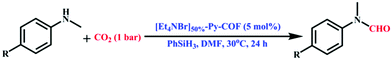Open Access Article
Open Access ArticleA study of contemporary progress relating to COF materials for CO2 capture and fixation reactions
Robius
Sani†
,
Tusar Kanto
Dey†
,
Mainak
Sarkar
,
Priyanka
Basu
and
Sk. Manirul
Islam
 *
*
Department of Chemistry, University of Kalyani, Kalyani, Nadia 741235, West Bengal, India. E-mail: manir65@rediffmail.com; Fax: +91-33-2582-8282; Tel: +91-33-2582-8750
First published on 9th June 2022
Abstract
Global warming is a great threat to the very survival of our planet. Among the different greenhouse gases, carbon dioxide is pre-eminently responsible for the occurrence of global warming. Therefore, to overcome this problem, covalent organic frameworks (COFs) have been developed as a new class of multifunctional crystalline organic catalyst constructed from organic monomers via robust covalent bonds. COF materials could be used for the chemical fixation of CO2 with different organic molecules to synthesize various valuable chemicals. The specific surface area of the covalent organic framework plays a vital role in the catalytic performance of a COF during chemical conversion involving CO2. The incorporation of various metals, such as Pd, Cu, Ag, and Ni, into a COF material can enhance its catalytic efficacy during carbon dioxide fixation reactions. Basically, a Schiff base reaction between an amine and aldehyde can result in the formation of a cyclic COF, e.g., TFPB–TAPB-COF, TFPA–TAPB-COF, BTMA–TAPA-COF, and TFPA–TAPA-COF, as reported by Donglin Jiang et al. Different kinds of valuable chemicals have been synthesized using these COFs, such as cyclic carbonates, oxazolidinones, N-formylated products, formic acid, formaldehyde, and methanol. In this review paper, we present recent advances in this area, including the development of various types of COFs and their application as catalysts for different types of CO2 fixation reactions. Herein we mainly describe synthesis schemes, some important characterization information (specifically N2-BET and XRD data), and examples of CO2 fixation reactions performed using these previously reported COFs. The excellent CO2 absorption capacities of COFs make them tremendous catalysts for CO2 conversion, and they can act as CO2 storage materials.
1. Introduction
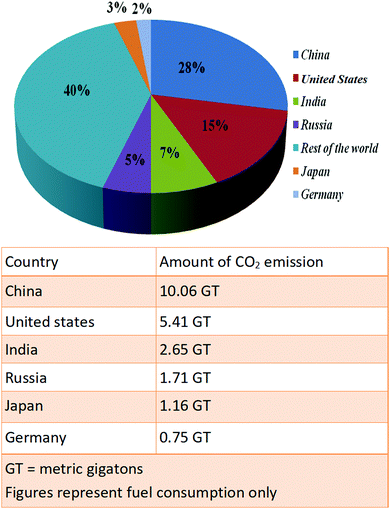
The continuous emission of greenhouse gases is causing global warming, resulting in a constant increase in the earth's temperature. Almost 60% of global warming is caused by carbon dioxide gas. 30 GT of carbon dioxide gas is emitted per year due to fossil fuel consumption, increasing the CO2 gas concentration in the atmosphere to 400 ppm, a drastic elevation over normal levels (300 ppm). The International Energy Agency, in 2012, predicted that the global temperature will have increased by 2 °C by 2100 and the CO2 concentration will have reached up to 500 ppm. A major concern of the present era is the increased concentration of carbon dioxide in the earth's atmosphere due to the incineration of fossil fuels. This enhanced amount of CO2 has a deadly impact on our surroundings.1 It also affects the global environment, making it hotter. The global temperature is increasing by 0.2 °C per decade, causing global climate change and the melting of icebergs. As a result, sea levels are also increasing every decade due to this melting. Fig. 1 shows the CO2 emission levels in 2020 from different countries.To save the environment, there is a very urgent need to decrease CO2 levels immediately, and atmospheric CO2 can be used to produce many valuable chemicals via different techniques.2 Researchers have found different protocols for the chemical fixation of carbon dioxide into different organic substrates.2,3 However, the chemical fixation of carbon dioxide is thermodynamically unfavorable.3 The chemical fixation of CO2 into organic substrates is very challenging due to the high activation energy. Thermodynamically, the Gibbs free energy changes in CO2 fixation reactions are positive, making the chemical fixation of CO2 thermodynamically unfavorable. For this reason, CO2 fixation reactions are generally performed with reagents that have high negative Gibbs free energy changes. To overcome the high activation energy, high CO2 pressures, elevated reaction temperatures, and prolonged reaction times are required. These reaction conditions are not profitable for the industrial large-scale synthesis of valued-added products via CO2 fixation reactions. These exertions can be overruled via designing a suitable catalytic system for the chemical conversion of CO2. CO2 is a non-toxic, renewable, inexpensive, and abundant raw C1 feedstock, and it can act as a C1 building block for the synthesis of various valuable chemicals with added economic value, such as carbonates, carbamates, urea, etc.4–7 Different types of catalysts can be designed for this purpose, like porous organic polymers (POPs), metal organic frameworks (MOFs), covalent organic frameworks (COFs), oxide-based compounds, ionic liquids, etc.4–9 The designed catalysts should be selective, stable, easily recoverable from the reaction medium, durable, and highly efficient for industrial applications. Lots of catalytic systems have been reported for the conversion of CO2 to value-added products, but at present they are unsuitable due to the need for harsh reaction conditions, facing issues like high reaction pressures, difficulties in recoverability, high production costs, etc. In the present situation, COFs are outstanding materials for CO2 storage and conversion due to their exceptional structures and porosity.7 These materials can be utilized effectively for different types of CO2 fixation reactions.
Covalent organic frameworks are an excellent class of crystalline porous polymer with two-dimensional or three-dimensional arrangements in an ordered manner, and they are formed from repeating organic precursors. They have excellent surface areas, periodic frameworks, suitable densities, and tuneable functional groups.8 The extraordinary structural characteristics of COFs make them outstanding versatile materials for use in heterogeneous catalysis, gas adsorption and storage, sensors, and optoelectronics.9 In 2005, Omar M. Yaghi and Adiren P. Cote first reported a 2-D COF at the University of Michigan.10 They used phenyl diboronic acid and hexahydroxytriphenylene as building blocks for the synthesis of their COF. Their synthesized COF has excellent thermal stability up to 600 °C, a high surface area, and high porosity. The most interesting aspect of COFs is that their structures can be designed according to the application requirements, and they have tuneable porosity and surface areas, making them exceptional crystalline solids versus other available solid crystalline materials.11 Depending on the building blocks used, COFs can be classified mainly into three categories: triazine-supported, imine-supported, and boron-containing COFs.12 Covalent triazine frameworks are usually synthesized from repeating nitrile building blocks and these COFs have an inferior crystalline nature, although they have outstanding thermal stability and chemically inertness.13 Imine-based COFs can be synthesized in two ways: either via Schiff base formation between an aldehyde and a ketone14 or via condensation between an aldehyde and a hydrazide.15 These imine-supported COFs are highly stable in a variety of solvents and many metals can be grafted onto these COFs in chelate form because of their nitrogen-enriched structures.16
The adsorption of CO2 in a COF depends on some factors, like the building blocks or framework of the COF, the type of linkages between the repeating units, and the porosity type. It has been observed that COFs having triarylamine in their structures are excellent CO2 storage materials, and the adsorption efficiency increases with an increase in the triarylamine content. Donglin Jiang et al. recently reported four imine-linked COFs, TFPB–TAPB-COF, TFPA–TAPB-COF, BTMA–TAPA-COF, and TFPA–TAPA-COF, with similar hexagonal structures and almost the same porosity.17 They used tris(4-aminophenyl)amine and tris(4-formylphenyl)amine as triarylamine blocks and 1,3,5-tris(4-formylphenyl)benzene, 1,3,5-tris(4-aminophenyl)benzene, and 4,4′,4′′-boranetriyltris(2,3,5,6-tetramethylbenzaldehyde) as non-triarylamine building blocks. The CO2 uptake capacities of the four types of COF are presented in Table 1. It was observed that TFPB–TAPB-COF acts as a very poor material for CO2 adsorption due to the lack of triarylamine units. TFPA–TAPB-COF contains three triarylamine units in its framework, and it showed a 2.75-fold increase at 298 K and a 3-fold increase at 273 K in terms of the CO2 adsorption capacity compared with TFPB–TAPB-COF, which does not have any triarylamine units. BTMA–TAPA-COF also contains the same number of triarylamine units (three), but due to the presence of boron in its framework it showed better CO2 adsorption capacity. The maximum CO2 adsorption capacity was shown by TFPA–TAPA-COF, which contains six triarylamine units in its framework, and it shows an almost 4.34-fold increase at 298 K and a 5.25-fold increase at 273 K in terms of CO2 adsorption capabilities compared with TFPB–TAPB-COF, which does not contain any triarylamine units. Therefore, increasing the number of triarylamine units increases the CO2 adsorption capabilities, and this type of COF can be used as an efficient catalyst for CO2 fixation reactions.
| COF | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) | CO2 uptake capacity (wt%) |
|---|---|---|---|---|
| TFPB–TAPB-COF | 410 | 0.19 | 1.91 | 1.2 at 298 K and 2.0 at 273 K |
| TFPA–TAPB-COF | 540 | 0.25 | 1.72 | 3.3 at 298 K and 6.1 at 273 K |
| BTMA–TAPA-COF | 630 | 0.32 | 1.59 | 4.5 at 298 K and 8.4 at 273 K |
| TFPA–TAPA-COF | 660 | 0.32 | 1.59 | 5.2 at 298 and 10.5 at 273 K |
Another important fact about COFs is that the presence of positively charged pores can effectively enhance the CO2 intake capacity. Ionic-liquid-immobilized [Et4NBr]50%-Py-COF can take up 164.6 mg g−1 CO2 at 0 °C under atmospheric pressure.18 Other functional groups like azo19 and carboxylic acid20 groups can also improve the CO2 intake capacity.
Through this review article, we try to present recent advances relating to different COF materials for CO2 fixation reactions producing cyclic carbonates, carbamates, oxazolidinones, N-formylated products, etc. We have tried to detail the structural features and some important characterization experiment results that will definitely help provide a better understanding of COF materials and the CO2 fixation reactions that are performed by the reported COFs.
2. Different COF-catalyzed CO2 fixation reactions
2.1 N-Formylation of aromatic amines
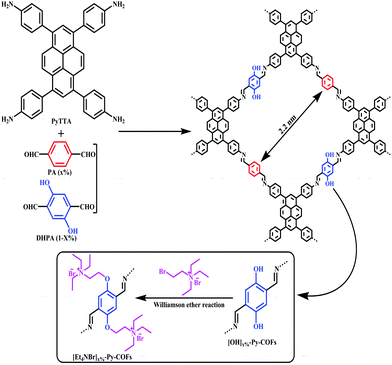
In 2016, Yanan Gao et al. presented the immobilization of the ionic liquid [Et4NBr]+Br− inside the framework of a COF. After synthesizing an ionic-liquid COF named [Et4NBr]50%-Py-COF, it was employed in the transformation of carbon dioxide into valuable formamides under mild reaction conditions.18 Different molar ratios of 2,5-dihydroxyterephthalaldehyde and 1,4-phthalaldehyde were used along with 1,3,6,8-tetraphenyl-1,8-dihydro-pyrene to synthesize COFs, referred to as [HO]X%-Py-COFs, and after immobilizing the ionic liquid [Et4NBr]+Br−, they acquired [Et4NBr]X%-Py-COFs via a Williamson ether reaction (Scheme 1).18 The authors found the PXRD patterns of [HO]0%-Py-COF, [HO]100%-Py-COF, and all other COFs with different ‘X’ values to be same, with peaks at 3.72, 5.23, 4.74, 8.67, 11.29, 15.17, and 23.69 degrees due to the (110), (020), (220), (030), (040), (060), and (001) planes, respectively (Fig. 2). They followed several protocols to confirm the formation of [Et4NBr]50%-Py-COFs, studying the ion density. The bromide content was determined with the help of ion chromatography (4.6% and 8.9% for X = 25 and 50, respectively). The research group showed the porous nature of the COFs based on N2 adsorption isotherms at 77 K. They observed that [HO]X%-Py-COFs showed type-IV isotherms, which are characteristic of mesoscopic materials. The N2-adsorption study showed that the surface coverage, pore volume, and pore size became smaller when the ionic liquid was introduced in the case of [HO]X%-Py-COFs.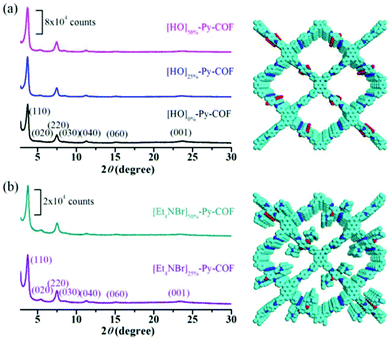 | ||
| Fig. 2 Powder X-ray diffraction analysis of (a) [HO]X%-Py-COFs (where X is 0, 25, and 50) and (b) [Et4NBr]X%-Py-COFs (where X is 25 and 50). Top views of [HO]50%-Py-COF and [Et4NBr]X%-Py-COF (X = 50) are given in the right-side panels. Reproduced with permission from ref. 18; copyright: 2016, Chemical Communications. | ||
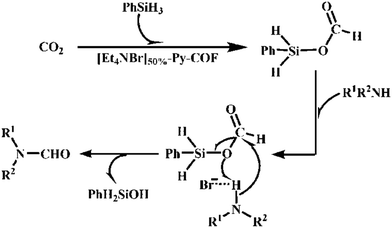
The Y. Gao group performed N-formylation reactions involving various amines efficiently using [Et4NBr]X%-Py-COFs as immiscible catalysts. Primarily, they executed the N-formylation of N-methylaniline under 1 bar CO2 pressure and at 30 °C taking DMF as the solvent and phenyl silane as a source of hydrogen (Scheme 2). The Gao research group acquired N-formylated N-methylaniline as the only product with an excellent yield of 94%. They also applied [HO]50%-Py-COF as the catalyst and observed a poor catalytic outcome, gaining a yield of only 32%. This research group studied different kinds of N-methylaniline substrates with electron-withdrawing and donating substituents, and they also responded in a similar way in the N-formylation reaction. A plausible reaction mechanism involving phenyl silane was also demonstrated (Scheme 3).
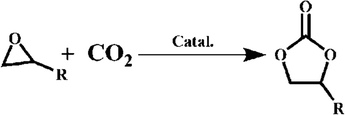
2.2 Cycloaddition reactions between CO2 and substituted epoxides
Recently, Ramanathan Vaidhyanathan and his team developed a resorcinol-phenylenediamine-based COF loaded with Ag nanoparticles, which was designated as IISERP-COF15 and applied to the chemical fixation of CO2.21 They prepared IISERP-COF15 via treating triformyl-resorcinol and phenylenediamine in a dioxane and mesitylene mixture at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) under solvothermal conditions, and then the silver nanoparticles were encapsulated into the COF (Fig. 3).
1 (v/v) under solvothermal conditions, and then the silver nanoparticles were encapsulated into the COF (Fig. 3).
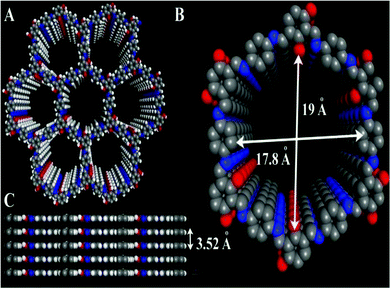 | ||
| Fig. 3 The 3D structure of Ag@COF. Reproduced with permission from ref. 21; copyright: 2019, Chemistry An Asian Journal. | ||
The research group carried out numerous characterization studies to establish the structure of the COF. Via PXRD analysis they identified that the COF has a crystalline structure, having a uniform pore size of 14 Å. The PXRD peaks of the Ag-nanoparticle-loaded COF appeared at 2θ values of 38.1°, 44.1°, 64.1°, and 77.1° for the (111), (200), (220), and (311) planes, respectively, due to the Ag nanoparticles, and it also showed the characteristic low-angle peaks of the COF. In addition, thermogravimetric analysis showed that the COF is stable up to 300 °C and that the COF can retain its crystalline structure up to 250 °C. Through a N2 adsorption–desorption study the research group determined that IISERP-COF-15 has significantly high BET and Langmuir surface areas of 1230 and 1540 m2 g−1, respectively. They observed that the volume of the COF pores is 0.6332 cm3 g−1 and their size is 12 Å with the help of non-local density functional theory (NLDFT) (Fig. 4).
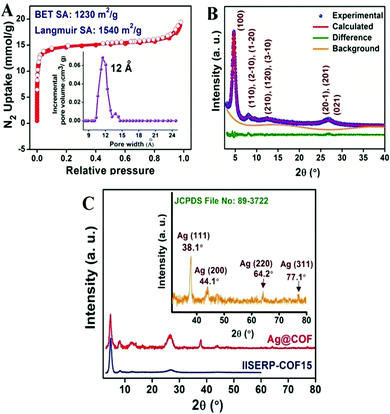 | ||
| Fig. 4 (A) The N2 adsorption isotherm of IISERP-COF-15. (B) The PXRD pattern of the COF. (C) The PXRD pattern of Ag@COF. Reproduced with permission from ref. 21; copyright: 2019, Chemistry An Asian Journal. | ||
Dr Vaidhyanathan and his research team treated 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and propargyl alcohol under a CO2 atmosphere using Ag@COF as the catalyst and DMF as the solvent for 4 h and obtained a cyclic carbonate yield of 90% and a TON greater than 150 (Scheme 4). The research group claimed that the microporous structure and high surface area of Ag@COF are highly effective for the conversion of CO2 into cyclic carbonates, and the COF can be reused up to five times with only a minute change in its catalytic activity.
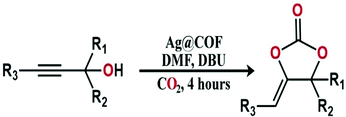 | ||
| Scheme 4 A synthetic diagram of the formation of cyclic carbonates. Reproduced with permission from ref. 21; copyright: 2019, Chemistry An Asian Journal. | ||
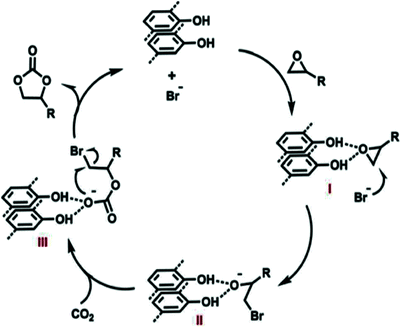
Miao Du et al. and his team designed a metal-free bi-functional COF catalyst for coupling CO2 with epoxides to form cyclic carbonates under gentle reaction conditions and they designated the catalyst as TpPa-Py-Br−-COF (Scheme 5). This unique bi-functional COF has both active nucleophilic sites and Brønsted acid catalytic sites.22 The research group synthesized TpPa-Py COF via treating 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde, 2,5-diaminopyridine, 1,4-dioxane, mesitylene, and aqueous acetic acid (6 M) in a sealed Pyrex pressure tube at 120 °C for three days. After that, they treated COF-TpPa-Py with a solution of saturated Br–CH2COOH under a N2 atmosphere for one day at 70 °C and finally obtained TpPa-Py-Br−-COF. The Br− content in TpPa-Py-Br−-COF is 1.25 wt%, with 0.149 mmol g−1 total acid sites.
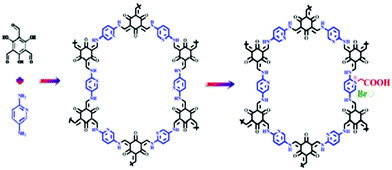 | ||
| Scheme 5 The synthesis of TpPa-Py-Br−-COF. Reproduced with permission from ref. 22; copyright: 2020, ChemCatChem. | ||
The research group confirmed the successful formation of TpPa-Py-Br−-COF via solid-state 13C-NMR (Fig. 5), FT-IR and PXRD (Fig. 6), and XPS analysis. The FT-IR spectrum confirms the successful formation of TpPa-Py-Br−-COF via the binding of BrCH2COOH to TpPa-Py-COF and it also confirms the presence of carboxylic groups in the COF structure. The PXRD patterns of TpPa-Py-COF and TpPa-Py-Br−-COF do not show much difference, revealing that there are no ordered long-range structures.
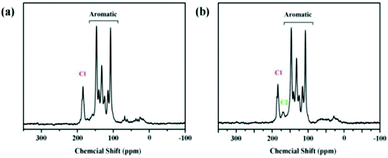 | ||
| Fig. 5 (a) The solid-state 13C-NMR spectrum of TpPa-Py-COF. (b) The solid-state 13C-NMR spectrum of TpPa-Py-Br−-COF. Reproduced with permission from ref. 22; copyright: 2020, ChemCatChem. | ||
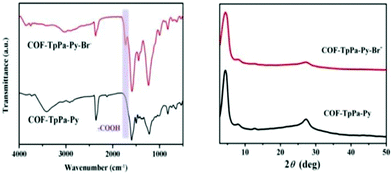 | ||
| Fig. 6 FT-IR and PXRD patterns of TpPa-Py-Br−-COF. Reproduced with permission from ref. 22; copyright: 2020, ChemCatChem. | ||
N2 absorption–desorption studies confirm the permanent porous nature of TpPa-Py-COF and TpPa-Py-Br−-COF (Fig. 7). The total calculated BET surface areas of TpPa-Py-COF and TpPa-Py-Br−-COF are found to be 514 m2 g−1 and 113 m2 g−1, respectively. The calculated P/P0 values range from 0.054099 to 0.295057 for TpPa-Py-COF, while for TpPa-Py-Br−-COF these values lie between 0.084401 and 0.294829. From thermogravimetric analysis, it was established that TpPa-Py-COF and TpPa-Py-Br−-COF are stable up to 450 °C.
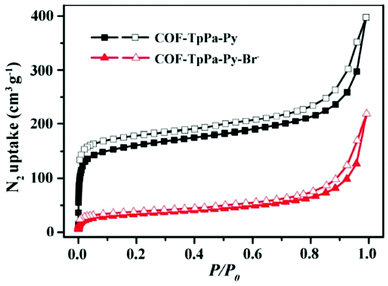 | ||
| Fig. 7 The N2 adsorption isotherm of TpPa-Py-Br−-COF. Reproduced with permission from ref. 22; copyright: 2020, ChemCatChem. | ||
Miao Du et al. and his research group used different substrates for CO2 cycloaddition reactions (Scheme 6). They treated epichlorohydrin under a CO2 atmosphere at 100 °C using TpPa-Py-Br−-COF as a catalyst and achieved a 99% cyclic carbonate yield. Their experimental observations showed that the yield was reduced to 1% from 99% upon lowering the temperature to 40 °C from 100 °C. They determined that the cyclic carbonate yield decreased from 99% to less than 1% via the non-catalytic path. The group also showed that this heterogeneous porous bi-functional COF can be reused five times without any significant reduction in catalytic activity. Therefore, all these experimental observations confirm that COF-TpPa-Py-Br− is an excellent catalyst for CO2 cycloaddition reactions involving epoxides.
 | ||
| Scheme 6 The synthesis scheme for cycloaddition involving CO2. Reproduced with permission from ref. 22; copyright: 2020, ChemCatChem. | ||
Another interesting material was reported by H. Wang et al. in the form of a metallo-porphin-based two-dimensional COF and this was applied to the preparation of cyclic carbonates via CO2 fixation reactions.23 The covalent organic framework they synthesized was denoted as COF-366-Zn, and this was used for the formation of 4-ethyl-[1,3]dioxolan-2-one, having activity of ∼99.2% under gentle reaction conditions. In this context, they showed that embedding Zn and Co metal into 2-dimensional COFs, i.e., COF-366 and COF-SQ, resulted in marvelous catalytic activity for the transformation of CO2 to cyclic carbonates. They processed COF-366 via applying a condensation method involving tetra-(amidogenphenyl)-porphine and 1,4-benzenedicarboxaldehyde. For the synthesis of COF-SQ, a squaric acid analog was used instead of BDA. COF-366 was combined with zinc acetate dihydrate and cobalt acetate dihydrate to prepare COF-366-Zn and COF-366-Co(1), respectively. The research group fabricated COF-366-Co(2) via linking BDA with Co(OAc)2·2H2O. Based on Fourier-transform infrared spectroscopy (Fig. 8), the structure of the COF was confirmed. They gathered information about the crystallinity of the COF-366-Co(1) and COF-366-M species, as shown in Fig. 8. N2 adsorption–desorption analysis of COF-366-Zn at 77 K reveals that zinc metal was incorporated into COF-366 with a 2 nm pore size.
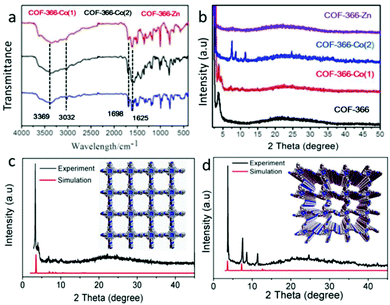 | ||
| Fig. 8 (a) FTIR and (b) PXRD analysis of COF-366-M catalysts, and PXRD patterns of (c) COF-366-Co(2) and (d) COF-366-Co(1). Reproduced with permission from ref. 23; copyright: 2017, Dalton Transactions. | ||
H. Wang and co-workers used COF-366-Zn, COF-366-Co(1), COF-366-Co(2), COF-SQ-Zn, and COF-SQ-Co catalysts to synthesize cyclic carbonates via CO2 fixation (Scheme 7) and found that the COF-366-Zn catalyst is more proficient (98.9% yield) than the others. They also observed that there was no effective impact when they used COF-366-Zn and phenyltrimethylammonium (PTAT) individually for cyclo-addition reactions involving CO2. However, if COF-366-Zn is used with phenyltrimethylammonium, TPPB, tetrabutylammonium bromide, or dimethylaminopyridine as an auxiliary catalyst in methylcyanide solvent, the product yield becomes 98.9%, 99.2%, 71.2%, or 19.6%, respectively; the acceleration in the reaction rate could be attributed to the strong nucleophile Br−, which works as an auxiliary metallic site during CO2 cycloaddition reactions.24
 | ||
| Scheme 7 The synthesis of cyclic carbonates via CO2 incorporation using COF-366-Zn. Reproduced with permission from ref. 23; copyright: 2017, Dalton Transactions. | ||
Xiaoming Liu et al., in 2018, studied metal-free COFs, namely COF-JLU6 and COF-JLU7, for their application in cycloaddition reactions of carbon dioxide with epoxides for the synthesis of cyclic carbonates.25 1,3,5-Tris-(4-aminophenyl)triazine (0.08 mmol) and 2,5-dihydroxy-benzene-1,4-dicarbaldehyde (0.12 mmol) were used as starting materials for the formation of COF-JLU6 under solvothermal conditions, and mesitylene/1,4-dioxane was used as the solvent. In a very similar way, COF-JLU7 was synthesized, but the solvent was different in this case: o-dichlorobenzene/n-butanol. One-dimensional oriented porous channels are present in both COF-JLU6 and COF-JLU7. The two synthesized COFs contain some heteroatoms and hydroxyl groups on their walls, which help to enrich the COFs with CO2 and facilitate CO2 fixation with epoxides (Fig. 9). The COFs possess a large BET surface area, approximately 1390 m2 g−1, with a suitable pore volume of 1.78 cm3 g−1. The carbon dioxide uptake capacity of COF-JLU7 is 151 g mg−1 at 273 K and 1 bar pressure. The characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency of the monomer at 1683 cm−1 also disappeared for both COFs. A strong stretching vibration was observed at 1584 cm−1 for COF-JLU6 and at 1582 cm−1 for COF-JLU7. They found a powder X-ray diffraction peak at a 2θ value of 2.82° for COF-JLU6 and some weak peaks at 2θ values of 4.82, 5.62, and 7.49 degrees, which correspond to the (100), (200), (210), and (220) reflection planes, respectively. Likewise, PXRD analysis of COF-JLU7 showed peaks at 2θ values of 2.67, 4.72, and 9.50 degrees.
O stretching frequency of the monomer at 1683 cm−1 also disappeared for both COFs. A strong stretching vibration was observed at 1584 cm−1 for COF-JLU6 and at 1582 cm−1 for COF-JLU7. They found a powder X-ray diffraction peak at a 2θ value of 2.82° for COF-JLU6 and some weak peaks at 2θ values of 4.82, 5.62, and 7.49 degrees, which correspond to the (100), (200), (210), and (220) reflection planes, respectively. Likewise, PXRD analysis of COF-JLU7 showed peaks at 2θ values of 2.67, 4.72, and 9.50 degrees.
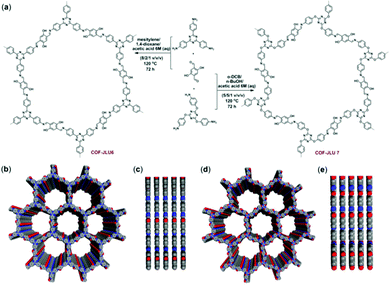 | ||
| Fig. 9 (a) A diagram of the synthesis of COF-JLU6 and COF-JLU7 via condensation. (b) Top and (c) side views of [COF-JLU6]. (d) Top and (e) side views of [COF-JLU7]. Reproduced with permission from ref. 25; copyright: 2018, Journal of Materials Chemistry A. | ||
This research group confidently used COF-JLU6 and COF-JLU7 as elegant catalytic systems for the cycloaddition of carbon dioxide and epoxides to make cyclic carbonates under ambient conditions, as represented in Scheme 8. Here, they used tetrabutylammonium bromide as an auxiliary catalyst for the cycloaddition reactions and observed enhanced rates and yields during the reactions (approx. 86% in the case of COF-JLU6 and 92% for COF-JLU7). They concluded that COF-JLU7 showed a more satisfactory catalytic outcome compared to COF-JLU6. The group demonstrated a suitable mechanistic path for the cycloaddition of CO2 and epoxides using a COF-JLU7 and TBAB pair (Scheme 9).
 | ||
| Scheme 8 COFs (JLU6 and JLU7) used in cycloaddition reactions. Reproduced with permission from ref. 25; copyright: 2018, Journal of Materials Chemistry A. | ||
 | ||
| Scheme 9 A self-explanatory mechanistic path supporting the cycloaddition of CO2 using COF-JLU7 and TBAB. Reproduced with permission from ref. 25; copyright: 2018, Journal of Materials Chemistry A. | ||
In 2019, our research group headed by Prof. S. M. Islam reported that CuxOy nanoparticles (Cu2O and Cu4O3) could be used as an effective catalyst in cycloaddition reactions involving CO2 when those nanoparticles were decorated on a covalent organic framework.26 We synthesized the COF via a slight modification to the literature report by R. Banerjee et al.27 To fabricate the COF, our group combined 2.5 mmol of para-toluenesulfonic acid with 0.45 mmol of 2-toluidine in a pestle and mortar and ground it for some time; then, 1,3,5-triformylphloroglucinol (0.3 mmol) was poured into the mixture. Lastly, the entire mixture was transferred into an autoclave and left at 100 °C overnight, and reddish-orange COF powder was obtained (Scheme 10). Later on, we added copper oxide (CuxOy) nanomaterial to the surface of the covalent organic framework. It was observed that the low-angle X-ray diffraction patterns of COF and CuxOy@COF show an acute peak at a 2θ value of 3.5° due to the (100) reflection plane, and it was observed that the COF remained unaltered even after decoration with copper oxide nanomaterial. The sharp peak at a 2θ value of ∼28.29° indicates the crystallinity of the substance, which remains unchanged even after CuxOy incorporation.28 The N2 adsorption–desorption isotherm revealed that the synthesized copper-oxide-loaded COF exhibited a type-IV isotherm, which indicates the mesoporous nature of the CuxOy@COF material. It was observed that the total calculated surface coverage of COF, pristine CuxOy@COF, and used CuxOy@COF were 460, 385, and 379 m2 g−1, respectively.
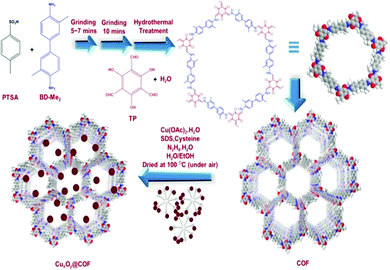 | ||
| Scheme 10 A schematic diagram of the synthesis of CuxOy@COF. Reproduced with permission from ref. 26; copyright: 2019, Journal of CO2 Utilization. | ||
The CuxOy@COF material has been successfully applied in the cycloaddition of CO2 to styrene oxide in the presence of the co-catalyst tetrabutylammonium bromide (5 mol%). A schematic representation of this reaction is given in Scheme 11. The research group carried out a time-on-stream (TOS) study, which revealed a gradual increase in the conversion of styrene oxide; after 12 h, the reaction yield became constant (approx. 92%). It was found that in the absence of CuxOy@COF and TBAB, the reaction did not proceed at all. A plausible reaction mechanism for the cycloaddition reaction was also demonstrated, as presented in Scheme 12.
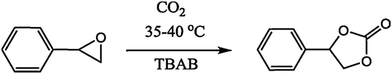 | ||
| Scheme 11 The cycloaddition of CO2 with styrene oxide using CuXOY@COF. Reproduced with permission from ref. 26; copyright: 2019, Journal of CO2 Utilization. | ||
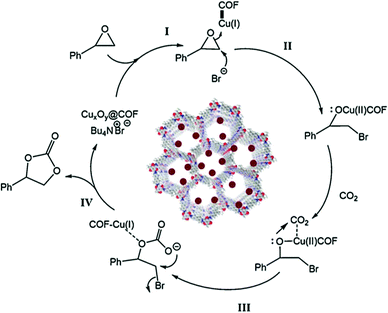 | ||
| Scheme 12 A probable mechanistic path for the synthesis of styrene carbonate via a cycloaddition reaction using the CuxOy@COF catalyst and TBAB as a co-catalyst. Reproduced with permission from ref. 26; copyright: 2019, Journal of CO2 Utilization. | ||
In 2019, Jianji Wang and his research team reported a newly fashioned azine-linked 3D-COF named 3D-HNU5 which was applied fruitfully as a catalyst to support the cycloaddition reaction of propargyl alcohol with carbon dioxide to make cyclic carbonates.29 Nowadays, 3D COFs receive huge attention due to their large surface areas and ample numbers of open sites.30 This research group synthesized an azine-linked 3D COF for the very first time. They synthesized the 3D-HNU5 COF via the condensation of tetrakis(4-formylphenyl)-methane in the ionic liquid [1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide], as represented in Scheme 13. X-ray diffraction and simulation studies revealed that 3D-HNU5 COF has an interpenetrating diamond-like topology, with excellent crystallinity and high thermal stability. An SEM study revealed that the COF has rod-shaped morphology with dimensions of 0.5–1 micrometer. They performed PXRD analysis, and peaks were obtained at 2θ values of 7.75, 9.67, 10.97, 13.29, 14.89, 17.42, and 19.16 degrees corresponding to the (101), (111), (102), (003), (202), (221), and (311) reflection planes, respectively. The type-I isotherm obtained via N2 adsorption–desorption studies at 77 K helped demonstrate the microporous properties of the COF. The N2-BET surface coverage was observed to be 864 m2 g−1 for 3D-HNU5-COF. The research team obtained an impressive pore volume for this COF of 0.89 cm3 g−1 at a P/P0 value of 0.99.
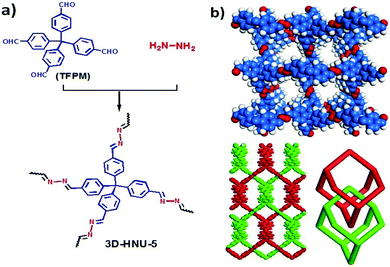 | ||
| Scheme 13 (a) The synthesis of 3D-HNU5 COF and (b) its 3D perforated structure and 2-fold interpenetrating diamond-like frame (blue: C, white: H, red: N). Reproduced with permission from ref. 29; copyright: 2019, Chemical Communications. | ||
Among different COFs, 3D COFs can be used for gas storage because of their great surface coverage, low densities, and many free sites.31 The same research group used the three-dimensional HNU5 COF as a support for Ag metal and used this silver-embedded COF for CO2 fixation using 2-methylbut-3-yn-2-ol under atmospheric pressure. It was found that the cycloaddition reaction did not work in the absence of Ag nanoparticles, but with Ag@3D-HNU5 COF, the yield was up to 99% with a TON of 990 (Fig. 10).
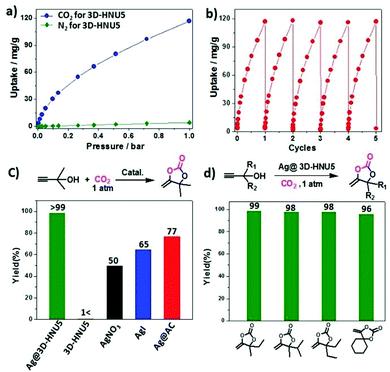 | ||
| Fig. 10 (a) CO2 and N2 isotherms for 3D-HNU5 COF at 273 K. (b) CO2 adsorption by 3D-HNU5 over multiple cycles at 273 K. (c) A study of catalysts for the formation of cyclic carbonates. (d) The yield percentages of different cyclic carbonates when Ag@3D-HNU5 is used as the catalyst. Reproduced with permission from ref. 29; copyright: 2019, Chemical Communications. | ||
Yu-Bin Dong et al., in 2019, reported the synthesis of the ionic-liquid-immobilized COF presented in Scheme 14 and a newly fashioned COF-chitosan aerogel, and they studied the catalytic outcomes during the fixation of CO2 with styrene oxide to form cyclic carbonates.32 They prepared 1,3,5-triformylphloroglucinol according to a reported process.33 Thiol-group-containing chitosan was prepared according to a reported method.34 They also synthesized the three required precursors for IL-ADH according to previously reported processes with slight modifications.35 The research group synthesized COF-IL@chitosan via blending COF-IL (1.35 g) and HMPP (150 μL) with an aqueous solution of acidic chitosan, as represented in Scheme 15. The group carried out 13C-NMR CP-MAS studies to show the formation of COF-IL. The PXRD pattern of COF-IL@chitosan showed that its crystallinity and integrity were suitably maintained in the sponge-like aerogel.
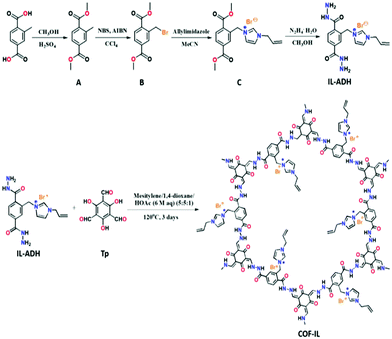 | ||
| Scheme 14 The synthesis of COF-IL and IL-ADH and their oriented structures. Reproduced with permission from ref. 32; copyright: 2019, Journal of Materials Chemistry A. | ||
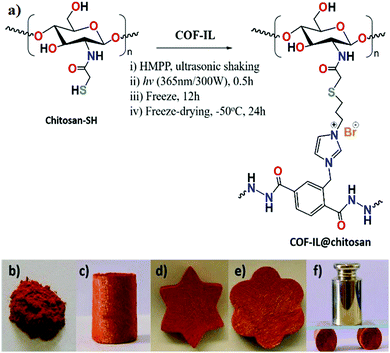 | ||
| Scheme 15 (a) The synthesis of COF-IL@chitosan. Photographs of (b) COF-IL (solid-crystals) and (c–e) COF-IL@chitosan (aerogel) with different shapes. (f) The pressure testing of an aerogel column done with a weight of 250 g. Reproduced with permission from ref. 32; copyright: 2019, Journal of Materials Chemistry A. | ||
They found COF-IL to be very efficient for use in CO2 cycloaddition reactions (Fig. 11). COF-IL could be applied to many types of epoxides, except for long hexadecyl-connected epoxides. The researchers observed that under the optimized conditions, epichlorohydrin and epibromohydrin provided high yields of 98% and 100%, respectively, within 48 h. For actual applications, they prepared a cuplike reactor in situ and used the advantages of the COF@chitosan aerogel.
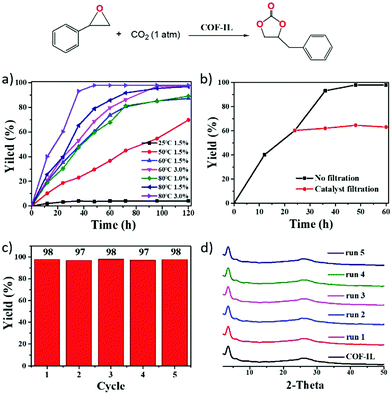 | ||
| Fig. 11 (a) The effects of temperature, reaction time, and catalyst amount on COF-IL-catalysed CO2 cycloaddition to styrene oxide. (b) Reaction time analysis (black line) and leaching testing (red line) during CO2 cycloaddition to styrene oxide catalyzed by COF-IL. (c) Recycling of the catalyst. (d) PXRD patterns from every catalytic cycle. Reproduced with permission from ref. 32; copyright: 2019, Journal of Materials Chemistry A. | ||
Another report was provided by Jianji Wang et al. in 2020. A standard acid–base reaction method was used to combine an ionic liquid (imidazolium-salt-based) and covalent organic framework, and this material was applied as a catalyst to promote the formation of cyclic carbonates from carbon dioxide.36 Firstly, they prepared an SO3H-activated COF named TpPa–SO3H, which has numerous Brønsted acid sites, and these sites could be utilized to immobilize the imidazolium salt, and eventually the COF was termed COF-HNU14 (Scheme 16). This COF-material showed very high crystallinity during nanoscale measurements of the pores. COF-HNU14 performed excellently in CO2 cycloaddition with epoxides. Condensation between 1,3,5-triformylphloroglucinol and 2,5-diaminobenzenesulphonic acid resulted in the formation of the TpPa–SO3H material. The group prepared red crystals with an isolated TpPa–SO3H yield of ∼82% via mixing Tp (0.5 mmol) and Pa–SO3H (183 mg, 0.75 mmol) under the catalytic acceleration of acetic acid at 120 °C for three days. They studied the PXRD patterns to clarify the structure of the TpPa–SO3H material. TpPa–SO3H showed peaks at 2θ values of 4.4, 8.1, and 25 degrees corresponding to the (100), (110), and (001) planes, respectively. The (001) reflection confirms the π–π stacking of the planes and the pattern resembled an AA stacking model.37 N2 adsorption–desorption isotherm studies revealed the permanent porous nature of TpPa–SO3H at 77 K. The research group calculated the surface area with the help of BET analysis and found that the coverage is 294 m2 g−1, and the pore volume of TpPa–SO3H is 0.41 cm3 g−1. They fabricated COF-HNU14 via mixing TpPa–SO3H with an aqueous solution of 1-aminopropyl-3-methylimidazolium bromide at room temperature. BET analysis revealed that COF-HNU14 has a lower surface area of 196 m2 g−1 compared to TpPa–SO3H.
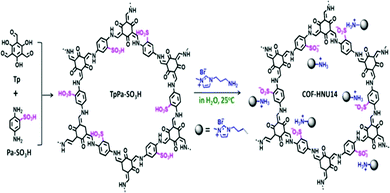 | ||
| Scheme 16 The synthesis of TpPa–SO3H and COF-HNU14. Reproduced with permission from ref. 36; copyright: 2020, ACS Sustainable Chemistry & Engineering. | ||
Wang et al. found that cycloaddition reactions between carbon dioxide and epoxides were highly atom-economical when COF-HNU14 was used as the catalyst material, as shown in Scheme 17. It was observed that the cyclic carbonate yield was up to 99% when COF-HNU14 was used as the catalyst in the absence of any co-catalyst. This enhanced catalytic performance of the COF is ascribed to the nano-range order of the catalyst.38 Cycloaddition between CO2 and various epoxide substrates was performed, and it was observed that in the case of 2-chloromethyl oxirane and 2-bromomethyl oxirane substrates, the yields were maximal (∼99%).
This research group proposed a three-step mechanistic route, based on the Gibbs free energy (Fig. 12), for cyclic carbonate synthesis, collecting information from some previous reports. They also proposed optimized geometries for the transition states TS1, TS2, and TS3 (Fig. 13).
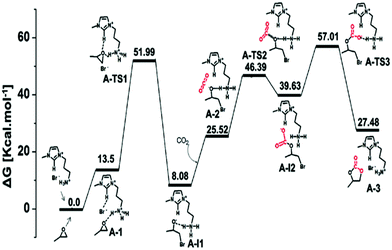 | ||
| Fig. 12 COF-HNU14-catalyzed free-energy profiles for cyclic carbonate synthesis. Reproduced with permission from ref. 36; copyright: 2020, ACS Sustainable Chemistry & Engineering. | ||
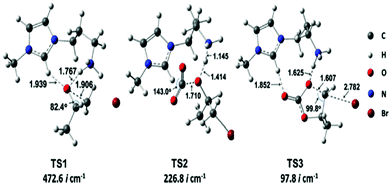 | ||
| Fig. 13 Optimized geometries for the transition states highlighted in Fig. 12. Reproduced with permission from ref. 36; copyright: 2020, ACS Sustainable Chemistry & Engineering. | ||
Long Chen et al., in 2020, reported different covalent organic frameworks with structural similarities that could be used as metal-free immiscible catalysts for CO2 cycloaddition.39 Four different types of blocks were used for the TPBP ring-core, including 2,2′-dihydroxyl-3,3′,5,5′-tetrakis(4-formylphenyl)biphenyl (OH-TFBP), OMe-TFBP, 2,2′,-dimethoxy-3,3′,5,5′-tetrakis(4-aminophenyl)biphenyl (OMe-TABP), and OH-TABP, and they were used as frames to build three separate COFs having different docking regions in a solvothermal environment and via a Schiff base poly-condensation method (Scheme 18). Solid-state 13C-NMR spectra were obtained to establish the formation of the imine linkages. PXRD measurements gave diffraction peaks at 2θ values of 5.52, 8.05, and 11.05 degrees which were indexed to the (110), (200), and (220) planes, respectively, for the OMe-OH-TPBP material (Fig. 14).
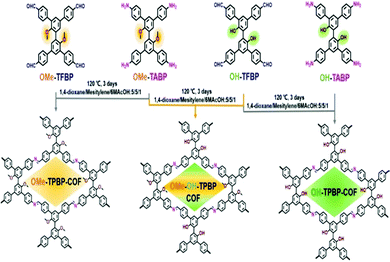 | ||
| Scheme 18 The synthesis of OMe-TPBP, OMe-OH-TPBP, and OH-TPBP COF. Reproduced with permission from ref. 39; copyright: 2020, Chemistry A European Journal. | ||
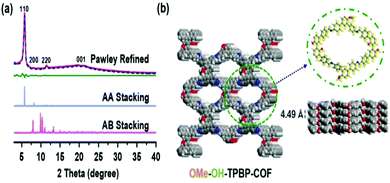 | ||
| Fig. 14 (a) The powder XRD patterns of OMe-OH-TPBP-COF: experimentally observed (red), Pawley refinement (blue), and the difference (green), and simulations of AA-stacking (light blue) and AB-stacking (pink), (b) top and side views of OMe-OH-TPBP-COF. Reproduced with permission from ref. 39; copyright: 2020, Chemistry A European Journal. | ||
This research group studied the cycloaddition reactions between CO2 and various ethylene oxides. The methoxy COF analogue (OMe-OH-TPBP) showed the best catalytic performance during CO2 fixation reactions to produce cyclic carbonates. Under the optimized conditions, OMe-OH-TPBP (COF) showed a 96% yield for CO2 cycloaddition with epibromohydrin. The reaction yield was about 90% for the commercially vital epoxypropane, and the yield was slightly lowered in the case of epoxides with long alkyl-chain lengths. They also studied a possible reaction mechanism for CO2 fixation with epoxides where the catalyst OMe-OH-TPBP-COF and tetrabutylammonium bromide were used, as presented in Scheme 19.
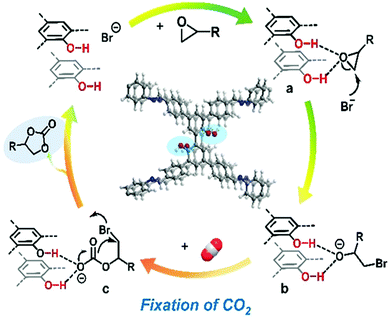 | ||
| Scheme 19 A probable mechanism for CO2 cycloaddition with ethylene oxides using methoxy-hydroxyl-TPBP-COF and tetrabutylammonium bromide. Reproduced with permission from ref. 39; copyright: 2020, Chemistry A European Journal. | ||
After that, in 2020, Ruihu Wang et al. reported an acceptor–receptor catalytic analog via combining an imidazolium-based ionic polymer and a nitrogen-filled covalent organic framework (TT-COF), and this supported CO2 cycloaddition.40 Among the available catalytic methodologies for CO2 cycloaddition with epoxides, this is an atom-economical and environmentally benign method.41 They showed that immobilizing the ionic liquid (imidazolium analog) on the wall of the COF improves the catalytic abilities in the absence of co-catalysts and other materials.42 The group prepared TT-COF via mixing 1H-[1,2,4]triazole-3,5-diamine and para-toluenesulfonic acid in a dichloromethane solution of 2,4,6-triformylphloroglucinol (TP). Then, they performed an ultrasonic reaction of TT-COF with 3-ethyl-1-vinyl-imidazolium salt dissolved in N,N-dimethylformamide, followed by polymerization in azobisisobutyronitrile to generate ImIP@TT-COF (Scheme 20). They ascertained the structure and composition via performing FT-IR spectroscopy, solid-state 13C-NMR, and powder X-ray diffraction studies. They observed the disappearance of the carbonyl peak of triformylphloroglucinol at 1647 cm−1 in the FT-IR spectrum of TT-COF, while C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–N stretching signals were observed at 1593 and 1261 cm−1, respectively. From 13C-NMR analysis, they observed peaks at 136, 122, 45, 37, 31, and 14 ppm in the ImIP@TT-COF spectrum, illustrating the unification of ImIP (ionic polymer) with TT-COF. The powder XRD pattern of TT-COF revealed a pattern similar to previously reported results.43
C and C–N stretching signals were observed at 1593 and 1261 cm−1, respectively. From 13C-NMR analysis, they observed peaks at 136, 122, 45, 37, 31, and 14 ppm in the ImIP@TT-COF spectrum, illustrating the unification of ImIP (ionic polymer) with TT-COF. The powder XRD pattern of TT-COF revealed a pattern similar to previously reported results.43
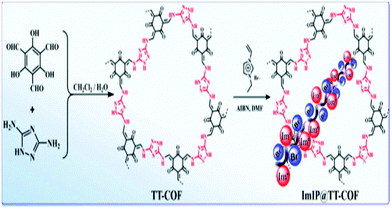 | ||
| Scheme 20 The synthesis of ImIP@TT-COF. Reproduced with permission from ref. 40; copyright: 2020, ChemSusChem. | ||
The kinetic curves obtained for ImIP@TT-COF during the catalytic reaction are demonstrated in Fig. 15. The authors observed simultaneous increases in the formation of 4-chloromethyl-[1,3]dioxolan-2-one and the consumption of 2-chloromethyloxirane, with completion within 8 h. They studied the temperature-dependency under atmospheric CO2 over a temperature range of 25 to 140 °C. It was observed that comparatively higher temperatures have a good effect on CO2 fixation. At 120 °C, conversion was the most effective. The impact of 3-alkyl-1-vinyl-imidazolium attachment to TT-COF on catalytic conversion was also studied. The percentage yield of 4-chloromethyl-1,3-dioxolan-2-one was increased when the ImIP content reached 7.2 wt%, and the approximately linear relationship between catalytic activity and electron-rich Br− species content was revealed.
 | ||
| Fig. 15 (a) The catalytic activities of ImIP@TT-COF, ImIP, TT-COF, and a blend of imidazolium salt and TT-COF. (b) Kinetic curves for the CO2 cycloaddition reaction. (c) The impact of ImIP content. (d) The recyclability of the COF. Reproduced with permission from ref. 40; copyright: 2020, ChemSusChem. | ||
Wei-Qiao Deng et al., in 2020, reported a COF named Zn-salen-COF-SDU113 which was developed through a ‘pore enrichment’ technique and applied to CO2 cycloaddition to epoxides.44 They previously showed that a salen-conjugated perforated polymer can accelerate CO2 fixation and can convert CO2 to value-added chemicals.45 In this article, they used a large-scale computational screening technique to determine the best candidate from a COF library and present the suitably predicted COF. Firstly, the authors built a virtual library of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994 COFs utilizing salen-metal as the active center. Then, they synthesized the best COF, named Zn-salen-COF-SDU113 (COF), and studied its activity as a catalyst for CO2 fixation. CO2 cycloaddition can also be done with terminal epoxides and internal epoxides (e.g., 2,3-epoxybutane) using Zn-salen-COF-SDU113 (COF) as the catalyst. This research group constructed a library of salen-COFs via choosing three kinds of linker groups to vary the structure of the COF. Thus, 27 metal elements were used as catalytic centers and different alkane substituents present in the salen molecule were taken into consideration during the formation of the salen-COFs (Fig. 16). The structures that were constructed helped with CO2 enrichment. For Zn-salen-COF-SDU113, the amount of CO2 adsorption is 3.99 mol g−1 (maximum value), and the second highest value of 3.94 mol g−1 is obtained with the Fe-salen compound.
994 COFs utilizing salen-metal as the active center. Then, they synthesized the best COF, named Zn-salen-COF-SDU113 (COF), and studied its activity as a catalyst for CO2 fixation. CO2 cycloaddition can also be done with terminal epoxides and internal epoxides (e.g., 2,3-epoxybutane) using Zn-salen-COF-SDU113 (COF) as the catalyst. This research group constructed a library of salen-COFs via choosing three kinds of linker groups to vary the structure of the COF. Thus, 27 metal elements were used as catalytic centers and different alkane substituents present in the salen molecule were taken into consideration during the formation of the salen-COFs (Fig. 16). The structures that were constructed helped with CO2 enrichment. For Zn-salen-COF-SDU113, the amount of CO2 adsorption is 3.99 mol g−1 (maximum value), and the second highest value of 3.94 mol g−1 is obtained with the Fe-salen compound.
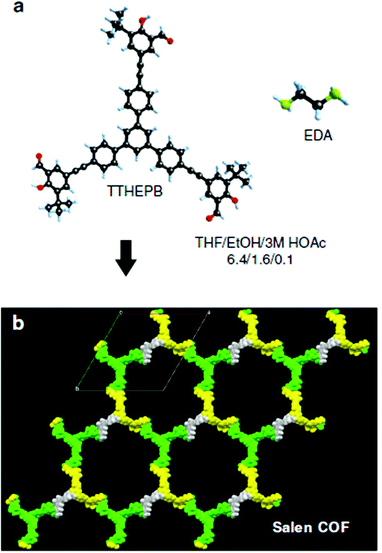 | ||
| Fig. 16 The synthesis of salen-COF. Reproduced with permission from ref. 44; copyright: 2020, Nature Communications. | ||
In the case of CO2 cycloaddition reactions with a terminal epoxide, Zn-salen-COF-SDU11 afforded a yield of 84.2% and a TON of 1142.7, while Zn-salen-COF-SDU113 had a yield of 98.2% and a TON of 3068.9 (Fig. 17).
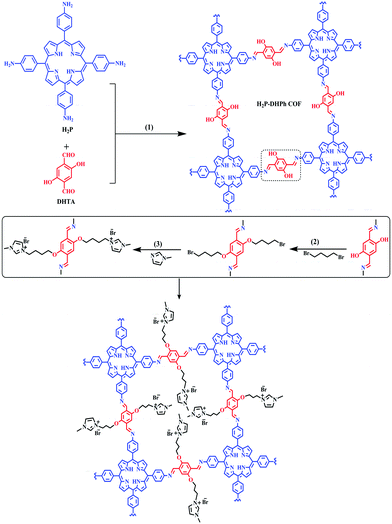
In 2020, Yanan Gao et al. reported a two-dimensional COF attached to a 3-methyl-1-alkyl-3H-imidazol-1-ium salt, which was named AMIMBr@H2P-DHPh-COF and used for cycloaddition reactions between epoxides and CO2.46 Among different available catalysts, various ionic liquids (ILs), e.g., those based on ammonium salts,47 phosphonium,48 imidazolium,49 and pyridinium cations,50 have already shown extraordinary catalytic performance during carbon dioxide fixation reactions. The authors performed the immobilization of the ionic liquid on the channel barriers of a H2P-DHPh COF via a specific method (Scheme 21).
The PXRD analysis and simulations illustrated the crystalline nature of the COF. X-ray diffraction peaks for H2P-DHPh COF were observed at 2θ values of 3.5, 7.1, and 22.9 degrees that were attributed to the (100), (200), and (001) reflection planes. The authors also investigated a similar COF named BuBr@H2P-DHPh COF.
This research group showed that the AMIMBr@H2P-DHPh COF is very efficient during the cycloaddition of CO2 and epoxides, and the yield is 91% for the substrate epichlorohydrin. However, using the catalyst H2P-DHPh COF, the yield with the same substrate (epichlorohydrin) is only 40%, while without any additives the cycloaddition yield between epichlorohydrin and CO2 is 38%. The authors have also studied different substrate variations (epoxide derivatives) and the catalytic performance of the AMIMBr@H2P-DHPh COF during these reactions (Scheme 22).
 | ||
| Scheme 22 Cycloaddition reactions using AMIMBr@H2P-DHPh COF. Reproduced with permission from ref. 46; copyright: 2020, Chinese Journal of Catalysis. | ||
In 2019, our research group synthesized a porous TFPG-DAAQ COF photocatalyst (Scheme 23) for the synthesis of formic acid from atmospheric CO2.51 Our group developed the COF via heating 1,3,5-triformylphloroglucinol and 2,6-diaminoanthraquinone (DAAQ) with p-toluenesulfonic acid (PTSA) in a steel autoclave with an inner Teflon lining at 60 °C for 6 h and for another 10 h at 90 °C under nondynamic conditions.
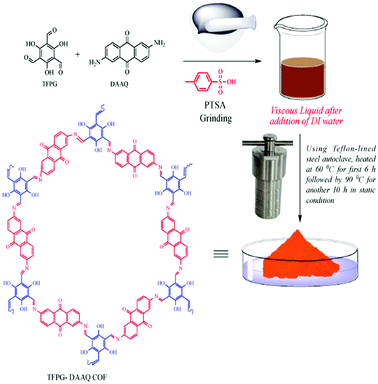 | ||
| Scheme 23 The synthesis of TFPG-DAAQ COF. Reproduced with permission from ref. 51; copyright: 2020, Molecular Catalysis. | ||
Our team has performed numerous characterization studies to ascertain the structure of TFPG-DAAQ COF (Fig. 18). There are two characteristic peaks present in the PXRD pattern of TFPG-DAAQ COF at 2θ values of 27.1° and 3.5°, confirming the presence of (001) and (100) diffraction planes, respectively. The peak at a 2θ value of 27.1° arises due to the π–π synergy of the (001) plane. From the FT-IR spectra of DAAQ, TFPG, and the TFPG-DAAQ COF catalyst, we can confirm the successful complexation reaction between DAAQ and TFPG.
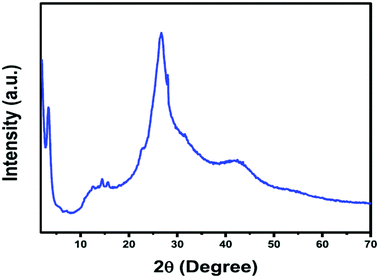 | ||
| Fig. 18 PXRD analysis of TFPG-DAAQ. Reproduced with permission from ref. 51; copyright: 2020, Molecular Catalysis. | ||
The TGA results for the TFPG-DAAQ COF show that the COF is thermally stable up to 425 °C. With the help of N2 sorption studies involving the TFPG-DAAQ COF catalyst, we showed that the TFPG-DAAQ COF has a pore diameter of 3.8 nm; the pores are narrow and have a uniform pore-size distribution. This classifies the catalyst as mesoporous, and the calculated BET surface area of the TFPG-DAAQ COF is observed to be 600 m2 g−1, with a total pore volume of 0.81 cm3 g−1.
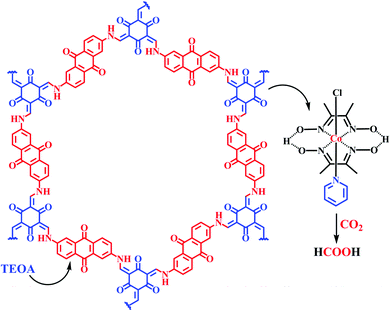
Our group demonstrated the conversion of triethanolamine with the assistance of the TFPG-DAAQ COF catalyst under a CO2 atmosphere and irradiation at 445 nm and 12 W cm−2 at room temperature, and we obtained an excellent yield of formic acid with a turnover number of 125. We also used Co(dmg)2 as a co-catalyst for this reaction. We proposed that the same reaction can be executed even in the presence of ambient sunlight at a slower rate, having a TON of 36. From this study, we concluded that the completely heterogeneous TFPG-DAAQ COF catalyst can be recycled up to five times without any prominent change in its catalytic activity. It was observed that the TON of the isolated product decreases from 125 to 0 in the absence of the TFPG-DAAQ COF catalyst. Based on all these experimental observations, this TFPG-DAAQ COF catalyst was identified as an excellent one for formic acid preparation (Scheme 24).
2.3 Carbamate syntheses
Our team, in 2019, reported a zinc-loaded COF material designated as Zn(II)@TFP-DAQ COF.52 We synthesized this COF base catalyst through the loading of anhydrous ZnCl2 on a TFP-DAQ COF in methanol solvent. Our team performed several characterization studies to establish the structure of this zinc-loaded COF (Scheme 25). The PXRD pattern of the zinc-loaded COF showed two characteristic peaks at 2θ values of 3.6° and 27.2°, which indicate the presence of the (100) and (001) planes, respectively, as represented in Fig. 19. Of these two intense peaks, the second arises due to π–π interactions involving the (001) plane.53 Our research group also performed XPS analysis, which showed that the oxidation state of zinc in the Zn(II)@TFP-DAQ COF is 2+. The total calculated BET surface area of the synthesized mesoporous Zn(II)@TFP-DAQ COF catalyst is 1117.375 m2 g−1 with a pore volume of 1.31 cm3 g−1. This admirable surface area allows this synthesized Zn(II)@TFP-DAQ COF catalyst to show tremendous performance during carbamate synthesis via CO2 fixation.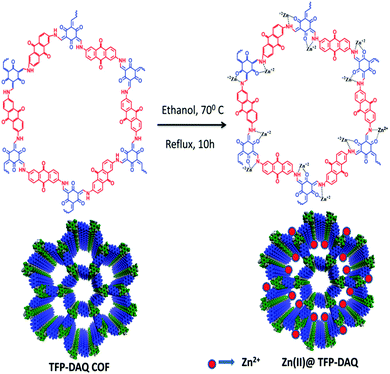 | ||
| Scheme 25 The synthesis of a zinc-loaded porous@TFP-DAQ COF. Reproduced with permission from ref. 52; copyright: 2020, New Journal of Chemistry. | ||
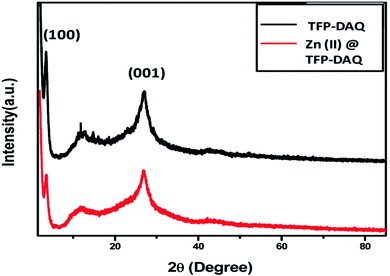 | ||
| Fig. 19 The PXRD analysis of TFP-DAQ COF and Zn(II)@TFP-DAQ COF. Reproduced with permission from ref. 52; copyright: 2020, New Journal of Chemistry. | ||
Our research group has synthesized both alkyl and aryl carbamates at room temperature under atmospheric pressure, starting from different aniline substrates, via CO2 fixation reactions using the Zn(II)@TFP-DAQ catalyst in DMSO solvent at room temperature and in the absence of base (Scheme 26). We reported that this reaction is completed within 8 h with an extraordinary carbamate yield of 97%. Aniline and alkyl bromide were used as starting materials during carbamate synthesis under CO2 at atmospheric pressure. We demonstrated that this completely heterogeneous catalyst can be recycled five times with almost no change in catalyst activity. We also carried out the same reaction in the absence of the catalyst and observed that there is a huge difference in yields of the catalytic and non-catalytic pathways, showing this catalyst to be superior during carbamate synthesis.
After that, in 2020, our research group reported a silver-loaded COF, designated as Ag/TFPG-DMB, for carbamate synthesis via CO2 fixation reactions.54 We prepared the TFPG-DMB COF from 1,3,5-triformylphloroglucinol (TFPG) and DMB using p-toluenesulfonic acid (PTSA) through hydrothermal treatment, and we then decorated the TFPG-DMB COF with silver nanoparticles via a previously reported method (Scheme 27).55
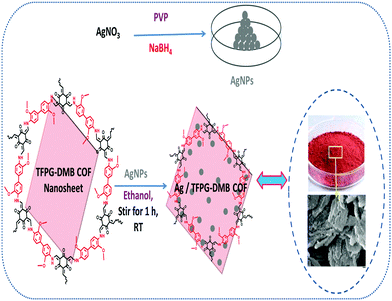 | ||
| Scheme 27 The synthesis of Ag/TFPG-DMB COF. Reproduced with permission from ref. 54; copyright: 2020, Molecular Catalysis. | ||
Our group has performed numerous characterization studies to determine the structure of the silver-loaded COF. The PXRD pattern (Fig. 20) of this silver-loaded COF showed a characteristic peak at a 2θ value of 3.5° due to the (100) plane and a peak at 26.39° which implies the presence of the (001) plane due to the π–π interactions of (001) planes. Four characteristic peaks from metallic Ag were also observed at 2θ values of 44.56°, 64.31°, 38.17°, and 77.31°, indicating the presence of the (220), (200), (111), and (311) crystal planes, respectively. With the help of N2 adsorption–desorption isotherms, we identified that this COF catalyst is a mesoporous one, having a pore diameter of 3.9 nm, and the calculated BET surface area and total pore volume of this COF are found to be 651.64 m2 g−1 and 0.36 cm3 g−1. This high surface area makes this catalyst an excellent one for carbamate synthesis.
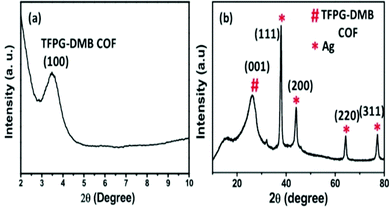 | ||
| Fig. 20 PXRD analysis of Ag/TFPG-DMB COF. Reproduced with permission from ref. 54; copyright: 2020, Molecular Catalysis. | ||
Our team treated various benzylamine derivatives in the presence of the Ag/TFPG-DMB COF as a catalyst, using Cs2CO3 as a base and methanol as a solvent under CO2 at atmospheric pressure at 80 °C for 8 h, and we obtained an excellent carbamate yield of 97%, as represented in Scheme 28. We proposed that this completely heterogeneous catalyst can be recycled five times with minimal changes in the activity of the catalyst. When we carried out the reaction in the absence of catalyst, it was observed that the yield of isolated product diminished to 2% from 97%, showing the catalyst to be superior for carbamate synthesis.
 | ||
| Scheme 28 Methyl benzyl carbamate synthesis via a CO2 fixation reaction. Reproduced with permission from ref. 54; copyright: 2020, Molecular Catalysis. | ||
After this successful work, we demonstrated another interesting work56 in which the silver-loaded COFs Ag@TpPa-1 and Ag@TpTta were fabricated and applied in carbamate synthesis. At first, we synthesized TpPa-1 via mixing exact quantities of triformylphloroglucinol and p-phenylene diamine in the presence of DMF solvent. TpTta, a covalent framework, was synthesized from (1,3,5-triazine-2,4,6-triyl)-trianiline (Tta), triformylphloroglucinol, and 4-aminobenzonitrile. A special characteristic of the synthesized COFs is that the materials are highly enriched with N atoms, which can hold Ag nanoparticles effectively, and the materials are also alkali free (Schemes 29 and 30).56 The PXRD patterns of both the catalysts revealed highly intense and exceptional peaks at 2θ values of ∼4.8 degrees for Ag@TpPa-1 and ∼5.8 degrees for the COF in the nanocatalyst (Ag@TpTta), corresponding to the (100) plane, as represented in Fig. 21.57 The two extra peaks at 2θ values of ∼15.1 and 10.0 degrees correspond to the (210) and (110) planes, respectively. According to the PXRD pattern, the peak at a 2θ value of 27.7° (for Ag@TpPa-1), attributed to the (001) plane, was observed due to π–π stacking between the benzene rings of two adjacent layers of the TpPa-1 cage. N2 adsorption measurements confirmed that the ‘Ag@TpPa-1’ COF has a hugely perforated orientation with a surface area of 243 m2 g−1.
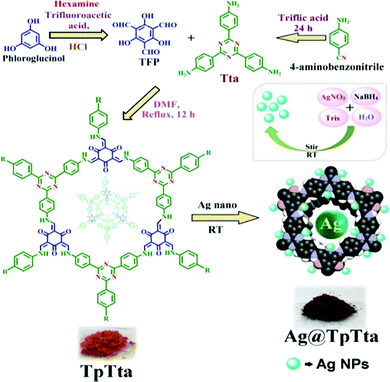 | ||
| Scheme 29 The synthesis of Ag@TpTta. Reproduced with permission from ref. 56; copyright: 2020, ACS Sustainable Chemistry & Engineering. | ||
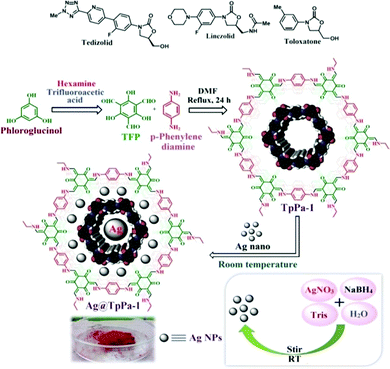 | ||
| Scheme 30 The synthesis of Ag@TpPa-1. Reproduced with permission from ref. 56; copyright: 2020, ACS Sustainable Chemistry & Engineering. | ||
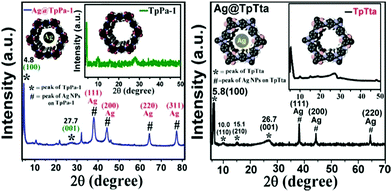 | ||
| Fig. 21 The powder XRD patterns of Ag@TpPa-1 and Ag@TpTta. Reproduced with permission from ref. 56; copyright: 2020, ACS Sustainable Chemistry & Engineering. | ||
The previously mentioned Ag@TpTta COF reported by our group was also used to perform various carbamate formation reactions with allylamine and NIS at room temperature using DMSO as a solvent, as represented in Scheme 31; we observed an excellent cyclic carbamate yield of 96%, whereas we got a satisfactory cyclic carbamate yield of 86% under the same conditions when using the Ag@TpPa-1 COF. However, under solvent-free conditions, our group obtained a carbamate yield of 89% using the Ag@TpPa-1 COF. From experimental observations, we have concluded that Ag@TpTta performed better than Ag@TpPa-1, and the Ag@TpTta COF was selected for this CO2-capture reaction. We showed in our research paper that there is a huge difference in the isolated product yields of the catalytic and noncatalytic pathways, making the catalyst superior for carbamate synthesis.
 | ||
| Scheme 31 The synthesis of carbamate using Ag@TpTta/Ag@TpPa-1 COFs. Reproduced with permission from ref. 56; copyright: 2020, ACS Sustainable Chemistry & Engineering. | ||
2.4 Oxazolidinone synthesis
Our research group also reported a copper-nanoparticle-loaded COF material, designated as Cu-NPs@COF, as represented in Scheme 32.58 In our study, we synthesized the COF support using 2,4,6-triformylphloroglucinol and 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline and performed different characterization studies to establish the structure of the copper-nanoparticle-loaded COF. From the XRD study, a broad peak was observed at a 2θ value of 26.71° due to π–π stacking involving the (001) reflection plane of the COF.59 Then, we also found that the particle size of the designed material is around 0.5 nm.60 It was clearly established from an XPS study that the oxidation state of copper is zero due to successful nanoparticle formation.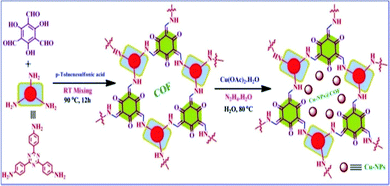 | ||
| Scheme 32 The synthesis of Cu-NPs@COF. Reproduced with permission from ref. 58; copyright: 2020, Journal of CO2 Utilization. | ||
The members of our team synthesized 2-oxazolidinones under atmospheric pressure, starting from different propargyl amine substrates, via CO2 fixation reactions at 50 °C using Cu-NPs@COF in MeCN solvent (Scheme 33). We reported that the reactions were completed after 12 h with excellent 2-oxazolidinone yields (95%). Our group found that using DBU as a base during 2-oxazolidinone synthesis provided maximum yields compared to other commercially available bases like Et3N, Cs2CO3, K2CO3, and DABCO. It was also established that an increase or decrease in the reaction temperature from the optimum level decreased the yield of the product. Our team specified in the study that the designed catalyst is recyclable for up to six cycles with only small changes in the product yields; this makes the catalyst excellent for 2-oxazolidinone synthesis.
 | ||
| Scheme 33 A general synthesis scheme for 2-oxazolidinones. Reproduced with permission from ref. 58; copyright: 2020, Journal of CO2 Utilization. | ||
In 2020, our research group reported another interesting work;61 we developed a silver-nanoparticle-loaded COF material, termed AgN@COF, as a competent catalytic system for CO2 fixation reactions. We designed the COF using 2,4,6-triformylphloroglucinol and 4-aminobenzoic acid hydrazide in the presence of p-toluenesulphonic acid. The Ag nanoparticles were synthesized (Scheme 34) via a green technique using leaf extract from Cantana Camara. We have used several characterization techniques to establish the structure of the COF embedded with silver nanoparticles. The PXRD spectrum of the COF revealed a peak at a 2θ value of 5.6° that corresponds to the (100) reflection plane and, in addition, a π–π stacking plane (001) signal was noticed over a wide 2θ range around 27.9°.62 We have performed high-resolution XPS analysis and showed that the 3d sub-shell of Ag suitably resembled the Ag 3d shell of Ag(0), which indicates that Ag(0) is embedded in the COF surface.63 From N2-BET adsorption–desorption isotherms, our team found that the total surface-areas of the synthesized COF and AgNPs@COF are 167 m2 g−1 and 247.53 m2 g−1, respectively. Also, from TGA analysis, it was demonstrated that the designed catalyst is stable up to 250 °C. We observed micropores with a size of ca. 1.6 nm in the overall sample based on HRTEM images of AgNPs@COF (Fig. 22).
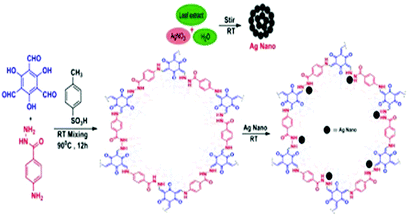 | ||
| Scheme 34 The synthesis of AgNPs@COF. Reproduced with permission from ref. 61; copyright: 2020, ChemNanoMat. | ||
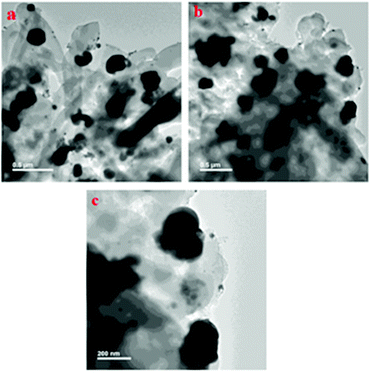 | ||
| Fig. 22 HRTEM images of the synthesized AgNPs@COF material. Reproduced with permission from ref. 61; copyright: 2020, ChemNanoMat. | ||
Our group synthesized 2-oxazolidinones from different propargyl amine substrates via CO2 fixation at 55 °C using AgNPs@COF in acetonitrile solvent and using DBU as a base under atmospheric pressure (Scheme 35). We have used benzylamines and propargyl bromide to synthesize propargyl amines, and then CO2 fixation reactions were performed to obtain 2-oxazolidinones. Before varying the substrate, we optimized several reaction conditions, including the catalyst amount, solvent, base, and reaction temperature. Our research group has shown that the reaction was completed within 10 h with a great 2-oxazolidinone yield of 96%. We have broadly studied substrate variations and yield differentiations. Based on a previous report, a mechanism for 2-oxazolidinone synthesis using AgNPs@COF as a catalyst was proposed, which is presented in Scheme 36.
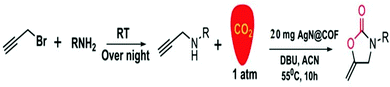 | ||
| Scheme 35 A general diagram of the synthesis of 2-oxazolidinones. Reproduced with permission from ref. 61; copyright: 2020, ChemNanoMat. | ||
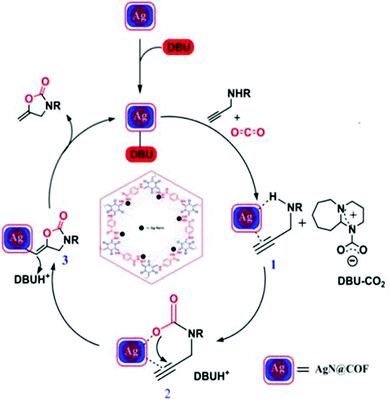 | ||
| Scheme 36 The reaction mechanism for 2-oxazolidinone synthesis using the synthesized AgNPs@COF material. Reproduced with permission from ref. 61; copyright: 2020, ChemNanoMat. | ||
2-Oxazolidinone synthesis56 was performed using Ag@TpTta and Ag@TpPa-1 nanocatalysts from propargylic amines and homoallyl amines. Earlier, we discussed the Ag@TpTta and Ag@TpPa-1 nanocatalysts. Via using different derivatives of propargylic amine and CO2 under pressure of 0.1 MPa, we synthesized 2-oxazolidinones under solvent-free conditions with excellent conversion rates (>99%) in a temperature range of 40–80 °C (Scheme 37). Our group gathered information related to the reaction time, temperature, conversion, and yield for both types of COF catalyst and established that the Ag@TpTta catalyst is more proficient for use in the said reaction than the Ag@TpPa-1 catalyst. Our designed catalysts are recyclable for up to five cycles with only negligible changes in catalytic activity.
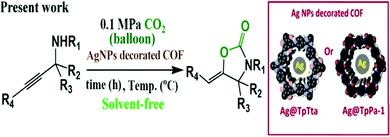 | ||
| Scheme 37 The synthesis of 2-oxazolidinones using a AgNP-decorated COF. Reproduced with permission from ref. 56; copyright: 2020, ACS Sustainable Chemistry & Engineering. | ||
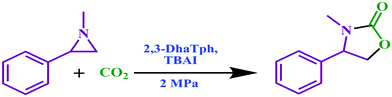
M. Bhanage et al. reported an excellent work on a highly perforated catechol-porphyrin-based COF.64 In that work, two covalent organic frameworks (2,3-DhaTph and 2,3-DmaTph) contained super-efficient sites and both acidic (catechol) and basic (porphyrin) moieties. They synthesized 2,3-DhaTph and 2,3-DmaTph COFs following a previously reported process via Schiff-base interactions using 2,3-dihydroxyterephthalaldehyde, 2,3-dimethoxyterephthalaldehyde, and 5,10,15,20-tetrakis(4-aminophenyl)porphin units.65 They carried out FT-IR, TGA, SEM, BET, and X-ray diffraction analysis to ascertain the properties of the synthesized COFs (Fig. 23). From thermogravimetric analysis, it was observed that these COFs have satisfactory surface areas of ∼1000 m2 g−1.
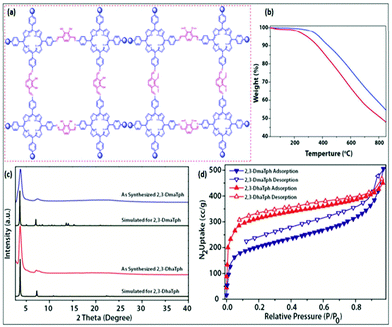 | ||
| Fig. 23 (a) The structure of a catechol-porphyrin COF; (b) TGA plots of (blue) 2,3-DhaTph and (red) 2,3-DmaTph; (c) PXRD analysis; and (d) N2-BET analysis. Reproduced with permission from ref. 64; copyright: 2016, Catalysis Science & Technology. | ||
The research group applied these COF-based catalysts to the synthesis of derivatives of oxazolidinone via the fixation of CO2 with aziridines.66 1-Methyl-2-phenyl-aziridines were made and used for the formation of 5-aryl-2-oxazolidinones at normal temperature and under 2 MPa pressure using 2,3-DhaTph COF as a catalyst in a solvent-deprived state, as represented in Scheme 38.
2.5 Carboxylation of ethynylbenzene
In 2021, En-Qing Gao and his research group reported an excellent Cu(I)-modified covalent organic framework enriched with N–N and N–O chelating groups for the attachment of CO2 to terminal alkynes.67 Here, a freshly prepared 2D-COF, named TpBpy, with attached Cu(I) was fabricated from 2,4,6-triformylphloroglucinol (Tp) and [2,2′]bipyridinyl-5,5′-diamine (Bpyda), as represented in Scheme 39.68 Cu(I) was buried inside TpBpy via a heterogeneous reaction at ambient temperature in acetonitrile solvent. The concerned COF has an eclipsed and hexagonal structure, which was ascertained based on the XRD pattern. Due to the 2D-structure, the π–π stacking pattern was disturbed when Cu(I) was incorporated. The authors observed a decrease in the crystallinity of the COF after Cu(I) attachment to the COF support.69 They obtained the interesting observation that the surface area decreases to 434 m2 per gram from 1480 m2 per gram when TpBpy was loaded with copper to form TpBpy-Cu-14. TpBpy also showed excellent CO2 absorption capabilities: 66.8 cm3 g−1 at 0 °C. Also, TpBpy-Cu-14 can absorb 47.2 cm3 g−1 CO2 at the same temperature. Thermogravimetric analysis helped them to discover that the thermal stability of the COF decreased after metal incorporation. They examined the surface morphology of TpBpy-Cu-14 through SEM analysis and found that the morphology of the COF is unaltered, even after loading with copper(I).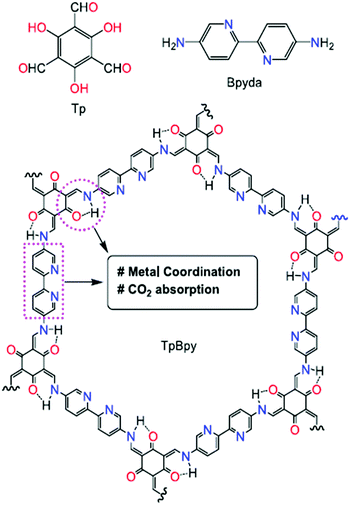 | ||
| Scheme 39 The structure of TpBpy COF. Reproduced with permission from ref. 67; copyright: 2021, Molecular Catalysis. | ||
En-Qing Gao and research team used ethynylbenzene as a sample substrate and let it react with carbon dioxide under atmospheric pressure using TpBpy-Cu-14 as a catalyst (Scheme 40). They studied various control reactions in the presence of the COF (TpBpy-Cu-14) and found that in the presence of Cs2CO3, phenylpropionic acid was formed, having yield percentages of 62% and 95% after 6 h and 24 h, respectively, at a controlled temperature of 60 °C. H-Bonding N/O active sites help reactions to occur and activate the terminal alkynes.70
In addition to COFs, MOF nanoporous organic polymers are also excellent catalytic materials for the conversion of CO2 into other valuable products or for the hydrogenation of CO2. Recently, A. Bhaumik and his research team reported some interesting review articles and research papers regarding this topic in which the use of organic-framework-based catalysts is demonstrated for the hydrogenation and conversion of CO2.71
3. Conclusions and future prospects
In the present era, COFs have become conspicuous materials in the area of catalysis due to their superior CO2 absorption capacities. As discussed earlier, metal-incorporating COFs or COFs without metal incorporation can be used effectively in different kinds of CO2 fixation reactions to obtain valuable organic products. In this review article, we have presented recently synthesized COFs and their applications in different types of CO2 fixation reactions. COFs are thermally stable compounds with excellent surface areas and pore volumes. In this article, most of the presented COFs are synthesized via Schiff-base mechanisms and are enriched with nitrogen sites. These nitrogen sites can hold and bind CO2 and different metallic nanoparticles or ions. For this reason, nitrogen-enriched COFs can be used for CO2 capture and storage and the catalytic fixation of CO2 with organic substrates. The COFs that are demonstrated show more excellent performance than other conventional catalysts due to their excellent responses towards CO2. COFs are now being employed for the conversion of CO2 to CO via photochemical or electrochemical reduction. More research is necessary in this field to develop fruitful applications. This review article will definitely encourage researchers to develop cost-effective COF materials in the near future which can be used for large-scale CO2 capture. Recently developed COFs should be restructured for the effective capture and binding of CO2. We hope that this field of research will decrease CO2 levels in the atmosphere via capturing CO2 more proficiently.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SMI acknowledges the Department of Science and Technology DST-SERB (project reference no. CRG/2020/000244), New Delhi, Govt. of India and Council of Scientific and Industrial and Research, CSIR (project reference no. 02(0453)/21/EMR-II dated 08/06/2021), New Delhi, Govt. of India for providing financial support. M. Sarkar is thankful to the University of Kalyani for his URS Fellowship. We acknowledge the DST for providing grants to the Department of Chemistry through FIST and PURSE grants.References
- (a) D. J. Darensbourg and A. D. Yeung, Polym. Chem., 2014, 5, 3949–3962 RSC; (b) A. Appel, J. Bercaw, A. Bocarsly, H. Dobbek, D. DuBois, M. Dupuis, J. Ferry, E. Fujita, R. Hille, P. Kenis, C. Kerfeld, R. Morris, C. Peden, A. Portis, S. Ragsdale, T. Rauchfuss, J. Reek, L. Seefeldt, R. Thauer and G. Waldrop, Chem. Rev., 2013, 113, 6621–6658 CrossRef CAS PubMed.
- (a) Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed; (b) M. R. Kember, A. Buchard and C. K. Williams, Chem. Commun., 2011, 47, 141–163 RSC; (c) M. Y. Wang, Y. Cao, X. Liu, N. Wang, L. N. He and S. H. Li, Green Chem., 2017, 19, 1240–1244 RSC; (d) L. Sun, J. H. Ye, W. J. Zhou, X. Zeng and D. G. Yu, Org. Lett., 2018, 20, 3049–3052 CrossRef CAS PubMed; (e) S. S. Islam, P. Bhanja, K. Ghosh, R. A. Molla, N. Yasmin, D. Das and S. M. Islam, Chem. Select, 2017, 2, 10595–10602 CAS; (f) N. Haque, S. Biswas, S. Ghosh, A. H. Chowdhury, A. Khan and S. M. Islam, ACS Appl. Nano Mater., 2021, 4, 7663–7674 CrossRef CAS.
- A. Bar-Even, A. Flamholz, E. Noor and R. Milo, Biochim. Biophys. Acta, 2012, 1817, 1646–1659 CrossRef CAS PubMed.
- (a) Y. Zheng, X. Wang, C. Liu, B. Yu, W. Li, H. Wang, T. Sun and J. Jiang, Inorg. Chem. Front., 2021, 8, 2880–2888 RSC; (b) I. H. Chowdhury, A. H. Chowdhury, P. Sarkar and S. M. Islam, ChemNanoMat, 2021, 7, 580–591 CrossRef; (c) A. H. Chowdhury, I. H. Chowdhury, S. Biswas and S. M. Islam, Mol. Catal., 2020, 493, 111050 CrossRef; (d) A. H. Chowdhury, I. H. Chowdhury and S. M. Islam, Ind. Eng. Chem. Res., 2019, 58, 11779–11786 CrossRef CAS; (e) S. Ghosh, R. A. Molla, U. Kayal, A. Bhaumik and S. M. Islam, Dalton Trans., 2019, 48, 4657–4666 RSC; (f) S. M. Islam, D. Mal, B. K. Palit and C. R. Saha, J. Mol. Catal. A: Chem., 1999, 142, 169–181 CrossRef CAS; (g) N. Haque, S. Biswas, P. Basu, I. H. Biswas, R. Khatun, A. Khan and S. M. Islam, New J. Chem., 2020, 44, 15446–15458 RSC; (h) A. Sahoo, A. H. Chowdhury, P. Singha, A. Banerjee, S. M. Islam and T. Bala, Mol. Catal., 2020, 493, 111070 CrossRef CAS; (i) P. Bhanja, K. Ghosh, S. S. Islam, A. K. Patra, S. M. Islam and A. Bhaumik, ACS Sustainable Chem. Eng., 2016, 4(12), 7147–7157 CrossRef CAS; (j) S. Roy, P. Bhanja, S. S. Islam, A. Bhaumik and S. M. Islam, Chem. Commun., 2016, 52, 1871 RSC; (k) S. Roy, B. Banerjee, A. Bhaumik and S. M. Islam, RSC Adv., 2016, 6, 31153–31160 RSC; (l) S. Bhunia, R. A. Molla, V. Kumari, S. M. Islam and A. Bhaumik, Chem. Commun., 2015, 51, 15732 RSC.
- (a) T. K. Pal, D. Deb and P. K. Bharadwaj, Coord. Chem. Rev., 2020, 408, 213173 CrossRef CAS; (b) P. Sarkar, A. H. Chowdhury, S. Riyajuddin, S. Biswas, K. Ghosh and S. M. Islam, New J. Chem., 2020, 44, 744–752 RSC; (c) P. Chakrabortty, A. Das, A. H. Chowdhury, S. Ghosh, A. Khan and S. M. Islam, New J. Chem., 2021, 45, 4738–4745 RSC; (d) P. Sarkar, A. H. Chowdhury, S. Biswas, A. Khan and S. M. Islam, Mater. Today Chem., 2021, 21, 100509 CrossRef CAS; (e) S. Biswas, R. Khatun, M. Sengupta and S. M. Islam, Mol. Catal., 2018, 452, 129–137 CrossRef CAS; (f) S. Roy, B. Banerjee, A. Bhaumik and S. M. Islam, RSC Adv., 2016, 6, 31153–31160 RSC; (g) S. Ghosh, P. Bhanja, R. A. Molla, R. Khatun and S. M. Islam, Chem. Select, 2017, 2, 2159–2165 CAS; (h) I. H. Chowdhury, A. H. Chowdhury, A. Das, A. Khan and S. M. Islam, New J. Chem., 2020, 44, 11720–11726 RSC; (i) P. Sarkar, S. Riyajuddin, A. Das, A. H. Chowdhury, K. Ghosh and S. M. Islam, Mol. Catal., 2020, 484, 110730 CrossRef CAS; (j) S. M. Islam, N. Salam, P. Mondal and A. S. Roy, J. Mol. Catal A: Chem., 2013, 366, 321–332 CrossRef CAS; (k) S. M. Islam, P. Mondal, S. Mukherjee, A. S. Roy and A. Bhaumik, Polym. Adv. Tech., 2011, 22(6), 933–941 CrossRef CAS; (l) M. Sengupta, A. Bag, S. Ghosh, P. Mondal, A. Bordoloi and S. M. Islam, J. CO2 Util., 2019, 34, 533–542 CrossRef CAS.
- (a) J. Lu, S. Liu, A. Xu, L. Jin, H. Chi, Y. Li, B. Li, L. Dong and M. Fan, Mater. Chem. Phys., 2021, 272, 124899 CrossRef CAS; (b) R. A. Molla, M. A. Iqubal, K. Ghosh and S. M. Islam, Green Chem., 2016, 18, 4649–4656 RSC; (c) R. Khatun, S. Biswas, I. H. Biswas, S. Riyajuddin, N. Haque and K. Ghosh, J. CO2 Util., 2020, 40, 101180 CrossRef CAS; (d) R. Khatun, P. Bhanja, R. A. Molla, S. Ghosh, A. Bhaumik and S. M. Islam, Mol. Catal., 2017, 434, 25–31 CrossRef CAS; (e) S. Biswas, R. Khatun, M. Dolai, I. H. Biswas, N. Haque, M. Sengupta and S. M. Islam, New J. Chem., 2020, 44, 141–151 RSC; (f) I. H. Biswas, S. Biswas, M. S. Islam, S. Riyajuddin, P. Sarkar and K. Ghosh, New J. Chem., 2019, 43, 14643–14652 RSC; (g) S. Ghosh, A. Ghosh, S. Riyajuddin, S. Sarkar, A. H. Chowdhury and K. Ghosh, ChemCatChem, 2020, 12, 1055–1067 CrossRef CAS; (h) S. Sarkar, S. Ghosh, J. Mondal and S. M. Islam, Chem. Commun., 2020, 56, 12202–12205 RSC; (i) T. K. Dey, P. Bhanja, P. Basu, A. Ghosh and S. M. Islam, Chem. Select, 2019, 4, 14315–14328 CAS; (j) S. Ghosh, A. Ghosh, S. Biswas, M. Sengupta, D. Roy and S. M. Islam, Chem. Select, 2019, 4, 3961–3972 CAS; (k) S. S. Islam, S. Biswas, R. Ali Molla, N. Yasmin and S. M. Islam, ChemNanoMat, 2020, 6, 1386–1397 CrossRef CAS; (l) P. Basu, T. K. Dey, S. Riyajuddin, S. Biswas, K. Ghosh and S. M. Islam, New J. Chem., 2020, 44, 12680–12691 RSC.
- (a) K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed; (b) R. Khatun, S. Biswas, S. Islam, I. H. Biswas, S. Riyajuddin, K. Ghosh and S. M. Islam, ChemCatChem, 2019, 11, 1303–1312 CrossRef CAS; (c) M. Sengupta, A. Bag, S. Ghosh, P. Mondal, A. Bordoloi and S. M. Islam, J. CO2 Util., 2019, 34, 533–542 CrossRef CAS; (d) A. H. Chowdhury, S. Ghosh and S. M. Islam, New J. Chem., 2018, 42, 14194–14202 RSC; (e) R. Khatun, P. Bhanja, P. Mondal, A. Bhaumik, D. Das and S. M. Islam, New J. Chem., 2017, 41, 12937–12946 RSC; (f) A. H. Chowdhury, U. Kayal, I. H. Chowdhury, S. Ghosh and S. M. Islam, ChemistrySelect, 2019, 4, 1069–1077 CrossRef CAS; (g) S. Ghosh, P. Bhanja, N. Salam, R. Khatun, A. Bhaumik and S. M. Islam, Catal. Today, 2018, 309, 253–262 CrossRef CAS; (h) R. A. Molla, K. Ghosh, K. Tuhina and S. M. Islam, New J. Chem., 2015, 39(2), 921–930 RSC; (i) M. Islam, A. S. Roy and S. Islam, Catal. Lett., 2016, 146(6), 1128–1138 CrossRef CAS; (j) S. M. Islam, N. Salam, P. Mondal, A. S. Roy, K. Ghosh and K. Tuhina, J. Mol. Catal. A: Chem., 2014, 387, 7–19 CrossRef CAS; (k) S. Roy, B. Banerjee, N. Salam, A. Bhaumik and S. M. Islam, ChemCatChem, 2015, 7(17), 2689–2697 CrossRef CAS; (l) S. M. Islam, R. A. Molla, A. S. Roy and K. Ghosh, RSC Adv., 2014, 4(50), 26181–26192 RSC; (m) S. Roy, T. Chatterjee, B. Banerjee, N. Salam, A. Bhaumik and S. M. Islam, RSC Adv., 2014, 4(86), 46075–46083 RSC; (n) S. Ghosh, T. S. Khan, A. Ghosh, A. H. Chowdhury, M. A. Haider and A. Khan, ACS Sustainable Chem. Eng., 2020, 8(14), 5495–5513 CrossRef CAS; (o) R. Khatun, P. Bhanja, P. Mondal, A. Bhaumik, D. Das and S. M. Islam, New J. Chem., 2017, 41(21), 12937–12946 RSC; (p) A. H. Chowdhury, S. Ghosh and S. M. Islam, New J. Chem., 2018, 42(17), 14194–14202 RSC.
- (a) B. Gole, V. Stepanenko, S. Rager, M. Grune, D. D. Medina, T. Bein, F. Wurthner and F. Beuerle, Angew. Chem., Int. Ed., 2018, 57, 846–850 CrossRef CAS PubMed; (b) T. Sick, A. G. Hufnagel, J. Kampmann, I. Kondofersky, M. Calik, J. M. Rotter, A. Evans, M. Doblinger, S. Herbert, K. Peters, D. Bohm, P. Knochel, D. D. Medina, D. Fattakhova-Rohlfing and T. Bein, J. Am. Chem. Soc., 2018, 140, 2085–2092 CrossRef CAS PubMed; (c) X. Zhao, P. Pachfule and A. Thomas, Chem. Soc. Rev., 2021, 50, 6871–6913 RSC; (d) Y. Chen, Q. Chen and Z. Zhang, Chin. J. Org. Chem., 2021, 41, 3826–3843 CrossRef.
- (a) R. P. Bisbey and W. R. Dichtel, ACS Cent. Sci., 2017, 3, 533–543 CrossRef CAS PubMed; (b) N. Huang, P. Wang and D. Jiang, Nat. Rev. Mater., 2016, 1, 1–19 Search PubMed; (c) M. S. Lohse and T. Bein, Adv. Funct. Mater., 2018, 28, 1705553 CrossRef; (d) C. S. Diercks and O. M. Yaghi, Science, 2017, 355, eaal1585 CrossRef PubMed; (e) S. L. Cai, W. G. Zhang, R. N. Zuckermann, Z. T. Li, X. Zhao and Y. Liu, Adv. Mater., 2015, 27, 5762–5770 CrossRef CAS PubMed; (f) Y. Song, Q. Sun, B. Aguila and S. Ma, Adv. Sci., 2019, 6, 1801410 CrossRef PubMed; (g) I. Omae, Coord. Chem. Rev., 2012, 256, 1384–1405 CrossRef CAS; (h) S. Ghosh, S. Riyajuddin, S. Sarkar, K. Ghosh and S. M. Islam, ChemNanoMat, 2020, 6, 160–172 CrossRef CAS; (i) S. Ghosh, P. Mondal, D. Das, K. Tuhina and S. M. Islam, J. Organomet. Chem., 2018, 866, 1–12 CrossRef CAS; (j) N. Salam, P. Paul, S. Ghosh, U. Mandi, A. Khan, S. M. Alam, D. Das and S. M. Islam, New J. Chem., 2020, 44, 5448–5456 RSC; (k) S. S. Islam, N. Salam, R. A. Molla, S. Riyajuddin, N. Yasmin, D. Das and K. Ghosh, Mol. Catal., 2019, 477, 110541 CrossRef; (l) R. K. Mondal, S. Riyajuddin, A. Ghosh, S. Ghosh, K. Ghosh and S. M. Islam, J. Organomet. Chem., 2019, 880, 322–332 CrossRef CAS; (m) S. Biswas, D. Roy, S. Ghosh and S. M. Islam, J. Organomet. Chem., 2019, 898, 120877 CrossRef; (n) A. Das, R. K. Mondal, P. Chakrabortty, S. Riyajuddin and A. H. Chowdhury, Mol. Catal., 2021, 499, 111253 CrossRef CAS.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzgerand and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- (a) P. J. Waller, Y. S. AlFaraj, C. S. Diercks, N. N. Jarenwattananon and O. M. Yaghi, J. Am. Chem. Soc., 2018, 140, 9099–9103 CrossRef CAS PubMed; (b) T. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L.-H. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun and O. M. Yaghi, Science, 2018, 361, 48–52 CrossRef CAS PubMed.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- S. Dey, A. Bhunia, D. Esquivel and C. Janiak, J. Mater. Chem. A, 2016, 4, 6259–6263 RSC.
- F. Haase, E. Troschke, G. Savasci, T. Banerjee, V. Duppel, S. Dorfler, M. M. J. Grundei, A. M. Burow, C. Ochsenfeld, S. Kaskel and B. V. Lotsch, Nat. Commun., 2018, 9, 2600 CrossRef PubMed.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, Chem. Sci., 2014, 5, 2789–2793 RSC.
- C. R. DeBlase, K. E. Silberstein, T. T. Truong, H. D. Abruna and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821–16824 CrossRef CAS PubMed.
- L. Zhai, N. Huang, H. Xu, Q. Chen and D. Jiang, Chem. Commun., 2017, 53, 4242–4245 RSC.
- B. Dong, L. Wang, S. Zhao, R. Ge, X. Song, Y. Wang and Y. Gao, Chem. Commun., 2016, 52, 7082–7085 RSC.
- S. Zhao, B. Dong, R. Ge, C. Wang, X. Song, W. Ma, Y. Wang, C. Hao, X. Guo and Y. Gao, RSC Adv., 2016, 6, 38774–38781 RSC.
- N. Huang, X. Chen, R. Krishna and D. Jiang, Angew. Chem., Int. Ed., 2015, 54, 2986–2990 CrossRef CAS PubMed.
- D. Chakraborty, P. Shekhar, H. D. Singh, R. Kushwaha, C. P. Vinod and R. Vaidhyanathan, Chem. – Asian J., 2019, 14, 4767–4773 CrossRef CAS PubMed.
- H. He, Q. Q. Zhu, W. W. Zhang, H. W. Zhang, J. Chen, C. P. Li and M. Du, ChemCatChem, 2020, 12, 5192–5199 CrossRef CAS.
- K. Xu, Y. Dai, B. Ye and H. Wang, Dalton Trans., 2017, 46, 10780–10785 RSC.
- A. Chen, Y. Zhang, J. Chen, L. Chen and Y. Yu, J. Mater. Chem. A, 2015, 3, 9807–9816 RSC.
- Y. Zhi, P. Shao, X. Feng, H. Xia, Y. Zhang, Z. Shi and X. Liu, J. Mater. Chem. A, 2018, 6, 374–382 RSC.
- M. Sengupta, A. Bag, S. Ghosh, P. Mondal, A. Bordoloi and S. M. Islam, J. CO2 Util., 2019, 34, 533–542 CrossRef CAS.
- S. Karak, S. Kandambeth, B. P. Biswal, H. S. Sasmal, S. Kumar, P. Pachfule and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 1856–1862 CrossRef CAS PubMed.
- Y. Han, M. Zhang, Y. Q. Zhang and Z. H. Zhang, Green Chem., 2018, 20, 4891–4900 RSC.
- P. Guan, J. Qiu, Y. Zhao, H. Wang, Z. Li, Y. Shi and J. Wang, Chem. Commun., 2019, 55, 12459–12462 RSC.
- (a) D. M. Fischbach, G. Rhoades, C. Espy, F. Goldberg and B. J. Smith, Chem. Commun., 2019, 55, 3594–3597 RSC; (b) T. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun and O. M. Yaghi, Science, 2018, 361, 48–52 CrossRef CAS PubMed; (c) C. Wang, Y. Wang, R. Ge, X. Song, X. Xing, Q. Jiang, H. Lu, C. Hao, X. Guo, Y. Hao and D. Jiang, Chem. – Eur. J., 2018, 24, 585–589 CrossRef CAS PubMed; (d) Q. Lu, Y. Ma, H. Li, X. Guan, Y. Yusran, M. Xue, Q. Fang, Y. Yan, S. Qiu and V. Valtchev, Angew. Chem., Int. Ed., 2018, 57, 6042–6048 CrossRef CAS PubMed; (e) R. Mercado, R.-S. Fu, A. V. Yakutovich, L. Talirz, M. Haranczyk and B. Smit, Chem. Mater., 2018, 30, 5069–5086 CrossRef CAS; (f) X. Han, J. Huang, C. Yuan, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2018, 140, 892–895 CrossRef CAS PubMed.
- (a) R. Mercado, R. S. Fu, A. V. Yakutovich, L. Talirz, M. Haranczyk and B. Smit, Chem. Mater., 2018, 30, 5069–5086 CrossRef CAS; (b) H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed; (c) L. A. Baldwin, J. W. Crowe, D. A. Pyles and P. L. McGrier, J. Am. Chem. Soc., 2016, 138, 15134–15137 CrossRef CAS PubMed.
- L. G. Ding, B. J. Yao, F. Li, S. C. Shi, N. Huang, H. B. Yin, Q. Guan and Y. B. Dong, J. Mater. Chem. A, 2019, 7, 4689–4698 RSC.
- F. Li, L. G. Ding, B. J. Yao, N. Huang, J. T. Li, Q. J. Fu and Y. B. Dong, J. Mater. Chem. A, 2018, 6, 11140–11146 RSC.
- C. Müller, D. Rahmat, F. Sarti, K. A. Leithner and B. Schnürch, J. Mater. Chem. A, 2012, 22, 3899–3908 RSC.
- B. J. Yao, L. G. Ding, F. Li, J. T. Li, Q. J. Fu, Y. J. Ban, A. Guo and Y. B. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 38919–38930 CrossRef CAS PubMed.
- Y. Zhao, Y. Zhao, J. Qiu, Z. Li, H. Wang and J. Wang, ACS Sustainable Chem. Eng., 2020, 8, 18413–18419 CrossRef CAS.
- K. Jeong, S. Park, G. Jung, S. Kim, Y. Lee, S. Kwak and S. Lee, J. Am. Chem. Soc., 2019, 141, 5880–5885 CrossRef CAS PubMed.
- J. Qiu, Y. Zhao, Z. Li, H. Wang, Y. Shi and J. Wang, ChemSusChem, 2019, 12, 2421–2427 CAS.
- F. Yang, Y. Li, T. Zhang, Z. Zhao, G. Xing and L. Chen, Chem. – Eur. J., 2020, 26, 4510–4514 CrossRef CAS PubMed.
- H. Zhong, J. Gao, R. Sa, S. Yang, Z. Wu and R. Wang, ChemSusChem, 2020, 13, 6323–6329 CAS.
- (a) S. Wang and C. Xi, Chem. Soc. Rev., 2019, 48, 382–404 RSC; (b) X. Sun, L. Lu, Q. Zhu, C. Wu, D. Yang, C. Chen and B. Han, Angew. Chem., Int. Ed., 2018, 57, 2427–2431 CrossRef CAS PubMed; (c) J. Qiu, Y. Zhao, Z. Li, H. Wang, M. Fan and J. Wang, ChemSusChem, 2017, 10, 1120–1127 CrossRef CAS PubMed; (d) F. D. Bobbink, W. Zhong, Z. Fei and P. J. Dyson, ChemSusChem, 2017, 10, 2728–2735 CrossRef PubMed.
- (a) S. Jayakumar, H. Li, Y. Zhao, J. Chen and Q. Yang, Chem. – Asian J., 2017, 12, 577–585 CrossRef CAS PubMed; (b) W. Zhang, F. Ma, L. Ma, Y. Zhou and J. Wang, ChemSusChem, 2020, 13, 341–350 CrossRef CAS PubMed; (c) M. B. Sogo, H. Garciab and C. Aprile, Catal. Sci. Technol., 2015, 5, 1222–1230 RSC.
- S. Haldar, K. Roy, S. Nandi, D. Chakraborty, D. Puthusseri, Y. Gawli, S. Ogale and R. Vaidhyanathan, Adv. Energy Mater., 2018, 8, 1702170 CrossRef.
- W. Zhou, Q. W. Deng, G. Q. Ren, L. Sun, L. Yang, Y. M. Li and W. Q. Deng, Nat. Commun., 2020, 11, 4481 CrossRef CAS PubMed.
- Y. Xie, T. T. Wang, X. H. Liu, K. Zou and W. Q. Deng, Nat. Commun., 2013, 4, 1960 CrossRef PubMed.
- Y. Zhang, H. Hu, J. Ju, Q. Yan, V. Arumugam, X. Jing and Y. Gao, Chin. J. Catal., 2020, 41, 485–493 CrossRef CAS.
- (a) J. Sun, S. Zhang, W. Cheng and J. Ren, Tetrahedron Lett., 2008, 49, 3588–3591 CrossRef CAS; (b) V. Caló, A. Nacci, A. Monopoli and A. Fanizzi, Org. Lett., 2002, 4, 2561–2563 CrossRef PubMed.
- J. M. Sun, S. Fujita and M. Arai, J. Organomet. Chem., 2005, 690, 3490–3497 CrossRef CAS.
- (a) J. Peng and Y. Deng, New J. Chem., 2001, 25, 639–641 RSC; (b) H. Kawanami, A. Sasaki, K. Matsui and Y. Ikushima, Chem. Commun., 2003, 896–897 RSC.
- H. Yang, Y. Gu, Y. Deng and F. Shi, Chem. Commun., 2002, 274–275 RSC.
- P. Sarkar, S. Riyajuddin, A. Das, A. H. Chowdhury, K. Ghosh and S. M. Islam, Mol. Catal., 2020, 484, 110730 CrossRef CAS.
- P. Sarkar, A. H. Chowdhury, S. Riyajuddin, S. Biswas, K. Ghosh and S. M. Islam, New J. Chem., 2020, 44, 744–752 RSC.
- S. Karak, S. Kandambeth, B. P. Biswal, H. Sekhar Sasmal, S. Kumar, P. Pachfule and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 1856–1862 CrossRef CAS PubMed.
- A. H. Chowdhury, I. H. Chowdhury, S. Biswas and S. M. Islam, Mol. Catal., 2020, 493, 111050 CrossRef.
- X. Ding, J. Guo, X. Feng, Y. Honsho, J. Guo, S. Seki, P. Maitarad, A. Saeki, S. Nagase and D. Jiang, Angew. Chem., Int. Ed., 2011, 50, 1289–1293 CrossRef CAS PubMed.
- S. Ghosh, T. S. Khan, A. Ghosh, A. H. Chowdhury, M. A. Haider, A. Khan and S. M. Islam, ACS Sustainable Chem. Eng., 2020, 8, 5495–5513 CrossRef CAS.
- S. Karak, S. Kandambeth, B. P. Biswal, H. S. Sasmal, S. Kumar, P. Pachfule and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 1856–1862 CrossRef CAS PubMed.
- R. Khatun, S. Biswas, I. H. Biswas, S. Riyajuddin, N. Haque, K. Ghosh and S. M. Islam, J. CO2 Util., 2020, 40, 101180 CrossRef CAS.
- S. Chandra, D. R. Chowdhury, M. Addicoat, T. Heine, A. Paul and R. Banerjee, Chem. Mater., 2017, 29, 2074–2080 CrossRef CAS.
- P. Puthiaraj and W. S. Ahn, Catal. Sci. Technol., 2016, 6, 1701–11709 RSC.
- S. S. Islam, S. Biswas, Dr. R. Ali Molla, Dr. N. Yasmin and S. M. Islam, ChemNanoMet, 2020, 6, 1386–1397 CrossRef CAS.
- (a) S. Karak, S. Kandambeth, B. P. Biswal, H. S. Sasmal, S. Kumar, P. Pachfule and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 1856–1862 CrossRef CAS PubMed; (b) L. Wang, C. Zeng, H. Xu, P. Yin, D. Chen, J. Deng, M. Li, N. Zheng, C. Gu and Y. Ma, Chem. Sci., 2019, 10, 1023–1028 RSC; (c) S. Ghosh, R. A. Molla, U. Kayal, A. Bhaumik and S. M. Islam, Dalton Trans., 2019, 48, 4657–4666 RSC; (d) M. Sengupta, A. Bag, S. Ghosh, P. Mondal, A. Bordoloi and S. M. Islam, J. CO2 Util., 2019, 34, 533–542 CrossRef CAS; (e) N. Haque, S. Biswas, S. Ghosh, A. H. Chowdhury, A. Khan and S. M. Islam, ACS Appl. Nano Mater., 2021, 4, 7663–7674 CrossRef CAS; (f) S. Ghosh, R. A. Molla, U. Kayal, A. Bhaumik and S. M. Islam, Dalton Trans., 2019, 48, 4657–4666 RSC.
- (a) Y. Wu, Z. Wang, S. Chen, J. Wu, X. Guo and Z. Liu, RSC Adv., 2015, 5, 87151–87156 RSC; (b) S. Li, Y. Shen, A. Xie, X. Yu, L. Qiu, L. Zhang and Q. Zhang, Green Chem., 2007, 9, 852–858 RSC.
- V. Saptal, D. B. Shinde, R. Banerjee and B. M. Bhanage, Catal. Sci. Technol., 2016, 6, 6152–6158 RSC.
- D. B. Shinde, S. Kandambeth, P. Pachfule, R. Kumar and R. Banerjee, Chem. Commun., 2015, 51, 310–313 RSC.
- (a) R. Watson, D. Batty, A. Baxter, D. Hannah, D. Owen and J. Montana, Tetrahedron Lett., 2002, 43, 683–685 CrossRef CAS; (b) L. Aurelio, R. Brownlee and A. Hughes, Chem. Rev., 2004, 104, 5823–5846 CrossRef CAS PubMed; (c) T. Mukhtar and G. Wright, Chem. Rev., 2005, 105, 529–542 CrossRef CAS PubMed; (d) T. Andreou, A. Costa, L. Esteban, L. Gonzalez, G. Mas and J. Vilarrasa, Org. Lett., 2005, 7, 4083–4086 CrossRef CAS PubMed; (e) R. Watile, D. Bagal, K. Deshmukh, K. Dhake and B. M. Bhanage, J. Mol. Catal. A: Chem., 2011, 351, 196–203 CrossRef CAS; (f) Y. N. Zhao, Z. Z. Yang, S. H. Luo and L. N. He, Catal. Today, 2013, 200, 2–8 CrossRef CAS.
- R. Bu, L. Zhang, L. L. Gao, W. J. Sun, S. L. Yang and E. Q. Gao, Mol. Catal., 2021, 499, 111319 CrossRef CAS.
- W. Zhong, R. Sa, L. Li, Y. He, L. Li, J. Bi, Z. Zhuang, Y. Yu and Z. Zou, J. Am. Chem. Soc., 2019, 141, 7615–7621 CrossRef CAS PubMed.
- D. Wu, Q. Xu, J. Qian, X. Li and Y. Sun, Chem. – Eur. J., 2019, 25, 3105–3111 CAS.
- (a) X. Lan, C. Du, L. Cao, T. She, Y. Li and G. Bai, ACS Appl. Mater. Interfaces, 2018, 10, 38953–38962 CrossRef CAS PubMed; (b) W. Zhang, Y. Mei, X. Huang, P. Wu, H. Wu and M. He, ACS Appl. Mater. Interfaces, 2019, 11, 44241–44248 CrossRef CAS PubMed; (c) M. Ding, R. W. Flaig, H. L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- (a) A. Modak, A. Ghosh, A. Bhaumik and B. Chowdhury, Adv. Colloid Interface Sci., 2021, 290, 102349 CrossRef CAS PubMed; (b) S. K. Das, A. Ghosh, S. Bhattacharjee, A. Chowdhury, P. Mitra and A. Bhaumik, New J. Chem., 2021, 45, 9189–9196 RSC; (c) A. Modak, A. Ghosh, A. R. Mankar, A. Pandey, M. Selvaraj, K. K. Pant, B. Chowdhury and A. Bhaumik, ACS Sustainable Chem. Eng., 2021, 9(37), 12431–12460 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |