Bifunctional chimera for ligand-directed photo-degradation of oncogenic microRNA†
Chen
Li
,
Jin
Wang
,
Yan
Wang
,
Yi
Feng
,
Jinbo
Li
 * and
Yan
Zhang
* and
Yan
Zhang
 *
*
State Key Laboratory of Analytical Chemistry for Life Sciences, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Chemistry and Biomedicine Innovation Center, Nanjing University, Nanjing 210023, China. E-mail: jinboli@nju.edu.cn; njuzy@nju.edu.cn
First published on 23rd May 2023
Abstract
Targeted inhibition of oncogenic microRNAs provides a promising anti-cancer approach. Here, we report a bifunctional chimera for ligand-directed regulation of target oncogenic precursor microRNA through photo-degradation. Chimeric TGP-210-Ppa with photosensitizer pyropheophorbide a (Ppa) linked with the ligand of the oncogenic precursor miR-210 was able to bind specifically to oncogenIc pre-miRNA and produce 1O2 under red light irradiation to degrade the target pre-miRNA. This bifunctional chimera-based modification of precursor microRNA serves as a unique method for target gene regulation since photo-irradiation was able to provide temporal–spatial resolution. We demonstrated that TGP-210-Ppa prevented the generation of functional miR-210 in breast cancer cells in a photocontrollable manner. This also successfully reversed the downstream oncogenic signaling pathway mediated by miR-210 to promote cancer cell death.
As a class of endogenous gene regulators, microRNAs (miRNAs) have been found to extensively participate in cancer progression.1 Suppression of oncogenic miRNAs thus represents a promising anti-cancer strategy.2,3 In addition to antisense oligonucleotides capable of blocking miRNA function through Watson–Crick base pairing-based hybridization,4 small molecules that directly bind to the secondary structures of miRNAs are emerging as a novel type of miRNA inhibitor.5 For example, xanthone derivatives,6 aminoglycosides,7,8 and ether-amide derivatives9 have been reported to selectively bind and inhibit target miRNAs in living cells. To further enhance the potency of small-molecule inhibitors, the Disney group developed bifunctional chimeras containing small-molecule ligands of precursor miRNAs for targeted miRNA degradation.10,11 Ribonuclease-targeting chimera (RIBOTAC) comprising a small-molecule miRNA ligand linked with an RNase recruiter is validated to induce miRNA degradation by bringing RNase in close proximity to the target miRNA.12,13 This approach has been demonstrated to degrade oncogenic miRNAs such as miR-96,14 miR-210,15 miR-17-92,16 and miR-21.12 These achievements highlight the promise of small-molecule chimeras for miRNA inhibition.
Singlet oxygen (1O2) is a reactive oxygen species (ROS) that is highly detrimental toward biomolecules, including nucleic acids, proteins and lipids.17,18 Exposure to 1O2 is known to cause oxidative damage to adjacent biomolecules and lead to malfunction of the oxidized biomolecules.19,20 Photosensitizers with strong absorption at long wavelengths have been widely adopted for biomedical applications to generate 1O2 in spatiotemporal- and dose-controllable manners.21,22 The integration of photosensitizers with photoirradiation to produce excessive 1O2 has led to the discovery of photodynamic therapy (PDT) as a powerful anti-cancer modality.23,24 A more deliberate approach, named chromophore-assisted light inactivation (CALI), has been developed to selectively inactivate proteins in living cells by pre-installation of photosensitizers onto the target followed by meticulous light illumination to generate 1O2.25,26 However, the possibility of using 1O2 to precisely inactivate miRNAs in living cells has been little reported.
Here we report a novel small-molecule chimera, TGP-210-Ppa, for targeted inhibition of miR-210 in living cancer cells (Scheme 1). TGP-210-Ppa consists of the small-molecule ligand, TGP-210, against precursor miR-210 (pre-miR-210) and the organic photosensitizer, pyropheophorbide a (Ppa). Upon ligand-directed binding of TGP-210-Ppa to pre-miR-210, red light irradiation is implemented to excite Ppa to produce 1O2 to oxidize riboguanosine (rG) into 8-oxo-rG, causing the mutation and dysfunction of pre-miR-210.
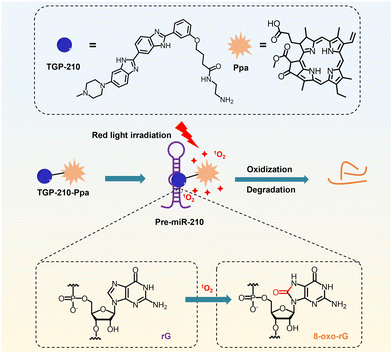 | ||
| Scheme 1 Schematic illustration on the working mechanism of the ligand-directed red-light regulation of oncogenic microRNA. | ||
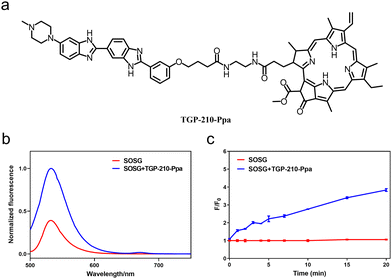
The chemical structure of TGP-210-Ppa is shown in Fig. 1a. The two functional units, TGP-210 and Ppa were readily conjugated together using an amide condensation strategy with ethylenediamine as the linker (Fig. S1, ESI†). The structure of the purified TGP-210-Ppa was confirmed by electrospray ionization-mass spectrometry (ESI-MS) (Fig. S2, ESI†). The ultraviolet-visible (UV-Vis) spectroscopy of TGP-210-Ppa shows the characteristic absorption of TGP-210 at ∼350 nm and two characteristic absorption peaks of Ppa at ∼400 nm and ∼660 nm (Fig. S3, ESI†). The fluorescence spectrum shows that TGP-210-Ppa has similar fluorescence emission to that of Ppa (Fig. S4, ESI†). These results suggest that the conjugation of TGP-210 to Ppa does not change the photophysical properties of the photo-sensitizer significantly.
We next examined whether TGP-210-Ppa was able to generate 1O2 upon red-light irradiation and induce photo damage to its target pre-miR-210. Using the 1O2 sensor green (SOSG) as the fluorescent indicator, we confirmed the capability of TGP-210-Ppa to generate 1O2 in Phosphate Buffered Saline (PBS) buffer upon red light irradiation. As shown in Fig. 1b, irradiation of TGP-210-Ppa in PBS buffer containing SOSG using light of wavelength around 670 nm increased the fluorescence signal around 530 nm, which is characteristic of the fluorescence signal enhanced by 1O2 on SOSG. Time dependence curve of the fluorescence enhancement versus irradiation time is shown in Fig. 1c. Subsequently, 9,10-anthracendipropionic acid (ADPA) was used as a capture probe to evaluate the efficiency of the 1O2 generation. As shown in Fig. S5 (ESI†), the absorption intensity of ADPA at 378 nm gradually decreased as the irradiation time increased in the presence of the TGP-210-Ppa and MB, suggesting ADPA decomposition. According to the decay curves of the ADPA absorption, the 1O2 quantum yield of the TGP-210-Ppa was about 0.327.27 We then chose red light irradiation for 5 min as the standard condition to induce 1O2-based oxidation.
Since nucleobases are known to be susceptible to 1O2,28 we evaluated the generation of oxidized nucleobases in pre-miR-210. Whether pre-miR-210 treated with TGP-210-Ppa upon red light irradiation results in the oxidation of nucleobases in pre-miR-210 was examined. We used LC-ESI-MS/MS to analyze the hydrolysis products of pre-miR-210 with or without TGP-210-Ppa under light irradiation (Fig. S6, ESI†). It is noteworthy that only 8-oxo-rG was detected as the oxidized nucleobase form in the hydrolyzed products of pre-miR-210 treated with TGP-210-Ppa and light irradiation. This is consistent with previous evidence that, among the four nucleobases in RNA, guanine is a major substrate of 1O2.29 Gel-shift assay revealed that excessive 1O2 generated in the system by the presence of 200 equivalent of TGP-210-Ppa to pre-miR-210, together with 15 min light irradiation induced strand breakage (Fig. S7, ESI†), which is in agreement with previously reported results.30,31 Overall, these results demonstrate that TGP-210-Ppa is able to cause oxidative damage to pre-miR-210 under photoirradiation.
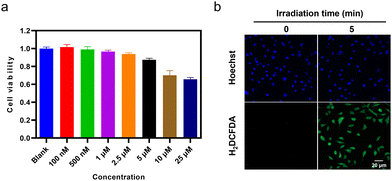
The intracellular applications of TGP-210-Ppa were then explored. An MTT assay showed that TGP-210-Ppa had no obvious cytotoxicity in the dark at the concentration up to 25 μM (Fig. 2a). Confocal fluorescence imaging and flow cytometry showed that TGP-210-Ppa was able to be taken up by cancer cell lines such as breast cancer MDA-MB-231 cells (Fig. S8, ESI†). Intracellular generation of 1O2 by TGP-210-Ppa upon exposure to red light irradiation was monitored using the 1O2-sensitive fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). As shown in Fig. 2b, the irradiation of MDA-MB-231 cells incubated with TGP-210-Ppa using light of 670 nm for 5 min significantly enhanced the fluorescence signal from the 1O2 indicator inside the cells. We next investigated whether the in-situ generation of 1O2 was able to regulate the intracellular pre-miR-210 since it was the target of TGP-210-Ppa for proximity-based regulation. Furthermore, to study the binding consequences of adding the Ppa moiety to TGP-210, binding affinities were measured by microscale thermophoresis (MST) to the targets with TGP-210 or TGP-210-Ppa.15,32 The results indicate that TGP-210-Ppa maintained selective binding to RNA with a Kd of 392 nM to pre-miR-210, which is modestly weaker compared with TGP-210 with a Kd of 265 nM (Fig. S9, ESI†).
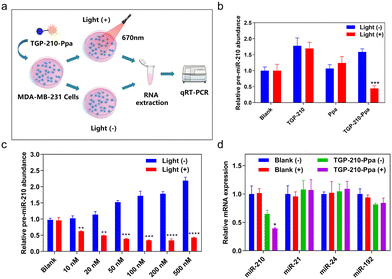
The protocol to evaluate the efficacy of the ligand-directed red-light regulation of intracellular pre-miR-210 is illustrated in Fig. 3a. Quantitative reverse transcription polymer chain reaction (qRT-PCR) results indicated the relative abundance of various RNAs in MDA-MB-231 cells treated with TGP-210-Ppa with or without red light irradiation. Cells treated by TGP-210 or Ppa were used as a control to exclude the effect caused by the ligand or photo-sensitizer alone (Fig. 3b). Without exposure to light irradiation, both TGP-210 and TGP-210-Ppa raised the intracellular level of pre-miR-210 dose-dependently, which could be attributed to the ligand-mediated blockade of pre-miR-210 processing due to the occupancy of enzymatic sites by TGP-210-Ppa.33 Cells treated by Ppa alone did not show obvious changes in the intracellular pre-miR-210 level even after red light irradiation.
For cells treated by TGP-210-Ppa with a concentration as low as 10 nM, red light irradiation induced a significant decrease of intracellular pre-miR-210 level (Fig. 3c). Higher concentrations of TGP-210-Ppa elevated the pre-miR-210 level before light irradiation. However, after the cells pre-treated with TGP-210-Ppa were exposed to red light irradiation to trigger the formation of 1O2, the ligand-directed modification or degradation of pre-miR-210 became predominant. These results suggest that the enhanced suppression of miR-210 by TGP-210-Ppa depends on both its chimeric effects. Also, TGP-210-Ppa further reduced the intracellular level of miR-210 in a dose-dependent manner under light irradiation, with similar effects observed for pre-miR-210 (Fig. S10, ESI†). Consequently, TGP-210-Ppa led to a more potent inhibition of miR-210 biogenesis after photoirradiation. Moreover, non-target miRNAs, including miR-21, miR-24 and miR-192, were not modulated by TGP-210-Ppa even after light irradiation (Fig. 3d), confirming a ligand-directed selective inhibition of miR-210.
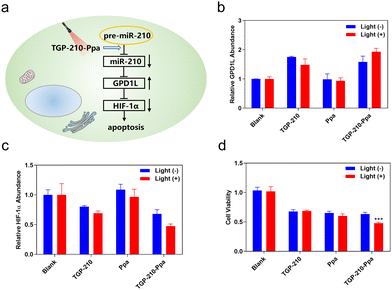
Finally, we investigated whether the ligand-directed photo-regulation of pre-miR-210 was able to further regulate relevant genes down-stream of miR-210. It has been well established that the overexpression of miR-210 promotes cancer cell growth by inhibiting the glycerol-3-phosphate dehydrogenase 1-like (GPD1L)-hypoxia inducible factor 1-alpha (HIF-1α) pathway (Fig. 4a).34 Therefore we examined the potential of TGP-210-Ppa for photo-regulating the expression level of GPD1L and HIF-1α. The results shown in Fig. 4b and c confirmed our assumption that TGP-210-Ppa in MDA-MB-231 cells upon photo-irradiation caused elevated expression of GPD1L and suppression of the HIF1α gene. MTT assay showed that TGP-210-Ppa with light irradiation elicited 52% cell apoptosis (Fig. 4d). Therefore, the ligand-directed photo-degradation of pre-miR-210 by TGP-210-Ppa could partially reverse the miR-210-involved signaling pathway to induce cancer apoptosis.
In summary, we reported a novel bifunctional chimera for photo-oxidation and degradation of target pre-miRNA that is related to cancer. TGP-210-Ppa that integrated the small-molecule ligand to the oncogenic pre-miR-210 with the photo-sensitizer Ppa was prepared, which demonstrated high efficacy for ligand-directed red-light degradation of intracellular pre-miR-210. TGP-210-Ppa could efficiently produce 1O2 upon red-light irradiation, which induced the oxidization of rG into 8-oxo-rG in pre-miR-210 and further degradation of intracellular pre-miR-210. Using TGP-210-Ppa and red-light irradiation, we demonstrated the possibility of reversing oncogenic signalling pathways downstream of miR-210 to promote cancer cell apoptosis. As more small-molecule ligands are being reported to bind with specific RNA targets, this proof-of-concept work holds promise to develop various photo-regulatable bifunctional chimeras for spatial and temporal degradation of disease-related precursor microRNAs. Further efforts to address the limit of this system for in vivo applications due to the penetration ability of red light into deep tissue are now underway in our group.
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20202004, BE2022835), the National Natural Science Foundation of China (22225703, 22137003, 22077063, 21877058, 21977043) and the Fundamental Research Funds for the Central Universities (020514380300).
Conflicts of interest
There are no conflicts to declare.References
- A. Abi, N. Farahani, G. Molavi and S. M. Gheibi Hayat, Cancer Gene Ther., 2020, 27, 280–293 CrossRef CAS PubMed.
- M. Garofalo, G. D. Leva and C. M. Croce, Curr. Pharm. Des., 2014, 20, 5328–5335 CrossRef CAS PubMed.
- M. Fan, M. Shan, X. Lan, X. Fang, D. Song, H. Luo and D. Wu, Front. Pharmacol., 2022, 13, 1033017 CrossRef CAS PubMed.
- S. T. Crooke, J. L. Witztum, C. F. Bennett and B. F. Baker, Cell Metab., 2018, 27, 714–739 CrossRef CAS PubMed.
- T. Felicetti and G. Manfroni, Future Med. Chem., 2021, 13, 1245–1248 CrossRef CAS PubMed.
- A. Murata, T. Fukuzumi, S. Umemoto and K. Nakatani, Bioorg. Med. Chem. Lett., 2013, 23, 252–255 CrossRef CAS PubMed.
- D. D. Vo, C. Staedel, L. Zehnacker, R. Benhida, F. Darfeuille and M. Duca, ACS Chem. Biol., 2014, 9, 711–721 CrossRef CAS PubMed.
- D. D. Vo, T. P. Tran, C. Staedel, R. Benhida, F. Darfeuille, A. Di Giorgio and M. Duca, Chemistry, 2016, 22, 5350–5362 CrossRef CAS PubMed.
- N. Ankenbruck, R. Kumbhare, Y. Naro, M. Thomas, L. Gardner, C. Emanuelson and A. Deiters, Bioorg. Med. Chem., 2019, 27, 3735–3743 Search PubMed.
- J. L. Childs-Disney, X. Yang, Q. M. R. Gibaut, Y. Tong, R. T. Batey and M. D. Disney, Nat. Rev. Drug Discovery, 2022, 21, 736–762 CrossRef CAS PubMed.
- P. Zhang, X. Liu, D. Abegg, T. Tanaka, Y. Tong, R. I. Benhamou, J. Baisden, G. Crynen, S. M. Meyer, M. D. Cameron, A. K. Chatterjee, A. Adibekian, J. L. Childs-Disney and M. D. Disney, J. Am. Chem. Soc., 2021, 143, 13044–13055 CrossRef CAS PubMed.
- S. M. Meyer, T. Tanaka, P. R. A. Zanon, J. T. Baisden, D. Abegg, X. Yang, Y. Akahori, Z. Alshakarchi, M. D. Cameron, A. Adibekian and M. D. Disney, J. Am. Chem. Soc., 2022, 144, 21096–21102 CrossRef CAS PubMed.
- Y. Tong, Q. M. R. Gibaut, W. Rouse, J. L. Childs-Disney, B. M. Suresh, D. Abegg, S. Choudhary, Y. Akahori, A. Adibekian, W. N. Moss and M. D. Disney, J. Am. Chem. Soc., 2022, 144, 11620–11625 Search PubMed.
- Y. Li and M. D. Disney, ACS Chem. Biol., 2018, 13, 3065–3071 CrossRef CAS PubMed.
- M. G. Costales, B. Suresh, K. Vishnu and M. D. Disney, Cell Chem. Biol., 2019, 26, 1180–1186.e1185 CrossRef CAS PubMed.
- X. Liu, H. S. Haniff, J. L. Childs-Disney, A. Shuster, H. Aikawa, A. Adibekian and M. D. Disney, J. Am. Chem. Soc., 2020, 142, 6970–6982 CrossRef CAS PubMed.
- P. Di Mascio, G. R. Martinez, S. Miyamoto, G. E. Ronsein, M. H. G. Medeiros and J. Cadet, Chem. Rev., 2019, 119, 2043–2086 CrossRef CAS PubMed.
- H. Sies, C. Berndt and D. P. Jones, Annu. Rev. Biochem., 2017, 86, 715–748 CrossRef CAS PubMed.
- W. K. Martins, N. F. Santos, C. S. Rocha, I. O. L. Bacellar, T. M. Tsubone, A. C. Viotto, A. Y. Matsukuma, A. B. P. Abrantes, P. Siani, L. G. Dias and M. S. Baptista, Autophagy, 2019, 15, 259–279 Search PubMed.
- K. Jakubczyk, K. Dec, J. Kaldunska, D. Kawczuga, J. Kochman and K. Janda, Pol. Merkuriusz Lek., 2020, 48, 124–127 Search PubMed.
- J. Shi, D. Wang, Y. Ma, J. Liu, Y. Li, R. Reza, Z. Zhang, J. Liu and K. Zhang, Small, 2021, 17, e2104722 CrossRef PubMed.
- Y. Jiao, Y. Gao, J. Wang, H. An, Y. X. Li and X. Zhang, Int. J. Pharm., 2022, 621, 121805 CrossRef CAS PubMed.
- J. Wang, H. He, X. Xu, X. Wang, Y. Chen and L. Yin, Biomaterials, 2018, 171, 72–82 CrossRef CAS PubMed.
- S. Nath and K. Moore, Bio-Protoc., 2019, 9, e3314 CAS.
- K. Takemoto, Nihon Yakurigaku Zasshi, 2022, 157, 238–243 CrossRef CAS PubMed.
- K. Miura, Y. Wen, M. Tsushima and H. Nakamura, ACS Omega, 2022, 7, 34685–34692 CrossRef CAS PubMed.
- J. Ge, Q. Jia, W. Liu, M. Lan, B. Zhou, L. Guo, H. Zhou, H. Zhang, Y. Wang, Y. Gu, X. Meng and P. Wang, Adv. Healthcare Mater., 2016, 5, 665–675 CrossRef CAS.
- C. Fimognari, Oxid. Med. Cell. Longevity, 2015, 2015, 358713 Search PubMed.
- A. M. Fleming, O. Alshykhly, J. Zhu, J. G. Muller and C. J. Burrows, Chem. Res. Toxicol., 2015, 28, 1292–1300 Search PubMed.
- P. I. Moreira, A. Nunomura, M. Nakamura, A. Takeda, J. C. Shenk, G. Aliev, M. A. Smith and G. Perry, Free Radical Biol. Med., 2008, 44, 1493–1505 CrossRef CAS PubMed.
- W. Martinet, G. R. de Meyer, A. G. Herman and M. M. Kockx, Eur. J. Clin. Invest., 2004, 34, 323–327 CrossRef CAS PubMed.
- M. H. Moon, T. A. Hilimire, A. M. Sanders and J. S. Schneekloth, Jr., Biochemistry, 2018, 57, 4638–4643 CrossRef CAS PubMed.
- M. G. Costales, C. L. Haga, S. P. Velagapudi, J. L. Childs-Disney, D. G. Phinney and M. D. Disney, J. Am. Chem. Soc., 2017, 139, 3446–3455 CrossRef CAS PubMed.
- S. Grosso, J. Doyen, S. K. Parks, T. Bertero, A. Paye, B. Cardinaud, P. Gounon, S. Lacas-Gervais, A. Noel, J. Pouyssegur, P. Barbry, N. M. Mazure and B. Mari, Cell Death Dis., 2013, 4, e544 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06687d |
| This journal is © The Royal Society of Chemistry 2023 |