Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A synopsis of progressive transition in precursor inks development for metal halide perovskites-based photovoltaic technology
Cuc Mai
Thi Kim
a,
Lahoucine
Atourki
b,
Mouad
Ouafi
cd and
Syed Ghufran
Hashmi
 *a
*a
aMicroelectronics Research Unit, Faculty of Information Technology & Electrical Engineering, University of Oulu, P. O. Box 8000, FI-90014, Finland. E-mail: ghufran.hashmi@oulu.fi
bMANAPSE Lab, Faculty of Science, Mohammed V University in Rabat, Morocco
cCBS, Mohammed VI Polytechnic University, Ben Guerir, Morocco
dLMER, Faculty of Science, Ibn Zohr University, Agadir, Morocco
First published on 20th September 2021
Abstract
Perovskite solar cell (PSC) technology has received considerable attention due to the rapid escalation of their solar-to-electrical energy conversion, which has recently surpassed 25% for lab-sized solar cells. Other benefits such as their fabrication through solution processing enable new opportunities for scaling up and rapid production. These features may play a key role in realizing quick installations worldwide, helping to meet the global energy production and consumption demand with a realistic energy pay-back time. This report provides an overview of the progress in developing liquid precursor inks for producing a variety of organic–inorganic halide perovskite-based light absorbing layers. In recent years, a variety of configurations for PSC technology have been reported, where intelligent inks of perovskite precursors have been formulated to facilitate novel designs with impressive solar-to-electrical energy conversions and promising stability. This report highlights the evolution of these novel perovskite precursor ink formulations, and discusses the emerging trends in developing efficient, scalable, and robust PSC technology. Moreover, the classification, advantages, and limitations of various types of perovskite precursor ink are addressed. Specifically, single- and multi-cation-based ink formulations are discussed in relation to their impact on producing efficient solar cells, which provides an overview of the recent progress in the development of this emerging and low-cost solar cell technology. Overall, this synopsis provides the current state of the art in designing novel perovskite precursor inks to be used in producing high performance, efficient, scalable, and stable configurations of perovskite solar cell technology.
1. Introduction
The growing population and resulting human activity continue to increase energy consumption, imposing new challenges for energy supply and production. Solutions must meet sustainable production criteria, especially under the current climate change scenarios.1,2 These stringent conditions have become a source of intense motivation for researchers worldwide to develop clean and alternative energy production schemes focused on reducing greenhouse gas effects and environmental pollution.3,4Among the various renewable energy sources, photovoltaics has been considered as a potential source for providing clean, silent, and affordable energy with high solar-to-electrical energy conversion.5–7 Despite the increase in installations of dominant Si-based photovoltaic systems, these have various limitations such as saturated performance for bulk outdoor electricity production, limited aesthetics for modern architecture-based building integrated photovoltaic (BIPV) applications, and non-eco-friendly and high energy consumption-based production methods. Moreover, they display limited performance under low light intensity conditions; efficient conversion of electrical energy under simulated light is needed to energize maintenance-free internet of things (IoT) devices and portable electronics.8–11
In this regard, third generation-based photovoltaic (PV) technologies such as dye-sensitized solar cells (DSSCs), organic solar cells (OSCs) and perovskite solar cells (PSCs) offer numerous advantages over Si-based PV systems.9–11 For example, they provide flexibility in designing solar cells and modules in a variety of sizes, their fundamental materials are abundantly available, and they are fabricated with non-vacuum-based scalable production methods such as established slot-die coating, screen printing or inkjet printing-based material deposition technologies.12,13 Such unique features make these next generation-based PV technologies a potential source for energizing modern electronics and utilizing their integration in the next generation of BIPV products. They also provide the possibility to produce bulk electricity with much higher solar-to-electrical energy conversion efficiencies compared to stand-alone Si-based PV systems, for example when smartly used in tandem configurations or installed outdoors under natural climatic conditions.14–16
Among these next generation-based PV technologies, PSCs have received considerable attention due to the rapid escalation of their solar-to-electrical energy conversion, which has recently surpassed 25%.17 In addition to these reported improvements in conversion efficiencies, other benefits such as their fabrication through solution processing enable new opportunities for scaling up and rapid production.18 Importantly, this may play a key role in realizing quick installations worldwide, thus providing a realistic way of meeting the global energy production and consumption demand.19,20
This report provides an overview of the progress in developing liquid precursor inks for producing a variety of organic–inorganic halide perovskite-based light absorbing layers in emerging PSC technology. Many configurations for perovskite solar cell technology have been reported in recent years. Specifically, intelligent inks of perovskite precursors have been formulated to facilitate novel designs with impressive solar-to-electrical energy conversions and promising stability.21–24 This report highlights the evolution of these novel perovskite precursor ink formulations and discusses the emerging trends towards developing efficient and robust PSC technology. In addition, the classification, advantages, and limitations of various types of perovskite precursor ink are addressed. Single- and multi-cation-based ink formulations are discussed in light of their impact on producing efficient solar cells. Progress in developing lead (Pb)-free precursor inks is also reviewed, as well as the scalable precursors that have been identified for producing large-area solar modules in a variety of configurations with popular scalable fabrication methods. Overall, this review provides the most up-to-date advancements being made in designing novel perovskite precursor inks for producing high performance, efficient, scalable, and stable configurations of perovskite solar cell technology.
2. Role of precursor inks in establishing the current state of the art for conversion efficiencies of PSC technology
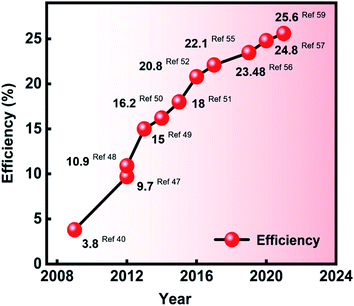
The progressive advances in PSC solar-to-electrical conversion efficiencies (Fig. 1) may be attributed to many factors, for example, the known knowledge of design principles for producing solid state DSSCs (ssDSSCs),25,26 optimizing the active layer thicknesses for improving the diffusion lengths of carriers,27–29 or even proposing intelligent strategies for minimizing both the radiative and non-radiative losses observed in device architectures.30–35The advances in designing novel perovskite precursor inks36–39 have played a key role in addressing the aforementioned key issues, thus leading to extraordinary improvements in solar-to-electrical energy conversions in recent years (Fig. 1).
The striking discovery of potential applications of perovskite-based light harvesters in photovoltaics was preliminarily reported by formulating two types of precursor ink.40 Briefly, CH3NH3Br and PbBr2 were dissolved in N,N-dimethylformamide (DMF) solvent, while CH3NH3I and PbI2 were mixed in γ-butyrolactone (GBL) as a high viscosity solvent, cast on a TiO2 nanocrystalline film via the spin coating method (also known as the single-step deposition method).41–43 The solar cells produced in a liquid junction DSSC assembly fashion resulted in 3.81% solar-to-electrical energy conversion when tested under full sunlight illumination.40
Following these findings, several additional reports have assessed other novel precursor inks including lead-based compounds. For example, lead iodide (PbI2) or lead chloride (PbCl2) have been combined with other organic–inorganic salts such as methylammonium iodide (CH3NH3I) or cesium iodide (CsI) and a variety of solvents to further improve the solar-to-electrical conversion efficiencies of the fabricated solar cells.44–46
Among these investigations, the study by Kim et al. mimics the solid-state dye-sensitized solar cell (ssDSSC) device design, in which the TiO2-based electron transport layer (ETL) was first infiltrated with MAI and PbI2 containing a perovskite precursor ink with a GBL solvent kept overnight while stirring at 60 °C.47 The solid-state cell architecture was further processed via casting a hole transporting layer (HTL), i.e. Spiro-OMeTAD, followed by thermal evaporation of gold to produce the final contact layer. Interestingly, the fabricated solar cells not only exhibited far higher conversion efficiencies (>9%) compared to previous reports, but they also exhibited striking photovoltaic performance stability during periodic measurements when tested without encapsulation for a period of 500 hours in air combined with room temperature (RT)-based ecological conditions.
Building on the outcomes of the aforementioned research, >10% conversion efficiencies were promptly demonstrated for lab-sized PSC devices, also by incorporating a mixed cation-based perovskite precursor solution ink that was used to achieve the perovskite light absorbing layer through a single-step spin coating method.48
Unlike the single-step processing-based precursor inks, the pioneering work reported by Burschka et al. used a two-step processing scheme. In this approach, the perovskite-based light absorbing layer in the PSC device structure was achieved by fabricating two individual solutions.49 The first precursor ink was obtained by dissolving PbI2 in DMF and was kept at 70 °C before the infiltration in step 1 through spin coating. Next, glass electrodes containing the PbI2-infiltrated electron transport layer (ETL, TiO2) were dipped into a solution of MAI containing 2-propanol solvent for 20 seconds to obtain the desired perovskite crystal-based light harvesting layer. Striking solar-to-electrical conversion efficiencies reached 15% due to achieving a controlled perovskite crystal morphology in response to the previously reported single-step mixed halide perovskite inks.49
Since the description of these initial techniques, progressive enhancements in conversion efficiencies have continued. The solar-to-electrical energy conversion milestone of 25% was recently surpassed for lab-sized PSCs17 due to the tremendous efforts being made in developing individual components of this solution-processed PV technology. Designing and developing novel precursor inks for producing high quality perovskite-based light absorbing active layers has remained the focus of reaching high performance and striking stability under several simulated and natural climatic conditions. Fig. 1 summarizes the solar-to-electrical energy conversion evolution. Table 1 provides a list of various perovskite precursor inks used to contribute towards enhancing the conversion efficiencies of lab-sized PSCs in recent years.
| Perovskite precursor ink recipe | Method of deposition | Cell structure | Active area (cm2) | η (%) | Ref. |
|---|---|---|---|---|---|
| a Two-step deposition: step 1 = spin coating, step 2 = dipping. b Two-step deposition: step 1 = spin coating, step 2 = spin coating. PVK = perovskite. | |||||
| MAPbI3-8 wt% of MAI and PbI2 in GBL | Spin-coating | DSSC liquid cell fashion | 0.238 | 3.81 | 40 |
| MAPbBr3-20 wt% of MABr and PbBr2 in DMF | Glass/FTO/TiO2/PVK/thermo-plastic separator film/Pt-coated FTO-glass | ||||
| 0.395 g synthesized MAI and 1.157 g PbI2 mixed in GBL (2 mL) at 60 °C overnight with stirring | Spin-coating | All-solid-state PSC: glass/FTO/c-TiO2/m-TiO2/PVK/Spiro-OMeTAD/Au | 0.207 | 9.7 | 47 |
MAI and PbCl2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) dissolved in anhydrous DMF → MAPbI2Cl (20 wt%) 1 molar ratio) dissolved in anhydrous DMF → MAPbI2Cl (20 wt%) |
Spin-coating | Glass/FTO/c-TiO2/m-Al2O3/PVK/Spiro-OMeTAD/Au | 0.09 | 10.9 | 48 |
| PbI2 in DMF (462 mg mL−1) (stirring at 70 °C) (spin coating) | Two-step depositiona | Glass/FTO/c-TiO2/m-TiO2/PVK/Spiro-OMeTAD/Au | 0.285 | 15 | 49 |
| MAI in IPA (10 mg mL−1) (dipping) | |||||
0.8 M MAPb(I1−xBrx)3 (x = 0.1–0.15) stirred in a mixture of GBL and DMSO (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) at 60 °C for 12 h 3, v/v) at 60 °C for 12 h |
Spin-coating | Glass/FTO/c-TiO2/m-TiO2-PVK nanocomposite layer/PVK upper layer/PTAA/Au | 0.094 | 16.2 | 50 |
| H2O (0.5–4 wt% vs. DMF) added to PbI2/DMF | Two-step depositionb | Glass/ITO/PEDOT:PSS/PVK/PC71BM/Ca/Al | 0.1 | 18 | 51 |
| (0.8 M) MAI in IPA (50 mg mL−1) | |||||
FAI, PbI2, MABr, and PbBr2 in mixed solvent DMF and DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
Spin-coating | Glass/FTO/c-TiO2/m-TiO2/PVK/Spiro-OMeTAD/Au | 0.16 | 20.8 | 52 |
| R PbI2/FAI (molar ratio) varies from 0.85 to 1.54 | |||||
| R PbI2/PbBr2 fixed at 5.67 | |||||
R
MABr/PbBr2 fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||||
Best: RPbI2=FAI = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05, corresponding to a 3% weight excess of PbI2 in the perovskite 05, corresponding to a 3% weight excess of PbI2 in the perovskite |
|||||
(FAI)0.81(PbI2)0.85(MAPbBr3)0.15 precursor solution prepared in a glovebox from 1.35 M Pb2+ (PbI2 and PbBr2) in a mixed solvent of DMF, NMP and DMSO (where the molar ratio of DMF/DMSO is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the molar ratio of Pb2+/[(DMSO)0.8(NMP)0.2] is 1 1, and the molar ratio of Pb2+/[(DMSO)0.8(NMP)0.2] is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Spin-coating | Glass/FTO/c-TiO2/m-TiO2/PVK/Spiro-OMeTAD/Au | 0.1051 | 21.6 | 53 |
RbI, pre-dissolved as a 1.5 M stock solution in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO 4 DMSO 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), added to the Cs/FA/MA triple cation perovskite to achieve the desired quadruple composition 1 (v/v), added to the Cs/FA/MA triple cation perovskite to achieve the desired quadruple composition |
Spin-coating | Glass/FTO/c-TiO2/m-TiO2/PVK/Spiro-OMeTAD/Au | 0.16 | 21.6 (stabilized) | 54 |
PbI2 (1.30 M) containing 2.5 mol% PbBr2 dissolved in a 1 mL mixture of DMF and DMSO (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) at 80 °C 2, v/v) at 80 °C |
Two-step depositionb | Glass/FTO/c-TiO2/m-TiO2-PVK nanocomposite layer/PVK upper layer/PTAA/Au | 0.096 | 22.1 | 55 |
| → Spin coating to form a PbI2(PbBr2)–DMSO film | |||||
| In the second step, a solution of FAI (80 mg) and MABr (10 mg), containing iodide ions (I−) (0–5 mM) dissolved in 1 mL IPA was spin coated | |||||
Mixing 1.139 mg mL−1 FAPbI3 (0.8 M solution of PbI2 and FAI in GBL) in a mixture of DMF and DMSO in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, v/v. MACl added in the range of 0–50 mol% 1 ratio, v/v. MACl added in the range of 0–50 mol% |
Spin-coating | Glass/FTO/c-TiO2/m-TiO2/PVK/Spiro-OMeTAD/Au | 0.0804 | 23.48 | 56 |
1.550 mg mL−1 FAPbI3 and 61 mg MACl dissolved in a mixture of DMF and DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
Spin-coating | Glass/FTO/c-TiO2/m-TiO2/PVK/HTM/Au | 0.0819 | 24.8 and 22.31 (a large area of 1 cm2 on the active area) | 57 |
| HTMs: two fluorinated isomeric analogs of Spiro-OMeTAD (Spiro-mF and Spiro-oF) | |||||
Perovskite solution was prepared by mixing 1.53 M PbI2, 1.4 M FAI, 0.5 M MACl (as an additive), and 0.0122 M − 0.153 M MAPbBr3 in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 8 DMSO = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Spin-coating | Glass/FTO/SnO2/PVK/Spiro-OMeTAD/Au | 0.0937 | 25.2 | 58 |
| Bulk perovskite layer is passivated with a 2D perovskite | |||||
| For the 2D perovskite passivation, 10 mM of alkylammonium bromide was deposited at 3000 rpm for 30 s | |||||
The reference perovskite precursor solution was prepared by mixing 1.139 mg FAPbI3 and 35 mol% MACl in a mixture of DMF and DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For the formate-doped FAPbI3 perovskite film, extra FAHCOO was added to the reference solution in the range of 1–4 mol% 1). For the formate-doped FAPbI3 perovskite film, extra FAHCOO was added to the reference solution in the range of 1–4 mol% |
Spin-coating | Glass/FTO/c-TiO2/m-TiO2/PVK/octylammonium iodide/Spiro-OmeTAD/Au | 0.0804 | 25.6 | 59 |
3. Emerging research trends in perovskite precursor ink development
There are a variety of precursor inks that have been developed by incorporating various novel materials to produce perovskite-based light absorbing active layers. These precursor inks (Fig. 2) can be categorized as classical single-cation based,60–62 multi-cation based,63–65 additive based,53,66–68 or lead-free.69–71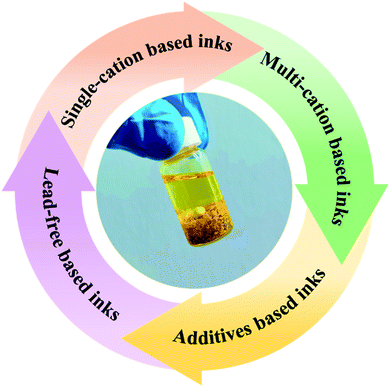 | ||
| Fig. 2 General classification trends of perovskite precursor inks that have emerged in recent years. | ||
Traditional PSC devices have been initially reported with a classical ABX3-based crystalline perovskite active layer. Here, A generally represents methyl-ammonium CH3NH3+, formamidinium NH2CH = NH2+ or cesium (Cs)-based monovalent organic or inorganic cations.40,46 B typically represents Pb2+, Sn2+ or even Ge2+-based divalent metal cations, and X3 shares I−, Cl−, Br− or Fl− as monovalent anions during the crystal formation of the traditional perovskite-based light absorbing layer.72,73
Due to the inherited characteristics such as stable crystal formations,74–78 good chemical compatibility with other active layers, high efficiency and robust stability under initially simulated environmental conditions,79,80 single cation-based perovskite precursor inks have been the focus of developing the first generation of lab-sized PSC devices.81–83 Interestingly, these first-generation precursor inks exhibited strong processing compatibility when incorporated utilizing both the single-step and two-step fabrication schemes to produce lab-sized PSC devices.84–88 Nevertheless, single-step processable precursor inks have remained the preferred choice for scaling up various device designs of PSC technology.89,90
Recently, trends of multi-cation-based ink formulations have also emerged, not only for suppressing non-radiative losses but also for achieving high performance and better stability. This has mainly been achieved through large grain boundary-based crystal formulations, along with physical and chemical stabilities of perovskites as light harvesters in PSCs.63,91–94
Bi et al. demonstrated a unique multi-cation-based halide precursor ink by incorporating multi-cations such as formamidinium iodide (FAI) and methylammonium bromide (MABr) salts with lead bromide (PbBr2) and PbI2. These were dissolved in a mixed solvent system containing dimethyl formamide (DMF) and dimethyl sulfoxide (DMSO) solvents. This recipe of perovskite precursor ink resulted in PSC devices with >20% conversion efficiency.52
In addition to multi-cations, other novel additives have also been introduced in precursor inks to overcome/achieve some of the key bottlenecks and milestones related to technological challenges and long-term performance stabilities necessary for the successful commercialization of PSC technology.95–99 In this regard, one ground-breaking research study in terms of designing a novel perovskite precursor ink was reported by Mei and co-workers. The striking mesoscopic carbon-based printable perovskite solar cell (CPSC) device design featured the introduction of an additive, i.e. 5-ammonium valeric acid iodide (5-AVAI), in the solution recipe of the traditional perovskite precursor ink.21 The additive (5-AVAI) induces control over the crystallization process during the infiltration of the formulated perovskite ink in a much thicker and more porous structure compared to the traditional PSC device configurations.100–102 As a result, the formulated ink contributed to surpassing the first >1000 hours stability test of these carbon-based printable perovskite solar cells when exposed to ambient air under full sunlight intensity conditions.21 The promising characteristics of this device design have yielded beneficial features, including successful scaling up and promising conversion efficiencies demonstrated in PSCs ranging from lab-sized to various module sizes, along with robust stability when deployed under various natural and simulated climatic conditions.103–105 Interestingly, 5-AVAI containing a novel precursor ink formulation has also been incorporated with scalable material deposition methods such as inkjet printing or slot-die coating-based technologies.106–108
In contrast to CPSCs, Bi and co-workers used polymer poly(methyl methacrylate) (PMMA) as the templating agent-based additive in the multi-cation-based precursor ink. They demonstrated perovskite films of high electronic quality for producing very high conversion efficiency (>21%) in one of the traditional mesoporous n–i–p PSC devices.53 With the impressive solar-to-electrical energy conversion, the photovoltaic parameters were shown to have an influence upon adjusting the concentration of PMMA, where the champion efficiencies (21.6%) were recorded with a controlled nucleation and crystal growth based light absorbing layer when a specific concentration (0.6 mg mL−1) was used in the fabricated perovskite precursor ink.53
Moreover, the concept of introducing ionic liquids (ILs) in perovskite precursor inks as one of the other promising additive components has also received attention for achieving high performance and robust stability when tested in several configurations.99,109–113 For example, Bai and co-workers introduced 1-butyl-3-methylimidazolium tetrafluoroborate (BMIMBF4) ionic liquid in the multi-cation perovskite precursor ink. They observed striking efficiencies of 20% at the steady state when fabricated as p–i–n configuration-based PSC devices containing this IL-based perovskite light absorbing layer.109 The inclusion of BMIMBF4 IL as an additive shows promise for influencing the crystallinity and reducing the defects in the incorporated perovskite-based light absorbing layer in the fabricated PSCs. This also contributed to the stability results when exposed to full-spectrum sunlight in air (RH 40–50%) at 60–65 °C, or full-spectrum sunlight at 70–75 °C based on the environmental testing conditions.109
Importantly, incorporating ILs in perovskite precursor inks not only offers control over crystallization dynamics and improved charge transport in the perovskite light absorbing layers, but it also provides a possibility for using them as green solvents for realizing the steps towards making PSCs an eco-friendly technology.114–117 For example, Chao and co-workers demonstrated a novel precursor ink utilizing an eco-friendly IL, i.e. methylammonium acetate (MAAc), as one of the possible green solvents for producing an efficient and pinhole-free perovskite light absorbing layer. This method revealed promising environmental stability for a period of more than 3000 hours under high humidity (60–80%) conditions.115 Motivated by such promising findings, both n–i–p and p–i–n configuration-based PSCs were fabricated by incorporating this novel precursor ink that showed promising solar-to-electrical energy conversions (∼16–20%). Notably, 20.05% is the champion conversion efficiency marked for the n–i–p based planar PSC device design under full sunlight illumination. More impressively, the non-sealed fabricated PSCs also exhibited striking long-term photovoltaic performance stability by retaining 93% of the initial device efficiency under air for more than 1000 hours. A minor deviation (<20%) in the initially achieved efficiency was observed after 700 hours of testing under light stress under the conditions maintained in the glovebox.115
Despite the numerous striking features acknowledged for this emerging low-cost PV technology, one of the key concerns raised relates to incorporating hazardous and toxic compounds such as lead (Pb)-based compounds, which have thus far remained an essential element in producing high performance PSCs.118–120 Use of Pb in a PSC, however, restricts its deployments in many portable and consumer electronics-based applications since it may leak from such devices and cause bodily harm.121 Similarly, deploying PSCs outdoors for bulk electricity generation also requires robust encapsulation procedures due to the solubility of Pb as a heavy metal in water. This may generate a negative environmental impact as indicated in several life cycle assessment reports for PSC technology.122–124 Hence, efforts are being made to either reduce or completely replace Pb-based compounds from the next generation of PSC devices.125,126
In this regard, the emerging trends for demonstrating lead-free precursor formulations include tin (Sn)-127–129 or germanium (Ge)-based halide perovskite precursor inks.130,131 Similarly, trivalent antimony (Sb3+)-132–134 or bismuth (Bi)-135–137 based perovskite precursor formulations have also been explored as possible alternatives for replacing hazardous and toxic lead-based halide perovskite precursor inks.125,126,138
Among these formulations, Sn-based formulations have thus far contributed to a gradual increase in the solar-to-electrical conversion efficiencies,128,129,139–141 which has recently exceeded above 13% when tested in inverted p–i–n device design-based PSCs under full sun illumination.127 Nevertheless, the long-term photovoltaic performance stability was not demonstrated, hence further investigations are needed for producing robust lead-free PSCs in the near future. Table 2 summarizes versatile ink formulations that have been incorporated in many device designs in recent years to produce high performance perovskite solar cell devices.
| Perovskite precursor inks | Deposition method | Perovskite | η (%) | Stability | Ref. | |
|---|---|---|---|---|---|---|
| Single cation | 1.6 M MAPbI3 (MAI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbI2 molar ratio 1 PbI2 molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in GBL, stirred overnight at 60 °C 1) in GBL, stirred overnight at 60 °C |
Solution space-limited inverse-temperature crystal growth method | MAPbI3 single crystal | 21.09 | — | 142 |
663 mg of the synthesized FAPbI3 powder and 71 μL of DMSO (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 568 μL of DMF 1) in 568 μL of DMF |
Spin coating | FAPbI3 | 21.07 (certified) | 80% of the original is retained after 30 days (in the dark, RH 20%) and 92% of the original is retained after 100 min (1 sun, RH 30%, without encapsulation) | 143 | |
The 0.6 M CsPbI3 perovskite precursor was prepared by dissolving stoichiometric CsI, PbI2, and xDMAI in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x molar ratio in DMF (x = 0.5, 0.7, 1.0 and 1.5) x molar ratio in DMF (x = 0.5, 0.7, 1.0 and 1.5) |
Spin coating | Phenyltrimethylammonium chloride (PTACl) passivated CsPbI3 | 19.03 | 90% of the original is retained after 500 h continuous white light LED illumination (100 mW cm−2) in a N2 glove without encapsulation | 144 | |
| Multi cations | Mixing FAI (1 M), PbI2 (1.1 M), MABr (0.2 M) and PbBr2 (0.2 M) in anhydrous DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO 4 DMSO 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v). Then CsI, pre-dissolved as a 1.5 M stock solution in DMSO, was added to the mixed perovskite precursor to achieve the desired triple cation composition 1 (v/v). Then CsI, pre-dissolved as a 1.5 M stock solution in DMSO, was added to the mixed perovskite precursor to achieve the desired triple cation composition |
Spin coating | Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | 21.1 | The device efficiency drops from 20% to 18% where it stays relatively stable for at least 250 h (in a N2 atmosphere and room temperature under continuous illumination at MPP) | 145 |
889 mg mL−1 of FAPbI3, 33 mg mL−1 of MAPbBr3 and 33 mg mL−1 of MACl in DMF/DMSO (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixed solvent 1, v/v) mixed solvent |
Spin coating | (FAPb3)0.95(MAPbBr3)0.05 | 22.7 (certified) | 95% of the initial efficiency retained after 1370 h under 1-sun illumination at room temperature (with encapsulation) | 146 | |
1.53 M PbI2, 1.4 M FAI, 0.11 M MAPbBr3, 0.5 M MACl in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 8 DMSO = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio 1 volume ratio |
Spin coating | (FAPbI3)0.92(MAPbBr3)0.08 | 23.4 | The device maintained 85% of its efficiency after 500 h under full solar illumination (AM 1.5G, 100 mW cm−2) without a UV-filter at MPP tracking (with encapsulation) in ambient air | 147 | |
0.02 M GAI, 0.09 M CsI, 0.2 M MABr, 1.1 M FAI, 1.2 M PbI2, and 0.3 M PbBr2 in anhydrous DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (4 DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixed solvent 1, v/v) mixed solvent |
Spin coating | GA0.015Cs0.046MA0.152FA0.787Pb(I0.815Br0.185)3 | 20.96 | — | 148 | |
| Additives | 1.35 M Pb2+(PbI2 and PbBr2) and 0.6 mg mL−1 BrPh-ThR in the mixed solvent of DMF and DMSO (volume ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Spin coating | (FAI)0.81(PbI2)0.85(MABr)0.15(PbBr2)0.15; (Lewis base BrPh-ThR and Lewis acid bis-PCBM (N-(4-bromophenyl)thiourea)) | 21.7 | The unsealed devices remained at 93% of the initial efficiency value in ambient air (10–20% relative humidity) after 3600 h at 20–25 °C, and dropped by 10% after 1500 h under continuous operation at 1-sun illumination and 55 °C in nitrogen with MPPT | 149 |
| Two-step solution process: | Spin coating | (Cs0.05FA0.54MA0.41)Pb(I0.98Br0.02)3; (NaF) | 21.46 (21.43% certified) | The unencapsulated device retained over 90% of its initial PCE after 1000 h under MPP tracking and continuous light irradiation with a white LED lamp, 100 mW cm−2 in a nitrogen atmosphere | 150 | |
Mixing 1.3 M PbI2 in DMF/DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with 5% CsI 1, v/v) with 5% CsI |
||||||
| Note: for the NaX-containing perovskite film, NaX was added | ||||||
| Mixing 0.12 M MAI, 0.05 M MABr, 0.07 M MACl, and 0.23 M FAI in IPA | ||||||
1.139 mg mL−1 FAPbI3 in a mixture of DMF and DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). MACl was added in the range 0–50 mol% 1, v/v). MACl was added in the range 0–50 mol% |
Spin coating | MACl additive (40 mol%) was applied in FAPbI3 | 24.02 (certified 23.48%) | The stability at RT showed 90% retention of the device's initial performance over 1200 h. The 40 °C thermal stability test showed 90% retention of the device's initial performances over 300 h | 56 | |
| 573.0 mg PbI2, 197.5 mg MAI, and 13.70 mg 5-AVAI in 1.0 mL GBL | Slot-die coating | 5-AVAI in MAPbI3 | 12.87 | — | 106 | |
| Lead free | Mixing the GeI2 doped FA0.98EDA0.01SnI3 and GeI2 doped EA0.98EDA0.01SnI3 precursor solution at a molar ratio of x = 0, 0.05, 0.1, 0.2 | Spin coating | GeI2 doped (FA0.9EA0.1)0.98EDA0.01SnI3. | 13.24 | — | 127 |
First dissolving 129 mg FAI, 39.7 mg MAI, 372.5 mg SnI2, and 15.7 mg SnF2 in 875 μL DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 μL DMSO. Meanwhile, 55.8 mg GeI2 was dissolved in 200 μL DMF. Different concentrations of GeI2 (5 mol% and 10 mol%) were subsequently added into the bulk precursor 125 μL DMSO. Meanwhile, 55.8 mg GeI2 was dissolved in 200 μL DMF. Different concentrations of GeI2 (5 mol% and 10 mol%) were subsequently added into the bulk precursor |
Spin coating | 5 mol% germanium doped FA0.75MA0.25Sn1−xGexI3 | 7.9 | 90% of the unencapsulated device's initial efficiency retained over 600 min under constant illumination under ambient conditions | 151 | |
Dissolving AgI, BiI3, and [Bi(S2CAr)3] at the desired ratios in a mixed solvent system (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HI, 3 HI, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), to which HI (0.12 vol.) was slowly added dropwise 1, v/v), to which HI (0.12 vol.) was slowly added dropwise |
Spin coating | Ag3BiI5.92S0.04 | 5.44 | When stored in air at room temperature (≈22–24 °C) at RH of 40% under diffuse light, the device showed a promising stability and retained more than 90% of the initial PCE after 45 days | 152 |
4. Research and development trends for advanced scalable precursor inks
Motivated by the successes achieved from solution processed lab-sized PSCs with traditional perovskite precursor inks, efforts have also been made towards scaling up PSC technology on a variety of substrates by designing novel precursor inks with key characteristics as shown in Fig. 3.153–158 In this regard, spin coating has remained the foremost choice for perovskite precursor ink deposition, as well as for the initial scaling up demonstrations of PSC technology (Table 3).159–161| Ink recipe (PVK) | Method | Device structure | Active area (cm2) | Module area (cm2) | η (%) | Ref. |
|---|---|---|---|---|---|---|
| a PVK = perovskite. b PCE based on active area. c Not mentioned. d PCE based on aperture area. | ||||||
MAI and PbCl2 (molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in DMF 1) in DMF |
Spin coating | Glass/FTO/TiO2/PVK/HTM/Au | 16.8 | 5 × 5 | 5.1b | 162 |
MAI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbI2 (molar ratio 1 PbI2 (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in GBL 1) in GBL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (3 DMSO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
Spin coating | Glass/ITO/PEDOT:PSS/PVK/PCBM/LiF/Al | 60 | 10 × 10 | 8.7c | 163 |
| PbI2 in DMF (285 mg mL−1) | Spin coating and dipping | Glass/FTO/c-TiO2/graphane + m-TiO2/Go-Li/PVK/Spiro/Au | 50.6 | 10 × 10 | 12.6b | 175 |
| MAI solution in IPA (10 mg mL−1) | ||||||
PbI2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAI (1 MAI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in GBL 1 molar ratio) in GBL |
Drop casting | Glass/FTO/TiO2/ZrO2/carbon/PVK | 70 | 10 × 10 | 10.75b | 176 |
5-AVA-I added to the perovskite solutions in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (AVA-I to MAI) 20 (AVA-I to MAI) |
||||||
| 1.2 M MAI and 1.2 M PbI2 dissolved in GBL | Drop casting | Glass/FTO/TiO2/ZrO2/carbon/PVK | 47.6 | 10 × 10 | 11.16b | 177 |
| For 2D perovskite: 1.2 M AVAI and 1.2 M PbI2 dissolved in GBL | ||||||
| Mixing 0.07 g 5-AVAI, 4.61 g PbI2 and 1.59 g MAI in 10.5 mL GBL | Drop casting | Glass/FTO/TiO2/ZrO2/carbon/PVK | 49 | 10 × 10 | 10.4b | 170 |
PbI2 and MAI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in GBL prepared by adding 5-AVAI to obtain a 3% molar ratio between 5-AVAI and MAI 1 molar ratio) in GBL prepared by adding 5-AVAI to obtain a 3% molar ratio between 5-AVAI and MAI |
Drop casting | Glass/FTO/TiO2/ZrO2/carbon/PVK | 198 | 435.6 (aperture) | 6.3b (stabilized) (after 2 months since fabrication) | 178 |
| Blade coating of PbI2 in DMF (330 mg mL−1) | Blade coating | Glass/FTO/c-TiO2/mp-TiO2/PVK/P3HT/Au | 100 | 16 × 11 | 4.3b | 164 |
| Dipping into MAI solution (in IPA) (10 mg mL−1) | ||||||
30 wt% and 45 wt% equimolar ratio of MAI and PbI2 precursors with different amounts of MACl additive in a mixed solvent (NMP/DMF 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v) 8, v/v) |
Blade coating | Glass/FTO/c-TiO2/PVK/Spiro-OMeTAD/Au | 11.09 | 12.6 | 13.3b (stabilized) | 179 |
| 0.8 M MAPbI3 in DMF (496 mg mL−1) and 0.25 M LP surfactant in DMF (0.2 mg mL−1) | Blade coating | Glass/ITO/PTAA/PVK/C60/BCP/Cu | 57.2 | 13 × 8.5 | 14.6d (stabilized) | 180 |
276.7 mg PbI2 in 1 mL DMF or mixed solvent (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) additives) (0.6 M) additives) (0.6 M) |
Blade coating | Glass/FTO/SnO2/PVK/Spiro-OMeTAD/Au | 16.54% for 5 × 5 cm2 perovskite solar modules, and 13.32% for 10 × 10 cm2 modules | 167 | ||
The solution mixture of FAI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MABr MABr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MACl (60 mg MACl (60 mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mg 6 mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mg in 1 mL IPA) was bladed onto the PbI2 films 6 mg in 1 mL IPA) was bladed onto the PbI2 films |
||||||
The solid MAPbI3 crystals changed to liquid via MA gas-assisted solid–liquid transition. The viscous liquid was diluted with ACN in a weight ratio of viscous liquid to ACN = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
D-bar coating | Glass/FTO/SnO2/PVK/Spiro-OMeTAD/Au | 10 × 10 | 10 × 12 | Many PCEsb were reported (∼16-18%) | 181 |
| PbI2 in DMF (0.7 M, 322 mg mL−1) | Slot-die coating | PET/ITO/ZnO/PVK/P3HT/Ag | 5 × 8 | 10 × 10 | — | 165 |
| MAI (10 mg mL−1) in IPA | ||||||
| 40% MAPbI3 in DMF | Slot-die coating | Glass/FTO/ZnO/PVK/home-made carbon paste | 17.6 | 5 × 5 | 10.6b | 182 |
1 mM of lead compounds PbCl2 and Pb(CH3COO)·3H2O at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 3 mM of MAI per 1 mL of DMF 4 and 3 mM of MAI per 1 mL of DMF |
Slot-die coating | Glass/ITO/c-TiO2/PVK/Spiro-OMeTAD/Au | 151.88 | 12.5 × 13.5 | 11.1b | 183 |
| 573.0 mg PbI2, 197.5 mg MAI, and 13.70 mg 5-AVAI in 1.0 mL GBL | Slot-die coating | Glass/FTO/m-TiO2/ZrO2/carbon/PVK | 60.08 | 80.55 (aperture area) | 12.87b | 106 |
| 0.8 M MAPbI3−xClx in DMF/GBL mixed solvent | Spray coating | Glass/FTO/TiO2/PVK/PTAA/Au | 40 | 10 × 10 | 15.5b | 184 |
0.15 M Cs0.17FA0.83PbI3 in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) 2, v/v) |
Spray coating | Glass/ITO/NiO/PVK/C60/BCP/Au | 5.9 | — | 15.5b | 185 |
| 0.53 g of PbI2, 0.19 g of MAI and 0.0176 g of 5-AVAI in 1 mL of GBL | Ink-jet printing | Glass/FTO/TiO2/ZrO2/carbon/printed PVK | — | 10 × 10 (18 cells) | — | 107 |
| 1.2 M PbI2, 1.2 M MAI and 0.06 M 5-AVAI in 1 mL of GBL | Ink-jet printing | Glass/FTO/TiO2/ZrO2/carbon/printed PVK | 0.64 | 1.5 | 9.1b | 108 |
| L-α-Phosphatidylcholine and methylammonium hypophosphite were added into ≈1.45 M MAPbI3/2-ME solution at a concentration of ≈0.3 mg mL−1 and ≈0.15 vol%, respectively | Blade coating | Willow glass/ITO/PTAA/PVK/C60/BCP/Cu | Geometric fill factor 92% | 42.9 (aperture area) | 15.86d | 186 |
1.26 mM ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbI2, 1.26 mM PbI2, 1.26 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FAI, 0.06 mM MAPbBr3 and 0.5 mM MACl in DMF/DMSO (8 FAI, 0.06 mM MAPbBr3 and 0.5 mM MACl in DMF/DMSO (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v/v) mixed solvent v/v) mixed solvent |
Spin coating | PEN/ITO/np-SnO2 and porous-ZSO (Zn2SnO4)/PVK/Spiro-OMeTAD/Au | PCE of 15.5%, 12.9% and 11.8% on an aperture aread of 100 cm2, 225 cm2 and 400 cm2, respectively | 187 | ||
1.3 M organic cation (0.85 FAI and 0.15 MABr) and 1.4 M mixture of metal lead salts (0.85 PbI2 and 0.15 PbBr2) in a solvent mixture of DMF/DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
Spin coating | PET/ITO/SnO2/PVK/Spiro-OMeTAD/Au | 16.07 | 30 | 15.22b | 188 |
| 460 mg mL−1 PbI2 in DMF | Spin coating | Cu foil/CuI/PVK/ZnO/Ag | 0.8 | — | — | 189 |
| 40 mg mL−1 MAI in 2-propanal | ||||||
Among these initial pioneering efforts, Matteocci et al. demonstrated the first n–i–p structure-based solid-state monolithic module162 with organometal halide perovskite CH3NH3PbI3−xClx ink, produced by dissolving methylammonium iodide CH3NH3I and lead chloride PbCl2 in a N,N-dimethylformamide (DMF)-based solvent. The ink was spin-coated at 2000 rpm for 40 s over the nanocrystalline TiO2-based electron transport layer (ETL) in air and successively heated at 120 °C for 45 minutes to obtain the final crystalline structure. The fabricated modules exhibited similar (∼5.1%) conversion efficiencies when tested with two types of hole transporting layer (Spiro-OMeTAD and poly(3-hexylthiophene-2,5-diyl) (P3HT) polymer) and thermally evaporated gold (Au)-based contact layers over an active area of 16.8 cm2.
As a further advancement, Seo et al. reported an inverted (p–i–n) structure-based module design with a perovskite ink composed of CH3NH3I and PbI2, which were stirred in a mixture of dimethyl sulfoxide (DMSO) and γ-butyrolactone (GBL)-based solvents at 60 °C for 12 h.163 The ink was deposited onto a PEDOT:PSS/ITO substrate by a consecutive two-step spin-coating process at 1000 and 4000 rpm for 20 and 60 s, respectively. The toluene in the final spin-stage was dripped onto the substrate during spin coating. The perovskite precursor-coated substrate was then dried on a hot plate at 100 °C. The fabricated module with the phenyl-C61-butyric acid methyl ester (PCBM)-based ETL and Al coated contact layer showed an impressive conversion efficiency (8.7%) over a far larger active area (60 cm2) when tested under full sunlight illumination.
In addition to these earlier reports of precursor ink development with the spin coating-based material deposition method, advanced single- and two-step processable precursor inks have also been developed and tested with more established scalable approaches (as depicted in Fig. 4), such as blade coating and slot-die coating to fabricate large area perovskite-based solar modules.164–167 For example, Razza et al. used a two-step approach where the pre-heated PbI2 precursor ink was first coated on ETL layers using a blade coating method followed by rapid evaporation of its solvent (DMF) through an airflow.164 Next, the perovskite crystalline layer was achieved by directly dipping the substrates containing PbI2-coated ETL layers into the MAI solution with different dipping times. The perovskite modules fabricated with this approach exhibited 4.3% conversion efficiency under full sunlight illumination over an active area of 100 cm2.
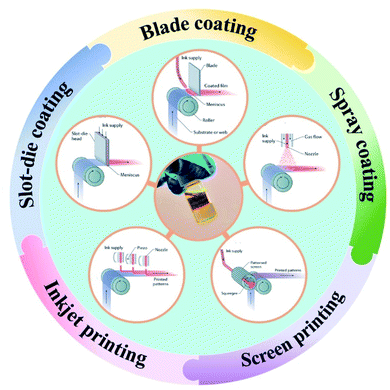 | ||
| Fig. 4 Some of the popular fabrication schemes opted to produce large area perovskite solar cells and modules. Reproduced from ref. 158 with permission. | ||
Deng et al. introduced the novel concept of adding a surfactant to the perovskite precursor ink and demonstrated impressive control over the crystallization morphology of the large-area perovskite-based light absorbing layer when deposited with a blade coating scheme.168 The fabricated modules showed very high (∼15%) conversion efficiency when tested under full sunlight illumination with a p–i–n device design. Similarly, Giacomo et al. demonstrated slot-die coating of a precursor solution for a perovskite layer, which was prepared under room temperature conditions in an inert N2 environment.169 The solution was prepared by dissolving 1 mM of lead compounds and 3 mM of CH3NH3I in 1 mL of DMF solvent. The solution was stirred for at least 10 minutes before final use. Slot-die coating of this perovskite precursor ink was performed inside a glovebox, which allowed deposition of a wide range of thicknesses. The wet layer thickness of the perovskite was in the range of 3–4 μm to get a dry layer thickness of 350 nm. With these optimizations, a 12.5 × 13.5 cm2 module with power conversion efficiencies above 10% over an active area of approximately 152 cm2 was achieved in the planar n–i–p configuration.169
Recently, slot-die coating of a novel perovskite precursor ink containing MAI, PbI2 and 5-ammonium valeric acid (5-AVAI) in GBL (tested with the drop-casting170 and inkjet printing methods107,108) has also been demonstrated.106 The fabricated triple mesoscopic printable perovskite solar modules showed an impressive conversion efficiency of 12.87%, which was attained over an active area of 60.08 cm2 under full sunlight illumination.106
Similar scalable demonstrations of organic–inorganic lead halide perovskite-based novel inks with the techniques discussed above have also been utilized to scale up PSC technology on flexible substrates. Flexible modules in various device designs have been developed and reported both by research labs and commercial players in recent years.171–174 Therefore, there is promising evidence of successful fabrication of the novel organic–inorganic lead halide-based precursor ink formulations for achieving module production, where several device designs can be potentially achieved on both rigid and flexible-based substrates according to the targeted applications. Table 3 provides a summary of the recently reported scalable device configurations of perovskite solar modules designed using scalable perovskite precursor ink formulations on rigid glass and flexible substrates.
5. Forthcoming directions in precursor inks, technological research, and development of next-generation PSC technology
The progressive research and development of PSCs over the past decade provide future directions and guidelines to overcome the remaining key challenges necessary for reaching commercial breakthroughs imagined for this efficient PV technology as envisioned in Fig. 5.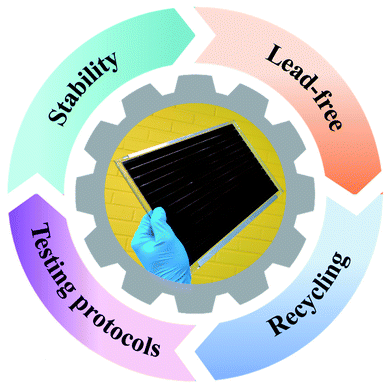 | ||
| Fig. 5 Summary of the key futuristic directives envisioned for the emerging perovskite solar cell technology. | ||
Among them, the toxicity and environmental impact of traditional lead-based precursor inks pose a challenge for the safe deployment of PSCs. In this regard, not only should the safety protocols but also the effective recycling procedures be chosen for the safe removal of hazardous and toxic materials such as PbI2 with high yield from the installed panels of PSC technology after their end of life. Interestingly, notable progress has been reported in many scientific reports where effective recycling of PbI2, methylammonium iodide (MAI) and expensive contact layers has been successfully demonstrated for the lab-sized PSCs.190–192 These proof-of-concept based striking demonstrations provide pathways in designing effective recycling protocols for large area perovskite solar modules, which may significantly suppress the environmental concerns, which have been heavily debated during the emergence of this low-cost PV technology.193–195 Moreover, the abundance, availability, cost and sustainability of potential lead compound substitutes and their respective precursor inks must be further investigated to justify their bulk-scale usage to produce the so-called large area eco-friendly perovskite-based PV panels.196
On the other hand, despite the growing number of reports on achieving stable photovoltaic performance under various environmental conditions while incorporating advanced perovskite precursor inks,103,104,197 realistic assessments of PSC device stability based on recently suggested stability testing protocols197 have yet to be established. Thus far, there are several reports of either stable performances or degradation of initial efficiencies in various device designs of PSCs containing novel precursor inks, using the suggested stability testing protocols for PSC technology. This again raises concerns due to the uncertainty in deploying this emerging PV technology in many suitable applications.
In addition, new consensus statements need to be developed to address advanced stability testing protocols from the perspective of precisely controlled indoor ecological conditions and some severely cold outdoor climatic conditions, which have not been specifically covered in the current stability testing protocols.8 The possible upcoming deployments of PSC technology in batteryless and maintenance-free IoT devices198–200 inside modern buildings with precisely controlled ecological conditions or stand-alone communication gadgets in severely cold outdoor climatic conditions call for new testing standards. Notably, degradation rates could vary under such environmental conditions, but these are not known or have rarely been reported to date.
Interesting research questions may provide future research and development directives towards the forthcoming generation of PSC devices over the next decade. Considering the progress that has already been made, it is expected that future technological developments would resolve key research problems of achieving eco-friendly and robust PSC technology with certified integration in vast applications.
6. Summary and conclusions
The development of progressive precursor inks reviewed in this work provides evidence for their contributions towards achieving striking milestones such as high-performance or versatile configuration designs and highlights unique integration possibilities of emerging PSC technology. Moreover, the intelligent precursor inks designed with novel materials reported in the recent past have offered pathways for scalability over both rigid glass and flexible polymer foil-based substrates. As a result, activities related to commercializing PSC technology have greatly accelerated as its integration in smart sensors, charging stations and BIPV based applications continues to be demonstrated.201Nevertheless, additional materials screening is needed for the development of the next generation of novel precursor inks of the perovskite light absorbing layer in order to meet the growing demand, especially from the construction industry where there is keen interest in aesthetically appealing solar panels for smart buildings and nature-friendly ecological architecture.202–209 This demand may potentially be met by creating precise and flexible patterning that allows individual PSCs to be assembled into aesthetic structures based on solar modules for energy generation.
The current methods such as slot-die coating, spraying or blade coating techniques offer limited flexibility, and have mostly been used to produce serially connected pattern-based modules in traditional n–i–p or p–i–n based device configurations.188,210,211 Notably, alternative schemes such as drop on demand inkjet printing may provide more freedom of design, since advanced precursor inks of both perovskite-based materials and other active materials can be fabricated to create individual PSCs. These PSCs can be designed with aesthetically attractive arbitrary patterns, which may be intelligently connected to create electricity at targeted watt scales.
Surprisingly, except for the few demonstrations of printing individual active layers212–215 (including the perovskite-based light absorbing layer107,216), inkjet printing has been rarely reported in fabricating complete device designs of PSC technology, despite knowing its potential for fabricating large area-based active layers at high speed. In this regard, one of the most critical limitations realized in producing active layers through inkjet printing is when the nozzles choke the cartridges, which severely limits drop volumes (1–10 picolitres). This in turn makes it difficult to print nanoparticle-based solution processable inks with the necessary precision and resolution. Moreover, the delicate cartridges frequently exhibit chemical incompatibility and often react with the fabricated inks that are composed of novel materials and solvents, thus making it difficult to achieve the desired patterns with ease and accurate precision.217–220
These key challenges call for the development of advanced precursor inks to be used with high chemical compatibility, not only to achieve precisely patterned perovskite-based light absorbing layers but also for other active layers to produce fully printed PSC technology with pattern flexibility. One of the potential precursor inks to achieve a perovskite light absorbing layer with high precision has been demonstrated utilizing an inkjet printing technique to produce carbon-based printable perovskite solar cells.107 The precursor ink showed great compatibility during inkjet printing when fabricated with a novel additive (5-ammonium valeric acid iodide; 5-AVAI), which significantly slows the perovskite crystallization formation and allows the fabricated ink to pass through the inkjet nozzles without reacting with them. Thus far, this stable device configuration103,105 of PSCs has been demonstrated with schemes such as inkjet printing and screen-printing methods. There is immense potential to achieve further module fabrication with customized designs to generate appealing patterns based on individual cells as discussed earlier in this section.221
Hence, a transformation from the first generation of serially connected carbon-based printable perovskite solar modules106,178 to aesthetically appealing solar panels can be anticipated, which could provide guidelines to produce the next generation of traditional n–i–p and p–i–n configuration-based PSCs, especially when considering evolved design principles. This could assuredly enable realistic outcomes such as the rapid deployment of PSCs worldwide and the resulting positive influence on climate change expected to arise from this promising photovoltaic technology.
Author contributions
C. M. T. K contributed with manuscript planning and communication, writing Sections 1, 2 and 4, drafting tables and figures, screening, and furnishing the manuscript text and references. L. A and M. O contributed with writing and drafting Sections 3 and 5. S. G. H. supervised the research work and contributed with funding acquisition, outline drafting and writing sections, reviewing, and editing the overall manuscript.Conflicts of interest
The authors have no conflicts to declare.Acknowledgements
This work was financed by the CAPRINT project (Decision # 2430354811). CMTK and SGH are grateful to the Technology Industries of Finland Centennial Foundation and to the Jane and Aatos Erkko Foundation for financing the doctoral research work and awarding the project funding, respectively. Printed Intelligence Infrastructure is also acknowledged for this work.References
- S. Ghasemian, A. Faridzad, P. Abbaszadeh, A. Taklif, A. Ghasemi and R. Hafezi, Int. J. Environ. Sci. Technol., 2020 DOI:10.1007/s13762-020-02738-5.
- A. S. World Energy Council and P. S. Institute, World Energy Resources, World Energy Counc. Co. Ltd, 2016, pp. 1–138 Search PubMed.
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2016, 16, 16–22 CrossRef PubMed.
- Ms. Fitria, J. Chem. Inf. Model., 2013, 53, 1689–1699 CrossRef PubMed.
- D. Gielen, F. Boshell, D. Saygin, M. D. Bazilian, N. Wagner and R. Gorini, Energy Strategy Reviews, 2019, 24, 38–50 CrossRef.
- J. Khan and M. H. Arsalan, Renewable Sustainable Energy Rev., 2016, 55, 414–425 CrossRef.
- T. M. David, P. M. Silva Rocha Rizol, M. A. Guerreiro Machado and G. P. Buccieri, Heliyon, 2020, 6, e04452 CrossRef PubMed.
- M. Kokkonen, P. Talebi, J. Zhou, S. Asgari, S. A. Soomro, F. Elsehrawy, J. Halme, S. Ahmad, A. Hagfeldt and S. G. Hashmi, J. Mater. Chem. A, 2021, 9, 10527–10545 RSC.
- J. Park, H. Yoo, V. Karade, K. S. Gour, E. Choi, M. Kim, X. Hao, S. J. Shin, J. Kim, H. Shim, D. Kim, J. H. Kim, J. Yun and J. Hyeok Kim, J. Mater. Chem. A, 2020, 8, 14538–14544 RSC.
- S. Biswas and H. Kim, Polymers, 2020, 12, 1338 CrossRef CAS.
- X. Hou, Y. Wang, H. Ka, H. Lee, R. Datt, N. U. Miano, D. Yan, M. Li, F. Zhu, B. Hou, W. C. Tsoi and Z. Li, J. Mater. Chem. A, 2020, 21503–21525 RSC.
- N. G. Park and K. Zhu, Nat. Rev. Mater., 2020, 5, 333–350 CrossRef CAS.
- A. S. Gertsen, M. F. Castro, R. R. Søndergaard and J. W. Andreasen, Flexible Printed Electron., 2020, 5, 014004 CrossRef CAS.
- E. Aydin, T. G. Allen, M. De Bastiani, L. Xu, J. Ávila, M. Salvador, E. Van Kerschaver and S. De Wolf, Nat. Energy, 2020, 5, 851–859 CrossRef CAS.
- E. Bellini, Solliance, MiaSolé hit 26.5% efficiency on tandem CIGS/perovskite solar cell, accessed on 16.09.2021, https://www.pv-magazine.com/2021/02/15/solliance-miasole-hit-26-5-efficiency-on-tandem-cigs-perovskite-solar-cell/.
- A. Aslam, U. Mehmood, M. H. Arshad, A. Ishfaq, J. Zaheer, A. Ul, H. Khan and M. Sufyan, Sol. Energy, 2020, 207, 874–892 CrossRef CAS.
- Best Research-Cell Efficiency Chart, accessed on 16.09.2021, https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200104.pdf.
- A. E. Shalan, Mater. Adv., 2020, 1, 292–309 RSC.
- J. Yeo and Y. Jeong, Energy Rep., 2020, 6, 2075–2085 CrossRef.
- I. Hussain, H. P. Tran, J. Jaksik, J. Moore, N. Islam and M. J. Uddin, Emergent Mater., 2018, 1, 133–154 CrossRef CAS.
- A. Mei, X. Li, L. Liu, Z. Ku, T. Liu, Y. Rong, M. Xu, M. Hu, J. Chen, Y. Yang, M. Grätzel and H. Han, Science, 2014, 345, 295–298 CrossRef CAS PubMed.
- J. Su, H. Cai, J. Yang, X. Ye, R. Han, J. Ni, J. Li and J. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 3531–3538 CrossRef CAS PubMed.
- A. Giuri, S. Masi, A. Listorti, G. Gigli, S. Colella, C. Esposito and A. Rizzo, Nano Energy, 2018, 54, 400–408 CrossRef CAS.
- J. Li, J. Dagar, O. Shargaieva, M. A. Flatken, H. Köbler, M. Fenske, C. Schultz, B. Stegemann, J. Just, D. M. Többens, A. Abate, R. Munir and E. Unger, Adv. Energy Mater., 2021, 11, 2003460 CrossRef CAS.
- N. Vlachopoulos, A. Hagfeldt, I. Benesperi, M. Freitag, G. Hashmi, G. Jia, R. A. Wahyuono, J. Plentz and B. Dietzek, Sustainable Energy Fuels, 2021, 5, 367–383 RSC.
- I. Benesperi, H. Michaels and M. Freitag, J. Mater. Chem. C, 2018, 6, 11903–11942 RSC.
- M. Rai, L. H. Wong and L. Etgar, J. Phys. Chem. Lett., 2020, 11, 8189–8194 CrossRef CAS PubMed.
- Z. Chen, Q. Dong, Y. Liu, C. Bao, Y. Fang, Y. Lin, S. Tang, Q. Wang, X. Xiao, Y. Bai, Y. Deng and J. Huang, Nat. Commun., 2017, 8, 1–7 CrossRef PubMed.
- A. Bag, R. Radhakrishnan, R. Nekovei and R. Jeyakumar, Sol. Energy, 2020, 196, 177–182 CrossRef CAS.
- Y. Hou, W. Chen, D. Baran, T. Stubhan, N. A. Luechinger, B. Hartmeier, M. Richter, J. Min, S. Chen, C. O. R. Quiroz, N. Li, H. Zhang, T. Heumueller, G. J. Matt, A. Osvet, K. Forberich, Z. G. Zhang, Y. Li, B. Winter, P. Schweizer, E. Spiecker and C. J. Brabec, Adv. Mater., 2016, 28, 5112–5120 CrossRef CAS PubMed.
- J. Chen and N. G. Park, Adv. Mater., 2019, 31, 1–56 Search PubMed.
- C. M. Wolff, P. Caprioglio, M. Stolterfoht and D. Neher, Adv. Mater., 2019, 31, 1902762 CrossRef CAS PubMed.
- D. Luo, R. Su, W. Zhang, Q. Gong and R. Zhu, Nat. Rev. Mater., 2020, 5, 44–60 CrossRef CAS.
- B. Wang, H. Li, Q. Dai, M. Zhang, Z. Zou, J.-L. Brédas and Z. Lin, Angew. Chem., Int. Ed., 2021, 60, 17664–17670 CrossRef CAS PubMed.
- T.-S. Su, F. T. Eickemeyer, M. A. Hope, F. Jahanbakhshi, M. Mladenović, J. Li, Z. Zhou, A. Mishra, J.-H. Yum, D. Ren, A. Krishna, O. Ouellette, T.-C. Wei, H. Zhou, H.-H. Huang, M. D. Mensi, K. Sivula, S. M. Zakeeruddin, J. V Milić, A. Hagfeldt, U. Rothlisberger, L. Emsley, H. Zhang and M. Grätzel, J. Am. Chem. Soc., 2020, 142, 19980–19991 CrossRef CAS.
- D. Liu, C. J. Traverse, P. Chen, M. Elinski, C. Yang, L. Wang, M. Young and R. R. Lunt, Adv. Sci., 2018, 5, 1700484 CrossRef.
- M. Qin, J. Cao, T. Zhang, J. Mai, T. K. Lau, S. Zhou, Y. Zhou, J. Wang, Y. J. Hsu, N. Zhao, J. Xu, X. Zhan and X. Lu, Adv. Energy Mater., 2018, 8, 1–9 Search PubMed.
- X. Wu, Y. Jiang, C. Chen, J. Guo, X. Kong, Y. Feng, S. Wu, X. Gao, X. Lu, Q. Wang, G. Zhou, Y. Chen, J. M. Liu, K. Kempa and J. Gao, Adv. Funct. Mater., 2020, 30, 1908613 CrossRef CAS.
- J. Kim, B. W. Park, J. Baek, J. S. Yun, H. W. Kwon, J. Seidel, H. Min, S. Coelho, S. Lim, S. Huang, K. Gaus, M. A. Green, T. J. Shin, A. W. Y. Ho-Baillie, M. G. Kim and S. Il Seok, J. Am. Chem. Soc., 2020, 142, 6251–6260 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- G. Wang, D. Liu, J. Xiang, D. Zhou, K. Alameh, B. Ding and Q. Song, RSC Adv., 2016, 6, 43299–43303 RSC.
- S. M. H. Qaid, M. S. Al Sobaie, M. A. Majeed Khan, I. M. Bedja, F. H. Alharbi, M. K. Nazeeruddin and A. S. Aldwayyan, Mater. Lett., 2016, 164, 498–501 CrossRef CAS.
- N. Yantara, F. Yanan, C. Shi, H. A. Dewi, P. P. Boix, S. G. Mhaisalkar and N. Mathews, Chem. Mater., 2015, 27, 2309–2314 CrossRef CAS.
- P. Docampo, J. M. Ball, M. Darwich, G. E. Eperon and H. J. Snaith, Nat. Commun., 2013, 4, 1–6 Search PubMed.
- J. M. Ball, M. M. Lee, A. Hey and H. J. Snaith, Energy Environ. Sci., 2013, 6, 1739–1743 RSC.
- H. Choi, J. Jeong, H.-B. Kim, S. Kim, B. Walker, G.-H. Kim and J. Y. Kim, Nano Energy, 2014, 7, 80–85 CrossRef CAS.
- H. S. Kim, C. R. Lee, J. H. Im, K. B. Lee, T. Moehl, A. Marchioro, S. J. Moon, R. Humphry-Baker, J. H. Yum, J. E. Moser, M. Grätzel and N. G. Park, Sci. Rep., 2012, 2, 1–7 Search PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- J. Burschka, N. Pellet, S. J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. Il Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- C. G. Wu, C. H. Chiang, Z. L. Tseng, M. K. Nazeeruddin, A. Hagfeldt and M. Grätzel, Energy Environ. Sci., 2015, 8, 2725–2733 RSC.
- D. Bi, W. Tress, M. I. Dar, P. Gao, J. Luo, C. Renevier, K. Schenk, A. Abate, F. Giordano, J. P. C. Baena, J. D. Decoppet, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel and A. Hagfeldt, Sci. Adv., 2016, 2, e1501170 CrossRef PubMed.
- D. Bi, C. Yi, J. Luo, J. D. Décoppet, F. Zhang, S. M. Zakeeruddin, X. Li, A. Hagfeldt and M. Grätzel, Nat. Energy, 2016, 1, 1–5 Search PubMed.
- M. Saliba, T. Matsui, K. Domanski, J.-Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J.-P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Science, 2016, 354, 206–209 CrossRef CAS PubMed.
- W. S. Yang, B. W. Park, E. H. Jung, N. J. Jeon, Y. C. Kim, D. U. Lee, S. S. Shin, J. Seo, E. K. Kim, J. H. Noh and S. Il Seok, Science, 2017, 356, 1376–1379 CrossRef CAS PubMed.
- M. Kim, G. H. Kim, T. K. Lee, I. W. Choi, H. W. Choi, Y. Jo, Y. J. Yoon, J. W. Kim, J. Lee, D. Huh, H. Lee, S. K. Kwak, J. Y. Kim and D. S. Kim, Joule, 2019, 3, 2179–2192 CrossRef CAS.
- M. Jeong, I. W. Choi, E. M. Go, Y. Cho, M. Kim, B. Lee, S. Jeong, Y. Jo, H. W. Choi, J. Lee, J. H. Bae, S. K. Kwak, D. S. Kim and C. Yang, Science, 2020, 369, 1615–1620 CrossRef CAS PubMed.
- J. J. Yoo, G. Seo, M. R. Chua, T. G. Park, Y. Lu, F. Rotermund, Y. K. Kim, C. S. Moon, N. J. Jeon, J. P. Correa-Baena, V. Bulović, S. S. Shin, M. G. Bawendi and J. Seo, Nature, 2021, 590, 587–593 CrossRef CAS PubMed.
- J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat, A. Mishra, Y. Yang, M. A. Hope, F. T. Eickemeyer, M. Kim, Y. J. Yoon, I. W. Choi, B. P. Darwich, S. J. Choi, Y. Jo, J. H. Lee, B. Walker, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, A. Hagfeldt, D. S. Kim, M. Grätzel and J. Y. Kim, Nature, 2021, 592, 381–385 CrossRef CAS PubMed.
- K. Zhao, Y. Li, H. Cheng, K. Hu and Z. S. Wang, J. Power Sources, 2020, 472, 9–12 CrossRef.
- S. Yang, H. Zhao, M. Wu, S. Yuan, Y. Han, Z. Liu, K. Guo, S. (Frank) Liu, S. Yang, H. Zhao, S. Yuan, Y. Han, Z. Liu, S. Liu, M. Wu and K. Guo, Sol. Energy Mater. Sol. Cells, 2019, 201, 110052 CrossRef CAS.
- C. C. Tseng, L. C. Chen, L. B. Chang, G. M. Wu, W. S. Feng, M. J. Jeng, D. W. Chen and K. L. Lee, Sol. Energy, 2020, 204, 270–279 CrossRef CAS.
- M. I. Saidaminov, K. Williams, M. Wei, A. Johnston, R. Quintero-Bermudez, M. Vafaie, J. M. Pina, A. H. Proppe, Y. Hou, G. Walters, S. O. Kelley, W. A. Tisdale and E. H. Sargent, Nat. Mater., 2020, 19, 412–418 CrossRef CAS PubMed.
- S. Karthick, H. Hawashin, N. Parou, S. Vedraine, S. Velumani and J. Bouclé, Sol. Energy, 2021, 218, 226–236 CrossRef CAS.
- N. K. Pendyala, S. Magdassi and L. Etgar, ACS Appl. Mater. Interfaces, 2021, 13, 30524–30532 CrossRef CAS PubMed.
- H. T. Hussein, R. S. Zamel, M. S. Mohamed and M. K. A. Mohammed, J. Phys. Chem. Solids, 2021, 149, 109792 CrossRef CAS.
- B. Yu, L. Zhang, J. Wu, K. Liu, H. Wu, J. Shi, Y. Luo, D. Li, Z. Bo and Q. Meng, J. Mater. Chem. A, 2020, 8, 1417–1424 RSC.
- X. Li, S. Ke, X. X. Feng, X. Zhao, W. Zhang and J. Fang, J. Mater. Chem. A, 2021, 9, 12684–12689 RSC.
- L. Xu, X. Feng, W. Jia, W. Lv, A. Mei, Y. Zhou, Q. Zhang, R. Chen and W. Huang, Energy Environ. Sci., 2021, 14, 4292–4317 RSC.
- T. Ye, K. Wang, Y. Hou, D. Yang, N. Smith, B. Magill, J. Yoon, R. R. H. H. Mudiyanselage, G. A. Khodaparast, K. Wang and S. Priya, J. Am. Chem. Soc., 2021, 143, 4319–4328 CrossRef CAS PubMed.
- K. D. Jayan and V. Sebastian, Adv. Theory Simul., 2021, 4, 1–11 Search PubMed.
- Z. Xiao and Y. Yan, Adv. Energy Mater., 2017, 7, 1–20 CAS.
- H. Li, Q. Wei and Z. Ning, Appl. Phys. Lett., 2020, 117, 060502 CrossRef CAS.
- J. K. Nam, M. S. Jung, S. U. Chai, Y. J. Choi, D. Kim and J. H. Park, J. Phys. Chem. Lett., 2017, 8, 2936–2940 CrossRef CAS PubMed.
- A. Bonadio, C. A. Escanhoela, F. P. Sabino, G. Sombrio, V. G. De Paula, F. F. Ferreira, A. Janotti, G. M. Dalpian and J. A. Souza, J. Mater. Chem. A, 2021, 9, 1089–1099 RSC.
- T. Chen, B. J. Foley, C. Park, C. M. Brown, L. W. Harriger, J. Lee, J. Ruff, M. Yoon, J. J. Choi and S. H. Lee, Sci. Adv., 2016, 2, 1–7 Search PubMed.
- G. Murugadoss, P. Arunachalam, S. K. Panda, M. Rajesh Kumar, J. R. Rajabathar, H. Al-Lohedan and M. D. Wasmiah, J. Mater. Res. Technol., 2021, 12, 1924–1930 CrossRef CAS.
- L. Guo, G. Xu, G. Tang, D. Fang and J. Hong, Nanotechnology, 2020, 31, 225204 CrossRef CAS PubMed.
- G. Tong, D. Y. Son, L. K. Ono, H. B. Kang, S. He, L. Qiu, H. Zhang, Y. Liu, J. Hieulle and Y. Qi, Nano Energy, 2021, 87, 106152 CrossRef CAS.
- D. Huang, P. Xie, Z. Pan, H. Rao and X. Zhong, J. Mater. Chem. A, 2019, 7, 22420–22428 RSC.
- S. Wang, Z. Ma, B. Liu, W. Wu, Y. Zhu, R. Ma and C. Wang, Sol. RRL, 2018, 2, 1–10 CAS.
- N. Ahn, D. Y. Son, I. H. Jang, S. M. Kang, M. Choi and N. G. Park, J. Am. Chem. Soc., 2015, 137, 8696–8699 CrossRef CAS PubMed.
- Y. Liu, I. Shin, I. W. Hwang, S. Kim, J. Lee, M. S. Yang, Y. K. Jung, J. W. Jang, J. H. Jeong, S. H. Park and K. H. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 12382–12390 CrossRef CAS PubMed.
- L. A. Frolova, A. I. Davlethanov, N. N. Dremova, I. Zhidkov, A. F. Akbulatov, E. Z. Kurmaev, S. M. Aldoshin, K. J. Stevenson and P. A. Troshin, J. Phys. Chem. Lett., 2020, 11, 6772–6778 CrossRef CAS PubMed.
- J. W. Lee, D. J. Seol, A. N. Cho and N. G. Park, Adv. Mater., 2014, 26, 4991–4998 CrossRef CAS PubMed.
- M. B. Johansson, L. Xie, B. J. Kim, J. Thyr, T. Kandra, E. M. J. Johansson, M. Göthelid, T. Edvinsson and G. Boschloo, Nano Energy, 2020, 78, 105346 CrossRef CAS.
- B. A. Al-Asbahi, S. M. H. Qaid, M. Hezam, I. Bedja, H. M. Ghaithan and A. S. Aldwayyan, Opt. Mater., 2020, 103, 109836 CrossRef CAS.
- T. Niu, J. Lu, M. C. Tang, D. Barrit, D. M. Smilgies, Z. Yang, J. Li, Y. Fan, T. Luo, I. McCulloch, A. Amassian, S. Liu and K. Zhao, Energy Environ. Sci., 2018, 11, 3358–3366 RSC.
- H.-C. Liao, P. Guo, C.-P. Hsu, M. Lin, B. Wang, L. Zeng, W. Huang, C. M. M. Soe, W.-F. Su, M. J. Bedzyk, M. R. Wasielewski, A. Facchetti, R. P. H. Chang, M. G. Kanatzidis and T. J. Marks, Adv. Energy Mater., 2017, 7, 1601660 CrossRef.
- L. Tzounis, T. Stergiopoulos, A. Zachariadis, C. Gravalidis, A. Laskarakis and S. Logothetidis, Mater. Today: Proc., 2017, 4, 5082–5089 Search PubMed.
- X. Dong, D. Chen, J. Zhou, Y. Z. Zheng and X. Tao, Nanoscale, 2018, 10, 7218–7227 RSC.
- H. Kim, H. R. Byun and M. S. Jeong, Sci. Rep., 2019, 9, 1–7 Search PubMed.
- H. X. Dang, K. Wang, M. Ghasemi, M. C. Tang, M. De Bastiani, E. Aydin, E. Dauzon, D. Barrit, J. Peng, D. M. Smilgies, S. De Wolf and A. Amassian, Joule, 2019, 3, 1746–1764 CrossRef CAS.
- S. Moghadamzadeh, I. M. Hossain, T. Duong, S. Gharibzadeh, T. Abzieher, H. Pham, H. Hu, P. Fassl, U. Lemmer, B. A. Nejand and U. W. Paetzold, J. Mater. Chem. A, 2020, 8, 24608–24619 RSC.
- Y. Yu, C. Wang, C. R. Grice, N. Shrestha, D. Zhao, W. Liao, L. Guan, R. A. Awni, W. Meng, A. J. Cimaroli, K. Zhu, R. J. Ellingson and Y. Yan, ACS Energy Lett., 2017, 2, 1177–1182 CrossRef CAS.
- S. Ham, Y. J. Choi, J. W. Lee, N. G. Park and D. Kim, J. Phys. Chem. C, 2017, 121, 3143–3148 CrossRef CAS.
- G. Tang, P. You, Q. Tai, R. Wu and F. Yan, Sol. RRL, 2018, 2, 1–9 CAS.
- J. Chen, S. G. Kim, X. Ren, H. S. Jung and N. G. Park, J. Mater. Chem. A, 2019, 7, 4977–4987 RSC.
- M. J. Choi, Y. S. Lee, I. H. Cho, S. S. Kim, D. H. Kim, S. N. Kwon and S. I. Na, Nano Energy, 2020, 71, 104639 CrossRef CAS.
- A. Mishra and Z. Ahmad, J. Mater. Sci.: Mater. Electron., 2019, 30, 20320–20329 CrossRef CAS.
- D. Zheng, C. Tong, T. Zhu, Y. Rong and T. Pauporté, Nanomaterials, 2020, 10, 1–15 Search PubMed.
- S. M. P. Meroni, C. Worsley, D. Raptis and T. M. Watson, Energies, 2021, 14 Search PubMed.
- G. Grancini, C. Roldán-Carmona, I. Zimmermann, E. Mosconi, X. Lee, D. Martineau, S. Narbey, F. Oswald, F. De Angelis, M. Graetzel and M. K. Nazeeruddin, Nat. Commun., 2017, 8, 1–8 CrossRef PubMed.
- Y. Sheng, W. Ji, Y. Chu, Y. Ming, A. Mei, Y. Hu, Y. Rong and H. Han, Sol. RRL, 2020, 4, 1–7 Search PubMed.
- A. Mei, Y. Sheng, Y. Ming, Y. Hu, Y. Rong, W. Zhang, S. Luo, G. Na, C. Tian, X. Hou, Y. Xiong, Z. Zhang, S. Liu, S. Uchida, T.-W. Kim, Y. Yuan, L. Zhang, Y. Zhou and H. Han, Joule, 2020, 4, 2646–2660 CrossRef CAS.
- M. Xu, W. Ji, Y. Sheng, Y. Wu, H. Cheng, J. Meng, Z. Yan, J. Xu, A. Mei, Y. Hu, Y. Rong and H. Han, Nano Energy, 2020, 74, 1–8 Search PubMed.
- S. G. Hashmi, D. Martineau, X. Li, M. Ozkan, A. Tiihonen, M. I. Dar, T. Sarikka, S. M. Zakeeruddin, J. Paltakari, P. D. Lund and M. Grätzel, Adv. Mater. Technol., 2017, 2, 4–9 Search PubMed.
- A. Verma, D. Martineau, S. Abdolhosseinzadeh, J. Heier and F. Nüesch, Mater. Adv., 2020, 1, 153–160 RSC.
- S. Bai, P. Da, C. Li, Z. Wang, Z. Yuan, F. Fu, M. Kawecki, X. Liu, N. Sakai, J. T. W. Wang, S. Huettner, S. Buecheler, M. Fahlman, F. Gao and H. J. Snaith, Nature, 2019, 571, 245–250 CrossRef CAS PubMed.
- L. Zhou, Z. Lin, Z. Ning, T. Li, X. Guo, J. Ma, J. Su, C. Zhang, J. Zhang, S. Liu, J. Chang and Y. Hao, Sol. RRL, 2019, 3, 1–10 Search PubMed.
- C. Gao, H. Dong, X. Bao, Y. Zhang, A. Saparbaev, L. Yu, S. Wen, R. Yang and L. Dong, J. Mater. Chem. C, 2018, 6, 8234–8241 RSC.
- F. Liu, X. Zuo, K. Wang, H. Bao, L. Liu, Z. Guo, S. Wang and S. F. Liu, Sol. RRL, 2021, 5, 1–10 Search PubMed.
- L. Chao, T. Niu, H. Gu, Y. Yang, Q. Wei, Y. Xia, W. Hui, S. Zuo, Z. Zhu, C. Pei, X. Li, J. Zhang, J. Fang, G. Xing, H. Li, X. Huang, X. Gao, C. Ran, L. Song, L. Fu, Y. Chen and W. Huang, Research, 2020, 2020, 2616345 CrossRef CAS PubMed.
- T. Niu, L. Chao, W. Gao, C. Ran, L. Song, Y. Chen, L. Fu and W. Huang, ACS Energy Lett., 2021, 6, 1453–1479 CrossRef CAS.
- L. Chao, Y. Xia, B. Li, G. Xing, Y. Chen and W. Huang, Chem, 2019, 5, 995–1006 CAS.
- N. Park, Nature Sustainability, 2021, 4, 192–193 CrossRef.
- T. Bu, L. Wu, X. Liu, X. Yang, P. Zhou, X. Yu, T. Qin, J. Shi, S. Wang, S. Li, Z. Ku, Y. Peng, F. Huang, Q. Meng, Y.-B. Cheng and J. Zhong, Adv. Energy Mater., 2017, 7, 1700576 CrossRef.
- Q. Zhang, F. Hao, J. Li, Y. Zhou, Y. Wei and H. Lin, Sci. Technol. Adv. Mater., 2018, 19, 425–442 CrossRef CAS PubMed.
- R. Guo, B. Dahal, A. Thapa, Y. R. Poudel, Y. Liu and W. Li, Nanoscale Adv., 2021, 3, 2056–2064 RSC.
- H. Zhu, Y. Ren, L. Pan, O. Ouellette, F. T. Eickemeyer, Y. Wu, X. Li, S. Wang, H. Liu, X. Dong, S. M. Zakeeruddin, Y. Liu, A. Hagfeldt and M. Grätzel, J. Am. Chem. Soc., 2021, 143, 3231–3237 CrossRef CAS PubMed.
- J. Li, H.-L. Cao, W.-B. Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Nat. Commun., 2020, 11, 310 CrossRef CAS PubMed.
- M. Krebs-Moberg, M. Pitz, T. L. Dorsette and S. H. Gheewala, Renewable Energy, 2021, 164, 556–565 CrossRef CAS.
- T. Ibn-Mohammed, S. C. L. Koh, I. M. Reaney, A. Acquaye, G. Schileo, K. B. Mustapha and R. Greenough, Renewable Sustainable Energy Rev., 2017, 80, 1321–1344 CrossRef CAS.
- R. Vidal, J. Alberola-Borràs, N. Sánchez-Pantoja and I. Mora-Seró, Advanced Energy and Sustainability Research, 2021, 2, 2000088 CrossRef.
- L. Gollino and T. Pauporté, Sol. RRL, 2021, 5, 1–28 Search PubMed.
- M. Wang, W. Wang, B. Ma, W. Shen, L. Liu, K. Cao, S. Chen and W. Huang, Lead-Free Perovskite Materials for Solar Cells, Springer, Singapore, 2021, vol. 13 Search PubMed.
- K. Nishimura, M. A. Kamarudin, D. Hirotani, K. Hamada, Q. Shen, S. Iikubo, T. Minemoto, K. Yoshino and S. Hayase, Nano Energy, 2020, 74, 104858 CrossRef CAS.
- T. Wu, X. Liu, X. He, Y. Wang, X. Meng, T. Noda, X. Yang and L. Han, Sci. China: Chem., 2020, 63, 107–115 CrossRef CAS.
- Z. Jin, B. Bin Yu, M. Liao, D. Liu, J. Xiu, Z. Zhang, E. Lifshitz, J. Tang, H. Song and Z. He, J. Energy Chem., 2021, 54, 414–421 CrossRef.
- I. Kopacic, B. Friesenbichler, S. F. Hoefler, B. Kunert, H. Plank, T. Rath and G. Trimmel, ACS Appl. Energy Mater., 2018, 1, 343–347 CrossRef CAS.
- N. Ito, M. A. Kamarudin, D. Hirotani, Y. Zhang, Q. Shen, Y. Ogomi, S. Iikubo, T. Minemoto, K. Yoshino and S. Hayase, J. Phys. Chem. Lett., 2018, 9, 1682–1688 CrossRef CAS PubMed.
- A. Singh, P. T. Lai, A. Mohapatra, C. Y. Chen, H. W. Lin, Y. J. Lu and C. W. Chu, Chem. Eng. J., 2021, 419, 129424 CrossRef CAS.
- N. Giesbrecht, A. Weis and T. Bein, J. Phys Energy, 2020, 2, 024007 CrossRef CAS.
- Y. Yang, C. Liu, M. Cai, Y. Liao, Y. Ding, S. Ma, X. Liu, M. Guli, S. Dai and M. K. Nazeeruddin, ACS Appl. Mater. Interfaces, 2020, 12, 17062–17069 CrossRef CAS PubMed.
- W. Hu, X. He, Z. Fang, W. Lian, Y. Shang, X. Li, W. Zhou, M. Zhang, T. Chen, Y. Lu, L. Zhang, L. Ding and S. Yang, Nano Energy, 2020, 68, 104362 CrossRef CAS.
- D. B. Khadka, Y. Shirai, M. Yanagida and K. Miyano, J. Mater. Chem. C, 2019, 7, 8335–8343 RSC.
- F. Bai, Y. Hu, Y. Hu, T. Qiu, X. Miao and S. Zhang, Sol. Energy Mater. Sol. Cells, 2018, 184, 15–21 CrossRef CAS.
- J. Li, J. Duan, X. Yang, Y. Duan, P. Yang and Q. Tang, Nano Energy, 2021, 80, 105526 CrossRef CAS.
- X. Liu, T. Wu, J. Y. Chen, X. Meng, X. He, T. Noda, H. Chen, X. Yang, H. Segawa, Y. Wang and L. Han, Energy Environ. Sci., 2020, 13, 2896–2902 RSC.
- Z. Yang, M. Zhong, Y. Liang, L. Yang, X. Liu, Q. Li, J. Zhang and D. Xu, Adv. Funct. Mater., 2019, 29, 1903621 CrossRef CAS.
- W. Ke, P. Priyanka, S. Vegiraju, C. C. Stoumpos, I. Spanopoulos, C. M. M. Soe, T. J. Marks, M. C. Chen and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 140, 388–393 CrossRef CAS PubMed.
- Z. Chen, B. Turedi, A. Y. Alsalloum, C. Yang, X. Zheng, I. Gereige, A. Alsaggaf, O. F. Mohammed and O. M. Bakr, ACS Energy Lett., 2019, 4, 1258–1259 CrossRef CAS.
- Y. Zhang, S. Seo, S. Y. Lim, Y. Kim, S. G. Kim, D. K. Lee, S. H. Lee, H. Shin, H. Cheong and N. G. Park, ACS Energy Lett., 2020, 360–366 CrossRef CAS.
- Y. Wang, X. Liu, T. Zhang, X. Wang, M. Kan, J. Shi and Y. Zhao, Angew. Chem., Int. Ed., 2019, 58, 16691–16696 CrossRef CAS PubMed.
- M. Saliba, T. Matsui, J. Y. Seo, K. Domanski, J. P. Correa-Baena, M. K. Nazeeruddin, S. M. Zakeeruddin, W. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Energy Environ. Sci., 2016, 9, 1989–1997 RSC.
- E. H. Jung, N. J. Jeon, E. Y. Park, C. S. Moon, T. J. Shin, T. Y. Yang, J. H. Noh and J. Seo, Nature, 2019, 567, 511–515 CrossRef CAS PubMed.
- J. J. Yoo, S. Wieghold, M. C. Sponseller, M. R. Chua, S. N. Bertram, N. T. P. Hartono, J. S. Tresback, E. C. Hansen, J. P. Correa-Baena, V. Bulović, T. Buonassisi, S. S. Shin and M. G. Bawendi, Energy Environ. Sci., 2019, 12, 2192–2199 RSC.
- E. Jung, K. Budzinauskas, S. Öz, F. Ünlü, H. Kuhn, J. Wagner, D. Grabowski, B. Klingebiel, M. Cherasse, J. Dong, P. Aversa, P. Vivo, T. Kirchartz, T. Miyasaka, P. H. M. Van Loosdrecht, L. Perfetti and S. Mathur, ACS Energy Lett., 2020, 5, 785–792 CrossRef CAS.
- F. Zhang, D. Bi, N. Pellet, C. Xiao, Z. Li, J. J. Berry, S. M. Zakeeruddin, K. Zhu and M. Grätzel, Energy Environ. Sci., 2018, 11, 3480–3490 RSC.
- N. Li, S. Tao, Y. Chen, X. Niu, C. K. Onwudinanti, C. Hu, Z. Qiu, Z. Xu, G. Zheng, L. Wang, Y. Zhang, L. Li, H. Liu, Y. Lun, J. Hong, X. Wang, Y. Liu, H. Xie, Y. Gao, Y. Bai, S. Yang, G. Brocks, Q. Chen and H. Zhou, Nat. Energy, 2019, 4, 408–415 CrossRef CAS.
- C. H. Ng, K. Nishimura, N. Ito, K. Hamada, D. Hirotani, Z. Wang, F. Yang, S. likubo, Q. Shen, K. Yoshino, T. Minemoto and S. Hayase, Nano Energy, 2019, 58, 130–137 CrossRef CAS.
- N. Pai, J. Lu, T. R. Gengenbach, A. Seeber, A. S. R. Chesman, L. Jiang, D. C. Senevirathna, P. C. Andrews, U. Bach, Y. B. Cheng and A. N. Simonov, Adv. Energy Mater., 2019, 9, 1–11 Search PubMed.
- F. D. Giacomo, H. Fledderus, H. Gorter, G. Kirchner, I. d. Vries, I. Dogan, W. Verhees, V. Zardetto, M. Najafi, D. Zhang, H. Lifka, Y. Galagan, T. Aernouts, S. Veenstra, P. Groen and R. Andriesse, in 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), 2018, pp. 2795–2798 Search PubMed.
- X. Li, D. Bi, C. Yi, J.-D. Décoppet, J. Luo, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Science, 2016, 353, 58–62 CrossRef CAS PubMed.
- Q. He, K. Yao, X. Wang, X. Xia, S. Leng and F. Li, ACS Appl. Mater. Interfaces, 2017, 9, 41887–41897 CrossRef CAS PubMed.
- C. Li, J. Yin, R. Chen, X. Lv, X. Feng, Y. Wu and J. Cao, J. Am. Chem. Soc., 2019, 141, 6345–6351 CrossRef CAS PubMed.
- G. Li, Y. Jiang, S. Deng, A. Tam, P. Xu, M. Wong and H.-S. Kwok, Adv. Sci., 2017, 4, 1700463 CrossRef PubMed.
- Z. Li, T. R. Klein, D. H. Kim, M. Yang, J. J. Berry, M. F. A. M. van Hest and K. Zhu, Nat. Rev. Mater., 2018, 3, 18017 CrossRef CAS.
- P. Zhao, B. J. Kim, X. Ren, D. G. Lee, G. J. Bang, J. B. Jeon, W. Bin Kim and H. S. Jung, Adv. Mater., 2018, 30, 1802763 CrossRef PubMed.
- W. Qiu, T. Merckx, M. Jaysankar, C. Masse de la Huerta, L. Rakocevic, W. Zhang, U. W. Paetzold, R. Gehlhaar, L. Froyen, J. Poortmans, D. Cheyns, H. J. Snaith and P. Heremans, Energy Environ. Sci., 2016, 9, 484–489 RSC.
- T. Bu, X. Liu, R. Chen, Z. Liu, K. Li, W. Li, Y. Peng, Z. Ku, F. Huang, Y.-B. Cheng and J. Zhong, J. Mater. Chem. A, 2018, 6, 6319–6326 RSC.
- F. Matteocci, S. Razza, F. Di Giacomo, S. Casaluci, G. Mincuzzi, T. M. Brown, A. D'Epifanio, S. Licoccia and A. Di Carlo, Phys. Chem. Chem. Phys., 2014, 16, 3918–3923 RSC.
- J. Seo, S. Park, Y. Chan Kim, N. J. Jeon, J. H. Noh, S. C. Yoon and S. Il Seok, Energy Environ. Sci., 2014, 7, 2642–2646 RSC.
- S. Razza, F. D. Giacomo, F. Matteocci, L. Cinà, A. L. Palma, S. Casaluci, P. Cameron, A. D’Epifanio, S. Licoccia, A. Reale, T. M. Brown and A. D. Carlo, J. Power Sources, 2015, 277, 286–291 CrossRef CAS.
- K. Hwang, Y.-S. Jung, Y.-J. Heo, F. H. Scholes, S. E. Watkins, J. Subbiah, D. J. Jones, D.-Y. Kim and D. Vak, Adv. Mater., 2015, 27, 1241–1247 CrossRef CAS PubMed.
- G. Cotella, J. Baker, D. Worsley, F. De Rossi, C. Pleydell-Pearce, M. Carnie and T. Watson, Sol. Energy Mater. Sol. Cells, 2017, 159, 362–369 CrossRef CAS.
- J. Zhang, T. Bu, J. Li, H. Li, Y. Mo, Z. Wu, Y. Liu, X.-L. Zhang, Y.-B. Cheng and F. Huang, J. Mater. Chem. A, 2020, 8, 8447–8454 RSC.
- Y. Deng, X. Zheng, Y. Bai, Q. Wang, J. Zhao and J. Huang, Nat. Energy, 2018, 3, 560–566 CrossRef CAS.
- F. Di Giacomo, S. Shanmugam, H. Fledderus, B. J. Bruijnaers, W. J. H. Verhees, M. S. Dorenkamper, S. C. Veenstra, W. Qiu, R. Gehlhaar, T. Merckx, T. Aernouts, R. Andriessen and Y. Galagan, Sol. Energy Mater. Sol. Cells, 2018, 181, 53–59 CrossRef CAS.
- Y. Hu, S. Si, A. Mei, Y. Rong, H. Liu, X. Li and H. Han, Sol. RRL, 2017, 1, 1600019 CrossRef.
- NEDO and Toshiba develops world's largest film-based perovskite photovoltaic module – 703 cm2 module achieves 11.7% power conversion efficiency, accessed on 16.09.2021, https://www.global.toshiba/ww/technology/corporate/rdc/rd/topics/18/1806-03.html.
- G. Overton, Saule prints flexible perovskite solar modules with consistent 10% efficiency, accessed on 16.09.2021, https://www.laserfocusworld.com/detectors-imaging/article/14035450/saule-prints-flexible-perovskite-solar-modules-with-consistent-10-efficiency.
- T. Bu, S. Shi, J. Li, Y. Liu, J. Shi, L. Chen, X. Liu, J. Qiu, Z. Ku, Y. Peng, J. Zhong, Y.-B. Cheng and F. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 14922–14929 CrossRef CAS PubMed.
- S. Hong, G. Kim, B. Park, J.-H. Kim, J. Kim, Y. Pak, J. Kim, S. Kwon and K. Lee, J. Mater. Chem. A, 2020, 8, 18659–18667 RSC.
- A. Agresti, S. Pescetelli, A. L. Palma, A. E. Del Rio Castillo, D. Konios, G. Kakavelakis, S. Razza, L. Cinà, E. Kymakis, F. Bonaccorso and A. Di Carlo, ACS Energy Lett., 2017, 2, 279–287 CrossRef CAS.
- A. Priyadarshi, L. J. Haur, P. Murray, D. Fu, S. Kulkarni, G. Xing, T. C. Sum, N. Mathews and S. G. Mhaisalkar, Energy Environ. Sci., 2016, 9, 3687–3692 RSC.
- G. Grancini, C. Roldán-Carmona, I. Zimmermann, E. Mosconi, X. Lee, D. Martineau, S. Narbey, F. Oswald, F. De Angelis, M. Graetzel and M. K. Nazeeruddin, Nat. Commun., 2017, 8, 15684 CrossRef CAS PubMed.
- F. De Rossi, J. A. Baker, D. Beynon, K. E. A. Hooper, S. M. P. Meroni, D. Williams, Z. Wei, A. Yasin, C. Charbonneau, E. H. Jewell and T. M. Watson, Adv. Mater. Technol., 2018, 3, 1800156 CrossRef.
- M. Yang, Z. Li, M. O. Reese, O. G. Reid, D. H. Kim, S. Siol, T. R. Klein, Y. Yan, J. J. Berry, M. F. A. M. van Hest and K. Zhu, Nat. Energy, 2017, 2, 17038 CrossRef CAS.
- Y. Deng, X. Zheng, Y. Bai, Q. Wang, J. Zhao and J. Huang, Nat. Energy, 2018, 3, 560–566 CrossRef CAS.
- D. N. Jeong, D. K. Lee, S. Seo, S. Y. Lim, Y. Zhang, H. Shin, H. Cheong and N. G. Park, ACS Energy Lett., 2019, 4, 1189–1195 CrossRef CAS.
- L. Cai, L. Liang, J. Wu, B. Ding, L. Gao and B. Fan, J. Semicond., 2017, 38, 014006 CrossRef.
- F. Di, S. Shanmugam, H. Fledderus, B. J. Bruijnaers, W. J. H. Verhees, M. S. Dorenkamper, S. C. Veenstra and W. Qiu, Sol. Energy Mater. Sol. Cells, 2018, 181, 53–59 CrossRef.
- J. H. Heo, M. H. Lee, M. H. Jang and S. H. Im, J. Mater. Chem. A, 2016, 4, 17636–17642 RSC.
- N. Rolston, W. J. Scheideler, A. C. Flick, J. P. Chen, H. Elmaraghi, A. Sleugh, O. Zhao, M. Woodhouse and R. H. Dauskardt, Joule, 2020, 4, 2675–2692 CrossRef CAS.
- X. Dai, Y. Deng, C. H. Van Brackle, S. Chen, P. N. Rudd, X. Xiao, Y. Lin, B. Chen and J. Huang, Adv. Energy Mater., 2020, 10, 1903108 CrossRef CAS.
- J. Chung, S. S. Shin, K. Hwang, G. Kim, K. W. Kim, D. S. Lee, W. Kim, B. S. Ma, Y.-K. Kim, T.-S. Kim and J. Seo, Energy Environ. Sci., 2020, 13, 4854–4861 RSC.
- T. Bu, J. Li, F. Zheng, W. Chen, X. Wen, Z. Ku, Y. Peng, J. Zhong, Y.-B. Cheng and F. Huang, Nat. Commun., 2018, 9, 4609 CrossRef PubMed.
- B. Abdollahi Nejand, P. Nazari, S. Gharibzadeh, V. Ahmadi and A. Moshaii, Chem. Commun., 2017, 53, 747–750 RSC.
- F. Yang, S. Wang, P. Dai, L. Chen, A. Wakamiya and K. Matsuda, J. Mater. Chem. A, 2021, 9, 2612–2627 RSC.
- F.-W. Liu, G. Biesold, M. Zhang, R. Lawless, J.-P. Correa-Baena, Y.-L. Chueh and Z. Lin, Mater. Today, 2021, 43, 185–197 CrossRef CAS.
- B. J. Kim, D. H. Kim, S. L. Kwon, S. Y. Park, Z. Li, K. Zhu and H. S. Jung, Nat. Commun., 2016, 7, 11735 CrossRef CAS PubMed.
- S.-Y. Bae, S. Y. Lee, J. Kim, H. N. Umh, J. Jeong, S. Bae, J. Yi, Y. Kim and J. Choi, Sci. Rep., 2019, 9, 4242 CrossRef PubMed.
- A. Urbina, J. Phys. Energy, 2020, 2, 22001 CrossRef CAS.
- R. Vidal, J.-A. Alberola-Borràs, N. Sánchez-Pantoja and I. Mora-Seró, Advanced Energy and Sustainability Research, 2021, 2, 2000088 CrossRef.
- G. Schileo and G. Grancini, J. Mater. Chem. C, 2021, 9, 67–76 RSC.
- M. V. Khenkin, E. A. Katz, A. Abate, G. Bardizza, J. J. Berry, C. Brabec, F. Brunetti, V. Bulović, Q. Burlingame, A. Di Carlo, R. Cheacharoen, Y. B. Cheng, A. Colsmann, S. Cros, K. Domanski, M. Dusza, C. J. Fell, S. R. Forrest, Y. Galagan, D. Di Girolamo, M. Grätzel, A. Hagfeldt, E. von Hauff, H. Hoppe, J. Kettle, H. Köbler, M. S. Leite, S. (Frank) Liu, Y. L. Loo, J. M. Luther, C. Q. Ma, M. Madsen, M. Manceau, M. Matheron, M. McGehee, R. Meitzner, M. K. Nazeeruddin, A. F. Nogueira, Ç. Odabaşı, A. Osherov, N. G. Park, M. O. Reese, F. De Rossi, M. Saliba, U. S. Schubert, H. J. Snaith, S. D. Stranks, W. Tress, P. A. Troshin, V. Turkovic, S. Veenstra, I. Visoly-Fisher, A. Walsh, T. Watson, H. Xie, R. Yıldırım, S. M. Zakeeruddin, K. Zhu and M. Lira-Cantu, Nat. Energy, 2020, 5, 35–49 CrossRef.
- S. Castro-Hermosa, G. Lucarelli, M. Top, M. Fahland, J. Fahlteich and T. M. Brown, Cell Rep. Phys. Sci., 2020, 1, 100045 CrossRef.
- X. He, J. Chen, X. Ren, L. Zhang, Y. Liu, J. Feng, J. Fang, K. Zhao and S. Liu, Adv. Mater., 2021, 2100770, 1–10 Search PubMed.
- Versatility of Solar PV Technology: Saule Technologies to Provide Perovskite Solar Cells for Animal Tracking Devices under WWF Project to Save European Bison, accessed on 16.09.2021, http://taiyangnews.info/technology/wwf-project-to-use-solar-cell-powered-animal-trackers/.
- Products-Saule Technologies, accessed on 16.09.2021, https://sauletech.com/product/.
- Y. Zhu, L. Shu, Q. Zhang, Y. Zhu, S. Poddar, C. Wang, Z. He and Z. Fan, EcoMat, 2021, 3, e12117, DOI:10.1002/eom2.12117 , accessed on 16.09.2021..
- S. Kim, J. Yi and J. Kim, Sol. RRL, 2021, 5, 2100162 CrossRef.
- H. Alaa and N. Gharib, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 974, 12020, DOI:10.1088/1757-899X/974/1/012020.
- A. Y. Foo, M. H. Saw, Y. S. Khoo and S. E. R. Tay, in 2021 IEEE 48th Photovoltaic Specialists Conference (PVSC), 2021, pp. 272–277, accessed on 16.09.2021, https://ieeexplore.ieee.org/document/9518916 Search PubMed.
- D. Hardy, in PLEA 2013 Sustainable Architecture for a Renewable Future, 2013, accessed on 16.09.2021, https://www.researchgate.net/publication/284639897_Improving_the_Aesthetics_of_Photovoltaics_Through_Use_of_Coloured_Encapsulants Search PubMed.
- S. Conejos, M. Y. L. Chew, K. Tay, S. Tay and S. Safiena, Int. J. Build. Pathol. Adapt. DOI:10.1108/IJBPA-04-2019-0038.
- S. A. Al-Janahi, S. G. Al-Ghamdi, Environmental Impact Associated with the Performance of Building Integrated Photovoltaics: Life-Cycle Assessment Perspective, in Energy Systems EvaluationEnergy and Technology, 2021, ed. J. Ren, Green Energy and Technology, Springer, Cham, vol. 1 Search PubMed.
- P. Corti, P. Bonomo, F. Frontini, P. Mace, E. Bosch, Building Integrated Photovoltaics: A practical handbook for solar buildings' stakeholders, 2020 Search PubMed.
- L.-H. Chou, Y.-T. Yu, I. Osaka, X.-F. Wang and C.-L. Liu, J. Power Sources, 2021, 491, 229586 CrossRef CAS.
- S. Razza, F. Di Giacomo, F. Matteocci, L. Cinà, A. L. Palma, S. Casaluci, P. Cameron, A. D'Epifanio, S. Licoccia, A. Reale, T. M. Brown and A. Di Carlo, J. Power Sources, 2015, 277, 286–291 CrossRef CAS.
- F. Schackmar, H. Eggers, M. Frericks, B. S. Richards, U. Lemmer, G. Hernandez-Sosa and U. W. Paetzold, Adv. Mater. Technol., 2021, 6, 2000271 CrossRef CAS.
- A. Verma, D. Martineau, S. Abdolhosseinzadeh, J. Heier and F. Nüesch, Mater. Adv., 2020, 1, 153–160 RSC.
- N. K. Pendyala, S. Magdassi and L. Etgar, ACS Appl. Mater. Interfaces, 2021, 13, 30524–30532 CrossRef CAS PubMed.
- F. Mathies, H. Eggers, B. S. Richards, G. Hernandez-Sosa, U. Lemmer and U. W. Paetzold, ACS Appl. Energy Mater., 2018, 1, 1834–1839 CrossRef CAS.
- L. Zhang, S. Chen, X. Wang, D. Wang, Y. Li, Q. Ai, X. Sun, J. Chen, Y. Li, X. Jiang, S. Yang and B. Xu, Sol. RRL, 2021, 5, 2100106 CrossRef CAS.
- J. R. Castrejón-Pita, W. R. S. Baxter, J. Morgan, S. Temple, G. D. Martin and I. M. Hutchings, Atomization Sprays, 2013, 23, 571–595 Search PubMed.
- A. A. Castrejón-Pita, J. R. Castrejón-Pita and G. D. Martin, Rev. Sci. Instrum., 2012, 83, 115105 CrossRef PubMed.
- T. Lamminmäki, J. Kettle, H. Rautkoski, A. Kokko and P. Gane, Ind. Eng. Chem. Res., 2011, 50, 7251–7263 CrossRef.
- M. Makrygianni, A. Milionis, C. Kryou, I. Trantakis, D. Poulikakos and I. Zergioti, Adv. Mater. Interfaces, 2018, 5, 1800440 CrossRef.
- H. Sebastian, Master thesis, Aalto University, 2021, accessed on 16.09.2021, https://aaltodoc.aalto.fi/handle/123456789/107031.
| This journal is © The Royal Society of Chemistry 2021 |