Open Access Article
Open Access ArticleA review on phytochemical constituents and pharmacological potential of Calotropis procera
Barkha Darra Wadhwani
 a,
Deepak Mali
a,
Pooja Vyas
a,
Deepak Mali
a,
Pooja Vyas
 a,
Rashmy Nair
a,
Rashmy Nair
 b and
Poonam Khandelwal
b and
Poonam Khandelwal
 *a
*a
aDepartment of Chemistry, Mohanlal Sukhadia University, Udaipur-313001, India. E-mail: poonamkhandelwal@mlsu.ac.in
bDepartment of Chemistry, S.S. Jain Subodh P.G. College, Jaipur-302004, India
First published on 4th November 2021
Abstract
Calotropis procera is locally known as Aak or Madar in Hindi, milk weed in English and belongs to the family Apocynaceae and subfamily Asclepiadoideae. Although a wasteland plant, it is of sacred use as its flowers are offered for worshipping Lord Shiva, a Hindu God. Tribes all over the world use the plant in treatment of various diseases like snake bite, body pain, asthma, epilepsy, cancer, sexual disorders, skin diseases and many more. This plant contains various phytoconstituents such as flavonoids, terpenoids, cardenolides, steroids oxypregnanes etc. Though literature searches reveal many reviews about ethnomedicinal uses, chemical composition and pharmacological activities, no recent papers are available that provide an overview of the therapeutic potential and toxicity of Calotropis procera. Hence, the insight of this review is to provide a systemic summary of phytochemistry, pharmacology, toxicology and therapeutic potential of Calotropis procera and to highlight the gaps in the knowledge so as to offer inspiration for future research.
1. Introduction
Calotropis belongs to the Apocynaceae family, which is commonly known as milkweed or Aak. Plants of this genus are known as milkweeds due to the exudation of white and sticky latex from different plant parts. Genus Calotropis has two common species viz. Calotropis procera (Rakta arka) and Calotropis gigantea (Sweata arka), which are described as possessing vital pharmacological properties in Ayurvedic toxicology and therapeutics. Other species are C. sussuela and C. acia.Calotropis procera (Aiton) W. T. Aiton is an erect, soft wooded, evergreen perennial shrub and commonly known as ‘Sodom apple’ or ‘Madar shrub’. In Bengali, it is known as ‘Akanda’ and in Hindi as ‘Aak’. It manifests its wide utilization in Indian, Arabic and Sudanese traditional medicinal systems for healing global range of diseases.
The Dangas tribe in Gujarat,1 Singhum tribe in Bihar,2 tribes of Ghatigaon forest in Gwalior,3 tribes of Andhra Pradesh4 have been using this plant in the treatment of various disorders such as ear pain, cough, fever, abdominal pain, dysentery and elephantiasis.
Calotropis procera is more toxic than Calotropis gigantea and assumed to be even more poisonous than cobra venom. It is interesting that the cobra and other poisonous snakes cannot even bear its smell; hence snake charmers of Bengal use this plant for controlling or taming cobras.5
Earlier reviews6–16 have discussed on phytochemistry, ethnobotany and pharmacological potential of Calotropis procera. Review on Calotropis species17–20 comparing procera and gigantea have deliberated their therapeutic importance. The present review summarizes the phytochemistry, pharmacology, commercial aspects, traditional medicinal uses, toxicology and recent studies on Calotropis procera. The future scope of Calotropis procera has also been affirmed with a view to establish its multiple biological activities and mode of action.
2. Unique properties of Calotropis procera
2.1 Toxicity
C. procera finds its widespread distribution over many regions of the globe. What makes its phytochemistry interesting is the exudation of milky and toxic latex from all the plant parts. The latex is referred to as vegetable mercury as it shows mercury like effects on human body.21Every part of this plant is toxic, but stem (latex) and roots are more poisonous than leaves. The leaves of this plant have three toxic glycosides calotropin, calotoxin and uscharin, whereas its latex contains calotropin, calotoxin and calactin, which are caustic and considered poisonous in nature. Besides this, the concentration of calactin, which is a toxic glycoside, gets increased as defense mechanism on encounter of grasshopper or insect attack and this is the rationale behind the plant not being consumed by cattles or other grazing animals.22 Other than this, osmotin, a laticifer protein purified from latex also provides protection to plant against phytopathogens.23 Its milk is irritant, neurotoxic and has anticholinergic activity, which causes toxicity and fatal complications. Madar juice and latex has bitter taste and a burning pain which causes salivation, stomatitis, vomiting, diarrhoea, dilated pupils, titanic convulsion, collapse and death. The fatal period varies from half an hour to eight hours.24 If latex enters into the eye, it causes kerato-conjunctivitis, corneal edema and dimness of vision without any pain.25–27 Some cases showed permanent endothelial cell damage, which was evident after three weeks.5,28 C. procera was found toxic at the dose of 100 mg kg−1 to chick embryo. Its toxicity caused hepatocellular degeneration in liver, brain congestion, dilation of central veins, sinusoids, underdeveloped lung and kidneys.29 Hence, bearing in mind the toxic effects of certain extracts and glycosides, further studies should be focused to explain toxicity and safe use of C. procera.
2.2 Ability to survive under extreme climatic conditions
Another interesting aspect of this plant is its ability to tolerate adverse environmental conditions like scarcity of water, arid environment or any kind of harsh climate. To understand this, Akhkha30 studied the effect of stress caused due to water scarcity and found that photosynthetic machinery remained uninfluenced, infact rate of photosynthesis gets raised at mild water regime (50%) which can be considered as a compensatory mechanism. Further Ramadana et al.31 studied the influence of light and irrigation on cumulation of β-sitosterol in C. procera. They hypothesized that β-sitosterol biosynthesis pathway supported the plant to bear drought and light intensity stress.2.3 Commercial prospective
2.4 Industrial prospective
3. Ethnomedicinal uses
An insight into Ayurveda, Unani and folk uses of different parts of C. procera and C. gigantea to cure various ailments was compiled by Misra et al.43 Ethnomedicinal uses of plant parts of C. procera in curing various diseases have been summarized in Table 1.| Plant part | Disease | Preparation/administration | References |
|---|---|---|---|
| Root/root bark | Amoebic dysentery | Paste with/without opium taken orally | 44–46 |
| Cholera | Powder orally taken or paste along with black pepper and ginger juice | 44 | |
| Dysentery | Powder orally taken | 47 | |
| Elephantiasis and hydrocele | Paste mixed with fermented rice water applied on the affected area | 48–50 | |
| Epilepsy | Grounded with goat milk and used as nasal drops | 46 | |
| Indigestion | Powder orally taken | 47 | |
| Jaundice | Taken with rice in grounded form | 51 | |
| Neuritis | Orally administered with cow butter | 46 | |
| Rheumatism | Powder taken with milk and sugar | 48 | |
| Snake bite | Powder orally taken. Paste applied on wounds and internally taken with ghee | 47 and 52 | |
| Spider and insect bite | Powdered and taken with vinegar | 48 | |
| Syphilis | Root bark powder taken orally | 46 | |
| Latex | Boils | Applied externally | 46 |
| Black scar on the face | Applied along with turmeric paste | 44 | |
| Ascites | Applied externally | 47 | |
| Liver and spleen disorder | Taken after dilution | 47 | |
| Leprosy | Applied on the affected area | 47 | |
| Migraine | Applied on the affected side vein of forehead | 44 | |
| Piles (haemorrhoids) | Applied externally | 44 | |
| Dog/jackal bite | Applied on wound | 44 and 48 | |
| Ring worm | Applied externally | 46 | |
| Scabies | Applied externally | 46 | |
| Snake bite | Applied on wounds or taken orally (20–30 drops for adults and 15–20 for infants) | 46 | |
| Five drops with 50 drops of distilled water injected hypodermally | 46 | ||
| Syphilis, leprosy and odema | Applied externally with sesame oil | 48 and 50 | |
| Tooth ache | Applied on affected tooth | 48 and 50 | |
| Vertigo | Applied on affected parts | 53 | |
| Leaf | Cold, cough, asthma and bronchitis | Warmed along with ghee and bandaged on the chest of infants | 44 |
| Calculus, liver and spleen disorder | Powder taken orally | 48 | |
| Ear ache or ear troubles | Juice along with fermented boiled rice water used as ear drops | 50 | |
| Eczema and skin eruptions | Applied externally along with turmeric and sesame oil | 48, 50 and 53 | |
| Enlargement of abdominal viscera and spleen | Oral administration of powder | 48 and 51 | |
| Gonorrhoea | Decoction used for washing and taken orally | 51 | |
| Inflammatory swellings | Covered on affected part after warming | 51 | |
| Joint pain | Powder taken | 47 | |
| Malaria and intermittent fever | Oral administration of fresh juice | 46, 49 and 51 | |
| Body pain | Paste applied after warming | 51 | |
| Paralysis and sciatica | Massaged after preparing decoction with sesame oil | 47 | |
| Snake bite | Oral administration of fresh juice | 50 | |
| Ulcers, wounds, sores | Powder orally administered or external application | 47, 49 and 51 | |
| Flowers | Health tonic | Oral administration of powder | 47 |
| Cough | Burnt to produce ash, then taken with honey | 44 | |
| Rat bite | Oral administration of powder | 47 and 49 | |
| Dog/jackal bite (rabies) | Seven tepals chewed with fine rice on seventh day of biting, continued for seven days decreasing one tepal everyday | 44 | |
| Feet pain | Decoction used for fomentation | 46 | |
| Epilepsy | Oral administration of paste with black pepper | 46 | |
| Asthma and bronchitis | Fruit taken with jaggery | 3 | |
| Liver and spleen disorder | Administered along with milk | 46 | |
| Fruit | Eye disorder | Decanted ash water applied on eye lids | 44 |
| Anemia | Mixed with same quantity of red chilli, mineral salt and taken with milk. | 46 | |
| Whole plant | Rheumatic pain and hyperacidity | Paste directly taken | 44 |
| Young twigs | Purgative | Juice taken | 54 |
4. Major milestone of Calotropis phytochemistry
Phytochemistry of Calotropis procera has always attracted the attention of researchers because despite its toxicity, it employs wide applications in traditional medicinal system till date. Dating back to 1936, Hesse et al.55 identified calotropin as the first compound from this plant. Further Hesse and his coworkers56,57 isolated heart poisons or cardiac glycosides namely calotropin, calotoxin, calactin, uscharin, voruscharin and uscharidin.58 Root powder of this plant is used in tribes to induce abortion in women and as an uterotonic since ancient period. Later it was found that it was due to the compound calotropin. Gupta et al.59 administered calotropin to gerbils and rabbits and observed reduction in spermatids count by 65% and 94% respectively.In 1955, Rajagopalan et al.60 identified chemical constituents of seed viz. coroglaucigenin, corotoxigenin and frugoside (cardenolides). Later Bruschweiler et al.61 identified three additional cardenolides viz. uzarigenin, syriogenin and procerosid. A novel cardenolide, 2′′-oxovoruscharin was isolated from the root bark by Quaquebeke et al.62 and modified into its semisynthetic derivative, i.e., UNBS1450. Akhtar and Malik63 isolated a new cardenolide named proceragenin from the hexane-insoluble fraction of C. procera.
