 Open Access Article
Open Access ArticleQuinoxaline-based Y-type acceptors for organic solar cells
Meiling
Xie
ab,
Zhixiang
Wei
 *ab and
Kun
Lu
*ab and
Kun
Lu
 *ab
*ab
aCAS Key Laboratory of Nanosystem and Hierarchical Fabrication, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing 100190, China
bUniversity of Chinese Academy of Sciences, Beijing 100049, China. E-mail: weizx@nanoctr.cn; lvk@nanoctr.cn
First published on 7th May 2024
Abstract
Minimizing energy loss plays a critical role in the quest for high-performance organic solar cells (OSCs). However, the origin of large energy loss in OCSs is complicated, involving the strong exciton binding energy of organic semiconductors, nonradiative charge-transfer state decay, defective molecular stacking network, and so on. The recently developed quinoxaline (Qx)-based acceptors have attracted extensive interest due to their low reorganization energy, high structural modification possibilities, and distinctive molecular packing modes, which contribute to reduced energy loss and superior charge generation/transport, thus improving the photovoltaic performance of OSCs. This perspective summarizes the design strategies of Qx-based acceptors (including small-molecule, giant dimeric and polymeric acceptors) and the resulting optoelectronic properties and device performance. In addition, the ternary strategy of introducing Qx-based acceptors as the third component to reduce energy loss is briefly discussed. Finally, some perspectives for the further exploration of Qx-based acceptors toward efficient, stable, and industry-compatible OSCs are proposed.
1. Introduction
Organic solar cells (OSCs) represent a promising photovoltaic technology for multi-scene applications due to their various advantages, such as lightweight, flexibility, and semitransparency.1–3 Benefiting from a deeper understanding of material design and device engineering, the state-of-the-art single-junction OCSs have achieved power conversion efficiencies (PCEs) of over 19%.4–7 Specifically, the PCE is determined by three critical parameters, including open-circuit voltage (VOC), short-circuit current density (JSC), and fill factor (FF). However, the inferior VOC of OSCs compared to perovskite and inorganic solar cells, caused by larger energy losses (Eloss), is still a major obstacle to further improvement of device performance.8–10 Therefore, it is necessary to investigate the origin of Eloss and to develop effective OSC systems with low Eloss.According to Shockley–Queisser (SQ) limit theory, the Eloss in photovoltaic cells could be divided into three parts (Fig. 1a): radiative energy loss above the bandgap (ΔE1), radiative energy loss below the bandgap (ΔE2) due to non-step function absorption for practical devices, and nonradiative energy loss (ΔE3).11 In addition, ΔE3 can be calculated from the equation: ΔE3 = −kBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(EQEEL), where kB, T, and EQEEL are the Boltzmann constant, temperature, and external electroluminescence quantum efficiency of the device, respectively.12 Considering the bandgap dependent ΔE1 and the negligible ΔE2 in the emerging non-fullerene acceptor (NFA)-based OSCs, the reduction of Eloss is mainly focused on minimizing ΔE3.13–16 To this end, chemical modifications of acceptor materials have been widely applied to optimize their electronic structures and aggregation behaviors, resulting in effective hybridization of local exciton (LE) and charge-transfer (CT) states, decreased energetic disorder, and increased photoluminescence quantum yield (PLQY).17–20 Nevertheless, numerous studies have shown that a better trade-off between VOC and JSC is essential for high-efficiency OSCs.21–23 Only under the premise of efficient charge generation is the rational pursuit of low Eloss conducive to the further development of OSCs to a new milestone, which requires the precise design of organic semiconductors and fine-tuning of the film morphology.
ln(EQEEL), where kB, T, and EQEEL are the Boltzmann constant, temperature, and external electroluminescence quantum efficiency of the device, respectively.12 Considering the bandgap dependent ΔE1 and the negligible ΔE2 in the emerging non-fullerene acceptor (NFA)-based OSCs, the reduction of Eloss is mainly focused on minimizing ΔE3.13–16 To this end, chemical modifications of acceptor materials have been widely applied to optimize their electronic structures and aggregation behaviors, resulting in effective hybridization of local exciton (LE) and charge-transfer (CT) states, decreased energetic disorder, and increased photoluminescence quantum yield (PLQY).17–20 Nevertheless, numerous studies have shown that a better trade-off between VOC and JSC is essential for high-efficiency OSCs.21–23 Only under the premise of efficient charge generation is the rational pursuit of low Eloss conducive to the further development of OSCs to a new milestone, which requires the precise design of organic semiconductors and fine-tuning of the film morphology.
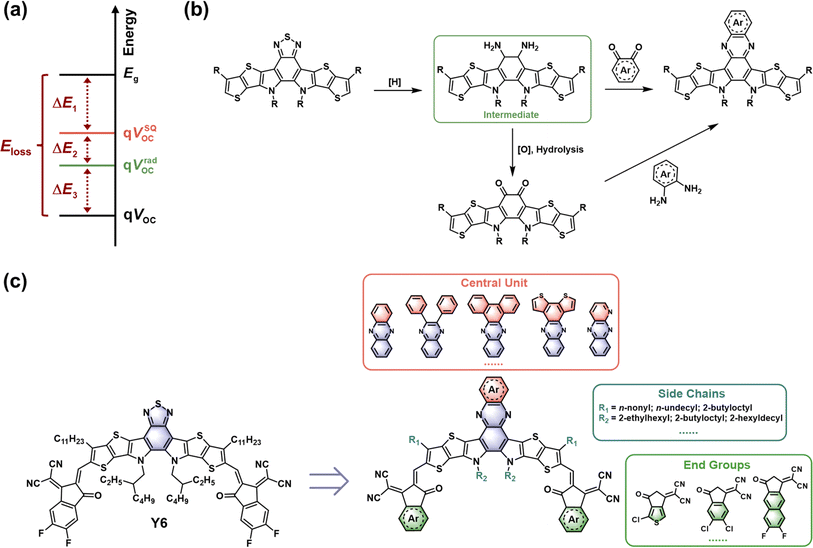 | ||
| Fig. 1 (a) Energy loss diagram of photovoltaic cells. (b) Typical synthetic routes of Qx central units. (c) Schematic diagram of the structural modification of Qx-based acceptors. | ||
As mentioned above, the significant progress in the PCE of OSCs can largely be attributed to the innovation of NFAs, such as ITIC, Y6, and their derivatives.24–30 Among them, the efficient Y-series acceptors show unique molecular packing behaviors to form a three-dimensional (3D) stacking network, which enables effective charge transport/collection and low Eloss in relevant OSCs.31,32 Such a distinctive molecular packing is attributed not only to terminal group interactions, but also to the interaction between the central units, highlighting the critical role of central-unit modification in the construction of efficient NFAs. Significantly, the recently developed quinoxaline (Qx)-based acceptors with multiple substitution sites on the central unit have attracted considerable interest because of their reduced energy losses, abundant structural modification possibilities, diverse molecular packing modes, and favorable film morphology, showing great potential for achieving a better balance among VOC, JSC, and FF.33–35 In this perspective, we summarize the molecular design strategies and progress of Qx-based acceptors for OSCs, including small-molecule, giant dimeric, and polymeric acceptors. The development of ternary OSCs with Qx-based acceptors as the third component is then briefly discussed. Finally, several potential directions for further exploration of Qx systems towards efficient and stable OSCs are proposed with the aim of promoting their commercial applications.
2. The development of Qx-based acceptors
In general, Y-series NFAs with an A–DA′D–A framework (where A is an electron-deficient acceptor moiety and D is an electron-rich donor moiety) are composed of a central unit, end groups, and stretched side chains. The Qx moiety and its derivatives, which possess a quinoid resonance effect and additional modification sites, have been introduced to replace the A′ moiety (benzothiadiazole, BT) in Y6 via a one- or two-step reaction (Fig. 1b and c), which is beneficial for fine-tuning the optoelectronic properties and intermolecular packing behaviors of acceptors, thus enhancing the photovoltaic performance of relevant OSCs. In 2019, Zhu and co-workers first reported a pair of NFAs, AQx-1 and AQx-2 (Fig. 2), with Qx-containing central units. The AQx-1 based device shows an extremely small highest occupied molecular orbital (HOMO) offset (0.02 eV) and low energy loss (0.52 eV). To balance the trade-off between the VOC and charge generation, AQx-2 was developed by removing the electron-donating methyl substituents in the Qx moiety, resulting in a stronger π–π interaction and a reduced phase separation size when blended with the donor PM6. Due to more balanced charge transport and suppressed recombination, AQx-2-based OSCs deliver a significantly improved PCE of 16.64% (Table 1) compared to AQx-1-based OSCs (13.31%).33 This is followed by the development of a range of Qx-based acceptors with innovative structures, resulting in a champion PCE of over 19.5% and an Eloss of 0.518 eV for binary OSCs.36 In this section, we discuss the progress of Qx-based acceptors for efficient OSCs from a molecular design perspective, focusing on the effects of conjugated extension, halogenation, and side-chain modification in small-molecule acceptors (SMAs). Furthermore, the recent advances in Qx-based giant dimeric and polymeric acceptors are concisely summarized. The ternary strategy of incorporating Qx-based acceptors as the third component to reduce Eloss is also presented.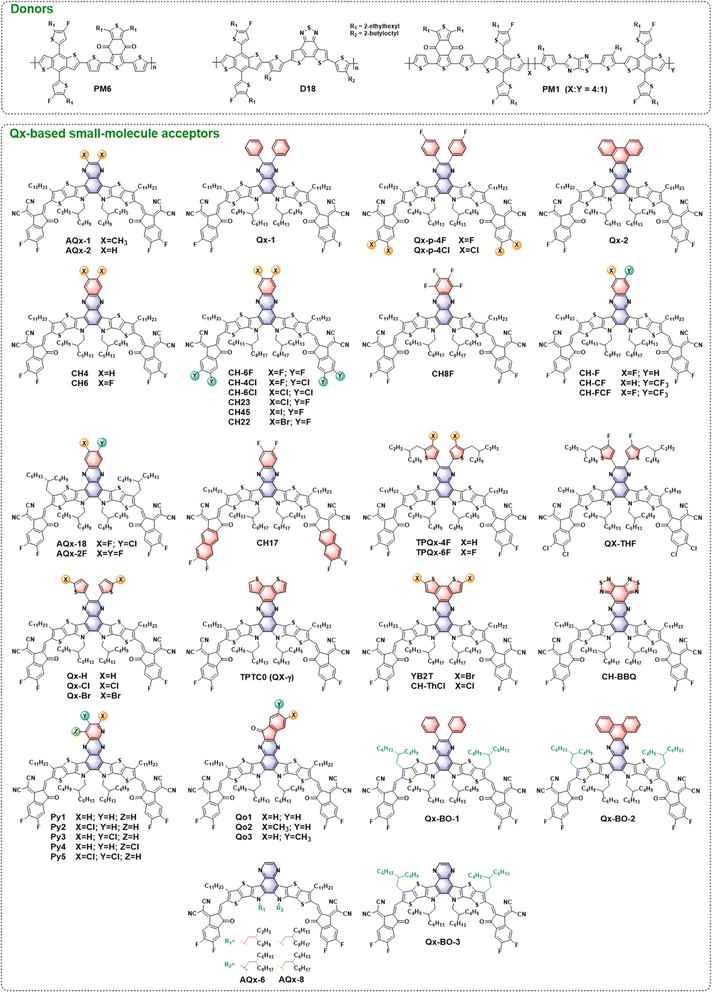 | ||
| Fig. 2 Chemical structures of donors and Qx-based small-molecule acceptors mentioned in this perspective. | ||
| Acceptor | HOMO/LUMO [eV] | E optg [eV] | Donor | V OC [V] | J SC [mA cm−2] | FF [%] | PCE [%] | E loss [eV] | ΔE3 [eV] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| AQx-1 | −5.59/−3.85 | 1.37 | PM6 | 0.89 | 22.18 | 67.14 | 13.31 | 0.52 | 0.23 | 33 |
| AQx-2 | −5.62/−3.88 | 1.36 | PM6 | 0.86 | 25.38 | 76.25 | 16.64 | 0.54 | 0.24 | 33 |
| Qx-1 | −5.58/−3.98 | 1.36 | PM6 | 0.911 | 26.1 | 75.5 | 17.9 | 0.508 | 0.214 | 34 |
| Qx-2 | −5.54/−3.86 | 1.36 | PM6 | 0.934 | 26.5 | 73.7 | 18.2 | 0.482 | 0.190 | 34 |
| Qx-o-4F | −5.73/−3.92 | 1.44 | PM6 | 0.928 | 23.64 | 75.46 | 16.55 | 0.522 | 0.202 | 23 |
| Qx-m-4F | −5.69/−3.82 | 1.45 | PM6 | 0.898 | 24.55 | 76.89 | 16.95 | 0.549 | 0.229 | 23 |
| Qx-p-4F | −5.76/−3.91 | 1.47 | PM6 | 0.877 | 24.89 | 79.46 | 17.34 | 0.560 | 0.246 | 23 |
| Qx-p-4Cl | −5.65/−3.96 | 1.41 | PM6 | 0.879 | 25.63 | 80.17 | 18.06 | 0.535 | 0.225 | 23 |
| CH4 | −5.64/−3.83 | 1.37 | PM6 | 0.888 | 26.11 | 71.1 | 16.49 | 0.51 | 0.21 | 22 |
| CH6 | −5.68/−3.85 | 1.40 | PM6 | 0.875 | 26.62 | 78.4 | 18.33 | 0.53 | 0.23 | 22 |
| CH-6F | −5.70/−3.85 | 1.41 | PM6 | 0.872 | 25.31 | 75.99 | 16.77 | 0.557 | 0.232 | 37 |
| CH-4Cl | −5.74/−3.91 | 1.39 | PM6 | 0.872 | 26.50 | 76.68 | 17.72 | 0.537 | 0.224 | 37 |
| CH-6Cl | −5.79/−3.97 | 1.36 | PM6 | 0.866 | 26.07 | 76.28 | 17.22 | 0.533 | 0.231 | 37 |
| CH23 | −5.74/−3.82 | 1.39 | PM6 | 0.876 | 26.64 | 80.45 | 18.77 | 0.538 | 0.237 | 38 |
| CH8F | −5.78/−3.80 | — | D18 | 0.899 | 26.01 | 80.38 | 18.80 | — | 0.177 | 39 |
| CH45 | −5.66/−3.85 | 1.37 | PM6 | 0.910 | 25.57 | 78.0 | 18.15 | 0.526 | 0.212 | 40 |
| CH22 | −5.67/−3.83 | 1.39 | PM6 | 0.884 | 26.74 | 80.62 | 19.06 | 0.530 | 0.230 | 35 |
| CH-F | −5.62/−3.86 | 1.33 | PM6 | 0.875 | 26.21 | 75.59 | 17.34 | 0.525 | 0.212 | 41 |
| CH-CF | −5.65/−3.85 | 1.37 | PM6 | 0.886 | 26.07 | 76.30 | 17.62 | 0.534 | 0.240 | 41 |
| CH-FCF | −5.67/−3.84 | 1.38 | PM6 | 0.897 | 26.02 | 78.86 | 18.41 | 0.545 | 0.242 | 41 |
| AQx-18 | −5.63/−3.85 | 1.40 | D18 | 0.938 | 25.0 | 77.8 | 18.2 | 0.537 | 0.200 | 42 |
| AQx-2F | −5.62/−3.88 | — | D18 | 0.937 | 26.1 | 80.4 | 19.7 | 0.518 | 0.194 | 36 |
| CH17 | −5.65/−3.86 | 1.36 | PM6 | 0.883 | 26.19 | 77.2 | 17.84 | 0.50 | 0.19 | 43 |
| TPQx-4F | −5.57/−3.74 | 1.41 | PM6 | 0.94 | 14.96 | 54.41 | 7.72 | — | — | 44 |
| TPQx-6F | −5.61/−3.78 | 1.43 | PM6 | 0.92 | 21.83 | 70.94 | 14.30 | — | — | 44 |
| QX-THF | −5.81/−4.08 | 1.40 | PM6 | 0.902 | 24.49 | 78.99 | 17.45 | — | — | 45 |
| Qx-H | −5.59/−3.92 | 1.41 | PM6 | 0.922 | 20.78 | 73.77 | 14.11 | 0.521 | 0.205 | 46 |
| Qx-Cl | −5.65/−3.95 | 1.44 | PM6 | 0.913 | 24.13 | 78.32 | 17.25 | 0.533 | 0.217 | 46 |
| Qx-Br | −5.64/−3.95 | 1.44 | PM6 | 0.915 | 24.27 | 78.47 | 17.42 | 0.526 | 0.214 | 46 |
| TPTC0 | −5.64/−3.86 | 1.39 | PM6 | 0.913 | 25.40 | 76.35 | 17.70 | 0.503 | 0.193 | 47 |
| YB2T | −5.73/−3.89 | 1.38 | PM6 | 0.918 | 24.48 | 75.85 | 17.05 | 0.556 | 0.236 | 48 |
| CH-ThCl | −5.67/−3.78 | — | PM6 | 0.934 | 25.43 | 76.45 | 18.16 | 0.519 | 0.211 | 49 |
| CH-BBQ | −5.83/−3.88 | 1.35 | PM6 | 0.881 | 26.15 | 78.9 | 18.19 | 0.567 | 0.242 | 50 |
| Py1 | −5.66/−4.04 | 1.33 | PM1 | 0.736 | 25.29 | 63.3 | 11.78 | — | — | 51 |
| Py2 | −5.73/−3.98 | 1.33 | PM1 | 0.871 | 26.83 | 75.9 | 17.73 | — | — | 51 |
| Py3 | −5.73/−4.00 | 1.34 | PM1 | 0.869 | 25.65 | 72.5 | 16.15 | — | — | 51 |
| Py4 | −5.71/−4.02 | 1.35 | PM1 | 0.817 | 18.04 | 50.9 | 7.40 | — | — | 51 |
| Py5 | −5.78/−3.95 | 1.35 | PM1 | 0.890 | 23.32 | 76.5 | 15.87 | — | — | 51 |
| Qo1 | −5.69/−4.02 | 1.41 | PM6 | 0.861 | 24.5 | 74.7 | 15.8 | — | — | 52 |
| Qo2 | −5.73/−4.01 | 1.41 | PM6 | 0.894 | 26.6 | 77.3 | 18.4 | — | — | 52 |
| Qo3 | −5.75/−4.00 | 1.44 | PM6 | 0.955 | 23.8 | 73.5 | 16.7 | — | — | 52 |
| Qx-BO-1 | −5.56/−3.74 | 1.46 | PM6 | 0.956 | 18.21 | 60.64 | 10.57 | 0.503 | 0.189 | 53 |
| Qx-BO-2 | −5.50/−3.80 | 1.47 | PM6 | 0.963 | 19.18 | 61.49 | 11.34 | 0.507 | 0.186 | 53 |
| Qx-BO-3 | −5.64/−3.84 | 1.41 | PM6 | 0.889 | 24.76 | 77.39 | 17.03 | 0.550 | 0.229 | 53 |
| AQx-6 | −5.61/−3.86 | 1.32 | D18 | 0.892 | 26.8 | 77.8 | 18.6 | 0.518 | 0.198 | 14 |
| AQx-8 | −5.60/−3.85 | 1.33 | D18 | 0.913 | 24.7 | 75.7 | 17.1 | 0.493 | 0.178 | 14 |
2.1 Qx-based small-molecule acceptors
By replacing the central BT moiety in Y6 with more conjugated Qx derivatives, Wei and co-workers developed a pair of acceptors, named Qx-1 and Qx-2, to investigate the factors affecting the Eloss in devices.34 Compared to Y6, these two acceptors show reduced reorganization energies due to the suppressed molecular vibration of the C–C bond stretching and thus decreased ΔE2 and ΔE3 (Fig. 3a–c). Moreover, the better aggregation patterns and longer exciton diffusion length of Qx-2 promote charge generation and transport in the corresponding device, thus a high PCE of 18.2% with a low ΔE3 of 0.190 eV and an Eloss of 0.482 eV is achieved in the PM6:Qx-2-based OSCs. This work highlights the importance of the reorganization energy in achieving small energy loss and paves the way for obtaining high-performance OSCs. Since halogenation plays a crucial role in optimizing the properties of NFAs, they further developed a series of acceptors (Qx-o-4F, Qx-m-4F, Qx-p-4F, and Qx-p-4Cl) by performing precise central unit fluorination and end-group chlorination.23 Different substitution positions of central fluorine atoms on these acceptors would significantly affect the local dipole moments and lead to distinct molecular packing behaviors, which are attributed to the interaction between the 1,1-dicyanomethylene-3-indanone (IC) terminal group and the fluorinated phenyl ring, the interaction between the IC terminal group and the thienothiophene (TT) unit, and the interaction between two IC terminal groups (Fig. 4d and e). Although Qx-o-4F exhibits more packing modes, it cannot form a well-connected charge transport network due to the large steric hindrance of the non-planar phenyl ring. In contrast, Qx-m-4F has good one-dimensional charge transport channels with strong π–π interaction. It is suggested that Qx-p-4F and Qx-p-4Cl can form a better charge transport network due to the enhanced local dipole moment, which is conducive to efficient charge transport. Furthermore, the end-group chlorination helps to broaden the absorption spectrum and decrease the energy loss, resulting in a PCE of 18.06% (18.78% after interlayer optimization) and a relatively low Eloss of 0.535 eV for PM6:Qx-p-4Cl-based OSCs. These results highlight the critical role of selective halogenation of central units and end groups in manipulating molecular packing, contributing to a better balance between VOC and JSC in OSCs.
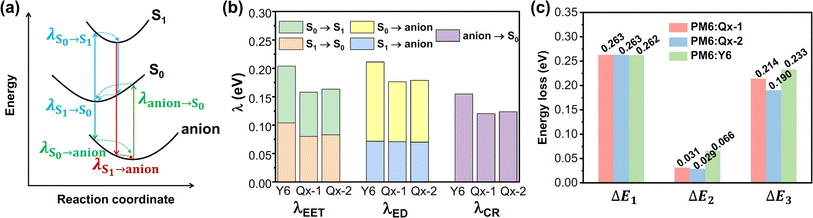 | ||
| Fig. 3 (a) Illustration of the transitions among the ground state (S0), the lowest singlet excited state (S1), and the anionic state during the photoelectric conversion processes, taking the acceptor as an example. (b) The calculated reorganization energies for exciton diffusion (λEET, excitation energy transfer), exciton dissociation (λED), and nonradiative charge recombination (λCR) at the tuned-ωB97XD/6-31G(d,p) level for Y6, Qx-1, and Qx-2. (c) Energy losses for OSCs based on different acceptors. Reproduced from ref. 32 with permission from the Nature Publishing Group, copyright [2022]. | ||
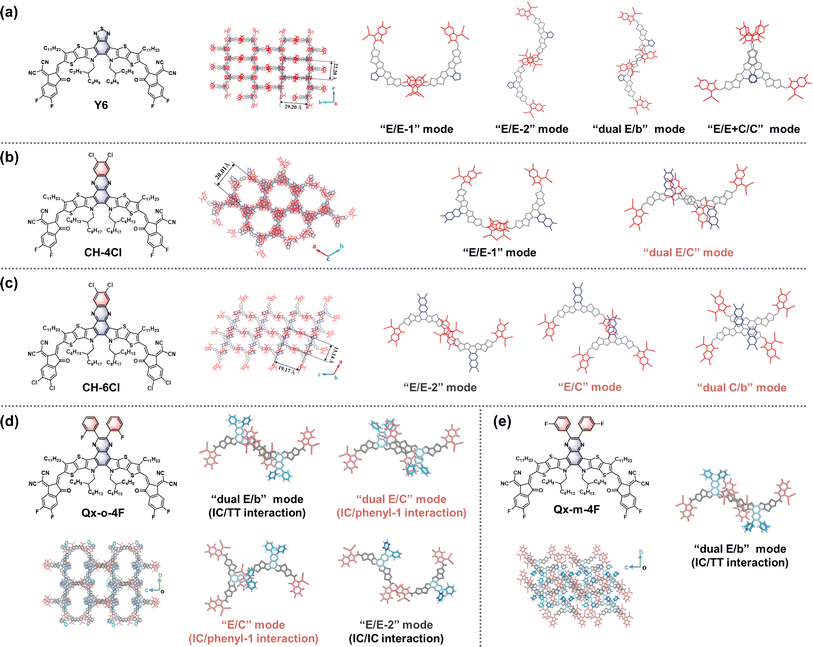 | ||
| Fig. 4 Chemical structures, single crystal packing diagrams, and intermolecular packing modes of (a) Y6, (b) CH-4Cl, (c) CH-6Cl, (d) Qx-m-4F, and (e) Qx-o-4F. Reproduced from ref. 37 with permission from the Royal Society of Chemistry, copyright [2022]. Reproduced from ref. 23 with permission from the Royal Society of Chemistry, copyright [2023]. | ||
Chen and co-workers have also extended the central Qx moiety to a phenazine moiety and developed a new acceptor CH4.22 Although a small Eloss of 0.51 eV is achieved in the PM6:CH4-based device, a moderate PCE of 16.49% is delivered with an inferior FF of 71.1%. Inspired by the success of the end-group fluorination strategy in other Y-series acceptors, CH6 with a difluorophenazine central unit was developed. The fluorinated CH6 exhibits enhanced crystallinity and superior blend morphology, resulting in a significantly improved PCE of 18.33% with an Eloss of 0.53 eV for PM6:CH6-based OSCs. Based on the phenazine central unit, they developed a series of acceptors (CH-series) with different peripheral halogens in their subsequent studies to systematically investigate the effect of central unit halogenation of acceptors on their intermolecular packing behavior, film morphology, and the resulting photovoltaic performance in OSCs. For example, the regulation of fluorination and chlorination on both the central and end units gives rise to four acceptors, CH-6F, CH-4Cl, CH-6Cl, and CH23, with different molecular packing modes.37,38 Among them, CH-4Cl, CH-6Cl, and CH23 exhibit several distinctive packing modes, including end unit to central unit (“E/C” mode), dual end unit to central unit (“dual E/C” mode), and dual end unit to bridge unit (“dual E/b” mode), which are not observed in the Y6 single crystal (Fig. 4a–c). Benefiting from their unique and superior packing modes, these acceptors, when blended with PM6, show favorable film morphologies, small energetic disorder, and more effective hybridization of CT and LE states, thus suppressing ΔE3 in the relevant devices. In particular, CH23, with its increased packing density and dielectric properties, realizes a remarkable PCE of 18.77% along with a ΔE3 of 0.237 eV and an Eloss of 0.538 eV in the resulting OSCs. Furthermore, they developed a new acceptor, CH8F, with a perfluorinated central unit.39 Due to enhanced fluorine-induced secondary interactions and larger dipole moment, CH8F exhibits stronger and more ordered molecular packing compared to CH-6F, resulting in a larger dielectric constant and lower exciton binding energy. Consequently, OSCs based on D18:CH8F with favorable nanoscale morphology yield an excellent PCE of 18.80%. The iodization of the central unit has been used to enhance the intermolecular interaction and provides a novel acceptor, CH45.40 Due to its increased molecular polarizability and reduced exciton binding energy, a PCE of 18.15% with a high VOC of 0.910 V is realized in PM6:CH45-based OSCs. Considering that bromine has the additional advantages of greater crystallinity and easier polarization than fluorine and chlorine, they have recently developed a new acceptor, CH22, with a brominated central unit.35 The bromination on the central unit contributes to enhancing the crystallinity and dielectric constant of the acceptor without compromising the favorable intermolecular packing created by end groups. Consequently, PM6:CH22-based binary OSCs afford an impressive PCE of 19.06% and an acceptable Eloss of 0.530 eV, along with decent thermal stability.
The asymmetric halogenation strategy has also been applied to the subtle modification of the Qx-based central unit. Lu and co-workers carried out direct fluorination and indirect trifluoromethylation on the central phenazine unit and constructed three acceptors, CH-F, CH-CF, and CH-FCF.41 Among them, CH-FCF with both the fluorine and trifluoromethyl substitutions on the central unit shows enhanced intermolecular interactions and crystallinity, contributing to the formation of fibrillar network morphology with superior charge generation/transport. As a result, OSCs based on PM6:CH-FCF realize a high PCE of 18.41%. On the basis of L8BO, Zhu and co-workers synthesized a two-dimensional (2D) conjugated acceptor (AQx-18) with fluorine and chlorine atoms on the central unit, which exhibits ordered and compact molecular packing, resulting in a PCE of 18.2% when blended with D18.42 In addition, they have recently developed an extended conjugated acceptor (AQx-2F) with a selectively fluorinated central unit.36 Benefiting from an appropriate energy offset and a favorable fibril morphology with superior exciton/charge carrier dynamics, D18:AQx-2F-based binary OSCs achieve a fine balance between VOC and JSC, realizing a record PCE of 19.7% with a high VOC of 0.937 V and a low Eloss of 0.518 eV, while exhibiting decent operational stability and retaining 83% of their initial PCE under maximum power point tracking for 500 hours.
The π-conjugated extension on both the central unit and end groups is another available method to manipulate the intermolecular stacking of acceptors. For example, Chen and co-workers reported a conjugation-extended acceptor, CH17, featuring a phenazine-based core and naphthalene-based terminal groups.43 Compared to Y6, CH17 with bi-directional extended conjugation exhibits a more compact 3D molecular packing arrangement, leading to better charge transport, reduced energetic disorder, and suppressed ΔE3. As a result, PM6:CH17-based OSCs yield a PCE of 17.84% along with a low Eloss of 0.50 eV and a ΔE3 of 0.19 eV.
The introduction of halogenated thiophene into the Qx central core is a practical approach to tailoring the photoelectronic properties and aggregation behavior of acceptors. For example, Zou and co-workers developed two Qx-containing acceptors (TPQx-4F and TPQx-6F) with thiophene substituents on the central unit.44 Compared to TPQx-4F, TPQx-6F with fluorine atoms on the thiophene substituent shows more uniform nanophase aggregation when blended with PM6, resulting in a higher PCE of 14.30% in binary OSCs (7.72% for PM6:TPQx-4F-based OSCs). Similarly, based on efficient BTP-eC9, Ge and co-workers replaced the central unit with a fluorinated thiophene-substituted Qx core and synthesized QX-THF.45 Due to enhanced intermolecular interaction and optimized film morphology, PM6:QX-THF-based OSCs yield a PCE of 17.45%. These results demonstrate that the fluorinated thiophene ring is a promising building block for Qx-based acceptors. In addition, Wei and co-workers have developed three acceptors (Qx-H, Qx-Cl, and Qx-Br) with different halogenated thiophene substitutions on the Qx core.46 Compared to Qx-H without halogen atoms on the thiophene moiety, Qx-Br and Qx-Cl show enhanced absorption, improved crystallinity, and favorable film morphology, contributing to efficient charge transport. Consequently, a PCE of 17.42% with a VOC of 0.915 V is realized in PM6:Qx-Br-based OSCs. Apart from the conjugated extension via a rotatable single bond, the thiophene ring was fused into the central Qx moiety to build large 2D conjugated acceptors. For example, Wei and co-workers reported an acceptor (TPTC0) with fused thiophene rings on the central unit.47 The extended and rigid planar structure is conducive to molecular aggregation, resulting in high crystallinity and appropriate phase separation, giving a PCE of 17.70% with a low Eloss of 0.503 eV for PM6:TPTC0-based OSCs. The halogen atoms have also been introduced into the thiophene-fused Qx-containing central unit to optimize the molecular aggregation properties. For example, He and co-workers synthesized a 2D conjugated acceptor, YB2T, with fused bromothiophene rings on the central core.48 The ordered and tight intermolecular stacking of YB2T enables the formation of a suitable phase separation structure when blended with a donor, contributing to efficient exciton dissociation and charge transport. Accordingly, a PCE of 17.05% is achieved in PM6:YB2T-based OSCs. Chen and co-workers also incorporated chlorothiophene rings into the phenazine-fused core and developed a new 2D conjugated acceptor, CH-ThCl.49 The more extended conjugated central unit with chlorine substitution enhances the acceptor's PLQY and suppresses ΔE3 in the resulting device. As a result, the PM6:CH-ThCl-based device achieves an excellent PCE of 18.16% with a high VOC of 0.934 V, due to its relatively low ΔE3 of 0.21 eV and Eloss of 0.519 eV.
In addition to the benzene and thiophane moieties, various heteroaromatic rings have been incorporated into the central Qx moiety, resulting in novel conjugation-extended central units that are advantageous for regulating the energy levels, intramolecular stacking, and crystallization behavior of acceptors. For example, Chen and co-workers developed a conjugation-extended acceptor (CH-BBQ) with a benzobisthiadiazole-fused Qx core that exhibits suitable energy levels, enhanced crystallinity, and desirable film morphology, resulting in a PCE of 18.19% for binary OSCs blended with PM6.50 Moreover, Yang and co-workers reported a series of acceptors (Py-series, Py1–Py5) containing a pyridoquinoxaline core with chlorine substitution at different positions.51 Compared to the non-chlorinated Py1, the chlorinated acceptors show enlarged optical band gaps. Among them, the ortho-chlorinated Py2 exhibits a larger dipole moment and tighter molecular stacking than other chlorinated isomers, contributing to favorable aggregation behaviors, resulting in a PCE of 17.73% for PM1:Py2-based OSCs. In addition, the introduction of intermolecular hydrogen-bonding interactions is a feasible strategy for tuning molecular packing patterns. Luo and co-workers incorporated methylated indanone moieties into the Qx core and synthesized three acceptors called Qo1, Qo2, and Qo3.52 Among them, Qo2 shows multiple and stronger hydrogen-bonding interactions, contributing to a superior 3D charge transport network and achieving an excellent PCE of 18.4% for PM6:Qo2-based OSCs. Significantly, the optimal device exhibits satisfactory thermal stability, retaining 83% of its initial PCE after thermal aging at 65 °C for 250 hours.
To sum up, the strategy of π-conjugated extension combined with halogenation for Qx-based acceptors plays a crucial role in adjusting their intermolecular packing, optimizing the film morphology and suppressing energy loss. The binary OSCs based on acceptors with a precisely halogenated central unit have achieved a champion PCE of 19.7% with a low Eloss of 0.518 eV (ΔE3 < 0.20 eV).36 Therefore, subtle modification of the central unit has great potential to construct novel acceptors and further promote the development of efficient OSCs.
Inspired by the successful design of L8-BO, we have recently reported three Qx-based acceptors, Qx-BO-1, Qx-BO-2, and Qx-BO-3, containing 2-butyloctyl side chains at the β-positions of the outer thiophene units.53 The introduction of branched alkyl chains enables high VOC values of over 0.95 V with a low Eloss of around 0.50 eV for OSCs based on PM6:Qx-BO-1 and PM6:Qx-BO-2. However, their large central units and branched alkyl chains exhibit undesirable steric hindrance, resulting in inferior film morphology, which affects charge generation/transport and the resulting PCE (<12%). In contrast, Qx-BO-3 with a smaller Qx core shows suitable aggregation morphology and more efficient charge transport, resulting in a better balance among JSC, VOC, and FF and thus a higher PCE of about 17.0% in the PM6:Qx-BO-3-based device despite the largest Eloss (0.550 eV). This work demonstrates the importance of fine-tuning the size of the central unit in order to achieve rational side-chain modification.
In addition, Zhu and co-workers proposed that prolonged alkyl side chains contribute to a reduced free volume ratio (FVR), which restricts molecular motion in the solid state.14 Specifically, they developed two acceptors, AQx-6 and AQx-8, with gradually extended alkyl chains at the nitrogen position of the pyrrole rings. The decreased FVRs for these two acceptors are beneficial for reducing electron-phonon coupling and suppressing ΔE3 in the relevant devices. However, the side-chain length also affects the phase separation and crystallinity in blend films, thus influencing photophysical processes in OSCs. Significantly, AQ-6 with asymmetric side chains shows good miscibility with the donor D18, resulting in favorable film morphology with suitable separation and compact molecular packing, achieving a better balance between low ΔE3 for high VOC and efficient charge generation/transport for high JSC. Consequently, D18:AQx-8-based OSCs deliver an impressive PCE of 18.6% with an Eloss of 0.518 eV and a small ΔE3 of 0.198 eV.
2.2 Qx-based giant dimeric acceptors
The newly developed giant dimeric acceptors have attracted wide attention because of their unique advantages of a well-defined molecular structure, high efficiency, and excellent long-term stability.54–56 Generally, giant dimeric acceptors are synthesized by linking the end groups of two Y-series SMAs via a π-spacer (“End group coupling”). In order to tune the molecular packing and improve the device performance, massive efforts have been devoted to π-spacer modification to improve the backbone planarity of giant dimeric acceptors.20,57 In addition to the π-spacer, the resulting performance is largely related to the monomer. Therefore, Qx-based acceptors are promising candidates for giant units due to their multiple modification sites on the central unit, which are advantageous for optimizing the aggregation properties of acceptors and allow a novel coupling approach by linking the central units (“Central unit coupling”).Recently, Deng and co-workers reported two novel Qx-based giant dimeric acceptors, Dimer-QX and Dimer-2CF, without/with trifluoromethyl substitution on the central unit (Fig. 5a).58 The introduction of trifluoromethyl with strong electronegativity reduces the surface energy of Dimer-2CF and enhances its electrostatic interaction with the donor PM6, contributing to favorable vertical phase distribution, suitable phase separation (Fig. 5b), and ordered molecular packing. The superior morphology endows Dimer-2CF-based OSCs with efficient exciton dissociation and charge transport, resulting in an excellent PCE of 18.12% (19.02% after interlayer optimization) with a high FF of 80.62% (Table 2). Significantly, both Dimer-QX and Dimer-2CF possess a high glass transition temperature (ca. 115 °C), which imparts excellent thermal stability to the resulting OSCs (Fig. 5c), retaining above 90% of their initial efficiencies after continuous heating at 80 °C for 1000 hours (extrapolated T80 ≈ 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h). Moreover, both Dimer-based OSCs exhibit good photostability, with an extrapolated T80 lifetime of around 2500 h. This work highlights the importance of controlling hetero-molecular interactions and demonstrates the great potential of Qx-based giant dimeric acceptors for the construction of highly efficient and stable OSCs.
000 h). Moreover, both Dimer-based OSCs exhibit good photostability, with an extrapolated T80 lifetime of around 2500 h. This work highlights the importance of controlling hetero-molecular interactions and demonstrates the great potential of Qx-based giant dimeric acceptors for the construction of highly efficient and stable OSCs.
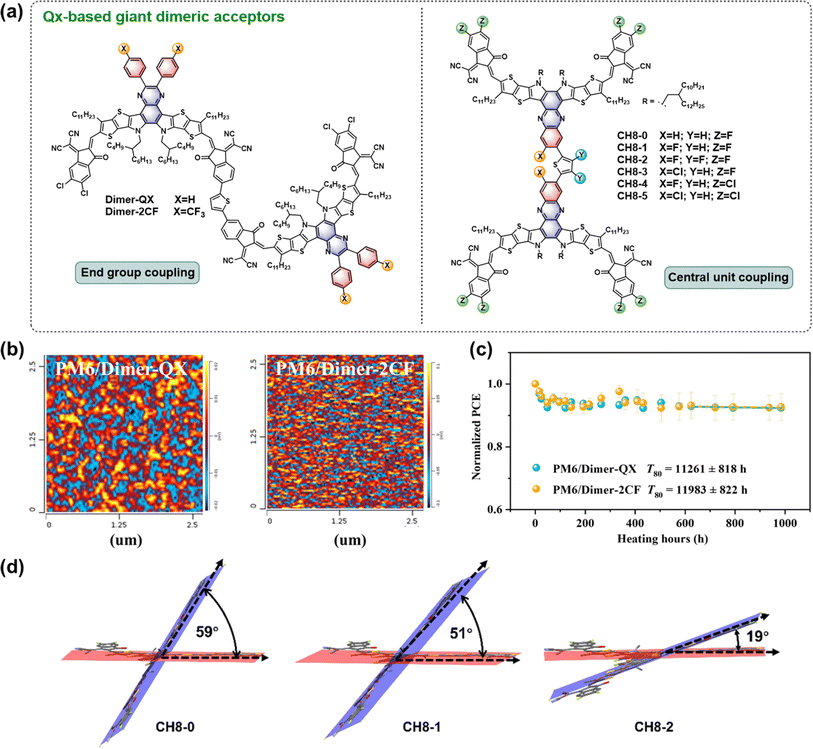 | ||
| Fig. 5 (a) Chemical structures of Qx-based giant dimeric acceptors. (b) Atomic force microscopy-infrared spectroscopy (AFM-IR) images of PM6:Dimer-QX and PM6:Dimer-2CF blend films (measured at a characteristic wavenumber of these two dimeric acceptors, 1532 cm−1). (c) Normalized PCEs of PM6:Dimer-QX and PM6:Dimer-2CF-based devices heating at 80 °C for 1000 hours. Reproduced from ref. 58 with permission from Wiley, copyright [2023]. (d) Ground-state geometries of CH8-0, CH8-1 and CH8-2 calculated by the DFT method, with the dihedral angles between two monomer planes marked. Reproduced from ref. 59 with permission from the Royal Society of Chemistry, copyright [2023]. | ||
| Acceptor | HOMO/LUMO [eV] | Egopt [eV] | Donor | V OC [V] | J SC [mA cm−2] | FF [%] | PCE [%] | E loss [eV] | ΔE3 [eV] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Dimer-QX | −5.52/−3.77 | 1.40 | PM6 | 0.933 | 22.57 | 69.26 | 14.59 | — | — | 58 |
| Dimer-2CF | −5.57/−3.78 | 1.41 | PM6 | 0.889 | 25.27 | 80.62 | 18.12 | — | — | 58 |
| CH8-0 | −5.61/−3.78 | 1.41 | PM6 | 0.936 | 22.61 | 72.1 | 15.26 | 0.490 | 0.182 | 59 |
| CH8-1 | −5.69/−3.80 | 1.42 | PM6 | 0.923 | 24.89 | 74.2 | 17.05 | 0.518 | 0.206 | 59 |
| CH8-2 | −5.71/−3.81 | 1.44 | PM6 | 0.928 | 24.24 | 74.9 | 16.84 | 0.529 | 0.213 | 59 |
| CH8-3 | −5.74/−3.76 | 1.44 | PM6 | 0.915 | 24.44 | 77.0 | 17.22 | 0.529 | 0.214 | 60 |
| CH8-4 | −5.75/−3.79 | 1.40 | PM6 | 0.894 | 26.05 | 75.5 | 17.58 | 0.518 | 0.193 | 60 |
| CH8-5 | −5.76/−3.79 | 1.41 | PM6 | 0.902 | 24.75 | 75.2 | 16.79 | 0.520 | 0.206 | 60 |
| PQx1 | −5.56/−3.75 | 1.43 | PM6 | 0.951 | 17.36 | 59.05 | 9.75 | 0.494 | 0.173 | 64 |
| PQx2 | −5.55/−3.72 | 1.44 | PM6 | 0.899 | 5.63 | 51.61 | 2.61 | 0.535 | 0.208 | 64 |
| PQx3 | −5.60/−3.76 | 1.45 | PM6 | 0.932 | 24.79 | 76.17 | 17.60 | 0.518 | 0.191 | 64 |
| PZC16 | −5.61/−3.81 | 1.43 | PM6 | 0.861 | 18.98 | 56.65 | 9.22 | 0.599 | 0.291 | 65 |
| PZC17 | −5.60/−3.79 | 1.44 | PM6 | 0.926 | 23.35 | 75.54 | 16.33 | 0.534 | 0.236 | 65 |
| PZC1 | −5.69/−3.72 | 1.44 | PM6 | 0.923 | 22.10 | 67.88 | 13.83 | — | — | 66 |
| PZC2 | −5.62/−3.74 | 1.45 | PM6 | 0.936 | 24.61 | 74.93 | 17.30 | — | — | 66 |
| PZC3 | −5.64/−3.75 | 1.46 | PM6 | 0.953 | 22.34 | 73.97 | 15.75 | — | — | 66 |
| SH-1 | −5.68/−3.69 | 1.45 | PM6 | 0.979 | 21.03 | 68.67 | 14.14 | 0.522 | 0.203 | 67 |
| SH-2 | −5.64/−3.76 | 1.38 | PM6 | 0.908 | 12.87 | 55.94 | 6.55 | 0.529 | 0.224 | 67 |
In order to release more end groups for efficient molecular packing, Chen and co-workers developed a series of giant dimeric acceptors (CH8-series) by linking the central units of two CH-series SMAs via a thiophene spacer. Selective fluorination was applied to the central phenazine moiety and the thiophene spacer, leading to gradually decreased dihedral angles between the two monomers from CH8-0 to CH8-1 to CH8-2 (Fig. 5d), caused by different strengths of noncovalently conformational locks via F–S/F–H secondary interactions between the central moiety and the spacer.59 Benefiting from the low electron recombination energy, fibrillar network film morphology, and enhanced charge transport, OSCs based on PM6:CH8-1 achieve a PCE of 17.05%, along with decent thermal/photo-stability. Furthermore, selective chlorination was employed to the central unit and end groups of giant dimeric acceptors, giving CH8-3, CH8-4, and CH8-5.60 From the fluorinated end groups to the chlorinated end groups, the resulting acceptors (CH8-4 and CH8-5) exhibit red-shifted absorption and enhanced crystallinity, affording a champion PCE of 17.58% for PM6:CH8-4-based OSCs. Importantly, devices based on CH8-3/4/5 show satisfactory thermal and photostability, retaining around 90% and 80% of their initial efficiencies after thermal aging at 65 °C for 430 h and continuous one-sun illumination for 250 h, respectively. The decent device stability could be partially attributed to the excellent morphological stability induced by both the strong intermolecular π–π stacking and the weak noncovalent interactions. These results demonstrate a novel and feasible strategy to construct promising multidimensional acceptors via central unit coupling towards efficient and stable OSCs.
2.3 Qx-based polymeric acceptors
All-polymer solar cells (all-PSCs) have also drawn tremendous attention due to their superior morphological stability and great mechanical flexibility.61,62 However, limited by the lack of efficient polymeric acceptors, the efficiencies of all-PSCs still lag behind those of SMA-based OSCs. Impressively, polymerized small molecule acceptors (PSMAs) have achieved great success by inheriting the excellent properties of SMAs.63 To further improve the performance of all-PSCs, various structural modification strategies involving the SMA monomer and the linking unit have been developed. In particular, Qx-based polymeric acceptors have great potential to construct highly efficient all-PSCs by virtue of their unique molecular packing properties and abundant modification sites.In terms of the SMA monomer, its molecular structure, particularly the central unit substitutions, has a significant effect on the molecular interactions and aggregation behavior during the blend film formation process. Lu and co-workers have recently reported three Qx-based PSMAs, PQx1, PQx2, and PQx3 (Fig. 6a), featuring different central unit substitutions.64 As shown in Fig. 6b–d, the difference in the central unit affects the crystallinity of these polymeric acceptors and their miscibility with PM6. Among them, PQx-3 shows appropriate aggregation ability and intermolecular interaction, resulting in optimal blend morphology with suitable phase separation and superior charge transport properties. In contrast, PQx1 and PQx2 exhibit insufficient or excessive crystallinity, giving unsatisfactory blend morphologies. Consequently, PQx-3-based binary all-PSCs achieve an excellent PCE of 17.60% (9.75% and 2.61% for PQx1- and PQx-2-based devices, respectively), which is further improved to 18.82% in ternary all-PSCs. In addition, due to their superior morphological stability, PM6:PQx-3-based devices exhibit better thermal stability with a T80 lifetime of about 160 h compared to PQx-1/PQx-2-based devices (T80 ≈ 50 h).
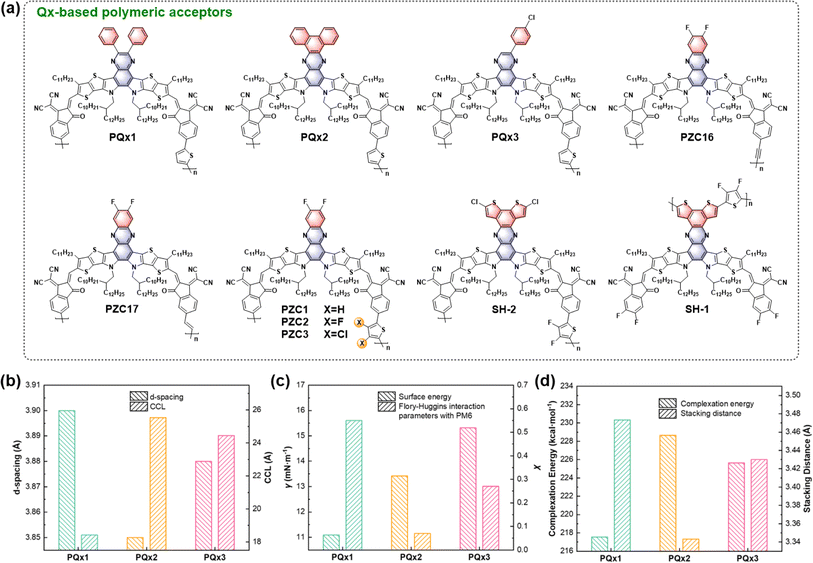 | ||
| Fig. 6 (a) Chemical structures of Qx-based polymeric acceptors. (b) π–π stacking d-spacing and crystalline coherent length (CCL) of PQx1, PQx2, and PQx3 neat films. (c) Surface energy of the three polymeric acceptors and the Flory–Huggins interaction parameters with PM6. (d) Complexation energy and stacking distances of three polymeric acceptors with PM6 according to the theoretical calculation. Reproduced from ref. 64 with permission from Wiley, copyright [2023]. | ||
In terms of the linking unit, Chen and co-workers have developed a range of Qx-based polymeric acceptors (PZC-series) to investigate the effect of linking unit modification on the optoelectronic and molecular aggregation properties of polymeric acceptors. To reduce the molecular torsion caused by the traditional aromatic linkers, they synthesized two PSMAs with non-aromatic linkers (ethynyl for PZC16 and vinylene for PZC17). Both polymeric acceptors show good planarity of the conjugated backbones and similar energy levels, while PZC17 shows better miscibility with the donor PM6, leading to a suitable phase separation in blend films. Therefore, PZC17-based all-PSCs deliver a higher PCE (16.33%) than PZC16-based devices (9.22%).65 In addition, three polymeric acceptors (PZC1, PZC2, and PZC3) were developed by introducing halogen atoms on the thiophene linker.66 Among them, PZC2 with a fluorinated thiophene linker exhibits a more planar molecular conformation and optimal morphology in blend films, contributing to efficient charge dynamics. As a result, PZC2-based all-PSCs achieve an excellent PCE of 17.30% (13.83% and 15.75% for PZC1- and PZC3-based devices, respectively), while exhibiting the best thermal stability, partly due to the stable morphology induced by the coplanar molecular conformation.
In addition to the polymerization between the end groups of two SMAs, the polymerization between the central units has been developed to produce novel Qx-based polymeric acceptors. Based on CH-series SMAs, Chen and co-workers reported a polymeric acceptor, SH-1, by central unit polymerization.67 Compared to the end-group polymerized SH-2, SH-1 exhibits more ordered intermolecular packing and favorable morphology in blend films, contributing to efficient exciton dissociation and charge transport. Therefore, PM6:SH-1-based devices deliver significantly higher PCE (14.14%) than PM6:SH-2-based devices (6.55%). This work provides an alternative pathway to construct efficient polymer acceptors through the delicate design of both the central building block and the linking unit.
2.4 Ternary strategy by introducing Qx-based acceptors
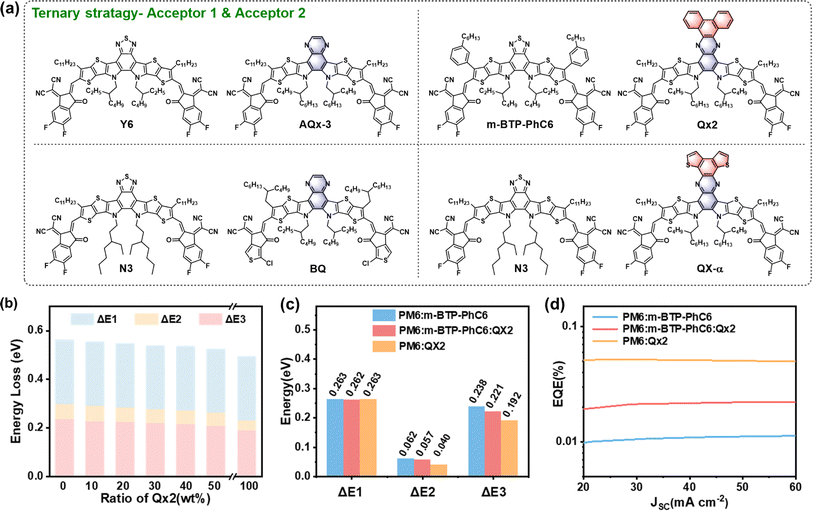
The ternary strategy, namely incorporating an additional donor or acceptor into the binary system, is an effective way to improve the photovoltaic performance of OSCs. The introduction of a suitable third component commonly contributes to complementary absorption coverage, morphology optimization, and energy loss mitigation, resulting in an outstanding PCE of over 19.5% in the state-of-the-art ternary organic solar cells (TOSCs).5,6 As previously discussed, Qx-based NFAs exhibit structural diversity, superior molecular aggregation properties, and reduced energy loss, enabling them to be a third component to construct efficient TOSCs with decreased Eloss.To fine-tune the optoelectronic properties and morphology of the typical PM6:Y6 binary system, Zhu and coworkers conceived a “two-in-one” strategy by incorporating a Qx-containing acceptor, AQx-3 (Fig. 7a).68 These two acceptors (AQx-3 and Y6), with high structural similarity and compatibility, form an alloy-like composite that is conducive to adjusting the HOMO offset and optimizing the film morphology, thereby enhancing charge generation/transport and reducing Eloss. Thus, the optimal TOSCs (PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6
Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AQx-3 = 1
AQx-3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4) realize an impressive PCE of over 18% (Table 3), which is the highest value reported at that time for Y6-based TOSCs.
0.4) realize an impressive PCE of over 18% (Table 3), which is the highest value reported at that time for Y6-based TOSCs.
 | ||
| Fig. 7 (a) Chemical structures of acceptors of ternary systems discussed in this perspective. (b) Variation tendency in the energy losses of devices with different Qx2 ratios. (c) Statistical diagram of detailed energy loss terms for the binary and optimal ternary devices. (d) EQEEL curves of the binary and optimal ternary devices. Reproduced from ref. 69 with permission from the Royal Society of Chemistry, copyright [2023]. | ||
| Acceptor | HOMO/LUMO [eV] | Donor | V OC [V] | J SC [mA cm−2] | FF [%] | PCE [%] | E loss [eV] | Ref. |
|---|---|---|---|---|---|---|---|---|
| AQx-3 | −5.64/−3.86 | PM6 | 0.891 | 24.16 | 77.4 | 16.67 | 0.539 | 68 |
| Y6 | −5.70/−3.90 | PM6 | 0.856 | 25.73 | 76.8 | 16.94 | 0.554 | 68 |
| Y6:AQx-3 | −5.69/−3.88 | PM6 | 0.870 | 26.82 | 77.2 | 18.01 | 0.550 | 68 |
| Qx2 | −5.54/−3.86 | PM6 | 0.935 | 24.28 | 75.37 | 17.11 | 0.493 | 69 |
| m-BTP-PhC6 | −5.59/−3.86 | PM6 | 0.850 | 25.69 | 78.43 | 17.12 | 0.560 | 69 |
| m-BTP-PhC6:Qx2 | — | PM6 | 0.875 | 25.91 | 79.12 | 17.97 | 0.539 | 69 |
| BQ | −5.56/−4.17 | D18 | 0.959 | 21.27 | 69.35 | 14.14 | — | 70 |
| N3 | −5.70/−4.41 | D18 | 0.827 | 27.53 | 78.04 | 17.77 | 0.562 | 70 |
| N3:BQ | — | D18 | 0.846 | 27.92 | 79.95 | 18.89 | 0.547 | 70 |
| QX-α | −5.64/−3.88 | D18 | 0.944 | 24.67 | 69.9 | 14.30 | — | 71 |
| N3 | −5.79/−3.94 | D18 | 0.832 | 27.98 | 78.0 | 18.16 | 0.587 | 71 |
| N3:QX-α | — | D18 | 0.862 | 27.86 | 80.5 | 19.33 | 0.553 | 71 |
Encouraged by the PM6:Qx2 system with low energy loss, Wei and co-workers further constructed efficient TOSCs based on PM6:m-BTP-PhC6:Qx2 with simultaneously increased VOC, JSC, and FF.69 The third component Qx2 promotes the miscibility between the donor and the acceptor composite in the resulting ternary blend, leading to ideal film morphology and thus decreased energetic disorder, improved charge extraction, and reduced Eloss (Fig. 7b and c) in the optimum TOSCs. As a result, PM6:m-BTP-PhC6:Qx2-based TOSCs deliver a champion PCE of 17.97% (18.62% after interlayer optimization) with a significantly improved VOC of 0.875 V (0.850 V for the PM6:m-BTP-PhC6 binary system).
Designing an appropriate third component based on the Qx core is a feasible approach to further improve the performance of existing efficient binary systems. Ge and co-workers synthesized a novel acceptor, BQ, featuring a Qx central unit and chlorinated thiophene end groups.70 On the basis of the Qx core, the introduction of chlorinated thiophene on the end groups of the NFAs helps to regulate energy levels and suppress Eloss, leading to much-upshifted energy levels of BQ and thus a high VOC of 0.959 V when blended with D18. After the addition of BQ to the D18:N3 binary system, cascade-like model TOSCs are realized with undisturbed nanofiber network morphology, enhanced charge transfer/collection, and reduced energy loss. Eventually, the resulting TOSCs achieve an increased PCE of 18.89% with a decreased Eloss of 0.547 eV compared to the D18:N3-based binary OSCs (17.77% and 0.562 eV, respectively). In addition, they developed two isomeric acceptors (QX-α and QX-γ) with different orientations of fused thiophene rings on the Qx core.71 Compared to QX-γ, QX-α has S–N noncovalent interactions, giving a larger dipole moment, more ordered molecular stacking, and a higher surface energy. By incorporating QX-α into D18:N3, the resulting TOSCs show more balanced charge mobilities, enhanced charge collection, and suppressed energy loss than D18:N3-based binary OSCs, yielding a champion PCE of 19.33% with obviously increased VOC and FF. These results demonstrate that Qx-based acceptors with delicate modification could serve as a suitable third component for the construction of highly efficient TOSCs.
3. Conclusions and outlooks
This perspective summarizes the design strategies and advances of Qx-based acceptors, including small-molecule, giant dimeric, and polymeric acceptors. Extensive efforts have offered a better understanding of the structure–property relationship of Qx-based acceptors, contributing to the realization of high-performance OSCs with lower energy loss and more efficient charge generation/transport. Due to the huge space for structural modification, Qx-based acceptors have great potential to achieve efficient and stable OSCs for commercial applications. Therefore, several directions for further studies in molecular design and device optimization for Qx systems are proposed.(1) From a molecular design perspective, subtle structural modification can lead to significant improvements in device performance. Firstly, the incorporation of intermolecular noncovalent interactions via precise substituent engineering is a promising approach to regulating the molecular packing behavior of acceptors, which is conducive to further improving the charge transport properties. Secondly, other commercialized simple polycyclic aromatic/heteroaromatic moieties can be introduced into the central core to increase the photoluminescence quantum yield, which has great potential to reduce nonradiative energy loss. Synthetic complexity and scalability should also be considered. Additional side-chain or substituent engineering may be required to ensure sufficient solubility when extending the conjugated central core. Therefore, it is critical to control the size of the central unit and employ proper heteroatom replacement rather than overextending the rigid conjugated plane. Thirdly, bilateral substitution of aromatic or heteroaromatic rings on the Qx core can be changed to unilateral substitution to improve reaction yields and fine-tune energy levels and molecular stacking, which is expected to achieve lower energy losses and further performance improvements. Fourthly, developing novel Qx-based dimeric and trimeric acceptors with a higher glass transition temperature could simultaneously improve the charge transport and long-term stability of the devices, thus realizing highly efficient and stable OSCs.
(2) From a device optimization perspective, the matching of donor/acceptor materials and the construction of ternary devices are essential approaches to accessing the lower limit of energy losses for a given material without sacrificing charge generation. Firstly, in addition to the commonly used PM6 donor, it is necessary to introduce other efficient donors with matched energy levels and suitable miscibility with Qx-based acceptors to form the desired nanoscale phase separation and ordered molecular packing in blend films. Secondly, the planar and rigid skeleton is likely to result in reduced reorganization energies and thus decreased energy loss, making Qx-based acceptors a promising third component for the construction of highly efficient TOSCs. For existing Qx-based acceptors, efficient binary systems with appropriate interactions and miscibility should be sought to realize morphology control and energy loss reduction. Thirdly, the low energy loss Qx system provides an excellent platform for large-area roll-to-roll fabrication.72 However, most Qx-based acceptors with a large rigid conjugated central unit have a strong molecular aggregation ability, which would induce excessive crystallinity and unfavorable phase separation during the film formation process. It is therefore necessary to manipulate the film formation kinetics of Qx systems in green solvents, further expanding the application prospects of materials with high crystallinity.
Author contributions
M. X. and K. L. conceived the idea. M. X. prepared the manuscript. Z. W. and K. L. directed and supervised the project. All authors discussed and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support provided by the Beijing Natural Science Foundation (Grant No. Z230018) and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB36010200 and XDB0520102).References
- D. Wang, Y. Li, G. Zhou, E. Gu, R. Xia, B. Yan, J. Yao, H. Zhu, X. Lu, H.-L. Yip, H. Chen and C.-Z. Li, Energy Environ. Sci., 2022, 15, 2629–2637 RSC.
- Z. Wang, Y. Bo, P. Bai, S. Zhang, G. Li, X. Wan, Y. Liu, R. Ma and Y. Chen, Science, 2023, 382, 1291–1296 CrossRef CAS PubMed.
- J. Wang, Y. Wang, J. Li, Y. Yu, P. Bi, J. Qiao, Z. Chen, C. Wang, W. Wang, J. Dai, X. Hao, S. Zhang and J. Hou, Angew. Chem., Int. Ed., 2023, 62, e202314362 CrossRef CAS PubMed.
- T. Chen, S. Li, Y. Li, Z. Chen, H. Wu, Y. Lin, Y. Gao, M. Wang, G. Ding, J. Min, Z. Ma, H. Zhu, L. Zuo and H. Chen, Adv. Mater., 2023, 35, 2300400 CrossRef CAS.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C. C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Nat. Mater., 2022, 21, 656–663 CrossRef CAS PubMed.
- P. Bi, J. Wang, Y. Cui, J. Zhang, T. Zhang, Z. Chen, J. Qiao, J. Dai, S. Zhang, X. Hao, Z. Wei and J. Hou, Adv. Mater., 2023, 35, 2210865 CrossRef CAS.
- B. Pang, C. Liao, X. Xu, L. Yu, R. Li and Q. Peng, Adv. Mater., 2023, 35, 2300631 CrossRef CAS.
- X.-K. Chen, D. Qian, Y. Wang, T. Kirchartz, W. Tress, H. Yao, J. Yuan, M. Hülsbeck, M. Zhang, Y. Zou, Y. Sun, Y. Li, J. Hou, O. Inganäs, V. Coropceanu, J.-L. Bredas and F. Gao, Nat. Energy, 2021, 6, 799–806 CrossRef CAS.
- J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat, A. Mishra, Y. Yang, M. A. Hope, F. T. Eickemeyer, M. Kim, Y. J. Yoon, I. W. Choi, B. P. Darwich, S. J. Choi, Y. Jo, J. H. Lee, B. Walker, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, A. Hagfeldt, D. S. Kim, M. Gratzel and J. Y. Kim, Nature, 2021, 592, 381–385 CrossRef CAS PubMed.
- J. Yuan, H. Zhang, R. Zhang, Y. Wang, J. Hou, M. Leclerc, X. Zhan, F. Huang, F. Gao, Y. Zou and Y. Li, Chem, 2020, 6, 2147–2161 CAS.
- J. Yao, T. Kirchartz, M. S. Vezie, M. A. Faist, W. Gong, Z. He, H. Wu, J. Troughton, T. Watson, D. Bryant and J. Nelson, Phys. Rev. Appl., 2015, 4, 014020 CrossRef.
- M. Azzouzi, J. Yan, T. Kirchartz, K. Liu, J. Wang, H. Wu and J. Nelson, Phys. Rev. X, 2018, 8, 031055 CAS.
- S. Liu, J. Yuan, W. Deng, M. Luo, Y. Xie, Q. Liang, Y. Zou, Z. He, H. Wu and Y. Cao, Nat. Photonics, 2020, 14, 300–305 CrossRef CAS.
- Y. Jiang, Y. Li, F. Liu, W. Wang, W. Su, W. Liu, S. Liu, W. Zhang, J. Hou, S. Xu, Y. Yi and X. Zhu, Nat. Commun., 2023, 14, 5079 CrossRef CAS PubMed.
- D. Qian, Z. Zheng, H. Yao, W. Tress, T. R. Hopper, S. Chen, S. Li, J. Liu, S. Chen, J. Zhang, X. K. Liu, B. Gao, L. Ouyang, Y. Jin, G. Pozina, I. A. Buyanova, W. M. Chen, O. Inganas, V. Coropceanu, J. L. Bredas, H. Yan, J. Hou, F. Zhang, A. A. Bakulin and F. Gao, Nat. Mater., 2018, 17, 703–709 CrossRef CAS.
- S. M. Hosseini, S. Wilken, B. Sun, F. Huang, S. Y. Jeong, H. Y. Woo, V. Coropceanu and S. Shoaee, Adv. Energy Mater., 2023, 13, 2203576 CrossRef CAS.
- F. D. Eisner, M. Azzouzi, Z. Fei, X. Hou, T. D. Anthopoulos, T. J. S. Dennis, M. Heeney and J. Nelson, J. Am. Chem. Soc., 2019, 141, 6362–6374 CrossRef CAS PubMed.
- Y. Xu, H. Yao, L. Ma, L. Hong, J. Li, Q. Liao, Y. Zu, J. Wang, M. Gao, L. Ye and J. Hou, Angew. Chem., Int. Ed., 2020, 59, 9004–9010 CrossRef CAS PubMed.
- Z. Zhang, Y. Li, G. Cai, Y. Zhang, X. Lu and Y. Lin, J. Am. Chem. Soc., 2020, 142, 18741–18745 CrossRef CAS.
- X. Gu, Y. Wei, N. Yu, J. Qiao, Z. Han, Q. Lin, X. Han, J. Gao, C. Li, J. Zhang, X. Hao, Z. Wei, Z. Tang, Y. Cai, X. Zhang and H. Huang, CCS Chem., 2023, 5, 2576–2588 CrossRef CAS.
- C. Yang, Q. An, M. Jiang, X. Ma, A. Mahmood, H. Zhang, X. Zhao, H. F. Zhi, M. H. Jee, H. Y. Woo, X. Liao, D. Deng, Z. Wei and J. L. Wang, Angew. Chem., Int. Ed., 2023, 62, e202313016 CrossRef CAS.
- H. Chen, H. Liang, Z. Guo, Y. Zhu, Z. Zhang, Z. Li, X. Cao, H. Wang, W. Feng, Y. Zou, L. Meng, X. Xu, B. Kan, C. Li, Z. Yao, X. Wan, Z. Ma and Y. Chen, Angew. Chem., Int. Ed., 2022, 61, e202209580 CrossRef CAS.
- M. Xie, Y. Shi, L. Zhu, J. Zhang, Q. Cheng, H. Zhang, Y. Yan, M. Zhu, H. Zhou, K. Lu and Z. Wei, Energy Environ. Sci., 2023, 16, 3543–3551 RSC.
- Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed.
- Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C. J. Su, T. Li, J. Wang, J. Zhu, Y. Sun, C. Wang and X. Zhan, J. Am. Chem. Soc., 2016, 138, 2973–2976 CrossRef CAS PubMed.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- Y. Cui, H. Yao, J. Zhang, K. Xian, T. Zhang, L. Hong, Y. Wang, Y. Xu, K. Ma, C. An, C. He, Z. Wei, F. Gao and J. Hou, Adv. Mater., 2020, 32, 1908205 CrossRef CAS PubMed.
- C. Li, J. Zhou, J. Song, J. Xu, H. Zhang, X. Zhang, J. Guo, L. Zhu, D. Wei, G. Han, J. Min, Y. Zhang, Z. Xie, Y. Yi, H. Yan, F. Gao, F. Liu and Y. Sun, Nat. Energy, 2021, 6, 605–613 CrossRef CAS.
- D. Luo, Z. Jiang, C. Shan, L. Li, C. Duan, Q. Liu, Z. Wang, K. Wang, B. Xu and A. K. K. Kyaw, ACS Appl. Mater. Interfaces, 2022, 14, 24374–24385 CrossRef CAS PubMed.
- D. Luo, W. Jang, D. D. Babu, M. S. Kim, D. H. Wang and A. K. K. Kyaw, J. Mater. Chem. A, 2022, 10, 3255–3295 RSC.
- G. Zhang, X. K. Chen, J. Xiao, P. C. Y. Chow, M. Ren, G. Kupgan, X. Jiao, C. C. S. Chan, X. Du, R. Xia, Z. Chen, J. Yuan, Y. Zhang, S. Zhang, Y. Liu, Y. Zou, H. Yan, K. S. Wong, V. Coropceanu, N. Li, C. J. Brabec, J. L. Bredas, H. L. Yip and Y. Cao, Nat. Commun., 2020, 11, 3943 CrossRef CAS.
- F. Lin, K. Jiang, W. Kaminsky, Z. Zhu and A. K. Jen, J. Am. Chem. Soc., 2020, 142, 15246–15251 CrossRef CAS PubMed.
- Z. Zhou, W. Liu, G. Zhou, M. Zhang, D. Qian, J. Zhang, S. Chen, S. Xu, C. Yang, F. Gao, H. Zhu, F. Liu and X. Zhu, Adv. Mater., 2020, 32, 1906324 CrossRef CAS PubMed.
- Y. Shi, Y. Chang, K. Lu, Z. Chen, J. Zhang, Y. Yan, D. Qiu, Y. Liu, M. A. Adil, W. Ma, X. Hao, L. Zhu and Z. Wei, Nat. Commun., 2022, 13, 3256 CrossRef CAS PubMed.
- H. Liang, X. Bi, H. Chen, T. He, Y. Lin, Y. Zhang, K. Ma, W. Feng, Z. Ma, G. Long, C. Li, B. Kan, H. Zhang, O. A. Rakitin, X. Wan, Z. Yao and Y. Chen, Nat. Commun., 2023, 14, 4707 CrossRef CAS PubMed.
- K. Liu, Y. Jiang, G. Ran, F. Liu, W. Zhang and X. Zhu, Joule, 2024, 8, 835–851 CrossRef CAS.
- Y. Zou, H. Chen, X. Bi, X. Xu, H. Wang, M. Lin, Z. Ma, M. Zhang, C. Li, X. Wan, G. Long, Y. Zhaoyang and Y. Chen, Energy Environ. Sci., 2022, 15, 3519–3533 RSC.
- H. Liang, H. Chen, P. Wang, Y. Zhu, Y. Zhang, W. Feng, K. Ma, Y. Lin, Z. Ma, G. Long, C. Li, B. Kan, Z. Yao, H. Zhang, X. Wan and Y. Chen, Adv. Funct. Mater., 2023, 33, 2301573 CrossRef CAS.
- Z. Yao, X. Cao, X. Bi, T. He, Y. Li, X. Jia, H. Liang, Y. Guo, G. Long, B. Kan, C. Li, X. Wan and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202312630 CrossRef CAS PubMed.
- H. Chen, X. Cao, P. Wang, F. Huang, Y. Zhang, H. Liang, X. Bi, T. He, W. Feng, Y. Guo, Z. Ma, G. Long, Z. Yao, B. Kan, C. Li, X. Wan and Y. Chen, J. Mater. Chem. A, 2023, 11, 25368–25376 RSC.
- J. Wang, H. Chen, C. Li, Y. Lin, Y. Yang, Z. Ma and Y. Lu, Chem. Eng. J., 2023, 477 Search PubMed.
- K. Liu, Y. Jiang, F. Liu, G. Ran, F. Huang, W. Wang, W. Zhang, C. Zhang, J. Hou and X. Zhu, Adv. Mater., 2023, 35, 2300363 CrossRef CAS.
- H. Chen, Y. Zou, H. Liang, T. He, X. Xu, Y. Zhang, Z. Ma, J. Wang, M. Zhang, Q. Li, C. Li, G. Long, X. Wan, Z. Yao and Y. Chen, Sci. China: Chem., 2022, 65, 1362–1373 CrossRef CAS.
- Y. Zhao, H. Chen, C. Zhu, J. Yuan, Y. Li, J. Hai, Y. Hu, L. Jiang, G. Chen and Y. Zou, Mater. Chem. Front., 2020, 4, 3310–3318 RSC.
- Z. Chen, J. Ge, Y. Guo, M. Zhao, J. Shi, Y. Qiu, E. Zhou and Z. Ge, ACS Energy Lett., 2022, 7, 3432–3438 CrossRef CAS.
- D. Qiu, J. Zhang, K. Lu and Z. Wei, Nano Res., 2023, 16, 11630–11637 CrossRef CAS.
- L. Zhang, K. Yang, D. Qiu, J. Zhang, Z. Wei and K. Lu, Chem. Eng. J., 2024, 481, 148648 CrossRef CAS.
- Y. Ding, S. Xiong, M. Li, M. Pu, Y. Zhu, X. Lai, Y. Wang, D. Qiu, H. Lai and F. He, Small, 2023, 2309169, DOI:10.1002/smll.202309169.
- X. Si, Y. Huang, W. Shi, R. Wang, K. Ma, Y. Zhang, S. Wu, Z. Yao, C. Li, X. Wan and Y. Chen, Adv. Funct. Mater., 2023, 33, 2306471 CrossRef CAS.
- T. Duan, W. Feng, Y. Li, Z. Li, Z. Zhang, H. Liang, H. Chen, C. Zhong, S. Jeong, C. Yang, S. Chen, S. Lu, O. A. Rakitin, C. Li, X. Wan, B. Kan and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202308832 CrossRef CAS PubMed.
- T. Xu, Z. Luo, R. Ma, Z. Chen, T. A. Dela Pena, H. Liu, Q. Wei, M. Li, C. Zhang, J. Wu, X. Lu, G. Li and C. Yang, Angew. Chem., Int. Ed., 2023, 62, e202304127 CrossRef CAS.
- W. Wei, C. Zhang, Z. Chen, W. Chen, G. Ran, G. Pan, W. Zhang, P. Muller-Buschbaum, Z. Bo, C. Yang and Z. Luo, Angew Chem. Int. Ed. Engl., 2024, 63, e202315625 CrossRef CAS.
- X. Ran, Y. Shi, D. Qiu, J. Zhang, K. Lu and Z. Wei, Nanoscale, 2023, 15, 18291–18299 RSC.
- L. Zhang, Z. Zhang, D. Deng, H. Zhou, J. Zhang and Z. Wei, Adv. Sci., 2022, 9, e2202513 CrossRef.
- J.-W. Lee, C. Sun, C. Lee, Z. Tan, T. N.-L. Phan, H. Jeon, D. Jeong, S.-K. Kwon, Y.-H. Kim and B. J. Kim, ACS Energy Lett., 2023, 8, 1344–1353 CrossRef CAS.
- H. Zhuo, X. Li, J. Zhang, S. Qin, J. Guo, R. Zhou, X. Jiang, X. Wu, Z. Chen, J. Li, L. Meng and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202303551 CrossRef CAS PubMed.
- J. Wu, Z. Ling, L. R. Franco, S. Y. Jeong, Z. Genene, J. Mena, S. Chen, C. Chen, C. M. Araujo, C. F. N. Marchiori, J. Kimpel, X. Chang, F. H. Isikgor, Q. Chen, H. Faber, Y. Han, F. Laquai, M. Zhang, H. Y. Woo, D. Yu, T. D. Anthopoulos and E. Wang, Angew. Chem., Int. Ed., 2023, 62, e202302888 CrossRef CAS PubMed.
- M. Lv, Q. Wang, J. Zhang, Y. Wang, Z. G. Zhang, T. Wang, H. Zhang, K. Lu, Z. Wei and D. Deng, Adv. Mater., 2023, 36, 2310046 CrossRef.
- H. Chen, Z. Zhang, P. Wang, Y. Zhang, K. Ma, Y. Lin, T. Duan, T. He, Z. Ma, G. Long, C. Li, B. Kan, Z. Yao, X. Wan and Y. Chen, Energy Environ. Sci., 2023, 16, 1773–1782 RSC.
- H. Chen, B. Kan, P. Wang, W. Feng, L. Li, S. Zhang, T. Chen, Y. Yang, T. Duan, Z. Yao, C. Li, X. Wan and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202307962 CrossRef CAS.
- T. Zhang, Y. Xu, H. Yao, J. Zhang, P. Bi, Z. Chen, J. Wang, Y. Cui, L. Ma, K. Xian, Z. Li, X. Hao, Z. Wei and J. Hou, Energy Environ. Sci., 2023, 16, 1581–1589 RSC.
- R. Zeng, L. Zhu, M. Zhang, W. Zhong, G. Zhou, J. Zhuang, T. Hao, Z. Zhou, L. Zhou, N. Hartmann, X. Xue, H. Jing, F. Han, Y. Bai, H. Wu, Z. Tang, Y. Zou, H. Zhu, C. C. Chen, Y. Zhang and F. Liu, Nat. Commun., 2023, 14, 4148 CrossRef CAS PubMed.
- Z. G. Zhang, Y. Yang, J. Yao, L. Xue, S. Chen, X. Li, W. Morrison, C. Yang and Y. Li, Angew. Chem., Int. Ed., 2017, 56, 13503–13507 CrossRef CAS.
- D. Qiu, H. Zhang, C. Tian, J. Zhang, L. Zhu, Z. Wei and K. Lu, Adv. Mater., 2023, 35, 2307398 CrossRef CAS.
- Z. Zhang, Z. Li, P. Wang, H. Chen, K. Ma, Y. Zhang, T. Duan, C. Li, Z. Yao, B. Kan, X. Wan and Y. Chen, Adv. Funct. Mater., 2023, 33, 2214248 CrossRef CAS.
- Z. Zhang, Z. Li, B. Kan, T. Chen, Y. Zhang, P. Wang, Z. Yao, C. Li, B. Zhao, M. Li, T. Duan, X. Wan and Y. Chen, Nano Energy, 2023, 116, 108766 CrossRef CAS.
- Y. Huang, X. Si, R. Wang, K. Ma, W. Shi, C. Jiang, Y. Lu, C. Li, X. Wan and Y. Chen, J. Mater. Chem. A, 2023, 11, 14768–14775 RSC.
- F. Liu, L. Zhou, W. Liu, Z. Zhou, Q. Yue, W. Zheng, R. Sun, W. Liu, S. Xu, H. Fan, L. Feng, Y. Yi, W. Zhang and X. Zhu, Adv. Mater., 2021, 33, 2100830 CrossRef CAS.
- M. Wang, Y. Shi, Z. Zhang, Y. Shen, M. Lv, Y. Yan, H. Zhou, J. Zhang, K. Lv, Y. Zhang, H. Peng and Z. Wei, Nanoscale Horiz., 2023, 8, 1073–1081 RSC.
- H. Liu, Z. Chen, R. Peng, Y. Qiu, J. Shi, J. Zhu, Y. Meng, Z. Tang, J. Zhang, F. Chen and Z. Ge, Chem. Eng. J., 2023, 474, 145807 CrossRef CAS.
- Z. Chen, J. Zhu, D. Yang, W. Song, J. Shi, J. Ge, Y. Guo, X. Tong, F. Chen and Z. Ge, Energy Environ. Sci., 2023, 16, 3119–3127 RSC.
- Y. F. Shen, H. Zhang, J. Zhang, C. Tian, Y. Shi, D. Qiu, Z. Zhang, K. Lu and Z. Wei, Adv. Mater., 2023, 35, 2209030 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |
