substantial presence in renewables, contributing to nearly 85% of the nation's electricity supply, and has outlined plans for further expansion. Presently, the onshore wind capacity stands at 21 GW, with an anticipated increase to 33 GW by 2026, as indicated by the existing pipeline. An exceptional surge is expected, reaching between 110 and 194 GW by 2050.36 Despite the absence of operational offshore wind farms, Brazil possesses considerable offshore wind resources and actively develops offshore capabilities. Currently, 66 offshore wind farm projects are under environmental license analysis, encompassing 11,571 wind turbines and presenting a potential power output of 169 GW,37 surpassing the nation's initial offshore wind forecast of 16 GW by 2050.36
Furthermore, Brazil is actively advancing its hydrogen economy, leveraging natural resources for green electricity generation. A national hydrogen strategy is under development, intending to position Brazil as a significant hydrogen exporter, as shown in Fig. 6. At COP26, Brazil announced revised emissions reduction goals, targeting a 50% reduction by 2030 (using the 2005 baseline year) and striving for net-zero emissions by 2050.36 The efforts concentrate on several initiatives for experimental and large-scale industrial production of green hydrogen, which involves a process with zero or nearly zero CO2 emissions.
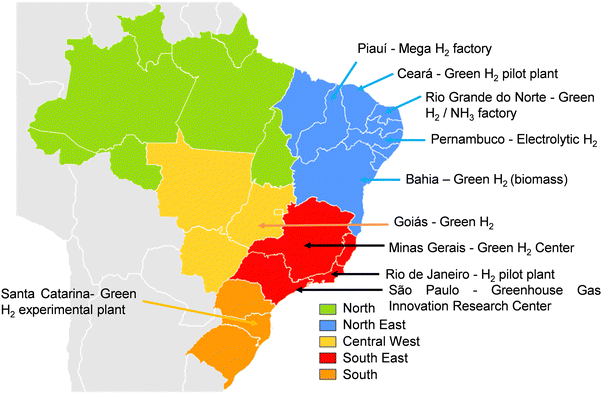
In the central-west region of Brazil, the pilot project for green hydrogen by Furnas Company at Itumbiara's hydroelectric plant in Goiás State was established two years ago, and it will produce 1.5 tons of green hydrogen in 2023. In the northeastern region, in the state of Rio Grande do Norte, there is a project in the licensing phase for the construction of a green hydrogen and ammonia factory in Macau. In Suape, Pernambuco, the company White Martins began in the year 2022, the production of electrolytic hydrogen using solar energy at the city's Industrial Complex. The effort consists of the first certified green hydrogen in the country and South America, and it can produce 156 tons of gas per year. In Bahia, the Unigel Fertilizer factory is set to inaugurate a plant in the Camaçari industrial complex in 2024, with the capacity to produce 10 thousand tons of gas, potentially quadrupling in two years. In the Pacém Thermal Power Complex in Ceará, EDP Brasil produces green hydrogen in a pilot plant, producing 197 tons of solar energy annually. In the southeast region of Brazil, in Minas Gerais, the Federal University of Itajubá inaugurated the Green Hydrogen Center in September 2023, intending to study fuel use in electricity generation, industry, and electric mobility.
In Rio de Janeiro state, a pilot plant with a production capacity of 3 tons per year has been operating at the Federal University of Rio de Janeiro in partnership with the German organization GIZ. In the state of São Paulo, there is a project at the Greenhouse Gas Innovation Research Center for hydrogen production from byproducts of the sugarcane industry. In the country's southern region, there is the Itaipu Technological Park production plant, which has been using wind and hydraulic energy since 2014 to generate green gas. In Santa Catarina, there have been investments from the German Government to build an experimental green hydrogen production plant at the Federal University of Santa Catarina.
There are also other robust projects in the course. A pilot plant will be inaugurated in 2025 in Açu Harbour, north of Rio de Janeiro state, with Shell as the leading partner. It will poise to engage in water electrolysis and use electricity from the national grid to facilitate the production of green hydrogen. Initially designed with a capacity of 10 MW, there are plans for potential expansion to 100 MW. A segment of the produced hydrogen will be allocated for storage and subsequent shipment to potential consumers, while the remaining portion will be directed to a renewable ammonia generation plant. This pilot initiative will catalyze the comprehensive development of the renewable hydrogen generation value chain, from technology suppliers and plant operation expertise to training specialized labor. Signifying the initial strides toward establishing a green hydrogen economy in Brazil, this endeavor holds the potential to shape the future trajectory of sustainable energy production.
In the northeastern region of Brazil, specifically in Paraíba on the coast of the state of Piauí, a mega-factory for hydrogen production is slated for installation. The plant is designed with a formidable capacity of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 megawatts (nearly equivalent to the Three Gorges Dam in China, boasting 22
000 megawatts (nearly equivalent to the Three Gorges Dam in China, boasting 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 megawatts). Spearheaded by the European companies Green Energy Park and Solatio, the venture aims to supply industries in southeastern Europe. These undertakings assume critical importance in Brazil's transition to green energy and have the potential to position the country as one of the foremost global producers of green hydrogen.
500 megawatts). Spearheaded by the European companies Green Energy Park and Solatio, the venture aims to supply industries in southeastern Europe. These undertakings assume critical importance in Brazil's transition to green energy and have the potential to position the country as one of the foremost global producers of green hydrogen.
Finally, is Brazil a reduced-scale laboratory for a total energy transition demonstration with exclusive H2 production and consumption? Maybe yes, since a complete energy-material chain is available. Let us implement biofuels/H2 production through further research and a good design for large-scale photocatalysis. In this view, the last paragraph discusses the idea of using the Langmuir–Blodgett approach for photoelectrode design with BiVO4 nanoparticles as an example in this view.
