Chemical modulation of AIREIIICIVQVI4 family compounds for band gap and optical anisotropy enhancement†
Hongshan
Wang
ab,
Xueting
Pan
a,
Shilie
Pan
 *ab and
Junjie
Li
*ab and
Junjie
Li
 *ab
*ab
aResearch Center for Crystal Materials; State Key Laboratory of Functional Materials and Devices for Special Environmental Conditions; Xinjiang Key Laboratory of Functional Crystal Materials; Xinjiang Technical Institute of Physics and Chemistry, CAS, 40-1 South Beijing Road, Urumqi 830011, China. E-mail: slpan@ms.xjb.ac.cn; lijunjie@ms.xjb.ac.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
First published on 14th August 2024
Abstract
Rare-earth (RE) compounds show wide applications in advanced photoelectric functional materials. Herein, by introducing [AgS3] trihedral and [NaQ6] (Q = S, Se) octahedral units into the AIREIIICIVQVI4 family for the first time, four new RE-based chalcogenides AIREIIISiQVI4 (AI = Ag, Na; REIII = La, Y; QVI = S, Se) were designed and successfully synthesized. With the increase of atomic radius from Ag, Li, Na, K, to Rb and Cs, the compounds show evident structural transitions from Ama2 (LiLaSiS4), P21/c (AgLaSiS4, NaLaSiS4), and P21 (KLaSiS4) to Pnma (RbLaSiS4, CsLaSiS4), highlighting that chemical modulations including atomic radius, coordination and bond length co-affected the structure transition. The title compounds exhibit wide band gaps (3.33 and 3.18 eV for AgLaSiS4 and AgYSiS4; 3.83 and 3.02 eV (HSE06) for NaLaSiS4 and NaLaSiSe4, respectively) that are higher than the Ag- and RE-based chalcogenides, as well as strong optical anisotropies (Δncal = 0.114–0.160@1064 nm). The theoretical calculations confirm the charge transfer enhanced band gap mechanism in the compounds and demonstrate that the layer distance influenced birefringence. The results enrich the chemical and structural diversity of RE compounds in the AIREIIICIVQVI4 family and give new insights into the design of new RE-based compounds with wide band gaps and large birefringence.
Introduction
The development of new functional materials is highly dependent on the discovery of new compounds with distinctive crystal structures and physicochemical properties.1–6 Chalcogenides show abundant structural and chemical diversities, wide infrared (IR) transmission ranges, and adjustable optical properties, which are widely used in the fields of photo-detection, nonlinear optical (NLO) applications, light polarization, and photo-catalysis, making them an essential natural treasure trove for the exploration of advanced photoelectric functional materials.7–11 Over the past few decades, by combining different fundamental building block groups,12–14 a large number of metal chalcogenides have been developed, such as Cs3In(In4Se7)(P2Se6),15 Sn7Br10S2,16 and SrBIIGeS4 (BII = Zn, Hg)17,18 with strong NLO response as well as Rb3NaSn3Se819 and Na2HgSn2Se620 with large optical anisotropy.To increase the functional diversity, introducing rare-earth (RE) elements with a unique f-electron configuration, strong positive charge, and multiple coordination environments into the structural design of chalcogenides has been demonstrated as a feasible strategy.21–24 More recently, Yu and coworkers reported the synthesis of the first stable La2+-based chalcogenide LaMg6Ga6S16, in which the La2+ is six-coordinated with S atoms.25 The compound exhibits a wide green emission band at 500 nm, as well as a strong NLO response (∼1 × AgGaS2), a wide band gap (Eg = 3.0 eV) and a high laser-induced damage threshold (∼5 × AgGaS2).25 Remarkably, the relatively large size of the standard RE3+ ion radius can accommodate higher coordination numbers (up to ∼12).23 The abundant coordination modes lead to multiple crystal structures in RE-contained chalcogenides, such as in REIII3GaS6 (REIII = Y, Dy, Ho, and Er),26 Ba2REIIIMIIISe5 (REIII = Y, Ce, Nd, Sm, Gd, Dy, and Er; MIII = Ga, In),27 La3LiMIVS7 (MIV = Ge, Sn),28 and AIREIIICIVQVI4 (AI = Li, K, Rb, Cs; REIII = Y, La–Nd, Sm–Yb; CIV = Si, Ge; QVI = S, Se).1,29–31
In the AIREIIICIVQVI4 family, many attempts have been made and more than 50 compounds have been synthesized. Among them, the AI-site atom is usually occupied by Li, K, Rb or Cs, while the syntheses of Na-/Ag-containing compounds have still not been achieved, similar to the case in the AIB4O6F (AI = NH4, Na, Rb, Cs) family (where the synthesis of Li-/KB4O6F is yet to be achieved) famous for their excellent NLO properties in the UV regions.32–34 In this work, Na- and Ag-containing systems were investigated, and four new RE chalcogenides AIREIIISiQVI4 (AI = Ag, Na; REIII = La, Y; QVI = S, Se) have been synthesized by the high temperature solution method in sealed quartz tubes. Based on structural investigations in the Inorganic Crystal Structure Database (ICSD version 5.0.0 (build 20230418-1517)), and to the best of our knowledge, they are the first series of Na- and Ag-containing compounds in the AIREIIICIVQVI4 family. The compounds crystallize in the centrosymmetric (CS) P21/c space group that is different from the commonly appearing non-centrosymmetric (NCS) P21, Ama2 and CS Pnma space groups in the Li-, K-, Rb-, Cs-containing compounds in this family. The compounds show wide band gaps (3.18 and 3.33 eV for AgYSiS4 and AgLaSiS4; 3.83 and 3.02 eV (HSE06) for NaLaSiS4 and NaLaSiSe4, respectively), strong optical anisotropy and large birefringence from 0.114@1064 nm for AgLaSiS4 to 0.160@1064 nm for NaLaSiSe4. In addition, theoretical calculations reveal that the wide band gap in the title compounds is mainly attributed to the charge transfer enhanced band gap mechanism between [REQ8] and [SiQ4] groups.
