 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality
Natalia
Manousi
 a,
Wojciech
Wojnowski
a,
Wojciech
Wojnowski
 bc,
Justyna
Płotka-Wasylka
cd and
Victoria
Samanidou
bc,
Justyna
Płotka-Wasylka
cd and
Victoria
Samanidou
 *a
*a
aLaboratory of Analytical Chemistry, School of Chemistry, Aristotle University of Thessaloniki, GR 54124 Thessaloniki, Greece. E-mail: samanidu@chem.auth.gr
bDepartment of Chemistry, University of Oslo, P.O. Box 1033-Blindern, 0315 Oslo, Norway
cDepartment of Analytical Chemistry, Faculty of Chemistry, Gdańsk University of Technology, 11/12 G. Narutowicza Street, 80-233 Gdańsk, Poland
dBioTechMed Center, Gdańsk University of Technology, 11/12 G. Narutowicza Street, 80-233 Gdańsk, Poland
First published on 22nd August 2023
Abstract
In this work, blue applicability grade index (BAGI) is proposed as a new metric tool for evaluating the practicality of an analytical method. BAGI can be considered complementary to the well-established green metrics, and it is mainly focused on the practical aspects of White Analytical Chemistry. This tool evaluates ten main attributes including the type of analysis, the number of analytes that are simultaneously determined, the number of samples that can be analyzed per hour, the type of reagents and materials used in the analytical method, the required instrumentation, the number of samples that can be simultaneously treated, the requirement for preconcentration, the automation degree, the type of sample preparation, and the amount of sample. Through the evaluation of these attributes, an asteroid pictogram is generated, together with the respective score. To facilitate the use of the metric a simple, open-source application was created (mostwiedzy.pl/bagi). It is accompanied by a web application available at bagi-index.anvil.app. The functionality of the tool was demonstrated by evaluating the applicability of five different analytical methods as case studies. All things considered, BAGI can be easily used to identify the weak and strong points of a method in terms of practicality and applicability, as well as to compare the performance of different analytical methods. We believe that BAGI metric tool will gain not only attention but also trust and acceptance from the chemical community.
1. Introduction
Green chemistry was originally proposed in the 1990s by Paul Anastas and John Warner as a way in which the skills, knowledge, and talents of chemists can be combined to avoid threats to human health and the environment in all types of chemical processes.1 Green Analytical Chemistry (GAC)2 is a concept that emerged from green chemistry in 2000 and it concerns the role of analytical chemists in making laboratory practices more environmentally friendly. GAC considers different aspects of an analytical method including the safety of solvents/reagents, the generation of toxic laboratory wastes, the safety of the analysts, and the energy demands, aiming to redefine and reevaluate the analytical methods. Moreover, the ten principles of Green Sample Preparation (GSP) were proposed aiming to chart the path towards the development of greener sample preparation methods, to minimize the environmental impact of this step and to enhance sample throughput.3 Thus, today compliance with green chemistry, GAC,2 and GSP3 principles has become a necessity in the development of analytical methods to reassure sustainability requirements. More recently, the Unified Greenness Theory was presented that merged the principles of green chemistry, GAC, and other sets of principles and introduced a novel set of hierarchal and universal statements.4Several green metric tools have been proposed and already implemented in recent publications to evaluate the green performance of an analytical method and its subsequent impact on the environment. These tools include the National Environmental Method Index (NEMI),5 analytical eco-scale,6 green analytical procedure index (GAPI),7 analytical greenness calculator (AGREE),8 complementary green analytical procedure index (ComplexGAPI),9 and analytical greenness metric for sample preparation (AGREEprep).10 Each of the above-mentioned tools has certain advantages and disadvantages; thus, some of them have prevailed since they provide a more quantitative description of the green character of the method.
However, none of these tools considers the practicality of the method, which is a very important parameter that is encountered by all routine analysis laboratories. This parameter has been already included in the concept of White Analytical Chemistry (WAC) that was introduced in 2021 by Nowak et al.11 WAC serves as an extension and complement to green analytical chemistry and combines the ecological, analytical, and practical perspectives of an analytical method according to the red-green-blue (RGB) model.12 The red colour of WAC is related to the analytical efficiency as described by the method's validation criteria (accuracy, precision, sensitivity, and others), while blue represents the productivity and practical/economic efficiency of the method. The four attributes of the ‘blue’ category correspond to cost-efficiency, time-efficiency, requirements, and operational simplicity.
In this article, we introduce a simple and fast metric tool for the evaluation of the practicality of any analytical method (e.g., conventional, state-of-the-art, newly developed, etc.). As such, the blue applicability grade index (BAGI) is developed and proposed herein. The blue colour is inspired by the RGB model, and the proposed index may be considered a complementary concept to the existing green metrics tools. To facilitate its use open-source desktop and web applications were developed, and their functionality was demonstrated based on various analytical methods. The target audience of this new tool includes but is not restricted to analytical method developers and users from academia, industry and routine analysis laboratories. BAGI tool has many advantages with the most important being complementary to the existing green assessment tools such as complexGAPI and AGREEprep. In addition, it is in line with the principles of environmental sustainability. We believe that the BAGI metric tool will gain not only attention but also trust and acceptance from the chemical community.
2. Characteristics of the BAGI attributes
To evaluate the applicability of an analytical method, the BAGI metric tool takes into consideration the following main attributes:1. The type of analysis.
2. The number of analytes that are simultaneously determined.
3. The analytical technique and required analytical instrumentation.
4. The number of samples that can be simultaneously treated.
5. The sample preparation.
6. The number of samples that can be analyzed per hour.
7. The type of reagents and materials used in the analytical method.
8. The requirement for preconcentration
9. The automation degree.
10. The amount of sample.
Attributes 1–3 correspond to the step of the analytical determination, attributes 4 and 5 correspond to the sample preparation step, while attributes 6–10 correspond to both steps. The selection of the main attributes and their respective levels was based on a consideration of a wide range of different analytical methods that were reported in the literature. To ensure the simplicity of the performance, four discrete scores of equal weights are used in the assessment. Each score corresponds to a different hue (for the qualitative evaluation of the method's applicability) and contributes to the final, overall score (for the quantitative evaluation of the method's applicability). In this sense, 10, 7.5, 5.0, and 2.5 points correspond to dark blue (#0c305b), blue (#3a89c1), light blue (#adcffd), and white (#FFFFFF), respectively. The BAGI tool also takes into consideration the field of application to adjust the bias and treat all methods at realistic ranges. For example, it distinguishes the differences between bioanalytical methods that can be applied to low sample amounts requiring low reagents amounts and food or environmental samples, where the sample amount can be easily increased to achieve the criteria required by legislation and the necessary sensitivity.
2.1 Description of attributes
Taking all into consideration, the analytical method gets 10 points in cases where common commercially available reagents (e.g. methanol, acetonitrile, HNO3, nitrogen or other common gases), are used. When commercially available reagents are required that are non-common in quality control laboratories (e.g., derivatization reagents, solid-phase extraction cartridges, solid-phase microextraction fibres), a score of 7.5 is attained. However, in cases where reagents are needed to be synthesized in the laboratory, a score of 5 or 2.5 points is added to the total, considering whether this can be performed in a simple way using common laboratory equipment or involves the use of advanced equipment/know-how (e.g., specially designed metal–organic frameworks), respectively.
The attributes along with their respective hues and score points are summarized in Table 1. It should be noted that when clear score cannot be given or the researcher are on the verge of two options, they have to choose the one that is closer to the available scores, based on their expertise.
| Criterion | Attribute | Dark blue (10 points) | Blue (7.5 points) | Light blue (5 points) | White (2.5 points) |
|---|---|---|---|---|---|
| a GC: gas chromatography, UV: ultraviolet spectrometry, HPLC-UV: high-performance liquid chromatography-ultraviolet detection, HPLC-DAD: high-performance liquid chromatography-diode array detection, UHPLC: ultra-high performance liquid chromatography, FAAS: flame atomic absorption spectrometry, ETAAS: electrothermal atomic absorption spectrometry, ICP-OES: inductively coupled plasma-optical emission spectrometry, GC-FID: gas chromatography-flame ionization detection, LC-MS: liquid chromatography-mass spectrometry, GC-MS: gas chromatography-mass spectrometry, ICP-MS: inductively coupled plasma-mass spectrometry, SFC: supercritical fluid chromatography, 2D-LC: two-dimensional liquid chromatography, 2D-GC: two-dimensional gas chromatography, LC-MS/MS: liquid chromatography-tandem mass spectrometry, GC-MS/MS: gas chromatography-tandem mass spectrometry. b SPME: solid phase microextraction, DLLME: dispersive liquid–liquid extraction, MEPS: microextraction by packed sorbents, SBSE: stir bar sorptive extraction, d-SPE: dispersive solid-phase extraction, FPSE: fabric phase sorptive, LLE: liquid–liquid extraction, SPE: solid-phase extraction. | |||||
| 1 | Type of analysis | Quantitative and confirmatory | Quantitative | Screening | Qualitative |
| 2 | Multi- or single-element analysis | Multi-element analysis for >15 compounds | Multi-element analysis for 6–15 compounds of the same chemical class or 2–15 compounds of different chemical classes | Multi-element analysis for 2–5 compounds of the same chemical class | Single element |
| 3 | Analytical techniquea | Simple in operation portable instrumentation (e.g., smart-phone based detectors, portable GC) | Simple instrumentation available in most labs (e.g., UV, HPLC-UV, HPLC-DAD, UHPLC, FAAS, ETAAS, ICP-OES, GC-FID) | Sophisticated instrumentation (e.g., LC-MS, GC-MS, ICP-MS, homemade interfaces, homemade automatic systems) | Instrumentation that is not commonly available in most labs (e.g., SFC, 2D-GC, 2D-LC, LC-MS/MS, GC-MS/MS) |
| 4 | Simultaneous sample preparation | >95 | 13–95 | 2–12 | 1 |
| 5 | Sample preparationb | Not required or on-site sample preparation if required | Simple low-cost sample preparation is required (protein precipitation etc.) | Miniaturized extraction sample preparation (e.g., SPME, DLLME, MEPS, SBSE, d-SPE, FPSE) | Multi-step sample preparation is required (e.g., LLE, SPE and/or derivatization) |
| 6 | Samples per h (sample preparation + analysis time) | >10 | 5–10 | 2–4 | ≤1 |
| 7 | Reagents and materials | Common commercially available reagents (methanol, acetonitrile, HNO3, nitrogen or other common gases etc.). | Commercially available reagents that are non-common in QC labs (e.g., derivatization reagents, SPE cartridges, SPME fibres) | Need to be synthesized in the lab with common instrumentation and in a simple way. | Need to be synthesized in the lab with advanced equipment or know-how (e.g., specially designed metal–organic frameworks, modified nanomaterials). |
| 8 | Preconcentration | No preconcentration is required. Required sensitivity and/or legislation criteria are directly met. | Preconcentration is required. Required sensitivity is met with one-step preconcentration. | — | Preconcentration is required. Legislation criteria are met after complicated stages (e.g., extraction, evaporation, and reconstitution). |
| 9 | Automation degree | Fully automated with novel technology advanced devices (e.g., robotics, lab-in-syringe) | Semi-automated with common devices (HPLC autosampler, etc.) | Semi-automated with non-common devices (homemade systems, etc.) | Manual treatment and analysis. |
| 10 | Amount of sample | ≤100 μL (or mg) bioanalytical samples | 101–500 μL (or mg) bioanalytical samples | 501–1000 μL (or mg) bioanalytical samples | >1000 μL (or mg) bioanalytical samples |
| ≤10 mL (or g) food/environmental/other. | 10.1–50 mL (or g) food/environmental. | 51–100 mL (or g) food/environmental. | >100 mL (or g) food/environmental. | ||
2.2 Explanation of the obtained results
Two different types of results can be obtained using the BAGI metric tool which are correlated to the obtained pictogram and the obtained score. The overall assessment result is an asteroid pictogram with the number in its centre. The hue scale of the pictogram shows the compliance of the method with the set criteria (i.e., dark blue for high compliance, blue for medium compliance, light blue for low compliance, and white for no compliance). The number in the inner part of the BAGI pictogram reveals the assigned overall score of the analytical method and it ranges between 25–100. The worst method performance in terms of applicability is assigned to a score value of 25, while a score value of 100 reveals the excellent performance of the method. In order to be considered practical”, it is recommended that the method attains at least 60 points. This score is recommendable, but not determinant. The ten parts of the asteroid pictogram are related to the different performance criteria, and they are considered of equal importance. The attributes 1–5 that correspond either to the step of the analytical determination or to the step of the sample preparation are in the inner part of the pictogram, while the attributes 6–10 that correspond to both steps are in the outer part. The central field containing the score is assigned a hue based on the value of the total score by sampling from the whole range of the matplotlib ‘blues’ sequential colourmap15 mapped to the 25–100 scale. The discrete hues for the individual fields of the pictogram were sampled from the same colour map. This allowed to obtain a perceptually uniform mapping of hues to values that is colourblind-safe and legible when reproduced in grayscale.16 This is akin to the approach used in the AGREE and AGREEprep tools.8,10Using the overall BAGI pictogram it is easy to find the weak and strong points of an analytical method by evaluating its applicability in terms of practicality to further improve them and to compare the performance of different methods.
3. Application of BAGI to selected methods
In this section, the utilization of the BAGI index web app is demonstrated. For this purpose, the new tool was used to evaluate the applicability of five different analytical methods dealing with the determination of antidepressants in post-mortem whole blood and cerebrospinal liquor,17 bisphenol A (BPA) in food contact materials’ leachates,18 androgens and progestogens in environmental water samples,19 ibuprofen in milk-containing simulated gastrointestinal media,20 and quinine in soft drinks.21 The BAGI index pictograms for these methods are depicted in Fig. 1.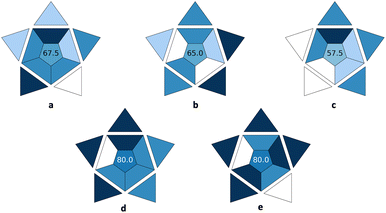 | ||
| Fig. 1 BAGI index pictograms for five different analytical methods for the (a) determination of antidepressants in post-mortem whole blood and cerebrospinal liquor,17 (b) bisphenol A (BPA) in food contact materials’ leachates,18 (c) androgens and progestogens in environmental water samples,19 (d) ibuprofen in milk-containing simulated gastrointestinal media,20 and (e) quinine in soft drinks.21 | ||
In the first case study, a fabric phase sorptive (FPSE) extraction method combined with high-performance liquid chromatography-diode array detection (HPLC-DAD) was used for the quantification of seven different antidepressant drugs in human whole blood, plasma, and urine.17 The information of the analysis was both quantitative and confirmatory due to the employment of the DAD detector that was set in the range of 200–400 nm. The determination enabled the quantification of seven compounds belonging to three different classes (i.e., serotonin and norepinephrine reuptake inhibitor; selective serotonin reuptake inhibitors and tricyclic antidepressants). Since the FPSE membranes are not commercially available, they need to be synthesized in the lab in a relatively simple and straightforward way using simple equipment. Regarding the instrumentation, simple equipment available in most labs was employed. The simultaneous sample preparation of almost 20 samples was assumed, which can be easily performed using two magnetic stirrers. Following the simultaneous sample preparation of the 20 samples that requires a time span of around 40 min, the total analysis time by HPLC-DAD is 20 min, resulting in a sample throughput of 2.7 h−1. As demonstrated by the results, no preconcentration was needed and the required sensitivity was directly achieved. Manual treatment and analysis were performed, which can be considered a drawback of the method, and it can be further improved by automating some steps of the analytical procedure. As for the sample preparation, miniaturized extraction was employed and the sample volume for the bioanalytical matrix was 500 μL. Thus, a BAGI score of 67.5 is attained for the method and the whole protocol shows good applicability potential.
In the second case study, an automatic lab-in-syringe sol–gel coated foam microextraction platform was used for monitoring BPA in food contact materials’ leachates during migration studies.18 The separation and determination of the target analyte were conducted using high-performance liquid chromatography-ultraviolet detection (HPLC-UV), resulting in quantitative analysis. Using this approach, a single element could be determined, which can be considered as a drawback of the method that can be potentially expanded to include other bisphenols. The sample throughput (sample preparation and analysis) was 5 h−1. The foam microextraction media were not commercially available, and their synthesis in the lab was required. Like the FPSE membranes, this can be performed in a relatively simple way using common reagents. The HPLC-UV system that is required belongs to the category of simple equipment which is available in most labs. Using the lab-in-syringe system, one sample can be treated at a time. The legislation criteria for the migration studies were achieved after the one-step extraction and preconcentration. The whole procedure was fully automatic, and it required the miniaturized extraction of BPA from 10 mL of sample solution. The assigned BAGI score for the developed method was 65, demonstrating its applicability.
In the third case study, the metal–organic framework UiO-66(Zr) was used as a sorbent for the porous membrane-protected micro-solid-phase extraction of androgens and progestogens from environmental water samples prior to their determination by liquid-chromatography tandem mass spectrometry (LC-MS/MS).19 Using this technique, both quantitative and confirmatory data can be obtained. As target analytes, four compounds belonging to more than one different class were included. Regarding MOF synthesis, advanced know-how and/or instrumentation are typically required for their preparation in the lab. Simultaneous sample preparation of around 10 samples was assumed for the micro-solid-phase extraction procedure that required around 90 min. Since a time span of 2.5 min was required for the LC-MS/MS analysis, the sample throughput (sample preparation and analysis) was between 5–10 h−1. Preconcentration was required after complicated stages including extraction, evaporation, and reconstitution. The LC-MS/MS systems are not commonly available in most labs. Finally, manual systems were used for the sample preparation and analysis, miniaturized extraction was proposed for sample preparation and 20 mL of water sample was required. The method had a BAGI score of 57.5, demonstrating that improvements are required to make it practical in laboratories.
In the fourth case study, the BAGI index was employed for the evaluation of an ultra-performance liquid chromatography-diode array detection (UPLC-DAD) analytical method for the determination of ibuprofen in milk-containing simulated gastrointestinal media.20 The rapid protein precipitation scheme and the analysis (2.5 min) resulted in a sample throughput of more than 10 h−1. Common, commercially available reagents were used, while instrumentation currently available in most labs was required. Simultaneous sample preparation of approx. 40 samples was assumed. No pre-concentration was required, manual treatment took place, an autosampler was used resulting in semi-automation, simple and low-cost sample preparation was chosen, and a sample volume of 200 μL was used. The BAGI score of 80 that was assigned to the method demonstrates its good applicability.
In the fifth case study, the applicability of an equipment-free paper-based fluorometric method for the determination of quinine in soft drinks was examined.21 The method was used for the quantification of a single analyte with only common, commercially available reagents and simple in operation instrumentation. The sample throughput of the method was higher than 10 h−1, while a simultaneous sample preparation of around 50 samples was assumed. No preconcentration was required to achieve the required sensitivity, as well as minimal sample preparation (i.e., dilution of the sample). An aliquot of only 1 μL of the sample was required for the analysis, and the whole operation was performed in manual mode. Thus, a BAGI score of 80 was assigned to the method demonstrating its superiority in terms of practicality and applicability.
4. Conclusions
In this work, a novel index that can efficiently assess the practicality and applicability of an analytical method is proposed. BAGI is complementary to the green assessment tools (e.g., GAPI, ComplexGAPI, AGREE, AGREEprep) and it revolves around the “blue” principles of white analytical chemistry, which are mainly related to practical aspects. BAGI considers ten criteria to produce a pictogram and a score that depicts the applicability and functionality of an analytical method. A sequential blue colour scale was used to represent the final score, with discrete hues of dark blue, blue, light blue, and white used to demonstrate high, medium, low, and no compliance of the method with the set criteria, respectively. Regarding the obtained total score, it is recommended to be higher than 60, so that the analytical method can be considered “practical”. The proposed index was used to evaluate the applicability in five different analytical methods. The assessment of the applicability of the analytical method is facilitated by using a desktop application (mostwiedzy.pl/bagi) or a corresponding web application (bagi-index.anvil.app) and enables the comparison of different analytical methods at a first glance. The biggest advantage of BAGI is the simplicity and ease of application, which was achieved by preparing an interesting web application that allows to quickly evaluate the method and copy a colourful pictogram presenting the results of the evaluation. We believe that the BAGI tool will gain attention and acceptance from the chemical community.Author contributions
N. Manousi: data curation, formal analysis, methodology, writing – original draft, W. Wojnowski: software, visualization, writing – review & editing, J. Płotka-Wasylka: methodology, supervision, visualization, writing – review & editing, V. Samanidou: conceptualization, data curation, formal analysis, methodology, supervision, visualization, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support of the study from Gdańsk University of Technology by the 32/WCh-BR/2021 grant under the Iconic Scholars-‘Excellence Initiative-Research University’ program is gratefully acknowledged by Justyna Płotka-Wasylka. Graphical abstract was created with BioRender.com.References
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- A. Gałuszka, Z. Migaszewski and J. Namieśnik, TrAC, Trends Anal. Chem., 2013, 50, 78–84 CrossRef.
- Á. I. López-Lorente, F. Pena-Pereira, S. Pedersen-Bjergaard, V. G. Zuin, S. A. Ozkan and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 148, 116530 CrossRef.
- P. M. Nowak, Green Chem., 2023, 25, 4625–4640 RSC.
- L. H. Keith, L. U. Gron, J. L. Young, C. Safety, C. Way, H. College and W. Avenue, Chem. Rev., 2007, 107, 2695–2708 CrossRef CAS PubMed.
- A. Gałuszka, Z. M. Migaszewski, P. Konieczka and J. Namieśnik, TrAC, Trends Anal. Chem., 2012, 37, 61–72 CrossRef.
- J. Płotka-Wasylka, Talanta, 2018, 181, 204–209 CrossRef PubMed.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed.
- J. Płotka-Wasylka and W. Wojnowski, Green Chem., 2021, 23, 8657–8665 RSC.
- W. Wojnowski, M. Tobiszewski, F. Pena-Pereira and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 149, 116553 CrossRef CAS.
- P. M. Nowak, R. Wietecha-Posłuszny and J. Pawliszyn, TrAC, Trends Anal. Chem., 2021, 138, 116223 CrossRef CAS.
- P. M. Nowak and P. Kościelniak, Anal. Chem., 2019, 91, 10343–10352 CrossRef CAS PubMed.
- Commission of the European Communities: European Commission Decision of 12 August 2002 Implementing Council Directive 96/23/ EC Concerning the Performance of Analytical Methods and the Interpretation of Results (2002/657/EC), Off J Eur Comm, L 221, 8–36.
- J. P. Hutchinson, L. Setkova and J. Pawliszyn, J. Chromatogr. A, 2007, 1149, 127–137 CrossRef CAS PubMed.
- J. D. Hunter, Comput. Sci. Eng., 2007, 9, 90–95 Search PubMed.
- K. D. Moreland, Diverging Color Maps for Scientific Visualization, 2009 Search PubMed.
- M. Locatelli, S. Covone, E. Rosato, M. Bonelli, F. Savini, K. G. Furton, I. Gazioglu, C. D'Ovidio, A. Kabir and A. Tartaglia, Forensic Chem., 2022, 31, 100460 CrossRef CAS.
- N. Manousi, I. Priovolos, A. Kabir, K. G. Furton, V. F. Samanidou and A. Anthemidis, Anal. Chim. Acta, 2023, 1268, 341400 CrossRef CAS PubMed.
- G. Gao, Y. Xing, T. Liu, J. Wang and X. Hou, Microchem. J., 2019, 146, 126–133 CrossRef CAS.
- A. Doumtsi, N. Manousi, C. Karavasili, D. G. Fatouros, P. D. Tzanavaras and C. K. Zacharis, J. Sep. Sci., 2022, 45, 3955–3965 CrossRef CAS PubMed.
- V. C. Tsaftari, M. Tarara, P. D. Tzanavaras and G. Z. Tsogas, Sensors, 2023, 23, 5153 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
