Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supercritical carbon dioxide as reaction medium for selective hydrogenation of fluorinated arenes†
Souha
Kacem‡
ac,
Yunxiang
Qiao‡
 ab,
Cornelia
Wirtz
b,
Nils
Theyssen
ab,
Cornelia
Wirtz
b,
Nils
Theyssen
 b,
Alexis
Bordet
b,
Alexis
Bordet
 *a and
Walter
Leitner
*a and
Walter
Leitner
 *ac
*ac
aMax Planck Institute for Chemical Energy Conversion, Stiftstr. 34-36, 45470 Mülheim an der Ruhr, Germany. E-mail: alexis.bordet@cec.mpg.de; walter.leitner@cec.mpg.de
bMax-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
cInstitut für Technische und Makromolekulare Chemie (ITMC), RWTH Aachen University, Worringer Weg 2, 52074 Aachen, Germany
First published on 7th September 2022
Abstract
The selective hydrogenation of fluorinated arenes with polar functionalities is achieved, opening a versatile pathway for the production of valuable substituted fluorocyclohexane derivatives from readily available substrates. The use of supercritical carbon dioxide (scCO2) as a hydrophobic and green solvent in combination with Rh nanoparticles immobilized on molecularly modified silica as catalysts favours hydrogenation over hydrodefluorination. The approach compares favourably in terms of green chemistry metrics relative to traditional synthetic routes as exemplified for the useful building block 4-fluorocyclohexan-1-ol.
Introduction
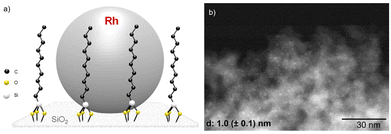
Fluorinated cyclohexanes are of great interest for the chemical industry where they are used as building blocks for the production of various materials,1 agrochemicals,2 and pharmaceuticals.3,4 To access these motifs, the selective hydrogenation of widely accessible fluorinated arenes5 is emerging as an attractive alternative to direct alkane fluorination methods that typically require several steps, the use of hazardous chemicals, and that are poorly atom economical (Fig. 1a).6 The broad application of the hydrogenation strategy relies, however, on the development of catalytic systems capable of hydrogenating fluorinated arenes while limiting the competing and parallel hydrodefluorination, which remains a great challenge.7–11 Recently, several groups, including ours, reported catalytic systems composed of Rh-based molecular complexes12 or nanoparticles13,14 showing good activity and selectivity for the hydrogenation of a range of fluorinated arenes to the corresponding fluorinated cyclohexane derivatives (Fig. 1b). The studies evidenced that the hydrophobicity of the environment of the active metal sites – as controlled by ligands, supports, and the reaction media – was especially important to limit hydrodefluorination. For example, Glorius et al. reported the use of a Rh-carbene pre-catalyst for the selective hydrogenation of various fluorinated arenes to cis-fluorocycloalkanes in presence of SiO2 or molecular sieves with hexane as a non-polar solvent.12,13 Following our organometallic approach to multifunctional catalytic systems,15 we showed that the selectivity of rhodium nanoparticles (NPs) could be significantly improved by immobilizing them on silica with a hydrophobic molecularly modified surface (MMS).14 In particular, materials composed of Rh NPs on SiO2 functionalized with decyl triethoxysilane (Rh@Si-Dec) proved highly effective when used in n-heptane as solvent together with CaO as HF scavenger. A range of fluorinated cyclohexane derivatives was accessed in high selectivity and yields (70–92%), with excellent catalyst stability and recyclability.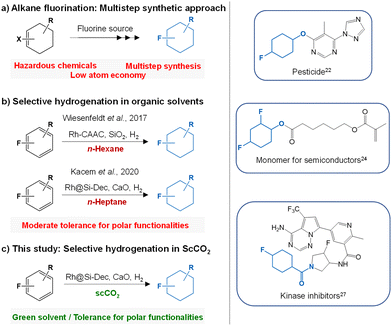 | ||
| Fig. 1 Synthetic strategies for the production of fluorinated cycloalkane derivatives, and examples of applications. | ||
However, despite these promising advances, fluorinated arenes possessing polar functional groups (i.e. –OH, –COOH, –COOR, etc.) proved to be challenging substrates, and the corresponding fluorinated cyclohexane derivatives, while being key building blocks, remained so far inaccessible through this route.
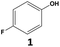
In this context, we report here the use of supercritical carbon dioxide (scCO2) as reaction medium to replace commonly used organic solvents (i.e. hexane, heptane) in the hydrogenation of fluorinated arenes (Fig. 1c). ScCO2 is non-flammable and non-toxic, and has a “generally recognized as safe (GRAS)” status in food processing.16,17 It was selected here for its environmental properties18 as well as for its hydrophobicity and capacity to solubilize fluorinated compounds,19,20 features of great importance for this transformation. Using the selective hydrogenation of 4-fluorophenol to 4-fluorocyclohexan-1-ol as model reaction, the potential of scCO2 as a reaction solvent was explored through a systematic comparison of the hydrogenation performance of Rh@Si-Dec in scCO2 and in heptane. This approach was then extended to a broad range of fluorinated arenes with polar functionalities, providing access to fluorinated cyclohexane derivatives that were so far out of reach through hydrogenation.
Results and discussion
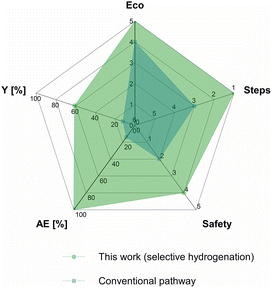
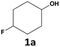
The synthesis of the Rh@Si-Dec (Fig. 2) catalyst was achieved following a previously reported procedure.14 In brief, the preparation of the molecularly functionalized silica supports (Si-Dec) was accomplished through the condensation of commercial decyltriethoxysilanes on dehydroxylated SiO2 (see ESI† for full synthetic and characterization details). The immobilization of Rh NPs on the support was accomplished through the wet impregnation of Si-Dec with a solution of [Rh(allyl)3] followed by reduction of the dried powder under an atmosphere of H2 (50 bar) at 100 °C for 18 h. The resulting Rh@Si-Dec material was characterized by N2 adsorption experiments, giving a BET surface area of 316 m2 g−1, slightly lower than for the starting SiO2 (332 m2 g−1) as expected due to the chemisorption of the decyltriethoxysilane groups. The chemisorption of the decyl chains by silanization was demonstrated by 29Si solid state NMR in a previous study.14 The Rh loading (0.9 wt%) determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) on Rh@Si-Dec was found well in agreement with the theoretical value (1 wt%). Scanning Transmission Electron Microscopy with High Angle Annular Dark Field (STEM-HAADF) evidenced the formation of small and well dispersed nanoparticles with an average size of 1.0 (±0.1) nm (Fig. 2b).The hydrogenation of 4-fluorophenol (1) was selected as model reaction for the catalytic study as this transformation has so far not been successfully achieved, and the corresponding 4-fluorocyclohexan-1-ol (1a) is an important building block for the production of pharmaceuticals,21 agrochemicals22 and functional materials such as resins.23 The catalytic performances of Rh@Si-Dec using heptane or scCO2 as solvents were systematically compared under a standard set of reaction conditions (see details in ESI†). The substrate-to-metal ratio was adjusted to 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and small amounts of CaO were added to trap potentially formed HF. The reactors were pressurized with H2 to 55 bar at room temperature and the reaction mixtures were agitated with a magnetic stir bar at 500 rpm for 1 h at a temperature of 80 °C. Reactions in heptane were performed using 1 mL of solvent in a 10 mL stainless steel autoclave. The experiments using scCO2 were carried out in a 30 mL autoclave at a density of 0.5 g mL−1 as determined by weighing in the corresponding amount of CO2 (Fig. S1†). The solubility of substrate 1 in scCO2 and in the mixture of scCO2 + H2 under the reaction conditions was confirmed by visual inspection using a window-equipped high-pressure reactor (Fig. S2†). No change in the physical state of the catalyst (fine powder) could be observed under supercritical conditions.
1 and small amounts of CaO were added to trap potentially formed HF. The reactors were pressurized with H2 to 55 bar at room temperature and the reaction mixtures were agitated with a magnetic stir bar at 500 rpm for 1 h at a temperature of 80 °C. Reactions in heptane were performed using 1 mL of solvent in a 10 mL stainless steel autoclave. The experiments using scCO2 were carried out in a 30 mL autoclave at a density of 0.5 g mL−1 as determined by weighing in the corresponding amount of CO2 (Fig. S1†). The solubility of substrate 1 in scCO2 and in the mixture of scCO2 + H2 under the reaction conditions was confirmed by visual inspection using a window-equipped high-pressure reactor (Fig. S2†). No change in the physical state of the catalyst (fine powder) could be observed under supercritical conditions.
Under neat conditions in the absence of solvent, 1 was nearly fully converted after one hour, however with a very poor selectivity towards the desired product 1a of only 20% whereas hydrodefluorination to cyclohexanol 1b was the major pathway (78% yield, Table 1, entry 1). Using heptane as a solvent, complete conversion was reached giving a similar product mixture composed of 4-fluorocyclohexan-1-ol (1a, 24%), cyclohexanol (1b, 63%), and cyclohexane (1f, 11%) (Table 1, entry 2). In sharp contrast, the selectivity and yield of 1a reached an average value of 55 ± 7% over a series of 25 experiments (see details in ESI†) in scCO2 indicating that hydrogenation could be favoured over hydrodefluorination in this medium (entry 3). Other products include 1b (27 ± 4%) and the intermediates 4-fluorocyclohexanone (1c, 8 ± 3%) and cyclohexanone (1d, 10 ± 3%). Interestingly, 1c and 1d were not observed in heptane. These results indicate that the use of scCO2 as reaction medium has the potential to improve very significantly the selectivity of the Rh@Si-Dec catalyst for the hydrogenation of challenging fluorinated arenes as compared to non-polar hydrocarbons as solvents.
| # | Solvent | T (°C) | CO2 density (g mL−1) | t (h) | X (%) | Y (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 1d | 1e | 1f | ||||||
| Reaction conditions: Catalyst (5 mg, 0.0005 mmol Rh), 4-fluorophenol (22.4 mg, 0.2 mmol, 400 eq.), n-heptane (750 mg, ≈1 mL) or scCO2 (0.5 g mL−1, ≈12.5 g), CaO (7 mg), 500 rpm.a Time required to reach the indicated temperature. X = conversion, Y = yield, determined by GC-FID using tetradecane as internal standard. | |||||||||||
| 1 | None | 80 | — | 1 | 97 | 19 | 78 | 0 | 0 | 0 | 0 |
| 2 | n-Heptane | 80 | — | 1 | >99 | 24 ± 1 | 63 ± 3 | 0 | 0 | 2 ± 1 | 11 ± 1 |
| 3 | scCO 2 | 80 | 0.5 | 1 | 99 ± 1 | 55 ± 7 | 27 ± 4 | 8 ± 3 | 10 ± 3 | 0 | 0 |
| 4 | scCO2 | 30 | 0.8 | 24 | 22 | 4 | 4 | 4 | 10 | 0 | 0 |
| 5 | scCO2 | 80 | 0.5 | 0.25a | 96 ± 3 | 50 ± 1 | 24 ± 2 | 11 ± 1 | 11 ± 1 | 0 | 0 |
| 6 | scCO2 | 80 | 0.61 | 1 | >99 | 58 ± 3 | 23 ± 1 | 10 ± 1 | 9 ± 2 | 0 | 0 |
| 7 | scCO2 | 80 | 0.75 | 1 | >99 | 61 ± 4 | 22 ± 1 | 10 ± 1 | 5 ± 1 | 0 | 0 |
| 8 | scCO2 | 50 | 0.5 | 1 | 95 ± 5 | 51 ± 2 | 25 ± 2 | 8 ± 1 | 10 ± 2 | 0 | 0 |
| 9 | scCO2 | 120 | 0.5 | 1 | >99 | 58 ± 3 | 34 ± 4 | 4 ± 2 | 9 ± 9 | 0 | 0 |
Low conversion was observed when running the reaction at 30 °C with selectivity for 1a in a similar range as in heptane (Table 1, entry 4). Rapid hydrogenation of 1 was observed at reaction temperatures above the critical temperature of CO2, reaching already 96% conversion during the heating time (15 min) required to reach 80 °C (Table 1, entry 5). The relative selectivity of ca. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 1a over 1b was maintained in this case. Increasing the CO2 density from 0.5 to 0.61 and 0.75 g mL−1 did not affect significantly the conversion nor the selectivity toward 1a (entries 6 and 7). Thus, a CO2 density of 0.5 g mL−1 was selected for further investigations as it allows working under mild total pressure, far away from the technical limitations of the reactor (Tmax = 200 °C, pmax = 400 bar). Varying the reaction temperature around 80 °C (50 °C entry 8, 120 °C entry 9) did not improve the selectivity toward the formation of 1a.
1 for 1a over 1b was maintained in this case. Increasing the CO2 density from 0.5 to 0.61 and 0.75 g mL−1 did not affect significantly the conversion nor the selectivity toward 1a (entries 6 and 7). Thus, a CO2 density of 0.5 g mL−1 was selected for further investigations as it allows working under mild total pressure, far away from the technical limitations of the reactor (Tmax = 200 °C, pmax = 400 bar). Varying the reaction temperature around 80 °C (50 °C entry 8, 120 °C entry 9) did not improve the selectivity toward the formation of 1a.
Furthermore, scCO2 was tested with several other Rh-based catalysts possessing similar Rh NPs size (Fig. S3–S8, Tables S1 and S2†). Typically, conversion and selectivity were lower and only Rh@SiO2 reached similar performance data. In order to maintain the combined effect of reaction medium and surface modification, the Rh@Si-Dec catalyst was chosen for further studies.
Motivated by these promising results, the scope of the reaction was explored by considering various fluorinated arenes bearing different functional groups (acid, ester, alcohol, phenol, ether, and amide). For all substrates, the performances of Rh@Si-Dec in heptane and scCO2 were compared under optimized conditions (Table 2).
| Substrate | Product | scCO2 | Heptane | ||||
|---|---|---|---|---|---|---|---|
| X (%) | S xa (%) | Y xa (%) | X (%) | S xa (%) | Y xa (%) | ||
| Reaction conditions: Rh@Si-Dec (5 mg, 0.0005 mmol Rh), substrate (0.2 mmol), scCO2 (0.5 g mL−1, ≈12.5 g), CaO (7 mg for monofluoroalkene, 14 mg for difluoroalkene and 21 mg for trifluoroalkene), 55 bar H2, 80 °C, 1 h, 500 rpm.a 20 mg Rh@Si-Dec.b 120 °C.c Substrate (0.1 mmol).d 10 mg Rh@Si-Dec.e 2 h. | |||||||
|
|
>99 | 55 | 55 | >99 | 24 | 24 |
|
|
89 | 78 | 69 | 98 | 11 | 11 |
|
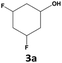
|
75 | 55 | 41 | 76 | 7 | 5 |
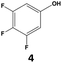
|
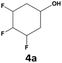
|
70 | 28 | 20 | 99 | 2 | 2 |
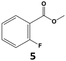
|
|
97 | 80 | 78 | >99 | 78 | 78 |
|
|
96 | 90 | 86 | 95 | 83 | 79 |
|
|
88 | 64 | 56 | 96 | 36 | 34 |
|
|
72 | 76 | 55 | >99 | 35 | 35 |
|
|
>99 | 80 | 80 | >99 | 75 | 75 |
|
|
>99 | 79 | 79 | >99 | 83 | 83 |
|
|
82 | 84 | 69 | >99 | 77 | 77 |
|
|
99 | 47 | 47 | >99 | 42 | 42 |
|
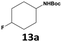
|
>99 | 72 | 72 | >99 | 70 | 70 |
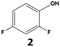
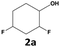
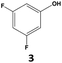
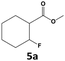
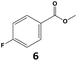
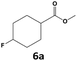
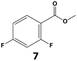
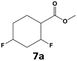
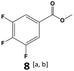
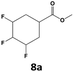
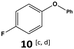
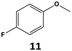
In all cases, the selectivity and yield of fluorocyclohexane derivatives were at least as good, and often much better when using scCO2 than when using heptane as solvent. The selectivity improvement in scCO2 was particularly striking for multi-fluorinated substrates (2, 3, 4, 7, and 8), providing products 2a, 3a, 4a, 7a and 8a in 20–78% yield (28–80% selectivity) against 2–35% yield (2–36% selectivity) in heptane. Interestingly, 2a and 4a can be used directly as building blocks for the synthesis of fluorine-containing polymers24 and liquid crystal materials.25 Methyl 2- and 4-fluorocylohexane-1-carboxylate (5a, 6a) were obtained in excellent yields from 5 and 6 in both reaction media, but with still slightly better selectivity in scCO2. With increasing numbers of fluorine substituents (7 and 8), the benefits of using scCO2 became more obvious in line with the known compatibility of fluorinated compounds and scCO2. 7a and 8a are key intermediates for the synthesis of bioactive molecules,26,27 and were produced in 56% and 55% yield under these conditions, respectively.
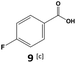
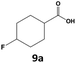
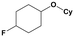
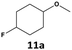
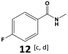
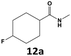
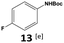
Furthermore, monofluorinated cyclohexanes with acid (9a), phenol (10a), ether (11a), amide (12a) and NBoc-protected amine (13a) substitutes were obtained in good yields using both solvents, with a slightly better selectivity in scCO2. To the best of our knowledge, this is the first report for the synthesis of compounds 3a, 7a, 8a, 10a and 12a by hydrogenation of the corresponding fluorinated arenes. The structures of these products were validated by GC-MS and NMR analysis (see ESI† for details).
To stress the significance of this work, a conventional multistep preparation method of 4-fluorocyclohexan-1-ol (Fig. S9†) via fluorination was systematically compared to the selective hydrogenation of readily available and cheap 4-fluorophenol to fluorocyclohexan-1-ol (Fig. S10†). For the comparison, five parameters based on the green chemistry principles28 were considered, i.e. the number of steps (Steps), the atom economy (AE), the overall reaction yield (Y), the hazardous nature of the reagents (Safety) and the economical aspect (Eco) (detailed description is provided in the ESI: Fig. S9–S10, Table S3†). The spider web-type graph in Fig. 3 shows that all the parameters are clearly in favour of the hydrogenation route proposed here, in particular considering atom economy, safety, and yield.
Conclusion
The reactivity of a molecularly modified Rh@Si-Dec catalyst was explored for the selective hydrogenation of fluorinated arenes using scCO2 as a green solvent, and compared to the use of n-heptane. The use of scCO2 generally favoured hydrogenation over hydrodefluorination, thus improving very significantly the selectivity of the reaction in a number of synthetically important applications. This improvement was particularly striking for substrates bearing polar functionalities (e.g. 4-fluorophenol) as well as multiple fluorine substituents (e.g. difluorophenol, difluorophenylacetate, etc.). As a result, a wide range of fluorinated arenes could be hydrogenated using Rh@Si-Dec in scCO2, giving highly valuable fluorocyclohexane derivatives in moderate to high yields in one step. This strategy was shown to be potentially more efficient and sustainable than conventional multistep fluorination approaches, paving the way towards the production of valuable fluorinated building blocks directly via catalytic hydrogenation of widely available fluorinated arenes.Author contributions
S. Kacem, A. Bordet and W. Leitner proposed the project. N. Theyssen, A. Bordet and W. Leitner co-supervised the project. S. Kacem prepared the catalytic material and performed the activity tests in heptane, while Y. X. Qiao performed the experiments in supercritical CO2. C. Wirtz performed all the NMR measurements and made full analysis. All authors discussed the results and edited the manuscript before submission.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support by the Max Planck Society and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – Exzellenzcluster 2186 “The Fuel Science Center” ID: 390919832. Furthermore, the authors thank Savarithai Jenani Louis Anandaraj and Julia Zerbe for their support with the experiments, Alina Jakubowski, Annika Gurowski, Justus Werkmeister, Natalia Kowalew, Norbert Pfänder, (MPI-CEC, Mülheim/Ruhr), Adrian Schlüter, Dr Christophe Farès (MPI-Kofo, Mülheim/Ruhr) for their support with the analytics. Open Access funding provided by the Max Planck Society.References
- M. Hird, Chem. Soc. Rev., 2007, 36, 2070–2095 RSC.
- T. Fujiwara and D. O'Hagan, J. Fluor. Chem., 2014, 167, 16–29 CrossRef CAS.
- J. Wang, M. Sanchez-Rosello, J. L. Acena, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- G. Backfisch, M. Bakker, G. Blaich, W. Braje, K. Drescher, T. Erhard, A. Haupt, C. Hoft, A. Kling, V. Lakics, H. Mack, F. Oellien, R. Peter, F. Pohlki and A. L. Relo, US20190062305A1, 2019.
- D. E. Yerien, S. Bonesi and A. Postigo, Org. Biomol. Chem., 2016, 14, 8398–8427 RSC.
- R. D. Chambers, Fluorine in Organic Chemistry, Blackwell Publishing Ltd, Oxford, 2004 Search PubMed.
- H. Yang, H. Gao and R. J. Angelici, Organometallics, 1999, 18, 2285–2287 CrossRef CAS.
- G. Haufe, S. Pietz, D. Wölker and R. Fröhlich, Eur. J. Org. Chem., 2003, 2166–2175 CrossRef CAS.
- K. J. Stanger and R. J. Angelici, J. Mol. Catal. A: Chem., 2004, 207, 59–68 CrossRef CAS.
- M. P. Wiesenfeldt, Z. Nairoukh, T. Dalton and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 10460–10476 CrossRef PubMed.
- T. Charvillat, P. Bernardelli, M. Daumas, X. Pannecoucke, V. Ferey and T. Besset, Chem. Soc. Rev., 2021, 50, 8178–8192 RSC.
- M. P. Wiesenfeldt, Z. Nairoukh, W. Li and F. Glorius, Science, 2017, 357, 908–912 CrossRef CAS PubMed.
- D. Moock, M. P. Wiesenfeldt, M. Freitag, S. Muratsugu, S. Ikemoto, R. Knitsch, J. Schneidewind, W. Baumann, A. H. Schäfer, A. Timmer, M. Tada, M. R. Hansen and F. Glorius, ACS Catal., 2020, 10, 6309–6317 CrossRef CAS PubMed.
- S. Kacem, M. Emondts, A. Bordet and W. Leitner, Catal. Sci. Technol., 2020, 10, 8120–8126 RSC.
- A. Bordet and W. Leitner, Acc. Chem. Res., 2021, 54, 2144–2157 CrossRef CAS PubMed.
- J. R. Hyde, P. Licence, D. Carter and M. Poliakoff, Appl. Catal., A, 2001, 222, 119–131 CrossRef CAS.
- P. Licence, J. Ke, M. Sokolova, S. K. Ross and M. Poliakoff, Green Chem., 2003, 5, 99–104 RSC.
- W. Leitner, Acc. Chem. Res., 2002, 35, 746–756 CrossRef CAS PubMed.
- D. A. Newman, T. A. Hoefling, R. R. Beitle, E. J. Beckman and R. M. Enick, J. Supercrit. Fluids, 1993, 6, 205–210 CrossRef CAS.
- P. G. Jessop and W. Leitner, Chemical Synthesis Using Supercritical Fluids, Wiley-VCH, 1999 Search PubMed.
- C. Qi, H. Tsui, Q. Zeng, Z. Yang and X. Zhang, WO2020/238900A1, 2020.
- Y. Nokura, H. Ikegami, H. Tomioka and D. Takaoka, WO2012/050237A1, 2012.
- T. Masuyama, S. Yamamoto and K. Ichikawa, JP2020015900A, 2020 CAS.
- T. Arai and T. Itokazu, JP2009197184A, 2009.
- D. Xue, C. Dong and G. Renwei, CN103627405, 2012.
- R. Shimono, D. Matsuda, S. Masuda and S. Yamamoto, JP2018199623A, 2018.
- S. H. Watterson, M. Andappan Murugaiah Subbaiah, C. D. Dzierba, H. Gong, J. M. Guernon, J. Guo, A. C. Hart, G. Luo, J. E. Macor, W. J. Pitts, J. Shi, B. L. Venables, C. A. Weigelt, Y.-J. Wu, Z. B. Zheng, S.-Y. Sit and J. Chen, WO2019US14918, 2019.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc02623f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |