Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ionic liquid-based aqueous biphasic systems as one-step clean-up, microextraction and preconcentration platforms for the improved determination of salivary biomarkers†
Raúl
González-Martín
 abc,
Francisca A.
e Silva
abc,
Francisca A.
e Silva
 c,
María J.
Trujillo-Rodríguez
c,
María J.
Trujillo-Rodríguez
 abc,
David
Díaz Díaz
de,
Jacob
Lorenzo-Morales
fgh,
Mara G.
Freire
abc,
David
Díaz Díaz
de,
Jacob
Lorenzo-Morales
fgh,
Mara G.
Freire
 *c and
Verónica
Pino
*c and
Verónica
Pino
 *abg
*abg
aLaboratorio de Materiales para Análisis Químico (MAT4LL), Departamento de Química, Unidad Departamental de Química Analítica, Universidad de La Laguna (ULL), Avda. Astrofísico Francisco Sánchez, s/n, 38206, San Cristóbal de La Laguna, Spain. E-mail: veropino@ull.edu.es; Tel: +34 922318990
bUnidad de Investigación de Bioanalítica y Medio Ambiente, Instituto Universitario de Enfermedades Tropicales y Salud Pública de Canarias (IUETSP), ULL, Avda. Astrofísico Francisco Sánchez, s/n, 38206, San Cristóbal de La Laguna, Spain
cCICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193, Aveiro, Portugal. E-mail: maragfreire@ua.pt
dDepartamento de Química Orgánica, ULL, Avda. Astrofísico Francisco Sánchez 3, 38206, San Cristóbal de La Laguna, Spain
eInstituto de Bio-Orgánica Antonio González, ULL, Avda. Astrofísico Francisco Sánchez 2, 38206, San Cristóbal de La Laguna, Spain
fIUESTP, ULL, Avda. Astrofísico Francisco Sánchez, s/n, 38206, San Cristóbal de La Laguna, Spain
gCentro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III, 28029, Madrid, Spain
hDepartamento de Obstetricia y Ginecología, Pediatría, Medicina Preventiva y Salud Pública, Toxicología, Medicina Legal y Forense y Parasitología, ULL, 38200, San Cristóbal de La Laguna, Spain
First published on 8th September 2023
Abstract
Although saliva is a convenient human fluid for biomonitoring, current analytical set-ups for its analysis exhibit technical, sensitivity and instrumental compatibility constraints. To overcome these drawbacks, while following the Green Analytical Chemistry trends, this study is the first to propose the use of aqueous biphasic systems (ABSs) comprising the low cytotoxic butylguanidinium chloride ionic liquid (IL) and different salts as a saliva clean-up, microextraction and preconcentration tool. Among the developed and characterized ABSs, the one composed of the IL and K2HPO4 was selected to develop an integrated analytical procedure in saliva. Sample clean-up is achieved by removing 70% of the salivary proteins through precipitation as a solid interphase of the ABS – creating a three-phase partitioning (ABS/TPP) system – while a miniaturized extraction and preconcentration approach is simultaneously performed. The ABS/TPP, together with high-performance liquid chromatography and fluorescence detection (HPLC-FD), was optimized for bisphenols as representative biomarkers in saliva. Optimum conditions included 0.35 g of IL, 0.60 g of salt, 1.1 g of saliva, 1 min of stirring, and centrifugation. The ABS/TPP-HPLC-FD method exhibited enrichment factors up to ca. 3, extraction efficiencies higher than 80.5% despite using a miniaturized technique, and limits of detection down to 0.40 ng mL−1. The inter-day precision, expressed as relative standard deviation, was lower than 9.1%, achieving average relative recoveries of 106%. The method was successfully performed when analyzing male and female saliva. The green nature of the method as compared to other state-of-the-art techniques was demonstrated using several green metrics, scoring 0.63 in AGREEprep.
Introduction
Saliva has become a useful alternative to other biofluids for assessing human exposure to a wide variety of hazardous compounds, taking advantage of the continuous exchange of compounds between the oral cavity and human plasma.1 Thus, the monitoring of a variety of endocrine disrupting chemicals, contaminants (including those of emerging concern), and biomarkers in saliva, is a hotspot trend to assess the human risk of developing different diseases.2,3The analysis of saliva offers several advantages, as it is easier to collect than blood4 and is not limited to the determination of polar metabolites that are urinary excreted.5 Despite the abovementioned advantages of using saliva for biomonitoring, current methods for its analysis face two important issues: (i) the concentration levels of the target compounds are usually lower than those found in blood and urine, limiting determination; and (ii) the volume available of saliva is normally low (∼2–3 mL at maximum per collection).6 Thus, saliva analysis normally requires steps of preconcentration prior to the analytical quantification; therefore, miniaturized approaches have been proposed.7,8
However, attaining adequate preconcentration in a microsample is highly challenging. Most analyses of saliva need to combine microextraction strategies with highly sensitive instrumental techniques, normally with liquid chromatography (LC) or gas chromatography (GC) instruments with mass spectrometry (MS) detection.6 Nevertheless, even if MS detection is used, these methods necessarily require the removal of salivary proteins prior to the analysis to avoid damage to the instrumentation and inaccurate analysis. To cope with these analytical constraints, deproteinization steps are commonly implemented throughout the analytical set-up.9,10 Nevertheless, some of these steps do not follow the Green Chemistry requirements, as they usually involve the use of volatile hazardous solvents in tedious, lengthy, and energy-consuming procedures. Thus, it is urgent to develop methods that overcome the abovementioned sensitivity and instrumental compatibility challenges, while ensuring a simpler approach for the analysis of saliva.
In light of this background, aqueous biphasic systems (ABSs) are viable candidates to fulfill the requirements claimed for salivary bioanalysis. ABSs stand among the mildest extraction strategies as they are mostly based on water, whereas conventional extraction and even microextraction techniques may involve toxic and/or volatile organic solvents.11 ABSs are formed by mixing in water a minimum of two water-soluble components that split into two immiscible phases above certain concentrations and under specific temperature and pH conditions.12 This feature creates a plethora of possibilities to perform efficient extractions by simply tuning the ABS components, compositions, and operating conditions.
Taking advantage of this tunability, and through an adequate design, the incorporation of ionic liquids (ILs) as phase-forming components of ABSs has been a step forward to improve extraction performance and selectivity, while meeting some of the Green Chemistry principles.13 ILs are structurally versatile compounds that are composed of asymmetric organic cations and organic or inorganic anions, presenting high thermal and chemical stability, and high solvation ability for a wide number of compounds. Despite often possessing negligible vapor pressure at room temperature, low volatility, and non-flammability, attention should also be given to the toxicity and biodegradability of ILs to certify their real safety.14 Thus, the use of ILs presenting low environmental and health impacts is desirable, preferentially those that incorporate bio-inspired moieties in their structure. Following this trend, ILs with morpholinium,15 cholinium,16 and guanidinium17 cations have been recently explored as alternative phase-forming components of ABSs.
From a Green Analytical Chemistry perspective,18 IL-based ABSs can be promising platforms for developing alternative extraction methods for the analysis of biological samples, which conventionally resort to complex methods. IL-based ABSs have been successfully used for the analysis of biofluids, especially urine19–21 and blood byproducts.22,23 Despite their successful use as bioanalytical platforms, to the best of our knowledge, IL-based ABSs have not been explored for the analysis of saliva. This mostly relies on the low available volume of saliva that makes its pretreatment with IL-based ABSs quite challenging, as ABSs intended for preconcentration normally benefit from the scalability of the sample (i.e., the possibility of using high volumes of sample).24,25
Hence, this study aims to shift for the first time the use of an IL-based ABS method to the analysis of human saliva, while ensuring adequate enrichment factors despite the low volume of saliva analyzed. To this end, IL-based ABSs were prepared using the low cytotoxic butylguanidinium chloride (C4Gu-Cl) IL, K2HPO4 as the salting-out agent and non-diluted human saliva. Given their worsening endocrine disrupting effects, five bisphenols were selected as representative contaminants of emerging concern to monitor in saliva using high-performance liquid chromatography and fluorescence detection (HPLC-FD).6,26 The proposed method is intended to be the first one-step analytical procedure comprising saliva clean-up, microextraction and preconcentration. It is shown that it allows the precipitation of most salivary interfering proteins at the interphase of the ABS, creating a three-phase partitioning (TPP) platform based on the ABS technique (ABS/TPP). Finally, the green character of the proposed set-up for the analysis of saliva as compared to others previously reported was addressed using green metrics.
Experimental
Chemicals, solutions, reagents, and materials
Five bisphenols (BPs) were selected as target analytes: bisphenol A (BPA), bisphenol B (BPB), bisphenol C (BPC), bisphenol F (BPF), and bisphenol P (BPP). All BPs were supplied by Sigma-Aldrich (San Luis, MO, USA) with purity higher than 98%. The chemical structures and main physicochemical properties of the target BPs are included in Table S1 in the ESI.† Individual stock solutions of each analyte were prepared by dissolving the commercial solids in acetonitrile (ACN, ≥99.0%) LC-MS Chromasolv™ grade, provided by Honeywell Riedel-de Haën (Charlotte, NC, USA). Stock solutions were prepared at the following concentrations: 1477 mg L−1 for BPA, 909 mg L−1 for BPB, 1026 mg L−1 for BPC, 941 mg L−1 for BPF, and 1002 mg L−1 for BPP. Intermediate standard solutions containing all analytes were prepared at 10 mg L−1 and 1 mg L−1 in ACN, by mixing the proper amount of each stock solution. All solutions were stored in a refrigerator protected from light at 4 °C.The reagents used for the preparation of the C4Gu-Cl IL were 1H-pyrazole-1-carboxamidine hydrochloride (99%) and butylamine (99.5%), both supplied by Sigma-Aldrich. Readers are referred to the ESI† for more details regarding the synthesis and characterization of the IL. The chemicals used for the development of ABSs were all purchased from Sigma-Aldrich, including citric acid monohydrate (C6H8O7·H2O, ≥99.0%), potassium citrate monohydrate (C6H5K3O7·H2O, ≥99.0%), and the following phosphate salts: K3PO4 (≥98%), K2HPO4 (≥98%), and KH2PO4 (≥99.0%). Buffer solutions at different pH values were prepared in distilled water at 50 wt% for citrate buffers (C6H5K3O7/C6H8O7), and 40 wt% for phosphate buffer (K2HPO4/KH2PO4) (see Table S2 in the ESI†). Aqueous solutions of C6H5K3O7 (50 wt%), K3PO4 (40 wt%), K2HPO4 (40 wt%), and KH2PO4 (18 wt%), were also prepared to obtain the phase diagrams.
Ethanol (EtOH, ≥99.0%) LC grade was purchased from Millipore (Burlington, MA, USA). Methanol (MeOH, ≥99.8%) and acetic acid (≥99.7%) were supplied by Honeywell Riedel-de Haën. ACN and ultrapure water, with a resistivity of 18.2 MΩ cm, produced by a Milli-Q water purification system (Bedford, MA, USA), were used to prepare the chromatographic mobile phases, together with formic acid LC-MS LiChropur™ (98–100%), supplied by Sigma-Aldrich. Mobile phases were filtered through 0.22 μm Millipore filters.
Bovine Serum Albumin (BSA) solution (30% (w/v) in phosphate buffered saline) was required for the protein assay, as well as the Bradford regent (for 0.1–1.4 mg mL−1 of protein), both supplied by Sigma-Aldrich. The mPAGE® 4 × LDS Sample Buffer (Laemmli buffer) was acquired from Millipore. The Brilliant Blue R dye was purchased from Sigma-Aldrich and was used for the preparation of the Coomasie solution by dissolving 0.2 g of this dye in a mixture MeOH/acetic acid/water (40/10/50, v/v/v).
Pyrex® (Staffordshire, UK) centrifuge tubes of 15 mL (9.5 cm L × 2 cm O.D.) were used for the development of the microextraction method, and 250 μL Hamilton syringes with flat needles were used for the phase separation. Stir bars (15 × 4.5 mm) from Sigma-Aldrich were required for magnetic stirring. Eppendorf™ (Hamburg, Germany) polypropylene microtubes of 1.5 and 2.0 mL were used to obtain the ABS tie-lines (TLs).
Instrumentation and equipment
An RV 10 digital rotary evaporator with temperature control purchased from IKA® (Staufen, Germany), in combination with a vacuum pump VP 2 Autoyac from Vacuubrand (Wertheim, Germany), was used during the synthesis of the IL.The liquid–liquid ternary phase diagrams were determined using a magnetic stirring plate from P Selecta® (Barcelona, Spain). A Heidolph® (Schwabach, Germany) vortex stirring system and a 5702-centrifuge from Eppendorf™ were used in the microextraction procedure. A GLP21 pH-meter from Crison® (Alella, Barcelona) was employed.
The characterization of the IL was performed by proton nuclear magnetic resonance (1H-NMR) using an Avance™ NMR spectrometer (300 MHz). A 96-well plate with an assay volume of 300 μL per well was used in the Bradford Colorimetric Assay (BCA), for which an EnSpire multimode plate reader from PerkinElmer (Waltham, MA, USA) was required. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a Pierce Precise™ Protein Gel at 8 wt%, acquired from ThermoFisher Scientific™ (Waltham, MA, USA). This gel was used following the recommendations of the suppliers. Further details related to the experimental procedure followed for both BCA and SDS-PAGE are included in the ESI.†
The separation of the target BPs was accomplished in a HPLC system from Agilent Technologies (Santa Clara, CA, USA) coupled with a Waters™ 474 fluorescence detector (FD) and equipped with a Rheodyne 7725i injection valve with a 20 μL loop supplied by Supelco (Bellefonte, PA, USA). The chromatographic column was an InfinityLab Poroshell 120 EC-C18 (100 mm L × 4.6 mm I.D, 4.0 μm particle size) from Agilent, protected by a Pelliguard LC-18 guard column from Supelco.
The HPLC elution gradient for the proper separation of BPs was optimized using a mobile phase composed of ACN and ultrapure water (water acidified with 0.02% (v/v) of formic acid) at a constant flow of 1 mL min−1. The optimum gradient started at 20% (v/v) of ACN, keeping this percentage for 3 min. Then, it was increased up to 35% (v/v) in 1 min, kept at this percentage for 2 min, and finally reached 55% (v/v) in 14 min. This percentage was then kept for 8 min. The fluorescence program for the detection of the analytes was optimized by registering each bisphenol at the excitation (λex) and emission (λem) wavelengths that provided the highest sensitivity. Table S3 in the ESI† includes the retention times of the studied BPs obtained at the optimum separation conditions, as well as their optimum FD conditions.
Liquid–liquid ternary phase diagrams of IL-based ABSs
The binodal curves of the IL-based ABSs were developed following the cloud-point titration method at 25 °C and atmospheric pressure. An aqueous solution of the IL at 70 wt% was prepared and placed in a glass vial containing a stirring bar. The aqueous solution of the salt (18 wt%, 40 wt% or 50 wt%, depending on the salt) was added dropwise to the IL solution under constant stirring until turbidity was appreciated. Then, the dropwise addition of ultrapure water was carried out until the mixture became limpid. This procedure was repeated for each point of the binodal curve, and the system compositions were obtained by weight quantification of the components added to the mixture. The experimental binodal data were fitted using eqn (1):[IL] = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(B[salt]0.5) − (C[salt]3)] exp[(B[salt]0.5) − (C[salt]3)] | (1) |
The TLs were obtained using the gravimetric method proposed by Merchuk et al.27 A mixture point belonging to the biphasic region of each liquid–liquid phase diagram was selected and prepared gravimetrically. The mixture was stirred by vortex, and the system was incubated at 25 °C for 12 h to reach the equilibrium. Subsequently, both top (IL-rich) and bottom (salt-rich) phases were separated, accurately weighed, and each TL was determined through the level-arm rule. Further information regarding the equations for determining the TLs is provided in the ESI.†
Saliva collection
Saliva samples were provided by healthy volunteers (two male and one female) who signed an individual informed consent. All the saliva samples were collected by direct passive spitting in a glass tube, at a minimum of 1 h after eating, drinking, or brushing the teeth, and after rinsing the mouth properly with water.Microextraction procedure under optimum conditions
The microextraction method based on the ABS/TPP was performed using real saliva following the optimum procedure shown in Fig. 1. The composition of the system, once optimized, involved 18 wt% of the C4Gu-Cl IL, 30 wt% of K2HPO4, and 52 wt% of non-diluted saliva. Briefly, 0.35 g of the IL were placed in a glass centrifuge tube. Then, 0.60 g of K2HPO4 were added, followed by the addition of 1.1 g of either a saliva sample or a saliva standard solution containing the bisphenols at concentrations ranging from 0.20 to 500 ng g−1, depending on the experiment. The solution (total mass of ABS/TPP ∼2 g) became immediately turbid, and 1 min of vortex was applied to ensure the proper mixing of all components. Subsequently, the system was centrifuged for 20 min at 3000 ×g to promote the phase separation. Finally, 0.22 g of IL-rich phase were carefully collected with a Hamilton syringe, followed by a dilution with 25 μL of the HPLC initial mobile phase, that is, the mixture ACN/H2O (20/80, v/v). Such diluted IL-rich phase was then directly injected in HPLC-FD.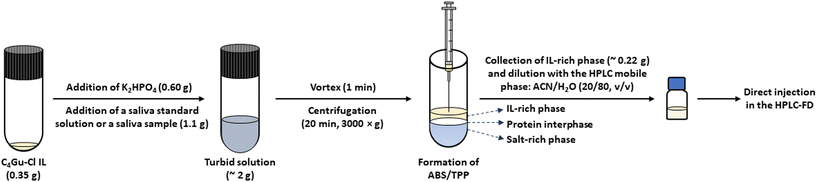 | ||
| Fig. 1 Microextraction procedure based on the ABS/TPP performed using saliva under optimum conditions: 18 wt% of the C4Gu-Cl IL, 30 wt% of K2HPO4, and 52 wt% of non-diluted saliva. | ||
Results and discussion
Development of C4Gu-Cl-based ABSs
An IL comprising the guanidinium cation (C4Gu-Cl) was selected in this study to develop ABSs.28 This IL belongs to a generation of ILs specifically designed to contain bio-inspired cations derived from natural sources. Due to this structural particularity, guanidinium ILs present low cytotoxicity when compared with conventional imidazolium ILs.29,30 Also, in the case of the C4Gu-Cl used in this study, the alkyl chain of the cation was tuned to ensure a shorten length (only 4 carbon atoms), thus decreasing the hydrophobicity and cytotoxicity of the IL when compared with that of other guanidinium ILs with longer alkyl chain lengths (i.e., 8 and 10 carbon atoms).29,30 Moreover, this combination of the guanidinium cation with the chloride anion avoids the formation of fluorides, commonly reported during the decomposition of ILs with fluorinated anions. C4Gu-Cl was combined with two groups of salts to form ABSs: organic salts based on citrate anions and inorganic salts based on phosphate anions. These salts were selected because they are widely used salting-out agents in the development of IL-based ABS, also granting the possibility to undergo phase separation in water at different pH values and under mild conditions.31,32 Furthermore, the salts with citrate anions have low toxicological impact, and they are fully biodegradable.31 The selection of salts with different anions was performed in order to deeply assess the behavior of C4Gu-Cl as ABS phase-forming component depending on: (i) the chemical structure of the salt (i.e., nature of the anion); and (ii) the pH. Therefore, this rational design of both the IL and the different salts allowed to obtain ABSs with green characteristics and satisfactory tuneability.Table S4 in the ESI† summarizes the salts that were tested and which of them were able to form ABSs with C4Gu-Cl. Fig. 2 display the binodal curves obtained for all systems, while Table S5 in the ESI† includes the regression parameters obtained by fitting the experimental data to eqn (1). The detailed experimental weight fraction data for each liquid–liquid ternary phase diagram can be found in the ESI (from Tables S6 to S12†). Successful correlation parameters were obtained for all systems, showing correlation coefficients between 0.984 and 0.998.
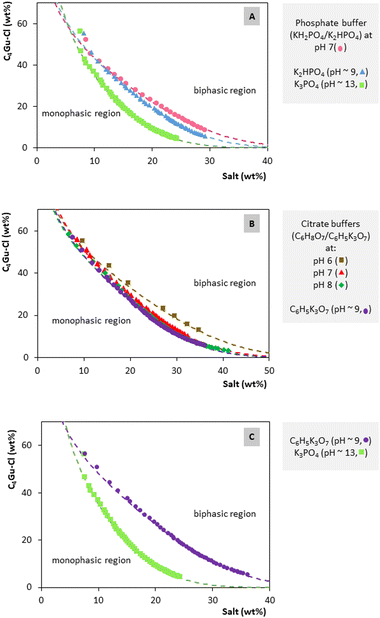 | ||
| Fig. 2 Binodal curves of the ABSs composed of C4Gu-Cl IL + salt + H2O at 25 °C: (A) effect of the salt anion in the ABS formation ability using phosphate salts, (B) effect of the pH in the ABS formation ability using citrate salts, (C) effect of the salt nature in the ABS formation ability using phosphate versus citrate salts. Dashed lines represent the adjusted binodal data using eqn (1) (–). | ||
Fig. 2(A) shows the effect of the salt anion in the ABS formation ability when testing different potassium phosphate salts with the C4Gu-Cl IL. The larger the biphasic region (above the binodal curve) is, the higher the ability of the IL to undergo phase separation, and thus, the stronger is the salt as salting-out agent. The salting-out ability of phosphate salts follows the order: KH2PO4/K2HPO4 < K2HPO4 ≪ K3PO4. Representing the phosphate salt with the lowest valence anion under appraisal (i.e., H2PO4−versus HPO42−versus PO43−), KH2PO4 failed to form ABS. These results agree with the Hofmeister series,33 as salts with high charge density anions are generally more easily hydrated, thus leading to the preferential dehydration of the IL and its migration to a non-soluble IL-rich phase.
Fig. 2(B) shows the effect of the pH on the formation of C4Gu-Cl-based ABSs using citrate salts/buffers. C4Gu-Cl was able to generate ABSs at pH values higher than 5 (see Table S4 in the ESI†). The ABS formation ability order is as follows: pH 6 < pH 7 < pH 8 < pH 9. Thus, alkaline pH environments are more favorable to promote the separation of two phases than acidic pH values, as also observed using phosphate salts, i.e., KH2PO4/K2HPO4 (pH 7) < K2HPO4 (pH ∼ 9) ≪ K3PO4 (pH ∼ 13). Such a behavior follows that observed for ABSs composed of the same citrate salts and other IL families.34 According to the species distribution diagram of the citrate anion, these results can be explained following the Hofmeister series. Trivalent citrate anions become increasingly more predominant in solution at pH values above 5, while at pH 5 the divalent and monovalent citrate anions are the most prevalent species in solution.
Fig. 2(C) compares the binodal curves of the ABSs with C6H5K3O7 and K3PO4 allowing to capture the effect of the salt nature on ABS formation. K3PO4 has a higher ability to form ABSs with C4Gu-Cl than C6H5K3O7. Due to the wider biphasic regions that enable higher preconcentration factors and the use of lower amounts of IL/salts, phosphate salts can be considered the most propitious salting-out agents to develop an ABS-based preconcentration platform.
All C4Gu-Cl-based ABSs were successfully characterized by determining three TLs per system. Table S13 in the ESI† shows the experimental data for each TL, along with the tie-line lengths (TLLs) and α values (i.e., ratio between the IL-rich phase weight and the total mixture weight). One of the TLs (TL1) was determined using the same mixture composition for all systems (30 wt% of IL and 25 wt% of salt), allowing a proper comparison, and further confirming the higher potential of phosphate salts for preconcentration purposes due to the longer TLLs obtained. Indeed, for citrate salts, the TLLs for the TL1 range from 53.57 to 81.96, while for phosphate salts these TLL values are between 85.10 and 97.26. The other two TLs (TL2 and TL3) are specific for each system, being determined by fixing the percentage of salt and modifying that of IL in the mixture composition. The proper determination of the TLs allowed to establish concentration ranges in the biphasic region in which the weight composition of both the IL-rich and the salt-rich phases can be obtained by applying the level-arm rule. Based on the determination and characterization of the liquid–liquid phase diagrams, analytical features such as the enrichment factors or the extraction efficiencies can be tuned through the selection of the TL.
Shifting C4Gu-Cl-based ABSs to the analysis of human saliva
To test the developed C4Gu-Cl-based ABSs for the analysis of human saliva, a mixture composition belonging to the biphasic region of all systems (30 wt% IL + 25 wt% salt) was gravimetrically prepared. These systems were prepared using non-diluted, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diluted and 1
2 diluted and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 diluted healthy male saliva instead of ultrapure water. Given the low volume of saliva available, the mixture was prepared for a total weight of 2 g, and thus only 0.9 g of either non-diluted or diluted saliva were required for each experiment. After vortex and centrifugation, the formation of a solid interphase was observed, which created an ABS/TPP regardless of the salt and the sample dilution factor applied. Fig. S2 in the ESI† shows photos of the ABS/TPP obtained with a representative salt (K2HPO4), providing visual evidence of the solid interphase formation. In these systems, the top phase corresponds to the IL-rich phase, while the bottom phase is the salt-rich phase. Furthermore, it is assumed that several components from the saliva matrix precipitated in the interphase, as no interphase was formed when performing the ABS using ultrapure water (see Fig. S2 in the ESI†). This led us to test the possibility of developing a one-step clean-up, microextraction and preconcentration method using the C4Gu-Cl-based ABS/TPP. It is important to highlight that the ability of the ABS/TPP to directly analyze saliva relies on the efficient removal of salivary interfering compounds that precipitate at the interphase. In turn, the IL-rich phase is the phase to be analyzed as it contains a priori the target analytes, as shown below. Thus, the IL-rich phase must be free of any substance that can hinder the instrumental analytical determination due to incompatibility issues.
10 diluted healthy male saliva instead of ultrapure water. Given the low volume of saliva available, the mixture was prepared for a total weight of 2 g, and thus only 0.9 g of either non-diluted or diluted saliva were required for each experiment. After vortex and centrifugation, the formation of a solid interphase was observed, which created an ABS/TPP regardless of the salt and the sample dilution factor applied. Fig. S2 in the ESI† shows photos of the ABS/TPP obtained with a representative salt (K2HPO4), providing visual evidence of the solid interphase formation. In these systems, the top phase corresponds to the IL-rich phase, while the bottom phase is the salt-rich phase. Furthermore, it is assumed that several components from the saliva matrix precipitated in the interphase, as no interphase was formed when performing the ABS using ultrapure water (see Fig. S2 in the ESI†). This led us to test the possibility of developing a one-step clean-up, microextraction and preconcentration method using the C4Gu-Cl-based ABS/TPP. It is important to highlight that the ability of the ABS/TPP to directly analyze saliva relies on the efficient removal of salivary interfering compounds that precipitate at the interphase. In turn, the IL-rich phase is the phase to be analyzed as it contains a priori the target analytes, as shown below. Thus, the IL-rich phase must be free of any substance that can hinder the instrumental analytical determination due to incompatibility issues.
It should be noted that the composition used for these initial experiments involved a high amount of IL. This was performed to ensure enough volume of both IL and salt-rich phases for their proper visual assessment in the presence of saliva, which would be initially difficult if using low amounts of IL. Subsequent experiments are devoted to optimize the ABS composition by reducing the amount of IL to ensure not only microextraction but also preconcentration.
Aiming to evaluate the efficiency of the ABS/TPP as a platform for the direct analysis of saliva, 5 bisphenols (BPs) were selected as target analytes for the development of a microextraction and preconcentration method. Table S1 in the ESI† shows that the selected BPs have relatively basic pKa values (all higher than 9.91). Therefore, ABSs with pH values lower than 9.91 would provide better extraction performance towards those BPs (that are neutral at pH values < pKa). More basic pH values would promote the generation of the alkoxide form of the analytes (negatively charged), and thus their solubility in an aqueous medium would be increased, hindering their migration (and therefore extraction) to the IL-rich phase. Taking this into account, the proper C4Gu-Cl-based ABS for the extraction of BPs should meet the following pH requirements: (i) lower than 9.91; and (ii) as high as possible, as it was previously concluded that the more basic the pH, the larger the biphasic region. Despite the larger biphasic region granted by K3PO4 as the salting-out agent, only K2HPO4 and C6H5K3O7 meet the described pH requirements. Due to the larger biphasic region provided by K2HPO4 over C6H5K3O7, C4Gu-Cl IL + K2HPO4 ABS was selected to proceed with the development of the clean-up, microextraction and preconcentration platform for determining BPs in saliva.
Coupling the ABS/TPP with HPLC-FD
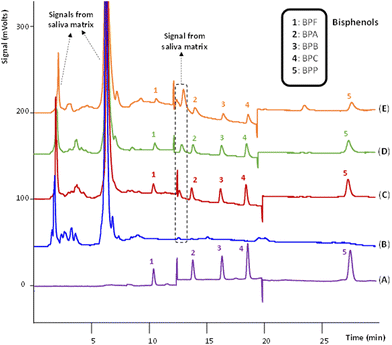
Given the native fluorescence of the target BPs, reversed phase (RP)-HPLC with fluorescence detection (FD) was the analytical determination technique selected to ensure the highest sensitivity. The elution gradient and the fluorescence conditions (see Experimental section) were optimized, and the chromatographic method was fully validated using standards of the analytes dissolved in the initial composition of the mobile phase (ACN/H2O, 20/80, v/v). Fig. 3(A) shows a representative chromatogram obtained after the injection of a standard of 80 ng g−1 of the analytes. Table S14 in the ESI† includes the quality analytical parameters of the chromatographic method.One of the main challenges for the direct coupling of the ABS/TPP method with HPLC-FD is the compatibility of the IL-rich phase with the HPLC system. Preferentially, the IL-rich phase (containing a priori the preconcentrated analytes) should be directly injected in HPLC to avoid additional steps of evaporation and/or reconstitution with a HPLC-compatible solvent. Such tedious steps would increase the extraction time and the solvent and energy consumption of the method. In this sense, the viscosity and solubility of the IL-rich phase were carefully addressed.
For direct injection in HPLC, the IL-rich phase should have a low viscosity and be completely soluble in the entire range of the HPLC mobile phase compositions to avoid the precipitation of any substance (proteins or salts) that could damage the chromatographic column, or even clogging the system.
Hence, the composition of the ABS was optimized to ensure compatibility with the analytical instrument as well as a proper preconcentration of BPs in the IL-rich phase. By fixing the weight percentage of salt at 30 wt%, three different IL percentages were tested, each one belonging to a different TL: 18 wt% (TL1), 13 wt% (TL2), and 9.1 wt% (TL3) (cf.Fig. 4). This allows for operation in TL regions that provide low IL-rich phase![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salt-rich volume phase ratios, thus providing higher enrichment factors, while keeping the IL-rich phase at compositions compatible with the HPLC-FD. It is important to highlight that it was necessary to achieve a compromise solution between the requirement of a high preconcentration and the compatibility of the IL-rich phase with HPLC, as a very low volume of this phase may lead to the saturation of the solution by the salt or by any protein extracted in this phase, provoking the precipitation of any of these compounds, and thus making direct injection in the HPLC impossible.
salt-rich volume phase ratios, thus providing higher enrichment factors, while keeping the IL-rich phase at compositions compatible with the HPLC-FD. It is important to highlight that it was necessary to achieve a compromise solution between the requirement of a high preconcentration and the compatibility of the IL-rich phase with HPLC, as a very low volume of this phase may lead to the saturation of the solution by the salt or by any protein extracted in this phase, provoking the precipitation of any of these compounds, and thus making direct injection in the HPLC impossible.
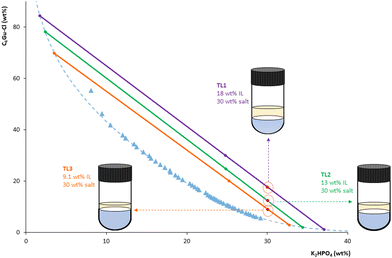 | ||
| Fig. 4 ABS/TPP compositions assessed in the optimization of the C4Gu-Cl IL weight percentage, using a fixed 30 wt% of K2HPO4: 18 wt% of IL (TL1), 13 wt% of IL (TL2) and 9.1 wt% of IL (TL3). | ||
Considering these instrumental compatibility requirements, together with our previous experience in developing preconcentration platforms using IL-based ABS, the selected optimal TL and mixture composition were TL1 and 18 wt% of IL + 30 wt% of salt, respectively. This selection relies on the following rationale: (i) TL1 was the TL with the longest TLL (90.56, see Table S13†), and thus with the higher IL percentage in the IL-rich phase (∼85 wt%, see Table S13†), leading a priori to the highest extraction efficiencies for this system; (ii) 18 wt% of IL was the minimum IL percentage required to experimentally collect the IL-rich phase without contamination from the interphase; (iii) the IL-rich phase obtained is completely soluble once diluted with the HPLC mobile phase (using both the initial and the final compositions), thus ensuring its compatibility with HPLC-FD (cf. Fig. S3(A) in the ESI†).
Optimization of the ABS/TPP-HPLC-FD method as a clean-up and preconcentration platform in saliva
Once the optimum composition of the ABS was set according to the requirement of directly coupling the ABS/TPP with HPLC, the remaining parameters affecting the ABS/TPP-based microextraction method were evaluated. Non-diluted saliva was selected as the matrix, as further dilutions of saliva would lead to significant losses in preconcentration. Regarding the vortex time, 1 min was selected, as it was the minimum time required to ensure the proper mixing of the components to form the ABS. With respect to centrifugation, different times (5, 10, 15 and 20 min) were tested at the maximum speed of the centrifuge (3000 ×g). Centrifugation is a very important step in promoting the phase separation of the ABS/TPP. 20 min was the minimum time required to ensure the proper separation, as the lower tested times were not sufficient to achieve the formation of the interphase. Finally, the collection of the IL-rich phase was performed immediately after centrifugation to enable a proper separation of the phases. Otherwise, the phases became turbid, making the proper separation infeasible. Therefore, no equilibration time was needed, which is an additional advantage in terms of process speed.Under these conditions, several ABS/TPP samples were prepared in real non-diluted saliva (without spiking the analytes) to assess the reproducibility of the IL-rich phase formation and collection, and the maximum preconcentration ability of the ABS/TPP. It is important to highlight that the amounts of the phase-forming components were adjusted for a total mass of the system of ∼2 g, given the low volume of saliva sample available after each collection. To prepare the ABS/TPP, 18 wt% of C4Gu-Cl IL (0.35 g), 30 wt% of K2HPO4 (0.60 g), and 52 wt% of real non-diluted saliva (1.1 g) were needed. The total weight of IL-rich phase obtained in the ABS/TPP was both theoretically (applying the level-arm rule) and experimentally determined. Thus, 0.41 ± 0.02 g (n = 4) of IL-rich phase was obtained, agreeing with the theoretical amount (0.40 g). The maximum enrichment factor (EFmax) with respect to the total weight of the system was calculated by applying the eqn (2).
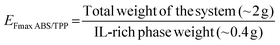 | (2) |
The calculated EFmax was 4.9 ± 0.3, close to the theoretical value of 5.0. Furthermore, this value could also be expressed with respect to the mass of saliva added to the ABS/TPP by applying the eqn (3).
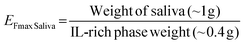 | (3) |
In this case, the EFmax was 2.6 ± 0.3. The ABS/TPP is therefore able to preconcentrate nearly 3 times (maximum) the BPs with respect to their initial concentration in the saliva sample, and even the concentration can be increased up to 5 times with respect to the initial concentration of the analytes diluted in the overall system. This demonstrates the ability of the ABS/TPP as a preconcentration platform in saliva, despite the low weight of both the sample and the entire system.
To guarantee that the collected IL-rich phase was not contaminated with the interphase, only a portion of the entire phase was collected to perform the analytical method. Thus, a reproducible collection could be obtained by sampling 0.22 g of the IL-rich phase. To this sampled phase, 25 μL (0.025 g) of HPLC mobile phase (ACN/H2O, 20/80, v/v) were added as the minimum volume required prior to the direct injection in HPLC-FD (total mass of extract ∼0.25 g). The final dilution factor (i.e., total mass of the IL-rich phase/total mass of the extract subjected to analysis) was 1.67. Thus, by dividing the EFmax of the ABS/TPP by the dilution factor required for the analysis, it was determined that the EFmax of the entire ABS/TPP-HPLC-FD method was 2.9 ± 0.2.
Under optimum conditions, a microextraction procedure was accomplished by subjecting a real healthy male saliva sample (without spiking the analytes) to the entire ABS/TPP-HPLC-FD method. Comparing the chromatograms of BPs standard solution and the diluted IL-rich phase in Fig. 3(A) and (B), respectively, it is shown that the IL-rich phase is free of BPs. Thus, it was selected to perform the entire analytical method. Subsequently, the method was applied to the same healthy male saliva, but after being spiked with 30 ng g−1 of the BPs (a concentration referred to the total amount of ABS/TPP). Fig. 3(C) shows the presence of the analytes in the diluted IL-rich phase by comparing this chromatogram with one of a standard of the BPs dissolved in ACN/H2O (20/80, v/v), without performing any ABS/TPP step (shown in Fig. 3(A)). The 5 BPs were successfully extracted, without any signal from the IL or the saliva that interfered with the analytical determination. Indeed, only 2 signals from the saliva appeared before the elution of the first BP, in the first 10 min of chromatogram, and between the first and the second BP (approximately 12.0 min). Furthermore, the peak areas were totally comparable to those of the standard of 80 ng g−1 shown in Fig. 3(A). As this standard had a concentration of more than up to 2 times than the spiked concentration in Fig. 3(C), the preconcentration of BPs was preliminarily confirmed with these experiments. Thus, the feasibility of the ABS/TPP as a preconcentration platform for the determination of BPs in human saliva was demonstrated, together with its direct compatibility with HPLC-FD.
Before proceeding with the analytical performance of the ABS/TPP-HPLC-FD method, the clean-up of the saliva sample under the optimum conditions of the microextraction procedure was verified. Even if total salivary protein removal was not achieved, the insoluble proteins that could precipitate in the mobile phase (due to the presence of ACN) were totally removed, as the diluted IL-rich phase was completely soluble in both the initial and final composition (20% and 55% of ACN, respectively). This was first visually confirmed by testing the solubility of the saliva sample, without any pretreatment, in both the initial and final composition of the mobile phase (cf. Fig. S3(B) in the ESI†). Saliva was completely insoluble in both cases and could not be directly analyzed by HPLC unless the developed ABS/TPP method was applied (cf. Fig. S3(A) in the ESI†).
The total protein content of the healthy male saliva selected for the analytical performance (without any pretreatment), and of both the top and bottom phases of the ABS/TPP, were obtained by the BCA. Non-diluted saliva, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 diluted saliva, were analyzed, obtaining an average content of 880.9 μg mL−1 in the non-diluted sample and 477.2 μg mL−1 for the 1
10 diluted saliva, were analyzed, obtaining an average content of 880.9 μg mL−1 in the non-diluted sample and 477.2 μg mL−1 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dilution. The protein content of the 1
2 dilution. The protein content of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 diluted saliva was negligible (under the calibration range). With respect to the ABS/TPP phases, collected after performing the microextraction method under optimum conditions, the solid interphase could not be dissolved in any of the commonly used solvents for BCA, and thus it could not be analyzed. A ∼1
10 diluted saliva was negligible (under the calibration range). With respect to the ABS/TPP phases, collected after performing the microextraction method under optimum conditions, the solid interphase could not be dissolved in any of the commonly used solvents for BCA, and thus it could not be analyzed. A ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dilution of the IL-rich phase was performed prior to the BCA, until having 1.1 g of final solution was obtained for comparative purposes, considering that the amount of real saliva added for the ABS/TPP was 1.1 g. Under such conditions, the salt-rich phase was completely free of proteins, and the IL-rich phase had 273.8 μg mL−1, a 31% of the total protein content of the initial saliva. Therefore, it was estimated that 69% of the salivary proteins precipitated in the solid interphase.
3 dilution of the IL-rich phase was performed prior to the BCA, until having 1.1 g of final solution was obtained for comparative purposes, considering that the amount of real saliva added for the ABS/TPP was 1.1 g. Under such conditions, the salt-rich phase was completely free of proteins, and the IL-rich phase had 273.8 μg mL−1, a 31% of the total protein content of the initial saliva. Therefore, it was estimated that 69% of the salivary proteins precipitated in the solid interphase.
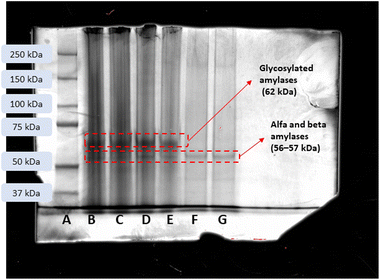
Aiming to identify the proteins present in the saliva and in the IL-rich phase and further corroborate the ABS/TPP clean-up efficiency, a SDS-PAGE study was performed. Fig. 5 shows the gel after the electrophoresis with the main protein bands identified. Non-diluted saliva and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diluted saliva have bands associated to alpha and beta amylases (approximately 56–57 kDa), and glycosylated amylases (62 kDa), which are proteins commonly found in human saliva.35 The bands observed in the IL-rich phase sample showed a preferential extraction of non-glycosylated amylases from the saliva by the C4Gu-Cl IL. These salivary proteins are a minority, and they are soluble and compatible with HPLC-FD analysis. Remarkably, most of the protein content that could interfere in the analytical determination was removed at the interphase, reinforcing the role of ABS/TPP as an efficient clean-up strategy.
2 diluted saliva have bands associated to alpha and beta amylases (approximately 56–57 kDa), and glycosylated amylases (62 kDa), which are proteins commonly found in human saliva.35 The bands observed in the IL-rich phase sample showed a preferential extraction of non-glycosylated amylases from the saliva by the C4Gu-Cl IL. These salivary proteins are a minority, and they are soluble and compatible with HPLC-FD analysis. Remarkably, most of the protein content that could interfere in the analytical determination was removed at the interphase, reinforcing the role of ABS/TPP as an efficient clean-up strategy.
Analytical performance of the ABS/TPP-HPLC-FD method in real saliva
Matrix-matched calibrations of the overall ABS/TPP-HPLC-FD method were obtained in the healthy male saliva 1, as it was free of the target BPs (see Fig. 3(B)). Table 1 includes the quality analytical parameters of the entire method: calibration range, calibration slopes, determination coefficients (R2), standard deviation of the residuals (Sy/x), limits of detection (LODs) and limits of quantification (LOQs). The concentrations of the saliva standard solutions subjected to the method were expressed in ng of analyte per g of the ABS/TPP. LODs were obtained by decreasing the concentration of saliva standards until a S/N of 3 was obtained for each BP, while LOQs were calculated as 10/3 times the LODs and experimentally verified. LODs and LOQs were also expressed in ng of analyte per mL of saliva in Table 1, considering the amount of saliva in the ABS/TPP (1.1 g of saliva for each 2 g of ABS/TPP) and the density of the sample (experimentally measured, d = 1.00 g mL−1). LODs ranged from 0.40 ng mL−1 for BPA and BPB to 1.4 ng mL−1 for BPF. In addition, BPA and BPB were the analytes with the widest calibration ranges (0.500–500 ng g−1). R2 values were also adequate, all of them higher than 0.9912 for all the BPs.| Bisphenol | Calibration range (ng g−1) | (b ± t·Sb)a × 10−6 |
R
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
S
y/x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × 10−4
× 10−4 |
LODd (ng g−1) | LODe (ng mL−1) | LOQf (ng g−1) | LOQg (ng mL−1) |
|---|---|---|---|---|---|---|---|---|
| a Slope and uncertainty of the slope within the calibration range (n = 7) for a confidential level of 95%. b Determination coefficient. c Standard deviation of the residuals. d Limit of detection (expressed in ng of analyte per g of ABS/TPP), determined by decreasing the concentration of the saliva standards until a S/N ratio of 3 was obtained. e Limit of detection expressed in ng of analyte per mL of saliva. f Limit of quantification (expressed in ng of analyte per g of ABS/TPP), estimated as 10/3 times the LOD, and experimentally verified by applying the method to saliva standards at the predicted concentrations. g Limit of quantification expressed in ng of analyte per mL of saliva. | ||||||||
| BPF | 2.50–500 | 8 ± 1 | 0.9915 | 15 | 0.75 | 1.4 | 2.5 | 4.8 |
| BPA | 0.500–500 | 9.5 ± 0.4 | 0.9990 | 6.0 | 0.20 | 0.40 | 0.50 | 1.0 |
| BPB | 0.500–500 | 13.0 ± 0.2 | 0.9938 | 19 | 0.20 | 0.40 | 0.50 | 1.0 |
| BPC | 1.00–500 | 20 ± 2 | 0.9912 | 36 | 0.30 | 0.60 | 1.0 | 1.9 |
| BPP | 2.50–500 | 25 ± 2 | 0.9948 | 35 | 0.50 | 1.0 | 2.0 | 3.8 |
The method was also studied in terms of preconcentration (enrichment factor), extraction efficiency (ER, %), precision, and relative recovery (RR). Table 2 summarizes the preconcentration assessment of both the ABS/TPP without the analytical quantification step (detailed above in previous sections) and of the entire ABS/TPP-HPLC-FD method. The real enrichment factors of the method (EF Method) were calculated by the ratio of calibration slopes (slope of the entire ABS/TPP-HPLC-FD method/slope of the chromatographic HPLC-FD method). The EF values ranged from 2.31 for BPP to 2.77 for BPC. It is important to highlight that these values are highly adequate considering the low volume of saliva analyzed, making its preconcentration extremely difficult.
| Bisphenol | ABS/TPP preconcentration | ABS/TPP-HPLC-FD method preconcentration |
E
R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (%)
(%) |
|
|---|---|---|---|---|
| (EFmax ABS/TPP ± SD)a | (EFmax Method ± SD)b | E F Method | ||
| a Average maximum enrichment factor of the ABS/TPP (expressed as total system weight/IL-rich phase weight ratio) and its standard deviation (n = 4). The EFmax of the ABS/TPP (expressed as saliva weight/IL-rich phase weight ratio) is 2.6 ± 0.3. b Maximum enrichment factor of the overall method (considering the dilution performed prior to the injection in the HPLC-FD) and its standard deviation (n = 4). c Enrichment factor of the overall method, obtained by dividing the slope of the ABS/TPP-HPLC-FD calibration curve (see Table 1) between that of the chromatographic method (see Table S14†). d Extraction efficiency of the overall method. | ||||
| BPF | 4.9 ± 0.3 | 2.9 ± 0.2 | 2.72 | 94.8 |
| BPA | 2.42 | 84.2 | ||
| BPB | 2.76 | 96.2 | ||
| BPC | 2.77 | 96.6 | ||
| BPP | 2.31 | 80.5 | ||
The extraction efficiency (ER) values were calculated following eqn (4).
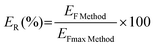 | (4) |
Table 3 includes the precision and RR data obtained by subjecting saliva standard solutions (using two different concentration levels) to the entire ABS/TPP-HPLC-FD method. The selected concentrations belonged to the calibration range of all BPs but were not used for obtaining the calibration curves. Precision was assessed with relative standard deviation (RSD, %) values, obtained in both intra-day and inter-day studies. Intra-day RSD values were below 8.8% for the low concentration level and below 3.0% for the high concentration level. Intermediate precision was assessed on 3 non-consecutive days, obtaining RSD values between 5.1% and 9.1% at the level of 30 ng g−1 and ranging from 3.8% to 7.5% at the level of 300 ng g−1. Aiming to assess the adequacy of the RSD values obtained, the Horwitz equation was applied.37 The selected concentration levels, expressed with respect to the volume of saliva sample, were 57.1 ng mL−1 and 571 ng mL−1. Horwitz RSD values were between 22.6% and 32.0% for the low concentration level and ranged between 16.0% and 22.6% for the level of 571 ng mL−1. Thus, the precision of the method was adequate, with RSD values even lower than those acceptable for the concentration levels evaluated. RR values were calculated with eqn (5).
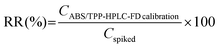 | (5) |
| Bisphenol | Low concentration level (30 ng g−1) | High concentration level (300 ng g−1) | ||||
|---|---|---|---|---|---|---|
| Intra-day RSD rangea (%) | Intermediate precision RSDb (%) | (RR ± SD)c (%) | Intra-day RSD rangea (%) | Intermediate precision RSDb (%) | (RR ± SD)c (%) | |
| a Range (day 1 to day 3) of relative standard deviation for intra-day precision (n = 3). b Relative standard deviation for intermediate precision (n = 12, 3 non-consecutive days). c Average relative recovery and its standard deviation (n = 12). | ||||||
| BPF | 2.0–8.8 | 9.1 | 108 ± 4 | 1.5–2.8 | 4.6 | 104 ± 4 |
| BPA | 2.9–7.7 | 5.1 | 98 ± 7 | 1.2–2.5 | 4.2 | 115 ± 3 |
| BPB | 1.7–6.7 | 8.3 | 115 ± 3 | 1.4–2.6 | 4.2 | 102 ± 4 |
| BPC | 0.8–1.7 | 7.1 | 105 ± 4 | 1.8–3.0 | 3.8 | 102 ± 2 |
| BPP | 1.1–6.8 | 8.6 | 109 ± 7 | 1.6–1.8 | 7.5 | 102 ± 2 |
Compared with literature methods, the analytical features of the developed ABS/TPP-HPLC-FD method are improved in terms of the toxicity/safety of the extraction medium and the total number of steps required for the analysis of saliva (cf. Table S15 in the ESI†). Most of the already reported studies use hazardous commercial solvents as extractants,8–10 while the proposed ABS/TPP-HPLC-FD is based on the use of a low cytotoxic IL, salts, and water. Furthermore, aiming to address the high complexity of saliva, most of the studies require a sample pretreatment step, mainly devoted to remove interfering salivary proteins from the sample.9,10,38 Instead, the present work reports for the first time a one-step procedure in which the clean-up of saliva, microextraction and preconcentration are simultaneously accomplished. These common steps performed prior to the extraction usually lead to tedious and time-consuming methods. Consequently, the entire time of the method is substantially increased by more than 1 h due to deproteinization procedures.9,10 In contrast, the proposed ABS/TPP-HPLC-FD method is simple, with centrifugation being the most time-consuming step. All reported studies deal with low amounts of saliva, ranging between 500 μL (ref. 8) and 2 mL,10 which makes it quite challenging to achieve adequate preconcentration. For this reason, all reported studies require highly sensitive analytical techniques (i.e., LC or GC with MS detection). This instrumentation is expensive, and it is not easily available for all laboratories. In our proposed method, BPs are properly determined in saliva by using fluorescence detection, which is more cost-effective than MS detection, and more sensitive and selective than UV detection. Furthermore, the literature methods use several additional steps to ensure the compatibility of the extract with the analytical instrument (specially with the MS equipment), and thus evaporation and reconstitution steps are often reported.8,9,38 In this regard, the proposed method allows for energy savings as the direct injection of the diluted IL-rich phase is possible. With respect to sensitivity, previously reported LODs range from 0.010 ng mL−1 (ref. 8) to 3.0 ng mL−1.10 Despite this study does not report the lowest LODs, they are in the ng mL−1 order, which is suitable for the detection of BPs in saliva,6 and comparable to LODs sometimes obtained with MS detection.9,10
None of the studies included in Table S15 in the ESI† report the EF and ER values. Since most of the studies require evaporation and reconstitution of the extract with an organic solvent prior to the injection in the analytical instrument, the preconcentration is achieved with such solvent-exchange, but not within the microextraction approach. Here, microextraction and preconcentration are achieved in one step, emphasizing the technological simplicity of the proposed ABS/TPP-HPLC-FD method as compared to state-of-the-art methods.
Analysis of different saliva samples
The optimized ABS/TPP-HPLC-FD method was applied for the analysis of saliva samples from two volunteers: a healthy female and a healthy male (different from the one who provided the saliva for the analytical performance of the method).Both samples (without spiking the analytes) were subjected to the entire method to assess their content in BPs. Table 4 includes the obtained concentration of the analytes in both the healthy male and the healthy female saliva. All BPs were detected in the female sample, whereas only BPB was detected in the male sample. Among the detected analytes, BPA and BPB were successfully quantified in female saliva, with concentrations of 7.8 ± 0.4 and 14 ± 2 ng mL−1, respectively, and a concentration of 12 ± 5 ng mL−1 of BPB was quantified in the male saliva. The developed ABS/TPP-HPLC-FD method had sufficient sensitivity to detect and even quantify the target BPs in several cases, despite (i) the low concentration levels of these analytes in saliva with respect to the levels found in serum or urine,6 and (ii) the inherent difficulty to preconcentrate the BPs when analyzing a low volume of saliva. Furthermore, the BP levels are in accordance with previous reported levels in human saliva.6 However, the wide range of concentrations reported shows that the BP levels are highly donor-dependent, with a difference of 100 times between the lowest and the highest level of BPA (as a representative analyte) in different saliva samples.
| Bisphenol | Healthy male saliva | Healthy female saliva | ||||
|---|---|---|---|---|---|---|
| Content ± SDa (ng mL−1) | RSDb (%) | RR ± SDc (%) | Content ± SDa (ng mL−1) | RSDb (%) | RR ± SDc (%) | |
| n.d.: non-detected. n.q.: detected but with contents below the LOQ, and thus non-quantified.a Concentration found of the analytes (ng of analyte per mL of saliva), considering the amount of saliva in the ABS/TPP and the saliva density, together with its standard deviation (n = 3).b Intra-day precision as relative standard deviation (n = 3), for a spiked level of 30 ng g−1.c Average relative recovery and its standard deviation (n = 3), for a spiked level of 30 ng g−1. | ||||||
| BPF | n.d. | 13 | 102 ± 13 | n.q. | 11 | 113 ± 11 |
| BPA | n.d. | 11 | 164 ± 17 | 7.8 ± 0.4 | 8.5 | 126 ± 5 |
| BPB | 12 ± 5 | 8.8 | 100 ± 9 | 14 ± 2 | 1.9 | 111 ± 2 |
| BPC | n.d. | 0.10 | 99.3 ± 0.1 | n.q. | 1.9 | 107 ± 2 |
| BPP | n.d. | 18 | 141 ± 26 | n.q. | 4.6 | 120.2 ± 0.3 |
To assess the suitability of the developed ABS/TPP-HPLC-FD method for the analysis of saliva samples different from that used for the matrix-matched calibration curves, both samples were spiked with 30 ng g−1 of the analytes and subjected to analysis. Fig. 3(D) and (E) show the chromatograms of the diluted IL-rich phase of the female saliva and the male saliva, respectively. The 5 BPs were successfully extracted, without interfering signals from the matrix. Moreover, the chromatograms show a similar profile to that obtained for the healthy male saliva in which the method was performed (see Fig. 3(C)).
Precision and RR values were obtained in both samples at the 30 ng g−1 level. Table 4 shows that the intra-day RSD values ranged from 0.10% to 18% for the male saliva and between 1.9% and 11% for the female saliva. These results demonstrate an adequate precision of the method despite the high complexity of the matrices. RR values ranged from 99.3% to 164%, but considering the standard deviations these values fell within the 80–120% range, except for BPA in male saliva. Despite these good results, statistical matrix effect studies were needed to assess the differences between the RR values obtained in the healthy male saliva used for the calibration and those obtained in the two additional samples analyzed. Table S16 in the ESI† includes the results of the statistical tests for the RRs obtained at the level of 30 ng g−1. First, a F statistical test was developed to compare the variances of the RRs data groups. Depending on the result of the F test (whether the variances are homogeneous or heterogeneous) different Student's t tests were performed to compare the RR means. According to Table S16,† a statistical matrix effect was confirmed only for BPA in the healthy male saliva. Thus, the developed ABS/TPP-HPLC-FD method can be applied for the determination of BPs in different saliva matrices using the matrix-matched calibration method performed in a real healthy male saliva.
Evaluation of the ABS/TPP-HPLC-FD method using green metrics
Aiming to assess the greenness of the proposed ABS/TPP-HPLC-FD method, Analytical GREENess Metric (AGREE) and AGREE metrics of environmental impact of sample preparation (AGREEprep) were used. AGREE evaluates the entire analytical procedure, from sampling to the analytical detection/quantification step, considering the 12 principles of Green Analytical Chemistry (GAC) as criteria;39 whereas AGREEprep focuses on the assessment of the sample preparation step and evaluates 10 parameters associated to the 10 Green Sample Preparation (GSP) principles.40 The results of both metrics are displayed in a colored diagram in which each assessed parameter has different colors depending on their relative score (green for the maximum score and red for the minimum). The total quantitative score, obtained out of 1, is also given at the center of the diagram, with 1 being the score assigned to the greenest methods.Fig. 6 shows the diagrams obtained by applying AGREE and AGREEprep to the ABS/TPP-HPLC-FD method. With respect to AGREE, the obtained score was 0.53. The greener aspects included the non-invasive analysis of saliva (criterium 1); the use of 1.1 g (low amount) of sample (criterium 2); the involvement of only 5 steps (criterium 4); the absence of a derivatization step (criterium 6); and the safety of the operator (criterium 12), with the ACN used for the HPLC analysis being the unique hazardous solvent (highly flammable).
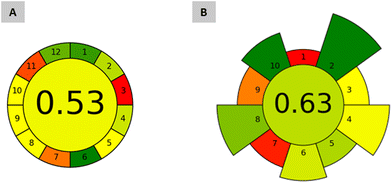 | ||
| Fig. 6 Greenness assessment of the proposed method through analytical metrics: (A) AGREE for the entire ABS/TPP-HPLC-FD method; (B) AGREEprep for the ABS/TPP-based microextraction approach. | ||
Most of the remaining parameters obtained non-green scores due to the analytical quantification step by HPLC. These comprise large volume of waste if considering the mobile phase required per each injection (criterium 7), a high energy input associated with the instrument (criterium 9), and a relatively low score in criterion 11, assigned due to the toxicity of ACN. However, the green aspects of the instrumentation are out of the control of the analysts, because HPLC is intrinsically needed for the analysis. A more representative conclusion regarding the green character of the proposed method can be appreciated with the AGREEprep evaluation (cf.Fig. 6). A total score of 0.63 was obtained. Most of the parameters obtained highly adequate punctuations, with criterion 1 (an ex situ sample preparation placement), criterion 7 (a non-automated sample preparation strategy), and criterion 9 (the post-sample preparation configuration based on the analysis by HPLC) being the least green parameters of the ABS/TPP method. Nevertheless, those parameters with the highest default weights (and thus with the highest impact in the greenness of the microextraction procedure) obtained green scores. These are the greenest features of the proposed ABS/TPP method according to AGREEprep:
(i) the use of non-hazardous materials (criterium 2), as this method only involves a low cytotoxic IL and a safe inorganic salt as reagents, which also leads to a green score in the operator's safety (criterium 10);
(ii) the waste (criterium 4), as the analysis of a miniaturized volume of saliva automatically leads to the generation of low amounts of waste (∼2 g), and also promotes a green score in the size economy of the sample (criterium 5); and
(iii) the energy consumption (criterium 8), with only vortex and centrifugation as the energy-consuming steps (an average energy input of 26.5 Wh).
For comparison purposes, Fig. S4 in the ESI† shows the diagrams of both the AGREE and AGREEprep applied to two representative studies reported in the literature.9,10 One of the studies utilized deproteinization and lyophilization of saliva as pretreatment steps (involving ∼9 h), two steps of ultrasound-assisted extraction with acetone and ethanol as extraction solvents, and LC with tandem MS as an analytical instrumental technique (for which evaporation and reconstitution are required).9 The second study utilized centrifugation and enzymatic digestion for the pretreatment of saliva (∼3.5 h), a dispersive liquid–liquid microextraction approach with ACN as extraction solvent, and GC-MS for the analytical quantification (preceded by a derivatization step).10 The proposed ABS/TPP-HPLC-FD method obtained higher green scores in both AGREE and AGREEprep than those obtained by other methods. A higher difference in the scores was observed for AGREEprep, in which the ABS/TPP procedure scored 0.63, while these studies only scored 0.11 and 0.26. These differences in the scores are related to the common use of hazardous chemicals (highly flammable organic solvents) as extractants, and the requirement of tedious and time-consuming steps of deproteinization prior to the extraction. This technological complexity leads to overconsumption of solvents, excessive waste generation, and high energy input in the overall sample preparation stage, when compared with the ABS/TPP-based microextraction.
Finally, it is interesting to assess the different greenness features of the guanidinium IL employed as solvent in the ABS/TPP approach, as this is one of the key points justifying such green scores obtained with the metrics. With respect to the toxicity and safety of the IL, the C4Gu-Cl IL is characterized by presenting low cytotoxic behavior when compared with guanidinium ILs with longer alkyl chain lengths.29 Thus, the reported 50% cytotoxic concentration (CC50) for this butylguanidinium IL is 680 ± 99 mg L−1, whereas the CC50 values for octyl- and decyl-guanidinium ILs are ∼11 and ∼4 mg L−1, respectively. Furthermore, the guanidinium core guarantees low cytotoxic profile when compared with ILs with other conventional cations of imidazolium and pyridinium.41 This low cytotoxicity also ensures the safety associated to the usage of this solvent.
With respect to the biodegradability, the guanidinium core of this IL has an amino-acid-inspired structure, and thus it is a bio-based moiety easily biodegradable when compared with ILs with benzene rings in the cation (i.e., imidazolium or pyridinium-based cations).42 Finally, with respect to the reusability, the guanidinium IL cannot be reused in this proposed application, as the IL-rich phase obtained after performing the microextraction is directly injected in the HPLC system. However, this lack of reusability does not imply a disadvantage in terms of greenness, as the direct injection allows to decrease the solvent and energy consumption and simplifies the procedure in terms of timing. Besides, the amount of IL involved limits to 0.22 g.
To sum up, it is interesting to mention that C4Gu-Cl meets the main requirements established for the design of safe and green ILs, in terms of the nature of the cation and its side chain (involving a guanidinium cation with short chain), and the incorporation of a halide instead of a fluorine-containing anion.42
Aiming to assess the suitability of the C4Gu-Cl IL not only from a green point of view, but also under a profitability perspective, the costs of using this IL were compared with the costs involved in the using of other ILs and conventional materials commonly reported as ABSs phase-forming components. Table S17 of the ESI† includes the different costs of the IL per gram, as well as an estimation of the costs per extraction, considering the amount of IL employed in the ABS/TPP method (0.35 g). The benchmark materials covered for the comparison include a conventional polymer (polypropylene glycol) and three conventional ILs with structural similarities to C4Gu-Cl (all with the chloride anion and similar alkyl chain lengths in the cation), but differing in the cation core, including imidazolium-, pyridinium- and pyrrolidinium-based ILs. As it can be observed in Table S17,† the guanidinium IL prepared in this study involves a similar cost than the one obtained with the polymer and the pyrrolidinium IL. Furthermore, the costs of the guanidinium IL were lower than those associated to the use of the imidazolium and the pyridinium IL. Besides, the procedure for the preparation of C4Gu-Cl does not require any purification step after its synthesis. On contrast, the purification of non-commercial imidazolium ILs usually involve liquid–liquid extraction protocols with halogenated organic solvents, or distillation,43 thus increasing the cost of the synthesis and also hindering the greenness associated to the preparation and usage of these ILs.
Therefore, it is important to highlight that the guanidinium IL not only ensures greener and safer features but also it does not involve a significant increase (and even in some cases it ensures a decrease) in the costs associated, thus supporting its feasibility as solvent for sustainable and affordable ABSs strategies.
Conclusions
A strategy comprising the simultaneous clean-up, microextraction and preconcentration of human saliva was successfully developed, for the first time, using IL-based ABSs. The developed platform takes advantage of the low cytotoxic C4Gu-Cl IL and the K2HPO4 salt to form a miniaturized ABS, while respecting some of the recommendations of Green Analytical Chemistry. Saliva clean-up is achieved through the formation of protein-enriched solid interphase (leading to the formation of an ABS/TPP), while target analytes can be extracted and preconcentrated in the IL-rich phase. The developed ABS/TPP strategy achieved the precipitation of nearly 70% of the salivary proteins in the interphase, thus ensuring the direct combination of the method with HPLC-FD for the determination of bisphenols as representative biomarkers in saliva.The entire ABS/TPP-HPLC-FD method, directly validated using human saliva, showed improved enrichment factors, extraction efficiencies, and intermediate precisions, despite the low volume of saliva sample available and miniaturized conditions adopted. The method shows high performance when determining bisphenols at relevant levels in human saliva. Finally, the proposed ABS/TPP-HPLC-FD approach was evaluated through different analytical greenness metrics, obtaining higher green scores than previously reported studies.
Ongoing work is being focused on developing other biocompatible ILs to be applied as ABS/TPP constituents to improve the preconcentration achieved. Furthermore, the applicability of these systems for the determination of other hazardous chemicals of wide occurrence in human saliva is expected to be expanded.
Author contributions
Conceptualization, R. G. M., F. A. e. S., M. J. T. R., M. G. F. and V. P.; methodology R. G. M., F. A. e. S., M. J. T. R. and J. L. M.; writing – original draft preparation, R. G. M.; writing – review and editing, R. G. M., F. A. e. S., M. J. T. R., D. D. D., J. L. M., M. G. F. and V. P.; supervision, F. A. e. S., M. J. T. R., D. D. D., M. G. F. and V. P.; funding acquisition, M. G. F. and V. P.; project administration, M. G. F. and V. P. All authors listed have made a substantial, direct, and intellectual contribution to the work and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was developed within the scope of the project ref. PID2020-115004RB-I00, funded by the Spanish Ministry of Science and Innovation, the project ref. ProID2020010089 of the Research Canary Agency “ACIISI”, the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC), and the project PTDC/EMD-TLM/3253/2020, funded by national funds (OE), through FCT/MCTES. This study was also funded by Consorcio Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III, Spain (CB21/13/00100), Cabildo Insular de Tenerife 2023–2028 and Ministerio de Sanidad, Gobierno de España. R. G.-M. thanks the Spanish Ministry of Universities for his FPU fellowship. F. A. e Silva acknowledges FCT – Fundação para a Ciência e a Tecnologia, I.P. for the researcher contract CEECIND/03076/2018 under the Scientific Employment Stimulus - Individual Call 2018. M. J. T.-R. thanks her former Excellence Junior research contract with Fundación La Caixa – Fundación CajaCanarias, which covered her research stay in Portugal, together with her current Ramón y Cajal contract (ref. RYC2021-032502-I) at Universidad de La Laguna, contract with funding of the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033 and the European Union “NextGenerationEU”/PRTR.References
- G. Sousa, C. Delerue-Matos, X. Wang, F. Rodrigues and M. Oliveira, Chapter Potential of Saliva for Biomonitoring of Occupational Exposure: Collection of Evidence from the Literature, in Occupational and Environmental Safety and Health IV, ed. P. M. Arezes, J. S. Baptista, R. B. Melo, J. C. Branco, P. Carneiro, A. Colim, N. Costa, S. Costa, J. Duarte, J. C. Guedes and G. Perestrelo, Springer, Switzerland, Studies in Systems, Decision and Control, 2023, vol. 449, pp. 587–598 Search PubMed.
- I. T. Gug, M. Tertis, O. Hosu and C. Cristea, TrAC, Trends Anal. Chem., 2019, 113, 301–316 CrossRef CAS.
- Y. Huang and M. Fang, Environ. Sci. Technol., 2020, 54, 14793–14796 CrossRef CAS PubMed.
- F. G. Bellagambi, T. Lomonaco, P. Salvo, F. Vivaldi, M. Hangouët, S. Ghimenti, D. Biagini, F. D. Francesco, R. Fuoco and A. Errachid, TrAC, Trends Anal. Chem., 2020, 124, 115781 CrossRef CAS.
- K. Vorkampa, A. Castaño, J.-P. Antignac, L. D. Boada, E. Cequier, A. Covaci, M. E. López, L. S. Hauge, M. Kasper-Sonnenberg, H. M. Koch, O. P. Luzardo, A. Osīte, L. Rambaudi, M.-T. Pinorini, G. Sabbioni and C. Thomsen, Environ. Int., 2021, 146, 106082 CrossRef PubMed.
- J. Marín-Sáez, R. López-Ruiz, M. Sobral, R. Romero-González, A. G. Frenich and I. M. P. L. V. O. Ferreira, TrAC, Trends Anal. Chem., 2023, 158, 116853 CrossRef.
- H. Kataoka, R. Inoue, K. Yagi and K. Saito, J. Pharm. Biomed. Anal., 2009, 49, 108–114 CrossRef CAS PubMed.
- M. L. de Oliveira, B. A. Rocha, V. C. O. Souza and F. Barbosa Jr., Talanta, 2019, 196, 271–276 CrossRef PubMed.
- I. Moscoso-Ruiz, Y. Gálvez-Ontiveros, S. Cantarero-Malagón, A. Rivas and A. Zafra-Gómez, Microchem. J., 2022, 175, 107122 CrossRef CAS.
- T. H. V. Vu, H.-H. Lim and H.-S. Shin, Bull. Korean Chem. Soc., 2020, 41, 424–432 CrossRef CAS.
- N. Saha, B. Sarkar and K. Sen, J. Mol. Liq., 2022, 363, 119882 CrossRef CAS.
- M. Iqbal, Y. Tao, S. Xie, Y. Zhu, D. Chen, X. Wang, L. Huang, D. Peng, A. Sattar, M. A. B. Shabbir, H. I. Hussain, S. Ahmed and Z. Yuan, Biol. Proced. Online, 2016, 18, 18 CrossRef PubMed.
- A. Basaiahgari and R. L. Gardas, Curr. Opin. Green Sustainable Chem., 2021, 27, 100423 CrossRef CAS.
- A. R. P. Gonçalves, X. Paredes, A. F. Cristino, F. J. V. Santos and C. S. G. P. Queirós, Int. J. Mol. Sci., 2021, 22, 5612 CrossRef PubMed.
- V. P. Priyanka and R. L. Gardas, Sep. Purif. Technol., 2020, 234, 116048 CrossRef CAS.
- C. P. Song, R. N. Ramanan, R. Vijayaraghavan, D. R. MacFarlane, E.-S. Chan and C.-W. Ooi, ACS Sustainable Chem. Eng., 2015, 3, 3291–3298 CrossRef CAS.
- G. Zhu, X. Ma, Q. Huang, L. Zhao, R. Zhang, X. Yang and S. Wang, ACS Sustainable Chem. Eng., 2022, 10, 1633–1643 CrossRef CAS.
- M. Tobiszewski, A. Mechlinska and J. Namiesnik, Chem. Soc. Rev., 2010, 39, 2869–2878 RSC.
- S. Sadeghi and A. Z. Moghaddam, J. Mol. Liq., 2016, 221, 798–804 CrossRef CAS.
- M. G. Bogdanov and I. Svinyarov, J. Chromatogr. A, 2018, 1559, 62–68 CrossRef CAS PubMed.
- M. M. Pereira, J. D. Calixto, A. C. A. Sousa, B. J. Pereira, A. S. Lima, J. A. P. Coutinho and M. G. Freire, Sci. Rep., 2020, 10, 14931 CrossRef CAS PubMed.
- F. C. Flora, S. B. Relvas, F. A. e Silva, M. G. Freire, V. Chu and J. P. Conde, Biosensors, 2023, 13, 334 CrossRef CAS PubMed.
- J. Flieger and A. Czajkowska-Zelazko, Food Chem., 2015, 166, 150–157 CrossRef CAS PubMed.
- T. B. V. Dinis, H. Passos, D. L. D. Lima, V. I. Esteves, J. A. P. Coutinho and M. G. Freire, Green Chem., 2015, 17, 2570–2579 RSC.
- H. F. D. Almeida, M. G. Freire and I. M. Marrucho, Green Chem., 2017, 19, 4651–4659 RSC.
- Y.-X. Wang, C. Liu, Y. Shen, Q. Wang, A. Pan, P. Yang, Y.-J. Chen, Y.-L. Deng, Q. Lu, L.-M. Cheng, X.-P. Miao, S.-Q. Xu, W.-Q. Lu and Q. Zeng, Environ. Int., 2019, 123, 301–309 CrossRef CAS PubMed.
- J. C. Merchuk, B. A. Andrews and J. A. Asenjo, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 711, 285–293 CrossRef CAS PubMed.
- S. P. F. Costa, A. M. O. Azevedo, P. C. A. G. Pinto and M. L. M. F. S. Saraiva, ChemSusChem, 2017, 10, 2321–2347 CrossRef CAS PubMed.
- R. González-Martín, I. Pacheco-Fernández, J. H. Ayala, A. M. Afonso and V. Pino, Talanta, 2019, 203, 305–313 CrossRef PubMed.
- A.-K. Amsel, O. Olsson and K. Kümmerer, Chemosphere, 2022, 299, 134385 CrossRef CAS PubMed.
- E. Gómez, I. Domínguez, Á. Domínguez and E. A. Macedo, J. Chem. Eng. Data, 2018, 63, 1103–1108 CrossRef.
- C. U. Mussagy, N. L. Tabanez, F. O. Farias, K. A. Kurnia, M. R. Mafra and J. F. B. Pereira, Chem. Phys. Lett., 2020, 754, 137623 CrossRef CAS.
- S. Shahriari, C. M. S. S. Neves, M. G. Freire and J. A. P. Coutinho, J. Phys. Chem. B, 2012, 116, 7252–7258 CrossRef CAS PubMed.
- T. E. Sintra, R. Cruz, S. P. M. Ventura and J. A. P. Coutinho, J. Chem. Thermodyn., 2014, 77, 206–213 CrossRef CAS.
- S. Z. Fisher, L. Govindasamy, C. Tu, M. Agbandje-McKenna, D. N. Silverman, H. J. Rajaniemi and R. McKenna, Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun., 2006, 62, 88–93 CrossRef CAS PubMed.
- M. J. Trujillo-Rodríguez, P. Rocío-Bautista, V. Pino and A. M. Afonso, TrAC, Trends Anal. Chem., 2013, 51, 87–106 CrossRef.
- I. Taverniers, M. D. Loose and E. V. Bockstaele, TrAC, Trends Anal. Chem., 2004, 23, 535–552 CrossRef CAS.
- E. Romera-García, N. Caballero-Casero and S. Rubio, Talanta, 2019, 204, 465–474 CrossRef PubMed.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed.
- W. Wojnowski, M. Tobiszewski, F. Pena-Pereira and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 149, 116553 CrossRef CAS.
- V. Thamke, P. Singh, S. Pal, M. Chaudhary, K. Kumari, I. Bahadur and R. S. Varma, J. Environ. Chem. Eng., 2022, 10, 107303 CrossRef CAS.
- S. Magina, A. Barros-Timmons, S. P. M. Ventura and D. V. Evtuguin, J. Hazard. Mater., 2021, 412, 125215 CrossRef CAS PubMed.
- B. Clare, A. Sirwardana and D. R. MacFarlane, Chapter Synthesis, Purification and Characterization of Ionic Liquids, in Topics in current Chemistry, ed. V. Balzani, A. de Meijere, K. N. Houk, H. Kessler, J.-M. Lehn, S. V. Ley, M. Olivucci, S. Schreiber, J. Thiem, B. M. Trost, P. Vogel, F. Vögtle, H. Wong and H. Yamamoto, Springer, Switzerland, 2010, vol. 290, pp. 1–40 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc02046k |
| This journal is © The Royal Society of Chemistry 2023 |