Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Adsorptive separation of saccharides and polyols over materials functionalized with boronate groups
Irina
Delidovich
 * and
Valérie
Toussaint
* and
Valérie
Toussaint

Institute of Chemical, Environmental and Bioscience Engineering, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria. E-mail: irina.delidovich@tuwien.ac.at
First published on 13th December 2023
Abstract
Saccharides and polyols play key roles in emerging biomass-based value chains, though recovery of these compounds from product streams remains challenging. This Review addresses the application of materials bearing boronate groups for separation and recovery of saccharides and polyols via affinity adsorption processes. First, the representative, industrially important product mixtures are considered, along with the design of separation processes exploiting the affinity adsorption. Thereafter, the structure-performance correlations are critically discussed for gel-type functionalized polymers, macroporous resins, molecularly imprinted polymers, and materials bearing grafted phenylboronate moieties and grafted polymer brushes. Finally, the separation processes based on functional materials bearing boronate groups are compared with the state-of-the-art chromatographic separation over Ca2+ ion-exchange resins. This analysis aims at identifying guidelines for rational material synthesis and knowledge-driven process design for novel economically viable and environmentally benign separation processes.
1. Motivation
Transition from exhaustible fossil resources to renewable feedstocks presents a key aspect of sustainable development. Lignocellulose is not digestible by humans and belongs thus to biomass sources of the second generation. Lignocellulose is available as a component of waste streams in forestry, agriculture, paper, and pulp production. On average, a cell wall consists of 35–50% cellulose, 20–40% hemicelluloses, 5–30% lignin, and 1–10% other extracted substrates.1 An interest in lignocellulose as industrial organic raw material is caused by its abundance, availability from waste streams, and high carbohydrate content of 60–70%.Comparison of typical naphtha components, mainly represented by linear alkanes CnH2n+2, with lignocellulose of an average formula C3H4O2,2 suggests significant differences in chemical structures, physical and chemical properties of the fossil and bio raw materials. Transformation of alkanes into custom products requires functionalization, whereas biomass-based raw materials and intermediates frequently require rather de-functionalization than functionalization.3 These compounds contain high content of oxygen, exhibit high boiling points, low vapor pressures, and/or decompose upon heating. A simple and energy-efficient integration of biomass into existing value chains to produce conventional base chemicals and intermediates poses a challenge.
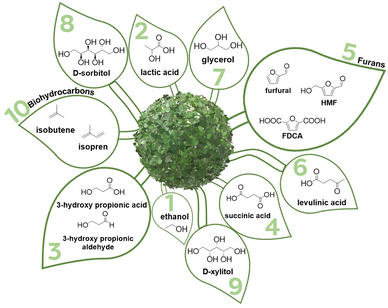
A complete re-design of the value chains upon the shift from naphtha to biomass feedstocks presents an alternative for an integration into the existing value chains. A concept of converting sugars into fuels and chemicals via novel 12 “building block chemicals” rather than conventional base chemicals was proposed by the US Department of Energy (DoE) in 2004.4 In 2010, this list was revisited, and only 10 “building block chemicals”, which are also referred to as “platform chemicals”, were proposed (Fig. 1).5 This approach combines depolymerization of the complex biomass-derived polymers and selective defunctionalization of the obtained monomers into novel platform chemicals.4–15 High density of the functional groups available in the platform molecules opens numerous possibilities for tailored chemical transformations.16
 | ||
| Fig. 1 Structures of ten molecules indicated by DoE as platform chemicals.5 | ||
Remarkably, separation and purification costs account for the major production and operating costs in biorefineries. Robust separation technologies are therefore critical to have biomass-based industry economically viable.17 Diluted and complex product streams are responsible for costly separations in biorefineries. Liquid crude oil, which is currently used as the main feedstock in industry, requires no dilution for processing. In contrast, pulp mill receives a feedstock that is solid and consists out of 50% water. To process the fiber into desired products, size reduction, and further dilution are required. Finally, multi-component streams containing the target product in the concentration range of 1–5 wt% are obtained. High dilution rates result in high recovery costs, which are usually inversely proportional to the product concentration in streams.18 The products and intermediates in biorefineries frequently exhibit high polarity, high boiling points along with low thermal stability. Consequently, application of conventional separation methods, such as distillation19,20 or liquid–liquid extraction,21 is either impossible or not economically feasible.
This Review Article focuses on recovery of sugar alcohols and saccharides from product streams in biorefineries. Section 2 addresses the role of saccharides and sugar alcohols in novel value chains. Processes which are based on adsorptive uptake of materials bearing boronate moieties are considered in section 3. Advances in preparation of adsorbents with boronate functionalities are summarized in section 4. Finally, section 5 provides a critical comparison of the currently used chromatographic separation over Ca2+ ion-exchange resins with adsorptive separation exploiting boronate-bearing materials and lists further steps which would facilitate application of adsorbents with boronate functionalities for separation in biorefineries.
2. Polyols and saccharides as elements of value chains
The current list of DoE “building block chemicals” contains three polyols, namely glycerol, D-sorbitol, and D-xylitol (Fig. 1).- Glycerol is a co-product of fatty acid methyl ester (FAME) obtained as crude glycerol, a solution containing water and salts. Costly vacuum distillation is the state-of-the-art separation technology commercially used for purification of glycerol.22,23
- Starch is used as a raw material for production of D-sorbitol. First, starch is enzymatically hydrolyzed to yield D-glucose, which is further hydrogenated in the presence of a Ni catalyst. Purification of D-sorbitol includes numerous stages of treatment with ion-exchange resins to perform desalination.24
- Production of D-xylitol resembles the production of D-sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-xylose is obtained from hydrolysates of hemicelluloses and is catalytically hydrogenated in the presence of Ni.24 Strikingly, D-xylitol is significantly more costly than D-sorbitol, i.e. their prices were estimated as 3900 $ per ton vs. 650 $ per ton, respectively.25 Hemicelluloses present heteropolymers, whose hydrolysis results in a number of monosaccharides: D-xylose, D-galactose, L-arabinose, D-glucose, and D-mannose as well as glucuronic acid and acetic acids.26 Two alternatives to produce D-xylitol are possible: either the monosaccharides are separated to isolate D-xylose prior to hydrogenation or a mixture of the hemicellulosic monosaccharides is hydrogenated and the obtained sugar alcohols are thereafter separated to yield pure D-xylitol. These chromatographically performed separations are mainly responsible for a high price of D-xylitol.25
D-xylose is obtained from hydrolysates of hemicelluloses and is catalytically hydrogenated in the presence of Ni.24 Strikingly, D-xylitol is significantly more costly than D-sorbitol, i.e. their prices were estimated as 3900 $ per ton vs. 650 $ per ton, respectively.25 Hemicelluloses present heteropolymers, whose hydrolysis results in a number of monosaccharides: D-xylose, D-galactose, L-arabinose, D-glucose, and D-mannose as well as glucuronic acid and acetic acids.26 Two alternatives to produce D-xylitol are possible: either the monosaccharides are separated to isolate D-xylose prior to hydrogenation or a mixture of the hemicellulosic monosaccharides is hydrogenated and the obtained sugar alcohols are thereafter separated to yield pure D-xylitol. These chromatographically performed separations are mainly responsible for a high price of D-xylitol.25
Noteworthily, potentially industrially relevant polyols are not limited to the platform chemicals designated by DoE. Further examples of commercially relevant saccharides and sugar alcohols, whose separation presents a bottleneck of the process, are known. For instance, biotechnologically produced 2,3-butanediol27,28 or ethylene glycol and propylene glycol29 as products of hydrogenolysis can hardly be recovered from the obtained aqueous product streams.
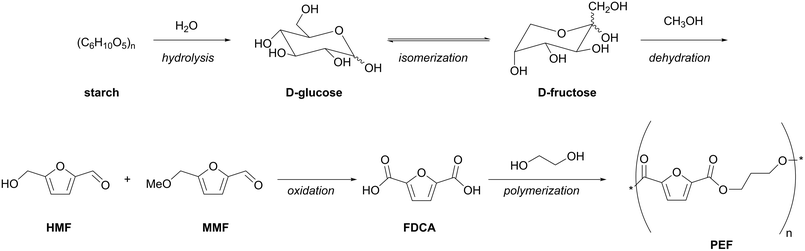
Separation of monosaccharides as intermediates for production of platform chemicals is another important challenge. For example, commercial production of furanics as versatile platform molecules requires certain saccharide substrates dissolved in an appropriate solvent in a high concentration. Numerous attempts to develop a profitable method for synthesis of 5-(hydroxymethyl)furfural (HMF) have been undertaken. Acid-catalyzed dehydration of D-glucose and D-fructose in water as solvent results in 10%30 and 50%31,32 yields of HMF, respectively. A method for acid-catalyzed rehydration of D-fructose to obtain a mixture of HMF and 5-(methoxymethyl)furfural (MMF) in up to 70% combined yield using methanol as a solvent was recently developed. Application of methanol instead of water results in suppression of HMF dehydration into levulinic acid and formic acid.33
Avantium explored D-fructose-based production of MMF and HMF as well as selective oxidation of these molecules to produce 2,5-furandicarboxylic acid (FDCA) on a pilot plant scale. Moreover, Avantium provided promising results for polymerization of FDCA with ethylene glycol – the latter is already available on an industrial scale based on bioethanol25 – to obtain polyethylene 2,5-furnadicarboxylate (PEF), a polyester suitable for packaging, fibers, and films (Fig. 2). Recently, an upscaling to Flagship Plant based on D-fructose as a starting material was announced by Avantium.34
D-Fructose is produced nowadays as a part of high fructose corn syrup (HFCS; 42–55% fructose, rest glucose, and 1–4% oligosaccharides), a liquid alternative sweetener to sucrose made from corn. This is by far the largest synthetic biocatalytic process.35 The major applications of HFCS are now carbonated beverages and raised bakery products.36 Starch is nowadays used as a starting material for production of HFCS. Comparing the scales of polyester production – 24.23 Mt polyethylene terephthalate (PET) were produced in 2021 – with the available supply of 14 Mt of HFCS, it becomes obvious that an increase in demand of D-fructose is expected in the near future.
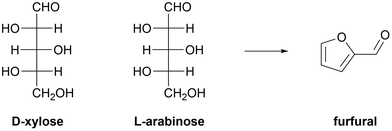
Profitable conversion of hemicellulosic pentoses (D-xylose and L-arabinose) into furfural shown in Fig. 3 presents another difficult task. Acid pre-treatment of lignocellulose results in highly diluted streams of hemicellulosic saccharides, whose concentration typically does not exceed 2 wt%. Economically efficient separation and concentration of saccharides from hydrolysates of hemicelluloses poses another challenge.37
Currently, saccharides are not recovered from industrial streams, with a few exceptions for separation of value-added products, such as D-fructose or L-arabinose. For instance, molasses consist of concentrated aqueous sugar solutions – sucrose and sometimes invert sugar – available as by-products at sugar mills. Molasses are composed of up to 50% of sugars and up to 17% of dissolved salts and are used without separation as animal feed or feedstock for fermentation.38 Stepwise hydrolysis of starch is performed via gelatinization, liquefaction, and enzymatic hydrolysis giving rise to highly pure D-glucose syrup, which is in demand in food industry.39 Hydrolysis of cellulose starts with the pre-treatment of lignocellulosic feedstock to break apart the tightly connected composite of cellulose, hemicelluloses, and lignin. Enzymatic fermentation of the pre-treated lignocellulose results in saccharification of cellulose yielding an aqueous solution of D-glucose. The obtained D-glucose hydrolysate is not as pure as the one prepared based on starch due to the presence of degradation products formed during the pre-treatment. Since these impurities inhibit fermentation of D-glucose, they are removed via so-called detoxification procedure. Finally, D-glucose hydrolysate is fermented without an intermediate separation of D-glucose. Cellulosic bioethanol is mainly the target fermentation product at lignocellulosic biorefineries, though production of other value-added compounds is also possible.40,41
3. Interaction of phenylboronates with saccharides
Boronic acids are remarkable in such a way that they do not donate protons in aqueous solution but rather act as Lewis acids, accepting OH− and releasing H+ (Fig. 4a).42 Acidity constant of a boronic acid depends on its structure and determines the concentration ratio of a neutral boronic acid to boronate anion at a certain pH value. The neutral and the anionic forms contain trigonal (sp2-hybridized) and tetragonal (sp3-hybridized) boron, respectively. For the simplest phenylboronic acid, a pKa of 8.8 was reported.43 Boronates are capable of reversible esterification with molecules bearing vic-diol moieties as shown in Fig. 4. The complexation constant depends primarily on the stereochemistry of a substrate: polyols and saccharides with cis-diol moieties form stronger complexes than the substrates with trans-moieties. The pKa value of a boronic acid enables an approximate prediction of an optimum pH value for formation of a boronate-substrate complex. According to the scheme in Fig. 4a, a pH value above the pKa value of a boronic acid facilitates its conversion into the boronate anion, which in turn reacts with the substrate to yield the boronate-substrate complex. Nevertheless, several publications report optimal complexation at pH below the pKa value of the boronic acid. The reason for this discrepancy is complexation of a substrate with neutral boronic acid moiety to form a boronic acid-substrate complex as depicted in Fig. 4b. Acidity constants of boronic acid-substrate complexes, where the substrate is a saccharide or a polyol, is frequently below the pKa value of a parent boronic acid. This results in the formation of a boronate-substrate complex according to the equation in Fig. 4b which takes place at pH values somewhat below the pKa values of the boronic acid.43–46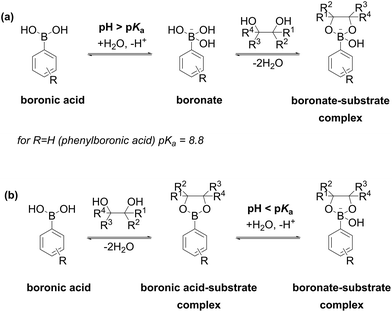 | ||
| Fig. 4 Two modes of complexation via (a) ionization of the boronic acid and (b) its direct complexation with a diol. | ||
Quick and highly specific complexation of boronate with molecules bearing cis-diol moieties became the basis of numerous applications. For example, carbohydrate sensors with boronic acid groups were developed.47–49 Boronic acids have been incorporated in polymeric particles, both as stimuli-responsive functional group and as targeting ligand.42,48 Boronate affinity chromatography is used to isolate a wide variety of cis-diol containing compounds in life science: catechols, nucleosides, nucleotides, nucleic acids, glycoproteins, and enzymes.48,50–53 Organoboron compounds are used as activating groups for diol motifs found in carbohydrates.48 Extraction via anionic complexation with boronic acids has been explored as means to recover saccharides from the biorefinery streams.53,54
Immobilization of boronic acid groups onto cellulose as support to obtain stationary phases for boronate-affinity adsorption was first performed in the 1970s.55 Since then, various boronate-functionalized materials were prepared and tested for separation of sugar alcohols and saccharides in biorefineries (Table 1).
| Entry | Process description | Adsorbent | Boron content | Adsorbate loading | Ref. |
|---|---|---|---|---|---|
| a Boron content estimated based on data provided in reference.85 b Boron content estimated based on data provided in ref. 71. c DVB designates divinylbenzene, EDMA refers to ethylene glycol dimethacrylate. d Boron content estimated based on data provided in reference.76 e Estimated by the authors based on energy-dispersive X-Ray spectroscopy (EDS) analysis in reference.61 f Estimated based on the composition of the polymer provided by the authors of reference.77 g PBS designates phosphate-buffered saline. h MOF refers to metal–organic framework, MIL refers to Materials of Institute Lavoisier. | |||||
| Gel-type materials | |||||
| 1 | Chromatographic separation of sugars (L-rhamnose, D-mannose, D-galactose, D-glucose, D-ribose, maltose, isomaltose, lactose, and raffinose) using 0.1% aqueous ammonia at pH 10.5 as eluate | “Boric acid gel” 3-Acrylamidophenylboronic acid cross-linked with tetramethylene dimethacrylate | Ca. 1.3 mmolB gpolymer−1a | No data | 74 |
| 2 | Chromatographic separation of sugar mixture (D-glucose, D-mannose, D-fructose, D-xylose) with NaOH at pH 9.6 or with pure water | Gel 4-vinylbenezeneboronic acid-divinylbenzene co-polymer | Ca. 4.4 mmolB gpolymer−1b | No data | 71 |
| 3 | Recovery of L-erythrulose from fermentation broths using frontal chromatography at pH 9.5 | Affi-Gel 601 for adsorption | Ca. 0.13 mmolB mLgel−1 | 6 mgL-erythrulose mLgel−1 | 67 |
| 4 | Recovery of D-fructose from its mixture with D-glucose; adsorption at pH 10, desorption with CO2 or acid | Cross-linked 4-vinylphenylboronic acid with 5–40 mol% of a cross-linker | 2.9–4.7 mmolB gpolymer−1 | 540 mgD-fructose gpolymer−1 | 58 |
| 5 | Recovery of diols, polyols, and saccharides; adsorption at high pH, desorption with acid or water | Cross-linked 4-vinylphenylboronic acid with 20 mol% of DVB or EDMA as a cross-linkerc | 3.5–3.7 mmolB gpolymer−1 | Up to 600 mg gpolymer−1 | 59 |
| 6 | Recovery of 2,3-butanediol from fermentation broths | Cross-linked 4-vinylphenylboronic acid with 20 mol% of DVB as a cross-linkerc | 2.6 mmolB gpolymer−1 | 250 mg2,3-butanediol gpolymer−1 | 60 |
| 7 | Chromatographic separation of D-glucose, D-fructose, and oligosaccharides | Commercial boronate Affi-Gel | 1.2–2.5 mmolB gpolymer−1 | No data | 75 |
| Macroporous resins | |||||
| 8 | Recovery of lactulose and D-tagatose; adsorption at pH > 10, desorption at pH 1.5 | Poly(3-acrylamidophenylboronic acid-co-ethylene dimethacrylate), 71 or 83 mol% cross-linker |
Ca. 1.1–1.45 mmolB gpolymer−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
120–170 mglactulose gpolymer−1, 54 mgD-tagatose gpolymer−1 | 68, 69, 72, 76 and 69 |
| 9 | Recovery of 1,2,4-butanetriol from fermentation broths; adsorption in NaOH solution, desorption with EtOH or water | Macroporous resin with phenylboronic acid, 2- and 4-naphtalene boronic acid or 4-biphenylboronic acid as functional monomers with 1,3,5-tris(bromomethyl)benzene as a cross-linker, 50 mol% cross-linker | 5.5–6.4 mmolB gpolymer−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
Up to 148 mg gpolymer−1 | 61 |
| Molecularly imprinted polymers | |||||
| 10 | Separation of D-glucose from mixtures with D-xylose; adsorption at pH 11, desorption with 0.5 M HCl | Molecularly imprinted polymer based on methacrylamidophenylboronic acid as functional monomer, methacrylic acid as monomer, ethylene glycol dimethacrylate and methylene-bis-acrylamide as cross-linkers | 0.25 mmolB gpolymer−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
Up to 236 mgD-glucose gpolymer−1 | 77 |
| Grafted boronate moieties | |||||
| 11 | Chromatographic separation of sugars (sucrose, D-glucose, D-ribose, and D-fructose) and polyols (erythritol, ribitol, L-arabitol, xylitol, D-mannitol, galactitol, and sorbitol) | Boronate acid groups grafted at cellulosic matrix | 0.2–0.6 mmolB gcellulose−1 | No data | 55 and 78 |
| 12 | Recovery of lactulose; adsorption at pH 8, desorption at pH 1.5 | Grafted boronate groups groups on amino macroporous resin AR-0 | 2.0 mmolB gpolymer−1 (AR-1M), 0.66 mmolB gpolymer−1 (AR-2M) | 85 mglactulose gpolymer−1 | 79 |
| 13 | Recovery of D-glucose at pH 8.5 | Boronic acid functionalized iron oxide | No data | Up to 260 mgD-glucose gmaterial−1 | 80 |
| 14 | Recovery of D-fructose from its mixture with D-glucose; adsorption at pH 8, desorption at pH 2 | Boronic acid grafted at magnetic nanoparticles | No data | Up to 14.4 mgD-fructose gmaterial−1 | 81 |
| 15 | In situ recovery of D-fructose and D-xylulose during isomerization of D-glucose and D-xylose, respectively | 3-Amino phenylboronic acid grafted onto Eupergit C | No data | 17 μmolD-fructoseose mLpacked bed−1 | 46 |
| 16 | Chromatographic separation of D-glucose and D-fructose using sodium pyrophosphate at pH 8.25 as eluate | Silica particles bearing grafted boronate groups | 0.25 mmolB gsilica−1 | No data | 82 |
| Grafted polymer brushes | |||||
| 17 | Recovery of D-glucose and D-xylose | Grafted 3-aminophenylboronic acid and grafted brushes with boronate moieties on SBA-15 | 0.26 mmolB gmaterial−1 (grafted boronate); 1.60 mmolB gmaterial−1 (grafted polymer brushes) | Maximum loadings for grafted boronates: 32 mgD-xylose gmaterial−1, 45 mgD-glucose gmaterial−1; Maximum loadings for grafted polymer brushes: 372 mgD-xylose gmaterial−1, 381 mgD-glucose gmaterial−1 | 56 |
| 18 | Adsorption of D-fructose in PBS at pH 7.4g | Polymer brushes on silica particles | 0.78 mmolB gmaterial−1 | 72 mgD-fructose gmaterial−1 | 83 |
| Miscellaneous | |||||
| 19 | Sugars (D-glucose, D-mannose, D-galactose, D-xylose); adsorption at pH 9, desorption by 0.1 M HNO3 | Cr-based MOF MIL-100 with 5-boronobenzene-1,3-dicarboxylic acid ligandh | Up to 0.65 mmolB gmaterial−1 | Up to 85 mgD-galactose gmaterial−1 | 84 |
The adsorbents bearing boronate groups were tested for separation under different modes:
Simultaneous recovery of few sugars or sugar alcohols from complex solution presents one example. For instance, D-glucose and D-xylose are simultaneously adsorbed from the hydrolysate of lignocellulose. D-Glucose and D-xylose exist predominantly in pyranose form possessing identical structures.56 As a result, D-glucose and D-xylose exhibit similar complexation constants with phenylboronate: 65 and 158.57 Cyclic processes are typically considered for such applications, i.e. adsorption at high pH value which favours complexation (Fig. 4 and the description in section 2) followed by desorption taking place under acidic conditions, i.e. at pH below pKa.
Adsorption of substrates with high and medium complexation constants – for instance, ketoses, aldoses, pentitols, or hexitols – can be readily performed under pH values slightly above pKa values of a boronic acid. Desorption of these substrates requires addition of molecular acids or at least CO2.58,59 Adsorption of substrates with low affinity – such as diols or triols – require addition of more equivalents of NaOH. However, these weakly bonded substrates can be readily desorbed by washing with water, ethanol, or water–ethanol mixtures.59–61
Isolation of a target product from a mixture of similar molecules has been the goal of numerous studies. Most frequently, the separation is based on differences of complexation constants of the adsorbates.59 Separation of an aldose and a corresponding 2-ketose presents a classic example of substrates suitable for separation by adsorption on boronate-bearing materials. Isomerization of D-glucose into D-fructose is of great interest as a method for production of HFCS (Fig. 5a). Isomerization of D-glucose into D-fructose results in ca. 42% product yield in a commercial process performed at 60 °C owing to thermodynamic and kinetic limitations; consequently, the obtained reaction mixture requires separation.36D-Glucose is an aldose which takes up pyranose forms in aqueous solution. Aldopyranoses in general and D-glucose in particular do not exhibit any cis-diol moieties, which leads to weak complexation of aldopyranoses with boronate moieties. Complexation of ketoses is typically significantly stronger than of aldoses (cf. complexation constants of 65 and 1698 for D-glucose and D-fructose, respectively, since β-D-fructofuranose and β-D-fructopyranose conformers exhibit cis-diol moieties, Fig. 5b).59 The presence of cis-diol groups enables a selective adsorption of D-fructose, whereas D-glucose remains in solution.58 Exploration of molecular boronate complexes suggested formation of 2,3-fructofuranose and 1,2-fructopyranose complexes (Fig. 5c).62 In addition to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between D-fructose and the boronate moiety, MAS NMR of the adsorbed D-fructose species suggested complexation of saccharide and boronate moiety with 1
1 complexes between D-fructose and the boronate moiety, MAS NMR of the adsorbed D-fructose species suggested complexation of saccharide and boronate moiety with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry.58
2 stoichiometry.58
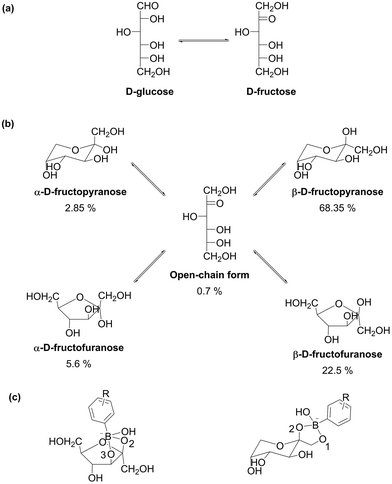 | ||
| Fig. 5 (a) Isomerization of D-glucose into D-fructose; (b) equilibrium forms of [2-13C]-D-fructose determined by 13C NMR at 25 °C in D2O as solvent;70 (c) complexes between boronate anions and anomers of D-fructose.62 | ||
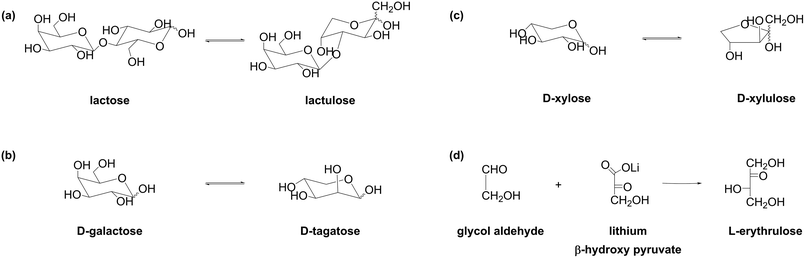
Ketoses of commercial interest which can be selectively recovered from the corresponding reaction mixtures are shown in Fig. 6. The aldodisaccharide lactose is readily available as by-product from dairy industry. Isomerization of lactose (Fig. 6a) into the corresponding ketodisaccharide lactulose has received considerable attention in recent years due to its increasing commercial value in the pharmaceutical, nutraceutical, and food industries. Lactulose has been registered in over 100 countries as a medicinal drug and is on the World Health Organization Model List of Essential Medicines, a database of the most important medications required in a basic health system.63,64D-Galactose (Fig. 6b) presents an aldohexose readily available as a monomer of hemicellulosic polysaccharides26 or a disaccharide lactose.65 Isomerization of D-galactose gives rise to a rare sugar D-tagatose with sweetness similar to that of sucrose. Importantly, D-tagatose presents a low-caloric sweetener, exhibits a low glycemic index, and shows prebiotic effect. Isomerization of D-xylose results in D-xylulose, which is in demand for synthesis of fine chemicals and can be considered as intermediates for synthesis of bio-based bulk chemicals such as furfural (Fig. 6c).11,66L-Erythrulose is a rare C4 ketose which can be produced via the Escherichia coli transketolase-catalyzed condensation of glycolaldehyde and β-hydroxypyruvate (Fig. 6d).67
The separation of ketoses is often performed in a cyclic batch adsorption mode: at elevated pH values in the range of 7.5–12, ketoses are adsorbed, whereas the rest of the components, i.e. the remaining substrates, catalysts, and eventually auxiliaries, remain in the liquid phase. Desorption of the ketoses under acidic conditions is possible. Frequently, small portions of aldose are co-adsorbed with ketose. Thus, desorption of such a binary mixture can be performed in two steps to improve their purity. Wang et al. explored desorption of less strongly adsorbed D-galactose and more strongly adsorbed D-tagatose. The first step of desorption presents treatment of the loaded resin with distilled water at pH ca. 6; under these conditions, D-galactose (complexation constant with phenylboronate 126)57 is readily desorbed, whereas D-tagatose (complexation constant with phenylboronate 1995)57 remains mostly adsorbed. The second step of desorption is treatment with an acid at pH below 2, leading to desorption of D-tagatose of high purity.68 The same approach was applied to produce high-purity lactulose.69
Selective adsorptive uptake of ketoses offers an opportunity for process intensification via development of adsorption-assisted synthetic methods. In situ product removal facilitates an equilibrium shift towards the product and mitigates the product inhibition of biocatalyzed processes. Materials bearing boronate moieties can be added directly into a reactor: improvement of yield of D-fructose from below 30% to 50.1% upon isomerization catalyzed by NaOH at pH 12 was reported.71 Similarly, a 56% yield of D-fructose was obtained by performing isomerization-adsorption cycles.58In situ and recycling experiments enabled adsorption-assisted processes with high yields of lactulose of up to 72%69,72 and of D-tagatose of 53%.68 A circulating set-up containing a reactor operating at elevated temperature and an adsorption column at room temperature enabled 56.7% yield of D-fructose.71 Adsorption onto boronate-bearing materials was used for in situ product isolation for isomerization of D-glucose into D-fructose and D-xylose into D-xylulose catalyzed by glucose isomerase. In situ adsorption improved the yields of D-fructose from 45% to 73% and of D-xylulose from 23% to 52%.46L-Erythrulose was adsorbed by in situ product removal during the Escherichia coli transketolase-catalyzed condensation of glycolaldehyde and lithium β-hydroxypyruvate resulting in improved productivity of the bioconversion.67
Importantly, not only affinity of a particular substrate towards boronate moieties but also other factors, for instance, a porous structure of an adsorbate, can be employed to control the selectivity of uptake. The concept of molecular imprinting implies creation of an artificial keyhole which exhibits an exact match to geometrical dimensions of the target molecule.73 A selective recovery of D-glucose from its mixture with D-fructose and D-xylose via selective adsorption on a molecularly imprinted polymer functionalized with phenylboronate moieties has been recently reported. Section 4.3 provides more details on synthesis and structural peculiarities of molecularly imprinted polymers.
Chromatographic separation over materials functionalized with boronate groups as stationary phases was investigated.74 A potential separation and recovery of all components of the complex mixture is a significant advantage of chromatographic separation compared to the adsorption method described above. In general, chromatographic separation remains the most frequently used separation technique for recovery of sugar alcohols and sugars in industry.25 Dilution of the recovered product with an eluate, which should be removed by evaporation, presents the major drawback of chromatography. A few authors reported a correlation between the substrate structure and its tendency to complexation. An increase in the number of cis-hydroxyl groups results in more extensive complex formation and, consequently, larger retention times at the stationary phases bearing boronate groups.55,71,74
4. Adsorbent
Table 1 summarizes different boronate-functionalized adsorbent materials and the corresponding separation procedures. The adsorbent types are discussed in detail in this section.4.1. Gel-type polymers
Gel-type polymers (Table 1, entries 1–7) can be readily prepared via radical polymerization of 4-vinylphenylboronic acid with addition of a small amount of a cross-linker. Commercial availability of 4-vinylphenylboronic acid as well as of the cross-linkers presents an advantage of this method (Fig. 7). Moreover, gel-type polymers contain high amount of boron and exhibit therefore high maximal adsorption capacity: the boron content of up to 4.7 mmolboron gpolymer−1 was reported.58 Alternatively, boron-containing Affi-Gel with as high boron content as 1.2–2.5 mmolboron gpolymer−1 in dry state is commercially available from Bio rad.75 Though these gels are produced via immobilization of boronate moieties, they exhibit a gel-type matrix and will be considered in this section.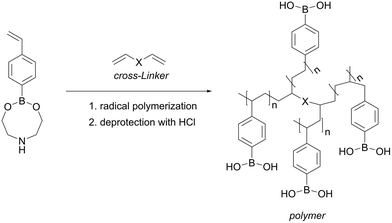 | ||
| Fig. 7 Synthesis of a cross-linked gel-type polymer. This figure has been adapted from ref. 58 with permission from the Royal Society of Chemistry, copyright 2020. | ||
Adsorption affinity of diols, polyols, and saccharides can be readily predicted based on their well-documented molecular complexation constants with phenylboronic acid.57 In line with this, elution order of the saccharides on the column was reported to be dependent on the complexation ability.
Gel-type polymers are nonporous in dry state and can swell in different solvents. Schroer et al. synthesized gel-type polymers with various types of linkers (Fig. 8a) and examined adsorption of D-fructose and D-glucose on the adsorbed polymers. D-Fructose presents an adsorbate with high affinity towards complexation (complexation constant is 1698).57 It is readily adsorbed on the outer shell of the polymer particle, and the polymer chains get negatively charged. Repulsion of the negatively charged polymer chains results in further swelling, making the boronate groups in the polymer core accessible, and further adsorption of D-fructose occurs. Maximum capacity of the polymer depends on the structure of the cross-linker: long aliphatic or stiff aromatic cross-linkers were favourable for an efficient transport of D-fructose towards the boronate sites (Fig. 8b). Thus, equimolar uptakes of D-fructose were possible for the materials bearing these cross-linkers, i.e. 1 mol D-fructose for 1 mol boron (Fig. 9). It is noteworthy that the capacity depends on the amount of a cross-linker: an increase of DVB content from 5 mol% to 40 mol% resulted in a decrease of a maximum uptake of D-fructose of 1 molD-fructose molboron−1 to 0.5 molD-fructose molboron−1.56 This result is expected since the pore size of gel-type microporous polymers is determined by the percentage of the used cross-linking monomer.86 Smaller pore size leads to lower uptake owing to worse accessibility of the boronate sites.
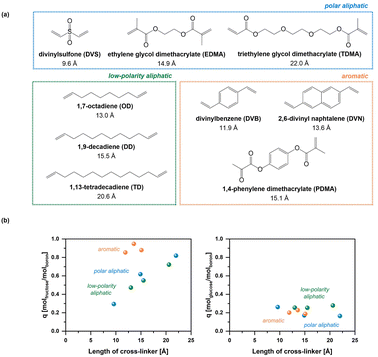 | ||
| Fig. 8 (a) Structures of the cross-linkers used to synthesize gel-type polymers with boronate moieties and (b) dependency of loadings of D-fructose (left) and D-glucose (right) on the nature of the cross-linker. This figure has been adapted from ref. 58 with permission from the Royal Society of Chemistry, copyright 2020. | ||
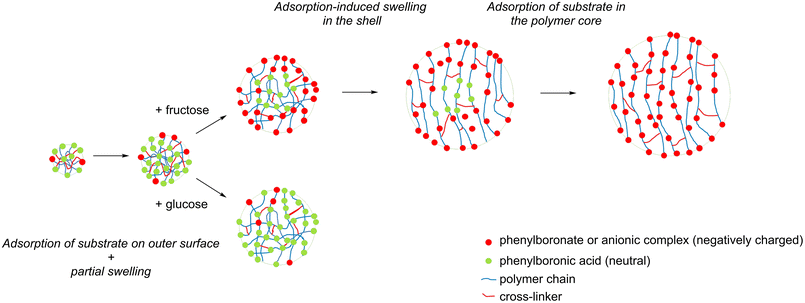 | ||
| Fig. 9 Scheme of swelling upon adsorption of D-fructose and D-glucose. This figure has been adapted from ref. 58 with permission from the Royal Society of Chemistry, copyright 2020. | ||
D-Glucose exhibits a significantly lower affinity to polymers functionalized with boronate moieties, which corroborates with its lower molecular complexation constant with phenylboronic acid of 65.57 Consequently, D-glucose is adsorbed on the outer shell of polymer in lower amount, leading to a lower charge of the polymer chains and, consequently, a lower swelling degree. As a result, most of the boronate sites remain inaccessible for D-glucose (Fig. 9).58
Schroer et al. investigated adsorption of 18 diols, sugar alcohols, and saccharides on gel-type poly(4-vinylphenylboronic acid) cross-linked with 20 mol% DVB or 20 mol% EDMA. The authors concluded that the adsorption mechanism schematically shown on Fig. 9 can be generalized: the adsorbates with high complexing constants readily induce swelling of the polymers and exhibit loadings of 1 mol substrate per 1 mol boronate group which corresponds to maximum loadings of up to 600 mgsubstrate gpolymer−1 in carbonate buffer at pH 10, estimated using Langmuir adsorption model. Very high loadings were observed for instance, for D-sorbitol (complexation constant 10232), D-xylitol (complexation constant 2399), D-mannitol (complexation constant 2089), or D-tagatose (complexation constant 1995).59
The adsorbates with low complexation constants adsorb on the outer shell of the polymer particle and low density of the negative charge on the polymer chains does not cause a significant swelling. An example of such adsorbates is glycerol with a complexation constant of 16, which exhibits a moderate loading of ca. 80 mgglycerol gpolymer−1 in carbonate buffer at pH 10. It is nevertheless possible to reach high loadings of adsorbates with low affinity by adding more equivalents of a base. As Fig. 4a suggests, increase of OH− concentration results in a shift of the equilibrium to the right. For 2,3-butanediol, the complexation constant is 3.5. In carbonate buffer at pH 10, only 160 mg2,3-butanediol gpolymer−1 was obtained. However, the capacity could be increased to 250 mg2,3-butanediol gpolymer−1 corresponding to an equimolar loading with respect to boron content upon addition of at least 1 equivalent of NaOH to one equivalent of substrate.60
Cyclic adsorption operations with boronate materials typically imply adsorption under basic and desorption under acidic conditions. However, placing the swollen loaded gel-type polymers into an acidic or neutral solution causes shrinkage of the materials.87 As a result, the adsorbate molecules located at the core of the polymer bead experience significant diffusional hindrances which leads to “trapping” of part of the desorbed adsorbate in the core of the shrunk resin and hinders a full recovery.58,59
A narrow peak shape and a good separation factor at high loading of the adsorbate are important factors for preparative chromatography. Barker et al. explored elution of D-glucose and D-fructose on a gel-type column using NaOH solution at pH 9.6 as a mobile phase. For low-concentration samples, sugars were eluted as symmetrical peaks, but the peak of D-fructose was broad with respect to D-glucose and D-mannose. Increase of a sugar loading from 250 μg to 500 mg resulted in a significant decrease of the retention factor of D-fructose and a peak tailing. The authors explained this effect by a considerable local increase in acidity due to complex formation at high concentration of D-fructose (Fig. 4b).71 At the same time, neutralization owing to injection of highly-concentrated D-fructose, which also exhibits acidic properties, cannot be excluded. Very high swelling degrees of the functionalized with boronates loaded gel-type polymers are well documented. Vente et al. observed volume increase of an adsorbent swollen in NaOH by a factor of 2.57 for commercial Boronate Affi-Gel.75 Schroer et al. reported 212% swelling degree in the presence of D-fructose in carbonate buffer at pH 10 for a gel-type 4-vinylphenylboronic acid polymer cross-linked with 20 mol% DVB.58 Such high swelling degrees suggest high elasticities and poor mechanical strength hampering large scale applications. Moreover, Vente et al. conducted column separation using gel-type Boronate Affi-Gel as a stationary phase, which was pre-swollen by washing with a NaOH solution at pH 9. Injection of D-fructose resulted in a decrease of pH to 6, a partial conversion of boronate groups into boronic acid groups (Fig. 4), and a shrinkage of the chromatographic bed.75
Though gel-type polymers with an optimized structure and an appropriate content of cross-linker are capable of high loadings of adsorbates with high complexation affinities, the rates of the adsorption are low. Thus, it takes typically 5–7 h to reach saturation loading of D-fructose on the poly(4-vinylphenylboronic acid) cross-linked with 20 mol% DVB at room temperature. The unfavourable kinetics may be explained by the time required for the polymer to swell as well as a slow substrate diffusion in the micropore structure of the swollen gel.58,59
Gel-type polymers bearing phenylboronic acid moieties are hydrophobic and not wettable by aqueous solutions of the adsorbates. Batch adsorption experiments required addition of an organic solvent, e.g. ethanol, as a co-solvent to improve wettability.58,59 Vente et al. performed column experiments and reported on improvement of hydrophilicity upon transformation of boronic acid groups into boronate groups.75
4.2. Macroporous resins
Macroporous resins are prepared with higher contents of cross-linkers than gel-type polymers (Table 1, entries 8 and 9). Macroporous (also referred to as macroreticular or isoporous) polymer particles constitute of an agglomerate made of secondary particles representing agglomerates of microspheres themselves.73The adsorption sites located inside of these microspheres become unavailable due to a high degree of cross-linking88 resulting in lower capacities of macroporous resins compared to gel-type materials. Transport of adsorbates into meso- and macropores between the agglomerates is quicker than into the microporous gel. Moreover, higher mechanic stability of macroporous resins than of highly elastic microporous gels is an advantage for their large-scale application.
The groups of Hua and Yang synthesized macroporous resins by radical polymerization using 3-acrylamidophenylboronic acid as a functional monomer, ethylene dimethacrylate as a cross-linker, and DMSO as a porogen (Fig. 10).76 A series of adsorbents varying the content of a cross-linker in the range of 50 to 93 mol% was prepared. The authors reported that the two materials containing 71 and 83% of the cross-linker, respectively, exhibited the best performance in terms of lactulose loading. Boron content was in the range of ca. 1.1 to 1.45 mmolboron gpolymer−1, whereas the maximal loading of lactulose was 120–170 mmollactulose gpolymer−1. This suggests that 30–50% of the total boronate groups are complexed at maximum loading of lactulose. Very quick adsorption of lactulose required less than 15 minutes at pH 10 and an almost instant (≤5 min) desorption at pH below 2 was reported. The prepared macroporous materials were used for in situ recovery of lactulose from the mixture obtained after isomerization of lactose catalyzed by Na2HPO4 + NaOH as well as for separation of D-tagatose from a reaction mixture after isomerization of D-galactose with NaOH at pH 12.68,69,72,76
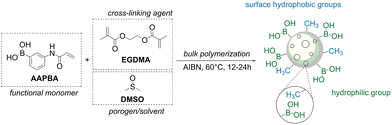 | ||
| Fig. 10 A method to produce a macroporous resin bearing boronic acid groups developed by Wang et al. This figure has been reproduced from ref. 76 with permission from the Elsevier, copyright 2019. Abbreviations: 3-acrylamidophenylboronic acid (AAPBA), ethylene glycol dimethylacrylate (EGMA), and dimethylsulfoxide (DMSO). | ||
Meng et al. explored Friedl–Crafts alkylation reaction to prepare macroporous resins functionalized with boronate moieties. The authors used phenylboronic acid, 1-naphthaleneboronic acid, 4-biphenylboronic acid, or 2-naphthalene boronic acid as functional monomers and 1,3,5-methylboronate benzene as a cross-linker (Fig. 11).
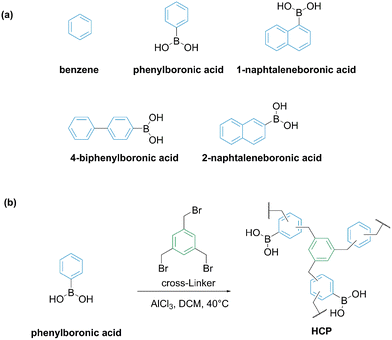 | ||
| Fig. 11 (a) Functional monomers for the manufacture of the polymers; (b) Friedel–Crafts method to synthesize boronate containing microporous resins.61 | ||
A functional monomer and a cross-linker were taken in equimolar amounts and polymerized in DCM using AlCl3 as a catalyst. The resin prepared based on 2-naphtaleneboronic acid exhibited the highest saturated adsorption capacity of 1,2,4-butanetriol of 148.2 mg gpolymer−1. The authors estimated the boron content based on Energy-dispersive X-ray spectroscopy (EDS) analysis as 6–7%. Thus, for the resin based on 2-naphtaleneboronic acid, the highest loading of 1,2,4-butanetriol corresponded to 0.25 mol adsorbate per mol boron. The materials exhibited permanent porosity and specific surface area of 800–1200 m2 g−1. A quick establishment of an equilibrium in a time shorter than 10 minutes was reported. 1,2,4-Butanetriol could be recovered by desorption with water or ethanol.61
4.3. Molecular imprinting
The concept of molecular imprinting was introduced by Mosbach and Wullf in the 1970s.73 Molecularly imprinted materials are prepared via cross-linking of a functional monomer complexed with a target adsorbate. Washing out the adsorbate enables formation of a cavity of certain geometry resulting in a shape selectivity for adsorption. Keeping the geometrical parameters of the artificial keyhole constant under adsorption and desorption conditions requires a high rigidity of the polymer matrix, which can be reached by a very high content of a cross-linker. Nevertheless, application of molecularly imprinted polymers found limited application in preparative chromatography owing to a few disadvantages. High content of a cross-linker results in a low content of a functional monomer and, consequently, in low adsorption capacities. Typical adsorption capacities of molecularly imprinted materials are in the range of 0.5–10 mgD-fructose gpolymer−1.89,90 Moreover, a highly cross-linked matrix of the polymer leads to serious diffusion limitations resulting in significant peak tailing.73Interestingly, Liu et al. recently reported a polymer design based on the monomers shown in Fig. 12 (Table 1, entry 10). The authors used D-glucose (1 mmol) as a template, methacrylamidophenylboronic acid (MAAPBA, 1.2 mmol) as a functional monomer, methacrylic acid (MAA, 6.8 mmol) as a monomer, ethylene glycol dimethacrylate (EGDMA, 19 mmol) as a hydrophobic cross-linker, and methylene-bis-acrylamide (MBAA, 1 mmol) as a hydrophilic cross-linker. The ratio of hydrophilic-to-hydrophobic cross-linkers was found to influence the adsorbate uptake. The material was molecularly imprinted with D-glucose, and a higher adsorption selectivity for D-glucose than for D-xylose despite similar complexation constants (complexation constants for D-glucose and D-xylose are 65 and 158, respectively) was reported.59 Rates of adsorption were low as the adsorption required 4 hours. A remarkable uptake of D-glucose of 236 mgD-glucose gpolymer−1 for adsorption at pH 11 was reported, being significantly higher than typically observed for molecularly imprinted polymers. Interestingly, estimation of boron content based on the ratio of monomers provided by the authors (0.25 mmolboron gpolymer−1) suggests the theoretical capacity of D-glucose of ca. 46 mgD-glucose gpolymer−1. Moreover, unexpectedly high loadings of D-glucose on the molecularly imprinted polymer of 76–79 mgD-glucose gpolymer−1 at acidic pH values of 3–5 were reported. Understanding the reasons of the observed high capacities under different adsorption conditions remains of great interest. Liu et al. tested a hollow-fiber membrane system containing the synthesized molecularly imprinted polymer aiming at a selective capture of D-glucose from cellulose hydrolysates. A higher adsorption capacity of D-glucose under static than under dynamic conditions was reported which was explained by slow kinetics of adsorption.77
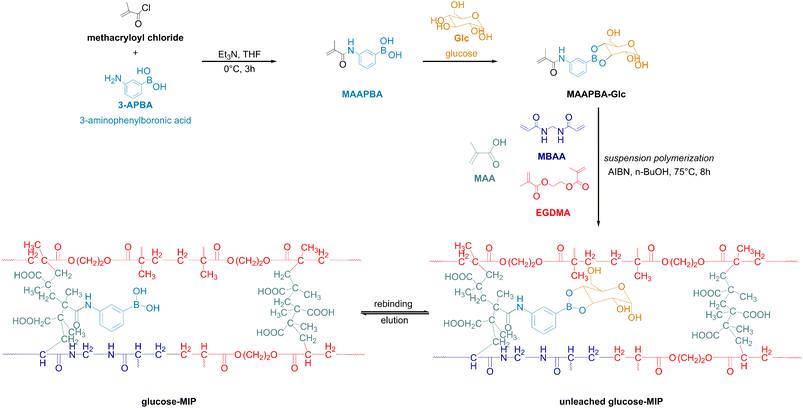 | ||
| Fig. 12 Molecularly imprinted polymer for a selective capture of D-glucose from cellulose hydrolysates. This figure has been reproduced from ref. 77 with permission from the John Wiley and Sons, copyright 2022. Abbreviations: 3-acrylamidophenylboronic acid (AAPBA), methacrylic acid (MAA), N,N′-methylenebisacrylamide (MBAA), ethylene glycol dimethylacrylate (EGMA), azobisisobutyronitrile (AIBN). | ||
4.4. Grafted boronate moieties
Historically, phenylboronate groups grafted on an inert core (Table 1, entries 11–16) constructed the first boronate-containing stationary phase fabricated by Weith et al. in 1970 for separation of saccharides, polyols, nucleosides, and deoxynucleosides.55 The authors chose cellulose as an insoluble matrix to avoid diffusion limitations and proposed two multistep methods for grafting dihydroxyboryl groups shown in Fig. 13. First, commercially available carboxymethylcellulose was converted in its hydrazide and then to azide. Treatment of the obtained material with m-aminobenzeneboronic acid resulted in formation N-(m-dihydroxyborylphenyl)-carbamylmethylenecellulose containing 0.2 mmol boronic acid groups per gram of dry cellulose (Route 1, Fig. 13). To obtain the second material, m-aminobenzeneboronic acid was converted into N-(m-dihydroxyborylphenyl)succinamic acid by reaction with succinic anhydride, and the condensation of this acid in aqueous solution with aminoethylcellulose in the presence of N-cyclohexyl-N′-β-(4-methylmorpholinium)ethylcarbodiimide p-toluenesulfonate as the activation agent to yield N-[N′(m-dihydroxyborylphenyl)succinamyl]aminoethylcellulose bearing 0.6 mmol boronic acid groups per gram of dry cellulose (Route 2, Fig. 13). Interestingly, the material prepared based on carboxymethylcellulose (Route 1, Fig. 13) contained some amount of the negatively charged COO− groups. On the contrary, utilization of aminoethylcellulose as a starting material gave rise to the stationary phase contained some amount of positively charged NH3+ groups (Route 2, Fig. 13). The authors performed column separation of saccharides, polyols, nucleolsides, and deoxynucleosides on a column filled with the synthesized stationary phases using solutions at constant pH values of 7.5 or 8.5. The results suggested that the grafted negatively charged COO− caused an increase of pKa of the grafted dihydroxyboryl groups, whereas the grafted positively charged NH3+ groups led to decrease of the pKa values of boronic acid moieties. Interestingly, increase of the ionic strength of an eluate led to diminishing of these effects.55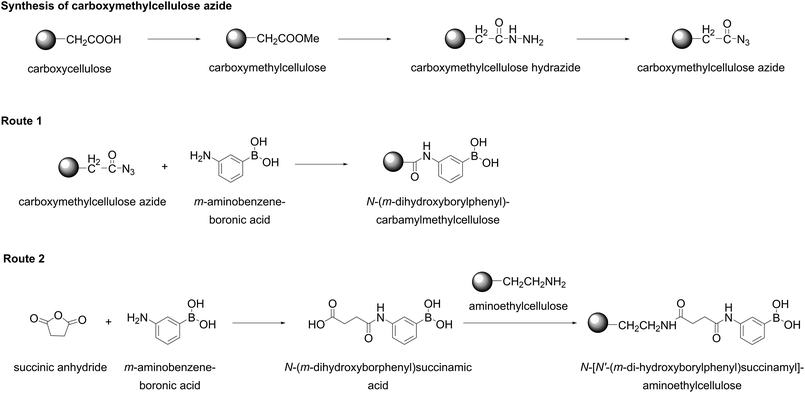 | ||
| Fig. 13 Two different routes for the grafting of boronic acid onto cellulose proposed by Weith et al. This figure has been reproduced from ref. 55 with permission from the American Chemical Society, copyright 1970. | ||
Wang et al. recently prepared two materials via grafting boronic acid groups on a macroporous NH2-containing resin AR-0 with a content of –NH2 functional groups of ca. 6.4 mmol–NH2 gdry resins−1. The synthetic methods and the boron contents reported by the authors are shown in Fig. 14. The resin was pre-swollen in DMF prior to grafting of the boronate functionalities. The resin AR-1M was obtained by covalent bonding of p-formylphenylboronic acid to the surface of AR-0 producing the Schiff′s base which was reduced by addition of sodium cyanohydride powder in methanol. In the second route, glutaraldehyde was first grafted to AR-0 resin followed by addition of 3-aminophenylboronic acid yielding the Schiff′s base. The latter was reduced by addition of sodium cyanohydride powder in methanol resulting in the resin AR-2M. The boron contents of AR-1M and AR-2M were 2.0 and 0.66 mmolboron gpolymer−1, respectively. The resins were tested for adsorption of lactulose, the maximum substrate loading was reported for the AR-1M material amounting to 85 mg gresin−1. This corresponds to 0.12 equivalents of the adsorbate to 1 equivalent of boron. For AR-2M polymer, a higher complexation degree of 22% could be attained. The reasons of rather low loadings of lactulose per boron equivalent were not discussed by the authors and can probably be explained by swelling of the AR-0 resin in DMF prior to the grafting. Probably, shrinking in aqueous phase led to low accessibility of the grafted adsorption sites. Slow kinetics of adsorption and acid-induced desorption requiring 3–4 h and 1–2 h, respectively, support the hypothesis of not readily accessible boronate groups. Adsorption under basic conditions resulted in a selective adsorption of lactulose with separation factors of 2–16. Dependency of lactulose uptake by AR-1M and AR-2M resins on pH value is remarkable: the highest lactulose capacity were observed at pH values of 6 and 8 for the materials synthesized via grafting of aldehyde and amine, respectively. Further increase of the pH value till 10 did not result in improvement of the uptake. The authors explained this effect by the presence of residual surface -NH2 groups responsible for alkaline microenvironment and capable of forming B–N-coordination complexes similar to these in Wulff type phenylboronic acids (Fig. 14).79
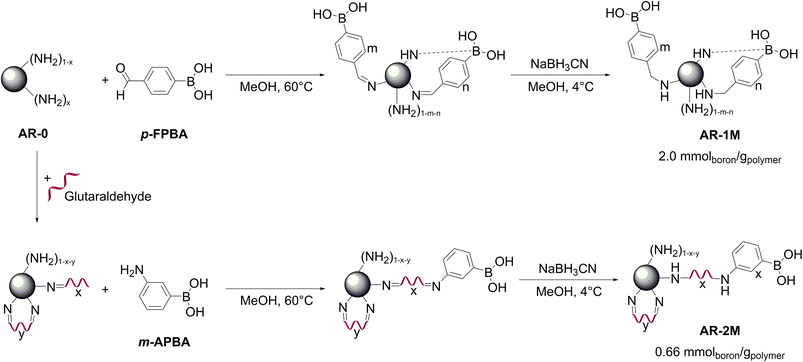 | ||
| Fig. 14 Synthetic routes for grafting phenylboronic acid moieties onto an amino microporous resin proposed by Wang et al. This figure has been reproduced from ref. 79 with permission from the American Chemical Society, copyright 2018. Abbreviations: aminated macroporous poly(styrene-co-divinylbenzene) resin (AR-0), p-formylphenylboronic acid (p-FPBA), 3-aminophenylboronic acid (m-APBA). | ||
Functionalization of Fe3O4 to prepare magnetically separable nanoparticles bearing boronate groups was conducted according to the scheme shown in Fig. 15. 3-Aminophenylboronic acid was allowed to react with 3,4-dihydroxybenzaldehyde; the obtained derivative was grafted onto Fe3O4 nanoparticles to yield functionalized particles of the size of 8 ± 2 nm. The boron content was not reported, but the synthesized nanoparticles exhibited a high uptake of D-glucose of up to 260 mgD-glucose gmaterial−1 in phosphate buffer at pH 8.5. Unfortunately, the authors did not report the results of desorption, which would be of interest since desorption at low pH values could result in leaching of iron.80
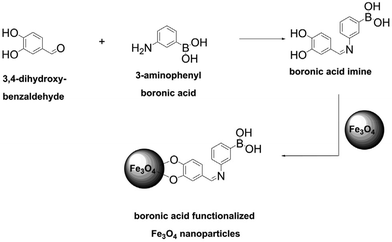 | ||
| Fig. 15 Functionalization of Fe3O4 nanoparticles with boronic acid moieties performed by Mohapatra et al. This figure has been reproduced from ref. 80 with permission from the Elsevier, copyright 2009. | ||
Gu et al. also prepared functionalized Fe3O4 magnetic nanoparticles. For these purpose, Fe3O4 nanoparticles were first coated with SiO2 using sol–gel reaction, thereafter a thiol-containing moiety was immobilized followed by grafting of 4-vinylphenylboronic acid via a thiol–ene click reaction. The nanoparticles had a mean diameter of 195 nm. Unfortunately, the authors did not provide information on boron content. The functionalized nanoparticles exhibited a moderate maximal adsorption capacity of D-fructose of ca. 14.4 mgD-fructose gmaterial−1 at pH 8.0. D-Glucose could be desorbed at pH 2 and the nanoparticles could be reused 6 times.81 Immobilization of 3-aminophenylboronic acid on a commercial Eupergit Gel also resulted in a binding capacity of D-fructose corresponding to 17 μmol mL−1 bed or 3.06 mg mL−1 bed at pH 8.0.46 Glad et al. proposed a method to load 3-aminophenylboronic acid on small 10 μm silica particles according to the scheme shown in Fig. 16.
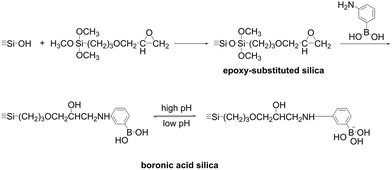 | ||
| Fig. 16 Grafting of boronate moieties onto silica support. This figure has been reproduced from ref. 82 with permission from the Elsevier, copyright 1980. | ||
First, silica was modified with γ-glycidopropyltrimethoxysilane; 3-aminobenzene boronic acid was grafted onto the modified surface. The authors fabricated small particles for HPLC rather than large particles applied for low-pressure chromatography (typically, the particles size of stationary phases applied in low-pressure chromatography is in the range of 50 to 200 μm)91 aiming at reduction of diffusion contributions. HPLC enables higher flow rates of an eluate associated with larger pressure drops. The obtained material contained 0.250 mmol boron per gram. The authors reported a baseline HPLC separation of D-glucose and D-fructose as peaks without tails with a buffer solution at pH 8.3 used as eluate.82
In summary, grafting of boronate groups results in highly accessible adsorption sites on tailored support. Low content of boron and, consequently, low capacity of an adsorbent present the major disadvantages of this preparative method.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of adsorbate-to-boron supporting the hypothesis of an improved accessibility of boron grafted as polymer brushes. Interestingly, the adsorption was not quick, and ca. 3–4 hours were required until the equilibrium was reached. It can be explained by location of the polymer brushes in pores of SBA-15, leading to drop of specific surface area of SBA-15 from 940 m2 g−1 for the unfunctionalized material to ca. 0 m2 g−1 for the material with grafted polymer brushes. Apparently, pore blockage hampers diffusion into the pores of SBA-15 filled with the grafted polymers.56
1 stoichiometry of adsorbate-to-boron supporting the hypothesis of an improved accessibility of boron grafted as polymer brushes. Interestingly, the adsorption was not quick, and ca. 3–4 hours were required until the equilibrium was reached. It can be explained by location of the polymer brushes in pores of SBA-15, leading to drop of specific surface area of SBA-15 from 940 m2 g−1 for the unfunctionalized material to ca. 0 m2 g−1 for the material with grafted polymer brushes. Apparently, pore blockage hampers diffusion into the pores of SBA-15 filled with the grafted polymers.56
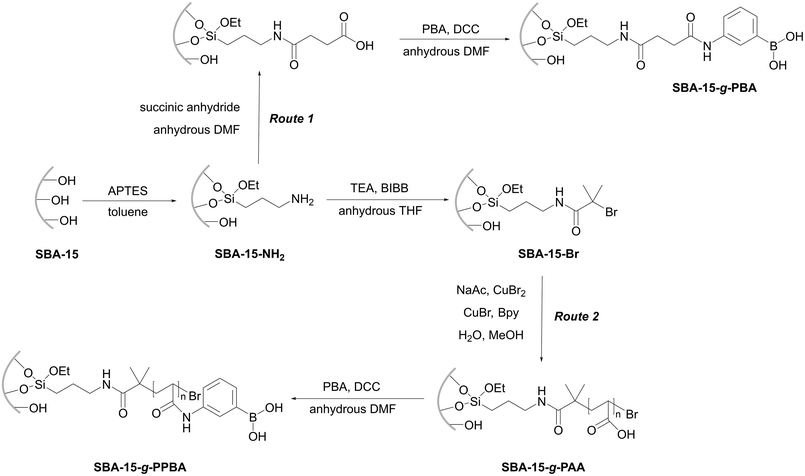 | ||
| Fig. 17 Grafting 3-aminophenyl boronic acid (Route 1) or polymer brushes (Route 2) with boronate moieties onto SBA-15 proposed by Zhao et al. This figure has been reproduced from ref. 56 with permission from the American Chemical Society, copyright 2011. Abbreviations: 3-aminophenylboronic acid (PBA), N,N′-dicyclohexylcarbodiimide (DCC), N,N-dimethylformamide (DMF), mesoporous silica Santa Barbara Amorphous-15 (SBA-15), (3-aminopropyl)triethoxysilane (APTES), triethylamine (TEA), 2-bromoisobutyryl bromide (BIBB), tetrahydrofuran (THF), and 2,2′-bipyridyl (bpy). | ||
Xu et al. also prepared polymer brushes on the silica particles using ATRP. The authors aimed at preparation of a fluorescent grafted polymer brush bearing boronic acid polymer. For this purpose, a fluorescent boronic acid monomer was first prepared from an azide-tagged boronic acid and an alkyne-containing acrylate using a CuAAC click chemistry reaction. Next, the boronic acid monomer was grafted on the surface of silica gel modified with an ATRP initiator (Fig. 18). The authors reported boron loading of 0.78 mmolboron gmaterial−1 and observed 72 mg g−1 loading of D-fructose in phosphate buffered saline (PBS) at pH 7.4.83
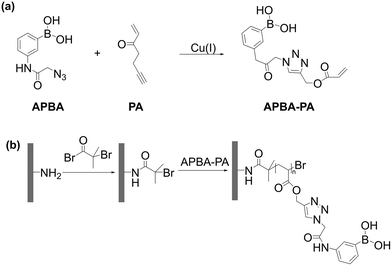 | ||
| Fig. 18 (a) Synthesis of boronic acid monomer and (b) its polymerization using surface-initiated ATRP. Reproduced with permission from ref. 83. Abbreviations: 3-(2-azido-acetylamino)phenylboronic acid (APBA) and propargyl acrylate (PA). | ||
5. Performance of boronate-functionalized materials vs. Ca2+ ion-exchanged resins: state of the art and outlook
Materials bearing boronate moieties are not the only stationary phases capable of adsorptive separation of sugar alcohols and saccharides. Chromatographic separation of saccharides using other adsorbates, such as materials functionalized with amino groups,92–94 ion-exchange resin in HCO3− form,95 or anion-exchange resin in bisulfite form,93,96 is well known. Nevertheless, these types of stationary phases – as well as the materials with boronate moieties – are not used for large-scale preparative processes. The reasons for limited application of these separation methods to laboratory scale are organic co-eluates required for separation on amine stationary phases93 or low stability of bisulfites against oxidation by molecular oxygen leading to conversion of bisulfite groups into sulfate groups.75 Development of material science opens further opportunities for synthesis of stationary phases. For instance, separation of D-glucose and D-fructose by pore exclusion of zirconium-based metal–organic frameworks was recently reported.97Gel-type slightly cross-linked cation exchange resins in Ca2+ form are most frequently used for recovery of sugars and polyols,98,99 for instance, for separation of D-fructose and D-glucose after the isomerization (Fig. 5a) as one of the largest separation processes of biomass-based compounds. Separation of saccharides over Ca2+ – containing materials was occasionally uncovered by Felicetta et al. in 1959: separating the sugars from spent sulfite liquors from the lignin sulfonates using ion-exchange resins, the authors observed separation of L-arabinose and D-xylose.99 In the 1970s, Ca2+ exchanged resins were explored and industrialized as stationary phases for large-scale chromatographic separation of D-fructose and D-glucose. Ligand exchange presents the main mechanism responsible for the separation. In aqueous solution, hydroxyl groups of saccharides compete with the coordinated water molecules at Ca2+ ion. Single OH− groups of carbohydrates cannot compete with the solvent, only a suitably arranged combination of two (axial-equatorial) or three (axial-equatorial-axial) hydroxyl groups results in a significant complex formation with Ca2+.100 The isomers bear the following numbers of the axial-equatorial pairs of OH−-groups: glucopyranose (1/0 for α/β), fructopyranose (1/2 for α/β), and fructofuranose (1/0 for α/β).98 Consequently, stronger complexation of D-fructose than of D-glucose results in a larger retention volume of the former. Tiihonen et al. explored complexation of the saccharides with Ca2+ and concluded on a weak complexation of D-fructose with Ca2+ and a very weak complexation of D-glucose with Ca2+.101 This result corroborates with other publications, which suggest partition into gel matrix of the ion-exchange resin as the main mechanism responsible for retention of D-glucose, implying that D-glucose is not really adsorbed in a formal sense but merely occupies the intraparticle pore space in direct proportion to its concentration in the fluid.102
Application of pure water as eluate presents a remarkable advantage of the separation over the Ca2+ ion exchange resins. It implies a low-cost and an environmentally benign eluate which requires no desalination. Warm water, typically of 55–60 °C, is used: increase of temperature is necessary to reduce pressure drop when operating at high concentrations of the sugars.102 Further advantages of Ca2+ ion exchange resins are low cost and commercial availability of the stationary phase, high long-term stability of the phase without regeneration, and a high column capacity.99 Moreover, the equilibrium isotherms of D-glucose and D-fructose are linear up to high concentrations of ca. 260 g L−1.103 This is of utmost importance for preparative chromatography since high concentrations and high loadings are typical of preparative mode. Linear adsorption isotherms are prerequisite for symmetric peaks without tailing.104 Noteworthily, moderate separation factors of D-glucose and D-fructose in the range of 2.5–3.5 present a disadvantage of Ca2+ ion exchange resins.105,106
Traditional batch chromatography implies injection of a solution to be separated followed by elution. Batch chromatography is usually characterized by high dilution of the obtained fractions which requires an energy-intensive evaporation of eluate. In order to reduce the consumption of eluate, an advanced simulated moving bed (SMB) mode is used on an industrial scale for separation of D-glucose and D-fructose. SMB presents a continuous countercurrent fluid-solid separation developed by UOP under the name of “Sorbex” in the 1970s.105,107
The first reports on materials bearing boronate groups as stationary phases for separation of sugar alcohols and saccharides were published at the same time as the publications on separations using Ca2+ ion exchange resins. However, adsorbents functionalized with boronates have not been commercialized yet. A necessity to use buffer solutions rather than pure water presents a crucial point limiting applicability of boronate-containing stationary phases. This challenge was addressed by Barker et al. who examined a boronate gel as a stationary phase and water as eluate and reported sharp peaks for non-adsorbed sugars, such as D-glucose and D-mannose, tailed peaks for D-fructose and D-xylose, and a very broad peak for D-ribose. The tailing was explained by acidification of the solution due to complexation of sugars with dihydroxyboronogroups (Fig. 4b).71 Interaction of a specific boronic acid with cis-diol groups is highly dependent on pH and pKa of the boronic acid. In most of the applied materials, phenylboronate or naphthalene boronate were used as adsorption sites (Table 1), i.e. the acids with pKa of ca. 9. Consequently, the pH value of ca. 9 for a suitable ionization degree is expected, whereas in pure water a pH value of ca. 6 is observed. Moreover, ionization of a complex of a substrate with a boronic acid moiety (Fig. 4b) can be responsible for local pH drop. Barker et al. suggested application of boronic acids with lower pKa values as functional monomers in order to keep a significant part of the boronate ionized at pH close to 6.7. Ionization constants of boronic acids depend on their structures, and phenylboronic acids with low pKa values are commercially available.52 A lack of functionality enabling polymerization of these commercial phenylboronic acids presents a major challenge. Fortunately, rapid development of x-omics, particularly proteomics, metabolomics, and glycomics in recent years, resulted in an increased interest to boronate affinity materials. Consequently, syntheses of novel functional monomers, a few examples of which are shown in Fig. 19 and whose synthetic routes were recently reviewed,52 were proposed. Barker et al. suggested that a half-neutralized solution of a boronic acid will act as a buffer and thus it is possible for a resin to be self-buffering if the eluent pH is equal to the pKa value for the acid.71 In addition to application of monomers with lower pKa values, modification of the materials with amine functionalities holds promise to decrease the pH of adsorption.79
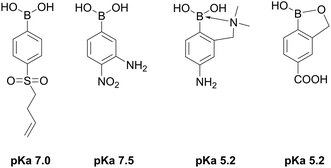 | ||
| Fig. 19 Examples of boronate monomers exhibiting low pKa values.108–111 | ||
Furthermore, optimization of the porous structure of the adsorbents bearing phenylboronic acid groups to enable a quick access to all the boron adsorption sites are required (Table 1). Gel-type polymers exhibit high swelling degrees of up to 250% and are therefore of limited mechanical strength; macroporous resins do not enable access to all the adsorption sites; and supported boronate groups exhibit low boron loadings resulting in low capacities. Polymer brushes of polymerized functional monomers appear to be promising adsorbent offering high boron loading along with accessibility of the adsorption centers. However, this type of materials remains underexplored. Shaping and pelletizing of the adsorbent beads has been rarely addressed in the literature.69,79,82 At the same time, shape and size distribution of the adsorbent particles used in a fixed bed is of great importance for hydrodynamics and diffusion contribution and, consequently, for efficiency of the separation.
Process design based on adsorption over materials bearing boronate groups presents another crucial milestone to develop convincing and competitive separation processes for biorefineries. Current research activity is mainly focused on cyclic adsorption–desorption processes with adsorption under basic and desorption under acidic conditions. Advances enabling a continuous separation process, such as SMB chromatography or cyclic batch adsorption process, and minimizing desalting of the eluates are necessary for further development. Aqueous solutions of saccharides and sugar alcohols are commercially used nowadays in food, cosmetic, or pharmaceutical industries. Production of saccharides and sugar alcohols as intermediates in novel value chains will probably require other solvents for fractionation demanding a re-design of production and separation processes. Conversion of D-fructose into a mixture of HMF and MMF using methanol with a few per cents of water as a solvent in Avantium process (Fig. 2) presents one example of saccharide transformation in an organic solution.
6. Conclusion
Saccharides and sugar alcohols are expected to play important roles in novel value chains based on biomass raw materials. Separation of these compounds from complex and frequently diluted product streams poses a challenge which can be tackled via selective adsorption of saccharides and sugar alcohols on tailored materials. Chromatographic separations on Ca2+ ion exchange resins are the state-of-the-art recovery techniques which sometimes suffer from low separation efficiency of the components over these stationary phases. Development of novel materials exhibiting higher separation factors provides opportunities for design of more efficient separation methods. In this regard, material bearing phenylboronic acid moieties are of utmost interest owing to high affinity of the boronate sites towards saccharides and polyols. An ideal adsorbent should exhibit a large boron content along with high accessibility of the adsorption sites, high mechanical stability owing to low tendency to swell, and a tailored porous structure enabling quick diffusion rates. Molecular structure of an adsorption site determines the pKa values of the boronic acid moieties. Synthesis of materials with low pKa values of the boronic acid sites are of utmost interest since these adsorbents are potentially capable of adsorption in pure water. Engineering processes of the adsorptive separation without a need for desalination of an eluate presents another crucial task to be solved for development of economically beneficial and environmentally benign separations. Overall, great versatility of materials bearing boronate moieties opens numerous opportunities for development of advanced separation processes.Author contributions
Irina Delidovich: conceptualisation, formal analysis, writing – original draft, funding acquisition. Valérie Toussaint: conceptualisation, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG Project 450360023).References
- A. Duwe, N. Tippkötter and R. Ulber, in Biorefineries, ed. K. Wagemann and N. Tippkötter, Springer International Publishing, Cham, 2019, pp. 177–215 Search PubMed.
- N. Dahmen, E. Henrich and T. Henrich, in Biorefineries, ed. K. Wagemann and N. Tippkötter, Springer International Publishing, Cham, 2019, pp. 217–245 Search PubMed.
- A. Jess and P. Wasserscheid, Chemical Technology: an Integrated Textbook, Wiley, 2013 Search PubMed.
- T. Werpy and G. R. Petersen, Top value added chemicals from biomass Volume I- Results of screening for potential candidates from sugars and synthesis gas, Washington, DC, 2004 Search PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef CAS.
- J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164 CrossRef CAS PubMed.
- R. Rinaldi and F. Schuth, Energy Environ. Sci., 2009, 2, 610–626 RSC.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- M. Yabushita, H. Kobayashi and A. Fukuoka, Appl. Catal., B, 2014, 145, 1–9 CrossRef CAS.
- I. Delidovich, P. J. C. Hausoul, L. Deng, R. Pfützenreuter, M. Rose and R. Palkovits, Chem. Rev., 2016, 116, 1540–1599 CrossRef CAS.
- J. Artz and R. Palkovits, Curr. Opin. Green Sustainable Chem., 2018, 14, 14–18 CrossRef.
- L. T. Mika, E. Cséfalvay and Á. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef CAS PubMed.
- A. Jaswal, P. P. Singh and T. Mondal, Green Chem., 2022, 24, 510–551 RSC.
- L. Goswami, R. Kayalvizhi, P. K. Dikshit, K. C. Sherpa, S. Roy, A. Kushwaha, B. S. Kim, R. Banerjee, S. Jacob and R. C. Rajak, Chem. Eng. J., 2022, 448, 137677 CrossRef CAS.
- I. Delidovich, K. Leonhard and R. Palkovits, Energy Environ. Sci., 2014, 7, 2803–2830 RSC.
- S. Venkatesan, in Sep. Purif. Technol. Biorefineries, 2013, pp. 101–148 Search PubMed.
- G. P. van Walsum, in Sep. Purif. Technol. Biorefineries, 2013, pp. 513–532 Search PubMed.
- S.-T. Yang and C. Lu, in Sep. Purif. Technol. Biorefineries, 2013, pp. 409–437 Search PubMed.
- Z. Lei and B. Chen, in Sep. Purif. Technol. Biorefineries, 2013, pp. 37–60 Search PubMed.
- J. Zhang and B. Hu, in Sep. Purif. Technol. Biorefineries, 2013, pp. 61–78 Search PubMed.
- M. Ayoub and A. Z. Abdullah, Renewable Sustainable Energy Rev., 2012, 16, 2671–2686 CrossRef CAS.
- A. A. Babadi, S. Rahmati, R. Fakhlaei, B. Barati, S. Wang, W. Doherty and K. Ostrikov, Biomass Bioenergy, 2022, 163, 106521 CrossRef CAS.
- H. Schiweck, A. Bär, R. Vogel, E. Schwarz, M. Kunz, C. Dusautois, A. Clement, C. Lefranc, B. Lüssem, M. Moser and S. Peters, in Ullmann's Encyclopedia of Industrial Chemistry, 2012 Search PubMed.
- E4tech, RE-CORD and WUR, “From the Sugar Platform to biofuels and biochemicals”. Final report for the European Commission, Contract No. ENER/C2/423-2012/SI2.673791, 2015.
- L. Negahdar, I. Delidovich and R. Palkovits, Appl. Catal., B, 2016, 184, 285–298 CrossRef CAS.
- B. G. Harvey, W. W. Merriman and R. L. Quintana, ChemSusChem, 2016, 9, 1814–1819 CrossRef CAS PubMed.
- P. Drabo, T. Tiso, B. Heyman, E. Sarikaya, P. Gaspar, J. Förster, J. Büchs, L. M. Blank and I. Delidovich, ChemSusChem, 2017, 10, 3252–3259 CrossRef CAS PubMed.
- J.-P. Lange, ChemSusChem, 2017, 10, 245–252 CrossRef CAS PubMed.
- R.-J. van Putten, J. C. van der Waal, E. de Jong and H. J. Heeres, Carbohydr. Res., 2017, 446, 1–6 CrossRef PubMed.
- B. F. M. Kuster, Starch – Stärke, 1990, 42, 314–321 CrossRef CAS.
- H. E. van Dam, A. P. G. Kieboom and H. van Bekkum, Starch – Stärke, 1986, 38, 95–101 CrossRef CAS.
- R.-J. van Putten, J. C. van der Waal, M. Harmse, H. H. van de Bovenkamp, E. de Jong and H. J. Heeres, ChemSusChem, 2016, 9, 1827–1834 CrossRef CAS PubMed.
- E. De Jong, H. Stichnothe, G. Bell and H. Jorgensen, Biobased chemicals: a 2020 update, 2020 Search PubMed.
- S. Wu, R. Snajdrova, J. C. Moore, K. Baldenius and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2021, 60, 88–119 CrossRef CAS.
- L. M. Hanover and J. S. White, Am. J. Clin. Nutr., 1993, 58, 724S–732S CrossRef CAS PubMed.
- J.-P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150–166 CrossRef CAS PubMed.
- K. Sriroth and K. Piyachomkwan, Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers, 2013, pp. 27–46 Search PubMed.
- A. Läufer, Biorefineries, 2019, 137–152 Search PubMed.
- V. Menon and M. Rao, Prog. Energy Combust. Sci., 2012, 38, 522–550 CrossRef CAS.
- A. K. Chandel, V. K. Garlapati, A. K. Singh, F. A. F. Antunes and S. S. da Silva, Bioresour. Technol., 2018, 264, 370–381 CrossRef CAS PubMed.
- A. Stubelius, S. Lee and A. Almutairi, Acc. Chem. Res., 2019, 52, 3108–3119 CrossRef CAS PubMed.
- G. Springsteen and B. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205–11209 CrossRef CAS.
- R. P. Singhal, B. Ramamurhy, N. Govindraj and Y. Sarwar, J. Chromatogr. A, 1991, 543, 17–38 CrossRef CAS.
- A. Dukler and A. Freeman, Biotechnol. Bioeng., 2001, 75, 25–28 CrossRef CAS.
- S. D. Bull, M. G. Davidson, J. M. H. van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y.-B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312–326 CrossRef CAS PubMed.
- C. A. McClary and M. S. Taylor, Carbohydr. Res., 2013, 381, 112–122 CrossRef CAS.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Angew. Chem., Int. Ed. Engl., 1996, 35, 1910–1922 CrossRef.
- X.-C. Liu and W. H. Scouten, in Affinity Chromatography: Methods and Protocols, ed. P. Bailon, G. K. Ehrlich, W.-J. Fung and W. Berthold, Humana Press, Totowa, NJ, 2000, pp. 119–128 Search PubMed.
- M. B. Espina-Benitez, J. Randon, C. Demesmay and V. Dugas, Sep. Purif. Rev., 2018, 47, 214–228 CrossRef CAS.
- D. Li, Y. Chen and Z. Liu, Chem. Soc. Rev., 2015, 44, 8097–8123 RSC.
- P. J. Duggan, Aust. J. Chem., 2004, 57, 291–299 CrossRef CAS.
- H. C. Ferraz, L. T. Duarte, M. Di Luccio, T. L. M. Alves, A. C. Habert and C. P. Borges, Braz. J. Chem. Eng., 2007, 24, 101–118 CrossRef CAS.
- H. L. Weith, J. L. Wiebers and P. T. Gilham, Biochemistry, 1970, 9, 4396–4401 CrossRef CAS.
- Y.-H. Zhao and D. F. Shantz, Langmuir, 2011, 27, 14554–14562 CrossRef CAS PubMed.
- J. A. Peters, Coord. Chem. Rev., 2014, 268, 1–22 CrossRef CAS.
- G. Schroer, J. Deischter, T. Zensen, J. Kraus, A.-C. Pöppler, L. Qi, S. Scott and I. Delidovich, Green Chem., 2020, 22, 550–562 RSC.
- G. Schroer, V. Toussaint, S. Bachmann, A.-C. Pöppler, C. H. Gierlich and I. Delidovich, ChemSusChem, 2021, 14, 5207–5215 CrossRef CAS.
- G. Schroer, V. Toussaint, B. Heyman, J. Büchs, A.-C. Pöppler and I. Delidovich, Curr. Res. Green Sustainable Chem., 2022, 5, 100297 CrossRef CAS.
- Q. Meng, M. Rong, H. Xing, J. Yu, Y. Wang, X. Wei, R.-A. Chi, C. Chen, H. Liu and L. Yang, Sep. Purif. Technol., 2023, 314, 123436 CrossRef CAS.
- I. Delidovich and R. Palkovits, Green Chem., 2016, 18, 5822–5830 RSC.
- M. Wang, L. Wang, X. Lyu, X. Hua, J. M. Goddard and R. Yang, Biotechnol. Adv., 2022, 60, 108021 CrossRef CAS PubMed.
- P. Khuwijitjaru, N. Milasing and S. Adachi, Sci. Eng. Health Stud., 2018, 12, 159–167 Search PubMed.
- S. Cheng, L. E. Metzger and S. I. Martínez-Monteagudo, Sci. Rep., 2020, 10, 2730 CrossRef CAS.
- V. Choudhary, A. B. Pinar, S. I. Sandler, D. G. Vlachos and R. F. Lobo, ACS Catal., 2011, 1, 1724–1728 CrossRef CAS.
- R. P. Chauhan, L. W. Powell and J. M. Woodley, J. Appl. Biotechnol. Bioeng., 1997, 56, 345–351 CrossRef CAS.
- Z. Wang, M. Wang, X. Lyu, C. Wang, Y. Tong, X. Hua and R. Yang, Chem. Eng. J., 2022, 446, 137089 CrossRef CAS.
- M. Wang, L. Wang, X. Hua and R. Yang, Food Chem., 2023, 429, 136935 CrossRef CAS PubMed.
- P. Drabo, M. Fischer, M. Emondts, J. Hamm, M. Engelke, M. Simonis, L. Qi, S. L. Scott, R. Palkovits and I. Delidovich, J. Catal., 2023, 418, 13–21 CrossRef CAS.
- S. A. Barker, B. W. Hatt, P. J. Somers and R. R. Woodbury, Carbohydr. Res., 1973, 26, 55–64 CrossRef CAS.
- X. Zhu, M. Wang, X. Hua, C. Yao and R. Yang, Chem. Eng. J., 2021, 406, 126751 CrossRef CAS.
- M. Schulte, in Preparative Chromatography, 2020, pp. 49–157 Search PubMed.
- K. Reske and H. Schott, Angew. Chem., Int. Ed. Engl., 1973, 12, 417–418 CrossRef.
- J. A. Vente, H. Bosch, A. B. de Haan and P. J. T. Bussmann, Adsorpt. Sci. Technol., 2006, 24, 771–780 CrossRef CAS.
- M. Wang, F. Ye, H. Wang, H. Admassu, M. A. A. Gasmalla, X. Hua and R. Yang, J. Chem. Eng., 2019, 370, 1274–1285 CrossRef CAS.
- J. Liu, Q. Song, W. Zheng, W. Jia, H. Jia, Y. Nan, F. Ren, J. J. Bao and Y. Li, J. Sep. Sci., 2022, 45, 2415–2428 CrossRef CAS PubMed.
- J. C. Dore and P. C. Wankat, Chem. Eng. Sci., 1976, 31, 921–927 CrossRef CAS.
- M. Wang, F. Ye, H. Wang, H. Admassu, Y. Feng, X. Hua and R. Yang, J. Agric. Food Chem., 2018, 66, 9269–9281 CrossRef CAS PubMed.
- S. Mohapatra, N. Panda and P. Pramanik, Mater. Sci. Eng., C, 2009, 29, 2254–2260 CrossRef CAS.
- L. Gu, Y. Wang, J. Han, L. Wang, X. Tang, C. Li and L. Ni, New J. Chem., 2017, 41, 13399–13407 RSC.
- M. Glad, S. Ohlson, L. Hansson, M.-O. Mánsson and K. Mosbach, J. Chromatogr. A, 1980, 200, 254–260 CrossRef CAS.
- Z. Xu, K. M. A. Uddin, T. Kamra, J. Schnadt and L. Ye, ACS Appl. Mater. Interfaces, 2014, 6, 1406–1414 CrossRef CAS PubMed.
- X. Zhu, J. Gu, J. Zhu, Y. Li, L. Zhao and J. Shi, Adv. Funct. Mater., 2015, 25, 3847–3854 CrossRef CAS.
- H. Schott, Angew. Chem., Int. Ed. Engl., 1972, 11, 824–825 CrossRef CAS.
- L. L. Lloyd and J. F. Kennedy, in Process Scale Liquid Chromatography, 1994, pp. 99–130 Search PubMed.
- D. C. Sherrington, Chem. Commun., 1998, 2275–2286 RSC.
- M. Berrios, J. A. Siles, M. A. Martín and A. Martín, in Separation and Purification Technologies in Biorefineries, 2013, pp. 149–165 Search PubMed.
- R. Rajkumar, A. Warsinke, H. Möhwald, F. W. Scheller and M. Katterle, Talanta, 2008, 76, 1119–1123 CrossRef CAS PubMed.
- S. Schumacher, F. Grüneberger, M. Katterle, C. Hettrich, D. G. Hall, F. W. Scheller and N. Gajovic-Eichelmann, Polymer, 2011, 52, 2485–2491 CrossRef CAS.
- H. Schmidt-Traub and A. Susanto, in Preparative Chromatography, 2020, pp. 525–600 Search PubMed.
- M. Yamaguchi, T. Asano, M. M. Yama and I. Iwami, J. Food Sci., 1978, 43, 1620–1621 CrossRef CAS.
- M. Verzele, G. Simoens and F. Van Damme, Chromatographia, 1987, 23, 292–300 CrossRef CAS.
- S. C. Churms, J. Chromatogr. A, 1996, 720, 75–91 CrossRef CAS.
- Y. S. Ghim and H. N. Chang, Ind. Eng. Chem. Fundam., 1982, 21, 369–374 CrossRef CAS.
- S. Adachi and H. Sugawara, Arch. Biochem. Biophys., 1963, 100, 468–471 CrossRef CAS.
- T. Xin, M. Chen, Z. Liu, R. Luo, Q. Xing, P. Bai, X. Guo and J. Lyu, Sep. Purif. Technol., 2023, 319, 124038 CrossRef CAS.
- C. Nobre, J. A. Teixeira and L. R. Rodrigues, Crit. Rev. Food Sci. Nutr., 2015, 55, 1444–1455 CrossRef CAS.
- S. J. Angyal, G. S. Bethell and R. J. Beveridge, Carbohydr. Res., 1979, 73, 9–18 CrossRef CAS.
- S. J. Angyal, in Advances in Carbohydrate Chemistry and Biochemistry, ed. R. S. Tipson and D. Horton, Academic Press, 1989, vol. 47, pp. 1–43 Search PubMed.
- J. Tiihonen, I. Markkanen and E. Paatero, Chem. Eng. Commun., 2002, 189, 995–1008 CrossRef CAS.
- C. Ho, C. B. Ching and D. M. Ruthven, Ind. Eng. Chem. Res., 1987, 26, 1407–1412 CrossRef CAS.
- J. Nowak, K. Gedicke, D. Antos, W. Piątkowski and A. Seidel-Morgenstern, J. Chromatogr. A, 2007, 1164, 224–234 CrossRef CAS.
- A. Seidel-Morgenstern, in Preparative Chromatography, 2020, pp. 9–48 Search PubMed.
- H. J. Bieser and A. J. De Rosset, Starch – Stärke, 1977, 29, 392–397 CrossRef CAS.
- Y. A. Beste, M. Lisso, G. Wozny and W. Arlt, J. Chromatogr. A, 2000, 868, 169–188 CrossRef CAS PubMed.
- J. A. Johnson, in Adsorption: Science and Technology, ed. A. E. Rodrigues, M. D. LeVan and D. Tondeur, Springer Netherlands, Dordrecht, 1989, pp. 383–395 Search PubMed.
- X. Li, J. Pennington, J. F. Stobaugh and C. Schöneich, Anal. Biochem., 2008, 372, 227–236 CrossRef CAS.
- F. Li, X. Zhao and G. Xu, Chin. J. Anal. Chem., 2006, 34, 1366–1370 CAS.
- H. Li, H. Wang, Y. Liu and Z. Liu, Chem. Commun., 2012, 48, 4115–4117 RSC.
- G. Wulff, Pure Appl. Chem., 1982, 54, 2093–2102 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |