Open Access Article
Open Access ArticleM/TiO2 (M = Fe, Co, Ni, Cu, Zn) catalysts for photocatalytic hydrogen production under UV and visible light irradiation†
L.
Díaz
 ab,
V. D.
Rodríguez
*cb,
M.
González-Rodríguez
ab,
V. D.
Rodríguez
*cb,
M.
González-Rodríguez
 ab,
E.
Rodríguez-Castellón
ab,
E.
Rodríguez-Castellón
 *d,
M.
Algarra
*d,
M.
Algarra
 d,
P.
Núñez
d,
P.
Núñez
 ab and
E.
Moretti
e
ab and
E.
Moretti
e
aDepartamento de Química, U.D. Química Inorgánica, Universidad de La Laguna, 38206 La Laguna, Tenerife, Spain
bInstituto Universitario de Materiales y Nanotecnología, Universidad de La Laguna, 38206 La Laguna, Tenerife, Spain
cDepartamento de Física, Universidad de La Laguna, 38206 La Laguna, Tenerife, Spain. E-mail: vrguez@ull.edu.es
dDepartamento de Química Inorgánica, Facultad de Ciencias, Universidad de Málaga, 29010 Málaga, Spain. E-mail: castellon@uma.es
eDipartimento di Scienze Molecolari e Nanosistemi, Università Ca’ Foscari Venezia, Via Torino 155, 30172 Mestre Venezia, Italy
First published on 28th May 2021
Abstract
In order to improve the photocatalytic response of TiO2 to UV and visible light for hydrogen photoproduction, low cost M/TiO2 semiconductor catalysts were prepared by the impregnation method of five different first row transition metals (M = Fe, Co, Ni, Cu or Zn) on a commercial titania support. The maximum hydrogen production efficiency was achieved for the Cu/TiO2 photocatalyst, with ∼5000 and ∼220 μmol h−1 g−1 H2 production rates for UV and visible irradiation, respectively. Ni/TiO2 and Co/TiO2 also showed a significant photocatalytic activity when UV light was used. The best performing catalyst, Cu/TiO2, was characterized by TEM and XPS measurements. The data showed that Cu was highly dispersed over the TiO2 support and the copper species existed as both reduced Cu0/Cu+ and oxidized Cu2+ on TiO2. Besides, during the hydrogen production reaction, the reduced Cu was partially oxidized to Cu2+ by the transfer of photogenerated holes under UV or visible light irradiation. With UV and visible lamps, the H2 production rates were higher than those obtained with non-impregnated TiO2 by factors of 16 and 3, respectively. These results demonstrated that a Cu/TiO2 photocatalyst could be considered a promising low-cost alternative to the well-known Pt/TiO2 system for hydrogen production, making the Cu-based catalyst an ideal cost-effective candidate for this reaction.
Introduction
Minimizing environmentally harmful emissions and reducing the dependence on fossil fuels can be achieved by sustainable energy sources. Hydrogen can be an ideal sustainable energy carrier because it is readily available, has a high energy density and has a minimal environmental impact compared with fossil fuels.1,2 Moreover, the storage capabilities, energy density versatility, transportability and environmental impacts of hydrogen are considered to be very important aspects in the assessment of its viability and use.3 However, for hydrogen to become a common source of energy there need to be technological developments that facilitate the reduction of costs with innovative renewable hydrogen production methods.4Hydrogen can be obtained from many different sources that include fossil resources, such as coal and natural gas, as well as renewable resources, such as biomass and water. Currently, the most developed and most used technology for hydrogen production is the reforming of hydrocarbon fuels. Nonetheless, to decrease the dependence on fossil fuels, several hydrogen generation technologies are being developed, including chemical, biological, electrolytic, photolytic, and thermo-chemical generation.5,6
Splitting water into hydrogen and oxygen could be the key technology in future hydrogen production. Photocatalytic water splitting is one of the most attractive methods since hydrogen can be produced from water and sunlight. In this regard, TiO2 is the most used photocatalyst for hydrogen production from water. The main concern is the lack of photocatalytic activity under visible light irradiation. In fact, TiO2 works only under UV light irradiation due to the wide band gap (3.2 eV for the anatase polymorph and 3.0 eV for the rutile one) and its photocatalytic performance is not sufficient for utilization since the amount of UV light contained in solar light is low (ca. 5%).7 Consequently, many efforts have been devoted to the synthesis of photocatalysts responding to visible light. Some research groups have reported that TiO2-based photocatalysts modified with transition metal compounds on the surface respond to visible light.8–11
Certain metals, such as Pt, Au, Pd and Ag, can be deposited on TiO2 to increase the hydrogen production rate by photocatalysis, but these metals are expensive. Fig. 1 shows the scheme of the reaction of photocatalytic water splitting through irradiation of Pt/TiO2. Photons are absorbed by the semiconductor-based photocatalyst to form electron–hole pairs. If the energy of the incident light is higher than the band gaps of the semiconductors, electrons and holes can be separated into the conduction (CB) and valence (VB) bands, respectively.
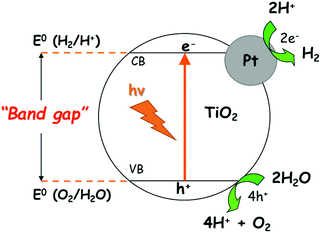 | ||
| Fig. 1 Schematic diagram of the water-splitting reaction on Pt/TiO2 as a heterogeneous photocatalyst. | ||
Photoexcited carriers are transferred to surface active sites and subsequently consumed by surface redox reactions; mass diffusion of reactants and products should concurrently proceed. Recombination of photoexcited carriers occurs along with these reactions. Hence, the charge separation in photocatalyst particles and the redox reactions on their surface must proceed within the lifetime of photoexcited carriers for successful water splitting.12,13 However, the addition of organic compounds (alcohols, organic acids and azo-dyes) or biomass derivatives (glycerol and carbohydrates) as sacrificial reagents in the catalyst dissolution favours the photocatalytic activity. In this reforming process, the oxidation of organic molecules and the reduction of H+ to H2 occur. For example, when methanol is used as a sacrificial reagent, methanol photoreforming occurs (CH3OH + H2O → 3H2 + CO2), where H2 is obtained, and the sacrificial agents are mineralized.14,15 However, most of these transition metal oxide-containing photocatalysts are generally found to remove contaminants from water.
Loading TiO2 with noble metals such as Pt, Pd or Au is an active research field for hydrogen production,16,17 as it is an effective way to enhance the photocatalytic efficiency of TiO2 by reducing the fast recombination of photogenerated charge carriers. But unfortunately, it is unsuitable for large-scale energy production due to the high cost of the precious metals and their limited availability. Therefore, the modification of TiO2 with non-noble metals (Cr, Fe, Co, Ni, Cu, and Zn) could be a viable alternative, decreasing the band gap of TiO2 and extending the photoresponse range of the TiO2 matrix to the visible region.18–22 Different modification methods have been used over TiO2 with different phases, morphologies, and microstructures (as nanocrystals or nanofibers).
Recent experimental results have demonstrated that Cu is a good candidate to replace Pt as an efficient and cost-effective co-catalyst for H2 production. Copper species have been incorporated into TiO2 by many different methodologies, such as sol–gel, impregnation, microemulsion and electroless plating.23–26 It is difficult to control the active species and their size during the synthesis and post treating processes, and thus, the optimal Cu loading amount can vary from one process to another, and the active species are still in discussion.27 However, it was found that the hydrogen-through-copper production was increased with respect to bare TiO2 by a factor of 4.23
On the other hand, the efficiency reached is usually expressed in terms of the hydrogen generation rate by the mass unit of the catalyst. But this rate depends on the experimental setup and cannot be used to compare the results obtained by different authors for different photocatalysts. In this sense, we consider it useful to compare the experimental results obtained with a new photocatalyst with those obtained with a well-known commercial one, such as TiO2 P25 from Degussa. Moreover, some authors present the results in terms of the obtained quantum yield, which is given by the ratio of hydrogen generated (2 × hydrogen molecules) to the number of incident photons. Thus, Montoya et al. obtained a quantum efficiency of 0.4% for TiO2 P25 by using a 450 W Hg lamp.19 This parameter depends on the experimental setup, the lamp spectrum, etc. Therefore, it is not useful to compare the results of different authors.
In a previous paper, we modified TiO2 nanoparticles by photodeposition of different percentages of Pt to obtain the best performance for hydrogen production. The effects of the reaction temperature and the sacrificial reagent (methanol, ethanol, or isopropanol) on the photocatalytic activity of Pt/TiO2 were also studied.28 In this work, a series of M/TiO2 photocatalysts (M = Fe, Co, Ni, Cu and Zn) were prepared by an impregnation method to improve the photoresponse of TiO2 to UV and visible radiation for hydrogen photoproduction and also by replacing noble metal co-catalysts, such as Pt, which are relatively scarce and very expensive. In this sense, Earth-abundant elements, such as the proposed transition metals, could be appropriate alternatives if they show reasonable photocatalytic activity. The results obtained with TiO2 P25 by using the same experimental setup are presented as a reference of the accomplished efficiency. Moreover, the results for Pt/TiO2 obtained with the same experimental setup are also presented for comparison purposes.
Results and discussion
Influence of the transition metals supported on TiO2
Traditionally, noble metal nanoparticles (Pt, Pd, Au, Rh, and Ag) have been used as cocatalysts in the photoreforming process for H2 production. It is well known that the presence of a cocatalyst can enhance photoactivity, providing additional reaction sites and favouring charge migration and separation. The latter promotional effect can be related to the formation of a Schottky barrier originating from the difference in the Fermi levels between the metal nanoparticles and the semiconductor (i.e. TiO2). This allows the injection of photogenerated electrons from the conduction band of the semiconductor into the metal.29 Until now, few studies on the use of non-noble metals as cocatalysts have been reported.30–33 To analyse the photocatalytic behaviour of the prepared M/TiO2 photocatalysts (M = Fe, Co, Ni, Cu, and Zn) under both visible and UV light irradiation, TiO2 was loaded with 2 wt% of selected transition metals.Hydrogen production profiles for TiO2 impregnated catalysts are shown in Fig. 2 and compared with the TiO2 P25 photocatalyst, without impregnation, using in both cases the same reactor system and the same operating conditions. As expected, bare titania did not show any remarkable activity, neither under UV nor under visible light irradiation. Overall, these impregnated photocatalysts presented a better photocatalytic activity for hydrogen production under UV light than that observed under visible light. As shown in Fig. 2, when UV light was employed, the highest hydrogen production was obtained for Cu/TiO2, followed by Ni/TiO2 and Co/TiO2, which showed much higher H2 production than the bare TiO2. However, Fe/TiO2 and Zn/TiO2 showed a very poor photocatalytic activity, even lower than that displayed by pure TiO2.
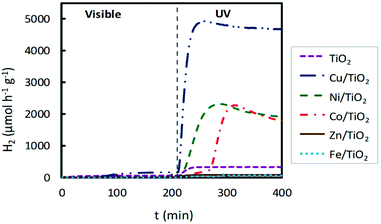 | ||
| Fig. 2 Hydrogen production for impregnated M/TiO2 photocatalysts (M = Fe, Co, Ni, Cu, Zn), 2 wt% M, under both visible and UV light irradiation. | ||
When visible light was used, Cu/TiO2 emerged again as the system providing the best photocatalytic activity (Fig. 3). The H2 production obtained with this material is about three times higher than that obtained with bare TiO2. Conversely, the materials impregnated with Ni, Co, Zn and Fe exhibited lower hydrogen production than the pure TiO2. Broadly, the Cu/TiO2 photocatalyst showed the best performance for hydrogen production, under both UV and visible light irradiation, achieving hydrogen production rates of 5000 and 220 μmol h−1 g−1, respectively.
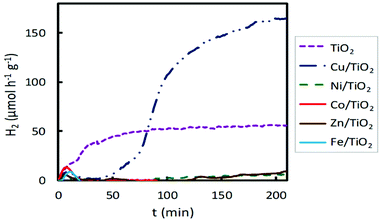 | ||
| Fig. 3 Hydrogen production for 2 wt% impregnated M/TiO2 photocatalysts (M = Fe, Co, Ni, Cu, Zn) under visible lamp irradiation. | ||
With both lamps, UV and visible, these H2 production rates are higher than those obtained with non-impregnated TiO2 by factors of 16 and 3, respectively. Furthermore, H2 production rates using UV light with Ni/TiO2 and Co/TiO2 photocatalysts were also high, see Fig. 2, achieving values of 2300 μmol h−1 g−1 and 2250 μmol h−1 g−1, respectively.
Effect of Cu loading on TiO2
The effect of the Cu content impregnated on the TiO2 matrix was examined by varying the percentage of Cu between 0.1 and 9 wt% to identify the optimal loading of the best performing cocatalyst. Usually there is an optimum cocatalyst loading towards enhanced photoactivity which varies depending on the cocatalyst used. Catalytic activity, in the beginning, increases with increasing cocatalyst amount on the surface of the semiconductor support.Then a further increase in the cocatalyst loading normally results in a decrease in the overall photocatalytic activity. This fact has been attributed both to shading effects, caused by the excess cocatalyst loading, and to an increase of the particle size with increasing cocatalyst content, with detrimental effects in charge recombination and specific surface area.34
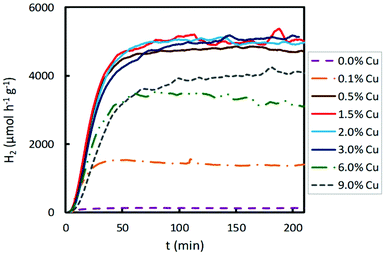
The results obtained for hydrogen production under UV irradiation over Cu-based photocatalysts are shown in Fig. 4. As reported before, bare TiO2 presents an extremely low hydrogen production, which is limited by the rapid recombination of the photogenerated electron–hole pairs. When a small amount of copper, 0.1 wt%, was loaded on the semiconductor surface, a significant improvement in photocatalytic activity was achieved. The increase in the Cu content, from 0.1 to 0.5 wt%, resulted in a remarkable enhancement in hydrogen production. Then, between 0.5 and 3.0 wt% of Cu content, hydrogen production for these Cu loadings is possibly due to the fact that the active sites on the TiO2 are sufficient and the cocatalyst can trap the photogenerated charges and assist in the charge separation appropriately. However, a further increase in the Cu content beyond 3 wt% (namely, 6.0 and 9.0 wt%) led to a decrease in the hydrogen production, probably due to several combined effects: (a) the percolation effects are over, that is, the active sites on the TiO2 surface begin to decrease because they are highly occupied by the Cu species, and (b) opacity and light scattering by the excessive Cu species agglomeration result in a decreased passage of irradiation.35,36 Therefore, when UV irradiation was employed for photocatalytic hydrogen production, the optimum Cu content on TiO2 was in the range of 0.5–3.0 wt%, achieving the highest hydrogen production (4700–5100 μmol h−1 g−1).
The effect of the Cu content of Cu/TiO2 samples on photocatalytic hydrogen production using visible light was also studied. The results are presented in Fig. 5. The hydrogen production was two-fold when TiO2 was impregnated with 0.1 wt% Cu. When the Cu content was increased from 0.1 to 1.5 wt%, the hydrogen production enhanced, achieving a maximum production for a Cu content of 1.5 wt%. For a copper loading higher than 1.5 wt%, the photocatalytic activity decreased. With higher Cu contents, from 1.5 to 9.0 wt%, lower H2 production was obtained. As discussed above, an excessive titania coverage by copper did not improve the ability of TiO2 to photogenerate hydrogen since a too large amount of Cu species led to agglomeration on the semiconductor surface, decreasing the light absorption of TiO2 and partially blocking its sensitization.37 Furthermore, it is known that excessive metal sites may act as recombination centres.38 Consequently, when visible light was used for the photocatalytic hydrogen production, the optimum Cu content was 1.5 wt%, achieving the highest hydrogen production of about 220 μmol h−1 g−1, a value much lower than that obtained under UV light.
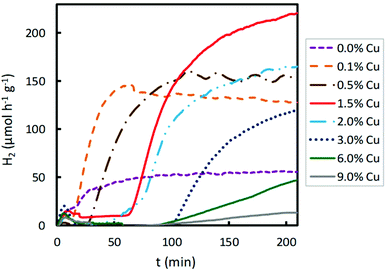 | ||
| Fig. 5 Effect of Cu impregnated content of TiO2 on photocatalytic hydrogen production under visible light irradiation. | ||
A noticeable delay of H2 production onset in Cu impregnated TiO2 compared to bare TiO2 is observed in Fig. 4 and 6, which increases with the Cu content. This delay could be associated with an initial photoreaction caused by the visible lamp, which is not appreciable with the UV lamp. Also, in Fig. 2, Co impregnated TiO2 shows delay under the UV lamp.
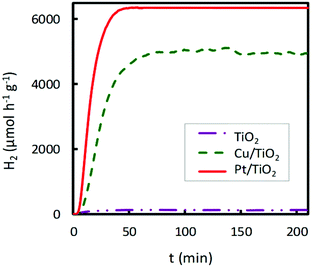 | ||
| Fig. 6 Comparison of the catalytic activity of bare commercial TiO2, 2 wt% Cu/TiO2 and 2 wt% Pt/TiO2 in the hydrogen photoproduction under UV irradiation. | ||
In Fig. S1† it is clear that 1.5% of the Cu sample shows the highest hydrogen production under visible light irradiation, and that high Cu contents (6.0 and 9.0%) give rise to low hydrogen production, even lower than the pristine support, as explained above. Under ultraviolet irradiation, samples with medium Cu contents (0.5, 1.0, 2.0 and 3.0%) show similar high hydrogen productivity; however, higher Cu contents (6.0 and 9.0%) give rise to lower productivity.
As mentioned earlier, Pt is the most efficient cocatalyst on TiO2 for photocatalytic hydrogen production. For this reason, comparative studies of the hydrogen production rate under UV irradiation of 2.0 wt% Cu or Pt impregnated TiO2 were performed. The obtained results are shown in Fig. 6. The Pt-TiO2 photocatalyst was prepared by photodeposition, following the procedure proposed by several authors.39,40 The highest catalytic activity was obtained for Pt/TiO2; however, Cu/TiO2 achieved ∼80% of the H2 production rate of Pt/TiO2. This fact suggests the great potential of the Cu/TiO2 photocatalyst in hydrogen production if the metal prices are compared.41
Cu/TiO2 characterization
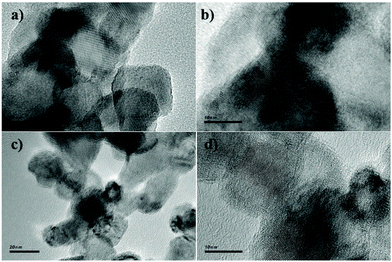
The TEM images of 2 wt% Cu/TiO2 and 9 wt% Cu/TiO2 samples, chosen for a comparative study, are very similar, as shown in Fig. 7. They show the starting TiO2 crystalline nanoparticles, without evidence of Cu-associated nanoparticles. The clear lattice fringes indicate the crystallinity of the samples and a d-spacing of 0.35 nm in all TEM images, in agreement with that of the (101) plane of anatase TiO2.42 The lack of nanoparticles corresponding to the agglomeration of Cu species observed from the TEM images confirms the high dispersion of Cu species on the titania surface. EDX analysis of both Cu/TiO2 systems shows two strong fluorescence signals (Fig. 8a and c), indicating the presence of Cu species. In addition, to clarify the distribution of the Cu species on the TiO2 surface, X-ray elemental mapping analysis was carried out, as shown in Fig. 8b and d. These figures show that the spatial distribution of Cu is in good agreement with the O and Ti mappings, confirming that the Cu species are highly dispersed on the surface of TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 according to that reported by Chen et al.,44 who prepared a CuO/TiO2 system by a complex precipitation method and suggested that a CuO monolayer was deposited on the titania surface. Under these conditions, electron diffraction, carried out in the HRTEM equipment, does not reveal the presence of the Cu species, which are localized as thin films over TiO2 nanoparticles.
43 according to that reported by Chen et al.,44 who prepared a CuO/TiO2 system by a complex precipitation method and suggested that a CuO monolayer was deposited on the titania surface. Under these conditions, electron diffraction, carried out in the HRTEM equipment, does not reveal the presence of the Cu species, which are localized as thin films over TiO2 nanoparticles.
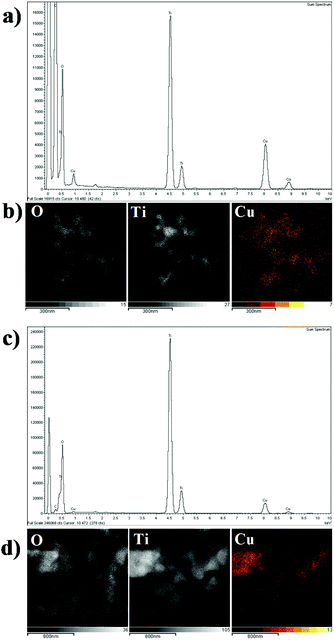 | ||
| Fig. 8 EDX analysis of (a) 2 wt% Cu/TiO2 and (c) 9 wt% Cu/TiO2 element mapping of O, Ti and Cu for (b) 2 wt% Cu/TiO2 and (d) 9 wt% Cu/TiO2. | ||
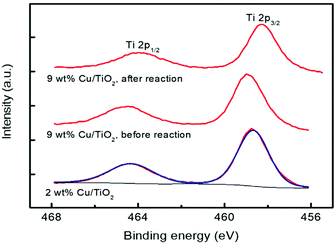
The chemical states of Ti and Cu elements on the TiO2 surface were analysed by X-ray photoelectron spectroscopy (XPS). Two catalytic materials were studied before the photocatalytic reaction: 2 and 9 wt% Cu/TiO2; the latter was also studied after the reaction. For this purpose, the material was separated from the reaction medium by centrifugation and then dried for 24 hours in air at room temperature. As shown in Fig. 9, all the catalysts show the characteristic doublet Ti 2p3/2 and Ti 2p1/2 at binding energies of 458.7 and 464.3 eV, respectively, revealing that titanium atoms exist as Ti4+ in the lattice of TiO2.45,46 No significant variation was observed between the Ti 2p binding energies of TiO2 and Cu/TiO2 catalysts, indicating the Cu was deposited on the TiO2 surface rather than being incorporated into the TiO2 lattice.46 Furthermore, the Cu 2p core level spectra of these materials are shown in Fig. 10.
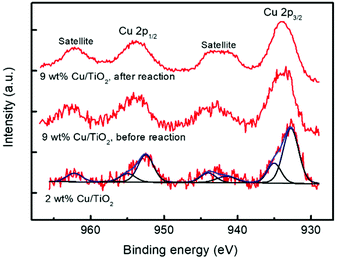 | ||
| Fig. 10 XPS spectra of Cu 2p for 2 wt% and 9 wt% Cu/TiO2 samples. Deconvolution into the main and satellite peaks is shown. | ||
The two catalysts present two peaks at 932.7 and 952.5 eV, both of them corresponding to reduced Cu0/Cu+, together with peaks at 935.0 and 954.9 eV (main peaks) and 941.5, 944.0 and 962.2 eV (satellites) due to Cu2+.47,48
The deconvolution into these peaks for the 2% Cu/TiO2 spectrum is shown in Fig. 10. The ratios of reduced to oxidized Cu are 1.6 and 0.7 for 2.0 and 9.0 wt% Cu/TiO2, respectively, and the last one decreases to 0.4 after the reaction. These results indicate that Cu is partially oxidized from the reduced Cu species to Cu2+ by the photocatalytic reaction. Short irradiation times were used when acquiring the Cu 2p spectra to avoid the photoreduction of the Cu species. For this reason, the Cu LMM signal is very noisy and the assigning of the oxidation state for the reduced copper species is not possible. The XPS spectra provide us with very valuable information since the characterization of the surface confirms the simultaneous presence of Cu+/Cu2+ species. The existence of these two species on the surface of the samples suggests that anionic vacancies are generated.
These vacancies could generate electronic gaps, which could determine the separation of charges, allowing greater methanol degradation with respect to the bare TiO2.
The Cu metal and Cu2O semiconductor have been reported as enhancers of the H2 production on TiO2 nanoparticles.24,47,49 These species are present in the catalysts investigated before the photocatalytic reaction and could be responsible for the large increase of the H2 production observed in our Cu/TiO2 catalysts, with respect to the bare TiO2. However, different authors have proposed that CuO clusters also increase the H2 production on TiO2.44,48 Therefore, the observed CuO clusters, which are enlarged by the photocatalytic reaction, could also contribute to the increased H2 production. CuO and Cu2O present band gaps, of 1.3 and 2.1 eV, shorter than TiO2, which lead to absorption at 950 and 590 nm, respectively.
Therefore, these semiconductors present absorption of visible light. Moreover, metallic Cu nanoparticles also present absorption in the visible range associated with surface plasmon resonance. Visible absorption by the different species of copper present on the samples would be responsible for the observed enhancement of the visible-light photoactivity.
The possible mechanisms for the redox reactions with photoreforming are as follows:19
| TiO2 + photon → e− + h+ | (1) |
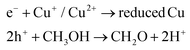 | (2) |
| 2e− + reduced Cu + 2H+ → H2 + reduced Cu | (3) |
The band gaps obtained for different Cu-containing tested materials are shown in Table 1. These were determined from the UV-Vis diffuse reflectance spectra (Fig. S2 and S3†).
| Cu/TiO2 | Band gap (eV) |
|---|---|
| P25 | 3.20 |
| P25-Cu0.1 | 3.12 |
| P25-Cu0.5 | 3.03 |
| P25-Cu1.5 | 3.05 |
| P25-Cu2 | 3.10 |
| P25-Cu3 | 3.05 |
| P25-Cu6 | 3.14 |
| P25-Cu9 | 3.16 |
The impregnation of the TiO2 structure with the Cu species led to the reduction of the band gap, achieving a lower value of 3.03 eV for the sample with 0.5 wt% of Cu.
In Fig. 11, a comparison of the photoluminescence spectra of bare titania and Cu/TiO2 photocatalysts with copper loadings of 2 and 9 wt% is reported. After irradiation at 340 nm, it is observed that the material displays a broad emission band centered at ca. 420 nm, regardless of whether the system contains Cu or not (Fig. 11). This level identified as Fermi level, which did not show any shift for doping effect, with an energy of 2.952 eV, was observed in the size control of Cu nanoparticles (Fig. 7).50 It can be noticed that when the Cu concentration is optimized at 2 wt%, the Fermi level shows higher energy as evidenced by the very intense band in terms of fluorescence emission, which can be converted into electrons photogeneration for higher H2 production.
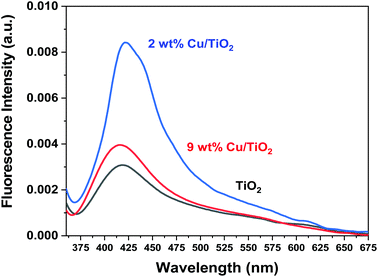 | ||
| Fig. 11 Photoluminescence spectra of the obtained bare TiO2 and titania modified with Cu (2 and 9 wt%), when excited at 340 nm. | ||
It has been reported in the literature that the hydrogen photoproduction using copper-based systems is strongly affected by the complex structural, morphological, and chemical features of copper species26 and that the occurrence of the highly dispersed Cu+/Cu2+ species is directly related to the higher performance of the system for the H2 production reaction.51,52
In our work, it seems that, in the 2 wt% Cu/TiO2 sample, both the optical properties and the structural-chemical properties contribute to enhancing the photocatalytic activity. This copper loading, besides displaying a much higher fluorescence band than the 9 wt% Cu/TiO2 sample, is also advantageous for the investigated reaction since an excessive coverage of the titania surface by the active phase has detrimental effects on the ability of TiO2 to photogenerate hydrogen. In fact, a too large amount of copper species could decrease the light absorption ability of TiO2 and partially block its sensitization.37 This, together with the fact that an excessive amount of metal particles may act as recombination centers,38 makes the sample containing 2 wt% of copper the most suitable system for hydrogen photogeneration under UV light irradiation.
Experimental
Preparation of M/TiO2 photocatalysts
M/TiO2 (M = Fe, Co, Ni, Cu, Zn) catalysts were prepared by the impregnation method. In a typical synthesis, a suitable amount of the M precursor of Fe(NO3)3·9H2O (≥98%), Co(NO3)2·6H2O (≥98%), Ni(NO3)2·6H2O (99.999%), Cu(NO3)2·3H2O (≥99%) and Zn(NO3)2·6H2O (98%), supplied by Sigma-Aldrich, was used without further purification. To obtain 2.0 wt%, the nitrate metal loading was weighed and dissolved in ethanol (1.0 mL). As for copper, the loading was varied between 0.1 and9.0 wt%. Each one of these solutions was well mixed with 0.3 g of the solid photocatalyst titanium dioxide (Degussa P25 TiO2), composed of rutile 20 wt% and anatase 80 wt%. As a result, a paste was formed, which was dried in an oven at 70 °C for 1 h and calcined at 350 °C for 8 h in a box furnace. Pt/TiO2, used for comparison purposes, was prepared as previously reported.28Photocatalytic hydrogen production
Photocatalytic experiments for hydrogen production were carried out in a novel and innovative confocal homemade system (Fig. 12). The reactor and lamps are located at the focus of an elliptic cylinder polished stainless-steel reflector. In this way, all the light coming from the lamp is reflected towards another focus, where the reactor is placed, achieving maximum radiation on the reactor. The reactor has a borosilicate double wall, which allows controlling the photocatalyst temperature by water circulation from a thermostatic bath. This setup includes two lamps: a UV lamp (Philips, HPA Synergy 300 W) and a visible lamp (Leuci, Classic Linear Halogen, 500 W), which can be switched on independently. A fan blade was placed beside the lamps to prevent overheating. In a typical photocatalytic experiment, 150 mL of water and 50 mL of MeOH (25 vol%), as a sacrificial reagent, were added into the reactor equipped with magnetic stirring. Besides, 0.2 g of photocatalyst were also added to study its efficiency for hydrogen production. The reactor temperature was fixed at 20 °C by a continuous water flux through the reactor jacket during the experiment.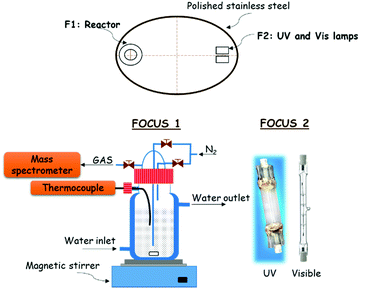 | ||
| Fig. 12 Scheme of the reaction system used for photocatalytic hydrogen production under UV and visible light irradiation at room temperature (20 °C) and atmospheric pressure. | ||
A continuous nitrogen flux of 13.2 mL min−1 through the reactor was fixed by a mass flow controller (Bronkhorst, EL-FLOW Select). For dynamical measurements of hydrogen production, a mass spectrometer Omnistar of Pfeiffer Vacuum Co. (Aßlar, Germany) was used that was connected in line with the reactor.
Characterization of Cu/TiO2 photocatalysts
The morphology of Cu/TiO2 photocatalysts was characterized using a transmission electron microscope (TEM 200 kV, Jeol JEM 2100). Prior to the measurement, the samples were dispersed in absolute ethanol followed by ultrasonication for 15 min. Then a drop of the resulting dispersion was placed on holey carbon coated copper TEM grids for analysis. XPS studies were carried out on a physical electronics spectrometer (PHI Versa Probe II Scanning XPS Microprobe) with monochromatic X-ray Al Kα radiation (100 μm, 100 W, 20 kV, 1486.6 eV) and a dual beam charge neutralizer. The spectrometer was calibrated using Cu 2p3/2, Ag 3d5/2, and Au 4f7/2 photoelectron lines at 932.7, 368.2 and 84.0 eV, respectively. Under a pass energy of 23.5 eV, the Au 4f7/2 line was recorded with 0.73 eV FWHM at a binding energy (BE) of 84.0 eV. The multiregion spectra were referenced with the C 1s signal of adventitious carbon at 284.8 eV. The recorded spectra were always fitted using Gauss–Lorentz curves. The atomic concentration percentages of the characteristic elements of the surfaces were determined considering the corresponding area sensitivity factor for the different measured spectral regions. Photoluminescence spectra were obtained by excitation of the TiO2 tablets with light from a 300 W Xe lamp passed through a 0.25 m double grating Spex monochromator. Fluorescence was detected using a 0.25 m Spex monochromator with a Hamamatsu R928 photomultiplier.Conclusions
M/TiO2 modified-photocatalysts (where M = Fe, Co, Ni, Cu and Zn) were prepared by an impregnation method in order to replace the expensive noble metals with other cheaper transition metals for hydrogen production under both UV and visible light irradiation at room temperature and atmospheric pressure. When these transition metal elements were added to a benchmark TiO2 (commercial P25), the maximum hydrogen production efficiency was achieved for the Cu/TiO2 photocatalyst, with ∼5000 μmol h−1 g−1 and ∼220 μmol h−1 g−1 H2 production rates for UV and visible irradiation, respectively. These values were much higher than those obtained with the bare TiO2 by factors of 16 and 3 for UV and visible light irradiation, respectively. Ni/TiO2 and Co/TiO2 also showed a significant photocatalytic activity when UV light was used. The best performing photocatalyst, Cu/TiO2, was further investigated by varying the copper amount from 0.1 to 9.0 wt% to identify the optimal cocatalyst loading. Two selected samples, containing 2.0 and 9.0 wt% of copper, were characterized by TEM and XPS. The results showed that Cu was highly dispersed over the TiO2 support and Cu species existed as both reduced Cu0/Cu+ and oxidized Cu2+ on the surface of the titania matrix. Besides, during the hydrogen production reaction the ratio of the reduced to oxidized Cu decreased, most probably due to the transfer of photogenerated holes under UV or visible light. In the case of 2 wt% Cu/TiO2 sample, both the optical properties, studied by photoluminescence, and the structural-chemical properties contribute to enhance the photocatalytic activity. A comparison was made with a Pt/TiO2 system, which is considered by far the most efficient cocatalyst on TiO2 for photocatalytic hydrogen production. However, it is worth noting that Cu/TiO2 achieved ∼80% of the H2 production rate of Pt/TiO2, suggesting the great potential of such a photocatalyst as a very promising low cost alternative to Pt-based systems for hydrogen production.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Ministerio de Economía y Competitividad of Spain (CTQ2015-68951-C3-3-R and MAT2016-80933-R), Ministerio de Ciencia, Innovación y Universidades of Spain (Project RTI2018-099668-B-C22) and FEDER funds.Notes and references
- I. Dincer and C. Acar, Review and evaluation of hydrogen production methods for better sustainability, Int. J. Hydrogen Energy, 2015, 40, 11094 CrossRef CAS.
- F. Yilmaz, M. Tolga Balta and R. Selbaş, A review of solar based hydrogen production methods, Renewable Sustainable Energy Rev., 2016, 56, 171 CrossRef CAS.
- H. Caliskan, I. Dincer and A. Hepbasli, Exergoeconomic and environmental impact analyses of a renewable energy based hydrogen production system, Int. J. Hydrogen Energy, 2013, 38, 6104 CrossRef CAS.
- I. Dincer, Exergetic and Sustainability Aspects of Green Energy Systems, Clean: Soil, Air, Water, 2007, 35, 311 CAS.
- J. D. Holladay, J. Hu, D. L. King and Y. Wang, An Overview of Hydrogen Production Technologies, Catal. Today, 2009, 139, 244 CrossRef CAS.
- S. E. Hosseini and M. A. Wahid, Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development, Renewable Sustainable Energy Rev., 2016, 57, 850–866 CrossRef CAS.
- S. Kitano, A. Tanaka, K. Hashimoto and H. Kominami, Metal ion-modified TiO2 photocatalysts having controllable oxidative performance under irradiation of visible light, Appl. Catal., A, 2016, 521, 202 CrossRef CAS.
- K. Chang, H. Xiao and Y. Jinhua, Transition Metal Disulfides as Noble–Metal–Alternative Co–Catalysts for Solar Hydrogen Production, Adv. Energy Mater., 2016, 6, 1502555 CrossRef.
- Q. Jin, M. Fujishima, A. Iwaszuk, M. Nolan and H. Tada, Loading Effect in Copper(II) Oxide Cluster-Surface-Modified Titanium(IV) Oxide on Visible- and UV-Light Activities, J. Phys. Chem. C, 2013, 117, 23848 CrossRef CAS.
- Q. Jin, H. Yamamoto, K. Yamamoto, M. Fujishima and H. Tada, Simultaneous induction of high level thermal and visible-light catalytic activities to titanium(iv) oxide by surface modification with cobalt(iii) oxide clusters, Phys. Chem. Chem. Phys., 2013, 15, 20313 RSC.
- O. Kerkez-Kuyumcu, E. Kibar, K. Dayıoglu, F. Gedic, A. N. Akın and S. Özkara-Aydınoglu, A comparative study for removal of different dyes over M/TiO2 (M = Cu, Ni, Co, Fe, Mn and Cr) photocatalysts under visible light irradiation, J. Photochem. Photobiol., A, 2015, 311, 176 CrossRef CAS.
- T. Hisatomi, J. Kubota and K. Domen, Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting, Chem. Soc. Rev., 2014, 43, 7520 RSC.
- Y. Yu, G. Chen, Y. Zhou and Z. Han, Recent advances in rare-earth elements modification of inorganic semiconductor-based photocatalysts for efficient solar energy conversion: A review, J. Rare Earths, 2015, 33, 453 CrossRef CAS.
- M. Ni, M. K. H. Leung, D. Y. C. Leung and K. Sumathy, A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production, Renewable Sustainable Energy Rev., 2007, 11, 401 CrossRef CAS.
- Y. Tamaki, A. Furube, M. Murai, K. Hara, R. Katoh and M. Tachiya, Dynamics of efficient electron–hole separation in TiO2nanoparticles revealed by femtosecond transient absorption spectroscopy under the weak-excitation condition, Phys. Chem. Chem. Phys., 2017, 9, 1453 RSC.
- K. Shimura and H. Yoshida, Heterogeneous photocatalytic hydrogen production from water and biomass derivatives, Energy Environ. Sci., 2011, 4, 2467 RSC.
- G. Colón, Towards the hydrogen production by photocatalysis, Appl. Catal., A, 2016, 518, 48 CrossRef.
- M. C. Wu, P. Y. Wu, T. H. Lin and T. F. Lin, Appl. Surf. Sci., 2018, 430, 390 CrossRef CAS.
- A. T. Montoya and E. G. Gillan, Enhanced Photocatalytic Hydrogen Evolution from Transition-Metal Surface-Modified TiO2, ACS Omega, 2018, 3, 2947 CrossRef CAS PubMed.
- K. K. Mandari, J. Y. Do, S. V. P. Vattikuti, A. K. R. Police and M. Kang, Solar light response with noble metal-free highly active copper(II) phosphate/titanium dioxide nanoparticle/copper(II) oxide nanocomposites for photocatalytic hydrogen production, J. Alloys Compd., 2018, 750, 292 CrossRef CAS.
- M. K. Kumar, J. Y. Do, P. A. K. Reddy and M. Kang, Natural solar light-driven preparation of plasmonic resonance-based alloy and core-shell catalyst for sustainable enhanced hydrogen production: Green approach and characterization, Appl. Catal., B, 2018, 231, 137 CrossRef.
- S. Velázquez-Martínez, S. Silva-Martínez, C. A. Pineda-Arellano, A. Jiménez-González, I. Salgado-Tránsito, A. A. Morales-Pérez and M. I. Peña-Cruz, Modified sol-gel/hydrothermal method for the synthesis of microsized TiO2 and iron-doped TiO2, its characterization and solar photocatalytic activity for an azo dye degradation, J. Photochem. Photobiol., A, 2018, 359, 93 CrossRef.
- M. Hinojosa-Reyes, R. Camposeco-Solís, R. Zanella and V. Rodríguez González, Hydrogen production by tailoring the brookite and Cu2O ratio of sol-gel Cu-TiO2 photocatalysts, Chemosphere, 2017, 184, 992 CrossRef CAS PubMed.
- Y. Liu, Z. Wang and W. Huang, Influences of TiO2 phase structures on the structures and photocatalytic hydrogen production of CuOx/TiO2 photocatalysts, Appl. Surf. Sci., 2016, 389, 760 CrossRef CAS.
- Z. Wang, K. Teramura, T. Shishido and T. Tanaka, Characterization of Cu Nanoparticles on TiO2 Photocatalysts Fabricated by Electroless Plating Method, Top. Catal., 2014, 57, 975 CrossRef CAS.
- A. Kubacka, M. J. Muñoz-Batista, M. Fernández-García, S. Obregón and G. Colón, Evolution of H2 photoproduction with Cu content on CuOx-TiO2 composite catalysts prepared by a microemulsion method, Appl. Catal., B, 2015, 163, 214 CrossRef CAS.
- D. Ni, H. Shen, H. Li, Y. Ma and T. Zhai, Synthesis of high efficient Cu/TiO2 photocatalysts by grinding and their size-dependent photocatalytic hydrogen production, Appl. Surf. Sci., 2017, 409, 241 CrossRef CAS.
- J. J. Velázquez, R. Fernández-González, L. Díaz, E. Pulido Melián, V. D. Rodríguez and P. Núñez, Effect of reaction temperature and sacrificial agent on the photocatalytic H2-production of Pt-TiO2, J. Alloys Compd., 2017, 721, 405 CrossRef.
- A. L. Linsebigler, G. Lu and J. T. Yates, Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- J. Si, L. Yu, Y. Wang, Z. Huang, K. Homewood and Y. Gao, Colour centre-controlled formation of stable sub-nanometer transition metal clusters on TiO2 nanosheet for high efficient H2 production, Appl. Surf. Sci., 2020, 511, 145577 CrossRef CAS.
- P. D. Tran, L. F. Xi, S. K. Batabyal, L. H. Wong, J. Barber and J. S. Loo, Enhancing the photocatalytic efficiency of TiO2 nanopowders for H2 production by using non-noble transition metal co-catalysts, Phys. Chem. Chem. Phys., 2012, 14, 11596 RSC.
- J. Si, Y. Wang, X. Xia, S. Peng, S. Xiao, L. Zhu, Y. Bao, Z. Huang and Y. Gao, Novel quantum dot and nano-sheet TiO2 (B) composite for enhanced photocatalytic H2-Production without Co-Catalyst, J. Power Sources, 2017, 360, 353 CrossRef CAS.
- J. Liu, G. Hodes, J. Yan and S. Liu, Metal-doped Mo2C (metal = Fe, Co, Ni, Cu) as catalysts on TiO2 for photocatalytic hydrogen evolution in neutral solution, Chin. J. Catal., 2021, 47, 205 CrossRef.
- K. C. Christoforidis and P. Fornasiero, Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply, ChemCatChem, 2017, 9, 1523 CrossRef CAS.
- G. D. Moon, J. B. Joo, I. Lee and Y. Yin, Decoration of size-tunable CuO nanodots on TiO2 nanocrystals for noble metal-free photocatalytic H2 production, Nanoscale, 2014, 6, 12002 RSC.
- J. Yu, X. Zhao and Q. Zhao, Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method, Thin Solid Films, 2000, 379, 7 CrossRef CAS.
- P. Khemthong, P. Photai and N. Grisdanurak, Structural properties of CuO/TiO2 nanorod in relation to their catalytic activity for simultaneous hydrogen production under solar light, Int. J. Hydrogen Energy, 2013, 38, 15992 CrossRef CAS.
- I. Rossetti, Hydrogen Production by Photoreforming of Renewable Substrates, ISRN Chem. Eng., 2012, 964936 Search PubMed.
- E. P. Melián, C. R. López, A. O. Méndez, O. G. Díaz, M. N. Suárez, J. M. Doña-Rodríguez, J. A. Navío and D. Fernández-Hevia, Hydrogen production using Pt-loaded TiO2 photocatalysts, Int. J. Hydrogen Energy, 2013, 38, 11737 CrossRef.
- M. C. Hidalgo, M. Maicu, J. A. Navío and G. Colón, Photocatalytic properties of surface modified platinised TiO2: Effects of particle size and structural composition, Catal. Today, 2007, 129, 43 CrossRef CAS.
- https://www.lme.com/ .
- E. Guo and L. Yin, Nitrogen doped TiO2-CuxO core-shell mesoporous spherical hybrids for high-performance dye-sensitized solar cells, Phys. Chem. Chem. Phys., 2015, 17, 563 RSC.
- S. Zhu, S. Liang, Y. Tong, X. An, J. Long, X. Fu and X. Wang, Photocatalytic reduction of CO2 with H2O to CH4 on Cu(I) supported TiO2 nanosheets with defective {001} facets, Phys. Chem. Chem. Phys., 2015, 17, 976 Search PubMed.
- W. T. Chen, V. Jovic, D. S. Waterhouse, H. Idriss and G. I. N. Waterhouse, The role of CuO in promoting photocatalytic hydrogen production over TiO2, Int. J. Hydrogen Energy, 2013, 38, 15036 CrossRef CAS.
- J. L. Cao, G. S. Shao, T. Y. Ma, Y. Wang, T. Z. Ren, S. H. Wu and Z. Y. Yuan, Hierarchical meso-macroporous titania-supported CuO nanocatalysts: preparation, characterization and catalytic CO oxidation, J. Mater. Sci., 2009, 44, 6717 CrossRef CAS.
- H. Tian, L. Hu, C. Zhang, W. Liu, Y. Huang, L. Mo, L. Guo, J. Sheng and S. Dai, Retarded Charge Recombination in Dye-Sensitized Nitrogen-Doped TiO2 Solar Cells, J. Phys. Chem. C, 2010, 114, 1627 CrossRef CAS.
- M. Jung, J. N. Hart, J. Scott, Y. H. Ng, Y. Jiang and R. Amal, Exploring Cu oxidation state on TiO2 and its transformation during photocatalytic hydrogen evolution, Appl. Catal., A, 2016, 521, 190 CrossRef CAS.
- J. Yu, Y. Hai and M. Jaroniec, Photocatalytic hydrogen production over CuO-modified titania, J. Colloid Interface Sci., 2011, 357, 223 CrossRef CAS PubMed.
- D. P. Kumar, N. L. Reddy, B. Srinivas, V. Durgakumari, V. Roddatis, O. Bondarchuk, M. Karthik, Y. Ikuma and M. V. Shankar, Stable and active CuxO/TiO2 nanostructured catalyst for proficient hydrogen production under solar light irradiation, Sol. Energy Mater. Sol. Cells, 2016, 146, 63 CrossRef.
- V. Subramanian, E. E. Wolf and P. V. Kamat, Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration, J. Am. Chem. Soc., 2004, 126, 4943 CrossRef CAS PubMed.
- J. M. Valero, S. Obregón and G. Colón, Active Site Considerations on the Photocatalytic H2 Evolution Performance of Cu-Doped TiO2 Obtained by Different Doping Methods, ACS Catal., 2014, 4, 3320 CrossRef CAS.
- K. Czelej, J. C. Colmenares, K. Jabłczyńska, K. Ćwieka, Ł. Werner and L. Gradoń, Sustainable hydrogen production by plasmonic thermophotocatalysis, Catal. Today, 2021 DOI:10.1016/j.cattod.2021.02.004.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qi01311k |
| This journal is © the Partner Organisations 2021 |