3. Langmuir–Blodgett approach to scale up photoelectrode: case of BiVO4
In order to demonstrate the potential of the Langmuir Blodgett (LB) technique to design photoelectrodes, we chose to focus on the BiVO4 photocatalyst as a meaningful case study. In Fig. 7, we imagine a type of identity card for this material with the main properties and characteristics observed until today and based on the literature data. BiVO4 is found in nature in the pucherite mineral, but the photoactive materials are synthesized mainly in the laboratory and lead to two main structures: Zircon-type and scheelite.15 The scheelite structure is the most photoactive because a band gap of 2.4 eV absorbs an essential part of visible light, and the literature demonstrates a close link between this property and the crystal structure, as explained by Park et al.15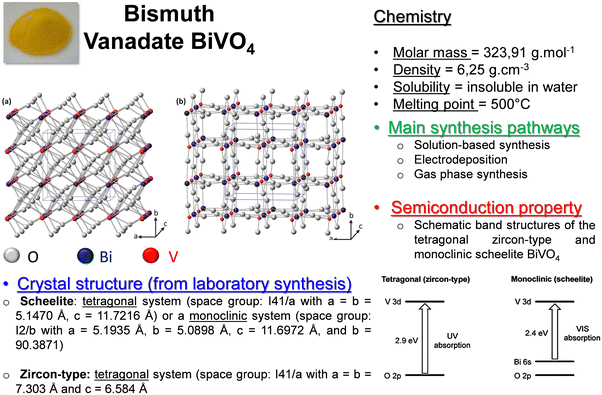 | ||
| Fig. 7 The identity of the BiVO4 compound with its main physico-chemical characteristics and properties, crystal structures, and band gap diagram is from Park et al.15 | ||
It is expected to boost semiconducting photoactivity with other compounds such as tungsten (WO3),19,38 thin SnO239 graphene oxide (GO),40 Bi4V2O1141–43 or V2O5.44–47 Multiple objectives include covering a wider absorption band, ensuring charge separation efficiency, and transport processes. According to a study by dos Santos et al.,43 the combination of BiVO4 with ferroelectric Bi4V2O11 can create virtual p–n junctions in the bulk film, resulting in photoelectrodes with spontaneous photovoltages as high as 1.39 V. This enhancement is sufficient to improve the separation of photogenerated charges in the bulk of the semiconductor film, and consequently, the photocurrent. On the other hand, Oliveira et al.44 demonstrated that V2O5 nanorods could increase the light absorption and hole transport in BiVO4 films, achieving photoelectrodes with a water oxidation photocurrent as high as 6.6 mA cm−2 at 1.23 V vs. RHE This value is very close to the maximum theoretically expected photocurrent for BiVO4 films, which is 7.5 mA cm−2 at 1 sun illumination. Despite the progress made in developing BiVO4-based photoelectrodes, their stability remains a critical issue that needs to be addressed. Some studies have suggested that incorporating electrocatalysts, such as CoPi48,49 and FeNiOx,50,51 on the surface of BiVO4 can enhance both the kinetics of oxygen evolution from water and the stability of the electrode by reducing corrosion. However, fabricating stable BiVO4 photoelectrodes in aqueous environments remains an open question. Recently, Liu et al.52 reported the use of photo-assisted electrodeposition to deposit vanadium oxide (VOx) enriched with oxygen vacancies on the surface of BiVO4 films, resulting in a photocurrent of 6.29 mA cm−2 and a photocurrent retention rate of approximately 88% after 40 h.
Recent recommendations by Melchionna and Fornasiero53 indicate critical perspectives to qualify and compare photocatalysts. To quote a few: a rigorous characterization dataset with evidence of negligible impurities impeding the excellent process of photocatalysis, a comparison of catalysts in terms of quantum yield (QY) instead of turnover frequency (TOF), or reporting both the catalyst activity in terms of mass and surface area per unit. Among the reviews and studies encountered in the specific case of BiVO4,15–21 we observed an excellent characterization procedure with deep scrutiny of the catalyst surface. The latter reveals the encouraging performance of the BiVO4 particles (scheelite) designed for a given photoelectrode, with a systematic photocurrent density between 3 and 6 mA cm−2, low resistance (below 10 Ω), and high purity of the designed material. Long-term studies demonstrating prototype stability and scale-up potential fail to observe any performance degradation regarding the benchtop. Even if the recent study by Kunturu et al.22 is an excellent example of scaling up BiVO4 photoelectrodes, as we have commented above, more work should be implemented to provide a statistical and robust opinion for the potential to industrialize BiVO4 photoelectrodes, following, if possible, the good practices proposed with the data reporting,53 particularly in the case of catalyst comparison.
As supplementary proof of relevance, today's dense literature demonstrates the potential of BiVO4 photoelectrodes for biomass valorization in biofuels and hydrogen. After some interesting proofs of concept, we comment here on the relevance of using and shaping photoelectrodes with the BiVO4 materials for such applications.
Almost ten years ago, Gil and Choi54 elaborated a photoelectrochemical cell (PEC) with a photoanode composed of BiVO4 to oxidize the 5-hydroxymethylfurfural (HMF) into 2,5-furandicarboxylic acid (FDCA). The HMF is frequently encountered in biomass matter (a derivative of C6 monosaccharides) and is used as a reference compound for the study. The cathode of their PEC is a platinum electrode where H2 production is observed. The authors revealed an FDCA yield close to 100%, making the BiVO4 a promising material for integrating a photoelectrode structure to convert biomass and produce H2 through this process instead of conventional water splitting.
More recently, Wang et al.55 proposed an exciting work on the conversion of tartaric acid, a very common low-cost biomass, as a reference compound through a PEC made of BiVO4 photoanodes (two kinds of photoanodes were tested: BiVO4 nanoparticles and nanoporous BiVO4). The authors aimed at promoting H2 production with this PEC instead of boosting biomass conversion. Their work demonstrated an excellent faradaic efficiency of close to 100% in H2 production. The authors documented the BiVO4 nanoparticle and nanoporous BiVO4 characterization and made a dense effort to explain the origin of such efficiency. As Gil and Choi,54 a part of the mechanism relies on the strong complexation between the BiVO4 nanoparticle surface and the organic matter, exacerbated when the surface area increases, which was observed with the nanoporous BiVO4 photoanode. The authors showed the importance of having a nanostructure photoanode and a nanoBiVO4 to perform well in biomass conversion and H2 production.
In the same trend, Han et al.56 used a surface modified BiVO4 photoanode with NiFe phenolic networks in a PEC to convert glycerol into formic acid. The authors focused on improving the selectivity of the photoelectrode to boost the H2 production efficiency. Even if the shaping of such electrode is quite complex (multi-step material synthesis before deposition on FTO substrates), the study managed to obtain a 94% faradaic efficiency and a formic acid production with 85% of selectivity, representing 573 mmol m−2 h−1 at the electrode interface.
Finally, a recent guide for photoelectrode development was proposed by Harris Lee et al.,57 which shows the pros and cons of using BiVO4 as a photoelectrode. Among the advantages of BiVO4 are its high stability, non-toxic nature, and bandgap in the visible range, which consists of crucial properties that can be integrated into a photoelectrochemical system. The BiVO4, however, must be doped to overcome the poor charge carrier mobility. The photocurrent stability is also a challenge for this material, but the articles above mentioned and the literature we have already commented on demonstrate the possibility of using protective layers in this view.
Thus, BiVO4 is justified as an example of scaling up efficient and stable photoelectrodes using the Langmuir–Blodgett technique.
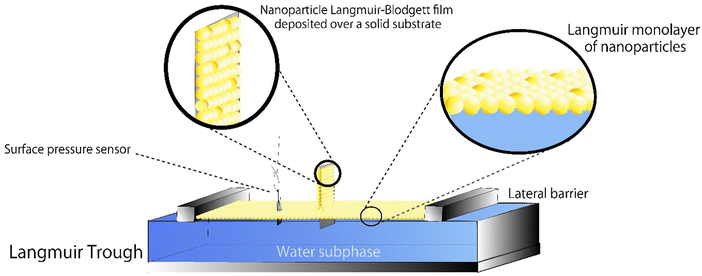
The LB method is one of the oldest deposition techniques for producing thin films. Since the beginning of the XIX century, devices and techniques have constantly evolved to produce high-quality thin films for numerous applications, including surface-interface systems such as solar cells, drug delivery, sensors and biosensors, tissue engineering, electronic devices, batteries, and fuel cell electrodes. However, the best applications encountered today for LB are the structuration of biosensors and, generally speaking, all chemistry closely linked with biology, particularly cell science. Ariga58 and Oliveira et al.59 accurately explained the principle and reviewed remarkable studies in the field. Fig. 8 depicts a typical LB experiment in which a Langmuir monolayer (insoluble substance adsorbed at air–water interface) of nanoparticles is submitted to a transference to a solid substrate.
Fatty acids and phospholipids were the first substances used as Langmuir monolayers. However, there are numerous possibilities, such as surface-modified nanoparticles, which make them amphiphilic systems for deposition. After several years of development, the LB is better adapted to be scaled up with biological or bio-inspired monolayers to design related applications (biosensors, tissue engineering, etc.), as dense reviews explain.58,59 Theoretically, the LB method can be used with any system concerning the air–liquid activity nature of the inorganic or organic substance. The significant obstacles to monolayer development in biological applications are the mechanical resistance of the layer and the homogeneity of the films obtained with inorganic species.
Notwithstanding, we note a new tentative of less common monolayer to produce LB films composed of inorganic–organic moieties, such as black phosphorus nanosheets (BPNS), metal–organic frameworks (MOFs), covalent organic frameworks (COFs), metallic nanoparticles, ZnO nanowires, and carbon-based materials (fullerenes, graphene, and nanotubes). These new monolayers open a new spectrum of energy applications for the design of accurate layers of catalysts for photocatalytic applications. Regarding our subject study with BiVO4 nanoparticles designed in a photoelectrode using the Langmuir–Blodgett approach, we noted two studies by Zhang et al.60,61
In the first work,60 the authors elaborated on a photoanode including a (040)-facet-oriented mono-grain layer of BiVO4 by combining the LB method and a particle transfer technique. They also explored a basic deposition technique for comparison with the LB technique and tested the influence of an electron transfer layer (Au nanoparticles). The process used is illustrated in Fig. 9. Finally, they studied three types of thin films to highlight the best system, promoting attractive charge separation efficiency for further applications. The main results are as follows:
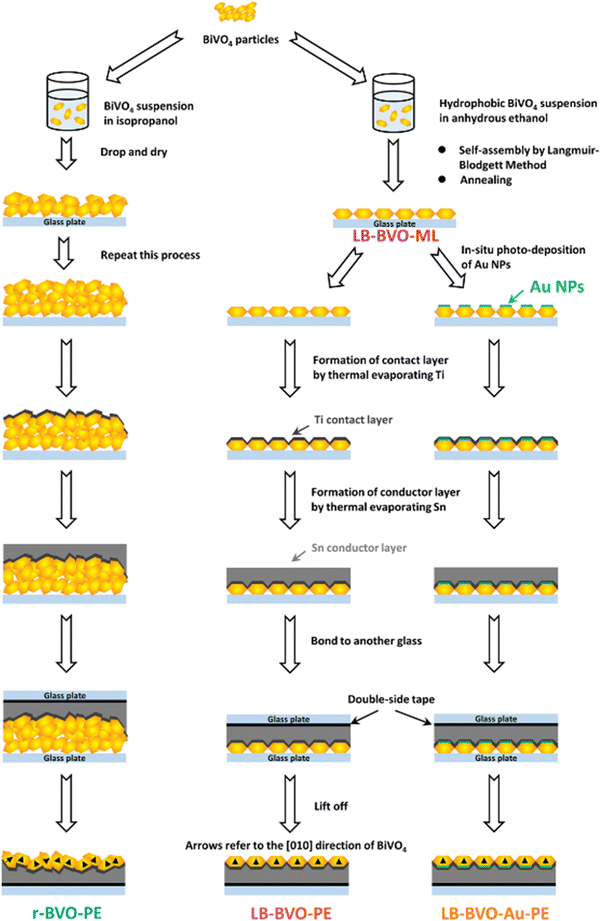 | ||
| Fig. 9 Schematic diagram of the particle transfer (PT) method for preparation of r-BVO-PE, LB-BVO-PE, and LB-BVO-Au-PE.60 | ||
• The SEM results show that the best homogeneity for photoelectrode surface is obtained when the LB method is used,
• Significant increase of the photocurrent at 1.23 V vs. RHE with the LB methods: +60% increase regarding the raw material,
• Spectrum absorbance is between the UV and 520 nm, which is an expected result. The quality of the material synthesized is well-proved,
• Low resistance from PEIS, these results demonstrate the impact of Au (see Fig. 10 from the article),
• Unsuccessful co-catalysis using the Co layer included in the LB-shaped materials.
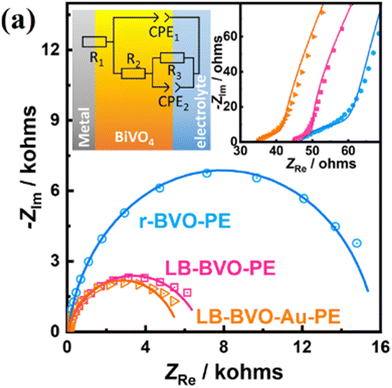 | ||
| Fig. 10 PEIS spectra of r-BVO-PE, LB-BVO-PE, and LB-BVO-Au-PE measured at 0.6 V vs. RHE and under AM 1.5G irradiation (with a 420 nm cutoff filter). Fig. 4(a) from the article.60 | ||
However, no long-term material stability has been studied here, and reproducibility has not been demonstrated, suggesting that one-shot good results could have been obtained. Moreover, the authors supported the preferential orientation of BiVO4 according to the (040) facet scheelite. However, it would have been great to determine which facet was the most active for better design of the material in the future to expose only the most active facet. Finally, there are no comments about particle transfer after the LB method, which could have contributed to a better understanding of the processability. The idea of co-catalysis is good, but the choice of material could have been more explored with other moieties, such as Fe/N/C materials, for instance, with the contribution of carbon as a supplementary electron transport layer.62
The same team implemented another work61 as an objective for water-splitting applications. The exciting part of this paper is to see how the LB method can improve the homogeneity of the photoelectrodes and the observed performance. The photoelectrode preparation procedure used is illustrated in Fig. 11 from their article. In the present work, to integrate the merits of PEC (Photoelectrocatalytic) and PC (Photocatalytic) techniques, a p–n conjugated two-electrode water-splitting system was miniaturized into one particle (denoted as an electrode particle) for PC reactions. As a bit of a recall, PEC is the direct electrolysis of water through the light absorption by catalysts, generating electricity and splitting H2O into H2. When the photocatalyst absorbs light with energy equal to or larger than the bandgap, the redox reaction occurs at the catalyst's surface, and we are in PC conditions. Specifically, a p-type Rh-doped strontium titanate (Rh:SrTiO3) photocathode material was selectively deposited on the electron-accumulated facet of a particulate n-type Mo-doped bismuth vanadate (Mo:BiVO4) photoanode by inserting a partially oxidized In@InOx interlayer as a grain binder and charge conductor. Once again, the authors demonstrated the relevance of using the LB approach to elaborate a complex nanostructuration. As shown in Fig. 12, they accumulated several textures of cubic BiVO4, Sr, and In elements. They obtained a homogeneous surface with a good connection between the elements. The authors also found that the thickness of the active layer, annealing temperature, quantity of catalyst used for the electrode, and measurement conditions affect the efficiency of H2 and O2 production. In brief, the LB method can be used to manage the thickness and quantity of the catalyst, but it does not seem to be responsible for other influencing parameters. This work is proof of concept for a highly efficient thin surface for water-splitting applications, but some doubts remain regarding potential scale-up. There are several compounds (Mo, BiVO4, In, InOx, Rh, Sr, TiO3) to manage and organize at the surface, and it is not very clear whether In or even Sr or Mo improves the water splitting efficiency. They also tested the co-catalysis with Co; as previously seen, the results are not encouraging, and the complex system elaborated is even worse than those using Au–BiVO4 presented above.
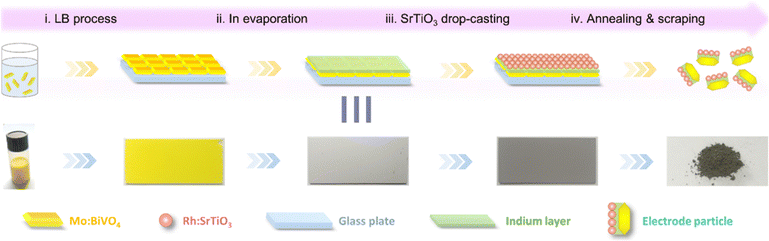 | ||
| Fig. 11 Schematic illustration for the preparation procedures of Mo:BiVO4/In@InOx/Rh:SrTiO3 electrode particles.61 | ||
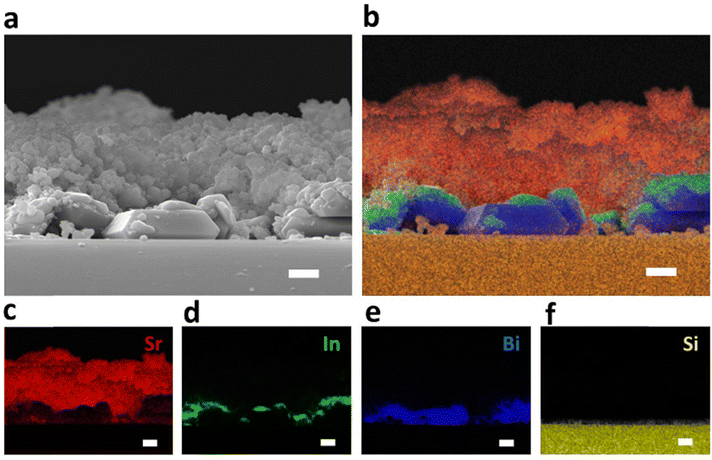 | ||
| Fig. 12 Cross-sectional SEM (a) and (b)–(f) corresponding EDS mapping images of annealed electrode particle before being scraped from the glass plate. Scale bars, 1 μm.61 | ||
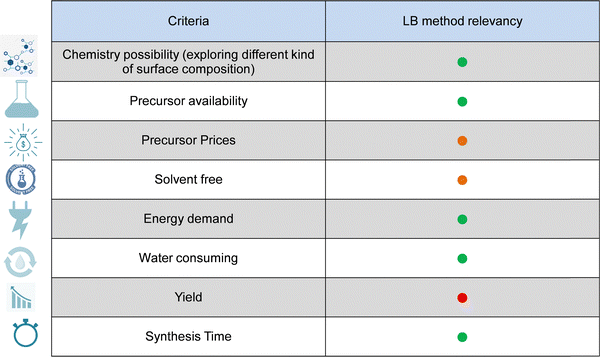
These recent studies have strengthened the LB method to satisfy the surface structuration of very complex materials. However, it should be better to reduce the system to no more than three materials of different natures to manage a surface prepared using an LB approach. Looking closer now, how efficient could a good photoelectrode structuration LB be? We tried to gather elements in Table 2 that could satisfy the criteria for the photoelectrodes. The LB method partially answers the green chemistry requirements with reduced water consumption, low energy demand, and the possibility of using available precursors at reduced cost. The nanochemistry and combinations are large even if some precursors are not readily available or constitute rare elements (e.g., rare earths or precious metals). The LB method is often followed by post-treatment: annealing, particle transfer, chemical treatment, new deposition, and complexification of the whole process, but this is frequently encountered in industry, and there is no exception for this technique.
Recent advances in the Langmuir–Blodgett (LB) film deposition technique enable scale production to surpass conventional laboratory methods, facilitating enhanced film preparation, particularly involving nanomaterials. New approaches are pivotal for positioning LB technology among industrial methods for manufacturing ultra-thin films for commercial and research purposes, especially concerning semiconductors whose catalytic, conversion, and energy storage properties are optimized when manipulated and organized on a nanoscale. The LB method's scalability is neither a dream nor an unreachable perspective since several proofs of concept demonstrated the possibility of depositing organic and inorganic materials on small and large surfaces.63–65
Several years ago, Lu et al.63 had already been able to deposit polystyrene particles of 700 nm on irregular surfaces (metallic earings) through the LB approach and obtained a diversity of colors for these jewels. Back in the day, this work, even limited to perfect regular shaped material (polystyrene spheres), appeared encouraging to develop and spread the LB technique for surface treatment with a variety of nanoparticles (organics, inorganics, composites) for several areas: optics, photonics, anticorrosive applications and so forth.
Ito et al.64 worked on a high-temperature LB machine that produced thin films of polymeric semiconductors on large surfaces. In this work, the authors used an atypical LB machine at high temperatures (above 100 °C), required for such material preparation to entail copolymerization reactions. The newly designed LB setup comprises a working area of 100 × 50 mm2, letting deposition on various sized and shaped supports of centimetric dimensions. The LB trough for this novel machine is W132 × D82 × H15 mm. Such a system showed the possibility of synthesizing and shaping a semiconductor item with adequate dimensions in a single step, which is ready to be characterized and tested in several applications. The typical support is an EAGLE XG glass with dimensions between 0.5 mm and 10.5 mm thick.
Recently, Cossi et al.65 designed an improved concept for auto-assembled nano and microparticles on 10 cm diameter silicon wafers. Even if they did not use the LB for the deposition, the authors prepared several dispersions of core–shell SiO2@TiO2@PS (polystyrene) particles (300 to 750 nm size). They used an electron spray device to spread them onto an air–water interface before transferring them to the silicon wafers. This work combining aerosol and surface-interface science is highly inspired by LB for deposition, maximizing the possibility of surface interaction to lead to well-organized monolayers on subphase before transferring on support. In Fig. 13, we can observe the perfectly organized monolayer from their work. If we turn over the work of Cossi et al. inspired by the LB, the LB could also be inspired by aerosol science using the electrospray technique.
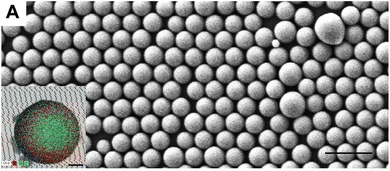 | ||
| Fig. 13 Scanning electron microscope (SEM) image of self-assembled core–shell SiO2@TiO2@PS nanoparticles (NPs) after deposition on the silicon wafer (down left frame). Scale bar 1.5 μm (figure from Cossi et al.65 work). | ||
One notable advancement is the Langmuir monolayer preparation method proposed by the Huang group,66 using the electrospray technique to disperse nanomaterials on the water surface for subsequent transfer to a solid substrate via the LB technique. Compared to the traditional approach, this method overcomes several limitations, where a syringe is manually manipulated to form droplets at the needle's tip, subsequently touching the subphase surface. On the other hand, nanomaterials, especially metal oxide semiconductors, struggle to maintain the interface for monolayer formation when subjected to manual dispersion spreading. One reason for that is the microliter volume of the droplet formed at the syringe tip, causing the liquid to drag the nanomaterials to the bottom of the Langmuir trough, preventing material loss upon contacting the water surface. Furthermore, nanomaterials often lack the inherently amphiphilic nature required for spontaneous maintenance at the air–liquid interface.
The electrospray spreading method for monolayer formation at such an interface simultaneously addresses these issues, allowing for the replacement of chloroform with other polar solvents, including water. Even liquids with high surface tension experience reduced droplet size under a high-magnitude electric field (∼10 kV). Nanoscale drops can be formed, enabling nanomaterial to rest at the interface and minimizing insertion into the liquid bulk. This approach also offers alternatives for toxic organic solvents like chloroform, enhances reproducibility, allows for automation, and enables large-scale LB film production. It may also eliminate the need to functionalize nanomaterials with hydrophobic ligands, which is often necessary to obtain a stable monolayer at the water surface. Huang's methodology can also be combined with roll-to-roll assembly techniques for large-scale colloid film production,67,68 utilizing equipment such as the MicroTM LT211 model69 and the Biolin Scientific KN 2008 KSV NIMA,70 which can be scaled to meet large-scale production demands for LB films of catalytic nanomaterials.
To summarize the scalability perspectives for LB, general data are given in Table 3. For the specific applications in photoelectrocatalysis as well as photovoltaics, the dimensions of the support aforementioned for the LB are really typical of the semi or fully-industrial scale as recently seen for the atomic layer deposition technique dealing with photelectrodes of large area (256 cm2) and silicon wafer (166 cm2).71 Dilger et al.72 also tried to design 40 cm2 of LaTiO2N photoanodes for water splitting by electrophoretic process, followed by a dip coating and thermal treatment. The volume used for all these depositions by LB or other approaches remains under 100 mL and, most of the time, is in the μL or mL range. A challenging piece of information is the cost of the whole operation since this latter is never mentioned in the different works encountered. However, the reference silicon wafer has a cost between 300–350 US$ (for 200 mm of diameter), and the chemical products being involved are generally at reasonable prices (<500 $ except for rare earth precursors or precious metals). Then, in a prospective scenario, the cost for one LB photoelectrode fabrication could be under 1000 US$ without including the acquisition of the LB setup, the post-processing after deposition, and the tiny volumes of nanoparticles dispersions involved. This value is obviously inaccurate, but it has the merits of giving a primary idea about the processability of the LB and the investments involved. Finally, this value does not sound as expensive as other deposition approaches with the same objectives.
| Surface area of the substrates | Volume of dispersion (mL) | Cost | Work |
|---|---|---|---|
| 100 cm2 | 1 | Not indicated | 63 |
| <5 cm2 | 1 | Not indicated | 64 |
| <10 cm2 | <1 | Not indicated | 65 |
| 256–276 cm2 | <100 | Not indicated | 71 |
| 1 to 40 cm2 | <100 | Not indicated | 72 |
4. Conclusion and outlooks
The Langmuir–Blodgett method demonstrates significant promise for the mass production of photoelectrodes, which are essential for the green hydrogen and liquid biofuel economy. This technique allows the deposition of single- or multilayered films onto electrodes, which enables the precise control of material properties critical for efficient photoelectrochemical processes. The Langmuir–Blodgett technique can significantly enhance the overall performance of photoelectrochemical devices by optimizing aspects such as light absorption, charge carrier transport, and catalytic activity.In order to fabricate efficient nanomaterial photoelectrodes using the LB technique, various strategic considerations should be considered. First, optimizing the monolayer formation by controlling the surface pressure, subphase composition, and temperature is crucial for ensuring a uniform and well-organized monolayer at the air–water interface. Secondly, precise layer-by-layer deposition of LB films is necessary, which can be accomplished by managing the lifting speed and optimizing the transfer conditions. Third, the selection and engineering of materials play a vital role in achieving optimal photoelectrochemical properties, which require careful choice of semiconductors, sensitizers, and catalysts. Fourth, surface functionalization and the introduction of catalytic sites can improve charge separation and reduce recombination losses. Finally, incorporating advanced nanomaterials into LB films is a fifth strategy to enhance photoconversion efficiency significantly. Additionally, conducting in situ characterization and monitoring during LB film deposition is essential for understanding film formation and enabling optimal-quality adjustments by utilizing surface pressure-area isotherms and spectroscopy techniques.
It is essential to prioritize the reproducibility of the LB film fabrication methods to ensure the scalability of optimized LB films for large-scale manufacturing. By precisely controlling the properties of LB films, it is possible to customize the material characteristics and enhance the adaptability of photoelectrodes to specific solar-driven fuel production processes and overall device performance. Incorporating advanced nanomaterials into LB films further drives innovation, potentially significantly increasing solar-driven fuel production efficiency. Ultimately, using efficient LB films in photoelectrochemical devices facilitates the transition to clean energy by enabling the production of liquid biofuels and green hydrogen, reducing the dependence on fossil fuels, and mitigating environmental impacts.
The primary objective in the future is to expand the Langmuir–Blodgett process for substantial energy generation, which demands elaborated strategies to enhance the stability and endurance of photoelectrodes, particularly under prolonged solar exposure and harsh environments. Moreover, investigating new materials with enhanced photoconversion efficiencies and long-term stability is crucial for this technology's successful and sustainable implementation. Incorporating advanced nanomaterials, such as quantum dots, single atoms, or nanostructured electrocatalysts, into Langmuir–Blodgett films opens new avenues for boosting the efficiency of solar-driven fuel production. Collaboration among material scientists, electrochemists, and engineers is critical for overcoming obstacles and converting laboratory breakthroughs into practical applications.
Finally, the development of LB films holds significant potential for promoting the utilization of renewable and clean energy sources, moving us closer to a sustainable future powered by solar-driven hydrogen or biofuels. Brazil is well placed to enhance its standing in clean energy production and decrease its dependence on fossil fuels. By transitioning towards green hydrogen and sustainable biofuels, Brazil aligns with global environmental objectives and positions itself as a pioneer in innovative and environmentally responsible energy solutions.
Author contributions
Claire Dazon: conceptualization, investigation, writing – original draft. Márcio César Pereira: funding acquisition, review writing, editing, and validation. Douglas Santos Monteiro: supervision, funding acquisition, review writing, editing, and validation.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
FAPEMIG: grant numbers REDE-113/10; RED00520-16; CEX-112-10; CEX-RED-00010-14; PPM-00104-17, APQ-03149-17. CNPq: grant number 307746/2021-6; INCT MIDAS. Finep/MCTI: Finep 01.22.0271.00. The authors are immensely grateful to CNPq, FAPEMIG, and FINEP/MCTI for their financial support and research grants.References
- IPCC (Intergovernmental Panel on Climate Change), 2021, Climate change, The physical science basis. Working Group I contribution to the IPCC Sixth Assessment Report, Cambridge University Press, Cambridge, United Kingdom.
- M. C. Sarofim, S. Saha, M. D. Hawkins, D. M. Mills, J. Hess, R. Horton, P. Kinney, J. Schwartz and A. St. Juliana, Temperature-related death and illness, The impacts of climate change on human health in the United States: A scientific assessment, US Global Change Research Program, 2016, ch. 2 Search PubMed.
- NOAA (National Oceanic and Atmospheric Administration), 2021, US Climate Extremes Index, Accessed March 2021.
- J. Scott, Grid Edge: Artificial Intelligence for Energy Systems, Presentation delivered at International Energy Agency Workshop on Modernising Energy Efficiency through Digitalisation, Paris, 2019 Search PubMed.
- S. Khan, M. Naushad, J. Iqbal, C. Bathula and A. H. Al-Muhtaseb, Challenges and perspectives on innovative technologies for biofuel production and sustainable environmental management, Fuel, 2022, 325, 124845 CrossRef CAS.
- C. Chantre, S. A. Eliziário, F. Pradelle, A. C. Católico, A. M. Branquinho Das Dores, E. T. Serra, R. C. Tucunduva, V. B. P. Cantarino and S. L. Braga, Hydrogen economy development in Brazil: An analysis of stakeholders' perception, Sustainable Prod. Consum., 2022, 34, 26–41 CrossRef.
- S. F. Macedo and D. Peyerl, Prospects and economic feasibility analysis of wind and solar photovoltaic hybrid systems for hydrogen production and storage: A case study of the Brazilian electric power sector, Int. J. Hydrogen Energy, 2022, 47(19), 10460–10473 CrossRef CAS.
- F. Carvalho, F. T. F. da Silva, A. Szklo and J. Portugal-Pereira, Potential for biojet production from different biomass feedstocks and consolidated technological routes: a georeferencing and spatial analysis in Brazil, Biofuels, Bioprod. Biorefin., 2019, 13, 1454–1475 CrossRef CAS.
- N. Khan, K. Sudhakar and R. Mamat, Role of Biofuels in Energy Transition, Green Economy and Carbon Neutrality, Sustainability, 2021, 13, 12374 CrossRef CAS.
- https://www.ifpenergiesnouvelles.com/article/biofuels-dashboard-2022, accessed October 2023.
- J. Incer-Valverde, A. Korayem, G. Tsatsaronis and T. Morosuk, “Colors” of hydrogen: Definitions and carbon intensity, Energy Convers. Manage., 2023, 291, 117294 CrossRef CAS.
- L. F. M. Rosa, K. Röhring and F. Harnisch, Electrolysis of medium chain carboxylic acids to aviation fuel at technical scale, Fuel, 2024, 356, 129590 CrossRef CAS.
- M. Z. Rahman, F. Raziq, H. Zhang and J. Gascon, Key Strategies for Enhancing H2 Production in Transition Metal Oxide Based Photocatalysts, Angew. Chem., Int. Ed., 2023, e202305385 CAS.
- A. Krishnan, A. Swarnalal, D. Das, M. Krishnan, V. S. Saji and S. M. A. Shibli, A review on transition metal oxides based photocatalysts for degradation of synthetic organic pollutants, J. Environ. Sci., 2024, 139, 389–417 CrossRef PubMed.
- Y. Park, K. J. McDonald and K.-S. Choi, Progress in bismuth vanadate photoanodes for use in solar water oxidation, Chem. Soc. Rev., 2013, 42(6), 2321–2337 RSC.
- Y. Qiu, et al., Efficient solar-driven water splitting by nanocone BiVO4-perovskite tandem cells, Sci. Adv., 2016, 2, e1501764 CrossRef PubMed.
- I. D. Sharp, J. K. Cooper, F. M. Toma and R. Buonsanti, Bismuth Vanadate as a Platform for Accelerating Discovery and Development of Complex Transition-Metal Oxide Photoanodes, ACS Energy Lett., 2017, 2(1), 139–150 CrossRef CAS.
- N. Ghani, J. Iqbal, S. Sadaf, H. N. Bhatti and M. Asgheret, Comparison of Photo-Esterification Capability of Bismuth Vanadate with Reduced Graphene Oxide Bismuth Vanadate (RGO/BiVO4) Composite for Biodiesel Production from High Free Fatty Acid Containing Non-Edible Oil, ChemistrySelect, 2020, 5, 9245–9253 CrossRef CAS.
- T. S. Andrade, B. A. C. Sá, A. T. Oliveira, C. G. O. Bruziquesi, P. E. A. Salomão, M. Rodriguez, F. G. E. Nogueira, L. C. A. de Oliveira and M. C. Pereira, W:BiVO4-WO3-V2O5 heterostructures increase light absorption and charge transport in photoanodes for water splitting, J. Environ. Chem. Eng., 2022, 10(2), 107278 CrossRef CAS.
- C. Li, W. Fan, S. Chen and F. Zhang, Effective Charge Carrier Utilization of BiVO4 for Solar Overall Water Splitting, Chem. – Eur. J., 2022, 28, e202201812 CrossRef CAS PubMed.
- Z. Wang, et al., Boosting H2 Production from a BiVO4 Photoelectrochemical Biomass Fuel Cell by the Construction of a Bridge for Charge and Energy Transfer, Adv. Mater., 2022, 34, 2201594 CrossRef CAS PubMed.
- P. Patil Kunturu, M. Lavorenti, S. Bera, H. Johnson, S. Kinge, M. C. van de Sanden and M. N. Tsampas, Scaling up BiVO4 Photoanodes on Ti Porous Transport Layers for Solar Hydrogen Production, ChemSusChem, 2023, e202300969 Search PubMed.
- B. Lovins, Soft Energy Paths: Towards a Durable Peace, Harper & Row, New York, 1979 Search PubMed.
- https://www.climatehubs.usda.gov/hubs/northwest/topic/biofuel-production, accessed in October 2023.
- R. S. Capaz, E. M. de Medeiros, D. G. Falco and J. E. A. Seabra, Environmental trade-offs of renewable jet fuels in Brazil: Beyond the carbon footprint, Sci. Total Environ., 2020, 714, 136696 CrossRef CAS PubMed.
- I. A. Cruz, L. R. S. Andrade, R. N. Bharagava, A. K. Nadda, M. Bilal, R. T. Figueiredo and L. F. R. Ferreira, Valorization of cassava residues for biogas production in Brazil based on the circular economy: An updated and comprehensive review, Clean. Eng. Technol., 2021, 4, 100196 CrossRef.
- F. Toldrá-Reig, L. Mora and F. Toldrá, Trends in Biodiesel Production from Animal Fat Waste, Appl. Sci., 2020, 10, 3644 CrossRef.
- F. de Oliveira Gonçalves, R. Firmani Perna, E. Savioli Lopes, L. Plazas Tovar, R. Maciel Filho and M. Savioli Lopes, Strategies to Ensure Fuel Security in Brazil Considering a Forecast of Ethanol Production, Biomass, 2023, 3, 1–17 CrossRef.
- E. P. R. Alves, O. Salcedo-Puerto, J. Nuncira, S. Emebu and C. Mendoza-Martinez, Renewable Energy Potential and CO2 Performance of Main Biomasses Used in Brazil, Energies, 2023, 16, 3959 CrossRef CAS.
- A. dos Santos, et al., Feedstock diversification for biodiesel production in Brazil: Using the Policy Analysis Matrix (PAM) to evaluate the impact of the PNPB and the economic competitiveness of alternative oil seeds, Energy Policy, 2017, 109, 297–309 CrossRef.
- A. Dessalle, J. Quílez-Bermejo, V. Fierro, F. Xu and A. Celzard, Carbon, 2023, 203, 237–260 CrossRef CAS.
- E. B. Cahoon and L. B. Yonghua, Plant unusual fatty acids: learning from the less common, Curr. Opin. Plant Biol., 2020, 55, 66–73 CrossRef CAS PubMed.
- J. C. C. Santana, P. G. Machado, C. A. O. D. Nascimento and C. D. O. Ribeiro, Economic and Environmental Assessment of Hydrogen Production from Brazilian Energy Grid, Energies, 2023, 16, 3769 CrossRef CAS.
- T. Butburee, P. Chakthranont, C. Phawa and K. Faungnawakij, Beyond Artificial Photosynthesis: Prospects on Photobiorefinery, ChemCatChem, 2020, 12, 1873–1890 CrossRef CAS.
- Energy Research Office, ‘BEN Summary Report 2021’, 2021, https://www.epe.gov.br/sites-en/publicacoes-dados-abertos/publicacoes/Paginas/Brazilian-Energy-Balance-2021.aspx, accessed in December 2023.
- https://unfccc.int/sites/default/files/NDC/2022-06, accessed in December 2023.
- https://www.ibama.gov.br/phocadownload/licenciamento/2022-08-11_Usinas_Eolicas_Offshore_Ibama.pdf, accessed December 2023.
- X. Shi, I. Herraiz-Cardona, L. Bertoluzzi, P. Lopez-Varo, J. Bisquert, J. H. Park and S. Gimenez, Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation, Energy Environ. Sci., 2011, 4(5), 1781–1787 RSC.
- S. Byun, B. Kim, S. Jeona and B. Shin, Effects of a SnO2 hole blocking layer in a BiVO4-based photoanode on photoelectrocatalytic water oxidation, J. Mater. Chem. A, 2017, 5(15), 6905–6913 RSC.
- H. Chen, S. Wang, J. Wu, X. Zhang, J. Zhang, M. Lyu, B. Luo, G. Qian and L. Wang, Identifying dual functions of rGO in a BiVO 4/rGO/NiFe-layered double hydroxide photoanode for efficient photoelectrochemical water splitting, J. Mater. Chem. A, 2020, 8(26), 13231–13240 RSC.
- W. S. dos Santos, et al., Bismuth vanadate photoelectrodes with high photovoltage as photoanode and photocathode in photoelectrochemical cells for water splitting, ChemSusChem, 2018, 11(3), 589–597 CrossRef CAS PubMed.
- W. S. dos Santos, et al., Photoelectrochemical water oxidation over fibrous and sponge-like BiVO4/β-Bi4V2O11 photoanodes fabricated by spray pyrolysis, Appl. Catal., B, 2016, 182, 247–256 CrossRef CAS.
- W. S. Dos Santos, et al., A hole inversion layer at the BiVO4/Bi4V2O11 interface produces a high tunable photovoltage for water splitting, Sci. Rep., 2016, 6(1), 31406 CrossRef CAS PubMed.
- A. T. Oliveira, et al., High water oxidation performance of W-doped BiVO4 photoanodes coupled to V2O5 rods as a photoabsorber and hole carrier, Sol. RRL, 2018, 2(8), 1800089 CrossRef.
- A. R. S. Neto, et al., Solid-state magic: How Bi2O3 and V2O5 combine to create a high-performance photoanode for water oxidation, Int. J. Hydrogen Energy, 2024, 51(Part C), 300–314 CrossRef.
- T. S. Andrade, et al., Decreasing the charging voltage of a zinc-air battery using a bifunctional W: BiVO4/V2O5 photoelectrode and sulfite as a sacrificial agent, Mater. Today Commun., 2021, 28, 102546 CrossRef CAS.
- I. C. Sena, et al., Photo-assisted chemical energy conversion into electricity using a sulfite-iron photocatalytic fuel cell, J. Electroanal. Chem., 2021, 881, 114940 CrossRef CAS.
- X. Yao, et al., Scale-Up of BiVO4 Photoanode for Water Splitting in a Photoelectrochemical Cell: Issues and Challenges., Energy Technol., 2018, 6(1), 100–109 CrossRef CAS.
- A. Singh, B. S. De, S. Karmakar and S. Basu, Large-Area WO3/BiVO4-CoPi Photoanode for Efficient Photoelectrochemical Water Splitting: Role of Patterned Metal Microgrid and Electrolyte Flow, ACS Appl. Energy Mater., 2023, 6(9), 4642–4656 CrossRef CAS.
- B. Zhang, et al., Nitrogen-incorporation activates NiFeOx catalysts for efficiently boosting oxygen evolution activity and stability of BiVO4 photoanodes, Nat. Commun., 2021, 12(1), 6969 CrossRef CAS PubMed.
- T. W. Kim and K.-S. Choi, Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting, Science, 2014, 343(6174), 990–994 CrossRef CAS PubMed.
- B. Liu, et al., A BiVO4 photoanode with a VOx layer bearing oxygen vacancies offers improved charge transfer and oxygen evolution kinetics in photoelectrochemical water splitting, Angew. Chem., Int. Ed., 2023, 62(10), e202217346 CrossRef CAS PubMed.
- M. Melchionna and P. Fornasiero, Updates on the Roadmap for Photocatalysis, ACS Catal., 2020, 10, 5493–5501 CrossRef CAS.
- H. Gil Cha and K.-S. Choi, Combined biomass valorization and hydrogen production in a photoelectrochemical cell, Nat. Chem., 2015, 7(4), 328–333 CrossRef PubMed.
- Z. Wang, et al., Boosting H2 Production from a BiVO4 Photoelectrochemical Biomass Fuel Cell by the Construction of a Bridge for Charge and Energy Transfer, Adv. Mater., 2022, 34, 2201594 CrossRef CAS PubMed.
- Y. Han, M. Chang, Z. Zhao, F. Niu, Z. Zhang, Z. Sun, L. Zhang and K. Hu, Selective Valorization of Glycerol to Formic Acid on a BiVO4 Photoanode through NiFe Phenolic Networks, ACS Appl. Mater. Interfaces, 2023, 15, 11678–11690 CrossRef CAS PubMed.
- T. R. Harris-Lee, et al., A chemist’'s guide to photoelectrode development for water splitting – the importance of molecular precursor design, EES Catal., 2023, 1, 832 RSC.
- K. Ariga, Don't Forget Langmuir−Blodgett Films 2020: Interfacial Nanoarchitectonics with Molecules, Materials, and Living Objects, Langmuir, 2020, 36, 7158–7180 CrossRef CAS PubMed.
- O. N. Oliveira, L. Caseli and K. Ariga, The Past and the Future of Langmuir and Langmuir−Blodgett Films, Chem. Rev., 2022, 122, 6459–6513 CrossRef PubMed.
- B. Zhang, et al., Fabrication of a Facet-Oriented BiVO4 Photoanode by Particle Engineering for Promotion of Charge Separation Efficiency, ACS Appl. Energy Mater., 2021, 4, 4259–4268 CrossRef CAS.
- B. Zhang, et al., Facet-Oriented Assembly of Mo:BiVO4 and Rh:SrTiO3 Particles: Integration of p−n Conjugated Photoelectrochemical System in a Particle Applied to Photocatalytic Overall Water Splitting, ACS Catal., 2022, 12, 2415–2425 CrossRef CAS.
- K. Li, C. Sun, Z. Chen, H. Qu, H. Xie and Q. Zhong, Fe-carbon dots enhance the photocatalytic nitrogen fixation activity of TiO2@CN heterojunction, Chem. Eng. J., 2022, 429, 132440 CrossRef CAS.
- G. Cossio, R. Barbosa, B. Korgel and E. T. Yu, Massively Scalable Self-Assembly of Nano and Microparticle Monolayers via Aerosol Assisted Deposition, Adv. Mater., 2024, 36, 2309775 CrossRef CAS PubMed.
- Z.-C. Lu, et al., Fabrication of Large-scale Nanostructure by Langmuir-Blodgett Technique, J. Bionic Eng., 2006, 3(2), 59–62 CrossRef.
- M. Ito, et al., Hyper 100 °C Langmuir−Blodgett (Langmuir−Schaefer) Technique for Organized Ultrathin Film of Polymeric Semiconductors, Langmuir, 2022, 38, 5237–5247 CrossRef CAS PubMed.
- H.-L. Nie, X. Dou, Z. Tang, H. D. Jang and J. Huang, High-Yield Spreading of Water-Miscible Solvents on Water for Langmuir–Blodgett Assembly, J. Am. Chem. Soc., 2015, 137(33), 10683–10688 CrossRef CAS PubMed.
- I. T. Chen, E. Schappell and X. Zhang, et al., Continuous roll-to-roll patterning of three-dimensional periodic nanostructures, Microsyst. Nanoeng., 2020, 6, 22 CrossRef CAS PubMed.
- M. Parchine, J. McGrath, M. Bardosova and M. E. Pemble, Large Area 2D and 3D Colloidal Photonic Crystals Fabricated by a Roll-to-Roll Langmuir–Blodgett Method, Langmuir, 2016, 32(23), 5862–5869 CrossRef CAS PubMed.
- https://microtm.com/lt211/lt211e.htm, accessed in December 2023.
- https://www.biolinscientific.com/ksvnima/fabrication-and-deposition-of-thin-films/langmuir-and-langmuir–blodgett-troughs#troughs .
- X. Wang, X. Wang, G. Zhang, B. Liu, Y. Wang, C. Zhao, C. Pei, H. Deng, W. Han, T. Wang and J. Gong, Scaling-Up of Thin-Film Photoelectrodes for Solar Water Splitting Based on Atomic Layer Deposition, ACS Appl. Mater. Interfaces, 2023, 15(1), 1138–1147 CrossRef CAS PubMed.
- S. Dilger, M. Trottmann and S. Pokrant, Scaling Up Electrodes for Photoelectrochemical Water Splitting: Fabrication Process and Performance of 40 cm2 LaTiO2N Photoanodes, ChemSusChem, 2019, 12, 1931 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |