pH-Responsive wound dressings: advances and prospects
Zeyu
Han†
ab,
Mujie
Yuan†
ab,
Lubin
Liu
b,
Kaiyue
Zhang
ab,
Baodong
Zhao
ab,
Bin
He
 c,
Yan
Liang
c,
Yan
Liang
 *d and
Fan
Li
*d and
Fan
Li
 *ab
*ab
aDepartment of Oral Implantology, The Affiliated Hospital of Qingdao University, Qingdao 266000, China. E-mail: lifan911017@qdu.edu.cn
bSchool of Stomatology, Qingdao University, Qingdao 266000, China
cNational Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China
dDepartment of Pharmaceutics, School of Pharmacy, Qingdao University, Qingdao 266000, China. E-mail: liangyan072@foxmail.com
First published on 28th February 2023
Abstract
Wound healing is a complex and dynamic process, in which the pH value plays an important role in reflecting the wound status. Wound dressings are materials that are able to accelerate the healing process. Among the multifunctional advanced wound dressings developed in recent years, pH-responsive wound dressings, especially hydrogels, show great potential owing to their unique properties of adjusting their functions according to the wound conditions, thereby allowing the wound to heal in a regulated manner. However, a comprehensive review of pH-responsive wound dressings is lacking. This review summarizes the design strategies and advanced functions of pH-responsive hydrogel wound dressings, including their excellent antibacterial properties and significant pro-healing abilities. Other advanced pH-responsive materials, such as nanofibers, composite films, nanoparticle clusters, and microneedles, are also classified and discussed. Next, the pH-monitoring functions of pH-responsive wound dressings and the related pH indicators are summarized in detail. Finally, the achievements, challenges, and future development trends of pH-responsive wound dressings are discussed.
1. Introduction
The skin is a human organ that is in direct contact with the external environment. Its functions of temperature regulation and the sensing of external stimuli are significant for protecting the body from external damage.1–3 Skin injuries are one of the most common types of injuries in the human body, causing pain and discomfort, and even death.4–6 After injury, wound healing is a dynamic and complex process that is usually divided into four phases: hemostasis, inflammation, proliferation, and remodeling (Fig. 1).7–9 The healing process is disturbed by adverse effects occurring at any stage, such as infections, inflammation, diabetes, pH, and temperature10–13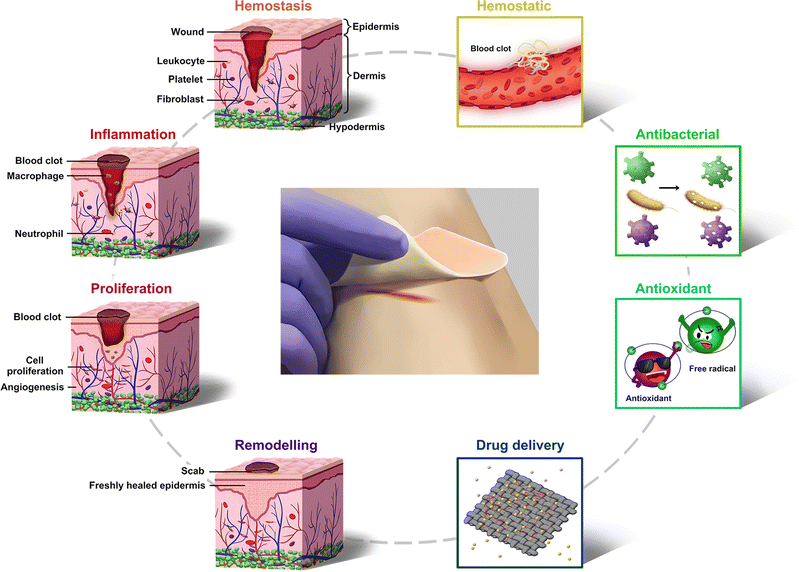 | ||
| Fig. 1 Schematic of the four stages of wound healing and the main advanced functions of pH-responsive wound dressings. | ||
Wound dressings act as barriers to cover wounds and reduce the risk of infection.14 In recent years, hydrogels,15 electrospun films,16 sponges,17 and other novel wound dressings have been developed. Compared with traditional wound dressings, they have more advanced functions to promote wound healing, such as inhibiting bacterial growth, maintaining moisture retention, absorbing exudate, and releasing drugs.18,19 However, several new ideas in wound dressings are to be explored further. The process of wound healing is dynamic; therefore, the function of wound dressings is to be regulated according to wound status.20 Furthermore, progress should be made in the fields of wound-status clarification and warning of infection risks.21
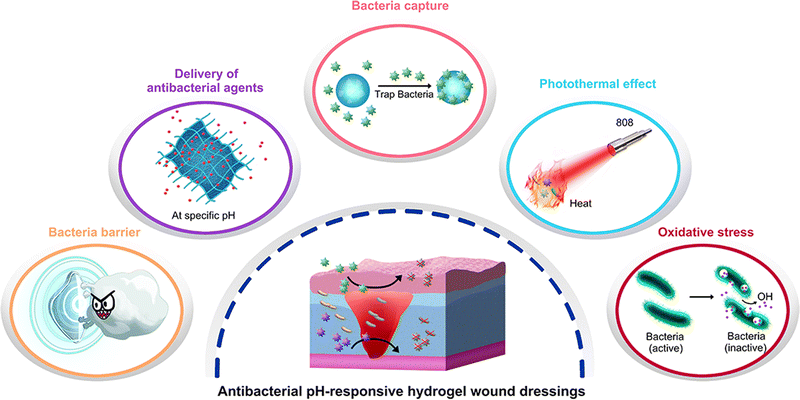
pH-Responsive wound dressings provide solutions to the aforementioned issues since the pH of the skin is closely related to the wound healing process. The pH of healthy skin is between 4 and 6 due to the products of fatty acids and amino acids secreted by keratinocytes, which provides a slightly acidic environment to inhibit bacterial proliferation.22 Generally, wounds are classified as acute wounds (wounds that heal in an orderly and timely manner and result in sustained restoration of anatomic and functional integrity) and chronic wounds (wounds that fail to heal through an orderly and timely process to achieve anatomic and functional integrity). When acute wounds occur, internal tissue and interstitial fluid with a pH of approximately 7.4 was exposed, changing the acidic milieu of normal skin, and the pH will return to acidic as wound healing. Chronic wounds have a pH between 7 and 9 because of the existence of blood, interstitial fluid, ammonia, etc.23 In addition, when bacterial infection occurs in a wound, the pH of the wound will decrease due to the acidic substances such as lactic acid and carbonic acid generated by bacterial growth.24 Therefore, pH is considered a reliable indicator of the wound status, and the variation in pH predicts a tendency for wound healing/deterioration.25 It is to be noted that pH-responsive wound dressings have unparalleled advantages in wound care because of their excellent biochemical and mechanical properties and their ability to tune their morphology,26 volume,27 and drug release behavior.28 Such materials are able to monitor the wound status based on the pH of the wound, heal the wound in a controlled way, reduce the infection risks, and shorten the healing time. However, a systematic review of the pH-responsive wound dressings still has not been reported.
In this article, a comprehensive overview of pH-responsive wound dressings, from the most common and widely used hydrogel to other wound dressings is presented, showing their characteristics and advantages for wound healing (Fig. 1). The article begins by providing the design strategies and advanced functions of pH-responsive hydrogel wound dressings. Other types of pH-responsive wound dressings are then classified and discussed, including nanofibers, composite films, nanoparticle clusters, microneedles, etc. A detailed summary of the pH-monitoring functions of pH-responsive wound dressings and related pH indicators follows. Finally, the review is concluded with a discussion on the challenges and future development trends of pH-responsive wound dressings.
2. pH-Responsive hydrogel wound dressings
2.1. Design strategies for pH-responsive hydrogel wound dressings
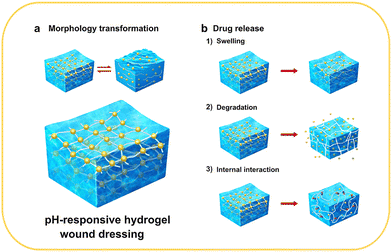
pH-Responsive hydrogel wound dressings are able to receive changes in the pH of the local environment of the wound and adjust their functions to meet the needs of wound healing.20 In this section, we briefly summarize the design strategies for pH-responsive hydrogel wound dressings, which are mainly classified into pH-responsive morphology, size, degradation, and drug release behavior (Fig. 2).pH-Responsive morphology implies that under specific conditions, the morphological properties of the dressings are functional or nonfunctional (Fig. 2(a)). When replacing wound dressings, the adhesion between the dressing and wound often causes pain and even secondary injury to the wound.14 The liquid-to-solid reversible morphological conversion of hydrogels has a unique effect in solving this problem, and this type of response is usually designed through imine bonds, Schiff-base bonds, and catechol–Fe coordinate bonds.26,29–32 For instance, Liang et al. developed hydrogels with excellent pro-healing and on-demand dissolution or removal properties via dual-dynamic-bond cross-linking of catechol–Fe coordinate bond and dynamic Schiff base bonds. After the treatment, the adhesive force of the dressing can be significantly reduced with the intervention of acid solution or deferoxamine mesylate solution, thereby easily removing the dressing and reducing patient suffering and the risk of unnecessary injury.32
Changes in the pH-responsive size mainly refer to the swelling behavior of the hydrogels. The swelling ratios of hydrogels vary significantly with pH, causing a change in the pore size inside the materials that perform pH-responsive drug delivery or signal transmission (Fig. 2(b1)). The different swelling behaviors are generally attributed to protonation, deprotonation, and charge repulsion of the functional groups in the material components.33–35 For example, pH-sensitive composite hydrogels prepared by Al-Arjan et al. showed a high curcumin release at pH 7.4. At this pH, the hydrophilic groups on bacterial cellulose (BC), polyvinyl alcohol (PVA), and graphene oxide (GO) caused swelling owing to hydrogen bonds and electrostatic repulsion forces.36
pH-Responsive degradation is an effective strategy for controlling drug release (Fig. 2(b2)). Ideally, when bacteria proliferate, pH-responsive degradation occurs, and then the drug is released to kill the bacteria. When the pH of the wound area returns to normal, the degradation process is terminated. The strength of the three-dimensional network of the hydrogels is weakened with an increase in the degradation rate, leading to larger internal pore size. For example, methylcellulose-based hydrogel prepared by Bonetti was crosslinked by ester bonds, and ester bonds would hydrolyze in an alkaline environment, which leads to hydrogel network expansion and drug release.37 In addition, the degradation of the loaded nanoparticles contributes to the pH-responsive behavior. The hydrogel–microgel composite prepared by Du et al. displayed elevated drug release owing to the higher degradation rate of the hydrogel networks.38 This method has also been reported in other studies on pH-responsive hydrogel wound dressings.39–41
pH-Responsive drug release is the ultimate goal of most pH-responsive hydrogel wound dressings. In addition to the aforementioned swelling and degradation behaviors, the pH-responsiveness is also influenced by other factors, such as the type of medium, concentration of ions in the medium, and interactions between drugs and the other components (Fig. 2(b3)).34,42,43 Therefore, the influence of various factors should be carefully considered when designing pH-responsive and controlled drug release systems. Furthermore, the sensitivity of pH-responsiveness must be examined in conditions that simulate the wound environment to ensure its applicability. A summary of the types, applications, synthetic methods, pH response mechanisms, and other relevant information about pH-responsive hydrogel wound dressings is presented in Table 1.
| Application | Composition | Synthetic methods | pH range | pH-Responsive mechanism | Ref. |
|---|---|---|---|---|---|
| Delivery of antibiotic: tetracycline hydrochloride (TH) | PVA, glutaraldehyde, glycerol | Chemical crosslinking | Higher release at basic conditions | Ionization of the hydroxyl and carboxyl groups | 44 |
| Delivery of antibacterial agent: triclosan | Peptide-based bis-acrylate, acrylic acid | Radical polymerization | Higher swelling ratios at basic conditions | Hydrogen bonds break in neutral or basic conditions | 45 |
| Delivery of antibiotic: gentamycin sulphate | Chitosan (CS), PVP, glycerol, PNIPAm | Chemical crosslinking | Higher swelling ratios at acidic conditions | Deprotonation of the –NH2 group and –NH group | 27 |
| Delivery of antibiotic: silver-sulphadiazine | Arabinoxylan, CS, reduced graphene oxide, tetraethyl orthosilicate | Chemical crosslinking | Higher swelling ratios at neutral conditions | The alcoholic and carboxylic acid functional groups at acidic conditions, the influence of Na+ at basic conditions | 43 |
| Delivery of antibiotic: silver-sulphadiazine | Arabinoxylan, carrageenan, reduced graphene oxide, tetraethyl orthosilicate | Chemical crosslinking | Higher swelling ratios at neutral conditions | The alcoholic and carboxylic acid functional groups at acidic conditions, the influence of Na+ at basic conditions | 46 |
| Delivery of antibiotic: silver-sulphadiazine | Carboxymethyl chitosan, oxidized carboxymethyl cellulose | Schiff-base reaction | Higher release at pH 5.5 and pH 9.5 | The stronger degradation rate in acid and intensive electrostatic repulsion in alkali | 38 |
| Release of tobramycin and borneol | Dialdehyde carboxymethyl cellulose, tobramycin, β-cyclodextrin derivative | Chemical crosslinking | High degradation at acidic conditions | Gradual hydrolysis of the imine bond in a weakly acidic environment | 39 |
| Release of graphene oxide quantum dots | Tannic acid, keratin | Chemical crosslinking | Higher swelling ratios at neutral alkaline conditions | High deprotonation and repulsion between the acidic chains in basic solutions | 47 |
| Release of AgNPs | Methylcellulose, citric acid | Chemical crosslinking | High degradation at alkaline conditions | Network expansion and release of AgNPs were due to the alkaline hydrolysis of ester bonds | 37 |
| Release of antibiotic and ZnS nanoparticles | 2-(Dimethylamino)ethyl methacrylate/polyethylene oxide, ZnS nanoparticles | γ-Irradiation polymerization | Higher swelling ratios at pH 4–7 | Decrease of protonation and intermolecular hydrogen bonds lead to a high swelling ratio (pH 4–7) | 34 |
| Release of silver nanoparticles (AgNPs) and deferoxamine | Oxidized dextran, dopamine | Schiff-base reaction | Higher degradation at acidic conditions | Gradually destroyed network at acidic conditions | 48 |
| Release of antibiotic and lysozyme | Gelatin methacryloyl, hyaluronic acid-aldehyde | Photo-crosslinking | High release at acidic conditions | The breakage of Schiff base bonds and the electrostatic interaction. | 49 |
| Release of antibiotic and basic fibroblast growth factor (bFGF) | Alginate, CaCO3 microparticles | Microfluidic technology | Slower release rate at pH 6.4 | Presence of CaCO3 and strong interactions between CaCO3 microspheres and the alginate network | 50 |
| Release of antibiotic | Silica nanoparticles, alginates | Chemical crosslinking | High release at alkaline conditions | The degradation of silica nanoparticles | 51 |
| Release of natural antibacterial substance: honey | PVA, chitosan, montmorillonite | Freezing–thawing method | Lower swelling ratios at acidic conditions | Protonation of amine groups to NH3+ at acidic conditions | 52 |
| Release of natural antibacterial substance: resveratrol | Poly(vinylalcohol)-borax (PB), resveratrol grafted cellulose nanofibrils (RPC) | Crosslinked by dynamic borate bonds and hydrogen bonds | Higher release at acidic conditions | Faster degradation of RPC/PB hydrogels at acidic conditions | 40 |
| Release of natural antibacterial substance: curcumin | Quaternized chitosan (QCS), benzaldehyde-terminated Pluronic®F127 | Chemical crosslinking | Higher release at acidic conditions | Faster degradation of QCS/PF hydrogels at acidic conditions | 41 |
| Release of natural antibacterial substance: curcumin | Polyaspartic acid crosslinked by graphene nanosheets, poly(acrylamide-co-acrylic acid), copper oxide, zinc oxide nanoparticles | Chemical crosslinking | Low release at pH 2.1. | Compressed hydrogel network at pH 2.1 | 53 |
| Release of natural antibacterial substance: curcumin | Blending bacterial nanocellulose, GO, PVA | Chemical crosslinking | Higher release at pH 7.4 | Hydrophilic groups and more electrostatic repulsion forces | 36 |
| Release of natural antibacterial substance: curcumin | Methacrylated gelatin, methacrylated pectin | Photo-crosslinking | Higher release at pH 7.4 | The ionization of carboxyl groups both in pectin and gelatin | 54 |
| Release of natural antibacterial substance: tannic acid (TA) | Carboxylated agarose, TA, zinc salts | Chemical crosslinking | Higher release at acidic conditions | Disruption of ionic interactions and protonation | 55 |
| Release of natural antibacterial substance: TA | Hydroxypropyl chitin, tannic acid, ferric ion | Chemical crosslinking | Higher release at acidic conditions | Stronger complexation due to the deprotonation of pyrogallol/catechol groups | 56 |
| Release of natural antibacterial substance: TA | Tannin–europium coordination complex, Eu3+ | Chemical crosslinking | Sustained release at acidic conditions | The metal–phenolic coordination and the protonation of phenolic hydroxyl groups | 57 |
| Release of natural antibacterial substance: TA | Gelatin (GTU), TA | One-pot physical hydrogen bonding | Higher release at high pH | Deprotonation of polymer and charge repulsion | 58 |
| Release of natural antibacterial substance: magnolol | Carboxyl-bearing magnolol derivative, chitosan hydrochloride | Chemical crosslinking | Higher release at acidic conditions | High degradation rate of magnolol-loaded chitosan nanocapsules at acidic conditions | 59 |
| Inherent antibacterial properties derived from CS | CS, PVA, guar gum, tetraethyl orthosilicate | Chemical crosslinking | Higher swelling at acidic conditions | Protonation of cationic groups, mainly –NH3 | 33 |
| Inherent antibacterial properties derived from CS | Collagen, CS, dialdehyde-terminated polyethylene glycol | Crosslinking by dynamic imine bonds | Sol state at acidic environment and hydrogel state at basic conditions. | Formation and breakage of imine linkages | 26 |
| Inherent antibacterial properties derived from Schiff-base bonds | Tetrabenzaldehyde-functionalized pen-taerythritol, CS | Schiff-base reaction and hydrogen linkages | Sol state at acidic environment and hydrogel state at basic conditions | Hydration of hydrogen bonds and the dissociation of Schiff bases | 29 |
| Bacterial trap behavior and Fenton reaction | N-Isopropyl acrylamide, acrylamide, N-[3-(dimethylamino)propyl]methacrylamide | Free-radical polymerization and electrostatic adsorption | In acidic conditions, CP@Tf-hy shows in situ self-supplied H2O2 and pH-responsive release of Fenton catalytic copper ions with ˙OH | Intrinsic properties of CP@Tf | 60 |
| Photothermal therapy | Poly(glycerol sebacate)-co-poly(ethylene glycol)-g-catechol prepolymer 2 (PEGSD2), FeCl3, GTU | Physical double-network linkages: catechol Fe3+ coordination and hydrogen bonding | Sol state at acidic environment | — | 31 |
| Photothermal therapy | Oxidized hyaluronic acid, QCS, berberine, epidermal growth factor, poly(styrene sulfonate) | Schiff-base reaction | Higher release at high pH | The protonation of the carboxyl group of oxidized hyaluronic acid | 61 |
| Photothermal therapy and pro-healing effect | Ferric iron, protocatechualdehyde containing catechol and aldehyde groups, QCS | Catechol–Fe coordinate bond and Schiff base bonds | Low adhesion at acid solution or deferoxamine mesylate solution | Catechol–Fe coordinate bond and Schiff base bonds | 32 |
| Enhance the resistance to bacteria | PVA, CMC, polyethylene glycol (PEG) | Freeze–thaw process and phase separation method | High swelling ratios at neutral conditions | Degree of dissociation of carboxylic groups | 62 |
| Release of the anti-inflammatory drug: diclofenac sodium | PVA, alginate-g-N-isopropyl acrylamide | Freeze thaw technique | High swelling ratios at neutral conditions | Repulsive interaction between the carboxyl group of alginate and diclofenac | 63 |
| Release of anti-inflammatory bioactive factors: SCF | Collagen, aldehyde polyethylene | Condensation between the primary amines of collagen and aldehydes of APG | Reversible morphological changes at acidic/basic conditions | Formation and breakage of imine linkages | 30 |
| Release of DNA-bearing polyplex | PEG, poly(sulfamethazine ester urethane) (PSMEU) | Physically crosslinking | Sol state at high pH and room temperature and gel state at the body conditions | Ionized sulfonamide groups | 64 |
| Release of anti-inflammatory drug: resolvin D1 | Cystamine, N,N0-bis(a-cryloyl)cystamine, acetalized cyclodextrin | Double-network crosslinking | Higher release at low pH | The pH-responsiveness of acetalized cyclodextrin nanoparticles | 65 |
| Release of the anti-inflammatory drug: insulin | N-Carboxyethyl chitosan, hyaluronic acid-aldehyde, adipic acid dihydrazide | Chemical crosslinking | Faster degradation rate at acidic conditions | Labile acylhydrazone bonds at acidic pH | 66 |
| Release of insulin and fibroblasts | Phenylboronic-modified chitosan, polyvinyl alcohol, benzaldehyde-capped poly-ethylene glycol | Cross-linking of Schiff's base and phenyl boronate ester | Higher release at acidic and glucose conditions | Unstable Schiff's base at acidic conditions. Preferred combination between the phenylboronic group and glucose over the hydroxyl group of PVA | 67 |
| Release of the metformin and GO | Dihydrocaffeic acid and L-arginine cografted chitosan, phenylboronic acid and benzal-dehyde bifunctional polyethylene glycol-co-poly(glycerol sebacic acid), polydopamine-coated reduced graphene oxide | Double dynamic bond of the Schiff-base and phenylboronate ester | Higher release at acidic and glucose conditions | Unstable Schiff's base at acidic conditions. Phenylboronate ester structure with a phenylboronic acid group is glucose-responsive | 68 |
| Release of tobramycin | QCS, oxidized dextran, tobramycin, polydopamine-coated polypyrrole nanowires | Schiff-base reaction | Higher release at acidic conditions | Unstable Schiff's base at acidic conditions | 24 |
2.2. Advanced properties of pH-responsive hydrogel wound dressings
Hydrogels are three-dimensional networks formed by the physical or chemical cross-linking of hydrophilic polymers.19 These are widely used in wound dressings because of their ability to maintain the cleanliness and wettability of wounds, thereby preventing infections and promoting wound healing.69 In addition, hydrogels are excellent carriers for loading cells, antimicrobials, growth factors, and biological macromolecules.30,50,64 pH-Responsive hydrogels play an important role in wound regeneration. Depending on composition, hydrogels are able to change their morphology, swelling behavior, degradation, or drug delivery efficiency based on the variation in pH; therefore, the wound healing process could be better regulated.26,45,60 In this section, we discuss recent advances in pH-responsive hydrogel wound dressings from the perspective of function.2.2.1.1. Delivery of synthetic antimicrobial agents. The use of antibiotics is the most common and effective strategy to treat wound infections in clinics.73 In recent years, a large number of antibiotics have been encapsulated in hydrogels to prevent infections, such as aminoglycosides,74 novobiocin,75 vancomycin, gentamicin, and minocycline.76 pH-Responsive hydrogels could accurately regulate the release behavior to achieve controlled and sustained release of antibiotics. Hosseini and Mohammad synthesized a hydrogel film based on basil seed mucilage and using various ratios of PVA, glutaraldehyde as a cross-linker, and glycerol as a plasticizer. The hydrogel film demonstrated excellent control over the release of antibiotics. The release efficiency of tetracycline hydrochloride (TH) was approximately 80% under alkaline conditions (pH 7.4/8.5, 120 h). However, under acidic conditions (pH 6.5, 120 h), the migration of TH from the TH-loaded film was difficult, and the drug release rate was only 46.5%.44 The design of the hydrogel membrane can adjust the release process and amount of TH according to the wound conditions, making the use of antibiotics more intelligent. Similarly, Zhu et al. synthesized a hydrogel that released an antimicrobial agent (triclosan) more rapidly in neutral or alkaline environments. In addition, the hydrogel can be biodegraded by enzymes owing to the presence of peptidic bonds.45 Temperature- and pH-responsive gentamycin sulfate-loaded chitosan-based hydrogel films constructed by Mohammad and Fehmeeda showed better antibacterial activity at high pH and temperature because of the higher release of gentamycin sulfate.27 Furthermore, the use of pH-responsive hydrogels as drug delivery systems can improve the bioavailability antimicrobials that are poorly water-soluble. For example, silver sulfadiazine is widely used for the treatment of various wounds, but its application is limited by its poor solubility in aqueous solutions.38 Khan et al. addressed this problem by building a pH-responsive hydrogel (composed of arabinoxylan, CS, reduced graphene oxide, tetraethyl orthosilicate) to load silver sulfadiazine and control its release. The controlled and sustained release of silver sulfadiazine was achieved, with release rates of 93.1, 58.3, and 53.71% at pH 7.4, 6.4, and 8.4, respectively. This controlled drug release system successfully enhanced the bioavailability of silver sulfadiazine.43 Their other study also reached similar conclusions by replacing CS with carrageenan.46 Delivering antibiotics is an effective strategy for pH-responsive wound dressings, but only using antibiotics to fight bacterial infections may not be enough in some cases.
In addition to delivering antibiotics only, other adjuvant drugs or antibacterial materials can also be encapsulated in hydrogels to enhance their ability to fight infections. For example, in a study by Fan et al., tobramycin was not directly encapsulated in hydrogels but was used as a cross-linking agent to react with carboxymethyl cellulose to form a hydrogel (the linking was designed through imine bonds, which gradually hydrolyze in a weakly acidic environment). The use of tobramycin as a cross-linking agent can reduce the toxicity and cost of supererogatory crosslinkers, and this method is worthy of reference in the pH-responsive design strategy of hydrogels. Meanwhile, the use of adjuvant borneol promoted wound healing.39 The addition of nanoparticles can also enhance the antibacterial ability, especially metal and nonmetal nanoparticles, such as silver, copper, zinc oxide, silicon, and graphene oxide quantum dots.34,37,47,77 Mohamed et al. added antibiotics to the hydrogel and ZnS nanoparticles simultaneously. According to their results, the minimum inhibitory concentration decreased by 2–16 times after adding ZnS, which effectively increased the ability to inhibit infection.34 Similar results were also presented by Hu et al., where the addition of silver nanoparticles (AgNPs) significantly improved the antibacterial properties of the hydrogel. Compared with the control group, the hydrogel@AgNPs exhibited a better antibacterial effect (survival rates of 17.8 ± 2.2% for S. aureus and 19.7 ± 2.8% for E. coli), and the antibacterial ability was increased by more than five times. In addition, deferoxamine was added to promote angiogenesis by increasing the expression of hypoxia-inducible factor-1 alpha and vascular endothelial growth factor.48 This strategy, which has both antibacterial and growth-promoting properties, was also reported by Shi et al., where a hydrogel loaded with both rifamycin and basic fibroblast growth factor (bFGF) successfully achieved the rapid release of rifamycin and sustained release of bFGF (Fig. 4(a)). The hydrogel not only inhibited the growth of S. aureus (Fig. 4(b)) but also promoted cell proliferation and migration, accelerating the wound healing process (Fig. 4(c)).50 Compared with the antibacterial effect of metal nanoparticles, non-metallic nanoparticles can be used as pH-responsive carriers of antibacterial drugs. Pan et al. developed silica nanoparticles that achieved a targeted chlorhexidine release on alkaline wounds and verified their antibacterial ability to both Gram-negative and -positive bacterial pathogens, then silica nanoparticles were further formulated into alginate hydrogels. Based on this strategy, pH-responsive hydrogel wound dressing was prepared.51 In general, metal and metal oxide nanoparticles are mainly used as antibacterial agents, while non-metallic nanoparticles can be used as drug carriers.
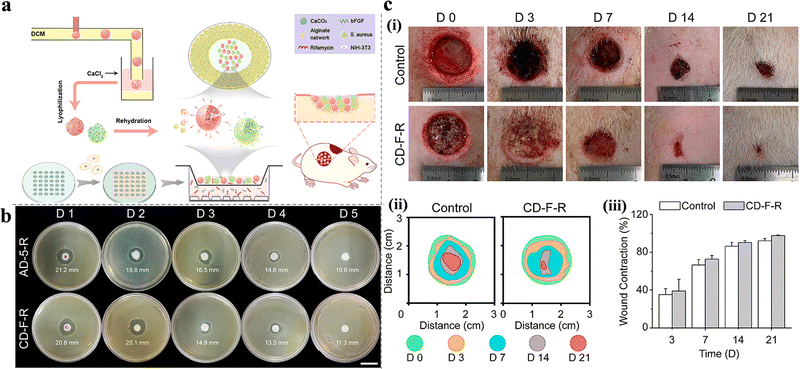 | ||
| Fig. 4 (a) Preparation process and applications of alginate/CaCO3 composite microparticles. (b) Evaluation of the sustained antibacterial activity of AD-5-R and CD-F-R. (c) In vivo performance of the CD-F-R on wound healing. (i) Typical digital images of the wound at predetermined time points. (ii) Traces of wound-bed closure during 21 days. (iii) Wound contraction for control and CD-F-R group. This figure has been reproduced from ref. 50 with permission from American Chemical Society, copyright 2019. | ||
2.2.1.2. Delivery of natural antibacterial agents. At present, the use of antibiotics is the most common approach to treating wound infections. However, their overuse causes long-term side effects and drug resistance. Furthermore, it is risky to only rely on antibiotics to resist future bacterial infections.78,79 Therefore, many researchers have focused on the use of natural antibacterial compounds to replace the frequent use of antibiotics.80 In addition, a number of natural antibacterial compounds also possess antioxidant properties that scavenge free radicals, such as honey, resveratrol, curcumin, and tannic acid (TA), which have a synergistic effect on wound healing.36,40,41,52,53,55,56,58
Honey is a common antimicrobial and antioxidant agent in food, and according to reports, it has been widely used in the treatment of wounds, including traumatic wounds, surgical incisions, and burns.81,82 Noori et al. synthesized a pH-responsive nanocomposite hydrogel based on chitosan/PVA/nanoclay using a freeze–thaw method. The maximum cumulative release of honey occurred at pH 7, whereas the wound size reduction in the PCMH group was 72.60% compared to that in the control group (55.23%, 6 days). In addition, the antibacterial activity of PCMH reached 99%, demonstrating its potential as an excellent wound dressing.52 To promote the healing process under acidic wound conditions, Yang et al. used resveratrol (RSV). The natural antibiotic was grafted onto cellulose nanofibrils and poly(vinylalcohol)-borax (PB) to produce a novel pH-responsive hydrogel with antibacterial (Fig. 5(a)), antioxidant and excellent adhesion properties (Fig. 5(c)). The scavenging efficiency of 1,1-diphenyl-2-picrylhydrazyl free radicals (DPPH˙) was used to evaluate the antioxidant activity of RSV-grafted cellulose nanofibril (RPC)/PB hydrogels. The groups containing RSV exhibited an increased DPPH˙ clearance rate, 10–20 times more compared to the groups without RSV, with the rate increasing upon addition of RSV. The inhibition zone method exhibited similar RSV dose-dependent results; the survival rates of RPC/PB-0.2, RPC/PB-0.5, and RPC/PB-0.8 were 29.4, 19.2, and 15.7%, respectively (Fig. 5(b)).83
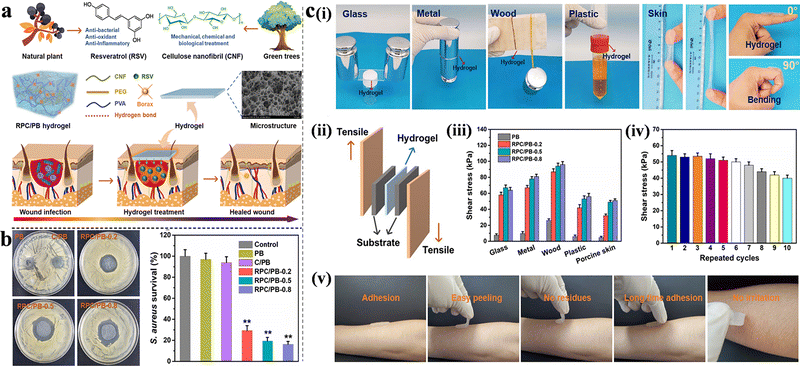 | ||
| Fig. 5 (a) Schematic illustration, construction and applications of RPC/PB hydrogels in wound healing. (b) Antibacterial activities of the PB, C/PB-0.5 and RPC/PB hydrogels against S. aureus. (c) Characterization of adhesion properties and quantitative data analysis of the hydrogel. (i) The adhesion to different surfaces and moved with the finger joint. (ii) Schematic of the hydrogel adhesion measurements. (iii) Adhesive strength to various substrates. (iv) Repeatable adhesion properties on pigskin. (v) No residue after removal on human skin. This figure has been reproduced from ref. 83 with permission from Springer Nature, copyright 2022. | ||
Similar to resveratrol and honey, curcumin is a natural antibacterial and antioxidant agent in food, approved by the Food and Drug Administration. In addition, curcumin has anti-tumor, anti-viral, and anti-inflammatory properties.84,85 Moreover, curcumin has been reported to accelerate fibroblast migration, collagen deposition, and epithelial regeneration.36,41,53 Therefore, curcumin is an ideal natural drug for wound dressings owing to its outstanding properties. Qu et al. studied the antimicrobial and free radical scavenging properties of QCS/PF hydrogels, as well as their ability to release curcumin in a pH-dependent manner, using in vitro experiments. In vivo experiments revealed a faster wound-healing rate with less inflammatory infiltration, thicker granulation tissue, higher fibroblast density, and higher collagen deposition (full-thickness skin defect model).41 Sattari et al., Al-Arjan et al. and Nazlı et al. also constructed pH-sensitive antibacterial hydrogels loaded with curcumin.36,53,54 It is worth mentioning that in a previous study, copper oxide and zinc oxide nanoparticles were used cooperatively to enhance the antibacterial properties. The addition of metal nanoparticles to polymer network hydrogels resulted in a more controlled nanoparticle release and increased mechanical toughness of the structure. Notably, in the latter study, the hydrogel showed antibacterial as well as anti-tumor activity. After treatment with curcumin-BSG-4 for 72 h, nearly 87.58% of the U87 cells were non-viable. Such results demonstrate the potential of this wound dressing as a biomaterial for wound care and the treatment of cancer patients.
Tannic acid (TA) is a typical plant-derived hydrolyzable tannin. It is considered an antioxidative, antimicrobial, anti-viral, and anti-inflammatory agent.86,87 It has been used to treat skin ulcers and burns, and its safety has been evaluated by the Food and Drug Administration. Compared with curcumin, resveratrol, and honey, TA itself can additionally act as a crosslinker to form hydrogels because of the interaction between its multiple pyrogallol/catechol groups and macromolecules, forming hydrogen bonds, ionic bonds, coordinate bonds, etc.86,88 Ninan et al. developed a novel antibacterial, anti-inflammatory, thermosetting, and pH-sensitive hydrogel based on carboxylate agarose and TA and ionically cross-linked with zinc salts. The hydrogel exhibited antimicrobial activity against E. coli, similar to that of gentamicin (the diameters of the inhibition zones of CTZ2 and gentamicin were 8 and 9 mm, respectively). Furthermore, TA presented antimicrobial activity at much lower concentrations than other TA-containing wound dressings. In addition, a nitric oxide (NO) assay was conducted to verify the anti-inflammatory activity of TA, and the results showed that CTZ2-conditioned media could inhibit NO production in a concentration-dependent manner in LPS-activated U-937 cells.55 Similarly, Ma et al. prepared a hydrogel composed of hydroxypropyl chitin, TA, and ferric ions, with a similar pH-responsive release of TA. Both hydrogels were crosslinked with metal salts, and they showed higher release under acidic conditions.56 In addition to chemical crosslinking, TA can also form hydrogels via physical crosslinking. Ahmadian et al. developed a gelatin-GT hydrogel with abundant hydrogen bonding between the functional groups of gelatin (GTU) and TA for the treatment of non-healing chronic and infected wounds (higher TA release at pH 7.4). GTU contains an arginine–glycine–aspartate (RGD) peptide sequence, which provides good cell adhesion and higher hemostatic ability; however, its poor mechanical properties and water sensitivity limit its application. The incorporation of TA overcomes the disadvantages of GTU and endows the hydrogels with inherent anti-inflammatory, antioxidant, and antimicrobial properties. Such a combination is an effective strategy for building multifunctional pH-responsive hydrogels.58
It is also an effective way to improve the utilization of natural agents by grafting strategy and then crosslinking with other components to form hydrogels. Magnolol is a natural antioxidant and antibacterial compound, but its poor water solubility leads to low bioavailability. Wang et al. synthesized magnolol-grafted-chitosan hydro-chloride via EDC/NHS coupling reaction between carboxyl-bearing magnolol derivative and chitosan hydrochloride, and then crosslinked with genipin, forming hydrogel (CSM-H) with dual effects of antibacterial and antioxidant activities. The results showed that the CSM-H hydrogel not only achieved the pH response release of magnolol but also significantly enhanced the antibacterial and antioxidant activities.59
2.2.1.3. Other antibacterial mechanisms. Hydrogels are often used as a delivery system for the controlled release of antimicrobial drugs. However, in addition to delivering synthetic/natural antimicrobial agents, hydrogels exhibit various interesting antibacterial mechanisms.
For example, pentaerythritol (PER-TBA) and chitosan CS have been used to synthesize CPT hydrogels with pH responsiveness and intrinsic antibacterial properties via the formation of Schiff-base linkages and hydrogen bonds. The antimicrobial mechanisms of CPT hydrogels are summarized as follows: first, the bacteria are inactivated by the interactions between the positively charged CS and negatively charged cell membranes (this antibacterial effect of CS was also confirmed by Khan et al. (Fig. 6(a)) and Ding et al.)26,33 Second, the large number of Schiff-base bonds formed by the cross-linking of PER-TBA and CS further enhances the antibacterial properties. The increased PER-TBA dose results in the formation of more Schiff-base bonds, which improves the antimicrobial activity. This was confirmed by the confocal laser scanning microscopy results. In addition, CPT hydrogels are converted to liquid under acidic conditions and can return to a hydrogel state after alkali addition. This pH-responsive morphological change may facilitate the replacement of wound dressings as well as reduce the patients’ pain and secondary injuries.29 Zu et al. developed a pH-responsive wound dressing hydrogel composed of transferrin-conjugated copper peroxide nanoparticles (CP@Tf-hy), presenting multiple antimicrobial mechanisms. The positively charged hydrogel carrier is formed by N-isopropyl acrylamide, acrylamide, and N-[3-(dimethylamino)propyl] methacrylamide via free radical polymerization. It can trap and eliminate bacteria with trapping efficiencies of 20.40 and 25.35% against E. coli and B. subtilis, respectively. Furthermore, under weak acidic conditions, Cu2+ is released from CP@Tf-hy, and it decomposes the self-supplied H2O2 into ˙OH. The membrane is damaged by both Fenton reactions and GSH oxidation, and 98.78% of E. coli and 92.02% of B. subtilis are eliminated, confirming a strong antibacterial activity (Fig. 6(b)).60
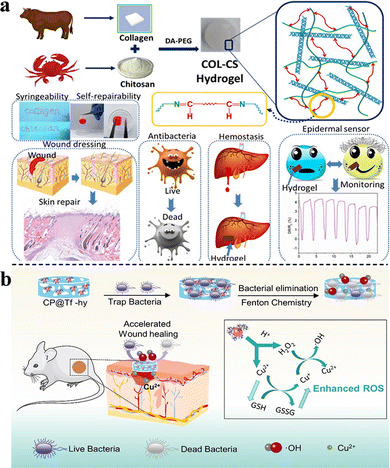 | ||
| Fig. 6 (a) Construction and applications of COL-CS hydrogels. This figure has been reproduced from ref. 26 with permission from American Chemical Society, copyright 2020. (b) The synthesis of CP@Tf NPs and wound infection treatment via bacterial elimination. This figure has been reproduced from ref. 60 with permission from American Chemical Society, copyright 2022. | ||
The denaturation of enzymes in bacteria is observed at temperatures above 50 °C, where the proteins and lipids of cell membranes are also damaged, causing bacterial death. Therefore, photothermal therapy has recently emerged as a means of combating bacteria. Hydrogel PEGSD2/GTU showed broad absorption in the near-infrared region, owing to its catechol–Fe3+ coordination. After 10 min of NIR irradiation, the highest ΔT was 36.6 °C. Antimicrobial tests showed that after 1 min of NIR irradiation, the killing ratios for E. coli and S. aureus were 83.9% and 85.0%, respectively.31 Similarly, Xue et al. developed a polysaccharide-based hydrogel to achieve photothermal-assisted bacterial inactivation, using QCS, oxidized hyaluronic acid, photo-thermal agent of poly(styrene-sulfonate), natural antibacterial agent of berberine, and epidermal growth factor. Besides the inherent antibacterial activity of QCS and berberine in hydrogels, the photothermal effect greatly improved the antibacterial properties of hydrogels, reducing the number of colonies of S. aureus and E. coli to 3.5% and 4.3%.61 The photothermal effect has attracted much attention in recent years. Hydrogels with photothermal effect can not only help enhance their antibacterial ability, but also broaden the application range of hydrogels by designing temperature sensitivity based on the photothermal effect.
Variations in the pore size of hydrogels may also enhance their antibacterial effect. The PVA-CMC-PEG hydrogel prepared through the freeze–thaw process and phase separation method exhibited different pore sizes from the top to the lower surface, as reported by Li et al. During the phase separation process, PEG floated on the surface of the solution, thereby forming larger pores on the top surface. This pore size distribution may strengthen resistance to bacterial infection, with the exact mechanism requiring further investigation.62
Inflammation plays an important role in wound healing. Appropriate inflammation is indispensable for removing dead tissue and resisting invasive pathogens. However, excessive inflammation leads to congestion and swelling of the tissue around the wound, thus delaying the healing process.89–91 Therefore, the suppression of excessive inflammation is an effective way to control wound restoration. Nonsteroidal anti-inflammatory drugs can eliminate inflammation by upregulating anti-inflammatory molecules and inhibiting inflammatory pathways.92 Montaser et al. prepared thermosensitive and pH-responsive hydrogels based on PVA/SA-g-NIPAM via a freeze–thaw technique. Diclofenac sodium was successfully loaded into the mixed solution, and its release was examined in vitro. The release of diclofenac sodium was controlled and sustained compared to that of the control group.63 The umbilical cord stem cell factor (SCF) is an anti-inflammatory bioactive factor secreted by umbilical cord stem cells and has been demonstrated to accelerate the wound healing process by activating M2 macrophages and promoting granulation tissue formation and re-epithelialization. To accelerate wound healing, SCF was loaded into Col/APG hydrogels, and it increased the number of activated M2 macrophages. It promoted angiogenesis and collagen deposition, which displayed a strong pro-healing ability.30 Therapeutic genetic materials, including DNA, can be loaded into hydrogels to eliminate proteolytic and enzymatic degradation. A PEG-PSMEU copolymer hydrogelator with superior adhesive, mechanical, and bioresorbable properties was successfully developed by Minh et al., and it demonstrated excellent adhesion to various hydrophilic, hydrophobic, and metal substrate surfaces. Quantification of the kinetics of wound closure showed that after one week of treatment, only 30% of the wound area had healed in the control group, whereas 70% had healed in the PEG-PSMEU group and 100% in the polyplex-loaded PEGPSMEU group. The same results were confirmed by hematoxylin and eosin staining, proving the powerful pro-healing properties of PEG-PSMEU and DNA polyplexes.64 In addition to drug delivery, specific crosslinking bonds may also enhance therapeutic effectiveness on inflammation. Lu et al. developed double-network hydrogels based on bioadhesive catechol-chitosan hydrogels. Disulfide bonds formed by the crosslinking of cystamine and N,N0-bis(a-cryloyl)cystamine enabled redox-responsiveness to the hydrogel; and acetalized cyclodextrin nanoparticles were used to release anti-inflammatory agent Resolvin E1 in a pH-responsive manner. This provided a new strategy for the hydrogel wound dressing to control the inflammation of hard-to-heal wounds and accelerate wound healing.65
Apart from common acute and chronic wounds, some special wounds that are difficult to heal have also attracted attention. For example, diabetic chronic wounds, especially diabetic foot ulcers (DFUs), are one of the most serious complications of diabetes, causing long-term pain and even amputation.93,94 According to the pathophysiology, the two main factors that inhibit DFU healing are diabetic peripheral vasculopathy and neuropathy.95 pH-responsive hydrogels are effective in healing DFUS and can provide a better method for the treatment of DFUS.66,67 Li et al. were the first to develop a diabetic microenvironment-responsive, self-healing, injectable, and insulin-loaded hydrogel for diabetic wound therapy. In addition, more detailed studies have been conducted to investigate the mechanisms of wound repair, such as re-epithelialization, neovascularization, and the expression of TGF-β1 and VEGF. The hydrogel not only shortened the inflammation phase and enhanced the formation of granulation tissue, neovascular tissue, and epithelium but also improved diabetic peripheral neuropathy, displaying highly promising therapeutic potential for DFUs.66 Zhao et al. synthesized pH and glucose dual-responsive injectable hydrogels that were loaded with insulin and fibroblasts (Fig. 7(a)), which further shortened the healing time (Fig. 7(b)) and enhanced their curative effect against DFUs (Fig. 7(c)). The simultaneous use of bioactive factors and cells is rarely reported in the field of wound dressings, and this may provide an effective strategy for wound dressing.67 Similarly, Liang et al. also constructed pH and glucose dual-responsive hydrogel wound dressings.68 Different from the above studies, other typical antidiabetic drugs metformin and GO were encapsulated in adhesion-enhanced self-healing easy-removable antibacterial antioxidant conductive hemostasis multifunctional hydrogel wound dressings. This multifunctional hydrogel wound dressing was more suitable for the microenvironment characteristics and wound healing process of diabetic foot, which may have guiding significance for future research. Burns is also a common health problem that is highly susceptible to bacterial infections. Hydrogel wound dressings for burns were prepared by Huang et al. by using QCS, oxidized dextran, tobramycin, and polydopamine-coated polypyrrole nanowires. Schiff base bonds between the oxidized dextran and tobramycin achieved the on-demand release of TOB due to the lower pH caused by the bacteria growth. In addition, the incorporation of polydopamine-coated polypyrrole nanowires endowed the hydrogel with NIR irradiation-assisted bactericidal activity, further enhancing the inhibition of drug-resistant bacteria, and leading to more ideal healing of burn wounds.24 In general, pH-responsive wound dressings for specific wounds show a trend of multifunctional, through the integration of multiple functions to make the wound healing process more controllable.
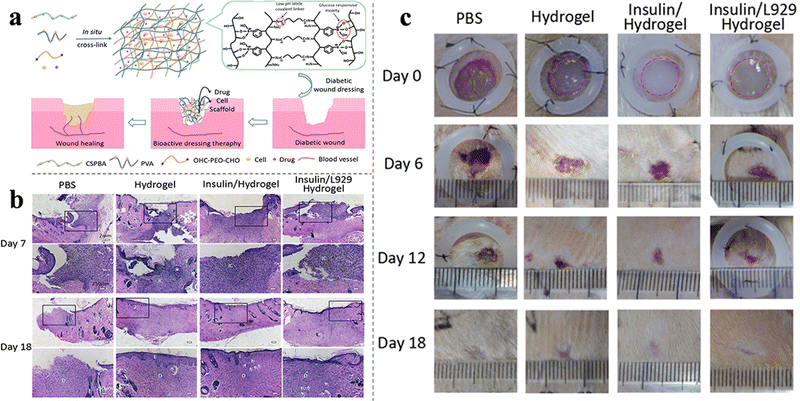 | ||
| Fig. 7 (a) Synthetic process and applications of insulin- and cell-loaded hydrogels. (b) Images of H&E-stained histopathological sections after 7/18 days of treatment. (c) Wound healing by the hydrogel in STZ-induced diabetic rats. This figure has been reproduced from ref. 67 with permission from American Chemical Society, copyright 2017. | ||
3. Other types of pH-responsive wound dressings
pH-Responsive hydrogels are the main type of pH-responsive wound dressings. Other advanced pH-responsive materials include nanofibers,96 composite films,97 nanoparticle clusters,98 and microneedles,99 each with unique characteristics and advantages for wound healing. In this section, the pH-responsive mechanisms and properties of other pH-responsive materials are discussed, and the relevant information is summarized in Table 2.| Classification | Application | Composition | Synthetic methods | pH range | pH-Responsive mechanism | Ref. |
|---|---|---|---|---|---|---|
| Nanofibers | Release of gentamycin/vitamin B12 | poly ε-caprolactone (PCL), nanostarch (NS) | Electrospinning technique | Higher release at high pH | Slower swelling and erosion of NS particles at low pH | 96 |
| Release of allicin | PVA, GO, CS | Electrospinning technique | Higher release at low pH | The protonation of amino-NH2 and acetylamino CH3CONH groups of CS | 100 | |
| Release of curcumin | PCL, nanochitosan | Electrospinning technique | Higher release at pH 5.8 | Faster swelling and dissolution of NC nanoparticles at pH 5.8 | 35 | |
| Release of curcumin | PVA, GO, AgNO3 | Electrospinning technique | Higher release at pH 5.4 | Protonated Ag+ groups and their interaction with other protonated components in an acidic medium | 101 | |
| Release of curcumin | Silk fibroin, CS, AgNO3 | Electrospinning technique | Higher release at pH 5.4 | The amino and hydroxyl groups in CS and hydroxyl group in curcumin | 102 | |
| Release of curcumin | Methyl methacrylate, N,N-diethylaminoethyl methacrylate | Free-radical polymerization | Higher release at pH 5 | — | 103 | |
| Release of chlorhexidine, rifampicin, thymol | Methacrylic acid copolymer Eudragit® L100-55, Eudragit® S100, Eudragit® RS100 | Electrospinning technique | L100-55 showed high release at pH > 5.5, S100 showed high release at pH > 7, and RS100 showed high release at alkaline conditions | L100-55 dissolves at pH > 5.5, S100 dissolves at pH > 7, and RS100 is pH-independent and slowly erodes and releases the drug | 104 | |
| Release of Ag+ | Bacterial cellulose (BC), silver nanoparticles | Chemical reduction | Higher release at acidic conditions | Higher H+ concentration leads to more Ag oxidation | 42 | |
| Release of AgSD | BC | Simple blending method | Higher release at acidic conditions | Higher H+ concentration causes more SD− releasing | 28 | |
| Release of ICG and DOX | Cellulose nanofiber, dopamine, polyethyleneimine (PEI) | Chemical modification | Higher release at acidic and NIR conditions | CNF-PEI is pH-responsive and CNF-DA fiber is NIR-responsive | 105 | |
| Release of ICG and DOX | Cellulose nanocrystal, dopamine, poly(N-isopropyl acrylamide), CS | Chemical modification | Higher release at acidic and NIR conditions | The breakage of dynamic imine bonds | 106 | |
| Composite films | ||||||
| Release of neomycin | CS, polyphenolic tannic acid | Chemical crosslinking | Higher release at acidic conditions | High solubility of CS at acidic conditions | 97 | |
| Release of epigallocatechin-3-gallate (EGCG) | Hydroxypropyl methylcellulose, EGCG | Formation of hydrogen bonds and hydrophobic interactions | Lower release at basic conditions | Weaker hydrogen bonding at high pH | 107 | |
| Release of minocycline | Halloysite nanotube, poly lactide-co-glycolide (PLGA), CS | Surface modification | Higher release at basic conditions | Decreased number of –NH3+ and increased repulsion of –COO− | 108 | |
| Release of levofloxacin | Porous silicon, poly(1,7-octadiene), poly(acrylic acid) | Surface modification | Higher release at acidic conditions | The dual plasma polymer layers of poly(1,7-octadiene) and poly(acrylic acid) | 109 | |
| Nanoparticle clusters | Release of Ag+ | PEG with ortho ester segment and –SH group, AgNO3 | Chemical binding | Higher release at acidic conditions | AgNCs hydrolyzed at acidic conditions | 98 |
| Microneedles | Smart drug delivery | Stainless steel microneedles with a porous poly actide-co-glycolide layer, Eudragit S100 | Surface coating | Higher release at basic conditions | Eudragit S100 is dissolvable at alkaline pH and insoluble at acidic pH | 99 |
| Sponge | Release of CuO2 | Copper hydroxide and gelatin sponge | Chemical reaction | Higher release at acidic conditions | Fenton reaction | 110 |
| Release of ibuprofen, gentamicin or ciprofloxacin | CS, microspheres | Chemical crosslinking | Cumulative release of gentamicin was higher at pH 4.5 while that of ciprofloxacin was higher at pH 8.0 | Different electrostatic interactions between the antibiotics and microspheres | 111 | |
| Polysaccharide-based dressing | Release of exosomes | Pluronic F127 grafting PEI and aldehyde pullulan | Schiff-base reaction | Higher release at acidic conditions | — | 112 |
| Foam | Release of protein drug | Polyurethane foam, alginate-bentonite hydrogel | Vacuum method | Higher release at basic conditions | Weaker crosslinking at high pH | 113 |
| Superabsorbent polymer | Release of peptide | Polyaspartic acid and 2-acrylamide-propane sulphonic acid | Free radical polymerization | Higher swell ratios at pH 2 and pH 10 | Protonation and dissociation at pH 2–4; protonated –SO3H and –COO− groups at pH 7–10 | 114 |
Recently, nanofibers have been widely used in biomedical fields, such as tissue engineering, drug delivery, and wound dressings, owing to their large surface area, customizability, biocompatibility, and modifiable properties.115,116 Thus, based on our investigation, the main application of pH-responsive nanofibers is the prevention or treatment of bacterial infections in wounds. In this section, a summary of the synthetic pathways and properties of pH-responsive nanofibers is presented.
Electrospun membranes are a common type of nanofiber materials. In addition to their primary drug delivery function, their random network arrangement and high porosity (60–90%) are also conducive to cell respiration, wound humidity, and blocking of invasion by external microorganisms.117 Reshmi et al. incorporated NS and poly ε-caprolactone (PCL) by direct electrospinning to form NS@PCL membranes with pH-responsive internal pore sizes. The 25NS@PCL membranes were loaded with gentamicin, and they showed evident bacterial inhibition zones against S. aureus and E. coli. In addition, 15 days of long-acting antimicrobial activity demonstrated an excellent controlled release effect.96 Electrospinning technology could realize intelligent drug delivery by adjusting of composition ratio. Liu et al. develop CS/PVA/GO nanofibrous membranes with strong antibacterial activity and sustained-release properties. The use of PVA enhanced the electro-spinnability of CS and the addition of 0.1 wt% GO enabled the nanofibrous membrane to a slow-release effect of allicin, thereby achieving the long-term therapeutic effect and improving the bioavailability of allicin.100 Similarly to hydrogel wound dressings, curcumin is also a potential drug embedded in nanofibers.35,101–103 For example, PCL/NC membranes synthesized from PCL and NC by Reshmi et al. successfully achieved a pH-responsive controlled release of curcumin, further increasing the bioavailability and action time of curcumin.35 Furthermore, Laura et al. prepared three methacrylic acid copolymers (L100-55, S100, and RS100) with different pH-responsiveness to adapt to the environments of different wounds. Specifically, the RIF loaded-L100-55 dressing is designed to prevent infection in intact skin with a pH of 5.5. CHXD-loaded L100-55 dressings are designed for use in infected chronic wounds, whereas the RIF-loaded S100 dressing is appropriate for acute wounds.104
Cellulose nanofibers are excellent delivery carriers for wound-repairing drugs owing to their high porosity, hydroxyl chemical reaction sites, and machinability. Chen et al. prepared pH-responsive CNS-PEI and NIR-responsive CNS-DA nanofibers and then mixed them to form a dual-responsive 3D nanocage structure loaded with ICG and DOX. The pH-activated and NIR-triggered nanocage wound dressings showed high drug delivery capability in the slightly acidic environments of wounds and tumors. In addition, CNF NWD@ICG&DOX, which could induce photothermal therapy, exhibited high antibacterial, antibiofilm, and anti-tumor abilities,105 a similar study was also carried out in their next work.106 Shao et al. successfully used BC and achieved pH-responsive, controlled release of AgNPs and AgSD. The antibacterial effect of the two BC nanofibers was demonstrated in their studies.28,42
Composite films are another type of advanced drug delivery system.109,115 Huang et al. developed a self-assembled multilayer and complex film through the formation of hydrogen bonds and hydrophobic interactions between hydroxypropyl methylcellulose and EGCG. In alkaline environments (near pH 8.0), EGCG transforms from phenol to phenolate ions and then quinone, thereby generating H2O2 to kill bacteria. The smart film was able to release antibacterial agents on demand, which is a strategy worth referring to.107 Chitosan is an excellent film-forming material owing to the presence of amino groups in its polysaccharide backbone, which can ionize at an acidic pH and form a 3D crosslinked film structure. A neomycin-loaded chitosan–tannic acid nanocomposite film was constructed via crosslinking. Its bacterial growth inhibition effect and pH-responsive drug release were demonstrated in vitro, and its antibacterial activity and drug release profiles were obtained.97 Furthermore, CS can also be used as a surface modifier to prepare pH-responsive drug delivery system.108
Besides hydrogels, nanofibers, and composite membrane materials, the field of pH-responsive wound dressings includes other materials, such as nanoparticle clusters,98 microneedles,118 sponges,110,111 polysaccharide,112 and foams,113 which present specific designs and functions. This section is a brief introduction to these materials.
AgNPs are typically used as loading drugs in wound dressings; however, Xie et al. employed chemical bonding to endow them with different pH-response characteristics. In particular, Ag nanoparticle clusters (AgNCs) were synthesized using functional polymers containing ortho ester segments as stabilizers (Fig. 8(a)). The ortho ester segment is hydrolyzed under acidic conditions caused by bacterial growth. The AgNCs will then collapse and release individual AgNPs around the bacteria, leading to the death of methicillin-resistant Staphylococcus aureus (MRSA).98 Microneedles (MNs) are minimally invasive delivery systems that painlessly deliver drugs into subcutaneous tissues and are mainly used for the controlled and sustained release of hydrophilic drugs.119–121 Ullah et al. created a PLGA layer on stainless steel MNs and then coated them with Eudragit S100 (a type of pH-responsive material mentioned above104), which enabled the pH-responsiveness of MNs.99 Gelatin sponge is a frequently-used degradable wound dressing, in the study of Cui et al., CuO2 was loaded into gelatin sponges. As wound infection occurred (low pH value), copper peroxide was released to kill bacteria through the Fenton reaction producing hydroxyl radicals. Meanwhile, the released Cu2+ would also stimulate angiogenesis and collagen deposition, speeding up the wound healing process.110 In addition to altering their function according to the pH of the wound environment, pH-responsive wound dressings can also act as response elements to artificially regulate drug release. Kiaee et al. developed an electronic wound dressing consisting of microfabricated electrodes and pH-sensitive poly(ethylene glycol)-diacrylate/LAPONITE® hydrogel, containing drug-loaded chitosan nanoparticles (ChPs). The pH around the anode increases after applying a DC voltage, resulting in drug release from the ChPs; however, under acidic conditions, the release is negligible, achieving controlled drug-release through this strategy (Fig. 8(b)).122
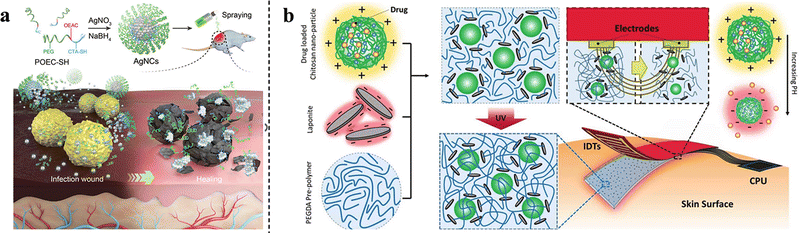 | ||
| Fig. 8 (a) Synthesis of AgNCs and in vivo wound-healing. This figure has been reproduced from ref. 98 with permission from Wiley-VCH, copyright 2020. (b) Schematic representation of the integrated electronic wound dressings. This figure has been reproduced from ref. 122 with permission from Wiley-VCH, copyright 2018. | ||
4. pH-Responsive wound dressings with monitoring function
Wound healing is a complicated process, with the pH of the wound environment being an important indicator of the wound state.123 Traditional wound dressings cannot provide information about wound healing, meaning that they must be manually removed or replaced to visually inspect the condition of the wound. This can lead to delayed dressing changes and loss of the opportunity to treat the infection and promote healing.124 It is therefore necessary to monitor the condition of the wound during treatment. To solve this problem, some researchers have added substances that reflect pH on gauze and fiber pads, such as Clitoria ternatea flower extract,125 red cabbage extract,126 curcumin,127 naphthalimide-rhodamine probe,128 and carbon quantum dots.129 However, the properties of wound dressings do not change with the addition of pH-displaying agents, and simple gauze or fiber pads are not efficient in meeting the needs of wound healing. Therefore, a combination of multifunctional pH-responsive wound dressings and pH-monitoring functions is essential. pH-responsive wound dressings with monitoring functions not only adjust their properties (antibacterial,130 hemostatic,131 and analgesic132) according to the condition of the wound but also reflect the state of the wound at the same time, successfully combining diagnosis and treatment, which coincides with the current research trend of nanomedicine.133 As Fig. 9 shows, the healing process can be observed through the color variation after applying the dressings. The orange arrow indicates that the wound is under normal healing process, while the blue arrow indicates the wound deterioration occurs, warning that the wound needs further treatment. | ||
| Fig. 9 Schematic of integrated diagnosis and treatment mode of pH-responsive wound dressings with monitoring function. | ||
In this section, the detailed functions of pH-responsive wound dressings with monitoring functions are discussed, and the pH indicators are summarized in Table 3.
| Classification | pH indicators | pH indication range | Ref. |
|---|---|---|---|
| Hydrogel | Prodigiosin | Yellow at pH 10 and purplish red at pH 4 | 134 |
| Red cabbage extract | pH values 4–9: red for pH < 7 and green for pH > 7 | 135 | |
| Cranberry extract | Visual color changes at pH 4–9 | 130 | |
| Curcuma Longa extract | pH values (3–13): yellow for pH ≤ 7 and dark red or reddish-brown for pH > 7 | 136 | |
| Litmus | Real-time pH monitoring at pH 4–9 | 131 | |
| Phenol red | Visible color change at pH 4–8 | 137 | |
| Methacrylate-modified phenol red | Visual color changes at pH 5–9 | 138 | |
| Cyanine 3 (Cy3) and cyanine 5 (Cy5) | Acidity and alkalinity are indicated by ratios of the fluorescence intensities of Cy5 and Cy3 | 139 | |
| Ammonium hydrogen citrate carbon dots | Calibration curve of pH values (2–12) and the related fluorescence intensity were plotted | 140 | |
| Chitosan–carbon quantum dots and phenol red | Visible color change was observed and the fluorescence intensity normalized curve (5–8) was established | 141 | |
| Nanofiber | Alizarin dye andanthocyanin dye | Reversible color response at pH 2–11 | 142 |
| Bromocresol green | Visual color changes at pH 2–10 | 132 | |
| Nanosheet bandage | Fluorescent magnesium hydroxide nanosheets (Mg(OH)2–NS) | Fluorescence intensity changes at pH 7–10 | 143 |
Extracts from certain plants act as pH indicators, and the incorporation of these extracts into multifunctional pH-responsive wound dressings is a common and effective strategy to build advanced wound dressings. Elkenawy et al. added prodigiosin to gamma-irradiated silver-sulfadiazine-embedded alginate hydrogels. In addition to their antibacterial, antioxidant, and anti-inflammatory properties, they were able to monitor the wound status through the variations in prodigiosin's color. The hydrogel changed from purplish red to yellow in response to bacterial colonization, and the reappearance of purplish red occurred after skin restoration.134 Similarly, Arafa et al. prepared multifunctional chitosan-based hydrogels using red cabbage extract as a natural pH-sensitive indicator.135 Cui et al. prepared smart calcium alginate fibers utilizing alizarin and anthocyanin dyes.142 Zepon et al. and Arafa et al. also prepared κC:LBG:CB hydrogel films and hydroxyethyl cellulose hydrogels using cranberry and Curcuma Longa extracts, respectively.130,136 It is worth mentioning that Wang et al. prepared a hydrogel that could be used as a 3D-printed wound dressing ink. They used a multifunctional hydrogel composed of polyacrylamide and chitosan quaternary ammonium salt (HACC-PAM) and added litmus as a pH indicator. The hydrogel displayed excellent antibacterial, hemostatic, and adhesion functions, and its 3D-printable feature enabled it to better assist wounds of different shapes, sizes, and depths. More interestingly, personalized wound management was achieved by utilizing a machine learning model based on the convolutional neural network algorithm, which could recognize and analyze the pH sensing map produced by the litmus in the hydrogel network.131
For complex wounds, pH-indicating dyes can be used in combination with other substances that reflect the state of the wound, making the wound assessment more accurate.124 For example, Zhu et al. used phenol red and two glucose-sensing enzymes, glucose oxidase and horseradish peroxidase (HRP), to construct a polycarboxybetaine (PCB) hydrogel and monitor the pH and glucose levels, to achieve better wound management for diabetic ulcers. The color variation (sensitive to changes in the pH of the wound environment due to phenol red) and fluorescence signals (generated by glucose oxidation) can be quantified with a spectrophotometer or smartphone for precise measurement (Fig. 10(a)).137 Liu et al. prepared an alginate/polyacrylamide (PAAm) hydrogel using phenol red as the pH indicator. Notably, phenol red was successfully modified with methacrylate, allowing copolymerization with the alginate/PAAm hydrogel matrix. The covalent attachment effectively reduced the dye leaching out of the matrix.138
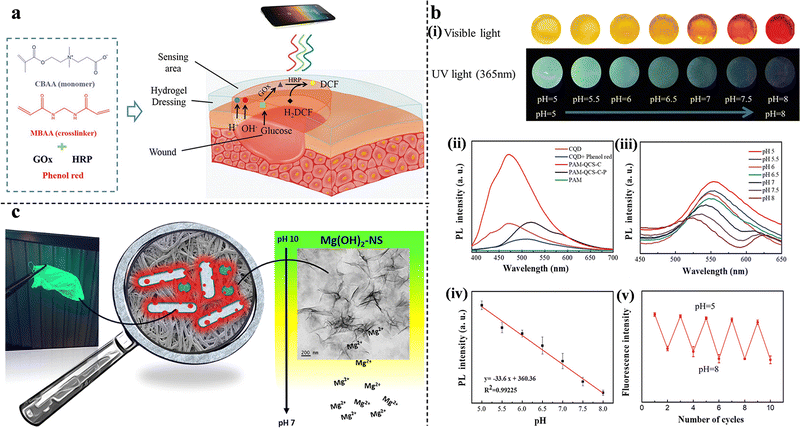 | ||
| Fig. 10 (a) A schematic representation of the detection of pH and glucose concentration by PCB hydrogels. This figure has been reproduced from ref. 137 with permission from Wiley-VCH, copyright 2019. (b) pH-Sensitivity of PAM-QCS-C-P hydrogels. (i) The color of the hydrogels under sunlight and ultraviolet radiation at different pH values. (ii) The fluorescence intensity of hydrogels, fluorescent hydrogels, and CQD solutions. (iii) The fluorescence emission spectra at different pH values. (iv) The relationship between the fluorescence intensity and pH value. (v) The reversible potential of hydrogels from pH 5–8. This figure has been reproduced from ref. 141 with permission from Wiley-VCH, copyright 2021. (c) Antimicrobial activity and pH-dependent fluorescence quenching of Mg(OH)2–NS electrospun fibers. This figure has been reproduced from ref. 143 with permission from American Chemical Society, copyright 2021. | ||
In addition to the combination of pH indicator dyes and multifunctional wound dressings, pH-sensitive fluorescent probes offer a good option. Qiao et al. designed a smart hybrid hydrogel capable of monitoring wound infections through pH-responsive fluorescence resonance energy transfer changes in the bacterial environment. Such a hydrogel can provide on-demand therapy for infections via NIR-triggered antibiotic release. The pH-monitoring ability was attributed to the energy donor cyanine 3 (Cy3) moiety and the energy acceptor cyanine 5 (Cy5)-loaded silica nanoparticles. Under neutral conditions, stronger Cy5 fluorescence was observed, owing to the fluorescence resonance energy transfer effect between Cy3 and Cy5. When the hydrogels were soaked in an acidic solution, Cy5 was released because of the breakage of the Schiff-base bond between Cy5 and SNP, thereby emitting strong Cy3 fluorescence.139 Carbon quantum dots (CQDs) have shown promising potential for bioimaging owing to their unique optical properties, biocompatibility, and photoluminescence. Omidi et al. added CQDs to chitosan nanocomposite films and successfully monitored the pH of the wound environment (from 4 to 9).140 Furthermore, to improve the responsiveness and accuracy of wound-state monitoring, Zheng et al. synthesized multifunctional double-colorimetry-integrated polyacrylamide-quaternary ammonium chitosan-CQD-phenol red hydrogels. The hybridization of CQDs and phenol red with the hydrogel simultaneously reflected the wound conditions in both ultraviolet and visible light. In addition, real-time remote evaluation can be achieved via signals collected by smartphones (Fig. 10(b)).141 Unlike the previous studies, Truskewycz et al. were able to achieve both therapeutic effects and wound status monitoring with a single component. A novel fluorescent magnesium hydroxide nanosheet (Mg(OH)2–NS) was synthesized and incorporated into PCL/polyethylene oxide polymeric fibers. In infected wounds with increased pH, Mg(OH)2–NS would persist and kill bacteria. At decreased pH, when the wound starts to heal, Mg(OH)2–NS would ionize, causing the loss of fluorescence; therefore, the fibers would indicate the pH and predict the wound-healing process (Fig. 10(c)).143
5. Conclusions and perspectives
Wound dressings play a significant role in the wound healing process by defending against external microorganisms, maintaining humidity, and releasing drugs. pH-Responsive wound dressings can tune their properties and functions according to the wound status, thereby allowing wounds to heal in a controlled manner and accelerating the healing process. This review summarizes the recent advances in pH-responsive wound dressings, mainly involving three aspects: hydrogels, other materials, and pH-responsive materials. Innovative pH-responsive wound dressings are expected to emerge through rational design and development and achieve excellent clinical performances. Although some preliminary success has been achieved with pH-responsive wound dressings, some challenges remain. Here, we presented the main problems and development trends of pH-responsive wound dressings.5.1. Multifunctionality
Wound repair is a dynamic and complex process, requiring more than one wound dressing for the entire process. At present, most pH-responsive wound dressings only focus on one or several functions (such as antibacterial, hemostatic, and antioxidant) and do not involve the whole procedure of skin tissue regeneration. Therefore, in the future, pH-responsive wound dressings will develop in the direction of multi-function. Additionally, pH-responsive wound dressings are usually designed based on a single aspect, such as pH-responsive drug release or pH-responsive liquid-to-gel transition. The development of a comprehensive pH-responsive design requires further research.5.2. Pertinence
Wound types are complex and diverse. The microenvironments of acute wounds, chronic wounds, infected wounds, and special wounds (diabetic ulcers and burns) are significantly different. In addition, the functional emphasis of pH-responsive wound dressings is different for certain wounds. Therefore pH-responsive wound dressings must be prepared depending on the application. Furthermore, pH-responsive wound dressings have been used only in skin wounds, and no studies have been conducted on mucous membranes, especially on the vulnerable oral and nasal mucosa. Mucosal injury is also very common in daily life and medical activities, and the mucosal environment is more complex compared with skin, which makes the development of pH-responsive wound dressings more difficult.5.3. Integration of diagnosis and treatment
The integration of diagnosis and treatment is a promising strategy for pH-responsive wound dressings. The pH-monitoring function allows physicians to assess the state of the wound while simultaneously monitoring the therapeutic effect of the dressings. Based on the results, it can be decided whether the dressing needs to be replaced or whether further surgical measures should be taken. To date, the pH-monitoring function is mainly attributed to pH indicator dyes that reflect the pH by the variation of visible light/fluorescence; in addition to optical detection, other monitoring mechanisms are awaiting further development.5.4. Personalization
Personalized customization is also needed in the clinic because of differences in wound characteristics, size, and depth. The wound could be better covered and wrapped by pH-responsive wound dressings that could be trimmed or shaped. 3D printing technology may provide a new strategy to personalize pH-responsive wound dressings. It is reported that 3D-printed hydrogels and nanofibers have been developed, and 3D printing technology will add new vitality to the development of pH-responsive wound dressing in the near future.Overall, pH-responsive wound dressings have great potential for promoting wound healing owing to their multifunctional merits, such as antibacterial, anti-inflammatory, hemostasis, adhesion, on-demand drug release, and pH monitoring. With the continuous development of interdisciplinary research, it is anticipated that pH-responsive wound dressings will become an important diagnostic and therapeutic tool for wound healing.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank for the financial support of National Science Foundation of China (51803098), Shandong Provincial Natural Science Foundation (ZR2022QE273) and Youth Scientific Research Foundation from the Affiliated Hospital of Qingdao University (QDFYQN202102046). We would like to express our gratitude to Xinqing Fu for the exquisite drawings of Fig. 1, 2, 3 and 9.References
- E. M. Tottoli, R. Dorati, I. Genta, E. Chiesa, S. Pisani and B. Conti, Pharmaceutics, 2020, 12, 1–30 CrossRef PubMed.
- H. S. Kim, X. Sun, J. H. Lee, H. W. Kim, X. Fu and K. W. Leong, Adv. Drug Delivery Rev., 2019, 146, 209–239 CrossRef CAS PubMed.
- K. Kwiecien, A. Zegar, J. Jung, P. Brzoza, M. Kwitniewski, U. Godlewska, B. Grygier, P. Kwiecinska, A. Morytko and J. Cichy, Cytokine Growth Factor Rev., 2019, 49, 70–84 CrossRef CAS PubMed.
- A. Argenta, L. Satish, P. Gallo, F. Liu and S. Kathju, PLoS One, 2016, 11, 1–16 CrossRef PubMed.
- K. Woo, G. Sibbald, K. Fogh, C. Glynn, D. Krasner, D. Leaper, J. Österbrink, P. Price and L. Teot, Int. Wound J., 2008, 5, 205–215 CrossRef PubMed.
- A. Popescu and R. S. Salcido, Adv. Skin Wound Care, 2004, 17, 14–22 CrossRef PubMed.
- N. Pazyar, R. Yaghoobi, E. Rafiee, A. Mehrabian and A. Feily, Skin Pharmacol. Physiol., 2014, 27, 303–310 CrossRef CAS PubMed.
- R. F. Pereira and P. J. Bártolo, Adv. Wound Care, 2016, 5, 208–229 CrossRef PubMed.
- H. Sorg, D. J. Tilkorn, S. Hager, J. Hauser and U. Mirastschijski, Eur. Surg. Res., 2017, 58, 81–94 CrossRef PubMed.
- S. A. Eming, T. Krieg and J. M. Davidson, J. Invest. Dermatol., 2007, 127, 514–525 CrossRef CAS PubMed.
- D. Leaper, O. Assadian and C. E. Edmiston, Br. J. Dermatol., 2015, 173, 351–358 CrossRef CAS PubMed.
- J. Clark and A. Sharp, Art Sci. Diabetes., 2011, 25, 41–47 Search PubMed.
- G. Power, Z. Moore and T. O’Connor, J. Wound Care, 2017, 26, 381–397 CrossRef CAS PubMed.
- T. Abdelrahman and H. Newton, Surgery, 2011, 29, 491–495 Search PubMed.
- E. A. Kamoun, E. R. S. Kenawy and X. Chen, J. Adv. Res., 2017, 8, 217–233 CrossRef CAS PubMed.
- M. S. Khil, D. Il Cha, H. Y. Kim, I. S. Kim and N. Bhattarai, J. Biomed. Mater. Res., Part B, 2003, 67, 675–679 CrossRef PubMed.
- Y. Feng, X. Li, Q. Zhang, S. Yan, Y. Guo, M. Li and R. You, Carbohydr. Polym., 2019, 216, 17–24 CrossRef CAS PubMed.
- R. S. Ambekar and B. Kandasubramanian, Eur. Polym. J., 2019, 117, 304–336 CrossRef CAS.
- S. Tavakoli and A. S. Klar, Biomolecules, 2020, 10, 1–20 CrossRef PubMed.
- Y. Liang, J. He and B. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS PubMed.
- F. Mariani, M. Serafini, I. Gualandi, D. Arcangeli, F. Decataldo, L. Possanzini, M. Tessarolo, D. Tonelli, B. Fraboni and E. Scavetta, ACS Sens., 2021, 6, 2366–2377 CrossRef CAS PubMed.
- N. Østergaard Knudsen and G. Pommergaard Pedersen, Curr. Probl. Dermatol., 2018, 54, 143–151 Search PubMed.
- L. A. Wallace, L. Gwynne and T. Jenkins, Ther. Delivery, 2019, 10, 719–735 CrossRef CAS PubMed.
- Y. Huang, L. Mu, X. Zhao, Y. Han and B. Guo, ACS Nano, 2022, 16, 13022–13036 CrossRef CAS PubMed.
- L. A. Schneider, A. Korber, S. Grabbe and J. Dissemond, Arch. Dermatol. Res., 2007, 298, 413–420 CrossRef PubMed.
- C. Ding, M. Tian, R. Feng, Y. Dang and M. Zhang, ACS Biomater. Sci. Eng., 2020, 6, 3855–3867 CrossRef CAS PubMed.
- M. A. Qureshi and F. Khatoon, Polym.-Plast. Technol. Eng., 2015, 54, 573–580 CrossRef CAS.
- W. Shao, H. Liu, J. Wu, S. Wang, X. Liu, M. Huang and P. Xu, J. Taiwan Inst. Chem. Eng., 2016, 63, 404–410 CrossRef CAS.
- L. Fan, Z. He, X. Peng, J. Xie, F. Su, D. X. Wei, Y. Zheng and D. Yao, ACS Appl. Mater. Interfaces, 2021, 13, 53541–53552 CrossRef CAS PubMed.
- L. Zhang, Y. Zhou, D. Su, S. Wu, J. Zhou and J. Chen, J. Mater. Chem. B, 2021, 9, 5887–5897 RSC.
- X. Zhao, Y. Liang, Y. Huang, J. He, Y. Han and B. Guo, Adv. Funct. Mater., 2020, 30, 1–18 Search PubMed.
- Y. Liang, Z. Li, Y. Huang, R. Yu and B. Guo, ACS Nano, 2021, 15, 7078–7093 CrossRef CAS PubMed.
- M. U. A. Khan, I. Iqbal, M. N. M. Ansari, S. I. A. Razak, M. A. Raza, A. Sajjad, F. Jabeen, M. R. Mohamad and N. Jusoh, Molecules, 2021, 26, 5937 CrossRef CAS PubMed.
- M. M. Ghobashy, A. M. Elbarbary, D. E. Hegazy and N. A. Maziad, J. Drug Delivery Sci. Technol., 2022, 73, 103399 CrossRef CAS.
- C. R. Reshmi, P. S. Suja, O. Manaf, P. P. Sanu and A. Sujith, Int. J. Biol. Macromol., 2018, 108, 1261–1272 CrossRef PubMed.
- W. S. Al-Arjan, M. U. A. Khan, H. H. Almutairi, S. M. Alharbi and S. I. A. Razak, Polymers, 2022, 14, 1949 CrossRef CAS PubMed.
- L. Bonetti, A. Fiorati, A. D’agostino, C. M. Pelacani, R. Chiesa, S. Farè and L. De Nardo, Gels, 2022, 8, 298 CrossRef CAS PubMed.
- S. Du, X. Chen, X. Chen, S. Li, G. Yuan, T. Zhou, J. Li, Y. Jia, D. Xiong and H. Tan, Macromol. Chem. Phys., 2019, 220, 1–11 Search PubMed.
- X. Fan, L. Yang, T. Wang, T. Sun and S. Lu, Eur. Polym. J., 2019, 121, 109290 CrossRef CAS.
- G. Yang, Z. Zhang, K. Liu, X. Ji, P. Fatehi and J. Chen, J. Nanobiotechnol., 2022, 1–16 Search PubMed.
- J. Qu, X. Zhao, Y. Liang, T. Zhang, P. X. Ma and B. Guo, Biomaterials, 2018, 183, 185–199 CrossRef CAS PubMed.
- W. Shao, H. Liu, X. Liu, H. Sun, S. Wang and R. Zhang, Int. J. Biol. Macromol., 2015, 76, 209–217 CrossRef CAS PubMed.
- M. U. A. Khan, S. Haider, M. A. Raza, S. A. Shah, S. I. A. Razak, M. R. A. Kadir, F. Subhan and A. Haider, Int. J. Biol. Macromol., 2021, 192, 820–831 CrossRef CAS PubMed.
- M. S. Hosseini and M. R. Nabid, Int. J. Biol. Macromol., 2020, 163, 336–347 CrossRef CAS PubMed.
- J. Zhu, H. Han, T. T. Ye, F. X. Li, X. L. Wang, J. Y. Yu and D. Q. Wu, Molecules, 2018, 23, 3383 CrossRef PubMed.
- M. U. A. Khan, S. I. Abd Razaq, H. Mehboob, S. Rehman, W. S. Al-Arjan and R. Amin, Polymers, 2021, 13, 3703 CrossRef CAS PubMed.
- Y. Ren, X. Yu, Z. Li, D. Liu and X. Xue, J. Photochem. Photobiol., B, 2020, 202, 111676 CrossRef CAS PubMed.
- C. Hu, L. Long, J. Cao, S. Zhang and Y. Wang, Chem. Eng. J., 2021, 411, 128564 CrossRef CAS.
- M. Du, J. Jin, F. Zhou, J. Chen and W. Jiang, Colloids Surf., B, 2023, 222, 113063 CrossRef CAS PubMed.
- M. Shi, H. Zhang, T. Song, X. Liu, Y. Gao, J. Zhou and Y. Li, ACS Appl. Mater. Interfaces, 2019, 11, 22730–22744 CrossRef CAS PubMed.
- F. Pan, G. Giovannini, S. Zhang, S. Altenried, F. Zuber, Q. Chen, L. F. Boesel and Q. Ren, Acta Biomater., 2022, 145, 172–184 CrossRef CAS PubMed.
- S. Noori, M. Kokabi and Z. M. Hassan, J. Appl. Polym. Sci., 2018, 135, 1–12 CrossRef.
- S. Sattari, A. D. Tehrani and M. Adeli, Polymers, 2018, 10, 660 CrossRef PubMed.
- N. S. Bostancı, S. Büyüksungur, N. Hasirci and A. Tezcaner, Biomater. Adv., 2022, 134, 112717 CrossRef PubMed.
- N. Ninan, A. Forget, V. P. Shastri, N. H. Voelcker and A. Blencowe, ACS Appl. Mater. Interfaces, 2016, 8, 28511–28521 CrossRef CAS PubMed.
- M. Ma, Y. Zhong and X. Jiang, Carbohydr. Polym., 2020, 236, 116096 CrossRef CAS PubMed.
- M. Fu, Y. Zhao, Y. Wang, Y. Li, M. Wu, Q. Liu, Z. Hou, Z. Lu, K. Wu and J. Guo, Small, 2022, 2205489 Search PubMed.
- Z. Ahmadian, A. Correia, M. Hasany, P. Figueiredo, F. Dobakhti, M. R. Eskandari, S. H. Hosseini, R. Abiri, S. Khorshid, J. Hirvonen, H. A. Santos and M. A. Shahbazi, Adv. Healthcare Mater., 2021, 10, 1–19 Search PubMed.
- M. Wang, H. Huang, C. Huang, S. Liu and X. Peng, Carbohydr. Polym., 2022, 292, 119643 CrossRef CAS PubMed.
- Y. Zu, Y. Wang, H. Yao, L. Yan, W. Yin and Z. Gu, ACS Appl. Bio Mater., 2022, 5, 1779–1793 CrossRef CAS PubMed.
- C. Xue, X. Xu, L. Zhang, Y. Liu, S. Liu, Z. Liu, M. Wu and Q. Shuai, Colloids Surf., B, 2022, 218, 112738 CrossRef CAS PubMed.
- Y. Li, C. Zhu, D. Fan, R. Fu, P. Ma, Z. Duan, X. Li, H. Lei and L. Chi, Int. J. Polym. Mater. Polym. Biomater., 2020, 69, 505–515 CrossRef CAS.
- A. S. Montaser, M. Rehan and M. E. El-Naggar, Int. J. Biol. Macromol., 2019, 124, 1016–1024 CrossRef CAS PubMed.
- T. M. D. Le, H. T. T. Duong, T. Thambi, V. H. Giang Phan, J. H. Jeong and D. S. Lee, Biomacromolecules, 2018, 19, 3536–3548 CrossRef CAS PubMed.
- B. Lu, X. Han, D. Zou, X. Luo, L. Liu, J. Wang, M. F. Maitz, P. Yang, N. Huang and A. Zhao, Mater. Today Bio, 2022, 16, 100392 CrossRef CAS PubMed.
- Z. Li, Y. Zhao, H. Liu, M. Ren, Z. Wang, X. Wang, H. Liu, Y. Feng, Q. Lin, C. Wang and J. Wang, Mater. Des., 2021, 210, 110104 CrossRef CAS.
- L. Zhao, L. Niu, H. Liang, H. Tan, C. Liu and F. Zhu, ACS Appl. Mater. Interfaces, 2017, 9, 37563–37574 CrossRef CAS PubMed.
- Y. Liang, M. Li, Y. Yang, L. Qiao, H. Xu and B. Guo, ACS Nano, 2022, 16, 3194–3207 CrossRef CAS PubMed.
- M. Li, Y. Liang, J. He, H. Zhang and B. Guo, Chem. Mater., 2020, 32, 9937–9953 CrossRef CAS.
- L. Liu, Z. Han, F. An, X. Gong, C. Zhao, W. Zheng, L. Mei and Q. Zhou, J. Nanobiotechnol., 2021, 19, 1–23 CrossRef PubMed.
- Y. C. Yeh, T. H. Huang, S. C. Yang, C. C. Chen and J. Y. Fang, Front. Chem., 2020, 8, 1–22 CrossRef PubMed.
- X. Hao, L. Huang, C. Zhao, S. Chen, W. Lin, Y. Lin, L. Zhang, A. Sun, C. Miao, X. Lin, M. Chen and S. Weng, Mater. Sci. Eng., C, 2021, 123, 111971 CrossRef CAS PubMed.
- G. Gao, Y. W. Jiang, H. R. Jia and F. G. Wu, Biomaterials, 2019, 188, 83–95 CrossRef CAS PubMed.
- J. Hu, C. Zhang, L. Zhou, Q. Hu, Y. Kong, D. Song, Y. Cheng and Y. Zhang, Sci. China Mater., 2021, 64, 1035–1046 CrossRef CAS.
- Y. Fan, M. Lüchow, Y. Zhang, J. Lin, L. Fortuin, S. Mohanty, A. Brauner and M. Malkoch, Adv. Funct. Mater., 2021, 31, 2006453 CrossRef CAS.
- K. Nuutila, J. Grolman, L. Yang, M. Broomhead, S. Lipsitz, A. Onderdonk, D. Mooney and E. Eriksson, Adv. Wound Care, 2020, 9, 48–60 CrossRef PubMed.
- F. Pan, G. Giovannini, S. Zhang, S. Altenried, F. Zuber, Q. Chen, L. F. Boesel and Q. Ren, Acta Biomater., 2022, 145, 172–184 CrossRef CAS PubMed.
- J. Dai, R. Han, Y. Xu, N. Li, J. Wang and W. Dan, Bioorg. Chem., 2020, 101, 103922 CrossRef CAS PubMed.
- Y. Yan, X. Li, C. Zhang, L. Lv, B. Gao and M. Li, Antibiotics, 2021, 10, 318 CrossRef CAS PubMed.
- Y. Wang, Y. Yang, Y. Shi, H. Song and C. Yu, Adv. Mater., 2019, 1904106, 1–21 Search PubMed.
- D. S. Lee, S. Sinno and A. Khachemoune, Am. J. Clin. Dermatol., 2011, 12, 181–190 CrossRef PubMed.
- J. Yupanqui Mieles, C. Vyas, E. Aslan, G. Humphreys, C. Diver and P. Bartolo, Pharmaceutics, 2022, 14, 1–36 CrossRef PubMed.
- G. Yang, Z. Zhang, K. Liu, X. Ji, P. Fatehi and J. Chen, J. Nanobiotechnol., 2022, 20, 1–16 CrossRef PubMed.
- A. Giordano and G. Tommonaro, Nutrients, 2019, 11, 2376 CrossRef CAS PubMed.
- S. J. Stohs, O. Chen, S. D. Ray, J. Ji, L. R. Bucci and H. G. Preuss, Molecules, 2020, 25, 1–12 CrossRef PubMed.
- B. Kaczmarek, Materials, 2020, 13, 3224 CrossRef CAS PubMed.
- W. Ge, S. Cao, F. Shen, Y. Wang, J. Ren and X. Wang, Carbohydr. Polym., 2019, 224, 115147 CrossRef CAS PubMed.
- W. Yan, M. Shi, C. Dong, L. Liu and C. Gao, Adv. Colloid Interface Sci., 2020, 284, 102267 CrossRef CAS PubMed.
- A. M. Szpaderska and L. A. DiPietro, Surgery, 2005, 137, 571–573 CrossRef PubMed.
- A. C. Lucchini, N. N. Gachanja, A. G. Rossi, D. A. Dorward and C. D. Lucas, Cells, 2021, 10, 1–19 CrossRef PubMed.
- S. Guo and L. A. DiPietro, J. Dent. Res., 2010, 89, 219–229 CrossRef CAS PubMed.
- Z. Xu, B. Liang, J. Tian and J. Wu, Biomater. Sci., 2021, 9, 4388–4409 RSC.
- J. Musuuza, B. L. Sutherland, S. Kurter, P. Balasubramanian, C. M. Bartels and M. B. Brennan, J. Vasc. Surg., 2020, 71, 1433–1446.e3 CrossRef PubMed.
- A. Rathnayake, A. Saboo, U. H. Malabu and H. Falhammar, World J. Diabetes, 2020, 11, 391–399 CrossRef PubMed.
- M. Chang and T. T. Nguyen, Acc. Chem. Res., 2021, 54, 1080–1093 CrossRef CAS PubMed.
- C. R. Reshmi, P. Sagitha and A. Sujith, Cellulose, 2022, 29, 427–443 CrossRef CAS.
- F. Chowdhury, S. Ahmed, M. Rahman, M. A. Ahmed, M. D. Hossain, H. M. Reza, S. Y. Park and S. M. Sharker, Mater. Today Commun., 2022, 31, 103310 CrossRef CAS.
- X. Xie, T. C. Sun, J. Xue, Z. Miao, X. Yan, W. W. Fang, Q. Li, R. Tang, Y. Lu, L. Tang, Z. Zha and T. He, Adv. Funct. Mater., 2020, 30, 1–10 Search PubMed.
- A. Ullah, M. Jang, H. Khan, H. J. Choi, S. An, D. Kim, Y. R. Kim, U. K. Kim and G. M. Kim, Sens. Actuators, B, 2021, 345, 130441 CrossRef CAS.
- Y. Liu, R. Song, X. Zhang and D. Zhang, Int. J. Biol. Macromol., 2020, 161, 1405–1413 CrossRef CAS PubMed.
- E. Rahmani, M. Pourmadadi, N. Zandi, A. Rahdar and F. Baino, Micromachines, 2022, 13, 1847 CrossRef PubMed.
- P. Heydari Foroushani, E. Rahmani, I. Alemzadeh, M. Vossoughi, M. Pourmadadi, A. Rahdar and A. M. Díez-Pascual, Nanomaterials, 2022, 12, 3426 CrossRef CAS PubMed.
- M. Gorji, D. Zarbaf, S. Mazinani, A. S. Noushabadi, M. A. Cella, A. Sadeghianmaryan and A. Ahmadi, J. Biomater. Sci., Polym. Ed., 2022, 0, 1–21 Search PubMed.
- L. Miranda-Calderon, C. Yus, G. Landa, G. Mendoza, M. Arruebo and S. Irusta, Int. J. Pharm., 2022, 624, 122003 CrossRef CAS PubMed.
- R. Chen, C. Zhao, Z. Chen, X. Shi, H. Zhu, Q. Bu, L. Wang, C. Wang and H. He, Biomaterials, 2022, 281, 121330 CrossRef CAS PubMed.
- Y. He, R. Chen, C. Zhao, Q. Lu, Z. Chen, H. Zhu, Q. Bu, L. Wang and H. He, ACS Appl. Mater. Interfaces, 2022, 51630–51644 CrossRef CAS PubMed.
- T. W. Huang, H. T. Lu, Y. C. Ho, K. Y. Lu, P. Wang and F. L. Mi, Mater. Sci. Eng., C, 2021, 118, 111396 CrossRef CAS PubMed.
- A. Mohebali and M. Abdouss, Int. J. Biol. Macromol., 2020, 164, 4193–4204 CrossRef CAS PubMed.
- R. B. Vasani, E. J. Szili, G. Rajeev and N. H. Voelcker, Chem. – Asian J., 2017, 12, 1605–1614 CrossRef CAS PubMed.
- H. Cui, M. Liu, W. Yu, Y. Cao, H. Zhou, J. Yin, H. Liu, S. Que, J. Wang, C. Huang, C. Gong and G. Zhao, ACS Appl. Mater. Interfaces, 2021, 13, 26800–26807 CrossRef CAS PubMed.
- A. B. Ozdabak-Sert, B. Sen and F. N. Kok, J. Appl. Polym. Sci., 2019, 136, 1–10 CrossRef.
- M. Wang, C. Wang, M. Chen, Y. Xi, W. Cheng, C. Mao, T. Xu, X. Zhang, C. Lin, W. Gao, Y. Guo and B. Lei, ACS Nano, 2019, 13, 10279–10293 CrossRef CAS PubMed.
- S. T. Oh, W. R. Kim, S. H. Kim, Y. C. Chung and J. S. Park, Fibers Polym., 2011, 12, 159–165 CrossRef CAS.
- S. Sharma, A. Dua and A. Malik, J. Polym. Res., 2017, 24, 1–8 CrossRef CAS.
- S. Chen, J. V. John, A. McCarthy and J. Xie, J. Mater. Chem. B, 2020, 8, 3733–3746 RSC.
- R. S. Ambekar and B. Kandasubramanian, Eur. Polym. J., 2019, 117, 304–336 CrossRef CAS.
- M. Abrigo, S. L. McArthur and P. Kingshott, Macromol. Biosci., 2014, 14, 772–792 CrossRef CAS PubMed.
- A. Ullah, M. Jang, H. Khan, H. J. Choi, S. An, D. Kim, Y. R. Kim, U. K. Kim and G. M. Kim, Sens. Actuators, B, 2021, 345, 130441 CrossRef CAS.
- J. H. Jung and S. G. Jin, J. Pharm. Invest., 2021, 51, 503–517 CrossRef PubMed.
- K. Ahmed Saeed AL-Japairai, S. Mahmood, S. Hamed Almurisi, J. Reddy Venugopal, A. Rebhi Hilles, M. Azmana and S. Raman, Int. J. Pharm., 2020, 587, 119673 CrossRef CAS PubMed.
- R. Jamaledin, C. K. Y. Yiu, E. N. Zare, L. N. Niu, R. Vecchione, G. Chen, Z. Gu, F. R. Tay and P. Makvandi, Adv. Mater., 2020, 32, 1–29 CrossRef PubMed.
- G. Kiaee, P. Mostafalu, M. Samandari and S. Sonkusale, Adv. Healthcare Mater., 2018, 7, 1–8 Search PubMed.
- S. L. Percival, S. McCarty, J. A. Hunt and E. J. Woods, Wound Repair Regen., 2014, 22, 174–186 CrossRef PubMed.
- M. S. Brown, B. Ashley and A. Koh, Front. Bioeng. Biotechnol., 2018, 6, 1–21 CrossRef PubMed.
- K. Kiti, C. Thanomsilp and O. Suwantong, Microchem. J., 2022, 177, 107277 CrossRef CAS.
- O. Alaysuy, R. M. Snari, A. A. Alfi, A. M. Aldawsari, S. Abu-Melha, M. E. Khalifa and N. M. El-Metwaly, Int. J. Biol. Macromol., 2022, 211, 390–399 CrossRef CAS PubMed.
- N. Pan, J. Qin, P. Feng, Z. Li and B. Song, J. Mater. Chem. B, 2019, 7, 2626–2633 RSC.
- J. Zhou, B. Jiang, C. Gao, K. Zhu, W. Xu and D. Song, Sens. Actuators, B, 2022, 355, 131310 CrossRef CAS.
- P. Yang, Z. Zhu, T. Zhang, W. Zhang, W. Chen, Y. Cao, M. Chen and X. Zhou, Small, 2019, 15, 1–11 Search PubMed.
- K. M. Zepon, M. M. Martins, M. S. Marques, J. M. Heckler, F. Dal Pont Morisso, M. G. Moreira, A. L. Ziulkoski and L. A. Kanis, Carbohydr. Polym., 2019, 206, 362–370 CrossRef CAS PubMed.
- L. Wang, M. Zhou, T. Xu and X. Zhang, Chem. Eng. J., 2022, 433, 134625 CrossRef CAS.
- M. Kurečič, T. Maver, N. Virant, A. Ojstršek, L. Gradišnik, S. Hribernik, M. Kolar, U. Maver and K. S. Kleinschek, Cellulose, 2018, 25, 7277–7297 CrossRef.
- P. Tan, X. Chen, H. Zhang, Q. Wei and K. Luo, Semin. Cancer Biol., 2023, 89, 61–75 CrossRef CAS PubMed.
- N. M. Elkenawy, H. M. Karam and D. S. Aboul-magd, Int. J. Biol. Macromol., 2022, 211, 170–182 CrossRef CAS PubMed.
- A. A. Arafa, A. A. Nada, A. Y. Ibrahim, P. Sajkiewicz, M. K. Zahran and O. A. Hakeim, Int. J. Biol. Macromol., 2021, 182, 1820–1831 CrossRef CAS PubMed.
- A. A. Arafa, A. A. Nada, A. Y. Ibrahim, M. K. Zahran and O. A. Hakeim, Eur. Polym. J., 2021, 159, 110744 CrossRef CAS.
- Y. Zhu, J. Zhang, J. Song, J. Yang, Z. Du, W. Zhao, H. Guo, C. Wen, Q. Li, X. Sui and L. Zhang, Adv. Funct. Mater., 2020, 30, 1–9 Search PubMed.
- L. Liu, X. Li, M. Nagao, A. L. Elias, R. Narain and H. J. Chung, Polymers, 2017, 9, 558 CrossRef PubMed.
- B. Qiao, Q. Pang, P. Yuan, Y. Luo and L. Ma, Biomater. Sci., 2020, 8, 1649–1657 RSC.
- M. Omidi, A. Yadegari and L. Tayebi, RSC Adv., 2017, 7, 10638–10649 RSC.
- K. Zheng, Y. Tong, S. Zhang, R. He, L. Xiao, Z. Iqbal, Y. Zhang, J. Gao, L. Zhang, L. Jiang and Y. Li, Adv. Funct. Mater., 2021, 31, 1–13 Search PubMed.
- L. Cui, J. Jing Hu, W. Wang, C. Yan, Y. Guo and C. Tu, Cellulose, 2020, 27, 6367–6381 CrossRef CAS.
- A. Truskewycz, V. K. Truong, A. S. Ball, S. Houshyar, N. Nassar, H. Yin, B. J. Murdoch and I. Cole, ACS Appl. Mater. Interfaces, 2021, 13, 27904–27919 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally in this work. |
| This journal is © The Royal Society of Chemistry 2023 |