Insights into the application of cerium dioxide nanoparticle-modified cobalt phosphide as an efficient electrocatalyst for high-performance lithium–sulfur batteries†
Xiaofei
Wang
 *a,
Ganfan
Zhang
a,
Yue
Li
a,
Yuanting
Wu
a and
Wei
Luo
*b
*a,
Ganfan
Zhang
a,
Yue
Li
a,
Yuanting
Wu
a and
Wei
Luo
*b
aSchool of Materials Science and Engineering, Shaanxi Key Laboratory of Green Preparation and Functionalization for Inorganic Materials, Shaanxi University of Science and Technology, Xi'an, 710021, PR China. E-mail: wangxiaof@sust.edu.cn
bCollege of Chemistry & Chemical Engineering, Yan'an University, Yan'an 716000, PR China. E-mail: luoweicxy@163.com
First published on 28th August 2024
Abstract
Developing high-efficiency catalysts is an effective strategy to boost the hysteretic polysulfide conversion behavior of lithium–sulfur (Li–S) batteries. Cobalt phosphide (CoP) is a typical promising catalyst due to its inherent advantages such as good electron conductivity, facile synthesis route and moderate catalytic capability and binding energy to polysulfide. However, the design and fabrication of highly active CoP remains a challenge. Herein, aiming to optimize the catalytic activity of CoP, the regulation of the electronic structure of CoP by cerium dioxide (CeO2) was explored, and the sulfur conversion capability and the electrochemical performance in Li–S batteries using the obtained CoP/CeO2 nanocomposites were demonstrated. Microstructure analysis demonstrates that CeO2 nanoparticles can embed in CoP and increase its exposed active sites, and the introduced CeO2 can adjust the electronic structure and optimize the charge transfer and polysulfide conversion behavior of CoP. Although DFT indicates a moderate adsorption energy of CoP/CeO2 towards Li2S6, practical catalytic activity depends strictly on the amount of CeO2, and the optimum amount is ∼10 mol%. A Li–S battery with a CoP/CeO2-10-modified separator exhibits a high specific capacity of 1400 mA h g−1 at 0.1C, an excellent rate performance of 722 mA h g−1 at 3C and a long-term cycling durability of 535 mA h g−1 at 1C after 1000 cycles. This work expands the range of CoP-based catalysts based on CeO2 in Li–S batteries.
Introduction
In order to drive electric vehicles to a long distance, the development of high-energy-density electrochemical storage devices has become an imperative and essential approach.1,2 Currently, over half of commercial energy storage systems employ Li-ion batteries; nevertheless, the practical specific capacity and energy density based on common anodes (such as graphite) and cathodes (such as NMC) almost reach the theoretical limit. In particular, energy density is still far from the targeted value (500 W h kg−1, proposed by a battery consortium).3 In sharp contrast, lithium–sulfur (Li–S) batteries, based on a novel sulfur conversion reaction, have gained ever-increasing interest due to their potential high energy density of 2600 W h kg−1.4,5 Meanwhile, the practical energy density of the constructed pouch-type Li–S battery has exceeded that of existing commercial Li-ion batteries.6 Moreover, sulfur, a cathode active material, has a low cost compared with commercial Li-ion cathodes, and the above advantages make Li–S batteries a potential candidate for the next-generation battery system.Although significant efforts have been made, the development of Li–S batteries is still in its early stage, and their practical application will face several scientific and technical bottlenecks: the insulating nature of sulfur, the dissolution and shuttling of lithium polysulfide (LiPS) intermediates, and the corrosion of Li metal.7,8 These issues will inevitably cause sluggish redox kinetics, limit sulfur utilization, cause fast capacity fading, and finally hinder the application of Li–S batteries. Researchers have witnessed the promising prosperity of Li–S batteries by exploring various kinds of catalysts (including heterogeneous, homogeneous, and semi-immobilized materials) to improve the electrochemical conversion reaction kinetics of LiPSs;9 for example, transition-metal-containing oxides,10,11 nitrides,12 sulfides13,14 and phosphides15,16 have been theoretically explored; developing polar catalysts can effectively capture polar polysulfides and inhibit the shuttle effect; and the synergistic effect of catalysis and adsorption to polysulfides can at least ensure high kinetic behavior.
It is worth mentioning that too strong adsorption interaction of catalysts to polysulfide may lead to the irreversibility of polysulfide conversion, while too weak interaction may result in insufficient binding force to the suppression of shuttle effect.17 For example, the binding energy between polysulfide and Co3O4 was 5 eV, which changed to 4.13 eV for CoP, and the battery using Co3O4 exhibited a lower rate performance than that of CoP.18 Although some researchers applied CoS2 as a catalyst, its binding energy to polysulfide was only 1.84 eV, hardly to completely prevent the loss of sulfur.19 On the premise of ensuring moderate binding energy to polysulfide, promoting the following conversion reaction of polysulfides is the focus. In this regard, the essential strategy is to regulate the binding energy of the catalyst towards catalytic intermediates; thus, electronic structure, an important factor in the decision of the concentration of active sites, attracts much attention and this usually has a close relationship with the Fermi level.20,21 For CoP, the unoccupied d orbitals of Co and p originate from P usually labeled as active sites,14 and the number of the valence electrons in their orbitals is a key factor in bonding and breaking bonds with other ions and can influence the catalyst effect toward polysulfides. Till date, although many efforts have been made to fabricate novel CoP-based catalysts in Li–S batteries,22–24 a single phase is hardly to regulate the valence electron and limits the number of active sites; therefore, the requirement for practical electric vehicles is still hard to achieve by applying single CoP due to its restricted electrocatalytic activity.
The construction of heterostructures provides a breakthrough point to enhance the catalytic activity, and the stitching of various phases can lead to the formation of heterostructured catalysts with rich heterointerfaces or heterojunctions, leading to the re-modulation of the electronic structure, and can finally regulate the catalytic activity, and thus, a serious of catalysts including CoP–Co3S4,25 CoP–Co2N,26 Co9S8–MoS2,27 Co–CoOOH,28 MoSe2–MXene29 and CoP–CeO230 were designed, which exhibited excellent electrocatalytic activity. Take cerium dioxide (CeO2), for example, in which Ce has a flexible valence shift between Ce3+ and Ce4+, and the valence-change via redox reaction provides an opportunity for the redistribution of electrons.31 Up to now, numerous transition metal phosphides have been coupled with CeO2 to adjust the active sites and electronic structure, and exhibited excellent performance in various kinds of aspects such as water splitting,32 Zn–air batteries,33 and supercapacitors.34 For example, Wang et al. and co-workers applied CeO2 to decorate CoP and successfully modulated the d-band center and achieved promising overall water splitting behavior.35 In the field of Li–S batteries, the electronic structure of CoP can be adjusted and rearranged by introducing CeO2, strong interactions at the interface of CoP and CeO2 will optimize the electronic environment around the active sites, which can theoretically reduce the LiPS conversion reaction energy barrier and thus enhance the conversion behavior of LiPSs. Besides, polar CeO2 exhibits strong capture ability to polysulfides, and even some researchers point out that CeO2 can catalyze the following conversion reaction of LiPSs.36 However, a deep understanding of the enhanced effect of CeO2-decorated CoP, including the shuttle effect and the corresponding electrochemical performance in Li–S battery, is still not clear.
Currently, there are two strategies for the application of highly efficient catalysts to Li–S batteries: by designing sulfur hosts or functional separators, theoretically, sulfur will experience dissolution and diffusion stages and the dissolved LiPSs can easily be captured by functional separator; meanwhile, the fabrication of functional separators is simple and can easily be scaled up and applied to various sulfur cathodes. In this work, we designed and fabricated a CoP/CeO2 heterostructure via a facile hydrothermal strategy, considering the inevitable dissolution of the polysulfides, and the fabricated CoP/CeO2 was coated onto a PP separator to obtain a modified separator to capture and catalyze the polysulfides. The microstructure characterization reveals that CeO2 can be embedded in CoP and increase the interface interaction. Meanwhile, the electronic structure can be regulated. Benefitting from sufficient interfaces and synergistic electron interaction between CoP and CeO2, the Li–S battery with a CoP/CeO2 heterostructure-modified separator exhibits dramatically enhanced sulfur redox kinetic behaviors compared with CoP. Meanwhile, the cycling stability can also be increased due to the enhanced adsorption capability to polysulfides, which can significantly inhibit the shuttling of polysulfides.
Experimental section
Chemicals
Cobaltous nitrate hexahydrate (Co(NO3)2·6H2O), cerium(III) nitrate hexahydrate (Ce(NO3)3·6H2O), sodium hypophosphite (NaH2PO2) and hexamethylenetetramine (HMT) were obtained from Aladdin. All compounds were used without further purification.Synthesis of CoP/CeO2-x
The CoP/CeO2 heterostructures were fabricated via a simple hydrothermal process followed by low-temperature phosphorization. In brief, 4 mmol mixture of metal salts (Ce(NO3)3·6H2O and Co(NO3)2·6H2O) and 12 mmol hexamethylenetetramine (HMT) were dissolved in 60 mL deionized water, and the molar ratios of Ce3+/Co2+ were adjusted to obtain optimized composites. The obtained clarified solution was then transferred to a 100 mL Teflon-lined stainless steel autoclave and heated at 100 °C for 8 h. The product was then washed three times with water and ethanol, and dried at 60 °C under vacuum overnight to obtain Co/Ce precursor. Then, 100 mg of the as-obtained precursor and 1 g NaH2PO2 were placed in two quartz vessels separately and NaH2PO2 was specifically placed upstream of the N2 stream. After experiencing a heating process at 350 °C for 2 h, the targeted sample (CoP/CeO2) was fabricated. To confirm the optimal amount of Ce(NO3)3·6H2O, 0, 0.2, 0.4, and 0.6 mmol Ce(NO3)3·6H2O were used and the corresponding Co(NO3)2·6H2O were 4, 3.8, 3.6 and 3.4 mmol. The fabricated samples were labeled as CoP/CeO2-0, CoP/CeO2-5, CoP/CeO2-10, and CoP/CeO2-15, respectively.Synthesis of CoP/CeO2-x modified separators
First, 1 wt% Super P, 1 wt% poly(vinylidene fluoride) (PVDF) and 8 wt% CoP/CeO2-x were thoroughly mixed in N-methyl-2-pyrrolidinone (NMP), and the obtained slurry was then coated onto a PP separator (Celgard 2500) dried at 60 °C for 12 h under vacuum to obtain the CoP/CeO2-x modified separators. The areal S loading was ∼0.6 mg cm−2 and the diameter of the modified separator was 18 mm.Material characterization
The crystallographic structures of the samples as produced were characterized using a powder XRD (Smart Lab 9 kW, Japan,) over a range of 10°–80°. The microstructure of the products was determined using a SEM (Regulus 8100) and a TEM (FEI Tecnai G2 F20 S-TWIN). The compositions of the elements and their valence states were determined using an XPS (Thermofisher Nexsa). The specific surface area of the samples was analyzed using a BET (Micromeritics ASAP 2460) model from the N2 adsorption isotherm obtained at a temperature of 77 K. These methods were employed in conducting a thorough investigation of the prepared samples.Electrochemical measurements
The CNT-S composite was constructed using a mass ratio of 7/3 of sublimed sulfur to carbon nanotubes (CNTs), which were heated at 155 °C for 12 h in an Ar atmosphere. Subsequently, a mixture of the obtained CNT-S composite, Super P and PVDF binder was prepared in NMP with a mass ratio of 8/1/1. This mixture was stirred for 4 h and then coated onto a carbon-coated Al foil, followed by vacuum-drying at 60 °C. The areal S loading was ∼2.0 mg cm−2 and the diameter of cathode was 12 mm. In addition, a high S loading of ∼4.0 mg cm−2 was prepared, and the difference was that the mass ratio of S to CNT was 75/25.The battery was evaluated using a CR2032 coin cell system with a piece of sulfur cathode, a piece of lithium anode, and a piece of CoP/CeO2-x-modified separator. The electrolyte was 1.0 M LiTFSI with 1 wt% LiNO3 in 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The electrolyte/sulfur (E/S) ratio of the battery using a low S loading cathode (2.0 mg cm−2) was 12 μL mg−1, and the E/S ratio of the battery using a high sulfur loading cathode (4 mg cm−2) was 6 μL mg−1. The electrochemical performance was evaluated within a voltage range of 2.8–1.7 V at different current densities (1C = 1675 mA h g−1). Cyclic voltammetry (CV) and electrochemical impedance spectrometry (EIS) measurements were performed using a CHI660E electrochemical workstation.
1). The electrolyte/sulfur (E/S) ratio of the battery using a low S loading cathode (2.0 mg cm−2) was 12 μL mg−1, and the E/S ratio of the battery using a high sulfur loading cathode (4 mg cm−2) was 6 μL mg−1. The electrochemical performance was evaluated within a voltage range of 2.8–1.7 V at different current densities (1C = 1675 mA h g−1). Cyclic voltammetry (CV) and electrochemical impedance spectrometry (EIS) measurements were performed using a CHI660E electrochemical workstation.
Adsorption and catalytic conversion tests of Li2S6
Li2S6 solution was prepared by dissolving Li2S and sulfur powder in DME/DOL (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using a molar ratio of 1/5 in an Ar-filled glove box. To assess the chemisorption capability, 20 mg CoP/CeO2-x powders were separately put in 10 mL Li2S6 solution (5 mM) to observe the adsorption behavior.
1) using a molar ratio of 1/5 in an Ar-filled glove box. To assess the chemisorption capability, 20 mg CoP/CeO2-x powders were separately put in 10 mL Li2S6 solution (5 mM) to observe the adsorption behavior.
To assemble a symmetric cell, 8 wt% CoP/CeO2-x, 1 wt% Super P and 8 wt% PVDF were dissolved into an NMP solution to form a homogeneous slurry, which was then coated onto Al foil and dried at 60 °C for 12 h. The fabricated electrode could be used as the cathode and anode, the areal mass loading of CoP/CeO2-x was ∼1.0 mg cm−2 and the diameter of cathode was 12 mm. To evaluate the catalytic conversion performance of CoP/CeO2-x to LiPSs, a symmetrical CR2032 coin cell was assembled using a piece of Celgard 2500 and 20 μL Li2S6 as the electrolyte, and CV tests were conducted at a sweep rate of 10 mV s−1 from −1 to 1 V.
Nucleation and dissolution of Li2S
A typical nucleation and dissolution test was also conducted using a CR2032 coin cell with a piece of PP separator, a piece of Li anode and a piece of CoP/CeO2-x cathode. The electrolyte was a Li2S8 solution (0.25 mol L−1), prepared by dissolving Li2S and sulfur powder in DME/DOL (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using a molar ratio of 1/7; note that 10 μL Li2S8 was added to the cathode side, while 10 μL pristine DME/DOL (v/v = 1
1) using a molar ratio of 1/7; note that 10 μL Li2S8 was added to the cathode side, while 10 μL pristine DME/DOL (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to the anode side. The assembled cell was discharged to 2.06 V at 0.112 mA to consume most of the high-order polysulfides, and then the cell was discharged at 2.05 V to induce nucleation and growth of Li2S until the current decreased to 10−5 A. For the dissolution of Li2S, the cell was first discharged to 1.7 V at 0.112 mA to generate Li2S, which was further dissolved into LiPSs by potentiostatically charging the cell at 2.4 V until the current decreased below 10−5 A.
1) was added to the anode side. The assembled cell was discharged to 2.06 V at 0.112 mA to consume most of the high-order polysulfides, and then the cell was discharged at 2.05 V to induce nucleation and growth of Li2S until the current decreased to 10−5 A. For the dissolution of Li2S, the cell was first discharged to 1.7 V at 0.112 mA to generate Li2S, which was further dissolved into LiPSs by potentiostatically charging the cell at 2.4 V until the current decreased below 10−5 A.
Computational details
The density functional theory (DFT) calculations were performed using the Vienna Ab initio Simulation Package (VASP), the exchange–correlation interactions were described by using the van der Waals force corrected Perdew–Burke–Ernzerhof (PBE) functional DFT-D3 method, and the Generalized Gradient Approximation (GGA) was applied to implement DFT.37 The CoP (011) and CeO2 (111) models were employed to simulate the interaction surface with Li2S6. The atom position was optimized by using the conjugated gradient optimization method, and the geometric convergence tolerance at each atom was set to below 0.03 eV Å−1, and the energy was converged within 10−5 eV. A vacuum layers were wet at least 15 Å along the z-direction to avoid interaction between planes. A cutoff energy of 400 eV was used. The binding strength of Li2S6 on the slab surface was calculated using the following expression and labeled as adsorption energy (ΔEads):38| ΔEads = ΔEads/base − ΔEads − ΔEbase |
Results and discussion
The CoP/CeO2 heterostructure's fabrication process is schematically illustrated in Fig. 1a; according to a previous report,34 the Co(OH)2/CeO2 precursor can be first produced after a facile hydrothermal reaction for 8 hours at 100 °C, and the alkaline environment can satisfy the demand of the growth of Co/Ce precursor, which can be adjusted via HMT. In practical applications, temperature conduction in the hydrothermal process itself is more uniform than that of other methods such as solid-phase calcination, which can ensure the reproducibility of the fabrication of samples. Meanwhile, hydrothermal method can also ensure the consistency of the preparation process even if the volume of the container is increased multiple times, facilitating a large-scale production. As the morphology of the heterostructure is a vital parameter for the creation and exposure of active sites, we investigated the morphology. Under the condition without Ce(NO3)3·6H2O, the precursor is composed of numerous nanosheets, which exhibit an average length of 2 μm, and these nanosheets are tightly interlaced and form clusters (Fig. S1a†). The size of the nanosheets decreases with the increase in the amount of Ce(NO3)3·6H2O, the average length decreases to ∼0.5 μm and even some nanoparticles appear after adding 5 mmol Ce(NO3)3·6H2O (Fig. S1b†). No significant nanosheets were found in the residue with the further increase in the amount of Ce(NO3)3·6H2O to 10 and 15 mmol (Fig. S1c and d†). It should be noted that other fabrication parameters were not changed, indicating that the addition of Ce(NO3)3·6H2O can regulate the morphology of Co(OH)3/CeO2 precursors. After experiencing a phosphorization treatment process, the morphology maintained its original shape (Fig. 1b–e), without any collapse or aggregation that usually confronted in most phosphating processes, indicating excellent microstructure stability of the precursor.39The detailed morphology of CoP/CeO2-0 is further explored using TEM (Fig. S2a†), and a relatively tight nanosheet can be observed, which is in sharp contrast to CoP/CeO2-5 (Fig. S2b†), where abundant microspores highlighted in yellow labels can be observed among the nanosheet, and this is also true for CoP/CeO2-10. As shown in Fig. 1f, numerous microspores accompany with many nanoparticles appear, and these characteristics are significantly different from that of CoP/CeO2-0. We then conducted N2 adsorption–desorption experiments to study the specific surface area and pore characteristic of various samples (Fig. S3†), and as expected, the specific surface area increases with the increase in the amount of CeO2, and the corresponding values are 9.8, 12.3, 12.7 and 16.4 m2 g−1 for CoP, CoP/CeO2-5, CoP/CeO2-10, and CoP/CeO2-15, respectively. Meanwhile, the pore-size characteristic appears with the introduction of CeO2 into CoP, and the pore sizes of the samples mainly focus in the range of 30–35 nm. Theoretically, the high surface area and microporous structure facilitate the adsorption of LiPSs on the sample. The high-resolution TEM (HRTEM) image of the edge of CoP/CeO2-10 shows that two types of lattice fringes appear (Fig. 1g); from the two-dimensional inverse fast Fourier transform (IFFT) pattern in Fig. 1h and 1i, it is clear that an interplanar distance of 0.283 nm corresponds to the (011) plane of the orthorhombic CoP, while an interplanar distance of 0.312 nm corresponds to the (111) plane of cubic CeO2.34 Furthermore, additional elemental mapping results of CoP/CeO2-10 suggest that Co, P, Ce, and O elements can be uniformly distributed on the sample (Fig. 1l), revealing the homogeneous embedding of CeO2.
The crystalline phase of the fabricated sample was then investigated by XRD, as shown in Fig. 2a; some characteristic peaks of CoP/CeO2-0 appear at 31.6°, 36.3°, 46.2°, 48.2° and 56.7°, corresponding to the (011), (111), (112), (211) and (301) crystal planes of CoP (JCPDS no. 29-0497), respectively. After the addition of Ce(NO3)3·6H2O, some new typical peaks at 28.5°, 33.1°, 47.5° and 56.3° appear and the corresponding peak intensity becomes stronger with the increase in the amount of Ce(NO3)3·6H2O; these peaks can be assigned to the (111), (200), (220), and (311) planes of CeO2 (JCPDS no. 34-0394), respectively.34 Meanwhile, the peak intensity of CoP gradually becomes weak with the increase in the content of CeO2. Combined with the TEM results, the above-mentioned results demonstrate the successful synthesis of CoP/CeO2 heterostructures.
The surface composition and chemical state of a catalyst have a close relationship with the catalytic activity, because some critical parameters such as electronic structure and oxygen vacancy will affect the adsorption/redox processes of an intermediate reaction product.40 Therefore, XPS is applied to synthesize the surface characteristic and the electronic interaction of the samples, involving the effect of CeO2 decorating on the elements of Co 2p and P 2p and the effect of Li2S6 adsorption via CoP and CoP/CeO2 on the elements of Ce 3d, Co 2p, P 2p and S 2p. From the fitted high-resolution XPS spectrum of Co 2p shown in Fig. 2b, it can be easily found that two pairs of peaks that belong to Co 2p3/2 (778.61 and 782.29 eV) and Co 2p1/2 (793.58 and 798.26 eV) appear, along with two shakeup satellites (corresponding to 786.50 and 802.78 eV, respectively). The peaks at 782.29 and 798.26 eV can be assigned to the Co species for Co–O, and the peaks at 778.61 and 793.58 eV correspond to the Co–P compound.41 After the introduction of CeO2, the shape of the fitted XPS spectrum of CoP/CeO2 exhibits significant differences from that of CoP, and the peak intensity around 778.61 and 793.58 eV becomes stronger than that around 782.29 and 798.26 eV. Meanwhile, the peak of Co 2p is notably shifted to a lower value than that of CoP, the fitted values of Co 2p3/2 located at 778.40 and 781.98 eV, and the Co 2p1/2 located at 793.39 and 798.12 eV, along with the shakeup satellites located at 785.51 and 802.78 eV, respectively.42 This phenomenon indicates the charge redistribution and partial electron loss of Co, referring to a previous report,35 and we believe the electronic concentration in the Fermi level of adsorbed LiPSs can be increased, which facilitates boosting the interfacial electronic transfer dynamics and promote the electrocatalytic reaction of LiPSs. Meanwhile, the electron transfer phenomenon (Co to CeO2) is also beneficial for the conductivity of CoP.43 The P 2p XPS spectra of both CoP and CoP/CeO2 are presented in Fig. 2c, and the broad peak around 134.13 eV can be assigned to oxidized P species,37 as expected. Although both types of samples exhibit a similar shape around 129.24 eV, the peak intensity at 130.08 and 129.24 eV that correspond to P 2p1/2 and P 2p3/2 is significantly different, and these peaks indicate the existence of Co–P, further confirming the fabrication of CoP and a strong interface interaction between CoP and CeO2.
After experiencing the Li2S6 adsorption, the high-resolution XPS of Co 2p exhibits a similar shape compared with that of pristine CoP/CeO2 except for a slight shift to a high binding energy of 0.4–0.6 eV, as shown in Fig. 2d, indicating that electrons can transfer from Li2S6 to Co, and this situation facilitates the decomposition of Li2S6. A similar condition can also be found from the high-resolution XPS of Ce 3d, as shown in Fig. 2e, where the peaks around 886.1 and 905.2 eV attribute to Ce 2d5/2 and Ce 2d3/2, respectively, and both can be fitted into three parts and belong to Ce3+, Ce3+ and Ce4+, respectively.44 These peaks exhibit a shift of 0.7–1.0 eV to a high binding energy due to the adsorption of Li2S6, suggesting that particle Ce3+ can be oxidized into Ce4+ and this can realize an electron transfer from Ce to Li2S6. For the P 2p XPS spectra of both CoP/CeO2 and CoP/CeO2–Li2S6, no significant shift can be detected and the shape of the fitted curves is nearly maintained, which may indicate that P does not play a critical role in the catalytic activity of CoP/CeO2 (Fig. 2f). Although the S 2p spectrum can be found in pristine CoP/CeO2, two peaks appear after the adsorption of Li2S6, which can be assigned to S 2p3/2 and S 2p1/2 (Fig. 2g), indirectly reflecting the existence of certain interaction between CoP/CeO2 and Li2S6.20 Therefore, the above-mentioned XPS results indicate a strong interaction between CoP/CeO2 and Li2S6, which facilitates the electron transfer from CoP/CeO2 to Li2S6 and can accelerate the electrochemical conversion reaction between Li2S6 and Li2S/Li2S2.45
In order to verify the electrocatalytic activity of the samples in Li–S batteries, symmetric cells are assembled with identical CoP/CeO2-x cathodes and anodes, as shown in Fig. 2h, and no significant CV peak can be observed in a Li2S6-free electrolyte. After adding Li2S6 to the electrolyte, the CV curve of the CoP/CeO2-x electrode shows one pair of sharp redox peaks, and the peak intensity becomes stronger with the increase in the amount of CeO2, indicating that the introduction of CeO2 can significantly enhance the current response and obtain a large peak area at 10 mV s−1. Generally speaking, the area enclosed by CV is proportional to the conversion ratio of polysulfides, which can also be used to analyze the catalytic activity, especially to study and compare the performance at the same CV scan rate. Apparently, these results point out the strong catalytic enhancement behavior of CeO2 to CoP, and this can boost the conversion kinetic of LiPSs in Li–S batteries. Furthermore, Fig. 2i shows the optimal configuration with the corresponding energetic information of Li2S6 on CoP/CeO2, and ΔEads was calculated to be −2.29 eV, indicating that a strong interaction exists between LiPSs and CoP/CeO2. Meanwhile, we also noted that the adsorption behavior is greatly affected by the content of CeO2. From the digital image of Li2S6 solutions before and after adding CoP/CeO2-x in Fig. S4,† it can be found that the initial Li2S6 solution exhibits a brown color, and the addition of 20 mg CoP/CeO2-x (x = 5, 10, 15) will lighten the color, especially CoP/CeO2-10, after equal mass of CoP/CeO2-x for 12 h, the color using CoP/CeO2-10 is almost transparent, while the color containing other amount of CeO2 exhibits certain degree of yellow, demonstrating the importance of CeO2 in anchoring polysulfides.
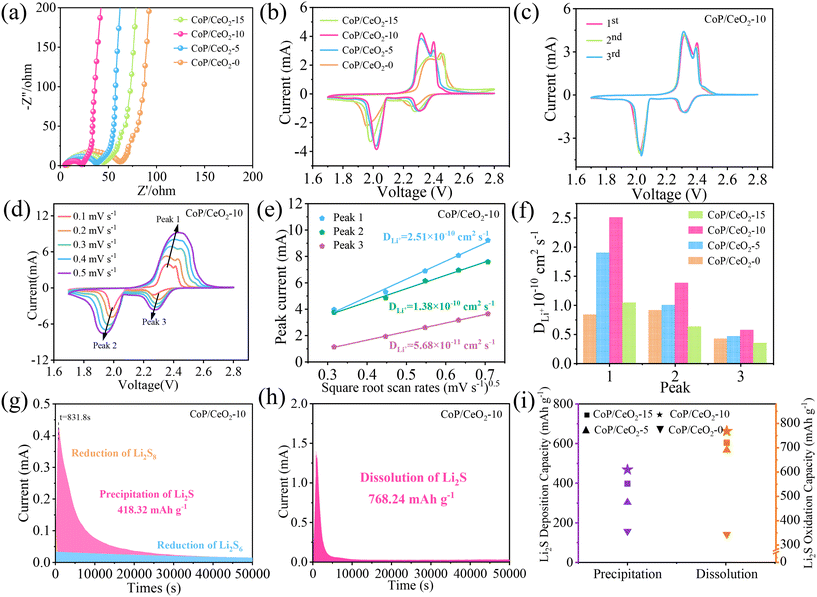
To investigate the interfacial charge transfer dynamics of the CoP/CeO2-x modified separator in the Li–S battery, EIS was used and the results are shown in Fig. 3a. All Nyquist diagrams contain a semicircle in the high-frequency region, which corresponds to charge transfer resistance at the interface of the electrolyte with the cathode, and a diagonal line appears in the low-frequency region corresponding to the diffusion behavior of ions.46 It is inspiring to find that the diameter of the semicircle exhibits the following order: CoP/CeO2-0 > CoP/CeO2-15 > CoP/CeO2-5 > CoP/CeO2-10, and the smallest value of the semicircle implies that the battery with the CoP/CeO2-10-modified separator can achieve the fastest reaction dynamics. CV was performed to investigate the electrochemical property of Li–S batteries, in the voltage range from 1.7 to 2.8 V at 0.1 mV s−1, as shown in Fig. 3b, in which two pairs of redox peaks appear for all kinds of batteries, which can be assigned to the multi-step reaction mechanism of LiPSs. The reduction peak around 2.31 V is ascribed to the reduction of solid S8 into soluble long-chain Li2Sx (x = 4, 6, and 8), and another peak around 2.02 V is assigned to the conversion of Li2Sx into solid short-chain sulfides (Li2S2/Li2S).47 Two anodic peaks appear around 2.32 and 2.4 V, which are ascribed to the reverse conversion of Li2S into Li2Sx and S8, respectively. The difference mainly focuses on the values of peak voltage and current. The battery using a CoP/CeO2-10-modified separator shows the highest current among all the batteries. Meanwhile, the peak current of the battery with the addition of CeO2 is always higher than that of pure CoP, and the corresponding voltage polarization (the difference between the oxidation peak voltage and the reduction peak voltage) of CeO2-added is always smaller than that of pure CoP. These results suggest that the introduction of CeO2 can greatly enhance the electrochemical kinetic of the Li–S battery, while this has a close relationship with the amount of CeO2, and here, the optimum value is CoP/CeO2-10.
We then investigated the first three CV curves of the Li–S battery with four kinds of modified separators (Fig. 3c and Fig. S5a–c†), and it can be found that the battery with CoP/CeO2-10 still presents the highest current density and all the curves nearly perfect overlap, suggesting excellent electrochemical reversibility of CoP/CeO2-10 in accelerating the redox reaction of LiPSs. The diffusion coefficient of Li+ is another major parameter to evaluate whether the separator is suitable to accelerate the sulfur dynamics of L–S batteries. The CV tests of the battery using various kinds of separators were carried out at different scanning rates (0.1–0.5 mV s−1) within the voltage range of 1.7–2.8 V, from the CV curves of the battery with CoP/CeO2-10, as shown in Fig. 3d, from which it can be found that all CV curves show two reduction peaks at 2.3 and 1.9 V during the cathodic scan, corresponding to two oxidation peaks at 2.3 and 2.4 V during the anodic scan. The peak current of the CV curve increases with the increase in the scan rates, the nearly unchanged curve shape indicates excellent electrochemical stability. The value of the peak current has a linear relation with the square root of the scanning rates, according to the Randles–Sevcik equation,48 and the diffusion coefficient can be obtained according to the following formula:
| Ip = (2.69 × 105)n1.5ADLi0.5νLi0.5CLi |
The conversion process of polysulfides can be further evaluated by monitoring the nucleation and decomposition processes of Li2S on CoP/CeO2-x. The nucleation of Li2S can be studied via a discharge curve with Li2S8 as the active material; during this process, the battery is set to galvanostatically discharge to 2.06 V and then potentiostatically discharge at 2.05 V, which can realize the conversion of long-chain LiPSs to Li2S.49 Generally, the integral parameters including current and time and the measured curve can be fitted mathematically to get the integral area, and the precipitation capacity of Li2S can be calculated according to Faraday's law. The nucleation curve of Li2S on CoP/CeO2-10 is shown in Fig. 3g, and for comparison, the nucleation of other kinds of CoP/CeO2-x is shown in Fig. S7a–c.† The nucleation capacities of Li2S on CoP/CeO2-0, CoP/CeO2-5, CoP/CeO2-10 and CoP/CeO2-15 are 157.18, 303.13, 418.32 and 397.41 mA h g−1, respectively; the high nucleation capacity suggests that CoP/CeO2-10 can capture maximum LiPSs and achieve an effective deposition of Li2S and high discharge capacity. The dissolution process of Li2S on CoP/CeO2-10 was further evaluated by a potentiostatic charging test, as shown in Fig. 3h, and the dissolution capacity contribution can be calculated to be 768.24 mA h g−1, which is still higher than that of CoP/CeO2-0 (343.81 mA h g−1), CoP/CeO2-5 (689.81 mA h g−1) and CoP/CeO2-15 (720.58 mA h g−1), as shown in Fig. S7d–f.† From the intuitive diagram in Fig. 3i, it is easy to find that the nucleation and dissolution capacities of Li2S exhibit the following order: CoP/CeO2-10 > CoP/CeO2-15 > CoP/CeO2-5 > CoP; clearly, this result further indicates the importance of CeO2 and the key role of the amount of CeO2 in CoP/CeO2-x in regulating the catalytic activity of the composite.
We then systematically studies the electrochemical performance of CoP/CeO2-x in a practical Li–S battery. From the rate capabilities shown in Fig. 4(a), it can be found that although the capacity of all the batteries decreases with the increase in current density, the battery with a CoP/CeO2-10-modified separator shows the smallest decrease in capacity; its specific capacity can achieve 1400, 1114, 992, 910, 806 and 722 mA h g−1 at 0.1, 0.2, 0.5, 1, 2 and 3C, respectively, which are higher than those of CoP/CeO2-5 (1300, 1028, 884, 786, 702 and 641 mA h g−1), CoP/CeO2-15 (1257, 948, 827, 747, 663 and 605 mA h g−1) and CoP/CeO2-0 (1211, 898, 771, 688, 573 and 131 mA h g−1). Meanwhile, the capacities can still reach 867 and 1077 mA h g−1 when the current density returns to 1 and 0.1C, respectively, verifying that CoP/CeO2-10 can maintain superior rate property. This excellent rate performance of the CoP/CeO2-10-modified separator can also be verified from the charge/discharge curves, as shown in Fig. 4b, and all the curves have two stable and typical voltage plateaus; however, the curves of other types of batteries nearly deform, especially at 3C (Fig. S8a–c†). We then compared the first galvanostatic charge/discharge curves at 0.2C, as shown in Fig. 4c, and the battery with a CoP/CeO2-10-modified separator exhibits the smallest polarization overpotential, and the value is about 140 mV, while the values of other types of batteries are as follows: CoP/CeO2-0 (270 mV), CoP/CeO2-5 (200 mV) and CoP/CeO2-15 (240 mV). The lowest polarization overpotential suggests that the battery using CoP/CeO2-10 can ensure the highest energy efficiency. Similar to the CV curve, the discharge process can also be divided into two parts: the first high voltage plateau can be assigned to the conversion of S8 to LiPSs, which can be denoted as ΔQ1, and the second low voltage plateau can be assigned to the conversion of LiPSs to Li2S, which can be denotes as ΔQ1. The battery with a CoP/CeO2-10-modified separator exhibits the largest values from either ΔQ1 or ΔQ2's respect, indicating that CoP/CeO2-10 can ensure the conversion of more S8 into LiPSs, allowing more LiPSs to take part in the following conversion process.
The Li–S battery with CoP/CeO2-10 further presents an excellent cycling stability at 0.2C. As shown in Fig. 4d, although the specific capacities of all the batteries decrease with the increase in the cycling number, it is also interesting to find that the battery with CoP/CeO2-10 always presents the largest value among them, and it can deliver a high initial discharge capacity of 1214.5 mA h g−1, and maintain 640.7 mA h g−1 after 500 cycles, corresponding to a capacity retention ratio of 51.4%; the coulombic efficiency during the whole cycling stage can be over 99%. While the performance based only on CoP exhibits the lowest electrochemical performance, it only exhibits the lowest capacity of 377.7 mA h g−1 after 500 cycles. We also note that the battery with a high sulfur loading of 4.0 mg cm−2 exhibits superior electrochemical performance to that of a low sulfur loading of 2.0 mg cm−2, at the same current density of 0.2C, the battery with CoP/CeO2-10 presents an initial capacity of 1041.6 mA h g−1 and the specific capacity can still reach 932.2 mA h g−1 after 100 cycles, with a capacity retention rate of 89.5% and a corresponding capacity decay rate of 0.010% per cycle, as shown in Fig. 4e. The main reason is that the mass ratio of S to CNT applied in the high-sulfur-loaded cathode is 75/25, while the low-sulfur-loaded S/C cathode uses a ratio of 7/3, and the thickness of the coated layer of the slurry is higher than that of the low sulfur loading. A little difference in the quality of the cathode may lead to this result, we believe that the role of the functional separator cannot be affected by the cathode, on the contrary, this result can further confirm that the CoP/CeO2-10-modified separator can play a very active role in Li–S batteries. After confirming the excellent electrochemical performance of CoP/CeO2-10 in Li–S batteries, we then investigated the stability of various kinds of CoP/CeO2-x during a long cycle number of 1000, as shown in Fig. 4f, and the initial capacity of battery with CoP/CeO2-10 reaches 1027.7 mA h g−1. It is worth noting that the current density is 1C, which is higher than that shown in Fig. 4d, and the capacity can be maintained at 535.0 mA h g−1 after 1000 cycles. About 52.1% of the capacity is maintained with a capacity decay rate of 0.047% per cycle, while the corresponding data of other kinds of batteries are as follows: CoP/CeO2-0 (717.9 mA h g−1, 218.8 mA h g−1, 30.5%, 0.070%), CoP/CeO2-5 (852.6 mA h g−1, 375.0 mA h g−1, 44.0%, 0.056%) and CoP/CeO2-15 (8817.4 mA h g−1, 326.8 mA h g−1, 40.0%, 0.060%). We further compare the electrochemical performance of Li–S battery using CoP or CeO2-based catalyst, as summarized in Table S1,† it is clear that the performance has surpassed most reported batteries. In addition, we provide a photograph of an LED sign lightened by a coin cell based on the Li–S battery with the CoP/CeO2-10-modified separator (inset in Fig. 4f), which highlights the promising practical application.
Conclusions
In summary, based on the unique electron transfer and ion exchange advantages of CeO2, we introduced CeO2 to regulate the catalytic activity of CoP, hoping to integrate the superiority of CeO2 and CoP to boost the conversion behavior of LiPSs in Li–S batteries. The results indicate that the introduction of CeO2 can not only induce electron redistribution but also accelerate the electron transfer between CoP/CeO2 and LiPSs. We also find that the introduction of CeO2 can create a porous structure in CoP, and the amount of CeO2 is a significant factor in determining the final catalytic activity of CoP/CeO2. As a result, the battery with CoP/CeO2-10 exhibits excellent adsorbability and catalytic activity to LiPSs, and the Li–S battery using a CoP/CeO2-10-modified separator exhibits high energy output, enhanced rate and excellent cycling performances. We hope this work can provide a reference for designing highly efficient catalysts in Li–S batteries based on the regulation effect of CeO2 on metal phosphides.Author contributions
Xiaofei Wang: methodology, data curation, writing – review & editing. Ganfan Zhang: resources, software. Yue Li: validation, conceptualization. Yuanting Wu: validation, funding acquisition. Wei Luo: investigation, supervision.Data availability
Data will be made available on request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51608412), the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2021JM-423), the Youth Innovation Team of Shaanxi Universities (No. 2022-70).References
- W. Lv, Z. Shen, X. Li, J. Meng, W. Yang, F. Ding, X. Ju, F. Ye, Y. Li, X. Lyu, M. Wang, Y. Tian and C. Xu, Discovering Cathodic Biocompatibility for Aqueous Zn-MnO2 Battery: An Integrating Biomass Carbon Strategy, Nano-Micro Lett., 2024, 16, 109 CrossRef CAS.
- M. Zhao, Y. Q. Peng, B. Q. Li, X. Q. Zhang and J. Q. Huang, Regulation of carbon distribution to construct high-sulfur-content cathode in lithium-sulfur batteries, J. Energy Chem., 2021, 56, 203–208 CrossRef CAS.
- C. Niu, H. Lee, S. Chen, Q. Li, J. Du, W. Xu, J.-G. Zhang, M. S. Whittingham, J. Xiao and J. Liu, High-energy lithium metal pouch cells with limited anode swelling and long stable cycles, Nat. Energy, 2019, 4, 551–559 CrossRef CAS.
- Z. Xie, B. Cao, X. Yue, R. Wang, Z. Xue, J. Wang, G. Guan and W. Chen, Metal organic frameworks-based cathode materials for advanced Li-S batteries: A comprehensive review, Nano Res., 2024, 17, 2592–2618 CrossRef CAS.
- J. T. Kim, H. Su, Y. Zhong, C. Wang, H. Wu, D. Zhao, C. Wang, X. Sun and Y. Li, All-solid-state lithium-sulfur batteries through a reaction engineering lens, Nat. Chem. Eng., 2024, 1, 400–410 CrossRef.
- Y. Liu, M. Zhao, L. P. Hou, Z. Li, C. X. Bi, Z. X. Chen, Q. Cheng, X. Q. Zhang, B. Q. Li, S. Kaskel and J. Q. Huang, An Organodiselenide Comediator to Facilitate Sulfur Redox Kinetics in Lithium-Sulfur Batteries with Encapsulating Lithium Polysulfide Electrolyte, Angew. Chem., Int. Ed., 2023, 62, e202303363 CrossRef CAS.
- R. Cheng, J. Liu, P. Manasa, M. Zhou, Y. Guan, K. Zhang, X. Lin, F. Rosei, A. A. Pimerzin, H. J. Seifert, F. Xu, L. Sun, D. Cai, J. Zeng, Z. Cao and H. G. Pan, Mechanism and thermal effects of phytic acid-assisted porous carbon sheets for high-performance lithium-sulfur batteries, Inorg. Chem. Front., 2023, 10, 7038–7053 RSC.
- P. Lin, B. Gao, X. Lan, M. Wang, J. Li and H. Fu, Advanced Separator Materials for Enhanced Electrochemical Performance of Lithium-Sulfur Batteries: Progress and Prospects, Langmuir, 2024, 40, 15996–16029 CrossRef CAS.
- M. Zhao, H. J. Peng, B.-Q. Li and J. Q. Huang, Kinetic Promoters for Sulfur Cathodes in Lithium-Sulfur Batteries, Acc. Chem. Res., 2024, 57, 545–557 CAS.
- Z. Zhao, Z. Yi, H. Li, R. Pathak, Z. Yang, X. Wang and Q. Qiao, Synergetic effect of spatially separated dual co-catalyst for accelerating multiple conversion reaction in advanced lithium sulfur batteries, Nano Energy, 2020, 81, 105621 CrossRef.
- Z. Zhou, H. Wang, L. Yang, C. Ma, J. Wang, W. Qiao and L. Ling, A review of the use of metal oxide/carbon composite materials to inhibit the shuttle effect in lithium-sulfur batteries, New Carbon Mater., 2024, 39, 201–220 CrossRef CAS.
- J. Fan, Z. Wang, J. Wang, Y. Tian and C. Wang, A Two-in-one host for High-loading cathode and Dendrite-free anode realized by activating metallic nitrides heterostructures toward Li-S full batteries, Chem. Eng. J., 2023, 460, 141862 CrossRef CAS.
- X. Y. Li, S. Feng, M. Zhao, C. X. Zhao, X. Chen, B. Q. Li, J. Q. Huang and Q. Zhang, Surface Gelation on Disulfide Electrocatalysts in Lithium-Sulfur Batteries, Angew. Chem., Int. Ed., 2022, 61, e202114671 CrossRef CAS.
- Q. Li, H. Liu, B. Jin, L. Li, Q. Sheng, M. Cui, Y. Li, X. Lang, Y. Zhu, L. Zhao and Q. Jiang, Anchoring polysulfides via a CoS2/NC@1T MoS2 modified separator for high-performance lithium-sulfur batteries, Inorg. Chem. Front., 2023, 10, 959–971 RSC.
- J. Zhang, D. Yang, C. Li, Q. Gong, W. Bi, X. Zheng, J. Arbiol, S. Li and A. Cabot, Two-Dimensional Transition Metal Phosphides As Cathode Additive in Robust Lithium-Sulfur Batteries, Nano Lett., 2024, 24, 7992–7998 CrossRef CAS PubMed.
- Y. Li, Y. Hao, U. Ali, B. Liu, Q. Zhang, Z. Jin, L. Li, C. Wang and L. Zhang, Ultrafine Ni12P5 nanoparticle-embedded carbon with abundant catalytic activity sites as separator modifiers in high-performance lithium-sulfur batteries, Inorg. Chem. Front., 2023, 10, 5719–5725 RSC.
- S. Yu, W. Cai, L. Chen, L. Song and Y. Song, Recent advances of metal phosphides for Li-S chemistry, J. Energy Chem., 2021, 55, 533–548 CrossRef CAS.
- J. Zhou, X. Liu, L. Zhu, J. Zhou, Y. Guan, L. Chen, S. Niu, J. Cai, D. Sun and Y. Zhu, Deciphering the Modulation Essence of p Bands in Co-Based Compounds on Li-S Chemistry, Joule, 2018, 2, 2681–2693 CrossRef CAS.
- S. Y. S. Jaberi, A. Ghaffarinejad, Z. Khajehsaeidi and A. Sadeghi, The synthesis, properties, and potential applications of CoS2 as a transition metal dichalcogenide (TMD), Int. J. Hydrogen Energy, 2023, 48, 15831–15878 CrossRef.
- Y. Z. Wang, M. Yang, Y. M. Ding, N. W. Li and L. Yu, Recent Advances in Complex Hollow Electrocatalysts for Water Splitting, Adv. Funct. Mater., 2021, 32, 2108681 CrossRef.
- S. Jiao, X. Fu and H. Huang, Descriptors for the Evaluation of Electrocatalytic Reactions: d-Band Theory and Beyond, Adv. Funct. Mater., 2021, 32, 2107651 CrossRef.
- H. Liu, X. Wang, Q. Wang, C. Pei, H. Wang and S. Guo, Dual-functional cobalt phosphide nanoparticles for performance enhancement of lithium-sulfur battery, J. Nanostruct. Chem., 2024, 14, 281–292 CrossRef CAS.
- H. Zhang, S. Xin, J. Li, H. Cui, Y. Liu, Y. Yang and M. Wang, Synergistic regulation of polysulfides immobilization and conversion by MOF-derived CoP-HNC nanocages for high-performance lithium-sulfur batteries, Nano Energy, 2021, 85, 106011 CrossRef CAS.
- X. Wang, G. Zhang, Q. Wang, Y. Li and S. Guo, Regulating the phase and catalytic activity of cobalt phosphide based on the morphological engineering of ZIF-67 to enhance the redox kinetics of polysulfides for high-performance lithium-sulfur batteries, J. Alloys Compd., 2023, 967, 171730 CrossRef CAS.
- D. Duan, K. Chen, C. Xing, X. Wang, X. Zhou and S. Liu, Enhancing the polysulfide redox conversion by a heterogeneous CoP-Co3S4 electrocatalyst for Li-S batteries, J. Alloys Compd., 2023, 961, 171099 CrossRef CAS.
- X. Zhao, T. Gao, W. Ren, C. Zhao, Z.-H. Liu and L. Li, Highly active CoP-Co2N confined in nanocarbon enabling efficient electrocatalytic immobilizing-conversion of polysulfide targeting high-rate lithium-sulfur batteries, J. Energy Chem., 2022, 75, 250–259 CrossRef CAS.
- B. Li, Q. Su, L. Yu, J. Zhang, G. Du, D. Wang, D. Han, M. Zhang, S. Ding and B. Xu, Tuning the Band Structure of MoS2 via Co9S8@MoS2 Core-Shell Structure to Boost Catalytic Activity for Lithium-Sulfur Batteries, ACS Nano, 2020, 14, 17285–17294 CrossRef CAS PubMed.
- K. Tan, Y. Liu, Z. Tan, J. Zhang, L. Hou and C. Yuan, High-yield and in situ fabrication of high-content nitrogen-doped graphene nanoribbons@Co/CoOOH as an integrated sulfur host towards Li-S batteries, J. Mater. Chem. A, 2020, 8, 3048–3059 RSC.
- W. Wang, X. Wang, J. Shan, L. Yue, W. Wang and Y. Li, Conductive few-layered 1T-MoSe2/MXene as a highly-efficient catalyst for accelerating bidirectional sulfur redox kinetics in Li-S batteries, J. Alloys Compd., 2023, 936, 168250 CrossRef CAS.
- L. Yang, R. Liu and L. Jiao, Electronic Redistribution: Construction and Modulation of Interface Engineering on CoP for Enhancing Overall Water Splitting, Adv. Funct. Mater., 2020, 30, 1909618 CrossRef CAS.
- C. I. Hiley, H. Y. Playford, J. M. Fisher, N. C. Felix, D. Thompsett, R. J. Kashtiban and R. I. Walton, Pair Distribution Function Analysis of Structural Disorder by Nb5+ Inclusion in Ceria: Evidence for Enhanced Oxygen Storage Capacity from Under-Coordinated Oxide, J. Am. Chem. Soc., 2018, 140, 1588–1591 CrossRef CAS PubMed.
- L. Zhang, Y. Lei, W. Xu, D. Wang, Y. Zhao, W. Chen, X. Xiang, X. Pang, B. Zhang and H. Shang, Highly active and durable nitrogen-doped CoP/CeO2 nanowire heterostructures for overall water splitting, Chem. Eng. J., 2023, 460, 141119 CrossRef CAS.
- J. Li, Y. Kang, Z. Lei and P. Liu, Well-controlled 3D flower-like CoP3/CeO2/C heterostructures as bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries, Appl. Catal., B, 2023, 321, 122029 CrossRef CAS.
- H. Xing, G. Long, J. Zheng, H. Zhao, Y. Zong, X. Li, Y. Wang, X. Zhu, M. Zhang and X. Zheng, Interface engineering boosts electrochemical performance by fabricating CeO2@CoP Schottky conjunction for hybrid supercapacitors, Electrochim. Acta, 2020, 337, 135817 CrossRef CAS.
- X. Song, T. Zhang, Y. Zhao, J. Ni, Y. Pan, Z. Tan and X. Wang, Heterostructure Interface Engineering in CoP/FeP/CeOx with a Tailored d-Band Center for Promising Overall Water Splitting Electrocatalysis, Inorg. Chem., 2023, 62, 8347–8356 CrossRef CAS.
- Y. Li, X. Zhang, X. Liang, H. H. Tan, Y. Yu, Y. Wu, J. Yan, J. Liu, Q. Zhang and J. Cui, Layer-by-Layer Assembly of CeO2@C-rGO Nanocomposites and CNTs as a Multifunctional Separator Coating for Highly Stable Lithium-Sulfur Batteries, ACS Appl. Mater. Interfaces, 2022, 14, 18634–18645 CrossRef CAS PubMed.
- Y. Li, P. Xu, G. Chen, J. Mou, S. Xue, K. Li, F. Zheng, Q. Dong, J. Hu, C. Yang and M. Liu, Enhancing Li-S redox kinetics by fabrication of a three dimensional Co/CoP@nitrogen-doped carbon electrocatalyst, Chem. Eng. J., 2020, 380, 122595 CrossRef CAS.
- C. Huang, Z. Q. Wang and X. Q. Gong, Activity and selectivity of propane oxidative dehydrogenation over VO3/CeO2(111) catalysts: A density functional theory study, Chin. J. Catal., 2018, 39, 1520–1526 CrossRef CAS.
- X. Wang, Y. Xie, W. Zhou, X. Wang, Z. Cai, Z. Xing, M. Li and K. Pan, The self-supported Zn-doped CoNiP microsphere/thorn hierarchical structures as efficient bifunctional catalysts for water splitting, Electrochim. Acta, 2020, 339, 135933 CrossRef CAS.
- M. A. Al-Tahan, Y. Dong, A. E. Shrshr, X. Liu, R. Zhang, H. Guan, X. Kang, R. Wei and J. Zhang, Enormous-sulfur-content cathode and excellent electrochemical performance of Li-S battery accouched by surface engineering of Ni-doped WS2@rGO nanohybrid as a modified separator, J. Colloid Interface Sci., 2022, 609, 235–248 CrossRef CAS.
- Z. Wu, S. Chen, L. Wang, Q. Deng, Z. Zeng, J. Wang and S. Deng, Implanting nickel and cobalt phosphide into well-defined carbon nanocages: A synergistic adsorption-electrocatalysis separator mediator for durable high-power Li-S batteries, Energy Storage Mater., 2021, 38, 381–388 CrossRef.
- P. Zhang, Y. Liu, T. Liang, E. H. Ang and Z. Dai, Nitrogen-Doped Carbon Wrapped Co-Mo2C Dual Mott-Schottky Nanosheets with Large Porosity for Efficient Water Electrolysis, Appl. Catal., B, 2020, 284, 119738 CrossRef.
- J. Li, C. Shu, A. J. Hu, Z. Ran, M. Li, R. Zheng and J. Long, Tuning Oxygen Non-stoichiometric Surface via Defect Engineering to Promote the Catalysis Activity of Co3O4 in Li-O2 Batteries, Chem. Eng. J., 2020, 381, 122678–122688 CrossRef CAS.
- X. Tao, Z. Yang, M. Cheng, R. Yan, F. Chen, S. Cao, S. Li, T. Ma, C. Cheng and W. Yang, Phosphorus modulated porous CeO2 nanocrystallines for accelerated polysulfide catalysis in advanced Li-S batteries, J. Mater. Sci. Technol., 2022, 131, 212–220 CrossRef CAS.
- H. Yu, X. Zhou, P. Zeng, C. Guo, H. Liu, X. Guo, B. Chang, M. Chen, J. Su and X. Wang, 3D Cellulose Graphene Aerogel with Self-Redox CeO2 as Li2S Host for High-Performance Li-S Battery, Energy Technol., 2022, 10, 2200616 CrossRef CAS.
- J. Wang, X. Yue, X. Cao, Z. Liu, A. M. Patil, J. Wang, X. Hao, A. Abudula and G. Guan, Metal organic frameworks derived CoS2/NiS2 heterostructure toward high-performance sodium storage anode materials, Chem. Eng. J., 2022, 431, 134091 CrossRef CAS.
- Y. Zuo, Y. Zhu, R. Wan, W. Su, Y. Fan, R. Liu, Y. Tang and Y. Chen, The Electrocatalyst based on LiVPO4F/CNT to enhance the electrochemical kinetics for high performance Li-S batteries, Chem. Eng. J., 2021, 415, 129053 CrossRef CAS.
- H. Wang, N. Li, J. Sun and P. Wang, Nitrogen-Doped CoP with optimized d-Band center as bidirectional electrocatalyst for high areal capacity of Li-S battery, J. Colloid Interface Sci., 2024, 665, 702–710 CrossRef CAS PubMed.
- L. Su, J. Zhang, Y. Chen, W. Yang, J. Wang, Z. Ma, G. Shao and G. Wang, Cobalt-embedded hierarchically-porous hollow carbon microspheres as multifunctional confined reactors for high-loading Li-S batteries, Nano Energy, 2021, 85, 105981 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi01349b |
| This journal is © the Partner Organisations 2024 |