Recent advances in carboxylate-based indium(III)–organic frameworks
Yong-Jie
Song
,
Yi-Hao
Zuo
,
Zi-Feng
Li
* and
Gang
Li
 *
*
College of Chemistry, Zhengzhou University, Zhengzhou 450001, Henan, P. R. China. E-mail: gangli@zzu.edu.cn; lzf2004@zzu.edu.cn
First published on 18th September 2024
Abstract
Indium-based metal–organic frameworks (In-MOFs) are gaining popularity as a research topic because of their distinct structural and practical advantages. Researchers understand that the indium atom has a comparatively big radius and is ecologically benign. Importantly, the In(III) cation has a distinct electronic configuration with a high cyclic p-block, and establishes strong coordination bonds with atoms such as O, N, and S in various Lewis acid environments. As a result, the stable In-MOFs with varied structural kinds and chemical properties deserve thorough exploration. The research progress must be properly evaluated to understand the future research trend of In-MOFs better. Furthermore, this analysis of In-MOFs will close gaps in the current literature, and a full assessment of such MOFs will immensely benefit future research. It should be noted that we are primarily interested in MOFs built using carboxylic acid ligands. Herein, we will discuss the preparation methodologies, structural features, and diverse applications of MOFs made up of indium(III) nodes and various linkages, as well as current and prospects.
1. Introduction
Metal–organic frameworks (MOFs) are a novel form of porous crystalline material that offers structural and functional tailorability.1–3 They are commonly employed in adsorption separation,4–6 chemical sensing,7–9 catalysis,10–12 and proton conduction.13–15 Although MOFs have been thoroughly studied for many years, there are still significant obstacles in exploring their designability and application to the real world. Researchers have learned that almost all metal elements in the periodic table can be used as nodes to produce MOFs, leading to a wide range of MOF structures and applications, thanks to the rapid advancements in crystal engineering and synthesis technologies. Divalent metal cations were the most widely used cations in the construction of MOFs for a long time, followed by trivalent or tetravalent cations (Fe3+, V3+, Cr3+, Zr4+, Hf4+, etc.) and lanthanide cations (Dy3+, Tb3+, Eu3+ and so on).16 Recently, the third main-group metals have gained popularity as an attractive family due to the advantages of low toxicity, relative abundance of reserves on earth, low cost, and structural stability and functional diversity.17 Indium as a representative element in the third main group, firstly, compared with other trivalent metals (Cr3+, Al3+, Sc3+, Ga3+, etc.) constructed MOFs, often requires the addition of strong acids or competing ligands to regulate crystal formation due to their kinetic inertness when bonding with ligands. The larger radius of In3+ ions (0.94 Å) greatly boosts the kinetic instability of the coordination, promotes the ligand exchange rate, and raises the likelihood of achieving a single-crystal structure. Secondly, based on the HSAB principle, in the same coordination environment, high valence metals (hard acids) with high charge density often preferentially bind to O (hard bases) on the ligand, forming MOFs with strong coordination bonds. Therefore, In-MOFs usually have good stability.17Based on their distinct structural advantages, indium-based MOFs (In-MOFs) have garnered a lot of attention in the development of diverse MOFs during the last two decades. As shown in Chart 1, from 2002 to 2024, the related reports on In-MOFs and their derivatives, while not significantly increasing, do show an upward trend, implying that there is still a large room for designing and preparing more In-MOFs to investigate their potential applications. Naturally, In-MOFs have similar general properties to other MOFs, such as high crystallinity, adjustable pore size/shape, permanent porosity, etc. More and more scientists are concentrating on the indium atom despite its relatively modest and dispersed distribution in the earth's crust18 since it is less poisonous than rare earth metals, which is more in line with the demands of green chemistry.19,20 The specific reasons are as follows: firstly, in terms of electronic structure, the In3+ center has high valence chemical properties and a large ionic radius.21–23 Furthermore, there is a valence electron on the p-orbital of metallic indium, while the valence layer electrons of transition metals are mainly concentrated on the s-orbital (such as Zn, Cd, Ni, Co and other metals) or d-orbital (such as Cu, Au, Ag and other metals). The high-energy p-orbital of the unsaturated In3+ cation makes it easier to acquire electrons in diverse Lewis acid reactions, which is conducive to the formation of coordination bonds during the reaction;24–28 secondly, by altering its valence state, the low-toxicity p-block metal In(III) may create structures with exceptional coordination and high stability in both air and moisture.29,30 These structures have found widespread application in the domains of chemical sensors,31–33 catalysis,34–36 adsorption, and separation.37–39 That's because In-MOFs have the advantages of diverse structures with different dimensionalities, high stability, and easy preparation, which are potentially utilized in many fields. For example, In-MOFs are celebrated for their exceptional porosity, making them ideal candidates for gas storage and separation applications. Moreover, indium MOFs have begun to make strides in catalysis and photocatalysis applications. The other explorations of biomedical and sensing applications on In-MOFs have attracted much attention as well. However, while there have been individual studies summarizing In-MOFs, they are outdated and insufficiently structured. As a result, we would want to give a complete examination of the setup, structural properties, and applications of these MOFs.
 | ||
| Chart 1 The number of publications per year dedicated to In-MOFs from 2002 to 2024 (from Web of Science up to August 2024). | ||
This review categorizes and analyzes In-MOFs based on the type of organic ligands. Given that the In-MOFs published thus far have primarily been assembled by carboxylic acid ligands,40 we will discuss and comment on the MOFs constructed by such ligands in the previous eight years, focusing on synthesis methodologies, structural features, properties, applications, and so on. We believe that a complete analysis of the most recent advances in In-MOFs would be extremely beneficial for future study. Table 1 summarizes the reported In-MOFs in recent years.
| MOF | Synthetic method | Structure | Property | Ref. |
|---|---|---|---|---|
| [Me2NH2][In(abtc)]·solvents (F29) | Solvothermal synthesis | 3D | Sensing performance | 21 |
| [Me2NH2][In(bptc)]·solvents (F30) | Solvothermal synthesis | 3D | Sensing performance | 21 |
| [In(BDC-NH 3 )(BDC-NH 2 )]·1.6DMF·1.9H 2 O | Solvothermal synthesis | 3D | — | 22 |
| (TPP)+[In(FcDCA)2]·5H2O | Hydrothermal synthesis | 2D | Redox activity | 25 |
| (Hbpy)+[In(FcDCA)(OX2−)]·H2O | Hydrothermal synthesis | 1D | Redox activity | 25 |
| (TPP)+2[In2(FcDCA)3(OX2−)]·5H2O | Hydrothermal synthesis | 3D | Redox activity | 25 |
| In-DM-BP | Solvothermal synthesis | 3D | Selective | 26 |
| In-DH-BP | Solvothermal synthesis | 3D | Selective | 26 |
| In-HMe-BPA | Solvothermal synthesis | 3D | Selective | 26 |
| In-TM-BF | Solvothermal synthesis | 3D | Selective | 26 |
| [In(2-Hqlca)2Cl3·3H2O] (T12) | Solvothermal synthesis | 3D | Iodine capture | 30 |
| [In(2-QLBM)(2-qlca)Cl2]2·CH3CN·2H2O] (T13) | Solvothermal synthesis | 3D | Iodine capture | 30 |
| JOU-24 | Solvothermal synthesis | 2D | Sensing performance | 33 |
| MIL-68(In) | Solvothermal synthesis | 3D | Catalyst | 34 |
| NUC-66 | Solvothermal synthesis | 3D | Catalyst | 35 |
| SNNU-120 | Solvothermal synthesis | 3D | Gas separation | 39 |
| SNNU-121 | Solvothermal synthesis | 3D | Gas separation | 39 |
| Na[In3(odpt)2(OH)2(H2O)2](H2O)4 (S1) | Hydrothermal synthesis | — | Dielectric behavior | 41 |
| {[In(btc)(H2O)2]·2H2O}n (S2) | Hydrothermal synthesis | — | Dielectric behavior | 41 |
| In 2 O 3 PHRs | Template method | — | Sensor | 43 |
| [In6(μ-OH)2L14(H2O)6]·(solv)x (AFMOF) | Hydrothermal synthesis | 3D | Luminescence | 44 |
| {In2L2(μ2-O)(H2O)3}n (S3) | Solvothermal synthesis | 3D | Photoluminescence | 45 |
| YCM-31 | Solvothermal synthesis | 2D | — | 47 |
| YCM-32 | Solvothermal synthesis | 3D | — | 47 |
| In/Gd-CBDA | Solvothermal synthesis | — | Gas adsorption | 48 |
| InPF-17 | Conventional heating and microwave synthesis | 2D | Catalyst | 52 |
| InPF-18 | Conventional heating and microwave synthesis | 3D | Catalyst | 52 |
| InPF-19 | Conventional heating and microwave synthesis | 3D | — | 52 |
| [In2(BTA)2μ-NO3·8H2O] (S4) | Solvothermal/oil bath/microwave-assisted synthesis | 3D | Sorption properties | 53 |
| MIL-68 | Oil bath synthesis | 3D | — | 56 |
| MIL-68-NO 2 | Oil bath synthesis | 3D | — | 56 |
| MIL-68-NH 2 | Oil bath synthesis | 3D | — | 56 |
| HKUST-1 | Electrochemical deposition | — | Microelectronic device | 59 |
| {[In2L32](Me2NH2)2(DMF)2(H2O)4.5}n (S6) | Solvothermal synthesis | 3D | Photoluminescent | 62 |
| {[In2L42](Me2NH2)2(DMF)4(H2O)3}n (S7) | Solvothermal synthesis | 3D | Photoluminescent | 62 |
| InPF-50 | Solvothermal synthesis | 2D | Catalysts | 63 |
| InPF-51 | Solvothermal synthesis | 2D | Catalysts | 63 |
| FJU-10 | Solvothermal synthesis | 3D | Proton conduction | 64 |
| [In(BTB)(2,2′-bipy)]·NMP (T8) | Solvothermal synthesis | 2D | Fluorescence | 65 |
| [In(BTB)(2,2′-bipy)]·NMP·2H2O (T9) | Solvothermal synthesis | 2D | Fluorescence | 65 |
| [In(BTB)(2,2′-bipy)]·NMP·0.5NMP (T10) | Solvothermal synthesis | 2D | Fluorescence | 65 |
| CAU-43 | Solvothermal synthesis | — | Redox activity | 69 |
| In-MIL-53-FcDC_a | Solvothermal synthesis | — | Redox activity | 69 |
| In-FcDC | Solvothermal synthesis | — | Redox activity | 69 |
| In-MIL-53-FcDC_b | Solvothermal synthesis | — | Redox activity | 69 |
| In(OH)CSA | Solvothermal synthesis | 2D | — | 70 |
| In(OH)PDG | Solvothermal synthesis | 2D | — | 70 |
| [(CH3)2NH2][In(EBDC)]·2DMF·2DMSO·H2O (T4) | Solvothermal synthesis | 3D | Gas sorption | 72 |
| In-soc-MOF-1b | Solvothermal synthesis | — | Hydrogen storage | 73 |
| In-soc-MOF-1c | Solvothermal synthesis | — | Hydrogen storage | 73 |
| MOF-In1 | Solvothermal synthesis | 3D | Drug release | 74 |
| MOF-In2 | Solvothermal synthesis | 3D | Drug release | 74 |
| NU-50 | Solvothermal synthesis | — | Sorption | 77 |
| UTSA-22 | Solvothermal synthesis | 3D | Storage gas | 78 |
| {[CH3NH3]-[In(TPTA)]·2(NMF)} (MOF 1) | Solvothermal synthesis | 3D | Gas sorption | 79 |
| {[In2(TPTA)(OH)2]·2(H2O)·(DMF)} (MOF 2) | Solvothermal synthesis | 3D | Gas sorption | 79 |
| [In(OH)bpydc] | Solvothermal synthesis | — | Fluorescence | 81 |
| InDCPN-Cl/Br/I | Solvothermal synthesis | 3D | CO2 fixation | 82 |
| In3L14·(CH3NH2CH3)3 (T14) | Solvothermal synthesis | 3D | Selective | 83 |
| In2L22·(CH3COO)2·(CH3)2NH2·(CH3)3NH·H2O (T15) | Solvothermal synthesis | 3D | Selective | 83 |
| (Me2NH2+){In(III)[Ni(C2S2(C6H4COO)2)2]}·3DMF·1.5H2O | — | 3D | Electrocatalysis | 88 |
| InOF-25 | Solvothermal synthesis | 3D | Electrocatalysis | 89 |
| InOF-26 | Solvothermal synthesis | 3D | Electrocatalysis | 89 |
| USTC-8(In) | Solvothermal synthesis | 3D | Photocatalysis | 93 |
| In-TPBD-20 | Solvothermal synthesis | — | Photocatalysis | 100 |
| In-TPBD-50 | Solvothermal synthesis | — | Photocatalysis | 100 |
| InPF-50 | Solvothermal synthesis | 2D | Catalytic reaction | 106 |
| InPF-51 | Solvothermal synthesis | 2D | Catalytic reaction | 106 |
| In12-GL | Solvothermal synthesis | 3D | Catalytic reaction | 107 |
| InOF-1 | Solvothermal synthesis | 3D | Catalytic reaction | 108 |
| NNU-32 | Solvothermal synthesis | — | Luminescence | 110 |
| V101 | Solvothermal synthesis | 3D | Luminescence | 113 |
| V102 | Solvothermal synthesis | 3D | Luminescence | 113 |
| [In(EBTC)][(CH3)2NH2](DMF)(H2O)5 (F9) | Solvothermal synthesis | 3D | Luminescence | 114 |
| [(CH3)2NH2][In(bpdc)2]·(DMF)2 (F10) | Solvothermal synthesis | 2D | Luminescence | 115 |
| [(CH3)2NH2]3[(In3Cl2)(bpdc)5]·(H2O)5(DMF)2.5 (F11) | Solvothermal synthesis | 2D | Luminescence | 115 |
| BUT-172 | Solvothermal synthesis | 3D | Luminescence | 116 |
| BUT-173 | Solvothermal synthesis | 3D | Luminescence | 116 |
| ZJU-28 | Solvothermal synthesis | — | Luminescence | 118 |
| InBTB-MOF | Solvothermal synthesis | — | Luminescence | 120 |
| JOU-25 | Solvothermal synthesis | 3D | Luminescence | 121 |
| BUT-205 | Solvothermal synthesis | 2D | Luminescence | 122 |
| HDU-1 | Solvothermal synthesis | Luminescence | 123 | |
| CPM-5 | Solvothermal synthesis | — | Xe separation | 127 |
| CPM-6 | Solvothermal synthesis | — | Xe separation | 127 |
| (Me2NH2)[In(SBA)2] (F15) | Solvothermal synthesis | — | Selective CO2 | 128 |
| (Me2NH2)[In(SBA)(BDC)] (F16) | Solvothermal synthesis | 2D | Selective CO2 | 128 |
| (Me2NH2) [In(SBA)(BDC-NH2)] (F17) | Solvothermal synthesis | 2D | Selective CO2 | 128 |
| (NH4)3[In3Cl2(BPDC)5] (F18) | Solvothermal synthesis | 2D | Selective CO2 | 128 |
| [In3(μ3O)(L)1.5(H2O)3][NO3]·5H2O·EtOH·1.5DMF (F19) | Solvothermal synthesis | 2D | Selective CO2 | 130 |
| InOF-23 | Solvothermal synthesis | 1D | Gas adsorption | 131 |
| [EMIM][In(ABDC)2]·DEF·H2O (In-ABDC-MOF) | Solvothermal synthesis | 3D | Gas adsorption | 132 |
| [In2(OH)2(TCPP)] (F20) | Solvothermal synthesis | — | NH3 capture | 133 |
| NUC-5 | — | 3D | Gas adsorption | 135 |
| In(aip) 2 | Solvothermal synthesis | 2D | Gas adsorption | 136 |
| FJI-H21 | Solvothermal synthesis | 3D | Adsorption dyes | 139 |
| JUC-210 | Solvothermal synthesis | 3D | Adsorption dyes | 140 |
| FJU-117 | Solvothermal synthesis | 3D | C2H2/CO2 separation | 149 |
| FJU-118 | Solvothermal synthesis | 3D | C2H2/CO2 separation | 149 |
| (Me2NH2)1.5[In1.5L92]·2DMF·2H2O (F28) | Solvothermal synthesis | 3D | C2H2/CO2 separation | 150 |
| ZJNU-121 | Solvothermal synthesis | 3D | C2H2/CH4 separation | 151 |
| JOU-23 | Solvothermal synthesis | 3D | Sensing ions | 155 |
| MFM-300(In) | Solvothermal synthesis | 3D | Sensing organic molecules | 156 |
| [In(BDC-NH2)(OH)]n (In1-NH2) | Solvothermal synthesis | 3D | Sensing organic molecules | 157 |
| MROF-1 | Solvothermal synthesis | 3D | Proton conduction | 164 |
| PCMOF-17 | Solvothermal synthesis | 2D | Proton conduction | 165 |
| FJU-16 | Solvothermal synthesis | 3D | Proton conduction | 166 |
| FJU-17 | Solvothermal synthesis | 3D | Proton conduction | 166 |
| [(CH3)2NH2][In(m-TTFTB)] (F32) | Solvothermal synthesis | 2D | Proton conduction | 168 |
| [(CH3)2NH2][In(TTFOC)] (F33) | Solvothermal synthesis | 2D | Proton conduction | 168 |
| In-BQ | Solvothermal synthesis | 2D | Proton conduction | 169 |
| [In2(OH)2(BPTC)]·6H2O (InOF-1) | Solvothermal synthesis | 3D | Proton conduction | 172 |
| FJU-302 | Solvothermal synthesis | — | Proton conduction | 173 |
| In-IPA | Solvothermal synthesis | — | Catalytic sites | 175 |
| MIL-68-In-NO 2 | Solvothermal synthesis | 3D | Proton conduction | 178 |
| MIL-68-In-Br | Solvothermal synthesis | 3D | Proton conduction | 178 |
2. Synthesis approaches of In-MOFs
According to the literature, the most common approach for In-MOF synthesis is hydrothermal/solvothermal. It also includes continuous flow synthesis, heating batch synthesis, ultrasonic-assisted batch synthesis, microwave approach, reflux technique, and electrochemical deposition, among other techniques. We'll go into more detail about each of these synthesis methods below.2.1. Hydrothermal/solvothermal approaches
One of the most common methods for producing MOFs is using hydrothermal procedures. Metal salts and organic ligands, as well as other materials that react poorly or not at all at ambient temperature, are usually mixed evenly in H2O before being sealed and heated in a reactor lined with PTFE. Because of this, the reactor is maintained for a considerable amount of time at a high enough temperature and pressure to enable the reactants to completely react before being cooled to produce single-crystal MOF products. Reactant concentration, temperature, reactant ratio, stirring duration, and reaction time are the primary parameters influencing the development of crystals. At the moment, this process for creating In-MOFs is more developed.For example, in 2020, Lu et al. reported two In-MOFs, Na[In3(odpt)2(OH)2(H2O)2](H2O)4 (S1) (odpt = 4,4′-oxydiphthalate) and {[In(btc)(H2O)2]·2H2O}n (S2) (btc = 1,2,3-benzenetricarboxylate), by hydrothermal method. They have different dielectric and bandgap properties due to the molecules’ varying degrees of polar solvation.41 By choosing In(NO3)3·xH2O/InCl3·4H2O as the metal salts to react with 1,1′-ferrocenedicarboxylic acid (H2FcDC) and oxalic acid (OX) linkages under hydrothermal conditions, Zhang and associates successfully prepared a range of ferrocene-based In-MOFs in 2023.25
Nowadays, solvothermal synthesis is the most popular approach for producing In-MOF. Although the reaction solvents are different, solvent-thermal synthesis operates on the same principles as hydrothermal synthesis. In addition to water, solvothermal synthesis can use various solvents such as CH3OH, CH3CH2OH, formic acid (HCOOH), and so on. Organic solvents are utilized to dissolve organic ligands because they are typically not easily soluble in water. One important observation is that organic solvents are occasionally used in coordination, which adds complexity to the relevant MOFs’ structures. In-MOFs are commonly synthesized using a variety of amide solvents, including N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMA), N-methylformamide (NMF), N,N-diethylformamide (DEF), and others.40 They were selected as reaction solvents for the following reasons: first, amides can improve ligand solubility and slowly break down at high temperatures to create amine bases and carboxylic acids, causing self-assembly. Due to the unique host–guest nature of MOFs, second, various solvents could play as templates to affect self-assembly and result in the formation of desired structures.
It is quite uncommon to use amide by itself as a solvent for the synthesis of In-MOFs, even though amide is typically selected as the reaction solvent. To our knowledge, the use of DMF alone as a solvent has only been limited to the synthesis of the MIL-68 series up to now. For example, in 2016, M. Oh's group utilized In(NO3)3 to react with benzenedicarboxylic acid (H2BDC), 2-bromoterephthalic acid (H2BDC-Br), or naphthalene-1,4-dicarboxylic acid (H2NDC), respectively, to prepare three In-MOFs, MIL-68, MIL-68-Br, and MOF-NDC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 (Fig. 1), where the reaction time is about 80 minutes.
42 (Fig. 1), where the reaction time is about 80 minutes.
 | ||
| Fig. 1 Preparation of three In-MOFs based on aromatic carboxylate ligands. Reproduced from ref. 42, with permission from American Chemical Society, Copyright 2016. | ||
In 2020, Ma and co-workers43 successfully prepared MIL-68(In) by dissolving In(NO3)3 and H2BDC in DMF and keeping it under 100 °C for 4 h. In comparison to the previous approach for synthesizing MIL-68,42 this synthesis protocol is time-consuming and unsuitable for high-volume production.
DMA can also be used as a reaction solvent for the synthesis of In-MOFs, and only one example has been successfully synthesized so far using only DMA as a solvent by Xue's group, in 2020.44 They prepared a rare tritiated triple MOF, [In6(μ-OH)2L14(H2O)6]·(solv)x (AFMOF) by dissolving In(NO3)3, oxalylbis(aza-nediyl)diisophthalic acid (H4L1) and benzoic acid in a DMA solution at 105 °C for 1.5 d.
In-MOFs are often made by adding one or more additional solvents to amide solvents at the same time. For instance, in 2017,45 Meng et al. adopted InCl3 to react with the tetracarboxylic acid derivative of 1,1′-biphenol (H4L2) in a combination of solvents including DMF, DMSO, THF, H2O, and HOAc to create a new indium-based chiral metal–organic skeleton, {In2L2(μ2-O)(H2O)3}n (S3). At 100 °C for 24 hours, it yielded up to 72%, a relatively high yield for In-MOF synthesis.
In 2019, Chen's team46 synthesized NH2-MIL-68 by reacting In(NO3)3 with NH2-BDC in a mixed solvent of DMF and pyridine. It was then used to create a MOF@COF hybrid material by reacting with tris(4-formylphenyl)amine and tris(4-aminophenyl)amine (Fig. 2). The material combined the advantages of COF and MOF, overcoming MOF's low aqueous stability.
 | ||
| Fig. 2 Preparation process of MOF@COF hybrid material. Reproduced from ref. 46, with permission from Springer Nature, copyright 2019. | ||
In the same year, Genna et al. added InCl3 and 4,4′,4′′-benzene-1,3,5-triyl-tribenzoic acid (H3BTB) to the spirocyclic ammonium cation spPPCl/spPPBr, respectively, and used a combination of DMF, dioxane, and water as the reaction solvent. Two MOFs, YCM-31 and YCM-32, backbone isomers of one another, were successfully synthesized.47
By dissolving In(NO3)3·4H2O, Gd(NO3)3·6H2O, and [5,5′-(carbonylbis(azanediyl))-diisophthalic acid] (CBDA) in a mixed solvent of DMA, NMF, and H2O in the presence of HNO3, Li's group developed heterogeneous metallic MOF In/Gd-CBDA in 2020.48 It was discovered that the kind and makeup of the solvent have a significant role in triggering the identification of carboxylic acid groups at various locations with various metal coordination sites.
Using DMF/dioxane solvent combinations, Genna's group49 synthesized several In-MOFs in 2021, including MIL-68, QMOF-2, ATF-1, and ZJU-28. A series of methods was employed to comprehend the mutually beneficial relationship between dioxane and DMF. Dioxane functions as a ligand for direct coordination with In and is essential to the native solvent, decreasing the dielectric constant. Additionally, InCl3(dioxane)2(H2O), a novel indium solvent complex polymer, was identified and demonstrated to be a productive source of In.
In conclusion, the current In-MOF synthesis relies heavily on the solvothermal approach, which, in addition to outstanding production efficiency, provides for regulated reaction steps that favor In-MOF crystallization.
2.2. Microwave approaches
The fundamental approach of microwave synthesis is to use microwave technology to swiftly absorb electromagnetic radiation from tiny molecules with unequal charge distribution, forcing them to rotate and collide rapidly.50,51 This enables polar molecules to oscillate in reaction to changes in the external electric field, resulting in thermal effects that quickly raise the temperature of the reactants. It differs from traditional heating methods in that it consumes less energy, is extremely efficient, and delivers uniform heating.For example, in 2017 Aguirre-Diaz's group prepared six novel In-MOFs by conventional solvothermal and microwave methods. The synthesis processes are shown in Fig. 3. When In(OAc)3 reacts with 5-(4-carboxy-2-nitrophenoxy)isophthalic acid (H3poph), 1,10-phenanthroline (1,10-phen), 1,7-phenanthroline (1,7-phen), 4,4′-bipy, or 2,2′-bipyridine (2,2′-bipy), respectively, to get six novel In-MOFs, [In8(OH)6(poph)6(H2O)4]·3H2O (InPF-16), [In(poph)(2,2′-bipy)]·3H2O (InPF-17), [In3(OH)3(poph)2(4,4′-bipy)]·4H2O (InPF-18), [In2(poph)2(4,4′-bipy)2]·3H2O (InPF-19), [In(OH)(Hpoph)]·0.5(1,7-phen) (InPF-20), and [In(poph)(1,10-phen)]·4H2O (InPF-21). Compared to the two synthesis processes, it can be seen that microwave synthesis only takes a few minutes, whereas solvothermal synthesis usually requires several days, indicating that microwave synthesis is a viable method for producing materials with a rapid reaction time and elevated yield.52
 | ||
| Fig. 3 Preparation of In-MOFs by the reaction of H3popha with In(OAc)3. Reproduced from ref. 52, with permission from Wiley-VCH GmbH, Copyright 2016. | ||
In 2023, Jangir's team53 prepared one In-MOF, [In2(BTA)2μ-NO3·8H2O] (S4), by three methods: microwave synthesis, oil bath in a round bottom flask (RBF), and solvent heat in a vial using In(NO3)3 and benzene-1,2,4,5-tetracarboxylic acid (H4BTA) (Fig. 4). All three procedures produced the predicted results, although the microwave-assisted method had a substantial benefit in terms of shorter reaction time. By reducing the 8-hour oil bath synthesis time to 30 minutes, the microwave approach confirmed its effectiveness and speed in the In-MOF synthesis.
 | ||
| Fig. 4 Synthesis of S4 in different conditions. Reproduced from ref. 53, with permission from Springer Nature, copyright 2023. | ||
Furthermore, In-MIL-68 can be produced utilizing both microwave-assisted and conventional solvothermal approaches. Microwave-assisted solvothermal approach is more efficient than traditional solvothermal methods. The microwave-assisted technique is effective because of its unique heating mechanism, which ensures that the entire reaction mixture is heated evenly and effectively. Shorter synthesis durations resulted in improved kinetics for the process. These findings provide valuable information regarding the synthesis of In-MOFs as well as potential real-world applications for the development of novel synthetic methodologies.
2.3. Oil bath approaches
Oil is used as a heat bath ingredient in the heating process known as “oil bath synthesis”. The hot-bath approach involves immersing a container in a hot material and gradually raising its temperature to a predefined level. The temperature of the hot substance is then held for a predetermined amount of time before being gradually lowered to enhance the material's homogeneity and the container's thermal conduction properties. These techniques have been used to manufacture MOFs based on indium.53–55The particular MIL-68 series that were synthesized using these techniques are the most indicative of these. As exhibited in Fig. 5, Ji's group created MIL-68, MIL-68-X (X = NH2 or NO2) in 2018, by reacting In(NO3)3·xH2O with H2BDC, H2BDC-NH2, and H2BDC-NO2, respectively, in an oil bath reaction at 100 °C in less than one hour.56 Furthermore, Wang et al.57 synthesized the crude products by dissolving H2BDC and indium nitrate hydrate in DMF solution, heating the mixture in an oil bath at 120 °C for 30 minutes, and then washing and purifying the mixture to produce MIL-68 with good crystallinity. While the reaction time was lowered, the yield was lower than with the conventional solvothermal technique.
 | ||
| Fig. 5 Schematic representation for the construction of MIL-68, MIL-68-NO2 and MIL-68-NH2. Reproduced from ref. 56, with permission from American Chemical Society, Copyright 2018. | ||
The oil bath approach has the advantage of making the response visible to the human eye, allowing for easy monitoring. This enables precise control of the reaction time and temperature as required.
2.4. Other approaches
Along with the many popular synthetic techniques listed above, some relatively specialized techniques can also be adapted to synthesize In-MOFs.58 These techniques include electrochemical deposition,59 heated batch synthesis (bs-th), ultrasound-assisted batch synthesis (bs-us), and unremitting flow synthesis (cf)60 as depicted in Fig. 6. | ||
| Fig. 6 Three methods for synthesizing S5. Reproduced from ref. 60, with permission from American Chemical Society, Copyright 2016. | ||
For instance, in 2016, Stock's group successfully prepared In-MOF [In2(OH)2(H2TCPP)]·3DMF·4H2O (S5) (H6TCPP = 4-tetracarboxyphenylporphyrin) using three approaches: ultrasound-assisted, heated batch synthesis, and unremitting flow preparation, respectively. The starting materials used were H6TCPP and In(NO3)3, and the solvents were DMF and H2O. Using these diverse synthetic approaches, one may efficiently control the nucleation rate and crystal size. Furthermore, HKUST-1 on indium tin oxide (ITO) was effectively created in 2017 by Tang et al.59 by a two-step electrochemical synthesis: electrodeposition of Cu on top of ITO, then electrooxidation of Cu/ITO to HKUST-1/ITO. Comparing this procedure to the usual method, it is easy to use, quick, gentle, and environmentally beneficial.
In 2020, Sinnwell et al.61 prepared sod-In MOF using solid-phase synthesis. The details are as follows: In(NO3)3·xH2O, imidazole (HIm), and 4,5-imidazoledicarboxylic acid (H3ImDC) were placed into the XRD reaction chamber in a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. To avoid air contact with the powdered sample combination, add the DMF reservoir (40 μL) immediately after sampling. Then it was gradually heated to 40 °C, and continuous in situ XRD detection began. This MOF is worth a continuous reaction lasting about 23 hours.
2. To avoid air contact with the powdered sample combination, add the DMF reservoir (40 μL) immediately after sampling. Then it was gradually heated to 40 °C, and continuous in situ XRD detection began. This MOF is worth a continuous reaction lasting about 23 hours.
A single-crystal of In-MOFs may only be formed after carefully selecting the solvent, taking into account its polarity, pH, and reaction temperature. These qualities complicate solvents’ involvement in synthesis since they might play as ligands, guests, templates, or all of the above. Moreover, acid modifiers (HF, HNO3, HCl, HCOOH, CH3COOH) must be included for the production of In-MOFs since they occasionally aid in the products’ crystallization.48,62
For example, in 2016, Chen et al.62 successfully synthesized two unusual indium-organic backbones by reacting In(NO3)3 with 5,5′-(butane-1,4-diylbis(oxy))diisophthalic acid (H4L3) and 5,5′-(1,4-phenylenebis(methylene))bis(oxy)diisophthalic acid (H4L4) in a mixed solvent of concentrated HNO3, DMF, and distilled water, respectively, namely, {[In2L32](Me2NH2)2(DMF)2(H2O)4.5}n (S6) and {[In2L42](Me2NH2)2(DMF)4(H2O)3}n (S7). It was discovered during the synthesizing process. Inorganic acid (HNO3) has a favorable effect on crystal formation. When inorganic acid was introduced, colorless crystals formed; when HNO3 was absent, only white turbidite formed. As a result, HNO3 helps to induce crystal assembly and regulates solution pH. Furthermore, the reaction temperature is critical for indium MOF self-assembly. In-MOFs are typically produced in organic solutions (DMF, DMA) at 80 to 150 °C.
3. Structural features of In-MOFs
MOFs containing p-blocked In(III) cations, as opposed to d- or f-blocked metal ions, have numerous and distinct coordination geometries that promote the formation of a range of building blocks, such as chained {In(O or OH)}n, trimeric {In3O(CO2)n(OH2)n}, and monomeric {In(CO2)n}, with the trivalent In(III) ions typically exhibiting high coordination numbers. The great majority of In-MOFs that are currently created have three-dimensional (3D) structures. In(III) is usually bonded to O in the form of an 8- or 6-coordinated dodecahedron or 6-coordinated octahedron in its structures. It also exists as InO6− or In(CO2)4− clusters, which assemble the unit by binding to carboxylate groups that are produced from organic ligands. Synthesized In-MOFs carry only negatively charged InO6− or In(CO2)4− building units and typically exhibit an anionic backbone. Protonated [Me2NH2]+ ions derived from DMF are usually required to offset the negative charge of the backbone during MOF formation.22,63 The trimeric [In3O(COO)6] cluster in ref. 64FJU-10, an indium cation cluster, is a secondary structural unit (SBU) that is still present. Six independent ligands connect the trimeric SBUs to form a cationic backbone with a 3D structure. Moreover, these three-dimensional constructions usually have large-diameter orifices with rhombic, triangular, hexagonal, and square channels.In addition to 3D structures, In-MOFs have 2D or 1D structures. The majority of 2D MOFs consist of indium halide and bipyridine N-donor ligands, such as 4,4′-bipy and bpe.27 In these 2D structures, indium is commonly connected to O by an 8-coordinated dodecahedron or a 7-coordinated monocapped trigonal prism. According to topological studies, they usually feature a planar structure with a zigzag chain composition and a (6,3) net hcb topology.65 Further investigation into the newly synthesized In-MOFs revealed that they rarely have a 1D structure. Typically, triazine and metal halides create 1D MOFs. Furthermore, because of indium's flexibility and structural change, some unique organic ligands, such as 1,1′-ferrocene dicarboxylic acid (H2FcDC), can be combined to generate 1D, 2D, and 3D MOFs.
Furthermore, we discovered that the frameworks of In-MOFs are primarily related to the structural type of ligands, so this work categorizes ligands based on their structural properties to describe the structures of In-MOFs. The goal is to encourage additional researchers to focus on such MOFs by demonstrating the various architectures of In-MOFs. As a result, we largely characterize the relevant MOFs in this review using the major backbone classification of the utilized organic molecules.
3.1. In-MOFs constructed with aliphatic carboxylate ligands
There are very few examples of In-MOFs made with aliphatic carboxylic acids. Fig. 7 displays the chemical structures of the aliphatic carboxylic compounds.Woschko and co-workers fabricated two metal–organic backbone single crystals, In-adc and In-fum, in 2023 via a solvothermal technique employing fumaric acid and acetylene dicarboxylic acid as organic ligands, and In(NO3)3·xH2O as a starting material.66 Among them, P4122 or P4322 crystallizes in enantiopure crystals (Fig. 8b), most likely racemic aggregates, while In-adc crystallizes in the chiral enantiomeric tetragonal space group. An In(III) cation, a μ-OH unit, and half of the adc2− unit make up its asymmetric structural unit. Each In(III) ion takes on an octahedral coordination. Furthermore, throughout the infinite {InO6} secondary building unit, each In3+ cation is coupled to two adjacent In3+ cations via four bridging carboxylate adc2− units and two cis-bridged μ-OH groups. Similar to Fig. 8a, this connection results in a crystal structure that displays a square channel network in three dimensions. MIL-53 and In-fum share the same structure. Each indium atom is coordinated by four O atoms of four separate fumarate ligands and two O atoms of two independently linking OH units. The fundamental octahedral secondary SBU in In-fum is the resulting InO6 building block. Fumarate linkers join the SBU's chains, creating a MOF structure with 1D rhombic channels.
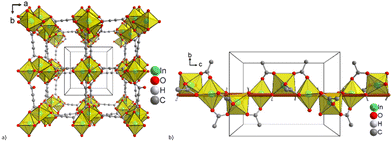 | ||
| Fig. 8 (a) 3D framework of In-adc; (b) right-handed 41 helix alongside the crystallographic 41 axis (brown) being colinear to the c-axis in In-adc. Reproduced from ref. 66, with permission from The Royal Society of Chemistry, Copyright 2023. | ||
3.2. In-MOFs constructed with aromatic carboxylate ligands
Polycyclic and monocyclic ligands are the two general groups into which aromatic carboxylic acid ligands can be divided when choosing them for the building of In-MOFs. The common features of these ligands are that almost all of them contain one or more rings, and these rings are stacked with each other, which makes the structure of indium-based MOFs more stable. At the same time, these rings all contain one or more substituents that can be coordinated with indium in a variety of ways, thus forming a variety of structurally novel indium-based MOFs.In 2018, Chen's group systematically studied the structural transformation from MIL-68 to MIL-53 or QMOF-2 by changing the ratio of metal salts to organic ligands [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 3
L = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 1
3 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6].67 The selected metal salt is In(NO3)3·xH2O, and the adopted ligands are H2BDC, H2BDC-NO2, H2BDC-Br or H2BDC-NH2. The results of the investigation demonstrated that the molar ratio of metal/ligand moles had a major impact on the creation of the products and that the structure altered as the number of ligands grew. In the presence of extra ligands, morphology changed from hexagonal needles to rhombic blocks. In3+ is hexa-coordinated with six O atoms for MIL-68 and MIL-53; two of these come from OH, and the other four are obtained from four distinct monodentate carboxylate ligands. On the other hand, eight O atoms, which come from four chelated bidentate carboxylate ligands, are octa-coordinated with In3+ in QMOF-2.
6].67 The selected metal salt is In(NO3)3·xH2O, and the adopted ligands are H2BDC, H2BDC-NO2, H2BDC-Br or H2BDC-NH2. The results of the investigation demonstrated that the molar ratio of metal/ligand moles had a major impact on the creation of the products and that the structure altered as the number of ligands grew. In the presence of extra ligands, morphology changed from hexagonal needles to rhombic blocks. In3+ is hexa-coordinated with six O atoms for MIL-68 and MIL-53; two of these come from OH, and the other four are obtained from four distinct monodentate carboxylate ligands. On the other hand, eight O atoms, which come from four chelated bidentate carboxylate ligands, are octa-coordinated with In3+ in QMOF-2.
In 2017, Stock et al.22 reported three In-MOFs, [In(BDC-NH3)(BDC-NH2)] (T1), H[In(BDC)0.57(BDC-NH2)0.43] (T2), and (DMA)2[In3(μ3-O)(BDC-NH2/NO2)4.5] (T3) using H2BDC-NH2, H2BDC-NO2, and 2-amino-5-nitro-terephthalic acid (H2BDC-NH2/NO2). For T1, the structure contains the tetrahedral [In(-CO2)4]− nodes. Each In(III) is linked with four other cations via organic ligands to build up a twisted quartz-like skeleton, which is interpenetrated by another skeleton, producing a 1D hexagonal channel (diameter: 4.4 Å). For T2, only BDC2− and BDC-NH22− anions were bound to In(III) cations. Therefore, BDC-NO22− could be reduced in situ using In3+/DMF. For T3, H2BDC-NH2/NO2 was used to form a bis-amino-functionalized MOF. The framework consists of InO6 polyhedra joined by μ3-O to form trinuclear clusters (Fig. 10a–d). The clusters were interconnected by tetragonal BDC-NH2/NO22− to constitute supertetrahedra (Fig. 10e). By confronting corners, the super-tetrahedron is shared throughout the construction of the complete framework. Structures with inaccessible cavities up to 10.4 Å in diameter are possible with this topology (Fig. 10e). Compared with the above literature reports,67 although the same raw materials were used for the synthesis of some MOFs, the structures obtained were very different. A comparison of the two reveals that different temperatures and solvents were utilized in MOF synthesis, which could be the most important explanation for the structural differences.
 | ||
| Fig. 10 (a) Explanation of the crystal structure of T3 with trinuclear clusters. (b) A supertetrahedron (yellow bar) formed by a four-toothed BDC-NH2/NO22− ion connection. (c) By attaching the three-toothed BDC-NH2/NO22− ion to each face of the hypertetrahedron (green bar). (d and e) The clusters interconnected by tetragonal BDC-NH2/NO22−. Reproduced from ref. 22, with permission from The Royal Society of Chemistry, Copyright 2017. | ||
Genna's lab created the unique MOFs, ATF-1, YCM-21, YCM-22, and YCM-23, using 2,5-thiophenedicarboxylic acid (TDC) and indium trichloride, which are regulated by adding ammonium salts.68ATF-1 is an anionic, 3D interpenetrating framework, in which a twisted tetrahedron with cis In–In–In angles of 83.7°, 97.7°, 98.7°, and 113.3° and trans angles of 131.0°, and 133.8°, respectively, makes up the In(III) center. The In(III) in YCM-21 was square planar with average cis In–In–In angles of about 90° and trans-angles of 180°. Both YCM-21-TEBA (triethylbutylammoniumbromide) and -TEA (triethylbutylammonium bromide) indicate cis In–In–In angles of 93.6° and 86.4°(TEA) and 97.0° and 83.0° (TEBA), respectively. YCM-22 bears a pseudotriangonal bipyramidal node and it coordinates with two TDC units and a Cl at the equatorial position, as well as two chlorides at the axial position. In addition, YCM-22 represents an unusual [InCl3(K2-O2CAr)2]2− node. It is hypothesized that sufficient chloride in the solution hinders the breakdown of the In–Cl bond, resulting in a five-coordinated In(III) core. YCM-23 is a novel 3D MOF bearing[In(μ-O2CR)2(μ-OH)]∞ chains. These endless chains are joined together by carboxylic esters of TDC, with each carboxylic acid binding two In(III) atoms and each organic connection connecting two distinct chains.
Three ferrocene-based In-MOFs, CAU-43, In-MIL-53-FcDC_a, and In-FcDC were made by the solvothermal technique using H2FcDC and In(III) salts.69 The fundamental distinction between these MOFs is how their inorganic building units (IBU) are linked. The IBU in CAU-43's structure is made up of alternating cis–trans junctions connecting the corner-sharing arrangement of InO6 octahedra, which creates a zigzag chain as a result of the joining pattern. For In-MIL-53-FcDC_a, the IBU is formed solely by trans-connected corner-sharing InO6 octahedra. Four of the six coordinating O atoms in each InO6 unit belong to the coordination carboxylate group of FcDC2−, whereas the O atoms of the shared corners come from the hydroxide group. This arrangement of the IBUs and linkers results in a windmill-type channel structure. For In-FcDC, the IBU is joined by a series of parallel-arranged, alternating trans–trans–cis corner-sharing InO6 octahedra. Moreover, four of the six coordination O atoms in its octahedral coordination are generated from FcDC2−, with the remaining two coming from hydroxide groups. As a result, the linker and IBU are arranged to produce “dimers” of inorganic chains in a ladder-like framework devoid of pores.
Haddad et al. have synthesized two amide-functionalized In-MOFs, In(OH)CSA and In(OH-PDG),70 employing flexible ligands, N-(4-carboxyphenyl)succinic acid (CSA) and N,N′-(1,4-phenylene dicarbonyl)diglycine (PDG), in 2017. The two MOFs contain {InO4(OH)2} octahedra forming trans-In(III)-OH chains (Fig. 11(a and b)) being joined across dicarboxylate ligands to build up a sheet (Fig. 11c).
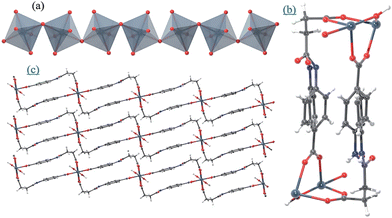 | ||
| Fig. 11 (a and b) Inorganic building units, the chain of {InO4(OH)2}, and the rectangular-shaped motif built by the L-shape CSA. (c) Sheet of In(OH)CSA. The red, white and gray atoms represent O, H, and In, respectively. Reproduced from ref. 70, with permission from The Royal Society of Chemistry, Copyright 2017. | ||
One year later, MIL-68(In) was synthesized by Xing's group using a solvothermal technique. Unlike the previous literature,67 which synthesized iso-configurational In-MOFs but did not go into detail about their structures, which are discussed below: an orthorhombic structure with the space group Cmcm is MIL-68(In).71 The kagome lattice in the ab-plane is formed by two chains of corner-sharing In-octahedra that are crystal-independent and joined by 1,4-H2BDC ligands. The lattice has four triangular channels parallel to the c-axis and one hexagonal channel.
In short, it was discovered that all of the ligands had either carboxyl or hydroxyl groups and that the majority of the core indium atoms were coordinated to carboxyl or hydroxyl oxygens to form a wide range of configurations.
3.2.2.1. In-MOFs constructed with polyphenyl carboxylate ligands. Polybenzene ring carboxylic acid ligands consist of multiple benzene rings and multiple carboxylic acid groups. These benzene rings contain a large number of π⋯π stacking interactions, which make the ring-to-ring contacts with each other tighter and more stable, while the large number of carboxylic acid groups contained in the benzene rings are coordinated with indium in various ways, resulting in the formation of structurally complex In-MOFs, and thus indium-based MOFs constructed from polybenzene-ring carboxylic acid ligands are potentially valuable to study. The chemical structures of the polybenzocyclic carboxylic acid ligands involved in this review are represented in Fig. 12.
In 2016, Eddaoudi's group constructed four In-MOFs, [(CH3)2NH2][In(EBDC)]·2DMF·2DMSO·H2O (T4), [(CH3)2NH2][In(EBDC)]·DMF (T5), [In3O(EBDC)1.5(H2O)3][NO3]·2DMF·2(MeCN)·0.75(H2O), (T6) [Me2NH2][In3O(EBDC)1.5·(H2O)3]2[In(EBDC)]3·8DMF·13(MeCN)·10(H2O) (T7) using 5,5′-(1,2-ethynediyl)bis(1,3-benzenedicarboxylic acid) (H4EBDC).72 The O atoms of 4 carboxylate units of 4 EBDC4− anions are octa-coordinated to In(III) center in T4 (Fig. 13a), in which [In(O2C)4]− and EBDC4− could be simplified as 4-linked nodes, ultimately forming an anionic backbone with a pts topology (Fig. 13b and c), additionally containing two kinds of cross-channel along the c- and the b-axes (Fig. 13d and e), with diameters of ca. 6.655 × 5.567 Å and 5.567 × 5.567 Å, respectively. T5's asymmetric structural unit is made up of two EBDC4− atoms and two In3+ atoms. A tetrahedron is formed when the In(III) is coordinated by four bidentate chelating carboxylic acids to create a 3D framework. For T6, its framework bears [In3O(O2C)6(H2O)3]+ units. These trimers are linked across six individual ligands, yielding a 3D structure. The structure of T7 includes two distinct In3+ conformations: a monomeric [In(O2C)4]− site and a trimeric [In3O(O2C)6(H2O)3]+ cluster. Wherein, each H4EBDC is connected by 2 monomeric In(III) atoms and 2 trimeric building units to form a new 3D ionic framework. Furthermore, T7 contains three types of channels, and the space-filling representation of the framework shows the channels along the [100], [110] and [111] directions, respectively.
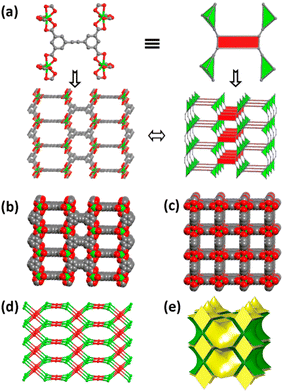 | ||
| Fig. 13 (a) [In(O2C)4]− units linked by EBDC4− to build up pts topology; (b and c) space-filling view of T4 demonstrating the channels along [010] and [001], respectively. (d and e) Ball-and-stick view of T4 with In(III) tetrahedral node. The green, red and gray atoms represent In, O, and C, respectively. Reproduced from ref. 72, with permission from American Chemical Society, Copyright 2016. | ||
In 2016, three In-MOFs, [In(BTB)(2,2′-bipy)]·NMP (T8), [In(BTB)(2,2′-bipy)]·NMP·2H2O (T9), and [In(BTB)(NMP)]·0.5NMP (T10) were created by Zhang et al.65T8 has an asymmetric unit with an In3+ ion, a BTB3− ligand, a coordinated 2,2′-bipy, and an uncoordinated NMP molecule. It has a 2D wave-layer structure. The three distinct BTB3− ligands each contain five oxygen atoms, which are the source of the atoms coordinated to the seven-coordination environment that surrounds the In3+ ion, and from the coordinated 2,2′-bipy unit with two nitrogen atoms with a twisted single-capped trigonal prism geometry. An analysis of the topology shows that the In3+ cation is taken as a 3-linked node and BTB is simplified as a 3-linked joint. After this simplification, the framework belongs to a (6,3)-connected hcb topology. T9 shows a 2D interpenetrating wave-layer skeleton. The asymmetric unit contains an In3+ ion, a BTB3− ligand, a coordinated 2,2′-bipy, an uncoordinated NMP molecule, and two uncoordinated water units. The coordination surrounding of In3+ is the same as that of coordinator T8 where each In3+ ion is linked to three carboxylic acid groups from three BTB ligands in the bc plane and each BTB ligand is attached to each of the three In3+ ions to form a 2D framework. For topological analysis, the 2D framework of T9 belongs to the 6,3-connected network with hcb topology. Harmony the orthorhombic Pbcn space group includes T10. An In3+ ion, a BTB3− ligand, a coordinated NMP, and one-half of an uncoordinated NMP molecule are all present in the asymmetric unit. With one O atom of a coordination NMP unit and six O atoms of three distinct BTB3− units, the In3+ ion is seven-coordinated (Fig. 14a). Moreover, a 2D framework (Fig. 14c and d) is formed by connecting each In3+ ion to three carboxylates from the BTB3− ligand in the ab plane, and each BTB3− ligand to three In3+ ions. This 6,3-connected network has the same hcb topology as T8 and T9 (Fig. 14b).
 | ||
| Fig. 14 (a) Complex surrounding of In3+ in T10. (b) 6,3-Connecting mode of T10. (c and d) 2D framework of T10. Reproduced from ref. 65, with permission from Chinese Chemical Society, Copyright 2016. | ||
In the same year, Eddaoudi's group synthesized two homogeneous microcrystalline compounds, [In3(C16N2O8H6)1.5(Cl)(H2O)2]·(H2O)5.35 (In-soc-MOF-1b) (for Cl−) and [In3(C16N2O8H6)1.5(Br)(H2O)2]·(H2O)6.25 (In-soc-MOF-1c) (for Br−) with cubic morphology.73 Remarkably, the so-called modified inorganic trinuclear molecular building block (MBBs) were disclosed, where eight [In3(μ3-O)(X)(H2O)2(O2C−)6] units (X = Cl or Br) and twelve ABTC4− anions define an anion-free cage.
One year later, [{H[In3(TPO)2(OH)4]·2H2O}n] (MOF-In1) (TPO = tris(para-carboxylphenyl)phosphine oxide) and [In(TPO)]n (MOF-In2), were made by Wang's team. The asymmetric unit of MOF-In1 has 1.5 In(III) ions, 0.5 neutral H2O guest molecules, 1 TPO3− anions, 2 OH− anions, and 0.5 H3O+ guest cations. In(III) adopts an octahedral 6-coordination mode with 4 independent TPO3− anions and 2 OH− connections, resulting in an 8-linked [In3(CO2)6(OH)4] units.74 For the TPO3− ligand, the two carboxylates are coordinated in a chelating fashion, connecting the two [In3(CO2)6(OH)4] units. Ligands that are usually connected in this way can be considered as 4-linked nodes with a quadrilateral surrounding. Thus, MOF-In1 shows a (4,8)-connected topological framework with a point symbol of (410·615·83)(45·6)2. MOF-In2 is a 3D diamond-like skeleton bearing [InO(CO2)3] SBUs (Fig. 15c and d). A TPO3− anion and an In(III) ion are present in the asymmetric unit. Four-connected [InO(CO2)3] SBUs are produced when In(III) ion is coupled to six O atoms of three carboxylate units and one O atom from the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit (Fig. 15a). In the meantime, the four [InO(CO2)3] units are connected by the ligands, which results in four connecting nodes. The bridging TPO3− ligands in SBUs-by-SBUs mode linkages connect the sheet of MOF-In2, resulting in a three-dimensional 2-fold diamond-like topology [(66)] (Fig. 15b).
O unit (Fig. 15a). In the meantime, the four [InO(CO2)3] units are connected by the ligands, which results in four connecting nodes. The bridging TPO3− ligands in SBUs-by-SBUs mode linkages connect the sheet of MOF-In2, resulting in a three-dimensional 2-fold diamond-like topology [(66)] (Fig. 15b).
 | ||
| Fig. 15 (a) [InO(CO2)3] SBUs of MOF-In2; (b) layer of MOF-In2; (c and d) three-dimensional 2-fold diamond-like topology of MOF-In2. The yellow, red and blue atoms represent In, O, and C, respectively. Reproduced from ref. 74, with permission from American Chemical Society, Copyright 2017. | ||
In 2017, Ren and co-workers75 prepared {[In2.5L52O(OH)1.5(H2O)2]·DMF·MeCN·2H2O} (T11) by a solvothermal technique using 2,2′,6,6′-tetramethoxy-4,4′-biphenyldicarboxylic acid (H2L5). Its asymmetric unit therein contains 2.5 In3+ ions, 2 L52− ligands, 1 bridged μ2-O atom, 1.5 bridged μ2-OH− anions, and 2 water units. All In(III) atoms adopt a six-coordination mode, forming a distorted octahedron. In1 is bound to four carboxylate O atoms of four L52− and two μ2-OH− ions, whereas In2 is bound to three L52−, one μ2-O atom, one μ2-OH− ion, and an H2O unit. For In3, In3+ is connected to two carboxylate O atoms from two L52−, two μ2-O atoms, and two H2O units. It is remarkable that, in contrast to earlier comparable findings, the In3 plays as the center to join two sets of In2–In1–In1–In2 chains as basic units from opposite places. These units are then further extended to constitute a 1D endless chain along the a-axis. Ultimately, a 3D framework is built by connecting these chains via L52− connections.
In 2018, utilizing solvothermal reactions of eight pre-designed concave tetracarboxylic acid linkers with In(III) salts, Zhang's group prepared four anionic In-MOFs with distinct topologies (dia, neb, lon, and pts) including In-DM-BP, In-DH-BP, In-TM-BP, In-HMe-BPA, In-TM-BF, In-DM-TP, In-DM-NTP, and In-DM-ATP.26 The networks in all of these MOFs are constituted by 4-connected tetrahedral inorganic and 4-connected tetrahedral/square plane organic building blocks.
PCN-167 was synthesized in 2019 by Zhou and colleagues adopting In(NO3)3·4H2O to react with H4SFTBA (4,4′,4′′,4′′′-(9,9′-spirobi[fluorene]-2,2′,7,7′-tetrayl)tetrabenzoic acid).76 It is fabricated by deprotonated SFTBA4− anions and [In3O(RCOO)6] units (Fig. 16a and b) being a cationic 3D network (Fig. 16c and d), and contains a cage-like SBU. This structure is composed of two adjacent triangular clusters along the c-axis, as well as three SFTBA4− sections that connect the two clusters (Fig. 16e and h). Simplifying the SFTBA4− units as planar 4-linked nodes and the trigonal units as 6-linked nodes, PCN-167 employs a 4,6-c stp network of {44·62}3{49·66}2.
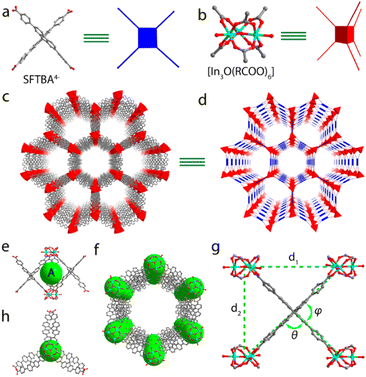 | ||
| Fig. 16 (a and b) The connection method of SFTBA4− ligand and a [In3O(RCOO)6] cluster of PCN-167. (c and d) The cationic 3D network of PCN-167. (e and h) Side and top view of building units of PCN-167. (g) Definition of the parameters d1, d2, θ, and φ. Color scheme: gray, C; red, O; light blue, In. (f) Arrangement of Cage A in the framework. Reproduced from ref. 76, with permission from American Chemical Society, Copyright 2019. | ||
A flexible and interpenetrating microporous In-MOF, NU-50,77 was described by Farha's group in 2020. It was fabricated by employing a tetratopic pyrene-based compound, 1,3,6,8-tetrakis(p-benzoic acid)pyrene (H4TBAPy) to react with indium(III) chloride under solvothermal conditions, and exhibited a 4,4-linked pts net. Half of the In(III) atoms and half of the H4TBAPy ligand are present in its asymmetric unit. To generate mononuclear [In(COO)4]− nodes, In(III) is bound by eight O atoms of the carboxylate units. Every H4TBAPy acts as a planar tetraconnected node, and every [In(COO)4]− monomer is a tetrahedral tetraconnected node. These two nodes work together to create a (4,4)-connected point-to-sociometric topology {42·84}.
Using 4,4′,4′′-((2,4,6-trimethylbenzene-1,3,5-triyl)tris(ethyne-2,1-diyl)) tribenzoic acid (H3TTETA) as starting materials, Chen et al. produced UTSA-22 single crystals by solvothermal reaction in 2022.78UTSA-22's asymmetric unit consists of a μ2-OH− group, 11/6 TTETA3− ligands, and three In3+ atoms (Fig. 17b). Among these, four distinct TTETA3− ligands and four carboxylate O atoms of the two μ2-OH− units in the apical position were coordinated to both In3+ atoms. The In–O and In–OH bond distances are between 2.050(4)–2.209(6) and 2.026(6)–2.094(6) Å, respectively. Two of the carboxylate groups in the TTETA3− ligand are coordinated in monodentate coordination to two nearby In3+ atoms, while the remaining carboxylate group is coordinated in a monodentate form to the In3+ atom. The μ2-OH− groups and the uncoordination carboxylate O-atom (O12) can interact by H-bonding. Infinite rod-like SBUs are produced when the In3+ atoms connect to the carboxylate and μ2-OH− units in the following order: “In1–In2–In3–In2–In1” (Fig. 17a). TTETA3− ligands bridge the SBUs to constitute a 3D structure (Fig. 17c and d).
 | ||
| Fig. 17 (a) One-dimensional rod-shaped SBUs. (b) Chemical structure of H3TTETA. (c and d) A 3D framework of UTSA-22. The turquoise, red and black atoms represent In, O, and C, respectively. Reproduced from ref. 78, with permission from The Partner Organisations, Copyright 2022. | ||
In 2022, Li's group79 successfully constructed two new heterogeneous In-MOFs, {[CH3NH3]·[In(TPTA)]·2(NMF)} (MOF 1) and {[In2(TPTA)(OH)2]·2(H2O)·(DMF)} (MOF 2) using tetracarboxylic acid, [1,1′:3′,1′′-terphenyl]-4,4′,4′′,6′-tetracarboxylic acid (H4TPTA). An indium atom, an H4TPTA ligand, and a [CH3NH3]+ equilibrium charge ion make up the asymmetric unit for MOF 1. In a double nodal state, each deprotonated ligand is coupled to four mononuclear indium, which reduces to a tetragonal form. Six carboxylate O atoms are joined to each mononuclear indium to form a tetrahedral shape. To summarize, the structure with a maximum hexagonal channel is created by connecting in the aforesaid method. {42·84} is the point symbol for this (4,4) connected network. Two In3+ ions, two μ2-OH− groups, and one H4TPTA ligand comprise the asymmetric unit for MOF 2. Every In3+ ion consists of two μ2-OH− and six O atoms from four distinct ligands, forming an octahedral coordination geometry. Following the aforementioned connections, four connected planar square lattice layers are supported by the In3+ ions and the four ligands. These layers are subsequently contacted by oxygen in the bridging μ2-OH− with repeating units of In3+ ions to build an endlessly long zigzag {In-OH-In}n chain that has a column-like appearance. One could think of this as a column-layer approach. When viewed as a whole, the layer forms a conventional 3D fsc architecture with many 1D open channels when combined with the columnar zigzag chain. It has both rhombic and rectangular channels based only on structure (Fig. 18a–f). H4TPTA could be considered as a 4-linked node and the In-OH-In cluster as a 6-linked node when evaluated topologically. As a result, it is a part of the fsc topology of {44·610·8}{44·62}.
 | ||
| Fig. 18 (a–f) Coordination fashions of H4TPTA and preparation procedure of MOF 1 and MOF 2 showing pts and fsc net topologies, respectively. Green, red and gray atoms represent In, O, and C, respectively. Reproduced from ref. 79, with permission from American Chemical Society, Copyright 2022. | ||
In 2024, Liu and his colleagues successfully prepared four In-MOFs with different topologies, InOF-1 (nti), InOF-2 (unc), InOF-12 (pts) and InOF-13 (nou), based on the structural characteristics of 3,3′,5,5′-biphenyl tetracarboxylic acid (BPTC) (since the diphenyl group can rotate around the C–C single bond, resulting in a variety of dihedral angles of the ligands, which increases the diversity of MOF structures with metal coordination) and the coordination properties of In(III) cation.80 Interestingly, InOF-2, InOF-12 and InOF-13 bearing mononuclear nodes demonstrated normal positive thermal expansion, while InOF-1 built by a one-dimensional chain of InO6 octahedrons manifested prominent negative thermal expansion. The authors believed that the twisting of the biphenyl rings in BPTC and the spring-like geometric deformation of the inorganic helical chain work together to produce negative thermal expansion, as demonstrated by a thorough in situ crystal structure and lattice dynamic studies.
3.2.2.2. In-MOFs constructed with polyheterocyclic carboxylic ligands. In addition to the previously published polybenzene ring carboxylic acid ligands, polyheterocyclic carboxylic acid ligands have been employed in the preparation of In-MOFs due to their high coordination capabilities and several coordination modes. Fig. 19 displays the chemical structures that are the subject of this review.
In 2016, Yan et al. presented a MOF, In(OH)bpydc, based on 2,2′-bipyridine-5,5′-dicarboxylic acid (H2bpydc) under solvothermal conditions,81 which is isostructural with MOF-253. It is a 3D framework consisting of an infinite indium-centered octahedron, InO6 linked to a bpydc2− ligand containing 1D channels.
In 2016, Yang's group30 solvothermally synthesized two In-MOFs, [In(2-Hqlca)2Cl3·3H2O] (T12) and {[In(2-QLBM)(2-qlca)Cl2]2·CH3CN·2H2O} (T13) (2-Hqlca = 2-quinolinecarboxylic acid) by adopting 2-(quinoline-2-yl)-benzimidazole (2-QLBM). T12's asymmetric unit bears one In3+ atom, two 2-Hqlca units, three Cl− ions and three water units. The In3+ is sited in a slightly distorted pentagonal bipyramid. The bond distances are 2.263(4)–2.422(4) Å for In–O and 2.457(1)–2.476(1) Å for In–Cl. An extensive H-bonded network exists in the structure and connects to the neighboring discrete monomers thus forming a 3D supramolecular structure. T13 denotes a rare (3,4)-linked 3D topology through a hydrogen-bonding interaction network. Its asymmetric unit is constituted by two In3+ cations, two 2-QLBM units, two 2-qlca units, four coordination Cl− ions, two crystallization water units, and one free MeCN unit. Both In1 and In2 atoms display the same six-coordination surrounding, where each metal centre consists of two N atoms of 2-QLBM, one N atom and one carboxyl O atom of 2-qlca−, and two coordination Cl− ions. The two mononuclear units built by the In1 cation are connected by C8–H8A⋯O1 and C16–H16A⋯O2 H-bonds to constitute a dimer. Additionally, there are intermolecular π⋯π interactions (3.672 Å) (Fig. 20a). These dimers linked each other to produce a layer in the ac plane (Fig. 20b and c). The 3D supramolecular structure is formed by extending these two 2D layers further, revealing a topological network connected in a (3,4) fashion with a point symbol of (63)(65·8).
 | ||
| Fig. 20 (a) Dimer built by π⋯π and C–H⋯O interactions in T13. (b and c) Chains formed by C–H⋯Cl interactions and sheets formed by chains and C–H⋯Cl interactions. Reproduced from ref. 30, with permission from The Partner Organisations, Copyright 2016. | ||
In 2019, Verma's group chose 5-(3′,5′-dicarboxyphenyl)nicotinic acid (H3DCPN) to prepare two kinds of unsaturated monomers, [In(OOC-)2(-N-)X(H2O)] and [In(OOC-)2(-N-)X2]− (X = Cl, Br, and I). There are two uncommon pyridyl-N-involved 7-coordination conformations with distinct terminal substituents in two indium monomers. Two pairs of carboxylate oxygens, a pyridyl N in the channel wall plane, and two more vertically coordinated terminal atoms are coupled to each central In(III) atom of both SBUs. This kind of coordination finally leads to the formation of pentagonal bipyramidal geometry. Furthermore, among them, all contain tetrahedral In(CO2)4− nodes. Consequently, the two monomers reacted with one trimeric cluster [In3O(OOC-)6(DMA)3]+ to integrate a 3D In-MOF,82 which contains 1D nanotube channels (pore size: about 18 Å).
In the same year, Geng's group83 prepared two In-MOFs, In3L14·(Me2NH2)3 (T14) [H3L1 = 5-(3′,5′-dicarboxyphenyl)nicotinic acid] and In2L22(CH3COO)2·Me2NH2·Me3NH·H2O (T15) [H3L2 = 3-(2,4-dicarboxyphenyl)-6-carboxypyridine]. The asymmetric structural unit of T14 is made up of two In3+ ions and an H3L1 ligand. In3+ is typically coupled to carboxylate O atoms; in the case of T14, 8 carboxylate O atoms are from four distinct L1 units. In turn, each carboxylate uses a bidentate chelation mechanism to attach to the In3+ center. Furthermore, each L1 is coordinated to three In3+ ions through three carboxylates, which produce a two-channel, three-dimensional framework (A and B). The asymmetric unit comprising an acetate molecule, one In3+ ion, and an L2 ligand is present in T15. In a seven-coordination mode, the In3+ center is joined to six carboxylate O atoms and one pyridyl N atom. Moreover, every L2 was bound to three In(III) cations forming a 2D layerlike structure.
In 2021, Gazit group presented a novel 4-fold interpenetrating In-MOF, TIF-1 (Fig. 21a),84 by coupling a binary carboxylate ligand (H2L2+(ClO4−)2) with a 4-conjugated In3+ node (Fig. 21b), in which each In3+ was linked to eight oxygen atoms. Coordination of the four ligands to In3+ results in a 4-linked 3D structure with an interpenetrating diameter topology. The inorganic In(III) building units could be seen as tetrahedral nodes, connected by ditopic organic ligands to constitute a 3-peridic dia framework. A more thorough examination demonstrates that the 3D network is made up of channels (28.6 × 31.5 Å) (Fig. 21c) resulting in the construction of a four-fold interpenetrating network of charged diameters (Fig. 21d).
 | ||
| Fig. 21 (a) Coordination mode of In3+ cation in TIF-1. (b) Photo of as-prepared crystal for TIF-1. (c) View of the 3D structure. (d) 4-Fold interpenetrated three-dimensional network. Reproduced from ref. 83, with permission from The Royal Society of Chemistry, Copyright 2021. | ||
In the same year, Jeevananthan group reported a three-dimensional In-MOF, namely SRMIST-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 by using In(NO3)3·xH2O and hexakis(4-carboxylatophenoxy)-cyclotriphosphazene (HCPCP), which consists of inorganic SBUs, {InO7} formed by seven-coordinated indium(III) cations in a pentagonal bipyramidal geometry. HCPCP ligands were coupled by indium(III) ions via (3-c)2(6-c) network of {4·82}2{42·813}.
85 by using In(NO3)3·xH2O and hexakis(4-carboxylatophenoxy)-cyclotriphosphazene (HCPCP), which consists of inorganic SBUs, {InO7} formed by seven-coordinated indium(III) cations in a pentagonal bipyramidal geometry. HCPCP ligands were coupled by indium(III) ions via (3-c)2(6-c) network of {4·82}2{42·813}.
This section describes the structures of In-MOFs made with aromatic carboxylic acid ligands. We discovered that the majority of the ligands chosen have carboxylic acid groups and coordinate with the majority of the central indium atoms to form new, intricate multidimensional structures. In particular, the MOFs constructed from polyheterocyclic aromatic carboxylic acid ligands are different from the aliphatic, monocyclic and polycyclic phenyl aromatic carboxylic acid ligands described in the previous section. The majority of heterocyclic ligands use N as the heteroatom, while some heterocycles contain S or O atoms, which can significantly boost coordination activity. In addition to the different functional units on heterocyclic ligands, their heteroatoms are excellent active sites for metal coordination. This also results in more complicated and diversified architectures for In-MOFs. Therefore, heterocyclic aromatic carboxylic acid ligands are unquestionably the greatest alternative for the future building of In-MOFs.
4. Applications of In-MOFs
Because of the complex interaction between indium and carboxylic acid ligands, In-MOFs can be produced in some configurations. These MOFs are widely employed in diverse applications, covering catalysis, luminescence, adsorption and separation, and electrochemistry, which are all covered in detail below.4.1. Catalysis
Main-group metal ions, particularly those with large radii such as Sr2+, Ba2+, Ga3+, and In3+, have many coordination numbers, making them ideal candidates for Lewis acid catalysts. In(III) cation has a higher charge density, which makes it more likely to construct thermally and chemically stable MOFs. Such MOFs are suitable for Lewis acid chemical catalysis and photocatalysis in organic transformations. In this review, we categorize them into three groups based on their catalytic properties: electrocatalysis, photocatalysis, and catalyze the organic functional group transition reaction.In 2021, Han and colleagues reported that CO2 can be electrically reduced to CO using atomic In-catalysts,87 which were obtained by pyrolysis of In-MOFs and dicyandiamide, anchored to nitrogen-doped carbon (InA/NC). InA/NC was found to have excellent CO generation and selection properties in ionic liquid/MeCN hybrid electrolytes.
Ding's team, using [Ni(C2S2(C6H4COOH)2)2] and In(III) made a 3D MOF with high stability, (Me2NH2+){In(III)-[Ni(C2S2(C6H4COO)2)2]}·3DMF·1.5H2O (F1) which has excellent performance in electrocatalytic CO2 reduction.88 By replicating the active [NiS4] sites of formate dehydrogenase and CO-dehydrogenase, the conversion and Faraday efficiency (FE) were significantly improved, with an increase in FEHCOO− from 54.7% to 89.6% (at −1.3 V vs. RHE, jHCOO− = 36.0 mA cm−2). Further studies confirmed that [NiS4] can act as a CO2 binding site and an effective catalytic center.
In 2023, Qian's team89 utilized one In-MOF (InOF-25/26) for conversion treatment to prepare phosphorus-doped multi-defect carbon nanoribbons for effective anchoring of iron phthalocyanine. This work demonstrates that phosphating the carbon surface, enhancing the tight binding with transition metal phthalocyanines, and causing charge transfer to boost electrocatalytic kinetics can all enhance ORR performance. Simultaneously, this synthesis approach provides a viable method for the creation of high-performance electrochemical catalysts through the combination of metal macrocyclic molecules and structurally changeable MOFs.
In 2023, Zhai's group90 described a MOF namely InCo-ABDBC-HIN (HIN = isonicotinic) by modulating the metal type and replacing the ligand, which was tested and found to have a FE (FEC1) of 81.5% and a current density of 21.20 mA cm−2 for the C1 product at a potential of −2.2 V vs. Ag/Ag+. Theoretical calculations show that its high catalytic activity is mainly ascribed to the doping of Co atoms, Co substitution of In greatly transforms the electronic structure of the In group this offers abundant unpaired electrons for CO2RR and improves the charge transfer efficiency. Meanwhile, the bimetallic InCo clusters altered the electronic structure to promote CO2 adsorption and CO2 activation, leading to excellent electrocatalytic CO2RR activity.
Additionally, in 2023, Liu et al. converted 2D indium MOFs into elemental indium nanosheets by a straightforward electrochemical reduction approach, creating a high-performance In catalyst (In-NSs) for effective CO2RR formate synthesis.91 Testing revealed that the rebuilt metal indium had a maximum partial current density of more than 360 mA cm−2 and a high FE of 96.3% for formates. Nearly no degradation happened after 140 h of operation in a 1 M KOH solution, outperforming the majority of cutting-edge indium-based electrocatalysts.
Currently, In-MOFs realize electrocatalysis mainly through three ways: first, the conversion of In-MOFs into In atoms and the utilization of the Lewis acid sites of In atoms to realize electrocatalysis. Secondly, In-MOFs are doped with another metal during the preparation of In-MOFs, which improves the electrocatalytic ability by changing the structural features (e.g., favorable porous structure, increasing the electrochemical surface area). In addition, electrocatalysis can be achieved by introducing catalytic groups (e.g., NiS4 groups or other groups).
 | ||
| Fig. 22 (a and b) Photocatalytic properties of AMX at variable conditions. (c) Photodegradation efficiency MIL-68(In)-NH2/GrO. (d) Photocatalytic properties of MIL-68(In)-NH2/GrO for the degradation of AMX at different pH values. Reproduced from ref. 92, with permission from Elsevier B.V. Copyright 2016. | ||
Jiang and co-workers (2018) used the In(OH)3 precursor to control metal ion release and successfully prepared an unusual exoplanar porphyrinyl MOF with high stability,93 which demonstrated unexpectedly high photocatalytic hydrogen production activity, far exceeding that of isomorphic MOF based on endoplanar porphyrin. It was revealed that when exposed to light, the In(III) ions at the outer porphyrins decreased and soon separated from the porphyrin ring, inhibiting reverse electron transport and impeding eh recombination. Further studies confirmed that USTC-8 species with different metal ions in the porphyrin core had dramatically varying photocatalytic hydrogen production capacities (Fig. 23c and d). USTC-8(In) had the maximum photocatalytic activity (341.3 μmol g−1 h−1) when cocatalyzed with H2PtCl6 under visible light irradiation (Fig. 23a and b). Because of their excellent stability, all of these MOFs maintain good skeleton integrity and crystallinity during photocatalysis. This is the first study on MOF photocatalysis based on the behavior of particular metal porphyrins.
 | ||
| Fig. 23 (a and b) Photocatalytic process of USTC-8. (c and d) Nyquist spectra of USTC-8(M) (M = Cu, In) in the absence or existence of light irradiation and UV–vis plots of USTC-8(In) suspension solution with and without light irradiation. Reproduced from ref. 93, with permission from American Chemical Society, Copyright 2018. | ||
In the same year, MIL-68-In was used as a precursor by Han et al.94 to successfully manufacture trapped hexagonal In2S3 nanorods with a hollow structure, which were then further vulcanized. First, under visible light irradiation, samples with varying vulcanization periods were used to assess the photodegradation capabilities of methyl orange (MO) dye. Next, to test the activity of photocatalytic removal of antibiotics, In2S3 was employed to degrade tetracycline hydrochloride (TC). Consequently, the resulting In2S3-8h manifested outstanding catalytic activity in the photodegradation of MO and TC.
In 2020, Lu et al. described a porphyrin-based In-MOF, In(H2TCPP)(1−n)[Fe(TCPP)(H2O)](1−n)[DEA](1−n) (F2). Owing to the mutually beneficial relationship between the highly catalytic iron-active sites and the photoactive porphyrin,95 the framework can be applied to high-efficiency visible light-induced CO2 to CO conversion. In just one day, a high CO yield of 3469 μmol g−1 with good CO selectivity (99.5%) was achieved. Experimental evidence has demonstrated that this catalytic activity is significantly greater than that of the iron-free pure In-MOF or its cobalt analog. It might be because the MOF matrix's porphyrin-loaded iron center functions as a useful active site for taking in electrons from photoexcited MOFs to facilitate CO2 reduction.
In 2020, Zeng et al. created bimetallic InαFe1−α-based NH2-MIL-68 photocatalysts by replacing part of the In with Fe.96NH2-MIL-68(InαFe1−α) outperformed NH2-MIL-68(In) in charge separation and transport efficiency. Additionally, under visible light irradiation, it demonstrated improved photocatalytic activity for Cr(VI) reduction and TC-HCl oxidation (Fig. 24a and b).
 | ||
| Fig. 24 (a and b). Photocatalytic properties of NH2-MIL-68(InαFe1−α) and NH2-MIL-68(In). Reproduced from ref. 96, with permission from Elsevier B.V. Copyright 2020. | ||
In 2021, Ni's group produced MOF-derived In2O3 nanorods, In2O3NS, and In2O3rods by using In-BDC MOFs as precursors before calcination.97 They showed better performance than commercial In2O3 in the photocatalytic decomposition of perfluoroctanoic acid (PFOA). After UV irradiation, PFOA and intermediates were completely degraded by In2O3NS or rods within 8 h, whereas commercial In2O3 removed only 35% of PFOA (Fig. 25a). The relatively small contact angle of In-BDC-derived In2O3 compared to commercial In2O3 indicates its enhanced hydrophilicity (Fig. 25b). Because of the porous shape, the liquid medium can more easily enter the interior of the photocatalysts, producing a more hydrophilic surface. The hydrophilicity of PFOS increases its breakdown efficiency by facilitating its interaction with In2O3 photocatalysts.
 | ||
| Fig. 25 (a) Adsorption of PFOA onto In2O3 samples. (b) The contact angles of three In2O3 samples. Reproduced from ref. 97, with permission from The Royal Society of Chemistry, Copyright 2021. | ||
In the same year, Yang et al. also created an effective visible-light-driven In2O3/CdZnS heterojunction photocatalyst the same year.55 They did this by combining ultrafine CdZnS nanoparticles with spindle-shaped In2O3 mesoporous nanorods produced from NH2-MIL-68. Under visible light irradiation, the catalysts manifested high photocatalytic activity without the need for any co-catalysts, and the rate of H2 generation reached 1110 μmol g−1 h−1. They produced H2 at a rate that was more than 185 times faster than pure In2O3 nanorods and faster than other In2O3-based photocatalysts that have been reported to date when exposed to visible light. This implies a bright future for them in sophisticated photocatalysts.
In addition, Dai's group designed a photocatalyst,98 namely In2O3/ZnIn2S4, that can be used for photocatalytic hydrogen evolution (PHE) reaction by growing a thin layer of ZnIn2S4 on the surface of carbon-coated hollow tubular In2O3(C/HT-In2O3) derived from In-MOF. This work presents an optimal photoactivated catalyst for PHE reactions in water.
In 2022, Yi and colleagues chose In(III) to react with functional tetracarboxylic acids,36 to construct an In-MOF (F3). The as-synthesized F3 could be further used as a photocatalyst for producing H2 by H2O decomposition. The test results show that F3 has a H production efficiency of 777.65 μmol g−1 h−1 (Fig. 26). The H2 yield of F3 is greatly higher than that of some previous catalysts, USTC-8(In), etc.93
 | ||
| Fig. 26 Photocatalytic H2 production of five samples. Reproduced from ref. 36, with permission from American Chemical Society, Copyright 2022. | ||
The excellent photocatalytic hydrogen production ability of 2D indium-based porphyrin-MOF cubic nanosheets (In-TCPP NS) was reported by Duan et al. in the same year.58 Due to its greatly improved electron–hole separation capabilities, 2D In-TCPP NS demonstrated an activity improvement in photocatalytic hydrogen production assays of almost an order of magnitude when compared to 3D native In-TCPP MOF. Furthermore, in an aqueous solution, it demonstrated good chemical stability in the pH range of 2–11. The activity of 2D In-TCPP NS barely diminished even after 40 h of continuous photocatalytic testing, indicating its enormous potential for useful commercial applications.
In 2022, Yao et al. obtained MOF, MIL-68(In)-X (X = NH2, OH, Br, NO2, CH3)99 and investigated their visible light photocatalytic ammonia preparation performance. Among them, MIL-68(In)-NH2 photocatalytic performance was superior to that of MIL-68(In), which exhibited an increased N2 photofixation rate of 140.34 μmol gcat−1 h−1 at 420 nm with the quantum efficiency of 5.69%. Furthermore, the adsorption energy of the N2 molecule onto the –NH2 group is seen to be larger (1.819 eV) than that of MIL-68(In) (1.342 eV) (Fig. 27a). Both the amine group content and the intermittent solvothermal reactor's size had an impact on the photocatalytic activity. Theory and experimentation indicate that amines modify the chemisorption of nitrogen molecules by acting as substituents and that there are two pathways via which nitrogen is converted to ammonia: the Mars–van Krevilen process and the ligand–metal charge-transfer mechanism (Fig. 27b–d).
 | ||
| Fig. 27 (a) Structures of N2-adsorbed MIL-68(In) and MIL-68(In)-NH2 and the adsorption energy. (b–d) A suggested mechanism for photocatalytic N2 reduction over MIL-68(In)-NH2. Reproduced from ref. 99, with permission from American Chemical Society, Copyright 2022. | ||
Two MOFs, 2-fold interpenetrating In-TPBD-20, and 4-fold interpenetrating In-TPBD-50, were created by Duan's group in 2022.100 The authors examined how the degree of MOF interpenetration affected the characteristics of light harvesting and O2 activation. Adding the photoactive push–pull chromophore H4TPBD to the interpenetrating In-MOFs not only improved light absorption and hole–electron separation, but also facilitated O2 adsorption and activation. In addition, ROS measurements showed that both In-MOFs could produce O2˙− or 1O2, with In-TPBD-20 exhibiting a stronger ability to make O2˙− but a weaker ability to create 1O2 compared with In-TPBD-50. TD-DFT calculations further indicate that the oxygen adsorbed on In-TPBD-50 is easily converted by spin-triggered O2˙− to 1O2via a spin-up electron transfer process. Therefore, In-TPBD-20 possesses moderate light trapping ability, remarkable O2˙− generation rate, and high backbone stability, and it can be used as an efficient and stable multiphase photocatalyst for a variety of photocatalytic aerobic oxidation reactions.
Using indium MOF (In3-TCA) as a template, Yang's group successfully created a novel robust MOF (Fe2In-TCA) in 2023 by achieving single-crystal to single-crystal conversion via a post-synthesis approach.101Fe2In-TCA has superior charge separation in addition to having a higher specific surface area, photocatalytic activity and migration efficiency. For instance, Fe2In-TCA has shown strong photocatalytic activity in both peroxydisul fate (PDS) activation and ofloxacin (OFL) degradation, according to experimental investigations. Fe2In-TCA could be utilized five times and eliminate 98% of OFL in 30 minutes under ideal test circumstances. As a result, it offers more alternatives as a fresh photocatalyst for removing OFL from wastewater. Fe2In-TCA exhibited higher photocatalytic activity compared with In3-TCA. It was confirmed that adding Fe3+ significantly hindered the complexation of photogenerated carriers in MOF. Fe2In-TCA currently has the shortest fluorescence lifetime and the fastest photogenerated electron migration rate, making it ideal for speeding photocatalytic reactions.
In 2023, by growing Cu-MOF nanosheets in situ around three-dimensional In-MOF nanorods, Du et al. created a “dimensionally mixed” heterostructure on MOF (Cu-MOF@In-MOF Z-scheme heterojunction).102 When exposed to visible light, it functions as a potent photocatalyst that effectively removes Cr(VI) ions from wastewater systems. Its huge specific surface area (718.1 m2 g−1) and heightened light absorption properties in the visible light band, along with greatly improved electron transport and photocarrier separation capabilities, are the key reasons for this. The 2D/3D Cu-MOF@In-MOF Z-scheme heterojunction exhibited a high photocatalytic reduction efficiency (98%) for Cr(VI) under visible light irradiation (50 mg L−1, pH = 5.4) for 1 h.
Hu et al.103 designed a series of Zn–In–S photocatalysts (Zn-doped In2S3, ZnIn2S4, and ZnS/ZnIn2S4), using one-step vulcanization of Zn/In-MOFs. The prepared Zn–In–S with microtubule structure showed the large surface area (107.61–127.26 m2 g−1), porosity (0.25–0.52 cm3 g−1), and tetracycline degradation rate (≥86%) within 120 minutes. Its extremely high photocatalytic degradation efficiency is mostly owing to the direct Z-type heterojunction generated between ZnIn2S4 and ZnS.
It is clear that the MIL-68 series, which are currently primarily used for photocatalysis, are more widely used as derived materials. This is because the indium MOFs-derived In2O3 can completely retain the MOFs’ high specific surface area and porous skeleton, supplying a great number of reaction sites for photocatalytic reactions and thus improving photocatalytic activity. Furthermore, the inherited or produced pores of MOF-derived In2O3 can act as guests or hosts for the in situ inclusion of other exotic semiconductor nanostructures, hence increasing photocatalytic activity via heterojunctions. Several studies have shown that the resulting heterojunctions have many reaction sites, high charge transfer efficiency, and consequently increased photocatalytic activity.
In 2017, Phan's group reported that MIL-68(In) can be used as a multiphase catalyst for the preparation of 2-nitro-3-arylimidazo [1,2-a] pyridines.104 It was experimentally verified that the catalyst could be reused to produce 2-nitro-3-arylimidazo [1,2-a] pyridines without a significant decrease in catalytic efficiency. Importantly, homogeneous catalysts have never been utilized before for the synthesis of 2-nitro-3-arylimidazo [1,2-a] pyridines.
In 2019, Cui and coworkers successfully prepared three chiral porous indium-based metal–organic frameworks, [Me2NH2][In(ODDA)]·H2O·2DMF (F4), [Me2NH2]2[In(ODDP)2]·2H2O·DMF (F5), and [In3(ODDM)2]·[NO3]·6.7H2O·4DMF (F6) with different networks using three 1,1′-biphenyl-phosphate-derived tetracarboxylic acids,105 which were doped by chiral phosphoric acid by spatially preventing them from coordinating. Doping chiral phosphoric acid into MOFs can significantly increase their Brønsted acidity and promote Brønsted acid-catalyzed asymmetric condensation, amine addition, and imine reduction. Modifying the 3,3′-substituent changes their enantioselectivity. DFT calculations show that the framework's limited milieu of catalytically active phosphoric acids is responsible for its high enantioselectivity. By pushing the boundaries of conventional solid-state Brønsted acid catalysts, this work opens the door to the development of a novel multiphase acid catalyst for the environmentally benign synthesis of fine molecules.
In 2019, MOFs, InPF-50 and InPF-51, were created by Reinares-Fisac et al., and it was demonstrated that these were efficient catalysts for the 4-component Ugi reaction.106 The two MOFs contain Lewis basic sites, which aid in synchronizing substrate activation and boost reaction yield. The authors discovered that the presence of Lewis basic sites, along with an IBU that appears to be made up of a single indium atom, produces a highly active catalyst for the multicomponent Ugi reaction. They explain this high activity not only by the easy access to acidic and basic sites inside the MOF structure but also by their location.
Furthermore, Wang and colleagues discovered a discrete tetrahedral In-cage, In12-GL, with Lewis acidic sites from In(III) cations and uncoordinated O atoms from the μ1-HCOO fraction.107 All of these In(III) cations are visible and readily available to the reactive substrate on the surface of In12-GL, which provides an additional benefit in that it increases the potential Lewis acid active sites within the cage and eliminates the need for a unique activation procedure for catalytic reactions occurring on the cage surface. For instance, In12-GL demonstrates efficient multiphase catalysis in the Strecker reaction of aminonitriles and the cycloaddition of CO2 with epoxides. Experimental research has shown that the catalytic efficiency of In12-GL is low for large-size substrates (2-(phenoxymethyl)oxirane), but more than doubles as the temperature is raised to 80 °C. Nevertheless, these catalytic effects are rather selective. The ligand and formate groups’ spatial limitations could be the cause of this size-selective catalysis.
In 2022, a team led by Jeong reported the development of an indium-based MOF (InOF-1) linked to combustion catalysis.108 This MOF generates transient open metal sites in In(III) MOFs by taking advantage of the semi-stability of metal–carboxylate linkages. Without losing activity or crystallinity, the transient open metal sites catalyze the Strecker reaction across several cycles. Spectroscopic and computational approaches were used to confirm the formation of open metal sites caused by the brief breakage of In(III)-carboxylate bonds. Moreover, temperature can influence the number of transitory open metal sites, as well as the following catalytic properties.
Ekhwan et al.109 produced MIL-68(In)-NHTr, an In-MOF catalyst, by attaching a triazole to the backbone of MIL-68(In)-NH2. For the preparation of cyclic carbonates, the epoxide was synthesized in high yield and selectivity at solvent-free ambient conditions (CO2 pressure of 1 bar, temperature of 50 °C, and reaction duration of 6 h). The catalytic performance of MIL-68(In)-NHTr is better than that of the original MIL-68(In)-NH2, thanks to the synergistic acid–base impact of indium SBU and triazole. Moreover, following six consecutive catalytic cycle tests, there was no discernible decrease in MOF's crystallinity or catalytic efficiency, indicating that the newly synthesized catalyst is non-leachable.
In 2023, Zhang and colleagues reported a In-MOF of {[In4(CPDD)2(μ3-OH)2(DMF)(H2O)2]·2DMF·5H2O}n (NUC-66).35 Because of its major composition of In3+ Lewis acid sites and N sites, Lewis alkaline μ2-OH− groups, and macropores microstructural properties, NUC-66a functions as a non-homogeneous phase catalyst that can significantly speed up the Knoevenagel condensation reaction of aldehydes with malononitrile (Fig. 28). These properties render NUC-66a highly catalytic for the cycloaddition reaction of epoxides with CO2 under mild circumstances. Its strong chemical stability and wide surface area are noteworthy. Thus, their study shows that it is promising to construct stiff nanoporous cluster-based MOFs based on large radius-ratio, highly charged metal ions and use them in fields like catalysis.
 | ||
| Fig. 28 The reaction mechanism of catalytic cyclization reaction. Reproduced from ref. 35, with permission from The Royal Society of Chemistry, Copyright 2023. | ||
In 2024, Jeevananthan group85 synthesized a multiphase Lewis acid catalyst SRMIST-1, which exhibited excellent reactivity for the preparation of 2,3-dihydroquinazolin-4(1H)-ones and 3,4-dihydro-2H-1,2,4-benzothiadiazine-1,1-dioxides. Even at low catalyst loading (1–5 mol%), mild reaction conditions, choice of ethanol as the green solvent, and simple treatment, good reaction yields were still achieved. Furthermore, the catalyst may be recycled five times, indicating that it has a wide range of applications as a green recyclable catalyst.
In short, the catalytic activity of In-MOFs makes them potentially efficient and effective catalysts. As a result, the majority of In-MOFs are now used as photocatalysts. Meanwhile, applications in areas such as electrocatalytic CO2 conversion and catalyzing the organic functional group conversion reaction have been widely reported.
4.2. Luminescence
Apart from their application as catalysts, In-MOFs have been thoroughly investigated for their intrinsic fluorescence. Sun et al.110 presented the results of a visible-light-responsive In-MOF, NNU-32, in 2016. The long-lived charge creation inside the framework is often realized under visible light irradiation by the ligand's production of free radicals. Experimental research on NNU-32 has shown that it can be employed as a photosensitizer for the atom transfer radical polymerization (ATRP) reactions triggered by visible light, which is useful for the production of polymers. NNU-32-mediated ATRP of methacrylate monomers occurs in a controllable manner, resulting in narrow molecular weight distributions and high-chain end-group retention. Kinetic studies indicate that the reaction is characterized by controlled free radical polymerization. Also, this work suggests that it is possible to incorporate photoactive MOF materials into a new polymer for photochemical reactions.Ln-In-TATB (F7) and Ln-In-BTC (F8) are two Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu co-doped In-MOFs that Fu's group synthesized in the same year.111 Additionally, the impacts of guest solvent units and ligands on the energy transfer process between Eu and Tb were examined. First, they looked into the luminescence characteristics of F7 and F8 (Fig. 29a). Both samples demonstrated the usual luminous characteristics of lanthanide ions with good control over the emission color (Fig. 29b). As opposed to the BTC ligand, the triplet state bridging ligand 4,4′,4′′-s-triazine-2,4,6-triyltribenzoate (TATB), which is positioned between the excited states of Tb and Eu, effectively facilitates the energy transfer between them. Polar guest solvent molecules affect energy transfer efficiency as well. Whereas for F8, the emission intensity ratio stays nearly constant with varying solvent polarity (Fig. 29d), the typical peaks of Tb
Eu co-doped In-MOFs that Fu's group synthesized in the same year.111 Additionally, the impacts of guest solvent units and ligands on the energy transfer process between Eu and Tb were examined. First, they looked into the luminescence characteristics of F7 and F8 (Fig. 29a). Both samples demonstrated the usual luminous characteristics of lanthanide ions with good control over the emission color (Fig. 29b). As opposed to the BTC ligand, the triplet state bridging ligand 4,4′,4′′-s-triazine-2,4,6-triyltribenzoate (TATB), which is positioned between the excited states of Tb and Eu, effectively facilitates the energy transfer between them. Polar guest solvent molecules affect energy transfer efficiency as well. Whereas for F8, the emission intensity ratio stays nearly constant with varying solvent polarity (Fig. 29d), the typical peaks of Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu emission intensity ratio declines with increasing solvent polarity (Fig. 29c). This demonstrates that although solvent polarity has an impact on the luminous characteristics of F7, it does not affect those of F8.
Eu emission intensity ratio declines with increasing solvent polarity (Fig. 29c). This demonstrates that although solvent polarity has an impact on the luminous characteristics of F7, it does not affect those of F8.
 | ||
Fig. 29 (a) Luminescence spectra of F8 with variable content ratios of Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu. (b) Photo of F8 with variable content ratios of Tb Eu. (b) Photo of F8 with variable content ratios of Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu under a UV light. (c) A plot of the raw content ratio vs. the real content ratio of Tb Eu under a UV light. (c) A plot of the raw content ratio vs. the real content ratio of Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu in F8. (d) The plot of the characteristic peak intensity ratio of Tb Eu in F8. (d) The plot of the characteristic peak intensity ratio of Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu vs. the raw content ratio in F8. Reproduced from ref. 111, with permission from The Royal Society of Chemistry, Copyright 2016. Eu vs. the raw content ratio in F8. Reproduced from ref. 111, with permission from The Royal Society of Chemistry, Copyright 2016. | ||
In 2017, Yan et al. prepared an In-MOF post-functionalized with Eu3+, namely In-MOF-Eu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112 in which both the central ligand and Eu3+ have strong emission peaks at an excitation wavelength of 360 nm; therefore, this excitation wavelength can be chosen as a basis for further investigation of benzene homologues (BTEX) sensing luminescence. Furthermore, testing was done on the aromatic hydrocarbons in gas-phase and liquid-phase settings, respectively, and the findings demonstrated their ability to be differentiated. To make their practical employment easier, a fluorescent paper was made to immediately differentiate between liquid BTEX. The powdered material's fluorescence intensity was found to differ significantly from that of the solution when exposed to BTEX vapor. As a result, it can be applied to BTEX substances in both solution and vapor phases as a fluorescent probe. The different BTEX compounds emit different colors of light in two primary regions: green and blue when they are in a BTEX solution. Furthermore, it might be a novel luminous sensor for identifying benzene congeners in the environment as poisons and organic contaminants.
112 in which both the central ligand and Eu3+ have strong emission peaks at an excitation wavelength of 360 nm; therefore, this excitation wavelength can be chosen as a basis for further investigation of benzene homologues (BTEX) sensing luminescence. Furthermore, testing was done on the aromatic hydrocarbons in gas-phase and liquid-phase settings, respectively, and the findings demonstrated their ability to be differentiated. To make their practical employment easier, a fluorescent paper was made to immediately differentiate between liquid BTEX. The powdered material's fluorescence intensity was found to differ significantly from that of the solution when exposed to BTEX vapor. As a result, it can be applied to BTEX substances in both solution and vapor phases as a fluorescent probe. The different BTEX compounds emit different colors of light in two primary regions: green and blue when they are in a BTEX solution. Furthermore, it might be a novel luminous sensor for identifying benzene congeners in the environment as poisons and organic contaminants.
In 2018, two anionic In-based MOFs, namely V101 and V102,113 were reported by Zhao et al. The differences in the solvents used led to two different structures, 2-fold interpenetration and non-interpenetration, respectively. Firstly, the liquid luminescence of the two MOFs and the related free ligand H4BCP in aqueous solution were determined at 25 °C. The emission of V101 and V102 was detected at 372 nm (λex = 300 nm), which is in line with the emission of H4BCP at 374 nm. Consequently, the π* → H4BCP π-leap could be responsible for the luminescence of V101 and V102. Additional luminescence research revealed that V102 is capable of finding nitrofurazone (NZF) traces in water. V101, however, was unable to identify NZF. The various interpenetrating structures may be the cause of the significant variation in NZF luminescence detection. Importantly, V102 has high stability and recoverability in water as a luminescent probe.
In 2019, Ren's group synthesized a super-stable compound, [In(EBTC)][Me2NH2](DMF)(H2O)5 (F9).114 Under UV light, its crystal and powder sample emit a vivid blue color; in the daytime, they are colorless (Fig. 30a). The highest emission of F9 is centered at 406 nm (λex = 335 nm) (Fig. 30b) being attributed to π* → π-electron leaps and luminescent quantum yield up to 61.4%. Interestingly, MOF materials are rarely found to produce pure blue light that is both steady and efficient. Even though there have been several reports of luminous MOFs, their applications are typically restricted by things like low quantum yield (QY) and infrequently perfect blue light emission, such as “The quantum yields of blue-emitting HSB-W1, ZnBDCA and PCN-94, respectively, were 31.1% and 53% and 76%, Nevertheless, the QY has not been given for ZJU-28, and only a QY of 60.72% has been indicated for the hybrid of the fluorescent dye 4-(p-dimethylaminostyryl)-1-methylpyridinium loaded into ZJU-28”. But in contrast to most previously reported luminous MOF materials, the QY found in F9 is substantially greater and emits pure blue light.
 | ||
| Fig. 30 (a) Luminescence plots of F9. Inset: photos of F9 under daylight (up) and UV light. (b) Time-resolved emission decay spectra of F9 under 406 nm. Reproduced from ref. 114, with permission from The Royal Society of Chemistry, Copyright 2019. | ||
In 2019, Li's team prepared two In-based MOFs, [Me2NH2][In(bpdc)2]·(DMF)2 (F10) and [Me2NH2]3[(In3Cl2)(bpdc)5]·(H2O)5(DMF)2.5 (F11),115 and found that neither of the compounds exhibited FL sensing behavior toward nitro-containing explosives, CS2, or trace Fe3+ ions. However, F11 activated a sensitive FL to thiol-functionalized molecules after being stripped into many layers of FL material. Furthermore, a post-synthesis cation-exchange procedure could bind Eu3+ to F11, and the produced F11@Eu may be employed as a ratiometric FL probe that exhibits a discernible color shift with temperature. In addition to serving as the backbone of the blue emitting body, F11 can accept cationic red and green emitting dyes such as aprazine (AF) and 4-(p-dimethylaminostyryl)-1-methylpyridinium (DSM) to produce white emission.
In 2020, Li and colleagues synthesized two heterostructured In-MOFs, BUT-172 and BUT-173,116 which denoted similar fluorescence emission at 380 nm (λex = 280 nm) to the relevant ligand, 5′-(4-carboxy-phenyl)-2′,4′,6′-trimethyl-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylic acid (H3CTTA). So the fluorescence was ligand-derived luminescence. Moreover, the fluorescence detection property of BUT-172 to different antibiotics in H2O was investigated. Three fluoroquinolone drugs were observed to exhibit noticeably bigger quenches than the other antibiotics, and the fluorescence intensity of the MOF samples dropped as soon as antibiotics were added. Following the addition of 300 μL of antibiotic (500 μM), the antibiotic norfloxacin (NOR) had the best quenching impact (91.8%) among such antibiotics. Antibiotics present in water can therefore be identified by variations in fluorescence intensity.
In addition, Li et al.117 described an anionic In-MOF (F12) that can photodegrade dyes via anionic and cationic dyes as photosensitizers. This MOF demonstrates remarkable quench response sensitivity to CrO42−, Cr2O72−, and Pb2+, as well as outstanding selectivity. The detection limits in aqueous solution were 31.4, 1.2, and 0.52 ppb, respectively. They are the smallest values among the MOFs that have been reported. They also offered a speculative explanation for the fluorescence emission and quenching mechanism of F12. Furthermore, after seven successive detection cycles, it was discovered that F12 had the quantitative detection ability for Cr(VI), which provides a solid basis for its practical application.
In 2020, Wang et al. created CsPbX3@ZJU-28 materials with dual-emission characteristics,118 which were achieved by growing CsPbX3 quantum dots in situ within a mesoporous, blue-emitting In-MOF (ZJU-28). The emission spectra of CsPbBr3@ZJU-28 vary depending on the wavelength of excitation used. The tiny peak is due to the exciton emission of the CsPbX3 quantum dot, while the large blue emission is observed when CsPbBr3@ZJU-28 is excited (254 nm). Interestingly, the excitation wavelength- and temperature-dependent fluorescence features of the CsPbX3@ZJU-28 composite show promise for temperature monitoring and anti-counterfeiting applications. Furthermore, ZJU-28 matrix encapsulation significantly increases CsPbX3 quantum dot stability, which is appealing for LED applications.
Furthermore, an anionic indium-based framework was constructed by Zhu's group, in which the inner cavity (F13) is occupied by [(CH3)2NH2]+ ions.119 With plenty of Lewis-basic sites for securing Cu(II) and Fe(III) cations as well as unsaturated and uncoordinated carboxylate O atoms and free N atoms in benzimidazole, the polybenzene ring linker gave F13 exceptional photosensitivity. Among the very low values of all reported photochemical sensors for MOFs, this anionic framework notably displays excellent fluorescence quench response and high photoluminescence performance to the target analytes with high selectivity (Fig. 31a and b). The sensitivity and detection limits for Cu(II) and Fe(III) ions, respectively, are 3.88 and 4.2 ppb. In addition, even in the presence of other anions or cations, F13 can identify objects quickly and accurately. More importantly, detailed investigations of potential photoluminescence and fluorescence quench processes were conducted. The coordination interaction between Cu2+/Fe3+ cations (Lewis acidic sites) and imidazole N and carboxyl O atoms of the backbone is primarily responsible for the effective fluorescence quench.
 | ||
| Fig. 31 (a) Luminescence intensities at 371 nm for F13 after adding various cationic or anionic (b) analytes. Reproduced from ref. 119, with permission from Elsevier B.V. Copyright 2020. | ||
In 2021, Huh's group prepared viologen@InBTB by encapsulating three different viologen cations in [Et2NH2]3[In3(BTB)4]·10DEF·14H2O (InBTB-MOF) using the cation exchange method. Three of the viologen cations were methyl viologen (MV2+), ethyl viologen (EV2+), and benzyl viologen (BV2+).120 Both EV@InBTB and BV@InBTB exhibited photoluminescence (PL) emission quenches in the range between 280 and 500 nm. However, MV@InBTB shows good PL emission at 306.6 nm.
In 2022, Zhang and colleagues reported a luminescence In-based MOF (JOU-25).121 The similar fluorescence emission of free ligand, 4,4′,4′′-triphenylamine tricarboxylic acid (TCA) at 439 nm (λex = 340 nm) and of JOU-25 at 443 nm (λex = 340 nm), suggesting that the emission of JOU-25 originates from ligand-based fluorescence. JOU-25 acts as a fluorescence sensor for detecting Cu2+, Fe3+, and NB in water.
Li et al.122 presented a twofold interpenetrating indium-based metal organoskeleton (BUT-205) with high H2O-stability. The room-temperature fluorescence emission of BUT-205 appeared at 404 nm (λex = 300 nm), which is similar to the free ligand. Thus, the emission bands of BUT-205 might be due to ligand-based fluorescence (n–π* and π–π*). The authors further explored its application for sensing Fe3+ (Fig. 32a and b). The detection limit for Fe3+ ions was 1.3 μmol L−1 and this feature remained after four cycles of testing, making it highly promising for practical applications.
 | ||
| Fig. 32 (a) UV-Vis plots of tested cations. (b) Anions in comparison with the excitation and emission plots of BUT-205. Reproduced from ref. 122, with permission from Chinese Chemical Society, Copyright 2022. | ||
A stable anionic In-MOF, [(CH3)2NH2]2In2[(TATAB)4(DMF)4](DMF)4(H2O)4 (HDU-1) was fabricated by Kong's group two years later.123 The cation-exchange technique was used to generate the fluorescent probe of Tb@HDU-1, which is a fluorescent probe that may be used to examine Fe(III) and Cu(II) with limits of detection of 1.52 and 8.91 μM, respectively. Also, Tb@HDU-1 was utilized as a ratiometric fluorescent probe for Cu(II) cations, while only a single-peak detection was performed for Fe(III) cations. This could be explained by variations in the competitive absorption and interactions between the metal ions and the backbone.
In conclusion, the luminescence of In-MOFs is primarily caused by the ligand or the introduction of rare-earth metals; however, luminescence research on In-MOFs is still required because their fluorescence characteristics can be employed to probe drugs and heavy metal ions in water, which is useful.
4.3. Adsorption and separation
Given the large amount of current research on adsorption/separation properties of In-based MOFs. These are further classified into three types: simple organics, dye molecules, and gas adsorption and separation. In the next sections, we will go into greater detail about them.In the same year, Li et al. chose H4TCPBDA (H4TCPBDA = N,N,N′,N′-tetrakis(4-carboxyphenyl)-biphenyl-4,4′-diamine) and InCl3·4H2O as the reactants to synthesize an In-MOF with a double interpenetrating microporous structure by the solvothermal method,125i.e., In(TCPBDA)(MeNH3)·6H2O (F14). The flexibility of its structure makes it exhibit an irreversible dynamic response to N2, Ar and CO2 adsorption. Further studies show that this is due to the occurrence of conformational changes of the ligands and pronounced mobile leaps of the neighboring networks, leading to pore swelling upon gas adsorption. This study will motivate more researchers to conduct more studies on the response property of flexible MOFs.
In 2017, Ibarra and colleagues reported the synthesis of InOF-1, followed by thermal activation of the as-prepared InOF-1. A small amount of residual DMF units were retained in InOF-1, forming the DMF@InOF-1. DMF@InOF-1 shows about 1.5 times higher CO2 capture capacity than fully activated InOF-1.126 This is due to the existence of residual DMF units, which build up a strong H-bonded interaction with the In2(μ-OH) unit and DMF molecules. This hydrogen bond hinders DMF leaching and maintains CO2 chelating activity even after 10 cycles.
Furthermore, three anionic porous MOFs (CPM-5, CPM-6, and Co2+-exchanged analog, Co2+-CPM-6) were prepared by Wang's group.127Co2+-CPM-6 was demonstrated to have much higher Xe adsorption ability and Xe/Kr selectivity than CPM-5 and CPM-6. Small and polarised Co2+ ions can boost the pore size or accessible microporous volume and increase the electrical properties within the pore space inducing strong interactions with Xe while decreasing the affinity for Kr.
In 2018, Lin and colleagues synthesized four new layer MOFs,128 ((CH3)2NH2)[In(SBA)2] (F15), ((CH3)2NH2)[In(SBA)(BDC)] (F16) (H2SBA = 4,4′-sulfonyldibenzoic acid), ((CH3)2NH2)[In(SBA)(BDC-NH2)] (F17), and (NH4)3[In3Cl2(BPDC)5] (F18) (H2BPDC = 4,4′-biphenyldicarboxylic acid). The Brunauer–Emmett–Teller (BET) surface area of F15 was 207 m2 g−1. The CO2 adsorption at 1 atmospheric pressure was discovered to be 0.8 and 0.52 mmol g−1 at 273 and 298 K, respectively. F15 was also able to adsorb CO2 at liquid nitrogen temperature with a 0.32 wt% uptake of hydrogen adsorption.
Moreover, Hmadeh's team reported an In-MOF (AUBM-1) showing pts topology. Owing to its excellent chemical stability, it was used as an adsorbent for arsenic from H2O, and its arsenate adsorption can reach 103.1 mg g−1. The adsorption kinetics accorded with a quasi-second-order kinetic model, and thermodynamic investigations manifested that the adsorption of As was a heat-absorptive process.129
In 2019, by using (5-(3,5-dicarboxybenzamido)-isophthalic acid) (H4L6), Wang et al. reported an In-MOF, [In3(μ3-O)(L6)1.5(H2O)3][NO3]·5H2O·CH3CH2OH·1.5DMF (F19).130 Owing to the metal sites, and amide-functionalized pores in the framework, F19 exhibits a high CO2 uptake rate (119.0 cm3 g−1 at 1 bar and 0 °C). In addition, it exhibits superior adsorption selectivity for CO2 (23.0 at 273 K) compared to N2 adsorption. Importantly, the higher water stability of F19 makes it a promising material for water adsorption.
In 2019, Hong's team synthesized a flexible bilayer interpenetrating MOF, InOF-23.131 As shown in Fig. 33a, InOF-23 manifested an unusual adsorption behavior, which was divided into two steps. At first, the activated InOF-23 adsorbed only a small amount of N2 at a low relative pressure (42.66 cm3 g−1 at 0.31). When the P/P0 was suddenly reduced to 0.2, the amount of N2 adsorbed increased dramatically to 282.82 cm3 g−1. This phenomenon suggests a dynamic structural transition from the narrow pore form (InOF-23-n) to the macroporous form (InOF-23-l) caused by N2 adsorption. Meanwhile, to further investigate the dynamic adsorption behavior of InOF-23 its adsorption capacity for Ar was tested at 87 K (Fig. 33b). The Ar adsorption isotherm is similar to the N2 adsorption isotherm. First, the amount of Ar adsorbed was 9.91 cm3 g−1 until P/P0 reached 0.85 when the adsorption fast reached 55.62 cm3 g−1. In addition, they also investigated the CO2 adsorption at 195 K. The CO2 adsorption was only 9.82 cm3 g−1 at P/P0 = 0.3, while the CO2 uptake could reach 54.92 cm3 g−1 at 0.81 bar. In conclusion, InOF-23 exhibits unusual gas adsorption behaviors: at low temperatures, it shows a dynamic response to N2, Ar, and CO2 adsorption stimulated by different pressures, and at high temperatures, the InOF-23 exhibits superior CO2 adsorption to N2 and CH4 adsorption, thus, InOF-23 has a broad application in the field of gas adsorption.
 | ||
| Fig. 33 (a) N2-sorption/desorption isotherms of InOF-23 (77 K). (b) Ar-sorption isotherm of InOF-23 (87 K). Reproduced from ref. 131, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2019. | ||
In 2019, Kim's group fabricated a 3D-In-ABDC-MOF using the ionic liquid, 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]) reacting with [EMIM][In(ABDC)2]·DEF·H2O.132 The N2 adsorption test (77 K) indicates that it is a microporous material. The presence of large-sized [EMIM]+ countercations resulted in a relatively low measured BET surface area. Notably, the presence of Lewis basic azo groups facilitates the enhancement of the interaction between the framework and CO2 units through acid–base interactions, this gives it an excellent CO2 adsorption capacity. Also, it demonstrated a good adsorption capacity with a moderately high isothermal heat of H2 adsorption.
In the same year, Farha's team synthesized an In-MOF, [In2(OH)2(TCPP)] (F20).133 Its BET surface area is 1610 m2 g−1. The NH3 uptake of F20 at 1 bar was 9.41 and 7.83 mmol g−1 in the first and second cycle determinations, respectively. This can be explained by the existence of a stronger interaction of NH3 with Brønsted –OH sites in F20 in the first cycle. These results may contribute to the future development of improved MOF-based NH3 adsorbents.
By utilizing 2,2′-(pyridine-2,6-diyl)diterephthalic acid (H4L7) as an organic ligand, Hong's team synthesized a microporous MOF, [(CH3)2NH2]2[In2L72]·3DMF·8H2O (InOF-18)134 in 2019. The saturation absorption at 1.0 bar was 396.61 cm3 g−1, which belongs to the typical properties of microporous materials (Fig. 34a). Its Langmuir surface area (SBET) (1708 cm2 g−1) and BET (1214 cm2 g−1) were used. The MOF showed a wide range of micropores from 11.0 to 22.0 Å. Tests of their H2 adsorption properties showed that the saturated H2 uptake reached 179.77 cm3 g−1 (77 K and 1.0 bar) (1.60 wt%) and 108.73 cm3 g−2 (0.97 wt%) (87 K and 1.0 bar) (Fig. 34b and c), thus indicating that InOF-18 has a lofty H2 adsorption capacity and a large H2 binding affinity. Furthermore, it exhibits higher selectivity for CO2 in comparison to N2 and CH4.
 | ||
| Fig. 34 (a) Nitrogen sorption curve of InOF-18 (77 K). (b) Hydrogen adsorption curves of InOF-18 (77 and 87 K). (c) Isosteric heats of hydrogen. Reproduced from ref. 134, with permission from American Chemical Society, Copyright 2019. | ||
In 2020, Ma's group135 designed a heterogeneous MOF {((CH3)2NH2)[InTb2(HTDP)2]·3DMF·3H2O}n (NUC-5) with a unique nanocage-like framework using [In(CO2)4] and [Tb2(CO2)8] units. Adsorption experiments on the activated NUC-5 showed that it exhibited type-I adsorption–desorption isotherm, with a calculated BET surface area of 1594 cm2 g−1 and a pore volume of 0.52 cm3 g−1. Moreover, its maximum CO2 uptake at 1 bar reached 69.07 and 43.88 cm3 g−1 at 0 and 25 °C, respectively, which may be ascribed to the structural properties of NUC-5 (high porosity, abound Lewis and Brønsted acidic sites). Also, the MOF denoted the efficient and reversible iodine trapping performance, indicating that it is also a potential adsorbent for radioactive products.
In 2022, Wang's team reported a 2D stacked In-MOF, In(aip)2.136 Its CO2 screening properties were systematically investigated. Static adsorption of a single gas shows that the MOF demonstrates a good uptake of CO2 at room temperature (1.27 mmol g−1 at 101 kPa), while no adsorption is found for N2 and CH4. The separation performance of CO2 from CH4 and N2 was affirmed by real-time breakthrough determinations. This is due to the presence of an uninvolved coordination –NH2 group in the ligand, which can play as a H-bond donor and Brønsted basic site to improve CO2 affinity.
One year later, Zhai et al.90 synthesized a bimetallic MOF, i.e., InCo-ABDBC-HIN. The introduction of Co(II) and functional groups was found to increase the specific surface area (SSA) by N2 adsorption property tests. Then its CO2 adsorption performance at low pressure was investigated. The maximum CO2 uptake of In-ABDBC-HIN and InCo-ABDBC-HIN were 68.5 and 101.7 cm−3 g−1, respectively, at a low pressure of 273 K. This suggests that the change in the electronic structure due to the inserted Co effectively contributes to the CO2 gas uptake capacity.
In 2024, Testing Left et al.137 synthesized two In-MOFs with high stability, namely (NH2Me2)3[In3(BTB)4]·12DMA·4.5H2O (In-MOF-1) and (NH2Me2)9[In9O6(BTB)8(H2O)4(DMSO)4]·27DMSO·21H2O (In-MOF-2). Among them, In-MOF-1 is a mononuclear structure, while In-MOF-2 exhibits a complex structure. Firstly, the N2 adsorption properties of the activated samples were tested and it was found that the activated In-MOF-2 had a high BET surface area of 998.8 m2g−1, while the BET of In-MOF-1 was only 6.88 m2g−1. Then, the adsorption capacities of In-MOF-2 for CH4, CO2 and N2 were determined. At 273 K (under 1 bar), its highest adsorption capacity for CO2 is 14.8 wt%. The CH4 uptakes are 1.60 wt% and the N2 uptakes are 0.80 wt%. Thus, InMOF-2 exhibits highly selective CO2 adsorption for CO2/CH4 and CO2/N2, respectively. The results were also verified by theoretical simulations.
In-MOFs are now mainly used for adsorptive separation of CO2, H2 and rare gases, among other things, due to their distinct structural properties, which include accessible open metal sites, a high ionic framework, and tiny channels. At the same time, compared with other adsorbents, the adsorption performance of In-MOFs is in an advantageous position; in particular, the adsorption performance of In-MOFs with a 2D structure is about one order of magnitude greater than that of other adsorbents.
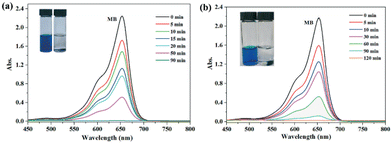 | ||
| Fig. 35 (a and b) UV-vis plots of MB in CH3OH at variable times during the adsorption with F21 and F22, respectively. Reproduced from ref. 138, with permission from The Royal Society of Chemistry, Copyright 2019. | ||
In the same year, Hong's group139 prepared an In-MOF, FJI-H21, which exhibits highly selective storage of C2–C3 hydrocarbons relative to CH4 at ambient conditions due to its intrinsic microporosity. Additionally, it can absorb cationic dyes preferentially including MB, ethyl violet (EV), and cationic red (GTL), as well as anionic or neutral dyes. The second-order kinetic constant (k2) of the dye MB reached 2.03 × 10−1 g mg−1 min−1 when the crystal size was smaller than 10 μm. This increased the adsorption rate of cationic dyes by nearly 100 times compared to the kinetic constant obtained using blocked crystals, indicating that sample size has a significant impact on adsorption performance.
In 2021, Sun and colleagues, using spirobifluorene-based ligands synthesized an anionic In-MOF (JUC-20),140 which has an anionic backbone with a bilayered interpenetrating pts framework for the separation of dyes, and can effectively adsorb MLB in DMF, but not the anionic dyes (Orange II and MO), and the neutral dyes, neutral red (NR) and Sudan I (SD I). When the solvent was altered to EtOH, JUC-210 could selectively adsorb MLB from the large cationic dyestuffs RhB, the anionic dyestuffs Orange II and MO, and the neutral dyestuffs SD I. It was evident from the data that solvent effects, size exclusion effects, and electrostatic interactions primarily impacted JUC-210's ability to selectively adsorb organic dyes.
In 2021, Chu group141 reported an In-MOF [(CH3)2NH2]1.5[In1.5(CPTA)2]·5.5NMF·6H2O (F23) with a 3D anionic framework under solvothermal conditions using the asymmetric pyridinium carboxylate, 2-(2-carboxypyridin-4-yl)terephthalic acid (H3CPTA). Its unique pore properties offer a proper environment for interfacial interactions between adsorbed units and pore matrix. Studies have confirmed that it exhibits good adsorption capacity for cationic dyes, with maximum adsorption capacities of up to 169.4, 170.8 and 202.8 mg g−1 for MB, NR and MV (methyl violet), respectively.
In-MOFs for the adsorption of dyes mainly utilize an anionic framework with a microporous structure. Because it has a high void space with a proper pore size and is also based on ion exchange and size repulsion, it usually denotes highly selective adsorption of positively charged cationic dyes.
In the same year, Zhang and co-workers143 presented a new 3D In-MOF with a polar channel, H3O[In3(dcpy)4(OH)2]·3DMF·4H2O (F24), through the use of the pyridinyl-modified dicarboxylate ligand, 3-(2′,5′-dicarboxyphenyl)pyridonic acid (H2dcpy). It exhibits good selectivity for C2H2, C2H4, and CO2, with maximum adsorption of the three gases reaching, respectively, 68, 51, and 61 cm3g−1 at 273 K and 1 atm. This high selectivity may be attributed to the pore environment's high polarity, in which the anionic skeleton, uncoordination carboxylate O atoms, and pyridine N atoms generate an electric field that enhances the skeleton's interaction with the quadrupolar C2H2, C2 H4, and CO2 units.
In 2019, Zhai and colleagues designed two isostructural In-MOFs,39 [In(OH)(BDC)] (SNNU-120) and [In(OH)(OHBDC)] (SNNU-121). According to the ideal adsorbed solution theory (IAST) calculations, both SNNU-120 and SNNU-121 exhibit extremely good gas adsorption properties and substantial selectivity for CO2 and C2 hydrocarbon phases for CH4. The alteration of the pore surface with –OH Lewis basic sites resulted in much greater CO2 and light hydrocarbon adsorption for SNNU-121 compared to SNNU-120. The suitable porosity and functional units may improve the adsorption/separation performance of MOFs for gases.
In 2020, Chu group144 gave a novel stable F-modified In-MOF, (Me2NH2)1.5[In1.5(FBDC)(BDC)]·2.5NMF·CH3CN (F25) with a 3D cross-pore system by employing a mixed carboxylate ligand synthesis strategy. The adsorption of C2H2 for this MOF was studied at ambient temperatures up to 1 atm based on proper pore sizes and polar pore surfaces modified with free F atoms. The uptake was up to 53 cm3 g−1 at 298 K. Its C2H2 uptake capacity is higher than that of MOF-5 (26 cm3 g−1)145 and ZIF-8 (25 cm3 g−1),146 and is comparable to that of previously described MOF BSF-1 (53 cm3 g−1).147
In the same year, Zhang et al. constructed two In-MOFs, [In2(L1′′)(OH)2]·2DMF·2H2O (F26) and [(CH3)2NH2][In(L1′′)]·2.5NMF·4H2O (F27), with the help of the pyridinium tetracarboxylate compound, 2,5-bis(2′,5′-dicarboxyphenyl) pyridine (H4L1′′),148 in different solvent systems that exhibited a high adsorption capacity toward C2H2, C2H4, CO2, and CH4 all showed high adsorption capacity. The maximum adsorption amounts of F26 for the four gases can reach 125, 88, 79, and 24 cm3 g−1 at 273 K (under 1 bar), while the sorption amounts of F27 are 85, 59, 59, and 12 cm3 g−1, respectively. The gas adsorption behaviors indicate that they have excellent separation selectivity for both C2Hx/CH4 and CO2/CH4.
In 2022, Zhang et al. reported two In-MOFs, [(CH3)2NH2]·[In(BDTA)] 5.5DMF·4H2O (FJU-117) and [In(4Me-BDTA)·0.5H2O]·6DMF·2H2O (FJU-118).149 In3+ in FJU-117 adopts a tetragonal coordination mode, whereas the In3+ ion in FJU-118 adopts seven coordination due to the spatial site-blocking effect of 4Me-BDTA. Accessible sites with partial residual charge in FJU-118 could offer electrostatic forces to fix the lattice H2O. The electrostatic force-driven lattice H2O connection stabilizes the FJU-118 structure. As shown in Fig. 36a, the BET and SBET of one of the activated samples, FJU-118, were 1860 and 2106 m2 g−1, respectively. The absorption uptakes of C2H2 and CO2 at 296/273 K were 88.6/155.1 and 35.6/66.9 cm3 g−1, respectively (Fig. 36b). Further studies showed that some of the remaining charges on the indium sites have electrostatic forces on the alkyne group of the C2H2 molecule for selective separation of C2H2/CO2 mixtures (Fig. 36c and d).
 | ||
| Fig. 36 (a) N2 adsorption isotherm of FJU-117a and FJU-118a (77 K). (b) CO2, C2H6, C2H4 and C2H2 adsorption spectra of FJU-118a (296 K). (c and d) Separation spectra of FJU-118a for C2H2/CO2/He gas mixtures and preferential adsorption sites for C2H2. Reproduced from ref. 149, with permission from The Royal Society of Chemistry, Copyright 2022. | ||
By employing 5-(ethyloxamate)-isophthalic acid (H2EtL9), an innovative porous In-MOF, ((CH3)2NH2)1.5[In1.5L92]·2DMF·2H2O (F28) was constructed by Wang's group in 2022. F28's structure includes two different types of pores that have been altered by amide groups. This allows F28 to exhibit good adsorption capacity for C2 hydrocarbons and CO2 at room temperature. The effective purification of CH4 and C2H2 was made possible by F28's good adsorption capacity for C2 hydrocarbons and CO2.150 Moreover, at mild conditions, F28 exhibits good conversion and size selectivity for the catalytic reaction of CO2 and epoxides owing to the existence of strong Lewis acid In3+ and Brønsted acid Me2NH2+ sites.
In the same year, He and colleagues synthesized an organic coordination skeleton, ZJNU-121, with a rare bi-directional rod packing structure using a tailor-made assembly of bis-thiophene-functionalized tetracarboxylate ligands.151 Impressively, not only does it have unique structural features, but it also exhibits excellent adsorption capacity to capture C2 hydrocarbons from CH4 and CO2 for C2/C1 and C2/CO2 separation. Wherein the ideal adsorption solution theory predicts adsorption selectivity in the ranges of 8.4–13.8 (C2Hn/CH4) and 2.3–3.4 (C2Hn/CO2) for equimolar mixtures under ambient conditions. The structural variety of rod MOFs is enhanced by this work, which also shows that it is a potent adsorbent with potential uses in the separation of different hydrocarbons.
In 2023, Wang et al.152 created a MOF (In-TATB) with 2,4,6-tris(4-carboxyphenyl)-1,3,5-triazine (H3TATB) and In3+ cations. Two three-dimensional In-chains and TATB3− units interpenetrate between the triazine rings through strong π⋯π interactions. Utilizing the limited ultramicroporous space (3.8 Å × 4.1 Å), it has a high enthalpy of adsorption for C2H2 (36.6 kJ mol−1 at 298 K), thus exhibiting a good separation of C2H2/CO2 mixtures.
To sum up, the adsorption of organic molecules, heavy metal ions, and dye molecules has received less attention than the adsorption of gases, which is the primary usage of In-MOFs at the moment. As a result, there is more room for growth and application of In-MOFs as adsorbents.
4.4. Chemical sensing
To make luminous In-MOFs, the indium(III) ion is commonly coupled with carboxylates with large conjugated double bonds (π-bonds), rigid planar structures, or electron-donating substituents. Photoluminescence is a key feature of In-MOFs, which are commonly employed in sensor probes to detect small biological units, gasses, metal cations and anions, and explosives.In 2019, Ding's team reported a MOF, AuNCs/MIL-68(In)-NH2/Cys by doping the fluorescent gold nanoclusters (AuNCs) into MIL-68(In)-NH2,154 which acts as a fluorescent nanosensor to detect mercury ions (Hg2+). When utilized as a nanosensor, it emitted bright pink fluorescence, showing that AuNCs were uniformly dispersed on MIL-68(In)-NH2. The sensor produced double fluorescence emission at 438 and 668 nm (λex = 370 nm). In contrast, when Hg2+ was present, its fluorescent peak at 668 nm burst, while the peak at 438 nm changed (Fig. 37A and B). AuNCs/MIL-68(In)-NH2/Cys demonstrated two linear ranges for detecting Hg2+: 20 pM to 0.2 μM and 0.2 μM to 60 μM (Fig. 37C and D). The sensor has been utilized to detect Hg2+ in actual H2O samples.
 | ||
| Fig. 37 (A and B) Fluorescence emission plots of AuNCs/MIL-68(In)-NH2/Cys in the presence of Hg2+ at variable concentration. (C and D) Linearity between FL668 nm/FL438 nm and Hg2+ concentration at different scales and concentration ranges. Reproduced from ref. 154, with permission from The Royal Society of Chemistry, Copyright 2019. | ||
Lee and colleagues synthesized two microporous In-MOFs,21 [(CH3)2NH2][In(abtc)]·solvents (F29) and [(CH3)2NH2][In(bptc)]·solvents (F30). Owing to the anionic backbones and channel size, they could be used for the selective uptake and separation of cationic dyes and show reversible dye uptake and release capabilities. The luminescence intensity of F29 at 402 nm was decreased only when Fe(III) was added. F30 can be adopted as an outstanding dual-emission channel sensor for selective detection of Fe3+ ions.
In 2021, Zhang's team constructed a three-dimensional 2-fold interpenetrating MOF (JOU-23),155 which denotes an anionic framework. JOU-23 can be a fluorescent sensor for sensing Fe(III) cations and nitroaromatic molecules. Among them, the detection limit for Fe3+ was as low as 2 × 10−6 M (KSV = 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 755 M−1), while JOU-23 can detect nitrobenzene (NB) with high sensitivity. Even when the concentration of NB reached only 4.175 × 10−5 M, the quenching efficiency still reached 86.4%.
755 M−1), while JOU-23 can detect nitrobenzene (NB) with high sensitivity. Even when the concentration of NB reached only 4.175 × 10−5 M, the quenching efficiency still reached 86.4%.
In 2022, Zhang and co-workers synthesized an indium-based metal organoskeleton, [In(HTCA)(OH)]·2DMF·H2O (JOU-24) with high stability using 4,4′,4′′-tricarboxytriphenylamine (H3TCA),33 which could be a fluorescent sensor for selective detection of Co(II) and NB in H2O owing to its high stability and fluorescent properties. Where for Co2+ sensing, the high burst content was 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 with a low detection limit of 4.9 × 10−6 mol L−1. For NB sensing, the burst content was 10
500 with a low detection limit of 4.9 × 10−6 mol L−1. For NB sensing, the burst content was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230.
230.
In 2024, Zuo et al.137 synthesized two In-MOFs, In-MOF-1 and In-MOF-2. In-MOF-1 has a mononuclear 3D skeleton, whereas In-MOF-2 has a 3D skeleton with three different multinuclear In-clusters. They exhibited excellent fluorescence sensing performance in the detection of Fe3+ cations with high sensitivity, excellent selectivity, and recoverability, which were attributed to the combined effects of competitive energy absorption and competitive energy adsorption and energy transfer, respectively.
In summary, these In-MOFs for fluorescent sensors usually contain organic ligands with fluorescent properties or rare earth elements with luminescent properties introduced through post-modification. Furthermore, most of these In-MOFs are anionic backbones.
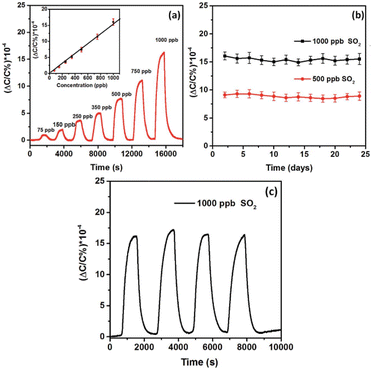 | ||
| Fig. 38 (a) Detection of sulfur dioxide from 75 to 1000 ppb. (b and c) MFM-300(In) cyclic and persistent detection of SO2 detection. Reproduced from ref. 156, with permission from The Royal Society of Chemistry, Copyright 2018. | ||
In 2021, Yang's team presented an In-MOF, [In(BDC-NH2)(OH)]n (In1-NH2)157 showing a high sensitivity identification and short response time for heavy metal ions and ClO− in aqueous solution. The LOD values are 0.11, 0.14, 0.64 and 1.15 μM for Fe3+, Cu2+, Pb2+, and ClO−, respectively. In addition, PBA-In1, which was produced by functionalizing In1-NH2 with phenylboronic acid through the PSM strategy, denoted the effective sensing performance of H2O2.
In 2021, SNNU-153 and related sensors were reported by Zhai's group.158 The synergistic effect of excited state intramolecular proton transfer signaling and the constrained MOF holes enables SNNU-153 to selectively sense hydrazine even in N-containing hydride analogues such as NH3, NH2OH and (CH3)2NNH2.
In 2022, Manzar Sohail et al.159 initially synthesized In-MOF [MIL-68(In)] using a solvothermal technique. They then inserted Ag+ by adding AgNO3 continuously, and finally, they calcined the mixture for three hours at 650 °C to produce Ag@In2O3. Using Ag@In2O3 as a modified NF, a non-enzymatic glucose sensor was utilized to measure the concentration of glucose in alkaline media.
In the same year, Wang's team prepared MOFs-derived In2O3/Ti3C2TxMXene composites by oil bath method and subsequent calcination treatment. It was found that In2O3/Ti3C2TxMXene exhibited excellent gas sensing performance,160 with a 60.6% response to 5 ppm NH3 gas and a detection limit of 5 ppm at room temperature. Furthermore, the sensor indicated outstanding linear response, ultra-fast response/recovery, and high stability over a range (5–100 ppm).
Also in 2022, Shen and colleagues prepared porous hollow hexagonal columns of pure and Co-doped In2O3 using MIL-68(In). In2O3 inherits the morphology of MIL-68(In) with a porous hollow hexagonal column structure and homogeneous size.32 When doped with Co2+, the gas sensitivity of the material will be greatly affected. The sensor prepared from cobalt-doped and pyrolyzed samples at 500 °C showed good gas sensitivity to ethanol gas, with a sensitivity of 14.6 and response/recovery times of 21 and 107 s for 100 ppm EtOH gas at 210 °C. This excellent gas sensitivity may be attributed to the fact that it has the morphology of a hollow hexagonal column and has a porous structure for gas diffusion as well as for the enrichment of active sites with a high specific area. Additionally, the increase in oxygen vacancies due to Co2+ doping also contributes to the increase in gas sensitivity.
Using Pd@MIL-68(In) MOFs as precursors, Wang's group produced Pd@In2O3-2 in 2023 by a hydrothermal technique and a calcination reduction process.161 Pd@In2O3-2 demonstrated the best methane detection performance, according to performance tests conducted on it. Furthermore, the Pd@In2O3-2PHTs demonstrated outstanding anti-interference capabilities by tracking methane selectively in the presence of interfering gasses. As a result, this work shows that Pd@In2O3 is a good option for creating methane sensors because of its high specific surface area, porous microstructure, elevated oxygen vacancies, and palladium nanoparticle catalytic action.
Following a post-calcination procedure, Volanti et al. prepared In-MIL-68-derived single hollow phase In2O3 micro rods using the microwave-assisted solvothermal method (MAS).162 This nanorod material can be utilized as a microbial volatile organic compounds (MVOC) sensor and exhibits a very high sensitivity to 1-pentanol, Fig. 39a shows a detection limit of 0.5 ppm for 1-pentanol at 350 °C and the response increases with increasing concentration of 1-pentanol, with a response of 6–390 being achieved for 2–100 ppm (Fig. 39b), which suggests that the sensor is highly sensitive to 1-pentanol even at low concentrations. Finally, the sensor based on hollow In2O3 microrods denotes promise for detecting 1-pentanol.
 | ||
| Fig. 39 (a) Measurement of 1-pentenal by MVOC sensors in different temperature ranges. (b) Response of different MVOCs to different substances at 350 °C at 100 ppm. Reproduced from ref. 162, with permission from Elsevier Ltd, Copyright 2023. | ||
In 2023, Yu and coworkers, synthesized MIL-68(In)@ZIF-8 precursors using oil bath and self-assembly methods and then obtained In2O3@ZnO S-type heterojunctions by heat treatment.163 In2O3/ZnO-5, formed by loading a certain proportion of ZnO on In2O3, showed wonderful sensing performance for HCHO. It was confirmed through a series of experimental tests that the splendid gas-sensitive performance of In2O3/ZnO-5 can be ascribed to the derived bi-MOFs In2O3@ZnO S-type heterojunctions, which provide a larger specific surface area, thus boosting the amount of adsorbed O on the surface.
Currently, sensing-selective organic molecules typically include derivatives of MIL-68(In) or MOFs, and derivatives of In-MOFs have a considerable specific surface area, which could offer many reactive active sites. In addition, as another MOF is loaded onto the surface of the substrate MOF, a MOF heterojunction on the MOF is generated, which can greatly boost the electrons for the sensing reaction and thus improve the sensing performance.
4.5. Electrochemical properties
In addition to the previously described applications in catalysis, adsorption, and chemical sensing, In-based MOFs can also be used in electrochemistry. In 2016, Xiang's group,164 reported an attractive MOF (MROF-1), which has interesting structural and topological characters with unique [In6(thb)6] metalloring nodes having the highest diameter of about 21 Å. In addition, owing to the abundant existence of (Me2NH2)+ cations and H2O units in the 1D channel and the ease with which the hydrophilic (Me2NH2)+ dissociates from the proton in the presence of H2O units. The proton conductivity (σ) of MROF-1 can attain 1.72 × 10−2 S cm−1 under 70 °C/97% relative humidity (RH) (Fig. 40a) being higher than that of many dimethylammonium-bearing compounds (Fig. 40b).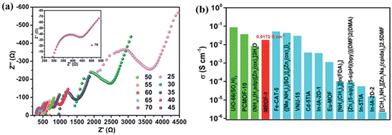 | ||
| Fig. 40 (a) Nyquist spectra of MROF-1 under 97% RH. (b) Comparison of the σ values. Reproduced from ref. 164, with permission from The Royal Society of Chemistry, Copyright 2016. | ||
One year later, Joarder et al. synthesized an In-sulfonated MOF, {[In(5-Hsip)2(Me2NH2)]·DMF·(H2O)1.4}n (PCMOF-17) (5-H3sip = 5-sulfoisophthalic acid),165 which contains H-bonded dimethylammonium cations and an anionic layered structure of water molecules, and the presence of all these groups facilitate proton transport. One of the impedance analyses of the powder showed the σ value >10−3 S cm−1 at RT and 40% RH. Because of this MOF's modest RH stability, AC measurements were done on a single-crystal, revealing that the crystals’ major proton transport channel is linked to a continuous H-bonded array.
In 2018, Xiang's team prepared two anionic MOFs with different degrees of interpenetration,166 [Me2NH2][In(PPTTA)]·2.5DMF·2H2O (FJU-16) and [Me2NH2][In(PPTTA)]·4.5DMF·16H2O (FJU-17) (H4PPTTA = 4,4′,4′′,4′′′-(1,4-phenylenbis(pyridine-4,2,6-triyl))-tetrabenzoic acid). Owing to the abundant existence of [Me2NH2]+ and H2O units in their pores, they attempted to examine their proton conductivity and found that, in the absence of additional humidity, FJU-17 showed a higher intrinsic proton conductivity compared to the 4-fold-interpenetrating FJU-16. Their study illustrates that proton conduction can be rationally improved by controlling the backbone interpenetration.
In 2019, Zhang's group reported a cationic MOF, {{[In3O(PPTTA)1.5(H2O)3](NO3)}·(DMA)3·(CH3CN)6·(H2O)30}n (FJU-10),64 and then encapsulated the dye, 8-hydroxy-1,3,6-stilbenesulfonic acid trisodium salt (HPTS), to get dye@FJU-10. Since HPTS is rich in –OH and –SO3H, it serves as a good proton carrier. A study of their intrinsic proton conductance without additional RH found that the σ of FJU-10 was 2.05 × 10−4 S cm−1 (30 °C). In contrast, the σ of dye@FJU-10 was 1.25 × 10−3 S cm−1. The highest σ of dye@FJU-10 was 7.50 × 10−3 S cm−1 (90 °C) being nearly 5 times higher than that of FJU-10, which suggests that encapsulated dyes in MOFs act as a key role in enhancing the σ.
An extremely thermally stable In-MOF, [In(EBTC)][Me2NH2](DMF)(H2O)5 (F31) was synthesized by Ren114 and co-workers. At 25 °C and 99% RH, it showed an outstanding σ of 3.49 × 10−3 S cm−1. The hydrogen bonds that form between the carboxylate groups in the framework, the lattice H2O units in the channels, and the [NH2Me2]+ cations are responsible for this occurrence. Proton transport is facilitated by the cooperation of the lattice. More opportunities for their practical uses in the future are provided by their strong hydrothermal stability and good electrochemical qualities.
Furthermore, Chu's group167 obtained porous carbon microspheres (PCMSs) with 3D pomegranate-like structures by assembly of hollow highly-graphitized thin-walled nanoballs across directly carbonizing In-MOFs (CPM-5). When the sulfur addition was 70 wt%, the S/PCMSs cathode provided a large initial discharge uptake of 1239 mA h g−1 at 0.2 C and exhibited an excellent high rate capacity of 742 mA h g−2 at 4 C. Excellent cycle stability was achieved at both low and high rates. Among them, a capacity decay rate of 0.067% per cycle over 700 cycles at 0.5 C and a capacity decay rate of 0.014% per cycle over 500 cycles at 4 C were achieved.
In 2020, Zuo's group,168 using [In(COO)4]− nodes and tetratopic tetrathiafulvalene (TTF)-based linkers, fabricated two two-dimensional proton-conducting MOFs, [Me2NH2][In(m-TTFTB)] (F32) and [Me2NH2][In(TTFOC)] (F33). (m-H4TTFTB = 3,3′,3′′,3′′′-([2,2′-bi(1,3-dithiolylidene)]-4,4′,5,5′-tetrayl)tetrabenzoic acid). As denoted in Fig. 41a and b, F33 has a higher σ (1.30 × 10−2 S cm−1 at 303 K and 98% RH) than that of F32 (6.66 × 10−4 S cm−1) due to the effective proton-conducting channel constituting of Me2NH2+, H2O, and the uncoordination carboxylate group. In addition to its excellent proton conductivity, it also has an interfacial pseudocapacitance, realized through its unique out-of-plane proton channel and redox-switchable ligands. In addition, by calculating the activation energy (Fig. 41c and d), the proton/pseudocapacitive coupling mechanism provides a novel approach for building interesting structures using the ionic conductivity of MOFs.
 | ||
| Fig. 41 (a and b) Nyquist spectra of F32 and F33 at 303 K and various RHs. (c and d) The activation energy values of F32 and F33. Reproduced from ref. 168, with permission from Elsevier Inc, Copyright 2019. | ||
In 2020, Zhang and co-workers synthesized169 a MOF, ((CH3)2NH2)((CH3)2NH)[In(mdhbqdc)2] (In-BQ) (H2mdhbqdc = dimethyl-3,6-dihydroxy-2,5-benzoquinone-1,4-dicarboxylicacid), which has a modified –OH unit anilicate ligand and dimethylamine cations. A large number of –OH units attached to the inner walls of the pores interacted with Me2NH and/or Me2NH2+ by N–H⋯O H-bonds. The high concentration of protons in the porous framework and the dynamics of protonated amines readily lead to a moderate conductivity (2.10 × 10−4 S cm−1) under 303 K and 95% RH and the Ea of 0.73 eV.
In 2020, Lu's team reported the synthesis of two In-MOFs,41S1 and S2. The conductivity was 6 and 7 μS cm−1 for S1 and S2 at 300 K and 1 MHz, respectively. Despite their low conductivities, the conductivities at various temperatures and frequencies remained at the same order of magnitude.
In 2021, Zhai and colleagues created CPM-200/CNT@S by mixing indium-based MOF-CPM-200 with multi-walled carbon nanotubes (CNTs) and sulfur.170 CNTs embedded in the framework of CPM-200 provided the compliant material with good conductive characteristics. CPM-200/CNT@S could be used as a cathode in Li-ion batteries, exhibiting high multiplicity performance and cycle stability, and has an initial discharge capacity of 0.1 C after 100 charge cycles and can reach a capacity of 1400 mA h g−1. Coulombic efficiency is close to 100%. Finally, the unique composite structure of lithium–sulfur battery cathode materials can reduce the “shuttle effect” while increasing electrical conductivity.
By inserting (Me2NH2)+-encapsulated IN-based anionic metal–organic skeleton (FJU-17) as a “capsule” into HTM, Zhang's group created a bifunctional layer of HTM-FJU-17.171 By releasing (Me2NH2)+ ions, FJU-17 capsules will passivate organic cationic vacancies. In addition, its anionic skeleton can stabilize the positively charged oxidized HTM to enhance hole transport. Power conversion efficiency (PCE) rose from 18.32% to 20.34% in the perovskite solar cells (PSCs), which also showed decreased charge complexation. Furthermore, after 1000 hours, HTM-FJU-17's hole mobility rose from 3.11 × 10−4 to 1.05 × 10−3 cm2 (V s)−1 and ambient circumstances. Stable devices were produced with 90% retention of the original PCE. This study confirms the promising use of anionic MOFs/HTM-based bifunctional layers to create PSC devices.
In 2021, Zhang's group prepared a helical IN-MOF, [In2(OH)2(BPTC)]·6H2O (InOF-1).172 Then, they proposed the elliptical substitution metal strategy, the elliptical substitution of In(III) by Ni(II), and succeeded in preparing the isostructural MOF, ([Ni2(BPTC)(HCOOH)2]·3H2O) (NiOF-1). The walls of the parent compound InOF-1 with –OH groups cover the channel surface, resulting in a σ of InOF-1 being 7.86 × 10−3 S cm−1 (328 K and 95% RH). Aliovalent-substituted and formic acid-modified NiOF-1 possesses stronger host–guest contacts and a richer H-bonding network, resulting in better σ of 3.41 × 10−2 S cm−1.
Also in 2021, they designed173 a carbazole-based non-interpenetrating In-MOF (FJU-302), which has a stable layer structure and contains negatively charged [In(cdc)(bpdc)]−. The σ was measured without additional humidity from −30 °C to 70 °C (Fig. 42a and b). The maximum σ of FJU-302 was 6.47 × 10−4 and 5.88 × 10−7 S cm−1 at 70 and −30 °C, respectively. Ea calculated from the antimetric measurements was 0.568 eV (Fig. 42c), which proves that the proton transport in FJU-302 follows a Vehicular mechanism.
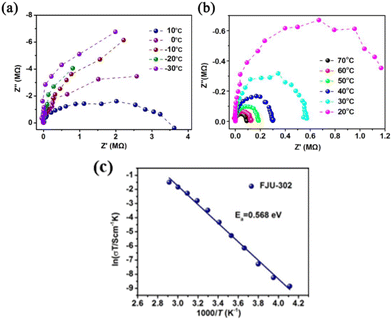 | ||
| Fig. 42 (a and b) Nyquist plots of FJU-302 at −30–70 °C without additional humidity. (c) The Ea of FJU-302. Reproduced from ref. 173, with permission from Chinese Chemical Society, Copyright 2021. | ||
In 2022, Farbod and colleagues designed nanocomposites of MIL-68(In) interwoven with CNTs to make MIL-68(In)-CNTs material. Batteries made from MIL-68(In)-CNTs nanoplatforms achieved a substantial capacitance of 314 F g−1 at a scan rate of 2 mV s−1, 15.3 W h kg−1 at a power density of 300 W kg−1, and maintained 95% of the capacitance after 4000 charge/discharge cycles.174MIL68(In)-CNTs material exhibits fast ion and electron transport. In this way, during charge and discharge, the porous cavities in this nanocomposite serve as electrolyte ion reservoirs.
Jiao et al. prepared In-IPA@rGO by hydrothermally coating three-dimensional In-IPA with rGO.175 The rGO networks not only greatly enhanced the conductivity of In-IPA but also improved the kinetic reaction reduction and facilitated electron transport. The appreciable initial capacity of In-IPA@rGO 1672.3 mA h g−1 at 0.2 C exhibited a reversible capacity of 898.7 mA h g−1 over 100 cycles. This study confirms that the non-transition MOFs in collaboration with rGOs have a potential application in lithium–sulfur batteries.
Sun's group thermally carbonized InOF-1 under inert argon gas and then formed indium particles through redox reactions. Then, a porous hollow carbon nanostraw (HCNS) was achieved by fusing and removing indium in a decarboxylation process, which has more charge active sites, and short and fast electron and ion transfer channels.176 In addition, the assembled zinc–iodine batteries (ZIBs) offer a high capacity of 234.1 mA h g−1 at 1 A g−1, and the multiplicity and cycling performance of the HCNS-based ZIBs are highly enhanced.
In 2023, Wen's team synthesized indium-MOFs with a hexagonal nanorod structure,177 then pyrolyzed the In-MOFs at 450 °C to prepare In/C hexagonal nanorods, and introduced Gr nanosheets to tune the electromagnetic parameters of the In/C nanorods. As the ratio of In/C nanorods to Gr nanosheets is 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a strong reflection loss of −43.7 dB at 14.0 GHz was obtained., which indicates that the In/C-Gr composites have significant applications.
1, a strong reflection loss of −43.7 dB at 14.0 GHz was obtained., which indicates that the In/C-Gr composites have significant applications.
In 2024, Li's group prepared five isostructural indium-based MOFs, MIL-68-In or MIL-68-In-X (X = NH2, OH, Br or NO2).178 All five MOFs have high σ values with the optimal σ being 2.37 × 10−4, 1.70 × 10−3, 1.72 × 10−3, 4.11 × 10−4, and 4.47 × 10−4 S cm−1 (100 °C and 98% RH), respectively.
In contrast to other domains where In-based MOFs are widely used, research into their usage in electrochemistry is still in its early stages. This is due to the strong affinity between the molecules and the backbone of In-MOFs, as well as their special structural stability and variation, and the fact that they form from tightly connected clusters. Its potential application in electrochemistry is thus quite promising. We believe that future studies into these MOFs will yield significant results for the development of innovative electrochemical materials.
5. Conclusions and perspectives
This review included the synthesis, design, and application of In-MOFs, as well as recent scientific breakthroughs. Based on this, a few common In-MOFs are illustrated, along with a brief discussion of their structures and properties. When it comes to MOFs in general, the In-MOF reports are only the tip of the iceberg. Despite indium's relatively low abundance and diffuse distribution in the earth's crust, an increasing number of In-based MOFs are being reported due to their low toxicity, environmental friendliness, and structural stability.We think that the following issues should be taken into consideration in future research on In-MOFs:
(I) To begin, in the case of currently available In-MOFs, the functionalization of organic ligands may have a significant impact on the final structure. In the presence of various Lewis acids, In3+'s peculiar electronic configuration with high-cycle p-blocking enables it to expediently receive electrons and form strong coordination bonds. Making tight connections between O, C, N, S, and other atoms can result in exceptionally stable structures. Simple monocyclic or polycyclic carboxylic acids are still the most widely investigated organic ligands. Imidazole, triazole, and tetrazole are examples of organic ligands with multiple nitrogen atoms; similarly, organic ligands with multiple oxygen atoms in phosphoric acid and sulfonic acid groups require special attention.
(II) Second, the most used technique for synthesizing In-MOFs is still hydrothermal/solvothermal. Nevertheless, this process is usually labor-intensive and unsuitable for large-scale manufacturing. Instead, more should be done to prepare In-MOFs using the recently developed microwave, mechanochemical, and acoustochemical technologies.
(III) In conclusion, because of the diverse architectures and exceptional stability of In-MOFs, a significant deal of research has already been done on the subject. Since the research's applicability is now somewhat narrow, it should be further broadened. At the moment, the primary areas of applied research include luminescence, adsorption and separation, and catalysis. However, more study is required in the fields of heavy metal removal, chemical sensors, proton conductivity, drug delivery—particularly in cancer therapy—and these applications.
If these studies continue, we think there is a lot of promise for improving material design in general and creating new, stable In-MOFs with improved performance characteristics in particular.
Abbreviations
| MOFs | Metal–organic frameworks |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 1D | One-dimensional |
| SBU | Structure building unit |
| σ | Proton conductivity |
| E a | Activation energy |
| RH | Relative humidity |
| DMA | N,N-Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| NMF | N-Methylformamide |
| DEF | N,N-Diethylformamide |
| MeOH | Methanol |
| EtOH | Ethanol |
| HCOOH | Formic acid |
| CH3COOH | Acetic acid |
| 1,10-Phen | 1,10-Phenanthroline |
| 1,7-Phen | 1,7-Phenanthroline |
| 2,2′-bipy | 2,2′-Bipyridine |
| 4,4′-bipy | 4,4′-Bipyridine |
| bpe | 1,2-Di(4-pyridyl)ethylene |
| bpa | 1,2-Di(4-pyridyl)ethane |
| H2FcDC | 1,1′-Ferrocenedicarboxylic acid |
| OX | Oxalic acid |
| HTM | Hole transporting material |
| HCNS | Hollow carbon nanosheet |
| ZIBs | Zinc–iodine batteries |
| rGO | Graphene oxide |
| CNTs | Carbon nanotubes |
| MAS | Microwave-assisted solvothermal |
| ESIPT | Intramolecular proton transfer |
| TEA | Triethylamine |
| AuNCs | Fluorescent gold nanoclusters |
| IAST | Ideal adsorbed solution theory |
| FL | Fluorescence |
| NZF | Nitrofurazone |
| BTEX | Benzenehomologues |
| ATRP | Atom transfer radical polymerization |
| ORR | Oxygen reduction reaction |
| RhB | Rhodamine B |
| MO | Methyl orange |
| MB | Methylene blue |
| OFL | Ofloxacin |
| FE | Faraday efficiency |
| PFOA | Perfluoroctanoic acid |
| OOP | Out-of-plane |
| QY | Quantum yield |
| TC | Tetracycline hydrochloride |
| H4FBDC | 2,5-Di(2′,5′-dicarboxylphenyl)-difluorobenzene |
| H2dcpy | 3-(2′,5′-Dicarboxyphenyl)pyridonic acid |
| SSA | Specific surface area |
| ODDA | 4,4′-((8r,11aR)-4,8-Bis(10-(4-carboxyphenyl)anthracen-9-yl)-6-hydoxy-1,11-dimethyl-6-oxidodibenzo[d,f][1,3,2]dioxaphosphepine-2,10-diyl)dibenzoic acid |
| ODDM | 4′,4′′′-(2,10-Bis(4-carboxyphenyl)-6-hydoxy-1,11-dimethyl-6-oxidodibenzo[d,f][1,3,2]dioxaphosphepine-4,8-diyl)bis(1,1′-biphenyl)-4-carboxylic acid |
| H2BDC | Terephthalic acid |
| H2NDC | Naphthalene-1,4-dicarboxylic acid |
| H4L1 | Oxalylbis(aza-nediyl)diisophthalic acid |
| H4L2 | 1,1′-Biphenol |
| H3BTB | 4,4′,4′′-Benzene-1,3,5-triyl-tribenzoic acid |
| CBDA | [5,5′-(Carbonylbis(azanediyl))-diisophthalic acid] |
| tpt | 2,4,6-Tris(4-pyridinyl)-1,3,5-triazine |
| H4BTA | Benzene-1,2,4,5-tetracarboxylic acid |
| H6TCPP | (4-Tetracarboxyphenylporphyrin) |
| H4L3 | 5,5′-(Butane-1,4-diylbis(oxy))diisophthalic acid |
| H4L4 | 5,5′-(1,4-Phenylenebis(methylene))bis(oxy)diisophthalic acid |
| TDC | 2,5-Thiophenedicarboxylic acid |
| CSA | N-(4-Carboxyphenyl)succinic acid |
| PDG | N,N′-(1,4-Phenylene dicarbonyl)diglycine |
| H4EBDC | 5,5′-(1,2-Ethynediyl)bis(1,3-benzenedicarboxylic acid) |
| H2L5 | 2,2′,6,6′-Tetramethoxy-4,4′-biphenyldicarboxylic acid |
| H4SFTBA | (4,4′,4′′,4′′′-(9,9′-Spirobi[fluorene]-2,2′,7,7′-tetrayl)tetrabenzoic acid) |
| H3popha | 5-(4-Carboxy-2-nitrophenoxy) isophthalic acid |
| H4TBAPy | (1,3,6,8-Tetrakis(p-benzoic acid)pyrene) |
| H4ABTC | (E)-5,5′-(Diazene-1,2-diyl)diisophthalic acid |
| H4TPTA | ([1,1′:3′,1′′-Terphenyl]-4,4′,4′′,6′-tetracarboxylic acid) |
| QLBM | (Quinoline-2-yl)-benzimidazole |
| H3DCPN | 5-(3′,5′-Dicarboxyphenyl)nicotinic acid |
| H3L1 | 5-(3′,5′-Dicarboxyphenyl)nicotinic acid |
| H3L2 | 3-(2,4-Dicarboxyphenyl)-6-carboxypyridine |
| TPyP | 5,10,15,20-Tetrakis(4-pyridyl)-21H,23H-porphyrin |
| H5CPDD | 4, 4′-(4-(4-Carboxyphenyl)pyridine-2,6-diyl)diisophthalic acid |
| TATB | 4,4′,4′′-s-Triazine-2,4,6-triyl tribenzoate |
| H4EBTC | 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylic acid |
| bpdcH2 | 4, 4′-Biphenyldicarboxylic acid |
| H3CTTA | 5′-(4-Carboxy-phenyl)-2′,4′,6′-trimethyl-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylic acid |
| H3TATAB | (4,4′,4′′-s-Triazine-1,3,5-triyltri-p-amino benzoic acid) |
| H2SBA | 4,4′-Sulfonyldibenzoic acid |
| ([EMIM][BF4]) | 1-Ethyl-3-methylimidazolium tetrafluoroborate |
| HTDP | 2,4,6-Tri(2,4-dicarboxyphenyl)pyridine |
| H2aip | 5-Aminoisophthalic acid |
| H3sip | 5-Sulfoisophthalic acid |
| H2mdhbqdc | Dimethyl-3,6-dihydroxy-2,5-benzoquinone-1,4-dicarboxylic acid |
| TTF | Tetratopic tetrathiafulvalene |
| Odpt | 4,4′-Oxydiphthalate |
| H4PPTTA | 4,4′,4′′,4′′′-(1,4-Phenylenbis(pyridine-4,2,6-triyl))-tetrabenzoic acid |
| H4bptc | 3,3′,5,5′-Biphenyl tetracarboxylate |
| IPA | Isophthalic acid |
| TCA | 4,4′,4′′-Triphenylamine tricarboxylic acid |
| DHBDC | 2,5-Dihydroxyterephthalic acid |
| H2fdc | Furan-2,5-dicarboxylic acid |
| H2TDC | Thiophene dicarboxylic acid |
| H3BTC | Benzene-1,2,3-tricarboxylic acid |
| H4TCPBDA | N,N,N′,N′-Tetrakis(4-carboxyphenyl)-biphenyl-4,4′-diamine |
| H3CPTA | 2-(2-Carboxypyridin-4-yl)terephthalic acid |
| H3TATB | 2,4,6-Tris(4-carboxyphenyl)-1,3,5-triazine |
| ODDP | 4,4′,4′′,4′′′-((11aR)-6-Hydoxy-1,11-dimethyl-6-oxidodibenzo[d,f][1,3,2]dioxaphosphepine-2,4,8,10-tetrayl)tetrabenzoic acid |
| HIN | Isonicotinic |
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the National Natural Science Foundation of China (grant 22071223).References
- G. Y. Ryu, S. J. An, S. Yu, K. J. Kim, H. Jae, D. Roh and W. S. Chi, Dual-sulfonated MOF/polysulfone composite membranes boosting performance for proton exchange membrane fuel cells, Eur. Polym. J., 2022, 180, 111601 CrossRef CAS.
- F. D. Wang, B. C. Wang, B. B. Hao, C. X. Zhang and Q. L. Wang, Designable guest-molecule encapsulation in metal-organic frameworks for proton conductivity, Chem. – Eur. J., 2022, 28, e202103732 CrossRef CAS.
- S.-L. Yang, G. Li, M.-Y. Guo, W.-S. Liu, R. Bu and E.-Q. Gao, Positive cooperative protonation of a metal–organic framework: pH-responsive fluorescence and proton conduction, J. Am. Chem. Soc., 2021, 143, 8838–8848 CrossRef CAS PubMed.
- D. Suttipat, S. Butcha, S. Assavapanumat, T. Maihom, B. Gupta, A. Perro, N. Sojic, A. Kuhn and C. Wattanakit, Chiral macroporous MOF surfaces for electroassisted enantioselective adsorption and separation, ACS Appl. Mater. Interfaces, 2020, 12, 36548–36557 CrossRef CAS.
- X. Dong, Q. Fan, W. Hao and Y. Chen, Adsorption and separation of hexane isomers in metal-organic frameworks (MOFs): A computational study, Comput. Theor. Chem., 2021, 1197, 113164 CrossRef CAS.
- C. Yang, J. Qi, A. Wang, J. Zha, C. Liu and S. Yao, Application of machine learning in MOFs for gas adsorption and separation, Mater. Res. Express, 2023, 10, 122001 CrossRef.
- S. F. Hammad, I. A. Abdallah, A. Bedair, R. M. Abdelhameed, M. Locatelli and F. R. Mansour, Metal organic framework-derived carbon nanomaterials and MOF hybrids for chemical sensing, TrAC, Trends Anal. Chem., 2024, 170, 117425 CrossRef.
- Y. Fei, K. Sun and L. Liu, Carbon-dots-referenced metal-organic frameworks for chemical sensing of tumor/mood biomarker 5-hydroxyindoleacetic acid in human urine: Covalent grafting blue-emitting carbon dots onto red-emitting MOF, Spectrochim. Acta, Part A, 2023, 290, 122244 CrossRef.
- J. F. Olorunyomi, S. T. Geh, R. A. Caruso and C. M. Doherty, Metal–organic frameworks for chemical sensing devices, Mater. Horiz., 2021, 8, 2387–2419 RSC.
- A. Ahmad, S. Khan, S. Tariq, R. Luque and F. Verpoort, Self-sacrifice MOFs for heterogeneous catalysis: Synthesis mechanisms and future perspectives, Mater. Today, 2022, 55, 137–169 CrossRef.
- I. Ahmed, M. M. H. Mondol, M. J. Jung, G. H. Lee and S. H. Jhung, MOFs with bridging or terminal hydroxo ligands: Applications in adsorption, catalysis, and functionalization, Coord. Chem. Rev., 2023, 475, 214912 CrossRef.
- K. Hemmer, M. Cokoja and R. A. Fischer, Exploitation of intrinsic confinement effects of MOFs in catalysis, ChemCatChem, 2021, 13, 1683–1691 CrossRef.
- Y. Tian, G. Liang, T. Fan, J. Shang, S. Shang, Y. Ma, R. Matsuda, M. Liu, M. Wang, L. Li and S. Kitagawa, Chem. Mater., 2019, 31, 8494–8503 CrossRef.
- K. Zhang, G.-H. Wen, S.-S. Bao, L.-Q. Wu and J.-G. Jia, Studying the proton conduction through the grain surface of UiO-66-NH2, ACS Appl. Energy Mater., 2020, 3, 8198–8204 CrossRef.
- E. O. Eren, N. Özkan and Y. Devrim, Preparation of polybenzimidazole/ZIF-8 and polybenzimidazole/UiO-66 composite membranes with enhanced proton conductivity, Int. J. Hydrogen Energy, 2022, 47, 19690–19701 CrossRef.
- L. M. Aguirre-Díaz, D. Reinares-Fisac, M. Iglesias, E. Gutiérrez-Puebla, F. Gándara, N. Snejko and M. Á. Monge, Group 13th metal-organic frameworks and their role in heterogeneous catalysis, Coord. Chem. Rev., 2017, 335, 1–27 CrossRef.
- W. Fan, K.-Y. Wang, C. Welton, L. Feng, X. Wang, X. Liu, Y. Li, Z. Kang, H.-C. Zhou, R. Wang and D. Sun, Aluminum metal–organic frameworks: From structures to applications, Coord. Chem. Rev., 2023, 489, 215175 CrossRef.
- L.-L. Kang, M. Xue, Y.-Y. Liu, Y.-H. Yu, Y.-R. Liu and G. Li, Proton conductive metal–organic frameworks based on main-group metals, Coord. Chem. Rev., 2022, 452, 214301 CrossRef.
- E. P. Gómez-Oliveira, D. Reinares-Fisac, L. M. Aguirre-Díaz, F. Esteban-Betegón, M. Pintado-Sierra, E. Gutiérrez-Puebla, M. Iglesias, M. Ángeles Monge and F. Gándara, Framework adaptability and concerted structural response in a bismuth metal-organic framework catalyst, Angew. Chem., Int. Ed., 2022, 61, e202209335 CrossRef.
- Q.-X. Wang and G. Li, Bi(iii) MOFs: synthesesstructures and applications, Inorg. Chem. Front., 2021, 8, 572–589 RSC.
- Y.-H. Luo, A. D. Xie, W.-C. Chen, D. Shen, D.-E. Zhang, Z.-W. Tong and C.-S. Lee, Multifunctional anionic indium–organic frameworks for organic dye separation, white-light emission and dual-emitting Fe3+ sensing, J. Mater. Chem. C, 2019, 7, 14897–14903 RSC.
- M. Krüger, M. Albat, A. K. Inge and N. Stock, Investigation of the effect of polar functional groups on the crystal structures of indium MOFs, CrystEngComm, 2017, 19, 4622–4628 RSC.
- C. Volkringer, T. Loiseau, N. Guillou, J. Marrot, G. Férey, M. Haouas, F. Taulelle, N. Audebrand and M. Latroche, The kagomé topology of the gallium and indium metal-organic framework types with a MIL-68 structure: synthesis, XRD, solid-State NMR characterizations, and hydrogen adsorption, Inorg. Chem., 2008, 47, 11901 CrossRef.
- Y.-B. Tian, Q.-H. Li, Z. Wang, Z.-G. Gu and J. Zhang, Coordination-induced symmetry breaking on metal-porphyrinic framework thin films for enhanced nonlinear optical limiting, Nano Lett., 2023, 23, 3062–3069 CrossRef PubMed.
- R. Zhang, B. Wang, F. Wang, S.-M. Chen and J. Zhang, Syntheses of ferrocene-functionalized indium-based metal–organic frameworks for third order nonlinear optical application, Inorg. Chem. Front., 2023, 10, 201–210 RSC.
- V. Kumar Maka, P. Tamuly, S. Jindal and J. Narasimha Moorthy, Control of In-MOF topologies and tuning of porosity through ligand structure, functionality and interpenetration: Selective cationic dye exchange, Appl. Mater. Today, 2020, 19, 100613 CrossRef.
- T. C. Schäfer, J. Becker, A. E. Sedykh and K. Müller-Buschbaum, 2D-Coordination polymers constituted from indium halides and dipyridyl N-donor ligands, Z. Anorg. Allg. Chem., 2021, 647, 1227–1233 CrossRef.
- J. Qian, P. Yu, K. Su, Y. Dong, S. Huang and M. Hong, Crystal structure, morphology and sorption behaviour of porous indium-tetracarboxylate framework materials, CrystEngComm, 2015, 17, 8512–8518 RSC.
- W. Dan, X. Liu, M. Deng, Y. Ling, Z. Chen and Y. Zhou, A highly stable indium phosphonocarboxylate framework as a multifunctional sensor for Cu2+ and methylviologen ions, Dalton Trans., 2015, 44, 3794–3800 RSC.
- X. Du, R. Fan, J. Fan, L. Qiang, Y. Song, Y. Dong, K. Xing, P. Wang and Y. Yang, Self-assembly of two supramolecular indium(III) metal–organic frameworks for reversible iodine capture and large band gap change semiconductor behavior, Inorg. Chem. Front., 2016, 3, 1480–1490 RSC.
- H. Zhang, J. Wu, W.-Y. Geng, Y.-H. Luo, Z.-J. Ding, Z.-X. Wang and D.-E. Zhang, An interpenetrated anionic In(III)-MOF for efficient adsorption/separation of organic dyes and selective sensing of Fe3+ ion and nitroaromatic compounds, J. Solid State Chem., 2021, 302, 122424 CrossRef.
- P. Yong, S. Wang, X. Zhang, H. Pan and S. Shen, MOFs-derived Co-doped In2O3 hollow hexagonal cylinder for selective detection of ethanol, Chem. Phys. Lett., 2022, 795, 139517 CrossRef.
- H. Zhang, Z.-X. Wang, Y.-H. Luo, F.-Y. Chen, C.-Y. Jia, X.-Q. Tan, Y.-Y. Zhang and D.-E. Zhang, Co2+ and nitrobenzene sensing using indium-based metal-organic framework, Polyhedron, 2022, 224, 116016 CrossRef.
- H. N. K. Lam, N. B. Nguyen, G. H. Dang, T. Truong and N. T. S. Phan, Three-component coupling of aldehyde, alkyne, and amine via C–H bond activation using indium-based metal–organic framework MIL-68(In) as a recyclable heterogeneous catalyst, Catal. Lett., 2016, 146, 2087–2097 CrossRef.
- H. Lv, L. Fan, T. Hu, C. Jiao and X. Zhang, A highly robust cluster-based indium(III)–organic framework with efficient catalytic activity in cycloaddition of CO2 and Knoevenagel condensation, Dalton Trans., 2023, 52, 3420–3430 RSC.
- C. Lu, D. Xiong, C. Chen, J. Wang, Y. Kong, T. Liu, S. Ying and F.-Y. Yi, Indium-based metal–organic framework for efficient photocatalytic hydrogen evolution, Inorg. Chem., 2022, 61, 2587–2594 CrossRef CAS PubMed.
- Y.-H. Li, S.-L. Wang, Y.-C. Su, B.-T. Ko, C.-Y. Tsai and C.-H. Lin, Microporous 2D indium metal–organic frameworks for selective CO2 capture and their application in the catalytic CO2-cycloaddition of epoxides, Dalton Trans., 2018, 47, 9474–9481 RSC.
- Y.-Z. Shi, Q.-H. Hu, X. Gao, L. Zhang, R.-P. Liang and J.-D. Qiu, A flexible indium-based metal-organic framework with ultrahigh adsorption capacity for iodine removal from seawater, Sep. Purif. Technol., 2023, 312, 123366 CrossRef CAS.
- J. Lei, Y.-P. Li, Y.-Y. Xue, Y. Wang, S.-N. Li, Y.-C. Jiang, M.-C. Hu and Q.-G. Zhai, Design of robust rod-packing [In(OH)(BDC)] frameworks and their high CO2/C2-hydrocarbons over CH4 separation performance, J. Solid State Chem., 2019, 279, 20936 CrossRef.
- B. Zhang, W. Wang, B. Liu and L. Hou, Indium metal–organic frameworks based on pyridylcarboxylate ligands and their potential applications, Dalton Trans., 2021, 50, 5713–5723 RSC.
- S. Kamal, K. R. Chiou, B. Sainbileg, A. I. Inamdar, M. Usman, A. Pathak, T.-T. Luo, J.-W. Chen, M. Hayashi, C.-H. Hung and K.-L. Lu, Thermally stable indium based metal–organic frameworks with high dielectric permittivity, J. Mater. Chem. C, 2020, 8, 9724–9733 RSC.
- S. Choi, T. Kim, H. Ji, H. J. Lee and M. Oh, Isotropic and anisotropic growth of metal–organic framework (MOF) on MOF: logical inference on MOF structure based on growth behavior and morphological feature, J. Am. Chem. Soc., 2016, 138, 14434–14440 CrossRef.
- J. Ma, H. Fan, X. Zheng, H. Wang, N. Zhao, M. Zhang, A. K. Yadav, W. Wang, W. Dong, S. Wang and J. Facile, Metal-organic frameworks-templated fabrication of hollow indium oxide microstructures for chlorine detection at low temperature, Hazard. Mater., 2020, 387, 122017 CrossRef.
- R.-R. Xie, X.-A. Guo, Z.-H. Zhang and D.-X. Xue, An oxamide-functionalized ternary indium-organic framework, Inorg. Chem. Commun., 2020, 113, 107762 CrossRef.
- C. L. Meng, Z. J. Li, Y. Liu, B. Z. Liu and Y. Cui, Synthesis, structure and characterization of a 3D chiral indium carboxylate metal-organic framework based on 1,1′-biphenol ligand, Chin. J. Struct. Chem., 2017, 12, 2086 Search PubMed.
- Z. Chen, C. Yu, J. Xi, S. Tang, T. Bao and J. Zhang, A hybrid material prepared by controlled growth of a covalent organic framework on amino-modified MIL-68 for pipette tip solid-phase extraction of sulfonamides prior to their determination by HPLC, Microchim. Acta, 2019, 186, 393 CrossRef PubMed.
- S. E. Springer, J. J. Mihaly, N. Amirmokhtari, A. B. Crom, M. Zeller, J. I. Feldblyum and D. T. Genna, Framework isomerism in a series of btb-containing In-derived metal–organic frameworks, Cryst. Growth Des., 2019, 19, 3124–3129 CrossRef CAS.
- Q. Chen, Y. Ying, L. Wang, Z. Guo, Y. Zhou, D. Wang and C. Li, Z. A Heterometallic MOF based on monofunctional linker by “one-pot” solvothermal method for highly selective gas adsorption, Anorg. Allg. Chem., 2020, 646, 437–443 CrossRef CAS.
- C. J. Tatebe, S. Yusuf, M. K. Bellas, M. Zeller, C. Arntsen and D. T. Genna, On the role of dioxane in the synthesis of In-derived MOFs, Cryst. Growth Des., 2021, 21, 6840–6846 CrossRef CAS.
- A. K. Inge, M. Köppen, J. Su, M. Feyand, H. Xu, X. Zou, M. O'Keeffe and N. Stock, Unprecedented topological complexity in a metal–organic framework constructed from simple building units, J. Am. Chem. Soc., 2016, 138, 1970–1976 CrossRef CAS PubMed.
- M. Köppen, O. Beyer, S. Wuttke, U. Lüning and N. Stock, Synthesis, functionalisation and post-synthetic modification of bismuth metal–organic frameworks, Dalton Trans., 2017, 46, 8658–8663 RSC.
- L. M. Aguirre-Díaz, M. Iglesias, N. Snejko, E. Gutiérrez-Puebla and M. Á. Monge, Synchronizing substrate activation rates in multicomponent reactions with metal-organic framework catalysts, Chem. – Eur. J., 2016, 22, 6654–6665 CrossRef PubMed.
- K. Maru, S. Kalla, A. K. Ghosh and R. Jangir, Synthesis of microcrystalline indium(III)-MOF and adsorptive and selective removal of dyes, Res. Chem. Intermed., 2023, 50, 147–174 CrossRef.
- S. Zhou, Q. Lu, M. Chen, B. Li, H. Wei, B. Zi, J. Zeng, Y. Zhang, J. Zhang, Z. Zhu and Q. Liu, Platinum-supported cerium-doped indium oxide for highly sensitive triethylamine gas sensing with good antihumidity, ACS Appl. Mater. Interfaces, 2020, 12, 42962–42970 CrossRef.
- H. Yang, J. Tang, Y. Luo, X. Zhan, Z. Liang, L. Jiang, H. Hou and W. Yang, MOFs-derived fusiform In2O3 mesoporous nanorods anchored with ultrafine CdZnS nanoparticles for boosting visible-light photocatalytic hydrogen evolution, Small, 2021, 17, 2102307 CrossRef.
- H. Ji, S. Lee, J. Park, T. Kim, S. Choi and M. Oh, Improvement in crystallinity and porosity of poorly crystalline metal–organic frameworks (MOFs) through their induced growth on a well-crystalline MOF template, Inorg. Chem., 2018, 57, 9048–9054 CrossRef.
- J. Sun, Y. Wang, P. Song, Z. Yang and Q. Wang, Metal-organic framework-derived Cr-doped hollow In2O3 nanoboxes with excellent gas-sensing performance toward ammonia, J. Alloys Compd., 2021, 879, 160472 CrossRef.
- Z. Zhang, Y. Wang, B. Niu, B. Liu, J. Li and W. Duan, Ultra-stable two-dimensional metal–organic frameworks for photocatalytic H2 production, Nanoscale, 2022, 14, 7146–7150 RSC.
- L.-L. Jiang, X. Zeng, M. Li, M.-Q. Wang, T.-Y. Su, X.-C. Tian and J. Tang, Rapid electrochemical synthesis of HKUST-1 on indium tin oxide, RSC Adv., 2017, 7, 9316–9320 RSC.
- T. Rhauderwiek, S. Waitschat, S. Wuttke, H. Reinsch, T. Bein and N. Stock, Nanoscale synthesis of two porphyrin-based MOFs with gallium and indium, Inorg. Chem., 2016, 55, 5312–5319 CrossRef CAS.
- M. A. Sinnwell, Q. R. S. Miller, L. Palys, D. Barpaga, L. Liu, M. E. Bowden, Y. Han, S. Ghose, M. L. Sushko, H. T. Schaef, W. Xu, M. Nyman and P. K. Thallapally, Molecular intermediate in the directed formation of a zeolitic metal–organic framework, J. Am. Chem. Soc., 2020, 142, 17598–17606 CrossRef CAS PubMed.
- X.-L. Chen, Y. Pan and X.-Z. Song, Two novel anionic indium–tetracarboxylate frameworks: Syntheses, structures and photoluminescent properties, Polyhedron, 2016, 117, 513–517 CrossRef CAS.
- D. Reinares-Fisac, L. M. Aguirre-Díaz, M. Iglesias, E. Gutiérrez-Puebla, F. Gándara and M. Á. Monge, Anionic and neutral 2D indium metal–organic frameworks as catalysts for the Ugi one-pot multicomponent reaction, Dalton Trans., 2019, 48, 2988–2995 RSC.
- L. Liu, Z. Yao, Y. Ye, C. Liu, Q. Lin, S. Chen, S. Xiang and Z. Zhang, Enhancement of intrinsic proton conductivity and aniline sensitivity by introducing dye molecules into the MOF channel, ACS Appl. Mater. Interfaces, 2019, 11, 16490–16495 CrossRef CAS.
- R. M. Zhang, M. H. Zhang, W. Wang, Y. W. Xu, Z. Y. Wen and Z. Y. Wang, Interpenetration of three 2D In-MOFs with (6, 3) topology: syntheses, structures and fluorescent properties, Chin. J. Struct. Chem., 2016, 35, 1722 Search PubMed.
- D. Woschko, S. Yilmaz, C. Jansen, A. Spieß, R. Oestreich, T. J. Matemb Ma Ntep and C. Janiak, Enhanced sorption in an indium-acetylenedicarboxylate metal–organic framework with unexpected chains of cis-μ-OH-connected {InO6} octahedra, Dalton Trans., 2023, 52, 977–989 RSC.
- L. Wu, W. Wang, R. Liu, G. Wu and H. Chen, Impact of the functionalization onto structure transformation and gas adsorption of MIL-68(In), R. Soc. Open Sci., 2018, 5, 181378 CrossRef CAS PubMed.
- J. J. Mihaly, M. Zeller and D. T. Genna, Ion-directed synthesis of indium-derived 2,5-thiophenedicarboxylate metal–organic frameworks: tuning framework dimensionality, Cryst. Growth Des., 2016, 16, 1550–1558 CrossRef CAS.
- J. Benecke, E. S. Grape, A. Fuß, S. Wöhlbrandt, T. A. Engesser, A. K. Inge, N. Stock and H. Reinsch, Polymorphous indium metal–organic frameworks based on a ferrocene linker: redox activity, porosity, and structural diversity, Inorg. Chem., 2020, 59, 9969–9978 CrossRef CAS.
- J. Haddad, G. F. S. Whitehead, A. P. Katsoulidis and M. J. Rosseinsky, In-MOFs based on amide functionalised flexible linkers, Faraday Discuss., 2017, 201, 327–335 RSC.
- Z. Liu, Q. Li, H. Zhu, K. Lin, J. Deng, J. Chen and X. Xing, 3D negative thermal expansion in orthorhombic MIL-68(In), Chem. Commun., 2018, 54, 5712–5715 RSC.
- B. Zheng, X. Sun, G. Li, A. J. Cairns, V. C. Kravtsov, Q. Huo, Y. Liu and M. Eddaoudi, Solvent-controlled assembly of ionic metal–organic frameworks based on indium and tetracarboxylate ligand: topology variety and gas sorption properties, Cryst. Growth Des., 2016, 16, 5554–5562 CrossRef CAS.
- A. J. Cairns, J. Eckert, L. Wojtas, M. Thommes, D. Wallacher, P. A. Georgiev, P. M. Forster, Y. Belmabkhout, J. Ollivier and M. Eddaoudi, Gaining insights on the H2-sorbent interactions: robust soc-MOF platform as a case study, Chem. Mater., 2016, 28, 7353–7361 CrossRef CAS.
- X. Du, R. Fan, L. Qiang, K. Xing, H. Ye, X. Ran, Y. Song, P. Wang and Y. Yang, Controlled Zn2+-triggered drug release by preferred coordination of open active sites within functionalization indium metal organic frameworks, ACS Appl. Mater. Interfaces, 2017, 9, 28939–28948 CrossRef CAS.
- J. Li, Y. Fan and Y. Ren, Structures and characterization of four metal-organic frameworks constructed by 2,2′,6,6′-tetramethoxy-4,4′-biphenyldicarboxylic acid, Z. Anorg. Allg. Chem., 2017, 643, 612–618 CrossRef CAS.
- J. Pang, M. Wu, J.-S. Qin, C. Liu, C. T. Lollar, D. Yuan, M. Hong and H.-C. Zhou, Solvent-assisted, thermally triggered structural transformation in flexible mesoporous metal–organic frameworks, Chem. Mater., 2019, 31, 8787–8793 CrossRef CAS.
- Y. Chen, X. Zhang, H. Chen, R. J. Drout, Z. Chen, M. R. Mian, R. R. Maldonado, K. Ma, X. Wang, Q. Xia, Z. Li, T. Islamoglu, R. Q. Snurr and O. K. Farha, Tuning the atrazine binding sites in an indium-based flexible metal–organic framework, ACS Appl. Mater. Interfaces, 2020, 12, 44762–44768 CrossRef CAS.
- H. Cui, Y. Ye, R. Lin, Y. Shi, Z. A. Alothman, O. Alduhaish, B. Wang, J. Zhang and B. Chen, An indium-based microporous metal–organic framework with unique three-way rod-shaped secondary building units for efficient methane and hydrogen storage, Inorg. Chem. Front., 2022, 9, 6527–6533 RSC.
- M. Feng, P. Zhou, J. Wang, X. Wang, D. Wang and C. Li, Two solvent-induced In(III)-based metal–organic frameworks with controllable topology performing high-efficiency separation of C2H2/CH4 and CO2/CH4, Inorg. Chem., 2022, 61, 11057–11065 CrossRef CAS PubMed.
- Z. Liu, C. Xing, S. Wu, M. Ma and J. Tian, Biphenyl tetracarboxylic acid-based metal–organic frameworks: a case of topology-dependent thermal expansion, Mater. Horiz., 2024, 11, 3345–3351 RSC.
- J.-X. Wu and B. Yan, Lanthanides post-functionalized indium metal–organic frameworks (MOFs) for luminescence tuning, polymer film preparation and near-UV white LED assembly, Dalton Trans., 2016, 45, 18585–18590 RSC.
- Y. Yuan, J. Li, X. Sun, G. Li, Y. Liu, G. Verma and S. Ma, Indium–organic frameworks based on dual secondary building units featuring halogen-decorated channels for highly effective CO2 fixation, Chem. Mater., 2019, 31, 1084–1091 CrossRef.
- Y.-W. Peng, R.-J. Wu, M. Liu, S. Yao, A.-F. Geng and Z.-M. Zhang, Nitrogen coordination to dramatically enhance the stability of In-MOF for selectively capturing CO2 from a CO2/N2 mixture, Cryst. Growth Des., 2019, 19, 1322–1328 CrossRef.
- S. Pappuru, K. B. Idrees, Z. Chen, D. Shpasser and O. M. Gazit, A rare 4-fold interpenetrated metal–organic framework constructed from an anionic indium-based node and a cationic dicopper linker, Dalton Trans., 2021, 50, 6631–6636 RSC.
- V. Jeevananthan, G. C. Senadi, K. Muthu, A. Arumugam and S. Shanmugan, Construction of indium(III)–organic framework based on a flexible cyclotriphosphazene-derived hexacarboxylate as a reusable green catalyst for the synthesis of bioactive aza-heterocycles, Inorg. Chem., 2024, 63, 5446–5463 CrossRef PubMed.
- W. Guo, X. Sun, C. Chen, D. Yang, L. Lu, Y. Yang and B. Han, Metal–organic framework-derived indium–copper bimetallic oxide catalysts for selective aqueous electroreduction of CO2, Green Chem., 2019, 21, 503–508 RSC.
- W. Guo, X. Tan, J. Bi, L. Xu, D. Yang, C. Chen, Q. Zhu, J. Ma, A. Tayal, J. Ma, Y. Huang, X. Sun, S. Liu and B. Han, Atomic indium catalysts for switching CO2 electroreduction products from formate to CO, J. Am. Chem. Soc., 2021, 143, 6877–6885 CrossRef CAS.
- Y. Zhou, S. Liu, Y. Gu, G.-H. Wen, J. Ma, J.-L. Zuo and M. Ding, In(III) metal–organic framework incorporated with enzyme-mimicking nickel bis(dithiolene) ligand for highly selective CO2 electroreduction, J. Am. Chem. Soc., 2021, 143, 14071–14076 CrossRef CAS PubMed.
- Q. Huang, S. Xu, J. Liu, Y. Guo, D. Chen, Q. Sun, L. Zhang, H. Nie, Z. Yang and J. Qian, Fe phthalocyanine stabilized on phosphorous-doped multi-defective carbon nanoribbons as oxygen reduction electrocatalysts, Appl. Catal., B, 2023, 339, 123172 CrossRef CAS.
- S. Wang, Y. Wang, J.-M. Huo, W.-Y. Yuan, P. Zhang, S.-N. Li and Q.-G. Zhai, Multivariate indium–organic frameworks for highly efficient carbon dioxide capture and electrocatalytic conversion, Inorg. Chem. Front., 2023, 10, 158–167 RSC.
- S.-H. Li, S. Hu, H. Liu, J. Liu, X. Kang, S. Ge, Z. Zhang, Q. Yu and B. Liu, Two-dimensional metal coordination polymer derived indium nanosheet for efficient carbon dioxide reduction to formate, ACS Nano, 2023, 17, 9338–9346 CrossRef PubMed.
- C. Yang, X. You, J. Cheng, H. Zheng and Y. Chen, A novel visible-light-driven In-based MOF/graphene oxide composite photocatalyst with enhanced photocatalytic activity toward the degradation of amoxicillin, Appl. Catal., B, 2017, 200, 673–680 CrossRef.
- F. Leng, H. Liu, M. Ding, Q.-P. Lin and H.-L. Jiang, Boosting photocatalytic hydrogen production of porphyrinic MOFs: the metal location in metalloporphyrin matters, ACS Catal., 2018, 8, 4583–4590 CrossRef.
- Y. Fang, S.-R. Zhu, M.-K. Wu, W.-N. Zhao and L. Han, MOF-derived In2S3 nanorods for photocatalytic removal of dye and antibiotics, J. Solid State Chem., 2018, 266, 205–209 CrossRef.
- S.-S. Wang, H.-H. Huang, M. Liu, S. Yao, S. Guo, J.-W. Wang, Z.-M. Zhang and T.-B. Lu, Encapsulation of single iron sites in a metal–porphyrin framework for high-performance photocatalytic CO2 reduction, Inorg. Chem., 2020, 59, 6301–6307 CrossRef PubMed.
- S. Wang, F. Meng, X. Sun, M. Bao, J. Ren, S. Yu, Z. Zhang, J. Ke and L. Zeng, Bimetallic Fe/In metal-organic frameworks boosting charge transfer for enhancing pollutant degradation in wastewater, Appl. Surf. Sci., 2020, 528, 147053 CrossRef CAS.
- X. Liu, B. Xu, X. Duan, Q. Hao, W. Wei, S. Wang and B.-J. Ni, Facile preparation of hydrophilic In2O3 nanospheres and rods with improved performances for photocatalytic degradation of PFOA, Environ. Sci.: Nano, 2021, 8, 1010–1018 RSC.
- Q. Zhang, J. Zhang, X. Wang, L. Li, Y.-F. Li and W.-L. Dai, In–N–In sites boosting interfacial charge transfer in carbon-coated hollow tubular In2O3/ZnIn2S4 heterostructure derived from In-MOF for enhanced photocatalytic hydrogen evolution, ACS Catal., 2021, 11, 6276–6289 CrossRef CAS.
- Q. Sun, Y. Zhu, X. Zhong, M. Jiang, Y. Fan and J. Yao, Tuning photoactive MIL-68(In) by functionalized ligands for boosting visible-light nitrogen fixation, ACS Appl. Mater. Interfaces, 2022, 14, 53904–53915 Search PubMed.
- L. Hou, X. Jing, H. Huang and C. Duan, Interpenetrating dye-functionalized indium–organic frameworks for photooxidative cyanation and oxidative cyclization, J. Mater. Chem. A, 2022, 10, 24320–24330 RSC.
- H. Zhang, F.-Y. Chen, Z.-Y. Liu, Y.-H. Luo, Z.-L. Zhou, X.-M. Jia, X. Wang and Y.-Q. Yang, Photocatalytic activation of peroxodisulfate using iron-containing MOFs synthesized by single-crystal-to-single-crystal transformation for ofloxacin degradation, New J. Chem., 2023, 47, 21081–21090 Search PubMed.
- J.-Y. Tian, W.-C. Lv, A.-S. Shen, Y. Ma, M. Wang, S. Zhang, X.-L. Liu, Z. Zhang and M. Du, Construction of the copper metal-organic framework (MOF)-on-indium MOF Z-scheme heterojunction for efficiently photocatalytic reduction of Cr(VI), Sep. Purif. Technol., 2023, 327, 124903 Search PubMed.
- X. Li, X. Guo, J. Niu, J. Lin, J. Cheng, Y. Hu, Y. Chen and W. Wen, Constructing self-assembled microtubular structured Zn–In–S and modulation mechanism towards efficient photocatalytic degradation of tetracycline, J. Cleaner Prod., 2023, 385, 135685 CrossRef CAS.
- P. T. M. Ha, T. N. Lieu, S. H. Doan, T. T. B. Phan, T. T. Nguyen, T. Truong and N. T. S. Phan, Indium-based metal–organic frameworks as catalysts: synthesis of 2-nitro-3-arylimidazo[1,2-a]pyridines via oxidative amination under air using MIL-68(In) as an effective heterogeneous catalyst, RSC Adv., 2017, 7, 23073–23082 Search PubMed.
- X. Chen, H. Jiang, X. Li, B. Hou, W. Gong, X. Wu, X. Han, F. Zheng, Y. Liu, J. Jiang and Y. Cui, Chiral phosphoric acids in metal–organic frameworks with enhanced acidity and tunable catalytic selectivity, Angew. Chem., Int. Ed., 2019, 58, 14748–14757 Search PubMed.
- D. Reinares-Fisac, L. M. Aguirre-Díaz, M. Iglesias, E. Gutiérrez-Puebla, F. Gándara and M. Á. Monge, Anionic and neutral 2D indium metal–organic frameworks as catalysts for the Ugi one-pot multicomponent reaction, Dalton Trans., 2019, 48, 2988–2995 RSC.
- G.-M. Liang, P. Xiong, K. Azam, Q.-L. Ni, J.-Q. Zeng, L.-C. Gui and X.-J. Wang, A discrete tetrahedral indium cage as an efficient heterogeneous catalyst for the fixation of CO2 and the strecker reaction of ketones, Inorg. Chem., 2020, 59, 1653–1659 CrossRef CAS.
- R. A. Peralta, M. T. Huxley, P. Lyu, M. L. Díaz-Ramírez, S. H. Park, J. L. Obeso, C. Leyva, C. Y. Heo, S. Jang, J. H. Kwak, G. Maurin, I. A. Ibarra and N. C. Jeong, Engineering catalysis within a saturated In(III)-based MOF possessing dynamic ligand–metal bonding, ACS Appl. Mater. Interfaces, 2022, 15, 1410–1417 Search PubMed.
- A. Helal, M. H. Zahir, A. Albadrani and M. M. Ekhwan, Triazole appended metal–organic framework for CO2 fixation as cyclic carbonates under solvent-free ambient conditions, Catal. Lett., 2022, 153, 2883–2891 CrossRef.
- X. Li, D. Chen, Y. Liu, Z. Yu, Q. Xia, H. Xing and W. Sun, Anthracene-based indium metal–organic framework as a promising photosensitizer for visible-light-induced atom transfer radical polymerization, CrystEngComm, 2016, 18, 3696–3702 RSC.
- W. Yan, L. Wang, K. Yangxiao, Z. Fu and T. Wu, Effects of ligand and guest solvent molecules on the luminescence properties of Tb:Eu-codoped indium-based MOFs, Dalton Trans., 2016, 45, 4518–4521 Search PubMed.
- J. X. Wu and B. Yan, A Europium ion post-functionalized indium metal–organic framework hybrid system for fluorescence detection of aromatics, Analyst, 2017, 142, 4633–4637 RSC.
- S.-L. Hou, J. Dong, X.-L. Jiang, Z.-H. Jiao, C.-M. Wang and B. Zhao, Interpenetration-dependent luminescent probe in indium-organic frameworks for selectively detecting nitrofurazone in water, Anal. Chem., 2018, 90, 1516–1519 Search PubMed.
- L. Zhai, J.-W. Yu, J. Zhang, W.-W. Zhang, L. Wang and X.-M. Ren, High quantum yield pure blue emission and fast proton conduction from an indium–metal–organic framework, Dalton Trans., 2019, 48, 12088–12095 RSC.
- Z.-F. Wu, B. Tan, E. Velasco, H. Wang, N.-N. Shen, Y.-J. Gao, X. Zhang, K. Zhu, G.-Y. Zhang, Y.-Y. Liu, X.-Z. Hei, X.-Y. Huang and J. Li, Fluorescent In based MOFs showing “turn on” luminescence towards thiols and acting as a ratiometric fluorescence thermometer, J. Mater. Chem. C, 2019, 7, 3049–3055 RSC.
- W.-B. Zhong, R.-X. Li, J. Lv, T. He, M.-M. Xu, B. Wang, L.-H. Xie and J.-R. Li, Two isomeric In(iii)-MOFs: unexpected stability difference and selective fluorescence detection of fluoroquinolone antibiotics in water, Inorg. Chem. Front., 2020, 7, 1161–1171 RSC.
- Q. Li, B.-B. Guan, W. Zhu, T.-H. Liu, L.-H. Chen, Y. Wang and D.-X. Xue, Highly selective and sensitive dual-fluorescent probe for cationic Pb2+ and anionic Cr2O72−, CrO42− contaminants via a powerful indium−organic framework, J. Solid State Chem., 2020, 291, 121672 CrossRef CAS.
- J. Ren, X. Zhou and Y. Wang, Dual-emitting CsPbX3@ZJU-28 (X = Cl, Br, I) composites with enhanced stability and unique optical properties for multifunctional applications, Chem. Eng. J., 2020, 391, 123622 CrossRef CAS.
- B.-B. Guan, Q. Li, Y.-T. Xu, L.-H. Chen, Z. Wu, Z.-L. Fan and W. Zhu, Highly selective and sensitive detection towards cationic Cu2+ and Fe3+ contaminants via an In-MOF based dual-responsive fluorescence probe, Inorg. Chem. Commun., 2020, 122, 108273 Search PubMed.
- S. Yoon, H. C. Kim, Y. Kim and S. Huh, Photophysical properties and electrochromism of viologen encapsulated viologen@InBTB metal–organic framework, Bull. Korean Chem. Soc., 2020, 42, 326–332 Search PubMed.
- H. Zhang, Z.-J. Ding, Y.-H. Luo, W.-Y. Geng, Z.-X. Wang and D.-E. Zhang, Assembly of a rod indium–organic framework with fluorescence properties for selective sensing of Cu2+, Fe3+ and nitroaromatics in water, CrystEngComm, 2022, 24, 667–673 Search PubMed.
- Y. L. Zhao, Y. B. Xie, L. Wang, L. H. Xie, X. Zhang and J. R. Li, An interpenetrated anionic In metal-organic framework for selective sensing of Fe3+ in water, Chin. J. Inorg. Chem., 2022, 38, 1824 Search PubMed.
- B. Wang, W. Zheng, J. Chen, Y. Wang, X. Duan, S. Ma, Z. Kong and T. Xia, A Tb3+ ion encapsulated anionic indium-organic framework as logical probe for distinguishing quenching Fe3+ and Cu2+ ions, Spectrochim. Acta, Part A, 2024, 304, 123388 CrossRef.
- R. A. Peralta, A. Campos-Reales-Pineda, H. Pfeiffer, J. R. Álvarez, J. A. Zárate, J. Balmaseda, E. González-Zamora, A. Martínez, D. Martínez-Otero, V. Jancik and I. A. Ibarra, CO2 capture enhancement in InOF-1 via the bottleneck effect of confined ethanol, Chem. Commun., 2016, 52, 10273–10276 RSC.
- X. Li, X. Chen, F. Jiang, L. Chen, S. Lu, Q. Chen, M. Wu, D. Yuan and M. Hong, The dynamic response of a flexible indium based metal–organic framework to gas sorption, Chem. Commun., 2016, 52, 2277–2280 RSC.
- E. Sánchez-González, E. González-Zamora, D. Martínez-Otero, V. Jancik and I. A. Ibarra, Bottleneck Effect of N,N-dimethylformamide in InOF-1: increasing CO2 capture in porous coordination polymers, Inorg. Chem., 2017, 56, 5863–5872 CrossRef.
- B. Y. Liu, Y. J. Gong, X. N. Wu, Q. Liu, W. Li, S. S. Xiong, S. Hu and X. L. Wang, Enhanced xenon adsorption and separation with an anionic indium–organic framework by ion exchange with Co2+, RSC Adv., 2017, 7, 55012–55019 RSC.
- Y.-H. Li, S.-L. Wang, Y.-C. Su, B.-T. Ko, C.-Y. Tsai and C.-H. Lin, Microporous 2D indium metal–organic frameworks for selective CO2 capture and their application in the catalytic CO2-cycloaddition of epoxides, Dalton Trans., 2018, 47, 9474–9481 RSC.
- H. Atallah, M. Elcheikh Mahmoud, F. M. Ali, A. Lough and M. Hmadeh, A highly stable indium based metal organic framework for efficient arsenic removal from water, Dalton Trans., 2018, 47, 799–806 RSC.
- H.-Y. Liu, G.-M. Gao, F.-L. Bao, Y.-H. Wei and H.-Y. Wang, Enhanced water stability and selective carbon dioxide adsorption of a soc-MOF with amide-functionalized linkers, Polyhedron, 2019, 160, 207–212 CrossRef.
- Z. Cao, L. Chen, S. Li, M. Yu, Z. Li, K. Zhou, C. Liu, F. Jiang and M. Hong, A flexible two-fold interpenetrated indium MOF exhibiting dynamic response to gas adsorption and high-sensitivity detection of nitroaromatic explosives, Chem. – Asian J., 2019, 14, 3597–3602 CrossRef.
- I.-H. Choi, S. B. Yoon, S.-Y. Jang, S. Huh, S.-J. Kim and Y. Kim, Gas sorption properties of a new three-dimensional In-ABDC MOF with a diamond Net, Front. Mater., 2019, 6, 7 CrossRef.
- S. Moribe, Z. Chen, S. Alayoglu, Z. H. Syed, T. Islamoglu and O. K. Farha, Ammonia capture within isoreticular metal–organic frameworks with rod secondary building units, ACS Mater. Lett., 2019, 1, 476–480 CrossRef.
- Z. Cao, L. Chen, F. Jiang, K. Zhou, M. Yu, T. Jing, S. Li, Z. Li and M. Hong, Incorporating three chiral channels into an In-MOF for excellent gas absorption and preliminary Cu2+ ion detection, Cryst. Growth Des., 2019, 19, 3860–3868 CrossRef.
- H. Chen, L. Fan, X. Zhang and L. Ma, Nanocage-based InIII{TbIII}2-organic framework featuring lotus-shaped channels for highly efficient CO2 fixation and I2 capture, ACS Appl. Mater. Interfaces, 2020, 12, 27803–27811 CrossRef PubMed.
- Y.-M. Gu, H.-F. Qi, S. Qadir, T.-J. Sun, R. Wei, S.-S. Zhao, X.-W. Liu, Z. Lai and S.-D. Wang, A two-dimensional stacked metal-organic framework for ultra highly-efficient CO2 sieving, Chem. Eng. J., 2022, 449, 137768 CrossRef.
- C. Zuo, Q. Li, M. Dai and C. Fan, Solvent-regulated formation of metal/metal-oxo Nodes in two indium metal-organic Frameworks: syntheses, structures, selective gas adsorption and fluorescence sensor properties, Chem. – Eur. J., 2024, e202402437 CrossRef.
- H. Liu, G. Gao, J. Liu, F. Bao, Y. Wei and H. Wang, Amide-functionalized ionic indium–organic frameworks for efficient separation of organic dyes based on diverse adsorption interactions, CrystEngComm, 2019, 21, 2576–2584 RSC.
- P. Huang, C. Chen, M. Wu, F. Jiang and M. Hong, An indium–organic framework for the efficient storage of light hydrocarbons and selective removal of organic dyes, Dalton Trans., 2019, 48, 5527–5533 RSC.
- X. Shi, Y. Zu, S. Jiang and F. Sun, An anionic indium–organic framework with spirobifluorene-based ligand for selective adsorption of organic dyes, Inorg. Chem., 2021, 60, 1571–1578 CrossRef.
- Q. Chu, B. Zhang, Z. Yang, H. Zhou, H. Mu, W. Zhang, B. Liu and Y.-Y. Wang, Stable indium pyridylcarboxylate framework with highly selective adsorption of cationic dyes and effective nitenpyram detection, Inorg. Chem., 2021, 60, 5232–5239 CrossRef.
- Y. Lv, R. Zhang, S. Zeng, K. Liu, S. Huang, Y. Liu, P. Xu, C. Lin, Y. Cheng and M. Liu, Removal of p-arsanilic acid by an amino-functionalized indium-based metal–organic framework: Adsorption behavior and synergetic mechanism, Chem. Eng. J., 2018, 339, 359–368 CrossRef.
- B. Zhang, S.-H. Zhang, B. Liu, K.-F. Yue, L. Hou and Y.-Y. Wang, Stable indium-pyridylcarboxylate framework: selective gas capture and sensing of Fe3+ ion in water, Inorg. Chem., 2018, 57, 15262–15269 CrossRef.
- Q. Chu, B. Zhang, H. Zhou, B. Liu, L. Hou and Y.-Y. Wang, Effective C2H2 separation and nitrofurazone detection in a stable indium–organic framework, Inorg. Chem., 2020, 59, 2853–2860 CrossRef PubMed.
- Z. Chen, S. Xiang, H. D. Arman, P. Li, S. Tidrow, D. Zhao and B. Chen, A microporous metal–organic framework with immobilized –OH functional groups within the pore surfaces for selective gas sorption, Eur. J. Inorg. Chem., 2010, 2010, 3745–3749 CrossRef.
- S. H. Xiang, W. Zhou, Y. Liu and B. L. Chen, Exceptionally high acetylene uptake in a microporous metal-organic framework with open metal sites, J. Am. Chem. Soc., 2009, 131, 12419 Search PubMed.
- Y. Zhang, L. Yang, L. Wang, S. Duttwyler and H. Xing, A microporous metal-organic framework supramolecularly assembled from a cuII dodecaborate cluster complex for selective gas separation, Angew. Chem., Int. Ed., 2019, 58, 8145–8150 CrossRef PubMed.
- B. Zhang, P.-Y. Guo, L.-N. Ma, B. Liu, L. Hou and Y.-Y. Wang, Two robust In(III)-based metal–organic frameworks with higher gas separation, efficient carbon dioxide conversion, and rapid detection of antibiotics, Inorg. Chem., 2020, 59, 5231–5239 CrossRef.
- Q. Song, Y. Yang, F. Yuan, S. Zhu, J. Wang, S. Xiang and Z. Zhang, Electrostatic force-driven lattice water bridging to stabilize a partially charged indium MOF for efficient separation of C2H2/CO2 mixtures, J. Mater. Chem. A, 2022, 10, 9363–9369 RSC.
- L.-N. Ma, L. Zhang, W.-F. Zhang, Z.-H. Wang, L. Hou and Y.-Y. Wang, Amide-functionalized In-MOF for effective hydrocarbon separation and CO2 catalytic fixation, Inorg. Chem., 2022, 61, 2679–2685 CrossRef.
- Y. Lv, X. Wang, C. Jian, L. Yue, S. Lin, T. Zhang, B. Li, D.-L. Chen and Y. He, A two-way rod-packing indium-based nanoporous metal–organic framework for effective C2/C1 and C2/CO2 separation, ACS Appl. Nano Mater., 2022, 5, 18654–18663 CrossRef.
- W. Wang, W. Yuan, C. Kong, Y. Yang, S. Xi, X. Liu and B. Liu, An ultramicroporous multi-walled metal–organic framework for efficient C2H2/CO2 separation under humid conditions, J. Mater. Chem. A, 2023, 11, 25605–25611 RSC.
- X. Du, R. Fan, L. Qiang, P. Wang, Y. Song, K. Xing, X. Zheng and Y. Yang, Encapsulation and sensitization of Ln3+ within indium metal–organic frameworks for ratiometric Eu3+ sensing and linear dependence of white-light emission, Cryst. Growth Des., 2017, 17, 2746–2756 CrossRef.
- X.-J. Wu, F. Kong, C.-Q. Zhao and S.-N. Ding, Ratiometric fluorescent nanosensors for ultra-sensitive detection of mercury ions based on AuNCs/MOFs, Analyst, 2019, 144, 2523–2530 RSC.
- H. Zhang, J. Wu, W.-Y. Geng, Y.-H. Luo, Z.-J. Ding, Z.-X. Wang and D.-E. Zhang, An interpenetrated anionic In(III)-MOF for efficient adsorption/separation of organic dyes and selective sensing of Fe3+ ion and nitroaromatic compounds, J. Solid State Chem., 2021, 302, 122424 CrossRef.
- V. Chernikova, O. Yassine, O. Shekhah, M. Eddaoudi and K. N. Salama, Highly sensitive and selective SO2 MOF sensor: the integration of MFM-300 MOF as a sensitive layer on a capacitive interdigitated electrode, J. Mater. Chem. A, 2018, 6, 5550–5554 RSC.
- X. Jiang, R. Fan, X. Zhou, K. Zhu, T. Sun, X. Zheng, K. Xing, W. Chen and Y. Yang, Mixed functionalization strategy on indium-organic framework for multiple ion detection and H2O2 turn-on sensing, Dalton Trans., 2021, 50, 7554–7562 RSC.
- J. Lei, B. Wang, Y.-P. Li, W.-J. Ji, K. Wang, H. Qi, P.-T. Chou, M.-M. Zhang, H. Bian and Q.-G. Zhai, A new molecular recognition concept: multiple hydrogen bonds and their optically triggered proton transfer in confined metal–organic frameworks for superior sensing element, ACS Appl. Mater. Interfaces, 2021, 13, 22457–22465 CrossRef CAS.
- D. Arif, Z. Hussain, A. D. Abbasi and M. Sohail, Ag functionalized In2O3 derived from MIL-68(In) as an efficient electrochemical glucose sensor, Front. Chem., 2022, 10, 10 Search PubMed.
- M. Liu, J. Wang, P. Song, J. Ji and Q. Wang, Metal-organic frameworks-derived In2O3 microtubes/Ti3C2Tx MXene composites for NH3 detection at room temperature, Sens. Actuators, B, 2022, 361, 131755 CrossRef CAS.
- X. Zuo, Z. Yang, J. Kong, Z. Han, J. Zhang, X. Meng, S. Hao, L. Wu, S. Wu, J. Liu, Z. Wang and F. Wang, Imbedding Pd nanoparticles into porous In2O3 structure for enhanced low-concentration methane sensing, Sensors, 2023, 23, 1163 CrossRef CAS PubMed.
- B. S. de Sá, T. M. Perfecto, D. S. de Oliveira and D. P. Volanti, Microwave-assisted solvothermal synthesis of In-MIL-68 derived hollow In2O3 microrods for enhanced 1-pentanol sensing performance, Mater. Today Commun., 2023, 37, 107174 CrossRef.
- Y. Peng, B. Cheng, L. Zhang, J. Liu and J. Yu, In2O3/ZnO S-scheme heterojunction nanocomposite hollow microtubes with highly sensitive response to formaldehyde, Sens. Actuators, B, 2023, 385, 133700 CrossRef CAS.
- Y.-H. Han, Y. Ye, C. Tian, Z. Zhang, S.-W. Du and S. Xiang, High proton conductivity in an unprecedented anionic metalloring organic framework (MROF) containing novel metalloring clusters with the largest diameter, J. Mater. Chem. A, 2016, 4, 18742–18746 RSC.
- B. Joarder, J.-B. Lin, Z. Romero and G. K. H. Shimizu, Single crystal proton conduction study of a metal organic framework of modest water stability, J. Am. Chem. Soc., 2017, 139, 7176–7179 CrossRef CAS.
- L. Liu, Z. Yao, Y. Ye, Q. Lin, S. Chen, Z. Zhang and S. Xiang, Enhanced intrinsic proton conductivity of metal–organic frameworks by tuning the degree of interpenetration, Cryst. Growth Des., 2018, 18, 3724–3728 CrossRef CAS.
- S. Liu, T. Zhao, X. Tan, L. Guo, J. Wu, X. Kang, H. Wang, L. Sun and W. Chu, 3D pomegranate-like structures of porous carbon microspheres self-assembled by hollow thin-walled highly-graphitized nanoballs as sulfur immobilizers for Li–S batteries, Nano Energy, 2019, 63, 103894 CrossRef CAS.
- J. Su, W. He, X.-M. Li, L. Sun, H.-Y. Wang, Y.-Q. Lan, M. Ding and J.-L. Zuo, High electrical conductivity in a 2D MOF with intrinsic superprotonic conduction and interfacial pseudo-capacitance, Matter, 2020, 2, 711–722 CrossRef.
- H. Gao, Y.-B. He, J.-J. Hou, Q.-G. Zhai and X.-M. Zhang, Enhanced proton conductivity by aliovalent substitution of cadmium for indium in dimethylaminium-templated metal anilicates, ACS Appl. Mater. Interfaces, 2020, 12, 41605–41612 CrossRef CAS.
- G. Han, T. Deng, X. Jiao, X. Men, J. Wang, Y. Wang and Q. Zhai, Indium-based MOFs and carbon nanotube embedded efficient cathodes for high-performance lithium-sulfur batteries, Ionics, 2021, 27, 5115–5125 CrossRef CAS.
- J. Zhang, S. Guo, M. Zhu, C. Li, J. Chen, L. Liu, S. Xiang and Z. Zhang, Simultaneous defect passivation and hole mobility enhancement of perovskite solar cells by incorporating anionic metal-organic framework into hole transport materials, Chem. Eng. J., 2021, 408, 127328 CrossRef CAS.
- H. Gao, Y.-B. He, J.-J. Hou and X.-M. Zhang, In situ aliovalent nickle substitution and acidic modification of nanowalls promoted proton conductivity in InOF with 1D helical channel, ACS Appl. Mater. Interfaces, 2021, 13, 38289–38295 CrossRef CAS.
- S. S. Guo, L. L. Huang, Y. X. Ye, L. Z. Liu, Z. Z. Yao, S. C. Xiang, J. D. Zhang and Z. J. Zhang, Carbazole based anionic MOF for proton conductivity, Chin. J. Struct. Chem., 2021, 40, 60 Search PubMed.
- F. Farbod, M. Mazloum-Ardakani, H. R. Naderi, A. Mirvakili, M. Wang, D. V. Shinde, S. Dante, P. Salimi, S. Lauciello and M. Prato, Indium based metal-organic framework/carbon nanotubes composite as a template for In2O3 porous hexagonal prisms/carbon nanotubes hybrid structure and their application as promising super-capacitive electrodes, J. Energy Storage, 2022, 51, 104238 CrossRef.
- X. Jiao, T. Deng, X. Men and Y. Zuo, Indium metal-organic framework with catalytic sites coated conductive graphene for high-performance lithium-sulfur batteries, Ceram. Int., 2022, 48, 16754–16763 CrossRef.
- L. Chai, X. Wang, Y. Hu, X. Li, S. Huang, J. Pan, J. Qian and X. Sun, In-MOF-derived hierarchically hollow carbon nanostraws for advanced zinc-iodine batteries, Adv. Sci., 2022, 9, 1–9 Search PubMed.
- R. Zhang, C. Mu, B. Wang, J. Xiang, K. Zhai, T. Xue and F. Wen, Composites of In/C hexagonal nanorods and graphene nanosheets for high-performance electromagnetic wave absorption, Int. J. Miner., Metall. Mater., 2023, 30, 485–493 CrossRef.
- Y.-J. Song, Y.-L. Sang, K.-Y. Xu, H.-L. Hu, Q.-Q. Zhu and G. Li, Ligand-functionalized MIL-68-type indium(III) metal–organic frameworks with prominent intrinsic proton conductivity, Inorg. Chem., 2024, 63, 4233–4248 CrossRef PubMed.
| This journal is © the Partner Organisations 2024 |