Synthesis and sequencing of informational poly(amino phosphodiester)s†
Ian
Roszak
a,
Laurence
Oswald
a,
Abdelaziz
Al Ouahabi
a,
Annabelle
Bertin
abc,
Eline
Laurent
a,
Olivier
Felix
 a,
Isaure
Carvin-Sergent
a,
Isaure
Carvin-Sergent
 d,
Laurence
Charles
d,
Laurence
Charles
 d and
Jean-François
Lutz
d and
Jean-François
Lutz
 *a
*a
aUniversité de Strasbourg, CNRS, Institut Charles Sadron UPR22, 23 rue du Loess, 67034 Strasbourg Cedex 2, France. E-mail: jflutz@unistra.fr
bBAM Federal Institute for Materials Research and Testing, Unter den Eichen 87, 12205 Berlin, Germany
cInstitute of Chemistry and Biochemistry − Organic Chemistry, Free University Berlin, Takustraße 3, 14195 Berlin, Germany
dAix Marseille Université, CNRS, UMR 7273, Institute of Radical Chemistry, 13397, Marseille Cedex 20, France
First published on 13th September 2021
Abstract
Sequence-defined poly(amino phosphodiester)s containing main-chain tertiary amines were synthesized by automated solid-phase phosphoramidite chemistry. These polymers were prepared using four monomers with different substituents. The formed polymers were characterized by HPLC and mass spectrometry. These methods evidenced preparation of molecularly-defined polymers. Furthermore, the presence of tertiary amines in the polymer backbones facilitates sequencing by tandem mass spectrometry.
The stepwise synthesis of sequence-defined polymers has recently received increasing attention in fundamental polymer science.1–4 Indeed, the precise control of comonomer sequences enables new macromolecular properties to be achieved. For instance, it allows preparation of synthetic informational polymers, in which comonomers are used as a molecular language.5 Such information-containing polymers are used in various applications,6 in which they are often decoded by a sequencing analytical method.7 We8–10 and others11–16 have reported examples of such oligomers and polymers. These macromolecules have a uniform chain-length (i.e. apparent polydispersity close to 1.0) and a perfectly controlled comonomer sequence. Yet, they are not necessarily stereoregular. Since informational polymers are often decoded by analytical methods that are not sensitive to tacticity (e.g. tandem mass spectrometry, MS/MS), racemic synthons can be used to construct them. For instance, our group has reported the synthesis of digital poly(phosphodiester)s (d-PPDE),17–19 in which a comonomer alphabet is used to write binary information.20 These polymers are prepared by solid-phase phosphoramidite polymer chemistry (PPC); a versatile method that can be used for synthesis of biological (i.e. DNA and RNA),21 abiological19,22–24 and bio-hybrid macromolecules.25–28 In all d-PPDE we have studied so far, the coded side-chains are attached to a main-chain carbon atom.19 In order to avoid the formation of atactic polymers, symmetric carbons were used in our initial design.17 Still, we have recently developed expanded alphabets, in which racemic building blocks were also used.29,30 Although these polymers can be accurately deciphered by MS/MS, the formed backbones contain asymmetric carbons and are therefore atactic. This could be more problematic for other sequencing methods such as nanopore sensing.31
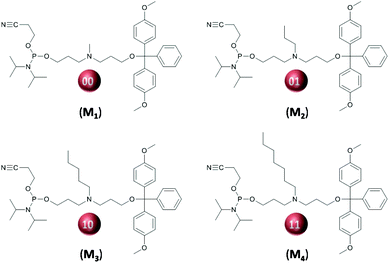
A convenient design to avoid tacticity issues is to install coded substituents on a main-chain nitrogen atom. This strategy is used for preparing achiral sequence-defined polymers such as peptide nucleic acids,32 peptoids33 and poly(N-substituted urethanes).34 Various amine-containing phosphoramidite monomers have been reported in the literature. Behr and coworkers have reported the synthesis of oligospermine by phosphoramidite chemistry.35,36 More recently, Sleiman and coworkers have described the synthesis of poly(phosphodiester)s containing main-chain tertiary amines that were used as a platform for side-chain functionalization.37 In the present work, a comparable design was applied for the first time to the synthesis of informational d-PPDE. Four phosphoramidite monomers containing main-chain nitrogen atom were synthesized and tested for the preparation of digitally-encoded poly(phosphodiester)s (Chart 1).
All the digital polymers investigated herein were synthesized by automated PPC on controlled pore glass solid supports.18 In this multistep synthesis, a cycle of four successive reactions, namely dimethoxytrityl (DMT) deprotection, phosphoramidite coupling, capping and oxidation, is repeated a certain number of times until a desired chain-length is reached. In order to store digital information in the polymer chains, a set of four phosphoramidite monomers was developed in this work (Chart 1). They all contain a main-chain nitrogen atom and alkyl side-chain substituents of increasing size, namely methyl (M1), propyl (M2), pentyl (M3) and heptyl (M4). Following our laboratory convention,19 monomers of increasing molar mass M1, M2, M3 and M4 represent binary dyads 00, 01, 10 and 11, respectively. Main-chain propyl spacers were intentionally placed between oxygen and nitrogen atoms in all these monomers. Attempts were also made with ethyl-based monomers that can be more easily derived from commercially-available products. However, as previously described,37 low yields and contaminated oligomers were obtained in these syntheses (data not shown). Table 1 and Scheme S1† list all the polymers that were synthesized in this work. At first homopolymers H1–H4 were synthesized in order to verify that all monomers M1–M4 can be efficiently used in PPC. In all cases, HPLC, ESI-MS and MS/MS measurements (Fig. S1–S4† and Table 1) indicated formation of uniform polymers with the expected chain-length and homopolymer sequence.
| Sequencea | m/zth | m/z | |
|---|---|---|---|
| a The letter T denotes a terminal thymidine that comes from the pre-loaded solid-support. b Detected as [M + 4H]4+. c Detected as [M + 3H]3+. d Detected as [M + 5H]5+. | |||
| H1 | (M1)10–T | 584.2341b | 584.2339 |
| H2 | (M2)10–T | 654.5631b | 654.5637 |
| H3 | (M3)10–T | 724.3906b | 724.3914 |
| H4 | (M4)10–T | 794.4688b | 794.4683 |
| P1 | (M2–M1)4–T | 676.6303c | 676.6305 |
| P2 | M1–(M2)3–(M1)4–T | 667.2865c | 667.2880 |
| P3 | M2–M1–(M2)2–M1–(M2)3–T | 695.3178c | 695.3181 |
| P4 | M1–(M2)4–(M1)6–(M2)4–M1–T | 763.3368d | 763.3375 |
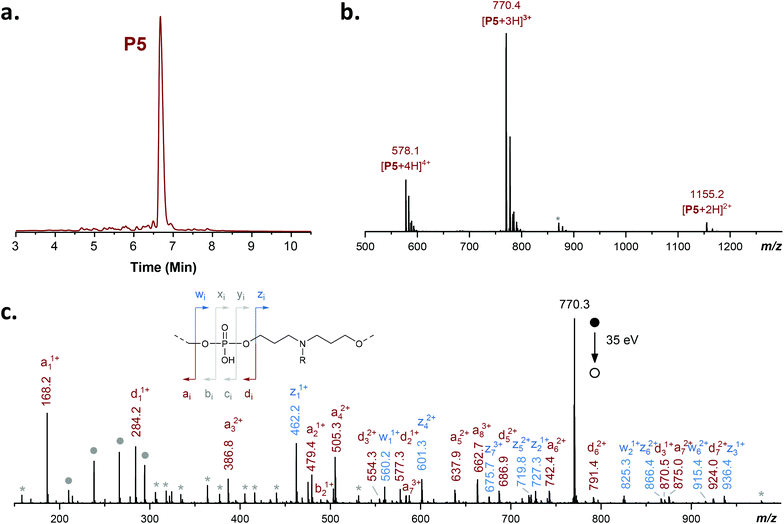
| P5 | M3–(M4)2–M2–M3–M1–M3–M2–T | 770.0680c | 770.0690 |
| P6 | (M4)2–M3–M1–M2–(M3)2–M4–T | 788.7555c | 788.7568 |
| P7 | (M1–M2–M3–M4)2–T | 751.3804c | 751.3813 |
| P8 | M1–M2–M3–(M4)2–M3–M2–M1–T | 751.3804c | 751.3819 |
Based on these promising results, different copolymers P1–P8 were synthesized and characterized (Table 1). Copolymers P1–P4 were synthesized using only comonomers M1 and M2, whereas P5–P8 were prepared using all four comonomers M1–M4. Overall, all copolymers appeared near-monodisperse, as evidenced by HPLC and ESI-MS (Fig. 1 and Fig. S5–S11†). Furthermore, all copolymers could be sequenced and decrypted by MS/MS (Fig. 1 and Fig. S5–S11†). While d-PPDE are usually exclusively sequenced by negative-mode MS/MS,38 the presence of a main-chain nitrogen atom in copolymers P1–P8 enables sequencing in both negative and positive mode. Moreover, it was found that dissociation of protonated species formed in the latter mode is highly beneficial to sequencing compared to the former one. Indeed, as schematized in the inset of Fig. 1c and detailed in Fig. S12,† phosphate repeat units usually lead to eight fragment ion series once deprotonated in the negative ion mode.38 Yet, in positive mode MS/MS, four of them (noted in grey in the inset) are either weakly or not detected. As a consequence, signal dilution is minimized and the other four series are detected with an unusually high intensity, thus allowing a perfect sequence coverage. As compared to conventional negative mode (Fig. S12†), it permits to envisage accurate sequencing of poly(amino phosphodiester)s from data recorded at very low concentrations or with very short acquisition times.
In summary, digitally-encoded poly(amino phosphodiester)s were successfully synthesized and decoded by MS/MS. The presence of a main-chain nitrogen atom in these informational copolymers brings substantial advantages. First of all, it is a convenient site for side-chain functionalization. In addition, it enables positive mode MS analysis, thus facilitating sequencing. Last but not least, such polymers, containing both main chain phosphates and tertiary amines, are potential polyampholytes and could therefore be useful for electrostatic self-assembly.39
Conflicts of interest
There are no conflicts to declare.Notes and references
- J.-F. Lutz, M. Ouchi, D. R. Liu and M. Sawamoto, Science, 2013, 341, 1238149 CrossRef PubMed.
- T. T. Trinh, C. Laure and J.-F. Lutz, Macromol. Chem. Phys., 2015, 216, 1498–1506 CrossRef CAS.
- S. C. Solleder, R. V. Schneider, K. S. Wetzel, A. C. Boukis and M. A. R. Meier, Macromol. Rapid Commun., 2017, 38, 1600711 CrossRef PubMed.
- P. Nanjan and M. Porel, Polym. Chem., 2019, 10, 5406–5424 RSC.
- J.-F. Lutz, Macromolecules, 2015, 48, 4759–4767 CrossRef CAS.
- M. A. R. Meier and C. Barner-Kowollik, Adv. Mater., 2019, 31, 1806027 CrossRef PubMed.
- H. Mutlu and J.-F. Lutz, Angew. Chem., Int. Ed., 2014, 53, 13010–13019 CrossRef CAS PubMed.
- T. T. Trinh, L. Oswald, D. Chan-Seng and J.-F. Lutz, Macromol. Rapid Commun., 2014, 35, 141–145 CrossRef CAS PubMed.
- R. K. Roy, A. Meszynska, C. Laure, L. Charles, C. Verchin and J.-F. Lutz, Nat. Commun., 2015, 6, 7237 CrossRef CAS PubMed.
- U. S. Gunay, B. E. Petit, D. Karamessini, A. Al Ouahabi, J.-A. Amalian, C. Chendo, M. Bouquey, D. Gigmes, L. Charles and J. F. Lutz, Chem, 2016, 1, 114–126 CAS.
- A. C. Boukis and M. A. R. Meier, Eur. Polym. J., 2018, 104, 32–38 CrossRef CAS.
- S. Martens, A. Landuyt, P. Espeel, B. Devreese, P. Dawyndt and F. Du Prez, Nat. Commun., 2018, 9, 4451 CrossRef PubMed.
- J. O. Holloway, F. Van Lijsebetten, N. Badi, H. A. Houck and F. E. Du Prez, Adv. Sci., 2020, 7, 1903698 CrossRef CAS PubMed.
- J. M. Lee, M. B. Koo, S. W. Lee, H. Lee, J. Kwon, Y. H. Shim, S. Y. Kim and K. T. Kim, Nat. Commun., 2020, 11, 56 CrossRef CAS PubMed.
- B. Liu, Q. Shi, L. Hu, Z. Huang, X. Zhu and Z. Zhang, Polym. Chem., 2020, 11, 1702–1707 RSC.
- K. S. Wetzel, M. Frölich, S. C. Solleder, R. Nickisch, P. Treu and M. A. R. Meier, Commun. Chem., 2020, 3, 63 CrossRef CAS.
- A. Al Ouahabi, L. Charles and J.-F. Lutz, J. Am. Chem. Soc., 2015, 137, 5629–5635 CrossRef CAS PubMed.
- A. Al Ouahabi, M. Kotera, L. Charles and J.-F. Lutz, ACS Macro Lett., 2015, 4, 1077–1080 CrossRef CAS.
- L. Charles and J.-F. Lutz, Acc. Chem. Res., 2021, 54, 1791–1800 CrossRef CAS PubMed.
- H. Colquhoun and J.-F. Lutz, Nat. Chem., 2014, 6, 455–456 CrossRef CAS PubMed.
- M. H. Caruthers, Science, 1985, 230, 281–285 CrossRef CAS PubMed.
- N. Appukutti and C. J. Serpell, Polym. Chem., 2018, 9, 2210–2226 RSC.
- M. Vybornyi, Y. Vyborna and R. Häner, Chem. Soc. Rev., 2019, 48, 4347–4360 RSC.
- N. Appukutti, J. R. Jones and C. J. Serpell, Chem. Commun., 2020, 56, 5307–5310 RSC.
- T. G. W. Edwardson, K. M. M. Carneiro, C. J. Serpell and H. F. Sleiman, Angew. Chem., Int. Ed., 2014, 53, 4567–4571 CrossRef CAS PubMed.
- T. Mondal, M. Nerantzaki, K. Flesch, C. Loth, M. Maaloum, Y. Cong, S. S. Sheiko and J.-F. Lutz, Macromolecules, 2021, 54, 3423–3429 CrossRef CAS.
- C. Loth, L. Charles, J.-F. Lutz and M. Nerantzaki, ACS Macro Lett., 2021, 10, 481–485 CrossRef CAS.
- M. Nerantzaki, C. Loth and J.-F. Lutz, Polym. Chem., 2021, 12, 3498–3509 RSC.
- E. Laurent, J.-A. Amalian, M. Parmentier, L. Oswald, A. Al Ouahabi, F. Dufour, K. Launay, J.-L. Clément, D. Gigmes, M.-A. Delsuc, L. Charles and J.-F. Lutz, Macromolecules, 2020, 53, 4022–4029 CrossRef CAS.
- E. Laurent, J. A. Amalian, T. Schutz, K. Launay, J.-L. Clément, D. Gigmes, A. Burel, C. Carapito, L. Charles, M.-A. Delsuc and J. F. Lutz, C. R. Chim., 2021, 24, 69–76 CrossRef.
- C. Cao, L. F. Krapp, A. Al Ouahabi, N. F. König, N. Cirauqui, A. Radenovic, J.-F. Lutz and M. D. Peraro, Sci. Adv., 2020, 6, eabc2661 CAS.
- M. Egholm, O. Buchardt, P. E. Nielsen and R. H. Berg, J. Am. Chem. Soc., 1992, 114, 1895–1897 CrossRef CAS.
- J. Sun and R. N. Zuckermann, ACS Nano, 2013, 7, 4715–4732 CrossRef CAS PubMed.
- T. Mondal, V. Greff, B. É. Petit, L. Charles and J.-F. Lutz, ACS Macro Lett., 2019, 8, 1002–1005 CrossRef CAS.
- E. Voirin, J.-P. Behr and M. Kotera, Nat. Protocols, 2007, 2, 1360–1367 CAS.
- R. Noir, M. Kotera, B. Pons, J.-S. Remy and J.-P. Behr, J. Am. Chem. Soc., 2008, 130, 13500–13505 CrossRef CAS PubMed.
- D. de Rochambeau, Y. Sun, M. Barlog, H. S. Bazzi and H. F. Sleiman, J. Org. Chem., 2018, 83, 9774–9786 CrossRef CAS PubMed.
- J. A. Amalian, A. Al Ouahabi, G. Cavallo, N. F. König, S. Poyer, J. F. Lutz and L. Charles, J. Mass Spectrom., 2017, 52, 788–798 CrossRef CAS PubMed.
- R. Szweda, M. Tschopp, O. Felix, G. Decher and J.-F. Lutz, Angew. Chem., 2018, 130, 16043–16047 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1py01052b |
| This journal is © The Royal Society of Chemistry 2021 |