A fascinating feature of the plant is its potential to curb Alzheimer's disease (AD), the most predominant root cause of dementia, a neurodegenerative disease. Its dried latex showed attenuation of β-amyloid deposition in mouse brain and cerebral protective activities.64 Hence, it is imperative to evaluate the mechanism of metabolites, so that it can lead to promising direction to search new scaffolds for AD treatment. In 2015, Mohamed et al. isolated three non-glycosidic cardenolides namely calactoprocin, procegenin A and procegenin B from the latex.65
A patent claimed that polar extract of C. procera showed anti-ulcerative colitis activity in dose-dependent manner in a subject mammal and was found to be more effective than the standard drug Prednisolone.66
5. Pharmacology
Over the last many years, researchers have carried out numerable pharmacological activities, which are summarized in Table 2.| S. no. | Pharmacological activities | Parts/extracts/possible chemical constituents | References |
|---|---|---|---|
| 1 | Wound healing potential | Latex: aqueous extract | 67 |
| Latex | 68 | ||
| Bark: ethanolic extract | 69 | ||
| Leaves: aqueous extract | 70 | ||
| Bark: aqueous extract | 71 | ||
| 2 | Anticoccidial activity | Dried leaves powder | 72 |
| 3 | Toxicity activity | Leaves: aqueous extract | 73 and 74 |
| Leaves and stem bark extracts | 75 | ||
| Leaves and stem: ethanolic extract | 29 | ||
| Leaves: ethanolic extract | 79 | ||
| 4 | Biopesticidal/insecticidal activity | Leaves: extract | 80 and 81 |
| Leaves: methanolic extract, latex protein fraction, flavonoids (quercetin-3-O-rutinoside) | 35 | ||
| 5 | Antimycoplasmal activity | Leaves: acetone extract | 82 |
| 6 | Hepatoprotective activity | Root bark: methanolic extract | 83 |
| Flowers: hydroethanolic extract | 84 | ||
| Roots: chloroform extract | 85 | ||
| 7 | Antimicrobial/antibacterial activity | Leaves: methanolic extract, flavonoids (quercetin-3-O-rutinoside) | 86 |
| Leaves and latex: ethanol, aqueous, and chloroform extract | 87 | ||
| Leaves and stem: aqueous, ethanolic, methanolic extract | 88 and 89 | ||
| Endophytic fungi of C. procera | 90 | ||
| Seeds: chloroform extract | 91 | ||
| Root: pet. ether, methanolic extract | 92 | ||
| Flowers: ethanolic extract | 93 | ||
| Latex | 94 | ||
| Leaves: methanolic extract | 95 | ||
| Leaves, flower, root bark: ethanolic extract | 96 | ||
| Leaves and latex: aqueous, ethanolic extract | 97 and 98 | ||
| Leaves: aqueous, methanolic extract | 99 | ||
| Latex: aqueous extract | 78 | ||
| 8 | Central nervous system activity | Latex proteins | 100 |
| 9 | Antioxidant activity | Leaves, flower, fruit, latex | 101 |
| Leaves: aqueous, methanolic extract, quercetin and its derivatives | 76 | ||
| Leaves: aqueous and methanolic extract | 102 | ||
| Leaves, flowers and fruits: methanolic extract | 103 | ||
| Bark: ethanolic extract | 69 | ||
| 10 | Antinociceptive activity | Latex protein | 104 |
| 11 | Antihelmintic activity | Flowers: crude powder, aqueous and methanolic extract | 105 |
| Latex: fresh, dried aqueous extract | 106 and 107 | ||
| 12 | Antiinflammatory activity | Dry latex | 108 and 109 |
| Stem bark: chloroform and hydro-alcoholic extract | 110 | ||
| Latex: hexane, dichloromethane, ethyl acetate, n-butanol and aqueous extract | 77 | ||
| Latex: pet. ether, acetone, methanol extract | 111 | ||
| Leaves: aqueous extract | 112 | ||
| Flowers: ethanolic extract | 93 | ||
| 13 | Antidiarroheal activity | Bark: Arkamula Tvarka (Ayurvedic preparation) | 45 |
| Latex | 113 | ||
| 14 | Antifungal activity | Aqueous bark extract | 114 |
| Leaves: aqueous, methanol, acetone and ethanol extract | 115 | ||
| Root bark | 116 | ||
| Antimycotic activity against dermatophytes | Latex | 117 | |
| Antimycofloral activity (fungi in wheat) | Fresh latex | 118 | |
| 15 | Larvicidal activity | Crude latex and ethanolic extract of leaf | 119 |
| Leaves: ethanolic extract | 120 | ||
| Leaves: aqueous extract | 121 | ||
| Flower, young bud, mature leaves and stems: ethanolic extract | 122 | ||
| Flowers: aqueous extract | 123 | ||
| 16 | Tobacco mosaic virus (TMV) inhibitor activity | Latex | 124 |
| 17 | Antifertility activity | Ethanolic extract of roots | 125 |
| Leaves: ethanolic extract | 79 | ||
| Roots (calotropin) | 59 | ||
| Abortifacient activity | Latex | 126 | |
| Antisperm activity | Root: chloroform extract | 127 | |
| Oestrogenic/antiovulatory activity | Roots: ethanolic and aqueous extract | 128 | |
| 18 | Plasma clotting activity | Protein fraction isolated from fresh latex | 129 |
| 19 | Antiplasmodial activity | Different plant parts: ethyl acetate, ethanolic and acetone extract | 130 |
| Leaves extract | 131 | ||
| 20 | Antipyretic activity | Dry latex: aqueous extract | 132 |
| Flowers: ethanolic extract | 93 | ||
| 21 | Antiasthmatic activity | Flowers | 133 |
| 22 | Anticonvulsant activity | Root extracts | 134 |
| 23 | Cytotoxic activity | Root (2′′-oxovoruscharin) | 62 |
| Laticifer proteins (LP) recovered from latex | 135 | ||
| Root: methanolic, aqueous, ethyl acetate, hexane extracts | 136 | ||
| Plant: methanolic extract | 137 | ||
| Stems: uzarigenin | 138 | ||
| Root bark: calotroprocerol A | 139 | ||
| Root: alcoholic, hydro-aqueous and aqueous | 140 | ||
| Leaf: ethanolic extract | 149 | ||
| 24 | Analgesic activity | Flowers: Ethanolic extract | 93 |
| 25. | Antihyperglycemic activity | Leaves: pet ether, methanol and aqueous extracts | 141 |
| 26 | Antiarthritis activity | Latex | 142 |
| Protein sub fraction of latex | 143 | ||
| 27 | Antimolluscicidal activity | Latex: 95% aqueous ethanol (uscharin) | 144 |
| 28 | Antitermites activity | Latex | 145 |
| 29 | Antimigraine activity | Dried terminal leaves | 146 |
| 30 | Anti-ulcer activity | Root: chloroform extract | 147 |
| Plant: 50% ethanolic extract | 148 | ||
| Leaf: ethanolic extract | 149 | ||
| Stem bark: chloroform and hydroalcoholic extract | 110 | ||
| 31 | Spasmolytic activity | Plant: aqueous extract | 150 |
| 32 | Allelopathic activity | Leaves: aqueous extract | 151 |
| 33 | Anti-keloidal activity | Latex | 68 |
| 34 | Anti-hyperbilirubinemic activity | Leaves: aqueous extract | 70 |
| 35 | Antiapoptotic activity | Latex | 152 |
The details enumerated in the Table 2 is indicative of the fact that the different plant parts demonstrate large number of pharmacological activities. Moreover, maximum number of activities were conducted at extract level, therefore horizons for further research is still bright, wherein the active principle constituents responsible for the activities may be identified. Here some of the very vital biological activites are being discussed in detail.
5.1 Cytotoxic potential
Various phytoconstituents and plant extracts were examined for their in vitro anticancer potential on various cancer cell lines, and showed significant cytotoxic activities as summarized in Table 3.| C. procera: plant part/chemical constituent | Cancer cell lines/model | Method of analysis/assay | Mechanism of action/investigation | Observation | References |
|---|---|---|---|---|---|
| Uscharin and its derivatives | Lung cancer (A549) | MTT colorimetric assay, intraperitoneal (ip) injection-related toxicity | Na+/K+-ATPase inhibition activity | Cardenolides derived from 2′′-oxovoruscharin exhibited significant in vitro antitumor activity and high in vivo tolerance | 62 |
| 2′′-Oxovoruscharin and its derivatives | Two glioblastoma (Hs683, U373) and two colon cancer (HCT-15 and LoVo) | ||||
| Laticifer proteins (LP) recovered from latex | HL60 (promoyelocytic leukemia), HCT-8 (colon), MDA-MB-435(breast), SF-295(brain) | 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide MTT | LP is a target for DNA topoisomerase I triggering apoptosis in cancer cell lines | IC50 values for LP ranged from 0.42 to 1.36 μg mL−1 to SF-295, MDA-MB-435 respectively | 135 |
| Root: methanolic, aqueous, ethyl acetate, hexane extracts (1, 5, 10, 25 μg mL−1) | Human Hep 2 | Tetrazolium bromide (MTT), colorimetry | Treatment initiated apoptotic mechanism by blocking the cell cycle at S-phase and thus preventing cells from entering proliferative (G2/M) phase | Ethyl acetate extract showed strongest cytotoxic effect | 136 |
| Plant: methanolic extract (0, 5, 10, 20 and 40 μg mL−1) | Human skin melanoma cells (SK-MEL-2) | Annexin-V FITC flow cytometry method, MTS assay | Methanolic extract induced apoptosis as shown by the accumulation of cells in the G2/M phase and the decrease of cell percentage in the G0/G1 phase | At 40 μg mL−1 late apoptotic cell percentage was increased up to 80%. C. procera exerted cytotoxic potential | 137 |
| 5-Hydroxy-3,7-dimethoxyflavone-4-O-β-glucopyranoside; uzarigenin; β-anhydroepidigitoxigenin; 2β,19-epoxy-3β,14β-dihydroxy-19-methoxy-5α-card-20(22)-enolide; β-anhydroepidigitoxigenin-3β-O-glucopyranoside | HT 29, HepG2 (human cancer cell lines), NIH-3T3 (mouse fibroblast cell line) | CellTiter-Blue® cell viability assay | — | Uzarigenin showed moderate cytotoxicity | 138 |
| Calotroprocerol A; calotroproceryl acetate A; calotroprocerone A, B; pseudo-taraxasterol acetate; taraxasterol; calotropursenyl acetate B; stigmasterol; (E)-octadec-7-enoic acid | A549 non-small cell lung cancer (NSCLC), the U373 glioblastoma (GBM) and the PC-3 prostate cancer cell lines | 3-(4,5-Dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay | Growth inhibition action | Calotroprocerol A exhibited in vitro growth inhibitory activity in all the three cancer cell lines with effects comparable to those of cisplatin and carboplatin | 139 |
| Calotroposide H; calotroposide I; calotroposide J; calotroposide K; Calotroposide L; calotroposide M; calotroposide N | A549 non-small cell lung cancer (NSCLC), U373 glioblastoma (GBM), and PC-3 prostate cancer cell lines | MTT colorimetric assay | Calotroposide K and M exhibited subnanomolar growth inhibition activity with IC50 ranging from 0.5 to 0.7 μM against U373 glioblastoma (GBM) and PC-3 prostate cancer cell lines | C. procera exhibited cytotoxic potential | 153 |
| Calotroposide S | PC-3 prostate cancer, A549 non-small cell lung cancer (NSCLC), and U373 glioblastoma (GBM) cell lines | MTT colorimetric assay | Calotroposide S showed potent anti proliferative activity | C. procera exerted anti-proliferative activity | 154 |
| Latex: hexane, chloroform, ethyl acetate and aqueous extract. Calactin; 15β-hydroxy calactin; afroside; uscharin; 15β-hydroxy uscharin; calotoxin; 12β-hydroxycoroglaucigenin; afrogenin; calactoprocin; procegenin A; procegenin B | A549 (lung) and hela (cervix) cancer cell lines using cisplatin as a positive control | MTT colorimetric assay | Growth inhibition action | Highest cytotoxic activity was displayed by chloroform extract. Amongst isolated compounds, calactin displayed highest cytotoxic activity | 65 |
| Root: alcoholic, hydro-aqueous and aqueous extracts(10 μg mL−1, 30 μg mL−1, 100 μg mL−1) | Human oral (KB) and central nervous system (SNB-78) cancer cell lines | Sulforhodamine-B (SRB) assay | Alcoholic extract showed significant growth inhibition action | C. procera roots exhibited in vitro cytotoxicity against oral and CNS human cancer cell lines | 140 |
Over past decade, cytotoxic activities of various extracts and chemical constituents of C. procera have been carried out. Majority of studies were conducted on various cancer cell line models in vitro, except the one conducted using UNBS1450. UNBS1450, a semi-synthesized cardenolide was compared to reference anticancer agents and classic cardenolides in prostate cancer cell line in vitro and in vivo following s.c. (subcutaneous) and orthotopic prostate cancer cell grafting into mice; it was found to be more effective than tested reference compounds, such as mitoxantrone, taxol, oxaliplatin, irinotecan and temozolomide and less toxic than cardenolides.155,156 Mechanism of UNBS1450 was studied and proven to be a potent sodium pump inhibitor as it inhibits NF-kB transactivation and triggers apoptosis by recruitment of pro-apoptotic Bak and Bax protein thereby leading to cell death.157,158 Carrying out further in vivo studies will play a crucial role in ascertaining the safer use of UNBS1450. Therefore, further studies are necessary to obtain the clinically important lead molecules for the development of potent anticancer drugs.
5.2 Wound healing potential
C. procera has folk medicinal reputation as a wound healing agent. In vivo studies proved its wound healing potential as summarized in Table 4.| Model | C. procera extract/dose/duration | Negative control | Investigation | Result | References |
|---|---|---|---|---|---|
| Guinea pigs | 20 mL of 1.0% sterile solution of the latex twice daily for 7 days | Excision wounds | Wounds exhibited marked dryness, no visual sign of inflammation | Significant prohealing property | 67 |
| Male albino-Wistar rats | Ethanolic extract of bark (50 mg per wound) | Incision and excision wounds | Extract demonstrated wound healing effect by accelerating wound closure and epithelialization | Excellent dermal wound healing potential | 69 |
| Wistar rats | Aqueous extract of C. procera (25 mg and 50 mg kg−1) | Incision and excision wounds | Significant (P < 0.05) increase in breaking strength and percentage wound contractions with decreased epithelization period was observed | Significant wound healing property | 70 |
These data strongly support its ethnomedicinal use in wound healing potential and skin problems. In vivo screening showed considerable results in dose-dependent manner when compared to positive controls. A future perspective of studying the side effects and toxicity of the extracts at the dose level can also be unravelled.
5.3 Anti-inflammatory potential
Anti-inflammatory potential of extracts from C. procera have been summarized in Table 5.| Model | C. procera extract/dose/duration | Negative control | Investigation | Result | References |
|---|---|---|---|---|---|
| Male albino rats and albino guinea pigs | 50 mg, 200 mg 500 mg and 1 g kg−1 dry latex | Carrageenan-induced oedema test, cotton pellet granuloma and vascular permeability etc. | Dry latex suppressed fluid exudation, due to its influence on vascular permeability and also delayed the onset and intensity of UV induced erythema | Significant anti-inflammatory potential | 108 |
| Male albino rats | Dry latex | Carrageenin and formalin-induced pedal oedema test | At dose 5 mg per rat, showed 71% inhibition in the case of the carrageenin-induced oedema (P < 0.005) and 32% inhibition for the formalin-induced oedema (P < 0.05). At higher dose (50 mg per rat), 96% and 98%, for carrageenin- and formalin-induced oedema groups respectively | Potent anti-inflam-matory activity | 109 |
| Albino rats of either sex | Stem bark: chloroform and hydro-alcoholic extract | Carrageenan-induced paw oedema | Significant reduction in the inflammation at 100, 200 and 400 mg kg−1 displayed by chloroform extract | Significant anti-inflammatory potential | 110 |
| Male Wistar rats | Dry latex: petroleum ether, acetone, methanol and aqueous extracts (50 mg per rat) | Carrageenan induced paw oedema | Maximum anti-inflammatory effect (59% and 53% inhibition) by the aqueous and acetone extracts respectively compared to (63%) inhibition exhibited by phenylbutazone | Latex of C. procera exerted anti-inflammatory property | 111 |
| Male Wistar rats | Crude latex: hexane, dichloromethane, ethyl acetate, n-butanol and aqueous fractions (1.0, 5.0 or 10.0 mg kg−1 and 0.2 mL) | Carrageenan-induced peritonitis | Dichloromethane, ethyl acetate, and aqueous fractions inhibited carrageenan-induced neutrophil migration in rats at the ratios 67%, 56%, and 72%, respectively | Latex of C. procera possess anti-inflammatory property | 77 |
On the basis of studies mentioned in Table 5, it can be concluded that the anti-inflammatory effect of dry latex needs to be further characterized as well as the nature of active principle leads responsible for anti-inflammatory activity remains to be identified.
5.4 Larvicidal/insecticidal potential
Aqueous and ethanolic extracts of leaves and other parts of C. procera showed significant larvicidal activities against various vector species as summarized in Table 6.| Vector species | C. procera extract/dose/duration | Observation | Result | References |
|---|---|---|---|---|
| Culex quinquefasciatus 3rd instar larvae | Crude latex and ethanolic extract of leaves | 100% larval mortality at 300 ppm concentration of latex and at 1000 ppm concentration of ethanolic leaf extract. LC50 values of the latex and ethanolic leaves extract were 57.3 and 388.7 ppm respectively | Crude latex exerted stronger larvicidal potential than ethanolic extract | 119 |
| Musca domestica 3rd instar larvae | Ethanolic extract of leaves (500 mg L−1) | 100% mortality at 500 ppm. LC50 value of the extract 282.5 ppm | Leaves exerted insecticidal potential | 120 |
| Anopheles arabiensis and Culex quinquefasciatus 2nd, 3rd, 4th instar larvae | Aqueous extract of leaves (1000, 500, 200 ppm) | LC50 value 273.53, 366.44, 454.99 ppm for 2nd, 3rd and 4th instar larvae | Leaves showed oviposition deterrent, larvicidal and adult emergence activity | 121 |
| Anopheles stephansi 3rd instar larvae | Ethanolic extracts of different parts viz. flower, young bud, mature leaves and stems (100 to 5000 ppm) | Mature leaves extract exhibited 100% mortality at 2000 ppm after 48 hours of incubation | Mature leaves showed high larvicidal activity against tested larvae | 122 |
| Culex species 4th instar | Aqueous extract of flowers (1%, 2.5% and 5%)/24 h | At 1% concentration, the mortality rate was 0%, 60% and 100% and at 2.5% concentration, mortality rate was 20%, 80% and 100% at the end of 1, 3 and 4 days of exposure, and at 5% concentration, 100% mortality was recorded at the end of third day | Flowers exhibited remarkable larvicidal properties against the pupae and late 4th instar larvae of Culex sp. | 123 |
Above studies indicated that aqueous and ethanolic extracts of leaves of C. procera possessed phenomenal oviposition deterrent and larvicidal effect, thus it can be developed as environment friendly alternative for the synthetic insecticides for mosquito control.
5.5 Anthelmintic potential
C. procera is used as an anthelmintic by ruminant farmers as proved by activities summarized in Table 7.| Model | C. procera extract/dose | Compared with drug | Observation | Result | References |
|---|---|---|---|---|---|
| In vivo: sheep infected with mixed species of nematodes in vitro: Haemonchus contortus | Crude powder (CP), crude aqueous (CAE) and crude methanolic extracts (CME) | Levamisole | 88.4%, 77.8% and 20.9% reduction in egg count percent for CAE, CP and CME respectively | Aqueous extract of C. procera has good anthelmintic potential | 105 |
| Earthworms | Aqueous extract of dry latex (5, 10, 50 and 100 mg mL−1) and fresh latex (1.45, 7.25, 29, 72.5 and 145 mg mL−1) | Piperazine | At 5 to 10 mg mL−1 concentration paralysis at 90 min, at 100 mg mL−1 death within 60 min. Fresh latex also showed dose-dependent paralysis | Latex showed wormicidal activity, hence can be used as an anthelmintic agent | 106 |
5.6 Antioxidant potential
Leaves of C. procera displayed highest antiradical activity as evident from activities summarized in Table 8.| C. procera part | Extract/dose/duration | Investigation | Result | References |
|---|---|---|---|---|
| Leaves, fruits, flowers and latex | Methanolic solution of dried extract | DPPH radical scavenging assay | Leaves exhibited maximum DPPH radical scavenging activity with IC50 = 0.18 mg mL−1, whereas latex showed minimum activity with IC50 = 0.42 mg mL−1 | 101 |
| Leaves | Aqueous and methanolic extract (1, 5, 10, 50, 100 and 500 μg mL−1) | DPPH radical scavenging assay | IC50 of the methanol extract was 110.25 μg mL−1, the aqueous extract showed mild antioxidant activity | 102 |
| Leaves | 2–100 mg mL−1 for quercetin in methanol and 20–100 mg mL−1 for AME and quercetin derivatives with different methoxy substitution | DPPH radical scavenging assay | Varying degrees of antioxidant activity was exerted by quercetin derivatives, but quercetin was found to be most active | 76 |
| Leaves, flowers and fruits | Methanolic extracts of the samples of different concentrations (100–1000 ppm) | DPPH radical scavenging assay | IC50 values in leaves, fruits and flowers were 16.08, 16.06 and 10.31 μg mL−1 respectively, showing strong antioxidant activity of C. procera | 103 |
Above activities proved that quercetin, aqueous and methanolic extracts of leaves of C. procera possessed remarkable antiradical activity. Evaluation of the in vivo antioxidant potential would be indispensable, so that it can be used as natural antioxidant ingredients in food and drug industries.
5.7 Antiplasmodial potential
Traditional practitioners use C. procera as antimalarial agent. Activity summarized in Table 9.| Model | C. procera extract/dose | Investigation | Result | References |
|---|---|---|---|---|
| Chloroquine sensitive strain, MRC 20 and a chloroquine resistant strain, MRC 76 of Plasmodium falciparum | Ethyl acetate, acetone, methanol fractions of flower, bud, root: (62–125 mg mL−1) | Percentage inhibition varied from 7.51 to 61.38% between the various fractions against MRC 20 and for MRC 76, percentage inhibition varied from 3.437 to 41.08% between the various fractions | At the lower dose range, the root extracts of C. procera found to be the most effective for both P. falciparum MRC 20 and MRC 76. Hence, C. procera exerted antiplasmodial potential | 130 |
Over past decades, reduction in efficiency of chloroquine has been observed, thus resistivity to antimalarial drugs can be a threat to control malaria. The hunt for analogues with reduced toxicity and improved antimalarial activity still prevails. The possibilities of finding active compounds and correlating with specific dose effective antimalarial activity, from those parts of the plant, which are used separately or together could be further pursued.
5.8 Hepatoprotective activity
In vivo experimental study proves that C. procera has hepatoprotective potential as summarized in Table 10.| Model | C. procera extract/dose | Negative control | Investigation | Result | References |
|---|---|---|---|---|---|
| Albino rats of either sex | Methanol extract (MCP) of root and its sub fractions viz. hexane (HCP), ethyl acetate (ECP) and chloroform (CCP) (200 mg kg−1) | Carbon tetra chloride | MCP and its sub fractions HCP, ECP displayed hepatoprotective effect by reducing the elevated serum levels of, serum glutamic pyruvic transaminase, alkaline phosphatase and serum glutamic oxaloacetic transaminase, it increased high density lipoprotein. CCP does not show effective results | C. procera exerted hepatoprotective potential | 83 |
| Wistar rats of either sex | Hydro-ethanolic extract of C. procera flowers (200 mg kg−1 and 400 mg kg−1) | Paracetamol-induced hepatitis | Improvement in the hepatic architecture was observed | C. procera flowers have hepatoprotective effect | 84 |
5.9 Miscellaneous activities
Antiapoptotic activity of latex of C. procera was carried out by Sayed et al. (2016) on catfishes exposed to (100 μg L−1) 4-nonylphenol as chemical pollutant. Significant (P < 0.05) decrease in apoptotic cells, enzymes (superoxidase dismutase, acetylcholinesterase cortisol etc.) and ions validified antiapoptotic activity of the crude latex against the toxicity of 4-nonylphenol.152 Hence, crude latex exerted antiapoptotic activities against the toxicity of 4-nonylphenol.Anti-hyperbilirubinemic activity of leaves was evaluated using phenylhydrazine and paracetamol induced Wistar rats. Significant (P < 0.05) decrease in concentrations of serum total bilirubin in hyperbilirubinemic rats proved bilirubin lowering activity of aqueous extracts of C. procera.70
Recent studies indicated that C. procera has significantly broader range of beneficial effects as it contains bioactive phytochemicals with therapeutic potential. By far only cytotoxic studies on cancer cell lines have been well established in clinical trials, whereas other activities have been evidenced by basic studies. Most of the studies are limited to in vitro studies which lack exploration of molecular mechanism of action. Therefore, mechanism based in vitro and in vivo studies should be carried out, which can lead to understanding of underlying mechanism related to traditional uses.
6. Phytochemistry
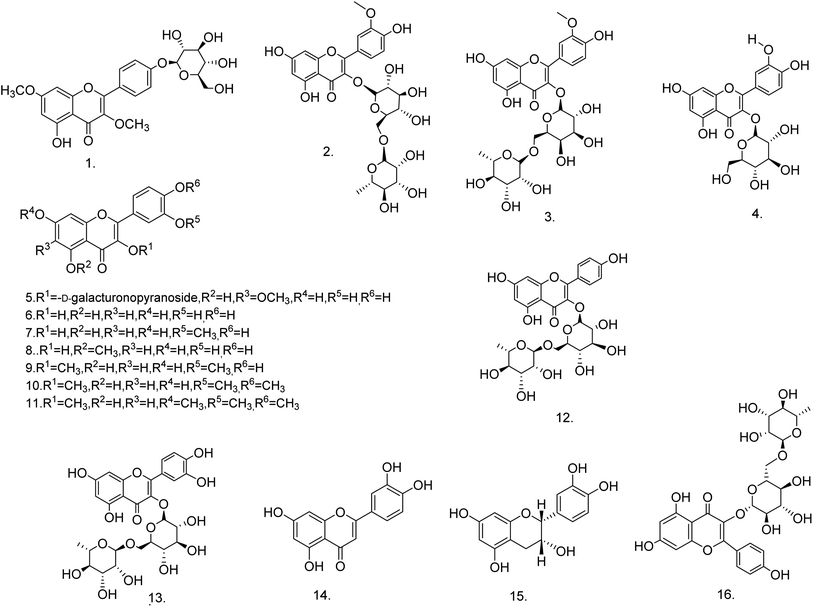
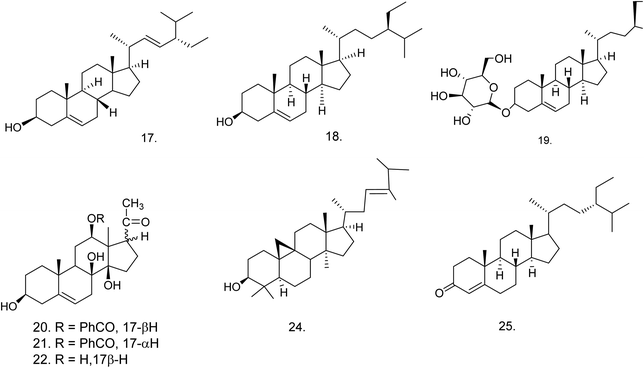
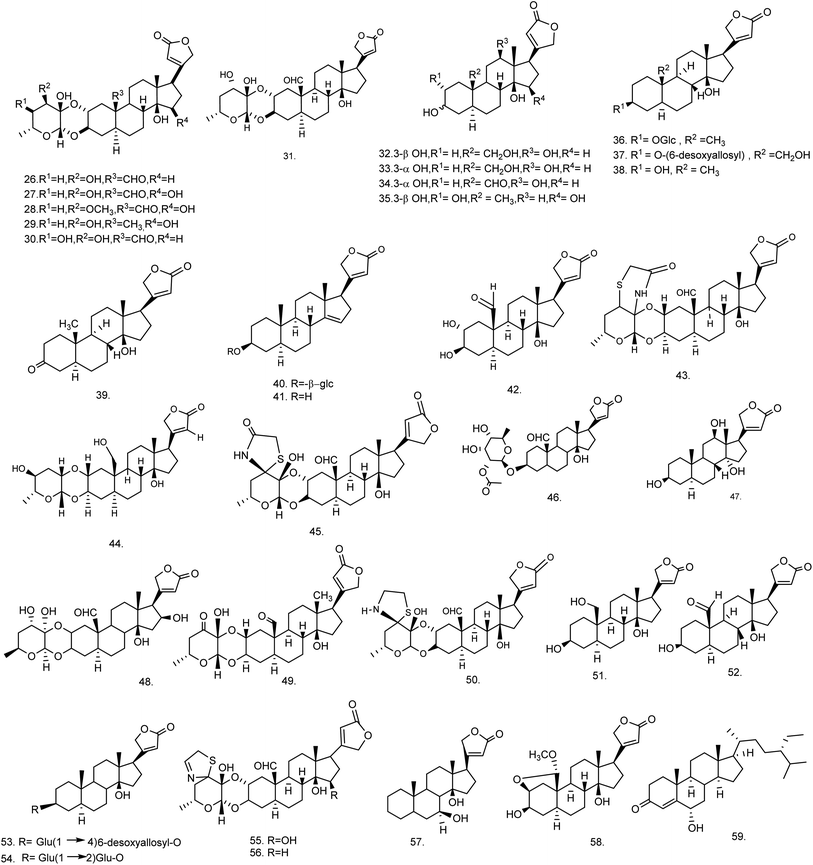
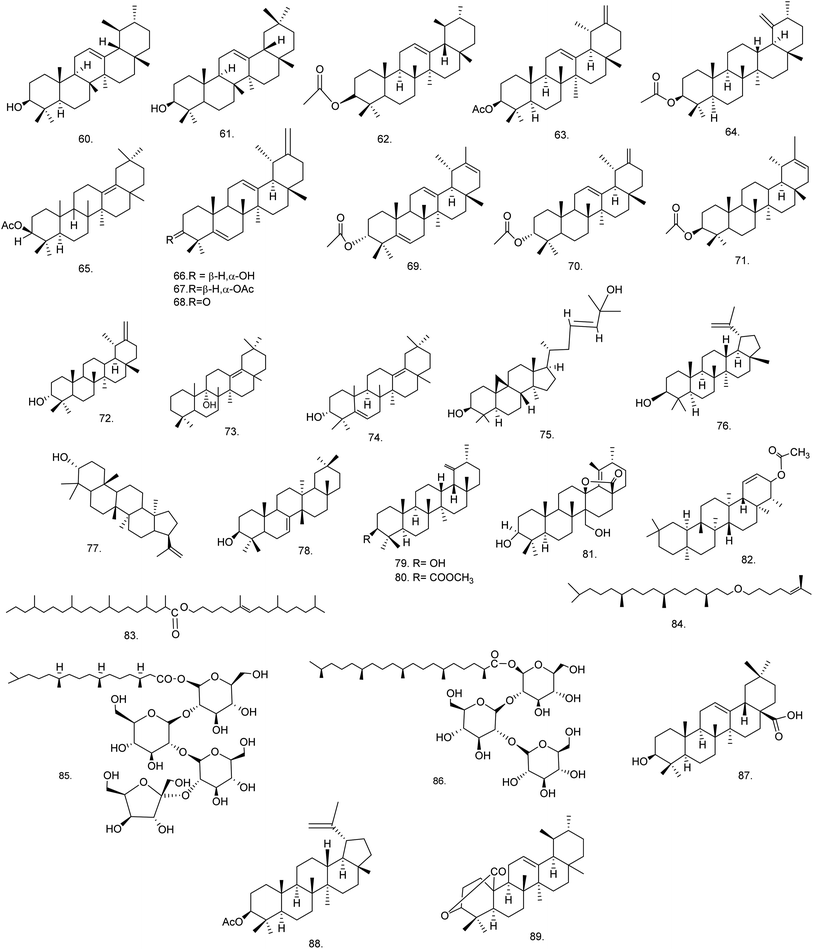
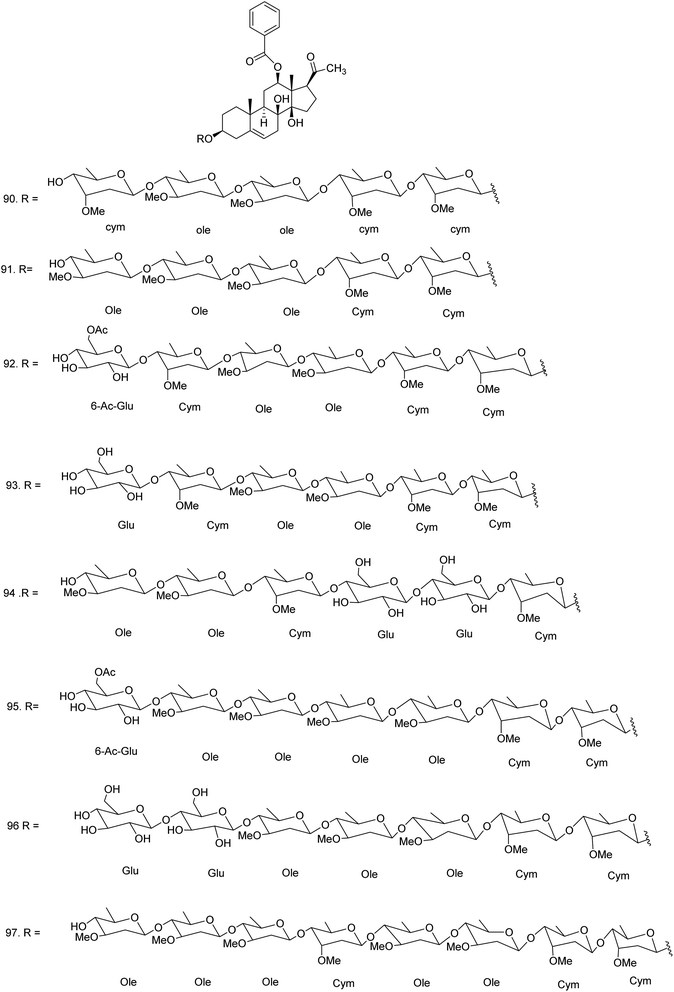
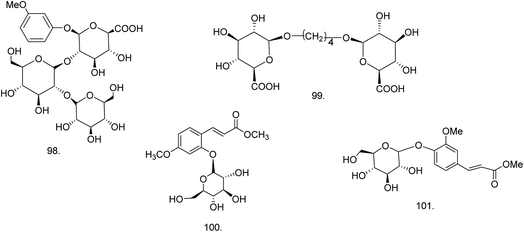
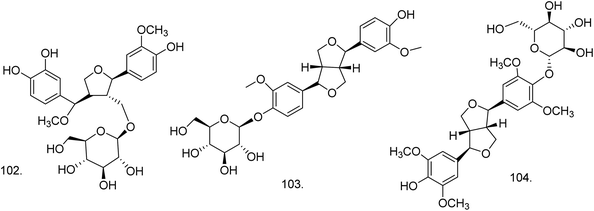
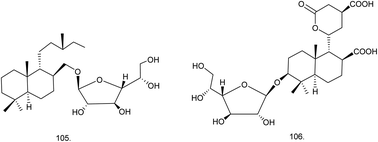
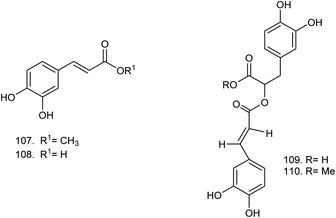
C. procera contains cardenolides, flavonoids, sterols, oxypregnanes triterpenoids, glycosides and other constituents as elaborated in Table 11.7 Flavonoid and its glycosides (Fig. 1) are the major compounds isolated from the leaves of C. procera. Steroids (Fig. 2) and cardenolides (Fig. 3) are the major secondary metabolites found in the latex. Cardenolides have also been reported from other plant genera of the family Apocynaceae or Asclepiadaceae like Strophanthus, Cerbera, Apocynum, Nerium, and Thevetia.159 Traditionally they are employed in curing of congestive heart failure.160 Cardenolides are C23 steroids with steroid nucleus having a glycoside moiety at C-3 and a lactone moiety at C-17.6 Cardiac glycosides can be novel antineoplastic agents as cancer cells are more prone to these compounds.159 Terpenoids (ursane, olenane type and pentacyclic triterpenes etc.) (Fig. 4) have been isolated from flowers, root bark and latex. Oxypregnane glycosides (Fig. 5) have recently been reported from root bark of this plant.153,154 They have steroidal skeleton containing a 2-deoxy sugar moiety. These oxypregnanes have benzoyl moiety at C-12 and a straight 5–7 units sugar chain connected to C-3 of the aglycone.6 Some glycosides (Fig. 6), lignan glycosides (Fig. 7), terpene glycosides (Fig. 8) and caffeic acid derivatives (Fig. 9) have also been isolated from this plant.A number of hydrocarbons, saturated and unsaturated fatty acids were also identified from C. procera extract by GC-MS.161,162 Similarly fatty acid ester, phthalate derivatives, and pentacyclic triterpenes were identified from chloroform extract of roots of Calotropis procera.163
| S. No. | Compound name (molecular formula) | Extract/fraction | Eluent | Plant part & references |
|---|---|---|---|---|
| Flavonoids | ||||
| 1 | 5-Hydroxy-3,7-dimethoxyflavone-4′-O-β-glucopyranoside (C23H24O11) | Ethanolic extract | Benzene-chloroform | Stem138 |
| 2 | Isorhamnetin 3-O-β-D-rutinoside (C28H32O16) | 85% methanolic extract | 10–40% methanol | Leaves76,164 |
| 3 | Isorhamnetin 3-O-β-D-robinoside (C28H32O16) | 85% methanolic extract | 10–40% methanol | Leaves76,164 |
| 4 | Isoquercitrin (C21H20O12) | 85% methanolic extract | 70% methanol | Leaves76 |
| 5 | Quercetagetin-6-methyl ether 3-O-β-D-4C1-galacturonopyranoside (C22H20O14) | 85% methanolic extract | 40–60% methanol | Leaves76 |
| 6 | Quercetin (C15H10O7) | 85% methanolic extract | 80% methanol | Leaves76 |
| 7 | Isorhamnetin (C16H12O7) | 85% methanolic extract | 80% methanol | Leaves76 |
| 8 | Azaleatin (C16H12O7) | 85% methanolic extract | 80% methanol | Leaves76 |
| 9 | 3,3′-Dimethoxy quercetin (C17H14O7) | 85% methanolic extract | 50–60% ethyl acetate | Leaves76 |
| 10 | 3,6,3′,4′-Tetramethoxy quercetin (C18H16O7) | 85% methanolic extract | 50–60% ethyl acetate | Leaves76 |
| 11 | 3,6,7,3′,4′-Pentamethoxy quercetin (C19H18O7) | 85% methanolic extract | 60–100% ethyl acetate | Leaves76 |
| 12 | Kaempferol-3-O-rutinoside (C27H30O15) | Methanolic extract | Ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formic acid formic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glacial acetic acid (100 glacial acetic acid (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, v/v) 11, v/v) |
Leaves86 |
| 13 | Quercetin-3-O-rutinoside (C27H30O16) | Methanolic extract | Ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formic acid formic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glacial acetic acid (100 glacial acetic acid (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, v/v) 11, v/v) |
Leaves86 |
| 14 | Luteolin (C15H10O6) | Ethanol–water extract (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40)/butanol fraction 40)/butanol fraction |
n-Hexane–acetone (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
Stem bark165 |
| 15 | Epicatechin (C15H14O6) | Ethanol–water extract (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40)/butanol fraction 40)/butanol fraction |
n-Hexane–acetone (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) 40) |
Stem bark165 |
| 16 | Kaempferol 3-O-α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranoside (C27H30O15) | Ethanolic extract | Water–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Fruits149 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Steroids | ||||
| 17 | Stigmasterol (C29H48O) | Methanolic extract/hexane fraction | Hexane–ethyl acetate | Flowers,166 root bark,139 latex167 |
| 18 | β-Sitosterol (C29H50O) | Ethanolic extract/chloroform fraction | Hexane–ethyl acetate | Flowers,166 latex,167 aerial part168 |
| 19 | Daucosterol or β-sitosterol glucoside (C35H60O6) | Ethanolic extract/chloroform fraction | 10% aq. methanol and hexane | Latex, aerial part,168 roots169 |
| 20 | Benzoyllineolone (C28H36O6) | Ether extract/chloroform fraction | Benzene–chloroform | Root bark170 |
| 21 | Benzoylisolineolone (C28H36O6) | Ether extract/chloroform fraction | Benzene–chloroform | Root bark170 |
| 22 | Lineolone (C21H32O5) | Ether extract | — | Root bark170 |
| 23 | Isolineolone (C21H32O5) | Ether extract | — | Root bark170 |
| 24 | Cyclosadol (C31H52O) | Methanolic extract | — | Flowers166 |
| 25 | β-Sitost-4-en-3-one (C29H48O) | Methanolic extract | n-Hexane–ethyl acetate (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
Flowers166 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
Steroids![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cardenolides cardenolides |
||||
| 26 | Calactin (C29H40O9) | Ethanolic extract/chloroform fraction | 10% aq. methanol and hexane | Roots,62 latex,65 aerial part168 |
| 27 | 15β-Hydroxycalactin (C29H40O10) | Ethanolic extract/chloroform fraction | — | Latex65 |
| 28 | Calactoprocin or 14β,15β-dihydroxy-19-oxo-2α,3β-[(2S,3S:4R,6R)-tetrahydro-3-hydroxy-4-methoxy-6-methyl-2H-pyran-2,3-diyl]bis(oxy)-5α-card-20(22)-enolide (3′β-methoxy-15β-hydroxy calactin) (C30H42O10) | Ethanolic extract/chloroform fraction | — | Latex65 |
| 29 | Afroside (C29H42O9) | Ethanolic extract/chloroform fraction | — | Latex65 |
| 30 | Calotoxin (C29H40O10) | Ethanolic extract/chloroform fraction | — | Aerial part,168 latex65 |
| 31 | Calotropin (C29H40O9) | Ethanolic extract/chloroform fraction | — | Root bark,62 latex and aerial part168 |
| 32 | 12β-Hydroxycoroglaucigenin (C23H34O6) | Ethanolic extract/chloroform fraction | — | Latex65 |
| 33 | Procegenin A or 3α,12β,14β-trihydroxy-19-hydroxymethyl-5α-card-20(22)-enolide or 3-epi,12β-hydroxycoroglaucigenin (C23H34O6) | Ethanolic extract/chloroform fraction | — | Latex65 |
| 34 | Procegenin B or 3α,12β,14β-trihydroxy-19-oxo-5α-card-20 (22)-enolide or 12β-hydroxy carpogenin (C23H32O6) | Ethanolic extract/chloroform fraction | — | Latex65 |
| 35 | Afrogenin (C23H34O6) | Ethanolic extract/chloroform fraction | — | Latex65 |
| 36 | Desglucouzarin (C29H44O9) | Ethanolic extract/chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate fraction ethyl acetate fraction |
Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Stem171 |
| 37 | Frugoside (C29H44O9) | Ethanolic extract/chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate fraction ethyl acetate fraction |
Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Seeds,60 stem,171 root bark172 |
| 38 | Uzarigenin (C23H34O4) | Ethanolic extract/chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate fraction ethyl acetate fraction |
Chloroform–methanol (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
Latex61 |
| Stem168,171,173 | ||||
| 39 | Uzarigenone (C23H32O4) | Ethanolic extract/benzene | Chloroform–methanol (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
Stem171 |
| 40 | β-Anhydroepidigitoxigenin-3β-O-glucopyranoside (C29H42O8) | Ethanolic extract/benzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform chloroform |
Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Stem138 |
| 41 | β-Anhydroepidigitoxigenin or 3β-hydroxy-5α-carda-14(15),20(22)-dienolide (C23H32O3) | Ethanolic extract → benzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform chloroform |
Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Stem138 |
| 42 | Calotropagenin (C23H32O6) | Chloroform extract | Hexane–diethyl ether (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11) 11) |
Aerial part174 |
| 43 | Ischarin (C31H41NO8S) | Ethanolic extract | Chloroform | Aerial part168 |
| 44 | Ischaridin (C29H42O8) | Ethanolic extract/10% aq. methanol and hexane fraction | Chloroform–methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Aerial part168 |
| 45 | 2′′-Oxovoruscharin (C31H41NO9S) | Methanolic extract | Dichloromethane–methanol (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Root bark62 |
| 46 | Proceraside A (C31H44O10) | Methanolic extract/ethyl acetate fraction | Chloroform–methanol | Root bark172 |
| 47 | Syriogenin (C23H34O5) | Methanolic extract | Water–methanol | Latex61 |
| 48 | Proceroside (C29H40O10) | Methanolic extract | Water–methanol | Latex61 |
| 49 | Uscharidin (C29H38O9) | Ethanolic extract | — | Aerial part56 |
| 50 | Voruscharin (C31H43NO8S) | Methanolic extract | Acetone–methanol (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Roots62 |
| 51 | Coroglaucigenin (C23H34O5) | Chloroform extract | — | Seeds60 |
| 52 | Corotoxigenin (C23H32O5) | Ether extract | — | Seeds60 |
| 53 | 3-[β-(4-O-β-D-Glucopyranosyl-β-D-6-desoxyallopyranosyl)oxy]uzarigenin (C35H54O13) | 70% ethanolic extract/benzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform chloroform |
Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) 1.5) |
Stem173 |
| 54 | Uzarin or 3-[β-(2-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy] uzarigenin (C35H54O14) | 70% ethanolic extract/benzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform chloroform |
Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Stem173 |
| 55 | 15β-Hydroxyuscharin (C31H41NO9S) | Ethanolic extract | Chloroform | Latex65 |
| 56 | Uscharin (C31H41NO8S) | Methanolic extract | Chloroform–methanol (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
Aerial part,168 latex65,168 |
| 57 | Proceragenin or 7β,14β-dihydroxy-5α-card-20(22)-enolide (C23H34O4) | Methanolic extract/chloroform fraction | Hexane–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
Aerial part63 |
| 58 | 2β,19-Epoxy-3β,14β-dihydroxy-19-methoxy-5-α-card-20(22)-enolide (C24H34O6) | Ethanolic extract/benzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform fraction chloroform fraction |
Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Stem138 |
| 59 | Procesterol or (24S)-24-ethyl-stigmast-4-en-6α-ol-3-one (C29H48O2) | Ethanolic extract/chloroform fraction | Hexane–chloroform (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Fresh and undried flowers176 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Terpenes/terpenoids | ||||
| 60 | α-Amyrin (C30H50O) | Methanolic extract/hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate gradients ethyl acetate gradients |
Dichloromethane–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Flowers176 |
| 61 | β-Amyrin (C30H50O) | Methanolic extract/hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate gradients ethyl acetate gradients |
Dichloromethane–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Flowers176 |
| 62 | α-Amyrin acetate (C32H52O2) | Methanolic extract | Pet. ether–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
Roots169 |
| 63 | Procerursenyl acetate or urs-18α-H-12,20(30)-diene-3β-yl acetate (C32H50O2) | Methanolic extract | Pet. ether–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots177 |
| 64 | Calotropenyl acetate or urs-19(29)-3β-yl acetate (C32H52O2) | Chloroform extract | Benzene–hexane (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) 40) |
Flower,175 latex and aerial part168 |
| 65 | Calotropoleanyl ester or olean-13(18)-en-3β-yl acetate (C32H52O2) | Ethanolic extract | Pet. ether | Root bark178 |
| 66 | Calotroprocerol A or ursa-5,12,20(30)-trien-18αH-3β-ol (C30H46O) | Methanolic extract | n-Hexane–ethyl acetate | Root bark139 |
| 67 | Calotroproceryl acetate A or ursa-5,12,20(30)-trien-18αH-3β-yl acetate (C32H48O2) | Methanolic extract | n-Hexane–ethyl acetate | Root bark139 |
| 68 | Calotroprocerone A or ursa-5,12,20(30)-trien-18αH-3-one (C30H44O) | Methanolic extract | n-Hexane–ethyl acetate | Root bark139 |
| 69 | Calotroproceryl acetate B or ursa-5,12,20-trien-18αH-3β-yl acetate (C32H48O2) | Methanolic extract | n-Hexane–ethyl acetate | Root bark139 |
| 70 | Calotropursenyl acetate B or urs-12,19(29)-diene-3β-yl acetate (C32H50O2) | Methanolic extract | n-Hexane–ethyl acetate | Root bark139,180 |
| 71 | Pseudo-taraxasterol acetate (C32H52O2) | Methanolic extract | n-Hexane–ethyl acetate | Root bark139 |
| 72 | Taraxasterol (C30H50O) | Methanolic extract | n-Hexane–ethyl acetate | Root bark139 |
| 73 | Proceroleanenol A or olean-13(18)-en- 9α-ol (C30H50O) | Ethanolic extract | Benzene–chloroform | Root bark178 |
| 74 | Proceroleanenol B or olean-5,13(18)-dien-3α-ol (C30H48O) | Ethanolic extract | Benzene–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Root bark178 |
| 75 | Cycloart-23-ene-3β,25-diol (C30H50O2) | Ethyl acetate extract | Hexane–ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Flowers166 |
| 76 | Lupeol (C30H50O) | Ethanolic extract | — | Latex179 |
| 77 | 3-epi-Moretenol (C30H50O) | Ethanolic extract | — | Latex179 |
| 78 | Multiflorenol (C30H50O) | Pet. ether fraction | Chloroform–ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Flowers,166 latex167 |
| 79 | Urs-19(29)-en-3-β-ol (C30H50O) | Acetone fraction | Pet. ether–acetone (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Latex167 |
| 80 | Calotropenyl acetate or urs-19(29)-en-3-yl acetate (C32H52O2) | Pet. ether fraction | Chloroform–ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
Latex167 |
| 81 | 3β,27-Dihydroxy-urs-18-en-13,28-olide (C30H46O4) | Ethyl acetate fraction | Benzene–ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Latex167 |
| 82 | Calotropfriedelenyl acetate or friedelin-1-ene-3 β-yl acetate (C32H52O2) | Ethanolic extract | — | Root bark180 |
| 83 | Calotropterpenyl ester or 6,10,14-trimethylpentadec-6-enyl-2′,4′,8′,12′,16′-pentamethyl nonadecane ester (C42H82O2) | Ethanolic extract | — | Root bark180 |
| 84 | Phytyl iso-octyl ether or 3,7,11,15-tetramethyl hexadecanyl-6′-methyl hept-5′-enyl ether (C28H56O) | Methanolic extract | Pet. ether–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Roots181 |
| 85 | Dihydrophytoyl tetraglucoside or 3,7,11,15 tetramethylhexadecanoyl-β-D-glucopyranosyl-(2 → 1)-β-D-glucopyranosyl-(2 → 1)-β-D-glucopyranosyl (2 → 1)-β-D-glucofuranoside (C44H80O22) | Methanolic extract | Chloroform–methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Roots181 |
| 86 | Procerasesterterpenoyl triglucoside or 2,6,10,14,18-pentamethylnonadecanoyl-β-D-glucopyranosyl-(2 → 1)-β-D-glucopyranosyl-(2 → 1)-β-D-glucopyranoside (C42H78O17) | Methanolic extract | Chloroform–methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots181 |
| 87 | Oleanolic acid (C30H48O3) | Chloroform extract/butanol fraction | Benzene–ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1 1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
Stem bark165 |
| 88 | Lupeol-3-O-acetate (C32H52O2) | Ethanolic extract | Chloroform–methanol (9.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7) 0.7) |
Leaves149 |
| 89 | Proceraursenolide or 18-αH-urs-12-en-3,25-olide (C30H46O2) | Ethanolic extract | Pet. ether–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Roots183 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Oxypregnane oligoglycosides | ||||
| 90 | Calotroposide H or 12-O-benzoylisolineolon-3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-cymaropyranosyl (C63H96O21) | Methanolic extract/n-butanol fraction | Chloroform–methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
Root bark153 |
| 91 | Calotroposide I or 12-O-benzoylisolineolon-3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl (C63H96O21) | Methanolic extract/n-butanol fraction | Chloroform–methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
Root bark153 |
| 92 | Calotroposide J or 12-O-benzoylisolineolon-3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl-( 1 → 4)-β-D-cymaropyranosyl-(1 → 4)-(6-O-acetyl)-β-D- glucopyranoside (C71H108O27) | Methanolic extract/n-butanol fraction | Chloroform–methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
Root bark153 |
| 93 | Calotroposide K or 12-O-benzoylisolineolon-3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D- glucopyranoside (C69H106O26) | Methanolic extract/n-butanol fraction | Chloroform–methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
Root bark153 |
| 94 | Calotroposide L or 12-O-benzoylisolineolon-3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-glucopyranosyl-(1 → 4)-β-D-glucopyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranoside (C68H104O28) | Methanolic extract/n-butanol fraction | Chloroform–methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
Root bark153 |
| 95 | Calotroposide M or 12-O-benzoylisolineolon-3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranoside-(1 → 4)-(6-O-acetyl)-β-D-glucopyranoside (C78H120O30) | Methanolic extract/n-butanol fraction | Chloroform–methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
Root bark153 |
| 96 | Calotroposide N or 12-O-benzoylisolineolon-3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D- oleandropyranosyl-(1 → 4)-β-D-glucopyranoside-(1 → 4)-β-D-gluopyranoside (C75H116O31) | Methanolic extract/n-butanol fraction | Chloroform–methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
Root bark153 |
| 97 | Calotroposide S or 12-benzoylisolineolon-3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-oleandropyranosyl-(1 → 4)-β-D-oleandro-pyranosyl-(1 → 4)-β-D-oleandropyranoside (C84H132O30) | Methanolic extract/n-butanol fraction | Chloroform–methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
Root bark154 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Aliphatic and phenolic glycoside | ||||
| 98 | Methyl resorcinyl triglycoside or O-methyl resorcinyl-β-D-glucuronopyranosyl (2 → 1)-β-D-glucopyranosyl-(2 → 1)-β-D-glucopyranoside (C25H36O18) (phenolic glycoside) | Methanolic extract | Chloroform–methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Roots169 |
| 99 | Butanediol diglucuronoside or (n-butan-1,4-diol-1,4-β-D-diglucuronopyranoside) (C16H26O14) (aliphatic glycoside) | Methanolic extract | Chloroform–methanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots169 |
| 100 | (E)-3-(4-Methoxyphenyl-2-O-β-D-4C1-glucopyranoside)-methyl propenoate (C17H22O9) | 85% methanolic extract | 40–60% aqueous methanol | Leaves76 |
| 101 | Methyl 4-O-β-D-glucopyranosyl ferulate (C17H22O9) | Ethanolic extract | Water–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Flowers149 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Lignan glycoside | ||||
| 102 | 7′-Methoxy-3′-O-demethyl-tanegool-9-O-β-D-glucopyranoside (C26H34O12) | Ethanolic extract | Water–methanol (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Flowers149 |
| 103 | Pinoresinol-4-O-glucoside (C26H32O11) | Ethanolic extract | Water–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Flowers149 |
| 104 | Syringaresinol-4-O-glucoside (C28H36O13) | Ethanolic extract | Water–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Fruits149 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Terpene glycoside | ||||
| 105 | Labdan-18-ol-β-D-galactofuranoside (C26H48O6) | Methanolic extract | Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots182 |
| 106 | Proceralabdanoside/labdan-3β-ol-11,15-olide-18,20-dioic acid-3β-D-galactofuranoside (C26H40O12) | Methanolic extract | Chloroform–methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots182 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Caffeic acid derivatives | ||||
| 107 | Methyl caffeate (C10H10O4) | 85% methanolic extract | 30–50% aqueous methanol | Leaves76 |
| 108 | Caffeic acid (C9H8O4) | 85% methanolic extract | 30–50% aqueous methanol | Leaves76 |
| 109 | Rosmarinic acid (C18H16O8) | Ethanolic extract | Chloroform–methanol (8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) 1.5) |
Flowers149 |
| 110 | Methyl rosmarinate (C19H18O8) | Ethanolic extract | Chloroform–methanol (8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) 1.5) |
Flowers149 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Others | ||||
| 111 | 2-Propenyl-2Z-hydroxyethyl carbonate | — | — | Leaves186 |
| 112 | Glyceryl mono-oleolyl-2-phosphate (C21H41O7P) | Methanolic extract | Pet. ether–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Roots177 |
| 113 | Methyl behenate (C23H46O2) | Methanolic extract | Chloroform–methanol (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots177 |
| 114 | N-Dotriacont-6-ene (C32H64) | Methanolic extract | Pet. ether–chloroform (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots177 |
| 115 | Methyl myrisate (C15H30O2) | Methanolic extract | Chloroform | Roots177 |
| 116 | Glyceryl-1,2-dicapriate-3-phosphate (C23H45O8P) | Methanolic extract | Chloroform–methanol (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Roots177 |
| 117 | (E)-Octadec-7-enoic acid (C18H34O2) | Methanolic extract/n-hexane fraction | n-Hexane–ethyl acetate | Root bark139 |
| 118 | Proceranol or n-triacontan-10β-ol (C30H62O) | Methanolic extract | Chloroform–methanol (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots177 |
| 119 | Methyl ferulate | Methanolic extract | Chloroform–methanol (8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) 1.5) |
Flowers149 |
| 120 | 1,2-Dihexadecanoyl-3-phosphatyl glycerol (C35H69O8P) | Methanolic extract | Chloroform–methanol (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots181 |
| 121 | n-Tetradecanyl palmitoleate/n-tetradecanyl n-hexadec-9-enoate (C30H58O2) | Methanolic extract | Pet. ether–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Roots183 |
| 122 | Tricapryl glyceride (C33H62O6) | Methanolic extract | Pet. ether | Roots183 |
| 123 | Oleodipalmityl glyceride (C53H100O6) | Methanolic extract | Pet. ether–chloroform (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots183 |
| 124 | Tribehenyl glyceride (C69H134O6) | Methanolic extract | Pet. ether–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots183 |
| 125 | Capryl glucoside/n-decanoyl-β-D-glucopyranoside (C16H31O7) | Methanolic extract | Chloroform–methanol (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots182 |
| 126 | Palmityl glucoside/n-hexacosanoyl- β-D-glucopyranoside (C22H43O6) | Methanolic extract | Chloroform–methanol (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Roots182 |
| 127 | Stearyl glucoside/n-octadecanoyl-β-D-glucopyranoside (C24H47O7) | Methanolic extract | Chloroform–methanol (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
Roots182 |
| 128 | n-Heptanoate/heptylate (C8H16O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 129 | n-Octanoate/caprylate (C9H18O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 130 | n-Nonanoate (C10H20O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 131 | n-Tridecanoate/tridecylat (C14H28O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 132 | n-Pentadecanoate/pantadecylate (C16H32O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 133 | n-Hexadecanoate/palmitate (C16H34O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 134 | n-Heptadecanoate/margorate (C18H36O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 135 | Methyl nonanotetracnoate (C10H12O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 136 | n-Decenoic acid (C10H18O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 137 | 9-Decenoate (C11H20O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 138 | Undecadienoate (C12H20O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 139 | 9-Dodecenoate (C13H24O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 140 | Tridecatrienoate (C14H22O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 141 | 2,4,5-Tetradecatrienoate (C15H24O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 142 | Hiragonate (C17H28O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 143 | Heptadecadienoate (C18H22O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 144 | Heptadecenoate (C18H38O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 145 | 9-Eicosenoate/gadoleate (C21H40O2) | Ethanolic extract | Hexane–chloroform | Aerial part162 |
| 146 | Gallic acid (C7H6O5) | Ethanolic extract | HPLC analysis | Aerial part184 |
| 147 | Ferulic acid (C10H10O4) | Ethanolic extract | HPLC analysis | Aerial part184 |
| 148 | p-Coumaric acid (C9H8O3) | Ethanolic extract | HPLC analysis | Aerial part184 |
| 149 | Vanillic acid (C8H8O4) | Ethanolic extract | HPLC analysis | Aerial part184 |
| 150 | Rutin (C27H30O16) | Ethanolic extract | HPLC analysis | Aerial part184 |
| 151 | 4-Hydroxy-4-methylpentan-2-one (C6H12O2) | Acetone extract | GC-MS analysis | Latex161 |
| 152 | 2,3,4-Trimethylhexane (C9H20) | Acetone extract | GC-MS analysis | Latex161 |
| 153 | Decane (C10H22) | Acetone extract | GC-MS analysis | Latex161 |
| 154 | n-Pentadecane (C15H32) | Acetone extract | GC-MS analysis | Latex161 |
| 155 | 2,6-Dimethyl tetra-1,5-decaene (C16H28) | Acetone extract | GC-MS analysis | Latex161 |
| 156 | n-Eicosane (C20H42) | Acetone extract | GC-MS analysis | Latex161 |
| 157 | 3,7,11-Trimethyl-2,6,10,12-pentadecatrien-1-ol (C18H30O) | Acetone extract | GC-MS analysis | Latex161 |
| 158 | 2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene (C30H50) | Acetone extract | GC-MS analysis | Latex161 |
| 159 | 1,3,5-Tri-isopropylbenzene (C15H24) | Acetone extract | GC-MS analysis | Latex161 |
| 160 | 6,10,14-Trimethyl-pentadecanone-2 (C18H36O) | Hexane extract | GC-MS analysis | Leaves185 |
| 161 | 9-Octadecenoic acid (Z)-(C18H34O) | Hexane extract | GC-MS analysis | Leaves185 |
| 162 | (6Z,9Z)-Pentadecadien-1-ol (C15H28O) | Hexane extract | GC-MS analysis | Leaves185 |
| 163 | Farnesol isomer (C15H26O) | Hexane extract | GC-MS analysis | Leaves185 |
| 164 | Tetratetracontane (C44H90) | Hexane extract | GC-MS analysis | Leaves185 |
| 165 | Ergost-5-en-3-ol (C28H48O) | Hexane extract | GC-MS analysis | Leaves185 |
Apart from the compounds mentioned in Table 11, terpenoids named α-calotropeol and β-calotropeol have been isolated from ethanolic extract of latex.179 A cardenolide named 19-dihydrocalotropagenin and flavonoid named 3′-O-methyl-quercetin-3-O-rutinoside have also been reported from ethanolic extract of aerial parts.168
7. Conclusion, discussion and future perspectives
In the present review, the research progress in phytochemistry and pharmacology of C. procera have been summarized. There have been acquirements in the research; still some gaps came across our studies which are as follows:(1) Folks and tribes have been using C. procera since ancient times; still investigations can be carried out on inception time of traditional uses of C. procera.
(2) Secondary metabolites of plant vary according to several factors like region, environment, quality of soil, age of plant etc. Moreover, latex and root bark seem to be exhaustively investigated for phytoconstituents, not much research on flowers, pods and seeds for phyoconstituentsis have been conducted. Further exploring these parts can lead to discovery of new phytoconsituents of interest.
(3) The plant can be employed commercially as scientific studies have proved its use as cheese making agent, dehairing of leather, natural surfactant, biopesticide and corrosion inhibitor.
(4) Numerous activities on validation of its cytotoxic and anti-inflammatory potential have been conducted. A few have been carried out on its antimigraine, antiplasmodial and anticonvulsant effects. Carrying out further scientific studies in these fields can provide medical science with effective and promising new drugs.
(5) Most of the cytotoxic activities conducted are in vitro except the one conducted on UNBS1450; a semi-synthesized cardenolide. Further studies should be carried out to examine its in vivo potential.
(6) Right route and right dose can convert a dreadful toxicant into an outstanding drug whereas even a drug in lack of proper dosage and route can become a fatal poison. Folk practitioners have been employing C. procera as antifertility and uterotonic agent. Further studies using positive controls, study of toxicity and side effects can lead to discovery of effective and natural contraceptive drugs.
(7) Active principles behind many of the activities are unknown, except the one known for cytotoxic, antibacterial, antifertility, antimolluscicidal and insecticidal activity. More research can be carried out to know the active principles so that potent drugs can be made.
(8) Replicable and environment benign sources of energy are the need of hour, Calotropis procera being rich source of various hydrocarbons, thus can prove to be a promising biofuel agent.
Overall, the pharmacology, toxicology, traditional uses, use of secondary metabolites, clinical trials and quality control has been reviewed in this paper. However, there seems to be a good correspondence between pharmacological activities and traditional uses. Further research in this field is essential to determine the active principles and the underlying mechanisms.
Author contributions
Barkha Darra Wadhwani: literature collection, evaluation and draft manuscript preparation. Deepak Mali and Pooja Vyas: literature collection: pharmacological activity and analyses of chemicals constituents of C. procera. Rashmy Nair: reviewing and editing. Poonam Khandelwal: concept development; idea generation; manuscript preparation; reviewing and editing.Conflicts of interest
The authors confirm that this article content has no conflict of interest.Acknowledgements
One of the authors (Barkha Darra Wadhwani) is thankful to DST, India for providing WOS-A project sanction no. SR/WOS-A/CS-24/2019(G).References
- M. C. Joshi, M. B. Patel and P. J. Mehta, Bull. Med.-ethno-bot. Res., 1980, 1, 8–24 Search PubMed.
- K. Chandra and U. N. Pandey, Some folk medicines of Singhbhum (Bihar), Sachitra Ayurveda, 1984, 37, 253–357 Search PubMed.
- L. S. Bhatnagar, V. K. Singh and G. Pandey, J. Res. Indian Med., 1973, 8(2), 67–100 Search PubMed.
- J. Venkateswarulu, P. V. Bhairavamurthy and N. Rao, The Flora of Visakhapatnam, Andhra Pradesh Academy of Sciences, Hyderabad, 1972, p. 128 Search PubMed.
- H. S. Al-Mezaine, A. A. Al-Rajhi, A. Al-Assiri and M. D. Wagoner, Am. J. Ophthalmol., 2005, 139, 199–202 CrossRef PubMed.
- E. W. C. Chan, N. I. Sweidan, S. K. Wong and H. T. Chan, Rec. Nat. Prod., 2017, 11(4), 334–344 CrossRef CAS.
- P. M. Ranjit, G. E. Rao, M. Krishnapriya, V. Nagalakshmi, P. Silpa and M. Anjali, FS J. Pharm. Res., 2012, 1, 18–25 Search PubMed.
- R. Sharma, G. Thakur, B. S. Sanodiya, A. Savita, M. Pandey, A. Sharma and P. S. Bisen, IOSR J. Pharm. Biol. Sci., 2012, 4(3), 42–57 Search PubMed.
- P. A. Karale and M. A. Karale, Asian J. Pharm. Clin. Res., 2017, 10, 27–34 CrossRef CAS.
- G. Parihar and N. Balekar, Thai J. Pharm. Sci., 2016, 40, 115–131 CAS.
- R. K. Upadhyay, Int. J. Green Pharm., 2014, 8(3), 135–146 CrossRef CAS.
- R. P. Mali, P. S. Rao and R. S. Jadhav, J. Drug. Deliv. Ther., 2019, 9, 947–951 CAS.
- H. S. Alzahrani, M. Mohamemd, S. Kulvinder and M. R. Rizgallah, J. Appl. Environ. Biol. Sci., 2017, 7(10), 232–240 Search PubMed.
- A. K. Khairnar, S. R. Bhamare and H. P. Bhamare, Adv. Res. Pharm. Biol., 2012, 2, 142–156 CAS.
- A. Ranade and R. Acharya, Glob. J. Res. Med. Plants Indig. Med., 2014, 3(12), 475–488 Search PubMed.
- Z. Yaniv and H. Koltai, Isr. J. Plant Sci., 2018, 65, 55–61 Search PubMed.
- S. M. Bairagi, P. Ghule and R. Gilhotra, Ars Pharm., 2018, 59(1), 37–44 Search PubMed.
- N. Ranjan, S. K. Singh and C. Kumari, Int. J. Curr. Microbiol. App. Sci., 2017, 6(4), 1640–1648 CrossRef.
- Poonam and G. Punia, Global J. Res. Med. Plants & Indigen. Med., 2013, 2(5), 392–400 Search PubMed.
- (a) S. Quazi, K. Mathur and S. Arora, Indian J. Drugs, 2013, 1(2), 63–69 Search PubMed; (b) A. Bera, S. Maiti and N. Banerjee, Int. J. Pharm. Sci. Res., 2020, 11(11), 5425–5433 CAS; (c) I. Pavani and S. Udayavani, World J. Pharm. Res., 2020, 9(14), 1381–1392 CAS; (d) A. Kaur, D. R. Batish, S. Kaur and B. S. Chauhan, Front. Plant Sci., 2021, 12, 690806, DOI:10.3389/fpls.2021.690806.
- P. Chandrawat and R. A. Sharma, Res. J. Recent Sci., 2016, 5(1), 61–70 Search PubMed.
- A. K. Meena, A. Yadav and M. M. Rao, Asian J. Tradit. Med., 2011, 6(2), 45–53 Search PubMed.
- C. D. T. de Freitas, J. L. Lopes, L. M. Beltramini, R. S. B. de Oliveira, J. T. A. Oliveira and M. V. Ramos, Biochim. Biophys. Acta, 2011, 1808, 2501–2507 CrossRef PubMed.
- P. J. Modi, Medical Jurisprudence and Toxicology, 2006, first reprint Dr Mathiharan, K., Dr Patnaik, A.K. Lexis Nexis, New Delhi, 23rd edn, 2007, pp. 234–238 Search PubMed.
- B. Biedner and L. R. A. Witztum, Isr. J. Med. Sci., 1977, 13, 914–916 CAS.
- W. Laukanjanaratand and M. Tovanich, Thai. J. Ophthalmol., 1997, 1, 87–90 Search PubMed.
- T. Devasari, Indian J. Pharmacol., 1965, 27, 272–275 Search PubMed.
- S. K. Basak, A. Bhaumik, A. Mohanta and P. Singhal, Indian J. Ophthalmol., 2009, 57(3), 232–234 CrossRef PubMed.
- H. Tavakkoli, A. Derakhshanfar, J. Moayedi, A. P. Fard, S. Behrouz, M. A. Piltan and M. N. Soltani-Rad, Comp. Clin. Pathol., 2019, 28, 195–202 CrossRef CAS.
- A. Akhkha, Biosci. Biotechnol. Res. Asia, 2009, 6(2), 653–658 CAS.
- M. A. Ramadana, A. A. Azeiz, S. Baabada, S. Hassanein, N. O. Gadalla, S. Hassan, M. Algandaby, S. Bakr, T. Khan, H. H. Abouseadaa, H. M. Ali, A. Al-Ghamdi, G. Osman, S. Edris, H. Eissa and A. Bahieldin, Steroids, 2019, 141, 1–8 CrossRef PubMed.
- A. S. Traore, Bioresour. Technol., 1992, 41, 105–109 CrossRef CAS.
- M. O. Barbosa, J. S. de Almeida-Cortez, S. I. da Silva and A. F. M. de Oliveira, J. Am. Oil Chem. Soc., 2014, 91, 1433–1441 CrossRef CAS.
- M. V. Ramos, C. D. T. Freitas and F. Staniscuaski, Plant Science, 2007, 173, 349–357 CrossRef CAS.
- G. E. Nenaah, Ind. Crops Prod., 2013, 45, 327–334 CrossRef CAS.
- O. C. Aworh and S. Nakai, J. Food Sci., 1986, 51, 1569–1570 CrossRef CAS.
- D. Raheem, N. Suri and P. E. Saris, Int. J. Food Sci. Technol., 2007, 42, 220–223 CrossRef CAS.
- C. K. Atal and P. D. Sethi, Planta Med., 1962, 10(1), 77–90 CrossRef CAS.
- D. A. R. Agossou Yao, Y. Sprycha, S. Porembski and R. Horn, Genet. Resour. Crop. Evol., 2015, 62, 863–878 CrossRef.
- M. Chandrashekar, H. Nagabhushana, S. C. Sharma, Y. S. Vidya, K. S. Anantharaju, D. Prasad, S. C. Prashantha, D. Kavyashree and P. S. Maiya, Mater. Res. Express, 2015, 2(4), 045402, DOI:10.1088/2053-1591/2/4/045402.
- P. B. Raja and M. G. Sethuraman, Pigm. Resin Technol., 2009, 38(1), 33–37 CrossRef CAS.
- L. Lopez, C. Viana, M. Errasti, M. L. Garro, J. E. Martegani, G. A. Mazilli, C. D. T. Freitas, I. M. S. Araujo, R. O. da silva and M. V. Ramos, Bioprocess Biosyst. Eng., 2017, 40, 1391–1398 CrossRef CAS PubMed.
- M. K. Misra, M. K. Mohanty and P. K. Das, Anc. Sci. Life, 1993, 13, 40–56 CAS.
- L. Misra, Sahaja Chikichcha (in Oriya), ed. K. Devi Puri, 1959 Search PubMed.
- P. K. Jain, R. Verma, N. Kumar and A. Kumar, Jour. Res. Ay. Sid., 1985, 6, 88–91 Search PubMed.
- M. Garg, Sudhanidhi (Hindi edition) and D. Karyalaya, Bijoygarh, Uttar Pradesh, 1986, vol. 5, pp. 165–202 Search PubMed.
- K. R. Kirtikar and B. D. Basu, Indian Medicinal Plants, ed. B. Singh and M. Singh, Dehra Dun, 1933, vol. 3, pp. 1606–1611 Search PubMed.
- B. Tripathy, Dravyaguna Kalpadruma (Oriya edition), ed. D. Tripathy, Nayagarh, 1953, pp. 22–28 Search PubMed.
- Anon., The wealth of India (Raw Materials), Council of Scientific and Industrial Research, New Delhi, 1959, vol. 2, pp. 20–23 Search PubMed.
- R. R. Pathak, Therapeutic guide of Ayurvedic medicines, Baidyanath Ayurveda Bhawan, Patna, 1970 Search PubMed.
- J. F. Dastur, Medicinal Plants of India and Pakistan, D. B. Taraporevalla Sons & Co., Bombay, 1970, pp. 43–44 Search PubMed.
- S. K. Jain, D. K. Banerjee and D. C. Pal, Medicinal Plants among certain Adivasis in India, Bull. Bot. Surv. India, 1973, 15, 85–91 Search PubMed.
- P. V. Sharma, Dravyaguna Vigyana, Choukamba Bharati Academy, Varanasi, India, 5th hindi edn, 1985 Search PubMed.
- P. K. Hajra and A. K. Baishya, Ethnobotanical notes on the Miris (Mishings) of Assam Plains, ed. S. K. Jain, Glimpses of Indian Ethnobotany, Oxford & IBH Publishing Co., New Delhi, 1981, pp. 161–169 Search PubMed.
- G. Hesse and F. Reicheneder, Justus Liebigs Ann. Chem., 1936, 526, 252–276 CrossRef CAS.
- V. G. Hesse, F. Reicheneder and H. Eysenbach, Justus Liebigs Ann. Chem., 1939, 537, 67–86 CrossRef.
- G. Hesse and G. Ludwig, Justus Liebigs Ann. Chem., 1960, 632, 158–171 CrossRef CAS.
- D. H. G. Crout, C. H. Hassall and T. L. Jones, J. Chem. Soc., 1964, 2187–2194 RSC.
- R. S. Gupta, N. Sharma and V. P. Dixit, Anc. Sci. life, 1990, 9(4), 224–230 CAS.
- S. Rajagopalan, Ch. Tamm and T. Reichstein, Helv. Chim. Acta., Fasciculus, 1955, 38(7), 1809–1824 CrossRef CAS.
- F. Bruschweiler, W. Stocklin, K. Atockel and T. Reichstein, Helv. Chem. Acta., 1969, 52, 2086–2106 CrossRef CAS PubMed.
- V. E. Quaquebeke, G. Simon, A. Andre, J. Dewelle, M. E. Yazidi, F. Bruyneel, J. Tuti, O. Nacoulma, P. Guissou, C. Decaestecker, J. C. Braekman, R. Kiss and F. Darro, J. Med. Chem., 2005, 48, 849–856 CrossRef PubMed.
- N. Akhtar and A. Malik, Phytochemistry, 1992, 31(8), 2821–2824 CrossRef CAS.
- H. Joshi, V. Havannavar, C. Gavimat, H. Pooja and P. Praveena, J. Alzheimer's Assoc., 2008, 4(4), T502 Search PubMed.
- N. H. Mohamed, M. Liu, W. M. Abdel-Mageed, L. H. Alwahibi, H. Dai, M. A. Ismail, G. Badr, R. J. Quinn, X. Liu, L. Zhang and A. A. M. Shoreit, Bioorg. Med. Chem. Lett., 2015, 25, 4615–4620 CrossRef CAS PubMed.
- A. S. Awaad, G. M. Zain, M. Reham, H. F. Alkanhal and V. D. Seshadri, Calotropis procera extracts as anti-ulcerative colitis agents, US Pat., 9533019B1, 2017 Search PubMed.
- A. M. Rasik, R. Raghubir, A. Gupta, A. Shukla, M. P. Dubey, S. Srivastava, H. K. Jain and D. K. Kulshrestha, J. Ethnopharmacol., 1999, 68, 261–266 CrossRef CAS PubMed.
- A. O. Aderounmua, A. E. Omonisib, J. A. Akingbasotec, M. Makanjuolad, R. A. Bejide, L. O. Orafidiya and K. A. Adelusolae, Afr. J. Tradit. Complement. Altern. Med., 2013, 10(3), 574–579 Search PubMed.
- D. A. Tsala, N. Nga, M. B. N. Thiery, M. T. Bienvenueand and D. Theophile, J. Intercult. Ethnopharmacol., 2015, 4(1), 64–69 CrossRef CAS PubMed.
- R. A. Patil and A. B. Makwana, Indian J. Pharmacol., 2015, 47(4), 398–402 CrossRef CAS PubMed.
- R. P. Samy and V. T. K. Chow, Evid. Based Complement. Alternat. Med., 2012, 294528, DOI:10.1155/2012/294528 , PMID: 22973400, .
- A. S. Seddek, A. A. El-Ghoneimy, M. W. Dina, S. El-hamd and E. G. Mahmoud, Egypt. J. Chem. Environ. Health, 2015, 1(1), 768–784 Search PubMed.
- J. D. Mbako, Z. Adamu, J. K. Afutu, A. Aliyu, S. David, M. B. Umar and C. Nduaka, Afr. J. Biotechnol., 2009, 8(19), 5071–5075 CAS.
- G. B. Pouokam, H. Ahmed, C. Dawurung, A. Atiku, S. David and O. Philipe, J. Toxicol. Environ. Health Sci., 2011, 3(5), 119–126 Search PubMed.
- A. M. Dieye, M. A. Tidjani, A. Diouf, E. Bassene and B. Faye, Dakar Med., 1993, 38(1), 69–72 CAS.
- M. A. Mohamed, M. M. Hamed, W. S. Ahmed and A. M. Abdou, Z. Naturforsch., C: J. Biosci., 2011, 66, 547–554 CrossRef CAS PubMed.
- T. L. Juca, M. V. Ramos, F. B. M. Batista Moreno, M. P. V. de Matos, J. D. B. Marinho-Filho, R. A. Moreira and A. C. de Oliveira Monteiro-Moreiro, Sci. World J., 2013, 615454, DOI:10.1155/2013/615454.
- E. A. A. Sadaqa and K. S. Ali, Int. J. Pharm. and Pharm. Res., 2019, 16(4), 400–407 Search PubMed.
- E. S. A. Toson, S. A. Habib, E. A. Saad and N. H. Harraz, Int. J. Biochem., 2014, 195, 328–338 Search PubMed.
- A. B. Abbasi, R. Bibi, A. A. Khan, M. S. Iqbal, J. Sherani and A. M. Khan, J. Biofertil. Biopestici., 2012, 3, 126 Search PubMed.
- P. S. Jahan, A. Mannan, A. R. Khan and P. Karmakar, Bangladesh J. Zool., 1991, 19(2), 261–262 Search PubMed.
- I. A. Muraina, A. O. Adaudi, M. Mamman, H. M. Kazeem, J. Picard, L. J. McGaw and J. N. Elof, Pharm. Biol., 2010, 48(10), 1103–1107 CrossRef CAS PubMed.
- R. Chavda, K. R. Vadalia and R. Gokani, Int. J. Pharmacol., 2010, 6(6), 937–943 Search PubMed.
- S. R. Setty, A. A. Quereshi and A. H. M. Viswanath Swamy, Fitoterapia, 2007, 78, 451–454 CrossRef PubMed.
- A. Basu, T. Sen, R. N. Ray and A. K. Nag-Chaudhuri, Fitoterapia, 1992, 63(6), 507–514 Search PubMed.
- G. Nenaah, World J. Microbiol. Biotechnol., 2013, 29, 1255–1262 CrossRef CAS PubMed.
- S. O. Kareem, I. Akpan and O. P. Ojo, Afr. J. Biomed. Res., 2008, 11, 105–110 Search PubMed.
- H. O. Oladimeji, R. Nia and E. E. Essien, Afr. J. Biomed. Res., 2006, 9, 205–211 Search PubMed.
- S. C. Jain, R. Sharma, R. Jain and R. A. Sharma, Fitoterpia, 1996, 67(3), 275–277 Search PubMed.
- T. L. Nascimento, Y. Oki, D. M. M. Lima, J. S. Almeida-Cortez, G. W. Fernandes and C. M. Souza-Motta, Fungal Ecol., 2015, 14, 79–86 CrossRef.
- V. H. Bhaskar, Asian J. Chem., 2000, 21(7), 5788–5790 Search PubMed.
- B. Desta, J. Ethnopharmacol., 1993, 39(2), 129–139 CrossRef CAS PubMed.
- N. Mascolo, R. Sharma, S. C. Jain and F. Capasso, J. Ethnopharmacol., 1988, 22(2), 211–221 CrossRef CAS PubMed.
- O. P. Shukla and C. R. Krishnamurti, J. Sci. Ind. Res., 1961, 20(8), 225–226 Search PubMed.
- M. S. Kumar and U. K. Chanhan, Geobios, 1992, 19, 135–137 Search PubMed.
- N. Nawazisht, I. Malik and M. I. D. Chugtai, Pak. J. Sci., 1979, 31, 127–129 Search PubMed.
- A. H. Kawo, A. Mustapha, B. A. Abdullahi, L. D. Rogo, Z. A. Gaiyaand and A. S. Kumurya, Bayero. J. Pure Appl. Sci., 2009, 2(1), 34–40 Search PubMed.
- P. O. Akindele, O. A. Fatunla, K. A. Ibrahim and C. O. Afolayan, J. Complement. Altern. Med. Res., 2017, 2(1), 1–14 CrossRef.
- V. Talsaniya, T. Patel, N. Saiyad, S. Desai, D. Patel and D. Meshram, Int. J. Pharm. Sci. Rev. Res., 2014, 25(2), 241–244 Search PubMed.
- R. Lima, N. Lima, E. Chaves, L. Leal, M. Patrocinio, R. Lobato, M. Ramos, F. C. F. Sousa, K. Carvalho and S. Vasconcelos, J. Complement. Integr. Med., 2010, 7, 1–9 Search PubMed.
- S. Gholamshahi, A. V. Mohammad, S. Fatemeh and A. Salehi, Int. J. Biosci., 2014, 4(7), 159–164 CAS.
- M. N. Yesmin, S. N. Uddin, S. Mubassara and M. A. Akond, American-Eurasian J. Agric. & Environ. Sci., 2008, 4(5), 550–553 Search PubMed.
- S. Loonker, W. A. Qadri and J. Singh, Int. J. Cur. Res. Rev., 2015, 7, 55–59 Search PubMed.
- P. M. Soares, S. R. Lima, S. G. Matos, M. M. Andrade, M. C. A. Patrocinio, C. D. T. de Freitas., M. V. Ramos, D. N. Criddle, B. A. Cardi, K. M. Carvalho, A. M. S. Assreuy and S. M. M. Vasconcelos, J. Ethnopharmocol., 2005, 99, 125–129 CrossRef PubMed.
- Z. Iqbal, M. Lateef, A. Jabbar, G. Muhammad and M. N. Khan, J. Ethnopharmacol., 2005, 102, 256–261 CrossRef PubMed.
- Y. M. Shivkar and V. L. Kumar, Pharm. Biol., 2003, 41(4), 263–265 CrossRef.
- A. A. Al-Qarawi, O. M. Mahmoud, M. A. Sobaih, E. M. Haroum and S. E. I. Adam, Vet. Res. Commun., 2001, 25, 61–70 CrossRef CAS PubMed.
- H. Sangraula, S. Dewan and V. L. Kumar, Inflammopharmacology, 2002, 9(3), 257–264 CrossRef.
- V. L. Kumar and N. Basu, J. Ethnopharmacol., 1994, 44, 123–125 CrossRef CAS PubMed.
- N. S. Tour and G. S. Talele, Rev. Bras. Farmacogn., 2011, 21(6), 1118–1126 CrossRef CAS.
- P. K. Majumdar and V. L. Kumar, Phytother. Res., 1997, 11(2), 166–167 CrossRef.
- C. R. Jangde, C. G. Raut and V. V. Bisan, Livestock Advisor, 1994, 19(3), 29–31 Search PubMed.
- S. Kumar, S. Dewan, H. Sangraula and V. L. Kumar, J. Ethnopharmacol., 2001, 76(1), 115–118 CrossRef CAS PubMed.
- O. J. Olaitan, S. U. R. Wasagu, A. A. Adepoju-Bello, K. U. Nwaeze and A. Olufunsho, Nig. Q. J. Hosp. Med., 2013, 23(4), 338–341 Search PubMed.
- D. Srivastav and P. Singh, World J. Pharm. Res., 2015, 4(3), 1123–1135 Search PubMed.
- M. Larhsini, M. Bonsaid, H. Lazrek, M. Jana and H. Amarouch, Fitoterapia, 1997, 68(4), 371–373 Search PubMed.
- R. M. Aliyu, M. B. Abubakar, Y. U. Dabai, N. Lawal, M. B. Bello and A. Y. Fardami, J. Intercult. Ethnopharmacol., 2015, 4(4), 314–317 CrossRef CAS PubMed.
- N. Pathak and R. K. Zaidi, Ann. Biol. Res., 2013, 4(4), 1–6 CAS.
- A. M. Mashlawi, M. K. H. Ali and E. S. Tarek, Int. J. Mosq. Res., 2017, 4(1), 1–6 Search PubMed.
- N. Begum, B. Sharma and R. S. Pandey, J. Biofertil. Biopestici., 2010, 1, 101 Search PubMed.
- A. M. Elimam, K. H. Elimalik and F. S. Ali, J. Biol. Sci., 2009, 16, 95–100 Search PubMed.
- H. Doshi, H. Satodiya, M. C. Thakur, F. Parabia and A. Khan, Int. J. Plant Res., 2011, 1(1), 29–33 CrossRef.
- N. M. Azmathullah, M. A. Sheriff and A. K. S. Mohideen, Int. J. Pharm. Biol. Arch., 2011, 26, 1718–1721 Search PubMed.
- S. M. P. Khurana and S. Singh, Phytopathol. Z., 1972, 73, 341–346 CrossRef.
- J. V. Kamath and A. C. Rana, Fitoterapia, 2002, 73(2), 111–115 CrossRef PubMed.
- S. M. A. El-Badwi and A. O. Bakhiet, Sci. Res. Essays, 2010, 5(17), 2404–2408 Search PubMed.
- M. A. Qureshi, N. M. Qureshi, R. Arshad and R. Begum, Pak. J. Zool., 1991, 23(2), 161–165 Search PubMed.
- C. Circosta, R. Sanogo and F. Occhiuto, IL Farmaco, 2001, 56, 373–378 CrossRef CAS PubMed.
- M. V. Ramos, C. A. Viana and A. F. Silva, Naunyn Schmiedebergs Arch. Pharmacol., 2012, 385(5), 455–463 CrossRef CAS PubMed.
- P. Sharma and J. D. Sharma, J. Ethnopharmacol., 1999, 68, 83–95 CrossRef CAS PubMed.
- S. Y. Mudi and A. Bukar, Biochemistry, 2011, 23, 29–34 Search PubMed.
- S. Dewan, S. Kumar and V. L. kumar, Ind. J. Pharmacol., 2000, 32, 252–253 Search PubMed.
- U. P. Upadhyay, J. Sci. Res. Plant. Med., 1979, 1(1), 52–55 Search PubMed.
- S. S. Jalalpure, Pharm. Biol., 2009, 47(2), 162–167 CrossRef.
- J. S. Oliveira, D. P. Bezerra, C. D. T. Freitas, J. D. B. Marinho-Filho, M. O. de Moraes, C. Pessoa, L. C. V. Costa-Lotufo and M. V. Ramos, Toxicol. In. Vitro., 2007, 21, 1563–1573 CrossRef PubMed.
- R. Mathur, S. K. Gupta, S. R. Mathur and T. Velpandian, Indian J. Exp. Biol., 2009, 47(5), 343–348 CAS.
- A. L. Joshi, P. H. Roham, R. Mhaske, M. Jadhava, K. Krishnadasa, A. Kharatb, B. Hardikarc and R. K. Kiran, Nat. Prod. Res., 2015, 29, 2261–2264 CrossRef CAS PubMed.
- K. H. Shaker, N. Morsy, H. Zinecker, J. F. Imhoff and B. Schneider, Phytochem. Lett., 2010, 3, 212–216 CrossRef CAS.
- S. R. M. Ibrahim, G. A. Mohamed, L. A. Shaala, L. M. Y. Banuls, G. V. Goietsenoven, R. Kiss and D. T. A. Youssef, Phytochem. Lett., 2012, 5(3), 490–495 CrossRef CAS.
- M. Bhagat, J. S. Arora and A. K. Saxena, Int. J. Green. Pharm., 2010, 4, 286–288 Search PubMed.
- V. H. Bhaskar and S. A. Sumant, Global J. Pharmacol., 2009, 3, 95–98 Search PubMed.
- V. L. Kumar and S. Roy, Phytother. Res., 2009, 23, 1–5 CrossRef CAS PubMed.
- P. Chaudhary, M. V. Ramos, Md S. Vasconcelos and V. L. Kumar, Pharmacogn. Mag., 2016, 12, 147–151 CrossRef CAS PubMed.
- H. T. Hussein, A. Kamel, M. Abou-Zeid, A. K. H. El-Sebae and M. A. Saleh, Uscharin, J. Chem. Ecol., 1994, 20(1), 135–140 CrossRef CAS PubMed.
- G. Giridhar, S. Santosh and P. Vesudevan, Pesticides, 1988, 22, 31–33 CrossRef.
- G. Prasad, J. Nat. Med. Assoc., 1985, 27, 7–10 Search PubMed.
- A. Basu, T. Sen, S. Pal, F. Capasso and A. Nagchaudhri, Phytother. Res., 1997, 11, 163–165 CrossRef.
- S. K. Bhatnagar and S. K. Verma, J. Econ. Taxon. Bot., 1986, 8, 489–490 Search PubMed.
- A. M. Al-Taweel, S. Perveen, G. A. Fawzy, A. U. Rehman, A. Khan, R. Mehmood and L. M. Fadda, Evid. Based Complement. Alternat. Med., 2017, 2017, 1–10 CrossRef PubMed.
- E. O. Iwalewa, A. O. Elujoba and A. Olanrewaju, Fitoterapia, 2005, 76(2), 250–253 CrossRef PubMed.
- S. B. S. Aliyu-Umar and Y. Mustapha, Unique. Res. J. Agric. Sci., 2014, 2(4), 37–41 Search PubMed.
- A. D. Sayed, N. H. Mohammed, M. A. Ismail, W. M. Abdel-Mageedand and A. A. Shoreit, Ecotoxicol. Environ. Saf., 2016, 128, 189–194 CrossRef CAS PubMed.
- S. R. M. Ibrahim, G. A. Mohamed, L. A. Shaala, L. M. Y. Banuls, R. Kiss and D. T. A. Youssef, Steroids, 2015, 96, 63–72 CrossRef CAS PubMed.
- S. R. M. Ibrahim, G. A. Mohamed, L. A. Shaala and D. T. A. Youssef, Rec. Nat. Prod., 2016, 10, 761–765 CAS.
- T. Mijatovic, F. Lefranc, V. E. Quaquebeke, F. V. Vynckt, F. Darro and R. Kiss, Drug Dev. Res., 2007, 68, 164–173 CrossRef CAS.
- T. Mijatovic, D. V. Neve, P. Gailly, V. Mathieu, B. Haibe-Kains, G. Bontempi, J. Lapeira, C. Decaestecker, V. Facchini and R. Kiss, Mol. Cancer Ther., 2008, 7, 1285–1296 CrossRef CAS PubMed.
- T. Juncker, M. Schumacher, M. Dicato and M. Diederich, Biochem. Pharmacol., 2009, 78, 1–10 CrossRef CAS PubMed.
- T. Juncker, C. Cerella, M. H. Teiten, F. Morceau, M. Schumacher, J. Ghelfi, F. O. Gaascht, M. Schnekenburger, E. Henry, M. Dicato and M. Diederich, Biochem. Pharmacol., 2011, 81, 13–23 CrossRef CAS PubMed.
- S. Wen, Y. Chen, Y. Lu, Y. Wang, L. Ding and M. Jiang, Fitoterapia, 2016, 112, 74–84 CrossRef CAS PubMed.
- I. Prassas and E. P. Diamandis, Nat. Rev. Drug. Discov., 2008, 7, 926–935 CrossRef CAS PubMed.
- H. V. Doshi, F. M. Parabia, F. K. Sheth, I. L. Kothari, M. H. Parabia and A. Ray, Int. J. Plant. Res., 2012, 2(2), 28–30 CrossRef.
- S. K. Khanzada, W. Shaikh, T. G. Kazi, S. Sofia, A. Kabir, K. Usmanghani and A. A. Kandhro, Pak. J. Bot., 2008, 40(5), 1913–1921 CAS.
- A. A. Ibrahim and E. H. Tuhami, Sci. J. Anal. Chem., 2019, 4(2), 20–24 Search PubMed.
- R. S. Gallegos-Olea, M. O. R. Borges, A. C. R. Borges, S. M. F. Freire, L. M. S. Silveira, W. Vilegas, C. M. Rodrigues, A. V. Oliveira and J. L. Costa, Rev. Bras. Pl. Med., Botucatu., 2008, 10(1), 29–33 CAS.
- N. S. Tour and G. S. Talele, Chem. Nat. Compd., 2012, 48(4), 708–709 CrossRef CAS.
- A. Q. Khan and A. Malik, Fitoterapia, 1990, 61(1), 89 CAS.
- S. J. Chundattu, V. K. Agrawal and N. Ganesh, Arab. J. Chem., 2016, 9, S230–S234 CrossRef CAS.
- N. I. Sweidan and M. H. Abu Zarga, J. Asian Nat. Prod. Res., 2015, 17, 900–907 CrossRef CAS.
- A. Mittal and M. Ali, Int. J. Pharmtech. Res., 2012, 4(1), 213–217 CAS.
- R. F. Chandler, R. G. Coombe and T. R. Watson, Aust. J. Chem., 1968, 21(6), 1625–1631 CrossRef CAS.
- M. H. A. Elgamal, A. G. Hanna, N. A. M. Morsy, H. Duddeck, A. Simon, T. Gati and G. Toth, J. Mol. Struct., 1999, 477, 201–208 CrossRef CAS.
- S. R. M. Ibrahim, G. A. Mohamed, L. A. Shaala, L. Moreno, Y. Banuls, R. Kiss and D. T. A. Youssef, Nat. Prod. Res., 2014, 28, 1322–1327 CrossRef CAS PubMed.
- A. G. Hanna, M. H. A. Elgamal, N. A. M. Morsy, H. Duddeck, J. Kovacs and G. Toth, Magn. Reson. Chem., 1999, 37, 754–757 CrossRef CAS.
- B. Singh and R. P. Rastogi, Phytochemistry, 1972, 11(2), 757–762 CrossRef CAS.
- A. Q. Khan, Z. Ahmed, S. N. Kazmi and A. Malik, J. Nat. Prod., 1988, 51, 925–928 CrossRef CAS.
- A. Q. Khan and A. Malik, Phytochemistry, 1989, 28, 2859–2861 CrossRef CAS.
- P. Alam and M. Ali, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2009, 48, 443–446 Search PubMed.
- S. H. Ansari and M. Ali, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2000, 39, 287–290 Search PubMed.
- R. Pant and K. Chaturvedi, Curr. Sci., 1989, 58, 740–724 CAS.
- S. H. Ansari and M. Ali, Pharmazie, 2001, 56(2), 175–177 CAS.
- A. Mittal and M. Ali, J. Saudi. Chem. Soc., 2015, 19, 59–63 CrossRef.
- A. Mittal and M. Ali, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2013, 52, 641–645 Search PubMed.
- A. Mittal and M. Ali, Int. Res. J. Pharm., 2011, 2(9), 52–54 CAS.
- M. A. Khasawneh, H. M. Elwy, N. M. Fawzi, A. A. Hamza, A. R. Chevidenkandy and A. H. Hassan, Res. J. Phytochem., 2011, 5(2), 80–88 CrossRef CAS.
- B. Dwivedi, A. Singh, S. Mishra, R. Singh, P. Pant, L. K. Thakur and M. M. Padhi, World J. Pharm. Res., 2014, 3, 708–715 CAS.
- R. S. Gallegos Olea, A. V. Oliveira, L. M. Silveira and E. R. Silveira, Fitoterapia, 2002, 73, 263–265 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |