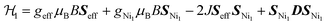Open Access Article
Open Access ArticleStrong magnetic exchange coupling in a radical-bridged trinuclear nickel complex†
Luming
Yang
,
Julius J.
Oppenheim
 and
Mircea
Dincă
and
Mircea
Dincă
 *
*
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: Mdinca@mit.edu
First published on 18th May 2022
Abstract
Reaction of 2,3,6,7,10,11-hexaaminotriphenylene hexahydrochloride (HATP·6HCl) and (TpPhNi)Cl (TpPh = tris(3,5-diphenyl-1-pyrazolyl)borate) produces the radical-bridged trinickel complex [(TpPhNi)3(HITP)] (HITP3−˙ = 2,3,6,7,10,11-hexaiminotriphenylene). Magnetic measurements and broken-symmetry density functional theory calculations reveal strong exchange coupling persisting at room temperature between HITP3−˙ and two of the three Ni2+ centers, a rare example of strong radical-mediated magnetic coupling in multimetallic complexes. These results demonstrate the potential of radical-bearing tritopic HITP ligands as building blocks for extended molecule-based magnetic materials.
Introduction
Radical-mediated coupling of paramagnetic spin centers is an effective strategy for developing molecule-based magnetic materials, including high-temperature multifunctional magnets,1–3 switchable porous magnetic sensors,4,5 and low-dimensional compounds with slow relaxation dynamics,6,7 because coupling in such compounds can give rise to strong and long-range magnetic interactions through the direct exchange mechanisms.3,8 To date, many ditopic radical-containing ligand bridges have been reported to mediate strong coupling persistent at room-temperature in transition-metal complexes.9–13 In comparison, trimetallic complexes with tritopic radical bridges, a common topological component for solid-state magnets as well as high-spin-state molecular magnets, are rare and often experience weaker coupling.3,6,14–16 One promising candidate for a tritopic radical bridge that could mediate strong coupling through π-type delocalized radicals is the trianionic 2,3,6,7,10,11-hexaiminotriphenylene (HITP3−˙),17 a tritopic congener of the radical anion o-benzosemiquinone diimine (BQDI) that is known for strong magnetic exchange coupling with transition metal centers.6Here, we report the synthesis of [(TpPhNi)3(HITP3−˙)], 1 (TpPh = tris(3,5-diphenyl-1-pyrazolyl)borate), a trimetallic cluster containing Ni2+ centers and an HITP-centered radical. Notably, magnetic measurements reveal strong Ni–HITP coupling between HITP3−˙ and two Ni2+ spins that persists at room temperature. This interaction is much stronger than the previously reported metal–ligand spin coupling in the oxygen analogs, [(TpPhNi)3(HOTP3−˙)] (2)18 and [(Me3TPANi)3(HOTP3−˙)] (3)19 (Me3TPA = N,N,N-tris[(6-methyl-2-pyridyl)methyl]amine), and is attributed to strong orbital delocalization between Ni2+ and the nitrogen atoms on HITP. These results provide inspiration for the rational design of new room-temperature magnetic materials.
