Open Access Article
Open Access ArticleBioinspired graphene-based metal oxide nanocomposites for photocatalytic and electrochemical performances: an updated review
Ajay K.
Potbhare
a,
S. K. Tarik
Aziz
b,
Mohd. Monis
Ayyub
 c,
Aniket
Kahate
a,
Rohit
Madankar
a,
Sneha
Wankar
c,
Aniket
Kahate
a,
Rohit
Madankar
a,
Sneha
Wankar
 d,
Arnab
Dutta
d,
Arnab
Dutta
 b,
Ahmed
Abdala
e,
Sami H.
Mohmood
b,
Ahmed
Abdala
e,
Sami H.
Mohmood
 *f,
Rameshwar
Adhikari
*g and
Ratiram G.
Chaudhary
*f,
Rameshwar
Adhikari
*g and
Ratiram G.
Chaudhary
 *a
*a
aPost Graduate Department of Chemistry, Seth Kesarimal Porwal College of Arts and Science and Commerce, Kamptee-441001, India. E-mail: chaudhary_rati@yahoo.com
bChemistry Department, Indian Institute of Technology, Bombay, Powai 400076, India
cNew Chemistry Unit, International Centre for Materials Science and Sheikh Saqr Laboratory, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India
dPost Graduate Teaching Department of Chemistry, Gondwana University, Gadchiroli 442605, India
eChemical Engineering Program, Texas A&M University at Qatar POB, 23784, Doha, Qatar
fDepartment of Physics, The University of Jordan, Amman 11942, Jordan. E-mail: s.mahmood@ju.edu.jo
gCentral Department of Chemistry and Research Centre for Applied Science and Technology (RECAST), Tribhuvan University, Kathmandu, Nepal. E-mail: nepalpolymer@yahoo.com
First published on 5th April 2024
Abstract
Considering the rapidly increasing population, the development of new resources, skills, and devices that can provide safe potable water and clean energy remains one of the vital research topics for the scientific community. Owing to this, scientific community discovered such material for tackle this issue of environment benign, the new materials with graphene functionalized derivatives show significant advantages for application in multifunctional catalysis and energy storage systems. Herein, we highlight the recent methods reported for the preparation of graphene-based materials by focusing on the following aspects: (i) transformation of graphite/graphite oxide into graphene/graphene oxide via exfoliation and reduction; (ii) bioinspired fabrication or modification of graphene with various metal oxides and its applications in photocatalysis and storage systems. The kinetics of photocatalysis and the effects of different parameters (such as photocatalyst dose and charge-carrier scavengers) for the optimization of the degradation efficiency of organic dyes, phenol compounds, antibiotics, and pharmaceutical drugs are discussed. Further, we present a brief introduction on different graphene-based metal oxides and a systematic survey of the recently published research literature on electrode materials for lithium-ion batteries (LIBs), supercapacitors, and fuel cells. Subsequently, the power density, stability, pseudocapacitance charge/discharge process, capacity and electrochemical reaction mechanisms of intercalation, and conversion- and alloying-type anode materials are summarized in detail. Furthermore, we thoroughly distinguish the intrinsic differences among underpotential deposition, intercalation, and conventional pseudocapacitance of electrode materials. This review offers a meaningful reference for the construction and fabrication of graphene-based metal oxides as effective photocatalysts for photodegradation study and high-performance optimization of anode materials for LIBs, supercapacitors, and fuel cells.
1. Introduction
Green nanotechnology is a laudable approach in the area of science and technology owing to the demand for cheaper, rapid, and non-hazardous biochemicals and their incredible properties. However, conventional techniques are being widely employed for the fabrication of nanomaterials (NMs) such as metals, metal oxides, quantum dots, carbon nanotubes (CNTs), and carbon-based NMs. Thus, the development of new manufacturing methods and evaluation of the atomic, molecular and macromolecular scales to create new materials with extraordinary trademarks that have excellent electrical, optical, magnetic, and catalytic properties are necessary.1,2 One of the extraordinary advanced materials discovered in the past two decades in the field of nanoscience and nanotechnology is graphene-based materials. Graphene is a promising and exciting material of the 21st century. It is densely packed in a honeycomb lattice with thin layers of sp2 hybridized carbon atoms (Fig. 1). Graphene composed of accurately arranged two-dimensional (2D) sheets displaying high crystallites with unique electronic qualities, thus emerging as an encouraging new NMs for a variety of stimulating applications despite of its short history. A. Geim and K. Novoselov (2004) were pioneers of this “miracle material”, which has attracted vast interest among scientists, resulting in an inundation of studies dedicated to the various features of graphene in the last few years.3 Recently, this (2D) single sheet of carbon dervatives of graphene has been proven to be highly well-organized for a wide range of applications, which is mainly attributed to its large surface area (2600 m2 g−1), high Young's modulus (1.0 TPa), and extraordinary thermal conductivity (∼5000 W m−1 K−1).4,5 It also displays single-molecule gas detection sensitivity,6 quantum captivity in nanoscale ribbons,7 extensive range of ballistic transport at RT8 and high optical transmittance (∼97.7%).9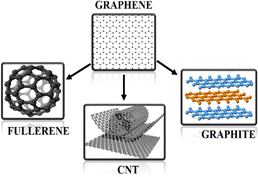 | ||
| Fig. 1 Graphene is a 2D building block for carbonaceous materials of all other dimensions. It can be wrapped into 0D buckyballs, rolled into 1D nanotubes or stacked into 3D graphite. | ||
These exclusive properties of graphene have revealed its extensive range of applications in various branches of nanotechnology (Fig. 2), for example, anti-bacterial activity,10in vitro toxicity to various cell lines, DNA degradation, photocatalytic activity, lithium-ion batteries,11–14 fuel cells, catalyst engineering,15,16 chemically derived sensors,17 biosensors,18 anti-microbial activity,19 flexible thin-film transistors,20 biomedical applications, drugs delivery,21,22 solar cells,23 photovoltaic devices,24 imaging,25 P–N junction diodes,26 graphene-based super capacitors,27 solar panels,28 electromagnetic shielding,29 water purification,30 absorption of non-aqueous liquids, e.g., oils, aromatic compounds, alkenes, dyes, and organic compounds, removal of organic pollutants and pharmaceutical drugs, and remediation of pesticides and heavy metal ionic solutions.31–35 In this case, large quantities of graphene materials are necessary to satisfy the vast demands of the above-mentioned applications.
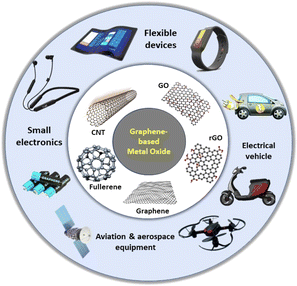 | ||
| Fig. 2 Overview of graphene-based materials for advanced energy storage devices and their potential applications in various fields. | ||
However, the fabrication of high-quality graphene via an economical route and its large-scale production are significant challenges. To date, numerous methodologies have been developed to fabricate graphene from pristine graphite, for instance epitaxial growth,36 vacuum-based thermal annealing,37 non-catalytic synthesis,38 Scotch tape method,39 micro-mechanical cleavage,40 (CVD),41 thermal exfoliation,42 CNT cutting,43 liquid-phase exfoliation,44 ultrasonication,45 and reduction of graphene oxide.46 There are also other alternative and harmful methods for the production of graphene oxide including chemical discharge,47 discharging arc48 and CNT unzipping.49 Epitaxial growth is capable of producing great-quality multilayer graphene but cannot separate single conductive layers/bilayers that are excellent for energy storage applications.50 A significant constraint of micromechanical cleavage is repetitive cycles of detaching throughout the layer exclusion and it is very difficult to maintain the number of isolated layers fabricated by this technique.51
Alternatively, thermal exfoliation requires high manufacturing prices.40 Meanwhile, in CVD, it is difficult to accomplish layer separation without the destruction of the material and effective exfoliation from the substrate is uncertain. In contrast, the reduction of graphene oxide (GO) is considered an encouraging methodology for the mass fabrication of graphene. The product achieved by this technique is usually designated as reduced graphene oxide (rGO) or nanostructure graphene (NSG).52 As an alternative method, the liquid-phase exfoliation technique, which involves the process of ultrasonication53 or mixing54 of a stabilizer containing graphite to preclude the accumulation of graphene to graphite, is also commonly employed by researchers due to its easy synthesis of graphene and wide flexibility in terms of the selected mixing device. Furthermore, the final product is a graphene solution after a centrifugation phase, and the application of liquid-exfoliated graphene can be additionally introduced into graphene paper by vacuum percolation,55 conductive ink56 or nanofluids.57
Alternatively, rGO/NSG can be synthesized from graphene oxide via green methods. Firstly, GO is fabricated from graphite powder through oxidative reaction, which comprehensively enriches the sheets with altered oxygen groups. The presence of abundant oxygen moieties functionalized on the basal plane of carbon makes GO electrically insulating, and also induces thermal instability.58 Therefore, the molecules of oxygen are reduced and significantly relocated to the π-lattice. This is beneficial to produce thermally established graphene and recapture electronic conductivity. Some GO reduction methods, e.g., electrochemical,59,60 thermal,61 photocatalytic,62 and chemical methods, have been described recently. Among them, electrochemical reduction is incompetent to heal the inherent deficiencies in the GO precursor. Also, in the case of the exfoliation of GO to single-layer sheets requires a lower content of oxygen in the GO precursor. Given that a low content of oxygen reduces the interaction within the oxygen functionalities of GO, stable species can be produced such as in-plane ether or out-of-plane carbonyl groups, which are reasonably tough to eliminate by the electrochemical reduction method.63 Presently, deoxygenation by the thermal method is economical. It is a complex phenomenon due to the thermal-energy prompted multi-step elimination process of introduced H2O and other oxide groups of –OH, –COOH and epoxy groups.64 Photocatalytic reduction involves dynamic activity of light-sensitive materials under UV emission.65 Hence, in contrast with the above-mentioned reduction methods, chemical reduction is considered the easiest technique to yield ultrathin graphene sheets with great crosswise dimensions and area.66 Furthermore, this technique is practically fast, inexpensive, facile, and appropriate for repeated handling and chemical variations.
Researchers have employing various types of reducing agents to transform GO to graphene via the reduction process. However, hydrazine, LiAlH4, NaBH4, hydroquinone, NaOH, etc. are noxious in nature, which are unfortunately harmful to the ecosystem. In particular, hydrazine has some drawbacks, for example, during the elimination of the oxygen functional groups, nitrogen tends to covalently interact with GO, forming hydrazones, amines, aziridines, etc. These C–N residuals are effective as n-type dopants and have a significant impact on the electronic structure of chemically derived rGO.67 These sheets have higher resistance, hindering their application. However, there are no simple techniques to remove these impurities, which may be present in a C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio of 16.1
N ratio of 16.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as reported by Stankovich et al. Therefore, as an alternative method, researchers have focused on the use of different types of biochemical constituents, amino acids, plant extract, bacteria, yeast, fungus cells, polysaccharides compounds and metal powders to prepare reduced graphene sheets from GO. These reducing agents are termed “green reducers”, given that they are free from carcinogenicity, corrosion, and toxicity. However, review articles are available on chemical or other reduction of GO,68,69 especially an overview of the green reduction of GO to graphene. Therefore, in this review, we summarize the reduction of GO using different environmentally friendly reducing agents reported recently.
1, as reported by Stankovich et al. Therefore, as an alternative method, researchers have focused on the use of different types of biochemical constituents, amino acids, plant extract, bacteria, yeast, fungus cells, polysaccharides compounds and metal powders to prepare reduced graphene sheets from GO. These reducing agents are termed “green reducers”, given that they are free from carcinogenicity, corrosion, and toxicity. However, review articles are available on chemical or other reduction of GO,68,69 especially an overview of the green reduction of GO to graphene. Therefore, in this review, we summarize the reduction of GO using different environmentally friendly reducing agents reported recently.
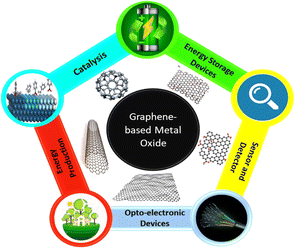
Presently, graphene/inorganic NP-based nanocomposites have attracted significant interest as an advanced era of nanohybrid materials.70,71 The interest in the scientific community in these materials is continuously increasing due to their individualities in combining the desirable properties of building blocks for application.72 The innovative magnetic, catalytic, and optoelectronic properties of graphene NCs based on hybridization with NPs have been exploited in various applications, including antibacterial coating, catalysis, sensors, detectors, electronics, imaging, and energy storage (Fig. 3).73–78
To date, metals, metal oxides and graphene have been combined to form unique nanomaterials with various properties due to the synergistic effect among these materials, which enhances their properties.79 These combinations of metal oxide NPs on graphene sheets restrict their re-stacking and agglomeration, thus improving the surface area of the prepared NMs. In addition to enhancing the properties of graphene, the NPs also act as stabilizers against the agglomeration of individual graphene sheets, which is caused by the strong van der Waals interactions between the graphene layers.80 Moreover, in several cases, the insertion of metallic NPs in graphene also simplifies the exfoliation of specific graphene layers from graphite. Thus, more efforts and development of new approaches to synthesize graphene-based nanocomposites (NCs) are indispensable. The decoration of metallic NPs on graphene sheets for the fabrication of graphene-based NCs can be achieved via two different methods, as follows:81 (i) post immobilization (ex situ hybridization) and (ii) in situ binding (in situ crystallization). Ex situ hybridization involves the mixing of separate graphene nanosheet solutions and pre-synthesized or commercially available NPs.82 Alternatively, in situ binding of NPs is carried out through the simultaneous reduction of respective metallic salts and graphene oxide (GO) with various reductants.83 Generally, NPs prepared via in situ binding methods have been broadly used due to their cost-effectiveness given that they reduce the number of steps to obtain the desired product.84 Several reduction techniques have been applied during the in situ preparation of graphene NCs, e.g. chemical, thermal, bioinspired, or green and electrochemical reduction. Among them, chemical reduction is the most promising and widely applied method for the large-scale production of graphene metallic NP-based NCs,85–87 but this method has several disadvantages due to the use of toxic chemicals in synthesis process. Therefore, recently, the trend of applying green reductants in the synthesis of NMs has gained popularity.88 For example, a variety of green reductants has been applied for the synthesis of metallic NPs, such as plant extracts, vitamin C, amino acids, ascorbic acid, glucose, bovine serum albumin and microorganisms (bacteria, fungi, and yeast).89–92 Among them, plant extracts have received remarkable interest due to their cost effectiveness, eco-friendly nature, easy availability and bulk quantities.93 Additionally, plant extracts not only act as green reducing agents but also function as capping and stabilizing agents, which limit the use of additional chemical stabilizers.94 Consequently, plant extracts have not only been employed to reduce metallic NPs, but in many cases, they have also been effectively applied for the reduction of GO.95 However, although a series of metallic NPs has been synthesis using plant extracts as a green reducing agents, their application in the synthesis of graphene-based NCs has been limited thus far.96–98
The enhancement of these advanced composite materials has facilitated their application in energy storage systems including Li-ion batteries, fuel cells and supercapacitors, or even energy systems such as photovoltaic devices and photocatalysis. These scopes were well-addressed in the review articles by Huang et al.99 and Chen et al.,100 respectively, from the standpoint of electrochemistry. They significantly discussed the up-to-date synthesis techniques, together with the electron transfer properties, involving the electronic structure and electron transport in the graphene composite NSs.
Presently, although the incredible developments in science and technology have certainly improved the quality and standard of human life and health, they have created a multitude of problems as well. Among them, water pollution and contamination are one of the biggest and the most alarming problems that demands formidable and effective solutions. Predominantly, in developing countries, the situation is worrisome, and the non-availability of inexpensive water treatment systems further aggravates the situation.101 Although huge initiatives are already underway to tackle this problem, further and highly rigorous research dedicated to this issue is still required. In recent times, graphene-functionalized semiconductor (metal oxide) photocatalysts have captivated significant attention towards the scientific community due to their large surface area, tunable band gap, good electron conductivity, and high capacity of light adsorption.102,103 The primary demonstration by Fujishima and Honda in 1972 of the electrochemical splitting of water on TiO2 electrodes using photons104 was a significant development. Since then, graphene-based metal oxide semiconductor photocatalysts have been employed in solar energy conversion, degradation of various water pollutants and environmental remediation, as shown in Fig. 4. Some semiconductor photocatalysts including TiO2, CuO, BiOCl, ZnO, CdO, CdS, ZnWO4, SnO2, NiFe2O4, Bi2O3/BiOCl, and MnFe2O4 can act as light-induced photocatalysts for the degradation of pollutants.
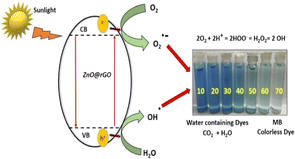 | ||
| Fig. 4 Electron transfer from the conduction band of MO to graphene through the percolation mechanism of dye degradation. Reproduced with permission from Elsevier. Copyright 2021. | ||
Abd Elkodous et al.105 synthesized a Co0.5Ni0.5Fe2O4/SiO2/TiO2 layer-by-layer matrix loaded with C-dots, rGO and SWCNTs, acting as an electron reservoir for photocatalytic degradation. The photocatalytic properties of the fabricated nanocomposites were compared with the standard TiO2 (P25). The kinetics of the photocatalysis and the role of charge scavengers were studied in detail.
Ecological energy creation, economically sustainable expansion, and reasonable healthcare are three key challenges of the present global society. Electrochemistry is the basis of many chemicals, and also biological sensors, energy storage devices and new-generation tools. Electrochemistry consumes energy in the sparkling form as electricity to assemble or introduce electrons through electrodes. New innovative materials are always pursued for existing devices to improve their performances or generate new technologies. Graphene and associated materials induce innovative perceptions and projections in electrochemical systems. The electrochemical devices established by graphene exhibit certain advantages. One of the objectives of this review is to offer a critical outline on the electrochemistry of graphene-based metal oxide NCs and related materials. Subsequently, we focus on numerous applications of graphene-based metal oxide NCs in electrochemical devices, including supercapacitors, fuel cells, rechargeable ion batteries, sensors and biosensors and the release of electrical energy via electrochemical practices in an efficient, reversible, and expedient way, which have been extensively used in several fields such as moveable electronics, electrical vehicles (EVs), and aviation/aerospace tools including sensors and biosensors, and then energy storage and new-generation applications.106 We critically discuss the advantages and drawbacks of graphene-based NCs and outline their future challenges.
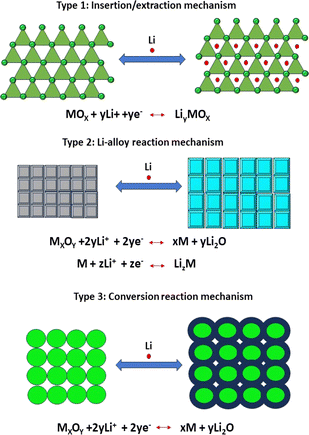
The selective illustration of electrode materials as NS materials has presented the opportunity to design novel energy-storage materials for next-generation devices such as high-performance LIBs, supercapacitors and fuel cells, which have played a prominent role among the sustainable energy technologies, and in particular have attracted significant research interest in the past few years owing to their elevated higher power density, higher energy density, and longer cycle life. Due to the high surface area and specific configuration of NS materials, these electrodes can provide high lithium-ion flux across the interface, short diffusion pathways for both Li ions and electrons, abundant active sites for Li storage, high freedom for volume changes during the electrochemical charge/discharge process, and long cycle stability with superior specific capacitance.107
Over the years, various types of materials, such as carbon-based NCs, have become readily accessible for electrochemical application. Especially, graphene-based metal oxides/hydroxides, mixed metal oxides, layered double hydroxides, carbonaceous materials, conducting polymers, inorganic carbides, nitrides, etc. have been extensively considered as electrodes materials. Graphene-based metal oxide electrodes are cost effective and have high permeability, allowing the simple penetration of electrolytes into electrodes and improving the capacitance. Besides, their large surface area, operative electrical conductivity and rich redox activity make them suitable for electric SCs with double-layer capacitance (electric double-layer capacitance).108,109
Similarly, Kumar et al.110 synthesized cost-effective novel N-rGO@CoO NCs via the microwave technique. In this work, CoO nanocrystals were decorated on 2D N-doped rGO nanosheets (NSs) as an excellent electrode material, which showed superior cyclic stability and specific capacitance. The optimized N-rGO@CoO electrodes exhibited a specific capacitance value of 744.1 F g−1. Also, the stability of NCs was demonstrated, with the electrode retaining about 91.3% of its original specific capacitance even after 5000 cycles. Also, Youssry et al. synthesized TrGO/Ni NCs using the thermal method using nickel foam (NF) as a substrate under the inert atmosphere of nitrogen gas.111 The as-synthesized TrGO/Ni electrode achieved a very high specific capacitance of 617 f g−1 at a scan rate of 2 mV s−1. The TrGO/Ni NCs exhibited long-term stability over 2000 cycles at a scan rate of 50 mV s−1 with a capacitance retention of 90.2%.
Kumar et al. studied the simple and cost-effective microwave synthesis of rGO NSs combined with iron oxide for supercapacitor electrodes. In the resulting rGO@Fe3O4 composites, the faceted-Fe3O4 NPs were homogeneously distributed on the surfaces of the rGO NSs. The material revealed a maximum value of specific capacitance (771.3 F g−1 at 5 mV s−1), with outstanding cyclic stability (95.1% after 5000 cycles). The results indicated that the homogeneous microwave-processed material is a promising candidate for SC applications.112
Also, Kumar et al.113 achieved highly dispersed TiO2 nanoparticles (NPs) on graphene NSs via hydrothermal treatment. The hybrid graphene TiO2 NS composite (H-GTN) showed enhanced optical and electrochemical properties for future application as a supercapacitor. The cyclic voltammetry test for H-GTN showed a very high specific capacitance value of up to 530 F g−1 at a scan rate of 3 mV s−1, and nearly stable capacitance of 400 F g−1 above 20 mV s−1, showing long cyclic stability. Similarly, Youssry et al.114 demonstrated a feasible way to fabricate Co–Ni LDH NS electrode materials on Ni foam via the electrochemical deposition method. The electrochemical deposition of Co–Ni LDH NSs was achieved by cyclic voltammetry. The Co–Ni LDH NS electrode materials synthesized at 8 cycles exhibited an enhanced electrochemical efficiency and provided a superior capacitance of 3130.8 F g−1. Furthermore, Co–Ni LDH NSs was stable over 3000 cycles with a capacitance retention of 72.4%.
Kumar et al.115 carried out a one-step rapid and facile microwave-assisted synthesis process for fabricating ZnOL@MpEG hybrids and investigated their application as electrode materials. In this synthesis process, the microwave-assisted deposition of ZnO layers occurred on microwave partially exfoliated graphene (MpEG) sheets to obtain the ZnOL@MpEG hybrids, which demonstrated enhanced electrochemical properties as supercapacitors. The ZnOL@MpEG hybrids are promising for application in supercapacitors, displaying a high specific capacitance of 347 F g−1 at a current density of 5.0 A g−1.
2. Overview and history of graphene-based metal oxide NCs
Since the 1990s, after the discovery of 0D buckminsterfullerene, other carbonaceous materials, e.g. fullerenes and CNTs, have attracted great interest because of their extraordinary mechanical and electronic properties. Formerly, it was proposed fullerene and CNTs can be derived from 2D graphene sheet. Nevertheless, as a matter of fact, they are not obtained from graphene, successively increasing the interest in research field of carbon-based materials.116 The discovery of graphene revolutionized and thoroughly redefined leading-edge technology with its exceptional thermal, mechanical, electronic and optical properties. Owing to these outstanding properties, graphene has been explored as major filling agent in NCs, where it can strengthen and improve the interfacial bonding between the host matrix and graphene.117 As far back as the 1840s, graphite oxide and graphite intercalation compounds were explored, which were precursor materials used primarily to obtained graphite nanoplatelets and graphene-based materials. However, many studies have been focused on the use of graphene derivatives (GO, rGO, frGO, etc) as alternatives to pristine graphene because of its high production cost. There are three different historical method for the synthesis of GO dating back to 1958. The first reported method was the Brodie technique, where KClO3 was added to a graphite and fuming HNO3 mixture, achieving the oxidation of graphite. Later, the Staudenmaier technique was developed, which is a modified Brodie method replacing HNO3 with a mixture of concentrated H2SO4 and HNO3. Thirdly, the Hummer's method was reported, where KMnO4 was added to a solution of NaNO3 and H2SO4. However, this method releases toxic gases, namely NO2, N2O4 and explosive ClO2. Thus, to control their release, an improved Hummers' method was investigated by the Tour group at Rice University by excluding NaNO3 for the environment-friendly synthesis of GO.118 Boehm et al. reported the use of various chemical reductants (such as hydrazine and hydroquinone) as well as thermal expansion and reduction of a GO dispersion.119 However, the toxicity of these chemical reducing agents poses a major risk to living organisms and the ecosystem. Thus, to overcome these issues, researchers recently reported an easy, cost effective and eco-friendly green alternative for reduction. Scientists reported a green synthesis method using plant extracts, fruit extracts, microbes, metal powder, vitamin C, organic acids, carrot juice, coconut water, and green tea.120–122A variety of applications has been envisioned for the use of graphene and graphene derivatives as reinforcing nanofillers. The advancement in the field of graphene-based NCs has been outlined in numerous review and research articles, either by considering particular materials including inorganic materials or polymers or specific applications for energy and environment.116 In the past few decades, most of the literature published on NCs of graphene with metal oxides and polymers reported a remarkable enhancement in their structural as well as functional properties. To date, different types of metal oxides including ZnO, MnO2, SnO2, TiO2, Fe2O3, Fe3O4, NiO, Co3O4, and Cu2O123 have been prepared and incorporated in graphene. Notably, very limited literature has been reported on NCs of group-5 elements such as niobium and tantalum. Furthermore, sometimes transition metals are also added to graphene-based metal oxide NCPs of indium and tantalum.124 Similarly, Wang et al.125 reported the preparation of NCs of graphene with metal oxides (SnO2, NiO, MnO2 and SiO2) and their potential in energy storage was demonstrated. SnO2/graphene NCs films exhibited a near theoretical specific energy density for Li-ion insertion without notable charge/discharge degradation. The NCs of TiO2, Mn3O4, and Co3O4 with rGO exhibited potential application in lithium-ion batteries.126 NCs of graphene with metal oxides have attracted extreme interest from researchers for the fabrication of well-organized supercapacitors. Anwar et al.127 in their research article described the supercapacitor behavior of graphene-based metal oxide (CO3O4, Cu2O, Fe2O3, Fe3O4, V2O5, MnO2, TiO2, NiO, Ni(OH)2, RuO2, SnO2, WO3, and ZnO) NCs. In their literature review, they also analyzed that nickel NCs poses a greater specific capacitance, while NCs with different forms of manganese exhibit higher relative stability. Zhang et al.128 in their feature article reviewed the electrochemical performance of NCs of graphene NSs with A3O4-type transition metal oxides (A = Mn/Fe/Co). The electrochemical properties of the synthesized NS-based Mn3O4, Fe3O4 and Co3O4 were investigated by cyclic voltammetry and galvanostatic charge–discharge tests. They exhibited a high energy density (20, 10 and 7 W h kg−1), large specific conductance (708, 358 and 240 F g−1) and good electrochemical stability (73%, 67.8% and 95.8%), respectively.
Graphene and metal oxide/polymer NCs have promising application as photocatalysts due to the advanced arrangement of their electronic structure, charge separation and transport and light absorption properties. A major breakthrough in photocatalysis research was reported in 1972, when Fujishima and Honda discovered that TiO2 together with a Pt electrode enabled the electrochemical photocatalysis of water under UV light radiation.114 A variety of inorganic nanoparticles including TiO2, CuO, BiOCl, ZnO, CdO, CdS, ZnWO4, SnO2, NiFe2O4, Bi2O3/BiOCl, and MnFe2O4 has been proposed to poses photocatalytic activity. However, they are associated with a few drawbacks such as poor absorption capacity, fast electron–hole recombination, low photocatalytic quantum yield, and low stability. Thus, various approaches have been employed to improve the photocatalytic performance of graphene NCs such as surface sensitization, doping, improving their electrical conductivity, increasing the number of catalytic active sites, coupling, constructing well-ordered NSs, and fabrication of different structures. Singh et al.129 discussed all these modification pathways briefly in their review. Functionalized graphene-reinforced metal oxide/polymer NCs have been shown to be promising in environmental sensing and remediation. Also, the photocatalytic activities of TiO2 NPs have been reported to be increased by the incorporation of graphene. Zhang et al.130 employed graphene/TiO2 NCs as a photocatalyst for the degradation of organic dyes such as methylene blue. Graphene/TiO2 showed the better photodegradation of dyes compared to CNTs/TiO2 and pure TiO2 due to the efficient charge separation and extended light absorption properties of graphene/TiO2 NCs. Besides TiO2, graphene NCs with ZnO and Cu2O were also explored for the photodegradation of pollutants.
3. Synthesis of graphene oxide nanosheets
In the synthesis of GO, the choice of graphite is important. Brief accounts on the reported processes are presented in the following sections. There are four methods for the synthesis of graphene oxide, as follows:(1) Synthesis of graphene oxide from graphite flakes.
(2) The original Hummers' method.
(3) Two-step modified Hummers' method.
(4) The improved Hummers' method/one-step modified Hummers' method.
Herein, we discuss the synthesis of GO by different methods and the most recent method for its synthesis, i.e. improved Hummers; method/one-step modified Hummers' method.
3.1. The original Hummers method
Generally, graphene oxide is synthesized by the Hummers' method (HM)131 and improved modified Hummers' method (MHM)132 (Fig. 5). In the Hummers' method, graphite is subjected to the oxidation process using sulphuric acid, sodium nitrate, and potassium permanganate (while hydrogen peroxide is used for the elimination of permanganate and manganese dioxide residue), which produces a bright-yellow colour suspension, and also terminates the reaction. The bright-yellow colour confirms the conversion of GO from pristine graphite. Sulphuric acid provides acidic condition in the reaction, while KMnO4 acts as a strong oxidizing agent in acidic condition. When KMnO4 is added to the sulphuric acid-containing pristine graphite, graphite is inserted in sulphuric acid. This results in the growth of graphite bisulfate ions, in which each single-layer of graphene is inserted through different layers of bisulphate ions.133,134 This effective intercalation facilitates the successful diffusion of KMnO4 into the graphene layers for the oxidation of graphite.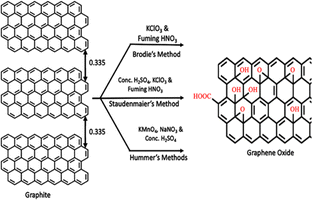 | ||
| Fig. 5 Representation of the synthesis of GO using different methods with the production of under an oxidized hydrophobic carbon material. | ||
The modified improved Hummers' method is either a one-step method or two-step method. The one-step modified Hummers' method was developed by Chen et al.89 In this method, also known as the “improved Hummers'” method, NaNO3 is not used in the oxidation process. Alternatively, in the two-step modified Hummers' method, H2SO4, K2S2O8 and P2O5 are used for the pre-oxidation of graphite powder. Then, the pre-oxidized graphite is exposed to oxidation using KMnO4 and H2SO4 by the Hummers, method. Marcano and co-workers135 developed a new method for the synthesis of GO, which they called the “improved synthesis” of GO. They used KMnO4 in a weight ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with an H2SO4 and H3PO4 mixture. In addition, Xu et al.136 developed another approach called “mild oxidation” by reducing the KMnO4/graphite ratio from 3
1 with an H2SO4 and H3PO4 mixture. In addition, Xu et al.136 developed another approach called “mild oxidation” by reducing the KMnO4/graphite ratio from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
4. Synthesis of reduced graphene oxide (rGO)
The reduction of GO can be accomplished by different approaches including chemical, electrochemical, thermal,137,138 and photochemical methods139 to achieve graphene-like materials, which are generally referred to in the literature as highly reduced graphene oxide (HrGO), reduced graphene oxide (rGO), chemically reduced graphene oxide (CrGO), chemically converted graphene (CCG), etc.4.1. Chemical methods
Chemically reduced graphene oxide or graphene using GO by applying different reducing agents such as NaBH4, hydrazine monohydrate, and many other chemicals.140–143 Among the reducing agents, hydrazine is the most significant and undoubtedly the most reported and extensively used reductant owing to its excellent stability and reactivity in aqueous medium. The reduction of GO through hydrazine reestablishes the π-conjugation inside the aromatic rings in graphite, resulting in an enhancement in electrical conductivity. Previously, the hydrazine-mediated reduction of graphene oxide was observed to yield the highest conductivity of about 99.6 S cm−1 and the C/O ratio was estimated to be 12.5; however, an electronic conductivity of up to 300 S cm−1 was perceived with the less noxious acetic acid and hydroiodic after replacing hydrazine.144,145 Although hydrazine efficiently eliminates oxide functional groups, it also leads to the addition of impurities such as nitrogen, which remains covalently bound on the surface of the graphene sheets in the form of hydrazones, amines, or other functionalities.141 Furthermore, it has been described that higher features of graphene can be achieved by reducing GO with hydrogen gas, but the requirement of inert condition and high temperature restricts its applications.4.2. Thermal methods
GO can be reduced by another operative technique, i.e., thermal method, where it can be prepared by the thermal exfoliation of graphene oxide followed by rapid heating (>200 °C min−1) of dehydrated GO under an inert atmosphere at high temperature (about 1050 °C).146 The quick heating of GO leads to the breakdown of the oxygen moieties convoluted on the GO plane of carbon, releasing poisonous gases such as CO and CO2. The free gas gradually evolves into the space present between the GO sheets and produces local pressure within the stacked layers, which ultimately aids the exfoliation process of GO. This thermal treatment not only exfoliates but also reduces GO to graphene, concurrently by eliminating oxygen-containing moieties, which is referred to as TRG (thermally reduced graphene). In the exfoliation process of GO, about ∼30% mass loss of GO occurs due to the disintegration of the oxygen-containing moieties and dehydration of water. Thus, these techniques only produce small-size and wrinkled graphene sheets with severe topological defects and the TRG sheets are damaged with etches holes all over the plane of the sheets.147 These defects reduce the carrier transport path length, and also induce numerous smattering spots in the TRG sheets, which shows adverse effects on their electronic properties.148However, although the thermal reduction techniques of GO are exceedingly effective, more efforts are required to attain the high-quality large-scale production of rGO at lower calcination temperatures. In essence, high-temperature annealing not only requires critical treatment conditions, but also needs larger energy consumption and demands sophisticated and expensive instruments. Moreover, the fabrication of HRG films on substrates containing inorganic/organic materials is difficult under the thermal reduction conditions, which requires electronic instruments for annealing at high temperature.
4.3. Microwave-assisted method
GO reduction by the microwave method involving high-temperature galvanizing is extremely effective and extensively applied, but it poses some noticeable disadvantages.149 Thermal annealing is generally carried out by thermal treatment; nevertheless, some unusual heating instruments have been studied for thermal reduction, such as microwave irradiation (MWI).150,151 In this technique, microwave energy is converted into heat. Compared to other established heating approaches, the reaction solution is homogeneously and speedily. Moreover, the reactant and solvent possess different dielectric constants, and therefore, selective dielectric heating throughout the reaction can be carried out by the MWI method. Recently, MWI has revealed admirable capacity to synthesized graphene from GO. However, the interaction between GO graphene with microwaves is not fully understood to date.Hu et al. described that for carbon-based materials, their MW assisted synthesis mostly relies on their chemical structures and configuration. In this study, the oxygen content in the GO and graphene materials was varied, where the absorption capability of GO was reduced remarkably with an increase in oxygen moieties because of the decrease in the sp2 hybridized structure. Moreover, the GO reduction process was speculated due to the small addition of pristine graphene, which was greatly enhances. Exfoliated graphite (EG) was preparing by this MWI method using a variety of graphite-intercalating graphite compounds (IGCs).152 EG was also synthesized by MWI very quickly (4 min); where a mixture of natural graphite and KMnO4 and HNO3 was directly heated in a microwave oven. The preparation of rGO also been described by concurrent GO exfoliation and reduction under a dry environment by rapid MWI in ∼1 min.153
4.4. Hydrothermal and solvothermal methods
The hydrothermal method is an extraordinary, potent and incipient chemical reduction process for the green fabrication of graphene and graphene-based composites.149 This one-step method can be employed to synthesize highly uniform crystalline NSs without further annealing/calcination process.154 The reduction of GO by the hydrothermal process is carried out in a sealed tube system in the presence of either water or a solvent (solvothermal process) at a certain temperature or beyond its boiling point to generate extremely high reduced pressure.155 Supercritical water (SCW) is considered an ecofriendly reducing agent and alternative to noxious chemical reducing agents. GO reduction can be achieved hydrothermally via SCW, where SCW not only removes the oxygen-containing functionalities partially but also recovers the aromaticity of the compound.156 During this reaction, SWC acts as a source of protonated H+ ions for the hydroxyl groups. This leads to the dehydration of rGO, thereby simplifying the reduction method. Moreover, the true pH may be different compared to other reduction procedures. The minimal pH of the solution plays an important role, i.e. a basic solution (pH = 11) yields a constant rGO solution, while the rGO sheets aggregate in an acidic solution (pH = 3).GO deoxygenation was carried out by solvothermal reduction. In this procedure, Wang et al. describes the reduction of GO using N,N-dimethylformamide (DMF) as the solvent at 180 °C and hydrazine monohydrate as the reducing agent.157 The rGO sheets were dispersed in DMF solvent, and the C/O ratio in the rGO sheets reached 14.3. However, the presence of nitrogen doping initiated by hydrazine resulted in poor conductivity in the rGO sheets. In a different study, Dubin et al. used N-methyl-2-pyrrolidinone (NMP) as the solvent for the solvothermal reduction reaction.158 Here, a sealed tube container was not used for the reduction of GO and the reaction temperature was less than (200 °C) the boiling point of NMP (202 °C at 1 atm). It was suggested that the oxygen-scavenging properties of NMP at higher temperature and the adequate thermal annealing aided the reduction of graphene oxide.159
4.5. The green reduction of graphene oxide
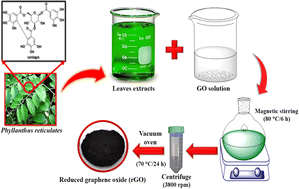
In a distinctive reduction of GO, firstly a GO suspension dispersed in an appropriate solvent and ecofriendly green reducing agents are blended to yield the reaction solution and in situ binding. Furthermore, under controlled different temperatures and altered times, the reaction mixture was preserved. In previous studies, the concentration of GO was about 0.1–1 mg mL−1, and the temperature of the reaction was observed to be 80–85 °C. Table 1 shows the GO concentration, temperature and reduction time of GO in different reports. The pH is a vital parameter for the reaction solution because a change in the pH will possibly change the reduction process in different solutions, i.e., both acidic and alkaline media.160 Especially, HCl, NH4OH and dilute NaOH are used to control the pH of the reaction solution.161 Merino et al. discussed that alkaline solution is vital for the reduction process in a shorter time. Therefore, the further use of any reducing agent can deoxygenate the GO sheet.162 It increases the colloidal durability of the GO sheet due to electrostatic repulsion. Bosch et al.163 clearly demonstrated that graphene sheets are affected by the use of different pH solutions. According to their conclusions, for large defects, acid pH is responsible for a higher concentration, extensive agglomeration and small size reduction of graphene sheets. An acidic pH may turn the graphene into others forms of graphite with a nano-size, for instance fullerene, nano-flowers, nano-onion and MWCNTs. This study disclosed that alkaline pH is more favorable for the fabrication of less defective and high-quality graphene.164 After the reduction, the GO solution turns from a brownish-yellow colour into a black slurry of graphene sheets. The obtained graphene sheets are extracted either filtration or by centrifugation.165 After centrifugation, the obtained black slurry should be washed numerous times with deionized water and alcohol to remove all the unwanted materials. After carrying out the washing process, the pH of the slurry should be neutral (pH = 7).166 Lastly, the solid and black rGO sediment can be dried in a vacuum oven at 50–100 °C.167| Reducing agent | Time | Temp (°C) | GO (mg mL−1) | Ref. |
|---|---|---|---|---|
| L-Valine | A few h | 90 °C | 0.1 | 168 |
| Melatonin | 3 | 40 °C, 60 °C, 80 °C | 0.1 | 169 |
| Aluminium powder | 3 | 100 °C, 200 °C | 4 | 170 |
| Pulicaria glutinosa | 24 h | 98 °C | 5 | 173 |
| Dalbergia latifolia | 174 | |||
| Gallic acid | 24 | RT | 4 | 175 |
| Carrot root | 48 | RT | 0.5 | 176 |
| Shewanella cells | 48 | RT | 300 | 177 |
| P. aeruginosa | 72 h | 37 °C | 0.35 | 178 |
| Asian red ginseng | 10 min | 80 °C | 0.1 | 179 |
| Syzygium aromaticum | 30 min | 100 °C | 1.6 | 180 |
| Chinese wolfberry | 24 h | 90 °C | 0.5 | 181 |
| E. coli | 48 h | 37 °C | 5 | 182 |
| Yeast | 72 h | 35–40 °C | 0.5 | 183 |
| Caffeic acid | 12 & 24 h | 95 °C | 0.1 | 184 |
| Hibiscus sabdariffa | 1 h | — | 0.4 | 185 |
| Allium sativum | 3 h | 100 °C | 5 | 186 |
| Eichhornia crassipes | 10 h | 180 °C | 0.1 | 187 |
| Spinach (Spinacia oleracea) | 30 min | RT | 1.6 | 188 |
| Seed extract of T. chebula | 24 h | 90 °C | 1 | 189 |
| Grapes (Vitis vinifera) | 1, 3, 6 h | 95 °C | 0.6 | 190 |
| Salvadora persica roots | 24 h | 98 °C | 5 | 191 |
| Pomegranate juice | 12, 18, 24 h | 60 °C | 2.5 | 192 |
| Caffeine | 12 h | 80 °C | 0.1 | 193 |
| Cinnamomum verum | 12 h | 100 °C | 1 | 194 |
| Chrysanthemum | 24 h | 95 °C | 0.1 | 195 |
| Ficus religiosa | 24 h | 50 °C | 0.1 | 196 |
| Mangifera indica | 24 h | 70 °C | N/A | 197 |
| Mangifera indica L. | 8 h | 70 °C | 1 | 198 |
| Azadirachta indica | 60 min | 11.1 | 199 | |
| Green tea extracts | 8 h | 80 °C | 0.5 | 200 |
| β-Carotene | 24 h | 95 | 201 | |
| Cinnamon | 45 min | RT | 1.6 | 202 |
| Cocos nucifera L. | 12, 24, 36 h | 100 °C | 0.66 | 203 |
| Curcumin | 120 min | 85 °C | 204 | |
| Tea polyphenols | 8 h | 80 °C | 1 | 205 |
| Tea polyphenols + iron | 10 min | 40 °C, 60 °C, 80 °C | 0.1 | 206 |
| Rosa damascena | 5 h | 95 °C | 7 | 207 |
| Sugar cane juice | 12 h | 150 °C | 1.25 | 208 |
| Marigold flower | 3 h | 95 °C | 0.625 | 209 |
| Ficus carica | 24 h | 80 °C | 5 | 210 |
| Lignin | 8 h | 90 °C | 1.0 | 211 |
| Annona squamosa | 12 h | 100 °C | 0.4 | 212 |
| Lemon extract | 24 h | 95 °C | 0.1 | 213 |
| Platanus orientalis | 10 h | 100 °C | 1 | 214 |
| Cherry, Magnolia, Platanus | 12 h | 95 °C | 0.5 | 215 |
| G. biloba | 30 °C | 0.5 | 216 | |
| Rose water | 5 h | 95 °C | 7 | 217 |
| Reducing sugar | 1 h | 95 °C | 0.1 | 218 |
| Tin powder | 0.5–3 h | RT | 1 | 219 |
| Vitamin C | 0.5–4 h | 95 °C | 0.1 | 220 |
| L-Ascorbic acid | N/A | 23 °C | 0.1 | 221 |
| L-Cysteine | 12–72 h | 26 ± 2 °C | 0.5 | 222 |
Xiao et al.168 synthesized functional graphene nanosheets, which were dried at room temperature. In this report, L-valine was used for the reduction of GO to rGO as a green biomolecule source. Furthermore, the quality of the functional graphene sheets vastly depend on the following features: (i) the properties of the original graphite, (ii) process of oxidation, and (iii) final deoxygenation method of GO to graphene.
Feng et al.141 established an ecofriendly technique for the large-scale fabrication of high-quality rGO with a small content of oxygen. A reducing agent Na-NH3 solution were used, where the solvated electrons assisted the GO reduction and the construction of a conjugated complex of rGO with an oxygen content of 5.6 wt%. Recently, Esfandiar et al.169 reported the reduction of GO by introducing melatonin as a replacement for hydrazine. Melatonin is a biocompatible antioxidant, which led to excellent results compared hydrazine under the same conditions. Moreover, due to the π–π stacking of the melatonin molecules on the reduced graphene sheets, the melatonin-reduced suspension of GO suspension was more stable compared with the hydrazine-reduced suspension, this is due to its greater aggregation.
Newly, metal-based GO reduction has been considered eco-friendly and leads to more rapid reduction of GO. Fan et al. described an efficient synthesis technique for the reduction of GO using aluminum powder.170 The reaction was completed within a short time of less than 30 min under the acidic medium. The graphene sheets showed a high bulk electrical conductivity of 2.1 × 103 S m−1. Similarly, Mei and Ouyang replaced Al with Zn, leading to the successful reduction of GO using the sonication method at room temperature in mild acidic medium.171 The reaction was completed in one minute, i.e. taking much less time than described for GO reduction using aluminum and with iron powder. The low reduction potential of Zn2+/Zn and ultrasonication facilitated the fast and efficient reduction of GO. Furthermore, the rGO sheets achieved by this technique displayed good electrical conductivity and effective thermal stability.
More recently, there has been a new trend of employing plant extracts that can act as reducing/capping/stabilizing agents during the synthesis of nanoparticles, where this property of plant extracts has fascinated noteworthy attention of the scientific community. Plant extracts (PE) are comparatively easy to handle, easily available, cost effective, and have been significantly exploited owing to their biocompatibility in the field of nanoscience and nanotechnology. Although different metallic NPs have been previously synthesized effectively using PE as bioreductants,172 their reducing capacities have been tested recently for the reduction of GO to rGO.
Khan et al. demonstrated the Pulicaria glutinosa (P. glutinosa) plant extract (PE)-assisted green synthesis of HRG via GO using efficient green root. The phytochemicals present in the PE of P. glutinosa were responsible for the reduction of GO and also functionalized the HRG nanosheets to stabilize different solvents on their surface, which eliminated the need for other harmful surfactants and reductants (Fig. 6).173 Nagaraj et al. developed a green route for the biosynthesis of rGO via a green approach using Dalbergia latifolia leaf extract act as a reducing, capping and stabilizing agent for the reduction of GO.174 Similarly, Li et al. described a green method employing gallic acid for the reduction of GO, which acted as both a stabilizing and reducing agent in the reduction process.175
Besides this, the microbial reduction of GO is a new trend that has widely been reported. Kuila et al. reported the carrot root-based reduction of GO to HRG. Here, the endophytic microorganism present in the carrot root acted as a biocatalyst, which helped the reduction to proceed at room temperature.176
Similarly, Wang et al. demonstrated the reduction of GO via the microbial inhalation of Shewanella cells under ambient condition in an aerobic experimental setup.177 Due to the metal-reducing property of Shewanella bacteria, they metabolically generate electrons, which transfer to the external parts of the metal oxide solids from the interior parts of the cells. In the GO reduction process, the extracellular electron transfer is mediated by both the self-secreted electrons and outer membrane containing c-type cytochromes (Mtr/Ornc). It has been described that microbially reduced GO possesses admirable electrochemical properties compared with that synthesized by chemical methods. Similarly, Potbhare et al. employed bacterial biomass extract (Pseudomonas aeruginosa) for the reduction of graphene oxide as well metal salts. The biomass extract acted as a stabilizing and reducing agent in the reaction.178
5. Synthesis of graphene-based metal oxide nanocomposites (GMONCs)
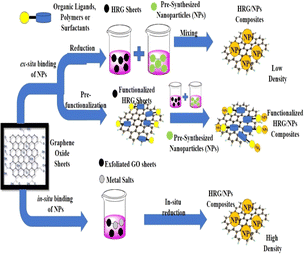
The anchoring of metal/metal oxides NPs on graphene sheets for the fabrication of graphene-based NCs can generally be performed via two different methodologies (Fig. 7), i.e., ex situ hybridization and in situ binding. Ex situ hybridization involves the mixing of separate pre-synthesized NPs and graphene sheets. Before blending both the NPs and graphene sheets, their surface functionalities can be enhanced using the sonication approach.223 The non-covalent π–π stacking and/or covalent C–C coupling reactions facilitate the functionalization of the conjugated graphene sheets. The functionalization of graphene-based NPs significantly enhances their solubility, facilitating the fabrication of graphene-based NCs. However, ex situ hybridization may result in the non-uniform distribution of NPs on the graphene sheets, which may also suffer from a low density. In this review, we discuss the in situ crystallization method for the fabrication graphene-based metal oxide NCs. In following sections, we present the most recent techniques for the fabrication of graphene/metal oxide NCs. Readers interested in the details of the in situ methods (e.g. functionalization and preparation of functionalized graphene-based metal oxide NCs) can refer to the brilliant reviews by Yang or Shi et al.223,224Different methods can be applied for the preparation of graphene-based metal/metal oxide NCs by in situ combining metal precursors such as AgNO3, HAuCl4, H2PdCl6, and K2PtCl4 with reductants such as NaBH4, hydrazine hydrate, and amines, which are commonly and widely applied. Alternatively, the preparation of NCs via the green reduction of metal/HrGO is a straightforward, cost-effective and single-pot method, and thus researchers are currently focused on finding green reducing agents for the fabrication of graphene-based NCs. A summary of the diverse graphene-based metal oxide NCs is given in Table 2. In this context, the green synthesis of Ag/HrGO is effective and environmentally friendly, where the in situ reduction process occurs by simultaneously adding both GO and AgNO3 in aqueous solution using tannic acid (as a green reducing agent).225 The obtained material demonstrated exceptional SERS (surface enhanced Raman scattering) activity as an SERS substrate, and remarkable catalytic property for the reduction of hydrogen peroxide.
| Graphene-based metal oxide | Method of synthesis | Graphene filler | Ref. |
|---|---|---|---|
| ZnO/rGO | Co-precipitation | rGO | 227 |
| rGO/SnO2/Au | Co-precipitation | rGO | 228 |
| Pd@graphene | Co-precipitation | Graphene | 229 |
| Graphene-TiO2 | Sol–gel | Graphene | 230 |
| rGO-SiO2 | Sol–gel | rGO | 231 |
| Graphene-BiFO3 | Sol–gel | Graphene | 232 |
| TiO2-graphene | Sol–gel | Graphene | 233 |
| ZnO@rGO | Microwave | rGO | 237 |
| rGO/TiO2 | Hydrothermal | rGO | 238 |
| g-Fe3O4/rGO | Hydrothermal | rGO | 240 |
| rGO-ZnO | Hydrothermal | rGO | 242 |
| MO-chitosan-GO | Solution cast | GO | 243 |
| rGO-Fe3O4 | Co-precipitation | rGO | 244 |
| NiO/rGO | Bath deposition | rGO | 245 |
| Graphene/Mn3O4 | Solvothermal | Graphene | 246 |
| rGO/WO3 | Ultrasonication | rGO | 247 |
| rGO/NiO | Decomposition | rGO | 248 |
| Fe3O4/ZnO-rGO | Dry method | rGO | 249 |
| Graphene/Pd/TiO2 | Hydrothermal | Graphene | 250 |
| CuO–ZnO-Fe2O3/rGO | Co-precipitation | rGO | 251 |
| Graphene-Mn2O3 | Sonochemical | Graphene | 252 |
| Graphene-TiO2/Fe3O4 | Solvothermal | Graphene | 253 |
| ZnFe2O4/graphene | Hydrothermal | Graphene | 254 |
| ZnO-rGO | Microwave | rGO | 255 |
| β-SnWO4-rGO | Microwave | rGO | 256 |
| Ag-Cu2O/rGO | Co-precipitation | rGO | 257 |
| Fe-doped TiO2 | Sol–gel | rGO | 258 |
| rGO@CuO | Co-precipitation | rGO | 259 |
| Ce-Bi2O3-rGO | Co-precipitation | rGO | 260 |
| MnO2/graphene | Hydrothermal | Graphene | 261 |
| Cu/Bi2Ti2O7/rGO | Co-precipitation | rGO | 262 |
| GO-modified TiO2 | Hydrothermal | GO | 263 |
| Co3O4/TiO2 GO | Sol–gel hydrothermal | GO | 264 |
| Fe3O4/Mn3O4-rGO | Polyol | rGO | 265 |
| Ce(MoO4)2/GO | Hydrothermal | GO | 266 |
| ZnO-G/TiO2-G | Hydrothermal | Graphene | 267 |
| Co/GO-TiO2 | Sol–gel | GO | 268 |
| Fe3O4/rGO | Co-precipitation | rGO | 270 |
| Graphene-LiMn2O4 | Solvothermal | Graphene | 271 |
| V2O3/rGO | Solvothermal | rGO | 272 |
| SnO2/rGO | Hydrothermal | rGO | 273 |
| ZnFe3O4/GO/Chitosan | Solution mixing | GO | 275 |
| rGO/ZnO | Co-precipitation | rGO | 276 |
| Graphene/Ni3V2O8 | Solvothermal | Graphene | 277 |
| ZnO–CuO/N-rGO | Hydrothermal | rGO | 278 |
| SnO2/Co3O4/rGO | Co-precipitation | rGO | 279 |
| Fe3O4 NPs/N-rGO | Hydrothermal | Graphene | 280 |
| Co3O4 NR/RGO | Hydrothermal | rGO | 281 |
Metallic NPs can be anchored to the surface of rGO sheets via two techniques, i.e., in situ and ex situ binding. A bulk yield of NPs can be achieved through in situ binding. Both rGO sheets and NPs can be functionalized either by C–C coupling covalent reactions or non-covalent π–π stacking. In addition to bimetallic NPs, graphene-based metal oxide NCs have been fabricated by in situ binding via bioinspired reduction (Fig. 8).226
5.1. Co-precipitation method
Elavarasan et al.227 demonstrates an economical and sustainable approach for the biogenic synthesis of ZnO/rGO NCs via a simple co-precipitation method using aqueous leaf extract of Dalbergia latifolia. TEM revealed that the typical particle size of the ZnO/rGO NCs was 12–18 nm, which were arbitrarily anchored on the edges and surfaces of the GO NSs. This study revealed the potential anticancer activity of the synthesized NCs on the MCF-7 cell line compared to the A-549 cancer cell line. Furthermore, the results indicated that the metal oxide NPs with graphene as excellent chemotherapeutic agents may have an even wider variety of applications in the treatment of cancer.rGO/SnO2/Au nanohybrid materials were fabricated using a dispersion of GO and SnCl2 aqueous solution by Meng et al.228 SnCl2 acted as a reducing agent in the reaction, which is the key step of the synthesis, and tin reduces both GO and HAuCl4 by the sonication-assisted method. This approach also has the potential for preparing other multicomponent NCs using different metal precursors.
Al-Marri et al.229 successfully synthesized palladium NPs loaded on graphene sheets via a green method using S. persica L. (miswak) root extract as a bioreductant. Using this technique, spherical and crystalline palladium NPs were homogeneously scattered on the surface of graphene sheets owing to the superior dispersibility of the synthesized NCs by the stabilization of the phytomolecules in the S. persica L. (miswak) root extract, where the as-prepared NCs exhibited outstanding catalytic properties toward the oxidation of different aromatic alcohols.
5.2. Sol–gel method
The sol–gel method enables the straightforward and economical synthesis of uniform NC materials with admirable compositional control, and therefore is used in many applications. Zhang and coworkers prepared graphene-TiO2 NCs via the sol–gel approach, where graphene oxide and tetra butyl titanate were employed as the starting materials and the fabricated NCs showed photocatalytic activity, which was affected by both the calcination temperature and graphene content.230 Hintze et al. fabricated rGO-SiO2 NCs using the sol–gel method, having a controlled carbon phase with significant electrical conductivity.231 Sol–gel-mediated graphene-BiFO3 NCs were synthesized by Nayak et al. and their electrochemical performance as a supercapacitor investigated. Graphene-BiFO3 NCs exhibited a specific capacitance of about 17–4 mF cm−2 and 95% retention of capacitance after 2000 cycles.232 Li et al. developed a sol–gel method for the synthesis of ultra-disperse TiO2 NPs on the surface of graphene sheets with extreme control, enabling the developed nanomaterial to achieve two times the specific capacity of mechanically mixed NCs.2335.3. Microwave-assisted method (MWI)
MWI is broadly used for the preparation of graphene-based NCs.234 Mostly, the in situ reduction of rGO/metal oxide NCs using GO and various metal precursors is highly effective. MWI techniques have attracted increasing attention due to their scalable and large-scale production, and also easy processing. Moreover, the fast and uniform heating by microwaves inhibit the agglomeration of the graphene layers. However, the major drawbacks of this technique are the poor homogeneity and distribution NPs on the surface of rGO sheets and inability to control the NPs size. Hassan et al. demonstrated that rGO/bimetallic NP composites can be prepared via the reduction of GO using the microwave technique.235 This method permitted the speedy chemical reduction of graphene oxide with a variety of reducing agents in both aqueous and organic solvents, where the instantaneous reduction of the metal salts and led to the formation of graphene sheet-supported metallic and bimetallic NPs. Dey et al.236 developed a novel, economical and green route for the in situ reduction of graphene/iron oxide NCs (GINC). An efficient material with an enhanced thermoelectric performance was developed using microwave irradiation. The fabricated NC film demonstrated a conductivity of 2.18 × 104 S m−1 with a Seebeck coefficient of 38.8 μV K−1. Hence, the power factor (PF) reached 32.90 μW m−1 K−2, which was folds higher than that of the thermoelectric material based on a PVAc-graphene composite. R. Kumar et al.237 successfully synthesized microwave-assisted ZnO NPs and loaded them on the surface of rGO NSs to produce ZnO@rGO, which was investigated as a supercapacitor electrode. This electrode showed a specific capacitance of 102.4 F g−1 at 30 mV s−1. Also, its cycling stability was demonstrated using CV for 3000 cycles, where its capacitance retention was maintained at 82.5%.5.4. Hydrothermal method
Hydrothermal or solvothermal reduction techniques have been widely applied for the fabrication of graphene-based metal oxide NCs.238 Hydrothermal treatment is not only preferred for the reduction of GO but also aids in the synthesis of metal oxide NPs. It becomes clear that hydrothermally fabricated graphene materials can be produced in large quantities with acceptable structures and properties. These techniques have numerous benefits. For example, the inexpensive raw material graphite is used for the production of graphene-like materials (rGO) on a large scale, making them very economical. Graphene/rGO achieved from hydrothermal methods is exceedingly processable and can be used for the production of devices and macroscopic nanostructures on a large scale via simple, economical and green reduction methods. Hence, these methods have gained noteworthy consideration by the scientific community, and the hydrothermal route is considered an exceptional tool for widespread research in the future. It is a big challenge to entirely eliminate the functional moieties from the surface of rGO sheets, and the restoration of the defects formed during the oxidation procedure is very challenging.Recently, Shen et al.239 reported the use of glucose as an eco-friendly reducing agent. It has been proposed that the reducing capability of glucose may enhance the yield of a reduced suspension of graphene (rGO) under hydrothermal conditions analogous to that acquired with hydrazine. The process of reduction was complemented by the construction of rGO/TiO2 NCs, whereas the photocatalytic efficiency of TiO2 NPs was superior due to the interface between TiO2 NPs and rGO.
Padhi et al.240 reported the novel, ecological, single-step Averrhoa carambola leaf extract-mediated fabrication of a stable photocatalyst and magnetically separable g-Fe3O4/rGO NCs. The hydrothermal route was adopted for the fabrication NCs, which resulted the excellent incorporation of g-Fe3O4 NPs with an average particle size of about 22 ± 2 nm in 2D rGO sheets. The as-fabricated NCs showed superior photocatalytic activity toward the reduction of Cr(VI) ions using 50 mg L−1 catalyst. Specifically, 97% degradation occurred within 1 h using 10 mg L−1 photocatalyst, while 76% phenol degradation was observed within 2 h a under visible light illumination at room temperature. Furthermore, the g-Fe3O4/rGO NCs showed better antibacterial activity towards Gram-positive bacteria compared with Gram-negative bacteria.
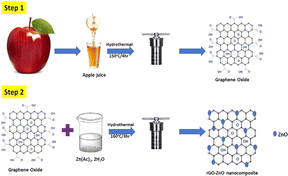
Chaudhary et al.241 designed the one-step in situ fabrication of graphene oxide-decorated ZnO NCs using the hydrothermal method. The estimated particle size from the TEM analysis was 15–20 nm, while the photocatalytic performances of NCs showed ∼85% efficiency against MB dye under visible light irradiation within 70 min.
The development of in situ rGO-ZnO NCs using the hydrothermal method was reported by Kumbhakar et al. (Fig. 9),242 where different concentrations of zinc precursors played an important role in the reduction of GO to rGO. The rGO-ZnO NCs showed ∼91% photodegradation efficiency under irradiation from UV-vis light and 60% under sunlight irradiation within 60 min in comparison to other NCs.
6. Characterization of graphene-based metal oxide NCs
The morphology of rGO-Fe3O4 NCs is shown in Fig. 10A and B, where the presence of magnetic NPs on the crumpled silk-shaped graphene sheets suggests the formation of an rGO-Fe3O4 nanohybrid (NH). The TEM image (Fig. 10B) displays that the rGO sheet is translucent in nature and the Fe3O4 NPs are well distributed on the surface of the sheet. Meanwhile, TEM revealed the average particle size of about 20 nm, which is consistent with the SEM images. Hence, there was no apparent change in the morphological behavior of rGO-Fe3O4, The XRD pattern of rGO-Fe3O4 NCs is similar to that of graphene oxide Fe3O4 NCs (Fig. 10C). The central peaks at 2θ = 30.21° (220), 35.71° (311), 43.31° (400), 53.7° (422), 57.35° (511), and 62.72° (440) show the presence of Fe3O4 in both GO-Fe3O4 and rGO-Fe3O4 NCs.244 The wide diffraction peaks reveal the small crystalline size of NPs. Given that the Fe3O4 NPs have an analogous crystal structure to γ-Fe2O3, it was very difficult to differentiate them based on the XRD study. The XRD analysis revealed the presence of agglomerated graphene sheets with no carbon peak, which may be due to the fact that the magnetite NPs reduced the agglomeration of the sheets, resulting in a greater number of graphene sheet monolayers. Therefore, a weaker peak was observed for graphene and strong signal observed for Fe3O4 compared with the weak carbon peaks.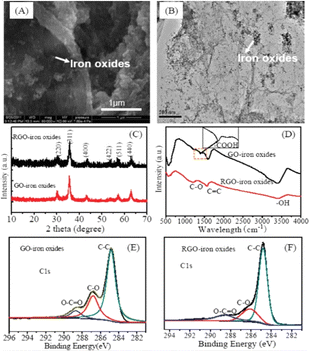 | ||
| Fig. 10 SEM (A) and TEM (B) images, XRD patterns (C), FTIR spectra (D), and high-resolution C 1s (E and F) XPS of GO-Fe3O4 NC and rGO-Fe3O4 NC hybrid materials. This figure has been reproduced from ref. 244 with permission from the Royal Society of Chemistry, Copyright 2012. | ||
The FTIR spectra assisted the identification of the functional groups present in the samples. Vibration stretching bands corresponding to O–H (3389 cm−1) of H2O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1578 cm−1) and epoxy C–O (1225 cm−1) were observed for GO-Fe3O4/rGO-Fe3O4 NCs (Fig. 10D). In the case of GO-Fe3O4, an additional band was observed at 1399 cm−1, which is attributed to the vibration stretching of the O–C
C (1578 cm−1) and epoxy C–O (1225 cm−1) were observed for GO-Fe3O4/rGO-Fe3O4 NCs (Fig. 10D). In the case of GO-Fe3O4, an additional band was observed at 1399 cm−1, which is attributed to the vibration stretching of the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. The peak at around 584 cm−1 is ascribed to Fe–O, and the increased intensity for the Fe–O band is ascribed to iron anchored on GO-Fe3O4/rGO-Fe3O4.
O group. The peak at around 584 cm−1 is ascribed to Fe–O, and the increased intensity for the Fe–O band is ascribed to iron anchored on GO-Fe3O4/rGO-Fe3O4.
The deconvolution of the C 1s peaks for GO-Fe3O4/rGO-Fe3O4 NCs is shown in Fig. 10E and F, respectively. The regions of the C 1s spectra could be deconvoluted into three constituents, as follows: (1) C–C (non-oxygenated) observed at 284.8 eV; (2) the C–O (carbon) peak displayed at 286.8 eV; and (3) the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carboxylate carbon) peak, which is located at 288.8 eV. The intensity of the O–C
O (carboxylate carbon) peak, which is located at 288.8 eV. The intensity of the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak declined in the C 1s spectrum of rGO-Fe3O4 as compared to that of GO-Fe3O4. The C/O ratio of GO-Fe3O4 and rGO-Fe3O4 is around 1.21 and 4.69, respectively, indicating that the oxygen functional groups in rGO-Fe3O4 were partially reduced.
O peak declined in the C 1s spectrum of rGO-Fe3O4 as compared to that of GO-Fe3O4. The C/O ratio of GO-Fe3O4 and rGO-Fe3O4 is around 1.21 and 4.69, respectively, indicating that the oxygen functional groups in rGO-Fe3O4 were partially reduced.
The morphological exploration of NiO and NiO/rGO hybrid films were done by SEM, as shown in Fig. 11. The NiO and NiO/rGO NC films displayed superior electrochromic properties with high coloring ability, fast exchanging speed and improved cycling performance, as described by Cai et al.245 The NiO NP film was porous in nature and exhibited a nanoscale interlocking network-like structure (Fig. 11a–c), which was synthesized by crumbly NiO with a particle size of about 10–15 nm. The morphological structure of both the synthesized NiO NPs and NiO/rGO hybrid film was nearly similar in nature (Fig. 11d–f). However, more open space was observed for the NiO/rGO hybrid film due to its more porous nature among all the synthesized materials, enabling the electrolyte to penetrate through the film and condense the proton dispersion tracks within the NiO bulk and the film thickness increased smoothly to 15–25 nm. The thickness of both the NP and NC films was 500 nm, as shown in Fig. 11c and f, respectively.
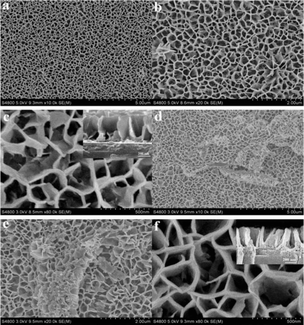 | ||
| Fig. 11 SEM images: (a)–(c) porous NiO film and (d)–(f) porous NiO/rGO hybrid film. Upper-right insets in (c) and (f) correspond to the side views of the films. Reproduced with permission from ref. 245. Copyright 2012, the Royal Society of Chemistry. | ||
Qian et al.246 described the fabrication of graphene/Mn3O4 NCs via the solvothermal method at 200 °C for 4 h, 8 h and 16 h, respectively, using a manganese(III) precursor. The stacking density of graphene was reduced to 70% from 100% and additionally up to 50–60%. These outcomes revealed an increase in particle size, i.e. 2 to 10 to 25 nm with an increase in the reaction time. The HR-TEM image revealed that the loading of the Mn3O4 NPs on the surface of graphene was in the crystallized form with the lattice spacings of about 0.25 nm and 0.29 nm (Fig. 12d), which are attributed to the (211) and (200) reflection planes of the tetragonal hausmannite Mn3O4 phase, respectively. The lattice fringes of graphene show that the number of graphene layers was about 4–5. The SAED pattern (Fig. 12e) shows deflection rings from the hausmannite Mn3O4 phase, with the reflection planes found to be at (112), (211), (220), (105), and (321). Fig. 12f shows the typical EDAX pattern of the graphene/Mn3O4 NCs, where the Mn3O4 NPs were anchored very well on the graphene sheet, proving the presence of Mn and O elements.
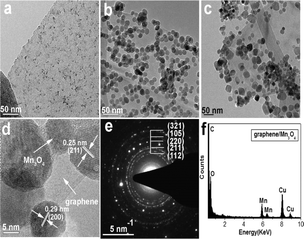 | ||
| Fig. 12 TEM analysis of graphene/Mn3O4 NCs: products prepared at reaction times of 4 h (a), 8 h (b), and 16 h (c); HRTEM image of attached Mn3O4 NPs (d) and corresponding SAED pattern (e); and (f) EDS pattern of Mn3O4 NPs. Reproduced with the permission from ref. 246. Copyright 2017, the Royal Society of Chemistry. | ||
Jeevitha et al. synthesized porous reduced graphene oxide (rGO/WO3) NCs. The pore size (particle distribution) and specific surface area of WO3 with different weight percentages (wt%) of rGO content were examined using the adsorption–desorption phenomenon. Fig. 13a–c show the surface area (BET) and pore size (BJH) of the pure WO3 and different (wt%) NCs (1% and 5% rGO/WO3). Astonishingly, all the samples exhibited type IV isotherms, demonstrating the mesoporous nature of the materials with a relative pressure (P/P0) between 0.1 and 0.9. The specific surface areas of 9.96, 12.53 and 21.464 m2 g−1 with an average pore size of 25.79, 31.68 and 29.41 nm were perceived for pure WO3 and 1% and 5% rGO/WO3, respectively.247 This result supports the improved performance of the 5% rGO/WO3 NCs, where the slight decrease in pore size may be due to the integration of a greater number of graphene layers.237
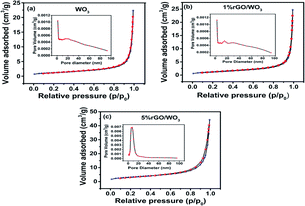 | ||
| Fig. 13 Nitrogen-adsorption/desorption isotherms and corresponding pore size distribution of (a) pure WO3, (b) 1% rGO/WO3 and (c) 5% rGO/WO3. This figure has been reproduced from ref. 247 with permission from the Royal Society of Chemistry, Copyright 2019. | ||
7. Photocatalytic applications
7.1. Photocatalysis
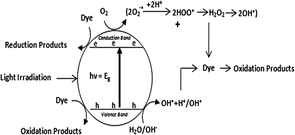
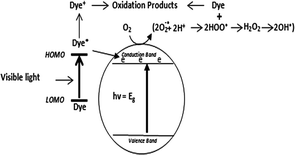
In recent years, a great deal of effort has been dedicated to solving the widespread pollution caused by effluents from urban and agricultural industries, which includes bio recalcitrant and organic pollutants.283 in the rapid industrialization and continuous progression of newer technologies coupled with poor environmental policies have led to the generation of toxic effluents in huge amounts, which are discarded into natural water bodies. The main components of this chemical discharge from the textile, chemical, petroleum and many other industries are azo dyes. These toxic dyes not only disturb the aquatic ecosystem but also have a devastating effect on human life and the terrestrial biota. These dyes are mutagenic, carcinogenic, highly toxic to the CNS and can cause respiratory diseases, cancer, genetic disorders, chromosomal abnormalities and many acute disorders. Moreover, these dyes remain in the environment for longer periods of time without degrading.284 Thus, in view of this situation, it has become imperative to reduce and remove these chemical pollutants (synthetic dyes) from the environment. In this regard, semiconductor-assisted photocatalysis has received global attention, given that the above-mentioned dyes are resistant to biological and physical treatment methods.285Heterogeneous semiconductor photocatalysts can absorb solar radiation to generate electron–hole pairs, which can accelerate the remediation of these dyes. A simple mechanism of heterogeneous photocatalysis includes absorption of light by a semiconductor, leading to the excitation of electrons from its valence band to conduction band, leaving holes in the VB. Fig. 14 and 15 depict the process of dye degradation on a semiconductor photocatalyst involving the indirect and direct pathways, respectively. In the indirect pathway, the dye is degraded by the reactive oxygen species formed on the catalyst surface. Subsequently, the photogenerated holes react with adsorbed water or hydroxyl ions to generate hydroxyl radicals, whereas the electrons in the CB react with oxygen to form superoxide radicals. These reactive oxygen species rapidly and non-selectively reduce pollutants, including dyes, antibiotics, drugs, and phenolic compounds. In the direct pathway using different sources of light irradiation, carbon-based dyes such as Rh–B, IC, TB, MO, CR and MB can also be excited in the photodegradation process, which is a phenomenon called photosensitization, and in this process, the dyes are excited from the ground state to the excited state. In the excited-state dyes, charge transfer occurs from the highest occupied molecular orbital (HOMO) to the conduction band of the photocatalyst, providing more electrons to produce superoxide radicals, significantly enhancing the photocatalytic degradation activity.286
However, the speedy recombination rate of photo-generated electron–hole pairs within photocatalytic materials results in their poor performance, which restrains their applications. Thus, to enhance the photodegradation property of semiconducting PCs, it is necessary to suppress the recombination of charge carriers. In addition, using the conventional metal doping and addition of surfactants, carbon-based semiconductor hybrid materials have emerged as a new era of PCs, which has attracted significant attention recently.286 Among the carbonaceous materials, graphene has received increasing attention due to its unique properties, such as high charge-carrier flexibility, high thermal and electrical conductivity, and extraordinary high surface area. These unique properties of graphene make it an ideal support material for semiconductor photocatalysts. Furthermore, the interaction of graphene with semiconductor materials give rise to unique properties such as comprehensive light absorption and decreased charge carrier recombination rates, accompanied by high stability.287 Therefore, graphene-semiconductor hybrid materials have been extensively used for the photodegradation of organic dyes, pharmaceutical drugs, antibiotics, organic phenolic compound, photocatalytic generation of hydrogen and disinfection. In this section, the main applications of graphene-assisted semiconducting photocatalysts such as NiO, TiO2, Fe3O4, and ZnO are briefly summarized.
7.2. Photocatalytic degradation of dyes
Nowadays, a significant challenge is the inadequacy of pure and potable water, and its accessibility is becoming increasingly luxurious on a daily basis. This is due to the disposal of several toxic wastes from many industries, pharmaceutical laboratories and agricultural field. Among these effluents, inorganic and organic dyes are a major issue of concern. Thus, scientists are focusing on biodegrading dyes and dye-derived components. Biopolymers are the most preferable choice for the degradation of dyes due to their enhanced catalytic activities, as widely reported thus far. Millions of people in developing countries have been affected by diseases due to the unavailability of clean and pure drinking water and a safe hygienic environment. Thus, it is necessary to develop ecofriendly, cheap and easy processes to biodegrade these toxic dyes for the purification of water as the sources of portable water are vanishing daily and becoming ponds of eutrophicated, toxic, dirty and polluted water bodies. The major pollutants found in water bodies include pesticides, herbicides, dyes, and surfactants.288 Thus, to overcome these problems, in the past few years, graphene-based photocatalysts have gained significant importance for photocatalytic applications. Due to their high photosensitivity, high surface area, excellent optical, mechanical and electrical properties, stability, eco-friendly and nontoxic nature, low cost and enhanced catalytic properties, they are being widely used in water cleansing and photodegradation of toxic dyes and chemical effluents.289Rahimi et al. developed rGO/NiO nanowires for the photocatalytic disintegration of methyl orange (MO), which required both hydroxide and superoxide radicals.248
In another study, Elshypany et al.249 described the solid-state-mediated magnetite zinc oxide (MZO) (Fe3O4/ZnO) with different ratios of rGO. The results revealed that the photodegradation activity of magnetite zinc graphene (MZG) was more than 98.5% against methylene blue. This was due to the synergistic effect of magnetite and zinc oxide in the presence of rGO.
Hamed et al.250 reported the preparation of Gr/Pd/TiO2 NPs and Gr/Pd/TiO2 nanowalls via the combination of both hydrothermal and photo-deposition methods. They compared the degradation capacity of TiO2 nanowalls, Gr/Pd/TiO2 NPs and Gr/Pd/TiO2-NWs towards Rhodamine B under UV light irradiation. The results showed that among them, the Gr/Pd/TiO2 nanowalls had the best photodegradation activity owing to their high surface area.
Similarly, Zhang et al.251 studied the photodegradation of MB in water by CuO–ZnO. Fe2O3/rGO and CuO–ZnO. Fe2O3/CNT. The degradation efficacy of the composite with CNT was better than rGO. Also, Chandra et al. demonstrated that graphene-Mn2O3 NCs showed almost 80% photodegradation activity against eosin, methylene blue, and rhodamine B.252 Benjwal et al. explained the enriched photocatalytic degradation of MB dye via rGO-based TiO2/Fe3O4 NCs with an efficiency of ∼100% within 5 min.253 Fu and co-workers synthesized magnetically separable graphene-based ZnFe2O4 PCs via a simple hydrothermal technique. The PCs revealed 88% degradation efficiency for MB, and further by adding H2O2 as a scavenging agent, the degradation activity was enhanced up to 99% at 90 min under visible light irradiation. It played a dual function as a photoelectron-chemical degrader and producer of hydroxyl radicals by the photo-electrochemical disintegration of H2O2.254 Lv et al. reported the preparation of a ZnO-rGO composite via a rapid and simple microwave-assisted technique for the photocatalytic degradation of MB. In this study, rGO played a vital role in enhancing the photocatalytic activity. The results suggested that the ZnO-rGO NCs with 1.1 wt% of rGO achieved a supreme degradation efficacy of 88% in a neutral solution under UV light illumination compared to p-ZnO NPs (68%), which is attributed to the increased absorption property towards light absorption and large reduction in charge recombination.255 Thangavel et al. demonstrated the photocatalytic behavior of synthesized β-SnWO4-rGO NCs. The photodegradation efficiency of 55% and 60% was achieved by β-SnWO4 NPs, whereas that of rGO-based NCs was found to be better at ∼90 and 91% for methyl orange and Rhodamine B dyes, respectively.256 Ag-Cu2O ternary NCs reinforced by glucose-rGO with enhanced stability and visible light-induced photocatalytic action were proposed by Sharma et al. via a simple and green methodology at room temperature using glucose and Benedict's solution. The fabricated Ag-Cu2O/rGO NCs showed excellent photocatalytic efficiency towards methyl orange dye, and their degradation rate was greater than the immaculate Cu2O and Cu2O/rGO NCs.257 Isari et al. prepared ternary NCs of Fe-TiO2 decorated on rGO via a simple sol–gel method. The DRS study of the fabricated PCs exhibited a tunable band gap, which introduces Fe ions into the titania structure. The maximum degradation efficiency for Rhodamine B was 91% in 120 min/sunlight using 0.6 g Fe-TiO2/rGO NCs, with an initial pH of 6 in the photodegradation study.258
7.3. Degradation of antibiotics and pharmaceutical drugs
Besides adsorption, antibiotics can be efficiently decayed or decomposed into non-toxic small molecular species under different wavelengths of light (such as sunlight, visible and ultraviolet (UV) light) due to the presence of molecular active groups (e.g. superoxide ions (O2) and hydroxyl radicals (˙OH)) produced by photocatalysts.249 Thus, the photocatalytic degradation technique is one of the most effective, operative, eco-friendly, and commonly used techniques for the degradation of antibiotic contaminants in the environment. Graphene, as an exciting material and encouraging PC, has been broadly explored for the photodegradation of antibiotic toxins in water due to its high surface area for uniform distribution, tunable band-gap, exceptional electrical conductivity, fast electron transport, and cost-effective fabrication for large-scale production.260 Generally, graphene-based photocatalysts are formed by adding other photocatalysts to overcome its disadvantages and improve its photocatalytic efficiency for degrading antibiotics. In the last few years, various efforts have been dedicated to developing and fabricating graphene-based semiconductor photocatalysts to increase the degradation capability of antibiotic pollutants. In this regard, Song et al. synthesized graphene/MnO2 NCs via an in situ one-step hydrothermal route, which successfully degraded ∼99.4% of the tetracycline residue in pharmaceutical wastewater.261 In another study, Shanavas et al. studied the photodegradation capacity for tetracycline and ibuprofen drugs proficiently under visible light illumination in 90 min using Cu/Bi2Ti2O7/rGO ternary NCs. The synthesized Cu/Bi2Ti2O7/rGO PCs showed excellent charge carrier capture and the suppression of e−–h+ pair recombination, and the rGO sheets and Cu NPs also played an important role in the photocatalytic performances.262 Lin et al. synthesized a TiO2-reduced graphene oxide series with side-glowing optical fibers coating via the polymer-mediated hydrothermal deposition technique. The fabricated NCs displayed significantly superior photodegradation activities compared to the purely synthesized TiO2. The utmost photocatalytic efficacy was perceived by 2.7% rGO, resulting in a degradation efficiency of about 54%, 81% and 92% for carbamazepine, ibuprofen, and sulfamethoxazole, respectively, under high-pressure UV illumination within 180 min, where the rate of mineralization of the pharmaceutical drugs was in the range of 52–59%.263 Pastrana-Martínez et al. synthesized a modified TiO2-GO composite via the liquid-phase deposition method for the degradation of diphenhydramine as a pharmaceutical drug, where the UV-visible light-assisted photodegradation of the drug was demonstrated. The modified TiO2-GO composites demonstrated total degradation activity and substantial mineralization of diphenhydramine waste product in less than 60 min under UV/vis illumination for the optimum 3.3–4.0 wt%/GO in the modified TiO2-GO composites. In another study, a family of cobalt NP-derived titania, functionalized by amine using graphene oxide as the base material was synthesized via the sol–gel and hydrothermal methods, revealing a remarkable degradation performance for oxytetracycline under both solar and visible light irradiation. The formation of a heterojunction between anatase phase titania and low concentration of Co3O4 NPs induced the photon-based oxidative degradation of oxytetracycline, and the addition of a small of amount GO enhanced its photodegradation activity. Amine-functionalized 2 wt% Co3O4/TiO2/GO revealed exceptional rates and stability toward the degradation of oxytetracycline under visible light irradiation.264 Similarly, Fe3O4/Mn3O4-rGO NCs were demonstrated to be an efficient photocatalyst for the photocatalytic degradation of an aqueous solution of sulfamethazine. The outcomes of the reaction shown 99% sulfamethazine photodegradation proficiency under the optimal conditions of 0.5 g L−1 concentration of Fe3O4/Mn3O4-rGO NCs, 0.07 mm L−1 sulfamethazine at pH 3 and temperature of 35 °C, and 6 mM concentration of H2O2.265 Karthik et al. fabricated GO-decorated cerium molybdate nanocubes (Ce (MoO4)2/GO) for the photodegradation of chloramphenicol under visible light illumination. The (Ce(MoO4)2/GO) NCs exhibited an excellent photodegradation efficacy of towards chloramphenicol drug with the highest degradation efficiency of 99% within 50 min compared with pure Ce(MoO4)2 nanocubes. The remarkable photocatalytic performance of the NCs was attributed to the excellent charge separation of the photogenerated electrons and holes.2667.4. Photocatalytic degradation of phenolic compounds
Fundamentally, organic dyes have been studied the most for the assessment of the photodegradation performance of graphene-based NCs.290 Among the toxic organic pollutants found in industrial wastes, compounds such as phenol and phenolic compounds are essential to remove from water bodies.291 The quantity of phenol in water must be retained at 0.1–1 mg L−1 (ppm) based on the environmental protection rules of the Pollution Control Board (1992). In this case, semiconductor PCs revealed enhanced photodegradation of phenol under stimulated solar/UV/visible irradiation after the integration of graphene.Graphene-based TiO2/ZnO NCs were prepared via a hydrothermal method by Malekshoar et al.,267 and the photocatalytic profile of the coupled NCs with an optimized ratio was explored. The results revealed that the combined graphene-ZnO/TiO2 NCs with a ZnO/TiO2 to graphene ratio of 0.95 to 0.005 was superior compared to the single composite by a factor of 2. Furthermore, for the optimization of the reaction conditions, different parameters were investigated. The experimental investigation revealed that 1 h and graphene-ZnO/TiO2 NCs were required for the degradation phenol (40 ppm) at neutral pH under solar irradiation of 100 mW cm−2. The GO-TiO2 NCs as a magnetic recyclable catalyst showed up to 99% degradation efficiency under UV irradiation within 60 min for carbamazepine and caffeine. Subsequently, the NCs were fully reusable and recoverable by magnetic separation. The additional advantages are that the GO-TiO2 NCs were fully reusable, recoverable, and easy to fabricate.268
8. Electrochemical performances
Currently, the energy and environmental crises, such as the exhaustion of fossil fuels, environmental pollution and global are significant concerns in society. Thus, it is necessary to develop renewable energy storage and conversion materials, as well as the corresponding devices. In this framework, these problems can be overcome in two ways. Firstly, energy can be effectually converted from endless sources, mainly by the storage and conversion of electric and light energy to provide alternative energy sources to fossil fuels, e.g. the oxygen reduction reaction (ORR), water and solar power to relevant forms, such as electricity and fuel. To accomplish this goal, fuel cells, solar cells and water splitting catalysts are required. Secondly, ecological benignity, cheap, and high performance are required by energy storage devices. This is essential because of the sporadic features of most renewable energy sources. Lithium-based batteries, including lithium ion, lithium–air and lithium–sulphur, are the most handy and capable devices for the storage of energy. Another encouraging and competent type device for energy storage is supercapacitors, which can store and release energy within a few seconds.292 In the fields of catalysis and energy storage, the replacement of precious and rare metal electrodes and catalysts with commercially available substitutes has attracted much consideration from both industrial and academic researchers. However, although numerous studies have been performed, including the development of non-precious metal electrodes and catalysts, the results lag far behind the practical values due to the inadequate performance of the discovered materials and environmental hazards. It is worth noting that both theoretical calculations and experimental values have demonstrated the considerable activity of low-cost metal-free materials with unique electronic and nanostructures properties in a wide range of heterogeneous, electrochemical and catalytic processes.Graphene, a material with outstanding properties, has gained popularity worldwide due to its applicability in an extensive range of applications, predominantly in the energy area. With the rapidly increasing population, an extreme necessity has arisen to explore alternative ways to meet the expanded energy demand, where the sources of non-renewable energy are limited. Especially, graphene-based metallic bimetallic and metal oxide NCs and graphene-based materials have gained immense popularity in the field of electrochemical energy storage. Because of their physico-chemical properties such as high thermal and chemical stability, large specific active surface area, excellent electrical conductive capability, good capacitance, and superior thermal and mechanical properties, graphene-based materials have been exploited as electrode materials in electrical energy storage devices.293 Moreover, their broad potential range and rich surface chemistry have allowed the properties of storage devices to be customized.294 Therefore, graphene-based metal oxides and polymer NCs have found wide applications in energy storage and energy conversion devices, such as batteries, supercapacitors, fuel and solar cells.295–297 In this feature review article, we mainly focus on the electrochemical properties of graphene and its application towards lithium-based ion batteries, supercapacitors and ORR for fuel cells (Table 3).
| Graphene-based metal oxide | Name of electrolyte | Capacity (mA h g−1)/capacitance (F g−1) | Stability (%) (cycles) | Power/energy density |
|---|---|---|---|---|
| Fe3O4/rGO | 1.0 M LiPF6 (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC, 1 DMC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1000 mA h g−1 | ||
| Graphene-LiMn2O4 | 1.0 M LiPF6 (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC, 1 DMC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
130 mA h g−1 | 87% (100) | |
| V2O3/rGO | 1.0 M LiPF6 (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC, 1 DMC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
823.4 mA h g−1 | 89.9% (100) | 4.0 |
| SnO2/rGO | 1.0 M LiPF6 (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC, 1 DMC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1718 mA h g−1 | 90.1% (50) | 500 |
| ZnFe3O4/GO/Chitosan | 1.0 M H2SO4 | 830 F g−1 | 650 | |
| rGO/ZnO | 1 M Na2SO4 | 312 F g−1 | 95.2% (1000) | |
| Graphene/Ni3V2O8 | 2 M aq. KOH | 748 F g−1 | 71% (3000) | 45.61 |
| ZnO–CuO/N-rGO | 1 M aq. KOH | 1075 F g−1 | 88% (5000) | 1601 |
| SnO2/Co3O4/rGO | 1 M H2SO4 | 317.2 F g−1 | 1.2 | |
| NiO@NiMoO4@PPy | 1 M aq. KOH | 941.6 F g−1 | 77.1% (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
|
| TrGO/Ni | 1 M KOH | 154.3 mA h g−1 | 90.2% (2000) | |
| H-GTN | 0.5 M BMIM-BF4 50/CH3CN | 530 F g−1 | 90.2% (100) | |
| Co–Ni/LDH nanosheets | 3130.8 F g−1 | 72.4% (3000) | ||
| rGO@Fe3O4 | 1 M KOH | 771.3 F g−1 | 95.1% (5000) | |
| ZnOL@MpEG | 1 M Na2SO4 | 347 F g−1 | 5.0 |
8.1. Lithium-ion batteries (LIBs)
The ever-increasing requirement for advanced energy-density storage devices for power storage (power levelling) and transportation (electric-vehicles) is exciting for the scientific community to develop rechargeable batteries with a cycling performance comparable to that of Li-ion batteries but with significantly higher capacity. Carbon-constructed rechargeable batteries have attracted wide broad attention, specifically after the commercialization of Li-ion batteries by Sony laboratories, where metallic Li-ion is substituted by a carbon host structure, whereas the lithium ions can be absorbed and released reversibly at low electrochemical potentials. LIBs are considering encouraging candidates for the effective storage of energy owing to their high energy density and extensive life cycle. Their high definite capacity, enormous power density, and extended cyclic life are capable power cradles for electric vehicles. Although LIBs are the dominant power sources, their cathode and anode materials limit their energy density due to their nature and different characteristics. The most common configuration of LIBs is composed of an intercalated Li compound cathode (e.g., LiCoO2 and LiFePO4), with a graphitic anode and corresponding organic carbonate electrolyte, with the graphitic anode attaining the highest energy densities id around 120–150 W h kg−1.At present, LIB technology is limited by the low theoretical specific capability of the standard graphite anodes as (372 mA h g−1). Thus, the present research is focused on developing alternative anode materials, for example Si (4200 mA h g−1), Sn (994 mA h g−1), and SnO2 (782 mA h g−1).
In the last two decades, due to the huge ‘surface to mass ratio’ beyond 2600 m2 g−1, with tremendous electrical conductivity and high and mechanical power with the extra value of large-scale production of graphene, it has emerged as an exciting anode material for LIBs. As a single layer, graphene has an inadequate competency to absorb Li-ions (surface coverage of 5%) due to the revulsion forces within the Li+ ions at each side of the graphene layer, and thus significant work has been devoted to the exploitation of chemically modified graphene such as (GO) and (RGO), both at the anode and cathode positions. Graphene has also been used to anchor electrochemically energetic transition metal oxides, sulfides, nitrides, phosphides, and elements with alloying/dealloying mechanisms, for instance semiconductors, metals, and others.296 Additionally, polymer electrolyte membranes (PEMs) are used for energy conversion and storage devices as an electrolyte or separator, which are essential components. PEMs play an important role in the devices prepared using functional polymers. Various PEMs comprised of several fillers have been established to accomplish the requirements of energy devices. Also, graphene-based NC anode materials have been synthesized for Li-ion batteries with enhanced performances. The enhancement in electrochemical performance of graphene-based NCs can be attributed to three aspects, as follows: (i) graphene layers possess flexibility, which suppresses the bulk expansion of these electrochemically energetic materials during the continuous process of charging/discharging and improves the accumulation/liquidation problems; (ii) the exceptional electrical conductivity of graphene ensures notable electrical interaction between neighboring heterogeneous particles. As discussed previously, graphene has a large-surface area, surface deficiencies and high porosity, giving rise to high Li storage capability, enhanced electrolyte accessibility and rapid Li-ion diffusion. Finally, (iii) confining electrochemically active NPs between graphene layers can mitigate the restacking of the graphene sheets layers, and subsequently retain their large surface area activity. These synergistic effects of both graphene sheets and NPs can combine the best qualities of both materials.
Tarascon's group reported the first study on metal oxides as anode materials with high capacity. Although metal oxides generally exhibit poor conductivity, properly tailored metal oxides on the nanoscale have demonstrated promising characteristics. The reaction mechanism of lithiation and delithiation in metal oxides can be generally classified into three main types (Fig. 16), as follows: (1) insertion/extraction, (2) alloying/dealloying, and (3) conversion mechanisms. The first mechanism is observed in different types of anode materials, MOx (including anatase TiO2). In fact, most cathode materials with layered or spinel structures also follow the insertion–extraction mechanism, as discussed previously. Alloy reactions exist in some main-group elements, including Si, Sb, Ge, Bi and Sn, which can alloy with lithium, forming LixM and providing a high specific capacity. However, the lithium alloying and dealloying processes are typically associated with huge volumetric expansion (as high as 300%) and shrinkage, or pulverization, leading to capacity fading upon cycling. The third mechanism of conversion is typically observed in transition metal oxides (MxOy, M = Mn, Fe, Co, Ni, Cu, etc.). These conversion-type materials have relatively high theoretical capacities because they can incorporate more than one Li per metal. Metal grains and Li2O are formed during the process of lithiation. In many cases, the metals can reversibly alloy with lithium. Metal oxides also have the issue of pulverization, huge first-cycle irreversible capacity loss, as well as poor conductivity. Thus, to overcome these problems, much effort has been focused on the preparation of nanoscale metal oxides and MxOy/carbon composites.297–299
The three types electrochemical reactions are as follows:
(1) Insertion reaction mechanism:
| MOx + yLi+ + ye− ↔ LiyMOx | (1) |
(2) Li-alloy reaction mechanism:
| MxOy + 2yLi+ + 2ye− → xM + yLi2O (e.g., SnO2) | (2) |
| M + zLi+ + ze − ↔ LizM | (3) |
(3) Conversion reaction mechanism:
| MxOy + 2yLi+ + 2ye− ↔ xM + yLi2O | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively, in an argon-filled glove box. Most significantly, the full cells assembled using these novel nanostructures as anodes and LiNi1/3Mn1/3Co1/3O2 as cathodes exhibited excellent capacity retention at various current rates. In another study, Kumar et al.271 developed a 2D GO sheet enveloping 1D LiMn2O4 as NCs. The working lithium ion battery electrode was prepared with 80% active material, 10% super phosphorus and 10% polyvinylidene fluoride on Al foil. The NCs 3D porous cathode showed a high specific charge capacity of 130 mA h g−1 at a rate of 0.05C and coulombic efficiency of ∼98% after 100 cycles in the potential window of 3.5–4.3 V vs. Li/Li+ with encouraging initial charge capacity retention of ∼87%, and exceptional structural stability even after 100 cycles, giving rise to a promising cathode NCs. Xiao et al.272 successfully fabricated (V2O3/rGO) NCs via a simplistic solvothermal method and annealing process. In this synthesis protocol, V2O3 NPs were encapsulated by rGO. A coin-type cell (CR2025) was used in the electrochemical measurement. A LAND battery cycler was used to analyze the charge–discharge and cyclic performances of the cells over the potential range cs. Li/Li+ at 25 °C. The Li+ ion storage behaviour of V2O3/rGO was explored in the potential range of 0.01–3.0 V. The V2O3/rGO NCs achieved a high reversible specific capacity of 823.4 mA h g−1 under the current density of 0.1 A g−1, and 407.3 mA h g−1 under the high current density of 4.0 A g−1. Naresh et al. prepared SnO2/rGO NCs via a microwave-mediated hydrothermal technique. The Li-ion batteries fabricated using pure globular-shape SnO2 NPs and crumpled nanosheet-like morphology of SnO2/rGO NCs, which acted as the anode material, exhibited the good initial discharge–charge capacity of 2128 and 1718 mA h g−1, respectively. The capacity retention after 50 cycles was found to be 349 mA h g−1 at a current density of 500 mA g−1 for the Li-ion battery fabricated using pure globular-shape SnO2 NPs and its capacity retention after 300 cycles was found to be 318 mA h g−1 at a current density of 500 mA g−1 for the Li-ion battery fabricated using SnO2/rGO NCs, which was much better than the reported values.273 A modified glassy carbon electrode (GCE) was prepared by mixing the synthesized NMs with carbon black and PVDF in the weight percentage of 80
10, respectively, in an argon-filled glove box. Most significantly, the full cells assembled using these novel nanostructures as anodes and LiNi1/3Mn1/3Co1/3O2 as cathodes exhibited excellent capacity retention at various current rates. In another study, Kumar et al.271 developed a 2D GO sheet enveloping 1D LiMn2O4 as NCs. The working lithium ion battery electrode was prepared with 80% active material, 10% super phosphorus and 10% polyvinylidene fluoride on Al foil. The NCs 3D porous cathode showed a high specific charge capacity of 130 mA h g−1 at a rate of 0.05C and coulombic efficiency of ∼98% after 100 cycles in the potential window of 3.5–4.3 V vs. Li/Li+ with encouraging initial charge capacity retention of ∼87%, and exceptional structural stability even after 100 cycles, giving rise to a promising cathode NCs. Xiao et al.272 successfully fabricated (V2O3/rGO) NCs via a simplistic solvothermal method and annealing process. In this synthesis protocol, V2O3 NPs were encapsulated by rGO. A coin-type cell (CR2025) was used in the electrochemical measurement. A LAND battery cycler was used to analyze the charge–discharge and cyclic performances of the cells over the potential range cs. Li/Li+ at 25 °C. The Li+ ion storage behaviour of V2O3/rGO was explored in the potential range of 0.01–3.0 V. The V2O3/rGO NCs achieved a high reversible specific capacity of 823.4 mA h g−1 under the current density of 0.1 A g−1, and 407.3 mA h g−1 under the high current density of 4.0 A g−1. Naresh et al. prepared SnO2/rGO NCs via a microwave-mediated hydrothermal technique. The Li-ion batteries fabricated using pure globular-shape SnO2 NPs and crumpled nanosheet-like morphology of SnO2/rGO NCs, which acted as the anode material, exhibited the good initial discharge–charge capacity of 2128 and 1718 mA h g−1, respectively. The capacity retention after 50 cycles was found to be 349 mA h g−1 at a current density of 500 mA g−1 for the Li-ion battery fabricated using pure globular-shape SnO2 NPs and its capacity retention after 300 cycles was found to be 318 mA h g−1 at a current density of 500 mA g−1 for the Li-ion battery fabricated using SnO2/rGO NCs, which was much better than the reported values.273 A modified glassy carbon electrode (GCE) was prepared by mixing the synthesized NMs with carbon black and PVDF in the weight percentage of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively, using NMP solvent and grinding in a mortar. Alternatively, for the pure NPs, a 70
10, respectively, using NMP solvent and grinding in a mortar. Alternatively, for the pure NPs, a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 weight percentage was used. Lithium metal was used as the reference electrode, polypropylene (Celgard 2400) used as the separator and a mixture of 1 M LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1
10 weight percentage was used. Lithium metal was used as the reference electrode, polypropylene (Celgard 2400) used as the separator and a mixture of 1 M LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the electrolyte for the fabrication of Swagelok-type lithium-ion cells. The CV and GCD capacities of the fabricated Swagelok-type lithium-ion cells were recorded using a BCS 810 potentiostat (Biologic Instrument).
1) as the electrolyte for the fabrication of Swagelok-type lithium-ion cells. The CV and GCD capacities of the fabricated Swagelok-type lithium-ion cells were recorded using a BCS 810 potentiostat (Biologic Instrument).
8.2. Supercapacitors
Supercapacitors are new higher energy storage devices with exclusive features such as high capacitance, high-power density, and very long cycle life, which are also called ultracapacitors/electrochemical capacitors. Supercapacitors have become a research hotspot among the new energy storage devices due to their unique characteristics of rapid charge–discharge rate, high-power density, long-term cycling stability, eco-friendly nature, light weight, low cost, flexibility, and high competence, which remain a challenging task in research. Ultracapacitors working based on electrochemical double layer capacitance (EDLC) are electrical energy storage devices storing and releasing energy by nanoscopic charge separation at the electrochemical interface between the electrode and electrolyte. In EDLC, energy is deposited through polarization, followed by the adsorption of ionic charges on the surface of the electrode, and hence the surface area of the electrode plays a significant role.300 Because of their high specific surface area, porous nature, chemical inertness, and good electrical conductivity, carbon-based electrodes are promising for the fabrication of high-performance supercapacitors.301 In contrast, graphene-based conducting polymers and metal oxides are favorable candidates for pseudocapacitance because of their electrochemically active nature and ability to carry out the faradaic redox reactions.302 Low voltage spaces are the main obstacle for carbon-based materials to fully exploit their large surface area to accrue maximum charge and show greater capacitance. Organic electrolytes have been applied to increase the operational voltage windows instead of aqueous electrolytes, but their low thermal stability causes capacity fading. In this regard, ionic electrolytes have shown exciting results with large organic ions, low vapor pressure, wide liquid range, thermal stability, good electrochemical, and high ionic conductivity, together with extensive potential windows (typically, between 3 and 7 V).303 Therefore, moisture adversely affects the performance of ionic electrolytes, and thus a highly sophisticated vapor-controlled process is required to utilize ionic electrolytes. However, this problem was successfully addressed by using hydrophobic ionic electrolytes. To improve the capacitance performance of carbon-based materials, scientists have employed different morphologies, e.g. wrinkled and 3D networks, and composites of various carbonaceous materials such as graphene/CNT and polymers.304Presently, three types of pseudocapacitive mechanisms are known, as follows: (i) underpotential deposition, (ii) redox pseudocapacitance, and (iii) intercalation pseudocapacitance. Underpotential deposition occurs when the one metal ion forms an adsorbed monolayer on the surface of another metal with a higher redox potential. One of the classical examples is lead ions stacked on the surface of a gold electrode (Fig. 17a).
| Au + xPb2+ + 2xe− ↔ Au·xPbads |
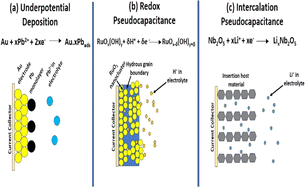 | ||
| Fig. 17 Different types of reversible redox mechanisms that give rise to pseudocapacitance: (a) underpotential deposition, (b) redox pseudocapacitance, and (c) intercalation pseudocapacitance. | ||
Redox pseudocapacitance the most predominant pseudocapacitive reaction that appears when ions are electrochemically engaged or adsorbed on the surface or near-surface of materials together with faradaic charge transfer (Fig. 18b). These two categories of pseudocapacitance are commonly applied in aqueous systems, which lead to a narrow potential window and limited energy density.
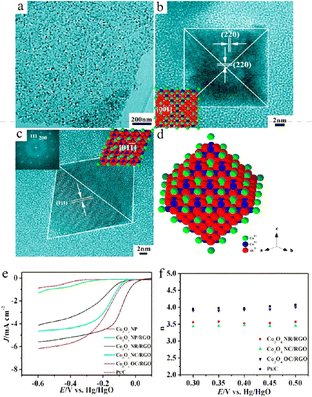 | ||
| Fig. 18 (a) Low- and (b and c) high-resolution TEM images of Co3O4 nano-octahedron (OC) supported on the rGO sheet, with (d) schematic image of Co3O4 OC bounded by eight {111} surfaces. (e) Rotating-disk voltammograms of the Co3O4/rGO electrocatalysts made of Co3O4 and (f) electron transfer number deduced from the Koutecky–Levich equation. Reproduced with permission from ref. 281. Copyright 2013, Springer-Nature. | ||
Herein, we discuss a representative structure (Fig. 17b), i.e., RuO2‚ xH2O nanocluster arrayed electrode, for this application. In this scheme, the crystalline RuO2‚ xH2O nanotubes (NTs) (and the RuO2‚ xH2O underlayer) with metallic conductivity provide electron “superhighways” for charge storage and delivery. The arrayed, porous architecture of the RuO2‚ xH2O NTs substantially reduces the resistance to electrolyte penetration/diffusion.305 Moreover, the hydrous nature of RuO2‚ xH2O promotes the rate of proton exchange (very different from the anodic aluminum oxide-based, anhydrous RuO2 nanotubes prepared by means of thermal decomposition) given that the superficial redox transitions of RuO2 involve a proton and electron double injecting/expelling process, which can be expressed as follows:
| RuOa·(OH)b + δH+ + δe− ↔ RuOa−δ (OH)b+δ |
Intercalation pseudocapacitance with a charge storage process takes place in the bulk material, exhibiting different electrochemical features from redox pseudocapacitance and underpotential deposition in a non-aqueous electrolyte system.
Conway previously suggested that this intercalation process is an intermediate behaviour between batteries and supercapacitors, where Li+ ions are actually accommodated in quasi-two-dimensional planes in the van der Waals gap of the host lattice material (Fig. 17c).
The proposed energy storage mechanism of “intercalation pseudocapacitance” has enormously expanded the research field of pseudocapacitive materials. The first to be uncovered was the pseudocapacitive mechanism of lithium-ion insertion into many polymorphic forms, such as pseudohexagonal (TT-Nb2O5), orthorhombic (T-Nb2O5), tetragonal (M-Nb2O5), and monoclinic phases (H-N Nb2O5). Amongst them, T-Nb2O5 is an orthorhombic phase crystal, which has been proven to be the most potential candidate for intercalation-type pseudocapacitive energy storage.306 T-Nb2O5 is mainly composed of highly distorted octahedral and pentagonal bipyramidal Nb environments. Its unit cell has sheets of edge- or corner-sharing distorted polyhedra lying parallel to the (001) plane, with each Nb5+ surrounded by either 6 or 7O2−. The mainly empty octahedral sites between the (001) planes offer suitable tunnels for Li+ transport throughout the ab plane. Charge storage from the intercalation of Li+ into Nb2O5 can be indicated as follows:
| Nb2O5 + xLi+ + xe− ↔ LixNb2O5 (0 ≤ x ≤ 2) |
Suneetha et al. prepared zinc-doped iron oxide/graphene oxide/chitosan NCs using an optimized quantity of constituents via a simple solution casting method. The bandgap was calculated to be 2.28 eV, which specifies the conducting nature of the composite. The formation of the composite in nano dimensions of around 20 nm was proven by XRD, SEM and HRTEM studies. The electrochemical profile of the composite was examined by cyclic voltammetry and capacitive performance by impedance studies (EIS). Three electrodes were used for the measurement cyclic voltammetry. The working electrode was made by coating a glassy carbon with the NCs, while 1.0 M sulphuric acid-containing NCs were casted on GC as the working electrode. The electrochemical studies showed the good adherent nature of the composite on the electrode surface at pH 1 and greater electrochemical stability with well-defined redox peaks. EIS showed that the NC-modified electrode revealed a good capacitance performance with a Bode phase angle of 87°, which verifies that it is a good candidate for supercapacitor applications.274,275 Jayachandrian et al.276 fabricated ZnO and rGO/ZnO NCs as electrode materials for application in supercapacitors. A straightforward and economical technique was used for the fabrication of rGO/ZnO NCs via ultrasonication. The electrode was prepared with 80 wt% of active material, 10 wt% of acetylene black and 10 wt% of PVDF mixed using NMP solvent and grinding in a mortar. The prepared electrode was examined with the help of by cyclic voltammetry, chromo-potentiometry (CP), cycling stability and electrochemical impendence spectroscopy (EIS). The electrochemical performance of rGO/ZnO NCs displayed that the synthesized composite has a strong specific capacitance (312 F g−1) in comparison with pure ZnO NPs (200 F g−1) with improved cycling stability up to 1000 cycles. Low et al.277 synthesized graphene/Ni3V2O8 NCs via the solvothermal method as a facile approach. Liquid-phase exfoliation is an ecofriendly approach for the fabrication of graphene sheets with the optimized volume ratio of ethanol to water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The electrochemical properties and the integrated advantages of the graphene sheet and pseudocapacitive nature of Ni3V2O8 resulted in a superior electrochemical performance to the pristine Ni3V2O8, demonstrating that it is a promising material. The working electrode was prepared with PVDF as the binder and carbon black as the conducting agent in the presence of NMP solvent, while 2 M KOH was used as the electrolyte for application in Li-ion batteries. CV and EIS were performed on an electrochemical workstation, and the GCD and cyclic stability test were carried on an Arbin instruments (BT-2000) using G 4NVO (graphene/Ni3V2O8 with the weight ratio of 1
3). The electrochemical properties and the integrated advantages of the graphene sheet and pseudocapacitive nature of Ni3V2O8 resulted in a superior electrochemical performance to the pristine Ni3V2O8, demonstrating that it is a promising material. The working electrode was prepared with PVDF as the binder and carbon black as the conducting agent in the presence of NMP solvent, while 2 M KOH was used as the electrolyte for application in Li-ion batteries. CV and EIS were performed on an electrochemical workstation, and the GCD and cyclic stability test were carried on an Arbin instruments (BT-2000) using G 4NVO (graphene/Ni3V2O8 with the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). The nanocomposite delivered the high specific capacitance of 748 F g1 at a current density of 0.5 Ag−1, remarkable energy density of 103.94 W h kg−1 at a power density of 45.61 kW kg−1, reasonable cycling stability with 71% capacitance retention and superior coulombic efficiency of 83% after 3000 charge–discharge cycles at 0.5 Ag−1. Maity et al.278 fabricated plate-like CuO and flower-like ZnO anchored on rGO and nitrogen-doped rGO following a simple large-scale production, economical, facile hydrothermal technique. XRD, FTIR and XPS studies authenticated the presence of the N-doped material on rGO sheets in all the synthesized composites. The electrochemical performance of all the composites was investigated by CV, GCD and EIS in a three-electrode configuration using 1 M aqueous KOH, platinum foil as the counter and Ag/AgCl as the reference electrode. The electroactive materials, dispersed in ethanol and mixed with 0.5% Nafion as binder, were mounted on a pre-acid-treated graphite rod (working electrode). They observed the high energy density (ED) value of the synthesized NCs to be 95.55 W h kg−1 with a power density (PD) of 399.97 W kg−1. The electrode made up of hybrid ZnO–CuO/N-rGO NCs revealed admirable cyclic stability of 88% specific capacitance retention after 5000 cycles. In another study, Nagarani et al.279 fabricated SnO2/Co3O4/rGO NCs via the conventional technique to estimate their enhanced electrochemical properties for supercapacitor applications. The modified glassy carbon electrode (GCE) was prepared by mixing the synthesized NMs with carbon black and PVDF in a weight percentage of 80
4). The nanocomposite delivered the high specific capacitance of 748 F g1 at a current density of 0.5 Ag−1, remarkable energy density of 103.94 W h kg−1 at a power density of 45.61 kW kg−1, reasonable cycling stability with 71% capacitance retention and superior coulombic efficiency of 83% after 3000 charge–discharge cycles at 0.5 Ag−1. Maity et al.278 fabricated plate-like CuO and flower-like ZnO anchored on rGO and nitrogen-doped rGO following a simple large-scale production, economical, facile hydrothermal technique. XRD, FTIR and XPS studies authenticated the presence of the N-doped material on rGO sheets in all the synthesized composites. The electrochemical performance of all the composites was investigated by CV, GCD and EIS in a three-electrode configuration using 1 M aqueous KOH, platinum foil as the counter and Ag/AgCl as the reference electrode. The electroactive materials, dispersed in ethanol and mixed with 0.5% Nafion as binder, were mounted on a pre-acid-treated graphite rod (working electrode). They observed the high energy density (ED) value of the synthesized NCs to be 95.55 W h kg−1 with a power density (PD) of 399.97 W kg−1. The electrode made up of hybrid ZnO–CuO/N-rGO NCs revealed admirable cyclic stability of 88% specific capacitance retention after 5000 cycles. In another study, Nagarani et al.279 fabricated SnO2/Co3O4/rGO NCs via the conventional technique to estimate their enhanced electrochemical properties for supercapacitor applications. The modified glassy carbon electrode (GCE) was prepared by mixing the synthesized NMs with carbon black and PVDF in a weight percentage of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively. The electrochemical properties of the modified electrode were investigated in a standard three-electrode cell, which also included a platinum electrode (counter) and Ag/AgCl (reference electrode). The electrochemical properties of the composites were studied be performing CV, GCD and EIS on an electrochemical workstation. BTMO/rGO NCs with a modified surface to volume ratio and enhanced electron conductivity were observed to have an enlarged integral area and current of 2.5117 × 10−4 A s−1 and 3.1686 × 10−4 A, respectively, in CV. The NCs also showed an increased specific capacitance value of 317.2 F g−1 at 1 A g−1. The increased specific capacitance value of BTMO/rGO NCs was largely due to the synergistic effect between SnO2/Co3O4 and rGO.
10, respectively. The electrochemical properties of the modified electrode were investigated in a standard three-electrode cell, which also included a platinum electrode (counter) and Ag/AgCl (reference electrode). The electrochemical properties of the composites were studied be performing CV, GCD and EIS on an electrochemical workstation. BTMO/rGO NCs with a modified surface to volume ratio and enhanced electron conductivity were observed to have an enlarged integral area and current of 2.5117 × 10−4 A s−1 and 3.1686 × 10−4 A, respectively, in CV. The NCs also showed an increased specific capacitance value of 317.2 F g−1 at 1 A g−1. The increased specific capacitance value of BTMO/rGO NCs was largely due to the synergistic effect between SnO2/Co3O4 and rGO.
8.3. Oxygen reduction reaction for fuel cells
Fuel cells are energy conversion devices, where chemical energy is converted to electrical energy using oxidizing fuel catalyzed by different catalysts, which are restrained on the electrodes. Fuel cells produce electricity by reactions occurring between the fuel at the anode and oxidant at the cathode, which are uninterruptedly delivered from external sources. Fuel cells have huge potential as clean and competent power sources for EVs because of their high energy translation efficiency, low operation temperature, low or zero emission, high energy and power density. However, the slow kinetics of the ORR (O2 + 4H+ + 4e/2H2O) is the main aspect limiting the energy conversion competence of fuel cells and their large-scale commercialization. Hence, developing capable electrocatalysts for the OR reaction at the cathode in fuel cells is necessary.307 In a fuel cell, polymer electrolyte membrane fuel cells (PEMFC) are considered exciting materials for electrochemical devices owing to their high power density and low greenhouse gas emissions.308 Among the numerous types of fuel cells, low-temperature PEMFCs have been widely investigated due to their extraordinary features such as high competence and speedy start-up.42 Graphene and its derivatives are of particular interest in PEMFCs.45–47 Among the oxidized forms of graphene, graphene oxide has been regularly used.309 However, pure graphene oxide is an electronic insulator with a differential conductivity in the range of 1 to 5 × 10−3 Scm−1 at a bias voltage of 10 V.310Wu et al. synthesized an N-doped rGO anchored Fe3O4 NP aerogel with enriched ORR activity compared to N-doped carbon soot and N-doped graphene sheets and sustained a close to 4-electron transfer even at a low voltage.280 To gain insight into the ORR activity of the Fe3O4 NPs supported on the 3D macroporous N-GAs, the electrocatalytic properties of Fe3O4/N-Gas were investigated in N2- and O2-saturated 0.1 M aqueous KOH electrolyte solution using cyclic voltammetry at a scan rate of 100 mV s−1. The restrained Fe3O4 NPs within the graphene layers were thought to enrich the interfacial charge transfer contact between the metal oxide and the graphene sheet, while protecting the NPs against dissolution and agglomeration. A similar structure of CoO on N-doped rGO showed excellent durability compared to Pt/C. By synthesizing Co3O4 nanocubes (NC), nanorods (NR), and nano-octahedra (OC) on rGO, where each shape was defined by different facets, Xiao et al.281 established that the ORR activity follows the sequence {111} > {100} ≫ {110} (Fig. 18). This demonstrated the surface structure-dependent electrocatalytic activity of Co3O4 NPs anchored on graphene sheets toward the oxygen reduction reaction. The ORR kinetics of the Co3O4/rGO composites was investigated using the rotating-disk electrode (RDE) technique in O2-saturated 0.1 M KOH electrolyte. Similarly, flower-shaped MnO2 NPs decorated on rGO showed great selectivity toward four-electron reduction and high tolerance toward methanol. By replacing Co3+ with Mn3+ in the spinel MnCo2O4/N-doped rGO, it was possible to achieve greater ORR activity than Pt/C at a medium overpotential with high selectivity toward the 4-electron transfer reaction. Other spinel mixed oxide composites such as NiCo2O4/rGO and CuCo2O4/N-doped rGO NCs have shown huge potential in ORR in terms of methanol tolerance and high durability, respectively.282
9. Conclusion, future perspectives and challenges
This review article presented the most recent literature with a focus on the recent research progress in graphene and graphene-based metal oxide NCs. Initially, we discussed various eco-friendly methods for the synthesis of GO and rGO sheets with, highlighting the latest available bottom-up approaches for the fabrication of bioinspired graphene/rGO using GO as an initiator. Thus, the precise control of surface defects and superficial-scale production of GO or rGO NSs are highly anticipated. Efforts should be devoted to the establishment of cost effective, eco-friendly, stable and recyclable NMs for large-scale application at the industrial level with significant electrochemical properties and photocatalytic performance. Furthermore, we discussed some of the most recently established advanced green methods for the large-scale synthesis of graphene-based metal oxide NCs, focusing on the conventional and bioinspired synthesis of graphene-based metal oxides NCs. Especially, the photosynthesis of graphene-based metal oxide NCs has emerged as a remarkably attractive methodology in the last decade. Numerous varieties of plant extracts (bio-constituents including plants, fungi, algae, bacteria, yeast and other plant-mediated extracts) have been considered as efficient resources for the synthesis/fabrication of several graphene-based materials. Among the aforementioned components, plant extracts were shown to exhibit supreme efficiency as reducing, stabilizing agent and capping agents for the controlled synthesis of materials, i.e., shapes, sizes, structures, surface area, crystallinity and other specific features. The controlled synthesis of these NMs inhibits the restacking of the graphene NSs and provides awesome templates for the preparation of porous networks with improved electronic and electrical properties. This wide range of graphene nanohybrid structures such as metal oxide-encapsulated, sandwiched, anchored, wrapped, mixed and layered structures with graphene has been studied. Furthermore, graphene nanohybrids with various nano-architectures and particle size, pore size and shape, conductive nano-channels, 2D to 3D interconnected network structures, spongy framework, etc. have huge potential for many industrial applications in the field of catalysis and energy storage system have also been demonstrated.Lastly, we concluded this review with inclusive descriptions of the applications of graphene-based NCs as semiconductor photocatalysts for the degradation of dyes, pharmaceutical drugs, antibiotics and phenolic compounds. In this photocatalytic study, graphene-based semiconductor photocatalysts, mechanistic properties, newest literature for considering the optimization parameters of the system such as photocatalyst dose, initial dye concentration, pH, temperature, light intensity, presence of oxidizing agents/electron acceptors and the presence of ionic compounds were examined accurately and the optimum degradation efficiency discussed. This review highlighted several morphologies connected with novel constructions based on NH materials, which greatly help in improving the electrochemical performance of graphene-based metal oxide NCs as electrodes with enhanced charge-storage capacity, increased rate capability, improved reliability of devices with cycling stability, and increased energy and power densities, galvanic charge discharge, pseudo-capacitance nature, and reaction mechanism of anode or electrode materials for LIBs and super-capacitors.
The major challenge in the synthesis of graphene and graphene-based NCs is their large-scale production, which needs tremendous efforts. The challenges are as follows: (i) how to prevent the accumulation of graphene in the matrix, (ii) how to regulate the surface defects in graphene; and (iii) how to enhance the desired application-based properties. There are different methodologies for the synthesis graphene. Although the green synthesis or reduction of graphene oxide is a cost-effective, environmentally friendly, and simple approach for the reduction of graphene oxide, it results in poor conductivity or defects in the graphene sheets, which are also major challenges in the large-scale production of reduced graphene oxide using phytoextracts. In case of energy devices, security is a main attention in anode design for viable batteries. Actual battery conditions are often more complicated than laboratory experimental conditions. The mechanical stability and safety of the battery cannot be overlooked. Instantaneously, the selection of materials for the production of battery electrode materials should avoid noxious and destructive substances. The use of eco-friendly or non-hazardous complexes and composites should be preferred, not only to protect consumers but also to reduce the risk to the surroundings, which is noble for reprocessing.
Author contributions
Conceptualization: A. K. P., S. K. T. A., M. M. A., S. R. W. and R. G. C.; visualization: A. K. P., S. K. T. A., A. S. K., R. S. M., and A. D.; supervision: S. H. W., S. H. M., A. A. A., R. A, and R. G. C.; writing–original draft: A. K. P., S. K. T. A., M. M. A., A. K., R. M., and R. G. C.; writing, reviewing & editing: A. D., A. A. A., S. H. M., R. A., and R. G. C.Consent for publication
All the consent has been reproduced from the publisher with permission for publishing the data.Conflicts of interest
The authors declare no conflict of interest, financial or otherwise.Acknowledgements
The authors are grateful to Principal, Seth Kesarimal Porwal College of Arts and Science and Commerce, Kamptee, for providing journal access facility. Moreover, we are heartily thankful to RGC, SHM and RA for their valuable inputs and continuous inspiration to write a review article.Notes and references
- C. E. E. Rao, A. E. Sood, K. E. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48(42), 7752–7777 CrossRef CAS PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS PubMed.
- A. Castro Neto, F. Guinea, N. Peres, K. Novoselov and A. Geim, Rev. Mod. Phys., 2009, 81, 109–162 CrossRef CAS.
- J. U. Lee, D. Yoon and H. Cheong, Nano Lett., 2012, 12, 4444–4448 CrossRef CAS PubMed.
- C. Xu, B. Xu and Y. Gu, Energy Environ. Sci., 2013, 6, 1388–1414 RSC.
- A. K. Potbhare, R. Bagade, P. B. Chouke, S. Zahra, T. Lambat, M. B. Bagade and R. G. Chaudhary, Mater. Today: Proc., 2019, 15, 454–463 CAS.
- A. Swain, L. Pradhan and D. Bahadur, ACS Appl. Mater. Interfaces, 2015, 7, 8013–8022 CrossRef CAS PubMed.
- S. Mukherjee, X. Meshik, M. Choi, S. Farid, D. Datta, Y. Lan, S. Poduri, K. Sarkar, U. Baterdene, C. E. Huang, Y. Y. Wang, P. Burke, M. Dutta and M. A. Stroscio, IEEE Trans. NanoBiosci., 2015, 14, 967–972 Search PubMed.
- V. N. Sonkusare, R. G. Chaudhary, G. S. Bhusari, A. Mondal, A. K. Potbhare, R. K. Mishra, H. D. Juneja and A. Abdala, ACS Omega, 2020, 5, 7823–7835 CrossRef CAS PubMed.
- X. Hu, G. Wang, B. Wang, X. Liu and H. Wang, Chem. Eng. J., 2019, 355, 986–998 CrossRef CAS.
- C. Liao and S. Wu, Chem. Eng. J., 2019, 355, 805–814 CrossRef CAS.
- H. Y. Hafeez, S. K. Lakhera, P. Karthik, M. Anpo and B. Neppolian, Appl. Surf. Sci., 2018, 449, 772–779 CrossRef CAS.
- C. Tian, L. Wang, F. Luan and X. Zhuang, Talanta, 2019, 191, 103–108 CrossRef CAS PubMed.
- M. Puiu and C. Bala, Bioelectrochem, 2018, 120, 66–75 CrossRef CAS PubMed.
- A. K. Potbhare, P. B. Chouke, A. Mondal, R. Thakare, S. Mondal, R. G. Chaudhary and A. R. Rai, Mater. Today: Proc., 2020, 29, 939–945 CAS.
- M. Zhang, C. Liao, Y. Yao, Z. Liu, F. Gong and F. Yan, Adv. Funct. Mater., 2014, 24, 978–985 CrossRef CAS.
- B. Zhang, C. Li, H. Zhang, Y. Chen, H. Jiang, L. Chen, F. Ur Rehman and X. Wang, J. Biomed. Nanotechnol., 2018, 14, 1277–1286 CrossRef CAS PubMed.
- P. J. Sephra, P. Baraneedharan, M. Sivakumar, T. D. Thangadurai and K. Nehru, Mater. Res. Bull., 2018, 106, 103–112 CrossRef CAS.
- S. Das, K. Sa, I. Alam and P. Mahanandia, Mater. Lett., 2018, 232, 232–236 CrossRef CAS.
- W. C. Dong, J. C. Hyun, F. Alan and B. B. Jong, J. Mater. Chem., 2014, 2, 12136–12149 RSC.
- P. P. A. Jose, M. S. Kala, N. Kalarikkal and S. Thomas, Res. Chem. Intermed., 2018, 44, 5597–5621 CrossRef CAS.
- D. Xu, B. Cheng, W. Wang, C. Jiang and J. Yu, Angew. Chem., Int. Ed., 2018, 231, 368–380 CAS.
- S. Qu, Y. Xiong and J. Zhang, Sep. Purif. Technol., 2019, 210, 382–389 CrossRef CAS.
- T. Chen, Y. Zhou, J. Zhang and Y. Cao, Int. J. Electrochem. Sci., 2018, 13(9), 8575–8588 CrossRef CAS.
- F. Gao, C.-L. Yang, M.-S. Wang, X.-G. Ma and W.-W. Liu, Spectrochim. Acta, Part A, 2019, 206, 216–223 CrossRef CAS PubMed.
- N. Yousef, X. Sun and X. Lin, Adv. Mater., 2014, 26, 5480–5487 CrossRef PubMed.
- G. Lui, J. Y. Liao and A. Duan, J. Mater. Chem. A, 2013, 1, 12255–12262 RSC.
- B. Li and H. Cao, J. Mater. Chem., 2011, 21, 3346 RSC.
- L. Zhu, Z. Liu, P. Xia, H. Li and Y. Xie, Ceram. Int., 2018, 44, 849–856 CrossRef CAS.
- S. Lathasree, A. N. Rao, B. SivaSankar, V. Sadasivam and K. Rengaraj, J. Mol. Catal. A: Chem., 2004, 223, 101 CrossRef CAS.
- C. Chen, J. Xi and E. Zhou, Nano-Micro Lett., 2017, 10, 26 CrossRef PubMed.
- D. Wang, W. Yan, S. H. Vijapur and G. G. Botte, Electrochim. Acta, 2013, 89, 732–736 CrossRef CAS.
- H. Chen, M. Müller, K. Gilmore, G. Wallace and D. Li, Adv. Mater., 2008, 20, 3557–3561 CrossRef CAS.
- V. C. Tung, M. J. Allen, Y. Yang and R. B. Kaner, Nat. Nanotechnol., 2008, 4, 25–29 CrossRef PubMed.
- B. Jayasena and S. Subbiah, Nanoscale Res. Lett., 2011, 6(1), 95–101 CrossRef PubMed.
- M. Yi and Z. Shen, J. Mater. Chem., 2015, 3, 11700–11715 RSC.
- J. Kwak, S. Y. Kim, Y. Jo, N. Y. Kim, S. Y. Kim, Z. Lee and S. Y. Kwon, Adv. Mater., 2018, 30, 1800022 CrossRef PubMed.
- M. Jin, H. Jeong, T. Kim, K. So, Y. Cui, W. Yu and J. Phy, Appl. Phys., 2010, 43, 275402 Search PubMed.
- C. Li, X. Wang, Y. Liu, W. Wang, J. Wynn and J. Gao, J. Nanopart. Res., 2012, 14, 1–11 Search PubMed.
- D. Parviz, F. Irin, S. A. Shah, S. Das, C. B. Sweeney and M. J. Green, Adv. Mater., 2016, 28, 8796–8818 CrossRef CAS PubMed.
- C. Chen, Z. Xu and Y. Han, ACS Appl. Mater. Interfaces, 2016, 8, 8000–8007 CrossRef CAS PubMed.
- S. Pei, J. Zhao, J. Du, W. Ren and H.-M. Cheng, Carbon, 2010, 48, 4466–4474 CrossRef CAS.
- G. Sun, X. Li, Y. Qu, X. Wang, H. Yan and Y. Zhang, Mater. Lett., 2018, 62, 703–760 CrossRef.
- K. Subrahmanyam, L. Panchakarla, A. Govindaraj and C. Rao, J. Phys. Chem. C, 2009, 113, 4257–4259 CrossRef CAS.
- I. Janowska, O. Ersen, T. Jacob, P. Vennégues, D. Bégin and M.-J. Ledoux, Appl. Catal., A, 2009, 371, 2–30 CrossRef.
- C. Wu, X. Huang and X. Wu, Nanoscale, 2013, 5, 3847–3855 RSC.
- M. Acik, G. Lee, C. Mattevi, A. Pirke, R. Wallace, M. Chhowalla, K. Cho and Y. Chabal, J. Phys. Chem. C, 2015, 115, 19761–19781 CrossRef.
- M. S. Umekar, G. S. Bhusari, A. K. Potbhare, A. Mondal, B. P. Kapgate, M. F. Desimone and R. G. Chaudhary, Curr. Pharm. Biotechnol., 2021, 22, 1759–1781 CAS.
- A. H. Abdullah, Z. Ismail, A. S. Z. Abidin and K. Yusoh, Mater. Chem. Phys., 2019, 222, 11–19 CrossRef CAS.
- Z. Ismail, N. F. A. Kassim, A. H. Abdullah, A. S. Z. Abidin, F. S. Ismail and K. Yusoh, Mater. Res. Express, 2017, 4, 075607 CrossRef.
- O. Sadak, A. K. Sundramoorthy and S. Gunasekaran, Carbon, 2018, 31, 108–117 CrossRef.
- E. Secor, T. Gao, A. E. Islam, R. Rao, S. G. Wallace, J. Zhu, K. W. Putz, B. Maruyama and M. Hersam, Chem. Mater., 2017, 19, 2332–2340 CrossRef.
- H. Akhavan-Zanjani, M. Saffar-Avval, M. Mansourkiaei, F. Sharif and M. Ahadi, Int. J. Therm. Sci., 2016, 100, 316–323 CrossRef CAS.
- W. Lv, D.-M. Tang, Y.-B. He, C.-H. You, Z.-Q. Shi and X.-C. Chen, ACS Nano, 2009, 3, 3730–3736 CrossRef CAS PubMed.
- K. Q. Deng, J.-h. Zhou and X.-F. Li, Colloids Surf., B, 2013, 101, 183–188 CrossRef CAS PubMed.
- M. A. Raj and S. A. John, Anal. Chim. Acta, 2013, 771, 14–20 CrossRef CAS PubMed.
- X. Liu, H. Kim and L. J. Guo, Org. Electron., 2013, 14, 591–598 CrossRef CAS.
- O. Akhavan, Carbon, 2011, 29, 11–18 CrossRef.
- S. Toh, K. Loh, S. Kamarudin and W. Daud, J. Chem. Eng., 2014, 251, 422–434 CrossRef CAS.
- A. K. Potbhare, T. S. Shrirame, V. Devthade, S. T. Yerpude, M. S. Umekar, R. G. Chaudhary and G. S. Bhusari, Biogenic Sustainable Nanotechnology: Trends and Progress, Elsevier, 2022, pp. 149–184 Search PubMed.
- P. Marchezi, G. Sonai and M. Hirata, J. Phys. Chem. C, 2016, 120, 23368–23376 CrossRef CAS.
- F. W. Low, C. W. Lai and S. B. A. Hamid, Ceram. Int., 2015, 41, 5798–5806 CrossRef CAS.
- D. Zhang, Y. Zhang and Y. Luo, Nano Res., 2018, 11, 1651–1663 CrossRef CAS.
- S. Thakur and N. Karak, Carbon, 2012, 50, 5331–5339 CrossRef CAS.
- S. Stankovich, D. Dinkin, R. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- N. Mahmood, C. Zhang, H. Yin and Y. Hou, J. Mater. Chem. A, 2014, 2, 15–32 RSC.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501–1246509 CrossRef PubMed.
- W. Li, F. Wang, Y. Liu, J. Wang, J. Yang, L. Zhang, A. A. Elzatahry, D. Al-Dahyan, Y. Xia and D. Zhao, Nano Lett., 2015, 15, 2186–2193 CrossRef CAS PubMed.
- R. G. Chaudhary, G. S. Bhusari, A. D. Tiple, A. R. Rai, S. R. Somkuvar, A. K. Potbhare, T. L. Lambat, P. P. Ingle and A. A. Abdala, Curr. Pharmaceut. Des., 2019, 25, 4013–4029 CrossRef CAS PubMed.
- R. Bagade, V. Sonkusare, A. K. Potbhare and R. G. Chaudhary, Mater. Today: Proc., 2020, 15, 566–574 Search PubMed.
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem. Rev., 2014, 114, 6179–6212 CrossRef CAS PubMed.
- B. Luo and L. Zhi, Energy Environ. Sci., 2015, 8, 456–477 RSC.
- S. K. Vashist and J. H. Luong, Carbon, 2015, 84, 519–550 CrossRef CAS.
- A. Kamyshny and S. Magdassi, Small, 2014, 10, 3515–3535 CrossRef CAS PubMed.
- K. Ullah, S. Ye, Z. Lei, K.-Y. Cho and W.-C. Oh, Catal. Sci. Technol., 2015, 5, 184–198 RSC.
- M. Tang, X. Wang, F. Wu, Y. Liu, S. Zhang, X. Pang, X. Li and H. Qiu, Carbon, 2014, 71, 238–248 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- B. Xia, Y. Yan, X. Wang and X. W. D. Lou, Mater. Horiz., 2014, 1, 379–399 RSC.
- C. Xu, X. Wang and J. Zhu, J. Phys. Chem. C, 2008, 112, 19841–19845 CrossRef CAS.
- A. Bhirud, S. Sathaye, R. Waichal, J. Ambekar, C. Park and B. Kale, Nanoscale, 2015, 7, 5023–5034 RSC.
- J. Leng, W.-M. Wang, L.-M. Lu, L. Bai and X.-L. Qiu, Nanoscale Res. Lett., 2014, 9(99), 1–8 Search PubMed.
- S. Stankovich, D. A Dikin, G. H. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- S. F. Adil, M. Assal, M. Khan, A. Al-Warthan, M. R. H. Siddiqui and L. M. Liz-Marzań, Dalton Trans., 2015, 44, 9709–9717 RSC.
- R. G. Chaudhary, A. K. Potbhare, P. B. Chouke, A. R Rai, R. K. Mishra, M. F Desimone and A. A. Abdala, Applications of Emerging Nanomaterials and Nanotechnology, Material Research Foundations, 2020, vol. 83, pp. 79–116 Search PubMed.
- P. B Chouke, A. K Potbhare, G. S Bhusari, S. Somkuwar, D. P. M. D. Shaik, R. K Mishra and R. G. Chaudhary, Adv. Mater. Lett., 2018, 10, 355–360 CrossRef.
- J. Chen, B. Yao, C. Li and G. Shi, Carbon, 2013, 64, 225–229 CrossRef CAS.
- M. N. Nadagouda and R. S. Varma, Cryst. Growth Des., 2007, 7, 2582–2587 CrossRef CAS.
- M. Bahram, F. Hoseinzadeh, K. Farhadi, M. Saadat, P. NajafiMoghaddam and A. Afkhami, Colloids Surf., A, 2014, 441, 517–524 CrossRef CAS.
- N. I. Hulkoti and T. Taranath, Colloids Surf., B, 2014, 121, 474–483 CrossRef CAS PubMed.
- M. S. Akhtar, J. Panwar and Y.-S. Yun, ACS Sustain. Chem. Eng., 2013, 1, 591–602 CrossRef CAS.
- A. K. Potbhare, R. G. Chaudhary, P. B. Chouke, S. Yerpude, A. Mondal, V. N. Sonkusare, A. R. Rai and H. D. Juneja, Mater. Sci. Eng., C, 2019, 99, 783–793 CrossRef CAS PubMed.
- P. B. Chouke, A. K. Potbhare, N. P. Meshram, M. M. Rai, K. M. Dadure, K. Chaudhary, A. R. Rai, M. F. Desimone, R. G. Chaudhary and D. T. Masram, ACS Omega, 2022, 7(8), 6869–6884 CrossRef CAS PubMed.
- M. Umekar, R. Chaudhary, G. Bhusari and A. Potbhare, Emerging Mater. Res., 2021, 10, 75–84 CrossRef.
- P. R. Bhilkar, R. S. Madankar, T. S. Shrirame, R. D. Utane, A. K. Potbhare, S. Yerpude, S. R. Thakare, A. A. Abdala and R. G. Chaudhary, Emerging Nanomaterials and Their Impact on 21st Century, Materials Research Forum, 2022, vol. 135, pp. 55–82 Search PubMed.
- N. B. Singh, R. G. Chaudhary, M. F. Desimone, A. Agrawal and S. K. Shukla, Clinic. Pharmac. Biotechnology, 2023, 24(1), 61–85 CrossRef CAS PubMed.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- D. Chen, L. Tang and J. Li, Chem. Soc. Rev., 2010, 39, 3157–3180 RSC.
- S. Pei, Q. Wei and K. Huang, Nat. Commun., 2018, 9, 145 CrossRef PubMed.
- M. S. Umekar, R. G. Chaudhary, G. S. Bhusari, A. Mondal, A. K. Potbhare and M. Sami, Mater. Today: Proc., 2020, 29, 709–714 CAS.
- R. Upadhyay, N. Soinb and S. Roy, RSC Adv., 2014, 4, 3823 RSC.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- M. Abd Elkodous, G. S. El-Sayyad, M. A. Maksoud, R. Kumar, K. Maegawa, G. Kawamura and A. Matsuda, J. Hazard. Mater., 2021, 410, 124657 CrossRef CAS PubMed.
- F. Zhang, X. Wang, H. Liu, C. Liu, Y. Wan and Y. Long, Appl. Sci., 2019, 9(12), 2489 CrossRef CAS.
- R. Kumar, S. Sahoo, E. Joanni, R. K. Singh and K. K. Kar, ACS Appl. Mater. Interfaces, 2021, 14(18), 20306–20325 CrossRef PubMed.
- R. Kumar, S. Sahoo, E. Joanni, R. Pandey and J. J. Shim, Chem. Commun., 2023, 59(41), 6109–6127 RSC.
- R. Kumar, S. Sahoo, E. Joanni and R. K. Singh, J. Energy Chem., 2022, 74, 252–282 CrossRef CAS.
- R. Kumar, S. Sahoo, E. Joanni, R. Pandey, W. K. Tan, G. Kawamura and A. Matsuda, J. Energy Storage, 2023, 68, 107820 CrossRef.
- S. M. Youssry, M. Abd Elkodous, R. Kumar, G. Kawamura, W. K. Tan and A. Matsuda, Electrochim. Acta, 2023, 463, 142814 CrossRef CAS.
- R. Kumar, S. M. Youssry, E. Joanni, S. Sahoo, G. Kawamura and A. Matsuda, J. Energy Storage, 2022, 56, 105896 CrossRef.
- R. Kumar, R. K. Singh, P. K. Dubey, D. P. Singh, R. M. Yadav and R. S. Tiwari, RSC Adv., 2015, 5(10), 7112–7120 RSC.
- S. M. Youssry, I. S. El-Hallag, R. Kumar, G. Kawamura, W. K. Tan, A. Matsuda and M. N. El-Nahass, J. Energy Storage, 2022, 50, 104638 CrossRef.
- R. Kumar, R. K. Singh, A. R. Vaz and S. A. Moshkalev, RSC Adv., 2015, 5(83), 67988–67995 RSC.
- H. Su and Y. H. Hu, Sci. Eng., 2020, 1, 1–26 Search PubMed.
- R. Arukula, M. Vinothkannan, A. Kim and D. Yoo, J. Alloys Compd., 2019, 771, 477–488 CrossRef CAS.
- C. Botas, P. Alvarez, P. Blanco and R. Menéndez, Carbon, 2013, 65, 156–164 CrossRef CAS.
- P. Boehm, R. Setton and E. Stumpp, Carbon, 1986, 24, 241 CrossRef.
- G. Long, C. Tang, K. Wong, C. Man, M. Fan, W. Lau, T. Xuc and B. Wang, Green Chem., 2013, 15, 821–828 RSC.
- K. K. H. De Silva, H. Huang, R. K. Joshi and M. Yoshimura, Carbon, 2017, 119, 190–199 CrossRef CAS.
- Y. Wang, Z. Shi and J. Yin, ACS Appl. Mater. Interfaces, 2011, 3, 1127–1133 CrossRef CAS PubMed.
- P. B. Chouke, A. K. Potbhare, K. M. Dadure, A. J. Mungole, N. P. Meshram, R. R. Chaudhary, A. R. Rai and R. G. Chaudhary, Mater. Today: Proc., 2022, 29, 815–821 Search PubMed.
- R. K. Upadhyay, N. Soinb and S. Roy, RSC Adv., 2014, 4, 3823–3851 RSC.
- D. Wang, ACS Nano, 2003, 4, 1587–1595 CrossRef PubMed.
- M. Chakrabarti, C. Low, N. Brandon, V. Yufit, M. Hashim, M. Irfan, J. Akthar, E. Ruiz-Trejo and M. hussain, Electrochim. Acta, 2013, 107, 425–440 CrossRef CAS.
- A. W. Anwar, J. Mater. Sci. Technol., 2015, 31, 699–707 CrossRef CAS.
- W. Zhang, F. Liu, Q. Li, Q. Shou and J. Cheng, Phys. Chem. Chem. Phys., 2012, 14, 16331–16337 RSC.
- P. Singh, P. Shandilya, P. Raizada, A. Sudhaik, A. Rahmani-sani and A. Hosseini-bandegharaei, Arabian J. Chem., 2020, 13, 3498–3520 CrossRef CAS.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- Y. Wang, Y. Li, L. Tang, J. Lu and J. Li, Electrochem. Commun., 2009, 11, 889–892 CrossRef CAS.
- J. Chen, B. Yao, C. Li and G. Shi, Carbon, 2013, 64, 225–229 CrossRef CAS.
- D. Dreyer, S. Park, C. Bielawski and R. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- N. Sorokina, M. Khaskov, V. Avdeev and I. Nikol'Skaya, Russ. J. Gen. Chem., 2005, 75, 162–168 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4(8), 4806–4814 CrossRef CAS PubMed.
- Y. Xu, K. Sheng, C. Li and G. Shi, J. Mater. Chem., 2011, 21, 7376–7380 RSC.
- M. Ajorloo, M. Fasihi, M. Ohshima and K. Taki, Mater. Des., 2019, 181, 108068 CrossRef CAS.
- P. V. Kumar, N. M. Bardhan, S. Tongay, J. Wu, A. M. Belcher and J. C. Grossmann, Nat. Chem., 2014, 6, 151–158 CrossRef CAS PubMed.
- B. Wang, Q. Liu, J. Han, X. Zhang, J. Wang, Z. Li, H. Yan and L. Liu, J. Mater. Chem. A, 2014, 2, 1137–1143 RSC.
- M. Rajabi, K. Mahanpoor and O. Moradi, Composites, Part B, 2019, 167, 544–555 CrossRef CAS.
- B. Feng, J. Xie, C. Dong, S. Zhang, G. Cao and X. Zhao, RSC Adv., 2014, 4, 17902–17907 RSC.
- V. H. Pham, T. T. Dang, K. Singh, S. H. Hur, E. W. Shin, J. S. Kim, M. A. Lee, S. H. Baeck and J. S. Chung, J. Mater. Chem. A, 2013, 1, 1070–1077 RSC.
- A. N. Abbas, G. Liu, A. Narita, M. Orosco, X. Feng, K. Müllen and C. Zhou, J. Am. Chem. Soc., 2014, 136, 7555–7558 CrossRef CAS PubMed.
- I. K. Moon, J. Lee, R. S. Ruoff and H. Lee, Nat. Commun., 2010, 1, 73–78 CrossRef PubMed.
- M. McAllister, J. Li, D. Adamson, H. Schniepp, A. Abdala, J. Liu, M. Herrera-Alonso, D. Milius, R. Car, R. Prud’homme and I. Aksay, Chem. Mater., 2007, 19, 4396–4404 CrossRef CAS.
- L. Song, F. Khoerunnisa, W. GaoGao, W. Dou and T. Hayashi, Carbon, 2013, 52, 608–612 CrossRef CAS.
- B. Shen, D. Lu, W. Zhai and W. Zheng, J. Mater. Chem. C, 2013, 1, 50–53 RSC.
- T. Kuila, A. K. Mishra, P. Khanra, N. H. Kim and J. H. Lee, Nanoscale, 2013, 5, 52–71 RSC.
- J. Yang, M. R. Jo, M. Kang, Y. S. Huh, H. Jung and Y. M. Kang, Carbon, 2014, 53, 106–113 CrossRef.
- J. A. Gerbec, D. Magana, A. Washington and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 15791–15800 CrossRef CAS PubMed.
- H. Hu, X. Wang, F. Liu, J. Wang and C. Xu, Syn. Met., 2011, 161, 404–410 CrossRef CAS.
- B. Tryba, A. W. Morawski and M. Inagaki, Carbon, 2005, 43, 2417–2419 CrossRef CAS.
- Z. Li, Y. Yao, Z. Lin, K.S. Moon, W. Lin and C. Wong, Mater. Chem., 2010, 20, 4781–4783 RSC.
- G. Demazeau, J. Mater. Chem., 1999, 9, 15–18 RSC.
- J. T. Robinson, X. Li and H. Dia, J. Mater. Chem., 2009, 131, 9910–9911 Search PubMed.
- Y. Zhou, Q. Bao, L. A. L. Tang, Y. Zhong and K. P. Loh, Chem. Mater., 2009, 21, 2950–2956 CrossRef CAS.
- R. Wang, Y. Wang, C. Xu, J. Sun and L. Gao, RSC Adv., 2013, 3, 1194–1200 RSC.
- S. Dubin, S. Gilje, K. Wang, V. C. Tung, K. Cha and A. S. Hall, RSC Adv., 2010, 4, 3845–3852 CAS.
- D. Zhou, Q.-Y. Cheng and B.-H. Han, Carbon, 2011, 49, 3920–3927 CrossRef CAS.
- J. Xiao, W. Lv and Z. Xie, J. Mater. Chem. A, 2016, 4, 12126–12135 RSC.
- O. A. Rahman, V. Chellasamy, N. Ponpandian, S. Amirthapandian, B. Panigrahi and P. Thangadurai, RSC Adv., 2014, 4, 56910–156917 RSC.
- M. Fernandez-Merino, L. Guardia, L. Paredes, S. Villar-Rodil, P. olis-Fernandez and A. Martinez-Alonso, J. Phys. Chem. C, 2010, 114, 6426–6432 CrossRef CAS.
- C. Bosch-Navarro, E. Coronado, C. Martí-Gastaldo, J. Sánchez-Royo and M. G. Gómez, Nano, 2012, 4, 3977–3982 CAS.
- Z. Bo, X. Shuai, S. Mao, H. Yang, J. Qian and J. Chen, Sci. Rep., 2014, 4(1), 4684 CrossRef PubMed.
- S. Liu, J. Tian, L. Wang and X. Sun, Carbon, 2011, 49, 3158–3164 CrossRef CAS.
- D. Wan, C. Yang, T. Lin, Y. Tang, M. Zhou and Y. Zhong, ACS Nano, 2012, 6(1), 9068–9078 CrossRef CAS PubMed.
- C. Chua, A. Ambrosi and M. Pumera, J. Mater. Chem. A, 2012, 22, 11054–11061 RSC.
- J. Xiao, W. Lv and Z. Xie, J. Mater. Chem. A, 2016, 4, 12126–12135 RSC.
- A. Esfandiar, O. Akhavan and A. Irajizad, J. Mater. Chem., 2011, 21, 10907–10914 RSC.
- X. Fan, W. Peng, Y. Li, X. Li, S. Wang and G. Zhang, Adv. Mater., 2008, 20, 4490–4493 CrossRef CAS.
- X. Mei and J. Ouyang, Carbon, 2011, 49, 5389–5397 CrossRef CAS.
- H. L. Guo, X. F. Wang and Q. Y. Qian, ACS Nano, 2009, 3, 2653–2659 CrossRef CAS PubMed.
- M. Khan, A. Al-Marri, M. Khan, N. Mohri, S. Adil, A. Al-Warthan, M. Siddiqui, H. Alkhathlan, R. Berger and W. Tremel, RSC Adv., 2014, 4, 24119–24125 RSC.
- E. Nagaraj, A. Al-Marri, S. Vignesh, M. Srinivasan, G. Venkatesh, G. Palanisamy, P. Ramasamy, B. Palanivel, M. Al-Enizi, M. Ubaidullah, V. Reddy and W. Kim, J. Alloys. Compd., 2022, 906, 164255 CrossRef.
- J. Li, G. Xiao, C. Chen and R. Li, J. Mater. Chem. A, 2013, 1, 1481–1487 RSC.
- T. Kuila, S. Bose, P. Khanra, A. K. Mishra, N. H. Kim and J. H. Lee, Carbon, 2012, 50, 914–921 CrossRef CAS.
- G. Wang, F. Qian, C. Saltikov, Y. Jiao and Y. Li, Nano Res., 2011, 4, 563–570 CrossRef CAS.
- A. K. Potbhare, M. S. Umekar, P. B. Chouke, M. B. Bagade, S. K. Tarik Aziz, A. A. Abdala and R. G. Chaudhary, Mater. Today: Proc., 2020, 29, 720–725 CAS.
- B. Feng, J. Xie, C. Dong, S. Zhang, G. Cao and X. Zhao, RSC Adv., 2014, 4, 17902–17907 RSC.
- P. Khanra, T. Kuila, N. H. Kim, S. H. Bae, D.-S. Yu and J. H. Lee, Chem. Eng. J., 2012, 183, 526–533 CrossRef CAS.
- Z. Bo, X. Shuai, S. Mao, H. Yang, J. Qian, J. Chen, J. Yan and K. Cen, Sci. Rep., 2014, 4, 4684 CrossRef PubMed.
- H.-J. Chu, C.-Y. Lee and N.-H. Tai, Carbon, 2014, 80, 725–733 CrossRef CAS.
- S. K. Srivastava, C. Ogino and A. Kondo, RSC Adv., 2014, 4, 5986–5989 RSC.
- M. J. Firdhouse and P. Lalitha, Int. Nano Lett., 2014, 4, 103–108 CrossRef CAS.
- D. Suresh, P. Nethravathi, H. Nagabhushana and S. Sharma, Ceram. Int., 2015, 41, 4810–4813 CrossRef CAS.
- S. B. Maddinedi, B. K. Mandal, R. Vankayala, P. Kalluru and S. R. Pamanji, Spectrochim. Acta, Part A, 2015, 145, 117–124 CrossRef CAS PubMed.
- R. K. Upadhyay, N. Soin, G. Bhattacharya, S. Saha, A. Barman and S. S. Roy, Mater. Lett., 2015, 160, 355–358 CrossRef CAS.
- M. Khan, A. H. Al-Marri, M. Khan, M. R. Shaik, N. Mohri, S. F. Adil, M. Kuniyil, H. Z. Alkhathlan, A. Al-Warthan and W. Tremel, Nanoscale Res. Lett., 2015, 10, 281 CrossRef PubMed.
- F. Tavakoli, M. Salavati-Niasari and F. Mohandes, Mater. Res. Bull., 2015, 63, 51–57 CrossRef CAS.
- T. Vu, T. Tran, H. Le, P. Nguyen, N. Bui and N. Essayem, Mater. Sci., 2015, 38, 667–671 CAS.
- W. Han, W.-Y. Niu, B. Sun, G.-C. Shi and X.-Q. Cui, J. Photochem. Photobiol., B, 2016, 165, 305–309 CrossRef CAS PubMed.
- D. Hou, Q. Liu, H. Cheng, K. Li, D. Wang and H. Zhang, Mater. Chem. Phys., 2016, 183, 76–82 CrossRef CAS.
- P. Chamoli, R. Sharma, M. K. Das and K. K. Kar, RSC Adv., 2016, 6, 96355–96366 RSC.
- X. Jiang, Z. Li, J. Yao, Z. Shao and X. Chen, Mater. Sci. Eng., C, 2016, 68, 798–804 CrossRef CAS PubMed.
- S. Sadhukhan, T. Ghosh, D. Rana, I. Roy, A. Bhattacharyya, G. Sarkar, M. Chakraborty and D. Chattopadhyay, Mater. Res. Bull., 2016, 79, 41–51 CrossRef CAS.
- C. Li, Z. Zhuang, X. Jin and Z. Chen, Appl. Surf. Sci., 2017, 422, 469–474 CrossRef CAS.
- X. Weng, J. Wu, L. Ma, G. Owens and Z. Chen, Chem. Eng. J., 2019, 359, 976–981 CrossRef CAS.
- R. Zaid, F. Chong, E. Teo, E. Ng and K. Chong, Arabian J. Chem., 2015, 8, 560–569 CrossRef CAS.
- D. Suresh, M. P. Kumar, H. Nagabhushana and S. Sharma, Mater. Lett., 2015, 151, 93–95 CrossRef CAS.
- B. Kartick and S. Srivastava, J. Nanosci. Nanotechnol., 2013, 13, 4320–4324 CrossRef CAS PubMed.
- S. Hatamie, O. Akhavan, S. K. Sadrnezhaad, M. M. Ahadian, M. M. Shirolkar and H. Q. Wang, Mater. Sci. Eng., C, 2015, 55, 482–489 CrossRef CAS PubMed.
- R. Liao, Z. Tang, Y. Lei and B. Guo, J. Phys. Chem. C, 2011, 115, 20740–20746 CrossRef CAS.
- O. Akhavan, M. Kalaee, Z. Alavi, S. Ghiasi and A. Esfandiar, Carbon, 2012, 50, 3015–3025 CrossRef CAS.
- B. Haghighi and M. A. Tabrizi, RSC Adv., 2013, 3, 13365–13371 RSC.
- A. Singh, B. Ahmed, A. Singh and A. K. Ojha, Spectrochim. Acta, Part A, 2018, 204, 603–610 CrossRef CAS PubMed.
- S. Shahane and D. Sidhaye, Int. J. Mod. Phys. B, 2018, 32, 1840068 CrossRef CAS.
- J. R. Anasdass, P. Kannaiyan, R. Raghavachary, S. C. Gopinath and Y. Chen, PLoS One, 2018, 13, e0193281 CrossRef PubMed.
- W. Ye, X. Li, J. Luo, X. Wang and R. Sun, Ind. Crops Prod., 2017, 109, 410–419 CrossRef CAS.
- B. Chandu, V. Mosali, B. Mullamuri and H. Bollikolla, Carbon Lett., 2017, 21, 74–80 CrossRef.
- D. Hou, Q. Liu, H. Cheng and K. Li, J. Nanosci. Nanotechnol., 2017, 17, 6518–6523 CrossRef CAS.
- F.-Y. Xing, L.-L. Guan, Y.-L. Li and C.-J. Jia, Environ. Toxicol. Pharmacol., 2016, 48, 110–115 CrossRef CAS PubMed.
- G. Lee and B. S. Kim, Biotechnol. Prog., 2014, 30, 463–469 CrossRef CAS PubMed.
- S. Gurunathan, J. W. Han, J. H. Park, V. Eppakayala and J. H. Kim, Int. J. Nanomed., 2014, 9, 363–377 CrossRef PubMed.
- B. Haghighi and M. A. Tabrizi, RSC Adv., 2013, 3, 13365–13371 RSC.
- C. Zhu, S. Guo, Y. Fang and S. Dong, ACS Nano, 2010, 4, 2429–2437 CrossRef CAS PubMed.
- N. H. Kim, P. Khanra, T. Kuila, D. Jung and J. H. Lee, J. Mater. Chem. A, 2013, 1, 11320–11328 RSC.
- B. Haghighi and M. A. Tabrizi, RSC Adv., 2013, 3, 13365–13371 RSC.
- J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang and S. Guo, Chem. Comm., 2010, 46, 1112–1114 RSC.
- D. Chen, L. Li and L. Guo, Nanotech, 2011, 22, 325601 CrossRef PubMed.
- Y. Wang, Z. Shi and J. Yin, ACS Appl. Mater. Interfaces, 2011, 3, 1127–1133 CrossRef CAS PubMed.
- K. Loh, Q. Bao, K. Ang and J. Yang, J. Mater. Chem., 2012, 20, 2277–2289 RSC.
- H. Bai, C. Li and G. Shi, Adv. Mater., 2011, 23, 1089–1115 CrossRef CAS PubMed.
- S. Yang, W. Yue, D. Huang, C. Chen, H. Lin and X. Yang, RSC Adv., 2012, 2, 8827–8832 RSC.
- H. Su and H. Hu, Energy Sci. Eng., 2021, 9, 958–983 CrossRef CAS.
- J. Bai, D. Liu, J. Yang and Y. Chen, ChemSusChem, 2019, 12, 2117–2132 CrossRef CAS PubMed.
- M. Iqbal, A. Rehman and S. Siddique, J. Energy Chem., 2019, 39, 217–234 CrossRef.
- N. Elavarasan, S. Prakasha, K. Kokilaa, C. Thirunavukkarasuc and V. Sujatha, New J. Chem., 2020, 44, 2166–2179 RSC.
- F. Meng, H. Zheng, Y. Chang, Y. Zhao, M. Li and C. Wang, IEEE Trans. Nanotechnol., 2018, 17, 212–219 CAS.
- H. Al-Marri, M. Khan, M. Shaik, N. Mohri, S. Adil, M. Kuniyil, H. Alkhathlan, A. Al-Warthan, W. Tremel, M. Tahir, M. Khan, M. Rafiq and H. Siddiqui, Arabian J. Chem., 2016, 9, 835–845 CrossRef.
- X. Zhang, J. Mater. Chem., 2010, 20, 2801–2806 RSC.
- C. Hintze, J. Eur. Ceram. Soc., 2016, 36, 2923–2930 CrossRef CAS.
- S. Nayak, A. Soam, J. Nanda, C. Mahender, M. Singh, D. Mohpatra and R. Kumar, J. Mater. Sci.: Mater. Electron., 2018, 29, 9361–9368 CrossRef CAS.
- W. Li, F. Wang, S. Feng, J. Wang, Z. Sun, B. Li, Y. Li, J. Yang, A. Elzatahry, Y. Xia and D. Zhao, J. Am. Chem. Soc., 2013, 135, 18300–18303 CrossRef CAS PubMed.
- R. K. Singh, R. Kumar, D. P. Singh, R. Savu and S. A. Moshkalev, Mater. Today Chem., 2019, 12, 282–314 CrossRef CAS.
- H. Hassan, V. Abdelsayed, R. Khder, K. AbouZeid, J. Terner, M. ElShall, S. Al-Resayes and A. El-Azhary, J. Mater. Chem., 2009, 19, 3832–3837 RSC.
- A. Dey, S. Panja, A. K. Sikder and S. Chattopadhyay, RSC Adv., 2015, 5(14), 10358–10364 RSC.
- R. Kumar, S. M. Youssry, M. M. Abdel-Galeil and A. Matsuda, J. Mater. Sci.: Mater. Electron., 2020, 31, 15456–15465 CrossRef.
- S. Chabi, C. Peng, Z. Yang, Y. Xia and Y. Zhu, RSC Adv., 2015, 5, 3999–4008 RSC.
- J. Shen, M. Shi, H. Ma, B. Yan, N. Li and M. Ye, Mater. Res. Bull., 2011, 46, 2077–2083 CrossRef CAS.
- D. K. Padhi, T. Panigrahi, K. Parida, S. K. Singh and P. M. Mishra, ACS Sustainable Chem. Eng., 2017, 5, 10551–10562 CrossRef CAS.
- R. G. Chaudhary, A. K Potbhare, S. K. Tarik Aziz, M. S. Umekar, S. S. Bhuyar and A. Mondal, Mater. Today: Proc., 2021, 36, 756–762 CAS.
- P. Kumbhakar, A. Pramanik, S. Biswas, A. K. Kole, R. Sarkar and P. Kumbhakar, J. Hazard. Mater., 2018, 360, 193–203 CrossRef CAS PubMed.
- M. Hamid, M. Rianna, W. R. Rangkuti, T. Sembiring and P. Sebayang, S. Afr. J. Chem. Eng., 2022, 42, 280–282 Search PubMed.
- X. Yang, C. Chen, J. Li, G. Zhao, X. Ren and X. Wang, RSC Adv., 2012, 2, 8821–8826 RSC.
- G. Cai, J. Tu, J. Zhang, Y. Mai, Y. Lu, C. Guab and X. Wang, Nanoscale, 2012, 4, 5724–5730 RSC.
- W. Qian, Z. Chen, S. Cottingham, W. Alexander, A. Natasja, A. M. Goforth, T. L. Clarec and J. Jiao, Green Chem., 2012, 14, 371–377 RSC.
- G. Jeevitha, R. Abhinayaa, D. Mangalaraj, N. Ponpandian, P. Meena, V. Mounasamy and S. Madanagurusamy, Nanoscale Adv., 2019, 1, 1799–1811 RSC.
- K. Rahimi, H. Zafarkish and A. Yazdani, Mater. Des., 2018, 144, 214–221 CrossRef CAS.
- R. Elshypany, H. Selim, K. Zakaria, A. H. Moustafa, S. A. Sadeek, S. I. Sharaa, P. Raynaud and A. A. Nada, Molecules, 2021, 14, 2269 CrossRef PubMed.
- S. Hamed, K. Hossain, S. Masoud, S. Niasari and D. Mortazavi, J. Colloid Interface Sci., 2017, 498, 423–432 CrossRef PubMed.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- S. Chandra, P. Das, S. B. P. Pramanik and P. Pramanik, Mater. Sci. Eng., B, 2012, 177, 855–861 CrossRef CAS.
- B. N. Benjwal, M. Kumar, P. Chamoli and K. K. Kar, RSC Adv., 2015, 5, 73249–73260 RSC.
- Y. Fu and X. Wang, Ind. Eng. Chem. Res., 2011, 50, 7210–7218 CrossRef CAS.
- T. Lv, L. Pan, X. Liu, T. Lu, G. Zhu and Z. Sun, J. Alloys Compd., 2011, 509, 10086–10091 CrossRef CAS.
- S. Thangavel, G. Venugopal and K. Jae, Mater. Chem. Phys., 2014, 145, 108–115 CrossRef CAS.
- K. Sharma, K. Maiti, N. H. Kim, D. Hui and J. H. Lee, Composites, Part B, 2018, 138, 35–44 CrossRef CAS.
- A. Isari, A. Payan, M. Fattahi, S. Jorfi and B. Kakavandi, Appl. Surf. Sci., 2018, 462, 549–564 CrossRef CAS.
- R. G. Chaudhary, V. Sonkusare, G. Bhusari, A. Mondal, A. Potbhare, H. Juneja and R. Sharma, Environ. Res., 2023, 222, 115363 CrossRef CAS PubMed.
- S. K. T. Aziz, M. Awasthi, S. Guria, M. Umekar, I. Karajagi, S. K. Riyajuddin, K. V. Siddhartha, A. Saini, A. Potbhare, R. G. Chaudhary and V. Vishal, STAR Protoc., 2023, 4(3), 102448 CrossRef CAS PubMed.
- Z. Song, Y.-L. Ma and C.-E. Li, Sci. Total Environ., 2019, 651, 580–590 CrossRef CAS PubMed.
- S. Shanavas, A. Priyadharsan, E. I. Gkanas, R. Acevedo and P. M. Anbarasan, J. Ind. Eng. Chem., 2019, 72, 512–528 CrossRef CAS.
- L. Lin, H. Wang and P. Xu, Chem. Ind. Eng. J., 2017, 310, 389–398 CAS.
- L. Pastrana-Martínez, S. MoralesTorres, V. Likodimos, J. Figueiredo, J. Faria and P. Falaras, Appl. Catal., B, 2012, 123, 241–256 CrossRef.
- Z. Wan and J. Wang, J. Hazard. Mater., 2017, 324, 653–664 CrossRef CAS PubMed.
- R. Karthik, V. J. Kumar, S.-M. Chen, C. Karuppiah, Y.-H. Cheng and V. A. Muthuraj, ACS Appl. Mater. Interfaces, 2017, 9, 6547–6559 CrossRef CAS PubMed.
- G. Malekshoar, K. Pal, Q. He, A. Yu and A. K. Ray, Ind. Eng. Chem. Res., 2014, 53, 18824–18832 CrossRef CAS.
- S. Jo W-K, S. Kumar, M. Isaacs, A. Lee and S. Karthikeyan, Appl. Catal., B, 2017, 201, 159–168 CrossRef.
- L. Ji, Z. Tan, T. R. Kuykendall, S. Aloni, S. Xun, E. Lin, V. Battaglia and Y. Zhang, Phys. Chem. Chem. Phys., 2011, 13, 7170 RSC.
- A. Staffolani, H. Darjazi, G. Carbonari, F. Maroni, S. Gabrielli and F. Nobili, Molecules, 2016, 26, 4316 CrossRef PubMed.
- N. Kumar, J. R. Rodriguez, V. G. Pol and A. Sen, Appl. Surf. Sci., 2019, 463, 132–140 CrossRef CAS.
- B. Xiao, B. Zhang, L. B. Tang, C. S. An, Z. J. He, H. Tong, W. J. Yu and J. C. Zheng, Ceram. Int., 2018, 44(13), 15044–15049 CrossRef CAS.
- N. Naresh, D. Narsimulu, P. Jena, S. R. E. S. Dhu and N. Satyanarayana, J. Mater. Sci.: Mater. Electron., 2018, 29, 14723–14732 CrossRef CAS.
- S. T. Aziz, M. Ummekar, I. Karajagi, S. K. Riyajuddin, K. V. R. Siddhartha, A. Saini, A. Potbhare, R. G. Chaudhary, V. Vishal, P. C. Ghosh and A. Dutta, Cell Rep. Phys. Sci., 2022, 3, 101106 CrossRef CAS.
- R. B. Suneetha, P. Selvi and C. Vedhi, Vacuum, 2019, 164, 396–404 CrossRef CAS.
- J. Jayachandiran, J. Yesuraj, M. Arivanandhan, A. Raja, S. A. Suthanthiraraj, R. Jayavel and D. Nedumaran, J. Inorg. Organomet. Polym. Mater., 2018, 28, 2046–2055 CrossRef CAS.
- W. H. Low, P. S. Khiew, S. S. Lim, C. W. Siong and E. R. Ezeigwe, J. Alloys Compd., 2018, 768, 995–1005 CrossRef CAS.
- C. K. Maity, G. Hatui, K. Verma, G. Udayabhanu, D. D. Pathak and G. C. Nayak, Vacuum, 2018, 157, 145–154 CrossRef CAS.
- S. Nagarani, G. Sasikala, K. Satheesh, M. Yuvaraj and R. Jayavel, J. Mater. Sci.: Mater. Electron., 2018, 29, 11738–11748 CrossRef CAS.
- Z. Wu, S. Yang, Y. Sun, K. Parvez, X. Feng and K. Müllen, J. Am. Chem. Soc., 2018, 134, 9082–9085 CrossRef PubMed.
- J. Xiao, Q. Kuang, S. Yang, F. Xiao, S. Wang and L. Guo, Sci. Rep., 2013, 3, 2300 CrossRef PubMed.
- R. Arukula, M. Vinothkannana, A. Rhan, K. Dong and J. Yoo, J. Alloys Compd., 2019, 771, 477–488 CrossRef CAS.
- B. Baruah, A. Kumar, G. Umapathy and S. Ojha, J. Electroanal. Chem., 2019, 840, 35–51 CrossRef CAS.
- Y. C. Cao, C. Xu and X. Wu, J. Power Sources, 2011, 196, 8377–8382 CrossRef CAS.
- N. A. Kotov, Nature, 2006, 442, 254–255 CrossRef CAS PubMed.
- M. Zhang, L. Su and L. Mao, Carbon, 2006, 44, 276–283 CrossRef CAS.
- D.-W. Wang, F. Li, M. Liu, G. Lu and H. Cheng, Angew. Chem., 2008, 120, 379–382 CrossRef.
- J. Liu, J. Shen, M. Li and L. Guo, Chin. Chem. Lett., 2015, 26, 1478–1484 CrossRef CAS.
- M. Khan, M. Khan and M. Cho, Nanoscale, 2018, 10, 9427–9440 RSC.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603–2636 RSC.
- X. Lü, J. Shen, J. Wang, Z. Cui and J. Xie, RSC Adv., 2015, 5, 15993–15999 RSC.
- A. Ajmal, I. Majeed, R. N. Malik, H. Idriss and M. A. Nadeem, RSC Adv., 2014, 4, 37003–37026 RSC.
- R. Molinari, C. Lavorato and P. Argurio, Chem. Eng. J., 2015, 274, 307–316 CrossRef CAS.
- S. K. Yadav, R. Kumar, A. K. Sundramoorthy, R. K. Singh and C. M. Koo, RSC Adv., 2016, 6(58), 52945–52949 RSC.
- S. Vadahanambi, J. H. Jung, R. Kumar, H. J. Kim and I. K. Oh, Carbon, 2013, 53, 391–398 CrossRef CAS.
- H. M. El-Bery, Y. Matsushita and A. Abdel-moneim, Appl. Surf. Sci., 2017, 423, 185–196 CrossRef CAS.
- T. T. Wei, P. Peng, Y. R. Ji, Y. R. Zhu, T. F. Yi and Y. Xie, J. Energy Chem., 2022, 71, 400–410 CrossRef CAS.
- T. F. Yi, L. Shi, X. Han, F. Wang, Y. Zhu and Y. Xie, Energy Environ. Mater., 2021, 4(4), 586–595 CrossRef CAS.
- H. Chang, Y. R. Wu, X. Han and T. F. Yi, Energy Mater., 2021, 1(3), 24 Search PubMed.
- B. Li and H. Cao, J. Mater. Chem., 2011, 21, 3346 RSC.
- L. Sun, Y. Zhang and D. Zhang, Nanoscale, 2017, 9, 18552–18560 RSC.
- C. Lin, L. Hu and C. Cheng, Electrochim. Acta, 2018, 260, 65–72 CrossRef CAS.
- L. Bai, Y. Zhang and L. Zhang, Nano Energy, 2018, 53, 982–992 CrossRef CAS.
- B. Kirubasankar, B. Murugadoss and V. Lin, Nanoscale, 2018, 10, 20414–20425 RSC.
- T. F. Yi, H. M. K. Sari, X. Li, F. Wang, Y. R. Zhu, J. Hu and X. Li, Nano Energy, 2021, 85, 105955 CrossRef CAS.
- T. F. Yi, L. Y. Qiu, J. Mei, S. Y. Qi, P. Cui, S. Luo and Y. B. He, Sci. Bull., 2020, 65, 546–556 CrossRef CAS PubMed.
- J. Han, L. L. Zhang and S. Lee, ACS Nano, 2013, 7, 19–26 CrossRef CAS PubMed.
- S. Xiong, B. Yang, D. Cai, G. Qiu and Z. Wu, Electrochim. Acta, 2015, 185, 52–61 CrossRef CAS.
- J. Zhang, C. Guo, L. Zhang and C. Li, Chem. Commun., 2013, 49, 6334–6336 RSC.
- A. Marinoiu, M. Raceanu, E. Carcadea and M. Varlam, Appl. Surf. Sci., 2018, 456, 238–245 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |