Direct immunoassay on a polyester microwell plate for colorimetric detection of the spike protein in swab and saliva samples†
Nikaele S.
Moreira
a,
Thaisa A.
Baldo
a,
Lucas C.
Duarte
 ae,
Leonardo
Lopes-Luz
bc,
Karoliny A.
Oliveira
a,
Paulo F. N.
Estrela
ae,
Leonardo
Lopes-Luz
bc,
Karoliny A.
Oliveira
a,
Paulo F. N.
Estrela
 a,
Amanda M.
Simões
a,
Samira
Bührer-Sékula
a,
Amanda M.
Simões
a,
Samira
Bührer-Sékula
 bc,
Gabriela R. M.
Duarte
bc,
Gabriela R. M.
Duarte
 ac and
Wendell K. T.
Coltro
ac and
Wendell K. T.
Coltro
 *acd
*acd
aInstituto de Química, Universidade Federal de Goiás, 74690-900, Goiânia, GO, Brazil. E-mail: wendell@ufg.br
bInstituto de Patologia Tropical e Saúde Pública, Centro Multiusuário de Bioinsumos e Tecnologias em Saúde, Universidade Federal de Goiás, 74605-050, Goiânia, GO, Brazil
cInnovation Hub in Point-of-Care Technologies, 74690-900, Goiânia, GO, Brazil
dInstituto Nacional de Ciência e Tecnologia de Bioanalítica, 13084-971, Campinas, SP, Brazil
eInstituto Federal de Educação, Ciência e Tecnologia de Goiás – Campus Inhumas, 75402-556, Inhumas, GO, Brazil
First published on 21st November 2023
Abstract
This study presents the development of a polyester microplate for detecting the S-protein of the SARS-CoV-2 virus in saliva and nasopharyngeal swab samples using direct enzyme-linked immunosorbent assay (ELISA) technology. The polyester microplate was designed to contain 96 zones with a 3 mm diameter each, and a volume of 2–3 μL. The experimental conditions including reagent concentration and reaction time were optimized. The microplate image was digitized and analyzed using graphical software. The linear range obtained between protein S concentrations and pixel intensity was 0–10 μg mL−1, with a correlation coefficient of 0.99 and a limit of detection of 0.44 μg mL−1. The developed methodology showed satisfactory intraplate and interplate repeatability with RSD values lower than 7.8%. The results achieved through immunoassay performed on polyester microplates were consistent with those of the RT-PCR method and showed a sensitivity of 100% and 90% and specificity of 85.71% and 100% for saliva and nasopharyngeal samples, respectively. The proposed direct immunoassay on polyester microplates emerges as an alternative to conventional immunoassays performed on commercial polystyrene plates, given the low cost of the device, low consumption of samples and reagents, lower waste generation, and shorter analysis time. Moreover, the immunoassay has shown great potential for diagnosing COVID-19 with precision and accuracy.
1 Introduction
The COVID-19 disease, caused by the new coronavirus (SARS-CoV-2), has become a grave threat to public and financial health globally. This virus is very contagious and has spread across the world, affecting over 762 million people and resulting in the deaths of more than 6.8 million people worldwide (as of December 2019, April 2023 data).1 To effectively combat the pandemic, it is crucial to have access to fast and accurate diagnostic testing due to the highly contagious nature of the disease and the potential for asymptomatic transmission. Although COVID-19 has been around for more than three years and is reasonably controlled in many countries, the emergence of new virus variants2 can cause new waves of contagion, which could put us back in a vulnerable situation at any time. Thus, there is a great need for diagnostic test development. In addition, many low- and middle-income countries still have a low testing rate. They only perform 27 tests per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people per day.3 In response to this problem, there has been a major effort to develop new point-of-care (POC) methods that can rapidly detect the disease in individuals who have been infected. This is especially important in areas with limited laboratory resources, where testing options are limited.4–6
000 people per day.3 In response to this problem, there has been a major effort to develop new point-of-care (POC) methods that can rapidly detect the disease in individuals who have been infected. This is especially important in areas with limited laboratory resources, where testing options are limited.4–6
RT-PCR (quantitative reverse transcription-polymerase chain reaction) molecular assays are known as the gold standard for clinical diagnostic detection of SARS-CoV-2. However, despite their accuracy, these assays are complex and demand specialized personnel and expensive laboratory equipment. This makes them difficult to use in places with limited resources.7,8 On the other hand, antigen assays that detect proteins from SARS-CoV-2 have become a viable option for diagnosing COVID-19. These tests are simple to use, rapid, cost-effective, and can be applied directly at the POC.9–11
One of the techniques commonly used for diagnosing COVID-19 biomarkers is the traditional enzyme-linked immunosorbent assay (ELISA). This method detects the viral spike (S) protein and/or nucleocapsid (N) protein by interacting with specific recognition biomolecules.12–17 The ELISA is performed using commercially available polymeric microplates and benchtop microplate readers. However, due to the reliance on these instruments, it is important to have highly trained laboratory technicians to ensure the accuracy of the method. It is worth noting that this method may have limitations when it comes to supporting the diagnosis of COVID-19 at the POC. Additionally, traditional ELISA test results can be time-consuming, taking more than 24 hours to obtain a response.
Lateral flow tests (LFTs) offer shorter response times and can be applied at the POC, enabling decentralized diagnosis in addition to self-testing. However, one barrier to accepting LFTs during the pandemic was their limited accuracy.18 Furthermore, the LFT response is qualitative, making it impossible, for example, to provide a relationship between the quantity of the target and the contagion time.
Developing new substrates for immunoassays can contribute to creating affordable and accurate biosensors that may allow quick diagnosis.4,7,8,19–22 Colorimetric immunoassays on inexpensive platforms have been demonstrated to be promising for POC detection of SARS-CoV-2. A recent example showing the development of wax-printed ELISA paper plate assay was successfully demonstrated to detect the S protein in saliva samples.23 To enhance the sensitivity of the immunoassay, the immunochain was supported by using magnetic beads (MBs).
Carrel and their colleagues utilized a PET film, a pressure-sensitive adhesive, and nitrocellulose to create an ELISA tool that can identify the N-protein antigen and help diagnose COVID-19.24 The device was created by using PET film-based capillary flow channels and nitrocellulose pumps to guide the flow and enable automatic wash steps in the assay. In this report, we describe the development of a polyester microwell plate for colorimetric detection of the S-protein through direct immunoassay for diagnosing COVID-19 in swab and saliva samples. The polyester microwell plate showed reasonable sensitivity and specificity, and the results obtained demonstrated its suitability for S-protein detection in clinical samples from SARS-CoV-2 infected patients as a proof of concept.
2 Materials and methods
2.1 Chemicals and materials
Sodium carbonate, sodium bicarbonate, sodium phosphate dibasic, potassium phosphate monobasic, sodium chloride, potassium chloride, Tween 20, bovine serum albumin (BSA), 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate ready to use solution, absolute ethanol, and streptavidin–horseradish peroxidase from Streptomyces avidinii (Strep-HRP) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The spike Sars-Cov-2 recombinant protein (S-protein) – full length (#IM 0862) and biotinylated monoclonal antibody (#IM-0827) were acquired from Rhea Biotech company (Campinas, SP, Brazil). All solutions were prepared with ultrapure water (resistivity 18 MΩ cm). The carbonate/bicarbonate buffer (pH 9.6; 50 mmol L−1) was prepared with 10 mmol L−1 sodium carbonate and 40 mmol L−1 sodium bicarbonate. Phosphate-buffered saline (PBS, pH 7.4; 10 mmol L−1) was prepared with 10 mmol L−1 of dibasic sodium phosphate, 1.8 mmol L−1 of potassium phosphate monobasic, 137 mmol L−1 of sodium chloride, and 2.7 mmol L−1 of potassium chloride. PBS solution containing 0.05% Tween-20 was used as a washing buffer (PBS-T).2.2 Fabrication of microwell plates in polyester films
The immunoassay for detecting the S-protein was developed in a microwell plate manufactured on a polyester film, which is an alternative and low-cost substrate. The fabrication was based on a protocol reported elsewhere.25 The device was designed in a rectangular shape (135 mm long × 90 mm wide) using the graphical software Silhouette Studio v. 4.2. The layout was designed containing 96 microwells (3 mm diameter each) distributed in 12 columns and 8 rows. Afterwards, 250 μm-thick polyester films were cut using a Silhouette Cameo cutting plotter (Silhouette, Belo Horizonte, MG, Brazil). Finally, the cut layers were thermally laminated at 140 °C (Gazela, Divinopolis, MG, Brazil), thus creating a polyester plate with microwells with a maximum capacity of 3 μL of solution for assays.2.3 Immunoassays on microwell plates
The microplates were washed with absolute ethanol and water and dried at 60 °C before direct immunoassay experiments were conducted The immunoassay assembly is shown in Fig. 1 and all steps involved are described in the following subheadings.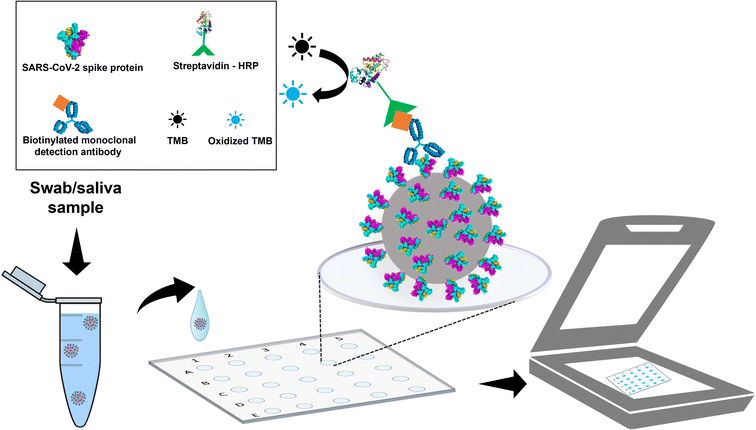 | ||
| Fig. 1 Scheme of the direct immunoassay for detecting SARS-CoV-2 in a polyester plate. The gray sphere represents the SARS-CoV-2 virus. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (v
1000 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v), and 2 μL was added to the wells. Incubation was performed for 1 hour, and the microwells were washed (4x).
v), and 2 μL was added to the wells. Incubation was performed for 1 hour, and the microwells were washed (4x).
2.4 Immunoassay application on polyester plate biological samples
To demonstrate the feasibility of the polyester plate immunoassay, a total of 37 samples from patients with suspected SARS-CoV-2 infection were collected using sterile rayon swabs for nasopharyngeal samples and cryotubes for self-collected saliva samples during the COVID-19 pandemic in Brazil. The samples were collected in February, March, and June of 2021 and from May to July of 2022. After collection, the swabs were placed in cryotubes containing 1 mL of 0.9% m/v sodium chloride saline solution. The samples were previously confirmed by RT-PCR. Among them, 10 of the swabs and 11 saliva samples were from positive patients, and 9 of the swabs and 7 of the saliva samples were from patients negative for SARS-CoV-2. All samples were stored in a freezer at −80 °C and were handled in a biosafety level 2 laboratory with personal protection equipment.All assays involving biological samples were performed according to the ethics and safety protocols confirmed by the Federal University of Goiás (protocols 50176621.7.0000.5083 and 5.027.133). All experiments complied with nationally required guidelines, followed the resolutions CNS 466/12 and CNS 441/11, and complied with institutional instructions. It is important to mention that biological samples were used with the consent of all patients.
The samples were diluted at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v
1 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) in carbonate/bicarbonate buffer, and 3 μL of the solution was added to the microwells and incubated for 4 h. The subsequent assays were carried out as described in previous subheadings.
v) in carbonate/bicarbonate buffer, and 3 μL of the solution was added to the microwells and incubated for 4 h. The subsequent assays were carried out as described in previous subheadings.
The GraphPad Prism software (version 8.1.0) was used to generate the receiver operator characteristic (ROC) curve and to evaluate the area under the curve (AUC) aiming to assess the sensitivity and specificity of the immunoassay. The diagnostic accuracy was evaluated by comparing the results obtained from the immunoassay with those of the RT-qPCR, using free online software MedCalc's Diagnostic Test Evaluation Calculator.
3 Results and discussion
3.1 Optimization of detection parameters
The detection conditions and reactional parameters were evaluated to develop the immunoassay for measuring the S-protein from the SARS-CoV-2 virus. Initially, the time for image acquisition through a benchtop scanner was studied (Fig. 1SA†) since the last step of the immunoassay consists of adding the chromogen agent TMB which undergoes rapid oxidation. For this reason, time control is essential to ensure the best detection conditions. The image's percentage scale was decreased to 100% to reduce the acquisition time, resulting in a scanning time of approximately 1 minute. This scanning condition was maintained for all subsequent experiments.The intensity of pixels was analyzed in various color channels of the image, including RGB and CMYK, aiming to achieve the highest level of sensitivity in detecting the S-protein from the SARS-CoV-2 virus using the color reaction between the enzyme (HRP) and substrate (TMB). It was somewhat expected that the cyan color channel of the CMYK system would display better linearity (as shown in Fig. 1SB and 1SC†), once the reaction product produces a light blue color due to the oxidation of TMB.
3.2 Optimization of immunoassay reaction parameters
The reaction parameters for the direct immunoassay were also optimized. This included optimization of the steps of sensitization, blocking, and addition of the detection antibody, as well as the addition of strep-HRP and chromogen. Briefly, the proposed immunoassay involves a direct enzyme-linked immunosorbent assay (ELISA) for S-protein detection, as illustrated in Fig. 1. The first step comprises the sensibilization of microplates with the S-protein, so an incubation time in the range of 15 to 120 min was investigated. In addition, the reaction time for image capture was also evaluated, ranging from 1 to 20 min. The data are presented in ESI Fig. S2.† A sensitization time of 30 min resulted in pixel intensity similar to that after longer times when the image was captured with up to 5 min of reaction. On the other hand, the captured image after 5 min, a more significant standard deviation was revealed in the measurements of pixel intensity, probably due to the evaporation of the solution in the microwells.Furthermore, a saturation of the response signal was noticed after 5 min of reaction when the incubation time was analyzed at 30 min. Therefore, 30 min and 5 min were considered the best conditions for the plate sensitization time and image capture, respectively. The conventional ELISA26 typically takes a long time for sensitization, which can be overnight, while the proposed assay has provided a noticeable improvement in this aspect.
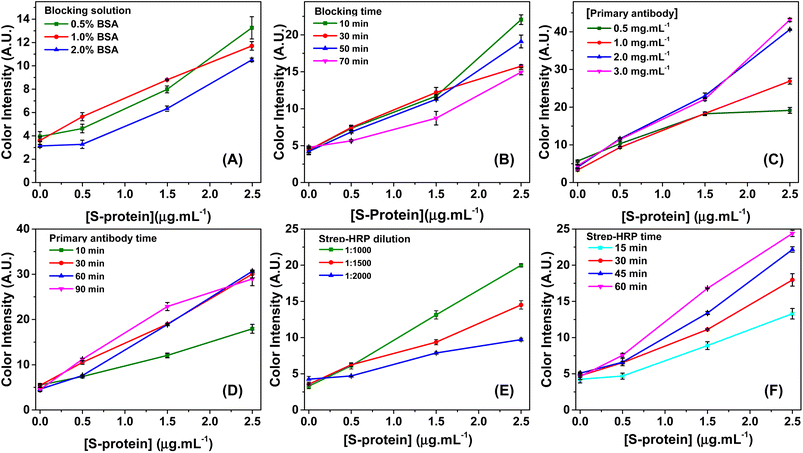
In the blocking step, the concentration of BSA solution (0.5–2.0%) and the blocking time (10–70 min) were evaluated. The results are presented in Fig. 2A and B, respectively. Fig. 2A shows that a BSA concentration of 0.5% did not provide effective blocking, as the blank (without antigen) revealed a color intensity similar to a concentration of 0.5 μg mL−1 of antigen, indicating the occurrence of nonspecific binding of the antibody of detection on the microwell surface. Based on the results shown in Fig. 2A, it is possible to note that blocking with BSA concentrations starting at 1% was suitable. In addition, a BSA concentration of 1% offered lower standard deviations between replicates and it decreased the blank's background signal without compromising the antigen detection sensitivity. The achieved sensitivity was 3.29 AU/μg mL−1, which is higher than the sensitivity values obtained for BSA concentrations of 0.5% (3.22 AU/μg mL−1) and 2.0% (2.92 AU/μg mL−1). Regarding the blocking period, the data presented in Fig. 2B reveal a lower color intensity at 50 and 70 min compared to that at 10 and 30 min. Thus, the shortest time (10 min) was considered optimum and kept constant for the subsequent experiments.
The concentration and incubation time of the detection antibody were optimized. The antibodies within a range of 0.5 to 3.0 μg mL−1 were evaluated. Fig. 2C illustrates a saturation point in color intensity that occurred when the antibodies were present at a concentration of 0.5 μg mL−1 and the antigen concentration exceeded 1.5 μg mL−1. This saturation can be likely due to the active sites of the antibodies being fully occupied, which prevented further increase in color intensity despite higher concentrations of the S protein.27,28 However, it is noteworthy that no saturation of color intensity was observed when the antibody concentration was 1 μg mL−1 or higher. The higher antibody concentrations (2.0 μg mL−1 and 3.0 μg mL−1) demonstrated a similar color intensity but exhibited a superior analytical signal compared to the concentration of 1.0 μg mL−1. The sensitivity values obtained with the antibody concentrations of 1.0, 2.0, and 3.0 μg mL−1 were 9.62, 14.50, and 15.06 AU/μg mL−1, respectively. Considering that the two higher concentrations showed similar intensity and sensitivity values, the 2.0 μg mL−1 concentration was deemed the ideal choice and selected for the following stages of the study. This decision was based on reliable results without resorting to an even higher concentration, which could contribute to increasing the assay costs without providing significant sensitivity benefits.
The antibody incubation time was evaluated, ranging from 10 to 70 min. Based on the results presented in Fig. 2D, an incubation time of or longer than 30 min resulted in a similar color intensity for all three antigen concentrations and provided the highest sensitivity values. In contrast, with an incubation time of only 10 min, low color intensity was observed in different concentrations from antigens. So, the incubation time of 30 min was selected for the subsequent steps of the study.
The optimization of the concentration and incubation time of the strep-HRP solution was carried out, and the results are presented in Fig. 2E and F, respectively. In Fig. 2E, it can be seen that the HRP ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (v
1000 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) showed greater sensitivity (6.75 AU/μg mL−1) when compared with the proportions of 1
v) showed greater sensitivity (6.75 AU/μg mL−1) when compared with the proportions of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 (4.25 AU/μg mL−1) and 1
1500 (4.25 AU/μg mL−1) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 (2.62 AU/μg mL−1). Based on Fig. 2F, a reaction time of strep-HRP of 60 min has been chosen because the colorimetric response has exhibited a linear and best behavior for color detection in polyester plates.
2000 (2.62 AU/μg mL−1). Based on Fig. 2F, a reaction time of strep-HRP of 60 min has been chosen because the colorimetric response has exhibited a linear and best behavior for color detection in polyester plates.
Lastly, the reaction time with the chromogen TMB was studied, and a reaction time of 5 min exhibited lower standard deviations when compared with times greater than or equal to 10 min (see Fig. S2†). Moreover, for longer incubation times, a saturation of the color intensity is perceived, as mentioned in the second paragraph of section 2.3. Therefore, the immunoassay was performed in 2 h and 15 min under the optimized reaction conditions with standard solution. This time is considered satisfactory since the traditional ELISA requires at least 12 hours. The shorter duration of the developed immunoassay demonstrates its efficiency and practicality, making it a promising alternative for faster and more reliable results.
3.3 Analytical performance of the colorimetric immunoassay for the detection of SARS-CoV-2 on a polyester plate
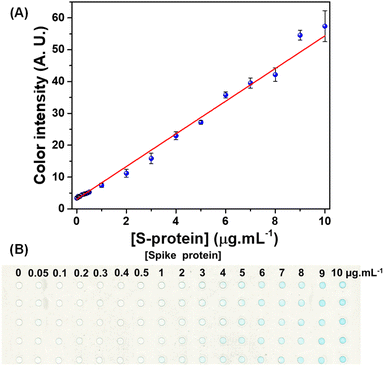
Quantitative detection of the S-protein was performed in microwell plates using the previously optimized conditions. The colorimetric response for the S-protein was obtained for standard solutions prepared in a concentration range between 0 and 10 μg mL−1, as shown in Fig. 3A. The increased pixel intensity was directly proportional to the concentration. This behavior was expected because the higher concentration of S-protein in the microwell results in a larger amount of monoclonal antibodies binding to it. Consequently, more HRP catalyzes the oxidation of TMB, thus resulting in the appearance of a blue color.The linear behavior of the colorimetric response revealed a determination coefficient of 0.991 and RSD (n = 5) ranging from 2.2 to 11.2%. The RSD values were considered satisfactory because the image was captured with a scanner and analyzed by graphic software, which offers reliable quantification without requiring the use of a spectrophotometer.29,30Fig. 3B displays the resulting image of the immunoassay performed in a polyester plate in the concentration range between 0 and 10 μg mL−1.
The sensitivity and the limit of detection (LOD) obtained for the immunoassay were 5.13 AU/μg mL−1 and 0.44 μg mL−1, respectively. The LOD value was calculated based on the ratio between three times the standard deviation for the blank and angular coefficient of the analytical curve. When comparing the LOD of the proposed colorimetric immunoassay for detecting the S-protein in polyester plates with those found in the literature (Table 1), a higher LOD value is observed. On the other hand, it is noteworthy that many of the devices mentioned in the literature were not tested for clinical samples.26,31–33
| Target | Device | Type | LODb | Sensitivity (%) | Specificity (%) | Samples | Device requirement | Volumec (μL) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a NR: not reported. b LOD: limit of detection. c The volume of washing steps in assays performed on microplates was not considered. | |||||||||
| S1 | Lateral flow immunoassay | Sandwich | 1.86 × 105 copies mL−1 | NR | NR | Nasopharyngeal and nasal swabs | Portable analyzer | ∼100 | 34 |
| S1 | Commercial microplate | Sandwich | 11 ng mL−1 | NR | NR | NR | Microplate reader | 550 | 26 |
| S | Quartz micro cuvettes | Direct | 48 ng mL−1 | NR | NR | Enriched saliva | Spectrophotometer | 200 | 30 |
| S | Polyurethane-polydiacetylene nanofiber composite | Direct | NR | NR | NR | NR | NR | NR | 31 |
| S | Paper-based immunoassay | Sandwich | 0.1 μg mL−1 | NR | NR | Saliva | Smartphone | 630 | 23 |
| S | Paper-based antigen assay | Sandwich | 0.03–0.56 nmol L−1 | NR | NR | Human saliva and serum | Scanner | ∼30 | 35 |
| S1 | Commercial microplate | Sandwich | 1.8 pg mL−1 | NR | NR | NR | Microplate reader | 500 | 32 |
| S, M and E | Commercial microplate | Direct | C t = 36.5 | 96% | 98% | Nasal and throat swabs | Microplate reader | 250 | 36 |
| S and N | PVDF strips | Sandwich | 5 pg μL−1 | NR | NR | NR | NR | 400 | 33 |
| S | Polyester microplate | Direct | 0.44 μg mL−1 | 90 | 100 | Swab | Scanner | 11 | This work |
| 100 | 85.71 | Saliva | |||||||
The study proposed by Fabiani et al.23 exhibits the closest LOD to our study, with an LOD of 0.1 μg mL−1. Their approach involves paper devices with 96 wells delimited by wax barriers, wherein a sandwich immunoassay was conducted using MBs coated with antibodies as a support for assembling a sandwich with S protein as the target. In this immunoassay, three different antibodies were used: one conjugated with the MBs, a monoclonal capture antibody, and a monoclonal detection antibody. Using different types of antibodies and magnetic nanoparticles can increase assay costs. Our direct immunoassay, which employs only one monoclonal capture antibody, exhibits a slightly higher LOD compared to the other method. On the other hand, fewer steps are involved, and the low-cost per assay is about $0.021. Additionally, lower LODs observed in studies using spectrophotometers30 or plate readers32 can also be attributed to the equipment used for detection. In terms of accessibility in obtaining results, the proposed immunoassay using a scanner is more practical and readily available to the community. Moreover, regarding clinical application, the proposed immunoassay has been demonstrated to be a promising tool for diagnosing COVID-19, as demonstrated in section 3.4.
In addition, as seen in Table 1, most reported studies23,26,30,32–36 use reagent volumes equal to or greater than 30 μL, while the proposed method uses only 11 μL. This is indicative that an analytical method proposed can be considered more ecological, with lower consumption of reagents and, consequently, lower waste generation.
Considering the aliquot added into each microwell, the LOD achieved in our report was converted to mass per well and compared again with the data presented in Table 1. For this purpose, using an aliquot of 11 μL, the LOD calculated was 4.84 ng per well. This value is better than that reported by Fabiani et al.,23 who used a paper-based immunoassay-based microplate, that uses 630 μL, giving a value of LOD of 63 ng per well. In addition, the LOD obtained in mass per well using the disposable microwell plates was comparable to those achieved by other authors employing commercial microplates26 and conventional spectrophotometry.30
Intra and inter-plate (n = 3) comparisons were performed to investigate the repeatability among them. The RSD values for the four analyzed concentrations (n = 5) were below 7.8%. The data are presented in Table S1, available in the ESI.† Furthermore, the intra-plate and inter-plate measurements revealed no significant difference in pixel intensity values based on analysis of variance (ANOVA) at a 95% confidence interval (CI), thus indicating consistent results across different plates.
3.4 Application of immunoassay on a polyester plate in clinical samples
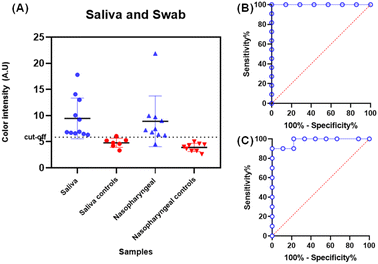
Detection of the SARS-CoV-2 virus in biological specimens is often performed on nasopharyngeal swabs and saliva.37 Thus, to assess the bioanalytical feasibility of the proposed immunoassay, human saliva and nasopharyngeal swab samples from positive and control patients were used to detect the S-protein (Table S2†). A cut-off color intensity value of 5.87 AU was obtained by the mean of the color intensity of the controls plus three times the standard deviation, and this value indicates the midpoint for which samples are classified as positive and negative for the SARS-CoV-2 virus.38,39Fig. 4A displays the color intensity for 11 positive saliva samples (blue dots) and 10 positive nasopharyngeal samples (blue triangles). Control samples, red dots, and triangles are below the cut-off. The ROC curve provides information regarding the performance of the assay for the accuracy of predictions in classifying positive and negative samples.40 Then, the analysis of the ROC curve (Fig. 4B and C) showed an area under the curve (AUC) of 1.0 (95% CI, 1.000 to 1.000, p < 0.0001) and of 0.9778 (95% CI, 0.9220 to 1.000, p = 0.0004), respectively, indicating that the proposed assay presents high precision in discriminating between positive and negative swab and saliva samples. Comparing the results of the immunoassay proposed with RT-PCR, the samples of saliva offered 100% (95% CI, 74.12% to 100.0%) sensitivity and 85.71% (95% CI, 48.69% to 99.27%) specificity, while nasopharyngeal immunoassay exhibited 90% (95% CI, 59.58% to 99.49%) sensitivity and 100% (95% CI, 70.09% to 100.0%) specificity.The diagnostic accuracy obtained by comparing the results obtained by the proposed method with RT-qPCR for saliva and swab samples was 94.74% (95% CI: 73.97–99.87%) and 95.00% (95% CI: 75.13–99.87%), respectively. This indicates that the proposed method could predict over 94% of the samples correctly.
The values obtained for sensitivity and specificity on applying the immunoassay in biological samples as a proof of concept were considered satisfactory. Based on the comparison presented in Table 1 and it is observed that few studies reported diagnostic sensitivity and specificity values. Ventura et al.36 developed a colorimetric biosensor based on a colloidal solution of gold nanoparticles (AuNPs) with 20 nm diameter functionalized with three antibodies corresponding to the three proteins S, M, and E. Thus, in the presence of viral particles, it formed a layer of AuNPs on the surface, causing a color change from red to purple. The biosensor showed higher values of sensitivity (96%) and specificity (98%) when compared to the mean sensitivity (95%) and specificity (92.85%) of the swab and saliva samples in this current study. These slightly better values can be attributed to the fact that they detect virus particles through antibody-antigen bindings of three SARS-CoV-2 proteins, enhancing its overall performance.
On the other hand, the proposed immunoassay presents several significant advantages over traditional detection methods. In terms of costs, this approach stands out for its cost-effectiveness using only one pair of antibody-antigen for detection and choosing the TMB (U$S 1.08/mL) chromogen, which is more accessible compared to AuNP (U$S 6.60/mL). This results in a considerable reduction in expenses related to reagents and microplate substrates. The manufacturing of polyester microplates is another strong point of this assay as the device was manufactured through a cutting printing protocol and it employs low-cost tools, such as a cutting printer, laminator, and polyester films, making large-scale production highly accessible. The estimated manufacturing cost of polyester microplates is approximately $0.02 per unit (Table S3†), representing significant savings compared to conventional microwell plates. In addition, it is important to mention the reduced consumption of reagents. The requirement of only 3 μL of solution to fill the microwells is quite advantageous when compared to conventional microplates with make use of a volume of 50 μL and represents a substantial advantage. Another crucial advantage is the high diagnostic accuracy of the microplate assay, with an accuracy rate of 94.6%; the results obtained through this technique exhibit a significant correlation with those obtained by the standard RT-qPCR technique. Such accuracy reinforces the reliability of the assay in detecting the SARS-CoV-2 virus and underscores its efficacy as a dependable diagnostic tool.
In summary, the microplate immunoassay represents a promising approach for pathogen detection, with a focus on identifying SARS-CoV-2. Its economic reagent consumption, ease of large-scale manufacturing, and high diagnostic accuracy make it a valuable option for enhancing diagnostic efforts and controlling infectious diseases, which can positively impact public health.
4 Conclusions
The developed immunoassay has demonstrated ease of use and accuracy for detecting the S-protein in saliva and swab samples from SARS-CoV-2 infected patients. Polyester microplates can be manufactured at a low cost ($0.021), making them ideal for future POC applications. This immunoassay presented a linear behaviour in the S-protein concentration range of 0–10 μg mL−1 (determination coefficient of 0.991) with a LOD of 0.44 μg mL−1. Additionally, good precision (RSD < 7.8%) in the assessment of intra-plate and inter-plate repeatability was also achieved. The method was applied to biological samples, and the results were consistent with those of RT-qPCR, presenting a diagnostic accuracy of 94.6%, with satisfactory sensitivity and specificity. The ROC curve analysis demonstrated the method's accuracy with an AUC value close to 1.0, representing high accuracy. The direct immunoassay for detecting S proteins of the COVID-19 virus developed in polyester microplates presented an alternative to conventional immunoassays performed in polystyrene plates, given the accessible cost of the device, low consumption of samples and reagents and lower generation of waste and analysis time. Therefore, this work represents an important contribution to developing new diagnostic technologies for COVID-19 that will allow accurate and affordable diagnosis to control the pandemic.Author contributions
Nikaele S. Moreira: formal analysis, investigation, methodology, software, validation, visualization, writing – original draft, writing – review & editing; Thaisa A. Baldo: conceptualization, investigation, methodology, validation, visualization, writing – review & editing; Lucas C. Duarte: writing – original draft, writing – review & editing; Leonardo Lopes-Luz: methodology, validation, writing – review & editing; Karoliny A. Oliveira: visualization, writing – review & editing; Paulo F. N. Estrela: methodology, validation, writing – original draft; Amanda M. Simões: methodology, writing – original draft; Samira Bührer-Sékula: resources, writing – review & editing; Gabriela R. M. Duarte: resources, writing – review & editing; Wendell K. T. Coltro: conceptualization, funding acquisition, project administration, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from CNPq (grants 307554/2020-1, 405620/2021-7, 402694/2020-1 and 382604/2022-9), CAPES (finance code 001 and grant 88882.385457/2007091), FAPEG (grants 202310267000258 and 202010267000273), INCTBio (grant 465389/2014-7) and Labor Prosecution Service. CNPq and CAPES are also thanked for the scholarships and researcher fellowships granted to the authors. Dr Thiago Paixão and William R. de Araújo are also recognized for their helpful discussions in the initial development stage of this project. Icaro Salgado Perovani is also recognized for his helpful discussions in the analysis of variance (ANOVA).References
- Coronavirus disease (COVID-19), https://www.who.int/emergencies/diseases/novel-coronavirus-2019, (accessed 21 September 2023) Search PubMed.
- A. M. Carabelli, T. P. Peacock, L. G. Thorne, W. T. Harvey, J. Hughes, T. I. de Silva, S. J. Peacock, W. S. Barclay, T. I. de Silva, G. J. Towers and D. L. Robertson, Nat. Rev. Microbiol., 2023, 21, 162–177 Search PubMed.
- A. X. Han, A. Toporowski, J. A. Sacks, M. D. Perkins, S. Briand, M. van Kerkhove, E. Hannay, S. Carmona, B. Rodriguez, E. Parker, B. E. Nichols and C. A. Russell, Nat. Genet., 2023, 55(1), 26–33 CrossRef PubMed.
- T. Ji, Z. Liu, G. Q. Wang, X. Guo, S. Akbar khan, C. Lai, H. Chen, S. Huang, S. Xia, B. Chen, H. Jia, Y. Chen and Q. Zhou, Biosens. Bioelectron., 2020, 166, 112455 CrossRef PubMed.
- W. Wang, Y. Xu, R. Gao, R. Lu, K. Han, G. Wu and W. Tan, JAMA, 2020, 323, 1843–1844 Search PubMed.
- B. C. Dhar, Anal. Bioanal. Chem., 2022, 414, 2903–2934 CrossRef PubMed.
- M. Leventopoulos, V. Michou, M. Papadimitropoulos, E. Vourva, N. G. Manias, H. P. Kavvadas, D. Nikolopoulos, V. Tsilivakos and G. Georgoulias, Diagn. Microbiol. Infect. Dis., 2022, 104, 115786 CrossRef CAS PubMed.
- Y. Kyosei, S. Yamura, M. Namba, T. Yoshimura, S. Watabe and E. Ito, Biophys. Physicobiol., 2021, 18, 28–39 CrossRef CAS.
- S. Smith, J. G. Korvink, D. Mager and K. Land, RSC Adv., 2018, 8, 34012–34034 RSC.
- R. W. Peeling, K. K. Holmes, D. Mabey and A. Ronald, Sex. Transm. Infect., 2006, 82, v1–v6 CrossRef.
- T. Bhardwaj, L. N. Ramana and T. K. Sharma, Biosens, 2022, 12, 357 CrossRef CAS.
- J. Van Elslande, E. Houben, M. Depypere, A. Brackenier, S. Desmet, E. André, M. Van Ranst, K. Lagrou and P. Vermeersch, Clin. Microbiol. Infect., 2020, 26, 1082–1087 CrossRef CAS.
- S. A. Ejazi, S. Ghosh and N. Ali, Immunol. Cell Biol., 2021, 99, 21–33 CrossRef CAS.
- F. Gong, H. X. Wei, Q. Li, L. Liu and B. Li, Front. Mol. Biosci., 2021, 8, 682405 CrossRef CAS PubMed.
- N. Tsurusawa, J. Chang, M. Namba, D. Makioka, S. Yamura, K. Iha, Y. Kyosei, S. Watabe, T. Yoshimura and E. Ito, J. Clin. Med., 2021, 10, 5197 CrossRef.
- T. Lagousi, J. Routsias and V. Spoulou, Diagnostics, 2021, 11, 1970 CrossRef.
- W. H. Khan, N. Khan, A. Mishra, S. Gupta, V. Bansode, D. Mehta, R. Bhambure, M. A. Ansari, S. Das and A. S. Rathore, Int. J. Biol. Macromol., 2022, 200, 428–437 CrossRef.
- J. Budd, B. S. Miller, N. E. Weckman, D. Cherkaoui, D. Huang, A. T. Decruz, N. Fongwen, G.-R. Han, M. Broto, C. S. Estcourt, J. Gibbs, D. Pillay, P. Sonnenberg, R. Meurant, M. R. Thomas, N. Keegan, M. M. Stevens, E. Nastouli, E. J. Topol, A. M. Johnson, M. Shahmanesh, A. Ozcan, J. J. Collins, M. Fernandez Suarez, B. Rodriguez, R. W. Peeling and R. A. McKendry, Nat. Rev. Bioeng., 2023, 1, 13–31 CrossRef.
- Y. Mei, L. Li, N. Chen, C. Zhong and W. Hu, Analyst, 2020, 145, 6395–6400 RSC.
- Q. Li, S. A. Bencherif and M. Su, Anal. Chem., 2021, 93, 10292–10300 CrossRef PubMed.
- S. Xue, H. Zeng, J. Yang, H. Nakajima and K. Uchiyama, Sensors, 2014, 14, 9132–9144 CrossRef PubMed.
- H. Singh, M. Shimojima, S. Fukushi, A. Le Van, M. Sugamata and M. Yang, Bio-Med. Mater. Eng., 2015, 26, S45–S53 Search PubMed.
- L. Fabiani, V. Mazzaracchio, D. Moscone, S. Fillo, R. De Santis, A. Monte, D. Amatore, F. Lista and F. Arduini, Biosens. Bioelectron., 2022, 200, 113909 CrossRef PubMed.
- C. Carrell, I. Jang, J. Link, J. S. Terry, M. S. Scherman, Z. Call, Y. Panraksa, D. S. Dandy, B. J. Geiss and C. S. Henry, DOI:10.26434/CHEMRXIV-2021-C4BMD-V3.
- N. S. Moreira, C. L. S. Chagas, K. A. Oliveira, G. F. Duarte-Junior, F. R. de Souza, M. Santhiago, C. D. Garcia, L. T. Kubota and W. K. T. Coltro, Anal. Chim. Acta, 2020, 1119, 1–10 CrossRef PubMed.
- Z. Fu, W. Zeng, S. Cai, H. Li, J. Ding, C. Wang, Y. Chen, N. Han and R. Yang, J. Colloid Interface Sci., 2021, 604, 113–121 CrossRef PubMed.
- J. E. Bishop and K. A. Davis, J. Immunol. Methods, 1997, 210, 79–87 CrossRef CAS PubMed.
- M. Nomura, M. Imai, S. Usuda, T. Nakamura, Y. Miyakawa and M. Mayumi, J. Immunol. Methods, 1983, 56, 13–17 CrossRef CAS PubMed.
- F. Wu, M. Mao, L. Cai, Q. Lin, X. Guan, X. Shi and L. Ma, ACS Biomater. Sci. Eng., 2022, 2022, 3932 Search PubMed.
- E. Karakuş, E. Erdemir, N. Demirbilek and L. Liv, Anal. Chim. Acta, 2021, 1182, 338939 CrossRef PubMed.
- A. Bhattacharjee, R. M. Sabino, J. Gangwish, V. K. Manivasagam, S. James, K. C. Popat, M. Reynolds and Y. V. Li, Vitr. Model., 2022, 1, 241–247 CrossRef.
- Y. Kyosei, M. Namba, S. Yamura, R. Takeuchi, N. Aoki, K. Nakaishi, S. Watabe and E. Ito, Diagnostics, 2020, 10, 594 CrossRef CAS PubMed.
- M. Di Domenico, A. De Rosa and M. Boccellino, Diagnostics, 2021, 11, 698 CrossRef CAS PubMed.
- J. H. Lee, M. Choi, Y. Jung, S. K. Lee, C. S. Lee, J. Kim, J. Kim, N. H. Kim, B. T. Kim and H. G. Kim, Biosens. Bioelectron., 2021, 171, 112715 CrossRef CAS PubMed.
- D. Hristov, H. Rijal, J. Gomez-Marquez and K. Hamad-Schifferli, Anal. Chem., 2021, 93, 7825–7832 CrossRef CAS PubMed.
- B. Della Ventura, M. Cennamo, A. Minopoli, R. Campanile, S. B. Censi, D. Terracciano, G. Portella and R. Velotta, ACS Sens., 2020, 5, 3043–3048 CrossRef.
- B. Pierri, M. Tafuro, M. C. Cuomo, D. Di Concilio, L. Vassallo, A. Pierri, A. Ferro, G. Rofrano, A. Gallo, A. Di Stasio, A. Mancusi, L. Galdi, A. Coppola, C. Buonerba, L. Atripaldi and P. Cerino, Front. Public Health, 2022, 10, 840996 CrossRef.
- D. Scheie, P. A. Andresen, M. Cvancarova, A. S. Bø, E. Helseth, K. Skullerud and K. Beiske, Am. J. Surg. Pathol., 2006, 30, 828–837 CrossRef PubMed.
- L. Lopes-Luz, I. C. Junqueira, L. A. da Silveira, B. R. de Melo Pereira, L. A. da Silva, B. M. Ribeiro and T. Nagata, Mol. Biol. Rep., 2020, 47, 7333–7340 CrossRef PubMed.
- B. Sharma and R. Jain, Asian J. Med. Sci., 2014, 5, 30–34 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ay01755a |
| This journal is © The Royal Society of Chemistry 2024 |