Results and discussion
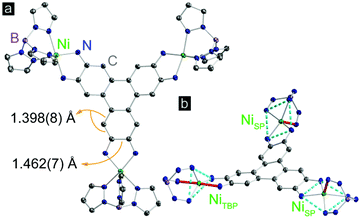
Complex 1 was synthesized by deprotonation of 2,3,6,7,10,11-hexaaminotriphenylene hexahydrochloride with tetrabutylammonium hydroxide under an atmosphere of N2, followed by reaction with (TpPhNi)Cl in air in a dichloromethane–methanol mixture. Dark purple crystals were obtained in 45% yield by layering n-hexane onto a solution of 1 in dichloromethane (Scheme S1†). High resolution electrospray ionization/mass spectrometry (ESI/MS) revealed an M+ peak at m/z = 2496.8, which together with microelemental analysis confirmed the identity and purity of 1 (Fig. S1†).Compound 1 crystallizes in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, and displays three crystallographically independent Ni atoms. Two of the Ni ions exhibit square pyramidal (SP) geometry, and the third one has trigonal bipyramidal geometry (TBP) (Fig. 1). All Ni centers experience slight distortion from the respective ideal geometries, reflected by the non-zero continuous-shape measurement parameters (Tables S5–S7† and related discussion).20 The bulky κ3-chelating TpPh capping ligands magnetically isolate individual Ni3HITP units from neighboring molecules. The average Ni–NTp bond length, 2.063(6) Å, is in line with the values reported for high-spin Ni2+ complexes capped by Tp derivatives.21 The average length of the C–C bonds forming Ni–N–C–C–N metallocycles with each phenylenediamine arm within HITP is 1.462(7) Å, much longer than the adjacent C–C bond within the same aromatic ring, 1.398(8) Å (Fig. 1). This reflects a significant distortion of each phenylenediamine ring and evidences the “bisallyl”-type configuration resulting from the oxidation of a fully deprotonated HITP6− moiety to HITP3−˙ (Scheme S2†).22 For metal–diamine complexes, the degree of oxidation on the diamine fragment is also reflected in the C–N bond lengths.22 In 1, the average C–NHITP bond length, 1.322(6) Å, lies between that of trimetallic complexes with the fully-reduced HITP6− (1.39–1.40 Å) and the more oxidized HITP2− (1.305(6) Å), as may be expected for an intermediate oxidation in HITP3−˙.23,24 Finally, although the Ni–N–C–C–N metallocycles are nearly planar, the triphenylene core of HITP3−˙ experiences significant distortion from planarity, with dihedral angles of 20.9°, 26.7°, and 40.1° between pairs of planes defined by the three Ni–N–C–C–N metallocycles (Fig. S2†). Such distortions are not uncommon in large aromatic systems and have been assigned to Jahn–Teller and crystal packing effects.25
space group, and displays three crystallographically independent Ni atoms. Two of the Ni ions exhibit square pyramidal (SP) geometry, and the third one has trigonal bipyramidal geometry (TBP) (Fig. 1). All Ni centers experience slight distortion from the respective ideal geometries, reflected by the non-zero continuous-shape measurement parameters (Tables S5–S7† and related discussion).20 The bulky κ3-chelating TpPh capping ligands magnetically isolate individual Ni3HITP units from neighboring molecules. The average Ni–NTp bond length, 2.063(6) Å, is in line with the values reported for high-spin Ni2+ complexes capped by Tp derivatives.21 The average length of the C–C bonds forming Ni–N–C–C–N metallocycles with each phenylenediamine arm within HITP is 1.462(7) Å, much longer than the adjacent C–C bond within the same aromatic ring, 1.398(8) Å (Fig. 1). This reflects a significant distortion of each phenylenediamine ring and evidences the “bisallyl”-type configuration resulting from the oxidation of a fully deprotonated HITP6− moiety to HITP3−˙ (Scheme S2†).22 For metal–diamine complexes, the degree of oxidation on the diamine fragment is also reflected in the C–N bond lengths.22 In 1, the average C–NHITP bond length, 1.322(6) Å, lies between that of trimetallic complexes with the fully-reduced HITP6− (1.39–1.40 Å) and the more oxidized HITP2− (1.305(6) Å), as may be expected for an intermediate oxidation in HITP3−˙.23,24 Finally, although the Ni–N–C–C–N metallocycles are nearly planar, the triphenylene core of HITP3−˙ experiences significant distortion from planarity, with dihedral angles of 20.9°, 26.7°, and 40.1° between pairs of planes defined by the three Ni–N–C–C–N metallocycles (Fig. S2†). Such distortions are not uncommon in large aromatic systems and have been assigned to Jahn–Teller and crystal packing effects.25
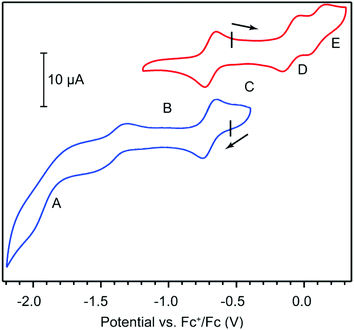
Cyclic voltammetry (CV) provided further evidence in assigning the formal oxidation state of HITP and offered clues on the electronic delocalization in 1. CV measurements in 0.15 M solution of tetrabutylammonium hexafluorophosphate (TBAPF6) in tetrahydrofuran (THF) under a N2 atmosphere reveal quasi-reversible electron transfer events centered at −1.41, −0.69, −0.10, and 0.10 V with respect to the ferrocenium/ferrocene couple (Fc+/Fc, Fig. 2 and Table S1†). These values are comparable to ligand-centered redox events for other trimetallic HXTP compounds (X = I, O), and are therefore assigned to the HITP4−/5−, HITP3−/4−, HITP2−/3−, and HITP−/2− redox couples.18,24,26 In the context of classical mixed-valence theory, which relates electrochemical redox potentials to the degree of electronic delocalization, the potential difference between HITP4−/5− and HITP3−/4− corresponds to a comproportionation constant (Kc) of 1012.2.27 This large value indicates full delocalization among the three phenylenediamine/BQDI arms of the HITP ligand. Additionally, Kc values of 1010.1 and 103.2 between the HITP3−/4−/HITP2−/3− and HITP2−/3−/HITP−/2− redox couples evidence less delocalization for the more oxidized HITP forms, suggesting that the middle oxidation state, HITP3−˙, is the more intriguing candidate for exploring magnetic interactions mediated by HITP.
Electron paramagnetic resonance (EPR) spectroscopy provided critical evidence for assigning the spin states in 1. X-band measurement of a frozen glass of 1 in toluene in the range 5–160 K reveals an axial pattern typical for an S = 1/2 spin with g∼2.26, suggesting a thermally-isolated spin state with significant Ni2+ character (Fig. S3a†). The absence of signals with g ≥ 4 suggests the absence of S > 1/2 excited states. Because Ni2+ possess integer spins (either S = 1 or 0), the observation of a half-integer spin state in 1 suggests the presence of a HITP-centered monoradical.
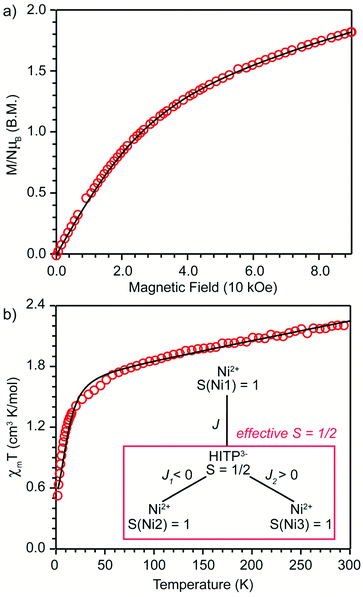
An estimation of the strength of magnetic coupling within 1 came from magnetometry. Variable-field magnetization measurements at 1.8 K reveal gradual increase of the magnetization to 1.83μB upon increasing the field to 90 kOe (Fig. 3a). This magnetization value is much higher than the value expected for an S = 1/2 spin (1.0μB with g = 2.0). The absence of a saturation plateau at high magnetic field or an inflection point are often associated with the presence of zero-field splitting. The temperature-dependent susceptibility data, χMT, at 1 and 10 kOe are superimposable (Fig. S4†), confirming the absence of ferromagnetic impurities. They reveal a gradual decrease from 300 K to 30 K, followed by a steeper drop down to 1.8 K, often also associated with zero-field splitting (Fig. 3b). The χMT value of 0.52 cm3 K mol−1 at 1.8 K is close to the value expected for an S = 1/2 spin with g = 2.26 (0.48 cm3 K mol−1), corroborating the ground state spin assignment based on EPR. Meanwhile, a χMT value of 2.21 cm3 K mol−1 at 300 K is much smaller than the value expected for three uncoupled S = 1 Ni2+ centers and an S = 1/2 ligand (3.375 cm3 K mol−1 with g = 2.0). Instead, it is closer to the value of 1.76 cm3 K mol−1 expected for a two-spin system comprising an S = 1 spin center and an S = 1/2 center with g = 2.26. In 1, such a two-spin system could arise if we consider strong magnetic exchanges between two of the three Ni2+ centers and the HITP-centered radical (denoted as Ni–HITP–Ni), together forming a single S = 1/2 effective spin center, which itself then couples to the third Ni2+ center with S = 1. With this proposed effective two-spin model, the magnetic data for 1 can be fitted to the following Hamiltonian:
 (Fig. 3b inset). Although the weak temperature dependence of χMT above 30 K precludes accurate determination of J1 and J2, lower bounds can be estimated under the assumption |J1| = |J2|, suggesting −J1 = J2 ≥ 1300 cm−1 (Fig. S6†). We note that these values should only serve as estimates, because the slope in the high-temperature region of χMT is sensitive to subtle changes in diamagnetic corrections.11 Nevertheless, the large estimates for J1,2 confirm that the tritopic radical bridge enables strong magnetic exchange interactions that persist at room temperature. Alternative spin models did not provide satisfactory fits (see discussion on page 12 in the ESI†).
(Fig. 3b inset). Although the weak temperature dependence of χMT above 30 K precludes accurate determination of J1 and J2, lower bounds can be estimated under the assumption |J1| = |J2|, suggesting −J1 = J2 ≥ 1300 cm−1 (Fig. S6†). We note that these values should only serve as estimates, because the slope in the high-temperature region of χMT is sensitive to subtle changes in diamagnetic corrections.11 Nevertheless, the large estimates for J1,2 confirm that the tritopic radical bridge enables strong magnetic exchange interactions that persist at room temperature. Alternative spin models did not provide satisfactory fits (see discussion on page 12 in the ESI†).
BS-DFT analysis provided additional insight into the magnetic interactions in 1. Electronic and spin ground states of 1 were evaluated by comparing the single-point energy of the states with S = 1 (termed “BS state”) or S = 0 (termed “diamagnetic-Ni state”) for the two Ni atoms in the Ni–HITP–Ni moiety (Fig. 3b and S7a†). The calculation revealed the BS state lying 1524.8 cm−1 below the diamagnetic-Ni state, supporting the assignment of all Ni2+ having S = 1 spin state in 1 (Fig. 4 and S7b†). Moreover, Löwdin spin density analysis for the BS state showed dominant positive spin density on two of the three Ni atoms, and negative density on HITP and the third Ni, contrasting the diamagnetic-Ni state (Tables S2 and S3†). Two antiferromagnetic and one ferromagnetic coupling pathways exist between HITP3−˙ and the three Ni2+ spins, in excellent agreement with our interpretation based on magnetometry and EPR. The HITP-centered delocalized spin is further strongly polarized by the Ni spins, an effect commonly observed for π-ligand bridges and radicals.19,28 The larger asymmetry in the metal–ligand exchange coupling constants in 1 compared with 2 and 3 is likely related to stronger geometric distortion, which results from the more diffuse orbital of the bridgehead atoms and stronger ligand–metal covalent interaction for the nitrogen-based HITP.6 Additionally, the BS-DFT analysis together with crystallographic and magnetic characterizations allow tentative assignment of the Ni identity. Ni1 (Fig. 3 and 4) can be assigned as the crystallographic site Ni3A (Fig. S8†) due to its unique TBP geometry. The weaker exchange coupling with HITP3−˙ is likely due to poorer orbital overlap resulted from the larger geometric distortion from an ideal geometry (Table S7†). Because no alternative BS state (for instance J1 > 0 and J2 < 0) could be converged, the two Ni with TBP geometry, Ni2 and Ni3, can be assigned as the crystallographic sites of Ni2A and Ni1A, respectively. This reflects the influence of global symmetry, beside local symmetry, on the nature of exchange coupling between the metal and the radical bridge, as can be observed in the structurally related compounds 2 and 3.18,19
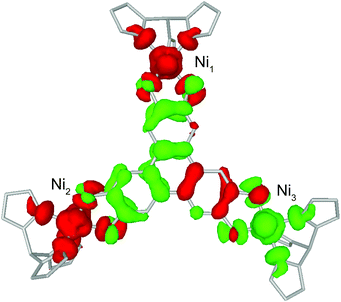 | ||
| Fig. 4 Calculated spin density isosurfaces (0.0015 a.u.) in the broken-symmetry state of 1. Red and green represent positive and negative spin densities. Labels for Ni are consistent with Fig. 3. | ||
Conclusions
Altogether, crystallographic, electrochemical, magnetic, and computation analyses demonstrate that an HITP radical bridge enables strong magnetic coupling, exemplified here in a trinickel complex. The two Ni–HITP exchange interactions are rare examples of radical-mediated coupling persistent at room temperature in complexes with a tritopic radical bridge, and are comparable to the metal–radical coupling in well-known dinickel bis(BQDI) complexes.29 These results also confirm that radical-mediated coupling with amine-based HITP in 1 is much stronger than with catechol-based HOTP in 2 and 3, and stronger too than with closed-shell HITP tritopic bridges.18,19,24,26 The enhanced coupling for nitrogenous ligands is in line with previous observations in diradical-bridged complexes,30 and is likely due to the more diffuse nitrogen orbitals of the radical bridge and better orbital energy match with the metal centers. These results provide motivation for using HITP3−˙ as a particularly attractive bridging ligand for other magnetic molecules and solids, where uniform strong coupling may be achieved through supramolecular design or crystal packing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Prof. T. D. Harris for valuable discussions. This research was funded by the National Science Foundation (Waterman Award to MD; DMR-1645232). We thank the Massachusetts Green High-Performance Computer C3DDB for providing computing resources.Notes and references
- P. Dechambenoit and J. R. Long, Chem. Soc. Rev., 2011, 40, 3249 RSC.
- G. Mínguez Espallargas and E. Coronado, Chem. Soc. Rev., 2018, 47, 533–557 RSC.
- A. E. Thorarinsdottir and T. D. Harris, Chem. Rev., 2020, 120, 8716–8789 CrossRef CAS PubMed.
- N. Motokawa, S. Matsunaga, S. Takaishi, H. Miyasaka, M. Yamashita and K. R. Dunbar, J. Am. Chem. Soc., 2010, 132, 11943–11951 CrossRef CAS PubMed.
- J. Zhang, W. Kosaka, K. Sugimoto and H. Miyasaka, J. Am. Chem. Soc., 2018, 140, 5644–5652 CrossRef CAS PubMed.
- S. Demir, I. R. Jeon, J. R. Long and T. D. Harris, Coord. Chem. Rev., 2015, 289–290, 149–176 CrossRef CAS.
- M. Atzori and R. Sessoli, J. Am. Chem. Soc., 2019, 141, 11339–11352 CrossRef CAS PubMed.
- A. Caneschi, D. Gatteschi, R. Sessoli and P. Rey, Acc. Chem. Res., 1989, 22, 392–398 CrossRef CAS.
- C. Benelli, A. Dei, D. Gatteschi and L. Pardi, Inorg. Chem., 1988, 27, 2831–2836 CrossRef CAS.
- V. I. Ovcharenko, E. V. Gorelik, S. V. Fokin, G. V. Romanenko, V. N. Ikorskii, A. V. Krashilina, V. K. Cherkasov and G. A. Abakumov, J. Am. Chem. Soc., 2007, 129, 10512–10521 CrossRef CAS PubMed.
- I.-R. Jeon, J. G. Park, D. J. Xiao and T. D. Harris, J. Am. Chem. Soc., 2013, 135, 16845–16848 CrossRef CAS PubMed.
- K. Chłopek, E. Bothe, F. Neese, T. Weyhermüller and K. Wieghardt, Inorg. Chem., 2006, 45, 6298–6307 CrossRef PubMed.
- N. Mavragani, D. Errulat, D. A. Gálico, A. A. Kitos, A. Mansikkamäki and M. Murugesu, Angew. Chem., Int. Ed., 2021 DOI:10.1002/anie.202110813.
- C. A. Gould, L. E. Darago, M. I. Gonzalez, S. Demir and J. R. Long, Angew. Chem., Int. Ed., 2017, 56, 10103–10107 CrossRef CAS PubMed.
- A. Datcu, N. Roques, V. Jubera, D. Maspoch, X. Fontrodona, K. Wurst, I. Imaz, G. Mouchaham, J.-P. Sutter, C. Rovira and J. Veciana, Chem. – Eur. J., 2012, 18, 152–162 CrossRef CAS PubMed.
- J. O. Moilanen, N. F. Chilton, B. M. Day, T. Pugh and R. A. Layfield, Angew. Chem., Int. Ed., 2016, 55, 5521–5525 CrossRef CAS PubMed.
- R. Breslow, B. Jaun, R. Q. Kluttz and C. Xia, Tetrahedron, 1982, 38, 863–867 CrossRef CAS.
- Y. Wang, F. Lambert, E. Rivière, R. Guillot, C. Herrero, A. Tissot, Z. Halime and T. Mallah, Chem. Commun., 2019, 55, 12336–12339 RSC.
- L. Yang and M. Dincă, Angew. Chem., Int. Ed., 2021, 60, 23784–23789 CrossRef CAS PubMed.
- J. Cirera, E. Ruiz and S. Alvarez, Organometallics, 2005, 24, 1556–1562 CrossRef CAS.
- D. J. Harding, P. Harding, H. Adams and T. Tuntulani, Inorg. Chim. Acta, 2007, 360, 3335–3340 CrossRef CAS.
- S. Pascal and O. Siri, Coord. Chem. Rev., 2017, 350, 178–195 CrossRef CAS.
- F. Zhong, X. Yang, L. Shen, Y. Zhao, H. Ma, B. Wu and X. J. Yang, Inorg. Chem., 2016, 55, 9112–9120 CrossRef CAS PubMed.
- L. Yang, X. He and M. Dincǎ, J. Am. Chem. Soc., 2019, 141, 10475–10480 CrossRef CAS PubMed.
- T. Kato and T. Yamabe, Chem. Phys. Lett., 2005, 403, 113–118 CrossRef CAS.
- N. Hoshino and T. Akutagawa, Chem. – Eur. J., 2018, 24, 19323–19331 CrossRef CAS PubMed.
- D. E. Richardson and H. Taube, Coord. Chem. Rev., 1984, 60, 107–129 CrossRef CAS.
- T. Glaser, M. Heidemeier, J. B. H. Strautmann, H. Bögge, A. Stammler, E. Krickemeyer, R. Huenerbein, S. Grimme, E. Bothe and E. Bill, Chem. – Eur. J., 2007, 13, 9191–9206 CrossRef CAS PubMed.
- K. Chłopek, E. Bothe, F. Neese, T. Weyhermuller and K. Wieghardt, Inorg. Chem., 2006, 45, 6298–6307 CrossRef PubMed.
- D. Schweinfurth, M. M. Khusniyarov, D. Bubrin, S. Hohloch, C.-Y. Su and B. Sarkar, Inorg. Chem., 2013, 52, 10332–10339 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2109142. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt01337a |
| This journal is © The Royal Society of Chemistry 2022 |