Experimental section
Raw materials
Raw materials utilized for the experimental syntheses, such as Na (99.9%), Ag (99.9%), La (99.99%), Y (99.99%), Si (99.9%), S (99.9%) and Se (99.9%), were purchased from the Shanghai Aladdin Biochemistry Technology Co., Ltd. To prevent oxidation and deliquescence of the materials, all starting materials were preserved and weighed in an Ar gas-filled glove box.Chemical syntheses
The single crystals of AgLaSiS4, AgYSiS4, NaLaSiS4, and NaLaSiSe4 for structural determinations were fabricated by the high temperature solution method as follows: (1) Ag/Na, La/Y, Si, and S/Se materials with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were mixed initially and loaded into quartz tubes with an inner diameter of 10 mm; (2) the quartz tubes were sealed with a hydrogen–oxygen flame under a high vacuum of 10−3 Pa; (3) the sealed samples were put into a Muffle furnace with the following programmed temperature: heated at 7 °C h−1 from room temperature to 900 °C, maintained at this temperature for 75 h, then slowly cooled to room temperature at 6 °C h−1. After that, the light yellow single crystals of AgLaSiS4, AgYSiS4, and NaLaSiS4, and the yellow single crystals of NaLaSiSe4 were harvested.
4 were mixed initially and loaded into quartz tubes with an inner diameter of 10 mm; (2) the quartz tubes were sealed with a hydrogen–oxygen flame under a high vacuum of 10−3 Pa; (3) the sealed samples were put into a Muffle furnace with the following programmed temperature: heated at 7 °C h−1 from room temperature to 900 °C, maintained at this temperature for 75 h, then slowly cooled to room temperature at 6 °C h−1. After that, the light yellow single crystals of AgLaSiS4, AgYSiS4, and NaLaSiS4, and the yellow single crystals of NaLaSiSe4 were harvested.
The polycrystalline powder samples of AgLaSiS4 and AgYSiS4 were synthesized by a similar procedure with the chemical stoichiometric ratios of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) La/Y
La/Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and the holding time at the temperatures (900 °C for AgLaSiS4 and 930 °C for AgYSiS4) was set to 100 h. It is worth noting that the syntheses of the polycrystalline NaLaSiQVI4 (QVI = S, Se) pure phase powder samples were also tried, but they failed.
4, and the holding time at the temperatures (900 °C for AgLaSiS4 and 930 °C for AgYSiS4) was set to 100 h. It is worth noting that the syntheses of the polycrystalline NaLaSiQVI4 (QVI = S, Se) pure phase powder samples were also tried, but they failed.
Structure determination
The single-crystal diffraction data of the title compounds were collected on a Bruker APEX II charge-coupled device (CCD) diffractometer equipped with monochromatic Mo Kα radiation (λ = 0.71073 Å) at room temperature.35,36 The absorption correction was performed by the multi-scan method. The crystal structures were determined directly and refined through a full matrix with least squares fit on F2 by the structure resolution program package SHELXTL on Olex2 v1.2.37,38 The PLATON program was used to detect possible missing symmetry elements, and no higher symmetries were found in the crystal structures.Powder X-ray diffraction (PXRD)
To check the purity of the obtained polycrystalline powder samples, the PXRD patterns of AgLaSiS4 and AgYSiS4 were collected on an automated Bruker D2 bit-phase diffractometer with Cu target Kα radiation (λ = 1.5418 Å). The diffraction data were recorded from 10 to 70° (2θ ranges) with a scan step width of 0.02° and a fixed counting time of 1 s per step at room temperature.Energy-dispersive X-ray spectroscopy (EDS) analyses
The EDS spectra and mappings of the title compounds were conducted on a field emission scanning electron microscope (FE-SEM, JEOL JSM-7610F Plus, Japan) at 298 K with an energy-dispersive X-ray spectrometer (Oxford, X-Max 50) at 5 kV.UV-vis-NIR diffuse reflectance spectroscopy
Diffuse-reflectance spectra of the polycrystalline AgREIIISiS4 (REIII = La, Y) powder samples were obtained on a Shimadzu SolidSpec-3700 DUV spectrophotometer over the wavelength range of 200–2600 nm at room temperature,39 and BaSO4 served as the reference. The experimental optical band gaps (Eg) were determined by converting the diffuse reflection data to absorption data through the Kubelka–Munk function, F(R) = K/S = (1 − R)2/2R, where R denotes the reflection coefficient, K is the absorption value, and S is the scattering coefficient.Raman spectra
The Raman spectra of the four compounds were measured on a LABRAM HR Evolution spectrometer (Japan) fitted with a CCD detector operating at 532 nm radiation and were acquired from 4000 to 100 cm−1 (2.5–100 μm).Theoretical calculations
The band structures, partial and total density of states (P/TDOS), and birefringence of the title compounds were computed by the plane wave pseudopotential method in CASTEP.40,41 The electronic structures of the compounds were analyzed by density functional theory (DFT) with the Perdew–Burke–Ernzerhof (PBE) exchange–correlation function in the generalized gradient approximation (GGA).42 Meanwhile, the band gaps of the title compounds were investigated by the PWmat code utilizing the Heyd–Scuseria–Ernzerhof (HSE06) hybrid functional, with a plane wave cutoff of 50 eV.43 The interactions between the ionic core and electrons were described by norm-conserving pseudopotentials (NCP). The valence electron configurations of Ag 4d105s1, Na 2p63s1, La 5d16s2, Y 5s24d1, Si 3s23p2, S 3s23p4 and Se 4s24p4 were considered during the calculations. A cutoff energy of 800 eV was utilized. The Monkhorst–Pack k-point in the Brillouin zone (BZ) was set to 0.035 Å−1. The refractive indices were theoretically derived from the real part of the dielectric function using the Kramers–Kronig transform.44Results and discussion
Crystal structures
Transparent AgLaSiS4, AgYSiS4, NaLaSiS4, and NaLaSiSe4 single crystals for structure determination were picked under an optical microscope. The results of single-crystal XRD show that the four compounds crystallize in the same space group (Table S1†) and show similar structural features (Fig. 1 and S1–S3†). Therefore, AgLaSiS4 is utilized as an example to illustrate their crystal structures herein. AgLaSiS4 crystallizes in the monoclinic P21/c (no. 14) space group with cell parameters a = 8.9451(8) Å, b = 10.5565(9) Å, c = 6.9470(6) Å, Z = 4. In its asymmetric unit, there is one crystallographically independent Ag, one La, one Si, and four S atoms, and the atoms are all located at Wyckoff 4e positions. The Ag atoms are coordinated with three S atoms to build the [AgS3] triangle planar units with the Ag–S bond lengths of 2.490–2.615 Å (Fig. 1a). The Si atoms are bonded with four S atoms to form [SiS4] tetrahedra with Si–S bond lengths of 2.086–2.140 Å (Fig. 1b). The La atoms are connected with eight S atoms to construct the [LaS8] polyhedral units with La–S bond lengths of 2.916–3.136 Å (Fig. 1c). The resulting [SiS4] and [LaS8] units are edge-shared into a [LaSiS10] dimer (Fig. 1d), which further extends by vertex-sharing into a serpentine [LaSiS10]∞ layer (Fig. 1g). Furthermore, the formed [AgS3] unit pseudo-layers are located between the serpentine [LaSiS10]∞ layers, constructing the final three-dimensional (3D) crystal structure of AgLaSiS4 (Fig. 1e and f). It is worth mentioning that NaLaSiQ4 (Q = S, Se) shows a similar crystal structure to AgREIIISiS4 (REIII = La, Y), but the Na atoms are 6-coordinated in the former that is different to the 3-coordinated Ag atoms in the latter, giving rise to an enhanced layer spacing in NaLaSiQVI4 (QVI = S, Se).The detailed crystal data, atomic coordinate equivalent isotropic displacement parameters, bond valence sum (BVS), selected bond lengths, and bond angle data of the title compounds are provided in Tables S1–S17,† respectively. The calculated bond valence sums (BVSs) and global instability indexes (GIIs) (Tables S2–S5†) verify the reasonability of the crystal structures. To check the chemical compositions, EDS spectra and element mappings of the four compounds were carried out. The results confirm the presence of elements Ag/Na, La/Y, Si, and S/Se in AIREIIISiQVI4 (AI = Ag, Na; REIII = La, Y; QVI = S, Se) and the element ratios (Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) La
La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1.10
S = 1.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.03
1.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.03
1.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.00 in AgLaSiS4, Ag
4.00 in AgLaSiS4, Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y
Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1.06
S = 1.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.09
1.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.11
1.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.00 in AgYSiS4, Na
4.00 in AgYSiS4, Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) La
La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1.16
S = 1.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.02
1.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.99
0.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.00 in NaLaSiS4, and Na
4.00 in NaLaSiS4, and Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) La
La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se = 0.97
Se = 0.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.98
0.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.96
0.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.00 in NaLaSiSe4) show good agreement with the values according to their chemical formulae (Fig. S4†). To further confirm the chemical bonding, the Raman spectra of the compounds were characterized. As shown in Fig. S5,† the peaks at 62.25–266.56 cm−1 in AgLaSiS4 (59.98–259.03 cm−1 in AgYSiS4) can be assigned to the vibrations of Ag–S and La–S (Ag–S and Y–S) bonds;11,25,45,46 while the peaks between 73.81–287.15 cm−1 in NaLaSiS4 (59.98–183.98 cm−1 in NaLaSiSe4) can be attributed to the vibrations of Na–S and La–S (Na–Se and La–Se) bonds;11,25,47 the peak at 397.67/408.01/399.07 cm−1 in AgLaSiS4/AgYSiS4/NaLaSiS4 (228.47 and 250.37 cm−1 in NaLaSiSe4) is related to the characteristic vibrations of Si–S or Si–Se bonds.48–50
4.00 in NaLaSiSe4) show good agreement with the values according to their chemical formulae (Fig. S4†). To further confirm the chemical bonding, the Raman spectra of the compounds were characterized. As shown in Fig. S5,† the peaks at 62.25–266.56 cm−1 in AgLaSiS4 (59.98–259.03 cm−1 in AgYSiS4) can be assigned to the vibrations of Ag–S and La–S (Ag–S and Y–S) bonds;11,25,45,46 while the peaks between 73.81–287.15 cm−1 in NaLaSiS4 (59.98–183.98 cm−1 in NaLaSiSe4) can be attributed to the vibrations of Na–S and La–S (Na–Se and La–Se) bonds;11,25,47 the peak at 397.67/408.01/399.07 cm−1 in AgLaSiS4/AgYSiS4/NaLaSiS4 (228.47 and 250.37 cm−1 in NaLaSiSe4) is related to the characteristic vibrations of Si–S or Si–Se bonds.48–50
Based on the structural investigations in the ICSD (version 5.0.0, build 20230418-1517), the A-site is usually occupied by K, Rb, or Cs atoms in the AIREIIICIVQVI4 family, and the title compounds are the first Na- and Ag-containing compounds in the family (Fig. 2a). Moreover, it can be seen that the AIREIIICIVQVI4 family compounds (61 cases) are generally crystallized in the P21 (no. 4) and P212121 (no. 19) space groups, and the number of compounds that crystallize in the P21/m (no. 11), P21/c (no. 14), Pnma (no. 62), and Ama2 (no. 40) space groups are quite rare (Table S18† and Fig. 2b).
 | ||
| Fig. 2 Statistical analyses showing the different A-site atoms (a) and space groups (b) in the AIREIIICIVQVI4 (AI = Ag, Li–Cs; REIII = Y, La–Nd, Sm–Yb; CIV = Si, Ge; QVI = S, Se) family compounds. | ||
To illustrate the A-site atom affected crystal structures in the AIREIIICIVQVI4 family, a detailed structural analysis on AILaSiS4 (AI = Li, Ag, Na, K, Rb, Cs) was carried out, as shown in Fig. 3. It can be seen that: (i) with the increase of atomic radius from Li, Na, K, to Rb and Cs, the compounds show evident structural transitions from Ama2 (LiLaSiS4),31P21/c (NaLaSiS4), and P21 (KLaSiS4)30 to Pnma (RbLaSiS4, CsLaSiS4)51,52 (Fig. 3a–d). Meanwhile, AILaSiS4 (AI = Ag, Na, K, Rb, Cs) shows a similar framework structure composed of [LaS8] and [SiS4], different from the [LaSiS11]∞ framework built by [LaS8] and [SiS4] in LiLaSiS4 (Fig. 3e–h and S6†); (ii) since the Ag atom is 3-coordinated with S atoms, the formed [AgS3] units in AgLaSiS4 are isolated from others to form a pseudo-layer structure, different to the formed Na/K/Rb/Cs–S layer structure built by [NaS6] octahedra or [K/Rb/CsS8] polyhedra in AILaSiS4 (AI = Na, K, Rb, Cs), confirming that the coordination mode affected crystal structures (Fig. S7†); (iii) compared to the shorter Ag–S bond length (2.4897–2.6149 Å) in AgLaSiS4, the longer Na–S bond length (2.780–3.267 Å) results in a larger layer spacing of 9.314 Å in NaLaSiS4 (8.945 Å for AgLaSiS4). RbLaSiS4 and CsLaSiS4 exhibit a similar phenomenon, and the layer spacing is increased from 8.642 Å in RbLaSiS4 to 8.932 Å in CsLaSiS4 (Fig. S8†). The results highlight that atomic radius, coordination number, and bond length (related to A-site atoms) co-affected the crystal structure in the AIREIIICIVQVI4 family.
Optical properties
To investigate the optical properties of the compounds, the pure-phase polycrystalline powder samples of AgLaSiS4 and AgYSiS4 were successfully prepared and characterized. The experimental XRD patterns of the two compounds match well with the theoretical results from their CIF files (Fig. 4a and b), confirming the single crystal structures and high purity for the obtained polycrystalline powder samples. To evaluate the experimental band gaps, UV-vis-NIR diffuse reflectance spectra were measured on the pure phase powder samples. Based on the Kubelka–Munk function, the experimental band gaps of AgLaSiS4 and AgYSiS4 were determined to be ∼3.33 eV and ∼3.18 eV, respectively (Fig. 4c and d), which are comparable to the results in recently developed wide band gap chalcogenides like Zn2HgP2S8 (3.37 eV),53 LiMGa8S14 (M = Rb/Ba, Cs/Ba) (3.24–3.32 eV),54 Ca2La(BS3)(SiS4) (3.38 eV),55 [Ba4(S2)][ZnGa4S10] (3.39 eV)56 and Na2Ba[Na2Sn2S7] (3.42 eV),57 but smaller than the RE-based oxychalcogenide Nd3[Ga3O3S3][Ge2O7] (4.35 eV).58 Interestingly, Ag-based chalcogenides like AgGaS2 (2.64 eV),59 Ag2In2SiS6 (2.41 eV),60 Ag2In2GeS6 (2.3 eV),61 Li/NaAgIn2GeS6 (2.47/2.40 eV),61 and AgHgPS4 (2.63 eV),62 as well as RE-based chalcogenides, such as REIII2BII3Sn3S12 (REIII = La, Sm, Gd; B = Ca, Sr)(1.51–1.71 eV),63 La6BIII2GeS14 (BIII = Ga, In) (2.54–2.61 eV),64 and EuBIICIVQVI4 (BII = Cd, Hg; CIV = Ge, Sn; QVI = S, Se) (1.97–2.50 eV),65,66 usually exhibit small band gaps that limit their applications, while both Ag and RE containing compounds AgLaSiS4 (3.33 eV) and AgYSiS4 (3.18 eV) in this work display wide band gaps, breaking through the “3.0 eV wall” of energy band gap in the Ag and RE coexisting chalcogenides. To check the physical and chemical stability, AIREIIISiQVI4 single crystals were picked and placed in water for 7 days. The optical images (Fig. S9†) confirm that the title compounds have a good chemical stability in water. Moreover, the polycrystalline AgREIIISiS4 powder samples were exposed in the air for 6 months, and no changes have been observed in the XRD patterns before and after the exposure (Fig. S10†), indicating the compounds are air stable. | ||
| Fig. 4 The experimental and calculated XRD patterns of (a) AgLaSiS4, and (b) AgYSiS4; the UV-vis-NIR diffuse reflectance spectra and experimental band gaps of (c) AgLaSiS4, and (d) AgYSiS4. | ||
Theoretical calculations
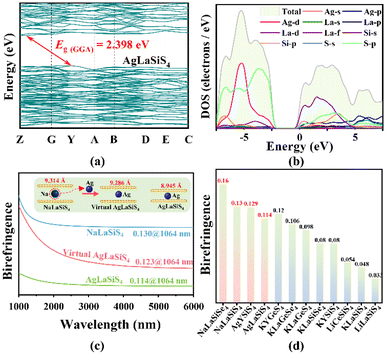
To clarify the origin of optical band gaps of the title compounds, first-principles calculations were carried out. The calculated band structures indicate that AIREIIISiQVI4 are indirect band gap compounds. The calculated GGA band gaps are 2.398 eV for AgLaSiS4, 2.274 eV for AgYSiS4, 2.873 eV for NaLaSiS4, and 2.282 eV for NaLaSiSe4 (Fig. 5a and S11a–c†). Due to the discontinuity of the exchange–correlation energy function, the GGA band gap is usually underestimated.67,68 Thus, the HSE06 functional was employed to guarantee the accuracy and consistency of the theoretical band gaps, and the resulted HSE06 band gaps are 3.56 eV for AgLaSiS4, 3.34 eV for AgYSiS4, 3.83 eV for NaLaSiS4, and 3.02 eV for NaLaSiSe4, which are close to the experimental values of the selected AgLaSiS4 (3.33 eV) and AgYSiS4 (3.18 eV). The results of T/PDOS indicate that the valence band (VB) maximum is mainly occupied by Ag-d, La-d, S-p orbitals in AgLaSiS4; Ag-d, Y-d, S-p orbitals in AgYSiS4; La-d, S-p orbitals in NaLaSiS4; and La-d, Se-p orbitals in NaLaSiSe4, and the conduction band (CB) minimum is mainly occupied by La-d, Si-p, S-p orbitals in AgLaSiS4; Y-d, Si-p, S-p orbitals in AgYSiS4; La-d, Si-p, S-p orbitals in NaLaSiS4; and La-d, Si-p, Se-p orbitals in NaLaSiSe4 (Fig. 5b and S11d–f†). It implies that the optical band gap is mainly determined by [AgS3] and [RES8] units in AgREIIISiS4, while [LaQ8] units determine the optical band gap in NaLaSiQVI4. Further analyses on the T/PDOS structures confirm the charge transfer enhanced band gap mechanism in the compounds.1 In contrast to La2S3, where the La-d and S-p orbitals show a strong hybridisation in the −4.5–0 and 2.5–5 eV regions (indicated by the yellow region in Fig. S12b†), the d–p orbital hybridisation between La and S is significantly reduced in the same region, and an evident orbital hybridisation between the S-p orbitals and Si-p orbitals in the 2.5 to 5.0 eV regions (indicated by the blue and red regions in Fig. S12d†) is observed in AgLaSiS4 (taking AgLaSiS4 as an example herein), giving rise to the decreased covalency of La–S bonds by transferring notably charge from [LaS8] to [SiS4] units, thus resulting in a wide band gap in AgLaSiS4. The other title compounds show similar charge transfer enhanced band gap mechanisms (Fig. S13 and 14†). In addition, compared to the hybridization regions (−0.43–0.71 eV) of Y-d, Si-p, and S-p orbitals near the bottom of the CB minimum in AgYSiS4, the regions of La-d, Si-p, and S-p orbitals in AgLaSiS4 show a slight shift to −0.09–0.97 eV, resulting in a wider band gap in AgLaSiS4 (3.33 eV) than AgYSiS4 (3.18 eV), similar to the case in KLaGeS4 (3.34 eV)69 and KYGeS4 (3.15 eV).1Moreover, due to the strong optical anisotropy in the crystal structures, the birefringence of the title compounds was calculated to be 0.114 (AgLaSiS4), 0.129 (AgYSiS4), 0.130 (NaLaSiS4), and 0.160 (NaLaSiSe4) at 1064 nm (Fig. 5c and S11g–i†). Among them, NaLaSiSe4 exhibits the largest known birefringence in the AIRECIVQVI4 family compounds (Fig. 5d). Moreover, the birefringence discrepancy between AgLaSiS4 and NaLaSiS4 could be related to the fluctuation of layer spacing between [LaSiS10]∞ layers (8.945 Å for AgLaSiS4, 9.314 Å for NaLaSiS4) induced by the fluctuation of Ag and Na atoms in the structures.70 To confirm this point, a virtual AgLaSiS4 atom model (Fig. S15†) with a larger [LaSiS10]∞ layer spacing of 9.286 Å was built from NaLaSiS4 by atomic replacement, and its birefringence was computed to be Δn = 0.123@1064 nm, as shown in Fig. 5c. It means that along with the increase of layer spacing from 8.945 (experimental AgLaSiS4 model) to 9.286 Å (virtual AgLaSiS4 model), the birefringence in the built model is calculated to be 0.123@1064 nm, larger than the value (0.114@1064 nm) in the experimental model, indicating that the larger layer spacing is beneficial for producing larger birefringence.
Conclusions
In summary, four new RE compounds AIREIIISiQVI4 (AI = Ag, Na; REIII = La, Y; QVI = S, Se) have been rationally designed, and synthesized by a high temperature solution method. To the best of our knowledge, the four compounds are the first series of Ag- or Na-containing compounds in the AIREIIICIVQVI4 family, enriching the chemical diversity of the family. The four compounds crystallize in the same P21/c space group and are constructed with [AgS3], [RES8], and [SiS4] units in AgREIIISiS4 and [NaQ6], [LaQ8], and [SiQ4] units in NaLaSiQVI4. Due to the fluctuations of the atomic radius and coordination numbers at the A-site, the compounds in the AIREIIICIVQVI4 family show a multiple structural transition from Ama2 (LiLaSiS4), P21/c (Na/AgLaSiS4), and P21 (KLaSiS4) to Pnma (Rb/CsLaSiS4). Moreover, the compounds exhibit wide band gaps (3.33 eV for AgLaSiS4, 3.18 for AgYSiS4, 3.83 eV (HSE06) for NaLaSiS4, and 3.02 eV (HSE06) for NaLaSiSe4) and large birefringence (0.114 for AgLaSiS4, 0.129 for AgYSiS4, 0.130 for NaLaSiS4, and 0.160 for NaLaSiSe4 at 1064 nm). It is worth noting that NaLaSiSe4 exhibits the largest known birefringence in the AIREIIICIVQVI4 family compounds. DFT calculations unveil that the band gaps are enhanced by the charge transfer from [REQ8] to [SiQ4] in the title compounds and highlight that the layer distance affected birefringence in the isostructural compounds, which are beneficial for the regulation of the band gap and birefringence of advanced functional materials by similar chemical modulations.Data availability statement
The authors declare that the main data that support the findings of this study are available within the article and the ESI.† Other relevant data are available from the authors upon reasonable request.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2024D01E30), the National Natural Science Foundation of China (22335007, 52002398, 61835014, 51972336) and the Xinjiang Key Laboratory of Electronic Information Materials and Devices (2017D04029).References
- D. Mei, W. Cao, N. Wang, X. Jiang, J. Zhao, W. Wang, J. Dang, S. Zhang, Y. Wu, P. Rao and Z. Lin, Breaking through the “3.0 eV wall” of energy band gap in mid-infrared nonlinear optical rare earth chalcogenides by charge-transfer engineering, Mater. Horiz., 2021, 8, 2330–2334 RSC.
- Y. Kang and Q. Wu, A review of the relationship between the structure and nonlinear optical properties of organic-inorganic hybrid materials, Coord. Chem. Rev., 2024, 498, 215458 CrossRef CAS.
- Y. Kang, C. Yang, J. Gou, Y. Zhu, Q. Zhu, W. Xu and Q. Wu, From Cd(SCN)2(CH4N2S)2 to Cd(SCN)2(C4H6N2)2: controlling sulfur content in thiocyanate systems significantly improves the overall performance of UV nonlinear optical materials, Angew. Chem., Int. Ed., 2024, 63, e202402086 CrossRef CAS PubMed.
- M. Zhu, Y. Tang, X. Chen, L. Xu and X. Fan, B-N bonds between SrBi2B2O7 and humic acid composites enhance polarized electric field for efficient piezocatalytic degradation of oxytetracycline in water, Chem. Eng. J., 2024, 481, 148379 CrossRef CAS.
- Y. Tang, X. Chen, M. Zhu, X. Liao, S. Hou, Y. Yu and X. Fan, The strong alternating built-in electric field sourced by ball milling on Pb2BO3X (X = Cl, Br, I) piezoelectric materials contributes to high catalytic activity, Nano Energy, 2022, 101, 107545 CrossRef CAS.
- J. Li and F. L. Deepak, In situ kinetic observations on crystal nucleation and growth, Chem. Rev., 2022, 122, 16911–16982 CrossRef CAS PubMed.
- X. Wang, J. Li, Z. Zhao, S. Huang and W. Xie, Crystal structure and electronic structure of quaternary semiconductors Cu2ZnTiSe4 and Cu2ZnTiS4 for solar cell absorber, J. Appl. Phys., 2012, 112, 023701 CrossRef.
- L. Wang, Q. Sun and J. Li, Recent progress on sulfide infrared nonlinear optical materials with large SHG response and wide band gap, Chin. J. Struct. Chem., 2023, 42, 100013 CrossRef CAS.
- G. Deokar, N. S. Rajput, J. Li, F. L. Deepak, W. Ou-Yang, N. Reckinger, C. Bittencourt, J. F. Colomer and M. Jouiad, Toward the use of CVD-grown MoS2 nanosheets as field-emission source, Beilstein J. Nanotechnol., 2018, 9, 1686–1694 CrossRef CAS PubMed.
- Q. Wu, L. Kang and Z. Lin, A machine learning study on high thermal conductivity assisted to discover chalcogenides with balanced infrared nonlinear optical performance, Adv. Mater., 2024, 36, 2309675 CrossRef CAS PubMed.
- J. Xu, K. Wu, B. Zhang, H. Yu and H. Zhang, LaAeAl3S7 (Ae = Ca, Sr): cairo pentagonal layered thioaluminates achieving a good balance between a strong second harmonic generation response and a wide bandgap, Inorg. Chem. Front., 2023, 10, 2045–2052 RSC.
- M. Yan, R. Tang, W. Xu, W. Liu and S. Guo, Centrosymmetric CaBaMF8 and Noncentrosymmetric Li2CaMF8 (M = Zr, Hf): Dimension Variation and Nonlinear Optical Activity Resulting from an Isovalent Cation Substitution-Oriented Design, Inorg. Chem., 2024, 63, 5260–5268 CrossRef CAS PubMed.
- S. Liu, C. Li, J. Jiao, Y. She, T. Zhang, D. Ju, N. Ye, Z. Hu and Y. Wu, Three in one: a cadmium bismuth vanadate NLO crystal exhibiting a large second-harmonic generation response and enhanced birefringence, Inorg. Chem. Front., 2024, 11, 2384–2391 RSC.
- W. Xie, R. Tang, S. Yan, N. Ma, C. Hu and J. Mao, Ba4B14O25: a deep ultraviolet transparent nonlinear optical crystal with strong second harmonic generation response achieved by a boron-rich closed-loop strategy, Small, 2024, 20, 2307072 CrossRef CAS PubMed.
- Z. Qian, H. Wu, Z. Hu, J. Wang, Y. Wu and H. Yu, Cs3In(In4Se7)(P2Se6): a multi-chromophore chalcogenide with excellent nonlinear optical property designed by group grafting, Angew. Chem., Int. Ed., 2024, 63, e202400892 CrossRef CAS PubMed.
- X. Li, Z. Shi, M. Yang, W. Liu and S. Guo, Sn7Br10S2: the first ternary halogen-rich chalcohalide exhibiting a chiral structure and pronounced nonlinear optical properties, Angew. Chem., Int. Ed., 2022, 61, e202115871 CrossRef CAS PubMed.
- Q. Liu, X. Liu, L. Wu and L. Chen, SrZnGeS4: a dual-waveband nonlinear optical material with a transparency spanning UV/Vis and Far-IR spectral regions, Angew. Chem., Int. Ed., 2022, 61, e202205587 CrossRef CAS PubMed.
- X. Zhang, H. Wu, Z. Hu, J. Wang, Y. Wu and H. Yu, AIIHgMIVS4 (AII = Sr, Ba, MIV = Si, Ge): a series of materials with large second harmonic generation response and wide band gaps, Adv. Opt. Mater., 2024, 12, 2301735 CrossRef CAS.
- X. Ji, H. Wu, B. Zhang, H. Yu, Z. Hu, J. Wang and Y. Wu, Intriguing dimensional transition inducing variable birefringence in K2Na2Sn3S8 and Rb3NaSn3Se8, Inorg. Chem., 2020, 60, 1055–1061 CrossRef PubMed.
- Z. Li, Y. Liu, S. Zhang, W. Xing, W. Yin, Z. Lin, J. Yao and Y. Wu, Functional chalcogenide Na2HgSn2Se6 and K2MnGe2Se6 exhibiting flexible chain structure and intriguing birefringence tunability, Inorg. Chem., 2020, 59, 7614–7621 CrossRef CAS PubMed.
- Z. Chen, W. Liu and S. Guo, A review of structures and physical properties of rare earth chalcophosphates, Coord. Chem. Rev., 2023, 474, 214870 CrossRef CAS.
- L. Dong, S. Zhang, P. Gong, L. Kang and Z. Lin, Evaluation and prospect of mid-infrared nonlinear optical materials in f0 rare earth (RE = Sc, Y, La) chalcogenides, Coord. Chem. Rev., 2024, 509, 215805 CrossRef CAS.
- P. Feng, J. Zhang, M. Ran, X. Wu, H. Lin and Q. Zhu, Rare-earth-based chalcogenides and their derivatives: an encouraging IR nonlinear optical material candidate, Chem. Sci., 2024, 15, 5869–5896 RSC.
- L. Gao, X. Wu, J. Xu, X. Tian, B. Zhang and K. Wu, Rational combination of multiple structural groups on regulating nonlinear optical property in hexagonal Ln3MGeS7 polar crystals, J. Alloys Compd., 2022, 900, 163535 CrossRef CAS.
- Y. Zhang, J. Chen, K. Li, H. Wu, Z. Hu, J. Wang, Y. Wu and H. Yu, LaMg6Ga6S16: a chemical stable divalent lanthanide chalcogenide, Nat. Commun., 2024, 15, 2959 CrossRef CAS PubMed.
- H. Chen, Y. Zhang, Y. Yang and S. Guo, Ternary rare-earth sulfides RE3GaS6 (RE = Ho, Er): Crystal chemistry, second-order nonlinear optical properties and theoretical investigation, J. Alloys Compd., 2021, 868, 159112 CrossRef CAS.
- W. Yin, K. Feng, W. Wang, Y. Shi, W. Hao, J. Yao and Y. Wu, Syntheses, structures, optical and magnetic properties of Ba2MLnSe5 (M = Ga, In; Ln = Y, Nd, Sm, Gd, Dy, Er), Inorg. Chem., 2012, 51, 6860–6867 CrossRef CAS PubMed.
- Y. Yang, Y. Chu, B. Zhang, K. Wu and S. Pan, Unique unilateral-chelated mode-induced d–p−π interaction enhances second-harmonic generation response in new Ln3LiMS7 family, Chem. Mater., 2021, 33, 4225–4230 CrossRef CAS.
- A. Choudhury, L. A. Polyakova, I. Hartenbach, T. Schleid and P. K. Dorhout, Synthesis, structures, and properties of layered quaternary chalcogenides of the general formula ALnEQ4 (A = K, Rb; Ln = Ce, Pr, Eu; E = Si, Ge; Q = S, Se), Z. Anorg. Allg. Chem., 2006, 632, 2395–2401 CrossRef.
- P. Wu and J. A. Ibers, Synthesis and structures of the quaternary chalcogenides of the type KLnMQ4 (Ln = La, Nd, Gd, Y; M = Si, Ge; Q = S, Se), J. Solid State Chem., 1993, 107, 347–355 CrossRef CAS.
- Y. Han, C. Hu, B. Li and J. Mao, LnLiSiS4 (Ln = La and Ce): promising infrared nonlinear optical materials designed by aliovalent substitution from SrCdSiS4, Mater. Today Phys., 2023, 31, 100987 CrossRef CAS.
- G. Shi, Y. Wang, F. Zhang, B. Zhang, Z. Yang, X. Hou, S. Pan and K. R. Poeppelmeier, Finding the next deep-ultraviolet nonlinear optical material: NH4B4O6F, J. Am. Chem. Soc., 2017, 139, 10645–10648 CrossRef CAS PubMed.
- X. Wang, Y. Wang, B. Zhang, F. Zhang, Z. Yang and S. Pan, CsB4O6F: a congruent-melting deep-ultraviolet nonlinear optical material by combining superior functional units, Angew. Chem., Int. Ed., 2017, 56, 14119–14123 CrossRef CAS PubMed.
- Z. Zhang, Y. Wang, B. Zhang, Z. Yang and S. Pan, Polar fluorooxoborate, NaB4O6F: a promising material for ionic conduction and nonlinear optics, Angew. Chem., Int. Ed., 2018, 57, 6577–6581 CrossRef CAS PubMed.
- A. Abudurusuli, J. Huang, P. Wang, Z. Yang, S. Pan and J. Li, Li4MgGe2S7: the first alkali and alkaline-earth diamond-like infrared nonlinear optical material with exceptional large band gap, Angew. Chem., Int. Ed., 2021, 60, 24131–24136 CrossRef CAS PubMed.
- X. Chen, Q. Jing and K. M. Ok, Pb18O8Cl15I5: a polar lead mixed oxyhalide with unprecedented architecture and excellent infrared nonlinear optical properties, Angew. Chem., Int. Ed., 2020, 59, 20323–20327 CrossRef CAS PubMed.
- Y. Chu, H. Wang, Q. Chen, X. Su, Z. Chen, Z. Yang, J. Li and S. Pan, “Three-in-One”: a new Hg-based selenide Hg7P2Se12 exhibiting wide infrared transparency range and strong nonlinear optical effect, Adv. Funct. Mater., 2024, 34, 2314933 CrossRef CAS.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: a complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- M. Mutailipu, J. Han, Z. Li, F. Li, J. Li, F. Zhang, X. Long, Z. Yang and S. Pan, Achieving the full-wavelength phase-matching for efficient nonlinear optical frequency conversion in C(NH2)3BF4, Nat. Photonics, 2023, 17, 694–701 CrossRef CAS.
- B. Zhang, G. Shi, Z. Yang, F. Zhang and S. Pan, Fluorooxoborates: beryllium-free deep-ultraviolet nonlinear optical materials without layered growth, Angew. Chem., Int. Ed., 2017, 56, 3916–3919 CrossRef CAS PubMed.
- M. Mutailipu, M. Zhang, B. Zhang, L. Wang, Z. Yang, X. Zhou and S. Pan, SrB5O7F3: the first asymmetric alkaline-earth fluorooxoborate with unprecedented [B5O9F3]6− functionalized chromophore, Angew. Chem., Int. Ed., 2018, 57, 6095–6099 CrossRef CAS PubMed.
- X. Lou, X. Jiang, B. Liu and G. Guo, Excellent nonlinear optical M[M4Cl][Ga11S20] (M = A/Ba, A = K, Rb) achieved by unusual cationic substitution strategy, Small, 2024, 20, 2305711 CrossRef CAS PubMed.
- H. Wang, X. Pan, W. Zhao, Y. Chu and J. Li, A new infrared nonlinear optical material BaZnGeS4 with wide band gap and large nonlinear optical response, Inorg. Chem. Front., 2023, 10, 6253–6261 RSC.
- L. Wang, D. Chu, Z. Yang, J. Li and S. Pan, Wide band gap selenide infrared nonlinear optical materials AIIMg6Ga6Se16 with strong SHG responses and high laser-induced damage thresholds, Chem. Sci., 2024, 15, 6577–6582 RSC.
- N. Pienack and W. Bensch, The new silver thiostannate (1, 4−dabH2) Ag2SnS4: solvothermal synthesis, crystal structure and spectroscopic properties, Z. Anorg. Allg. Chem., 2006, 632, 1733–1736 CrossRef CAS.
- Y. Wu and W. Bensch, Syntheses, crystal structures and spectroscopic properties of Ag2Nb[P2S6][S2] and KAg2[PS4], J. Solid State Chem., 2009, 182, 471–478 CrossRef CAS.
- A. Grzechnik, J. Z. Zheng, D. Wright and P. F. Mcmillan, LaSe2−x compounds: Vibrational and electrical properties, J. Phys. Chem. Solids, 1996, 57, 1625–1634 CrossRef CAS.
- H. Wang, Y. Chu, X. Pan, Z. Yang, S. Pan and J. Li, Double alkaline earth metals sulfide SrMgGeS4 with high laser-induced damage threshold and strong second-harmonic generation, Mater. Today Phys., 2023, 38, 101243 CrossRef CAS.
- J. Zhou, Z. Fan, K. Zhang, Z. Yang, S. Pan and J. Li, Rb2CdSi4S10: novel [Si4S10] T2-supertetrahedra-contained infrared nonlinear optical material with large band gap, Mater. Horiz., 2023, 10, 619–624 RSC.
- A. P. Litvinchuk, V. M. Dzhagan, V. O. Yukhymchuk, M. Y. Valakh, I. S. Babichuk, O. V. Parasyuk, L. V. Piskach, O. D. Gordan and D. R. Zahn, Electronic structure, optical properties, and lattice dynamics of orthorhombic Cu2CdGeS4 and Cu2CdSiS4 semiconductors, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 165201 CrossRef.
- I. Hartenbach and T. Schleid, Thiosilicate der selten-erd-eemente: III. KLa[SiS4] und RbLa[SiS4] – ein struktureller vergleich, Z. Anorg. Allg. Chem., 2005, 631, 1365–1370 CrossRef CAS.
- M. Usman, M. D. Smith, G. Morrison, V. V. Klepov, W. Zhang, P. S. Halasyamani and H.-C. zur Loye, Molten alkali halide flux growth of an extensive family of noncentrosymmetric rare earth sulfides: structure and magnetic and optical (SHG) properties, Inorg. Chem., 2019, 58, 8541–8550 CrossRef CAS PubMed.
- Y. Chu, H. Wang, T. Abutukadi, Z. Li, M. Mutailipu, X. Su, Z. Yang, J. Li and S. Pan, Zn2HgP2S8: a wide bandgap Hg-based Infrared nonlinear optical material with large second-harmonic generation response, Small, 2023, 19, 2305074 CrossRef CAS PubMed.
- M. Zhang, B. Liu, X. Jiang and G. Guo, Nonlinear optical sulfides LiMGa8S14 (M = Rb/Ba, Cs/Ba) created by Li+ driven 2D centrosymmetric to 3D noncentrosymmetric transformation, Small, 2023, 19, 2302088 CrossRef CAS PubMed.
- Y. Han, C. Hu and J. Mao, Ca2Ln(BS3)(SiS4) (Ln = La, Ce, and Gd): mixed metal thioborate–thiosilicates as well–performed infrared nonlinear optical materials, Small, 2023, 19, 2302088 CrossRef PubMed.
- K. Ding, H. Wu, Z. Hu, J. Wang, Y. Wu and H. Yu, [Ba4(S2)][ZnGa4S10]: design of an unprecedented infrared nonlinear salt-inclusion chalcogenide with disulfide-bonds, Small, 2023, 19, 2302819 CrossRef CAS PubMed.
- R. Li, Q. Liu, X. Liu, Y. Liu, X. Jiang, Z. Lin, F. Jia, L. Xiong, L. Chen and L. Wu, Na2Ba[Na2Sn2S7]: structural tolerance factor-guided NLO performance improvement, Angew. Chem., Int. Ed., 2023, 62, e202218048 CrossRef CAS PubMed.
- M. Ran, S. Zhou, W. Wei, B. Li, X. Wu, H. Lin and Q. Zhu, Rational design of a rare-earth oxychalcogenide Nd3[Ga3O3S3][Ge2O7] with superior infrared nonlinear optical performance, Small, 2023, 19, 2300248 CrossRef CAS PubMed.
- A. O. Okorogu, S. B. Mirov, W. Lee, D. I. Crouthamel, N. Jenkins, A. Y. Dergachev, K. L. Vodopyanov and V. V. Badikov, Tunable middle infrared downconversion in GaSe and AgGaS2, Opt. Commun., 1998, 155, 307–312 CrossRef CAS.
- W. Zhou, W. Yao, Q. Zhang, H. Xue and S. Guo, Introduction of Li into Ag-based noncentrosymmetric sulfides for high-performance infrared nonlinear optical materials, Inorg. Chem., 2021, 60, 5198–5205 CrossRef CAS PubMed.
- W. Zhou, M. Geng, M. Yan, N. Suen, W. Liu and S. Guo, Alkali metal partial substitution-induced improved second-harmonic generation and enhanced laser-induced damage threshold for Ag-based sulfides, Inorg. Chem. Front., 2022, 9, 3779–3787 RSC.
- W. Xing, N. Wang, C. Tang, C. Li, Z. Lin, J. Yao, W. Yin and B. Kang, From AgGaS2 to AgHgPS4: vacancy defects and highly distorted HgS4 tetrahedra double-induced remarkable second-harmonic generation response, J. Mater. Chem. C, 2021, 9, 1062–1068 RSC.
- J. Xu, Y. Xiao, K. Wu, B. Zhang, D. Lu, H. Yu and H. Zhang, Flexible anionic groups-activated structure dissymmetry for strong nonlinearity in Ln2Ae3MIV3S12 family, Small, 2024, 20, 2306577 CrossRef CAS PubMed.
- Y. Shi, Y. Chen, M. Chen, L. Wu, H. Lin, L. Zhou and L. Chen, Strongest second harmonic generation in the polar R3MTQ7 family: atomic distribution induced nonlinear optical cooperation, Chem. Mater., 2015, 27, 1876–1884 CrossRef CAS.
- W. Xing, C. Tang, N. Wang, C. Li, Z. Li, J. Wu, Z. Lin, J. Yao, W. Yin and B. Kang, EuHgGeSe4 and EuHgSnS4: two quaternary Eu-based infrared nonlinear optical materials with strong second-harmonic-generation responses, Inorg. Chem., 2020, 59, 18452–18460 CrossRef CAS PubMed.
- W. Xing, N. Wang, Y. Guo, Z. Li, J. Tang, K. Kang, W. Yin, Z. Lin, J. Yao and B. Kang, Two rare-earth-based quaternary chalcogenides EuCdGeQ4 (Q = S, Se) with strong second-harmonic generation, Dalton Trans., 2019, 48, 17620–17625 RSC.
- P. Wang, Y. Chu, A. Tudi, C. Xie, Z. Yang, S. Pan and J. Li, The combination of structure prediction and experiment for the exploration of alkali-earth metal-contained chalcopyrite-like IR nonlinear optical material, Adv. Sci., 2022, 9, e2106120 CrossRef PubMed.
- L. Luo, L. Wang, J. Chen, J. Zhou, Z. Yang, S. Pan and J. Li, AIBII3CIII3QVI8: a new family for the design of infrared nonlinear optical materials by coupling octahedra and tetrahedra units, J. Am. Chem. Soc., 2022, 144, 21916–21925 CrossRef CAS PubMed.
- Y. Liu, X. Li, S. Wu, M. Ma, X. Jiang, Y. Wu and D. Mei, A rare earth chalcogenide nonlinear optical crystal KLaGeS4: achieving good balance among band gap, second harmonic generation effect, and birefringence, Inorg. Chem., 2024, 63, 10938–10942 CrossRef CAS PubMed.
- L. Wang, C. Tu, H. Gao, J. Zhou, H. Wang, Z. Yang, S. Pan and J. Li, Clamping effect driven design and fabrication of new infrared birefringent materials with large optical anisotropy, Sci. China: Chem., 2023, 66, 1086–1093 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2368926–2368929. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi01738b |
| This journal is © the Partner Organisations 2024 |