A chelating coordination modulation method for the synthesis of Ti-MOF single crystals†
Hui-Zi
Li
ab,
Shangda
Li
 a,
Fei
Wang
a,
Fei
Wang
 *a and
Jian
Zhang
*a and
Jian
Zhang
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: wangfei04@fjirsm.ac.cn; zhj@fjirsm.ac.cn.
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 17th April 2024
Abstract
Even though titanium-based metal–organic frameworks (Ti-MOFs) are promising as efficient photocatalysts, the high reactivity of titanium ions makes the synthesis and structure determination of new Ti-MOFs quite challenging. In this study, we propose a chelating coordination modulation (CCM) method for the synthesis of Ti-MOF single crystals by using molecules with chelating coordination groups as the modulator. Thanks to this method, three Ti-MOFs (FIR-117–119) have been obtained and their structures were determined by single crystal X-ray diffraction (SCXRD), validating the universality of this approach. By capturing the intermediate and determining its single crystal structure, the role of the modulator in the growth of Ti-MOF single crystals is clarified: the use of a chelating coordination molecule as the modulator and a competitive ligand slows down the reaction rate by forming Ti–modulator key intermediates, which balance the formation of Ti-MOFs and growth of single crystals. Furthermore, FIR-119 exhibits excellent photocatalytic performance under visible light due to its good light absorption ability with a narrow bandgap. These results highlight the potential of the chelating coordination modulation method in the synthesis of new photoactive Ti-MOFs and their single crystals.
Introduction
Metal–organic frameworks (MOFs) have been extensively researched for gas separation and storage owing to their unique pore structure and high surface areas.1–7 Recently, they have emerged as promising photocatalysts that combine the merits of molecular catalysts and heterogeneous systems, showing potential as alternatives to traditional photocatalysts like TiO2.8 Among MOFs, titanium-based metal–organic frameworks (Ti-MOFs) have garnered special attention due to their excellent photocatalytic activity.9–21 Nevertheless, the scarcity of Ti-MOFs has limited the research in this area, with the focus mainly on two Ti-MOFs (MIL-125 and MIL-125-NH2) reported over ten years ago.22–24 Therefore, there is an urgent need to synthesize new Ti-MOFs.The main challenge in synthesizing new Ti-MOFs lies in the high activity and uncontrollable reactivity of Ti4+ ions,25 which lead to the formation of various byproducts, including TiO2, titanium-oxo clusters (TOCs),26,27 and even unidentified species. The mixing of different species in most cases presents significant difficulties in structure determination and performance study. Regarding the structure determination of compounds, powder XRD combined with theoretical calculations can be employed for pure-phase Ti-MOFs,14,16,28–32 whereas it becomes ineffective for mixed phases containing different species. Another method, namely the electron diffraction method using a cryo-electron microscope can also be used to determine the structure of some micro- and nano-sized microcrystalline Ti-MOFs.33,34 However, it should be noted that most MOFs are unstable under electron beam irradiation, making the application of this method very demanding for materials.35 Additionally, this instrument is expensive and not widely available. In comparison, single crystal XRD analysis remains the most popular and universal method used to determine the structure of substances at present. It is well-known that synthesizing single crystals is an important means to separate and purify different substances. Therefore, the synthesis of Ti-MOF single crystals not only allows for easy determination of the structure via single crystal XRD, but also realizes the separation and purification of Ti-MOFs, which is of great significance in research and practical applications. However, the uncontrollable reaction kinetics leads to a mismatch between the formation of Ti-MOFs and the growth of single crystals, making the synthesis of Ti-MOFs suitable for single crystal XRD measurement more challenging.
In 2014, Zhang and coworkers36 synthesized Ti-MOF (NTU-9) single crystals based on the 2,5-dihydroxyterephthalic acid (H4DOBDC) ligand by using acetic acid as the solvent. While Serre's group synthesized the intergrown crystals of MIL-167 from the same reactants in a diethylformamide (DEF)/methanol mixed solvent.37 Subsequently, Martí-Gastaldo constructed a Ti-MOF (MUV-11) in a dimethylformamide(DMF)/acetic acid mixed solvent using a chelating ligand, 1,4-phenylhydroxamic acid (p-H2bdh).38 These results suggest that monocarboxylic acids like acetic acid may play the role of a modulator in the formation of Ti-MOF single crystals, similar to that in the coordination modulation method of zirconium-based MOFs (Zr-MOFs).39 In 2015, Zhou and coworkers40 further developed this method for the synthesis of Ti-MOFs. The reaction of the hexameric titanium-oxo cluster precursor (Ti6) and the porphyrin carboxylic acid (TCPP) ligand with benzoic acid as a modulator gave single crystals of PCN-22. PCN-22 contained a new Ti7 cluster which was supposed to be rearranged from the original Ti6 cluster. Subsequently, using similar methods, different research groups successively synthesized single crystals of several Ti-MOFs, such as Ti-TBP,41 Ti3-BPDC,42 DGIST-1,43 and FIR-125.17 However, the successful examples based on this method are extremely limited, and the influence of the modulator on single crystal formation is poorly understood. In addition, compared with the original titanium-oxo cluster precursor, the Ti-based secondary building units (SBUs) in these Ti-MOFs changed without exception.44 This change highlights the uncontrollability of this Ti-MOF synthesis method and emphasizes the importance of developing new alternative synthesis strategies.
In this work, we propose a chelating coordination modulation (CCM) method for the synthesis of Ti-MOF single crystals. Inspired by the syntheses of NTU-9 and MIL-167, we chose the self-assembly of Ti and 2,5-dihydroxyterephthalic acid (H4DOBDC) as the model system and employed various molecules with chelating coordination groups as modulators. Thanks to this method, we successfully obtained three Ti-MOFs, namely FIR-117, FIR-118, and FIR-119 (FIR = Fujian Institute Research), and their structures were determined using single crystal X-ray diffraction (SCXRD). By capturing the intermediate and determining its single crystal structure, we clarify the role of the modulator in the growth of Ti-MOF single crystals. Initially, the four coordinated alcohols on Ti(iOPr)4 are replaced by three modulators in the form of chelation coordination, transforming the unstable tetrahedral configuration of the Ti center into a stable octahedral configuration. Subsequently, the coordinated modulator is replaced by bridging ligands to form a framework. The core aspect of this method lies in using chelating coordination molecules as the modulator and a competitive ligand, which slows down the reaction rate by forming Ti–modulator intermediates, thereby achieving a balance between the formation of Ti-MOFs and growth of single crystals. Furthermore, FIR-119 exhibits excellent photocatalytic performance under visible light due to its good light absorption ability with a narrow bandgap.
Results and discussion
NTU-9 is a typical mononuclear Ti-MOF with a 2D honeycomb-type (hcb) layer.36 The single crystal structural analysis of NTU-9 reveals that a chelating ligand (L) such as DODBC tends to chelate with Ti ions to form a mononuclear TiL3 subunit (SBU). In comparison with Ti-MOFs based on Ti-oxo cluster SBUs, its simple coordination mode provides certain advantages in the rational design and prediction of the structure of Ti-MOFs. In addition, this coordination mode also inhibits the excessive hydrolysis of Ti4+ ions, which could otherwise form complex titanium oxide clusters and other by-products. But things are not that simple. Serre and coworkers synthesized a 3D Ti-MOF (MIL-167) with a similar coordination mode and linkage, using the same reactants but only changing the solvent to diethylformamide (DEF) instead of acetic acid.37 The as-synthesized MIL-167 formed intergrown crystallites with a large crystal size, which prevented its structure determination by SCXRD. As a result, its structure was determined using the PXRD method.Based on the above analysis, we chose Ti(OiPr)4 as the Ti source and H4DOBDC as the ligand to explore the feasibility of synthesizing new Ti-MOF single crystals. The initial attempt using DEF and acetic acid as a mixed solvent resulted in the formation of intergrown crystals of MIL-167. Therefore, it is speculated that the reaction speed is too fast, which is not conducive to the growth of single crystals. Considering the strong chelating coordination ability of the DOBDC ligand, we further introduced molecules with chelating coordination groups as modulators and competitive ligands. The results showed that the addition of the modulator effectively improved the crystallinity of the Ti-MOF.
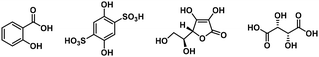
High-quality Ti-MOF single crystals of FIR-117 and FIR-118 were synthesized using salicylic acid and squaric acid as modulators, respectively (details in the Synthesis section). SCXRD analyses reveal that both FIR-117 and FIR-118 crystallize in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group but have different unit cell parameters (Table S1†). In both compounds, the Ti center is octahedrally coordinated by three different DOBDC ligands in the chelating mode (Fig. S1a†). Taking FIR-117 as an example, the bond length between Ti and the phenolic hydroxyl group O (denoted as OAr) is approximately 1.87 Å while the bond length between Ti and the carboxylate group O (denoted as OCO2) is ∼2.01 Å, both of which are close to those of NTU-9 and other reported TOCs.45
space group but have different unit cell parameters (Table S1†). In both compounds, the Ti center is octahedrally coordinated by three different DOBDC ligands in the chelating mode (Fig. S1a†). Taking FIR-117 as an example, the bond length between Ti and the phenolic hydroxyl group O (denoted as OAr) is approximately 1.87 Å while the bond length between Ti and the carboxylate group O (denoted as OCO2) is ∼2.01 Å, both of which are close to those of NTU-9 and other reported TOCs.45
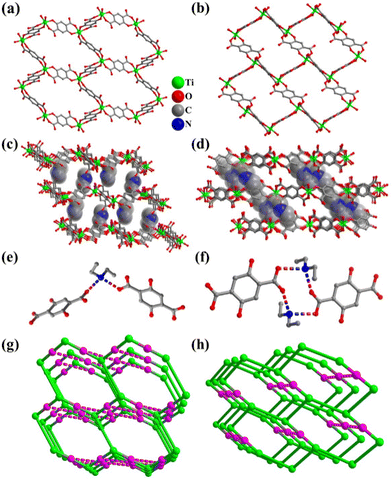
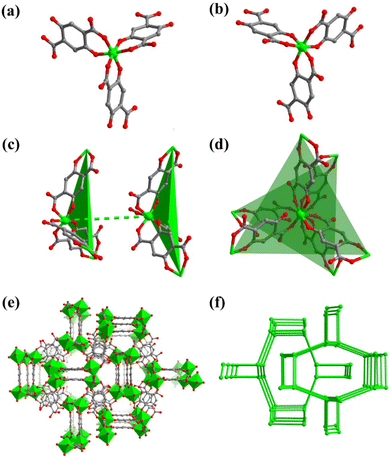
All the DOBDC ligands act as bridging ligands, while two types of ligands link Ti atoms to form an S-shaped 1D chain, which is then connected by the third ligand to form a wave-like 2D layer with a twisted hcb topology (Fig. 1a) in FIR-117. In comparison, in FIR-118, two kinds of DOBDC ligands link Ti atoms to form a zigzag 1D chain, which is connected by the third one to form a coplanar 2D layer with a twisted hcb topology (Fig. 1b).
These wave-like layers in FIR-117 stack in an AA manner along the a-axis. [NH2(C2H5)2]+ cations from the decomposition of DEF act as counterions and are located between these layers (Fig. 1c). One [NH2(C2H5)2]+ cation links two DOBDC ligands of two adjacent layers through strong hydrogen bond interactions (N⋯O distances are 2.720 and 2.849 Å) (Fig. 1e and S1b†). Therefore, adjacent layers are connected by [NH2(C2H5)2]+ cations to form a 3D hydrogen-bonded framework. As for FIR-118, the coplanar layers are also stacked in an AA manner along the a-axis, and the space between these layers is filled by ([NH2(C2H5)2]+) cations (Fig. 1d). In FIR-118, two [NH2(C2H5)2]+ cations link two DOBDC ligands of two adjacent layers through strong hydrogen bond interactions (N⋯O distances are 2.720 and 2.849 Å) (Fig. 1f and S1e†). The presence of multiple hydrogen bond interactions in FIR-118 is expected to endow it with good stability.
To better compare these two hydrogen-bonded frameworks, topological analysis was performed. In FIR-117, the DOBDC ligands with H-bond interactions act as 4-connected nodes, while others serve as linkers. Each Ti atom can be considered a 3-connected node (Fig. S1b and S1c†). The entire framework can be simplified as a 3,4-connected tfo topology (Fig. 1g). Similarly, the framework of FIR-118 can be simplified as a 3,4-connected tfc topology (Fig. 1h and S1e†). The topological results clearly showed that the coplanar layers vertically connect ligands (dashed pink line) through hydrogen bonds to form a 3D network of FIR-118, while the wave-like layer connected ligands by staggered hydrogen bonds to form a 3D network of FIR-117.
Encouraged by these results, we expanded our investigation to explore the feasibility of using other organic compounds containing chelating groups as modulators. In addition to the above-mentioned salicylic acid and squaric acid, we further examined other compounds containing O, O′ chelating groups, S, O chelating groups, and N, O chelating groups. The results showed that these alternative compounds can also be utilized as modulators to successfully synthesize Ti-MOF single crystals. The specific experimental results are summarized in Table 1.
In particular, by using thiosalicylic acid as a modulator, we successfully synthesized single crystals of FIR-119. SCXRD analysis reveals that FIR-119 crystallized in the cubic I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d space group (Table S1†) and is isostructural to MIL-167. However, its volume is ca. 13.5% smaller than that of MIL-167.
3d space group (Table S1†) and is isostructural to MIL-167. However, its volume is ca. 13.5% smaller than that of MIL-167.
In FIR-119, each Ti center is also octahedrally coordinated by three different DOBDC ligands in a chelating mode. The Ti-OAr and Ti-OCO2 bond lengths are 1.88 and 1.98 Å (Table S2†), falling within a reasonable range. Three DOBDC ligands coordinate with one Ti center to form a fan-type Ti(DOBDC)3 unit and two chiral Ti(DOBDC)3 units with Λ (Fig. 2a) and Δ (Fig. 2b) configurations coexisting in the same structure. A pair of Δ and Λ units are slightly staggered along the same direction (Fig. 2c and d). The Ti⋯Ti distance between adjacent units is 9.45 Å, which is larger than the Ti⋯Ti distances connected by DOBDC ligands (8.46 Å).
In FIR-119, all Λ-Ti(DOBDC)3 units link each other to form a 3D framework (Fig. 2e) with a chiral srs topology (Fig. 2f). Similarly, all Δ-Ti(DOBDC)3 units connect with each other to form the opposite chiral 3D framework. The interpenetration (2-fold) of opposite chiral frameworks results in the crystallization in the nonchiral space group. It is deduced that the disordered [NH2(C2H5)2]+ cations decomposed from DEF serve as counterions to balance the charge. This deduction is further supported by 1H NMR and 13C NMR results (Fig. S2 and S3†).
From the above discussion, it can be seen that the successful syntheses of FIR-117–119 single crystals benefit from the addition of compounds with chelating coordination groups. Previous reports have indicated that introducing proline as a modulator helps to improve the quality of Zr-MOF single crystals.46 In a series of comparative experiments, it was observed that both the carboxyl group and the N-heteroatom play important roles in the synthesis of Zr-MOF single crystals, but the specific roles are not yet clear.
Therefore, in order to clarify the roles of the modulators, we conducted comparative experiments. Previous experiments demonstrated that FIR-117 was obtained through a one-pot reaction of Ti(OiPr)4, salicylic acid and H4DOBDC. Interestingly, when the reaction time was shortened or the temperature was lowered, a Ti(salicylate)3 complex was obtained, suggesting that the Ti(salicylate)3 complex may serve as an intermediate in the formation of FIR-117. Additionally, the reaction of Ti(OiPr)4 with salicylic acid in the absence of H4DOBDC also resulted in the formation of the Ti(salicylate)3 complex. Furthermore, using the Ti(salicylate)3 complex as the Ti source and reacting it with the DOBDC ligand, FIR-117 single crystals could also be obtained. Therefore, we deduced that the Ti(salicylate)3 complex was the intermediate involved in the synthesis of FIR-117.
Based on these results, we propose a possible mechanism for the chelating coordination modulation (CCM) method to synthesize Ti-MOF single crystals (Fig. 3). First, in the presence of a large amount of chelating coordination modulators, tetrahedrally coordinated Ti(OiPr)4 with high reactivity reacts rapidly with modulators to form a more stable Ti(modulator)3 intermediate. The formation of these Ti(modulator)3 intermediates helps to inhibit the excessive hydrolysis of Ti4+ ions. Subsequently, DOBDC ligands with a similar coordination ability gradually replace the modulators on Ti in a controllable manner to form the final framework. The controllability is mainly reflected in the relatively reversible substitution reaction between the DOBDC ligand and the modulator, which slows down the formation of the framework and matches the growth speed of single crystals, thus realizing the synthesis of high-quality Ti-MOF single crystals.
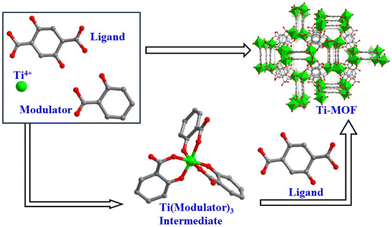 | ||
| Fig. 3 Possible mechanism of the chelating coordination modulation (CCM) method to synthesize Ti-MOF single crystals. | ||
The outcomes of our study illustrate that the chelating coordination modulation (CCM) method proves to be a highly effective approach for synthesizing single crystals of Ti-MOFs. The key aspect of the CCM method involves the use of molecules with chelating coordination groups as modulators, which play a crucial role in controlling the crystal growth process. Through the application of this method, we successfully synthesized high-quality Ti-MOF single crystals (FIR-117–119) and determined their structures using single crystal X-ray diffraction (SCXRD). The significance of the modulators in the growth of Ti-MOF single crystals was elucidated by capturing the intermediate species and determining their single crystal structures. Specifically, the modulators form stable mononuclear structures through chelating coordination with titanium ions, effectively inhibiting unwanted side reactions. This controllability ensures the successful synthesis of Ti-MOFs with desired structures and large-size single crystal growth. Our findings demonstrated the broad applicability of the CCM approach in synthesizing Ti-MOF single crystals with diverse structures and properties. This versatility opens up new possibilities for tailoring the properties of Ti-MOFs to suit specific applications. With the successful synthesis of Ti-MOF single crystals using various modulators, we anticipate that the CCM method holds promise for the rational design and synthesis of other metal–organic frameworks.
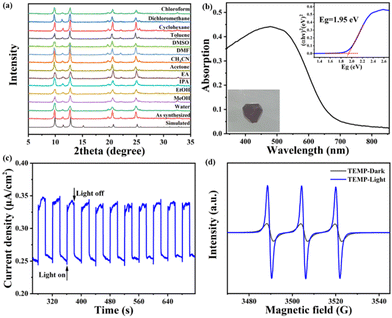
The powder XRD (PXRD) pattern of the as-synthesized crystal samples shows that most of the main peaks are well consistent with the simulated patterns derived from SCXRD data, which confirmed the phase purity of FIR-117, FIR-118 and FIR-119 (Fig. S5, S6† and 4a). The thermogravimetric analysis results of FIR-117 and FIR-118 showed structural stability below 200 °C (Fig. S7 and S8†). Meanwhile, thermogravimetric analysis showed that the framework of FIR-119 can be stable at approximately 300 °C (Fig. S9†). Additionally, the solvent stability of FIR-119 was also evaluated. Powder XRD measurements demonstrated that FIR-119 remained stable after soaking in common solvents such as water, various alcohols, ethyl acetate (EA), acetonitrile (CH3CN), amides, toluene, dichloromethane and chloroform at room temperature for 24 hours (Fig. 4a). Compared to that of the DOBDC ligand, the diffuse reflectance UV-Vis spectrum of FIR-117, 118 and 119 showed a broad absorption band in the visible light range. The calculated bandgaps of DOBDC and FIR-117–119 from the Tauc plot are 2.67, 1.90, 1.86 and 1.95 eV (Fig. 4b, S10, S11 and S12†). FIR-117–119 show a lower bandgap mainly due to ligand-to-metal charge transfer (LMCT) from the DOBDC ligand to high-valence titanium ions. The photocurrent profile of FIR-119 indicates that it is photoactive under visible light illumination (λ > 420 nm, Fig. 4c). The EDS spectrum and XPS spectrum of FIR-119 (Fig. S13 and S14a†) further confirm the presence of the elements titanium (Ti), nitrogen (N), oxygen (O), and carbon (C). The XPS Ti 2p region of FIR-119 shows two peaks with binding energies of 458.91 eV (Ti 2p3/2) and 464.71 eV (Ti 2p1/2), respectively, confirming that the valence state of Ti is +4 (Fig. S14b†). The electron paramagnetic resonance (EPR) test results showed that FIR-119 can produce 1O2 under visible light irradiation with 4-oxo-2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) as the trapping agent (Fig. 4d). Therefore, FIR-119 should be a very promising photocatalyst, especially in the area of aerobic catalytic reactions.
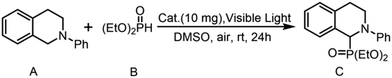
Due to its excellent stability, effective visible light absorption capability, and notable photoactivity, this mononuclear Ti-MOF is anticipated to emerge as a novel type of visible light catalyst. The cross-dehydrogenation coupling (CDC) reaction, facilitated by visible light, represents a strategy for directly activating C–H bonds and establishing C–C bonds from uncomplicated compounds.47 This reaction harnesses renewable energy, obviating the need for pre-functionalization of reactants, thereby synergistically promoting a green synthesis pathway. Consequently, we selected a representative aerobic CDC reaction involving N-phenyltetrahydroisoquinolines (A) and diethyl phosphite (B) to investigate the photocatalytic efficacy of FIR-119 (Table 2). When FIR-119 serves as a catalyst under visible light irradiation, the reaction yields 41% of product C (entry 3, Table 2 and Fig. S15†). The experimental findings further confirm FIR-119's commendable visible light and catalytic activity in CDC reactions. After 24 hours of CDC reaction, PXRD results (Fig. S16†) validate FIR-119's robust stability. As shown in Table 2, control experiments underscore the indispensability of both light and MOF photocatalysts in this typical CDC reaction. Under visible light conditions, devoid of a catalyst, product formation is nearly imperceptible. Likewise, in the absence of the FIR-119 catalyst, no product formation is observed under dark conditions (entry 2, Table 2).
| Entry | Catalyst | Light source | Yield |
|---|---|---|---|
| a Reaction conditions: substrate A (0.2 mmol), diethyl phosphite B (0.24 mmol), and catalyst FIR-119 (10 mg) in 2 mL DMSO exposed to air under different conditions (light source and catalyst) at room temperature for 24 h. | |||
| 1 | None | Visible light (λ > 420 nm) | Trace |
| 2 | FIR-119 | Without light | Trace |
| 3 | FIR-119 | Visible light (λ > 420 nm) | 41% |
Conclusions
In conclusion, we have developed the chelating coordination modulation (CCM) method, employing molecules with chelating coordination groups as modulators, for the synthesis of Ti-MOFs. Our study showcases the versatility and effectiveness of the chelating coordination modulation method as a controlled synthesis tool for obtaining high-quality Ti-MOF single crystals with tailored properties. The insights gained from this research can pave the way for the development of novel functional materials and advanced catalysts for various applications, including in the field of visible light photocatalysis and beyond.Author contributions
Hui-Zi Li: synthesis, characterization, data curation, formal analysis, investigation, visualization, methodology and writing – original draft. Shangda Li: data curation and investigation. Fei Wang: conceptualization, data curation, formal analysis, investigation, writing – original draft, writing – review and editing, and funding acquisition. Jian Zhang: conceptualization, methodology, supervision, project administration and funding acquisition.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21935010) and the Natural Science Foundation of Fujian Province (2022J01505).References
- Y. Su, K. I. Otake, J. J. Zheng, S. Horike, S. Kitagawa and C. Gu, Separating water isotopologues using diffusion-regulatory porous materials, Nature, 2022, 611, 289–294 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, Y. Su, X. Liu, P. Zhang, R.-B. Lin, S. Chen, Q. Deng, Z. Zeng, S. Deng and B. Chen, Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation, Nat. Commun., 2022, 13, 200 CrossRef CAS PubMed.
- H. Lin, Y. Yang, Y.-C. Hsu, J. Zhang, C. Welton, I. Afolabi, M. Loo and H.-C. Zhou, Metal-Organic Frameworks for Water Harvesting and Concurrent Carbon Capture: A Review for Hygroscopic Materials, Adv. Mater., 2023, 36, 2209073 CrossRef PubMed.
- Q. Yan, J. Wang, L. Zhang, J. Liu, M. Wahiduzzaman, N. Yan, L. Yu, R. Dupuis, H. Wang, G. Maurin, M. Hirscher, P. Guo, S. Wang and J. Du, A squarate-pillared titanium oxide quantum sieve towards practical hydrogen isotope separation, Nat. Commun., 2023, 14, 4189 CrossRef CAS PubMed.
- L. Yu, S. Ullah, K. Zhou, Q. Xia, H. Wang, S. Tu, J. Huang, H.-L. Xia, X.-Y. Liu, T. Thonhauser and J. Li, A Microporous Metal-Organic Framework Incorporating Both Primary and Secondary Building Units for Splitting Alkane Isomers, J. Am. Chem. Soc., 2022, 144, 3766–3770 CrossRef CAS PubMed.
- Y. Guo, K. Wang, Y. Hong, H. Wu and Q. Zhang, Recent progress on pristine two-dimensional metal–organic frameworks as active components in supercapacitors, Dalton Trans., 2021, 50, 11331–11346 RSC.
- C. Li, Y. Yuan, M. Yue, Q. Hu, X. Ren, B. Pan, C. Zhang, K. Wang and Q. Zhang, Recent Advances in Pristine Iron Triad Metal–Organic Framework Cathodes for Alkali Metal–Ion Batteries, Small, 2023, 2310373 Search PubMed.
- J. D. Xiao and H. L. Jiang, Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis, Acc. Chem. Res., 2019, 52, 356–366 CrossRef CAS PubMed.
- J. Zhu, P.-Z. Li, W. Guo, Y. Zhao and R. Zou, Titanium-based metal–organic frameworks for photocatalytic applications, Coord. Chem. Rev., 2018, 359, 80–101 CrossRef CAS.
- L. Li, X.-S. Wang, T.-F. Liu and J. Ye, Titanium-Based MOF Materials: From Crystal Engineering to Photocatalysis, Small Methods, 2020, 4, 2000486 CrossRef CAS.
- Y. Yan, C. Li, Y. Wu, J. Gao and Q. Zhang, From isolated Ti-oxo clusters to infinite Ti-oxo chains and sheets: Recent advances in photoactive Ti-based MOFs, J. Mater. Chem. A, 2020, 8, 15245–15270 RSC.
- C. Li, H. Xu, J. Gao, W. Du, L. Shangguan, X. Zhang, R.-B. Lin, H. Wu, W. Zhou, X. Liu, J. Yao and B. Chen, Tunable titanium metal–organic frameworks with infinite 1D Ti–O rods for efficient visible-light-driven photocatalytic H2 evolution, J. Mater. Chem. A, 2019, 7, 11928–11933 RSC.
- P. Salcedo-Abraira, A. A. Babaryk, E. Montero-Lanzuela, O. R. Contreras-Almengor, M. Cabrero-Antonino, E. S. Grape, T. Willhammar, S. Navalon, E. Elkaim, H. Garcia and P. Horcajada, A Novel Porous Ti-Squarate as Efficient Photocatalyst in the Overall Water Splitting Reaction under Simulated Sunlight Irradiation, Adv. Mater., 2021, 33, 2106627 CrossRef CAS PubMed.
- B. Bueken, F. Vermoortele, D. E. Vanpoucke, H. Reinsch, C. C. Tsou, P. Valvekens, T. De Baerdemaeker, R. Ameloot, C. E. Kirschhock, V. Van Speybroeck, J. M. Mayer and D. De Vos, A Flexible Photoactive Titanium Metal-Organic Framework Based on a [Ti(IV)3(mu3-O)(O)2(COO)6] Cluster, Angew. Chem., Int. Ed., 2015, 54, 13912–13917 CrossRef CAS PubMed.
- S. Wang, M. Cabrero-Antonino, S. Navalón, C.-C. Cao, A. Tissot, L. Dovgaliuk, J. Marrot, C. Martineau-Corcos, L. Yu, H. Wang, W. Shepard, H. García and C. Serre, A Robust Titanium Isophthalate Metal-Organic Framework for Visible Light Photocatalytic CO2 Methanation, Chem, 2020, 12, 3409–3427 Search PubMed.
- A. Cadiau, N. Kolobov, S. Srinivasan, M. G. Goesten, H. Haspel, A. V. Bavykina, M. R. Tchalala, P. Maity, A. Goryachev, A. S. Poryvaev, M. Eddaoudi, M. V. Fedin, O. F. Mohammed and J. Gascon, A Titanium Metal-Organic Framework with Visible-Light-Responsive Photocatalytic Activity, Angew. Chem., Int. Ed., 2020, 59, 13468–13472 CrossRef CAS PubMed.
- Y. Sun, D.-F. Lu, Y. Sun, M.-Y. Gao, N. Zheng, C. Gu, F. Wang and J. Zhang, Large Titanium-Oxo Clusters as Precursors to Synthesize the Single Crystals of Ti-MOFs, ACS Mater. Lett., 2021, 3, 64–68 CrossRef CAS.
- H. Z. Li, Y. Pan, Q. H. Li, Q. P. Lin, D. Y. Lin, F. Wang, G. Xu and J. Zhang, Rationally designed titanium-based metal-organic frameworks for visible-light activated chemiresistive sensing, J. Mater. Chem. A, 2023, 11, 965–971 RSC.
- D.-F. Lu, Y.-P. Han, Y. Sun, F. Wang and J. Zhang, Titanium Sulfonate-Based Metal–Organic Frameworks, Cryst. Growth Des., 2023, 23, 3778–3784 CrossRef CAS.
- D.-F. Lu, S. Li, M.-X. Guan, Y.-P. Han, Y. Sun, X. Wu, F. Wang and J. Zhang, Visible-Light-Active Titanium Sulfonate Framework for Photocatalytic Organic Synthesis, ACS Mater. Lett., 2023, 5, 2836–2842 CrossRef CAS.
- J. Castells-Gil, N. Almora-Barrios, B. Lerma-Berlanga, N. M. Padial and C. Martí-Gastaldo, Chemical complexity for targeted function in heterometallic titanium–organic frameworks, Chem. Sci., 2023, 14, 6826–6840 RSC.
- M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin, C. Sanchez and G. Ferey, A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate, J. Am. Chem. Soc., 2009, 131, 10857–10859 CrossRef CAS PubMed.
- C. Zlotea, D. Phanon, M. Mazaj, D. Heurtaux, V. Guillerm, C. Serre, P. Horcajada, T. Devic, E. Magnier, F. Cuevas, G. Ferey, P. L. Llewellyn and M. Latroche, Effect of NH2 and CF3 functionalization on the hydrogen sorption properties of MOFs, Dalton Trans., 2011, 40, 4879–4881 RSC.
- Y. Fu, D. Sun, Y. Chen, R. Huang, Z. Ding, X. Fu and Z. Li, An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction, Angew. Chem., Int. Ed., 2012, 51, 3364–3367 CrossRef CAS PubMed.
- H. Assi, G. Mouchaham, N. Steunou, T. Devic and C. Serre, Titanium coordination compounds: from discrete metal complexes to metal-organic frameworks, Chem. Soc. Rev., 2017, 46, 3431–3452 RSC.
- P. Coppens, Y. Chen and E. Trzop, Crystallography and properties of polyoxotitanate nanoclusters, Chem. Rev., 2014, 114, 9645–9661 CrossRef CAS PubMed.
- L. Zhang, X. Fan, X. Yi, X. Lin and J. Zhang, Coordination-Delayed-Hydrolysis Method for the Synthesis and Structural Modulation of Titanium-Oxo Clusters, Acc. Chem. Res., 2022, 55, 3150–3161 CrossRef CAS PubMed.
- H. L. Nguyen, F. Gandara, H. Furukawa, T. L. H. Doan, K. E. Cordova and O. M. Yaghi, A Titanium-Organic Framework as an Exemplar of Combining the Chemistry of Metal- and Covalent-Organic Frameworks, J. Am. Chem. Soc., 2016, 138, 4330–4333 CrossRef CAS PubMed.
- S. Wang, T. Kitao, N. Guillou, M. Wahiduzzaman, C. Martineau-Corcos, F. Nouar, A. Tissot, L. Binet, N. Ramsahye, S. Devautour-Vinot, S. Kitagawa, S. Seki, Y. Tsutsui, V. Briois, N. Steunou, G. Maurin, T. Uemura and C. Serre, A phase transformable ultrastable titanium-carboxylate framework for photoconduction, Nat. Commun., 2018, 9, 1660 CrossRef PubMed.
- C. Li, H. Xu, J. Gao, W. Du, L. Shangguan, X. Zhang, R.-B. Lin, H. Wu, W. Zhou, X. Liu, J. Yao and B. Chen, Tunable titanium metal–organic frameworks with infinite 1D Ti–O rods for efficient visible-light-driven photocatalytic H2 evolution, J. Mater. Chem. A, 2019, 7, 11928–11933 RSC.
- J. N. Chang, Q. Li, Y. Yan, J. W. Shi, J. Zhou, M. Lu, M. Zhang, H. M. Ding, Y. Chen, S. L. Li and Y. Q. Lan, Covalent-Bonding Oxidation Group and Titanium Cluster to Synthesize a Porous Crystalline Catalyst for Selective Photo-Oxidation Biomass Valorization, Angew. Chem., Int. Ed., 2022, 61, e202209289 CrossRef CAS PubMed.
- J. T. Bryant, M. W. Logan, Z. Chen, M. Djokic, D. R. Cairnie, D. A. Vazquez-Molina, A. Nijamudheen, K. R. Langlois, M. J. Markley, G. Pombar, A. A. Holland, J. D. Caranto, J. K. Harper, A. J. Morris, J. L. Mendoza-Cortes, T. Jurca, K. W. Chapman and F. J. Uribe-Romo, Synergistic Steric and Electronic Effects on the Photoredox Catalysis by a Multivariate Library of Titania Metal-Organic Frameworks, J. Am. Chem. Soc., 2023, 145, 4589–4600 CrossRef CAS PubMed.
- S. Smolders, T. Willhammar, A. Krajnc, K. Sentosun, M. T. Wharmby, K. A. Lomachenko, S. Bals, G. Mali, M. B. J. Roeffaers, D. E. De Vos and B. Bueken, A Titanium(IV)-based Metal-Organic Framework Featuring Defect-Rich Ti-O Sheets as an Oxidative Desulfurization Catalyst, Angew. Chem., Int. Ed., 2019, 58, 9160–9165 CrossRef CAS PubMed.
- S. Yuan, J. S. Qin, H. Q. Xu, J. Su, D. Rossi, Y. Chen, L. Zhang, C. Lollar, Q. Wang, H. L. Jiang, D. H. Son, H. Xu, Z. Huang, X. Zou and H. C. Zhou, [Ti8Zr2O12(COO)16] Cluster: An Ideal Inorganic Building Unit for Photoactive Metal-Organic Frameworks, ACS Cent. Sci., 2018, 4, 105–111 CrossRef CAS PubMed.
- Z. Huang, E. S. Grape, J. Li, A. K. Inge and X. Zou, 3D electron diffraction as an important technique for structure elucidation of metal-organic frameworks and covalent organic frameworks, Coord. Chem. Rev., 2021, 427, 213583 CrossRef CAS.
- J. Gao, J. Miao, P. Z. Li, W. Y. Teng, L. Yang, Y. Zhao, B. Liu and Q. Zhang, A p-type Ti(IV)-based metal-organic framework with visible-light photo-response, Chem. Commun., 2014, 50, 3786–3788 RSC.
- H. Assi, L. C. P. Pérez, G. Mouchaham, F. Ragon, M. Nasalevich, N. Guillou, C. Martineau, H. Chevreau, F. Kapteijn, J. Gascon, P. Fertey, E. Elkaim, C. Serre and T. Devic, Investigating the Case of Titanium(IV) Carboxyphenolate Photoactive Coordination Polymers, Inorg. Chem., 2016, 55, 7192–7199 CrossRef CAS PubMed.
- N. M. Padial, J. Castells-Gil, N. Almora-Barrios, M. Romero-Angel, I. da Silva, M. Barawi, A. Garcia-Sanchez, V. A. D. L. P. O’Shea and C. Marti-Gastaldo, Hydroxamate Titanium-Organic Frameworks and the Effect of Siderophore-Type Linkers over Their Photocatalytic Activity, J. Am. Chem. Soc., 2019, 141, 13124–13133 CrossRef CAS PubMed.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Modulated synthesis of Zr-based metal-organic frameworks: from nano to single crystals, Chem. – Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed.
- S. Yuan, T. F. Liu, D. Feng, J. Tian, K. Wang, J. Qin, Q. Zhang, Y. P. Chen, M. Bosch, L. Zou, S. J. Teat, S. J. Dalgarno and H. C. Zhou, A single crystalline porphyrinic titanium metal-organic framework, Chem. Sci., 2015, 6, 3926–3930 RSC.
- G. Lan, K. Ni, S. S. Veroneau, X. Feng, G. T. Nash, T. Luo, Z. Xu and W. Lin, Titanium-Based Nanoscale Metal-Organic Framework for Type I Photodynamic Therapy, J. Am. Chem. Soc., 2019, 141, 4204–4208 CrossRef CAS PubMed.
- X. Feng, Y. Song, J. S. Chen, Z. Li, E. Y. Chen, M. Kaufmann, C. Wang and W. Lin, Cobalt-bridged secondary building units in a titanium metal-organic framework catalyze cascade reduction of N-heteroarenes, Chem. Sci., 2019, 10, 2193–2198 RSC.
- Y. Keum, S. Park, Y. P. Chen and J. Park, Titanium-Carboxylate Metal-Organic Framework Based on an Unprecedented Ti-Oxo Chain Cluster, Angew. Chem., Int. Ed., 2018, 57, 14852–14856 CrossRef CAS PubMed.
- J. Castells-Gil, N. M. Padial, N. Almora-Barrios, I. da Silva, D. Mateo, J. Albero, H. García and C. Martí-Gastaldo, De novo synthesis of mesoporous photoactive titanium(iv)-organic frameworks with MIL-100 topology, Chem. Sci., 2019, 10, 4313–4321 RSC.
- M. Y. Gao, Z. R. Wang, Q. H. Li, D. J. Li, Y. Y. Sun, Y. H. Andaloussi, C. Ma, C. H. Deng, J. Zhang and L. Zhang, Black Titanium-Oxo Clusters with Ultralow Band Gaps and Enhanced Nonlinear Optical Performance, J. Am. Chem. Soc., 2022, 144, 8153–8161 CrossRef CAS PubMed.
- O. V. Gutov, S. Molina, E. C. Escudero-Adan and A. Shafir, Modulation by Amino Acids: Toward Superior Control in the Synthesis of Zirconium Metal-Organic Frameworks, Chem. – Eur. J., 2016, 22, 13582–13587 CrossRef CAS PubMed.
- A. K. Bagdi, M. Rahman, D. Bhattacherjee, G. V. Zyryanov, S. Ghosh, O. N. Chupakhin and A. Hajra, Visible light promoted cross-dehydrogenative coupling: a decade update, Green Chem., 2020, 22, 6632–6681 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2287493 (FIR-117), 2287494 (FIR-118) and 2287495 (FIR-119). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi00436a |
| This journal is © the Partner Organisations 2024 |