 Open Access Article
Open Access ArticleThe intrinsic piezoelectric properties of materials – a review with a focus on biological materials
Ratanak Lay
ab,
Gerrit Sjoerd Deijs
abc and
Jenny Malmström
 *ab
*ab
aDepartment of Chemical & Materials Engineering, Faculty of Engineering, The University of Auckland, Auckland, New Zealand. E-mail: j.malmstrom@auckland.ac.nz
bMacDiamid Institute for Advanced Materials and Nanotechnology, Wellington, New Zealand
cDepartment of Chemistry, Faculty of Science, The University of Auckland, Auckland, New Zealand
First published on 15th September 2021
Abstract
Piezoelectricity, a linear electromechanical coupling, is of great interest due to its extensive applications including energy harvesters, biomedical, sensors, and automobiles. A growing amount of research has been done to investigate the energy harvesting potential of this phenomenon. Traditional piezoelectric inorganics show high piezoelectric outputs but are often brittle, inflexible and may contain toxic compounds such as lead. On the other hand, biological piezoelectric materials are biodegradable, biocompatible, abundant, low in toxicity and are easy to fabricate. Thus, they are useful for many applications such as tissue engineering, biomedical and energy harvesting. This paper attempts to explain the basis of piezoelectricity in biological and non-biological materials and research involved in those materials as well as applications and limitations of each type of piezoelectric material.
Introduction
In addition to the rapid technological and social development of our societies, we intensively strive for a healthier life. Both areas are inherently linked; clean and renewable energy sources are needed to keep up with our worldwide demand sustainably and, at the same time, reduce the negative impacts of global warming and environmental pollution. Alternative renewable energy sources such as biomass, solar, wind and tidal energy are helping in the de-carbonization of the power sector.1–4 Mechanical energy harvesting is one of the front-runners to sustainably power micro-devices. These devices play a key role in our daily lives, not only for leisure but also to an increasing degree for monitoring/adjusting biological systems, including in vivo processes.5–7 This monitoring will be beneficial for diagnostics, for example, real time heart-beat observation,8 on time medical interventions9 and ultimately leading to longer and healthier lives.Mechanical energy harvesting research is gaining momentum, even at the nanoscale range.10–14 There is plenty of ‘wasted’ mechanical energy available in the environment waiting to be converted into useful electrical outputs. Daily human activities show possible energy harvesting examples such as talking, walking, running, heart pumping, knee bending, driving vehicles, etc.14–17 A promising way to harvest this wasted ambient mechanical energy is by using piezoelectricity. Piezoelectricity can provide a possible sustainable contribution, as part of a bigger solution scheme to the sustainability issues. It can help to minimise the reliance on non-renewable energy sources and ensure a more efficient use of natural resources.
Piezoelectric research started off by focusing on inorganic materials such as zinc oxide (ZnO),10 barium titanate (BaTiO3)18 and lead zirconate titanate (PbxZr1−xTiO3, known as PZT),19 but it later branched out to organic polymer materials such as polyvinylidene fluoride (PVDF) and polymer composites.20 In 2006, Wang et al. reduced the size of a ZnO piezoelectric device to create a nanogenerator. In this work, nanowires were deformed by small forces, which induced electrical charge generation at the lower and upper parts of the nanowires, confirming the possibility of using piezoelectricity for energy harvesting.10
Piezoelectricity is not only present in inorganic compounds, but it also plays a crucial role in biological organisms.21 It has been reported that piezoelectricity exists in various biological structures ranging from amino acids to tissues, and some of them have comparable piezoelectric strength to that of conventional piezoelectric materials.22 Furthermore, organic piezoelectric materials such as collagen hold several advantages over conventional piezoelectric ceramics including biocompatibility, biodegradability, high flexibility, low toxicity, and ease of fabrication.23
Piezoelectricity in biological structures has already been explored to fabricate energy harvesting nano-generators.24 However, its biggest potential lies within biomedical applications, due to relatively low energy demands and a need for biocompatible materials. Currently, batteries power various implantable devices that need to be replaced at the end of their lifespan. This requires surgery which can impose risk or complication to the patient.22 To overcome these limitations, it would be ideal to take advantage of energy that is wasted during the natural processes of the body. The origin of bio-piezoelectricity and its role in the biological structure are currently not fully understood, this impedes the development of bio-piezoelectric applications. Large heterogeneity within biological structures also limits precise control of the polarization's strength and direction.25 Therefore, understanding the mechanisms behind piezoelectricity in biological structures is key to developing and optimizing their applications further.
This review complements other published reviews on the topic21,26–29 by providing a careful examination of literature to gain a better grasp of the underlying mechanism of piezoelectricity with a particular focus on biological materials. It also attempts to provide a summary of key piezoelectric biological and traditional materials, their differences, applications and limitations, as well as a brief overview of the current state of piezoelectric development. This review provides a resource for the basic understanding of piezoelectricity to aid further development of piezoelectric applications in the future.
Fundamentals of piezoelectricity
Piezoelectricity is a linear electromechanical coupling phenomenon.30 This means that when mechanical stress is applied to a piezoelectric material, it deforms and generates electrical charges. The process of converting mechanical stress to electrical charge is known as the direct effect. In contrast, the converse effect refers to when an external electrical field is applied across a piezoelectric material causing the material to mechanically deform.30 Piezoelectricity exists in materials with non-centrosymmetric crystals. Out of the 32 crystal classes, 21 of them of lack a centre of symmetry and are said to be non-centrosymmetric and thus are piezoelectric.31Piezoelectricity is quantified by the piezoelectric coefficient d, which is the ratio between applied stress and charge (eqn (1)), or between strain and applied electric field (eqn (2)).
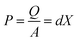 | (1) |
| x = dE | (2) |
The piezoelectricity of a material depends on both the orientation and symmetry of the material's crystal, and it can be described by third-rank tensors.32 The piezoelectric tensor can be expressed as a 3 × 6 matrix of component dij, where i represents the direction at which electrical field is applied or produced, and j represents the direction of applied stress or resulting strain.31 The subscripts 1–3 are used to represent X, Y and Z directional axes, and subscripts 4–6 are used to describe the shear planes perpendicular to each of those axes respectively. Since piezoelectricity is a direction-dependent property, the piezoelectric coefficient d has both magnitude and direction. The positive/negative sign indicates the direction of the resulting strain or induced polarization in respect to their reference axis.
For inorganic materials, the piezoelectricity is a result of asymmetrical charge distributions in the crystal under applied mechanical stress.30 The piezoelectricity of inorganic materials shows temperature dependent behaviours. Above a critical temperature known as the Curie temperature, a material dissipates its piezoelectric and ferroelectric properties.34 In other words, thermal motions can induce changes in the polarization of the material. On the other hand, piezoelectricity in organic materials arise mainly from reorientation of permanent molecular dipoles under an applied stress that results in alignment of the dipoles in a particular direction that yields a net polarization.23
Inorganic piezoelectricity
Well known piezoelectric ceramics include lead zirconate titanate (PZT),50–52 barium titanate (BaTiO3)18,47,48,53 and potassium sodium niobate (K0.5Na0.5NbO3, abbreviated as KNN).54–56 The high piezoelectric potential of PZT is what makes it a particularly attractive energy harvester (d33 = 500–600 pC N−1).57,58 It also exhibits ferroelectricity as observed by characteristic hysteresis loops. However, the brittle nature of PZT limits its applications. It is prone to fractures due to a high Young's modulus of 50 GPa and a tensile strain maximum of 0.2%.59 Practical testing under applied electric fields have indeed shown a tendency of PZT fatigue cracking in a brittle process with little or no plastic deformation.60 Owning to the high strain demand for converting ambient energy, energy harvesting devices must be both flexible and stretchable. Therefore, piezoceramics, such as PZT, are combined (often in a film configuration) with plastic or elastomeric substrates.50,52,61 One method utilized printing of PZT onto a pre-strained PDMS and created buckled PZT nano-thick ribbons upon release of the strain.61 This buckled PZT showed an order of magnitude larger strain endurance when compared to the flat non-treated PZT.61 Even though these composite devices are more durable, other mechanical properties should also be examined as it is still possible that slipping, cracks and delamination occurs.62 In addition, one major downfall of PZT is that it contains lead, which is not only toxic, but also environmentally unfavourable.
An alternative lead free piezoceramic is BaTiO3, another ferroelectric material. The piezoelectric capabilities of BaTiO3 were discovered in the early 1940s and the material was soon considered as a potential piezoelectric transducer.63 However, it was initially over-shadowed by PZT which showed better piezoelectric properties and a higher Tc.64 BaTiO3 came back into the picture when modifications of BaTiO3 with Ca2+ and Zr4+ enabled an increase of the piezoelectric coefficient to d33 > 500 pC N−1 with a maximum operational temperature of 90 °C.65
Several characteristics, for instance; crystallization,66 calcification,67 sintering,67 changing grain size,68 poling48 and doping69 can be used to enhance piezoelectric properties. Recently, one of the key piezo-enhancement factors have been identified to be morphotropic phase boundaries which are the transitions in the composition phase diagram, where the crystal structure changes rapidly and where the electromechanical properties are maximised.39,70 By carefully exploring such phase boundaries and optimising materials accordingly by varying chemical composition or mechanical pressure, it is possible to enhance the electromechanical coupling further.19,38,54 In one study of the morphotropic phase boundaries in BaTiO3-based ceramics, it was found that a high d33 (700 ± 30 pC N−1) could be induced in a specific region while in a broad range it showed >600 pC N−1.53 Even at the temperature between 10–40 °C, the optimised BaTiO3 surpassed the performance of popular lead-based systems.59 These steps make the employment of BaTiO3 for electronics more feasible. One crucial remaining factor that requires improvement for BaTiO3 is to increase the Curie temperature (about 130 °C for pure BaTiO3). To increase the Curie temperature, one recent study combined a ceramic (BaTiO3 + bismuth ferrite BiFeO3) with bismuth aluminate BiAlO3 (BA) with a high Tc of 527 °C.70 This study achieved an increase of the piezoelectric constant from d33 = 97 pC N−1 to 210 pC N−1 and a Tc of 400 °C.
As a result of a high Curie temperature and an impressive piezoelectric coefficient, KNN is another candidate to replacing PZT.55 Saito et al. demonstrated a d33 of 416 pC N−1 for KNN in 2004 by discovering a morphotropic phase boundary of the material and by processing into highly textured polycrystals.54 Optimisations, such as tailoring the phase fraction and chemical modifications71–73 have since produced KNN-based materials with even higher piezoelectric constants. However, KNN-based materials are also hampered by fatigue deterioration, an area where there is still a lot to be discovered and optimised for this material (reviewed by Genenko et al. 2015).74
Organic–inorganic metal halide perovskites (OMHPS)
Perovskites are materials with the crystal structure of calcium titanate which is known as the perovskite crystal structure.75 They consist of two cation types bonded together by an anion. Organic–inorganic metal halide perovskites (OMHPs) consist of; an organic cation (A), a metal cation (B) and a halide anion (X) with the general formula of ABX3.76Instead of the high fabrication temperature of ceramics, a low temperature OMHP fabrication was achieved with TMCM–MnCl3 (TMCM = trimethylchloromethyl ammonium). With ferroelectric properties, piezoelectric output of d33 = 185 pC N−1 and a Tc of 132 °C it is attractive for several applications.77 Further morphotropic phase boundary research resulted in a superlative OMHP with a d33 of ∼1540 pC N−1 consisting of trimethylfluoromethyl ammonium (TMFM), TMCM and xCdCl3 (x = different composites).78
Another interesting OMHP capability, namely illumination dependent piezoelectric response, was found in a methylammonium lead halide (MAPbI3) in which the piezoelectric output varied with different illumination.79 It was suggested that the illumination enhances the MAPbI3 dielectric constant and forms a considerable photo-induced piezoelectric dipole,79,80 which could play a role in energy harvesting by perovskite solar cells.76 Despite of the high piezoelectric output, the same ceramic disadvantages apply for OMHP; they show brittleness, inflexible properties when in bulk form. To overcome these limitations future studies could use thin film configurations on flexible substrates.
Organic piezoelectricity
Organic molecules such as polymers can exhibit complex dipole moments giving rise to polymer-based piezoelectricity.81,82 Polymers are excellent materials for piezoelectric devices due to; low-cost solution processing, low temperature processing, low toxicity and high chemical stability.83 This review divides piezoelectric polymers in two classes, namely bulk polymers (which normally require poling) and polymer composites.Piezoelectricity of PVDF was first reported in 1948, followed shortly after by reports of piezoelectric co-polymers of PVDF.84 PVDF is a polycrystalline (35–70% crystallinity) polymer with mainly ‘‘head-to-tail’’ arrangement of polymer chains in a zigzag form in the crystal grains. Consequently, dipoles are parallel to one another within adjacent chains.85 This dipole moment is what leads to the piezoelectricity in PVDF. There are four crystal forms of PVDF: α, β, γ and δ, with α being the simplest form to obtain. Unfortunately, only the β form has a piezoelectricity of ∼30–40 pC N−1.82 Despite this relatively low output, the intrinsic properties of PVDF creates an opportunity to overcome the disadvantages of bulk inorganic piezoelectric devices. These include: simple synthesis,86 high flexibility,87 and adaptable design.88 Fig. 1A shows the output voltage and the flexibility of a PVDF based thin film on a cellulose paper substrate.87 The device exhibited a maximum open circuit voltage of 1.5 V under periodic bending and releasing of force at ∼1 Hz.87
 | ||
| Fig. 1 PVDF based piezoelectric devices (A) open-circuit voltage of a flexible PVDF-TrFe thin film energy harvester. Reproduced from ref. 87 with the permission of AIP Publishing. (B) PVDF-TrFE nano-array formation. Reproduced from ref. 97, with the permission of Springer Nature. (C) Open-circuit voltage of PVDF/SM-KNN energy harvester. (D) TEM image of the PVDF/SM-KNN composite. Reproduced from ref. 108, with permission from Elsevier. | ||
As the β form is the crystal with the most prominent piezoelectricity, studies are focussing on increasing the amount of this form. Frequently used improvement methods are: stretching,89 polar additives87 and electro-spinning.20 One of the most promising PVDF copolymers explored for piezoelectric properties is PVDF-co-TrFE (trifluoroethylene). This copolymer is interesting because it demonstrates a higher piezoelectric power density (312.85 μW cm−3)90 when compared to PVDF (81.3 μW cm−3).91 The TrFE monomer adds an extra fluorine to the polymer, which induces a higher tendency for β form formation.92 PVDF-TrFE has been used in energy harvesters by spin coating,90 electro-spinning93–95 or bar coating91 of the polymer. The processing and deposition parameters of the polymer are varied to optimise the β crystal phase formation and thus increase the piezoelectric output.90 Electrospinning into nanofibers has been found to further increase the piezoelectric power density output of the polymer.96 During electrospinning, a high bias electric field is applied which leads to dipole alignment along the major axis direction of the nanofibers.96,97 Aligning PVDF-TrFE into a nanotube array (Fig. 1B) has also been shown to induce higher piezoelectric output (d33 = −35 pm V−1) when compared to conventional spin coated films (d33 = −17.8 pm V−1).97 All in all, PVDF-TrFE is a promising flexible polymeric material, not only for piezoelectric energy harvesting but also as pressure sensors98 and actuators.99
Amorphous polymers with high glass transition temperatures (Tg > 80 °C) are being studied for their piezoelectric responses as an alternative for high temperature processes. At the glass transition temperature, the amorphous polymer shifts from a glassy brittle state (limited molecular motion) to a flexible rubbery state (large scale molecular motion). Amorphous polymers generally rely on oriented dipole moments for their piezoelectricity.100 Such orientation is achieved by poling near the Tg. Examples of amorphous polymers explored for their piezoelectric properties are co-polymerized pyromellitic dianhydride with p-phenylenediamine81 and co-polymerized vinylidene cyanide and vinyl acetate.101
As an example of a successful composite, Mota et al. used rotating-disk electrospinning technique to create composite PVDF/BaTiO3 thin fibre meshes. The fibre alignment could be altered by changing the rotation speed, which was also found to change the amount of the piezoelectric β phase of the PVDF.106 The composite was also optimised in terms of composition and the authors achieved a 130 pC N−1 piezoelectric output at a 20/80 BaTiO3/PVDF composition.106 The authors additionally demonstrated preliminary evidence of biocompatibility in in vitro experiments, making this material interesting for use as implantable energy harvester.
PVDF/potassium sodium niobate (KNN) has also been successfully used as a piezoelectric composite. In one example, KNN nanorods were surface functionalised to form a composite with PVDF. The incorporation of rod-shaped KNN nanomaterial (Fig. 1D) was found to positively influence the number of dipoles aligned during electro-spinning.108 The optimised material contained 3% KNN and a fabricated nanogenerator of this was found to generate an output of ∼21 V (Fig. 1C) and ∼22 μA (compared to control PVDF: 0.5 V and ∼1 μA).108
Piezoelectricity in biological materials
It is thought that piezoelectricity of biological materials has physiological importance. For example, it has been shown that the piezoelectricity of bone influences its remodelling and growth according to Wolff's law, which describes the direct correlation between bone's structure and the stress that it is subjected to.109,110 In fact, it has been confirmed experimentally that under mechanical deformation, bone produces hydroxyapatite mineral, confirming that piezoelectricity in bone is linked to bone growth and remodelling.111 Piezoelectricity in the lung's elastin has been proposed to have a role in binding oxygen to haemoglobin during respiration.112 It is also believed that piezoelectricity plays a role in the nervous system for sensing external stimulation.113 Proteins such as collagen, elastin, actin and myosin have been shown to be piezoelectric.112,114–120 Their piezoelectric properties have been attributed to their constituent amino acids, electrical dipoles and the packing of the peptide chains.21,109,121 A summary of the reported piezoelectric coefficients for some protein/amino acid based biological materials is provided in Table 1.| Direction | Source | Piezoelectric coefficient | Measurement techniques |
|---|---|---|---|
| Longitudinal d11 | γ-Glycine single crystal121 | 1.7 pm V−1 | Piezometer |
| Longitudinal d22 | −1.1 pm V−1 | ||
| Longitudinal d33 | 9.93 pm V−1 | ||
| Shear d16 | β-Glycine microcrystals121 | 178 ± 11 pm V−1 | Resonance methods |
| Longitudinal | Tetragonal lysozyme aggregated film109 | 19.3 pm V−1 | PFM |
| Longitudinal | Tetragonal lysozyme aggregated film115 | 3.16 pC N−1 | Piezometer |
| Longitudinal | Monoclinic lysozyme aggregated film115 | 0.94 pC N−1 | |
| Longitudinal d31 | Rat tail collagen140 | −4.84 ± 2.96 pm V−1 | PFM |
| Longitudinal d33 | 0.89 ± 0.08 pm V−1 | ||
| Shear d14 | −12.00 ± 2.60 pm V−1 | ||
| Shear d15 | 6.21 ± 2.93 pm V−1 | ||
| Shear | Bone collagen141 | 0.1–0.3 pm V−1 | PFM |
| Longitudinal and shear | Murine lung elastin112 | 0.1 pm V−1 | PFM |
| Longitudinal | Human teeth enamel142 | 0.30 ± 0.04 pC N−1 | PFM |
| Longitudinal | Human teeth dentine142 | 0.51 ± 0.05 pC N−1 | PFM |
| Unspecified | Human eyes143 | 23 pC N−1 | Rheolograph solid |
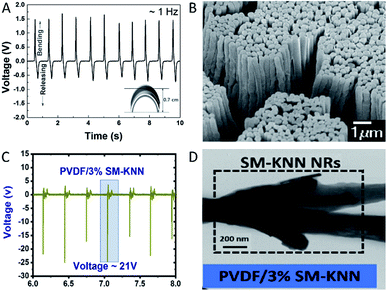
| Longitudinal d33 | Wild type M13 bacteriophage monolayer144 | 0.30 ± 0.03 pm V−1 | PFM |
| Longitudinal d33 | 4E engineered M13 phage monolayer144 | 0.70 ± 0.05 pm V−1 | PFM |
| Longitudinal d33 | Lateral aligned M13 phage film144 | 7.8 pm V−1 | PFM |
| Longitudinal d33 | Vertically aligned M13 phage film145 | 10.4 ± 0.5 pm V−1 | Quasi-static |
| Longitudinal d33 | Vertically aligned M13 phage film146 | 13.2 pm V−1 | PFM |
A common trend in biological materials is that the shear piezoelectric coefficient is typically higher than the longitudinal piezoelectric coefficient.109 This is also true for the amino acid crystals. The high shear piezoelectricity in amino acid crystals has been attributed to their relatively low elastic modulus as they tend to be softer when compared to inorganic crystals.109 This is because the piezoelectric strain coefficient is represented as the ratio between the piezoelectric charge coefficient and the elastic stiffness coefficient.109 Glycine and hydroxyproline crystals have been found to have the highest piezoelectric coefficients amongst the natural occurring amino acids.28 The shear piezoelectric coefficient of β-glycine crystals d16 has been measured to be as high as 178 pm V−1 when measured using piezometer.121 Such a high shear piezoelectricity was shown to be a result of efficient packing of glycine molecules within the crystal, enhancing the strength of electrical dipoles acting along a certain crystallographic direction.109 On the other hand, the longitudinal piezoelectric coefficient d22 of β-glycine was reported to be only around −5.7 pm V−1.121 The longitudinal piezoelectric coefficient d33 of γ-glycine is around 10 pm V−1, as measured by piezometer.121,147,148 In addition, ferroelectricity has also been demonstrated experimentally for both of the β- and γ-forms at the nanoscale using density functional theory (DFT), molecular dynamics and PFM.147,148 The ferroelectricity is higher in the γ-form due to more efficient arrangement of the amide group, which results in accumulation of dipole moment along the polarisation direction.147 However, the ferroelectricity starts to decrease at 630 K and disappear completely at 640 K as the glycine molecule transitions from being ferroelectric to paraelectric.147
Hydroxy-L-proline has the second highest shear piezoelectric coefficient d25 among the amino acids of around −28 pC N−1. And similar to glycine, hydroxy-L-proline is also ferroelectric and so its piezoelectric coefficient can be strengthened by applying an external field to align the polarization domains.109 Amino acids exhibit structural dependent piezoelectric properties.21,109,149 By adding –OH groups to proline to generate hydroxy-L-proline, the piezoelectric coefficient of it increases by two orders of magnitude.109 Hydroxy-L-proline and threonine were found to have no piezoelectricity in the longitudinal direction as single crystals, but when prepared as polycrystalline films, they were demonstrated to have a piezoelectric coefficient d33 of 1 pC N−1 and 0.1 pC N−1 respectively. It was proposed that the resultant longitudinal piezoelectricity in polycrystalline films is a result of inter-crystalline strain within the film which lowers the crystal symmetry of constituting single crystals making the polycrystals overall piezoelectric. In this case, the magnitude of the piezoelectricity is the vector summation of the piezoelectricity of strained randomly oriented single crystals that make up the films.149
 | ||
| Fig. 2 (A) Structure of M13 bacteriophage. (B) Piezoelectricity in the vertically aligned M13 phage structure. Reproduced from ref. 145, with the permission of Royal Society of Chemistry. (C) AFM topography image (top) and PFM amplitude image (bottom) of single collagen fibril. (D) AFM topography image (left) and PFM amplitude image (right) of bone. Reproduced from ref. 153, with the permission of American Chemistry Society. | ||
Another widely studied fibrous protein is elastin. It can be found in organs such as lung, skin and blood vessel walls.112,117,118,150 Both piezoelectricity and ferroelectricity has been confirmed in elastin.112,117,118 The piezoelectric coefficient of elastin has been found to be 0.1 pm V−1.112,117 While this is lower than that of collagen, elastin is ferroelectric while as collagen is not, and thus electrical poling can be used to enhance elastin piezoelectric strength. Similar to collagen, the piezoelectricity of elastin originates from its monomers, dipole moments and supramolecular packing.112
Lysozyme, a major globular protein in egg white and mammalian secretions has also been found to exhibit piezoelectricity. The longitudinal piezoelectric coefficient of tetragonal lysozyme monoclinic crystal measured using PFM was found to be 19.3 pm V−1, a relatively high value for biological material.116 However, since the lysozyme crystal belongs to the (422) crystal group, only shear piezoelectricity is permitted according to the classical theory of piezoelectricity.115 The origin of the measured longitudinal piezoelectricity was proposed to be a result of a structural defect that lowers the crystal symmetry of the crystal resulted from the crystal preparation.116 In addition, the longitudinal piezoelectric coefficient of a monoclinic lysozyme crystal film was found to be only around 0.94 pC/N, which is much lower than that of a tetragonal crystal.115 And because only limited number of studies have been performed on lysozyme, it is difficult to verify the accuracy of the measured values and whether the measured value was indeed deducted solely from piezoelectricity.
Similar to bone, teeth are also a bio-composite mainly comprised of hydroxyapatite nanocrystals and collagen with similar ratio to that of bone, though the piezoelectricity of teeth has not been studied as extensively as in bone. Out of the few studies done on teeth, most assumed that the hydroxyapatite is not piezoelectric as it belongs to the centrosymmetric spatial group P63/m.93,152 However, by studying human teeth enamel and dentine after removing collagen, Reyes-Gasga and others have shown that the hydroxyapatite is piezoelectric at both macroscale and nanoscale, but to a much lesser extent compared to collagen.142 It is possible that the hydroxyapatite present in teeth exists in more than one spatial group. It has been known that hydroxyapatite can also exists in the non-centrosymmetric P63 and P21 spatial groups and thus are piezoelectric.142 The other two teeth tissues, cementum and dentine have been shown to exhibit piezoelectricity across all the studies done on them but the piezoelectric coefficients vary quite significantly between studies.93,142,152 The nanoscale piezoelectric coefficient for teeth enamel and dentine have been measured by PFM to be 0.3 pC N−1 and 0.5 pC N−1 respectively.142
Tendon is comprised of highly ordered crystalline collagen. And just like any collagen-based material, the shear piezoelectric response is higher than that in the longitudinal direction for both macroscale and nanoscale measurements. In addition, the piezoelectric coefficients measured at the nanoscale using PFM have been found to be an order of magnitude higher than those measured at the macroscale.119,120,140,156 The shear piezoelectric coefficient d14 of rat tail tendon measured at the nanoscale was found to be −12 pm V−1, whilst the longitudinal piezoelectric coefficient d33 was measured to be 0.89 pm V−1.140 The shear piezoelectric coefficient d15 in bovine Achilles tendon has been found to be 1 pm V−1, significantly lower than that in rat tail tendon, likely due to the structural arrangement of the collagen fibres not being as efficiently packed as in rats.140,158
Sclera and cornea are two other collagen-based tissues in eyes. Just like other collagen-based tissues, their piezoelectricity is strongly dependent on the orientation at which pressure is applied.143,159,160 The piezoelectric coefficient for a middle circumferentially-cut sclera tissue has been found to be as high 23 pC N−1.143 But when the sclera tissue is cut in the anterior-posterior position, the piezoelectric coefficient was only around 7 pC N−1.143 It has also been found that regions with relatively higher elastic modulus corresponds to lower piezoelectricity as it is more difficult to deform mechanically stiff regions.160 In addition, it has been proposed that the water molecules in collagen help stabilise the structure. Thus, as the tissue dehydrates, the collagen fibres become disoriented which results in a reduction of piezoelectricity.143,159,160
Various parts of fish such as swim bladders and scales have been experimentally demonstrated to be piezoelectric.12,113,161 The fish swim bladder's source of piezoelectricity was attributed to be the self-aligned and ordered collagen nanofibrils.12,161 The same was said for the Catla catla fish scale.12,161 But the piezoelectricity in green carp scale was said to be from the hydroxyapatite crystals present in the fish scale. Interestingly, both of the Catla catla fish swim bladder and scale were found to be ferroelectric as suggested by the characteristic shape of the hysteresis loop.161 However, ferroelectricity was not demonstrated in collagen in other experiments.150 This suggest that piezoelectricity in both tissues may potentially also arise from other proteins in addition to collagen. Furthermore, the piezoelectric coefficient for both swim bladder and scale from the Catla catla fish were found to be higher than that of isolated collagen nanofibril. The enhanced piezoelectric coefficient was attributed to be a result of cooperative electromechanical interaction between the highly ordered packed and oriented collagen nano-fibrils under applied mechanical stress in both tissues.12,161
Piezoelectricity is not unique to animal tissues; it also exists in plants. For example, the longitudinal piezoelectric coefficient of electrically poled aloe vera films has been found to be −6.3 pm V−1, higher than that in collagen.162 The proteins and polysaccharides in Aloe vera plants were said to be responsible for the plant's overall piezoelectricity.162 Orange peel, a bio-waste that contains cellulose, polysaccharides, proteins and flavonoids has also been found to be piezoelectric.163 However, piezoelectricity in plants has not been as extensively investigated as in animals. Thus, further research is required to investigate the mechanisms and behaviours of plant piezoelectricity.
Applications of piezoelectric materials
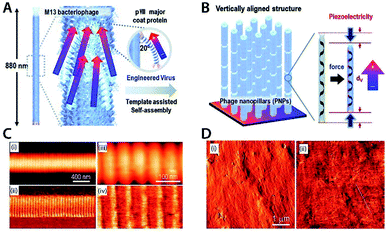
This section focusses on examples of different piezoelectric materials being used in applications. As the amount of papers on piezoelectric applications is immense,164 a handful of applications have been selected to represent the field. | ||
| Fig. 3 (A) Self-powered PVDF thin film based blood pressure sensor. (B) Circumferential and axial stress on aorta wall. Reproduced from ref. 166, with permission from Elsevier. (C) Schematic design of ZnO/PVDF based pressure and temperature sensor. (D) Top graph: mechanical impact of droplet onto sensor with unknown temperature. Bottom graph: corresponding resistance and recovery time upon impact of different temperature droplets. Reproduced from ref. 173, with permission from Springer Nature. | ||
Traffic-induced vibrations are another source of ‘wasted’ mechanical energy of interest for piezoelectric energy harvesting applications. Transportation infrastructures have been modified to hold piezoelectric devices and use the deformations and vibrations from moving vehicles to harvest energy.167,168 Examples of road infrastructures that have implemented such energy harvesting include railway,169 road pavement167 and walk pavement.170 Even though these projects are still in the experimental phase, piezoelectricity is becoming more commonly utilized in the transportation infrastructures. Interestingly, piezoelectric devices are also experimentally used as transducers to monitor damage in materials, for example, the steel rods in reinforced concrete.171 To achieve that, an ultrasonic transducer (emission) and a piezoelectric PZT transducer (receiver) were fixed to each end of the steel rod. The rod damage can then be assessed by analysing the change in ultrasonic amplitude, since the amplitude of the ultrasonic wave decreases gradually with the rod damage.172
Another example of interesting piezoelectric applications is temperature and pressure sensing. Lee et al. demonstrated a temperature/pressure sensor using a composite ZnO/PVDF film (Fig. 3C). To detect the pressure, the change in piezo-resistance of the material was measured. This piezo electrical resistance occurs upon application of mechanical stress (Fig. 3D). To detect the temperature, the recovery time of the piezo-resistance was measured and linked to temperature (Fig. 3D).173 The device was capable of detecting pressure differences of as little as 10 Pa within a measurement range of 10–140 Pa. Temperature could be measured in the range 20–120 °C.173
Finally, it is worthwhile to highlight sensory applications using piezoelectricity in robotics. The robotic field desires to mimic the flexibility and tactile capabilities of biological skin with electronic skin (e-skin). To even be considered for use as an e-skin, the material must be highly flexible and be superior in its ability to adapt to shape. An example of e-skin was made of electrospun PVDF, doped with graphene oxide and BaTiO3 nanoparticles.174 In this case the authors demonstrated the ability to accurately identify the shape of a hand touching the e-skin and the motion of human joints which might aid in the development of ‘smart’ prostheses.174
Due to their biocompatibility and biodegradability, bio-piezoelectric materials have particular potential as functional materials for biomedical and green energy niches.23 Various bio-based nano-generators have been fabricated to utilise to the direct piezoelectric effect to generate electricity.12,162,177,178 Those nano-generators (Fig. 4A–C) exploit the piezoelectricity in abundant biological materials or bio-waste, such as onion skin cell,14 eggshell membrane,178 orange peel,163 aloe vera gel,162 fish swim bladder,179 fish scale,12 bacteriophage and so on.144,146 Nanogenerators offer several benefits over conventional energy conversion techniques including ease of fabrication, high portability, high conversion efficiency and high sustainability.23 Nanogenerators can be used to harvest mechanical energy from various forms such as finger pressing, sound, walking and other simple motions that would be otherwise wasted as heat.
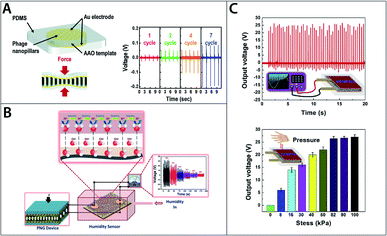 | ||
| Fig. 4 (A) Schematic of M13 bacteriophage based energy generator and generated voltage outputs. Reproduced from ref. 145, with permission from Royal Society of Chemistry. (B) Schematic of self-powered collagen-based humidity sensor. Reproduced from ref. 182, with permission from ACS publication. (C) Output voltage for eggshell membrane based energy generator. Reproduced from ref. 178, with permission from Elsevier. | ||
Fish scale is one example of a material with energy harvesting potential. Ghosh and Mandal demonstrated that up to 4 V of voltage could be produced under compressive stress of 0.17 MPa using a bio-piezoelectric nanogenerator fabricated from fish scale, which is rich in type-I collagen.12 Egg shell membrane, another collagen-rich biomaterial, has been used to fabricate a nanogenerator (Fig. 4C) which was able to produce up to 26.4 V per unit under 81.6 kPa of compressive stress. When connected in series and parallel, five units produced a total of 131 V, enough to power more than 90 green LEDs.178
In addition to being successfully used to fabricate nano-generators, collagen and collagen-based materials have been experimentally demonstrated that they can be used for various other biomedical and tissue engineering applications. Fish-skin collagen and gelatine were used to produce biosensors in the form of e-skin to monitor physiological signals that may provide information regarding medical conditions.180,181 Interestingly, those sensors could be powered by nano-generator produced from the same materials proving their ability to function as self-powered devices.180–182 Collagen also has the potential for tissue engineering applications as tissue scaffold and wound-healing dressing.183 It has been known that bio-piezoelectricity relates to growth and remodelling in tissues such as bone, hence it would be beneficial to exploit the piezoelectricity in materials such as collagen to develop tissue scaffolds that help promote a self-healing environment for wounds.180,181 Furthermore, collagen applications extend beyond biomedical applications and have been used to produce sensors measuring physical properties such as humidity and strain.182,184
As for the M13 bacteriophage, it has been used to fabricate nano-generator,144,146 sensor,185 and tissue engineering scaffolds.186,187 The phage is particularly versatile owing to its well-defined structure, narrow size distribution and the ability to modify the phage's properties with genetic and chemical modifications.144,145 Most importantly, the phage can be mass produced simply by infecting bacteria in culture where the phage co-opt the bacteria's metabolism releasing millions of its copies overnight.144 The ability to modify the phage's properties and ease of production might make the phage a more feasible option than the other materials mentioned.
One of the main challenges for bio-piezoelectric applications is that piezoelectric coefficients of biological materials are relatively low when compared to their inorganic counterparts.23 They can be modified or processed to enhance their piezoelectric strength for more energy intensive applications. For example, collagen has a relatively low longitudinal piezoelectric coefficient. However, it has been shown that cross-linking with EDC-NHS allows for the collagen fibres to self-assemble into a bundle more efficiently, with enhanced piezoelectricity in the longitudinal direction as a result.151 Electrospinning of chitin nano-fibre is another example of how processing can be used to improve material properties. In this case, the electrospinning was found to increase the crystallinity, and thereby the piezoelectric strength by 400%.188 Alternatively, bio-piezoelectric materials can also be combined with conventional piezoelectric materials such as PVDF to enhance their piezoelectricity.163 Gaur et al. combined the bio-waste orange peel with PVDF and produced a hybrid nanogenerator that was able to produce an output voltage of 90 V, three times higher than that of pure orange peel alone.163
Discussion/limitations
While piezoelectric materials are used in numerous applications already, there are still some challenges left to solve. The high piezoelectric output of lead-based piezoceramics (e.g. PZT) have made these materials a benchmark. However, it is important to note that even the best piezoelectric materials provide very low energy density compared to energy harvesting technology such as solar and wind. Therefore, the applications where piezoelectric energy harvesting is suitable are those where not much power is needed, and where other types are not practical. One such example is to power implanted medical devices with sensor capabilities. The most efficient, lead-based piezoceramics are also problematic environmentally, and have in fact been banned in many applications.189,190 Therefore, much of the research and optimisation of PZT based devices have been applied to non-lead ceramics. Consequently, several non-lead piezoceramics such as ZnO, BaTiO3 and KNN have gained momentum. However, as bulk materials they do not surpass PZT output. With complex modifications, non-lead ceramics can be competitive and occasionally even surpass lead-based ceramics.56,66–69For both lead and non-lead ceramics, the achieved output in volts is not the limiting factor; however, the current output is. Widespread application of piezoelectric energy harvesters is restricted due to this low current output. While volts transcends into the 100 V region, the reported current output is generally in the nano–micro amperages range.67,88,90,107 Consequently, enhancing this current output remains one of the toughest challenges. One possible solution to this problem is to manufacture piezoelectric devices in a multi-stacking design on a flexible substrate to use it as a bending energy harvester.191,192 In addition, time should be spent on creating a load and source impedance that are corresponding to each other. This impedance matching will aid in minimizing the losses of the energy harvesting.192,193
It could be argued that non-lead modified piezoceramics, in terms of piezoelectric output, are ready to be the benchmark in the electronics world. But while some of these materials have sufficient output, their inherent nature of being brittle and fragile still holds them back for certain applications. Especially biomedical applications need flexibility and stretchable energy harvesters due to energy conversion from soft tissues. The energy harvester should not only be completely adaptable to target organs or skin but also not restrict tissues in their normal function.166 In other words, no excessive strain should be created.
A possible solution for both flexible and stretchable energy harvesters may be found in biological piezoelectric materials. Piezoelectricity in biological materials have many potential applications in electromechanical, bio-medical and other areas. Even though these materials are flexible and stretchable, several factors still limit the development of biological piezoelectric applications. The main limiting factors are the lack of understanding of bio-piezoelectricity and a large discrepancy between experimental results from a pool of limited number of conducted experiments. The discrepancy between experimental could be a result from the lack of standardised testing method for bio-piezoelectricity which underpin development of bio-piezoelectricity.111 The understanding of the inherent properties and mechanism of piezoelectricity in each material is vital to reach an optimised device.
Various studies have quantified piezoelectric coefficients for biological materials at structural levels ranging from the amino acid level to tissue or organ level. Compared to traditional piezoelectric ceramics, piezoelectric coefficients for each biological material span over a much wider range, sometimes over several orders of magnitude.21,25,165 Since piezoelectricity is a material intrinsic property, the piezoelectric coefficient of a material measured at a condition should be consistent.25 A large discrepancy in the measured values from biological materials in particular raises a question of whether the reported piezoelectric coefficients originate solely from the piezoelectric properties of each material. Piezoelectricity is not the sole intrinsic electromechanical coupling process in a material. Electrostriction and flexoelectricity are the two other electromechanical coupling process that may interference with the measurements.25,150 Piezoelectricity couples mechanical and electrical domains of a material in a linear manner. Like piezoelectricity, electrostriction in a dielectric material is the deformation under an applied electric field. However, unlike piezoelectricity, electrostriction is a quadratic electromechanical coupling and it can occur without having the permanent dipoles aligning across all domains.25,150 Flexoelectricity is the polarisation induced in a dielectric material by a strain gradient. Unlike piezoelectricity, flexoelectricity is a size-dependent phenomenon and it is less prominent in a bulk material as it is relatively harder to induce a strain gradient in a large material.25,150 In addition, extrinsic factors such as ion migration and electrostatic interactions may also affect the measurements especially for hydrated samples.25,150 Since the direct and converse piezoelectric effects are thermodynamically equivalent, they can be measured to confirm if the measured value indeed truly corresponds to the piezoelectricity of the material.26 However, due to technical limits, especially in nano-materials, this may not always be possible. The converse effect can be, and has been, quantified at the nano-level using PFM, but it is challenging to quantify the direct effect at such scale as the detection sensitivity of currently existing instruments are not high enough to detect the low output signals from biological materials.25 In addition, noise signals, such as Johnson noise and contact electrification, can sometimes be as large or greater than the signals from the piezoelectricity themselves.25,165 This has been exemplified from measurements on hydroxyapatite crystals in bone. Although XRD and dynamic measurements both suggest that the crystals are piezoelectric, as they belong to a non-centrosymmetric crystal group, PFM results are inconclusive as the measured signals were barely above the background noise.155 It is therefore extremely important that the right sample preparation method and measurement technique are used to identify the electromechanical phenomenon so that the correct mechanism can be correctly identified for the development of its application.
Another available option to overcome the flexibility disadvantage of ceramics is using flexible piezoelectric polymers (often PVDF and its co-polymers) or creating composites of ceramics (often in nanoparticle form) and polymers. Optimisation and sometimes complex fabrication steps are needed to create a decent balance between mechanical characteristics and electrical output.106–108,194 Even though PVDF and its co-polymers are great options to increase flexibility of the composites, effort also needed to be directed towards increasing the β phase quantity in PVDF. Numerous studies have combined PVDF with a nanofiller non-lead piezoelectric ceramic.104–107 However, as shown by Bairagi et al., the hydrophobicity of PVDF does not always match with the polarity of chosen ceramics.108 Future studies should take the compatibility of PVDF or other polymers into account and optimize it to their chosen ceramic. While on the subject of PVDF compatibility, some piezoelectric devises use hydrophilic and rigid electrodes, for instance, Cu, Ag or Au based.82,89 Comparatively hydrophilic electrodes may suffer from surface contact issues, which affects the total energy harvesting capabilities. Rigid electrodes also suffer from mechanical issues, such as easy crack formation upon deformation and eventually breakage.74
The substrate supporting the piezoelectric material or film, also must be taken into consideration. In particular, in terms of flexibility. Common substrates, such as Pt/Si, Pt/Ti/SiO2/Si and Pt/TiO2/SiO2/Si are not flexible and are prone to cracks and breakage. Techniques that uses flexible polymers or fabrics as a sort of substrate are less prone to inflexibility problems.106,195 An alternative flexible substrate is a hybrid paper containing for example conducting polymers, multi-walled carbon nanotubes or ionic liquids for conductivity and the migration of ions.196 Flexible polyethylene terephthalate (PET) sheets107 have also been used for PVDF piezoelectric devices.
Another challenge lies in matching of the piezoelectric device with energy storage devices. For example, in energy harvesting from organs, the output pulses will be in sync with the pulsing organ. Consequently, energy storage is often needed. All the components of the total device should be investigated for long-term mechanical and chemical stability (protection against rust or oxidation). Not only in vitro but especially in vivo.
In vivo experiments are crucial for piezoelectric clinical applications and should not only focus on stability but predominately on biological safety, including implantation safety. As surgery is always a challenge for the body (anaesthesia, incisions, suturing and risk of infection) it is essential that a piezoelectric device and its components do not interfere with the normal functioning of the body. This can, for example, be investigated with inflammatory response or cytotoxicity tests etc.197 Many clinical applications would also benefit from linking a piezoelectric device with a wireless signal transmitting system such as telemetry.7 There are numerous examples of biological systems that are desirable to monitor real time. For instance, hypertensive patients would benefit a lot of a self-powered piezoelectric blood pressure sensor166 combined with a telemeter which transmits the signal to monitoring equipment.
Since piezoelectricity in a material is strongly affected by the material's structure, piezoelectricity at the nanoscale does not necessarily translate into piezoelectricity at the microscale. In principle, the alignment of polar domains leads to stronger piezoelectricity. Thermodynamically however, a random arrangement is favourable, which leads to randomly oriented polar domains being more common in nature.25,31 It is also important to note, that same material may be strongly piezoelectric at the nanoscale, but not at the microscale. This would be the case if the polarisation domains are randomly oriented at the micrometre length scale, leading to a reduction or cancellation of net polarisation.25 Most piezoelectric ceramics are ferroelectric, thus electrical poling can be used to align polarisation domain direction to enhance the strength of the polarisation. Most biological materials however are not ferroelectric, and electrical poling is not applicable. Methods like mechanical stretching have the potential to induce alignment in biological materials and to help strengthen the piezoelectric coefficient. However, stretching may also introduce non-uniform strain into the material, which makes extrinsic factors such as electrostatic interactions more prominent.25,165
In contrast to piezoelectricity, electrostatic induction or electromagnetics are known alternatives to piezoelectricity.198 Unfortunately, these methods have a disadvantage of requiring an external input. This limits the architectural boundaries of future devices. Real competition comes from triboelectric devices that also can be combined into a hybrid piezo-triboelectric device.199,200 In triboelectric a static electrical charge is generated due to friction of two materials. Certain piezo- and triboelectric devices share similar advantages such as flexibility, stretch-ability, relatively simple design and a general high energy output.3,81,96,201 Despite piezoelectric limitations, the potency of piezoelectric materials reaches great heights. It will not only be more profoundly available in our daily applications but also for monitoring health.
Author contributions
All authors listed have made substantial, direct and intellectual contributions to the work, and approved it for publication.Conflicts of interest
There are no conflicts to declare.References
- S. Ould Amrouche, D. Rekioua, T. Rekioua and S. Bacha, Int. J. Hydrogen Energy, 2016, 41, 20914–20927 CrossRef CAS.
- G. Mao, N. Huang, L. Chen and H. Wang, Sci. Total Environ., 2018, 635, 1081–1090 CrossRef CAS PubMed.
- S. A. Kale, Renewable energy systems, 2016 Search PubMed.
- B. Guo, D. Wang, J. Zhou, W. Shi and X. Zhou, Ocean Eng., 2020, 195, 106791 CrossRef.
- Y. Ma, Q. Zheng, Y. Liu, B. Shi, X. Xue, W. Ji, Z. Liu, Y. Jin, Y. Zou, Z. An, W. Zhang, X. Wang, W. Jiang, Z. Xu, Z. L. Wang, Z. Li and H. Zhang, Nano Lett., 2016, 16, 6042–6051 CrossRef CAS PubMed.
- Z. Liu, Y. Ma, H. Ouyang, B. Shi, N. Li, D. Jiang, F. Xie, D. Qu, Y. Zou, Y. Huang, H. Li, C. Zhao, P. Tan, M. Yu, Y. Fan, H. Zhang, Z. L. Wang and Z. Li, Adv. Funct. Mater., 2019, 29(3), 1807560 CrossRef.
- T. W. Emans, B. J. Janssen, M. I. Pinkham, C. P. C. Ow, R. G. Evans, J. A. Joles, S. C. Malpas, C. T. P. Krediet and M. P. Koeners, J. Physiol., 2016, 54, 6287–6300 CrossRef PubMed.
- Z. Li, G. Zhu, R. Yang, A. C. Wang and Z. L. Wang, Adv. Mater., 2010, 22(23), 2534–2537 CrossRef CAS PubMed.
- C. Dagdeviren, B. D. Yang, Y. Su, P. L. Tran, P. Joe, E. Anderson, J. Xia, V. Doraiswamy, B. Dehdashti, X. Feng, B. Lu, R. Poston, Z. Khalpey, R. Ghaffari, Y. Huang, M. J. Slepian and J. A. Rogers, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(5), 1927–1932 CrossRef CAS PubMed.
- Z. L. Wang and J. Song, Science, 2006, 312, 242–246 CrossRef CAS.
- Y. Qi and M. C. McAlpine, Energy Environ. Sci., 2010, 3, 1275–1285 RSC.
- S. K. Ghosh and D. Mandal, Appl. Phys. Lett., 2016, 109, 103701 CrossRef.
- J. Briscoe and S. Dunn, Nano Energy, 2015, 14, 15–29 CrossRef CAS.
- S. Maiti, S. Kumar Karan, J. Lee, A. Kumar Mishra, B. Bhusan Khatua and J. Kon Kim, Nano Energy, 2017, 42, 282–293 CrossRef CAS.
- Z. Zhao, T. Wang, J. Shi, B. Zhang, R. Zhang, M. Li and Y. Wen, Energy Sci. Eng., 2019, 7(6), 2741–2755 CrossRef.
- X. Li, C. Xu, C. Wang, J. Shao, X. Chen, C. Wang, H. Tian, Y. Wang, Q. Yang, L. Wang and B. Lu, Nano Energy, 2017, 40, 646–654 CrossRef CAS.
- F. Gao, G. Liu, B. L. H. Chung, H. H. T. Chan and W. H. Liao, Appl. Phys. Lett., 2019, 115, 033901 CrossRef.
- D. Berlincourt and H. Jaffe, Phys. Rev., 1958, 111, 143–148 CrossRef CAS.
- B. Jaffe, R. S. Roth and S. Marzullo, J. Appl. Phys., 1954, 25, 809–810 CrossRef CAS.
- S. Huang, W. A. Yee, W. C. Tjiu, Y. Liu, M. Kotaki, Y. C. F. Boey, J. Ma, T. Liu and X. Lu, Langmuir, 2008, 24(23), 13621–13626 CrossRef CAS PubMed.
- D. Kim, S. A. Han, J. H. Kim, J. H. Lee, S. W. Kim and S. W. Lee, Adv. Mater., 2020, 32, 1906989 CrossRef CAS PubMed.
- J. Al-Nabulsi, S. El-Sharo, N. Salawy and H. Al-Doori, J. Med. Eng. Technol., 2019, 43, 255–272 CrossRef PubMed.
- D. M. Shin, S. W. Hong and Y. H. Hwang, Nanomaterials, 2020, 10, 123 CrossRef CAS PubMed.
- F. Ali, W. Raza, X. Li, H. Gul and K. H. Kim, Nano Energy, 2019, 57, 879–902 CrossRef CAS.
- I. Chae, C. K. Jeong, Z. Ounaies and S. H. Kim, ACS Appl. Bio Mater., 2018, 1, 936–953 CrossRef CAS.
- Q. Xu, X. Gao, S. Zhao, Y. N. Liu, D. Zhang, K. Zhou, H. Khanbareh, W. Chen, Y. Zhang and C. Bowen, Adv. Mater., 2021, 33(27), 2008452 CrossRef CAS PubMed.
- N. Sezer and M. Koç, Nano Energy, 2021, 80, 105567 CrossRef CAS.
- S. Guerin, S. A. M. Tofail and D. Thompson, NPG Asia Mater., 2019, 11, 1–5 CrossRef.
- S. Banerjee, S. Bairagi and S. Wazed Ali, Ceram. Int., 2021, 47, 16402–16421 CrossRef CAS.
- P. Muralt, Encyclopedia of Materials: Science and Technology, 2001, DOI:10.1016/b0-08-043152-6/01600-4, pp. 8894–8897.
- R. Wojnar, in Piezoelectric Nanomaterials for Biomedical Applications, ed. G. Ciofani and A. Menciassi, Springer Berlin Heidelberg, Berlin, Heidelberg, 2012, pp. 173–185, DOI:10.1007/978-3-642-28044-3_6.
- J. F. Ihlefeld, in Ferroelectricity in Doped Hafnium Oxide: Materials, Properties and Devices, 2019, pp. 1–24, DOI:10.1016/B978-0-08-102430-0.00001-2.
- T. S. Narasimhamurty, in Photoelastic and Electro-Optic Properties of Crystals, ed. T. S. Narasimhamurty, Springer US, Boston, MA, 1981, pp. 333–344, DOI:10.1007/978-1-4757-0025-1_7.
- T. Stevenson, D. G. Martin, P. I. Cowin, A. Blumfield, A. J. Bell, T. P. Comyn and P. M. Weaver, J. Mater. Sci.: Mater. Electron., 2015, 26, 9256–9267 CrossRef CAS.
- J. Curie and P. Curie, Bulletin de la Société minéralogique de France, 1880, DOI:10.3406/bulmi.1880.1564.
- J. Valasek, Phys. Rev., 1922, 20, 639–664 CrossRef CAS.
- W. P. Mason, Phys. Rev., 1946, 69, 173–194 CrossRef CAS.
- M. Ahart, M. Somayazulu, R. E. Cohen, P. Ganesh, P. Dera, H. K. Mao, R. J. Hemley, Y. Ren, P. Liermann and Z. Wu, Nature, 2008, 451, 545–548 CrossRef CAS PubMed.
- A. Muliana, Int. J. Solids Struct., 2011, 48, 2718–2731 CrossRef CAS.
- H. Jaffe and D. A. Berlincourt, Proc. IEEE, 1965, 53, 1372–1386 Search PubMed.
- A. R. Hutson, Phys. Rev. Lett., 1960, 4, 505–507 CrossRef CAS.
- S. Goel and B. Kumar, J. Alloys Compd., 2020, 816, 152491 CrossRef CAS.
- H. A. Wahab, A. A. Salama, A. A. El-Saeid, O. Nur, M. Willander and I. K. Battisha, Results Phys., 2013, 3, 46–51 CrossRef.
- S. Jain, N. Karmakar, A. Shah and N. G. Shimpi, Mater. Sci. Eng. B Solid State Mater. Adv. Technol., 2019, 247, 114381 CrossRef CAS.
- T. Rueckes, K. Kim, E. Joselevich, G. Y. Tseng, C. L. Cheung and C. M. Lieber, Science, 2000, 289, 94–97 CrossRef CAS PubMed.
- K. Batra, N. Sinha, S. Goel, H. Yadav, A. J. Joseph and B. Kumar, J. Alloys Compd., 2018, 767, 1003–1011 CrossRef CAS.
- L. Egerton and S. E. Koonce, J. Am. Ceram. Soc., 1955, 38, 412–418 CrossRef CAS.
- J. P. Praveen, T. Karthik, A. R. James, E. Chandrakala, S. Asthana and D. Das, J. Eur. Ceram. Soc., 2015, 35, 1785–1798 CrossRef CAS.
- M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G. A. Rossetti and J. Rödel, Appl. Phys. Rev., 2017, 4, 041305 Search PubMed.
- Y. Wang, H. Cheng, J. Yan, N. Chen, P. Yan, F. Yang and J. Ouyang, Materialia, 2019, 5, 100228 CrossRef CAS.
- Y. Qi and M. C. McAlpine, Energy Environ. Sci., 2010, 3, 1275–1285 RSC.
- I. Kanno, H. Kotera and K. Wasa, Sens. Actuators, A, 2003, 107, 68–74 CrossRef CAS.
- C. Zhao, H. Wu, F. Li, Y. Cai, Y. Zhang, D. Song, J. Wu, X. Lyu, J. Yin, D. Xiao, J. Zhu and S. J. Pennycook, J. Am. Chem. Soc., 2018, 140, 15252–15260 CrossRef CAS PubMed.
- Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, T. Nagaya and M. Nakamura, Nature, 2004, 432, 84–87 CrossRef CAS PubMed.
- X. Lv, J. Wu, J. Zhu, D. Xiao and X. Zhangb, J. Eur. Ceram. Soc., 2018, 38, 85–94 CrossRef.
- P. Li, J. Zhai, B. Shen, S. Zhang, X. Li, F. Zhu and X. Zhang, Adv. Mater., 2018, 30, 1705171 CrossRef PubMed.
- B. Gamboa, A. Bhalla and R. Guo, Ferroelectrics, 2020, 555, 118–123 CrossRef CAS.
- M. Algueró, B. L. Cheng, F. Guiu, M. J. Reece, M. Poole and N. Alford, J. Eur. Ceram. Soc., 2001, 21, 1437–1440 CrossRef.
- T. Starner, IBM Syst. J., 1996, 35, 618–629 Search PubMed.
- A. G. Tobin and E. Pak, Proc. SPIE, 1993, 1916, 78–86 CrossRef CAS.
- Y. Qi, J. Kim, T. D. Nguyen, B. Lisko, P. K. Purohit and M. C. McAlpine, Nano Lett., 2011, 11(3), 1331–1336 CrossRef CAS PubMed.
- S. I. Park, J. H. Ahn, X. Feng, S. Wang, Y. Huang and J. A. Rogers, Adv. Funct. Mater., 2008, 18, 2673–2684 CrossRef CAS.
- H. Jaffe, J. Am. Ceram. Soc., 1958, 41, 494–498 CrossRef CAS.
- G. H. Haertling, J. Am. Ceram. Soc., 1999, 82, 797–818 CrossRef CAS.
- W. Liu and X. Ren, Phys. Rev. Lett., 2009, 103, 257602 CrossRef PubMed.
- I. Bhaumik, G. Singh, S. Ganesamoorthy, R. Bhatt, A. K. Karnal, V. S. Tiwari and P. K. Gupta, J. Cryst. Growth, 2013, 375, 20–25 CrossRef CAS.
- P. Wang, Y. Li and Y. Lu, J. Eur. Ceram. Soc., 2011, 31, 2005–2012 CrossRef CAS.
- Y. Tan, J. Zhang, Y. Wu, C. Wang, V. Koval, B. Shi, H. Ye, R. McKinnon, G. Viola and H. Yan, Sci. Rep., 2015, 5, 9953 CrossRef CAS PubMed.
- L. Zhao, B. P. Zhang, P. F. Zhou, L. F. Zhu and J. F. Li, J. Eur. Ceram. Soc., 2015, 35, 533–540 CrossRef CAS.
- S. A. Khan, F. Akram, J. Bae, T. Ahmed, T. K. Song, Y. S. Sung, M. H. Kim and S. Lee, Solid State Sci., 2019, 98, 106040 CrossRef CAS.
- J. Wu, H. Tao, Y. Yuan, X. Lv, X. Wang and X. Lou, RSC Adv., 2015, 5, 14575–14583 RSC.
- F. Rubio-Marcos, R. López-Juárez, R. E. Rojas-Hernandez, A. Del Campo, N. Razo-Pérez and J. F. Fernandez, ACS Appl. Mater. Interfaces, 2015, 7, 23080–23088 CrossRef CAS PubMed.
- Y. Huan, X. Wang, Z. Shen, J. Kim, H. Zhou and L. Li, J. Am. Ceram. Soc., 2014, 97, 700–703 CrossRef CAS.
- Y. A. Genenko, J. Glaum, M. J. Hoffmann and K. Albe, Mater. Sci. Eng. B Solid State Mater. Adv. Technol., 2015, 192, 52–82 CrossRef CAS.
- C. Zhou, H. Lin, Q. He, L. Xu, M. Worku, M. Chaaban, S. Lee, X. Shi, M. H. Du and B. Ma, Mater. Sci. Eng., R, 2019, 137, 38–65 CrossRef.
- D. Wang, M. Wright, N. K. Elumalai and A. Uddin, Sol. Energy Mater. Sol. Cells, 2016, 147, 255–275 CrossRef CAS.
- Y. M. You, W. Q. Liao, D. Zhao, H. Y. Ye, Y. Zhang, Q. Zhou, X. Niu, J. Wang, P. F. Li, D. W. Fu, Z. Wang, S. Gao, K. Yang, J. M. Liu, J. Li, Y. Yan and R. G. Xiong, Science, 2017, 357, 306–309 CrossRef CAS PubMed.
- W. Q. Liao, D. Zhao, Y. Y. Tang, Y. Zhang, P. F. Li, P. P. Shi, X. G. Chen, Y. M. You and R. G. Xiong, Science, 2019, 363, 1206–1210 CrossRef CAS PubMed.
- M. Coll, A. Gomez, E. Mas-Marza, O. Almora, G. Garcia-Belmonte, M. Campoy-Quiles and J. Bisquert, J. Phys. Chem. Lett., 2015, 6, 1408–1413 CrossRef CAS PubMed.
- X. Wu, H. Yu, N. Li, F. Wang, H. Xu and N. Zhao, J. Phys. Chem. C, 2015, 119, 1253–1259 CrossRef CAS.
- L. Yang, S. Chi, S. Dong, F. Yuan, Z. Wang, J. Lei, L. Bao, J. Xiang and J. Wang, Nano Energy, 2020, 67, 104220 CrossRef CAS.
- J. Gomes, J. S. Nunes, V. Sencadas and S. Lanceros-Mendez, Smart Mater. Struct., 2010, 19, 065010 CrossRef.
- M. Poulsen and S. Ducharme, IEEE Trans. Dielectr. Electr. Insul., 2010, 17, 1028–1035 CAS.
- C. A. Sperati and H. W. Starkweather Jr, Fortschr. Hochpolym.-Forsch., 1961, 2, 465–495 CrossRef CAS.
- M. Kutz, Applied Plastics Engineering Handbook: Processing, Materials, and Applications: Second Edition, 2016 Search PubMed.
- C. Chen, Z. Bai, Y. Cao, M. Dong, K. Jiang, Y. Zhou, Y. Tao, S. Gu, J. Xu, X. Yin and W. Xu, Compos. Sci. Technol., 2020, 192, 108100 CrossRef CAS.
- S. S. Won, M. Sheldon, N. Mostovych, J. Kwak, B. S. Chang, C. W. Ahn, A. I. Kingon, I. W. Kim and S. H. Kim, Appl. Phys. Lett., 2015, 107, 202901 CrossRef.
- R. A. Surmenev, T. Orlova, R. V. Chernozem, A. A. Ivanova, A. Bartasyte, S. Mathur and M. A. Surmeneva, Nano Energy, 2019, 62, 475–506 CrossRef CAS.
- A. Talbourdet, F. Rault, G. Lemort, C. Cochrane, E. Devaux and C. Campagne, Smart Mater. Struct., 2018, 27, 075010 CrossRef.
- S. S. Chauhan, U. M. Bhatt, P. Gautam, S. Thote, M. M. Joglekar and S. K. Manhas, Sens. Actuators, A, 2020, 304, 111879 CrossRef CAS.
- S. Park, Y. Kim, H. Jung, J. Y. Park, N. Lee and Y. Seo, Sci. Rep., 2017, 7, 17290 CrossRef PubMed.
- F. J. Baltá Calleja, A. González Arche, T. A. Ezquerra, C. Santa Cruz, F. Batallán, B. Frick and E. López Cabarcos, Journal, 1993, 108, X-48 Search PubMed.
- T. Wang, Z. Feng, Y. Song and X. Chen, Dent. Mater., 2007, 23, 450–453 CrossRef CAS PubMed.
- L. Persano, C. Dagdeviren, Y. Su, Y. Zhang, S. Girardo, D. Pisignano, Y. Huang and J. A. Rogers, Nat. Commun., 2013, 4, 1633 CrossRef PubMed.
- Z. H. Liu, C. T. Pan, L. W. Lin, J. C. Huang and Z. Y. Ou, Smart Mater. Struct., 2014, 23, 025003 CrossRef CAS.
- X. Wang, B. Yang, J. Liu, Y. Zhu, C. Yang and Q. He, Sci. Rep., 2016, 6, 36409 CrossRef CAS PubMed.
- W. H. Liew, M. S. Mirshekarloo, S. Chen, K. Yao and F. E. H. Tay, Sci. Rep., 2015, 5, 09790 CrossRef CAS PubMed.
- T. Sharma, S. S. Je, B. Gill and J. X. J. Zhang, Sens. Actuators, A, 2012, 177, 87–92 CrossRef CAS.
- Y. A. Yildirim, A. Toprak and O. Tigli, J. Microelectromech. Syst., 2018, 27, 86–94 CAS.
- Z. Ounaies, J. A. Young and J. S. Harrison, Journal, 1999, 726, 88–103 CAS.
- S. Miyata, M. Yoshikawa, S. Tasaka and M. Ko, Polym. J., 1980, 12, 857–860 CrossRef CAS.
- D. Singh, A. Choudhary and A. Garg, ACS Appl. Mater. Interfaces, 2018, 10, 2793–2800 CrossRef CAS PubMed.
- J. Li, C. Zhao, K. Xia, X. Liu, D. Li and J. Han, Appl. Surf. Sci., 2019, 463, 626–634 CrossRef CAS.
- N. A. Hoque, P. Thakur, S. Roy, A. Kool, B. Bagchi, P. Biswas, M. M. Saikh, F. Khatun, S. Das and P. P. Ray, ACS Appl. Mater. Interfaces, 2017, 9, 23048–23059 CrossRef CAS PubMed.
- S. Garain, T. Kumar Sinha, P. Adhikary, K. Henkel, S. Sen, S. Ram, C. Sinha, D. Schmeißer and D. Mandal, ACS Appl. Mater. Interfaces, 2015, 7, 1298–1307 CrossRef CAS PubMed.
- C. Mota, M. Labardi, L. Trombi, L. Astolfi, M. D'Acunto, D. Puppi, G. Gallone, F. Chiellini, S. Berrettini, L. Bruschini and S. Danti, Mater. Des., 2017, 122, 206–219 CrossRef CAS.
- C. K. Jeong, C. Baek, A. I. Kingon, K. I. Park and S. H. Kim, Small, 2018, 14, 1704022 CrossRef PubMed.
- S. Bairagi and S. W. Ali, Energy, 2020, 198, 117385 CrossRef CAS.
- S. Guerin, T. A. M. Syed and D. Thompson, Nanoscale, 2018, 10, 9653–9663 RSC.
- A. Gruverman, B. J. Rodriguez and S. V. Kalinin, Scanning Probe Microsc., 2007, 2, 615–633 Search PubMed.
- A. Šutka, P. C. Sherrell, N. A. Shepelin, L. Lapčinskis, K. Mālnieks and A. V. Ellis, Adv. Mater., 2020, 32, 2002979 CrossRef PubMed.
- P. Jiang, F. Yan, E. Nasr Esfahani, S. Xie, D. Zou, X. Liu, H. Zheng and J. Li, ACS Biomater. Sci. Eng., 2017, 3, 1827–1835 CrossRef CAS PubMed.
- H. Y. Jiang, F. Yen, C. W. Huang, R. B. Mei and L. Chen, AIP Adv., 2017, 7, 045215 CrossRef.
- H. Ueda and E. Fakada, Jpn. J. Appl. Phys., Part 1, 1971, 10, 1650–1651 CrossRef CAS.
- A. Stapleton, M. R. Noor, J. Sweeney, V. Casey, A. L. Kholkin, C. Silien, A. A. Gandhi, T. Soulimane and S. A. M. Tofail, Appl. Phys. Lett., 2017, 111, 142902 CrossRef.
- A. Stapleton, M. S. Ivanov, M. R. Noor, C. Silien, A. A. Gandhi, T. Soulimane, A. L. Kholkin and S. A. M. Tofail, Ferroelectrics, 2018, 525, 135–145 CrossRef CAS.
- Y. Liu, Y. Wang, M. J. Chow, N. Q. Chen, F. Ma, Y. Zhang and J. Li, Phys. Rev. Lett., 2013, 110, 1–5 Search PubMed.
- Y. Liu, H. L. Cai, M. Zelisko, Y. Wang, J. Sun, F. Yan, F. Ma, P. Wang, Q. N. Chen, H. Zheng, X. Meng, P. Sharma, Y. Zhang and J. Li, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E2780–E2786 CrossRef CAS PubMed.
- E. Fukada and I. Yasuda, Jpn. J. Appl. Phys., Part 1, 1964, 3, 502B CrossRef.
- E. Fukada and H. Ueda, Jpn. J. Appl. Phys., Part 1, 1970, 9, 844–845 CrossRef.
- S. Guerin, A. Stapleton, D. Chovan, R. Mouras, M. Gleeson, C. McKeown, M. R. Noor, C. Silien, F. M. F. Rhen, A. L. Kholkin, N. Liu, T. Soulimane, S. A. M. Tofail and D. Thompson, Nat. Mater., 2018, 17, 180–186 CrossRef CAS PubMed.
- E. Fukada, Wood Sci. Technol., 1968, 2, 299–307 CrossRef CAS.
- E. Fukada, J. Phys. Soc. Jpn., 1955, 10, 149–154 CrossRef.
- C. M. Lee, K. Kafle, D. W. Belias, Y. B. Park, R. E. Glick, C. H. Haigler and S. H. Kim, Cellulose, 2015, 22, 971–989 CrossRef CAS.
- K. Kafle, R. Shi, C. M. Lee, A. Mittal, Y. B. Park, Y. H. Sun, S. Park, V. Chiang and S. H. Kim, Cellulose, 2014, 21, 2219–2231 CrossRef CAS.
- B. Frka-Petesic, B. Jean and L. Heux, Europhys. Lett., 2014, 107, 28006 CrossRef.
- S. Rajala, T. Siponkoski, E. Sarlin, M. Mettänen, M. Vuoriluoto, A. Pammo, J. Juuti, O. J. Rojas, S. Franssila and S. Tuukkanen, ACS Appl. Mater. Interfaces, 2016, 8, 15607–15614 CrossRef CAS PubMed.
- S. Yun, J. H. Kim, Y. Li and J. Kim, J. Appl. Phys., 2008, 103, 083301 CrossRef.
- J. Wang, J. Wang, C. Carlos, Z. Zhang, J. Li, Y. Long, F. Yang, Y. Dong, X. Qiu, Y. Qian and X. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 26399–26404 CrossRef CAS PubMed.
- I. Chae, S. M. Q. Bokhari, X. Chen, R. Zu, K. Liu, A. Borhan, V. Gopalan, J. M. Catchmark and S. H. Kim, Carbohydr. Polym., 2021, 255, 117328 CrossRef CAS PubMed.
- G. Y. Yun, H. S. Kim, J. Kim, K. Kim and C. Yang, Sens. Actuators, A, 2008, 141, 530–535 CrossRef CAS.
- J. Leppiniemi, P. Lahtinen, A. Paajanen, R. Mahlberg, S. Metsä-Kortelainen, T. Pinomaa, H. Pajari, I. Vikholm-Lundin, P. Pursula and V. P. Hytönen, ACS Appl. Mater. Interfaces, 2017, 9, 21959–21970 CrossRef CAS PubMed.
- A. Hänninen, E. Sarlin, I. Lyyra, T. Salpavaara, M. Kellomäki and S. Tuukkanen, Carbohydr. Polym., 2018, 202, 418–424 CrossRef PubMed.
- E. S. Choi, H. C. Kim, R. M. Muthoka, P. S. Panicker, D. O. Agumba and J. Kim, Compos. Sci. Technol., 2021, 209, 108795 CrossRef CAS.
- O. M. Vanderfleet and E. D. Cranston, Nat. Rev. Mater., 2021, 6, 124–144 CrossRef CAS.
- D. Lasrado, S. Ahankari and K. Kar, J. Appl. Polym. Sci., 2020, 137, 48959 CrossRef CAS.
- P. K. Annamalai, A. K. Nanjundan, D. P. Dubal and J. B. Baek, Adv. Mater. Technol., 2021, 6(2021), 2001164 CrossRef CAS.
- D. Vasilescu, R. Cornillon and G. Mallet, Nature, 1970, 225, 635 CrossRef CAS PubMed.
- V. V. Lemanov, S. N. Popov and G. A. Pankova, Phys. Solid State, 2011, 53, 1191–1193 CrossRef CAS.
- D. Denning, J. I. Kilpatrick, E. Fukada, N. Zhang, S. Habelitz, A. Fertala, M. D. Gilchrist, Y. Zhang, S. A. M. Tofail and B. J. Rodriguez, ACS Biomater. Sci. Eng., 2017, 3, 929–935 CrossRef CAS PubMed.
- M. Minary-Jolandan and M. F. Yu, Appl. Phys. Lett., 2010, 97, 153127 CrossRef.
- J. Reyes-Gasga, M. Galindo-Mentle, E. Brès, N. Vargas-Becerril, E. Orozco, A. Rodríguez-Gómez and R. García-García, J. Phys. Chem. Solids, 2020, 136, 109140 CrossRef CAS.
- S. Ghosh, B. Z. Mei, V. Lubkin, J. I. Scheinbeim, B. A. Newman, P. Kramer, G. Bennett and N. Feit, J. Biomed. Mater. Res., 1998, 39, 453–457 CrossRef CAS PubMed.
- B. Y. Lee, J. Zhang, C. Zueger, W. J. Chung, S. Y. Yoo, E. Wang, J. Meyer, R. Ramesh and S. W. Lee, Nat. Nanotechnol., 2012, 7, 351–356 CrossRef CAS PubMed.
- D. M. Shin, H. J. Han, W. G. Kim, E. Kim, C. Kim, S. W. Hong, H. K. Kim, J. W. Oh and Y. H. Hwang, Energy Environ. Sci., 2015, 8, 3198–3203 RSC.
- J. H. Lee, J. H. Lee, J. Xiao, M. S. Desai, X. Zhang and S. W. Lee, Nano Lett., 2019, 19, 2661–2667 CrossRef CAS PubMed.
- P. Hu, S. Hu, Y. Huang, J. R. Reimers, A. M. Rappe, Y. Li, A. Stroppa and W. Ren, J. Phys. Chem. Lett., 2019, 10, 1319–1324 CrossRef CAS PubMed.
- A. Heredia, V. Meunier, I. K. Bdikin, J. Gracio, N. Balke, S. Jesse, A. Tselev, P. K. Agarwal, B. G. Sumpter, S. V. Kalinin and A. L. Kholkin, Adv. Funct. Mater., 2012, 22, 2996–3003 CrossRef CAS.
- S. Guerin, S. A. M. Tofail and D. Thompson, Cryst. Growth Des., 2018, 18, 4844–4848 CrossRef CAS.
- Y. Sun, K. Y. Zeng and T. Li, Sci. China: Phys., Mech. Astron., 2020, 63, 278701 CAS.
- M. Nair, Y. Calahorra, S. Kar-Narayan, S. M. Best and R. E. Cameron, Nanoscale, 2019, 11, 15120–15130 RSC.
- A. A. Marino and B. D. Gross, Arch. Oral Biol., 1989, 34, 507–509 CrossRef CAS PubMed.
- M. Minary-Jolandan and M. F. Yu, ACS Nano, 2009, 3, 1859–1863 CrossRef CAS PubMed.
- Z. Tylczyski, A. Sterczyska and M. Wiesner, J. Phys.: Condens. Matter, 2011, 23, 355901 CrossRef PubMed.
- Y. Zhang, A. A. Gandhi, J. Zeglinski, M. Gregor and S. A. M. Tofail, IEEE Trans. Dielectr. Electr. Insul., 2012, 19, 1151–1157 CAS.
- E. Fukada and I. Yasuda, J. Phys. Soc. Jpn., 1957, 12, 1158–1162 CrossRef.
- A. H. Rajabi, M. Jaffe and T. L. Arinzeh, Acta Biomater., 2015, 24, 12–23 CrossRef CAS PubMed.
- M. Minary-Jolandan and M. F. Yu, Nanotechnology, 2009, 20, 085706 CrossRef PubMed.
- A. C. Jayasuriya, J. I. Scheinbeim, V. Lubkin, G. Bennett and P. Kramer, J. Biomed. Mater. Res., 2003, 66, 260–265 CrossRef CAS PubMed.
- A. C. Jayasuriya, S. Ghosh, J. I. Scheinbeim, V. Lubkin, G. Bennett and P. Kramer, Biosens. Bioelectron.: X, 2003, 18, 381–387 CrossRef CAS.
- S. K. Ghosh and D. Mandal, Nano Energy, 2016, 28, 356–365 CrossRef CAS.
- N. R. Alluri, N. Prashanth, M. Joseph, G. Khandelwal, V. Vivekananthan and S.-j. Kim, Nano Energy, 2020, 73, 104767 CrossRef CAS.
- A. Gaur, S. Tiwari, C. Kumar and P. Maiti, Energy Rep., 2020, 6, 490–496 CrossRef.
- D. Jiang, B. Shi, H. Ouyang, Y. Fan, Z. L. Wang, Z. M. Chen and Z. Li, Mater. Today Energy, 2020, 16, 100386 CrossRef.
- J. Li, Y. Long, F. Yang and X. Wang, Curr. Opin. Solid State Mater. Sci., 2020, 24, 100806 CrossRef CAS PubMed.
- X. Cheng, X. Xue, Y. Ma, M. Han, W. Zhang, Z. Xu, H. Zhang and H. Zhang, Nano Energy, 2016, 22, 453–460 CrossRef CAS.
- C. Wang, S. Wang, Z. Gao and X. Wang, Appl. Energy, 2019, 251, 113383 CrossRef.
- L. Guo and Q. Lu, Renew. Sustain. Energy Rev., 2017, 72, 761–773 CrossRef CAS.
- Y. Tianchen, Y. Jian, S. Ruigang and L. Xiaowei, Smart Mater. Struct., 2014, 23, 125046 CrossRef.
- F. Faisal, N. Wu and K. Kapoor, Proc. SPIE, 2016, 9799, 97992Q CrossRef.
- P. Liu, Y. Hu, B. Geng and D. Xu, J. Mater. Res. Technol., 2020, 9, 3511–3519 CrossRef CAS.
- P. Liu, Y. Hu, Y. Chen, B. Geng and D. Xu, Constr. Build. Mater., 2020, 235, 117495 CrossRef.
- J. S. Lee, K. Y. Shin, O. J. Cheong, J. H. Kim and J. Jang, Sci. Rep., 2015, 5, 7887 CrossRef CAS PubMed.
- M. Zhu, M. Lou, I. Abdalla, J. Yu, Z. Li and B. Ding, Nano Energy, 2020, 69, 104429 CrossRef CAS.
- H. Yuan, T. Lei, Y. Qin, J. H. He and R. Yang, J. Phys. D: Appl. Phys., 2019, 51, 194002 CrossRef.
- V. Nguyen, S. Kelly and R. Yang, APL Mater., 2017, 5, 074108 CrossRef.
- W. A. Yee, M. Kotaki, Y. Liu and X. Lu, Polymer, 2007, 48, 512–521 CrossRef CAS.
- S. K. Karan, S. Maiti, S. Paria, A. Maitra, S. K. Si, J. K. Kim and B. B. Khatua, Mater. Today Energy, 2018, 9, 114–125 CrossRef.
- A. Maitra, S. K. Karan, S. Paria, A. K. Das, R. Bera, L. Halder, S. K. Si, A. Bera and B. B. Khatua, Nano Energy, 2017, 40, 633–645 CrossRef CAS.
- S. K. Ghosh and D. Mandal, ACS Sustainable Chem. Eng., 2017, 5, 8836–8843 CrossRef CAS.
- S. K. Ghosh, P. Adhikary, S. Jana, A. Biswas, V. Sencadas, S. D. Gupta, B. Tudu and D. Mandal, Nano Energy, 2017, 36, 166–175 CrossRef CAS.
- V. Vivekananthan, N. R. Alluri, Y. Purusothaman, A. Chandrasekhar, S. Selvarajan and S. J. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 18650–18656 CrossRef CAS PubMed.
- D. I. Zeugolis, G. R. Paul and G. Attenburrow, J. Biomed. Mater. Res., 2009, 89, 895–908 CrossRef PubMed.
- C. R. West and A. E. Bowden, Ann. Biomed. Eng., 2012, 40, 1568–1574 CrossRef PubMed.
- J. S. Moon, Y. Lee, D. M. Shin, C. Kim, W. G. Kim, M. Park, J. Han, H. Song, K. Kim and J. W. Oh, Chem. Asian J., 2016, 11, 3097–3101 CrossRef CAS PubMed.
- Y. C. Shin, C. Kim, S. J. Song, S. Jun, C. S. Kim, S. W. Hong, S. H. Hyon, D. W. Han and J. W. Oh, Nanotheranostics, 2018, 2, 144–156 CrossRef PubMed.
- I. S. Raja, C. Kim, S. J. Song, Y. C. Shin, M. S. Kang, S. H. Hyon, J. W. Oh and D. W. Han, Nanomaterials, 2019, 9, 1014 CrossRef CAS PubMed.
- R. M. Street, T. Huseynova, X. Xu, P. Chandrasekaran, L. Han, W. Y. Shih, W. H. Shih and C. L. Schauer, Carbohydr. Polym., 2018, 195, 218–224 CrossRef CAS PubMed.
- T. Ibn-Mohammed, I. M. Reaney, S. C. L. Koh, A. Acquaye, D. C. Sinclair, C. A. Randall, F. H. Abubakar, L. Smith, G. Schileo and L. Ozawa-Meida, J. Eur. Ceram. Soc., 2018, 38, 4922–4938 CrossRef CAS.
- J. Koruza, A. J. Bell, T. Frömling, K. G. Webber, K. Wang and J. Rödel, J. Materiomics, 2018, 4, 13–26 CrossRef.
- M. H. Chung, S. Yoo, H. J. Kim, J. Yoo, S. Y. Han, K. H. Yoo and H. Jeong, Sci. Rep., 2019, 9, 6581 CrossRef PubMed.
- L. Zhang, S. R. Oh, T. C. Wong, C. Y. Tan and K. Yao, IEEE Trans. Ultrason. Ferroelectrics Freq. Contr., 2013, 60, 2013–2020 Search PubMed.
- V. T. Rathod, Electronics, 2019, 8, 169 CrossRef.
- A. Mohebbi, F. Mighri, A. Ajji and D. Rodrigue, Polym. Adv. Technol., 2017, 28, 476–483 CrossRef CAS.
- N. Soin, T. H. Shah, S. C. Anand, J. Geng, W. Pornwannachai, P. Mandal, D. Reid, S. Sharma, R. L. Hadimani, D. V. Bayramol and E. Siores, Energy Environ. Sci., 2014, 7, 1670–1679 RSC.
- J. Kim, S. Yun, S. K. Mahadeva, K. Yun, S. Y. Yang and M. Maniruzzaman, Sensors, 2010, 10, 1473–1485 CrossRef CAS PubMed.
- A. Rosengren, L. Faxius, N. Tanaka, M. Watanabe and L. M. Bjursten, J. Biomed. Mater. Res., 2005, 75, 115–122 CrossRef PubMed.
- D. A. Wang and K. H. Chang, Microelectron. J., 2010, 41, 356–364 CrossRef CAS.
- S. Cherumannil Karumuthil, S. P. Rajeev and S. Varghese, Nano Energy, 2017, 40, 487–494 CrossRef CAS.
- Z. Li, Z. Saadatnia, Z. Yang and H. Naguib, Energy Convers. Manage., 2018, 174, 188–197 CrossRef.
- Q. Zheng, B. Shi, Z. Li and Z. L. Wang, Adv. Sci., 2017, 4, 1700029 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2021 |



