Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
3D direct ink printed materials for chemical conversion and environmental remediation applications: a review
Jeannie Z. Y.
Tan
 *ab,
Manuel Alejandro
Ávila-López
*ab,
Manuel Alejandro
Ávila-López
 c,
Amir
Jahanbakhsh
c,
Amir
Jahanbakhsh
 ab,
Xuesong
Lu†
a,
José
Bonilla-Cruz
ab,
Xuesong
Lu†
a,
José
Bonilla-Cruz
 c,
Tania E.
Lara-Ceniceros
c,
Tania E.
Lara-Ceniceros
 *c,
John M.
Andresen
*c,
John M.
Andresen
 ab and
M. Mercedes
Maroto-Valer
ab and
M. Mercedes
Maroto-Valer
 *ab
*ab
aResearch Centre for Carbon Solutions (RCCS), School of Engineering & Physical Sciences, Institute of Mechanical, Process & Energy Engineering, Heriot-Watt University, Edinburgh EH14 4AS, UK. E-mail: M.Maroto-Valer@hw.ac.uk
bIndustrial Decarbonisation Research and Innovation Centre (IDRIC), Heriot-Watt University, Edinburgh EH14 4AS, UK
cNano & Micro Additive Manufacturing of Polymers and Composite Materials Laboratory “3D LAB”, Advanced Functional Materials & Nanotechnology Group, Centro de Investigación en Materiales Avanzados S. C. (CIMAV-Subsede Monterrey), C. P. 66628 Apodaca, Nuevo León, Mexico. E-mail: tania.lara@cimav.edu.mx
First published on 28th February 2023
Abstract
3D printing technologies and continuous flow microreaction systems are rapidly gaining attention in the domain of heterogeneous catalysis. From a materials perspective, minimising material waste through simplified single (or few) step fabrication processes is attractive for commercial exploitation. Direct ink writing (DIW) has recently studied as potential printing technology for energy storage, such as batteries and capacitors. However, the use of DIW to develop catalysts and microreactors for photo-, photoelectro-, and electrocatalysis reactions for chemical conversion and environmental remediation applications has not been developed as much as reported for energy storage applications. Hence, this review summarises the most recent development of DIW for photo-, photoelectro-, and electrocatalysis reactions for chemical conversion and environmental remediation applications. Recent trends, such as fast prototyping of reactors via 3-D printing of flow channels, miniaturisation and use of multi-physics modelling, are also highlighted. Finally, a perspective of DIW development to accurately fabricate nanostructured catalysts as well as reactions for catalytic applications is presented.
1. Introduction
To preserve a liveable climate, greenhouse-gas emissions must be reduced to net zero by 2050.1 Hence, the development of clean energy plays an important role to tackle the climate emergency and build a sustainable world by driving the industry to decarbonized chemical manufacturing as well as remedy environmental issues. However, the commercialization of clean energy systems requires a significant improvement in their performance and efficiency.According to the definition standardised by Technical Committee of the American Society for Testing and Materials (ASTM) International in NF ISO/ASTM 52900, additive manufacturing (AM) is the process of joining materials to make objects from 3D model data, usually layer upon layer, as opposed to subtractive manufacturing methodologies.2 Unlike subtractive manufacturing processes, AM can directly produce complex 3D parts, with near-complete design freedom. Hence, it is advantageous products that have a high demand of customization, flexibility, design complexity and high transportation costs. It shows a great potential towards manufacturing novel designs of energy conversion and storage devices, which were previously inaccessible via traditional manufacturing methods. Other advantages of AM include reduced lead time and it is a more sustainable manufacturing technology as there is virtually no waste material. Extended discussions on the details and capabilities of a range of 3D printing methods can be found in recently published review articles.3–9
Catalysis is a driving force of the chemical industry. To intensify catalytic processes to make them more efficient and sustainable, the growth of AM for catalysis applications is growing exponentially with a myriad of strategies to obtain devices with catalytic properties. For example, a few studies and reviews have discussed the advantages and implications of 3D (three-dimensional) printed devices and materials for electrochemical energy storage6–8,10,11 and environmental remediation applications.12,13 Well-designed 3D structures were reported to have great potentials for increasing the performance of batteries, capacitors, fuel cells and advanced photovoltaic cells,14 as well as leading to improvements in reactor engineering and catalysis applications.15–17 For instance, by combining absorbers and reflectors with self-supporting 3D photovoltaic (3DPV) structures, which were achieved by mounting commercial Si cells on AM fabricated 3D plastic frames, the energy densities yielded were reported to be higher by a factor of 2–20 than stationary flat PV panels, compared to the increase by a factor of 1.3–18 for a flat panel with dual-axis sun tracking.18 AM can also be utilized during fabrication of microstructures that are capable of increasing the efficiency of single-junction solar cells.19–21
3D printing for photo-, electro-, and photo(electro)-chemical energy conversion and storage application is relatively new, only gaining momentum in the research world recently.5,6,22 To enable improvements in resource efficiency as well as in sustainable production and consumption, Gebler et al. suggested that replacing conventional manufacturing with AM has the potential to reduce total energy primary supply (TEPS) by 2.54–9.30 EJ and CO2 emissions by 130–525.5 Mt over its entire life-cycle.23 This implies that AM can transform the manufacturing system. Hence, AM is an attractive alternative to traditional subtractive fabrication techniques because it can rapidly produce cost effective and complex prototypes with varying degrees of functionality. Parts produced by AM have found applications in industries, such as aerospace,24 defense,25 construction,26 and biomedical.27,28
AM also termed 3D printing, has become a major driver of innovation in industrial manufacturing today.29,30 Today, 3D printing has become a fabrication tool for final products in addition to prototyping. The principles of AM have been explained in detail in numerous public articles.31,32 According to ASTM F2792-12a, 3D printing techniques can be categorised based on the printing methods, such as material extrusion (i.e., direct ink writing – DIW) and fused deposition modelling (FDM), powder bed fusion (i.e., selective laser sintering – SLS and direct metal laser sintering), vat photopolymerization (i.e., stereolithography – SLA), binder jetting, material jetting (i.e., inkjet printing – IJP), sheet laminate (i.e., laminated object manufacturing – LOM) and directed energy deposition.33 Overall, compared with the conventional subtractive methods, 3D printing provides a completely new bottom-up manufacturing technology to rapidly fabricate 3D-structured devices with complex architecture of materials, minimal waste and low cost. Among the printing techniques, FDM, DIW, SLA, LOM, and SLS are widely used techniques for catalyst preparation34 because they are capable to reach down to micro- and nano-scale structures.17
2. Direct ink writing (DIW) technology
Among the various AM techniques, DIW has emerged as the most versatile 3D printing technique for the broadest range of materials. DIW allows printing of practically any material, provided the precursor ink can be engineered to demonstrate appropriate rheological behaviour.35 The fabrication of catalytic materials for chemical conversion and environmental remediation applications using DIW is increasing gaining researchers' interest because this technique acts as a unique pathway to introduce design freedom, multifunctionality, and stability simultaneously into its printed structures.2.1 Tools
DIW has gained extensive attention, as it shows superiority due to free choices of materials, small amount of raw materials, open source and feasibility for multi-material printing.36,37 DIW is also called, robocasting, robot-assisted shape deposition, robotic deposition or 3D fibre deposition, and is a method associated with the material extrusion technique.38 It was initially established by Cesarani et al. at Sandia National Laboratories in 1997 for the fabrication of concentrated materials, such as ceramic pastes with some portion of organic binder. This technique is an easy, adaptable, and inexpensive methodology, appropriate for a broad range of materials, such as ceramic materials (monolithic and composites), polymers, alloys, and even food.39 It particular, DIW enables complex catalytic systems to be manufactured where traditional routes are not able to achieve the complexity of AM.The DIW AM technique can be categorized by the two main manufacturing approaches:
(1) Discontinuous droplet-based (also known as drop-on-demand inkjet printing, Fig. 1a.
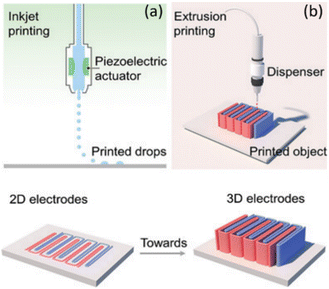 | ||
| Fig. 1 Direct writing: (a) drop-on-demand inkjet printing wherein the ink is jetted onto a support in droplet form and (b) extrusion 3D-printing wherein the ink has a paste-like consistency for printing self-supporting structures (reprinted with permission from ref. 42). | ||
• Printing heads eject discontinuous droplets onto the printing substrate to produce the image by means of the heat-induced explosion of ink bubbles (e.g., in bubble jet printers and thermal inkjets).40
(2) Continuous paste-based/filamentary ink approaches (Fig. 1b).36
• Pressurized ink is forced through a nozzle, and breaks up into uniform drops under the action of surface tension.41
The comparison of these two techniques is summarised in Table 1.
| Printing technique | Drop-on-demand inkjet printing | Extrusion printing |
|---|---|---|
| Characteristic | Generate individual droplets when required onto a substrate | Extension of conventional inkjet printing technology and can be used as a substrate-friendly method |
| Advantages | More economic in ink delivery | Highly versatile as a large variety of materials can be deposited |
| High printing resolution as dilute suspensions are used | Higher density of material deposited | |
| Capable of forming 3D structure | ||
| Disadvantages | Printing only 2D direction | Complicated post printing process (i.e., surfactant removal and post thermal annealing steps) |
| Low feature resolution |
Recent studies have reviewed the recent progress of inkjet printing.42–45 Furthermore, the ability of extrusion printing to fabricate self-standing 3D microstructure has received widespread attention because this resolves the adhesion concern between the printed materials and substrate and the compatibility of substrate for post printing treatment (e.g., post annealing step).45–47 In addition, self-standing 3D printed catalysts ease the catalyst recovery process. Hence, the following discussion will focus on extrusion-based printing. The extrusion-based DIW utilizes a non-Newtonian viscous slurry with composed rheological properties (discuss in Section 2.2.1) and configuration of liquid and solid phases as printing material at room temperature.48 The DIW head consists of a dispenser with a nozzle at the exit. 3D parts are printed by moving the robotic arm to promptly print the computer-aided design (CAD) model in a layer form. Once the printing is completed, the next step is de-binding and sintering treatment to remove the binder content and to obtain maximum densification.37,49,50
The characteristics of the slurry are very important and vital for the extrusion printing. The feedstock is developed by combining fine solid powder particles (10–50 μm) with the binder material normally dispersed in deionized water or organic solvents in the presence of a range of additives, including surfactants, dispersants and binders.51,52 Blending and mixing of powders are usually carried out using tumbler mixers.53 Furthermore, the viscosity is low enough to be extruded through the nozzle at comparatively low pressure. Meanwhile, the slurry must have sufficient yield strength and stiffness (storage modulus) to hold the shape. This is normally achieved by making the paste of binder/additives and ceramic powder with a volume fraction of the solid content higher than 50% to achieve maximum densification and to reduce dimensional inaccuracy in the final part. Shear-thinning behaviour is desired as it permits the slurry to be extruded through comparatively smaller diameter nozzles at relatively low pressure.37 The special usage of solid slurries with essential viscoelastic behaviour permits the production of parts that can retain their initial shape irrespective of the force caused by the freshly deposited layers on top of them.52 The beauty of DIW is its opportunity to fabricate structures with different configurations with complex parts and interconnecting cavities, to composite materials, solid monolithic components, and filaments with various cross-sectional forms.54,55
As previously discussed, the solid loading in DIW is normally greater than 50% in term of volume fraction to achieve maximum densification and to reduce dimensional inaccuracy in the final part, and typical ceramic slurries possess an average particle size from 1 to 100 μm.52 Therefore, it requires a relatively larger nozzle size of 100–1000 μm than the inkjet printing system (∼100 μm). According to Shahzad et al. the optimum diameter of the nozzle is generally 400–800 μm to avoid clogging of the slurry.52 During the preparation of the slurry, an uniform dispersion of micro/nanoparticles has an important impact on the quality and morphology accuracy of the printed parts. Generally, the key criteria for achieving excellent printability via extrusion printing are the pseudoplastic behaviour of the slurry, its setting speed after dispensing, and uniform dispersion of the particles. One of the primary issues with DIW is ensuring a high-density printing without clogging the nozzle. This is generally solved through the promotion of a homogenous dispersion of the particles in the slurry and the use of a narrow particle range. To obtain narrow particle size distribution, a vibratory sieve is used to help removing large agglomerates. To fabricate high-resolution microscale prints, the particles sizes in the slurry must be an order of magnitude smaller than the final part resolution.
There are three main types of inks utilized in the robocasting of ceramic-based items:
(I) Gels and hydrogels, which generally are composed of an organic polymer that has been polymerized to create a macro polymeric 3D network swollen in water and enriched with solid particles. The downside of solid gel-based inks is the post-processing multistep required, including a well temperature control and laborious routes to obtain the desired 3D item.
(II) Organogels, a class of gels named as semi-solid systems with an organic phase immobilized by a 3D network formed by particles; and.
(III) Colloidal inks, which are stable suspensions of highly concentrated solid particles (60–80 wt%) in aqueous media (or by using other low-viscosity solvent), with a small quantity of organic compounds ≤1 wt% as surfactants, dispersants or polymers.56 These inks need a significant amount of time to prepare them, involving the powder milling and stabilization of the colloidal powder required to achieve the desired rheology for having ink printability.57–59 Obtaining the optimal rheological range for printing monoliths structures has been a challenge because general rheological printability conditions for ceramic materials have not yet been devised.
The formulation of these ceramic-based inks significantly affects the printability and mechanical strength of the structures. Hence, it is paramount to understand the characteristics of the ink because they are stabilised and interacted via different mechanisms, namely electrostatic, steric and electrosteric mechanisms (Fig. 2). Further discussion about the ink characteristics and optimisation can be found in the recent publication by Lamnini et al.60
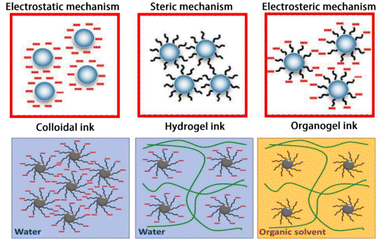 | ||
| Fig. 2 Schematic diagram illustrating the three main mechanisms for particle stabilisation: steric and electrostatic interactions are represented with black lines and negative red charges respectively. Colloidal inks involve an electro-steric stabilization mechanism, which gives rise to polyelectrolyte complexes. The green lines represent the polymer chains within gel-embedded inks (reprinted with permission from ref. 60). | ||
2.2 Ink properties
Various factors, including the viscosity and density of the slurry, diameter and shape of the nozzle, printing speed, sintering method, and drying technique can significantly affect the final part properties and surface finish.52 Among them, ink properties play the vital role in the printing process and finished product.2.2.1.1 The rheological behaviour of the slurry. The rheological behaviour of the slurry is the most critical attribute for printing 3D parts successfully, which means the desirable shape is retained as well as supporting the subsequent layers from collapsing under its weight (also known as slumping) without external assistance. The necessary rheological properties can be obtained through the appropriate choice of a binder-solid ratio. The flow behaviour of these slurries can be estimated by the model developed by Herschel–Bulkley. This model is based on ideal non-Newtonian fluids, which do not flow beneath a particular stress (the yield stress). When the stress is increased to a certain level, they obtain shear-thinning behaviour where their viscosity declines significantly as the shear rate rises. Mathematically, it can be explained by the Herschel–Bulkley equation.
τ = τy + K![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) n n | (1) |
For DIW, a low value of n is advantageous so that the pressure of extrusion is fairly low and to encourage mixing. A low value of K is also necessary for similar purposes, whereas a high value of τy is required to avoid failure of the final part after the 3D printing process. Unfortunately, most of the above factors are firmly reliant on one another. So, controlling any of these parameters in isolation is a major challenge. Finding appropriate values of these parameters for robocasting is difficult because no such universal requirements exist. They entirely depend upon the shape and scale of the printed part, the diameter and shape of the nozzle, and the slurry density.52,61 Hence, multi-physics simulation could provide a theoretical and optimization guidance in this regard (further discussed in Section 2.3).
2.2.2.1 Precursor sol–gel inks. Precursor sol–gel inks can be incorporated into the printing solution and post-treated to obtained crystalline ceramics. In this approach, the printed ink is based on sol–gel chemistry and uses an organometallic precursor in the form of a stable sol. The ink dries to form a xerogel layer that is further processed into oxide layers, usually by calcination. For precursor (sol–gel) inks, sol–gel chemistry parameters, such as precursor concentrations, type (e.g., organometallic, such as titanium isopropoxide), and solvent must be considered. These parameters, and the gelation period, allow the viscosity of sol–gel inks to be tuned. Sol–gel chemistry also allows tuning of the final microstructure (e.g., crystal phase or porosity). It should be noted that these systems differ from the inkjet printed sol-gels, which contain the preformed semiconductor nanoparticles.45,62
2.3 Multi-scale multi-physics simulation
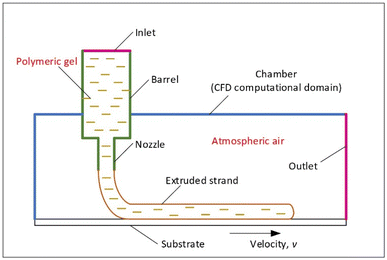
Multi-scale and multi-physics simulations are normally conducted using Computational Fluid Dynamics (CFD). CFD is a powerful tool to understand physical flow behaviour, such as pressure patterns, slurry velocity distribution and slurry viscosity changes in the extrusion nozzle or syringe. Simulation studies have been previously used to predict the cross-sectional morphology formed by the successive deposition of parallel strands in material extrusion.63 The simulation results provide a theoretical flow model that can be used to optimize for the apparatus design and operation. However, reported work did not consider the polymer chain folding during extrusion, but rather assumed the property uniform distribution in the extruded strand as well as the variation of mechanical properties, such as Young's moduli during extrusion (e.g., solidification process during fabrication). Thus, the multi-scale multi-physics modelling of ink for DIW are required to incorporate and simulate all the fundamental processes, including flow, heat transfer, viscoelastic properties, solidification and crystallization that occur across various length of scales.64(I) The die is driven by the ram with the constant velocity as shown in Fig. 3a.
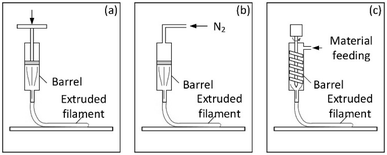 | ||
| Fig. 3 Types of direct-writing extruders: (a) constant ram velocity; (b) constant pressure; (c) screw extrusion (adapted with permission from ref. 65). | ||
(II) The die is driven by the high constant pressure gas e.g., N2 as shown in Fig. 3b.
(III) The gel, paste or slurry is deposited by a screw extruder as shown in Fig. 3c.
Currently, most of CFD modelling focused on the extruders with constant ram velocity or constant pressure as illustrated in Fig. 3a and b, respectively.
The gel for direct writing is a typical non-Newtonian fluid. Due to the relationship between shear rate (γ) and shear stress (τ), as outlined in eqn (1), printing fluids are either Newtonian or non-Newtonian fluids. The shear rate is the change of velocity along the direction of material or flow deformation. The shear stress is the applied parallel or tangential stress to cause the material or flow deformation. The Newtonian fluid has a linear relationship of shear stress to shear rate with a constant viscosity. The non-Newtonian fluid has a nonlinear relationship between shear rate and shear stress, including shear thinning pseudoplastic fluid, shear thickening dilatant fluid, Bingham plastic fluid or Bingham pseudoplastic fluid.
Generally, CFD is employed for modelling Newtonian fluids, such as air and water using the Navier–Stokes's equations. For simulation of non-Newtonian fluids, the viscosity in the momentum equation for generalized Newtonian fluids is substituted by the non-Newtonian fluid model. Two scenarios can be considered for CFD simulation of DIW:
(I) The focus of modelling is on the ink flows in the extrusion nozzle or syringe, then a single fluid is applied for simplifying the mathematic problems without losing the accuracy.
(II) If ink deposition process and deposited strand morphology are focused on, the two fluids including gel and atmospheric air are applied for modelling.
To obtain clear interface between two fluids, gel and air, generally the volume of fluid method (VOF) is employed. The general computational domains are shown in Fig. 4, including the extrusion barrel, extrusion nozzle and the operation chamber. The substrate is set as the moving wall with a velocity of v. The setting of the inlet boundary is based on the extrusion driven by the ram with constant velocity or by a constant high-pressure gas.
The extrusion governing equations are listed below.
Continuity equation:
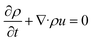 | (2) |
Momentum equation:
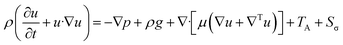 | (3) |
Volume fraction equation:
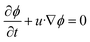 | (4) |
For non-Newtonian fluids, the viscosity, μ, in the momentum equation is
μ = f(![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) ) | (5) |
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) is the shear rate.
is the shear rate.
In the flow, the shear rate can be worked out by the following equations.
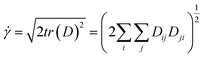 | (6) |
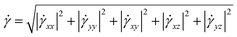 | (7) |
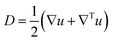 or
or ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = (∇u + ∇Tu).
= (∇u + ∇Tu).
There are two common methods to resolve these CFD governing equations, namely the finite volume method (FVM) and finite element method (FEM). Although FEM and FVM share some similarities, they have some differences in the ease of implementation. FVM is easier to implement that FEM according to the opinion of the majority of engineers. The FEM is a great method for Multiphysics analysis and complex geometries, however, the most significant advantage of FVM is that it needs to do flux evaluation for the cell boundaries. This makes FVM an excellent choice for solving non-linear conservative laws issues. The software developed based on these methods is divided into two categories: ANSYS FLUENT and open source OpenFOAM are based on FVM; whereas ANSYS POLYFLOW, FLOW-3D and COMSOL Multiphysics® are based on FEM.
Studies demonstrated that the cross-sectional morphology of the strand is well predicted by CFD modelling as shown in Fig. 5a. The cross-sectional morphology of the layered strands (Fig. 5b), the porosity of the deposited structure (Fig. 5c) and the strand overlapping (Fig. 5d) can be well simulated using CFD with the non-Newtonian fluid model.
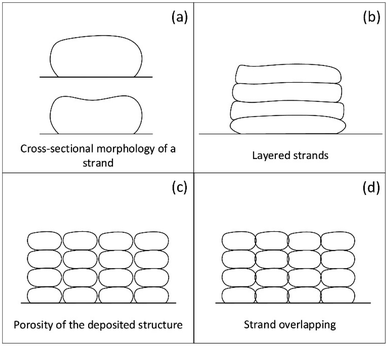 | ||
| Fig. 5 CFD predicted the (a) cross-sectional morphology, (b) layered strands, (c) porosity and (d) overlapping of strands. | ||
The non-Newtonian fluid models simulated with CFD for DIW are further discussed below.
2.3.1.1 Pseudo-Newtonian model. When the non-Newtonian fluid flow is in the creep region, which is below its onset shear rate, the viscosity could be assumed to be constant as the Newtonian flow, so the pseudo-Newtonian model can be described as:
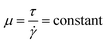 | (8) |
For instance, Serdeczny et al. numerically and experimentally investigated the polylactic acid (PLA) strand shape during the extrusion process.66 The PLA was extruded at 200 °C and deposited on a planar substrate at room temperature. The extrusion was treated to be in the creeping flow region. Since the viscosity was 1000 Pa s, the inertial effect was ignored. The simulation based on ANSYS FLUENT demonstrated that the predicted cross-sectional shape was in good agreement with the experimental measurements, showing the capability to predict the strand morphology for different extrusion conditions. Using the similar simulation approaches, the research group further investigating the relationship of the gap distance between the extrusion nozzle and the substrate, and the velocity ratio of the substrate to the average velocity of the flow inside the nozzle to the strand morphology.67 They concluded that the printing force applied by the extruded material on the substrate had a negative linear relationship with the velocity ratio at a constant printing distance. In addition, the simulation showed that by decreasing the layer thickness and the strand-to-strand distance led to a smaller pores and larger inter- and intra-layer bond line densities.66 Meanwhile, the surface roughness of the vertical and horizontal walls was improved with the decreasing of the layer thickness, in contrast, decreasing the strand-to-strand distance reduced the roughness of the horizontal surfaces. The quantitative predictions of the porosity, the bond line densities and the surface roughness opened the possibility of performing numerical optimizations of the process parameters. Furthermore, Narei et al. used ANSYS FLUENT to simulate the core–shell polymer strand in the extrusion process.68 The viscosity for the molten shell and core polymer were fixed at 1000 and 1500 Pa s, respectively. The cross-sectional morphology of the strand was then studied under different printing speeds to find out the highest volume fraction of the core through changing the printing parameters. However, this pseudo-Newtonian model is only suitable for very low shear rate, such as flowing in the creeping region. For higher shear rate, other models, such as the power law must be applied.
2.3.1.2 Herschel–Bulkley (power law) model. For most printing processes, the shear thinning fluid can be described by the power-law equation. The shear stress is linear to n times of shear rate. For Bingham pseudo plastic fluid, the viscosity can be presented by Herschel–Bulley (power law) model as eqn (8).
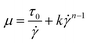 | (9) |
Recently, Liu et al. simulated a single pseudoplastic fluid of wheat gluten and potato granules extrusion process by ANSYS POLYFLOW using the power-law model.69 The pressure and viscosity distributions in the extrusion barrel were studied under different k and n values, which determines the flow field distribution. The validated model exhibited improved printing characteristic when the k value was between 1000 and 1800 Pa sn and n value between 0.15 and 0.3. Last year, Song et al. used ANSYS FLUENT to model the extrusion of the jammed gelatin microgel as the Bingham pseudoplastic fluid. The constitutive behaviour of the microgel composite ink was captured for the first time after fit with Herschel–Bulkley model.70 It was found that the cross-sectional shape turned into flat rectangular under a small normalized gap distance (less than 0.6 mm). Furthermore, the achievable maximum length (without collapse) of the jammed gelatin microgel-based composite ink, deposited both between two supporting substrates and over a supporting substrate, were evaluated and visualized. In addition, the self-supported filament printability was also evaluated using the same simulation method. Behdani et al., who used OpenFOAM with the power-law model, investigated the cross-sectional morphology of the extruded PLA strand, which is sensitive to the viscosity, and the gas size between deposition nozzle and the planar substrate.71 Papon et al. modelled the polymer fused filament fabrication through different nozzle geometries, such as circular, square and star shape, by the ANSYS FLUENT VOF method.72 The flow was assumed laminar, non-isothermal and non-Newtonian with the power-law model fitted. The results showed that the melting polymer spreading characteristic was affected by the nozzle geometry.
With the limitation of very slow shear rate and very high shear rate at which the flow has a nearly linear relationship between shear stress and shear rate, the Herschel–Bulley (power law) model is only suitable for the intermediate shear rate (0 < τ < ∞).
2.3.1.3 Cross model. In order to overcome the limitations of the power-law model, which fails to predict the Newtonian plateau at the viscosity under the very low and very high shear rates, the Cross model was introduced by M. M. Cross, in 1979 (ref. 73) as:
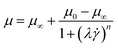 | (10) |
The Cross model is an empirical equation that is used to fit non-Newtonian data. More specifically, this model “describes pseudoplastic flow with asymptotic viscosities at zero (μ0) and infinite (μ∞) shear rates, and no yield stress”.74 The Cross model can describe many types of fluids, including dispersions, polymer melts, and polymeric solutions. Using this method, Gosset et al. employed OpenFOAM VOF models to simulate melting poly-lactic acid (PLA) extrusion process at the temperature of 215 °C.75 Through rheological experiments, the viscosity of melting PLA is better fitted with the Cross model compared to the power law model. The estimated extruded strand height and strand cross-sectional shape matched very well with the experimental measurement. In addition, Nikfarjam et al. introduced the cross model for non-Newtonian fluids into CFD simulation and developed Level-set-STAGgered (LS-STAG) cut-cell method, which showed its versatility even for the Xanthan flows in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 axisymmetric sudden expansion.76 Antonietti et al. undertook the mathematical modelling of the polymer extrusion of hollow and non-hollow yarns by ANSYS POLYFLOW.77 The flow was found to be non-Newtonian, incompressible and isothermal under these conditions. The cross rheological law was employed as constitutive model for the viscosity. The Cross model is popularly used for CFD simulations because it covers from very low shear rate to very high shear rate. However, since it is an empirical equation, the model may or may not be appropriate for various applications. Hence, the Bird–Carreau model was introduced in 1987.78
1 axisymmetric sudden expansion.76 Antonietti et al. undertook the mathematical modelling of the polymer extrusion of hollow and non-hollow yarns by ANSYS POLYFLOW.77 The flow was found to be non-Newtonian, incompressible and isothermal under these conditions. The cross rheological law was employed as constitutive model for the viscosity. The Cross model is popularly used for CFD simulations because it covers from very low shear rate to very high shear rate. However, since it is an empirical equation, the model may or may not be appropriate for various applications. Hence, the Bird–Carreau model was introduced in 1987.78
2.3.1.4 Bird–Carreau model. The Bird–Carreu model has more parameters than the Cross model and is also a more generalized form of the power law fluid model. For cases where there are significant variations from the Power Law model, such as at very low and very high shear rate, it becomes essential to incorporate the values of viscosity at zero shear (μ0) and at infinite shear (μ∞) into the formulations (eqn (11)).79
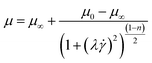 | (11) |
To provide industrial insight on the printability of 3D printed materials, Guo et al. employed Bird–Carreau model for single fluid and studied the grain gel extrusion process within the syringe.80 The simulations were performed using ANSYS POLYFLOW and clearly illustrated the transport behaviour, giving a more realistic understanding of the slurry flow during extrusion. Based on the simulation results, the piston pressure can be a criterion to evaluate the printing performance of different grain gels. However, Guo et al. suggested that the use of structural mechanics simulation would be helpful to evaluate the mechanical properties and structural stability of the printing materials. Ni et al. investigated the printing of flame retardant plastics, such as acrylonitrile–butadiene–styrene (ABS) and ammonium polyphosphate (APP), by applying a numerical approach using finite element (FEM)-based software, COMSOL Multiphysics®, aiming to overcome nozzle clogging and establish optimal three-dimensional (3D)-printing parameters.81 The fully melted ABS or APP was fitted to the Bird–Carreau viscosity model showing the temperature distribution, melting evaluation, pressure and velocity distribution in the nozzle for the 3D printed flame retardants. Yilmaz et al. proposed that the wall shear rate and shear stress, flow uniformity at the die exit and pressure drop through the dire were the critical factors for designing plastic extrusion dies.82 By employing ANSYS POLYFLOW and the Bird–Carreau model as the viscosity model for the polymer melt, the 3D CFD analyses provided a comprehensive understanding of the polypropylene (PP) melt flow through the conical spiral mandrel die.
Achieving a balanced flow is one of the major challenges during the profile design of extrusion dies.83 For this purpose, numerical modeling codes may be a very useful aid. Gonçalves et al. developed CFD code to model the flow of polymer melt to deal with unstructured meshes.84 The Bird–Carreau constitutive equation was employed to model the shear viscosity dependent on shear rate. The self-compiled code was verified by the problem of Lid-driven cavity. The developed code had been a valuable tool to aid the design of complex profile extrusion die with improved the flow distribution. Although the Bird–Carreau model has not been employed to simulate the extrusion process of DIW, it can be used for the wide range of shear rate. Therefore, the Bird–Carreau model can be employed in the CFD simulation of direct writing.
There is no doubt that modelling and simulation have significant contribution in characterizing and optimizing the whole process of 3D printing. However, it is crucial to validate numerical models via comparison to experimental results. Several studies have shown good agreement between modelling and experiment results. For instance, Luo et al. recently developed a heat transfer model, which provided an upper limit of the polymer feed rates.89 They also performed experiments on a modified extruder setup to obtain experimental results and validate their model. The modelling and experimental results matched well especially for a nozzle wall temperature range of 210–260 °C. Meanwhile, Coasey et al. used interlaminar fracture toughness measurements to validate a non-isothermal welding model, which predicted the healing over time by considering the effect of processing parameters and rheological properties.90 The model and the experimental results provided a good fundamental knowledge for future printer development.
A post-extrusion heating model was recently developed by Edwards and Mackay to minimize the formation of surface roughness called shark-skin defects for both amorphous and semi-crystalline polymers during material extrusion.91 Shark-skin defects can reduce the effectiveness of binding the polymer to the underlying substrate. They investigated one potential solution for this problem, which is reheating the polymer after it exits the hot end extrusion nozzle. This process allowed the polymer to relax more before deposition. They also performed experiments and observed that the shark-skin defects are reduced once the polymer has reached a certain temperature.
The heat transfer in the polymer melt is significantly affected by the rheological properties which control the flow characteristics.92 For instant, heat transfer can affect the differential pressure across the nozzle and reduce the printing speed as it was shown by a rheological behaviour modelling performed by Phan et al.93 It is essential to employ a multiphysics modelling approach to account for coupling effects of different effective physics in the 3D printing process.
3. Printing of functional materials by DIW
Global energy and environmental crises are among the most pressing challenges facing humankind. To overcome these challenges, recent years have seen an upsurge of interest in the development and production of renewable chemical fuels as alternatives to the non-renewable and high-polluting fossil fuels. Photocatalysis, photoelectrocatalysis, and electrocatalysis provide promising avenues for sustainable energy conversion.94 Various effort and design have been devoted for DIW extrusion-based printing to produce fine and complex 3D architectures as reviewed by Lewis.95 Recently, Fu et al. summarised the research efforts on the use of DIW and development of graphene oxide (GO) based inks to fabricated structures for energy storage, electronic circuits and thermal energy applications.39 Furthermore, Zhakeyev et al. highlighted the DIW-based studies on the development of 3D microstructured materials for different energy storage and conversion applications.173.1 Photocatalysts
Photocatalysis, which is the most typical wireless systems and is the easiest systems among different configurations (i.e., photocatalysis (PC), photoelectrocatalysis (PEC) and electrocatalysis (EC), offers new and timely opportunities for renewable energy generation, environmental remediation, and ‘green’ chemical synthesis.While the usage of photocatalysts has been growing during the last decades as summarized in Table 1, there are still relatively few cases reported of printed materials being used in this area of interest via robocasting,12,96–99 because the integration of AM and catalysis is still relatively new. The amount of published work only started to emerge in 2015.100 To date, different photocatalytic applications, such as photodegradation of pollutants in liquid or gas system, and chemical conversion to synthesize value-added molecules, like H2 or NH3, have been developed. This new trend of technological tailormade developments allowed users to obtain more specific materials, simplifying their design and application in complex reactor systems, and increasing their efficiency or equalling the photocatalytic activities of their powdered counterpart.101
A good photocatalytic material should involve a good charge separation, a fast charge transfer, absorption in the visible light spectrum of the UV-Vis range, high stability, low cost, and nontoxicity.94 Hence, the development of photocatalytic 3D printed materials should take into account all these properties. Furthermore, it is important to highlight that 3D printed photocatalytic designs should facilitate their use compared with conventional materials, including powdered materials, thin films, or coatings, and could be applied in pilot and/or industrial scales.
TiO2 powder-based systems have been extensively reported for PC, PEC and EC applications. However, the powder-based systems limit the recyclability and reusability of TiO2 materials. 3D-printed hierarchical porous TiO2 structures featuring a printed macrosized grid or cellular structures, porous microstructures, and a specific structural strength have shown great potential in many applications, including water treatment and catalyst carriers. The main aim of this section is to summarize the new trends and approaches related to 3D printing of catalysts and their photocatalytic applications for dyes degradation, H2 production, and air purification.
Compared to powder-based materials, thin films, or photocatalytic coating systems, the 3D-printed monolith structures are easy to reuse due to their high structural strength, which is critical for practical use. The design of the photocatalysts may take any shape once the necessary rheological properties of the inks have been achieved. However, the DIW technique is such a broad and virgin field for the development of photocatalyst 3D printed materials and is going to impose as an affordable tool to magnify the outstanding application in the very near future. The first monolithic structures created using 3D printers were solid support structures based on titanium using a direct manufacturing process called Robocasting. Duoss et al. created the first 3D printed TiO2 microstructures using sol–gel precursor solutions containing chelated titanium alkoxide and polyvinylpyrrolidone (PVP) as the polymer additive.102 Recently, a binder free design of TiO2 ceramic pastes was reported by Bonilla-Cruz et al.103 The study performed a systematic investigation on the rheological properties of TiO2 paste was conducted by adjusting the volumetric ratios of water and glycol, demonstrating for the first time a straightforward, facile, and environmentally friendly technique to produce a printable (viscosity range: 1 × 105 ≤ η ≤ 9.9 × 105 Pa s) and self-supporting monolith by DIW. The study emphasized that a shear-thinning behavior and a gel-point (G′ = G′′) > 1 × 103 Pa were crucial to obtain three dimensional and self-supported complex ceramic structures with excellent shape-retention. The binder-free three-dimensional ceramic structures exhibited good mechanical stability after 400 °C drying process.
TiO2 has been extensively used as a photocatalyst for the purification of water and air, and self-cleaning surfaces purposes. Furthermore, due to its high oxidation activity and super hydrophobicity, TiO2 can be employed as an antibacterial agent. TiO2 has a relatively high degree of reactivity and chemical stability when exposed to UV light (387 nm) with an energy greater than the band gap of the anatase crystalline phase, which is 3.3 eV.104 Recent development using TiO2 and other inorganic ceramic precursors for DIW for 3D printed monoliths and their photocatalyst performance are summarized in the Table 2.
| Material | Comments | Results/applications | Ref. |
|---|---|---|---|
| TiO2 | Mass: 0.5 g | Liquid degradation of triclosan 99.5% | 101 |
| Figure: square lattice | Gas degradation of n-butane 95.3% | ||
| Concentration of reactants: liquid 10 mg L−1 and gas 63% of propane, 7% of isobutane, and 30% of n-butane on a molar basis | Isobutane 93.7% | ||
| Light source: liquid, 150 mW cm−2 (380–780 nm); gas, 1680 μW cm−2 (200–280 nm) | Propane 52.9% | ||
| Adsorption time: 30 min equilibrium in dark | |||
| Degradation time: 4 h liquid; 15 h gas | |||
| pH: 7.0 | |||
| Mass: 1.25 g | 40–58%, acetaldehyde degradation | 98 | |
| Figure: monolith cylinder | |||
| Concentration of reactant: 5000 ppmv with a flow rate of 20 mL min−1 | |||
| Light source: 84 mW cm−2 | |||
| Adsorption time: 8 h equilibrium | |||
| Degradation time: 30 min | |||
| pH: not specified | |||
| Mass: 22 g | Degradation of acesulfame potassium (99.99%) | 105 | |
| Figure: square-lattice double-diagonal (13.5 × 13.5 cm) | |||
| Concentration: 20 mg L−1 of acesulfame potassium | |||
| Light source: solar simulator, 1500 W, Xe arc lamp, wavelength range 280–800 nm, 15 mW cm−2 | |||
| Adsorption time: not evaluated | |||
| Degradation time: 60 min | |||
| pH: 7.3 | |||
| Mass: not specified | NH3 production 7.4 nmol h−1 | 99 | |
| Figure: cube grille (10 mm × 10 mm) | |||
| Concentration of reactant: 30 mL min−1 of N2 | |||
| Lamp: 300 W Xe lamp, 100 mW cm−2 | |||
| Equilibrium time: 1 h in dark | |||
| Time reaction: 6 h | |||
| pH: not specified | |||
| Au/TiO2 (Au 1.0 w%) | Mass: not specified | H2 production 0.24 mol min−1 gAu−1 | 12 |
| Figure: circle mesh (24 cm2) | |||
| H2 production: water/ethanol | |||
| Concentration: 90/10 ratio | |||
| Light source: UV LEDs (58 mW cm−2) | |||
| Reaction time: 12 h | |||
| α-Fe2O3 | Mass catalyst: not specified | Degrade 60% of methylene blue | 96 |
| Figure: woodpile structure (14 mm × 14 mm and 8 mm) | |||
| Concentration: 2 mg L−1 of methylene blue | |||
| Lamp: 50 W (280–450 nm) | |||
| Adsorption time: not specified | |||
| Degradation time: 60 min | |||
| pH: not specified | |||
| g-C3N4/PEGDA | Mass catalyst: not specified | NO removal rate of 52.6% could be used continuously for more than 80 h | 97 |
| Figure: patterned mesh 3D air filter spacing of 0.8 mm and size of 2 × 2 cm2 | |||
| Concentration of reactant: 550 ppb NO (humidity RH 75%) 3.0 L min−1 | |||
| Lamp: LED lamps (420 nm, 20 mW cm−2) | |||
| Adsorption time: not specified | |||
| Degradation time: 60 min | |||
| pH: not specified | |||
| Fe2O3/GO | Mass: 7.2 mg | 97.8% of RhB after 120 min | 106 |
| Figure: Square length (7.5 mm and a thickness of 1.2 mm for 8 layers) | |||
| Concentration of reactant: 0.02 mmol L−1 of RhB | |||
| Lamp: 100 mW cm−2 solar simulator | |||
| Adsorption time: 4 h | |||
| Degradation time: 120 min | |||
| pH: 5.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppmv was around 8%. In the following year, Elkoro et al. used the same binder-free colloidal ink method to fabricate an Au/TiO2 monolith for hydrogen photoproduction.12 The monoliths impregnated with Au after 3D printing (i.e., post-impregnation) exhibited much higher efficiency compared to non- and pre-impregnated monoliths on the rates of hydrogen photoproduction. The best photoactivity was found at 0.24 mol min−1 gAu−1.
000 ppmv was around 8%. In the following year, Elkoro et al. used the same binder-free colloidal ink method to fabricate an Au/TiO2 monolith for hydrogen photoproduction.12 The monoliths impregnated with Au after 3D printing (i.e., post-impregnation) exhibited much higher efficiency compared to non- and pre-impregnated monoliths on the rates of hydrogen photoproduction. The best photoactivity was found at 0.24 mol min−1 gAu−1.
The ink development using environmental benign solvent, such as water, has been also reported. For instance, Mendez-Arriaga et al. developed the solid square-lattice double-diagonal structures (SLDD) using only water as a solvent to prepare the ink and evaluate the photocatalytic activity through the degradation of acesulfame potassium (ACE). The SLDD manufactured TiO2 showed 99% and 79% removal of 20 mg L−1 of ACE in solution within 60 min of UV-vis irradiation by using 0.5 and 22 g L−1 of TiO2 in slurry and SLDD form, respectively.105 Meanwhile, Ávila et al. fabricated the three-dimensional structures from TiO2 nanoparticles using a mixture of volumetric ratios of water and glycol 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and subsequently dried at 400 °C for 6 h.101 The binder-free monoliths were evaluated for the photodegradation of triclosan in liquid phase and light-weight hydrocarbons in gas phase. The 3D printed square-lattice monoliths shown 99.5% of triclosan photodegradation and >90% of total organic carbon mineralization after 240 min under UV-Vis LED light irradiation. Also, it is noteworthy to highlight that this material was used in 12 consecutive cycles without significant deterioration on the photocatalytic activity. On the other hand, in gas phase this material presents an efficiency of photodegradation around 95.3, 93.7 and 52.9% for n-butane, iso-butane and propane; respectively, demonstrating the versatility of these monolithic structures for liquid and gas phases photodegradation of emergent pollutants.
50, and subsequently dried at 400 °C for 6 h.101 The binder-free monoliths were evaluated for the photodegradation of triclosan in liquid phase and light-weight hydrocarbons in gas phase. The 3D printed square-lattice monoliths shown 99.5% of triclosan photodegradation and >90% of total organic carbon mineralization after 240 min under UV-Vis LED light irradiation. Also, it is noteworthy to highlight that this material was used in 12 consecutive cycles without significant deterioration on the photocatalytic activity. On the other hand, in gas phase this material presents an efficiency of photodegradation around 95.3, 93.7 and 52.9% for n-butane, iso-butane and propane; respectively, demonstrating the versatility of these monolithic structures for liquid and gas phases photodegradation of emergent pollutants.
Using 3D DIW printing technique, Xu et al. created a polymeric air filter made of g-C3N4/poly-(ethylene glycol) double acrylate (PEGDA) nanocomposite for nitrogen oxides (NOx) removal.96 The photocatalyst g-C3N4 serves as starting material, while the PEGDA served as the role of both dispersant and matrix to form an optimal ink. The as-printed structure was dried using a simple freeze-drying method. The fabricated air filter demonstrated excellent photocatalytic performance (52.6% of NOx removal) and long durability (up to 40 h).
Noticeably, compared to powder-based materials, thin films, or photocatalytic coating systems, the 3D-printed monolith structures are easy to reuse due to their high structural strength, which is critical for practical use. The design of the photocatalysts may take any shape once the necessary rheological properties of the inks have been achieved. However, the DIW technique is such a broad and virgin field for the development of photocatalyst 3D printed materials and is going to impose as an affordable tool to magnify the outstanding application in the very near future.
3.2 Photo(electro)catalysts
In recent years, TiO2 has been demonstrated to be able to perform photocatalytic nitrogen fixation due to its high photocatalytic activity, excellent chemical stability, easy preparation into versatile morphologies, environmental friendliness, and low cost.107,108 Li et al., demonstrated that the 3D printed hierarchical porous TiO2 structures could be used as an effective photoelectrode for N2 reduction without electric bias.109 The conversion efficiency of N2 to NH3 in the single photoelectrochemical cell was significantly enhanced when the 3D printed TiO2 substrate was modified with surface oxygen vacancies and decorated with gold nanoparticles, which exhibited a surface plasmon resonance effect. Xu et al. then prepared 3D hierarchical porous TiO2 structures (namely nanoparticles, micrometer-particles, and nano/microparticles inks in an aqueous solution of Pluronic F127, Fig. 6a) using powder-based inks for photoelectroreduction of N2.99 The use of powder-based inks offers good printing flexibility because the printing can be performed in an air environment without the need for an additional solidification treatment. The obtained porous TiO2 scaffold structure (Fig. 6b) printed with nano/microparticles inks exhibited the highest N2-to-NH3 conversion efficiency (7.4 nmol h−1); whereas the solid cube exhibits only 5.0 nmol h−1 of NH3 production (Fig. 6c).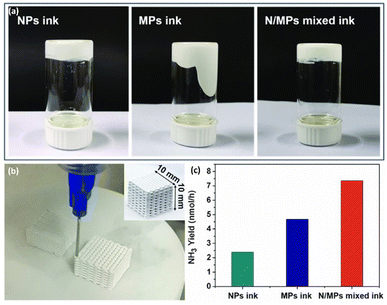 | ||
| Fig. 6 (a) Optical images of the nanoparticles (NP ink), micrometer-particles (MP ink), and nano/microparticles inks (N/MP mixed ink; contains 20 wt% of NPs with respect to MPs) with a TiO2 loading of 50 wt% in an upside-down vial). (b) Optical image of the 3D printing of the TiO2 scaffold. The inset is an optical image of a representative TiO2 cube scaffold with 20 layers and a side length of 10 mm. (c) NH3 production over 6 h obtained from different TiO2 scaffolds printed from different inks under light with an intensity of 100 mW cm−2 (AM1.5) (reproduced from ref. 99 with permission). | ||
3.3 Electrocatalysts
Energy storage in the form of hydrogen (H2) – a carbon free carrier with high energy density, has been proven to be a potential technique for intermittent energy storage. The development of active, durable, low-cost, earth-abundant electrocatalysts is vital to make this technology more viable and widespread.110 Although the catalyst development for H2 has been extensively conducted, the use of DIW technology to create 3D catalytic electrodes for electrochemical application in energy conversion is still at its infancy stage. A recent study demonstrated the use of DIW to fabricate a porous 3D printed graphene sheet/MoS2 aerogel as the cathodic material for H2 evolution reaction.111 The 3D printed porous network, which exhibited 1–2 nm of pore diameter, had shown to be a robust functionalised aerogel that provided multidimensional electron transport channels that improved the electronic conductivity; electrolyte dispersion that facilitated catalyst utilization.Integration of 3D printing and dealloying in the process of making monolithic nanoporous metals has improved the performance of the catalytic processes for energy applications. Monolithic nano-porous metals provide large surface area and high electrical conductivity due to their unique solid/void structures. However, their performance can be improved significantly with an engineered hierarchical macroporous network structure, which results in increased mass transport. Zhu et al. combined direct ink writing with alloying and dealloying processes to create hierarchical nanoporous metal architectures composed of nanoparticle Au with engineered nonrandom macroarchitectures.112 They managed to have both macro (10 to 1000 μm) and nano (30 to 500 nm) scales structures in the materials. Their printed nanoporous metals showed noticeable improvement mass transport and reaction rates for both liquids and gases. This has made the extrusion-based 3D printing DIW applicable to creating hierarchical nano-porous metal architectures.
Meanwhile, Ahn et al. utilised DIW to fabricate 3D graphene pyramids and then deposited Cu conductive layer and NiFe-LDH subsequently using electrochemical deposition method on the printed pyramid for electrochemical water splitting (Fig. 7a).113 The obtained catalysts, which showed homogeneous coating of NiFe-LDH on the pyramids (Fig. 7b), exhibited a comparable electrocatalytic activity when compared to conventional NiFe-LDH samples reported in the literature and excellent durability without significant potential decay for 60 h. The structured photo(electro)- and electrolytic materials printed by DIW are summarised in Table 3.
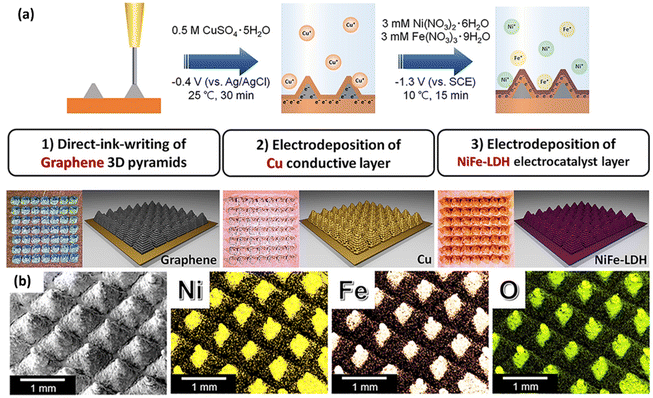 | ||
| Fig. 7 (a) Schematic illustration of the fabrication of 3D-printed pyramid electrodes for OER in three successive steps. (1) Direct-ink-writing of graphene 3D pyramid array, electrodeposition of (2) Cu conductive layer and (3) NiFe-layered double hydroxide (LDH) electrocatalyst layers. (b) Energy dispersive spectrometry (EDS) mapping images of NiFe-LDH pyramid electrode: Ni (yellow), Fe (apricot), and O (green) (reproduced from ref. 113 with permission). | ||
| Material | Fabricated structures | Applications | Ref. |
|---|---|---|---|
| Photo(electro)catalysis | |||
| TiO2 | Hierarchical porous | N2 photo(electro)reduction | 109 |
| Porous scaffolds | N2-to-NH3 photoelectroreduction | 99 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Electrocatalysis | |||
| Graphene sheet/MoS2 aerogel | Porous aerogel | H2 evolution reaction | 111 |
| Au | Nano-porous monolith | Selective oxidation of methanol | 112 |
| 3D graphene deposited with Cu and NiFe-LDH | 3D pyramid array | Electrochemical water splitting | 113 |
The use of DIW to fabricate the electrocatalysts and photo(electro)catalysts have not been extensively explored although many DIW-printed catalytic materials have been proposed and patterned for various applications, including thermochemical processes, chemical conversion and electricity production, as summarised previously.100 As discussed in Section 2.1, there is a high interest of developing DIW for photo-, photo(electro)-, electrocatalytic materials and this is due to its rapid prototyping, concise design and cost effective. However, the printed materials often showed inferior performance than the pristine powdered materials. Hence, the development of new hybrid formulations, in which printable catalysts or precursors that can be transformed into catalysts in successive stages, will be the key to resolve this bottleneck, preserving their catalytic performance.100
4. Reactor design and fabrication
AM technology is also called rapid prototyping or freeform fabrication to ‘print’ 3D from 3D digital models instead of cutting materials from an initial bulk.114,115 Due to its potential to offer reduced fabrication cost and time-efficient parts with complex 3D structure, AM has received significant attention to fabricate reactors for various energy conversion and environmental remediation applications.99 Their general features and significance in designing a reactor for a specialised, well-defined application include:116• Moderate capital and running costs, require low-cost components, a low cell potential difference (i.e., particularly for electrochemical cells) and a low-pressure drop over the entire cell including the inlet and outlet flow manifolds for the reaction solution (i.e., electrolyte for the case of an electrochemical cell); where possible, an undivided reactor that will simplify the engineering design and will lower the capital and running costs.
• Convenience and reliability, adequate design, installation, operation and maintenance and monitoring procedures.
• Appropriate engineering equipment for controlling and monitoring concentration, potential, current density (i.e., particularly for electrochemical cell) and adequate mass transport regime to provide and remove reactants and products respectively, via flow distributions.
• Simplicity and versatility are perhaps the least quantified and most overlooked, yet the most important, factors for achieving an elegant and long-lasting design to attract users. Provision for the future by designing a modular configuration that can be scale-up by adding unit cells or by increasing the size of each unit.
Microchannel reactors provide for the catalyst component and fine control over reactant flow.34 They have high surface to volume ratios, superior mass and heat transfer rates to conventional packed bed reactors, good laminar flow, small molecular diffusion distances, more homogeneous spatial illumination (i.e., in the case of photocatalysis and photo(electro)catalysis), and good reactant penetration throughout the catalyst bed.117,118 A few 3D printing approaches have been used to fabricate microchannel reactors, as outlined by Friedmann et al.44 Meanwhile, Yusuf et al. have reviewed the graphene-based materials in microfluidic photoreactors, which are potential efficient visible light photocatalysts. Zhakeyev et al. summarised the development of solar cells and photoreactors.17 The development of DIW-printed reactor has also been reported since last decade. For example, Potdar et al. designed porous milli-scale reactors for optical measurements and chemical system (Fig. 8). The porous reactor showed an enhanced mass transfer performance with an order of magnitude reduced energy dissipation compared to conventional milli-scale packed bed reactors.119
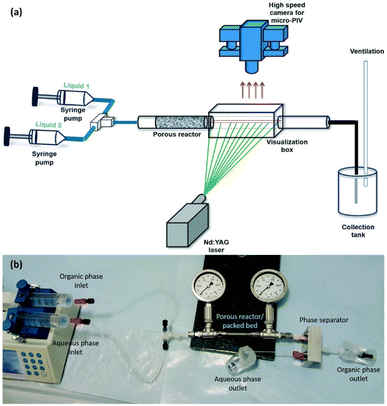 | ||
| Fig. 8 (a) Schematic representative of experimental setup of optical measurements and (b) photograph of experimental setup of chemical mass transfer experiments (reprinted with permission from ref. 119). | ||
5. Perspective
A range of new DIW applications have emerged over recent years, and it is envisioned that 3D printing will continue to be utilized in manufacturing tandem to traditional fabrication approaches.22 While 3D printing may offer an effective solution in the design of catalyst and rectors for performance testing, it is immensely beneficial for proof-of-concept and fundamental studies. Although such design may introduce further barriers to up-scaling and widespread adoption of the technology, the ability of DIW for rapid prototyping of the catalyst as well as microreactor provides a great research tool for industrial deployment especially in the fast emerging energy and environmental industries (Fig. 9). Once optimal designs are achieved, the technology be translated to processes, which are suitable for large-scale manufacturing. Typically, these processes require significant cost to set up (capital expense), but the operational expense of producing each unit is generally low. While today's additive fabrication technologies tend to have high operational expenses per unit, this is dropping and, depending upon the product, it is envisioned that manufacture will involve a combination of traditional and additive manufacturing technologies, while wholly printed structures will remain economically unviable for mass production in the short to medium term.22In theory, DIW process can be introduced to most of the materials, as long as these materials can be extruded or sintered. The active component distribution can also be highly designed through modelling and customized using DIW. Different catalysts can be distributed in different regions of the catalyst structures to complete complex reactions. This greatly enriched the traditional catalytic materials. For example, during some exothermic reactions (e.g., CO2 hydrogenation to methanol), heat accumulation may happen in certain region of the catalyst bed, which will reduce the yield of the objective product (e.g., methanol) in these reaction sites. Additionally, the 3D printing process achieves the preparation of fine structures with a relatively low cost. The structures can be customized and strictly controlled, and the complex manufacturing processes needed for conventional preparation methods are avoided. The prices of 3D printers and printing feedstocks are dropping rapidly in recent years. The cost of printed catalyst has the potential of being reduced to a reasonable range, which is crucial for industrial applications. At the same time, target structures can be carefully designed to fit the reaction system, such as monoliths, and an accurate rendering of the design can then be easily achieved. This makes the 3D printing process suitable for industrial use. Another potential use for DIW is in the preparation of microreactors. With 3D printing, the boundary between a catalyst, its support and reactor body are no longer very clear. The printed structure not only provides the catalyst component, but also controls the flow of the reactants. This may bring a significant enhancement to catalytic systems. Furthermore, the control of the microfluidic properties of the reactants helps to achieve good reaction performance with low cost. In addition, DIW, as a flexible approach, is also suitable for catalyst development. The printing process is applicable to small batch production and laboratory operations, which provides a great research tool for rapid prototyping, allowing to investigate and to optimize all kind of structures with a variety of porous materials. Accurate control of the structure and distribution of catalyst components is expected to bring a big improvement to the catalytic industry.
6. Summary
With the depletion of fossil fuel resources and increasing environmental concerns, advanced catalysts are playing more important roles in many areas. The market for catalysts with novel structures will continue to grow in the future. As a bottom-up method, 3D printing fabrication provides new solutions for preparing catalysts with new multi-scales structures in a more economical and energy-efficient way.34 As a new rapid fabrication method, DIW brings many new possibilities to the field of catalysis. Structural design, control of catalyst distribution, and the fabrication of monolithic reactors are becoming easier. However, further optimisation on the catalyst design and processing parameters are still needed to achieve required mechanical properties and even better catalytic performances for energy conversion and environmental remediation applications. Hence, insight on process simulation as well as artificial intelligent for DIW could probably aid the development of DIW with superior structure design and catalytic performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors/we would like to acknowledge that this work was supported by the UKRI ISCF Industrial Challenge within the UK Industrial Decarbonisation Research and Innovation Centre (IDRIC) award number: EP/V027050/1. Tania E. Lara-Ceniceros, José Bonilla-Cruz and Manuel Alejandro Ávila López would like to thank Centro de Investigación en Materiales Avanzados S.C. (CIMAV), for funding the Internal Project No. 23005 that supported this research. Manuel Alejandro Ávila López wants to thank CONACYT for the Postdoctoral scholarship, CVU 707526.References
- Summary for Policymakers of IPCC Special Report on Global Warming of 1.5°C approved by governments, https://www.ipcc.ch/2018/10/08/summary-for-policymakers-of-ipcc-special-report-on-global-warming-of-1-5c-approved-by-governments/#:%7E:text=Globalnethuman%2Dcausedemissions,removingCO2fromtheair.
- AF2792-12a, Standard Terminology for Additive Manufacturing Technologies (Withdrawn 2015), ASTM International, West Conshohocken, PA, 2012, DOI: DOI:10.1520/F2792-12A.
- S. A. M. Tofail, E. P. Koumoulos, A. Bandyopadhyay, S. Bose, L. O'Donoghue and C. Charitidis, Mater. Today, 2018, 22–37 CrossRef.
- Q. Yan, J. Li, J. Zhang and Z. Cai, Polymers, 2018, 10, 729 CrossRef PubMed.
- A. Ambrosi and M. Pumera, Chem. Soc. Rev., 2016, 45, 2740–2755 RSC.
- X. Tian, J. Jin, S. Yuan, C. K. Chua, S. B. Tor and K. Zhou, Adv. Energy Mater., 2017, 7, 1700127 CrossRef.
- C. Zhu, T. Liu, F. Qian, W. Chen, S. Chandrasekaran, B. Yao, Y. Song, E. B. Duoss, J. D. Kuntz, C. M. Spadaccini, M. A. Worsley and Y. Li, Nano Today, 2017, 15, 107–120 CrossRef CAS.
- F. Zhang, M. Wei, V. V. Viswanathan, B. Swart, Y. Shao, G. Wu and C. Zhou, Nano Energy, 2017, 40, 418–431 CrossRef CAS.
- T. D. Ngo, A. Kashani, G. Imbalzano, K. T. Q. Nguyen and D. Hui, Composites, Part B, 2018, 143, 172–196 CrossRef CAS.
- P. Chang, H. Mei, S. Zhou, K. G. Dassios and L. Cheng, J. Mater. Chem. A, 2019, 7, 4230–4258 RSC.
- Q. Zhang, J. Zhou, Z. Chen, C. Xu, W. Tang, G. Yang, C. Lai, Q. Xu, J. Yang and C. Peng, Adv. Eng. Mater., 2021, 23, 2100068 CrossRef CAS.
- A. Elkoro, L. Soler, J. Llorca and I. Casanova, Appl. Mater. Today, 2019, 16, 265–272 CrossRef.
- C.-Y. Lee, A. C. Taylor, S. Beirne and G. G. Wallace, Adv. Energy Mater., 2017, 7, 1701060 CrossRef.
- D. R. Rolison, J. W. Long, J. C. Lytle, A. E. Fischer, C. P. Rhodes, T. M. McEvoy, M. E. Bourg and A. M. Lubers, Chem. Soc. Rev., 2009, 38, 226–252 RSC.
- D. Christensen, J. Nijenhuis, J. R. van Ommen and M. O. Coppens, Ind. Eng. Chem. Res., 2008, 47, 3601–3618 CrossRef CAS.
- M.-O. Coppens, Curr. Opin. Chem. Eng., 2012, 1, 281–289 CrossRef CAS.
- A. Zhakeyev, P. Wang, L. Zhang, W. Shu, H. Wang and J. Xuan, Adv. Sci., 2017, 4, 1700187 CrossRef PubMed.
- M. Bernardi, N. Ferralis, J. H. Wan, R. Villalon and J. C. Grossman, Energy Environ. Sci., 2012, 5, 6880–6884 RSC.
- A. Polman and H. A. Atwater, Nat. Mater., 2012, 11, 174–177 CrossRef CAS PubMed.
- J. H. Atwater, P. Spinelli, E. Kosten, J. Parsons, C. V. Lare, J. V. d. Groep, J. G. d. Abajo, A. Polman and H. A. Atwater, Appl. Phys. Lett., 2011, 99, 151113 CrossRef.
- M. Gu, H. Lin and X. Li, Opt. Lett., 2013, 38, 3627–3630 CrossRef PubMed.
- C.-Y. Lee, A. C. Taylor, A. Nattestad, S. Beirne and G. G. Wallace, Joule, 2019, 3, 1835–1849 CrossRef CAS.
- M. Gebler, A. J. M. Schoot Uiterkamp and C. Visser, Energy Policy, 2014, 74, 158–167 CrossRef CAS.
- S. C. Joshi and A. A. Sheikh, Virtual Phys. Prototyping, 2015, 10, 175–185 CrossRef.
- M. Smith, Army Sustainment, 2014, 12–17 CAS.
- P. Wu, J. Wang and X. Wang, Autom. Constr., 2016, 68, 21–31 CrossRef.
- N. Tovar, L. Witek, P. Atria, M. Sobieraj, M. Bowers, C. D. Lopez, B. N. Cronstein and P. G. Coelho, J. Tissue Eng. Regener. Med., 2018, 12, 1986–1999 CrossRef CAS PubMed.
- Q. Yan, H. Dong, J. Su, J. Han, B. Song, Q. Wei and Y. Shi, Engineering, 2018, 4, 729–742 CrossRef CAS.
- C. Barnatt, 3D Printing, CreateSpace Independent Publishing Platform, 3rd edn, 2016 Search PubMed.
- I. Gibson, D. W. Rosen, B. Stucker and M. Khorasani, Additive Manufacturing Technologies, Springer, 2021 Search PubMed.
- B. C. Gross, J. L. Erkal, S. Y. Lockwood, C. Chen and D. M. Spence, Anal. Chem., 2014, 86, 3240–3253 CrossRef CAS PubMed.
- A. Waldbaur, H. Rapp, K. Länge and B. E. Rapp, Anal. Methods, 2011, 3, 2681–2716 RSC.
- J. Kim, R. Kumar, A. J. Bandodkar and J. Wang, Adv. Electron. Mater., 2017, 3, 1600260 CrossRef.
- X. Zhou and C.-j. Liu, Adv. Funct. Mater., 2017, 27, 1701134 CrossRef.
- M. A. S. R. Saadi, A. Maguire, N. T. Pottackal, M. S. H. Thakur, M. M. Ikram, A. J. Hart, P. M. Ajayan and M. M. Rahman, Adv. Mater., 2022, 34, 2108855 CrossRef CAS PubMed.
- L. Hao, D. Tang, T. Sun, W. Xiong, Z. Feng, K. E. Evans and Y. Li, Int. J. Precis. Eng. Manuf. – Green Technol., 2021, 8, 665–685 CrossRef.
- R. D. Farahani, M. Dubé and D. Therriault, Adv. Mater., 2016, 28, 5794–5821 CrossRef CAS PubMed.
- A. Shen, D. Caldwell, A. W. K. Ma and S. Dardona, Addit. Manuf., 2018, 22, 343–350 CAS.
- K. Fu, Y. Wang, C. Yan, Y. Yao, Y. Chen, J. Dai, S. Lacey, Y. Wang, J. Wan, T. Li, Z. Wang, Y. Xu and L. Hu, Adv. Mater., 2016, 28, 2587–2594 CrossRef CAS PubMed.
- Q. Huang and Y. Zhu, Adv. Mater. Technol., 2019, 4, 1800546 CrossRef.
- B.-J. de Gans, P. C. Duineveld and U. S. Schubert, Adv. Mater., 2004, 16, 203–213 CrossRef CAS.
- K.-S. Kwon, M. K. Rahman, T. H. Phung, S. Hoath, S. Jeong and J. S. Kim, Flexible Printed Electron., 2020, 5, 043003 CAS.
- J. Chunjiang and C. Guangxue, J. Part. Sci. Technol., 2013, 50, 160–170 Search PubMed.
- D. Friedmann, A. F. Lee, K. Wilson, R. Jalili and R. A. Caruso, J. Mater. Chem. A, 2019, 7, 10858–10878 RSC.
- P. Yang and H. J. Fan, Adv. Mater. Technol., 2020, 5, 2000217 CrossRef CAS.
- B. Nan, F. J. Galindo-Rosales and J. M. F. Ferreira, Mater. Today, 2020, 35, 16–24 CrossRef CAS.
- S. Yu, M. Xia, J. Sanjayan, L. Yang, J. Xiao and H. Du, J. Build. Eng., 2021, 44, 102948 CrossRef.
- Z. Chen, Z. Li, J. Li, C. Liu, C. Lao, Y. Fu, C. Liu, Y. Li, P. Wang and Y. He, J. Eur. Ceram. Soc., 2019, 39, 661–687 CrossRef CAS.
- J. Cesarano, R. Segalman and P. Calvert, Ceram. Ind., 1998, 148, 94–102 Search PubMed.
- C. Zhu, T. Liu, F. Qian, T. Y.-J. Han, E. B. Duoss, J. D. Kuntz, C. M. Spadaccini, M. A. Worsley and Y. Li, Nano Lett., 2016, 16, 3448–3456 CrossRef CAS PubMed.
- B. A. E. Ben-Arfa, A. S. Neto, I. M. Miranda Salvado, R. C. Pullar and J. M. F. Ferreira, J. Am. Ceram. Soc., 2019, 102, 1608–1618 CAS.
- A. Shahzad and I. Lazoglu, Composites, Part B, 2021, 225, 109249 CrossRef CAS.
- M. N. Rahaman and W. Xiao, Adv. Ceram. Compos., 2017, 38, 235 CAS.
- T. Schlordt, S. Schwanke, F. Keppner, T. Fey, N. Travitzky and P. Greil, J. Eur. Ceram. Soc., 2013, 33, 3243–3248 CrossRef CAS.
- J. E. Smay, G. M. Gratson, R. F. Shepherd, J. Cesarano III and J. A. Lewis, Adv. Mater., 2002, 14, 1279–1283 CrossRef CAS.
- E. Peng, D. Zhang and J. Ding, Adv. Mater., 2018, 30, 1802404 CrossRef PubMed.
- L. del-Mazo-Barbara and M.-P. Ginebra, J. Eur. Ceram. Soc., 2021, 41, 18–33 CrossRef CAS.
- Y.-Y. Li, L.-T. Li and B. Li, Mod. Phys. Lett. B, 2016, 30, 1650212 CrossRef CAS.
- L. Yang, X. Zeng and Y. Zhang, Mater. Lett., 2019, 255, 126564 CrossRef CAS.
- S. Lamnini, H. Elsayed, Y. Lakhdar, F. Baino, F. Smeacetto and E. Bernardo, Heliyon, 2022, 8, e10651 CrossRef CAS PubMed.
- B. A. Tuttle, J. E. Smay, J. Cesarano, J. A. Voigt, T. W. Scofield, W. R. Olson and J. A. Lewis, J. Am. Ceram. Soc., 2001, 84, 872–874 CrossRef CAS.
- M. R. Hartings and Z. Ahmed, Nat. Rev. Chem., 2019, 3, 305–314 CrossRef.
- M. Serdeczny, R. Comminal, D. Pedersen and J. Spangenberg, Presented in Part at the Euspen's 18th International Conference & Exhibition, 2018 Search PubMed.
- A. Das, C. McIlroy and M. J. Bortner, Prog. Addit. Manuf., 2021, 6, 3–17 CrossRef.
- C.-F. Guo, M. Zhang and B. Bhandari, Computers and Electronics in Agriculture, 2019, 162, 397–404 CrossRef.
- M. P. Serdeczny, R. Comminal, D. B. Pedersen and J. Spangenberg, Addit. Manuf., 2018, 24, 145–153 Search PubMed.
- R. Comminal, M. P. Serdeczny, D. B. Pedersen and J. Spangenberg, Addit. Manuf., 2018, 20, 68–76 Search PubMed.
- H. Narei, M. Fatehifar, A. H. Malt, J. Bissell, M. Souri, M. Nasr Esfahani and M. Jabbari, Polymers, 2021, 13, 476 CrossRef CAS PubMed.
- Q. Liu, N. Zhang, W. Wei, X.-j. Hu, Y. Tan, Y. Yu, Y.-J. Deng, C. Bi, L. Zhang and H. Zhang, J. Food Eng., 2020, 275, 109861 CrossRef CAS.
- K. Song, D. Zhang, J. Yin and Y. Huang, Addit. Manuf., 2021, 41, 101963 CAS.
- B. Behdani, M. Senter, L. Mason, M. Leu and J. Park, J. Manuf. Mater. Process., 2020, 4, 46 CAS.
- E. A. Papon, A. Haque and M. A. R. Sharif, Rapid Prototyp. J., 2021, 27(3), 518–529 CrossRef.
- M. M. Cross, Rheol. Acta, 1979, 18, 609–614 CrossRef CAS.
- V. A. Hackley and C. F. Ferraris, Guide to rheological nomenclature: measurement in ceramic particulate systems, Special Publication (NIST SP), 2001 Search PubMed.
- A. Gosset, D. Barreiro-Villaverde, J. C. Becerra Permuy, M. Lema, A. Ares-Pernas and M. J. Abad López, Polymers, 2020, 12, 2885 CrossRef CAS PubMed.
- F. Nikfarjam, Y. Cheny and O. Botella, Comput. Phys. Commun., 2018, 226, 67–80 CrossRef CAS.
- P. Antonietti, N. Fadel and M. Verani, Commun. Appl. Ind. Math., 2010, 1, 1 CrossRef.
- R. B. Bird, R. C. Armstrong and O. Hassager, Dynamics of Polymeric Liquids. Vol. 1: Fluid Mechanics, 1987 Search PubMed.
- G. Yong, Continuum Mechanics - Progress in Fundamentals and Engineering Applications, IntechOpen, London, 2012 Search PubMed.
- C. Guo, M. Zhang and S. Devahastin, J. Food Eng., 2020, 286, 110113 CrossRef CAS.
- A. Ni, M. N. Prabhakar, I.-H. Suk, J.-K. Park and J.-I. Song, Mech. Based Des. Struct. Mach., 2021, 1–17, DOI:10.1080/15397734.2021.1919525.
- O. Yilmaz, E. Kısasöz, F. Seniha Guner, C. Nart and K. Kirkkopru, Fibers Polym., 2014, 15, 84–90 CrossRef CAS.
- V. Hristov and J. Vlachopoulos, Adv. Polym. Technol., 2007, 26, 100–108 CrossRef CAS.
- N. D. Gonçalves, O. S. Carneiro and J. M. Nóbrega, J. Non-Newtonian Fluid Mech., 2013, 200, 103–110 CrossRef.
- T. J. Coogan and D. O. Kazmer, Rapid Prototyp. J., 2017, 23, 414–422 CrossRef.
- B. G. Compton, B. K. Post, C. E. Duty, L. Love and V. Kunc, Addit. Manuf., 2017, 17, 77–86 CAS.
- T. A. Osswald, J. Puentes and J. Kattinger, Addit. Manuf., 2018, 22, 51–59 CAS.
- P. Wang, B. Zou, S. Ding, L. Li and C. Huang, Chin. J. Aeronaut., 2021, 34, 236–246 CrossRef.
- C. Luo, X. Wang, K. B. Migler and J. E. Seppala, Addit. Manuf., 2020, 32, 101019 CAS.
- K. Coasey, K. R. Hart, E. Wetzel, D. Edwards and M. E. Mackay, Addit. Manuf., 2020, 33, 101140 CAS.
- D. A. Edwards and M. E. Mackay, J. Heat Transfer, 2020, 142, 052101 CrossRef.
- A. D'Amico and A. M. Peterson, Addit. Manuf., 2018, 21, 422–430 Search PubMed.
- D. D. Phan, Z. R. Swain and M. E. Mackay, J. Rheol., 2018, 62, 1097–1107 CrossRef CAS.
- H. Lu, J. Tournet, K. Dastafkan, Y. Liu, Y. H. Ng, S. K. Karuturi, C. Zhao and Z. Yin, Chem. Rev., 2021, 121, 10271–10366 CrossRef CAS PubMed.
- J. A. Lewis, Adv. Funct. Mater., 2006, 16, 2193–2204 CrossRef CAS.
- Y. Li, B. Li and L. Li, Mod. Phys. Lett. B, 2014, 28, 1450051 CrossRef.
- X. Xu, S. Xiao, H. J. Willy, T. Xiong, R. Borayek, W. Chen, D. Zhang and J. Ding, Appl. Catal., B, 2020, 262, 118307 CrossRef CAS.
- A. Elkoro and I. Casanova, 3D Printing and Additive Manufacturing, 2018, 5, 220–226 CrossRef.
- C. Xu, T. Liu, W. Guo, Y. Sun, C. Liang, K. Cao, T. Guan, Z. Liang and L. Jiang, Adv. Eng. Mater., 2020, 22, 1901088 CrossRef CAS.
- O. H. Laguna, P. F. Lietor, F. J. I. Godino and F. A. Corpas-Iglesias, Mater. Des., 2021, 208, 109927 CrossRef CAS.
- M. A. Ávila-López, T. E. Lara-Ceniceros, F. E. Longoria, A. A. Elguezabal, A. Martínez de la Cruz, M. A. Garza-Navarro and J. Bonilla-Cruz, ACS Appl. Nano Mater., 2022, 5, 11437–11446 CrossRef.
- E. B. Duoss, M. Twardowski and J. A. Lewis, Adv. Mater., 2007, 19, 3485–3489 CrossRef CAS.
- J. Bonilla-Cruz, M. A. Ávila-López, F. E. L. Rodríguez, A. Aguilar-Elguezabal and T. E. Lara-Ceniceros, J. Eur. Ceram. Soc., 2022, 42, 6033–6039 CrossRef CAS.
- A. Zaleska, Recent Pat. Eng., 2008, 2, 157–164 CrossRef CAS.
- F. Mendez-Arriaga, E. d. l. Calleja, L. Ruiz-Huerta, A. Caballero-Ruiz and R. Almanza, Mater. Sci. Semicond. Process., 2019, 100, 35–41 CrossRef CAS.
- Y. Wang, Y. Zhang, S. Wang, Y. Guan and Y. Zhang, Mater. Today Chem., 2021, 22, 100566 CrossRef CAS.
- Y. Zhao, Y. Zhao, R. Shi, B. Wang, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Mater., 2019, 31, 1806482 CrossRef PubMed.
- B. M. Comer and A. J. Medford, ACS Sustainable Chem. Eng., 2018, 6, 4648–4660 CrossRef CAS.
- C. Li, T. Wang, Z.-J. Zhao, W. Yang, J.-F. Li, A. Li, Z. Yang, G. A. Ozin and J. Gong, Angew. Chem., Int. Ed., 2018, 57, 5278–5282 CrossRef CAS PubMed.
- S. Lu, Y. Wang, H. Xiang, H. Lei, B. B. Xu, L. Xing, E. H. Yu and T. X. Liu, J. Energy Storage, 2022, 52, 104764 CrossRef.
- S. Chandrasekaran, J. Feaster, J. Ynzunza, F. Li, X. Wang, A. J. Nelson and M. A. Worsley, ACS Mater. Au, 2022, 2, 596–601 CrossRef CAS.
- C. Zhu, Z. Qi, V. A. Beck, M. Luneau, J. Lattimer, W. Chen, M. A. Worsley, J. Ye, E. B. Duoss, C. M. Spadaccini, C. M. Friend and J. Biener, Sci. Adv., 2018, 4, eaas9459 CrossRef CAS PubMed.
- J. Ahn, Y. S. Park, S. Lee, J. Yang, J. Pyo, J. Lee, G. H. Kim, S. M. Choi and S. K. Seol, Sci. Rep., 2022, 12, 346 CrossRef CAS PubMed.
- S. M. Thompson, L. Bian, N. Shamsaei and A. Yadollahi, Addit. Manuf., 2015, 8, 36–62 Search PubMed.
- W. E. Frazier, J. Mater. Eng. Perform., 2014, 23, 1917–1928 CrossRef CAS.
- F. C. Walsh, Pure Appl. Chem., 2001, 73, 1819–1837 CrossRef CAS.
- M. Krivec, K. Žagar, L. Suhadolnik, M. Čeh and G. Dražić, ACS Appl. Mater. Interfaces, 2013, 5, 9088–9094 CrossRef CAS PubMed.
- A. Yusuf, C. Garlisi and G. Palmisano, Catal. Today, 2018, 315, 79–92 CrossRef CAS.
- A. Potdar, L. N. Protasova, L. Thomassen and S. Kuhn, React. Chem. Eng., 2017, 2, 137–148 RSC.
Footnote |
| † Current address: Department of Chemical and Biological Engineering, University of Sheffield, Mappin Street, Sheffield, S1 3JD, United Kingdom. |
| This journal is © The Royal Society of Chemistry 2023 |